Abstract
MYB is a transcription factor which was identified in birds as a viral oncogene (v-MYB). Its cellular counterpart was subsequently isolated as c-MYB which has three functional domains - DNA binding domain, transactivation domain and negative regulatory domain. c-MYB is essential for survival, and deletion of both alleles of the gene results in embryonic death. It is highly expressed in hematopoietic cells, thymus and neural tissue, and required for T and B lymphocyte development and erythroid maturation. Additionally, aberrant MYB expression has been found in numerous solid cancer cells and human leukemia. Recent studies have also implicated c-MYB in the regulation of expression of fetal hemoglobin which is highly beneficial to the β-hemoglobinopathies (beta thalassemia and sickle cell disease). These findings suggest that MYB could be a potential therapeutic target in leukemia, and possibly also a target for therapeutic increase of fetal hemoglobin in the β-hemoglobinopathies.
Keywords: Hematopoiesis, Erythropoiesis, Fetal hemoglobin, C-MYB, Transcription factor
1. Introduction
v-MYB is an oncogene from avian myeloblastosis virus (AMV) (Hall et al., 1941) and E26 (another avian virus), implicated to be one of the oncogenes that cause myelomas and lymphomas in birds (Radke et al., 1982; Moscovici et al., 1983; Lipsick and Wang, 1999). c-MYB (MYB) was subsequently identified as a cellular homologue of the virus v-MYB (Klempnauer et al., 1982; Boyle et al., 1983; Klempnauer et al., 1983; Gonda et al., 1985; Majello et al., 1986) (Table 1). Other forms of MYB, A-MYB and B-MYB, exist in humans encoding transcription factors A-MYB and B-MYB, respectively, that share some homology, including the DNA binding domain, with c-MYB (Ganter and Lipsick, 1999; Bergholtz et al., 2001).
Table 1.
Orthologs of MYB.
| UniProt number | UniProt Entry name | Organism | Gene names | Protein names | Length (amino acids) | Mass (kD) |
|---|---|---|---|---|---|---|
|
| ||||||
| P10242 | MYB_HUMAN | Homo sapiens (Human) | MYB | Transcriptional activator MYB (Proto-oncogene c-MYB) | 640 | 72.35 |
| P06876 | MYB_MOUSE | Mus musculus (Mouse) | Myb | Transcriptional activator Myb (Proto-oncogene c-Myb) | 636 | 71.44 |
| A0A0G2JV86 | A0A0G2JV86_RAT | Rattus norvegicus (Rat) | Myb | Transcriptional activator Myb (Proto-oncogene c- Myb) | 626 | 70.63 |
| P01103 | MYB_CHICK | Gallus gallus (Chicken) | Myb | Transcriptional activator Myb (Proto-oncogene c-Myb) | 641 | 72.48 |
| P01104 | MYB_AVIMB | Avian myeloblastosis virus | v-Myb | Transforming protein Myb | 382 | 43.07 |
In this review MYB is used interchangeably with c-MYB and the encoded transcription factor referred to, as MYB. Since its discovery in the 1980s, MYB has been recognized as a crucial transcription factor in hematopoiesis and erythropoiesis. MYB is tightly regulated, deregulation of MYB is oncogenic; it has been shown to undergo rearrangement or translocation, leading to aberrant expression in human leukemias and lymphomas (Stenman et al., 2010; Pattabiraman and Gonda, 2013). MYB has also been shown to be highly expressed in colorectal, breast and pancreatic cancers (Biroccio et al., 2001; Persson et al., 2009). MYB has a critical physiological role in normal hematopoiesis; it is essential for definitive red cell maturation and its expression is precisely controlled during the different stages of hematopoiesis (Mucenski et al., 1991). More recently, studies have revealed that MYB has a role in regulating fetal hemoglobin gene expression (Jiang et al., 2006; Thein et al., 2007; Stadhouders et al., 2014), a major modifier of the severity of the beta hemoglobinopathies - beta thalassemia and sickle cell disease (Gardner and Thein, 2016; Thein, 2018).
While much has been learnt about MYB (see reviews, Ramsay and Gonda, 2008; Pattabiraman and Gonda, 2013; George and Ness, 2014; Paikari and Sheehan, 2018), a lot remains to be resolved about the regulatory control of MYB expression and how it impacts its function. MYB is considered as an oncogene, but the mechanism underlying this process is not clear. In some cancer cells, MYB expression is relatively higher, but it is not clear if the elevated expression is the cause of the oncogenic process. Forced over-expression of c-MYB did not cause the cells to become cancerous. Further, the key targeted gene(s) promoting oncogenesis regulated by MYB are still unknown, although many genes regulated by MYB have been investigated during differentiation, proliferation, apoptosis and development. While v-MYB and c-MYB have been studied for > 3 decades, the structure of the whole protein is not known. Although functional dissection of MYB has revealed three domains - DNA binding domain (DBD), transcription activation domain (TAD), and negative regulatory domain (NRD)- (Fig. 1), the functional roles of these domains, especially TAD and NRD, have yet to be fully determined. For instance, it is not clear if the NRD is required for all of its role in oncogenesis, apoptosis, proliferation and differentiation. Notably, v-MYB lacks NRD in the C-terminus, which is not surprising that it functions as a transcriptional activator all the time, a property that likely contributes to its role in leukemogenesis.
Fig. 1. MYB proteins.
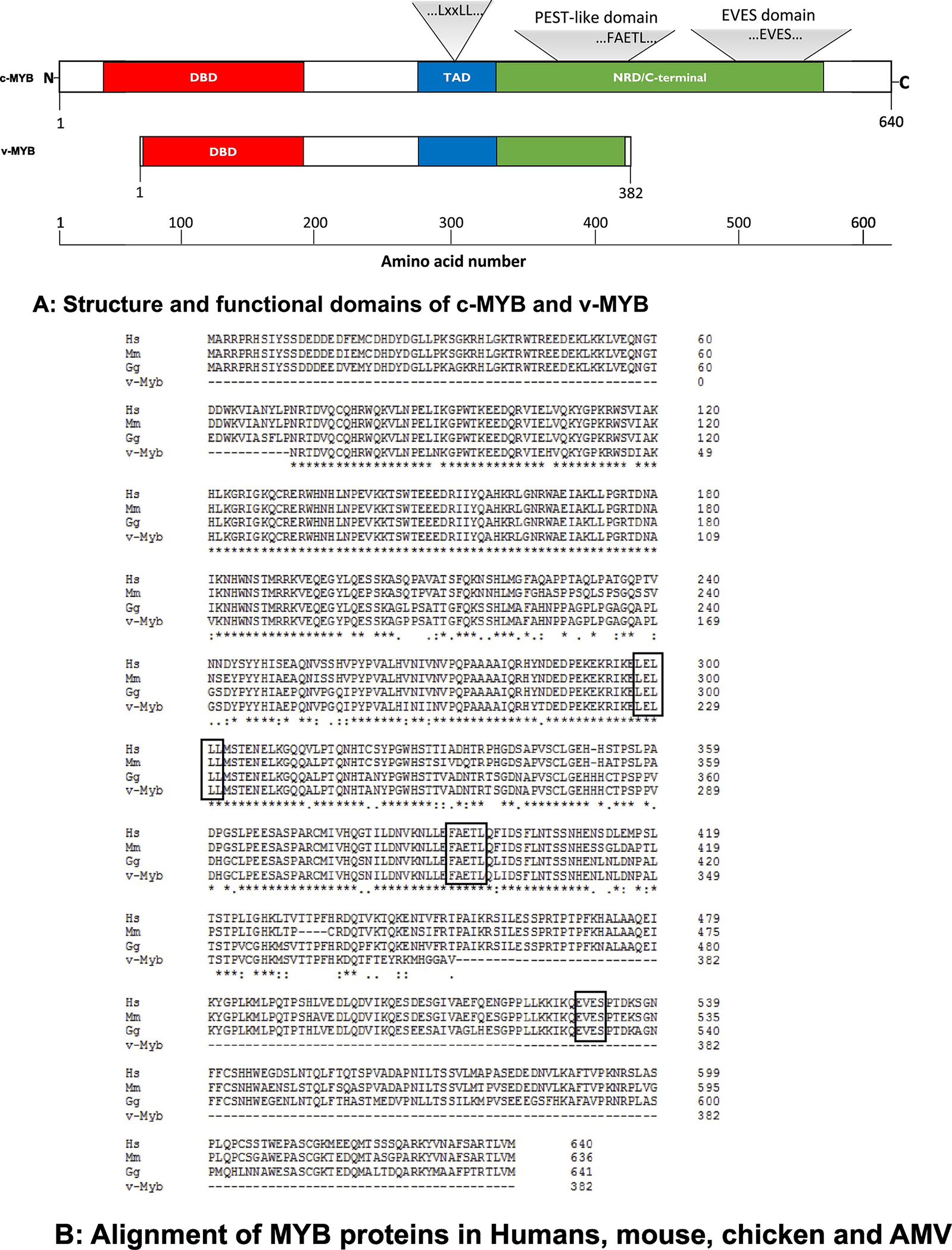
A: Structure and functional domains of c-MYB and v-MYB.
The schematic structure of c-MYB and its functional domains were drawn according to NCBI webpage, https://www.ncbi.nlm.nih.gov/protein/NP_005366.2? report=graph. DBD: DNA binding domain; TAD: transcription activation domain; NRD: negative regulatory domain. The locations for LxxLL, FAETL and EVES motives are depicted. v-MYB lacks NRD in the C-terminus, and hence, it is not surprising that it functions as a transcriptional activator all the time, a property that likely contributes to its role in leukemogenesis.
B: Alignment of MYB proteins in human, mouse, chicken and AMV.
The MYB proteins were aligned from Human (Hs for Homo sapiens, NP_005366.2), mouse (Ms for Mus musculus, NP_034978.3), chicken (Gg for Gallus gallus, P01103.1) and AMV (AAB31930.2). The identical residuals were marked as *, similar residuals were labeled as . or: The alignment was performed with software Clustal2.1. Motif sequences, “LxxLL”, “FAETL” and “EVES” are boxed.
Regulation of MYB expression is complex involving several levels, via its proximal promoter region (Sullivan et al., 1997) and microRNAs (miRNAs) at the posttranscriptional level (Lu et al., 2008; Zhao et al., 2009; Sankaran et al., 2011). Enforced miR-15a expression blocked both erythroid and myeloid colony formation in vitro, suggesting an important autoregulatory c-MYB-miR-15a circuit in human hematopoiesis (Zhao et al., 2009). A delayed HbF to HbA switch, along with persistently elevated HbF levels, in infants with trisomy 13 (Huehns et al., 1964) prompted further experiments that support involvement of miRNAs 15a and 16–1 in regulation of MYB expression. The gene encoding miRNAs 15a and 16–1 is localized on chromosome 13q14 that was unambiguously associated with the increased HbF trait in these infants (Sankaran et al., 2011). Recent experiments show that MYB is additionally controlled distally by enhancer elements > 80 kb upstream of its promoter (Stadhouders et al., 2014) and mouse studies show that the activity of Myb takes place within an active chromatin hub (Stadhouders et al., 2012).
2. MYB proteins and its transcripts
c-MYB is a transcription factor which is vital for survival. It is predominantly expressed in immature hematopoietic cells, and its expression remains precisely controlled throughout development. Knockout of the gene results in lethality at day 14 of embryogenic stage in mouse (Mucenski et al., 1991; Vegiopoulos et al., 2006).
As for other transcription factors, MYB proteins are able to bind DNA. The consensus sequence for DNA binding is 5′-YAACG/TG -3 (Howe and Watson, 1991); (Bergholtz et al., 2001), variation in the last 2 nucleotides of the consensus results in lower DNA binding. The MYB DNA binding domain is located at the N-terminal of the MYB protein. This region is also called SANT-like domain. A SANT domain is a protein domain that allows many chromatin remodeling proteins to interact with histones. SANT is an acronym for “Swi3, Ada2, N-Cor, and TFIIIB”. The SANT domain is highly conserved, and is similar to MYB DNA-binding domains. Although the structure for the DNA binding domain and the DNA binding form in solution has been solved (Ogata et al., 1995; Ogata et al., 1996; Tahirov et al., 2001) the structure of the whole protein is still not known.
