Abstract
Background:
High-risk neuroblastoma patients with end-induction residual disease commonly receive post-induction therapy in an effort to increase survival by improving response prior to autologous stem cell transplant (ASCT). We conducted a multi-center, retrospective study to investigate the efficacy of this approach.
Methods:
Patients diagnosed between 2008 and 2018 without progressive disease (PD) with ≤ partial response (PR) at end-induction were stratified according to post-induction treatment: i) no additional therapy prior to ASCT (Cohort 1); ii) post-induction “bridge” therapy prior to ASCT (Cohort 2); and iii) post-induction therapy without ASCT (Cohort 3). Chi-square tests were used to compare patient characteristics. Three-year event-free survival (EFS) and overall survival (OS) were estimated by the Kaplan-Meier method and survival curves were compared by log-rank test.
Results:
The study cohort consisted of 201 patients; Cohort 1 (n=123); Cohort 2 (n=51); and Cohort 3 (n=27). Although end-induction response was better for Cohort 1 than Cohorts 2 and 3, outcome for Cohort 1 and 2 was not significantly different (EFS; p=0.77 and OS; p=0.85). Inferior outcome was observed for Cohort 3 (EFS; p<0.001 and OS; p=0.06). Among patients with end-induction stable metastatic disease, 3-year EFS was significantly improved for Cohort 2 compared to Cohort 1 (p=0.04). Cohort 3 patients with complete response (CR) in metastatic sites following post-induction therapy had significantly better 3-year EFS compared to those with residual metastatic disease (p=0.01).
Conclusions:
Prospective studies to confirm the benefits of bridge treatment and the prognostic significance of metastatic response observed in this study are warranted.
Keywords: neuroblastoma, treatment response, prognosis, survival, autologous transplantation
Introduction
Approximately half of all patients diagnosed with neuroblastoma have aggressive, high-risk disease.1–3 Increasingly intensive, multi-modality approaches have led to improved survival,4, 5 and significantly increased event-free survival (EFS) has been observed with tandem cycles of high-dose therapy and autologous stem cell transplant (ASCT) compared to single transplant.6 However, approximately 40% of high-risk patients continue to relapse, and survival for this cohort is dismal.7, 8 Although outcome is better for patients with refractory neuroblastoma,9, 10 long-term survival remains poor.11, 12 Significantly inferior EFS was reported for patients enrolled on the Children’s Oncology Group (COG) A3973 high-risk clinical trial with post-induction meta-iodobenzylguanidine (MIBG) Curie scores >2 versus ≤2.12 Similarly, among patients enrolled on the International Society of Paediatric Oncology European Neuroblastoma Group (SIOPEN) high-risk trial HR-NBL1, worse EFS was associated with post-induction SIOPEN MIBG skeletal scores of >3 versus ≤3.13 Further, less than a partial response (PR) at end-induction was associated with significantly worse EFS and overall survival (OS) for patients enrolled on four consecutive high-risk COG trials.11
Based on these observations, we and others have hypothesized that the survival of high-risk patients with residual disease at end-induction will be enhanced if disease burden can be reduced with “bridge therapy” prior to consolidation with ASCT. Many providers are currently treating individual patients with end-induction residual disease with regimens shown to have anti-neuroblastoma activity in early phase clinical trials, including dinutuximab combined with irinotecan and temozolomide (DIT),14, 15 radiolabeled MIBG (131I-MIBG),16 or combinations of chemotherapeutic agents.17, 18 However, questions remain regarding the benefits of this approach and whether response to bridge therapy is associated with survival. To address these questions, we conducted a multi-center, retrospective study of high-risk neuroblastoma patients with end-induction residual disease and analyzed treatment approaches, response to post-induction therapy, and patient outcome.
Materials and Method
High-risk neuroblastoma patients without progressive disease (PD) diagnosed between January 1, 2008 and December 31, 2018 with ≤PR at end-induction were identified at the University of Chicago Comer Children’s Hospital, Children’s Hospital of Philadelphia, Ann & Robert H. Lurie Children’s Hospital of Chicago, Seattle Children’s Hospital, University of Michigan, and Texas Children’s Hospital. Patient and tumor characteristics,1 treatment, and outcome data were abstracted from electronic medical records. The patients were classified as high-risk based on 2006 COG risk criteria.3, 19 Responses to induction, bridge, and post-induction treatments were determined using the 2017 International Neuroblastoma Response Criteria (INRC),20 with a modification for the small number of patients who did not undergo bone marrow evaluation at these time points (end-induction, n=8; post-bridge, n=1; post-induction treatment=1). In those cases, overall response was determined based on primary tumor and metastatic soft tissue and bone disease response. In separate analyses, we evaluated metastatic response in International Neuroblastoma Staging System (INSS) stage 4 patients according to INRC for metastatic soft tissue and/or bone (component 1) and bone marrow (component 2).20 Metastatic complete response (CR) was defined as CR in both components; metastatic PR was defined as PR in component 1 and CR, PR, or minimal disease (MD) in component 2; metastatic SD was defined as SD in at least one component and no component with PD. Metastatic response for 10 patients with unknown bone marrow response was defined by response in component 1.
The study was approved by the Institutional Review Boards (IRB) at the University of Chicago, the primary study site, and each of the collaborating institutions according to the U.S. Common Rule ethical guidelines. Written informed consent was obtained for patients based on local IRB requirements (e.g. patients requiring prospective data collection).
Study Design
Eligibility for this retrospective, multi-institutional study was restricted to patients with ≤PR at end-induction. INRC response was confirmed by computed tomography (CT) or magnetic resonance imaging (MRI), bone marrow studies, and MIBG scan.20 For patients with MIBG non-avid tumors, extent of disease was evaluated with [18F] fluorodeoxyglucose (FDG)-positron emission tomography (FDG-PET) scans.21 MIBG scans performed at Seattle Children’s Hospital were reviewed by radiologists at the University of Chicago for Curie score calculation. All other institutions calculated Curie Scores locally. Investigators at each institution underwent virtual training to facilitate harmonized data abstraction, disease status criteria, and REDcap data entry. Patients were stratified into one of three cohorts according to the treatment they received following induction therapy. Treatment was based on physician, institutional, or family preferences. Clinicians from each institution were surveyed to evaluate how end-induction Curie Score and bone marrow disease influenced their current treatment strategies.
