Abstract
Introduction
Alport syndrome (AS) is a hereditary, progressive kidney disease characterized by structural abnormalities and dysfunction of the glomerular basement membrane (GBM). AS is classified as X-linked, autosomal, and digenic. The number of cases of digenic AS has increased, but the genotype-phenotype correlation of patient with digenic AS is still unclear. Here, we present a case of digenic AS with novel digenic missense variants in COL4A4 (c.827G>C, p.Gly276Ala) and COL4A5 (c.4369G>C, p.Gly1457Arg).
Case Presentation
The patient was a 29-year-old Japanese man suffering from persistent microscopic hematuria and proteinuria without kidney function impairment. Kidney biopsy showed focal interstitial foam cell infiltration, global and segmental glomerulosclerosis. Immunofluorescence staining for collagen IV α5 was almost negative in the GBM and Bowman’s capsule. Electron microscopy revealed irregular thickening with lamellation and segmental thinning of the GBM. Clinical and pathological findings were consistent with AS. Comprehensive next-generation sequencing revealed a heterozygous missense variant in COL4A4 (c.827G>C, p.Gly276Ala) in exon 1 and a hemizygous missense variant in COL4A5 (c.4369G>C, p.Gly1457Arg) in exon 49 on the patient’s paternal and maternal alleles, respectively. The same digenic variants were detected in his sister, and she also showed a similar phenotype. After treatment with angiotensin-converting enzyme inhibitors, proteinuria decreased from 2.3 to 1.1 g/g creatinine, but occult blood persisted. During follow-up, kidney function has been preserved.
Conclusion
The novel genotype of our case provides more information on the genotype-phenotype correlation of digenic XLAS, although long-term follow-up is required. The findings in the present case also indicate the importance of genetic tests for family members of a patient diagnosed with digenic AS.
Keywords: Digenic Alport syndrome, Alport syndrome, Novel variants
Introduction
Alport syndrome (AS) is a hereditary kidney disease with manifestations that include hematuria, proteinuria, progressive kidney failure, sensorineural hearing loss, and ocular lesions [1]. The pathological mechanism of AS is glomerular basement membrane (GBM) abnormalities due to pathogenic variants in genes encoding the α3, α4, and α5 chains of type IV collagen [2]. X-linked dominant hereditary type AS (XLAS) is most common and is caused by variants in COL4A5, located on chromosome Xq2. Autosomal recessive AS and autosomal dominant AS are caused by variants in COL4A3 and COL4A4, which are located at 2q36.3 [3].
In patients with XLAS, proteinuria becomes evident in early childhood. The median kidney survival period (age at onset of kidney failure) is 35 years, but the age at onset of kidney failure differs significantly among patients, based on genotype differences [4]. To date, >1,000 variants have been identified in XLAS [5]. However, there have been few reports of cases of male digenic AS caused by variants in COL4A5 and another. Here, we present a case of AS with novel digenic missense variants in COL4A4 (c.827G>C, p.Gly276Ala) and COL4A5 (c.4369G>C, p.Gly1457Arg) without kidney function impairment. The CARE Checklist has been completed by the authors for this case report, attached as online supplementary material (for all online suppl. material, see https://doi.org/10.1159/000535493).
