Abstract
SecA undergoes conformational changes during translocation, inserting domains into and across the membrane or enhancing the protease resistance of these domains. We now show that some SecA bound at SecYEG is accessible from the periplasm to a membrane-impermeant probe in cells with a permeabilized outer membrane but an intact plasma membrane.
Proteins to be exported to the periplasm or outer membrane of Escherichia coli are synthesized with a leader (signal) sequence (35) and bind to the polar, SecA subunit of preprotein translocase (17). Translocase is a complex enzyme, consisting of a dimer of SecA (1, 6, 14) bound to membrane-embedded heterotrimeric SecYEG or heterohexameric SecYEGDFyajC domains (15). SecA has binding sites for ATP (29, 30, 32) and for preprotein (26). ATP binding to SecA drives the translocation of an approximately 25-amino-acyl “loop” of the preprotein (37); ATP hydrolysis allows successive cycles of ATP-driven preprotein movement (16, 19). The membrane electrochemical potential translocates longer segments of the preprotein (13, 37).
Biochemical studies with purified inverted membrane vesicles, the precursor form of outer membrane protein A (proOmpA), and 125I- or 35S-SecA have shown that SecYEG-bound SecA undergoes a substantial conformational change upon binding preprotein and ATP (18, 20). An N-terminal 65-kDa domain and a C-terminal 30-kDa domain become resistant to even high concentrations of proteinase K or trypsin (20, 22). This resistance is lost upon membrane disruption by either freeze-thaw, sonication, or detergent extraction (18, 20, 22), suggesting that this represents SecA insertion and that these domains had become proteinase inaccessible rather than merely refolding to become protease resistant. Several independent kinds of data support this model of SecA function. (i) SecG, a small membrane-spanning subunit of translocase, inverts its topology during SecA insertion and resumes its original topology after SecA hydrolyzes ATP and deinserts (31). (ii) A preprotein arrested in translocation, with bound cross-linker in its membrane-transiting region, showed specific cross-linking to both SecA and SecY (25). (iii) Mutations in the membrane-spanning or periplasmic regions of SecY can dramatically alter the SecA insertion and deinsertion cycle (28). (iv) SecD and SecF, membrane-anchored translocase subunits which are largely exposed on the periplasmic surface of the plasma membrane, stabilize the inserted form of SecA (16, 19). (v) The preprotein chain itself moves forward and backward in conjunction with SecA insertion and deinsertion (16). (vi) Other complex bacterial transport systems have polar ATPase subunits which are exposed to the periplasm (2, 4, 39). (vii) The SecA which is recovered with right-side-out membrane vesicles has central (34) and C-terminal (41) regions exposed to membrane-impermeant probes. However, F1-ATP synthase, another larger peripheral membrane protein of E. coli, partially relocates to the periplasmic surface in such membrane vesicles (43). Soluble SecA, which can spontaneously bind to lipid (5, 40), might associate with both the outer and inner surfaces of the plasma membrane after cell lysis.
SecA exists in at least four states in the cell—in solution, bound to lipid, bound at SecYEG, and inserted in response to preprotein and ATP at SecYEG. Each yields distinct peptides upon proteolytic digestion, and this complexity has obscured the insertion-specific event (5, 6, 12, 22, 24, 40). Indeed, recent observations have called this model of SecA insertion into question (7, 8, 42). Thus, we have reexamined whether SecA actually inserts across the plasma membrane to reach the periplasm. This question is especially acute in that most prior studies employ broken cells or purified organelles with added SecA, which might not reflect the state of endogenous SecA in cells with an unbreached plasma membrane.