Statistical Methods
Chi-square tests were used to compare patient characteristics and treatment response according to cohort. The Mann-Whitney U test with Benjamini-Hochberg correction for multiple testing was used to compare year of diagnosis between treatment groups and Curie scores among patients at different time points during therapy. Three-year EFS (time from diagnosis to last follow up encounter, relapse, or death) and OS (time from diagnosis to last follow up or death) were estimated by the Kaplan-Meier method,22 and survival curves were compared using the log-rank test.23 Differences in EFS and OS between patient cohorts were analyzed using Cox proportional hazards models. The proportional hazards assumption was validated for all models. Models were not constructed for analyses of subgroups within a cohort given small sample sizes. Statistical analyses were performed using STATA 16.1, R 3.6.0, and PRISM 9.1.2.
Results
Patient Characteristics and Treatment
The entire cohort consisted of 201 high-risk neuroblastoma patients with ≤PR at end-induction. The patients were categorized into different cohorts based on post-induction treatment received. Cohort 1 (n=123) underwent consolidation with ASCT directly following completion of induction therapy. Cohort 2 (n=51) received bridge therapy prior to ASCT, and Cohort 3 (n=27) received post-induction therapy, but did not undergo ASCT (Figure 1A). Cohort profile according to end-induction INRC response is detailed in Figure 1B. Post-induction treatment changed over time, and significantly more patients received DIT in recent years compared to 131I-MIBG (p<0.001) (Supporting Figure 1). All clinicians currently consider bridge or post-induction therapy for patients with end-induction metastatic disease and Curie Scores >5 or bone marrows with >10% tumor cells. Bridge or post-induction therapy is also considered by >50% of the clinicians for patients with end-induction metastatic disease and Curie scores >2 but ≤5; a reduction in Curie Score by <50% regardless of absolute score; or bone marrows with >5% but ≤10% tumor cells. In Cohort 3, the decision to not consolidate with ASCT was based on poor metastatic response to post-induction therapy. For 3 of the 6 patients who achieved a metastatic CR, the decision to not proceed with ASCT was made by family members.
Figure 1:
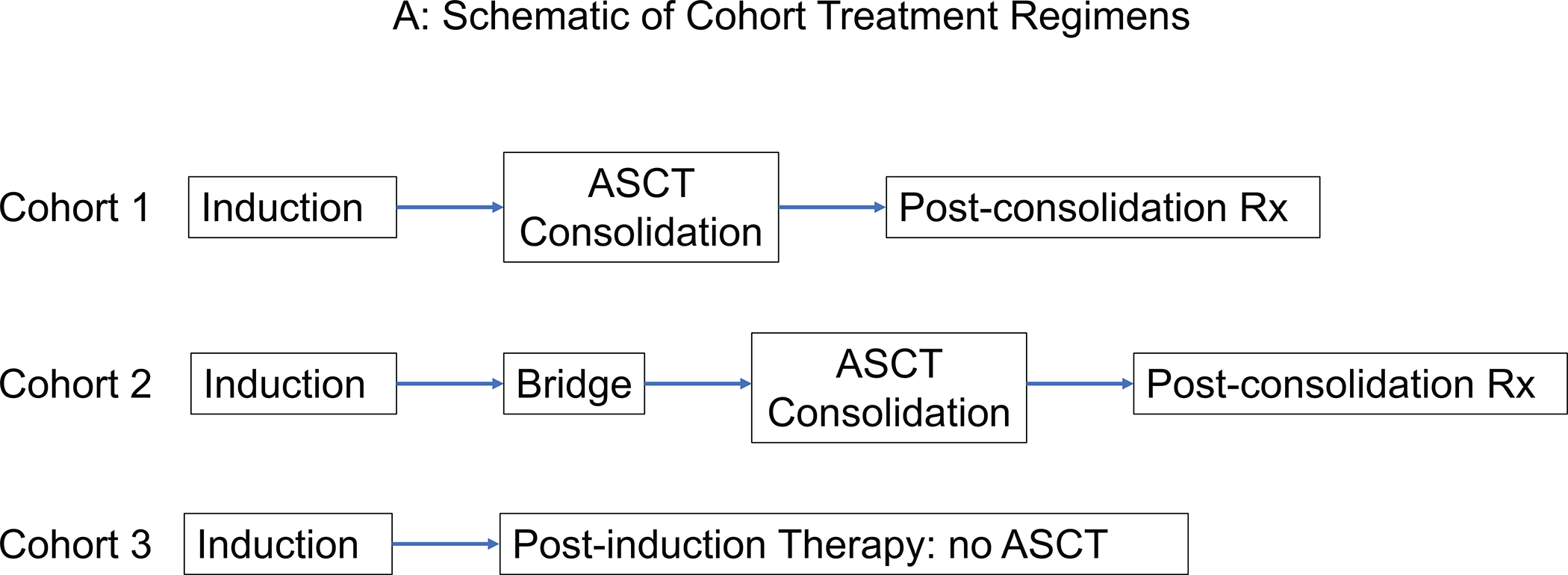
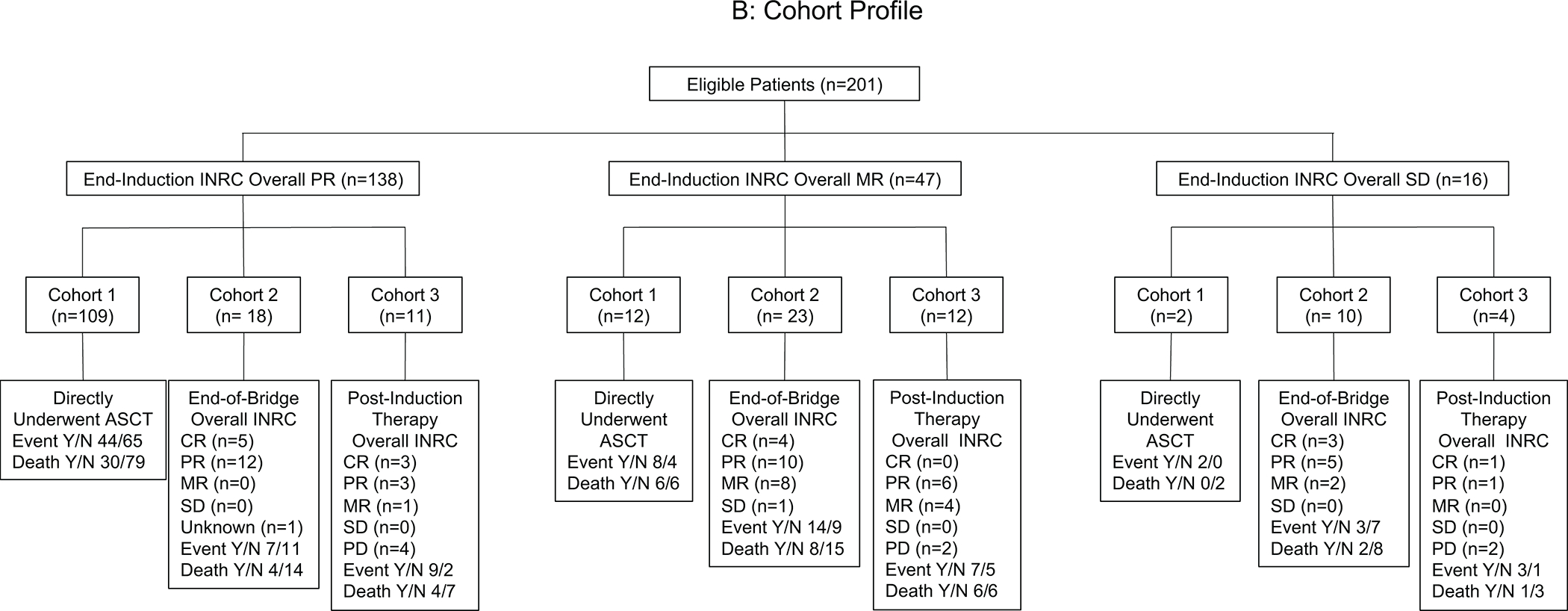
(A) Schematic of treatment cohorts and (B) Cohort profile according to end-induction INRC overall response.
ASCT indicates autologous stem cell transplantation; CR, complete response; INRC, International Neuroblastoma Response Criteria; MR, minor response; PD, progressive disease; PR, partial response; SD, stable disease.