Case Presentation
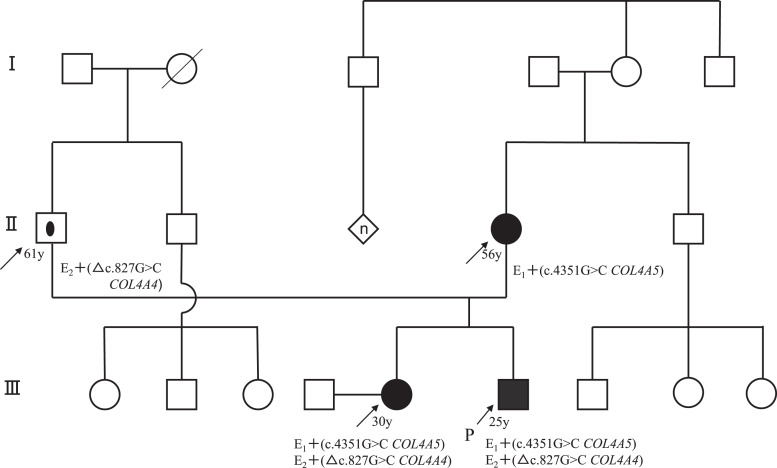
The patient was a 29-year-old Japanese man who was referred to our hospital because of persistent microscopic hematuria and proteinuria. He had a significant familial history of abnormal urinalysis. His maternal and paternal grandmothers had proteinuria and hematuria, his mother had both hematuria and proteinuria since she was a teenager, and his sister also presented with microscopic hematuria. However, none of the family members had kidney impairment. The pedigree of the patient’s family is shown in Figure 1.
Fig. 1.
Pedigree of the family. Black circles: hematuria, affected individual. Black squares: hematuria, proteinuria, proband. Circle within a square: carrier, asymptomatic individual.
Upon admission, body temperature was 36.8°C and blood pressure was 125/83 mm Hg. Optometry and otolaryngologic examinations revealed no abnormalities, although the patient subsequently developed sensorineural hearing loss. Physical examination findings were unremarkable for the heart, lungs, abdomen, and nervous system. Laboratory and urinary data at the time of admission are shown in Table 1. Urinalysis indicated proteinuria (3+) and occult blood (2+), and a spot urine protein/creatine ratio of 2.30 g/gCr. Serum creatinine was 0.90 mg/dL, estimated glomerular filtration rate was 87.45 mL/min/1.73 m2, blood urea nitrogen was 14 mg/dL, and albumin was 4.0 g/dL. Serum C3 and C4 were 111 and 20.5 mg/dL, respectively.
Table 1.
Laboratory findings on admission
| Parameter | Value (reference range) | Parameter | Value (reference range) |
|---|---|---|---|
| White blood cells, ×104/μL | 7,500 (4,300–8,000) | Hemoglobin A1c (NGSP), % | 5.5 (4.6–6.2) |
| Red blood cells, ×104/μL | 467 (450–510) | CH50, U/mL | 60.0≤ (31.6–57.6) |
| Hemoglobin, g/dL | 14.2 (12.4–17.2) | C3, mg/dL | 111 (65.0–135) |
| Hematocrit, % | 41.4 (38.0–54.0) | C4, mg/dL | 20.5 (13.0–35.0) |
| Platelet count, ×104/μL | 26.2 (18.0–34.0) | C-reactive protein, mg/dL | 2.04 (<5) |
