Abstract
Access to safe and effective antiretroviral therapy (ART) is a cornerstone in the global response to the HIV pandemic. Among people living with HIV, there is considerable interindividual variability in absolute CD4 T-cell recovery following initiation of virally suppressive ART. The contribution of host genetics to this variability is not well understood. We explored the contribution of a polygenic score which was derived from large, publicly available summary statistics for absolute lymphocyte count from individuals in the general population (PGSlymph) due to a lack of publicly available summary statistics for CD4 T-cell count. We explored associations with baseline CD4 T-cell count prior to ART initiation (n=4959) and change from baseline to week 48 on ART (n=3274) among treatment-naïve participants in prospective, randomized ART studies of the AIDS Clinical Trials Group. We separately examined an African-ancestry-derived and a European-ancestry-derived PGSlymph, and evaluated their performance across all participants, and also in the African and European ancestral groups separately. Multivariate models that included PGSlymph, baseline plasma HIV-1 RNA, age, sex, and 15 principal components (PCs) of genetic similarity explained ~26-27% of variability in baseline CD4 T-cell count, but PGSlymph accounted for <1% of this variability. Models that also included baseline CD4 T-cell count explained ~7-9% of variability in CD4 T-cell count increase on ART, but PGSlymph accounted for <1% of this variability. In univariate analyses, PGSlymph was not significantly associated with baseline or change in CD4 T-cell count. Among individuals of African ancestry, the African PGSlymph term in the multivariate model was significantly associated with change in CD4 T-cell count while not significant in the univariate model. When applied to lymphocyte count in a general medical biobank population (Penn Medicine BioBank), PGSlymph explained ~6-10% of variability in multivariate models (including age, sex, and PCs) but only ~1% in univariate models. In summary, a lymphocyte count PGS derived from the general population was not consistently associated with CD4 T-cell recovery on ART. Nonetheless, adjusting for clinical covariates is quite important when estimating such polygenic effects.
Keywords: HIV, Polygenic Scores, Lymphocyte Count, CD4 T-Cell Count, Pharmacogenomics
1. Introduction
1.1. Incomplete CD4 T-Cell Recovery in Response to Antiretroviral Therapy
Human immunodeficiency virus type 1 (HIV-1) is a global health challenge, with 38.4 million individuals worldwide living with HIV1, including nearly 1.2 million in the United States2. This virus depletes CD4 T lymphocytes (hereafter referred to as CD4 cells), a critical component of the immune system3. Effective antiretroviral therapy (ART) controls viral replication, improves health and prevents transmission4. With viral load reduction, CD4 cell counts may return to normal levels, but in many individuals this is not achieved5-7. Understanding the etiology of CD4 cell recovery is important because individuals with lower CD4 cell counts may be at increased risk for non-AIDS conditions such as hepatic cirrhosis, cardiovascular disease, kidney disease, and cancer8.
The etiology of incomplete CD4 cell recovery has not been fully elucidated, but many biological, demographic, treatment, and genetic factors have been associated9. Individuals who begin ART with CD4 cell counts <200 cells/mm3 are less likely to achieve normal CD4 cell counts >500 cells/mm5-7. Other biological factors associated with this treatment response include higher body mass index (BMI), lower naïve/memory CD4+ cell ratio, lower CD4/CD8 cell ratios, and other immunological factors9. Demographic factors have also been associated with poor CD4 cell recovery including older age, male sex, and Eastern African ancestry, as well as specific ART regimens9,10. Additionally, variants that influence the absorption, distribution, metabolism, and elimination of ART may also play a role11. Genes with single nucleotide polymorphisms (SNPs) reported to be associated with CD4 cell recovery on ART have included IL-2, IL-2Rβ, IL-2Rγ, IL-15, IL-15Rα, TRAIL, Bim, TNF-α, and IFN-γ12. One particular SNP (rs6897932) in IL7RA was associated with a faster CD4 cell count increase in individuals of both European and African ancestry, but another SNP in this gene (rs3194051) was only associated with this response in individuals of African ancestry13,14. Another study suggested that differences in CCR5 genotype and CCL3L1 dosage were associated with the extent and rate of CD4 cell recovery15. Additionally, HLA-Bw4 homozygosity was associated with impaired CD4 cell recovery16. Particular mitochondrial DNA haplogroups were associated with CD4 cell recovery in individuals of European and African ancestry17,18. More recently, whole exome sequencing associated 41 genes with CD4 cell response in females19.