It is interesting that MYB proteins have a unique DNA binding motif and lately, this motif has been found to be conserved among vertebrates and higher plants (Ambawat et al., 2013). The motif consists of imperfect repeats of about 50 amino acids. The conserved tryptophan is spaced between 18/19 amino acids and it participates in hydrophobic core formation. Each DNA binding domain consists of a structure of helix-turn-helix revealed by NMR (Ogata et al., 1992; Ogata et al., 1994; Ogata et al., 1995). Avian, mouse and human c-MYB has 3 DNA binding motives, while v-MYB from avian and E26 viruses has only two DNA binding motives (Fig. 1). The comparison between animal and v-MYB prompted the suggestion that the first DNA binding domain in animal MYB is not necessary for the DNA binding. Indeed, DNA binding assays confirmed that intact second and third DNA binding domains alone are able to bind DNA (Klempnauer and Sippel, 1987), and likely to contain the core DNA binding domains. All the MYB proteins, v-MYB from AMV and E26 virus, and animal c-MYB, are located in the nucleus and are able to bind the same DNA sequence. Although the first DNA binding domain in c-MYB is not required for DNA binding, it does facilitate its DNA binding, in that it mediates the interaction of other parts of the MYB protein and its associated proteins. Point mutation in this DBD of MYB changed the DNA-binding properties at a physiological target gene (Ivanova et al., 2007).
siRNA knockdown and global expression profiling in combination with chromatin immunoprecipitation (ChIP) assays, identified a set of c-MYB target genes in K562, an human erythroid cell line (Lorenzo et al., 2011). The genes included MYADM, LMO2, GATA2, STAT5A, and IKZF1. ChIP followed by high-throughput DNA sequencing (ChIPseq) relies on good antibodies, and currently available MYB antibodies interfere with ChIP assays. Bengtsen et al. (Bengtsen et al., 2015) used Digital Genomic Footprinting (DGF) to obtain a global picture of c-MYB occupancy in the human genome, the results showed that the predicted c-MYB specific binding sites vary strongly among hematopoietic cell types, but that a set of c-MYB footprints are common to all cell types analyzed.
The nuclear localization sequence of MYB has yet to be further confirmed. By sequential deletion experiments, Sakura et al. (Sakura et al., 1989) found that MYB protein lacking the first 2 DNA binding motives remained in the nucleus. This construct lacks transcription activation activity since it lacks DNA binding domain. Protein without the transactivation domain remained in the nuclei, while the construct with all three DNA binding motifs was found partially in the nuclei. These results suggest that the nuclear target sequence is dispersed over the N-terminal region and that the DNA binding domain has multiple functions. It can bind DNA, contains the nuclear localization signal, and is also involved in intramolecular interaction with other factors. Interestingly, Bengtsen et al. (Bengtsen et al., 2015) showed that MYB DNA binding was dynamic and varied among different hematopoietic cell types.
2.1. MYB trans-activation
The MYB transcriptional activation domain (TAD) is located in the middle of the protein (Fig. 1). By constructing vectors containing different part of c-MYB, Sakura et al. showed that the TAD domain was located in amino acids 241 to 325, although Kalkbrenner et al. (Kalkbrenner et al., 1990) defined the transactivation domain within MYB amino acids 275 to 327 by fusing the human c-MYB C-terminal regions to GAL4 DNA binding domain. Homologous comparison between v-MYB and its cellular counterpart revealed that v-MYB lacks the first 71 amino acids and 197 amino acids at C-terminus (Fig. 1). Since both v-MYB and c-MYB function as transcription factors, this suggests that the C-terminus in c-MYB is not required for transactivation. The dimerization domain is inferred from the different fusions, and may be located to amino acid 201 to 275, possibly up to 327 (Kalkbrenner et al., 1990). Nomura et al. reported that c-MYB can dimerize through its leucine zipper domain in the middle of the protein. c-MYB with mutated leucine zipper could not form dimers and interrupted transactivation of the wild type MYB protein (Nomura et al., 1993).
c-MYB plays a critical role in transactivation in hematopoietic cells and tissues, this role is mediated by interaction of TAD with CBP (CREB binding protein) and p300. LxxLL-motif is a highly conserved region on c-MYB interacting with p300, the partner domain on CBP/p300 is the KIX domain (Kasper et al., 2013). While the interaction between MYB TAD and CBP/p300 is required for its transcriptional regulation, this interaction could also directly repress target gene expression (Zhao et al., 2011). Apart from CBP and p300, MYB is able to interact with > 50 proteins in mice and human (Chatr-Aryamontri et al., 2017); (http://thebiogrid.org, Fig. 2).
Fig. 2. MYB interaction with other proteins.

For a detailed list of the 50 interacting proteins, please refer to webpage https://thebiogrid.org/. Coactivators are shown above MYB protein and corepressors, below. P: phosphorylation sites; Ac: acetylation; SUMO: SUMOylation. Only some of the 50 proteins interacting with MYB are indicated here; they include FLASH (Alm-Kristiansen et al., 2008), p100 (Leverson et al., 1998), Mi-2α (Saether et al., 2007), CBP/p300 (Pattabiraman et al., 2014), Menin (Nakata et al., 2010) Mybbp1a (Perrera et al., 2010), N-Cor, C-Ski, mSin-3a and TIF1β (Nomura et al., 2004). The modification sites are shown as phosphorylation (Luscher et al., 1990; Aziz et al., 1995), acetylation (Tomita et al., 2000) and SUMOylation (Bies et al., 2002).
Mutational studies of a region called FAETL domain (amino acid 296 to 371) of v-MYB showed that it is required for transcriptional activation and transformation of primary chicken myelomonocytic cells. Fu and Lipsick (Fu and Lipsick, 1996) showed that deletion of this region (amino acid 321–330) results in nonfunctional protein. Notably, they suggested that the leucines in this leucine rich region are not required for its function. However, alignment between v-MYB and c-MYB, A-MYB from different species revealed high conservation (Ganter and Lipsick, 1999).
EVES is another important motif at the C-terminus of MYB. This motif is highly conserved in vertebrate c-MYB and contains a known site for phosphorylation which has previously been implicated in the negative regulation of c-MYB. Dash et al. (Dash et al., 1996) demonstrated that c-MYB interacts through this motif with p100, a ubiquitously expressed transcriptional coactivator. In addition, the EVES motif also mediated intramolecular regulation of c-MYB by interacting with the N-terminus region (Dash et al., 1996). This interaction with p100 implicates its role in the expression of c-MYB, cell proliferation and differentiation. Since the intramolecular interaction influences the intermolecular interaction, this domain is named the negative regulatory domain.
Stability of transcription factors is another key factor for its transactivation role. Bies et al. (Bies and Wolff, 1997; Bies et al., 1999) found that c-MYB truncated 248 amino acids from the C-terminus has a fourfold increased half-life because of its ability to escape rapid degradation by the ubiquitin-26S proteasome pathway. What is clear is that the PEST (proline, glutamic acid, serine, threonine-rich region) region is not required for its stability but 87 amino acids at its C-terminus are essential. Kanei-Ishii et al. (Kanei-Ishii et al., 2004a; Kanei-Ishii et al., 2004b; Kanei-Ishii et al., 2008) reported that multiple targeted sites have been found at c-MYB C-terminus; Fbxw7 functions as an ubiquitin ligase targeting c-MYB through NLK (Nemo-like kinase)-induced degradation.
2.2. MYB transcripts
Dudek and Reddy first reported the existence of 2 isoforms of c-MYB proteins (Dudek and Reddy, 1989), both forms being present in normal and tumor cells. Since then, many isoforms of c-MYB have been found (Fig. 3). The major 2 isoforms in human are 89 kD (isoform 1) and 72 kD (isoform 2) in size. The p89-MYB has one extra spliced exon 9B (originally named 9A) which encoded 121 amino acids (Fig. 3). The dominant human MYB isoform (p72, isoform 2) is 72 kD (640 amino acids) (Fig. 3). Some publications reported that p89-MYB is more active in chickens (Woo et al., 1998) and in humans (O’Rourke and Ness, 2008). However, Baker et al. (Baker et al., 2010) demonstrated that the p89-MYB isoform is not required for hematopoietic development. The study generated a null-mutant mouse where exon 9B has been systemically deleted resulting in the absence of the p89-myb transcript and protein. Loss of the p89- encoding isoform does not have any deleterious effects on mammalian hematopoiesis and development. Given that the p89 isoform is a minor species in most published results and in our detection in HUDEP-2 and K562 erythroid cells (unpublished data), it would easy to conclude that p89 does not play a major role in hematopoiesis and development.
Fig. 3. Transcripts of c-MYB and its isoforms.
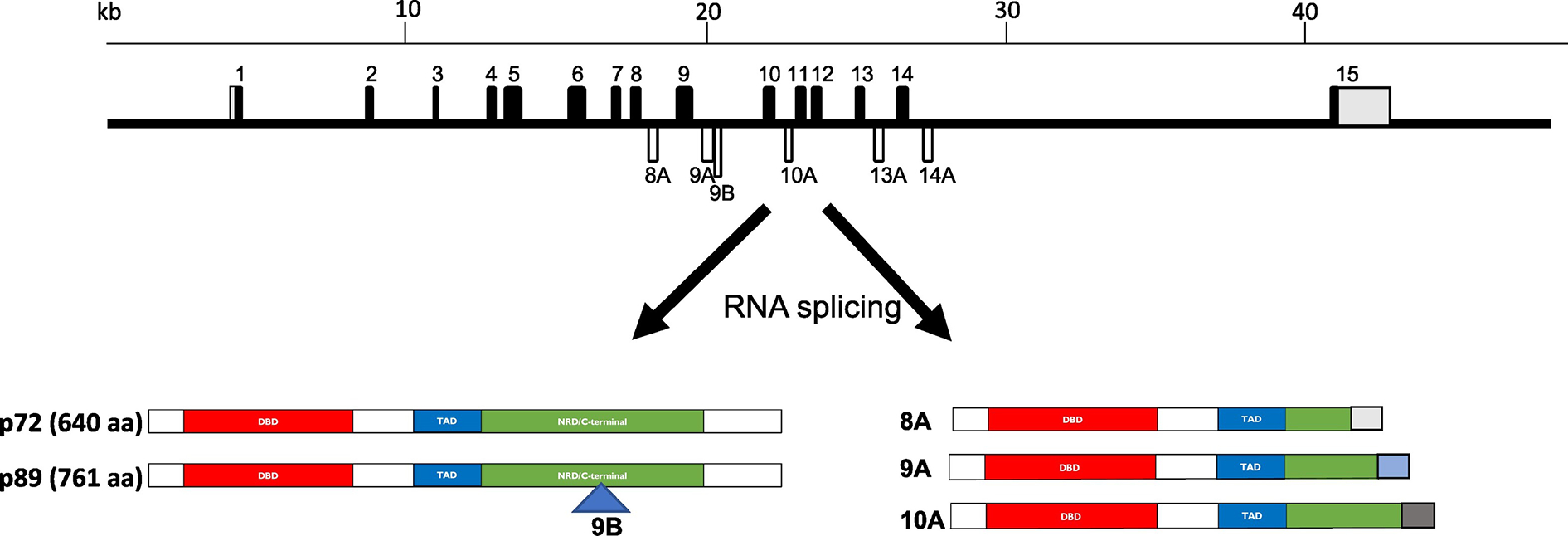
Transcripts of human c-MYB. The top part is schematic of human MYB gene structure. The black boxes are exons and the lines between exons are introns. The numbers indicated the exon numbers. The grey parts of exons are non-translated regions. The open boxes are alternative splicing exons. The lower part showed a few transcripts from different splicing (for more details, refer to (Zhou et al., 2011)). The major MYB isoform in human erythroid cells is 72 kD with 640 amino acids (NCBI Reference Sequence: NP_005366.2).
By comparing different platforms of RNA-seq, analysis of MYB alternative splicing detected at least 29 different transcript isoforms in human CD34+ progenitor and Jurkat-T cells (Brown et al., 2017). The functions of these minor isoforms in cell proliferation and differentiation are not very clear.
3. MYB is an oncogene and it regulates cell proliferation and differentiation
Being a cellular counterpart of v-MYB, c-MYB has been labeled an oncogene. c-MYB is a common integration site for avian and murine retroviruses, leading to a variety of leukemias (Oh and Reddy, 1999; Ramsay and Gonda, 2008; Zhang et al., 2012). Further studies indicated that c-MYB may be associated with some epithelial cancers and even some neural carcinomas where c-MYB expression is relatively higher in the malignant cells. However, overexpression of full length c-MYB in animal cells do not appear to be oncogenic (Grasser et al., 1991). Investigations showed that persistent expression of c-MYB blocks cellular differentiation. At present, the proposed mechanisms of MYB oncogenesis are: 1. overexpression; 2. Translocation leading to fusion with other genes, resulting in a hybrid protein which change the interaction with other proteins; 3. mutation in TAL-1 promoter creating a super MYB binding site in some leukemia (Emambokus et al., 2003; Carpinelli et al., 2004; Sandberg et al., 2005; Malaterre et al., 2008; Mansour et al., 2014).