| Total protein, g/dL | 6.3 (6.6–8.1) | IgG, mg/dL | 892 (870–1,700) |
| Albumin, g/dL | 4.0 (3.5–5.0) | IgA, mg/dL | 200 (93–393) |
| Blood urea nitrogen, mg/dL | 15 (8–20) | HBsAg | Negative |
| Creatinine, mg/dL | 0.90 (0.50–1.10) | HBsAb | Negative |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 87.45 (90-) | HBeAb | Negative |
| Sodium, mEq/L | 142 (138–145) | HCVAb | Negative |
| Potassium, mEq/L | 4.2 (3.6–4.8) | Urinalysis | |
| Chloride, mEq/L | 105 (101–108) | Protein | 3+ |
| Total bilirubin, mg/L | 1.3 (0.2–1.2) | Occult blood | 2+ |
| AST, IU/L | 16 (13–30) | Glucose | Negative |
| ALT, IU/L | 17 (8–42) | Red blood cells (/HPF) | 50–99 |
| Total cholesterol, mg/dL | 214 (130–220) | White blood cells (/HPF) | Negative |
| Triglyceride, mg/dL | 183 (50–150) | Protein excretion, g/gCr | 2.30 |
| HDL cholesterol, mg/dL | 37 (30–80) | Protein excretion, g/day | 1.74 |
| LDL cholesterol, mg/dL | 146 (70–139) | Selectivity Index | 0.15 |
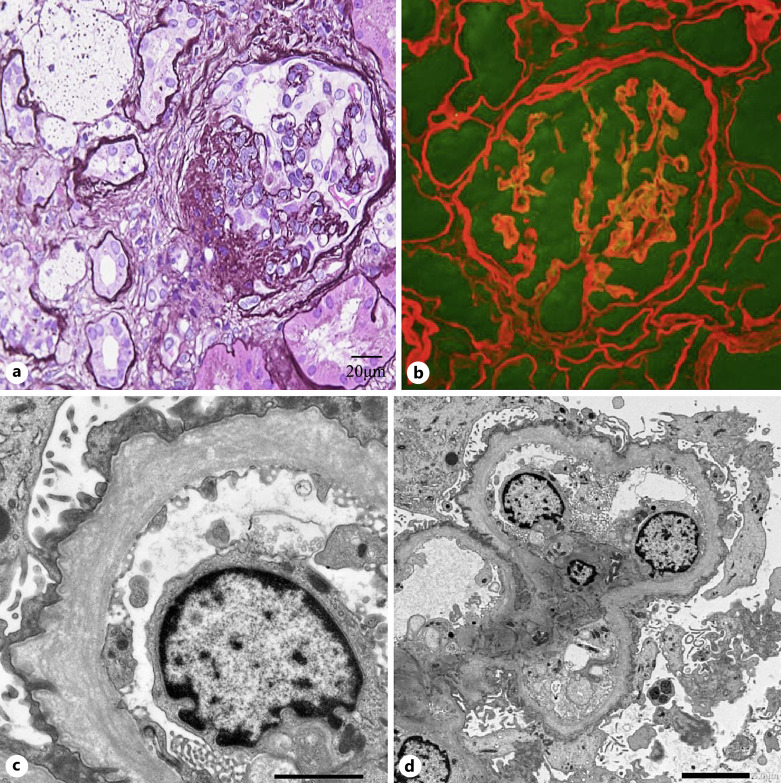
Due to these findings, a kidney biopsy was performed. Light microscopy revealed 21 glomeruli, of which 4 exhibited global glomerulosclerosis and 4 exhibited segmental sclerosis of the collapsed type. All other glomeruli showed no significant alterations. Interstitial fibrosis and foam cells were apparent on periodic acid-Schiff staining (Fig. 2a). Immunofluorescence staining for IgG, IgA, IgM, C1q, C3, and fibrinogen was not significant. Double immunofluorescence staining for α1 (red) and α5 (green) showed that the GBM and Bowman’s capsule were negative for α5 (Fig. 2b). Electron microscopy revealed irregular thickening with lamellation and segmental thinning of the GBM (Fig. 2c, d).
Fig. 2.
a Light microscopy revealed global glomerulosclerosis and segmental sclerosis. b Double immunofluorescence staining for α1 (red) and α5 (green) showed that the glomerular basement membrane (GBM) and Bowman’s capsule were completely negative for α5. c, d Electron microscopy revealed focal thickening with lamellation and segmental thinning of the GBM.