Patient clinical features and tumor biomarkers3 are summarized in Table 1. Although all patients were high-risk,19 Cohorts 2 and 3 had a higher proportion of patients with unfavorable age (≥18 months of age)24 and stage (INSS stage 4)6 compared to Cohort 1. In contrast, among patients with known MYCN status, MYCN amplification, an unfavorable genomic biomarker,25 was identified in a higher percentage of patients in Cohort 1 compared to Cohorts 2 and 3.
Table 1:
Patient and Tumor Characteristics According to Treatment Cohort
| Cohort 1 (N=123) n (%) |
Cohort 2 (N=51) n (%) |
Cohort 3 (N=27) n (%) |
P value | |
|---|---|---|---|---|
| Age at Diagnosis | ||||
| <18 months | 26 (21.5) | 3 (5.9) | 0 (0) | 0.002 |
| ≥18 months | 95 (78.5) | 48 (94.1) | 27 (100) | |
| Unknown | 2 | 0 | 0 | |
| Sex | ||||
| Male | 81 (65.8) | 31 (60.8) | 11 (40.7) | 0.053 |
| Female | 42 (34.2) | 20 (39.2) | 16 (59.3) | |
| INSS Stage | ||||
| 4 | 110 (89.4) | 51 (100) | 27 (100) | 0.028 |
| 3 | 12 (9.8) | 0 (0) | 0 (0) | |
| 2b | 1 (0.8) | 0 (0) | 0 (0) | |
| MYCN status | ||||
| Non-Amplified | 63 (58.9) | 32 (72.7) | 23 (88.5) | 0.008 |
| Amplified | 44 (41.1) | 12 (27.3) | 3 (11.5) | |
| Unknown | 16 | 7 | 1 | |
| Histology | ||||
| Favorable | 6 (5.9) | 1 (2.4) | 3 (13.0) | 0.23 |
| Unfavorable | 95 (94.1) | 40 (97.6) | 20 (87.0) | |
| Unknown | 22 | 10 | 4 | |
| Ploidy | 0.77 | |||
| Hyperdiploid | 34 (58.6) | 13 (59.1) | 9 (69.2) | |
| Diploid | 24 (41.4) | 9 (40.9) | 4 (30.8) | |
| Unknown | 65 | 29 | 14 | |
| Number of ASCT | 0.81* | |||
| 1 | 80 (65) | 33 (64.7) | 0 | |
| 2 | 42 (34.2) | 18 (35.3) | 0 | |
| >2 | 1 (0.8) | 0 (0) | 0 |
Abbreviations: ASCT, autologous stem cell transplantation; INSS, International Neuroblastoma Staging System.
p-value calculated for Cohort 1 compared to Cohort 2
Response to Induction Therapy Differed According to Cohort
End-induction response differed significantly among the Cohorts (p<0.001; Table 2), with a higher proportion of Cohort 1 patients achieving a better overall response compared to Cohorts 2 and 3. Metastatic response at end-induction was also better in Cohort 1. Only 3.2% of Cohort 1 patients had end-induction SD in metastatic soft tissue and bone compared to 51% in Cohort 2 and 48.2% in Cohort 3. Similarly, bone marrow SD at end-induction was detected in only 7.4% of Cohort 1 patients compared to 33.3% and 18.5% of Cohort 2 and Cohort 3 patients, respectively. Further, the median end-induction Curie Score was significantly lower in Cohort 1 compared to Cohort 2 (p<0.001) (Supporting Figure 2).