Early studies revealed that c-MYB plays a critical role in stem cell proliferation and differentiation (Gewirtz and Calabretta, 1988; Mucenski et al., 1991; Orlic et al., 1995). Knockdown of c-MYB in human bone marrow cells with antisense oligonucleotides results in decreased colony formation in both size and number (Gewirtz and Calabretta, 1988), while c-Myb knockout in mouse is lethal due to the impaired red cell development (Mucenski et al., 1991). A mutation, M303 V in Myb, blocked the T, B and red cell development with a remarkable increase in numbers of hematopoietic cells (Sandberg et al., 2005). The mutation resulted in the disruption between c-Myb and p300, further validating that the transactivation domain of c-MYB is encompassed within amino acids 275 to 327. It is interesting that amino acid M303 is next to the transactivation motif LxxLL (amino acid 298–302) which suggests that even one key residue change at that region could disrupt or alter its function.
c-MYB expression is precisely controlled during differentiation and proliferation; a conditional decrease promoted differentiation (Hogg et al., 1997; White and Weston, 2000), while forced expression of c-MYB inhibited cell differentiation in myeloid and erythroid cell lines (McMahon et al., 1988; Selvakumaran et al., 1992). Studies with v-myb showed that even a single amino acid change in the DNA binding domain would lead to different cell fates suggesting that different forms of MYB regulate different sets of differentiation-specific genes, leading to alternative directions of differentiation (Introna et al., 1990).
c-MYB is a stem cell regulator in multiple tissue compartments, including hematopoietic stem cells (HSC), neurons and vascular smooth muscle cells (Ramsay, 2005; Sandberg et al., 2005; Sakamoto et al., 2006; Malaterre et al., 2007; Malaterre et al., 2008; Lieu and Reddy, 2009; Shikatani et al., 2016), although most studies address its regulatory effects on hematopoietic stem cells. It is known that pluripotent stem cells express more c-MYB than relatively differentiated cells. One of the conventional transforming transcription factors for iPS cells is c-MYC which is a c-MYB target gene (Nakagoshi et al., 1992; Cogswell et al., 1993). Evidence that c-MYB binds to c-MYC promoter has been provided either by ChIP assays or by whole genome ChIP-seq (Berge et al., 2007; Ciznadija et al., 2009; Quintana et al., 2011a; Quintana et al., 2011b). However, the role of c-MYB regulating c-MYC is complicated by the fact that, while c-MYB can regulate c-MYC expression, it is not essential for this function. It is interesting that c-MYB also binds to KLF4 promoter, another iPS cell transformation factor (Quintana et al., 2011a). c-MYB also bound to NANOG promoter, and while NANOG cannot initiate the iPS cells reprogramming, it is required for the acquisition of pluripotency, implicating the role of c-MYB in reprogramming.
MYB also appears to have a role in cell cycling via its interaction with cyclins. Cyclin-dependent kinases (CDKs) are the catalytic subunits of a family of mammalian heterodimeric serine/threonine kinases in the control of cell-cycle progression, transcription and neuronal function (Malumbres and Barbacid, 2005). c-MYB has been shown to interact with cyclin D1 and D2 (Ganter et al., 1998; Lei et al., 2005). v-MYB and c-MYB appears to interact differently with the D-type cyclins; v-MYB could be inhibited by D-type cyclins, but not c-MYB (Ganter et al., 1998).
Accumulated evidence showed that c-MYB interacts with other cell cycle factors and plays a role in G2/M transition in cell cycle (Frampton et al., 1996); (Wasner et al., 2003; Nakata et al., 2007). c-MYB bound to the promoters of cyclin B1 and E1, and cyclin E1 expression seems to be c-MYB-dependent (Malaterre et al., 2007; Nakata et al., 2007). All these observations suggest that c-MYB is functional in multiple steps of the cell cycle, both in G1 and G2 phases.
Genome-wide binding studies showed that c-MYB bound > 10,000 promoters (Quintana et al., 2011a; Quintana et al., 2011b), many of which belong to cell cycle regulators, such as cyclin B1, CXCL4, KLF4. Studies of the c-MYB binding profile in human CD34+ cells and human Jurkat T cells, showed that c-MYB binding to gene promoters is highly dynamic during cell cycle. The specificity of c-MYB binding is not only dramatically different in small subpopulations of cells, but MYB also actively repositions itself in sub-sets of cells (Quintana et al., 2011b). For example, c-MYB associated with the promoter of the Cyclin E1 gene only in G2/M phase cells, while the protein was expressed at equal levels in the different cell cycle fractions. Given the DNA promoter sequence and DNA binding domain of c-MYB would not change during the cell cycle, it was suggested that the mechanism may derive from the protein interaction between c-MYB and other factors (Quintana et al., 2011b). It is this dynamic binding that leads to the different sets of gene expression by c-MYB during different phases of cell cycle.
3.1. Aberrant expression of MYB results in leukemias and lymphomas
It has been proposed that v-MYB originates from c-MYB, but its expression is driven by a virus LTR which has strong promoter activity, leading to a higher level of v-MYB. Both forms of avian v-MYB (v-MYB and E26) have proteins truncated from the C-terminus of c-MYB (Fig. 1), thus lacking the C-terminus domain of c-MYB, which is a negative regulatory domain (NRD). The higher levels together with the stability of the truncated MYB protein supports the active cell proliferation, blocking differentiation. This NRD domain may keep function of c-MYB in check so that cells can control their biological fate, either proceeding to differentiation or proliferation. Post-translational modifications have been found at C-terminus of c-MYB, including phosphorylation, sumoylation, acetylation and ubiquitinylation. The degradation of c-MYB is connected to the C-terminus domains. Changes of the phosphorylation sites to alanines considerately inhibited the efficient degradation by WNT-1 and NLK pathway (Kanei-Ishii et al., 2004a). FBXW7α has been shown to interact directly with c-MYB through its WD40 domain and induce the ubiquitination of c-MYB in the presence of NLK, indicating that FBXW7 targets c-MYB for degradation in response to WNT-1 signal pathway. Mutations in FBXW7 identified in T-ALL lines suggest the role of c-MYB in malignancies (Kanei-Ishii et al., 2004a; Kanei-Ishii et al., 2008).
> 60 alternative splice forms of c-MYB mRNAs have been found in primary leukemia samples (O’Rourke and Ness, 2008; Zhou et al., 2011). Some of the splice variants correlated with poor survival in a small cohort of precursor B-ALL samples (Zhou et al., 2011), suggesting their potential role as prognostic and diagnostic biomarkers. In another study, the 9A c-MYB isoform is overexpressed in Adult T-cell leukemia/lymphoma (ATL) (Nakano et al., 2016). c-MYB-9A induced significantly higher transactivation than wild type c-MYB on its regulated genes, including critical regulators for cell proliferation and NF-κB, implicating overexpression of this c-MYB variant is associated with disorders in cellular homeostasis and consequently, accelerated transformation, cell proliferation, and malignancy in ATL cells (Nakano et al., 2016).
3.2. High levels of MYB expression in cancer cells
Deregulated expression of MYB was first reported in human acute myelogenous leukemia (Westin et al., 1982); (Rosson and Tereba, 1983; Pattabiraman and Gonda, 2013) (Table 2), and subsequently also found in several other malignancies (Biroccio et al., 2001; Greco et al., 2001; Persson et al., 2009) (Table 2). MYB is highly expressed in breast, pancreatic and other cancers (Guerin et al., 1990; Biroccio et al., 2001; Drabsch et al., 2007; Zhang et al., 2013); in pancreatic cancer, aberrant expression of MYB was found in all malignant cases, but not detectable in normal pancreas (Srivastava et al., 2015). Forced expression of MYB promoted cell growth, cell-cycle progression, survival and malignant behavior. Gene expression profile revealed that MYB modulates the expression of genes associated with proliferation, survival and metastasis supporting the role of MYB in pancreatic cancer pathogenesis (Srivastava et al., 2015).
Table 2.
Aberrant/abnormal MYB in human leukemia and cancers.
| Leukemia/cancers | Abnormities | References |
|---|---|---|
|
| ||
| AML | MYB overexpression, detected by hybridization with v-amv nick translated DNA | (Westin et al., 1982) |
| Childhood myeloid leukemia | MYB overexpression, amplification in locus | (Rosson and Tereba, 1983) |
| Acute Myelogenous Leukemia (AML) | MYB overexpression, amplification in locus | (Pelicci et al., 1984) |
| AML, ALL | MYB overexpression | (Ferrari et al., 1985) |
| T-cell leukemia cell lines (PEER and MOLT-4) | MYB overexpression, amplification in locus | (Ohyashiki et al., 1988) |
| T-ALL-derived cell lines | MYB overexpression, amplification in locus | (Siegert et al., 1990) |
| Non-Hodgkin lymphoma | MYB overexpression | (Okada et al., 1990) |
| T-ALL cell line CCRF-CEM | MYB promoter rearrangement | (Jacobs et al., 1994) |
| Leukemia cell line TK-6 derived from CML patient with T-cell blast crisis | MYB truncation | (Tomita et al., 1998) |
| Pediatric T-ALL | Recurrent translocation involving MYB | (Sinclair et al., 2005) |
| T-ALL | Recurrent genomic duplication of MYB locus | (Clappier et al., 2007); (Lahortiga et al., 2007) |
| T-ALL | Recurrent translocation involving MYB | (Clappier et al., 2007) |
| T-ALL | Alu-mediated MYB tandem duplication | (O’Neil et al., 2007) |
| T-cell leukemia | Double minute chromosomes containing MYB | (Kawamata et al., 2009) |
| AML harboring MYST3-translocations | Genomic gain in MYB locus | (Murati et al., 2009) |
| Infant acute basophilic leukemia | Recurrent translocation involving MYB | (Quelen et al., 2011) |
| AML-M5 | Recurrent translocation involving MYB | (Belloni et al., 2011) |
| T-ALL | Mutation creates a super-enhancer upstream of the TAL1 by MYB | (Mansour et al., 2014) |
| T-ALL | Mutations at LMO promoter created MYB, ETS1 and RUNX1 binding | (Rahman et al., 2017); (Hu et al., 2017) |
| Colon carcinoma cell lines | MYB aberrantly overexpression | (Alitalo et al., 1984) |
| Colon adenocarcinoma cell line | MYB overexpression | (Winqvist et al., 1985) |
| Colon carcinoma | MYB overexpression | (Torelli et al., 1987) |
| Colorectal carcinoma cell lines | MYB overexpression | (Trainer et al., 1988) |
| Colon cancer cell lines, colon tumors | MYB aberrantly overexpression | (Untawale and Blick, 1988) |
| Colon carcinoma cell line Colo 205 | MYB overexpression | (Melani et al., 1991) |
| Colonic adenomatous polyps | MYB overexpression | (Ramsay et al., 1992) |
| Colon carcinoma cell lines | MYB overexpression from mutations at attenuator | (Thompson et al., 1997) |
| Colorectal cancer (CRC) | MYB deregulated | (Biroccio et al., 2001) |
| CRC | MYB aberrantly overexpression | (Biroccio et al., 2001) |
| Colon tumor | MYB deregulated | (Greco et al., 2001) |
| CRC | mutation in poly-T track of MYB intron 1 allowed its high expression | (Hugo et al., 2006) |
| CRC | MYB overexpression | (Tichy et al., 2016) |
| Esophageal adenocarcinoma | MYB overexpression | (Brabender et al., 2001) |
| Pancreatic cancer cells | MYB aberrantly overexpression | (Srivastava et al., 2015) |
| Breast cancer | MYB overexpression, 29% in BRCA1 mutations | (Kauraniemi et al., 2000) |
| Adenoid cystic carcinomas (ACC) (breast, head and neck) | MYB-NFIB gene fusions, lost miRNA target sequence | (Persson et al., 2009) |
| ACC (salivary gland, sinonasal cavity, tracheobronchial tree, larynx, breast, and vulva) | MYB-NFIB gene fusions | (Brill 2nd et al., 2011) |
| Breast denoid cystic carcinomas | MYB-NFIB gene fusion | (D’Alfonso et al., 2014) |
| Breast carcinoma | MYB overexpression | (Li et al., 2016) |
| Pediatric brain tumors | MYB rearrangements | (Zhang et al., 2013) |
| ACC (salivary gland) | MYB-NFIB gene fusion | (Hudson and Collins, 2014) |
| ACC (salivary gland and others) | MYB-NFIB rearrangements | (Togashi et al., 2018) |
Genetic rearrangements (translocations and duplications) of c-MYB is frequently found in tumors, including leukemia (Pattabiraman and Gonda, 2013) and adenoid cystic carcinoma (Brayer et al., 2016). These genetic changes are associated with higher c-MYB expression presumably due to juxtaposition of a new enhancer in the vicinity of c-MYB (Clappier et al., 2007) or a gene dosage effect (Lahortiga et al., 2007). A recurrent t(6;9) (q22–23; p23–24) translocation, which fuses the MYB proto-oncogene on chromosome 6q to the NFIB gene on chromosome 9p, potentially resulting in the expression of novel MYB – NFIB fusion oncogenes, has been reported (Persson et al., 2009; Mitani et al., 2011). Chimeric transcripts predominantly consisting of MYB exon 14 linked to the last coding exon(s) of NFIB have been detected. It has been proposed that the deletion of conserved target sites for miR-15a/16 and miR-150 microRNAs, important in negative regulation of MYB, and over expression of the fusion transcripts and protein, activate critical c-MYB targets including genes associated with apoptosis, cell cycle control, cell growth/angiogenesis, and cell adhesion, contributing to the oncogenic potential of MYB (Persson et al., 2009).