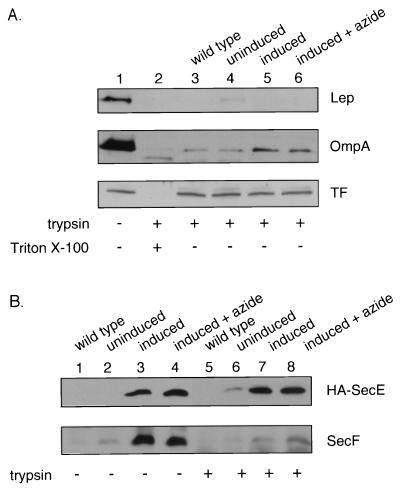
To explore the topology of SecA in cells with intact plasma membrane, we selectively permeabilized the outer membrane by a single freeze-thaw cycle and incubation for 1 h on ice in Tris-sucrose-EDTA, a technique previously characterized (11) for the study of E. coli membrane protein topology. Addition of trypsin to these outer membrane-permeabilized (OMP) cells digested both the large periplasmically oriented domain of leader peptidase (44) and the periplasmically exposed (10) OmpA (Fig. 1A). In contrast, trigger factor, a cytosolic protein (9), was inaccessible to digestion (Fig. 1A). OMP cells were also examined for the accessibility of integral membrane translocase subunits. E. coli BL21 cells with plasmids encoding the SecYEGDFyajC subunits under ara regulation were grown, and a portion of the culture was induced for ara expression. Induction resulted in strong overexpression (Fig. 1B). The SecE subunit, which only has periplasmically disposed basic residues that are quite near the membrane surface (36), is resistant to tryptic digestion, while the SecF subunit, with a large polar periplasmic domain (33), or the SecY subunit (data not shown) was readily digested by trypsin in OMP cell preparations. We conclude that OMP cells have an undisturbed plasma membrane permeability barrier and are suitable for examining whether the endogenous SecA is exposed to the periplasm.
FIG. 1.
The inner membrane remains intact in OMP cells. (A) The levels of the periplasmically oriented marker proteins leader peptidase (Lep) and outer membrane protein A (OmpA) and of the cytoplasmically localized trigger factor (TF) were determined by immunoblotting of OMP cells in the absence (lane 1) or presence (lane 2) of 1.0 mg of trypsin per ml and 1.0% Triton X-100 (Sigma). OMP cells were prepared (11) from BL21 wild-type cells (lane 3), uninduced (lane 4) and induced (lane 5) SecYEG-overproducing cells (12) (induced at an optical density at 600 nm of 0.5 with 1.5% arabinose for 2 h), and induced cells grown in the presence of 20 mM azide for the last 15 min (lane 6). Cells were harvested (5,000 rpm, 5 min, 4°C), resuspended in an equal weight of 10% sucrose–50 mM Tris-Cl (pH 7.5), and frozen in liquid nitrogen. Frozen cells were thawed, collected by centrifugation (5,000 rpm, 5 min, 4°C), and resuspended in 100 μl of 20% sucrose–10 mM EDTA–30 mM Tris-Cl (pH 8.1) for 60 min on ice. Cells were treated with 1.0 mg of trypsin per ml for 60 min on ice. The samples were then precipitated with 15% trichloroacetic acid and acetone washed, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with 15% polyacrylamide gels (12). For immunoblotting, proteins separated by SDS-PAGE were electrophoretically transferred to nitrocellulose (Bio-Rad, Hercules, Calif.) with a Genie electrophoretic blotter (Idea Scientific, Minneapolis, Minn.) at 250 mA for 1.25 h. (B) OMP cells were prepared from BL21 wild-type cells (lanes 1 and 5), uninduced (lanes 2 and 6) and induced (lanes 3 and 7) SecYEGDFyajC-overproducing cells, and induced cells grown in the presence of 20 mM azide (lanes 4 and 8). The level of SecE and SecF proteins was determined by immunoblotting of OMP cells incubated in the absence (lanes 1 to 4) or presence (lanes 5 to 8) of 1.0 mg of trypsin per ml. HA, hemagglutinin.