Although multiple genes and SNPs have been associated with poor CD4 cell count recovery on ART, these explain a small fraction of the variance. Previous studies considered effects of SNPs individually, which fails to consider whether combinations of many SNPs may explain a larger portion of the variance. Many conditions are polygenic (e.g., coronary artery disease), meaning that many genes and variants have impact20. It is conceivable that CD4 cell recovery on ART is also polygenic, so it is worth exploring whether polygenic scores may explain a larger portion of the genetic variance, which has never been investigated for this treatment response. Furthermore, understanding the pharmacogenomic underpinnings of treatment response has the potential to better individualize therapy21.
1.2. Polygenic Scores May Predict Complex Treatment Responses
One way to assess the contribution of many variants in combination is by applying Polygenic Scores (PGS), which are the mathematical, cumulative aggregation of risk derived from the total contribution of numerous variants in the genome22. PGS effectively predict phenotypes such as schizophrenia23-27, bipolar disorder23,28,29, breast cancer30-33, type 2 diabetes30,34,35, coronary artery disease 30,34,36, and atrial fibrillation30,34,37. Given their success in other disease areas, it is plausible that PGS could predict poor CD4 cell recovery in response to ART.
When using PGS, it is important to consider the potential for ancestral health disparity. Across many phenotypes, PGS is more predictive for individuals of European ancestry because this population has more readily available summary statistics from large genome-wide association studies (GWAS)38. An ultimate goal of PGS is clinical implementation so that patients can be informed of their genetic risk for disease38. However, clinical implementation could create a larger health disparity whereby individuals of European ancestry may more readily benefit from these risk prediction models38. Thus, it is important to improve risk prediction for global populations. This is particularly important for HIV given its global distribution of prevalence, particularly in Africa. We hope to better predict genetic risk in individuals of African ancestry by generating a PGS based on summary statistics generated in a dataset of individuals largely of African ancestry, in addition to a PGS generated in a dataset of individuals largely of European ancestry. Additionally, we plan to use PRScsx, a method that more effectively predicts polygenic risk in global populations39.
In this study, we assess whether the PGS generated from a general population is predictive of CD4 cell recovery in persons living with HIV (PWH). A similar approach used a body mass index PGS generated from a general population to study ART-associated weight gain40. As there are no large GWAS studies of CD4 cell count, either in the general population or in PWH, we generate statistical power by using summary statistics on total lymphocyte count from a general population, for which large sample sizes are publicly available. Finally, the principle of predicting phenotypic effects in a population affected by a health condition by using genetics from the general population was effective in one study that found that variants associated with cardiac QRS duration in individuals without cardiac diseases were also associated with arrhythmia and atrial fibrillation41. We assess whether this same principle applies to treatment response by testing whether the genetic underpinnings of lymphocyte count in a general population predicts CD4 cell recovery in PWH. We hypothesize that cumulative genetic variants that affect total lymphocyte count also affect recovery of the CD4 T cell subset in response to ART (i.e., that a lymphocyte count PGS [PGSlymph] generated from the general population will be associated with CD4 cell recovery on ART). We also hypothesize that PGSlymph will be associated with CD4 cell counts prior to initiating ART.
2. Methods
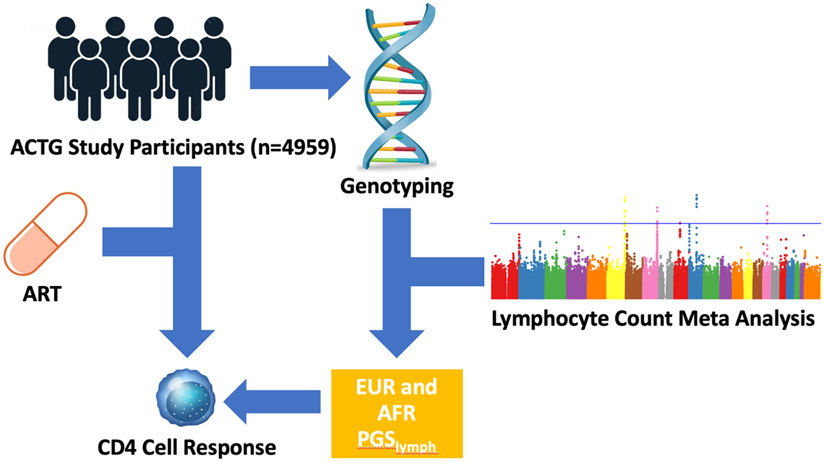
Figure 1: Study Overview:
EUR and AFR PGSlymph were trained using lymphocyte count GWAS summary statistics. Both PGSlymph were applied to individuals in the AIDS Clinical Trials Group (ACTG) to assess its predictability of CD4 cell response to ART.