4. MYB is essential for hematopoiesis and red cell differentiation
MYB is a key regulator of hematopoiesis and erythropoiesis (Ramsay and Gonda, 2008); (Mucenski et al., 1991). c-MYB plays an essential role in controlling the erythroid cellular proliferation/differentiation balance (Vegiopoulos et al., 2006), sustains proliferation, and a low MYB environment favors accelerated differentiation (Emambokus et al., 2003).
RNA interference (RNAi) and gene knockout experiments provided evidence that c-MYB is essential for hematopoiesis. In earlier studies, Gewirtz and Calabretta (Gewirtz and Calabretta, 1988) demonstrated that exposure of normal human bone marrow mononuclear cells to c-MYB antisense RNA resulted in a decrease in both colony size and number. Disruption of c-Myb (knockout of exon 6) in mouse by Mucenski et al. (Mucenski et al., 1991) was lethal in mice homozygous for the deletion, while heterozygous mice were phenotypically not distinguishable from wild type mice. c-Myb−/− mice exhibit embryonic (primitive) blood formation but die at about day 15 of gestation because of a failure to generate adult (definitive) hemopoiesis. Additional hematopoietic lineages were also affected in these c-Myb−/− mice. Clarke et al. differentiated c-Myb null ES cells in vitro and showed that primitive unilineage macrophage and erythroid precursor commitment could develop. However, no precursors of definitive hematopoiesis were detected, implicating maturation arrest at early multipotential stages (Clarke et al., 2000).
4.1. MYB in T and B lymphocyte development
Early studies demonstrated that knockout of c-Myb impaired development of all lineages of hematopoiesis (Mucenski et al., 1991). c-MYB is critical in T lymphocyte development, it promotes the development of helper T cells and blocks the development of cytotoxic T cells (Maurice et al., 2007). The study also showed that GATA-3 is a direct target of c-Myb, based on which Maurice et al. proposed that c-Myb is an important regulator of GATA-3. Nakata et al. (Nakata et al., 2010) provided more details on how c-MYB performs this role: it assembles a transcriptional complex with GATA-3, menin, and MLL that allows GATA-3 to auto-regulate its own expression and to regulate the development and maintain viability of Th2 cells in peripheral blood.
c-MYB plays an important role in the differentiation of lymphocytes from precursor stem cells and deletion of c-Myb blocks early T cell development (Allen 3rd et al., 1999). A conditional deletion model demonstrated that c-Myb is required for T cell differentiation during different stages T cell development (Bender et al., 2004).
Xiao et la. (Xiao et al., 2007) showed that miR-150 controls c-Myb expression in vivo in a dose-dependent manner over a narrow range of miRNA and this dramatically affects lymphocyte development and response. A key mediator in this role appears to be T-bet which is a transcription factor with essential roles in multiple immune lineages, and c-Myb has been demonstrated to have a key role in regulating the T-bet-mediated anti-viral program (Piovesan et al., 2017). Deletion of c-Myb in mature B cells significantly increased serum IgG2c and CXCR3 expression by upregulating T-bet. Increased expression of T-bet resulted in aberrant plasma cell differentiation within the germinal center. Chen et al. (Chen et al., 2017) found that miR-150 negatively regulates CD8 T cell memory in vivo by regulating c-Myb levels. Overexpression of miR-150 significantly reduced memory formation and fostered terminal differentiation with reduced c-MYB expression. Knocking out miR-150 resulted in higher levels of c-Myb and disrupted the balance between memory precursor and terminal effector CD8 T cells following acute viral infection. The study also showed that c-Myb regulated genes, such as Bcl-2 and Bcl-xL, were upregulated, suggesting a miR-150-c-Myb survival circuit during memory CD8 T cell development (Chen et al., 2017).
4.2. MYB enhancers regulate fetal hemoglobin (HbF) levels
A set of common single nucleotide polymorphisms (SNPs) at the HBS1L-MYB intergenic region in chromosome 6q23 has been consistently identified as highly associated with clinically important human erythroid traits (van der Harst et al., 2012). Prominent among these traits is the persistence of fetal hemoglobin in adults (HbF, measured as % HbF of total hemoglobin or as proportion of red blood cells carrying HbF, ‘% F cells’). A small number of these SNPs displayed an especially strong association, and are encompassed within a region of about 24 kb (originally termed HBS1L-MYB Intergenic Polymorphism block 2 or “HMIP-2”). The causal variants reside in two clusters within the block, at −84 and −71 kb respectively, upstream of MYB. The SNPs at these two regions disrupt binding of key erythroid enhancers affecting long-range interactions with MYB and MYB expression, providing a functional explanation for the genetic association of the 6q HBS1L-MYB intergenic region with HbF and F cell levels (Stadhouders et al., 2014) (Fig. 4).
Fig. 4.
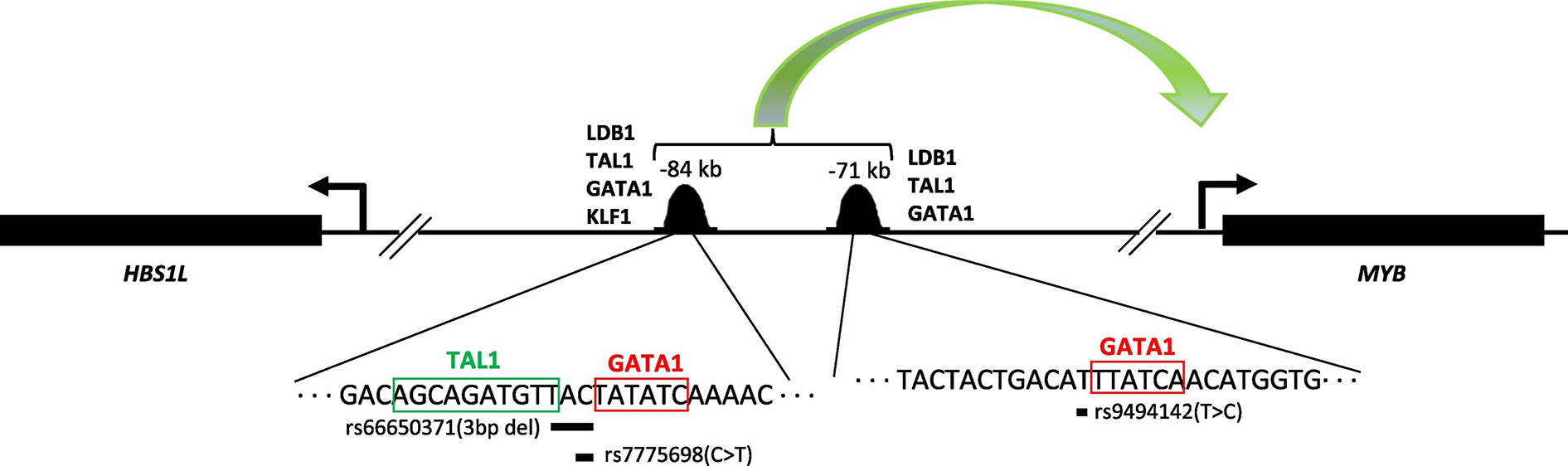
HBS1L – MYB intergenic region on chromosome 6q23 with the 2 enhancer sites encompassing the SNPs associated with fetal hemoglobin levels in adults. Schematic structure (not to scale) of HBS1L and MYB region is depicted. The transcription direction of HBS1L and MYB are indicated with black arrows. The peaks at −84 and − 71 are from transcription factor (as indicated) binding data (Stadhouders et al., 2014). The zoomed in parts show DNA sequence at −84 and − 71 upstream of the transcription start site of MYB. The consensus sequences for TAL1 and GATA1 are labeled and boxed. The three SNPs associated with fetal hemoglobin levels in adults are indicated. The green arrow shows the interactions between the enhancers and proximal promoter.
Mouse supporting studies were provided by Suzuki et al. (Suzuki et al., 2013) who reported a mouse model of hereditary persistence of fetal hemoglobin (HPFH) in which a transgene was randomly inserted into the orthologous murine Hbs1l-Myb locus. The transgene location corresponded to the −84 kb of human HMIP region. Homozygous transgenic mice (Hbs1l-MybTg/Tg) displayed a marked reduction of Myb expression in the megakaryocytic and erythroid lineages, and approximately 4- and 20-fold-higher levels of mouse embryonic εy- and βh1-globins, respectively, than control animals. Studies in mice utilizing ChIP-seq and Chromatin Conformation Capture (3C)-Sequencing showed that Myb activity occurs within an active chromatin hub (ACH) encompassing the enhancers in the Myb-Hbs1l intergenic region and the Myb promoter and first intron. Downregulation of Myb on erythroid differentiation leads to destabilization of the ACH (Stadhouders et al., 2012).
A systemic dissection applying saturating mutagenesis using multiple Cas9 CRISPR genome-editing approach identified 98 DNase I–hypersensitive sites (DHSs) in the HMIP region from erythroid precursors (Canver et al., 2017). The results confirmed the enhancer function of −84 kb and a potential element at −71 kb upstream of c-MYB, consistent with previous findings (Thein et al., 2007; Stadhouders et al., 2014). It also identified a novel c-MYB enhancer at position −36 and confirmed presence of the other potential elements at positions −7, −83 and −126 kb. Erythroid-specific transcripts were detected in the intergenic region (Wahlberg et al., 2009), that were subsequently shown to be long non-coding RNA (lncRNA) at the −84 region, in erythroid cells (Morrison et al., 2017). The mechanistics of how the lncRNA affects MYB expression was not shown in the study (Morrison et al., 2017). The conclusion from these studies is that, although regulation of MYB expression is via its proximal promoter region (Sullivan et al., 1997) and microRNAs (Lu et al., 2008; Zhao et al., 2009; Sankaran et al., 2011), it is additionally controlled distally by enhancer elements > 80 kb upstream of its promoter, illustrating the high degree of regulatory complexity that governs MYB expression.
These studies also established that MYB is the relevant gene in the HBS1L-MYB region regulating the erythroid traits and fetal hemoglobin levels. Compelling evidence has also been provided that the increased HbF effect is mediated, at least in part, through down-modulation of MYB as shown by targeting of its 3′UTR by microRNAs 15a and 16–1 (Sankaran et al., 2011). shRNA studies against c-MYB results in elevated fetal hemoglobin gene expression (Sankaran et al., 2011). Forced expression of c-MYB in K562 cells significantly inhibited γ-globin gene expression when compared with the parental cell line or vector controls (Jiang et al., 2006).
How c-MYB exactly controls HbF levels and the many other erythroid traits is not yet fully understood. A clear anti-correlation between MYB and HbF levels has emerged, which was further confirmed by the reduced MYB expression we observed in erythroid cells from high HbF individuals (Jiang et al., 2006). Lower MYB levels were reported to slow down cell cycle progression and to accelerate differentiation kinetics in later stages of erythroid development in mouse and human erythroid cells (Jiang et al., 2006; Bianchi et al., 2010). In accordance with these results, ChIP-seq experiments detected c-Myb binding to key cell cycle regulators (i.e. Bcl2, Cdk6, Myc) in murine erythroid progenitors. Furthermore, several of these genes were found to be misregulated in MYB loss-of-function studies in human erythroid progenitors. Accelerated differentiation in an environment of lower MYB levels could favor premature cell cycle termination during the proliferation cycles of adult erythropoiesis, producing more erythroid cells that synthesize predominantly HbF (“F-cells”) before the switch to adult hemoglobin synthesis occurs.
Alternatively, recent studies suggest that the c-MYB transcription factor plays an important role in the emerging TF network governing γ-globin expression, in which the BCL11A and KLF1 proteins play key repressive roles. Analysis of c-MYB loss-of-function studies (Bianchi et al., 2010; Sankaran et al., 2011; Suzuki et al., 2013) indeed showed that several of the c-MYB-bound γ-globin repressor genes (i.e. BCL11A, KLK1) are downregulated upon MYB depletion. These observations suggest that c-MYB directly activates key γ-globin repressor genes.
