Abstract
Endotoxins are high-molecular-weight complexes that contain lipopolysaccharide, protein, and phospholipid originating from the outer membrane of gram-negative bacteria. As gram-negative bacteria are naturally present in a variety of sources, endotoxins are commonly identified as contaminants in manufacturing environments. In industrial applications, endotoxin often is considered difficult to inactivate and to have a strong affinity with surfaces resulting from its hydrophobic chemical structure. This article describes the investigation of the true affinity of endotoxin, from various microbial sources in solution, for medical device material surfaces. In addition, endotoxin reduction was investigated with commonly used sterilization methods such as those based on ionizing radiation, dry and moist heat, and ethylene oxide sterilization. Endotoxin activity was found to be reduced following exposure to a range of sterilization modalities with the degree of activity reduction related to the source of endotoxin and the substrate material upon which it was present.
This research examined the affinity of bacterial endotoxins from various sources in water for the surfaces of representative medical device materials. The degree of affinity can be used to assess the risk of endotoxin remaining on a medical device after final cleaning and packaging. To further understand this risk, the effects of several common sterilization modalities were examined to determine the reduction in toxicity of bacterial endotoxin (if any) resulting from these processes.
Review of Literature
Bacterial endotoxins are the most prevalent microbial pyrogens.1 Their source is from the gram-negative bacterial cell wall. When introduced directly into blood or tissue, endotoxin can cause fever,2 meningitis, and decreased blood pressure at high doses (estimated to be ≥350 endotoxin units [EU] in a typical human [at 70 kg or ~5 EU/kg), though lower levels often are conservatively defined for certain applications (e.g., intrathecal exposure).3 The immune reaction includes an initial alarm system for infection, recognition by toll-like receptors (e.g., TLR4) on innate immune cells,4 and cytokine secretion (e.g., interleukin [IL]-1 and IL-8) to stimulate the immune system, but it is self-regulated. Higher concentrations can lead to signs of fever and inflammation. Exposure to low concentrations is known to lead to immune tolerance within the host initial alarm response, mild inflammation reaction and resolution, and increased neutrophil activity (phagocytosis).5
Although traditionally considered to be the lipopolysaccharide (LPS) component of the outer membrane of the gram-negative bacteria cell wall, the structure of endotoxin actually is more complex and may be defined as a high-molecular-weight complex that contains LPS, protein, and phospholipid originating from the outer membrane of gram-negative bacteria.6 In addition, humans are naturally exposed to endotoxin from various sources, including direct surface and airborne contact, as well as through the gut microbiome.7
The purpose of this article is to consider bacterial endotoxins in more detail from a chemistry perspective. This allows for the examination of the composition and structure of the bacterial endotoxin molecule and its expected properties when in contact with medical device materials.
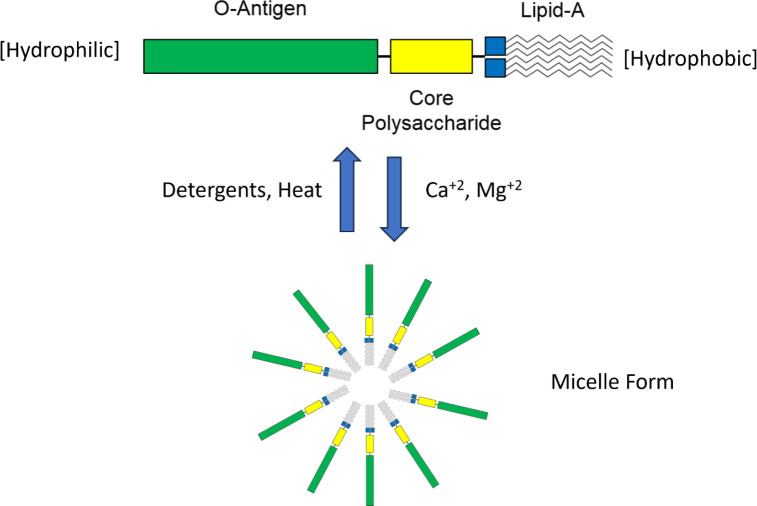
Bacterial endotoxin is associated with the outer cell wall of gram-negative bacteria, and a major component of endotoxin is the amphiphilic (or amphipathic) LPS molecule (Figure 1). The molecule consists of a hydrophobic lipid portion, as well as a hydrophilic polysaccharide chain and a hydrophilic O-antigenic oligosaccharide side chain. The molecular weight of LPS is in the range of 10 to 20 kDa,8 depending on its source. The lipid A core is made up of a β-glucosamine-(1–6)-glucosamine-1-phosphate base with fatty acid esters attached to both carbohydrates. The acyl chain length and number of acyl groups may vary among bacterial species but are constant within a given species. The inner polysaccharide core typically contains between one and four molecules of KDO (3-deoxy-α-D-manno-octulosonic acid) attached to the disaccharide core.9 Because LPS is heterogeneous and tends to form aggregates (micelles) of varying sizes, the molecular mass range for these aggregates has been reported as 1 to 4 million Dalton or greater (Figure 1). For example, when bacterial endotoxin is treated with sodium dodecyl sulfate and heat, the molecular mass was reported to be approximately 50 to 100 kDa.10
Figure 1.