Table 2:
2017 INRC Response of Primary Tumor, Soft Tissue and Bone Metastases, and Bone Marrow and Overall Response
| Cohort 1 (N=123) n (%) |
Cohort 2 (N=51) n (%) |
Cohort 3 (N=27) n (%) |
P value | |
|---|---|---|---|---|
|
End-Induction
Primary Tumor Response |
||||
| CR | 54 (43.9) | 25 (49.0) | 11 (40.7) | 0.001 |
| PR | 66 (53.7) | 15 (29.4) | 11 (40.7) | |
| SD | 2 (1.6) | 8 (15.7) | 5 (18.6) | |
| Not evaluable1 | 1 (0.8) | 1 (2.0) | 0 (0) | |
| Unknown response2 | 0 (0) | 2 (3.9) | 0 (0) | |
| End-Induction Metastatic Soft Tissue and Bone Disease Response | ||||
| CR | 37 (30.1) | 3 (5.9) | 4 (14.8) | <0.001 |
| PR | 68 (55.3) | 21 (41.2) | 10 (37.0) | |
| SD | 4 (3.2) | 26 (51.0) | 13 (48.2) | |
| Not evaluable1 | 14 (11.4) | 1 (1.9) | 0 (0) | |
| Unknown response2 | 0 (0) | 0 (0) | 0 (0) | |
| End-Induction Bone Marrow Metastasis Response | ||||
| CR | 41 (33.3) | 14 (27.5) | 7 (25.9) | <0.001 |
| MD | 33 (26.0) | 9 (17.7) | 8 (29.6) | |
| SD | 9 (7.4) | 17 (33.3) | 5 (18.5) | |
| Not evaluable1 | 41 (33.3) | 5 (9.8) | 5 (18.5) | |
| Unknown response2 | 0 (0) | 6 (11.8) | 2 (7.4) | |
| End-Induction Overall INRC Disease Response 3 | ||||
| PR | 109 (88.6) | 18 (35.3) | 11 (40.8) | <0.001 |
| MR | 12 (9.8) | 23 (45.1) | 12 (44.4) | |
| SD | 2 (1.6) | 10 (19.6) | 4 (14.8) |
Abbreviations: CR, complete response; INRC, International Neuroblastoma Response Criteria; MD, minimal disease; MR, minor response; PR, partial response; SD, stable disease.
Response not evaluable; site not involved at diagnosis and remains uninvolved
Unknown response of evaluable disease; required imaging or bone marrow was not done
Overall INRC response was determined using two components if bone marrow was not done
Survival According to Cohort
For Cohort 1, 3-year EFS and OS were 58.9% (95% CI 49.2–67.4%) and 80.2% (95% CI 71.7–86.4), respectively (Figures 2A and 2B). Although the proportion of patients with poor end-induction response was higher among patients in Cohort 2 compared to Cohort 1, EFS and OS were not significantly different (EFS; p=0.77 and OS; p=0.85). EFS but not OS was significantly inferior for Cohort 3 compared to Cohorts 1 and 2 (EFS; p<0.001 and OS; p=0.06). After accounting for stage and MYCN status, significantly inferior EFS (adjusted HR, 0.32; 95% CI, 0.19–0.54; p<0.001) and OS (adjusted HR, 0.49; 95% CI, 0.25–0.98; p<0.042) were observed for Cohort 3 patients compared to Cohorts 1 and 2. Median follow-up time for all patients was 44 months (IQR 25–74 months), with median follow-up of 50 months (IQR 25–84 months), 43 months (IQR 29–60 months), and 29 months (IQR 16–64 months) for Cohorts 1, 2, and 3, respectively.