2.1. Data and Study Participants
2.1.1. Lymphocyte Count Meta Analysis
We used publicly available summary statistics from a published meta-analysis of existing GWAS for lymphocyte count in populations of European and African ancestry in the general population42. The meta-analysis included 524,923 individuals of European ancestry with 47,264,266 SNPs, and 13,477 individuals of African ancestry with 34,121,887 SNPs42. The European ancestry summary statistics were subset to 1,120,498 SNPs that were present on the European linkage disequilibrium (LD) panels and the African ancestry summary statistics were subset to 1,225,091 SNPs that were present on the African LD reference panels.
2.1.2. AIDS Clinical Trials Group
Participants were ART-naïve individuals who had initiated ART in prospective, randomized clinical trials of the AIDS Clinical Trials Group (ACTG), and had consented to genetic research and provided DNA under ACTG protocol A512843. Data were generated by conducting a retrospective analysis of this cohort. Individuals had initiated ART in the United States in studies ACTG384, A5095 (NCT00013520), A5142 (NCT00050895), A5202 (NCT00118898), and A5257 (NCT25285539)44-47. All participants provided written, informed consent for genetic testing. Drug class components of regimens were randomly assigned except for nucleoside reverse transcriptase inhibitor (NRTI) choice in A5142. Included individuals had the following data: imputed genotype, sex, genetically inferred ancestry (GIA), lymphocyte count or CD4 cell count data. Additional eligibility criteria included HIV-1 RNA <400 copies/mL at week 48 on ART.
2.1.3. Penn Medicine BioBank
The Penn Medicine BioBank (PMBB) is an electronic health record (EHR)-linked biobank research program at the University of Pennsylvania48. PMBB participants included in this study provided consent for research including access to their medical records, blood sample collection, and generation of genetic data48. Individuals with both imputed genotype data from PMBB v2.0 and with lymphocyte count data were included in PGS analysis as a positive control. Included individuals had the following data: imputed genotype, lymphocyte count, sex, and GIA.
2.2. Genotyping and Quality Control
2.2.1. AIDS Clinical Trials Group
DNA extracted from whole blood was labeled with coded identifiers and genotyped in seven phases. Phases 1-3 were genotyped at the Broad Institute (Phases 1 and 2 with HumanHap650Yv3_A, and Phase 3 with Human1M-Duov3_B). Phases 4-7 were genotyped at the Vanderbilt Technologies for Advanced Genomics (VANTAGE) facility (Phase 4 using the Human Core Exome chip, phase 5 with the HumanOmni2.5Exome-8-v1.1_A1 chip, Phase 6 with the HumanOmni25-8v1-2_A1 chip, and phase 7 with the Illumina Infinium Multi-Ethnic Global BeadChip (MEGAEX).
Post-genotype quality control procedures utilizing PLINK v1.949 were conducted by Vanderbilt Technologies for Advanced Genomics Analysis and Research Design (VANGARD). Prior to imputation, samples with genotyping efficiency < 99% or with discordance between genotype sex and reported sex were removed. After completing these quality control procedures, each genotyping phase was imputed separately utilizing the TOPMed reference panel, which was parallelized by chromosome to increase computational efficiency50. During the imputation process, liftOver was used to transform genotype data to genome build 3850. After imputation, PLINK was used to merge the seven imputed datasets, and variants with imputation R2 scores < 0.3, genotyping call rates < 95%, or minor allele frequency (MAF) < 0.05 were dropped49. GIA was determined using principal component analysis (PCA) with 1000 Genomes as the reference, subsequently assigning each participant to one of six superpopulations: African (AFR), Admixed American (AMR), East Asian (EAS), European (EUR), South Asian (SAS), and Other.
2.2.2. Penn Medicine BioBank
DNA was extracted from blood samples. Approximately 80% of samples were genotyped by the Regeneron Genomics Center (RGC) using an Illumina Global Screening Array v.2.0 (GSAv2)48, while the remaining 20% were genotyped by the Center for Applied Genomics (CAG) at the Children’s Hospital of Philadelphia using the GSAv1 and GSAv2 genotyping array48.
Prior to imputation, sample level quality control was conducted48. Using PLINK v1.9, variants with genotyping call rates < 95%, individuals with sample call rates < 90%, and individuals with discordance between reported sex and genotype sex were dropped48. Autosomes were imputed utilizing a TOPMed version R2 genome build 38 reference panel48,50. After imputation, variants with imputation R2 scores < 0.3, genotype call rate < 99%, MAF < 1%, and/or were multi-allelic were dropped using PLINK v1.948. Individuals with sample call rate < 99% or discordant sex information were also dropped48. PCA was done to identify GIA using 1000 Genomes as the reference and subsequently separated individuals into six superpopulations: African (AFR), Admixed American (AMR), East Asian (EAS), European (EUR), South Asian (SAS), Other48.
