Abstract
Salmonella enterica Agona (S. Agona) and Salmonella enterica Saintpaul (S. Saintpaul) are among the emerging drug-resistant Salmonella in turkey production and processing. Rapid solutions to control emerging and uncommon serotypes such as S. Agona and S. Saintpaul are needed. This study tested pimenta essential oil (PEO) as a processing antibacterial against S. Agona and S. Saintpaul in experiments representative of different stages of turkey processing. The compound effectively reduced S. Agona and S. Saintpaul in nutrient broth studies and with mature biofilm assays. PEO was tested against a combination of S. Agona and S. Saintpaul in ground turkey meat and nonprocessed breast meat. In the first experiment with ground turkey, samples were inoculated with a mixture of S. Agona and S. Saintpaul (∼3 log10 CFU/g) and treated with PEO at different concentrations (0% PEO, 0.25% PEO, 0.5% PEO, 1% PEO, 2% PEO, and 2.5% PEO). In the second experiment with turkey breast, samples inoculated with ∼3 log10 CFU/g (SA+SP) were dipped in different concentrations of PEO with chitosan (CN) for 2 min. In both these experiments, samples were stored at 4°C, and Salmonella recovery was carried out at 0, 1, 3, 5, and 7 d. All experiments followed a completely randomized design and were repeated 6 times (n = 6). Statistical analysis was done using the PROC-ANOVA procedure of SAS. In the ground turkey meat, PEO at or above 2% reduced 2 log10 CFU/g of Salmonella by day 1. PEO at 2.5% in ground turkey meat resulted in enrichment-negative samples by 1 min, indicative of the rapid killing effect of the compound at a high concentration of PEO (P ≤ 0.05). A maximum reduction of 1.7 log10 CFU Salmonella/g of turkey breast meat was obtained after 2 min of dip treatment containing CN and 2.5% PEO. Results indicate that PEO could be used as a plant-based processing antibacterial against S. Agona and S. Saintpaul in turkey processing. Upscaling to plant-level studies is necessary before recommending its usage.
Key words: pimenta essential oil, Salmonella Agona, Salmonella Saintpaul, ground turkey, antibacterial
INTRODUCTION
Foodborne salmonellosis is a zoonotic illness caused by nontyphoidal Salmonella, a bacterial pathogen colonizing the gut of food animals, including poultry. Among the 15 most common foodborne illness-causing agents, Salmonella ranks first in causing the most economic burden, which imposes $4.7 billion annually (Hoffman et al., 2015; Scharff, 2020). Among the recent outbreaks, over 75% of foodborne salmonellosis was attributed to 7 food categories, including chicken, egg, and turkeys (CDC, 2021).
Several serotypes of Salmonella are circulating in turkey production, and many of them are emerging causes of foodborne outbreaks (Miller et al., 2020). Multiple Salmonella outbreaks linked to turkey products were reported in the past decade. Multidrug-resistant (MDR) S. Heidelberg and S. Hadar—associated outbreaks, involving contaminated ground turkey meat and turkey burgers, respectively, were reported during 2010 and 2011 (CDC, 2022). An S. Schwarzengrund-associated recall of approximately 78,000 pounds of ground turkey meat occurred in 2018 (CDC, 2022). MDR S. Reading serotypes were previously isolated from ground turkey meat samples involved in a multistate outbreak, reporting 358 illness cases and 1 death (CDC, 2022).
Industry predominantly focuses on controlling major serovars directly linked to the product's safety for many reasons, leaving the possibility of other circulating serovars causing future outbreaks distant. It is also thought that an antibacterial intervention applied against a major serovar could target other minor serovars. In an earlier investigation involving isolation and serotyping of Salmonella spp. from ground turkey and turkey meat parts, the researchers identified 15 serotypes, including Salmonella enterica Saintpaul (S. Saintpaul) and Salmonella enterica Agona (S. Agona). Salmonella Saintpaul was the second predominant serotype isolated from retail meats, exclusive to ground turkey meat from the 8 participating FoodNet (Connecticut, Georgia, Maryland, Minnesota, Oregon, Tennessee, California, and New York) sites and showed a good correlation with human isolates (Zhao et al., 2006). On the other hand, S. Agona was isolated from chicken breast, legs, and ground turkey with a constant presence observed in all 4 seasons and contributed 10% of total isolated serotypes (Erol et al., 2013).
In the United States, turkeys contributed 28.19 and 18.61% of nonclinical and clinical isolates of S. Agona from animal-related source pools (CDC, 2022). Salmonella in the ceca and fecal matter and those attached to the carcasses serve as a source of cross-contamination during poultry processing. The contamination can occur at any processing stage, starting from the transportation of flocks to the slaughterhouses until the final product is packaged. Even though appropriate scalding temperature, quality water, good airflow, good processing practices, and low water pH could reduce the Salmonella load on the carcass to a certain extent, appropriate interventions applied at various critical control points can ensure a better microbiologically safe product (USDA FSIS, 2021).
Essential oils could be a promising natural and environmentally friendly antimicrobial intervention to control multiple emerging and drug-resistant serotypes of Salmonella in animal production and processing, including turkeys (Nair and Kollanoor Johny, 2017; Dewi et al., 2021,2022; Nair et al., 2023). Pimenta essential oil (PEO), also known as allspice oil, approved as generally recognized as safe (GRAS) status (CFR, Title 21 §182.20) by the FDA (FDA, 2023), is extracted from the pimenta tree belonging to the Myrtaceae family. The oil contains eugenol, β myrcene, and E caryophyllene as 3 major antimicrobial components extracted from different plant parts (Samuel Mérida-Reyes et al., 2020). The oil exerts antibacterial activity against a broad spectrum of pathogens, including Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Salmonella spp., Streptococcus, Candida, Pseudomonas aeruginosa, Acinetobacter baumannii, and Enterococcus faecalis (Lowe et al., 2017; Lorenzo-Leal et al., 2019; Ismail et al., 2020; Milenković et al., 2020). The presence of phenolic compounds and flavonoids in PEO also could contribute to its antioxidant properties (Milenković et al., 2020). The oil has been proven for its pharmacological benefits, including anticancer, antimutagenic, antipyretic, and antihemorrhagic effects (Contreras-Moreno, 2018). It has been reported to be beneficial in the food industry when combined with chitosan (CN) against aflatoxins and Aspergillus flavus (Chaudhari et al., 2022). Recent reports show that PEO and its nanoemulsion can also be used in the turkey meat industry against MDR Salmonella Heidelberg (Nair and Kollanoor Johny, 2017). Although the mechanism of action of PEO is not fully understood, it is possible that, like many other essential oils, it could damage the cell membrane and its ATP machinery, resulting in bacterial cell death (Burt, 2004). Moreover, the primary component of PEO, eugenol, has been proven for its effect on downregulating virulence genes of S. Enteritidis (Kollanoor Johny et al., 2012; Kollanoor Johny et al., 2017).
The use of PEO as a direct antimicrobial additive is a least explored areas to improve the postharvest food safety of ground turkey meat. Targeting multiple MDR Salmonella serotypes in ground turkey meat and nonprocessed turkey breast meat is a unique aspect of this study as it represents a real-world situation of turkey meat contamination. In addition, we also explored different methods such as the use of coating agents to reduce the dip time of meat in PEO and to improve the contact time of the essential oil with Salmonella serotypes, utilizing the filming properties of coating agents. Hence, this study aimed to determine the efficacy of PEO on MDR S. Agona and S. Saintpaul in ground turkey meat. This study also determined the effect of a combination dip treatment of PEO with CN on S. Agona and S. Saintpaul attached to the nonprocessed turkey breast meat.
MATERIALS AND METHODS
Bacterial Strains and Culture Conditions
S. Agona and S. Saintpaul strains were obtained from a commercial turkey integrator. Each strain was cultured separately using a loopful of bacterial culture added into tryptic soy broth (TSB; catalog no. C7141, Criterion, Hardy Diagnostics, Santa Maria, CA) and incubated at 37°C for 24 h. After 3 successive subcultures in TSB, a stock culture was made by adding 15% glycerol (Glycerol; catalog no. J62399, Alfa Aesar, Haverhill, MA) to the bacterial culture and stored at −80°C until further use. Working cultures of S. Agona and S. Saintpaul were separately prepared by adding 100 µL of stock culture to 10 mL TSB and then incubated aerobically at 37°C for 24 h. Strains were induced resistance to nalidixic acid (NA) by culturing the strains in TSB containing NA sodium salt (CAS no. 3374-05-8, Alfa Aesar, Haverhill, MA) as previously published (Nair et al., 2019, Nair et al., 2021a, Nair et al., 2021b). Cultures were serially diluted using phosphate buffer saline (PBS; pH = 7.2) and plated with appropriate dilutions on xylose lysine desoxycholate agar (XLD; catalog no. C7322, Criterion, Hardy Diagnostics, Santa Maria, CA) plates containing 50 µg/mL NA and incubated at 37°C for 24 h. The growth of bacteria was identified by identifying black-colored colonies on the plates (Kollanoor Johny et al., 2012; Nair and Kollanoor Johny, 2017).