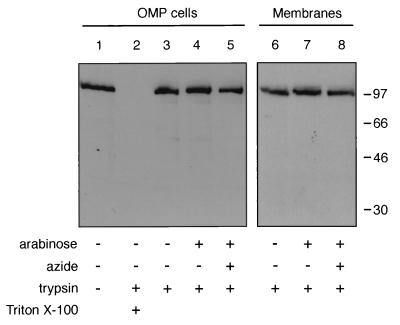
Most of the SecA in OMP cells was inaccessible to digestion by added trypsin (Fig. 2, lane 3), although it was completely susceptible when the membrane was dissolved by detergent (lane 2). Azide (32) or overexpression of SecYEG had little effect (lanes 4 and 5), and even the membrane fractions from these proteolyzed OMP cells had comparable levels of SecA (lanes 6 to 8). These studies suffer from the fact that they start from a signal of 100% of the cellular SecA, and thus they are unable to detect the digestion of even a moderate fraction of the protein. Nonetheless, they suggest that a substantial portion of the endogenous SecA is not exposed to the periplasm. This may represent the SecA which is cytoplasmic, lipid associated, or bound at SecYEG but not inserted, or it may show that the inserted SecA is not exposed to the periplasm.
FIG. 2.
Protease treatment of OMP cells does not affect the levels of SecA. OMP cells were treated with 1.0% Triton X-100 (lane 2) and/or 1.0 mg of trypsin per ml (lanes 2 to 5) and probed by immunoblotting with affinity-purified anti-SecA antibodies (lanes 1 to 5). To isolate total membranes, OMP cells were sonicated (Heat Systems-Ultrasonics, Inc., model 350; output 1.5, 50% duty cycle, 3 min, 0°C), cleared of unbroken cells and debris by centrifugation (5,000 rpm, 5 min, 4°C), and subjected to high-speed centrifugation (73,000 rpm, 10 min, 4°C, in a TL120.2 rotor; Beckman Optima TLX ultracentrifuge). The pellet, containing the membrane fraction, was resuspended in 100 μl of phosphate-buffered saline. The pellet, containing total membranes, was then resuspended and examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting (lanes 6 to 8). Antibody binding was detected by enhanced chemiluminescence. Samples were from BL21 wild-type cells (lanes 1 to 3 and 6), SecYEG-overproducing cells (lanes 4 and 7), and induced cells grown in the presence of 20 mM azide (lanes 5 and 8). Molecular mass markers (kilodaltons) are shown on the right.
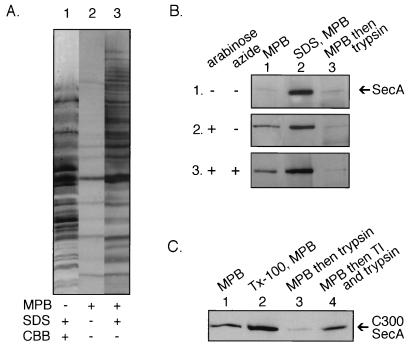
To search with a more sensitive, positive assay for inserted SecA, we exploited the fact that there are only four cysteine residues in SecA (38), with one buried and not accessible to derivatization (14) and the other three near the C terminus. Because disulfides are not involved in the formation of the SecA dimer (1), we treated OMP cells with the membrane-impermeant reagent 3-(N-maleimidylpropionyl)biocytin (MBP [3]) to test the accessibility of the cysteinyl residues of SecA from the periplasm. As reported for other membranes (27), few proteins were derivatized (Fig. 3A, lane 2) by MBP, unless the membranes were dissolved by detergent (lane 3). Only a small proportion of the SecA was accessible to MBP in OMP cells (Fig. 3B, panel 1, lane 1), unless the cells were lysed by detergent (lane 2). However, the induction of SecYEG, providing far more sites for membrane insertion (12, 15, 20), allowed a fraction of the SecA to become exposed to the periplasm (Fig. 3B, panel 2, lane 1), in agreement with earlier studies (41) using right-side-out inner membrane vesicles. All of this MBP-accessible SecA was exposed to proteolytic attack (lane 3), confirming its periplasmic orientation.
FIG. 3.