2.3. Polygenic Score Calculation
The PGSlymph was constructed using PRScsx (version released on July 29 2021), which integrates summary statistics and LD panels across genetically diverse populations to better predict polygenic risk in global populations39. 1000 Genomes phase 3 LD reference panels were used in the calculation51. Summary statistics from the lymphocyte count meta-analysis were used to train the PGSlymph42. The PGSlymph was applied to ACTG study participants with CD4 cell count data using PLINK2 “--score” function49. As positive controls, the PGSlymph was also applied to individuals with lymphocyte count data in ACTG as well as individuals with lymphocyte count data in PMBB.
2.4. Statistical Analysis
The results were analyzed to assess model predictability across all ancestries combined, and in European and African ancestries separately. Linear regressions were calculated, and performance was assessed with an R2 value generated from a multivariate linear regression between the phenotype of interest and the PGSlymph. Additionally, performance of individual covariates was assessed with effect sizes generated from these regressions. We used a p-value threshold of 0.05 to assess significance. Regressions were calculated in individuals of European and African ancestry only, as well as individuals of all superpopulations combined. PGSlymph was applied to two different cohorts, ACTG and PMBB. In ACTG, the predictability of the PGSlymph for three different phenotypes was assessed: the square root (SQRT) of CD4 cell count at study entry prior to ART (baseline), change in CD4 cell count from study entry to 48 weeks of ART (a measure of treatment response), and inverse normal lymphocyte count prior to ART (a control variable). We performed two regressions for each phenotype, one without correcting for any covariates, and one correcting for age, sex, principal components (PC) of genetic similarity 1-15, as well as log10-HIV-1 RNA (a measure of viral load). Additionally, we adjusted for SQRT of baseline CD4 cell count in regression models between PGSlymph and change in CD4 cell count on ART. In addition to these regressions, we also evaluated interactions between the PGSlymph and age, sex, viral load, and baseline CD4 cell count to identify whether PGSlymph interacts with any covariate. In PMBB, the predictability of PGSlymph for inverse normal lymphocyte count was assessed as a positive control and to understand predictability in a general medical biobank population. Similarly, two regressions were performed, one without correcting for covariates, and one correcting for age, sex, and PC1-15. These results were visualized using SynthesisView52.
3. Results
Table 1:
ACTG Participant Demographics at Baseline
| Lymphocyte Count Data |
Baseline CD4 Cell Count Data |
On-Treatment CD4 Cell Count Data |
|
|---|---|---|---|
| Total, N | 4680 | 4959 | 3274 |
| European ancestry, n (%) | 1835 (39.2%) | 1958 (39.4%) | 1319 (40.3%) |
| African ancestry, n (%) | 1721 (36.8%) | 1826 (36.8%) | 1154 (35.2%) |
| Male/Female, n (%) | 3824/856 (81.7%/18.3%) | 4051/908 (81.7%/18.3%) | 2715/559 (82.9%/17.1%) |
| Age, mean (range) | 37.9 (17.0-77.0) | 38.0 (17.0-77.0) | 38.2 (17.0-76.0) |
Table 2:
PMBB Demographics
| Lymphocyte Count Data | |
|---|---|
| Total, N | 37211 |
| European ancestry, n (%) | 25330 (68.1%) |
| African ancestry, n (%) | 10217 (27.5%) |
| Male/Female, n (%) | 18215/18996 (49.0%/51.0%) |
| Mean Age (Range) | 55.6 (13.9-101.7) |
Figure 2: Summary of Regression Results Between PGSlymph and Phenotype without Controlling for Covariates (Age, Sex, PC1-15, log10HIV-1 RNA (viral load), and SQRT of baseline CD4 cell count).
Figure 3: Summary of Regression Results Between PGSlymph and Phenotype While Controlling for Covariates (Age, Sex, PC1-15, log10HIV-1 RNA (viral load), and SQRT of baseline CD4 cell count).
4. Discussion
A lymphocyte count PGS trained in the general population did not effectively predict baseline CD4 cell count or change in CD4 cell count in response to ART, leading to rejection of our hypothesis that poor CD4 cell recovery in response to ART is dependent on each individual’s overall genetic predisposition to this outcome. When running regressions without correcting for covariates, R2 values were low across all ancestry groups and most regressions were not statistically significant (Figure 2, Supplementary Table 1. In contrast, clinical covariates were predictive of these phenotypes. When correcting for covariates, performance of the model improved markedly. Baseline regressions performed modestly (R2 = 0.278) while on-treatment regressions were not very predictive (R2 = 0.073), although all values were statistically significant (Figure 3, Supplementary Table 2). However, because the PGSlymph itself was not highly predictive, the success of this model was mostly due to the contribution of covariates. Additionally, when including covariates in the model, the model including the African PGSlymph better predicted change in CD4 cell count on-treatment in individuals of African ancestry than the model including the European PGSlymph (R2 was greater by 0.003) (Figure 3, Supplementary Table 2). This is the only case where we see improved performance by an AFR PGSlymph compared to a EUR PGSlymph. Interestingly, when considering effects of individual covariates in this model, the influence of the AFR PGSlymph is significant (p = 0.044) in individuals of African ancestry with an effect size of −2.062 (Supplementary Table 3). In comparison to other covariates, this effect size is minimal, but suggests that the AFR PGSlymph is playing a role. Furthermore, this shows that our methods improved PGSlymph performance in individuals of African ancestry, which was likely because of a combination of a PGSlymph based on African ancestry summary statistics and utilizing PRScsx for calculation.