Figure 2:
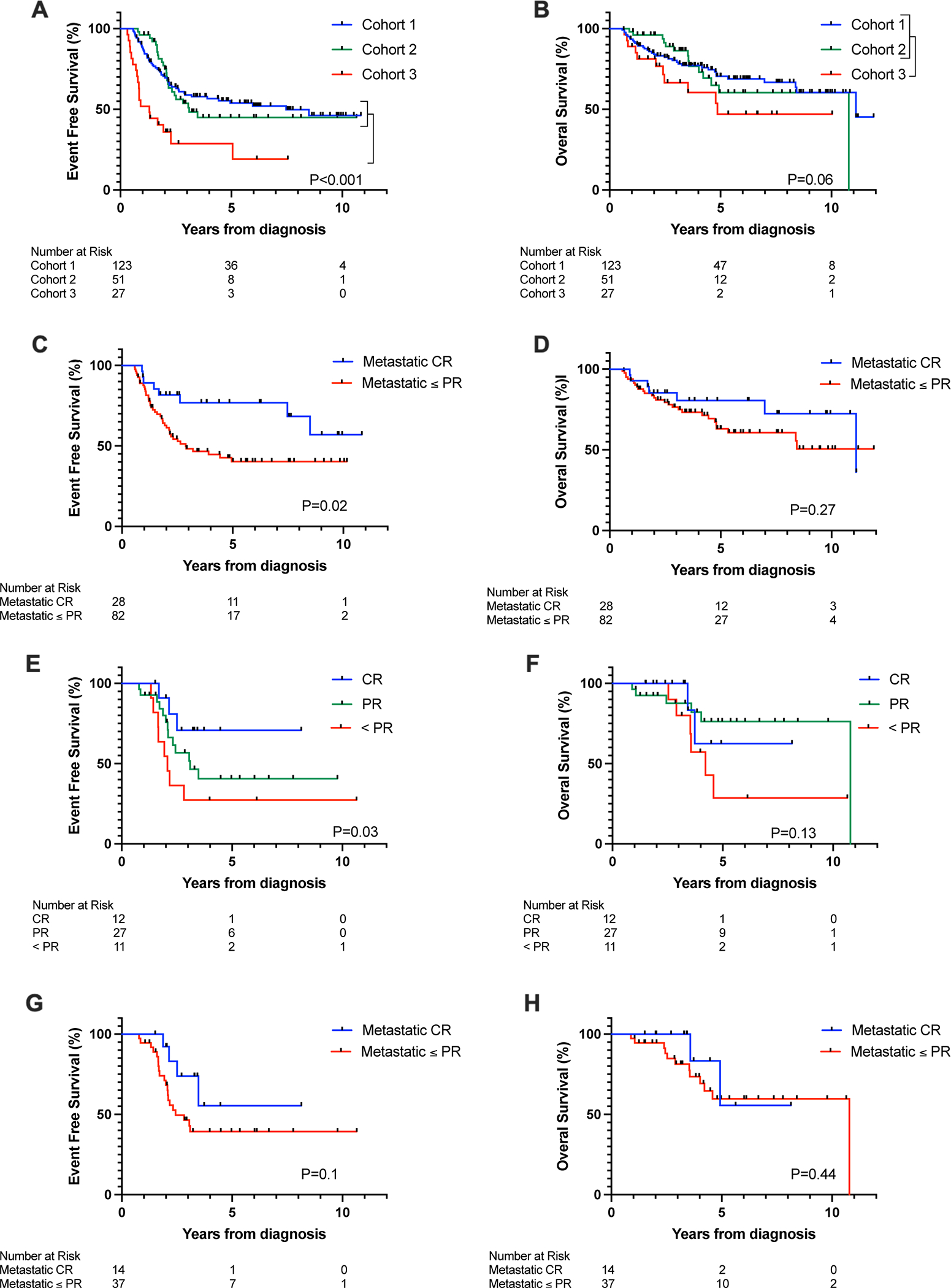
Probability of (A) EFS and (B) OS for patients according to the treatment Cohort, (C) EFS and (D) OS for stage 4 patients in Cohort 1 with and without an end-induction metastatic CR, (E) EFS and (F) OS for patients in Cohort 2 according to the overall INRC response at end-of-bridge therapy, and (G) EFS and (H) OS for patients in Cohort 2 with and without a metastatic CR at end-of-bridge therapy. CR indicates complete response; EFS, event-free survival; INRC, International Neuroblastoma Response Criteria; OS, overall survival; PR, partial response.
Outcome of Stage 4, Cohort 1 Patients According to End-Induction Metastatic Response
At end-induction, 28 (25.5%) of the 110 stage 4 patients in Cohort 1 met INRC for CR at metastatic sites. Bone marrow involvement was not detected at diagnosis or at end-induction in 38 (34.5%) patients. Significantly worse EFS was observed among the 82 (74.5%) patients with end-induction metastatic disease compared to those without end-induction metastatic disease (p=0.02; Figure 2C). However, OS did not significantly differ (p=0.27; Figure 2D).
Treatment, Response, and Outcome of Cohort 2 Patients
The bridge treatment regimens Cohort 2 patients received are shown in Tables 3 and 4. Of the 51 patients, 44 (86.3%) received therapies that included DIT and/or 131I-MIBG. Six patients were treated with combination chemotherapy with or without surgery and radiation, and one patient received radiation alone. Response to bridge therapy was determined from end-induction to end-of-bridge therapy. An overall CR following bridge therapy was observed in 12 (23.5%) patients who received DIT alone (n=7); DIT plus chemotherapy (n=1) or 131I-MIBG (n=1); 131I-MIBG alone (n=1); multi-agent chemotherapy (n=1); or chemotherapy plus cixutumumab (n=1) (Table 3). Sixteen patients (30.8%) with either minor response or SD at end-induction improved to PR. Significantly improved EFS was observed for patients who achieved an overall CR following bridge therapy (p=0.03; Figure 2E). Overall response to bridge treatment was not associated with OS (p=0.13; Figure 2F). In a separate analysis, the EFS of Cohort 2 patients who achieved a metastatic CR at end-of-bridge therapy was compared with the EFS of those who did not. A trend associating improved EFS was observed among patients with metastatic CR at end-of-bridge, but statistical significance was not reached (3-year EFS: 73.8% (95% CI 38.5–90.8) vs 46.5% (95% CI 29.1–62.2); log-rank p=0.1; Figure 2G). No difference in OS was seen (3-year OS: 100% vs 81.4% (95% CI 62.9–91.2); log-rank p=0.44; Figure 2H).