5. Summary
MYB is a transcription factor comprising 3 functional domains, DBD, TAD and NRD. It is required for hematopoiesis and essential for life. MYB can interact with many proteins, including p300, a multifunctional protein. p300 has several TAD domains and physically interact with many transcription factors. Both v-MYB and c-MYB are able to bind similar sequences of DNA and the functional difference may be from the protein interaction with other factors. Aberrant MYB expression, rare isoforms and truncated forms of MYB, have been found in human leukemias and solid tumors. Increasing fetal hemoglobin is beneficial for beta hemoglobinopathies, and recent investigations have provided some insight on the role of MYB in fetal hemoglobin gene expression. Dissection of the dynamic MYB expression and the dynamic interaction of MYB with a complex network of proteins is challenging but will provide insights on how it may control cell proliferation, differentiation and cell maturation.
Acknowledgements
We thank Rusinel Amarante for her help in preparation of the manuscript.
Financial support and sponsorship
This work was supported by the Intramural Research Program of the National Heart, Lungs, and Blood Institute, NIH.
Abbreviations:
- MYB
MYB protein
- MYB
gene
- v-MYB
AMV MYB protein
- c-Myb
Myb protein from either mouse or chicken
- c-Myb
Myb gene from mouse or chicken
- DBD
DNA binding domain
- TAD
transcription activation domain
- NRD
negative regulatory domain
- HMIP
HBS1L-MYB intergenic polymorphisms
- AMV
avian myeloblastosis virus
- HbF
fetal hemoglobin
- miRNA
microRNA
- SANT
Swi3, Ada2, N-Cor, and TFIIIB
- ChIPseq
ChIP sequencing
- PEST
proline, glutamic acid, serine, threonine
- ATL
Adult T-cell leukemia/lymphoma
- ALL
acute lymphoblastic leukemia
- AML
acute myelogenous leukemia
- CRC
colorectal cancer
- ACC
adenoid cystic carcinomas
- DHSs
DNase I–hypersensitive sites
Footnotes
Conflicts of interest
None declared for all authors.
References
- Alitalo K, Winqvist R, Lin CC, de la Chapelle A, Schwab M, Bishop JM, 1984. Aberrant expression of an amplified c-myb oncogene in two cell lines from a colon carcinoma. Proc. Natl. Acad. Sci. U. S. A. 81, 4534–4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RD 3rd, Bender TP, Siu G, 1999. c-Myb is essential for early T cell development. Genes Dev. 13, 1073–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm-Kristiansen AH, Saether T, Matre V, Gilfillan S, Dahle O, Gabrielsen OS, 2008. FLASH acts as a co-activator of the transcription factor c-Myb and localizes to active RNA polymerase II foci. Oncogene 27, 4644–4656. [DOI] [PubMed] [Google Scholar]
- Ambawat S, Sharma P, Yadav NR, Yadav RC, 2013. MYB transcription factor genes as regulators for plant responses: an overview. Physiol. Mol. Biol. Plants 19, 307–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz N, Miglarese MR, Hendrickson RC, Shabanowitz J, Sturgill TW, Hunt DF, Bender TP, 1995. Modulation of c-Myb-induced transcription activation by a phosphorylation site near the negative regulatory domain. Proc. Natl. Acad. Sci. U. S. A. 92, 6429–6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SJ, Kumar A, Reddy EP, 2010. p89c-Myb is not required for fetal or adult hematopoiesis. Genesis 48, 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belloni E, Shing D, Tapinassi C, Viale A, Mancuso P, Malazzi O, Gerbino E, Dall’Olio V, Egurbide I, Odero MD, Bertolini F, Pelicci PG, 2011. In vivo expression of an aberrant MYB-GATA1 fusion induces leukemia in the presence of GATA1 reduced levels. Leukemia 25, 733–736. [DOI] [PubMed] [Google Scholar]
- Bender TP, Kremer CS, Kraus M, Buch T, Rajewsky K, 2004. Critical functions for c-Myb at three checkpoints during thymocyte development. Nat. Immunol. 5, 721–729. [DOI] [PubMed] [Google Scholar]
- Bengtsen M, Klepper K, Gundersen S, Cuervo I, Drablos F, Hovig E, Sandve GK, Gabrielsen OS, Eskeland R, 2015. c-Myb binding sites in haematopoietic chromatin landscapes. PLoS One 10, e0133280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berge T, Matre V, Brendeford EM, Saether T, Luscher B, Gabrielsen OS, 2007. Revisiting a selection of target genes for the hematopoietic transcription factor c-Myb using chromatin immunoprecipitation and c-Myb knockdown. Blood Cells Mol. Dis. 39, 278–286. [DOI] [PubMed] [Google Scholar]
- Bergholtz S, Andersen TO, Andersson KB, Borrebaek J, Luscher B, Gabrielsen OS, 2001. The highly conserved DNA-binding domains of A-, B- and c-Myb differ with respect to DNA-binding, phosphorylation and redox properties. Nucleic Acids Res. 29, 3546–3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi E, Zini R, Salati S, Tenedini E, Norfo R, Tagliafico E, Manfredini R, Ferrari S, 2010. c-Myb supports erythropoiesis through the transactivation of KLF1 and LMO2 expression. Blood 116, e99–110. [DOI] [PubMed] [Google Scholar]
- Bies J, Wolff L, 1997. Oncogenic activation of c-Myb by carboxyl-terminal truncation leads to decreased proteolysis by the ubiquitin-26S proteasome pathway. Oncogene 14, 203–212. [DOI] [PubMed] [Google Scholar]
- Bies J, Nazarov V, Wolff L, 1999. Alteration of proteolytic processing of c-Myb as a consequence of its truncation in murine myeloid leukemia. Leukemia 13 (Suppl. 1), S116–7. [DOI] [PubMed] [Google Scholar]
- Bies J, Markus J, Wolff L, 2002. Covalent attachment of the SUMO-1 protein to the negative regulatory domain of the c-Myb transcription factor modifies its stability and transactivation capacity. J. Biol. Chem. 277, 8999–9009. [DOI] [PubMed] [Google Scholar]
- Biroccio A, Benassi B, D’Agnano I, D’Angelo C, Buglioni S, Mottolese M, Ricciotti A, Citro G, Cosimelli M, Ramsay RG, Calabretta B, Zupi G, 2001. c-Myb and Bcl-x overexpression predicts poor prognosis in colorectal cancer: clinical and experimental findings. Am. J. Pathol. 158, 1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle WJ, Lipsick JS, Reddy EP, Baluda MA, 1983. Identification of the leukemogenic protein of avian myeloblastosis virus and of its normal cellular homologue. Proc. Natl. Acad. Sci. U. S. A. 80, 2834–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabender J, Lord RV, Danenberg KD, Metzger R, Schneider PM, Park JM, Salonga D, Groshen S, Tsao-Wei DD, DeMeester TR, Holscher AH, Danenberg PV, 2001. Increased c-myb mRNA expression in Barrett’s esophagus and Barrett’s-associated adenocarcinoma. J. Surg. Res. 99, 301–306. [DOI] [PubMed] [Google Scholar]
- Brayer KJ, Frerich CA, Kang H, Ness SA, 2016. Recurrent fusions in MYB and MYBL1 define a common, transcription factor-driven oncogenic pathway in salivary gland adenoid cystic carcinoma. Cancer Discov. 6, 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill LB 2nd., Kanner WA, Fehr A, Andren Y, Moskaluk CA, Loning T, Stenman G, Frierson HF Jr., 2011. Analysis of MYB expression and MYB-NFIB gene fusions in adenoid cystic carcinoma and other salivary neoplasms. Mod. Pathol. 24, 1169–1176. [DOI] [PubMed] [Google Scholar]
- Brown RB, Madrid NJ, Suzuki H, Ness SA, 2017. Optimized approach for ion proton RNA sequencing reveals details of RNA splicing and editing features of the transcriptome. PLoS One 12, e0176675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canver MC, Lessard S, Pinello L, Wu Y, Ilboudo Y, Stern EN, Needleman AJ, Galacteros F, Brugnara C, Kutlar A, McKenzie C, Reid M, Chen DD, Das PP, M AC, Zeng J, Kurita R, Nakamura Y, Yuan GC, Lettre G, Bauer DE, Orkin SH, 2017. Variant-aware saturating mutagenesis using multiple Cas9 nucleases identifies regulatory elements at trait-associated loci. Nat. Genet. 49, 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpinelli MR, Hilton DJ, Metcalf D, Antonchuk JL, Hyland CD, Mifsud SL, Di Rago L, Hilton AA, Willson TA, Roberts AW, Ramsay RG, Nicola NA, Alexander WS, 2004. Suppressor screen in Mpl−/− mice: c-Myb mutation causes supraphysiological production of platelets in the absence of thrombopoietin signaling. Proc. Natl. Acad. Sci. U. S. A. 101, 6553–6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatr-Aryamontri A, Oughtred R, Boucher L, Rust J, Chang C, Kolas NK, O’Donnell L, Oster S, Theesfeld C, Sellam A, Stark C, Breitkreutz BJ, Dolinski K, Tyers M, 2017. The BioGRID interaction database: 2017 update. Nucleic Acids Res. 45, D369–D379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Stelekati E, Kurachi M, Yu S, Cai Z, Manne S, Khan O, Yang X, Wherry EJ, 2017. miR-150 regulates memory CD8 T cell differentiation via c-Myb. Cell Rep. 20, 2584–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciznadija D, Tothill R, Waterman ML, Zhao L, Huynh D, Yu RM, Ernst M, Ishii S, Mantamadiotis T, Gonda TJ, Ramsay RG, Malaterre J, 2009. Intestinal adenoma formation and MYC activation are regulated by cooperation between MYB and Wnt signaling. Cell Death Differ. 16, 1530–1538. [DOI] [PubMed] [Google Scholar]
- Clappier E, Cuccuini W, Kalota A, Crinquette A, Cayuela JM, Dik WA, Langerak AW, Montpellier B, Nadel B, Walrafen P, Delattre O, Aurias A, Leblanc T, Dombret H, Gewirtz AM, Baruchel A, Sigaux F, Soulier J, 2007. The C-MYB locus is involved in chromosomal translocation and genomic duplications in human T-cell acute leukemia (T-ALL), the translocation defining a new T-ALL subtype in very young children. Blood 110, 1251–1261. [DOI] [PubMed] [Google Scholar]
- Clarke D, Vegiopoulos A, Crawford A, Mucenski M, Bonifer C, Frampton J, 2000. In vitro differentiation of c-myb(−/−) ES cells reveals that the colony forming capacity of unilineage macrophage precursors and myeloid progenitor commitment are c-Myb independent. Oncogene 19, 3343–3351. [DOI] [PubMed] [Google Scholar]
- Cogswell JP, Cogswell PC, Kuehl WM, Cuddihy AM, Bender TM, Engelke U, Marcu KB, Ting JP, 1993. Mechanism of c-myc regulation by c-Myb in different cell lineages. Mol. Cell. Biol. 13, 2858–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alfonso TM, Mosquera JM, MacDonald TY, Padilla J, Liu YF, Rubin MA, Shin SJ, 2014. MYB-NFIB gene fusion in adenoid cystic carcinoma of the breast with special focus paid to the solid variant with basaloid features. Hum. Pathol. 45, 2270–2280. [DOI] [PubMed] [Google Scholar]
- Dash AB, Orrico FC, Ness SA, 1996. The EVES motif mediates both intermolecular and intramolecular regulation of c-Myb. Genes Dev. 10, 1858–1869. [DOI] [PubMed] [Google Scholar]
- Drabsch Y, Hugo H, Zhang R, Dowhan DH, Miao YR, Gewirtz AM, Barry SC, Ramsay RG, Gonda TJ, 2007. Mechanism of and requirement for estrogen-regulated MYB expression in estrogen-receptor-positive breast cancer cells. Proc. Natl. Acad. Sci. U. S. A. 104, 13762–13767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek H, Reddy EP, 1989. Identification of two translational products for c-myb. Oncogene 4, 1061–1066. [PubMed] [Google Scholar]
- Emambokus N, Vegiopoulos A, Harman B, Jenkinson E, Anderson G, Frampton J, 2003. Progression through key stages of haemopoiesis is dependent on distinct threshold levels of c-Myb. EMBO J. 22, 4478–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Torelli U, Selleri L, Donelli A, Venturelli D, Narni F, Moretti L, Torelli G, 1985. Study of the levels of expression of two oncogenes, c-myc and c-myb, in acute and chronic leukemias of both lymphoid and myeloid lineage. Leuk. Res. 9, 833–842. [DOI] [PubMed] [Google Scholar]