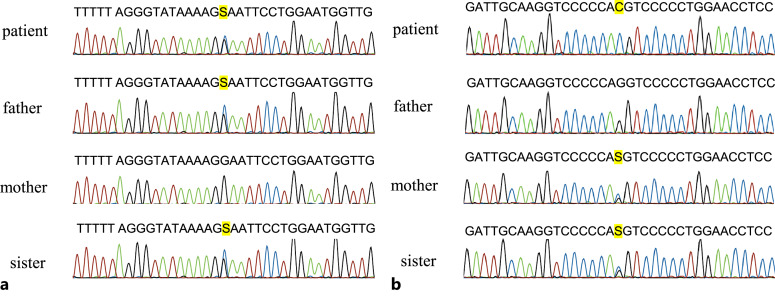
Based on his family history and findings suggestive of AS, a genetic evaluation was performed using comprehensive sequence analysis of multiple podocyte related genes, including COL4A3, COL4A4, and COL4A5, in the patient. Variants were validated using Sanger sequencing in the pedigree analysis, with sequencing of 193 genes. The target bases were covered at a depth of more than 100 folds, with 100% coverage. The mean base-read-depth was 460-fold in COL4A3, 447-fold in COL4A4, and 241-fold in COL4A5. This study was approved by the Institutional Review Board of Kobe University of Medicine, and written informed consent was obtained. Mutational analyses of COL4A3, COL4A4, and COL4A5 were performed using polymerase chain reaction and direct sequencing of genomic DNA for family members. The results showed that the patient had a compound heterozygous missense variant (c.827G>C; p.Gly276Ala) in exon 1 of COL4A4 and a hemizygous missense variant (c.4369G>C; p.Gly1457Arg) in exon 49 of COL4A5. Variants were also found in exon 1 of COL4A4 in his father, in exon 47 of COL4A5 in his mother, and in exons 1 and 47 of COL4A4 and COL4A5, respectively, in his sister (Fig. 3). According to the ACMG classification, the heterozygous missense variant c.827G>C was classified as pathogenic (PS1+PM1+PM2+PP2+PP3), and the hemizygous missense variant c.4351G>C was classified as likely pathogenic (PM1+PM2+PM5+PP2+PP3).
Fig. 3.
a Sequences of the heterozygous c.827G>C variant. b Sequences of the hemizygous c.4351G>C variant.
Discussion
Genetic analysis in our patient revealed a heterozygous missense variant c.827G>C in COL4A4, resulting in a glycine to alanine substitution at position 276 in exon 1, and a hemizygous missense variant c.4351G>C in COL4A5, resulting in a glycine to arginine substitution at position 1,457 in exon 49. Based on his family history and the results of genetic testing, our patient was diagnosed with digenic AS due to novel missense variants in COL4A4 and COL4A5.
AS is a hereditary disease caused by a genetic variant resulting in type IV collagen dysplasia [6]. Type IV collagen constitutes the basement membrane of systemic tissues and consists of three α chains in a triple helical structure. The combination of the three α chains is organ-specific, with α3-α4-α5 present in the GBM and α5-α5-α6 in the Bowman’s capsule membrane [7]. COL4A5 encodes collagen α5 chain and is the causative gene for XLAS.
α5 chain consists of an N-terminal domain, C-terminal domain, and collagenous domain, and includes a triple helix and a non-helical region [8, 9]. The collagen triple helix conformation requires a (Gly-X-Y)n repeat amino acid sequence, in which the glycine contributes to the stability of the triple helix [8, 10]. Therefore, missense variant in the collagenous domain of COL4A3, COL4A4, COL4A5 disrupt structural motifs in collagen comprising the (Gly-X-Y)n repeat amino acid sequence. There are significant clinical differences between patients with missense variants in COL4A5 and other genotypes [11]. Similar missense variant of glycine in exons 1–20 to that in the present case have been associated with onset of kidney failure at a median age of 30.1 years [12], and variants closer to the 5′ side were associated with a milder phenotype. Furthermore, patients with missense variants were recently reported to have a longer median kidney survival period of 40 years (35–45 years), compared to those with other truncating variants (nonsense variants, small insertion, or deletion) [4]. A case of a male patient with mild XLAS with a missense variant and presenting only with hematuria at age 38 has also been reported [13].