Table 3:
Cohort 2 Bridge Therapy and Disease Response from End-Induction to End-of-Bridge Therapy
| Bridge Therapy | Pt (n) | Number of Bridge Treatment Cycles (n) |
End-Induction Overall INRC Response (n) |
End-of- Bridge Overall INRC Response (n) |
End-of-Bridge Primary Tumor Response (n) |
End-of- Bridge Metastatic Bone and Soft Tissue Response (n) |
End-of- Bridge Bone Marrow Response (n) |
Event Y/N |
Death Y/N |
|---|---|---|---|---|---|---|---|---|---|
| DIT alone | 20 | 1–6 | CR: 0 PR: 7 MR: 10 SD: 3 |
CR: 7 PR: 10 MR: 3 SD: 0 |
CR: 13 PR: 4 SD: 2 1NE 1 2Unknown: 0 |
CR: 8 PR: 11 SD: 1 1NE: 0 2Unknown: 0 |
CR: 17 PR: 2 MD: 0 SD: 0 1NE: 1 2Unknown: 0 |
7/13 | 3/17 |
| DIT followed by surgery | 1 | 10 | SD: 1 | PR: 1 | CR: 1 | PR: 1 | CR: 1 | 1/0 | 0/1 |
| ICE followed by DIT | 1 | ICE: 2 DIT: 5 |
MR: 1 | CR: 1 | CR: 1 | CR: 1 | CR: 1 | 0/1 | 0/1 |
| MIBG Alone | 12 | MIBG: 1 (n=10) 2 (n=2) |
CR: 0 PR: 6 MR: 4 SD: 2 |
CR: 1 PR: 7 MR: 4 SD: 0 |
CR: 7 PR: 4 SD: 1 1NE: 0 2Unknown: 0 |
CR: 2 PR: 8 SD: 1 1NE: 1 2Unknown: 0 |
CR: 4 PR: 3 MD: 3 SD: 0 1NE: 2 2Unknown:0 |
7/5 | 5/7 |
| MIBG followed by DIT | 2 | MIBG: 1 (n=2) DIT: 5 (n=1) 3 (n=1) |
SD: 2 | CR: 1 PR: 1 |
CR: 2 | CR: 1 PR: 1 |
CR: 2 | 0/2 | 0/2 |
| I/T followed by MIBG | 4 | I/T: 1–6 MIBG : 2 (n=1) 1 (n=3) |
CR: 0 PR: 0 MR: 3 SD: 1 |
CR: 0 PR: 3 MR: 1 SD: 0 |
CR: 4 PR: 0 SD:0 1NE:0 2Unknown: 0 |
CR: 0 PR: 3 SD: 1 1NE:0 2Unknown: 0 |
CR: 2 PR: 1 MD: 1 SD: 0 1NE: 0 2Unknown: 0 |
2/2 | 2/2 |
| I + Bortezomib followed by MIBG | 1 | MIBG: 1 | MR: 1 | PR: 1 | CR: 1 | PR: 1 | CR: 1 | 1/0 | 0/1 |
| I/T followed by MIBG plus I/V | 1 | IT: 1 MIBG + I/V: 1 |
MR: 1 | PR: 1 | CR: 1 | PR: 1 | CR: 1 | 1/0 | 0/1 |
| Surgery and MIBG | 2 | MIBG: 1 (n=2) |
CR: 0 PR: 1 MR: 0 SD: 1 |
CR: 0 PR: 1 MR: 1 SD: 0 |
CR: 0 PR: 2 SD:0 1NE:0 2Unknown: 0 |
CR: 0 PR: 1 SD: 1 1NE:0 2Unknown: 0 |
CR: 0 PR: 2 MD:0 SD:0 1NE:0 2Unknown: 0 |
2/0 | 1/1 |
| Cyclo/ Topo |
3 | 2 (n=1) 1 (n=1) Unknown (n=1) |
CR: 0 PR: 3 MR: 0 SD: 0 |
CR: 1 PR: 1 MR: 0 SD: 0 2Unknown:1 |
CR: 2 PR: 0 SD: 0 1NE: 0 2Unknown: 1 |
CR: 2 PR: 0 SD: 0 1NE: 0 2Unknown: 1 |
CR: 1 PR: 1 MD: 0 SD: 0 1NE: 0 2Unknown: 1 |
1/2 | 1/2 |
| ICE followed by Cyclo/ Topo and surgery |
1 | ICE: 2 Cyclo/Topo: 2 |
MR: 1 | SD: 1 | CR: 1 | SD: 1 | CR: 1 | 0/1 | 0/1 |
| Cixutumu-mab and Temsiro-limus (ADVL1221) followed by surgery | 1 | 1 | MR: 1 | CR: 1 | CR: 1 | CR: 1 | CR: 1 | 1/0 | 1/0 |
| I/T followed by surgery | 1 | 1 | MR: 1 | MR: 1 | PR: 1 | SD: 1 | MR: 1 | 1/0 | 1/0 |
| External Beam Radiation |
1 | NA | PR: 1 | PR: 1 | PR: 1 | CR: 1 | PR: 1 | 0/1 | 0/1 |
Abbreviations: CR, complete response; Cyclo/Topo, cyclophosphamide and topotecan; DIT, dinutuximab, irinotecan, and temozolomide; ICE, ifosfamide, carboplatin, and etoposide; I, irinotecan; INRC, International Neuroblastoma Response Criteria; I/T, irinotecan and temozolomide; I/V, irinotecan and vincristine; MD, minimal disease; MIBG, meta-iodobenzylguanidine; MR, minor response; NA, not applicable; NE, not evaluable; PR, partial response; SD, stable disease; Y/N, yes/no.
Response not evaluable; site not involved at diagnosis and remains uninvolved
Unknown response of evaluable disease
Table 4:
Cohort 2 Disease Response and Outcome According to Bridge Regimen
| Bridge Regimens | Pt (n) | End-Induction Overall INRC Response (n) |
End-of- Bridge Overall INRC Response (n) |
2-year EFS 2-year OS (95%CI) |
3-year EFS 3-year OS (95%CI) |
5-year EFS 5-year OS (95% CI) |
|---|---|---|---|---|---|---|
| Regimen contains DIT without MIBG | 22 | CR: 0 PR: 7 MR: 11 SD: 4 |
CR: 8 PR: 11 MR: 3 SD: 0 |
67.8% (41.7–84.2) 95.2% (70.7–99.3) |
52.7% (26.3–73.6) 87.3% (56.4–96.8) |
N/A |
| Regimen contains MIBG without DIT | 20 | CR: 0 PR: 7 MR: 9 SD: 4 |
CR: 1 PR: 13 MR: 6 SD: 0 |
75.0% (50.0–88.7) 95% (69.5–99.3) |
50.0% (27.1–69.2) 85.0% (60.4–94.9) |
50.0% (27.1–69.2) 63.5% (38.0–80.8) |
| Regimen contains both MIBG and DIT | 2 | SD: 2 | CR: 1 PR: 1 |
100% 100% |
100% 100% |
N/A |
| Regimen without MIBG and DIT | 7 | CR: 0 PR: 4 MR: 3 SD: 0 |
CR: 2 PR: 2 MR: 1 SD: 1 1Unknown:1 |
85.7% (33.4–97.9) 100% |
57.1% (17.2–30.5) 85.7% (33.4–97.9) |
57.1% (17.2–30.5) 51.4% (11.8–81.3) |
Abbreviations: CR, complete response; DIT, dinutuximab, irinotecan, and temozolomide; EFS, event-free survival; INRC, International Neuroblastoma Response Criteria; MIBG, meta-iodobenzylguanidine; MR, minor response; NA, not applicable; PR, partial response; SD, stable disease.