- Frampton J, Ramqvist T, Graf T, 1996. V-Myb of E26 leukemia virus up-regulates bcl-2 and suppresses apoptosis in myeloid cells. Genes Dev. 10, 2720–2731. [DOI] [PubMed] [Google Scholar]
- Fu SL, Lipsick JS, 1996. FAETL motif required for leukemic transformation by v-Myb. J. Virol. 70, 5600–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganter B, Lipsick JS, 1999. Myb and oncogenesis. Adv. Cancer Res. 76, 21–60. [DOI] [PubMed] [Google Scholar]
- Ganter B, Fu S, Lipsick JS, 1998. D-type cyclins repress transcriptional activation by the v-Myb but not the c-Myb DNA-binding domain. EMBO J. 17, 255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner K, Thein SL, 2016. Genetic Factors Modifying Sickle Cell Disease Severity. In: Costa FF, Conran N (Eds.), Sickle Cell Anemia - from Basic Science to Clinical Practice. Springer International, Switzerland, pp. 371–397. [Google Scholar]
- George OL, Ness SA, 2014. Situational awareness: regulation of the myb transcription factor in differentiation, the cell cycle and oncogenesis. Cancers (Basel) 6, 2049–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz AM, Calabretta B, 1988. A c-myb antisense oligodeoxynucleotide inhibits normal human hematopoiesis in vitro. Science 242, 1303–1306. [DOI] [PubMed] [Google Scholar]
- Gonda TJ, Gough NM, Dunn AR, de Blaquiere J, 1985. Nucleotide sequence of cDNA clones of the murine myb proto-oncogene. EMBO J. 4, 2003–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasser FA, Graf T, Lipsick JS, 1991. Protein truncation is required for the activation of the c-myb proto-oncogene. Mol. Cell. Biol. 11, 3987–3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco C, Alvino S, Buglioni S, Assisi D, Lapenta R, Grassi A, Stigliano V, Mottolese M, Casale V, 2001. Activation of c-MYC and c-MYB proto-oncogenes is associated with decreased apoptosis in tumor colon progression. Anticancer Res. 21, 3185–3192. [PubMed] [Google Scholar]
- Guerin M, Sheng ZM, Andrieu N, Riou G, 1990. Strong association between c-myb and oestrogen-receptor expression in human breast cancer. Oncogene 5, 131–135. [PubMed] [Google Scholar]
- Hall WJ, Bean CW, Pollard M, 1941. Transmission of fowl leucosis through chick embryos and young chicks. Am. J. Vet. Res. 2, 272–279. [Google Scholar]
- Hogg A, Schirm S, Nakagoshi H, Bartley P, Ishii S, Bishop JM, Gonda TJ, 1997. Inactivation of a c-Myb/estrogen receptor fusion protein in transformed primary cells leads to granulocyte/macrophage differentiation and down regulation of c-kit but not c-myc or cdc2. Oncogene 15, 2885–2898. [DOI] [PubMed] [Google Scholar]
- Howe KM, Watson RJ, 1991. Nucleotide preferences in sequence-specific recognition of DNA by c-myb protein. Nucleic Acids Res. 19, 3913–3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Qian M, Zhang H, Guo Y, Yang J, Zhao X, He H, Lu J, Pan J, Chang M, Du G, Lin TN, Kham SK, Quah TC, Ariffin H, Tan AM, Cheng Y, Li C, Yeoh AE, Pui CH, Skanderup AJ, Yang JJ, 2017. Whole-genome noncoding sequence analysis in T-cell acute lymphoblastic leukemia identifies oncogene enhancer mutations. Blood 129, 3264–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JB, Collins BT, 2014. MYB gene abnormalities t(6;9) in adenoid cystic carcinoma fine-needle aspiration biopsy using fluorescence in situ hybridization. Arch. Pathol. Lab. Med. 138, 403–409. [DOI] [PubMed] [Google Scholar]
- Huehns ER, Hecht F, Keil JV, Motulsky AG, 1964. Developmental hemoglobin anomalies in a chromosomal triplication: D1 trisomy syndrome. Proc. Natl. Acad. Sci. U. S. A. 51, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo H, Cures A, Suraweera N, Drabsch Y, Purcell D, Mantamadiotis T, Phillips W, Dobrovic A, Zupi G, Gonda TJ, Iacopetta B, Ramsay RG, 2006. Mutations in the MYB intron I regulatory sequence increase transcription in colon cancers. Genes Chromosomes Cancer 45, 1143–1154. [DOI] [PubMed] [Google Scholar]
- Introna M, Golay J, Frampton J, Nakano T, Ness SA, Graf T, 1990. Mutations in v-myb alter the differentiation of myelomonocytic cells transformed by the oncogene. Cell 63, 1289–1297. [DOI] [PubMed] [Google Scholar]
- Ivanova O, Braas D, Klempnauer KH, 2007. Oncogenic point mutations in the Myb DNA-binding domain alter the DNA-binding properties of Myb at a physiological target gene. Nucleic Acids Res. 35, 7237–7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs SM, Gorse KM, Kennedy SJ, Westin EH, 1994. Characterization of a rearrangement in the c-MYB promoter in the acute lymphoblastic leukemia cell line CCRF-CEM. Cancer Genet. Cytogenet. 75, 31–39. [DOI] [PubMed] [Google Scholar]
- Jiang J, Best S, Menzel S, Silver N, Lai MI, Surdulescu GL, Spector TD, Thein SL, 2006. cMYB is involved in the regulation of fetal hemoglobin production in adults. Blood 108, 1077–1083. [DOI] [PubMed] [Google Scholar]
- Kalkbrenner F, Guehmann S, Moelling K, 1990. Transcriptional activation by human c-myb and v-myb genes. Oncogene 5, 657–661. [PubMed] [Google Scholar]
- Kanei-Ishii C, Ninomiya-Tsuji J, Tanikawa J, Nomura T, Ishitani T, Kishida S, Kokura K, Kurahashi T, Ichikawa-Iwata E, Kim Y, Matsumoto K, Ishii S, 2004a. Wnt-1 signal induces phosphorylation and degradation of c-Myb protein via TAK1, HIPK2, and NLK. Genes Dev. 18, 816–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanei-Ishii C, Nomura T, Tanikawa J, Ichikawa-Iwata E, Ishii S, 2004b. Differential sensitivity of v-Myb and c-Myb to Wnt-1-induced protein degradation. J. Biol. Chem. 279, 44582–44589. [DOI] [PubMed] [Google Scholar]
- Kanei-Ishii C, Nomura T, Takagi T, Watanabe N, Nakayama KI, Ishii S, 2008. Fbxw7 acts as an E3 ubiquitin ligase that targets c-Myb for nemo-like kinase (NLK)-induced degradation. J. Biol. Chem. 283, 30540–30548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper LH, Fukuyama T, Lerach S, Chang Y, Xu W, Wu S, Boyd KL, Brindle PK, 2013. Genetic interaction between mutations in c-Myb and the KIX domains of CBP and p300 affects multiple blood cell lineages and influences both gene activation and repression. PLoS One 8, e82684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauraniemi P, Hedenfalk I, Persson K, Duggan DJ, Tanner M, Johannsson O, Olsson H, Trent JM, Isola J, Borg A, 2000. MYB oncogene amplification in hereditary BRCA1 breast cancer. Cancer Res. 60, 5323–5328. [PubMed] [Google Scholar]
- Kawamata N, Zhang L, Ogawa S, Nannya Y, Dashti A, Lu D, Lim S, Schreck R, Koeffler HP, 2009. Double minute chromosomes containing MYB gene and NUP214-ABL1 fusion gene in T-cell leukemia detected by single nucleotide polymorphism DNA microarray and fluorescence in situ hybridization. Leuk. Res. 33, 569–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempnauer KH, Sippel AE, 1987. The highly conserved amino-terminal region of the protein encoded by the v-myb oncogene functions as a DNA-binding domain. EMBO J. 6, 2719–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempnauer KH, Gonda TJ, Bishop JM, 1982. Nucleotide sequence of the retroviral leukemia gene v-myb and its cellular progenitor c-myb: the architecture of a transduced oncogene. Cell 31, 453–463. [DOI] [PubMed] [Google Scholar]
- Klempnauer KH, Ramsay G, Bishop JM, Moscovici MG, Moscovici C, McGrath JP, Levinson AD, 1983. The product of the retroviral transforming gene v-myb is a truncated version of the protein encoded by the cellular oncogene c-myb. Cell 33, 345–355. [DOI] [PubMed] [Google Scholar]
- Lahortiga I, De Keersmaecker K, Van Vlierberghe P, Graux C, Cauwelier B, Lambert F, Mentens N, Beverloo HB, Pieters R, Speleman F, Odero MD, Bauters M, Froyen G, Marynen P, Vandenberghe P, Wlodarska I, Meijerink JP, Cools J, 2007. Duplication of the MYB oncogene in T cell acute lymphoblastic leukemia. Nat. Genet. 39 (5), 593. [DOI] [PubMed] [Google Scholar]
- Lei W, Liu F, Ness SA, 2005. Positive and negative regulation of c-Myb by cyclin D1, cyclin-dependent kinases, and p27 Kip1. Blood 105, 3855–3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverson JD, Koskinen PJ, Orrico FC, Rainio EM, Jalkanen KJ, Dash AB, Eisenman RN, Ness SA, 1998. Pim-1 kinase and p100 cooperate to enhance c-Myb activity. Mol. Cell 2, 417–425. [DOI] [PubMed] [Google Scholar]
- Li Y, Jin K, van Pelt GW, van Dam H, Yu X, Mesker WE, Ten Dijke P, Zhou F, Zhang L, 2016. c-Myb enhances breast cancer invasion and metastasis through the Wnt/beta-catenin/AXIN2 pathway. Cancer Res. 76, 3364–3375. [DOI] [PubMed] [Google Scholar]
- Lieu YK, Reddy EP, 2009. Conditional c-myb knockout in adult hematopoietic stem cells leads to loss of self-renewal due to impaired proliferation and accelerated differentiation. Proc. Natl. Acad. Sci. U. S. A. 106, 21689–21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsick JS, Wang DM, 1999. Transformation by v-Myb. Oncogene 18, 3047–3055. [DOI] [PubMed] [Google Scholar]
- Lorenzo PI, Brendeford EM, Gilfillan S, Gavrilov AA, Leedsak M, Razin SV, Eskeland R, Saether T, Gabrielsen OS, 2011. Identification of c-Myb target genes in K562 cells reveals a role for c-Myb as a master regulator. Genes Cancer 2, 805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Guo S, Ebert BL, Zhang H, Peng X, Bosco J, Pretz J, Schlanger R, Wang JY, Mak RH, Dombkowski DM, Preffer FI, Scadden DT, Golub TR, 2008. MicroRNA-mediated control of cell fate in megakaryocyte-erythrocyte progenitors. Dev. Cell 14, 843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Christenson E, Litchfield DW, Krebs EG, Eisenman RN, 1990. Myb DNA binding inhibited by phosphorylation at a site deleted during oncogenic activation. Nature 344, 517–522. [DOI] [PubMed] [Google Scholar]
- Majello B, Kenyon LC, Dalla-Favera R, 1986. Human c-myb protooncogene: nucleotide sequence of cDNA and organization of the genomic locus. Proc. Natl. Acad. Sci. U. S. A. 83, 9636–9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaterre J, Carpinelli M, Ernst M, Alexander W, Cooke M, Sutton S, Dworkin S, Heath JK, Frampton J, McArthur G, Clevers H, Hilton D, Mantamadiotis T, Ramsay RG, 2007. c-Myb is required for progenitor cell homeostasis in colonic crypts. Proc. Natl. Acad. Sci. U. S. A. 104, 3829–3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaterre J, Mantamadiotis T, Dworkin S, Lightowler S, Yang Q, Ransome MI, Turnley AM, Nichols NR, Emambokus NR, Frampton J, Ramsay RG, 2008. c-Myb is required for neural progenitor cell proliferation and maintenance of the neural stem cell niche in adult brain. Stem Cells 26, 173–181. [DOI] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M, 2005. Mammalian cyclin-dependent kinases. Trends Biochem. Sci. 30, 630–641. [DOI] [PubMed] [Google Scholar]