Most individuals with heterozygous variants in COL4A3 or COL4A4 result in thin basement membrane nephropathy without kidney dysfunction [14]. Autosomal recessive AS is caused by homozygous or compound heterozygous variants in the COL4A3 and COL4A4 genes, whereas autosomal dominant AS occurs due to heterozygous variants. Thus, the phenotypes of heterozygous variants vary from no urinary abnormality to kidney failure. Hirabayashi et al. [15] reported a woman with the same c.827G>C variant in COL4A4 as that in our case, who did not exhibit kidney impairment or proteinuria. Conversely, Imafuku et al. [16] described a man with the same variant who progressed to kidney failure at age 62. Additionally, Deltas et al. [17] found that 35% of patients with heterozygous COL4A3/A4 variants develop kidney failure.
It is difficult to determine if the effect of heterozygous variants in COL4A3 or COL4A4 in XLAS results in more severe disease because there are few reports of cases with digenic variants. A review of digenic AS in COL4A5 and another variant identified two cases with hypomorphic COL4A5 variants that caused severe disease [18–20]. However, digenic XLAS with COL4A5 variants in males was not associated with increased proteinuria or kidney function impairment [21]. These reports suggest that digenic XLAS with mild variants in COL4A5 and additional variants in COL4A3 or COL4A4 may not be associated with more severe disease in males. Our patient had mild digenic variants without kidney function impairment, hearing loss, or ocular abnormalities. We believe that digenic AS with missense variants is likely to be less severe; however, there are no reported cases of long-term renal survival with XLAS, and we are planning careful follow-up with attention to possible increased proteinuria and kidney function impairment.
There is currently no specific treatment for AS. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers (ARBs) significantly reduce urine protein and delay progression to kidney failure in AS [22, 23]. Bardoxolone methyl activates nuclear factor erythroid-2-related factor 2 (Nrf2) and suppresses nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). A phase 3, multicenter, double-blind, placebo-controlled, randomized trial showed that bardoxolone methyl improved the decline in estimated glomerular filtration rate but did not improve the time to onset of kidney failure in AS [24]. Moreover, exon-skipping therapy is under development for severe XLAS with severe variants, such as nonsense variants in males, using an antisense oligonucleotide to replace these variants with milder variants [25]. In our patient, treatment with an ARB resulted in a significant decrease in proteinuria from 2.3 to 1.1 g/gCr after kidney biopsy.
In conclusion, we have presented a case of digenic AS with novel missense variants. The novel genotype in this case provides more information on the genotype-phenotype correlation of digenic AS, but long-term follow-up is required. Further accumulation of cases is needed for understanding of digenic AS with missense variants.
Acknowledgments
We are indebted to the nephrologists and nursing staff at Osaka Metropolitan University Hospital and to the patient for their assistance with this case.
Statement of Ethics
This report does not include any experiments on humans or animals. This study protocol was reviewed and approved by Kobe University of Medicine, approval number [301]. Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images. All procedures involving human participants were in accordance with the ethical standards of the institution at which the work was conducted and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Conflict of Interest Statement
H.U. reported personal fees from Astellas Pharma Co. Ltd., Mitsubishi Tanabe Pharma Co. Ltd., Mochida Pharma Co. Ltd., Kyowa Kirin Co. Ltd., and Otsuka Pharmaceutical Co. Ltd. K.M. reported grants from Mitsubishi Tanabe Pharma Co. Ltd., Kyowa Kirin Co. Ltd., and Torii Pharmaceutical Co., Ltd. and personal fees from Astellas Pharma Co. Ltd., Bayer Yakuhin Co. Ltd., Chugai Pharmaceutical Co. Ltd., Kissei Pharmaceutical Co. Ltd., Kyowa Kirin Co. Ltd., Torii Pharmaceutical Co. Ltd., and Ono Pharmaceutical Co. Ltd. S.N. reported personal fees from Otsuka Pharmaceutical Co. Ltd., Astellas Pharma Co. Ltd., Kissei Pharmaceutical Co. Ltd., Kyowa Kirin Co. Ltd., Torii Pharmaceutical Co. Ltd., Novel Pharma, and Ono Pharmaceutical Co. Ltd. K.N. is a member of advisory groups for Kyowa Kirin Co. Ltd., Toa Eiyo Ltd., and Taisho Pharmaceutical Co. Ltd. He received speaker’s bureaus from Sumitomo Pharma Co. Ltd., Chugai Pharmaceutical Co. Ltd., and Kyowa Kirin Co., Ltd. He obtained a patent with Daiichi Sankyo Pharma Co., Ltd. for developing exon-skipping therapy for Alport syndrome patients. M.E. reported grants from Mitsubishi Tanabe, Kowa, Eisai, Chugai Pharmaceutical, and Bayer, personal fees from Novo Nordisk Pharma, Sanofi, and AstraZeneca, and grants and personal fees from Ono Pharmaceutical, Nippon Boehringer Ingelheim, Sumitomo Pharmaceutical, and Kyowa Kirin Co. Ltd. The authors declare that they have no other conflicts of interest regarding the study.