In univariate analyses, lymphocyte count PGS did not effectively predict baseline lymphocyte count in ACTG participants. R2 values were also low and insignificant (Figure 2, Supplementary Table 5). Performance improved when including covariates in this model, as R2 values rose to ~0.10 and regressions became statistically significant (Figure 3, Supplementary Table 6). Within the covariate models, the influence of the EUR PGSlymph is significant in individuals of European ancestry (p = 0.018) with a minimal effect size of 0.025 (Supplementary Table 7). However, as the effect size is small, though significant, the EUR PGSlymph is not adding much to this model. Still, this significant effect is exhibited as the R2 value of the EUR PGSlymph covariate model in individuals of European ancestry (0.103) is slightly higher than the R2 value of the AFR PGSlymph covariate model in individuals of European ancestry (0.101) (Figure 3, Supplementary Table 6). Additionally, in the multivariate model, the influence of the AFR PGSlymph is significant in the multi-ancestry group (p=8.7e-3) with an effect size of 8.3e-3 (Supplementary Table 9). Although this evidently did not have a large impact on the model, the effects of this are still present as the R2 value of the AFR PGSlymph covariate model in the multi-ancestry group (0.098) is slightly higher than the R2 value of the EUR PGSlymph covariate model in the multi-ancestry group (0.097) (Figure 3, Supplementary Table 6). Also, it is interesting that the R2 value did not increase as high as in CD4 cell count regressions, perhaps because viral load was the greatest contributing covariate (viral load had the lowest p-value of all variables in all CD4 cell count regressions), and total lymphocyte counts are not greatly affected by viral load, in contrast to CD4 cell counts53 (Supplementary Table 3).
Although this model did not perform well in PWH, it performed slightly better when applied to a general medical biobank population. The PGSlymph best predicted lymphocyte count in a general medical biobank population. Regressions were highly statistically significant, likely due to a large sample size (~37,000 individuals). In the univariate model, the African PGSlymph applied to the multi-ancestry group and the European PGSlymph applied to the European population had the highest R2 values (~0.01) (Figure 2, Supplementary Table 11). It is interesting that these regressions had the highest R2 values, as these are the only ACTG lymphocyte count regressions that had a significant contribution from PGSlymph in the multivariable model. Seeing these patterns across the general population and PWH shows that the AFR PGSlymph performs best in a multi-ancestry group and the EUR PGSlymph performs best in individuals of European ancestry. When controlling for covariates, performance of the model increased. R2 values rose to ~0.06-0.10 and p-values dropped even lower (Figure 3, Supplementary Table 12). This mirrors the impact of covariates seen in PWH. The effect size of the EUR PGSlymph was ~0.01 in all ancestry groups (Supplementary Table 13). It is interesting that without covariates, the EUR PGSlymph in individuals of European ancestry was the only regression mirroring this effect size (Figure 2, Supplementary Table 11). The effect size of the AFR PGSlymph was much lower, ~−5e-3 (Supplementary Table 14). This effect size was mirrored in the AFR PGSlymph regressions without covariates in European and African ancestry, as the R2 values were also low (~3e−3 or 8e−3), but interestingly the R2 value was higher when the AFR PGSlymph was applied to the multi-ancestry group (~0.01) (Figure 2, Supplementary Table 11).
Although these results showed that PGSlymph itself is not predictive of this treatment response, some results show that in combination with covariates, the impact of PGSlymph can become significant, suggesting a possible synergistic effect between PGSlymph and clinical covariates in the model. In the regressions between AFR PGSlymph and change in CD4 cell count in individuals of African ancestry, the impact of the PGSlymph was insignificant, but when including clinical covariates in the regression, the impact of the PGSlymph became significant (Supplementary Table 3). However, the AFR PGSlymph did not significantly interact with any covariates, eliminating the possibility of a synergistic effect (Supplementary Table 4). Additionally, in the regressions between the AFR PGSlymph and baseline lymphocyte count in PWH of all ancestry groups, as well as in the regressions between the EUR PGSlymph and baseline lymphocyte count in individuals of European ancestry, the same patterns were observed (Supplementary Table 7, Supplementary Table 9). Similarly, the AFR PGSlymph did not significantly interact with any covariates, but the EUR PGSlymph significantly interacted with age (Supplementary Table 8, Supplementary Table 10). Thus, it is possible that in PWH, there are synergistic effects between the EUR PGSlymph and covariates, thus leading the PGSlymph to become significant. These findings highlight the importance of including clinical covariates in PGS analyses, not only because the covariates themselves very predictive of treatment response, but also because they seem to interact with the PGSlymph in some way. Another explanation for this observation is that covariates with strong effects overshadow the effects of PGSlymph when not controlled for. Covariates such as viral load have such high significance and large effect sizes, that the effects of smaller impact variables such as PGSlymph are not seen unless these covariates were controlled for. Thus, it is important to consider clinical covariates when implementing PGS in a clinical setting.