Cysteine residues of SecA can be modified in OMP cells. (A) The total cellular protein content of cells incubated with 1.0% sodium dodecyl sulfate (SDS) was examined by SDS-polyacrylamide gel electrophoresis (PAGE) and revealed by Coomassie brilliant blue staining (lane 1). OMP cells were incubated with 0.05 mM MPB (from a 10 mM stock solution in dimethyl sulfoxide) for 4 min at 0°C (lane 2) or 1.0% SDS and 0.05 mM MPB (lane 3). Labeling was terminated by addition of 0.8 mM cysteine. Labeled proteins were examined by SDS-PAGE and detected with avidin-conjugated horseradish peroxidase (HRP) and enhanced chemiluminescence (ECL). (B) OMP cells were prepared from BL21 wild-type cells (panel 1), SecYEG-overproducing cells (panel 2), and SecYEG-overproducing cells incubated with 20 mM azide (panel 3) and incubated with 0.05 mM MPB (lane 1), 1.0% SDS, and 0.05 mM MPB (lane 2), or 0.05 mM MPB followed by 1.0 mg of trypsin per ml (lane 3). SecA was then immunoprecipitated with affinity-purified anti-SecA antibodies and detected with avidin-conjugated HRP and ECL. (C) OMP cells containing SecA with a single cysteine residue at position 300 were incubated with 0.05 mM MBP (lane 1), 1.0% Triton X-100 and 0.05 mM MBP (lane 2), 0.05 mM MBP followed by 1.0 mg of trypsin per ml (lane 3), or 0.05 mM MBP followed by an aliquot of a premixed trypsin and trypsin inhibitor (1:10) solution. SecA was then immunoprecipitated with affinity-purified anti-SecA antibodies and detected with avidin-conjugated HRP and ECL.
Since MBP reacts with cysteine residues (3) and the reactive cysteinyl residues are near the C terminus of SecA (14, 38), we repeated these studies with a well-characterized SecA mutant in which the four normal cysteinyl residues were removed by mutation and a unique cysteinyl residue was introduced at position 300 (34). Previous study of this C300 SecA, using right-side-out membrane vesicles, had shown its exposure to the periplasm. Periplasmic exposure was also seen for C300 in OMP cells (Fig. 3C) in which the plasma membrane had never been breached, establishing that a central part of some SecA molecules is also exposed to the periplasm.
Our studies show that a portion of the cell’s SecA is, at any one time, exposed to the periplasm, and this portion is dramatically enhanced by overproduction of SecYEG. The region near residue 300 and the C-terminal region are exposed to different extents, because a population of SecA molecules have the region near residue 300 exposed to the periplasm in wild-type cells (Fig. 3C), while exposure of the C-terminal region is only detectable under conditions of SecYEG overproduction (Fig. 3B). Whether partial exposure of SecA to the periplasm simply corresponds to its conformation when bound at SecYEG with high affinity, while exposure of other regions requires the conformational change of insertion, or whether exposure is indeed due to lipid association will require further study.
How might SecYEG accommodate the insertion of SecA? One possibility, supported by the SecG inversion during SecA insertion, is that SecYEG helices themselves undergo a major conformational change. It is also possible that SecYEG might oligomerize to accommodate SecA, much as the Sec61 complex is thought to oligomerize during preprotein translocation (23). In any case, studies have shown (21) that SecA in the inserted state is largely shielded from the fatty acyl phase of the bilayer.
Many salient questions remain about the function of SecA. It is not known whether each subunit of the dimer is capable of binding preprotein, binding and hydrolyzing ATP, and undergoing cycles of insertion and deinsertion, or whether the two subunits function at SecYEG in some alternating fashion. With a SecA dimer bound to SecYEGDFyajC, translocase has at least eight subunits; the dynamics of their associations and dissociation during translocation are only beginning to be explored.
Acknowledgments
We are grateful to D. Oliver for his gift of the C300 SecA strain.
This work was supported by NIH grant GM23377. J.E. received fellowship support from the Human Frontiers Science Program Organization.