Preparation of Bacterial Inoculum
Salmonella strains grown in TSB after incubating at 37°C for 24 h were centrifuged at 3600 rpm for 15 min. The supernatant was discarded, and the pellet was washed thrice using PBS. The pellet was resuspended using PBS and used as an inoculum for each experiment. Appropriate dilutions were done for obtaining the bacterial inoculum required for each experiment (Kollanoor Johny et al., 2012)
Construction of Comparative Growth Curves of S. Agona and S. Saintpaul
Bacterial survival and growth for both strains were studied separately by determining the bacterial growth at 0, 3, 6, 12, and 24 h of incubation in 10 mL TSB with 50 µg/mL NA containing 100 µL of bacterial culture at 37°C. At each time point, bacterial cultures were serially diluted, and appropriate dilutions were plated on XLD + NA agar plates and incubated the plates at 37°C for 24 h. Black colonies were counted, and dilution factors were applied to calculate bacterial count per mL of TSB (Kollanoor Johny et al., 2007).
Determining the Antibiotic Susceptibility of S. Agona and S. Saintpaul
Strains were cultured by adding 100 µL of working cultures into 10 mL of TSB and incubating at 37°C for 24 h. After 24 h of incubation, a loopful of culture was streaked on XLD agar, and plates were kept in an incubator at 37°C for 24 h. Salmonella colonies turned into black round colonies on XLD plates and were submitted to the Veterinary Diagnostic Lab (VDL), University of Minnesota (Saint Paul, Minnesota), to determine antibiotic susceptibility. Different categories of clinically relevant antibiotics were tested against these strains, and the minimum inhibitory concentration was determined for each antibiotic. The antibiotic susceptibility test was done using Sensititre plates (Trek Diagnostic System, Thermo Fisher Scientific, MA). The interpretation was done based on the criteria of the Clinical and Laboratory Standards Institute (CLSI) for evaluating minimum inhibitory concentration (Nair and Kollanoor Johny et al., 2018)
Determining the Sub-Inhibitory Concentration (SIC), Minimum Inhibitory Concentration (MIC), and Minimum Bactericidal Concentration (MBC) of PEO Against S. Agona and S. Saintpaul
The sub-inhibitory concentration (SIC), minimum inhibitory concentration (MIC), and minimum bactericidal concentration (MBC) of PEO (Spectrum Chemical, New Brunswick, NJ) against S. Agona and S. Saintpaul were determined (Kollanoor Johny et al., 2007). Different concentrations of PEO were prepared in 10 mL TSB containing 50 µg/mL NA. A 4 log10 CFU/mL bacterial inoculum was prepared from the diluted and resuspended bacterial pellet obtained after centrifugation of the overnight culture of Salmonella strains in TSB. A 100 µL portion of bacteria was added to each treatment and incubated at 37°C for 24 h. A control sample containing Salmonella inoculated TSB with no PEO was also included. Bacterial growth was enumerated at 0 and 24 h of incubation after plating appropriate dilutions of bacteria on XLD + NA agar plates. The study was repeated 6 times. The MIC of PEO against Salmonella is taken as the concentration at which the oil inhibits the growth of Salmonella strains in TSB at 37°C (no visible growth, however, bacterial survival and counts are expected). The MBC is taken as the concentration at which the oil causes the complete inactivation of Salmonella in TSB (no visible growth and at least >6 log10 CFU/mL reduction). The SIC is the concentration just below the MIC with no reduction of bacterial growth in TSB (no inhibitory effects due to the oil) (Kollanoor Johny et al., 2007, 2010, 2012).
Determining the Effect of PEO on Mature Biofilms Formed by S. Agona and S. Saintpaul
Twenty-four well plate assay was used to determine the effect of PEO on mature biofilms formed by both serotypes of Salmonella (Upadhyay et al., 2013). Each well was filled with 2 mL TSB + 50 µg/mL NA. After adding 100 µL of 5 log10 CFU/mL inoculum of Salmonella into the well, the plates were incubated at 37°C for 4 d and 4°C for 7 d to form mature biofilms. After the biofilm formation, the MBC, or >MBC of PEO, was added to the wells. To follow, TSB was diluted and plated on XLD + NA agar. Positive control was kept without adding PEO into the wells. The media was then removed, washed thrice with PBS, and removed from the well. The wells were scraped to loosen the bacteria attached to the mature biofilm and then plated again on XLD + NA after serially diluting it in PBS. The plates were incubated at 37°C for 24 h. The effects of PEO on mature biofilm film were determined by counting the bacterial colonies grown on XLD + NA plates and applying appropriate dilution factors to obtain the remaining viable bacterial populations.
Determining the Effect of PEO Against S. Agona and S. Saintpaul in Ground Turkey
This experiment followed the published protocols using ground turkey meat experiments (Dewi et al., 2021). Briefly, commercial ground turkey meat with 93% protein and 7% fat was purchased from a local grocery store and used in this study. Twenty g of ground turkey meat was weighed and packed separately in Whirl-Pak bags (Whirl-Pak, Nasco, Madison, WI) for each treatment. A 3 to 3.5 log10 CFU/mL S. Agona and S. Saintpaul in combination was added into the ground turkey meat and mixed well to distribute the inoculum. This experiment had 7 treatment groups, 5 different PEO concentrations (0.25%, 0.5%, 1%, 2%, and 2.5%), and 2 control groups. In all the essential oil groups, respective volumes of the oil were added directly to the meat and mixed well. The positive control group had Salmonella inoculated meat without PEO. The negative control group had neither the oil nor the Salmonella inoculum. Each Whirl-Pak bag was closed and stored at 4°C for 7 d. The bacterial counts were detected from the respective ground meat samples on storage days 0, 1, 3, 5, and 7. For bacterial recovery, samples were homogenized well for a minute using a homogenizer (Stomacher, 100/125V, 50/60 Hz, Neutec Group Inc., 200 Central Ave, Farmingdale, NY) and serially diluted in PBS. Appropriate dilutions were plated on XLD + NA plates and incubated at 37°C for 24 h before counting the bacterial colonies. One g sample was also enriched in 10 mL sodium selenite cystine (SCB; Criterion, Hardy Diagnostics, Santa Maria, CA) to maximize Salmonella’s detection limit.
Determining the Effect of PEO-CN Coating on Nonprocessed Turkey Breast Meat Against S. Agona and S. Saintpaul
Chitosan (Sigma Aldrich, St. Louis, MO) coating solution was prepared by adding (2% v/v) CN into distilled water and stirring overnight to make a homogenous mixture with a final concentration of 2%. After 12 h of stirring, different concentrations of PEO were added to each coating solution and allowed another 6 h to mix well (Upadhyay et al., 2015). For this study, nonprocessed, fresh turkey breast meat obtained from a local slaughter unit was used. Turkey meat was cut into samples of 20 g weight. A bacterial inoculum prepared using overnight culture of S. Agona and S. Saintpaul was used for inoculating the meat samples. An inoculum of 3 to 3.5 log10 Salmonella 2 serotype mix was inoculated over the meat samples and allowed to attach for 20 min at room temperature. Each meat sample was then dipped in coating solutions containing different concentrations of PEO in Whirl-Pak bags for 2 min. The positive control was the meat samples inoculated with Salmonella and dipped in PBS for 2 min. Inoculated samples dipped in CN coating solution without PEO served as the CN control to differentiate the effect of CN alone compared to CN added with different PEO concentrations. After the dip treatment, meat samples were stored at 4°C for 7 d. Bacterial recovery was done on days 0, 1, 3, 5, and 7 of storage. On respective days, samples were transferred to 20 mL PBS in a Whirl-Pak bag and homogenized well for a minute using a homogenizer. The samples were then serially diluted, plated on XLD + NA plates, and incubated at 37°C for 24 h.
Determining the Effect of PEO on L*, a*, and b* Values of Ground Turkey Meat
L* (lightness), a* (redness), and b* (yellowness) values of meat samples were measured using a spectrophotometer (Hunter Lab MiniScan EZ 4500S Spectrophotometer, Reston, VA). Samples were prepared as described in section 4.6 except inoculating with Salmonella. The instrument was calibrated per instructions and recorded on white and black platforms. L*, a*, and b* values were then measured from each meat sample on days 0, 1, 3, 5, and 7 of storage at 4°C. Each treatment and control group had 6 samples at a given time point.