Unknown response of evaluable disease
Outcome of Cohorts 1 and 2 Patients with Metastatic Disease at End-Induction
Metastatic disease was detected at end-induction in 82 (74.5%) Cohort 1 stage 4 patients and 49 (96.1%) Cohort 2 patients. Among those with SD at metastatic sites at end-induction, significantly improved EFS was observed for Cohort 2 compared to Cohort 1 (p=0.04; Figure 3A). OS was not significantly different (p=0.56; Figure 3B). Among Cohort 1 and 2 patients who achieved a metastatic PR at end-induction, there was no significant difference in EFS at 3 years (EFS: 52% (95% CI 39–63.5) and 44% (95% CI 19.2–66.5), respectively; log-rank p=0.9) or OS at 3 years (OS: 75.1% (95% CI 62.4–84) vs 69.2% (95% CI 40.4–86.1), log-rank p=0.8).
Figure 3:
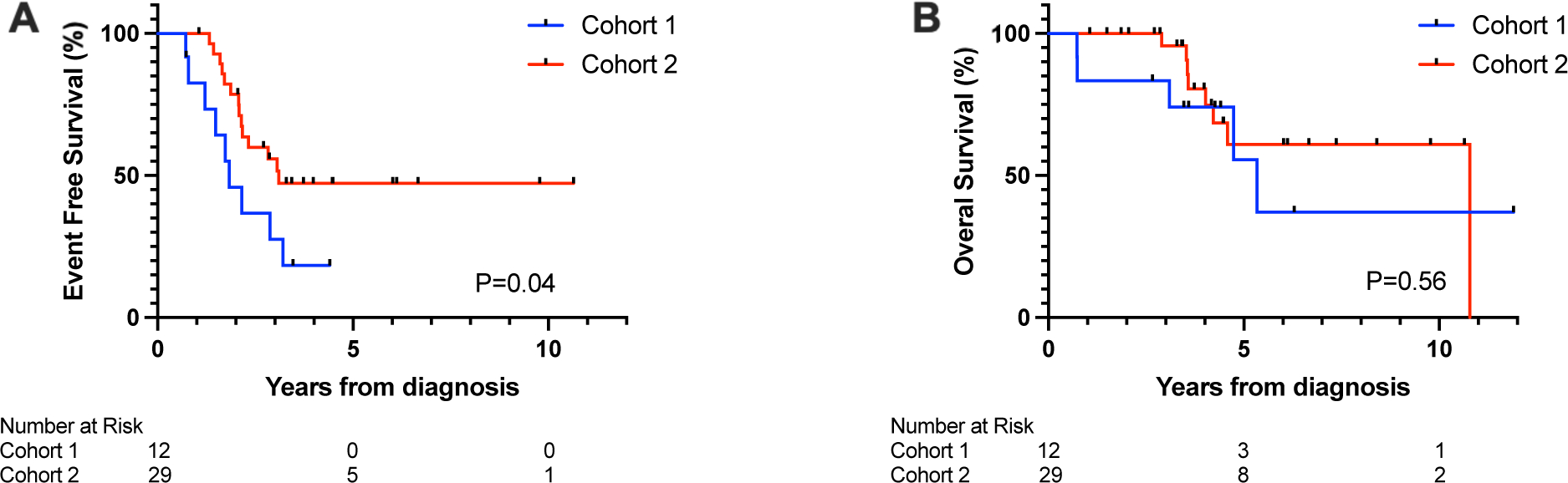
Probability of (A) event-free survival and (B) overall survival for Cohort 1 and Cohort 2 patients with stable disease at metastatic sites at the end of induction.
Treatment, Response, and Outcome of Cohort 3 Patients
The post-induction treatments Cohort 3 patients received are summarized in Supporting Table 1. More than 70% of the patients were treated with regimens that included DIT and/or 131I-MIBG. For 10 (37%) of the 27 patients, overall INRC disease response improved from end-induction with post-induction therapy. A total of 4 patients achieved an overall CR with post-induction therapy, including 3 who received DIT as part of their post-induction therapy, and 1 patient who was treated with combination therapy that included 131I-MIBG. Of the 23 patients with residual metastatic disease at end-induction, 6 achieved a metastatic CR with post-induction therapy, and these patients had significantly improved 3-year EFS compared to those with residual metastatic disease (p=0.01). A trend associating improved OS for patients who achieved a metastatic CR was observed, although statistical significance was not reached (p=0.057).
Discussion
In this retrospective study, the treatment for high-risk neuroblastoma patients with end-induction residual disease varied widely, largely based on response at metastatic sites. Thus, the percentage of Cohort 1 patients with residual metastatic disease at end-induction was less than Cohorts 2 and 3. Despite these differences in end-induction response, EFS and OS did not significantly differ for Cohort 1 and 2, suggesting that patients in Cohort 2 may have benefited from the bridge therapy. Among patients with end-induction stable metastatic disease, EFS was significantly improved for Cohort 2 compared to Cohort 1, further supporting the efficacy of bridge treatment. Although no difference in OS was observed, this may reflect the effects of additional treatments these patients may have received to treat relapsed disease.14, 26, 27 We did not detect differences in EFS for Cohort 1 and 2 patients with end-induction metastatic PR. Disease burden can vary significantly among patients classified as having a metastatic PR, and larger cohorts will need to be studied to determine if the effects of bridge therapy differ among patients with more versus less extensive metastatic disease and/or specific disease sites. We recognize that this study has potential sources of bias including a lead-time for Cohort 2 patients, as these patients underwent ASCT after receiving bridge treatments. Although the average number of cycles of bridge treatment was three, indicating that the lead-time was approximately two months for most Cohort 2 patients, future studies will be needed to confirm the efficacy of bridge therapy.