This study had several limitations. First, the sample size of the African ancestry summary statistics that were used to generate the African PGSlymph were small (~13,000 individuals), which is due to the lack of availability of lymphocyte count summary statistics for individuals of African ancestry. To improve these results, more lymphocyte count GWAS data are needed in future studies, as it is possible that the AFR PGSlymph could have performed better with a larger base sample size. Additionally, the ACTG sample size was modest (~4600 individuals) which was subset to even smaller groups when stratified by ancestry. It is possible that associations with PGSlymph may have become statistically significant with a larger sample size. Subsequent work in this area could investigate whether this model is predictive of other drug response traits, specifically other ART treatment responses.
Polygenic scores have the potential to leverage large, publicly available datasets to find novel genetic discoveries in pharmacogenomic cohorts. This study utilized a novel method to predict CD4 cell recovery in response to ART and illustrated the importance of including clinical covariates in a PGS model. As more associations or lack thereof are found, we continue to narrow down the biological underpinnings of responses to ART including suboptimal CD4 cell recovery.
Supplementary Material
Acknowledgements
The authors are grateful to the many persons living with HIV who volunteered for ACTG protocols ACTG384, A5095, A5142, A5202 and A5257. In addition, they acknowledge the contributions of study teams and site staff for these protocols. We thank Paul J. McLaren, PhD (Public Health Agency of Canada, Winnipeg, Canada) for prior involvement and collaborations that used these genome-wide genotype data. Study drugs were provided by Bristol-Myers Squibb, Inc., Gilead Sciences, Inc., GlaxoSmithKline, Inc.. The clinical trials were A5095 (NCT00013520), A5142 (NCT00050895), and A5202 (NCT00118898).
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number UM1 AI068634, UM1 AI068636 and UM1 AI106701. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Research reported in this publication was also supported in part by grants funded by the National Center for Research Resources and the National Center for Advancing Translational Sciences.
Grant support included TR000124 (to E.S.D.); AI110527, AI077505, TR000445, and AI069439 (to D.W.H.); AI077505 (to K.M.C, S.D., and M.D.R). This work was supported by the Tennessee Center for AIDS Research (P30) AI110527.
Clinical research sites that participated in ACTG protocols A5095, A5142 and/or A5202, and collected DNA under protocol A5128 were supported by the following grants from the National Institutes of Health (NIH): A1069412, A1069423, A1069424, A1069503, AI025859, AI025868, AI027658, AI027661, AI027666, AI027675, AI032782, AI034853, AI038858, AI045008, AI046370, AI046376, AI050409, AI050410, AI050410, AI058740, AI060354, AI068636, AI069412, AI069415, AI069418, AI069419, AI069423, AI069424, AI069428, AI069432, AI069432, AI069434, AI069439, AI069447, AI069450, AI069452, AI069465, AI069467, AI069470, AI069471, AI069472,AI069474, AI069477, AI069481, AI069484, AI069494, AI069495, AI069496, AI069501, AI069501, AI069502, AI069503, AI069511, AI069513, AI069532, AI069534, AI069556, AI072626, AI073961, RR000046, RR000425, RR023561, RR024156, RR024160, RR024996, RR025008, RR025747, RR025777, RR025780, TR000004, TR000058, TR000124, TR000170, TR000439, TR000445, TR000457, TR001079, TR001082, TR001111, and TR024160.
We acknowledge the Penn Medicine BioBank (PMBB) for providing data and thank the patient-participants of Penn Medicine who consented to participate in this research program. We would also like to thank the Penn Medicine BioBank team and Regeneron Genetics Center for providing genetic variant data for analysis. The PMBB is approved under IRB protocol# 813913 and supported by Perelman School of Medicine at University of Pennsylvania, a gift from the Smilow family, and the National Center for Advancing Translational Sciences of the National Institutes of Health under CTSA award number UL1TR001878.