Determining the effect of PEO on L*, a*, and b* values of nonprocessed turkey breast meat coated with food-grade edible CN. Samples were prepared as described previously, except these experiments did not use a Salmonella inoculum. L*, a*, and b* values were measured from meat samples using a spectrophotometer on days 0, 1, 3, 5, and 7 of storage at 4°C.
Determining the Effect of PEO on pH Values of Ground Turkey Meat
Samples were prepared as described previously, except these experiments did not use a Salmonella inoculum. The pH of each sample was measured using a pH meter (Apera Instruments Premium—Series PH60S Food pH Tester, Columbus, OH) on days 0, 1, 3, 5, and 7 of storage at 4°C.
Determining the Effect of PEO and CN Dip on the pH of Nonprocessed Turkey Meat
Samples were prepared as described previously, except these experiments did not use a Salmonella inoculum. The pH of each sample was measured using a pH meter on days 0, 1, 3, 5, and 7 of storage at 4°C.
Statistical Analysis
Each experiment followed a completely randomized design and was repeated 6 times (n = 6 samples/treatment). Bacterial counts were transformed into log10 values, and dilution factors were applied before the statistical analysis. The PROC-ANOVA procedure of SAS was used to detect the significant difference between groups. Mean separation was done using Tukey's test. Significance was tested at P < 0.05.
RESULTS
Growth Dynamics of S. Agona and S. Saintpaul
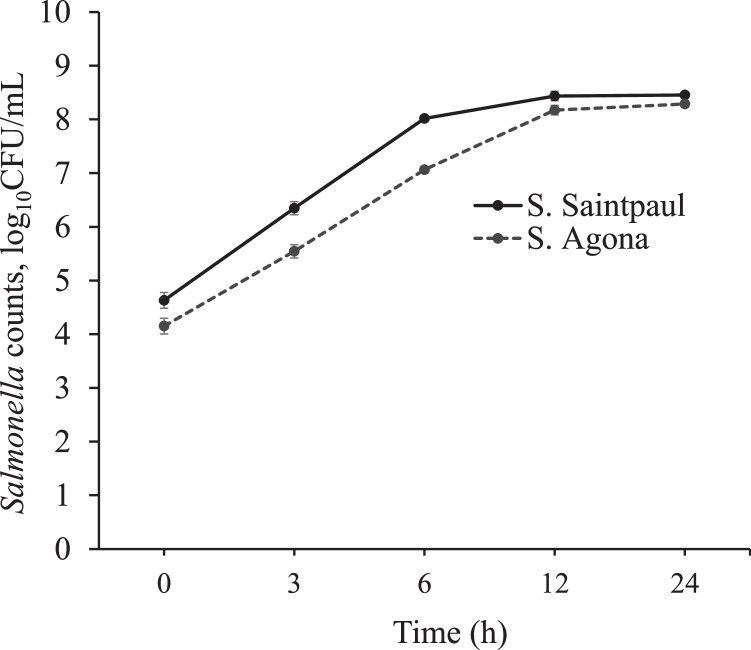
With an initial inoculum level of ∼4.5 log10 CFU/mL, S. Saintpaul reached 8.3 log10 CFU per mL at 6 h of incubation at 37°C in tryptic soy broth. Whereas, within the same time frame, S. Agona took 12 h to reach the same population levels (Figure 1).
Figure 1.
Growth curve of S. Agona (A) and S. Saintpaul (B) cultured in the TSB at 37°C for 24 h (n = 6).
Antibiotic Susceptibility of S. Agona and S. Saintpaul
Both S. Agona and S. Saintpaul strains were resistant to multiple clinically relevant antibiotics, including amoxicillin, clindamycin, gentamicin, novobiocin, oxytetracycline, penicillin, spectinomycin, streptomycin, sulfadimethoxine, sulphathiazole and tetracycline (Tables 1A and 1B).
Table 1A.
Antibiotic susceptibility profile of S. Agona strain.
| Antibiotics | MIC (µg/mL) |
|---|---|
| Amoxicillin | 001.00; resistant |
| Clindamycin | >004.00; resistant |
| Gentamicin | Resistant |
| Novobiocin | >004.00; resistant |
| Oxytetracycline | >008.00; resistant |
| Penicillin | 008.00; resistant |
| Spectinomycin | >064.00; resistant |
| Streptomycin | Resistant |
| Sulfadimethoxine | >256.00; resistant |
| Sulphathiazole | >256.00; resistant |
| Tetracycline | >008.00; resistant |
| Trimethoprim/sulphamethoxazole | </=005.00; resistant |
Table 1B.
Antibiotic susceptibility profile of S. Saintpaul strain.
| Antibiotics | MIC (µg/mL) |
|---|---|
| Amoxicillin | >016.00; resistant |
| Clindamycin | >004.00; resistant |
| Gentamicin | >008.00; resistant |
| Novobiocin | >004.00; resistant |
| Oxytetracycline | >008.00; resistant |
| Penicillin | >008.00; resistant |
| Spectinomycin | >064.00; resistant |
| Streptomycin | 064.00; resistant |
| Sulfadimethoxine | >256.00; resistant |
| Sulphathiazole | 256.00; resistant |
| Tetracycline | >008.00; resistant |
| Trimethoprim/sulphamethoxazole | <000.50; susceptible |
MIC, MBC, and SIC of PEO Against S. Agona and S. Saintpaul
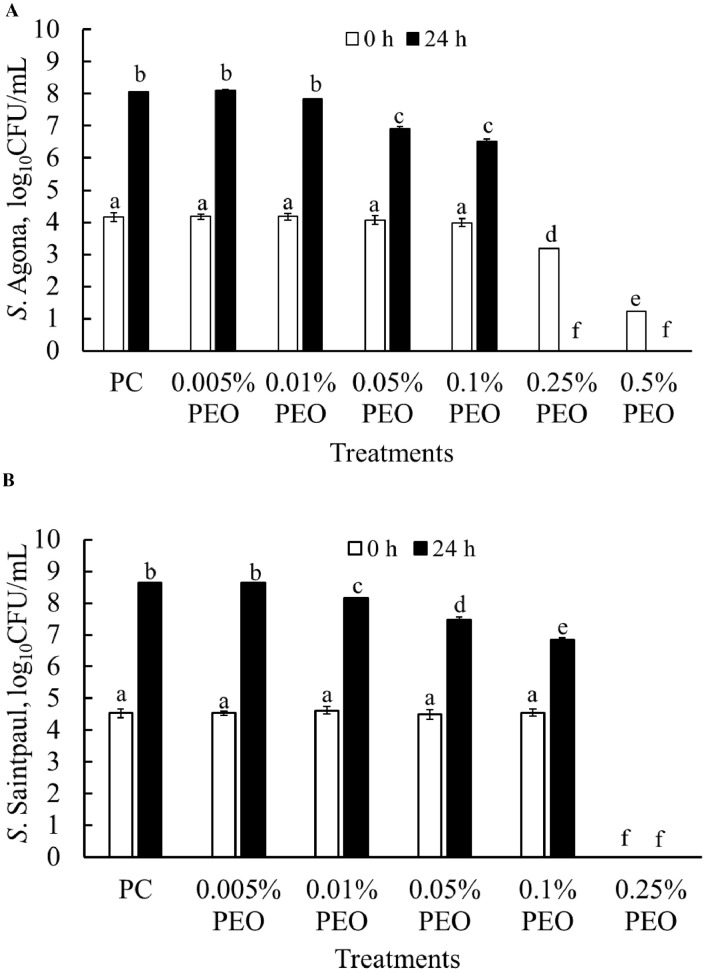
Approximately 4 log10CFU of S. Agona was recovered on XLD agar plates as initial inoculum from all the treatment groups at 0 h of incubation except for 0.25% and 0.5% groups (white bar; Figure 2A). An immediate reduction of S. Agona populations at 0 h was evident in treatment groups containing 0.25% and 0.5% PEO. At 24 h, PC and PEO at 0.005% and 0.01% had 8 log10 CFU/mL of S. Agona, whereas 0.05% and 0.1% of PEO had 6 log10 CFU/mL growth at 37°C (black bars; Figure 2A). Zero point two five percent and 0.5% PEO resulted in a complete reduction of Salmonella in TSB after 24 h.
Figure 2.
Effect of PEO against S. Agona (A) and S. Saintpaul (B) in tryptic soy broth culture at 37°C for 0 h, and 24 h (Mean ± SE; n = 6). a–f: bars with different superscripts are significantly different from each other at P < 0.05. Abbreviations: PC: positive control; PEO: pimenta essential oil.