REFERENCES
- 1.Akita M, Shinkai A, Matsuyama S-I, Mizushima S. SecA, an essential component of the secretory machinery of Escherichia coli, exists as a homodimer. Biochem Biophys Res Commun. 1991;174:211–216. doi: 10.1016/0006-291x(91)90507-4. [DOI] [PubMed] [Google Scholar]
- 2.Baichwal V, Liu D, Ames G F-L. The ATP-binding component of a prokaryotic traffic ATPase is exposed to the periplasmic (external) surface. Proc Natl Acad Sci USA. 1993;90:620–624. doi: 10.1073/pnas.90.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayer E A, Zalis M G, Wilchek M. 3-(N-maleimido-propionyl) biocytin: a versatile thiol-specific biotinylating reagent. Anal Biochem. 1985;149:529–536. doi: 10.1016/0003-2697(85)90609-8. [DOI] [PubMed] [Google Scholar]
- 4.Bliss J M, Silver R P. Evidence that KpsT, the ATP-binding component of an ATP-binding cassette transporter, is exposed to the periplasm and associates with polymer during translocation of the polysialic acid capsule of Escherichia coli K1. J Bacteriol. 1997;179:1400–1403. doi: 10.1128/jb.179.4.1400-1403.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breukink E, Demel R A, de Korte-Kool G, de Kruijff B. SecA insertion into phospholipids is stimulated by negatively charged lipids and inhibited by ATP: a monolayer study. Biochemistry. 1992;31:1119–1124. doi: 10.1021/bi00119a021. [DOI] [PubMed] [Google Scholar]
- 6.Cabelli R J, Dolan K M, Qian L, Oliver D B. Characterization of membrane-associated and soluble states of SecA protein from wild-type and SecA51(Ts) mutant strains of Escherichia coli. J Biol Chem. 1991;266:24420–24427. [PubMed] [Google Scholar]
- 7.Chen X, Xu H, Tai P C. A significant fraction of functional SecA is permanently embedded in the membrane. J Biol Chem. 1996;271:29698–29706. doi: 10.1074/jbc.271.47.29698. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Brown T, Tai P C. Identification and characterization of protease-resistant SecA fragments: SecA has two membrane-integral forms. J Bacteriol. 1998;180:527–537. doi: 10.1128/jb.180.3.527-537.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crooke E, Wickner W. Trigger factor: a soluble protein that folds pro-OmpA into a membrane-assembly-competent form. Proc Natl Acad Sci USA. 1987;84:5216–5220. doi: 10.1073/pnas.84.15.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalbey R E, Wickner W. Leader peptidase of Escherichia coli: critical role of a small domain in membrane assembly. Science. 1987;235:783–787. doi: 10.1126/science.3544218. [DOI] [PubMed] [Google Scholar]
- 11.Date T, Goodman J M, Wickner W T. Procoat, the precursor of M13 coat protein, requires an electrochemical potential for membrane insertion. Proc Natl Acad Sci USA. 1980;77:4669–4673. doi: 10.1073/pnas.77.8.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douville K, Price A, Eichler J, Economou A, Wickner W. SecYEG and SecA are stoichiometric components of preprotein translocase. J Biol Chem. 1995;270:20106–20111. doi: 10.1074/jbc.270.34.20106. [DOI] [PubMed] [Google Scholar]
- 13.Driessen A J. Precursor protein translocation by the Escherichia coli translocase is directed by the protonmotive force. EMBO J. 1992;11:847–853. doi: 10.1002/j.1460-2075.1992.tb05122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Driessen A J M. SecA, the peripheral subunit of the Escherichia coli precursor protein translocase, is functional as a dimer. Biochemistry. 1993;32:13190–13197. doi: 10.1021/bi00211a030. [DOI] [PubMed] [Google Scholar]