Funding Sources
While we have received honoraria and grants unrelated to this study, we have not received any specific funding for this research.
Author Contributions
H.U., K.M., S.N., K.W., R.N., F.M., K.S., C.O., J.H., A.T., N.M., T.S., and K.N. were responsible for the clinical management of the patient and for preparing the draft version of the manuscript. M.E. read and approved the manuscript. All authors participated in the writing of the manuscript and read and approved the final version.
Funding Statement
While we have received honoraria and grants unrelated to this study, we have not received any specific funding for this research.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Kashtan CE. Alport syndrome. An inherited disorder of renal, ocular, and cochlear basement membranes. Medicine. 1999;78(5):338–60. [DOI] [PubMed] [Google Scholar]
- 2. Savige J, Colville D, Rheault M, Gear S, Lennon R, Lagas S, et al. Alport syndrome in women and girls. Clin J Am Soc Nephrol. 2016;11(9):1713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rheault MN, Savige J, Randles MJ, Weinstock A, Stepney M, Turner AN, et al. The importance of clinician, patient and researcher collaborations in Alport syndrome. Pediatr Nephrol. 2020;35(5):733–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yamamura T, Horinouchi T, Nagano C, Omori T, Sakakibara N, Aoto Y, et al. Genotype-phenotype correlations influence the response to angiotensin-targeting drugs in Japanese patients with male X-linked Alport syndrome. Kidney Int. 2020;98(6):1605–14. [DOI] [PubMed] [Google Scholar]
- 5. Jais JP, Knebelmann B, Giatras I, Marchi M, Rizzoni G, Renieri A, et al. X-linked Alport syndrome: natural history in 195 families and genotype- phenotype correlations in males. J Am Soc Nephrol. 2000;11(4):649–57. [DOI] [PubMed] [Google Scholar]
- 6. Hudson BG. The molecular basis of Goodpasture and Alport syndromes: beacons for the discovery of the collagen IV family. J Am Soc Nephrol. 2004;15(10):2514–27. [DOI] [PubMed] [Google Scholar]
- 7. Kruegel J, Rubel D, Gross O. Alport syndrome: insights from basic and clinical research. Nat Rev Nephrol. 2013;9(3):170–8. [DOI] [PubMed] [Google Scholar]
- 8. Brodsky B, Persikov AV. Molecular structure of the collagen triple helix. Adv Protein Chem. 2005;70:301–39. [DOI] [PubMed] [Google Scholar]
- 9. Cosgrove D, Liu S. Collagen IV diseases: a focus on the glomerular basement membrane in Alport syndrome. Matrix Biol. 2017;57–58:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yeo J, Qiu Y, Jung GS, Zhang YW, Buehler MJ, Kaplan DL. Adverse effects of Alport syndrome-related Gly missense mutations on collagen type IV: insights from molecular simulations and experiments. Biomaterials. 2020;240:119857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bekheirnia MR, Reed B, Gregory MC, McFann K, Shamshirsaz AA, Masoumi A, et al. Genotype-phenotype correlation in X-linked Alport syndrome. J Am Soc Nephrol. 2010;21(5):876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gross O, Netzer KO, Lambrecht R, Seibold S, Weber M. Meta-analysis of genotype-phenotype correlation in X-linked Alport syndrome: impact on clinical counselling. Nephrol Dial Transplant. 2002;17(7):1218–27. [DOI] [PubMed] [Google Scholar]