Footnotes
Supplementary Tables
All supplemental data can be found at: https://ritchielab.org/files/PSB_supplemental_data/PSB_LymphocytePGSHIV_Cardone_2023_Supplementary.pdf
References
- 1.HIV. https://www.who.int/data/gho/data/themes/hiv-aids#cms.
- 2.Basic Statistics ∣ HIV Basics ∣ HIV/AIDS ∣ CDC. https://www.cdc.gov/hiv/basics/statistics.html (2022). [Google Scholar]
- 3.Vidya Vijayan KK, Karthigeyan KP, Tripathi SP & Hanna LE Pathophysiology of CD4+ T-Cell Depletion in HIV-1 and HIV-2 Infections. Front. Immunol 8, 580 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menéndez-Arias L & Delgado R Update and latest advances in antiretroviral therapy. Trends Pharmacol. Sci 43, 16–29 (2022). [DOI] [PubMed] [Google Scholar]
- 5.Kelley CF et al. Incomplete Peripheral CD4 + Cell Count Restoration in HIV-Infected Patients Receiving Long-Term Antiretroviral Treatment. Clin. Infect. Dis 48, 787–794 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lok JJ et al. Long-term increase in CD4+ T-cell counts during combination antiretroviral therapy for HIV-1 infection. AIDS 24, 1867–1876 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore RD & Keruly JC CD4+ Cell Count 6 Years after Commencement of Highly Active Antiretroviral Therapy in Persons with Sustained Virologic Suppression. Clin. Infect. Dis 44, 441–446 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Baker JV et al. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS 22, 841–848 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X et al. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: Challenges of immunological non-responders. J. Leukoc. Biol 107, 597–612 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geng EH et al. CD4+ T cell recovery during suppression of HIV replication: an international comparison of the immunological efficacy of antiretroviral therapy in North America, Asia and Africa. Int. J. Epidemiol 44, 251–263 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haas DW & Tarr PE Perspectives on pharmacogenomics of antiretroviral medications and HIV-associated comorbidities: Curr. Opin. HIV AIDS 10, 116–122 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haas DW et al. Immunogenetics of CD4 Lymphocyte Count Recovery during Antiretroviral Therapy: An AIDS Clinical Trials Group Study. J. Infect. Dis 194, 1098–1107 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Hartling HJ et al. Polymorphism in interleukin-7 receptor α gene is associated with faster CD4+ T-cell recovery after initiation of combination antiretroviral therapy. AIDS 28, 1739–1748 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Rajasuriar R et al. The role of SNPs in the α-chain of the IL-7R gene in CD4+ T-cell recovery in HIV-infected African patients receiving suppressive cART. Genes Immun. 13, 83–93 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Ahuja SK et al. CCL3L1-CCR5 genotype influences durability of immune recovery during antiretroviral therapy of HIV-1–infected individuals. Nat. Med 14, 413–420 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rauch A et al. HLA-Bw4 Homozygosity Is Associated with an Impaired CD4 T Cell Recovery after Initiation of Antiretroviral Therapy. Clin. Infect. Dis 46, 1921–1925 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Grady BJ et al. Mitochondrial Genomics and CD4 T-Cell Count Recovery After Antiretroviral Therapy Initiation in AIDS Clinical Trials Group Study 384. JAIDS J. Acquir. Immune Defic. Syndr 58, 363–370 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guzmán-Fulgencio M et al. European mitochondrial haplogroups are associated with CD4+ T cell recovery in HIV-infected patients on combination antiretroviral therapy. J. Antimicrob. Chemother 68, 2349–2357 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Greenblatt R et al. Genetic and clinical predictors of CD4 lymphocyte recovery during suppressive antiretroviral therapy: Whole exome sequencing and antiretroviral therapy response phenotypes. PLOS ONE 14, e0219201 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polygenic Risk Scores. Genome.gov https://www.genome.gov/Health/Genomics-and-Medicine/Polygenic-risk-scores (2022). [Google Scholar]
- 21.National Institute of General Medical Sciences. National Institute of General Medical Sciences (NIGMS) https://nigms.nih.gov/.