Representation of the structure of lipopolysaccharide (monomer) and formation of a micelle structure.
Many materials are used to fabricate medical devices. Their selection is based on two primary requirements: ability to perform the intended function and biocompatibility (i.e., not causing harm to the patient). Materials of choice can be metallic, polymeric, ceramic, or a combination (in the case of composite devices). Design considerations include strength, durability, stability, and, as importantly, manufacturability. In most cases, the manufacturing process results in contact between manufacturing materials or process aids and the substrate material of which the device is made. Examples of manufacturing materials or process aids include but are not limited to lubricants, coolants, abrasives, chemical surface finishing agents, and cleaning agents. As an example of the regulatory requirement: If such manufacturing materials could negatively affect product quality, procedures should be defined and maintained to remove them, and this should be documented.11
Gram-negative bacteria often are associated with water.12 Many manufacturing materials are water based or contain substantial amounts of water in the formulation. The combination of water and a source of carbon (as most of the solutions are organic) presents an opportunity for the colonization and propagation of bacteria, including the endotoxin-featuring gram-negative types. Water, particularly final-rinse water, is a potential source of bacterial endotoxin if not well controlled. This material exposure constitutes a vector for endotoxin to come in contact with medical devices during the manufacturing process. For these reasons, regulatory guidelines and requirements are provided for best practices in monitoring the risks of endotoxins in manufacturing environments. For example, Food and Drug Administration guidance for pyrogen and endotoxins testing suggests that endotoxin limits on medical devices depend on the intended use and contact of the device with various tissues, such as blood and cerebrospinal fluid and during device implantation.13,14 Using the methods defined in the guidance, limits of 0.5 EU/mL in a recommended extraction volume of 40 mL or 20 EU/device are recommended for products that directly or indirectly contact the cardiovascular system. Lower levels are defined for devices in contact with cerebrospinal fluid, at 0.06 EU/mL (in a similar extraction volume) or 2.15 EU/device.
Bacterial endotoxin is an amphipathic molecule (i.e., possesses both hydrophilic and hydrophobic domains). As such, there is an expectation that the interaction or affinity of this molecule for different medical device surfaces may not be intuitively obvious and that generalizations regarding expected behavior could be entirely incorrect. This points to the need for empirical evidence of the affinity behavior to determine the actual characteristics of bacterial endotoxin in the presence of an aqueous environment and the medical device material.
The polysaccharide portions of the molecule are thought to be difficult to assess regarding their surface energy.15 Hydrophilicity is thought to be governed primarily by the presence of hydroxyl groups on the polysaccharide moieties on this portion of the molecule. Of note, their presentation to the environment is sensitive to the nature of the solution in which they reside. This tertiary or quaternary structural rearrangement can profoundly affect the nature of the surface energetics of this portion of the molecule. Another contributor in this region of the molecule is the presence of weak acidic groups in the composition. Overall, however, the presence of these groups and the hydroxyls will contribute to the hydrophilic behavior of this portion of the molecule.
In contrast, the lipid portion of the molecule demonstrates marked hydrophobicity. Lipid A consists of aliphatic hydrocarbon chains. This portion of the molecule does not favor water, and as such, when bacterial endotoxin is suspended in water, aggregation—often regarded as micellization—occurs wherein the bacterial endotoxin molecules in solution arrange themselves16 to the lowest energy configuration (Figure 1). In this configuration, the polar polysaccharide groups face outward into the aqueous environment while the lipid portions face inward, away from the water. Disruption of this arrangement can occur for many reasons. A change in the nature of the environment from water to a less polar solution will cause the spontaneous rearrangement of the molecules to a more favorable configuration with the lowest overall energy.
We have found that the formation of such micelles can be observed spectrophotometrically. A surface may also attract and potentially retain the bacterial endotoxin molecule based on the surface energy of the substrate. As an example, LPS affinity for titanium biomaterials may be considered a function of surface energy primarily. For LPS to adhere, the implant biomaterials must exhibit greater surface energies than LPS.17 Surface energy is the work per-unit area done by the force that creates a new surface. Of note, regardless of the surface energy of the substrate, the amphipathic bacterial endotoxin molecule can attain an energetically favorable orientation. The degree to which this interaction can result in strongly retained bacterial endotoxin to a material surface is the subject of the present experiments.