Similarly, 4.5 log10 CFU/mL of S. Saintpaul was recovered in all treatment groups as well as in control groups at 0 h of incubation and reached up to 8.5 log10 after 24 h of incubation in both PC and 0.0005% PEO (Figure 2B). However, PEO at 0.01%, 0.05%, and 0.1% resulted in a growth of 8, 7.5, and 6.9 log10 S. Saintpaul CFU/mL after 24 h of incubation at 37°C. PEO at 0.25% resulted in a complete reduction of S. Saintpaul at 0 and 24 h of incubation. Briefly, MBC, MIC, and SIC of PEO against both S. Agona and S. Saintpaul were identified as 0.25%, between 0.1 and 0.25%, and at or below 0.005% of PEO, respectively (Figures 2A and 2B)
Effect of PEO on the Mature Biofilms Formed by S. Agona and S. Saintpaul
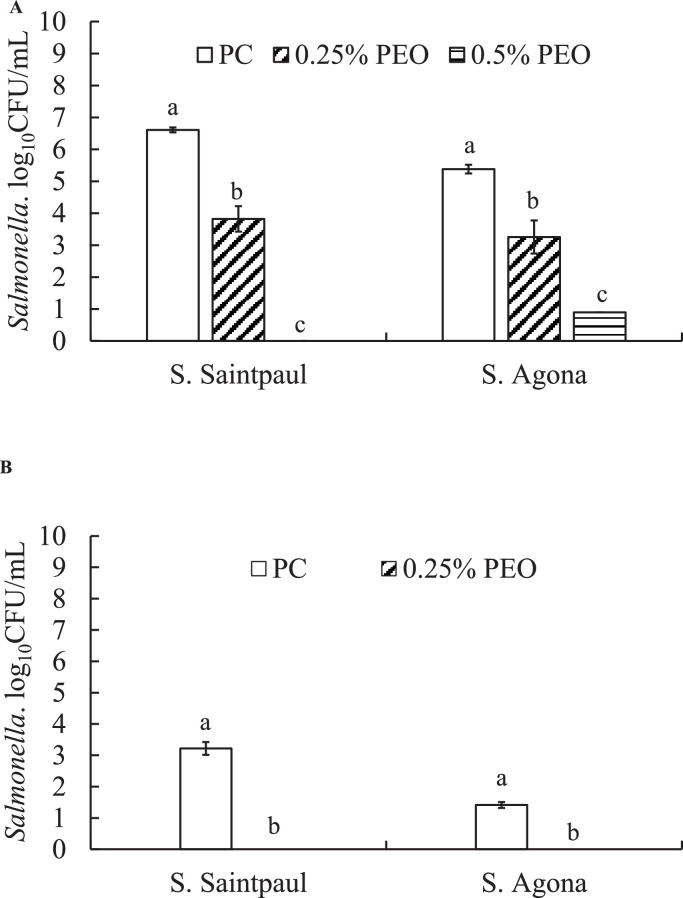
In the control groups, both S. Agona and S. Saintpaul had ∼5.4- and 6.6- log10 CFU/mL of bacteria associated with the mature biofilms at 37°C, respectively. PEO at 0.25% reduced S. Agona and S. Saintpaul by 2.1- and 2.8- log10CFU/mL of associated bacterial cells in the mature biofilms at 37°C (P < 0.05). A complete reduction of S. Saintpaul cells was observed when PEO was tested 0.5% (2X MBC) of PEO at 37°C. The maximum decrease of 4.5 log10 S. Agona/mL was observed when 0.5% of PEO was used on the mature biofilms (Figure 3A; second cluster). S. Agona and S. Saintpaul could also produce biofilms in polystyrene well plates even at 4°C and reached a count of 1.4- and 3.2- log10CFU/mL, respectively, after 7 d of incubation (Figure 3B). A complete reduction of Salmonella in the mature biofilms was observed with 0.25% PEO in polystyrene plates incubated at 4°C (Figure 3B).
Figure 3.
Effect of PEO on mature biofilm formed by S. Agona and S. Saintpaul at 37°C (A) and 4°C (B) (Mean ± SE; n = 6). a, b—bars with different superscripts are significantly different from each other at P < 0.05. Abbreviations: PC: positive control; PEO: pimenta essential oil.
Effect of PEO Against S. Agona and S. Saintpaul in Ground Turkey Meat
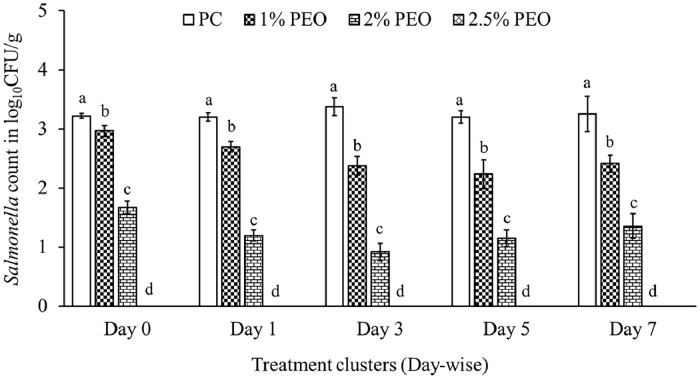
In the positive control groups, 3.2 log10 CFU of S. Agona and S. Saintpaul mixture were consistently recovered from the ground meat samples stored at 4°C from day 0 to 7. PEO at 0.25% and 0.5% concentrations were the least effective in reducing the Salmonella combination in the ground turkey meat with a maximum reduction of 0.36 and 0.5 log10 CFU/g, respectively at 4°C (data not shown). One percent PEO caused a maximum reduction of 0.9 log10 CFU/g at day 3 of storage at 4°C compared to PC. Two percent PEO was effective in reducing 1.5 log10 CFU/g immediately after inoculation, whereas 2 to 2.5 log10 CFU/g reduction was obtained in subsequent days of storage at 4°C. Two-point five percent PEO effectively reduced the Salmonella mix in ground turkey meat immediately after inoculation, and the magnitude of reduction was nearly 3.4 log10 CFU/g compared to PC (Figure 4).
Figure 4.
Effect of PEO on S. Agona and S. Saintpaul in ground turkey meat (Mean ± SE; n = 6). a, b, c, d—bars with different superscripts at a single time point significantly differ at P < 0.05. Abbreviations: PC: positive control; PEO: Pimenta essential oil.
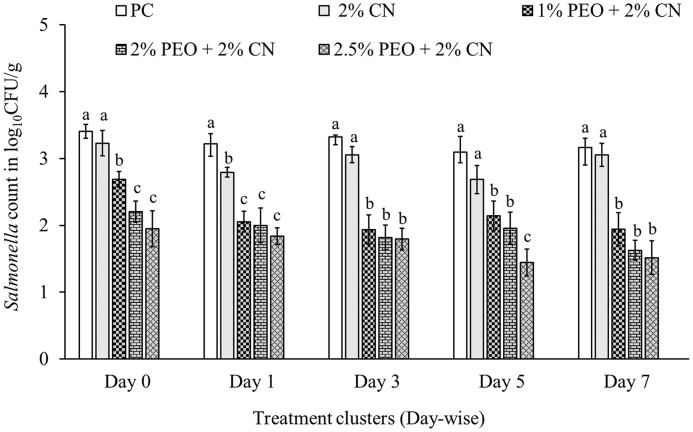
Effect of PEO With a Coating Agent, CN on S. Agona and S. Saintpaul Mix on Nonprocessed Turkey Meat
Salmonella recovery from the turkey breast meat samples from PC groups was between 3 to 3.4 log10 CFU/g during 7 d of storage at 4°C (Figure 5; white bars). It was found that 2% CN alone did not reduce Salmonella than PC (gray bars). The combination of 2% CN with 0.25% PEO was also ineffective in reducing Salmonella on turkey breast meat and resulted in 2.7 to 3 log10 CFU/g Salmonella recovery throughout the storage period (data not shown). Dip treatment in 0.5% and 1% PEO with 2% CN reduced 1- and 1.4- log10 CFU/g, respectively, on day 3 of storage at 4°C (data not shown). A maximum reduction of 1.54 log10 CFU/g was obtained on day 7 from turkey meat dipped in 2% CN with 2% PEO for 2 min. PEO at 2.5% PEO and CN at 2% effectively reduced 1.4 to 1.7 log10 CFU/g Salmonella from day 0 to day 7 of storage at 4°C (Figure 5).
Figure 5.
Effect of coating of PEO with CN on S. Agona and S. Saintpaul on turkey breast meat (Mean ± SE; n = 6). a, b, c, d—bars with different superscripts at a single time point significantly differ at P < 0.05. Abbreviations: PC: positive control; PEO: pimenta essential oil.