- 22.Choi SW, Mak TS-H & O’Reilly PF Tutorial: a guide to performing polygenic risk score analyses. Nat. Protoc 15, 2759–2772 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calafato MS et al. Use of schizophrenia and bipolar disorder polygenic scores to identify psychotic disorders. Br. J. Psychiatry J. Ment. Sci 213, 535–541 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jonas KG et al. Schizophrenia polygenic risk score and 20-year course of illness in psychotic disorders. Transl. Psychiatry 9, 300 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheutlin AB et al. Penetrance and Pleiotropy of Polygenic Risk Scores for Schizophrenia in 106,160 Patients Across Four Health Care Systems. Am. J. Psychiatry 176, 846–855 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alnæs D et al. Brain Heterogeneity in Schizophrenia and Its Association With Polygenic Risk. JAMA Psychiatry 76, 739–748 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mas-Bermejo P et al. Schizophrenia polygenic risk score in psychosis proneness. Eur. Arch. Psychiatry Clin. Neurosci (2023) doi: 10.1007/s00406-023-01633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mistry S, Harrison JR, Smith DJ, Escott-Price V & Zammit S The use of polygenic risk scores to identify phenotypes associated with genetic risk of bipolar disorder and depression: A systematic review. J. Affect. Disord 234, 148–155 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Hasseris S et al. Polygenic Risk and Episode Polarity Among Individuals With Bipolar Disorder. Am. J. Psychiatry 180, 200–208 (2023). [DOI] [PubMed] [Google Scholar]
- 30.Khera AV et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet 50, 1219–1224 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H et al. Breast cancer risk prediction using a polygenic risk score in the familial setting: a prospective study from the Breast Cancer Family Registry and kConFab. Genet. Med 19, 30–35 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mavaddat N et al. Polygenic Risk Scores for Prediction of Breast Cancer and Breast Cancer Subtypes. Am. J. Hum. Genet 104, 21–34 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts E, Howell S & Evans DG Polygenic risk scores and breast cancer risk prediction. Breast Edinb. Scotl 67, 71–77 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Sullivan JW, Ashley EA & Elliott PM Polygenic risk scores for the prediction of cardiometabolic disease. Eur. Heart J 44, 89–99 (2023). [DOI] [PubMed] [Google Scholar]
- 35.McCarthy MI & Mahajan A The value of genetic risk scores in precision medicine for diabetes. Expert Rev. Precis. Med. Drug Dev 3, 279–281 (2018). [Google Scholar]
- 36.Rao AS & Knowles JW Polygenic risk scores in coronary artery disease. Curr. Opin. Cardiol 34, 435–440 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Pulit SL et al. Atrial fibrillation genetic risk differentiates cardioembolic stroke from other stroke subtypes. Neurol. Genet 4, e293 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin AR et al. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat. Genet 51, 584–591 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruan Y et al. Improving polygenic prediction in ancestrally diverse populations. Nat. Genet 54, 573–580 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keat K et al. Leveraging Multi-Ancestry Polygenic Risk Scores for Body Mass Index to Predict Antiretroviral Therapy-Induced Weight Gain. Pac. Symp. Biocomput. Pac. Symp. Biocomput 28, 233–244 (2023). [PMC free article] [PubMed] [Google Scholar]
- 41.Ritchie MD et al. Genome- and Phenome-Wide Analyses of Cardiac Conduction Identifies Markers of Arrhythmia Risk. Circulation 127, 1377–1385 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen M-H et al. Trans-ethnic and Ancestry-Specific Blood-Cell Genetics in 746,667 Individuals from 5 Global Populations. Cell 182, 1198–1213.e14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haas DW et al. A Multi-Investigator/Institutional DNA Bank for AIDS-Related Human Genetic Studies: AACTG Protocol A5128. HIV Clin. Trials 4, 287–300 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Daar ES Atazanavir Plus Ritonavir or Efavirenz as Part of a 3-Drug Regimen for Initial Treatment of HIV-1: A Randomized Trial. Ann. Intern. Med 154, 445 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gulick RM Three- vs Four-Drug Antiretroviral Regimens for the Initial Treatment of HIV-1 InfectionA Randomized Controlled Trial. JAMA 296, 769 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Gulick RM et al. Triple-Nucleoside Regimens versus Efavirenz-Containing Regimens for the Initial Treatment of HIV-1 Infection. N. Engl. J. Med 350, 1850–1861 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Riddler SA et al. Class-Sparing Regimens for Initial Treatment of HIV-1 Infection. N. Engl. J. Med 358, 2095–2106 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verma A et al. The Penn Medicine BioBank: Towards a Genomics-Enabled Learning Healthcare System to Accelerate Precision Medicine in a Diverse Population. J. Pers. Med 12, 1974 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang CC et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience 4, 7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hinrichs AS The UCSC Genome Browser Database: update 2006. Nucleic Acids Res. 34, D590–D598 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.The 1000 Genomes Project Consortium et al. A global reference for human genetic variation. Nature 526, 68–74 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pendergrass SA, Dudek SM, Crawford DC & Ritchie MD Synthesis-View: visualization and interpretation of SNP association results for multi-cohort, multi-phenotype data and meta-analysis. BioData Min. 3, 10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.2.14 How CD4 and viral load are related ∣ Training manual ∣ HIV i-Base. https://i-base.info/ttfa/section-2/14-how-cd4-and-viral-load-are-related/. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.