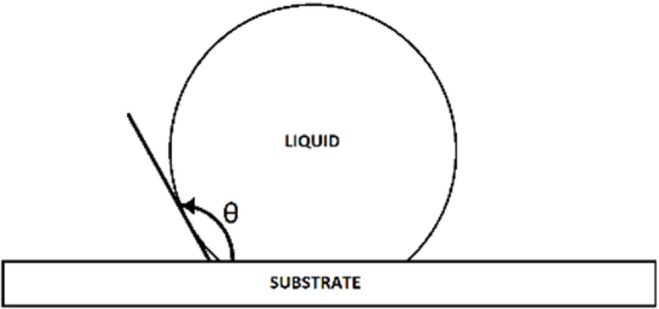
Medical device materials are selected based on their intended use and type of patient tissue contact, as well as whether they are single use or reusable. As such, a wide variety of materials are in common use. The chemical properties of medical device materials, particularly their surface energy, depend on their composition and surface condition. Of note, much of the literature in this regard represents materials prepared experimentally in pristine conditions. This may not effectively represent medical device materials, for which trace amounts (though technically clean by manufacturer standards) of organic residue are present. It has long been known that even insignificant amounts of hydrophobic contamination can increase the contact angles of metals (e.g., stainless steel, aluminum, titanium) considerably.18 The water contact angle (advancing; Figure 2) was determined to be between 3° and 42° for cleaned type 301 stainless steel, between 0° and 29° for cleaned 6061T6 aluminum alloy, and between 7° and 34° for cleaned grade 6 titanium. These contact angles represent very hydrophilic surfaces. A surface is hydrophobic when its static water contact angle is more than 90° and hydrophilic when the contact angle is less than 90°.19
Figure 2.
Representation of the contact angle (θ) between a liquid and associated surface.
For organically contaminated surfaces (e.g., with proteins or other carbon-based materials), the contact angles were noted to be much higher (into the range of hydrophobic surfaces). The water contact angle (advancing) was determined to be between 94° and 108° for soiled type 301 stainless steel, between 96° and 112° for 6061T6 aluminum alloy, and between 90° and 103° for grade 6 titanium. These contact angle results represent hydrophobic surfaces in the soiled condition. Due to the negative charge of the phosphate groups and the hydrophobicity of the LPS molecule, endotoxins will readily interact with cationic (positively charged) or hydrophobic materials.20 As an example, active endotoxin capture for clinical dialysis applications is performed using membranes prepared from polyether polymer alloys or polymethylmethacrylate. In these applications, the affinity for endotoxin depends on the high water contact angle, with efficient adsorption only on the hydrophobic side of the membrane.21
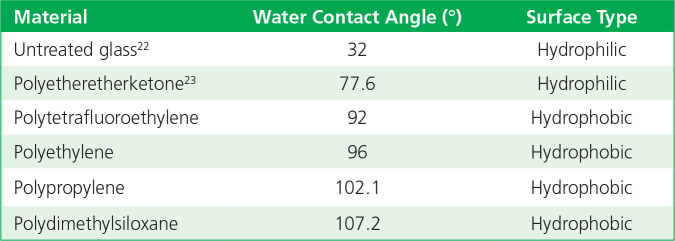
For polymer substrates used in medical devices, the general trend is for the material to be hydrophobic, thereby resulting in higher contact angles. For example, contact angles (Table 1) are reported24 for untreated clean glass and common polymers.
Table 1.
Contact angles of materials.
Based on the chemistry and the previously described experimental data for contact angles of common medical device materials, this section will discuss the nature of affinity and binding based on the type of molecular attraction associated with the materials and bacterial endotoxin. In contrast to bacterial endotoxin hydrophobic affinity, the basis of hydrophilic affinity is hydrogen bonding. Hydrogen bonding is a special type of dipole-dipole attraction between molecules rather than a covalent bond to a hydrogen atom. It results from the attractive force between a hydrogen atom covalently bonded to a very electronegative atom, such as a nitrogen, oxygen, or fluorine atom. Hydrogen bond strengths range from 4 to 50 kJ/mol of hydrogen bonds. An hydrogen atom in one molecule is electrostatically attracted to the nitrogen, oxygen, or fluorine atom in another molecule.25 For the case of many medical device materials, an abundance of oxygen atoms needs to be present at the surface of metals, as well as some polymers, for this behavior to be exhibited (e.g., the oxygen in the ether linkages in polyetheretherketone [PEEK], as well as the additional oxygen added during plasma treatment26,27 to improve wettability). The fundamental attraction is between the δ– associated with the oxygen atom and δ+associated with the hydrogen atom. δ+ and δ–, which are known as partial charges and more commonly called net atomic charges, result from the asymmetric distribution of electrons in chemical bonds.28
For the case of hydrophobic interactions, such as those occurring between the lipid portion of bacterial endotoxin and a hydrophobic surface in water, strong attraction is observed.29 Hydrophobic aggregation begins with a step in which water-coated nonpolar solutes approach one another due to longrange electrostatic forces.30 As such, the affinity of bacterial endotoxin for a hydrophobic surface is expected to be greater based on the lipophilic portion of the molecule and a nonpolar surface. Of note, wettability has the same definition as hydrophilicity, meaning a water contact angle less than 90°. Conversely, a non–water-wettable surface is the same as hydrophobicity, exhibiting a contact angle greater than 90°.31
Conceptual Framework
To investigate the affinity of bacterial endotoxin for material surfaces, these materials were incubated in aqueous preparations of bacterial endotoxin at approximately 100 EU/mL, followed by extraction in water for injection (WFI) and analysis of the extractable endotoxin by limulus amoebocyte lysate (LAL) assay.