Effect of PEO on L*, a*, and b* Values of Ground Turkey Meat
Table 2A depicts the L*, a*, and b* values of the ground turkey meat in response to PEO treatments. The L* values of meat samples from the control groups were between 49.55 and 58.53. The L* values of ground turkey meat samples mixed with different concentrations of PEO were compared with that of NC (control). PEO at 1, 2, and 2.5% concentrations in ground turkey meat showed L* values between 52.16 and 59.95, 56.22 and 63.80, 57.07 and 63.65, respectively, during storage at 4°C. PEO at 2 and 2.5% showed a significant difference from other groups from day 1 (P < 0.05). The a* values of ground turkey meat samples were 3.46 to 6.24, 3.96 to 6.65, 4.71 to 6.98, 5.74 to 7.04 in NC, 1% PEO, 2% PEO, and 2.5% PEO, respectively, during the storage period at 4°C. Day 7 a* values for all treatments significantly differed from the NC (P < 0.05). The b* values between 14.70 and 17.24, 15.31 and 17.46, 14.99 and 17.11, 16.24 and 17.24 were observed from the ground turkey meat samples in the NC group, 1% PEO, 2% PEO, and 2.5% PEO, respectively (Table 2A).
Table 2A.
Effect of PEO on L*, a*, and b* of ground turkey meat on days 0, 1, 3, 5, and 7 of storage at 4°C.
| L* | Day 0 | Day 1 | Day 3 | Day 5 | Day 7 |
|---|---|---|---|---|---|
| NC | 56.94 ± 2.33a | 49.55 ± 0.81a | 53.89 ± 1.03a | 57.65 ± 0.45a | 58.53 ± 1.72a |
| 1% PEO | 54.95 ± 1.87a | 52.16 ± 0.90a | 57.54 ± 1.11a | 59.95 ± 1.17a | 59.67 ± 0.60a,b |
| 2% PEO | 58.07 ± 0.84a | 56.22 ± 1.22b | 60.45 ± 1.13b | 63.92 ± 0.85b | 63.80 ± 0.92b |
| 2.5% PEO | 59.44 ± 1.75a | 57.07 ± 0.55b | 60.11 ± 1.02b | 63.65 ± 0.77b | 62.29 ± 0.95a,b |
| a* | Day 0 | Day 1 | Day 3 | Day 5 | Day 7 |
| NC | 5.40 ± 0.64a | 6.24 ± 0.45a | 5.22 ± 0.39a | 3.82 ± 0.65a | 3.46 ± 0.36a |
| 1% PEO | 5.82 ± 0.98a | 6.65 ± 0.25a | 6.03 ± 0.25a | 3.96 ± 0.74a | 5.53 ± 0.43b |
| 2% PEO | 6.21 ± 0.58a | 6.98 ± 0.60a | 5.60 ± 0.60a | 4.71 ± 0.81a | 5.98 ± 0.40b |
| 2.5% PEO | 5.78 ± 0.76a | 6.53 ± 0.34a | 6.28 ± 0.31a | 5.74 ± 0.37a | 7.04 ± 0.43b |
| b* | Day 0 | Day 1 | Day 3 | Day 5 | Day 7 |
| NC | 16.37 ± 0.71a | 15.47 ± 0.40a | 14.70 ± 0.53a | 17.24 ± 0.31a | 15.00 ± 0.98a |
| 1% PEO | 16.61 ± 1.18a | 16.35 ± 0.52a | 15.31 ± 0.45a | 17.46 ± 0.44a | 16.50 ± 0.16a |
| 2% PEO | 17.69 ± 0.65a | 17.11 ± 0.48a | 14.99 ± 0.81a | 16.46 ± 0.64a | 16.13 ± 0.54a |
| 2.5% PEO | 16.63 ± 1.26a | 17.24 ± 0.51a | 16.35 ± 0.46a | 16.35 ± 0.46a | 16.24 ± 0.46a |
The color values for each group are represented as Mean ± SE (n = 6).
Values with different superscripts at a single time point significantly differ at P < 0.05.
Effect of PEO on L*, a*, and b* Values of Nonprocessed Turkey Meat Coated With PEO Using CN
Table 3A depicts the L*, a*, and b* values of the ground turkey meat in response to PEO treatments with CN coating. The L* values for meat samples from NC were in the range of 43.99 to 51.11. The samples dipped in 2% CN showed L* values between 39.29 and 47.13. None of the dip treatments in PEO and CN combinations resulted in a significant change in L* values of meat samples over 7 d of storage at 4°C (P > 0.05). The a* values of meat samples from the NC control group and those dipped in CN were −0.17 to −1.03 and −0.37 to −1.74, respectively. a* values between −0.28 to −1.93, −0.43 to −1.22, and −0.40 to −0.92 were noticed with samples dipped in a combination of CN and 1%, 2%, and 2.5% PEO, respectively. The b* values for negative control samples range from 5.87 to 7.93 for 7 d storage period. Meat samples dipped in 2% CN showed b* values from 7.24 to 8.81. Dip treatments including 1% PEO + 2% CN, 2% PEO + 2% CN, and 2.5% PEO + 2% CN resulted in b* values of 6.67 to 7.85, 6.49 to 10.2 and 8.08 to 9.32, respectively (Table 3A).
Table 3A.
Effect of PEO and CN dip on L*, a*, and b* values of turkey meat at days 0, 1, 3, 5, and 7 of storage at 4°C.
| L* | Day 0 | Day 1 | Day 3 | Day 5 | Day 7 |
|---|---|---|---|---|---|
| NC | 51.11 ± 3.71a | 46.11 ± 0.99a,c | 44.44 ± 1.10a | 44.29 ± 1.22a | 43.99 ± 1.14a |
| 2% CN | 41.74 ± 1.29a | 39.29 ± 1.40b | 42.33 ± 1.22a | 45.66 ± 0.90a | 47.13 ± 2.97a,b |
| 1% PEO + 2% CN | 44.98 ± 1.38a | 43.27 ± 0.72a,b,c | 43.40 ± 1.07a | 46.91 ± 1.33a | 51.47 ± 1.80b |
| 2% PEO + 2% CN | 46.39 ± 2.57a | 41.99 ± 1.88a,b | 44.93 ± 1.77a | 44.00 ± 2.46a | 49.61 ± 1.52a,b |
| 2.5% PEO + 2% CN | 43.59 ± 4.10a | 47.47 ± 0.95c | 46.07 ± 0.70a | 51.74 ± 2.88a | 51.18 ± 0.62a,b |
| a* | Day 0 | Day 1 | Day 3 | Day 5 | Day 7 |
| NC | −1.03 ± 0.64a | −0.17 ± 0.24a | −0.95 ± 0.36a | −1.58 ± 0.23a | −0.81 ± 0.94a |
| 2% CN | −0.37 ± 0.32a | −0.54 ± 0.63a | −0.84 ± 0.27a | −1.74 ± 0.53a | −1.42 ± 0.39a |
| 1% PEO + 2% CN | −0.28 ± 0.41a | −0.38 ± 0.17a | −0.72 ± 0.24a | −1.19 ± 0.46a | −1.93 ± 0.33a |
| 2% PEO + 2% CN | −0.47 ± 0.56a | −0.43 ± 0.38a | −0.43 ± 0.52a | −0.92 ± 0.34a | −1.22 ± 0.22a |
| 2.5% PEO + 2% CN | −0.40 ± 0.53a | −0.86 ± 0.20a | −0.89 ± 0.20a | −0.55 ± 0.26a | −0.92 ± 0.40a |
| b* | Day 0 | Day 1 | Day 3 | Day 5 | Day 7 |
| NC | 6.99 ± 0.33a | 7.93 ± 0.83a | 6.45 ± 0.52a | 5.87 ± 0.60a | 6.48 ± 0.54a |
| 2% CN | 7.26 ± 1.04a | 8.31 ± 0.61a | 8.24 ± 0.71a | 8.81 ± 1.44a,b | 7.24 ± 0.79a,b |
| 1% PEO + 2% CN | 7.45 ± 0.64a | 7.85 ± 0.73a | 7.63 ± 0.69a | 6.67 ± 0.68a,b | 6.84 ± 0.64a |
| 2% PEO + 2% CN | 7.58 ± 0.58a | 7.14 ± 0.40a | 6.49 ± 0.45a | 9.97 ± 0.36b | 10.2 ± 0.54b |
| 2.5% PEO + 2% CN | 8.11 ± 0.44a | 8.08 ± 0.50a | 8.77 ± 0.45a | 8.61 ± 0.73a,b | 9.32 ± 1.08a,b |
The color values for each group are represented as Mean ± SE (n = 6).
values with different superscripts at a single time point significantly differ at P < 0.05.