- 15.Duong F, Wickner W. Distinct catalytic roles of the SecYE, SecG, and SecDFyajC subunits of preprotein translocase holoenzyme. EMBO J. 1997;16:2756–2768. doi: 10.1093/emboj/16.10.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duong F, Wickner W. The SecDFyajC domain of preprotein translocase controls preprotein movement by regulating SecA membrane cycling. EMBO J. 1997;16:4871–4879. doi: 10.1093/emboj/16.16.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duong F, Eichler J, Price A, Leonard M R, Wickner W. Biogenesis of the gram-negative bacterial envelope. Cell. 1997;91:567–573. doi: 10.1016/s0092-8674(00)80444-4. [DOI] [PubMed] [Google Scholar]
- 18.Economou A, Wickner W. SecA promotes preprotein insertion by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell. 1994;78:835–843. doi: 10.1016/s0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 19.Economou A, Pogliano J A, Beckwith J, Oliver D B, Wickner W. SecA membrane cycling at SecYEG is driven by distinct ATP binding and hydrolysis events and is regulated by SecD and SecF. Cell. 1995;83:1171–1181. doi: 10.1016/0092-8674(95)90143-4. [DOI] [PubMed] [Google Scholar]
- 20.Eichler J, Wickner W. Both an N-terminal 65-kDa domain and a C-terminal 30-kDa domain of SecA cycle into the membrane at SecYEG during translocation. Proc Natl Acad Sci USA. 1997;90:620–624. doi: 10.1073/pnas.94.11.5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eichler J, Brunner J, Wickner W. The protease-protected 30 kDa domain of SecA is largely inaccessible to the membrane lipid phase. EMBO J. 1997;16:2188–2196. doi: 10.1093/emboj/16.9.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eichler J, Rinard K, Wickner W. SecA catalysis of preprotein translocation occurs exclusively at SecYEG. J Biol Chem. 1998;273:21675–21681. doi: 10.1074/jbc.273.34.21675. [DOI] [PubMed] [Google Scholar]
- 23.Hanein D, Matlack K E, Jungnickel B, Plath K, Kalies K U, Miller K R, Rapoport T A, Akey C W. Oligomeric rings of the Sec61p complex induced by ligands required for protein translocation. Cell. 1996;87:721–732. doi: 10.1016/s0092-8674(00)81391-4. [DOI] [PubMed] [Google Scholar]
- 24.Hartl F-U, Lecker S, Schiebel E, Hendrick J P, Wickner W. The binding of SecB to SecA to SecY/E mediates preprotein targeting to the E. coli membrane. Cell. 1990;63:269–279. doi: 10.1016/0092-8674(90)90160-g. [DOI] [PubMed] [Google Scholar]
- 25.Joly J C, Wickner W. The SecA and SecY subunits of translocase are the nearest neighbors of a translocating preprotein, shielding it from phospholipids. EMBO J. 1993;12:255–263. doi: 10.1002/j.1460-2075.1993.tb05651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura E, Akita M, Matsuyama S-I, Mizushima S. Determination of a region in SecA that interacts with presecretory proteins in Escherichia coli. J Biol Chem. 1991;266:6600–6606. [PubMed] [Google Scholar]
- 27.Loo T W, Clarke D M. Membrane topology of a cysteine-less mutant of human P-glycoprotein. J Biol Chem. 1995;270:843–848. doi: 10.1074/jbc.270.2.843. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto G, Yoshihisa T, Ito K. SecY and SecA interact to allow SecA insertion and protein translocation across the Escherichia coli plasma membrane. EMBO J. 1997;16:6384–6393. doi: 10.1093/emboj/16.21.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuyama S, Kimura E, Mizushima S. Complementation of two overlapping fragments of SecA, a protein translocation ATPase of Escherichia coli, allows ATP binding to its amino-terminal region. J Biol Chem. 1990;265:8760–8765. [PubMed] [Google Scholar]