Similarly, the effect of different sterilization modalities on the inactivation of bacterial endotoxin applied to the surface of medical device materials was also examined following spiking of the test surfaces with bacterial endotoxin to achieve approximately 1,000 EU/device, then exposing them to different sterilization methods. The LAL assay was also performed on extracts of the test devices to determine the level of bacterial endotoxin activity following sterilization.
Design and Methods
For the affinity portion of the study, four representative implant-grade medical device materials were selected for evaluation:
316L stainless steel (passivated)
Titanium aluminum niobium alloy (Ti-6Al-7Nb; anodized)
PEEK
Ultra-high-molecular-weight polyethylene (UHMWPE)
The test samples were cleaned prior to the experiment with 70% 2-propanol followed by WFI to reduce native levels of contamination and endotoxin. The negative controls were similarly cleaned to establish the baseline level of endotoxin. All tests were performed in triplicate.
Two endotoxins were selected for the study:
Control standard endotoxin (CSE) (catalog no. E0005, lot 174; Associates of Cape Cod, Falmouth, MA)
Escherichia coli O55:B5 endotoxin (EC) (product no. L2637, batch no. 049M4095V; Sigma-Aldrich, St. Louis, MO).
The incubation solutions were prepared (based on the endotoxin supplier’s stated concentration) to approximately 100 EU/mL. The endotoxin unit indicates endotoxicity. An endotoxin unit is the endotoxin activity of 0.2 ng reference standard endotoxin (EC-2) or 5 EU/ng. To convert the current reference standard endotoxin (EC-6) from endotoxin units to nanograms, the conversion is 10 EU/ ng. Depending on the source of the endotoxin, the conversion from endotoxin units to nanograms will vary. Previous versions of the bacterial endotoxin test stated a suitable CSE has potency between 2 and 50 EU/ng.32 Following manufacturer’s recommendations, a CSE-based standard curve was generated and used for LAL EU/mL assay determinations. The Pyros Kinetix Flex instrument (Associates of Cape Cod) was used for LAL turbidimetric analysis following manufacturer’s instructions, and the limit of detection was 0.005 EU/mL.
Incubation solutions (100 EU/mL) were prepared from each stock solution and used to completely immerse the designated test samples. The test samples were placed onto a shaking platform within a 37°C ± 2°C incubator and incubated for 1 hour at approximately 120 rpm. Following incubation, the test samples were removed, rinsed with WFI to remove unbound bacterial endotoxin, placed in a sterile container, and dried in a 37°C ± 2°C incubator overnight. The postincubation solution was retained and evaluated using the LAL turbidimetric assay to determine if any endotoxin reduction was detectable following test sample incubation.
Aliquots of the incubation solution were obtained prior to sample exposure and evaluated using the LAL turbidimetric assay to determine the initial concentrations of endotoxin to serve as an experimental control. It is noted that the LAL assay is designed to perform best with CSE as the source of endotoxin. For other endotoxins, such as from EC used in this experiment, some variation in the response of the assay was expected.
The dried samples were placed in water free of detectable endotoxin, placed onto a shaking platform within a 37°C ± 2°C incubator, and extracted for 1 hour at 120 rpm. The ability of this method to extract endotoxin was previously validated in our laboratory (results not shown). The extracted sample solutions then were evaluated using the LAL turbidimetric assay.
For the evaluation of the effects of sterilization on detectable bacterial endotoxin, the following set of representative medical device materials were used:
M6–1.0 × 16 mm machine screws, six-lobe (Torx), pan head, 316 stainless steel (A4), ISO 14583 part no. 593260 (www.fastenersuperstore.com)
Glass microscope slides, item WL8117A (VWR International)
Cylindrical, socket head cap screw, no. 4-40, nylon, not graded, plain, 0.5 in length (Grainger)
Plastic press-fit binding barrels and screws, polyethylene, no. 90249A640 (McMaster-Carr)
M6 flanged 6 point drilled hex titanium bolt, 6Al4V/GR5 size: M6–1.0 × 10 mm length, SKU 126-00413-0510 (Ticon Industries)
All tests were performed at least in triplicate. The test material samples were inoculated directly with approximately 1,000 EU per device and dried overnight at ambient temperature. In addition to CSE and EC, a third endotoxin was examined: Pseudomonas aeruginosa PA10 (PA) (product no. L8643; Sigma-Aldrich). The devices then were appropriately packaged and sent for sterilization by the following methods:
Moist heat (autoclave, 134°C, 6 min, excluding polymers; Johnson & Johnson Microbiological Quality Sterility Assurance [JJMQSA] Raritan Services, Raritan, NJ)
Dry heat (200°C, 60 min, excluding polymers and glass; JJMQSA Raritan Services)
Electron beam (e-beam; 5 MeV and 40 kGy except for nylon [10 kGy]; Synergy Health AST, LLC, a STERIS Company, Petaluma, CA)
Ethylene oxide (EO; ~280 min dwell time, 53°C, 545 mg/L; JJMQSA Raritan Services)
The sterilized samples then were analyzed using the LAL assay and the results compared with noninoculated, unsterilized materials and inoculated materials that were not sterilized. Prior to analysis, all samples were placed in a suitable sterile container containing water free of detectable endotoxin and extracted as described previously at 37°C ± 2°C for 1 hour at 120 rpm. LAL analysis then was performed on the extracted sample solutions and log reductions determined from inoculated, unsterilized controls for each material.