- Mansour MR, Abraham BJ, Anders L, Berezovskaya A, Gutierrez A, Durbin AD, Etchin J, Lawton L, Sallan SE, Silverman LB, Loh ML, Hunger SP, Sanda T, Young RA, Look AT, 2014. Oncogene regulation. An oncogenic super-enhancer formed through somatic mutation of a noncoding intergenic element. Science 346, 1373–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice D, Hooper J, Lang G, Weston K, 2007. c-Myb regulates lineage choice in developing thymocytes via its target gene Gata3. EMBO J. 26, 3629–3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon J, Howe KM, Watson RJ, 1988. The induction of friend erythroleukaemia differentiation is markedly affected by expression of a transfected c-myb cDNA. Oncogene 3, 717–720. [PubMed] [Google Scholar]
- Melani C, Rivoltini L, Parmiani G, Calabretta B, Colombo MP, 1991. Inhibition of proliferation by c-myb antisense oligodeoxynucleotides in colon adenocarcinoma cell lines that express c-myb. Cancer Res. 51, 2897–2901. [PubMed] [Google Scholar]
- Mitani Y, Rao PH, Futreal PA, Roberts DB, Stephens PJ, Zhao YJ, Zhang L, Mitani M, Weber RS, Lippman SM, Caulin C, El-Naggar AK, 2011. Novel chromosomal rearrangements and break points at the t(6;9) in salivary adenoid cystic carcinoma: association with MYB-NFIB chimeric fusion, MYB expression, and clinical outcome. Clin. Cancer Res. 17, 7003–7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison TA, Wilcox I, Luo HY, Farrell JJ, Kurita R, Nakamura Y, Murphy GJ, Cui S, Steinberg MH, Chui DHK, 2017. A long noncoding RNA from the HBS1L-MYB intergenic region on chr6q23 regulates human fetal hemoglobin expression. Blood Cells Mol. Dis. 69, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovici MG, Jurdic P, Samarut J, Gazzolo L, Mura CV, Moscovici C, 1983. Characterization of the hemopoietic target cells for the avian leukemia virus E26. Virology 129, 65–78. [DOI] [PubMed] [Google Scholar]
- Mucenski ML, McLain K, Kier AB, Swerdlow SH, Schreiner CM, Miller TA, Pietryga DW, Scott WJ Jr., Potter SS, 1991. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell 65, 677–689. [DOI] [PubMed] [Google Scholar]
- Murati A, Gervais C, Carbuccia N, Finetti P, Cervera N, Adelaide J, Struski S, Lippert E, Mugneret F, Tigaud I, Penther D, Bastard C, Poppe B, Speleman F, Baranger L, Luquet I, Cornillet-Lefebvre P, Nadal N, Nguyen-Khac F, Perot C, Olschwang S, Bertucci F, Chaffanet M, Lessard M, Mozziconacci MJ, Birnbaum D, Groupe Francophone de Cytogenetique, H, 2009. Genome profiling of acute myelomonocytic leukemia: alteration of the MYB locus in MYST3-linked cases. Leukemia 23, 85–94. [DOI] [PubMed] [Google Scholar]
- Nakagoshi H, Kanei-Ishii C, Sawazaki T, Mizuguchi G, Ishii S, 1992. Transcriptional activation of the c-myc gene by the c-myb and B-myb gene products. Oncogene 7, 1233–1240. [PubMed] [Google Scholar]
- Nakano K, Uchimaru K, Utsunomiya A, Yamaguchi K, Watanabe T, 2016. Dysregulation of c-Myb pathway by aberrant expression of proto-oncogene MYB provides the basis for malignancy in adult T-cell leukemia/lymphoma cells. Clin. Cancer Res. 22, 5915–5928. [DOI] [PubMed] [Google Scholar]
- Nakata Y, Shetzline S, Sakashita C, Kalota A, Rallapalli R, Rudnick SI, Zhang Y, Emerson SG, Gewirtz AM, 2007. c-Myb contributes to G2/M cell cycle transition in human hematopoietic cells by direct regulation of cyclin B1 expression. Mol. Cell. Biol. 27, 2048–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata Y, Brignier AC, Jin S, Shen Y, Rudnick SI, Sugita M, Gewirtz AM, 2010. C-Myb, Menin, GATA-3, and MLL form a dynamic transcription complex that plays a pivotal role in human T helper type 2 cell development. Blood 116, 1280–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Sakai N, Sarai A, Sudo T, Kanei-Ishii C, Ramsay RG, Favier D, Gonda TJ, Ishii S, 1993. Negative autoregulation of c-Myb activity by homodimer formation through the leucine zipper. J. Biol. Chem. 268, 21914–21923. [PubMed] [Google Scholar]
- Nomura T, Tanikawa J, Akimaru H, Kanei-Ishii C, Ichikawa-Iwata E, Khan MM, Ito H, Ishii S, 2004. Oncogenic activation of c-Myb correlates with a loss of negative regulation by TIF1beta and ski. J. Biol. Chem. 279, 16715–16726. [DOI] [PubMed] [Google Scholar]
- Ogata K, Hojo H, Aimoto S, Nakai T, Nakamura H, Sarai A, Ishii S, Nishimura Y, 1992. Solution structure of a DNA-binding unit of Myb: a helix-turn-helix-related motif with conserved tryptophans forming a hydrophobic core. Proc. Natl. Acad. Sci. U. S. A. 89, 6428–6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata K, Morikawa S, Nakamura H, Sekikawa A, Inoue T, Kanai H, Sarai A, Ishii S, Nishimura Y, 1994. Solution structure of a specific DNA complex of the Myb DNA-binding domain with cooperative recognition helices. Cell 79, 639–648. [DOI] [PubMed] [Google Scholar]
- Ogata K, Morikawa S, Nakamura H, Hojo H, Yoshimura S, Zhang R, Aimoto S, Ametani Y, Hirata Z, Sarai A, et al. , 1995. Comparison of the free and DNA-complexed forms of the DNA-binding domain from c-Myb. Nat. Struct. Biol. 2, 309–320. [DOI] [PubMed] [Google Scholar]
- Ogata K, Kanei-Ishii C, Sasaki M, Hatanaka H, Nagadoi A, Enari M, Nakamura H, Nishimura Y, Ishii S, Sarai A, 1996. The cavity in the hydrophobic core of Myb DNA-binding domain is reserved for DNA recognition and trans-activation. Nat. Struct. Biol. 3, 178–187. [DOI] [PubMed] [Google Scholar]
- Oh IH, Reddy EP, 1999. The myb gene family in cell growth, differentiation and apoptosis. Oncogene 18, 3017–3033. [DOI] [PubMed] [Google Scholar]
- Ohyashiki K, Ohyashiki JH, Kinniburgh AJ, Toyama K, Ito H, Minowada J, Sandberg AA, 1988. Myb oncogene in human hematopoietic neoplasia with 6q-anomaly. Cancer Genet. Cytogenet. 33, 83–92. [DOI] [PubMed] [Google Scholar]
- Okada M, Tada M, Kanda N, Masuda M, Mizoguchi H, Kazuma M, Wada E, Kubota K, Nomura Y, 1990. C-myb gene analysis in T-cell malignancies with del (6q). Cancer Genet. Cytogenet. 48, 229–236. [DOI] [PubMed] [Google Scholar]
- O’Neil J, Tchinda J, Gutierrez A, Moreau L, Maser RS, Wong KK, Li W, McKenna K, Liu XS, Feng B, Neuberg D, Silverman L, DeAngelo DJ, Kutok JL, Rothstein R, DePinho RA, Chin L, Lee C, Look AT, 2007. Alu elements mediate MYB gene tandem duplication in human T-ALL. J. Exp. Med. 204, 3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlic D, Anderson S, Biesecker LG, Sorrentino BP, Bodine DM, 1995. Pluripotent hematopoietic stem cells contain high levels of mRNA for c-kit, GATA-2, p45 NF-E2, and c-myb and low levels or no mRNA for c-fms and the receptors for granulocyte colony-stimulating factor and interleukins 5 and 7. Proc. Natl. Acad. Sci. U. S. A. 92, 4601–4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke JP, Ness SA, 2008. Alternative RNA splicing produces multiple forms of c-Myb with unique transcriptional activities. Mol. Cell. Biol. 28, 2091–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paikari A, Sheehan VA, 2018. Fetal haemoglobin induction in sickle cell disease. Br. J. Haematol. 180 (2), 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattabiraman DR, Gonda TJ, 2013. Role and potential for therapeutic targeting of MYB in leukemia. Leukemia 27, 269–277. [DOI] [PubMed] [Google Scholar]
- Pattabiraman DR, McGirr C, Shakhbazov K, Barbier V, Krishnan K, Mukhopadhyay P, Hawthorne P, Trezise A, Ding J, Grimmond SM, Papathanasiou P, Alexander WS, Perkins AC, Levesque JP, Winkler IG, Gonda TJ, 2014. Interaction of c-Myb with p300 is required for the induction of acute myeloid leukemia (AML) by human AML oncogenes. Blood 123, 2682–2690. [DOI] [PubMed] [Google Scholar]
- Pelicci PG, Lanfrancone L, Brathwaite MD, Wolman SR, Dalla-Favera R, 1984. Amplification of the c-myb oncogene in a case of human acute myelogenous leukemia. Science 224, 1117–1121. [DOI] [PubMed] [Google Scholar]
- Perrera C, Colombo R, Valsasina B, Carpinelli P, Troiani S, Modugno M, Gianellini L, Cappella P, Isacchi A, Moll J, Rusconi L, 2010. Identification of Myb-binding protein 1A (MYBBP1A) as a novel substrate for aurora B kinase. J. Biol. Chem. 285, 11775–11785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson M, Andren Y, Mark J, Horlings HM, Persson F, Stenman G, 2009. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc. Natl. Acad. Sci. U. S. A. 106, 18740–18744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piovesan D, Tempany J, Di Pietro A, Baas I, Yiannis C, O’Donnell K, Chen Y, Peperzak V, Belz GT, Mackay CR, Smyth GK, Groom JR, Tarlinton DM, Good-Jacobson KL, 2017. c-Myb regulates the T-bet-dependent differentiation program in B cells to coordinate antibody responses. Cell Rep. 19, 461–470. [DOI] [PubMed] [Google Scholar]
- Quelen C, Lippert E, Struski S, Demur C, Soler G, Prade N, Delabesse E, Broccardo C, Dastugue N, Mahon FX, Brousset P, 2011. Identification of a transforming MYB-GATA1 fusion gene in acute basophilic leukemia: a new entity in male infants. Blood 117, 5719–5722. [DOI] [PubMed] [Google Scholar]
- Quintana AM, Liu F, O’Rourke JP, Ness SA, 2011a. Identification and regulation of c-Myb target genes in MCF-7 cells. BMC Cancer 11, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana AM, Zhou YE, Pena JJ, O’Rourke JP, Ness SA, 2011b. Dramatic repositioning of c-Myb to different promoters during the cell cycle observed by combining cell sorting with chromatin immunoprecipitation. PLoS One 6, e17362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke K, Beug H, Kornfeld S, Graf T, 1982. Transformation of both erythroid and myeloid cells by E26, an avian leukemia virus that contains the myb gene. Cell 31, 643–653. [DOI] [PubMed] [Google Scholar]
- Rahman S, Magnussen M, Leon TE, Farah N, Li Z, Abraham BJ, Alapi KZ, Mitchell RJ, Naughton T, Fielding AK, Pizzey A, Bustraan S, Allen C, Popa T, Pike-Overzet K, Garcia-Perez L, Gale RE, Linch DC, Staal FJT, Young RA, Look AT, Mansour MR, 2017. Activation of the LMO2 oncogene through a somatically acquired neomorphic promoter in T-cell acute lymphoblastic leukemia. Blood 129, 3221–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay RG, 2005. c-Myb a stem-progenitor cell regulator in multiple tissue compartments. Growth Factors 23, 253–261. [DOI] [PubMed] [Google Scholar]
- Ramsay RG, Gonda TJ, 2008. MYB function in normal and cancer cells. Nat. Rev. Cancer 8, 523–534. [DOI] [PubMed] [Google Scholar]
- Ramsay RG, Thompson MA, Hayman JA, Reid G, Gonda TJ, Whitehead RH, 1992. Myb expression is higher in malignant human colonic carcinoma and premalignant adenomatous polyps than in normal mucosa. Cell Growth Differ. 3, 723–730. [PubMed] [Google Scholar]
- Rosson D, Tereba A, 1983. Transcription of hematopoietic-associated oncogenes in childhood leukemia. Cancer Res. 43, 3912–3918. [PubMed] [Google Scholar]
- Saether T, Berge T, Ledsaak M, Matre V, Alm-Kristiansen AH, Dahle O, Aubry F, Gabrielsen OS, 2007. The chromatin remodeling factor mi-2alpha acts as a novel co-activator for human c-Myb. J. Biol. Chem. 282, 13994–14005. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Dai G, Tsujino K, Hashimoto K, Huang X, Fujimoto T, Mucenski M, Frampton J, Ogawa M, 2006. Proper levels of c-Myb are discretely defined at distinct steps of hematopoietic cell development. Blood 108, 896–903. [DOI] [PubMed] [Google Scholar]