- 30.Mitchell C, Oliver D. Two distinct ATP-binding domains are needed to promote protein export by Escherichia coli SecA ATPase. Mol Microbiol. 1993;10:483–497. doi: 10.1111/j.1365-2958.1993.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 31.Nishiyama K I, Suzuki T, Tokuda H. Inversion of the membrane topology of SecG coupled with SecA-dependent preprotein translocation. Cell. 1996;85:71–81. doi: 10.1016/s0092-8674(00)81083-1. [DOI] [PubMed] [Google Scholar]
- 32.Oliver D B, Cabelli R J, Dolan K M, Jarosik G P. Azide-resistant mutants of Escherichia coli alter the SecA protein, an azide-sensitive component of the protein export machinery. Proc Natl Acad Sci USA. 1990;87:8227–8231. doi: 10.1073/pnas.87.21.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pogliano J A, Beckwith J. SecD and SecF facilitate protein export in Escherichia coli. EMBO J. 1994;12:554–561. doi: 10.1002/j.1460-2075.1994.tb06293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramamurthy V, Oliver D. Topology of the integral membrane form of Escherichia coli SecA protein reveals multiple periplasmically exposed regions and modulation by ATP binding. J Biol Chem. 1997;272:23239–23246. doi: 10.1074/jbc.272.37.23239. [DOI] [PubMed] [Google Scholar]
- 35.Randall L L, Hardy S J S. Unity in function in the absence of consensus in sequence: role of leader peptides in export. Science. 1989;243:1156–1159. doi: 10.1126/science.2646712. [DOI] [PubMed] [Google Scholar]
- 36.Schatz P J, Riggs P D, Jacq A, Fath M J, Beckwith J. The secE gene encodes an integral membrane protein required for protein export in Escherichia coli. Genes Dev. 1989;3:1035–1044. doi: 10.1101/gad.3.7.1035. [DOI] [PubMed] [Google Scholar]
- 37.Schiebel E, Driessen A J M, Hartl F-U, Wickner W. ΔH+ and ATP function at different stages of the catalytic cycle of preprotein translocation. Cell. 1991;64:927–939. doi: 10.1016/0092-8674(91)90317-r. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt M G, Rollo E E, Grodberg J, Oliver D B. Nucleotide sequence of the secA gene and secA(Ts) mutations preventing protein export in Escherichia coli. J Bacteriol. 1988;170:3404–3414. doi: 10.1128/jb.170.8.3404-3414.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneider E, Hunke S, Tebbe S. The MalK protein of the ATP-binding cassette transporter for maltose of Escherichia coli is accessible to protease digestion from the periplasmic side of the membrane. J Bacteriol. 1995;177:5364–5367. doi: 10.1128/jb.177.18.5364-5367.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ulbrandt N D, London E, Oliver D B. Deep penetration of a portion of Escherichia coli SecA protein into model membranes is promoted by anionic lipids and by partial unfolding. J Biol Chem. 1992;267:15184–15192. [PubMed] [Google Scholar]
- 41.van der Does C, den Blaauwen T, de Wit J G, Manting E H, Groot N A, Fekkes P, Driessen A J M. SecA is an intrinsic subunit of the Escherichia coli preprotein translocase and exposes its carboxyl terminus to the periplasm. Mol Microbiol. 1996;22:619–629. doi: 10.1046/j.1365-2958.1996.d01-1712.x. [DOI] [PubMed] [Google Scholar]
- 42.van der Does C, Manting E H, Kaufmann A, Lutz M, Driessen A J M. Interaction between SecA and SecYEG in micellar solution and formation of the membrane-inserted state. Biochemistry. 1998;37:201–210. doi: 10.1021/bi972105t. [DOI] [PubMed] [Google Scholar]
- 43.Wickner W. Fractionation of membrane vesicles from coliphage M13-infected Escherichia coli. J Bacteriol. 1976;127:162–167. doi: 10.1128/jb.127.1.162-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolfe P B, Wickner W, Goodman J M. Sequence of the leader peptidase gene of Escherichia coli and the orientation of leader peptidase in the bacterial envelope. J Biol Chem. 1983;258:12073–12080. [PubMed] [Google Scholar]