Results
Postincubation endotoxin-containing solutions, following material exposure, were examined to determine the potential amount of endotoxin removed from the incubation solution due to material affinity. The starting concentrations in each case were targeted to be approximately 100 EU/ mL, but the actual levels determined in control samples were approximately 60 EU/ mL. The starting concentration endotoxin levels for CSE were similar for samples exposed to metallic and polymeric samples. The levels were not statistically different from those of the control solution, indicating extraordinarily little uptake of CSE by the test samples.
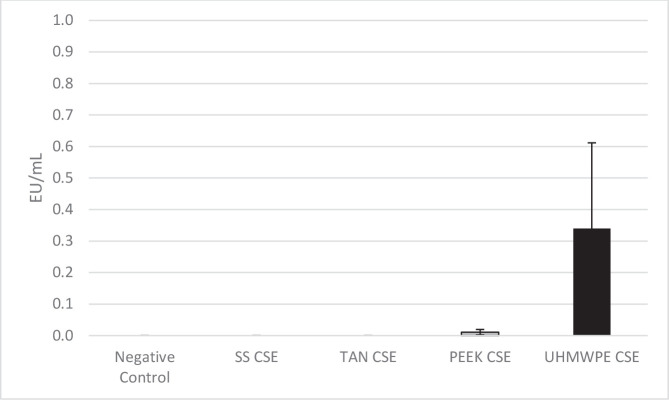
The results of the CSE extraction from the test surfaces are shown in Figure 3. For the negative control, stainless steel, and titanium alloy samples, the CSE levels of the extracts were reported as nondetected. Both PEEK and UHMWPE samples exhibited very minor levels of extractable CSE with UHMWPE, exhibiting the greatest amount of CSE and the most variability.
Figure 3.
Levels of control standard endotoxin (CSE) on each material type following incubation and extraction. Abbreviations used: PEEK, polyetheretherketone; SS, 316L stainless steel; TAN, titanium aluminum niobium alloy; UHMWPE, ultra-high-molecular-weight polyethylene.
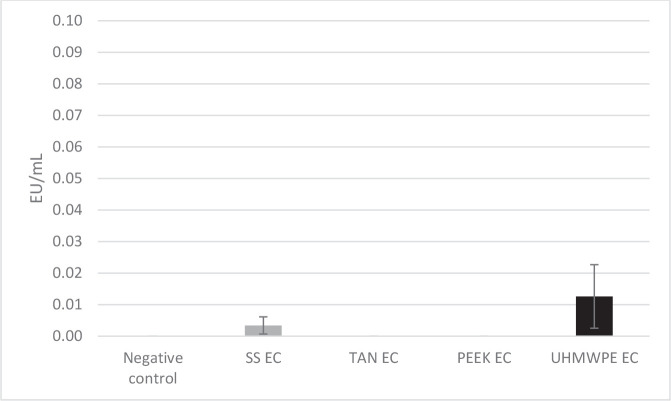
The second part of this experiment looked at affinity of a different source of endotoxin (EC) for the same substrates under the same testing conditions. In this case, the starting amounts in each case were also targeted to be approximately 100 EU/mL but were found to be higher than the estimate at 149.3 EU/ mL. No statistical difference was observed compared with the control solution, again suggesting little uptake of EC by the test substrates. This result was confirmed by the extraordinarily low levels of EC extracted from the test devices (Figure 4). The negative control, titanium alloy, and PEEK samples were reported as nondetected, while the stainless steel result was determined to be 0.003 EU/mL and the UHMWPE result reported as 0.013 EU/mL.
Figure 4.
Levels of Escherichia coli O55:B5 endotoxin (EC) on each material type following incubation and extraction. Abbreviations used: PEEK, polyetheretherketone; SS, 316L stainless steel; TAN, titanium aluminum niobium alloy; UHMWPE, ultra-high-molecular-weight polyethylene.