- Sakura H, Kanei-Ishii C, Nagase T, Nakagoshi H, Gonda TJ, Ishii S, 1989. Delineation of three functional domains of the transcriptional activator encoded by the c-myb protooncogene. Proc. Natl. Acad. Sci. U. S. A. 86, 5758–5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg ML, Sutton SE, Pletcher MT, Wiltshire T, Tarantino LM, Hogenesch JB, Cooke MP, 2005. c-Myb and p300 regulate hematopoietic stem cell proliferation and differentiation. Dev. Cell 8, 153–166. [DOI] [PubMed] [Google Scholar]
- Sankaran VG, Menne TF, Scepanovic D, Vergilio JA, Ji P, Kim J, Thiru P, Orkin SH, Lander ES, Lodish HF, 2011. MicroRNA-15a and -16-1 act via MYB to elevate fetal hemoglobin expression in human trisomy 13. Proc. Natl. Acad. Sci. U. S. A. 108, 1519–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvakumaran M, Liebermann DA, Hoffman-Liebermann B, 1992. Deregulated c-myb disrupts interleukin-6- or leukemia inhibitory factor-induced myeloid differentiation prior to c-myc: role in leukemogenesis. Mol. Cell. Biol. 12, 2493–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikatani EA, Chandy M, Besla R, Li CC, Momen A, El-Mounayri O, Robbins CS, Husain M, 2016. c-Myb regulates proliferation and differentiation of adventitial Sca1+ vascular smooth muscle cell progenitors by transactivation of myocardin. Arterioscler. Thromb. Vasc. Biol. 36, 1367–1376. [DOI] [PubMed] [Google Scholar]
- Siegert W, Beutler C, Langmach K, Keitel C, Schmidt CA, 1990. Differential expression of the oncoproteins c-myc and c-myb in human lymphoproliferative disorders. Eur. J. Cancer 26, 733–737. [DOI] [PubMed] [Google Scholar]
- Sinclair P, Harrison CJ, Jarosova M, Foroni L, 2005. Analysis of balanced rearrangements of chromosome 6 in acute leukemia: clustered breakpoints in q22-q23 and possible involvement of c-MYB in a new recurrent translocation, t(6;7)(q23;q32 through 36). Haematologica 90, 602–611. [PubMed] [Google Scholar]
- Srivastava SK, Bhardwaj A, Arora S, Singh S, Azim S, Tyagi N, Carter JE, Wang B, Singh AP, 2015. MYB is a novel regulator of pancreatic tumour growth and metastasis. Br. J. Cancer 113, 1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadhouders R, Thongjuea S, Andrieu-Soler C, Palstra RJ, Bryne JC, van den Heuvel A, Stevens M, de Boer E, Kockx C, van der Sloot A, van den Hout M, van Ijcken W, Eick D, Lenhard B, Grosveld F, Soler E, 2012. Dynamic long-range chromatin interactions control Myb proto-oncogene transcription during erythroid development. EMBO J. 31, 986–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadhouders R, Aktuna S, Thongjuea S, Aghajanirefah A, Pourfarzad F, van Ijcken W, Lenhard B, Rooks H, Best S, Menzel S, Grosveld F, Thein SL, Soler E, 2014. HBS1L-MYB intergenic variants modulate fetal hemoglobin via long-range MYB enhancers. J. Clin. Invest. 124, 1699–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman G, Andersson MK, Andren Y, 2010. New tricks from an old oncogene: gene fusion and copy number alterations of MYB in human cancer. Cell Cycle 9, 2986–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan J, Feeley B, Guerra J, Boxer LM, 1997. Identification of the major positive regulators of c-myb expression in hematopoietic cells of different lineages. J. Biol. Chem. 272, 1943–1949. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Yamazaki H, Mukai HY, Motohashi H, Shi L, Tanabe O, Engel JD, Yamamoto M, 2013. Disruption of the Hbs1l-Myb locus causes hereditary persistence of fetal hemoglobin in a mouse model. Mol. Cell. Biol. 33, 1687–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahirov TH, Sasaki M, Inoue-Bungo T, Fujikawa A, Sato K, Kumasaka T, Yamamoto M, Ogata K, 2001. Crystals of ternary protein-DNA complexes composed of DNA-binding domains of c-Myb or v-Myb, C/EBPalpha or C/EBPbeta and tom-1A promoter fragment. Acta Crystallogr. D Biol. Crystallogr. 57, 1655–1658. [DOI] [PubMed] [Google Scholar]
- Thein SL, 2018. Molecular basis of beta thalassemia and potential therapeutic targets. Blood Cells Mol. Dis. 70, 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thein SL, Menzel S, Peng X, Best S, Jiang J, Close J, Silver N, Gerovasilli A, Ping C, Yamaguchi M, Wahlberg K, Ulug P, Spector TD, Garner C, Matsuda F, Farrall M, Lathrop M, 2007. Intergenic variants of HBS1L-MYB are responsible for a major quantitative trait locus on chromosome 6q23 influencing fetal hemoglobin levels in adults. Proc. Natl. Acad. Sci. U. S. A. 104, 11346–11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MA, Flegg R, Westin EH, Ramsay RG, 1997. Microsatellite deletions in the c-myb transcriptional attenuator region associated with over-expression in colon tumour cell lines. Oncogene 14, 1715–1723. [DOI] [PubMed] [Google Scholar]
- Tichy M, Knopfova L, Jarkovsky J, Pekarcikova L, Veverkova L, Vlcek P, Katolicka J, Capov I, Hermanova M, Smarda J, Benes P, 2016. Overexpression of c-Myb is associated with suppression of distant metastases in colorectal carcinoma. Tumour Biol. 37, 10723–10729. [DOI] [PubMed] [Google Scholar]
- Togashi Y, Dobashi A, Sakata S, Sato Y, Baba S, Seto A, Mitani H, Kawabata K, Takeuchi K, 2018. MYB and MYBL1 in adenoid cystic carcinoma: diversity in the mode of genomic rearrangement and transcripts. Mod. Pathol. 10.1038/s41379-018-0008-8. [DOI] [PubMed] [Google Scholar]
- Tomita A, Watanabe T, Kosugi H, Ohashi H, Uchida T, Kinoshita T, Mizutani S, Hotta T, Murate T, Seto M, Saito H, 1998. Truncated c-Myb expression in the human leukemia cell line TK-6. Leukemia 12 (9), 1422. [DOI] [PubMed] [Google Scholar]
- Tomita A, Towatari M, Tsuzuki S, Hayakawa F, Kosugi H, Tamai K, Miyazaki T, Kinoshita T, Saito H, 2000. c-Myb acetylation at the carboxyl-terminal conserved domain by transcriptional co-activator p300. Oncogene 19, 444–451. [DOI] [PubMed] [Google Scholar]
- Torelli G, Venturelli D, Colo A, Zanni C, Selleri L, Moretti L, Calabretta B, Torelli U, 1987. Expression of c-myb protooncogene and other cell cycle-related genes in normal and neoplastic human colonic mucosa. Cancer Res. 47, 5266–5269. [PubMed] [Google Scholar]
- Trainer DL, Kline T, McCabe FL, Faucette LF, Feild J, Chaikin M, Anzano M, Rieman D, Hoffstein S, Li DJ, et al. , 1988. Biological characterization and oncogene expression in human colorectal carcinoma cell lines. Int. J. Cancer 41, 287–296. [DOI] [PubMed] [Google Scholar]
- Untawale S, Blick M, 1988. Oncogene expression in adenocarcinomas of the colon and in colon tumor-derived cell lines. Anticancer Res. 8, 1–7. [PubMed] [Google Scholar]
- van der Harst P, Zhang W, Mateo Leach I, Rendon A, Verweij N, Sehmi J, Paul DS, Elling U, Allayee H, Li X, Radhakrishnan A, Tan ST, Voss K, Weichenberger CX, Albers CA, Al-Hussani A, Asselbergs FW, Ciullo M, Danjou F, Dina C, Esko T, Evans DM, Franke L, Gogele M, Hartiala J, Hersch M, Holm H, Hottenga JJ, Kanoni S, Kleber ME, Lagou V, Langenberg C, Lopez LM, Lyytikainen LP, Melander O, Murgia F, Nolte IM, O’Reilly PF, Padmanabhan S, Parsa A, Pirastu N, Porcu E, Portas L, Prokopenko I, Ried JS, Shin SY, Tang CS, Teumer A, Traglia M, Ulivi S, Westra HJ, Yang J, Zhao JH, Anni F, Abdellaoui A, Attwood A, Balkau B, Bandinelli S, Bastardot F, Benyamin B, Boehm BO, Cookson WO, Das D, de Bakker PI, de Boer RA, de Geus EJ, de Moor MH, Dimitriou M, Domingues FS, Doring A, Engstrom G, Eyjolfsson GI, Ferrucci L, Fischer K, Galanello R, Garner SF, Genser B, Gibson QD, Girotto G, Gudbjartsson DF, Harris SE, Hartikainen AL, Hastie CE, Hedblad B, Illig T, Jolley J, Kahonen M, Kema IP, Kemp JP, Liang L, Lloyd-Jones H, Loos RJ, Meacham S, Medland SE, Meisinger C, Memari Y, Mihailov E, Miller K, Moffatt MF, Nauck M, et al. , 2012. Seventy-five genetic loci influencing the human red blood cell. Nature 492, 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegiopoulos A, Garcia P, Emambokus N, Frampton J, 2006. Coordination of erythropoiesis by the transcription factor c-Myb. Blood 107, 4703–4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlberg K, Jiang J, Rooks H, Jawaid K, Matsuda F, Yamaguchi M, Lathrop M, Thein SL, Best S, 2009. The HBS1L-MYB intergenic interval associated with elevated HbF levels shows characteristics of a distal regulatory region in erythroid cells. Blood 114, 1254–1262. [DOI] [PubMed] [Google Scholar]
- Wasner M, Haugwitz U, Reinhard W, Tschop K, Spiesbach K, Lorenz J, Mossner J, Engeland K, 2003. Three CCAAT-boxes and a single cell cycle genes homology region (CHR) are the major regulating sites for transcription from the human cyclin B2 promoter. Gene 312, 225–237. [DOI] [PubMed] [Google Scholar]
- Westin EH, Gallo RC, Arya SK, Souza LM, Baluda MA, Aaronson SA, Wong-Staal F, 1982. Differential expression of the amv gene in human hematopoietic cells. PNAS 79, 2194–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JR, Weston K, 2000. Myb is required for self-renewal in a model system of early hematopoiesis. Oncogene 19, 1196–1205. [DOI] [PubMed] [Google Scholar]
- Winqvist R, Knuutila S, Leprince D, Stehelin D, Alitalo K, 1985. Mapping of amplified c-myb oncogene, sister chromatid exchanges, and karyotypic analysis of the COLO 205 colon carcinoma cell line. Cancer Genet. Cytogenet. 18, 251–264. [DOI] [PubMed] [Google Scholar]
- Woo CH, Sopchak L, Lipsick JS, 1998. Overexpression of an alternatively spliced form of c-Myb results in increases in transactivation and transforms avian myelomonoblasts. J. Virol. 72, 6813–6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Calado DP, Galler G, Thai TH, Patterson HC, Wang J, Rajewsky N, Bender TP, Rajewsky K, 2007. MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell 131, 146–159. [DOI] [PubMed] [Google Scholar]
- Zhang J, Markus J, Bies J, Paul T, Wolff L, 2012. Three murine leukemia virus integration regions within 100 kilobases upstream of c-myb are proximal to the 5′ regulatory region of the gene through DNA looping. J. Virol. 86, 10524–10532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B, Orisme W, Punchihewa C, Parker M, Qaddoumi I, Boop FA, Lu C, Kandoth C, Ding L, Lee R, Huether R, Chen X, Hedlund E, Nagahawatte P, Rusch M, Boggs K, Cheng J, Becksfort J, Ma J, Song G, Li Y, Wei L, Wang J, Shurtleff S, Easton J, Zhao D, Fulton RS, Fulton LL, Dooling DJ, Vadodaria B, Mulder HL, Tang C, Ochoa K, Mullighan CG, Gajjar A, Kriwacki R, Sheer D, Gilbertson RJ, Mardis ER, Wilson RK, Downing JR, Baker SJ, Ellison DW, St. Jude Children’s Research Hospital-Washington University Pediatric Cancer Genome, P, 2013. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat. Genet. 45, 602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Kalota A, Jin S, Gewirtz AM, 2009. The c-myb proto-oncogene and microRNA-15a comprise an active autoregulatory feedback loop in human hematopoietic cells. Blood 113, 505–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Glazov EA, Pattabiraman DR, Al-Owaidi F, Zhang P, Brown MA, Leo PJ, Gonda TJ, 2011. Integrated genome-wide chromatin occupancy and expression analyses identify key myeloid pro-differentiation transcription factors repressed by Myb. Nucleic Acids Res. 39, 4664–4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YE, O’Rourke JP, Edwards JS, Ness SA, 2011. Single molecule analysis of c-myb alternative splicing reveals novel classifiers for precursor B-ALL. PLoS One 6, e22880. [DOI] [PMC free article] [PubMed] [Google Scholar]