Effect of PEO and/or CN Coating on pH Values Of Turkey Meat
The pH values of ground turkey meat samples without PEO (negative control) ranged from 5.93 to 6.28 during the storage period of 7 d. Ground turkey meat samples with 1% PEO and 2% PEO had pH values ranging between 6.05 and 6.47, 6.21 and 6.72, respectively, from day 0 to day 7 of storage at 4°C. The addition of 2.5% PEO into the ground turkey meat samples resulted in pH values of 6.17 to 6.53 on various days of storage at 4°C (Table 2B).
Table 2B.
Effect of PEO on pH of ground turkey meat on days 0, 1, 3, 5, and 7 of storage at 4C.
| Day 0 | Day 1 | Day 3 | Day 5 | Day 7 | |
|---|---|---|---|---|---|
| NC | 6.17 ± 0.03a | 6.26 ± 0.01a | 5.93 ± 0.01a | 6.23 ± 0.06a | 6.28 ± 0.05a |
| 1% PEO | 6.22 ± 0.03a,b | 6.29 ± 0.02a,b | 6.05 ± 0.03a | 6.47 ± 0.09a,b | 6.37 ± 0.05a |
| 2% PEO | 6.26 ± 0.01b | 6.31 ± 0.01b | 6.21 ± 0.04b | 6.55 ± 0.03b | 6.72 ± 0.06b |
| 2.5% PEO | 6.27 ± 0.01b | 6.38 ± 0.05b | 6.17 ± 0.04b | 6.53 ± 0.05b | 6.38 ± 0.05a |
The pH values for each group are represented as Mean ± SE (n = 6).
values with different superscripts at a single time point significantly differ at P < 0.05.
The pH values of nonprocessed turkey breast meat samples dipped in DI water (NC) for 2 min ranged from 5.94 to 6.89 during 7 d of storage at 4°C. Dipping the turkey meat samples in 2% CN coating solution with or without PEO for 2 min resulted in the pH values lower than the NC during storage (P < 0.05). CN with 1% PEO and 2% PEO dip treatments resulted in pH values ranging from 5.55 to 6 and 5.6 to 5.78, respectively, during 7 d of storage at 4°C. pH values between 5.6 and 5.94 were observed from the turkey breast meat samples stored in the refrigerator after dipping in 2.5% PEO with CN (Table 3B).
Table 3B.
Effect of PEO and CN dip on pH of turkey meat at days 0, 1, 3, 5, and 7 of storage at 4C.
| Day 0 | Day 1 | Day 3 | Day 5 | Day 7 | |
|---|---|---|---|---|---|
| NC | 5.94 ± 0.07a | 6.10 ± 0.16a | 6.34 ± 0.16a | 6.58 ± 0.11a | 6.89 ± 0.15a |
| 2% CN | 5.37 ± 0.03b | 5.63 ± 0.05b | 5.96 ± 0.14a,b | 6.01 ± 0.12b | 6.30 ± 0.14b |
| 1% PEO + 2% CN | 5.55 ± 0.10b | 5.72 ± 0.03b | 5.81 ± 0.10b | 5.81 ± 0.06b | 6.00 ± 0.15b,c |
| 2% PEO + 2% CN | 5.60 ± 0.12b | 5.66 ± 0.04b | 5.65 ± 0.05b | 5.77 ± 0.05b | 5.78 ± 0.08c |
| 2.5% PEO + 2% CN | 5.60 ± 0.05b | 5.73 ± 0.03b | 5.70 ± 0.03b | 5.94 ± 0.17b | 5.84 ± 0.05b,c |
The pH values for each group are represented as Mean ± SE (n = 6).
values with different superscripts at a single time point significantly differ at P < 0.05.
DISCUSSION
S. Agona and S. Saintpaul are commonly found serotypes in turkey meat products over many years (Erol et al., 2013). The consistent presence of these serotypes in turkeys indicates their hardiness to environmental factors and antimicrobials used in production and processing facilities. The results of comparative growth, antibiotic susceptibility and biofilm formation data of these serotypes obtained from this study also supports the persistence and emergence as potentially outbreak-causing strains of these serotypes in turkey production. Our goal in the study was to test the efficacy of plant-derived interventions against such serotypes of Salmonella that could co-exist on farm environments and in turkey products and devise control strategies before outbreaks occur.
Essential oils have been studied extensively for their manifold uses in the meat industry for the past few years due to their many benefits, including clean-label prospects (Dewi and Kollanoor Johny, 2022). The application of essential oil in meat industry has been explored at different stages of meat processing and packaging because of their multiple benefits including its use in food preservation and safety. Essential oils are considered as eco-friendly options for antimicrobial, antioxidant, and flavoring agents in the food industry (Maurya et al., 2021). It is imperative to choose the effective essential oil and the stage when targeting emerging serotypes of Salmonella in turkey production and processing.
Comminuted turkey products, including ground turkey, can contain portions from turkey skin, bone, and meat, potentially increasing the chances of Salmonella contamination in the final product (USDA FSIS, 2021). Combining these parts during the final stages of meat processing and the chances of contamination from grinding equipment demand the need for the direct addition of antimicrobials at or after grinding. Our study addressed this situation by adding and mixing PEO into the ground turkey meat inoculated with Salmonella cocktail. We found that PEO at 2.5% significantly reduced S. Agona and S. Saintpaul when used as a direct antimicrobial additive in ground turkey meat (Figure 4). Previously, Dewi and co-authors determined the efficacy of 3 plant-derived antimicrobials (PDAs), lemongrass essential oil (LGEO), citral, and trans-cinnamaldehyde (TC), against S. Heidelberg in ground turkey meat (Dewi et al., 2022). They also reported higher Salmonella reductions at 2% TC concentrations in ground turkey meat at 4°C on day 3 (Dewi et al., 2022), consistent with the findings of our study. A separate study tested 2,000 ppm lauric alginate and 1% carvacrol (CR) against S. Typhimurium in ground turkey. The compounds had a synergistic effect reducing the bacterial load to less than a log10CFU/g after 24 h of storage compared to the 5-log initial inoculum (Oladunjoye et al., 2013). In the same study, 2% CR reduced 2 to 3 log10CFU/g Salmonella in ground turkey meat when 3 strains of S. Typhimurium were used for the inoculation (Oladunjoye et al., 2013). These studies indicate the necessity of higher concentrations of essential oils for better efficacy against different Salmonella serotypes. The consistency in reducing Salmonella at higher concentrations of essential oils was also evident in this study. The magnitude of reduction could be maximized either by increasing the concentrations of essential oils or by combining them with other antimicrobials.
Exploring the right processing stage to implement essential oil interventions is challenging and can be affected by the type of final meat products. Previous studies show that developing a dipping model can best target pathogens attached to unprocessed meat. Similar to our research using multiple Salmonella serotypes, Nair and co-workers tested CR, TC, and thyme oil as 2 min dip treatments against a combination of S. Enteritidis, S. Typhimurium, and S. Heidelberg on turkey breast cutlets. They found that a 0.9 to 1.7 log10 CFU/mL reduction in Salmonella can be obtained with 2% essential oil dip treatment (Nair et al., 2014). A sequential dipping model with bacteriophage and essential oils (0.8% thymol and 1.6% CR) for 3 min also resulted in an additive effect on Salmonella reduction on chicken pieces stored at 4°C for 5 d compared to their individual treatments (Moon et al., 2020). Our previously reported study also explored the combination of caprylic acid and peracetic acid as scald additive against drug resistant S. Heidelberg. We found that 1% caprylic acid with 0.5% PAA in scalding water was in effective in reducing more than 2.5 log10 CFU/mL of S. Heidelberg attached to chicken skin (Manjankattil et al., 2021).
Pimenta essential oil has also been tested as scalding and chilling tank additives against S. Heidelberg. Three different concentrations of PEO were tested along 3 time points and found that the antibacterial effect is concentration and time-dependent. Nearly 2 log10 CFU/sq. inch reduction of Salmonella was obtained when PEO was used in the scalder water, whereas 2.4 log10 CFU/sq. inch reduction was obtained when used in chilled water for 5 min. The same study reported a decline in pathogen populations for 48 h at 4°C storage. It was also found that PEO nanoemulsion resulted in a comparable decrease in S. Heidelberg populations as with PEO (Nair and Kollanoor Johny, 2017).