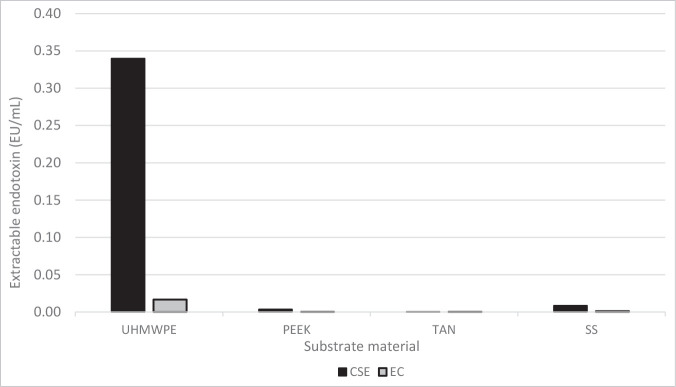
An overall comparison of the affinity of the various medical device materials for endotoxins is shown in Figure 5.
Figure 5.
Experimental affinity of bacterial endotoxin for different medical device materials. Abbreviations used: CSE, control standard endotoxin; EC, Escherichia coli O55:B5 endotoxin; PEEK, polyetheretherketone; SS, 316L stainless steel; TAN, titanium aluminum niobium alloy; UHMWPE, ultra-high-molecular-weight polyethylene.
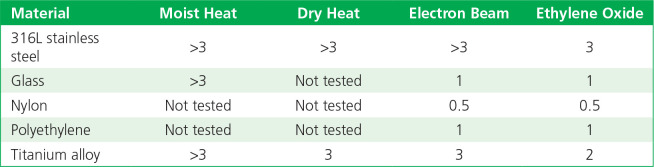
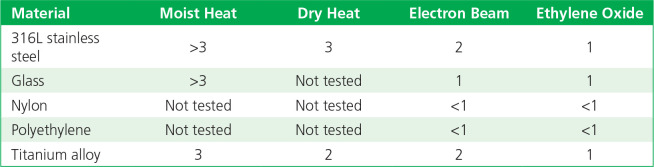
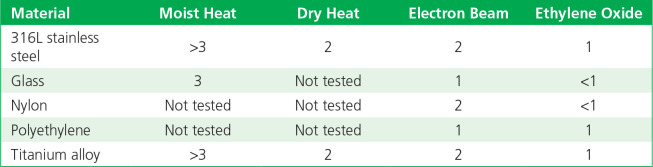
The log reductions of detectable bacterial endotoxin (from three different sources) when inoculated onto five types of materials following sterilization by different methods, as compared with controls, are summarized in Tables 2 to 4. Moist heat and dry heat generally were consistent in inactivating endotoxin, with more variable levels of inactivation found for radiation (e-beam) and EO gas. The results with e-beam and EO ranged depending on the material and endotoxin source.
Table 2.
Log10 reductions of endotoxin on exposure to sterilization: control standard endotoxin.
Table 4.
Log10 reductions of endotoxin on exposure to sterilization: Pseudomonas aeruginosa PA10.
Table 3.
Log10 reductions of endotoxin on exposure to sterilization: Escherichia coli O55:B5 endotoxin.
Discussion
Conventional wisdom regarding bacterial endotoxin is that it is a particularly difficult biological residue to remove/inactivate and has an affinity for surfaces, making it a risk to patients if not well controlled.17,33 Contrary to this, the data from the present experiments indicated that bacterial endotoxin has a low affinity for the tested metallic surfaces that were representative of medical device materials. A slightly higher affinity was noted for polymeric materials, such as PEEK and UHMWPE. Of note, although higher for polymeric materials, the levels noted were still extraordinarily low considering the starting concentration in the artificially contaminated water sources. Minor differences in the outcomes were noted between CSE and EC.
The active bacterial endotoxin adsorption methodology used in this study, consisting of substrate incubation for 1 hour followed by aseptic removal and testing of the remaining incubation solutions, was performed to mimic potential in-process material exposure conditions during medical device manufacturing. We purposely avoided the direct inoculation and drying of endotoxin onto substrate surfaces to avoid potential adsorption drying artifacts and to avoid the known limitations of reproducible endotoxin recovery from surfaces.34 Therefore, following incubation in the endotoxin solution and drying, the sample extraction results (performed in water free of detectable endotoxin) yielded low endotoxin recoveries. This observation supported the low active bacterial endotoxin adsorption results obtained in this study.
Endotoxin does not show the same affinity for all surfaces. Further, diverse types of endotoxin exhibit different degrees of affinity for the same surface. For the medical device materials studied, the affinity for endotoxin was low, with the highest observed affinity being for very nonpolar surfaces (e.g., UHMWPE). Of note, although PEEK is similar to UHMWPE from a hydrophobic point of view, it demonstrated the lowest observed affinity along with metallic materials (e.g., stainless steel [passivated], titanium alloy [anodized]). This may be related to differences in available oxygen at the surface. A moderate correlation also existed between contact angle and the affinity of a surface for endotoxin, with the interesting exception being glass. For UHMWPE—a material with a high contact angle (low surface energy)— the affinity for endotoxin (CSE) was greater.