- 13. Kaneko K, Tanaka S, Hasui M, Nozu K, Krol RP, Iijima K, et al. A family with X-linked benign familial hematuria. Pediatr Nephrol. 2010;25(3):545–8. [DOI] [PubMed] [Google Scholar]
- 14. Voskarides K, Pierides A, Deltas C. COL4A3/COL4A4 mutations link familial hematuria and focal segmental glomerulosclerosis. glomerular epithelium destruction via basement membrane thinning? Connect Tissue Res. 2008;49(3):283–8. [DOI] [PubMed] [Google Scholar]
- 15. Hirabayashi Y, Katayama K, Mori M, Matsuo H, Fujimoto M, Joh K, et al. Mutation analysis of thin basement membrane nephropathy. Genes. 2022;13(10):1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Imafuku A, Nozu K, Sawa N, Hasegawa E, Hiramatsu R, Kawada M, et al. Autosomal dominant form of type IV collagen nephropathy exists among patients with hereditary nephritis difficult to diagnose clinicopathologically. Nephrology. 2018;23(10):940–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deltas C, Savva I, Voskarides K, Papazachariou L, Pierides A. Carriers of autosomal recessive Alport syndrome with thin basement membrane nephropathy presenting as focal segmental glomerulosclerosis in later life. Nephron. 2015;130(4):271–80. [DOI] [PubMed] [Google Scholar]
- 18. Choi M, Anistan YM, Eckardt KU, Gollasch M, Nickel P. Possible digenic disease in a caucasian family with COL4A3 and COL4A5 mutations. Nephron. 2019;141(3):213–8. [DOI] [PubMed] [Google Scholar]
- 19. Zhang L, Sun BC, Zhao BG, Ma QS. An overview of the multi-pronged approach in the diagnosis of Alport syndrome for 22 children in Northeast China. BMC Nephrol. 2020;21(1):294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Domingo-Gallego A, Pybus M, Bullich G, Furlano M, Ejarque-Vila L, Lorente-Grandoso L, et al. Clinical utility of genetic testing in early-onset kidney disease: seven genes are the main players. Nephrol Dial Transplant. 2022;37(4):687–96. [DOI] [PubMed] [Google Scholar]
- 21. Savige J, Renieri A, Ars E, Daga S, Pinto AM, Rothe H, et al. Digenic Alport syndrome. Clin J Am Soc Nephrol. 2022;17(11):1697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Webb NJ, Lam C, Shahinfar S, Strehlau J, Wells TG, Gleim GW, et al. Efficacy and safety of losartan in children with Alport syndrome: results from a subgroup analysis of a prospective, randomized, placebo- or amlodipine-controlled trial. Nephrol Dial Transplant. 2011;26(8):2521–6. [DOI] [PubMed] [Google Scholar]
- 23. Gross O, Licht C, Anders HJ, Hoppe B, Beck B, Tönshoff B, et al. Early angiotensin-converting enzyme inhibition in Alport syndrome delays renal failure and improves life expectancy. Kidney Int. 2012;81(5):494–501. [DOI] [PubMed] [Google Scholar]
- 24. Warady BA, Pergola PE, Agarwal R, Andreoli S, Appel GB, Bangalore S, et al. Effects of bardoxolone methyl in Alport syndrome. Clin J Am Soc Nephrol. 2022;17(12):1763–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yamamura T, Horinouchi T, Adachi T, Terakawa M, Takaoka Y, Omachi K, et al. Development of an exon skipping therapy for X-linked Alport syndrome with truncating variants in COL4A5. Nat Commun. 2020;11(1):2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.