Salmonella surviving after chilling and antimicrobial wash steps can be a harmful risk for consumers purchasing turkey breast meat or whole carcasses. The strong attachment of Salmonella and less reduction associated with antimicrobial dip were established in our previous studies (Manjankattil et al., 2021). A shorter duration of dip treatment in PEO after chilling may not be sufficient to tackle the strongly attached Salmonella on turkey breast meat. This indicates the need for an edible coating agent with excellent filming properties to provide maximum contact time for antimicrobials to act on pathogens even with a 2-min dip treatment. In the current study, we tested an additive effect of PEO with an edible coating agent CN against 2 emerging serotypes of Salmonella. Previous studies show that 0.5% CN dip treatment effectively reduced less than 1 log of S. Typhimurium per cm2 of chicken skin. Zero point five percent CN effectively reduced aerobic gram-negative spoilage bacteria to an undetectable level over 12 d of storage at 4°C (Menconi et al., 2013). In contrast, 2% CN dip for 2 min was ineffective against S. Agona and S. Saintpaul on turkey breast meat in this study. A 2% CN (w/v) in combination with citrus extract showed an additive effect in reducing S. Typhimurium on turkey fillets to 4.5 and 5 log10 CFU/g at 4 and 10°C, respectively, after 21 d of storage (Vardaka et al., 2016). However, a combination treatment of CN and PEO resulted in only less than 2 log10 CFU/g reduction of target serotypes in this study.
Intervention-resistant Salmonella biofilms can be considered a biological hazard and a source of contamination of turkey meat and meat products from the processing facility. A biofilm can be defined as an assemblage of microbial cells that is irreversibly associated with a surface and enclosed in a matrix of primarily polysaccharide material (Donlan et al., 2002). Bacteria in biofilms are highly resistant to many environmental conditions including disinfectants compared to their planktonic counterparts. Among the many compounds explored against biofilms formed by Salmonella, essential oils have shown promising results in the past years. Essential oils like thyme oil, oregano essential oil, and CR effectively reduced the biofilm formation by S. Typhimurium. In the same study, the researchers found that 0.1% CR effectively reduced biofilms to undetectable levels on stainless steel within a contact time of 1 h (Soni et al., 2013). LGEO was also reported to have a pronounced effect in reducing biofilm formation by S. Heidelberg in microtiter plates (Dewi et al., 2021). TC has reported efficacy against S. Saintpaul in established biofilms on stainless steel (Piovezan et al., 2014).
The extent of biofilm formation can significantly vary with strains of Salmonella, and we found that both S. Agona and S. Saintpaul can produce biofilms at both 37 and 4°C. Consistent with other essential oils, PEO effectively eliminated mature biofilm-attached Salmonella in microtiter well plates (Figures 2A and 2B). We also noticed that S. Agona associated with mature biofilms was more resistant than S. Saintpaul and had comparatively lower reduction with PEO treatments (Figures 2A and 2B).
The mechanisms of action of essential oils were broadly studied in the past years because of its multiple use in the food industry. It is reported that the varieties of bioactive substances present in the essential oils contribute their antimicrobial activity by interacting with one or more molecular pathways in microorganisms. Cytotoxic effects of some essential oils could be attributed to their activity on disrupting cell membrane and interfering with energy metabolism (Li et al., 2022). Highly concentrated phenolic compounds in essential oils like PEO can also increase the fluidity of cells and leakage of intracellular materials (Li et al., 2022). It is also reported that some essential oils can cause cell cycle arrest and bacterial filamentation (Li et al., 2022).
Sensory analysis studies must be conducted before adopting the intervention in the processing units. It is also vital to conduct a cost-benefit analysis of PEO incorporation at different processing stages, finding economical sources of the essential oil, obtaining volume discounts, and consideration of added costs of the equipment, if such modifications are to be made (Viator et al., 2017). Overall, the results of this study indicate the potential of using PEO as an antimicrobial in various turkey processing stages to improve the microbiological safety of turkey meat and meat products. PEO was highly effective in reducing multiple emerging serovars of Salmonella in turkey breast meat and ground turkey meat without adversely affecting meat samples’ color parameters and pH values.
Acknowledgments
ACKNOWLEDGMENTS
The authors acknowledge the Minnesota Department of Agriculture for the Rapid Agricultural Response Grant # 81824 FY 20-21, awarded to Dr. Anup Kollanoor Johny for this research. We would also like to thank the Minnesota Discovery, Research, and InnoVation Economy—Global Food Venture (MnDRIVE-GFV) Graduate Fellowship awarded to Mrs. Shijinaraj Manjankattil during this study.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- Burt S. Essential oils: their antibacterial properties and potential applications in foods: a review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- CDC. 2021. Foodborne illness source attribution estimates for 2019 for Salmonella, Escherichia coli O157, Listeria monocytogenes, and Campylobacter using multi-year outbreak surveillance data, United States. accessed Mar. 2022. https://www.cdc.gov/foodsafety/ifsac/pdf/P19-2019-report-TriAgency-508.pdf
- CDC. 2022. Salmonella. accessed Mar. 2022. https://www.cdc.gov/salmonella/outbreaks.html
- Chaudhari A., Singh V., Das S., Dubey N.K. Fabrication, characterization, and bioactivity assessment of CN nanoemulsion containing allspice essential oil to mitigate Aspergillus flavus. Food Chem. 2022;372 doi: 10.1016/j.foodchem.2021.131221. [DOI] [PubMed] [Google Scholar]
- Contreras-Moreno B.Z. Potential of Essential Oils. IntechOpen Limited; London, UK: 2018. Chemical composition of essential oil of genus Pimenta (Myrtaceae) p. 21. [Google Scholar]
- Dewi G., Kollanoor Johny A. Lactobacillus in food animal production: a forerunner for clean label prospects in animal-derived products. Front. Sustain. Food Syst. 2022;6:128. [Google Scholar]
- Dewi G., Nair D.V., Peichel C., Johnson T.J., Noll S., Johny A.K. Effect of lemongrass essential oil against multidrug-resistant Salmonella Heidelberg and its attachment to chicken skin and meat. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewi G., Manjankattil S., Peichel C., Jia S., Nair D., Vickers Z., Johnson T.J., Cardona C., Noll S., Johny A.Kollanoor. Effect of plant-derived antimicrobials against multidrug-resistant Salmonella Heidelberg in ground turkey. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlan R.M. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 2002;8:881. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erol I., Goncuoglu M., Ayaz N.D., Ellerbroek L., Bilir Ormanci F.S., Kangal O.Iseri. Serotype distribution of Salmonella isolates from turkey ground meat and meat parts. Biomed Res. Int. 2013;2013 doi: 10.1155/2013/281591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA (Food and Drug Administration) CFR—Code of Federal Regulations Title 21, 2023. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=182&showFR=1
- Hoffmann S., Maculloch B., Batz M. United States Department of Agriculture; Washington, DC: 2015. Economic Burden of Major Foodborne Illnesses Acquired in the United States.http://www.ers.usda.gov/media/1837791/eib140.pdf [Google Scholar]
- Ismail M.M., Samir R., Saber F.R., Ahmed S.R., Farag M.A. Pimenta oil as a potential treatment for Acinetobacter baumannii wound infection: in vitro and in vivo bioassays in relation to its chemical composition. Antibiotics (Basel) 2020;9:679. doi: 10.3390/antibiotics9100679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollanoor, A., P. Vasudevan, M. Kumar, M. Nair, T. Hoagland, and K. Venkitanarayanan. 2007. Inactivation of bacterial fish pathogens by medium-chain lipid molecules (caprylic acid, monocaprylin and sodium caprylate). Aquac. Res. 38:1293-1300
- Kollanoor Johny A., Hoagland T., Venkitanarayanan K. Effect of subinhibitory concentrations of plant-derived molecules in increasing the sensitivity of multidrug-resistant Salmonella enterica serovar Typhimurium DT104 to antibiotics. Foodborne Pathog. Dis. 2010;7:1165–1170. doi: 10.1089/fpd.2009.0527. [DOI] [PubMed] [Google Scholar]