Several types of sterilization methods can have a beneficial effect on reducing the activity of endotoxin, in addition to the traditional depyrogenation method using dry heat. Our studies have shown that various sterilization modalities have an effect on reducing multiple sources of endotoxin, as well as on different medical device materials. E-beam sterilization shows substantial reduction in endotoxin activity for some substrate materials. Of note, differences exist between the endotoxin source and surface interaction with sterilization. We have yet to investigate the impact at lower doses. What can be concluded from these experiments is:
Moist heat (steam) shows considerable reductions in endotoxin activity across a spectrum of materials and endotoxin types.
Dry heat, which is suitable for some materials, shows a good reduction of endotoxin activity across a number of materials and endotoxin types. This is the classical example of depyrogenation, but the conditions tested in this case are considered lower than typical depyrogenation cycles. Additional investigations would also suggest that the onset of inactivation of endotoxin readily occurs at temperatures in excess of 150°C (unpublished results).
EO also exhibits a reduction in endotoxin but is more profoundly affected by the substrate material than other modalities in demonstrating effectiveness.
E-beam sterilization is most effective in reducing endotoxin activity on metallic substrates and to a lesser degree on nonmetallic materials.
Using other in vitro and in vivo (animal) models, further testing is needed regarding endotoxin structure, interaction, and inactivation. This will help elucidate the precise nature of the molecular structural contributions to endotoxin affinity (polysaccharide versus LPS versus lipid), as well as the susceptibility of the molecular structural components to sterilization modalities and confirmation in an animal model of the reduction or elimination of pyrogenicity. It can also be suggested that further investigations in affinity may include the use of naturally occurring bacterial endotoxin remaining bound to or an intrinsic part of the source gram-negative bacteria.
Acknowledgments
The following individuals contributed to this work: Allan Kimble, Romain Bolle-Reddat, James Hauschild, and Gerald McDonnell from Johnson & Johnson and Scott Weiss from Alcon.
References
- 1.Hagel L, Jagschies G, Sofer G. Analysis. In: Hagel L, Jagschies G, Sofer G, Eds. Handbook of Process Chromatography. 2nd ed. Cambridge, MA: Academic Press; 2008:133. [Google Scholar]
- 2. Roth J , Blatteis CM . Mechanisms of fever production and lysis: lessons from experimental LPS fever . Compr Physiol. 2014. ; 4 ( 4 ): 1563 – 604 . [DOI] [PubMed] [Google Scholar]
- 3. McCullough KZ . Calculating endotoxin limits for drug products . www.americanpharmaceuticalreview.com/featured-articles/353977-calculating-endotoxin-limits-for-drug-products/ . Accessed Nov. 16, 2023 .
- 4. Bowman JD , Surani S , Horseman MA . Endotoxin, toll-like receptor-4, and atherosclerotic heart disease . Curr Cardiol Rev. 2017. ; 13 ( 2 ): 86 – 93 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilson ME . Effects of bacterial endotoxins on neutrophil function . Rev Infect Dis. 1985. ; 7 ( 3 ): 404 – 18 . [DOI] [PubMed] [Google Scholar]
- 6. McDonnell G , Baseman H , Cordie-Bancroft L . Words matter: a commentary and glossary of definitions for microbiological quality . Biomed Instrum Technol. 2021. ; 55 ( 4 ): 143 – 64 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown GC . The endotoxin hypothesis of neurode-generation. J Neuroinflammation. 2019. ; 16 ( 1 ): 180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Petsch D , Anspach FB . Endotoxin removal from protein solutions . J Biotechnol. 2000. ; 76 ( 2-3 ): 97 – 119 . [DOI] [PubMed] [Google Scholar]
- 9. Sigma-Aldrich. Bacterial components: lipopolysaccharides . In: Glycobiology Analysis Manual: Tools for Glycoproteomics and Glycomics . 2nd ed. St. Louis, MO: : Sigma-Aldrich; ; 2008. . [Google Scholar]
- 10. Jann B , Reske K , Jann K . Heterogeneity of lipopolysaccharides: analysis of polysaccharide chain lengths by sodium dodecylsulfate-polyacrylamide gel electrophoresis . Eur J Biochem. 1975. ; 60 ( 1 ): 239 – 46 . [DOI] [PubMed] [Google Scholar]
- 11.Food and Drug Administration. 21 CFR Part 820: Quality System Regulation. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=820. Accessed Nov. 16, 2023.
- 12. Stojek NM , Szymańska J , Dutkiewicz J . Gram-negative bacteria in water distribution systems of hospitals . Ann Agric Environ Med. 2008. ; 15 ( 1 ): 135 – 42 . [PubMed] [Google Scholar]
- 13. Food and Drug Administration . Pyrogen and Endotoxins Testing : Questions and Answers . www.fda.gov/media/83477/download . Accessed Nov. 16, 2023 .
- 14. Food and Drug Administration . Submission and Review of Sterility Information in Premarket Notification (510 (k)) Submissions for Devices Labeled as Sterile . www.fda.gov/media/74445/download . Accessed Nov. 16, 2023 .
- 15.Wang Y, Dykes GA. Surface properties of polysaccharides. In: Ramawat K, Mérillon JM, Eds. Polysaccharides. Cham, Switzerland: Springer;2014:1–19. [Google Scholar]
- 16. Govorun EN , Khokhlov AR , Gusev AI . Micelle . https://eng.thesaurus.rusnano.com/wiki/article1199 .
- 17. Nelson SK , Knoernschild KL , Robinson FG , Schuster GS . Lipopolysacharide affinity for titanium implant biomaterials . J Prosthet Dent. 1997. ; 77 ( 1 ): 76 – 82 . [DOI] [PubMed] [Google Scholar]
- 18. Schwartz AM , Ellison AH . The effect of surface contamination on contact angles and surface potentials . Prepared for the National Aeronautics and Space Administration, Jan. 13 1966. Contract NAS 3 7104 . Washington, DC: : National Aeronautics and Space Administration; ; 1966 . [Google Scholar]
- 19. Law KY . Definitions for hydrophilicity, hydrophobicity, and superhydrophobicity: getting the basics right . J Phys Chem Lett. 2014. ; 5 ( 4 ): 686 – 8 . [DOI] [PubMed] [Google Scholar]
- 20. Lieder R , Petersen PH , Sigurjónsson ÓE . Endotoxins: invisible companion in biomaterials research . Tissue Eng Part B Rev. 2013. ; 19 ( 5 ): 391 – 402 . [DOI] [PubMed] [Google Scholar]
- 21. Takemoto Y , Nakatani T , Sugimura K , et al. Endotoxin adsorption of various dialysis membranes: in vitro study . Artif Organs. 2003. ; 27 ( 12 ): 1134 – 7 . [DOI] [PubMed] [Google Scholar]
- 22. Sumner AL , Menke EJ , Dubowski Y , et al. The nature of water on surfaces of laboratory systems and implications for heterogeneous chemistry in the troposphere . Physical Chemistry Chemical Physics. 2004. ; 6 : 604 – 13 . [Google Scholar]
- 23. Wang W , Luo CJ , Huang J , Edirisinghe M . PEEK surface modification by fast ambient-temperature sulfonation for bone implant applications . J R Soc Interface. 2019. ; 16 ( 152 ): 20180955 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Diversified Enterprises . Critical surface tension and contact angle with water for various polymers . www.accudynetest.com/polytable_03.html?sortby=contact_angle . Accessed Nov. 16, 2023 .
- 25. Purdue University . Hydrogen bonding . www.chem.purdue.edu/gchelp/liquids/hbond.html . Accessed Nov. 16, 2023 .
- 26. Comyn J , Mascia L , Xiao G , Parker BM . Plasma-treatment of polyetheretherketone (PEEK) for adhesive bonding . International Journal of Adhesion and Adhesives. 1996. ; 16 ( 2 ): 97 – 104 . [Google Scholar]
- 27. Donate R , Alemán-Domínguez ME , Monzón M . On the effectiveness of oxygen plasma and alkali surface treatments to modify the properties of polylactic acid scaffolds . Polymers (Basel). 2021. ; 13 ( 10 ): 1643 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Walsh A , Sokol AA , Buckeridge J , et al. Electron counting in solids: oxidation states, partial charges, and ionicity . J Phys Chem Lett. 2017. ; 8 ( 9 ): 2074 – 5 . [DOI] [PubMed] [Google Scholar]
- 29. Meyer EE , Rosenberg KJ , Israelachvili J . Recent progress in understanding hydrophobic interactions . Proc Natl Acad Sci USA. 2006. ; 103 ( 43 ): 15739 – 46 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Despa F , Berry RS . The origin of long-range attraction between hydrophobes in water . Biophys J. 2007. ; 92 ( 2 ): 373 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan Y, Lee TR. Contact angle and wetting properties. In: Bracco G., Holst B, Eds. Berlin, Germany: Springer; 2013 [Google Scholar]
- 32.Lonza. FAQ re: converting EU/mL (endotoxin unit) to ng/mL when performing endotoxin detection assay. https://knowledge.lonza.com/faq?id=795&search=. Accessed Nov. 16, 2023.
- 33. Tarafa PJ , Williams E , Panvelker S , et al. Removing endotoxin from metallic biomaterials with compressed carbon dioxide-based mixtures . J Supercrit Fluids. 2011. ; 55 ( 3 ): 1052 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bryans TD , Braithwaite C , Broad J , et al. Bacterial endotoxin testing: a report on the methods, background, data, and regulatory history of extraction recovery efficiency . Biomed Instrum Technol. 2004. ; 38 ( 1 ): 73 – 8 . [DOI] [PubMed] [Google Scholar]