- Kollanoor-Johny A.K., Frye J.G., Donoghue A., Donoghue D.J., Porwollik S., McClelland M., Venkitanarayanan K. Gene expression response of Salmonella serotype Enteritidis phage type 8 to subinhibitory concentrations of the plant-derived compounds trans-cinnamaldehyde and eugenol. Front. Microbiol. 2017;8:1828. doi: 10.3389/fmicb.2017.01828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollanoor-Johny A., Mattson T., Baskaran S.A., Amalaradjou M.A., Babapoor S., March B., Valipe S., Darre M., Hoagland T., Schreiber D., Khan M.I. Reduction of Salmonella enterica serovar Enteritidis colonization in 20-day-old broiler chickens by the plant-derived compounds trans-cinnamaldehyde and eugenol. Appl. Environ. Microbiol. 2012;78:2981–2987. doi: 10.1128/AEM.07643-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.X., Erhunmwunsee F., Liu M., Yang K., Zheng W., Tian J. Antimicrobial mechanisms of spice essential oils and application in food industry. Food Chem. 2022;382 doi: 10.1016/j.foodchem.2022.132312. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Leal A.C., Palou E., López-Malo A., Bach H. Antimicrobial, cytotoxic, and anti-inflammatory activities of Pimenta dioica and Rosmarinus officinalis essential oils. Biomed Res. Int. 2019;2019 doi: 10.1155/2019/1639726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe H.I.C., Daley D.K., Lindo J., Davis C., Rainford L., Hartley S.-A., Watson C., Chambers C., Reynolds-Campbell G., Foster S.R., Bahadoosingh P., Thoms-Rodriguez C. The antibacterial and antifungal analysis of crude extracts from the leaves and bark of Pimenta species found in Jamaica. J. Med. Plant Res. 2017;11:591–595. [Google Scholar]
- Manjankattil S., Nair D.V., Peichel C., Noll S., Johnson T.J., Cox R.B., Donoghue A.M., Johny A.K. Effect of caprylic acid alone or in combination with peracetic acid against multidrug-resistant Salmonella Heidelberg on chicken drumsticks in a soft scalding temperature-time setup. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurya A., Prasad J., Das S., Dwivedy A.K. Essential oils and their application in food safety. Front. Sustain. Food Syst. 2021;5 [Google Scholar]
- Menconi A., Hernandez-Velasco X., Latorre J.D., Kallapura G., Pumford N.R., Morgan M.J., Hargis B.M., Tellez G. Effect of CN as a biological sanitizer for Salmonella Typhimurium and aerobic gram-negative spoilage bacteria present on chicken skin. Int. J. Poult Sci. 2013;12:318–321. [Google Scholar]
- Milenković A., Stanojević J., Stojanović-Radić Z., Pejčić M., Cvetković D., Zvezdanović J., Stanojević L. Chemical composition, antioxidative and antimicrobial activity of allspice (Pimenta dioica (L.) Merr.) essential oil and extract. Adv. Technol. 2020;9:27–36. [Google Scholar]
- Miller E.A., Elnekave E., Flores-Figueroa C., Johnson A., Kearney A., Munoz-Aguayo J., Tagg K.A., L.Tschetter B.P.Weber, Nadon C.A., Boxrud D. Emergence of a novel Salmonella enterica serotype reading clonal group is linked to its expansion in commercial Turkey production, resulting in unanticipated human illness in North America. MSphere. 2020;5 doi: 10.1128/mSphere.00056-20. 00056-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon S.H., Waite-Cusic J., Huang E. Control of Salmonella in chicken meat using a combination of a commercial bacteriophage and plant-based essential oils. Food Control. 2020;110 [Google Scholar]
- Nair D.V.T., Johnson T.J., Noll S.L., Kollanoor Johny A. Effect of supplementation of a dairy-originated probiotic bacterium, Propionibacterium freudenreichii subsp. freudenreichii, on the cecal microbiome of turkeys challenged with multidrug-resistant Salmonella Heidelberg. Poult. Sci. 2021;100:283–295. doi: 10.1016/j.psj.2020.09.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair D.V.T., Kollanoor-Johny A. Food grade pimenta leaf essential oil reduces the attachment of Salmonella Heidelberg (2011 ground turkey outbreak isolate) on to turkey skin. Front. Microbiol. 2017;8:2328. doi: 10.3389/fmicb.2017.02328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair D.V.T., Kollanoor-Johny A. Characterizing the antimicrobial function of a dairy-originated probiotic, Propionibacterium freudenreichii, against multidrug-resistant Salmonella enterica serovar Heidelberg in turkey poults. Front. Microbiol. 2018;9:1475. doi: 10.3389/fmicb.2018.01475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair D.V.T., Manjankattil S., Peichel C., Martin W., Donoghue A.M., Venkitanarayanan K., Johny A.Kollanoor. Effect of plant-derived antimicrobials, eugenol, carvacrol, and β-resorcylic acid against Salmonella on organic chicken wings and carcasses. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair D.V., Nannapaneni R., Kiess A., Schilling W., Sharma C.S. Reduction of Salmonella on Turkey breast cutlets by plant-derived compounds. Foodborne Pathog. Dis. 2014;11:981–987. doi: 10.1089/fpd.2014.1803. [DOI] [PubMed] [Google Scholar]
- Nair D.V.T., Vazhakkattu Thomas J., Dewi G., Brannon J., Noll S.L., Johnson T.J., Cox R.B., Kollanoor Johny A. Propionibacterium freudenreichii freudenreichii B3523 reduces cecal colonization and internal organ dissemination of multidrug-resistant Salmonella Heidelberg in finishing turkeys. J. Appl. Poult. Res. 2021;30:100107. [Google Scholar]
- Nair D.V.T., Vazhakkattu Thomas J., Dewi G., Noll S., Brannon J., Kollanoor Johny A. Reduction of multidrug-resistant Salmonella serovar Heidelberg using a dairy-originated probiotic bacterium, Propionibacterium freudenreichii freudenreichii B3523, in growing turkeys. J. Appl. Poult. Res. 2019;28:356–363. [Google Scholar]
- Oladunjoye A., Soni K.A., Nannapaneni R., Schilling M.W., Silva J.L., Mikel B., Bailey R.H., Mahmoud B.S., Sharma C.S. Synergistic activity between lauric arginate and carvacrol in reducing Salmonella in ground turkey. Poult. Sci. 2013;92:1357–1365. doi: 10.3382/ps.2012-02620. [DOI] [PubMed] [Google Scholar]
- Piovezan M., Uchida N.S., da Silva A.F., Grespan R., Santos P.R., Silva E.L., Cuman R.K.N., Junior M.M., Mikcha J.M.G. Effect of cinnamon essential oil and cinnamaldehyde on Salmonella Saintpaul biofilm on a stainless-steel surface. J. Gen. Appl. Microbiol. 2014;60:119–121. doi: 10.2323/jgam.60.119. [DOI] [PubMed] [Google Scholar]
- Samuel Mérida-Reyes M., Muñoz-Wug M.Alejandro, Oliva-Hernández B.E., Gaitán-Fernández I.C., Luiz D., Simas R., Ribeiro Da Silva A.J., Francisco Pérez-Sabino J. Composition and antibacterial activity of the essential oil from Pimenta dioica (L.) Merr. from Guatemala. Medicines. 2020;7:59. doi: 10.3390/medicines7100059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff R.L. Food attribution and economic cost estimates for meat and poultry related illnesses. J. Food Prot. 2020;83:959–967. doi: 10.4315/JFP-19-548. [DOI] [PubMed] [Google Scholar]
- Soni K.A., Oladunjoye A., Nannapaneni R., Schilling M.W., Silva J.L., Mikel B., Bailey R.H. Inhibition and inactivation of Salmonella Typhimurium biofilms from polystyrene and stainless-steel surfaces by essential oils and phenolic constituent carvacrol. J. Food Prot. 2013;76:205–212. doi: 10.4315/0362-028X.JFP-12-196. [DOI] [PubMed] [Google Scholar]
- Upadhyay A., Upadhyaya I., Kollanoor Johny A., Venkitanarayanan K. Antibiofilm effect of plant derived antimicrobials on Listeria monocytogenes. Food Microbiol. 2013;36:79–89. doi: 10.1016/j.fm.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Upadhyay A., Upadhyaya I., Karumathil D.P., Yin H.B., Nair M.S., Bhattaram V., Chen C.H., Flock G., Mooyottu S., Venkitanarayanan K. Control of Listeria monocytogenes on skinless frankfurters by coating with phytochemicals. Food Sci. Technol. 2015;63:37–42. [Google Scholar]
- USDA FSIS. 2021. FSIS guidelines for con trolling Salmonella in raw poultry. accessed Mar. 2022 https://www.fsis.usda.gov/guidelines/2021-0005
- Vardaka V.D., Yehia H.M., Savvaidis I.N. Effects of Citrox and CN on the survival of Escherichia coli O157: H7 and Salmonella enterica in vacuum-packaged turkey meat. Food Microbiol. 2016;58:128–134. doi: 10.1016/j.fm.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Viator C.L., Muth M.K., Brophy J.E., Noyes G. Costs of food safety investments in the meat and poultry slaughter industries. J. Food Sci. 2017;82:260–269. doi: 10.1111/1750-3841.13597. [DOI] [PubMed] [Google Scholar]
- Zhao S., McDermott P.F., Friedman S., Abbott J., Ayers S., Glenn A., Hall-Robinson E., Hubert S.K., Harbottle H., Walker R.D., Chiller T.M. Antimicrobial resistance and genetic relatedness among Salmonella from retail foods of animal origin: NARMS retail meat surveillance. Foodborne Pathog. Dis. 2006;3:106–117. doi: 10.1089/fpd.2006.3.106. [DOI] [PubMed] [Google Scholar]