Our results also indicate that the presence of metastatic disease prior to ASCT is associated with inferior EFS. Cohort 1 patients with refractory metastatic disease at end-induction had significantly worse EFS compared to patients with only residual disease at the primary tumor site. A trend associating residual metastatic neuroblastoma at end-of-bridge with inferior EFS was also observed in Cohort 2 patients, but statistical significance was not reached, likely due to the small number of patients analyzed. The prognostic strength of end-induction metastatic disease highlights the limitation of studies evaluating the impact of extent of primary surgical resection on survival. Differences in metastatic response among the study cohorts likely contributed to the conflicting results and ongoing debate about the clinical value of near complete gross resection of the primary tumor.28–31
Cohort 3 patients had inferior EFS and OS compared to Cohorts 1 and 2. Ten patients (37%) developed PD while receiving post-induction therapy, and 6 (22%) had no improvement in disease response. Based on the poor response to post-induction therapy, these patients did not undergo consolidation with ASCT. Interestingly, 6 patients who achieved a metastatic CR with post-induction therapy also did not undergo ASCT, and all remain alive. Favorable outcome has also been reported for patients in CR at end-induction treated with anti-disialoganglioside (GD2) monoclonal antibody without ASCT in a single institutional study.32 High-dose therapy with ASCT is known to be associated with acute toxicities and late effects that negatively impact the long-term health of children with high-risk neuroblastoma,33, 34 highlighting the need for novel biomarkers to identify patients who may not require ASCT to achieve long-term survival.
Bridge therapy has previously been evaluated in the prospective, Phase 3 SIOPEN HR-NBL-1 study. In this trial, only patients with metastatic CR or PR limited to 123I-MIBG uptake in three abnormal skeletal areas on scintigraphy and no bone marrow disease were eligible to proceed to consolidation with high dose therapy and ASCT.5 Patients who did not achieve this response received two courses of post-induction therapy consisting of topotecan-vincristine-doxorubicin (TVD).35 However, in contrast to our results, the 23 patients who responded to bridge therapy and underwent ASCT had a significantly lower 5-year EFS compared to those who did not require this treatment.5 These conflicting results may reflect the differences in patient cohorts, due to the clinical trial eligibility criteria, as well as the disparate bridge regimens.
The results of this retrospective study suggest that bridge treatments that include DIT and/or 131I-MIBG prior to ASCT significantly improve the EFS of high-risk patients with SD in metastatic sites. Prospective clinical trials will be needed to validate these findings and identify patients most likely to benefit from bridge therapy. Additional studies to confirm the favorable outcome we observed among patients who achieved a metastatic CR with post-induction therapy without ASCT are also warranted.
Supplementary Material
Supporting Figure 1: Dot plot graph showing the distribution of bridge and post-induction regimens Cohort 2 (n=51) and 3 patients (n=27) respectively received between 2008–2019. *** = padj <0.001, * = padj <0.05
Supporting Figure 2: Dot plot graph showing the distribution of Curie Scores at diagnosis and end-induction for Cohort 1 (n=42) and Cohort 2 (n=37) patients with end-induction metastatic disease and available scores at both time points. Cohort 1 and 2 Curie scores were not statistically different at diagnosis (p=0.87). At end-induction, the Curie scores of patients in Cohort 2 Curie scores were significantly higher than patients in Cohort 1 (p<0.001). ns=not significant, *=p<0.001
Support:
This work was supported in part by the Matthew Bittker Foundation (to Susan L. Cohn), a gift from the MacRitchie Family (to Susan L. Cohn), and the National Institutes of Health/National Cancer Institute (K08CA226237; to Mark A. Applebaum; P30CA014599; Cancer Center Support Grant, Biostatistical Core Facility, to Theodore G. Karrison).
Footnotes
Conflicts of interest disclosures: Ami V. Desai: stock ownership in Pfizer and Viatris; consultancy/advisory board fees from Merck, Ology Medical Education, and YMabs Therapeutics; travel and accommodation expenses from GlaxoSmithKline. Mark A. Applebaum: consultancy/advisory board fees from Fennec Pharmaceuticals and Illumina Radiopharmaceuticals. Jennifer H. Foster: served on an advisory board for YMabs Therapeutics. Rochelle Bagatell: uncompensated member of a Y-Mabs Therapeutics advisory board. Susan L. Cohn: stock ownership in Pfizer, Merck, and Lilly; served on an advisory board for YMabs Therapeutics. The remaining authors made no disclosures.
References
- 1.Pinto NR, Applebaum MA, Volchenboum SL, et al. Advances in Risk Classification and Treatment Strategies for Neuroblastoma. J Clin Oncol. 2015;33: 3008–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagatell R, Cohn SL. Genetic discoveries and treatment advances in neuroblastoma. Curr Opin Pediatr. 2016;28: 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang WH, Federico SM, London WB, et al. Tailoring Therapy for Children With Neuroblastoma on the Basis of Risk Group Classification: Past, Present, and Future. JCO Clin Cancer Inform. 2020;4: 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irwin MS, Park JR. Neuroblastoma: paradigm for precision medicine. Pediatr Clin North Am. 2015;62: 225–256. [DOI] [PubMed] [Google Scholar]
- 5.Ladenstein R, Potschger U, Pearson ADJ, et al. Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): an international, randomised, multi-arm, open-label, phase 3 trial. Lancet Oncol. 2017;18: 500–514. [DOI] [PubMed] [Google Scholar]
- 6.Park JR, Kreissman SG, London WB, et al. Effect of Tandem Autologous Stem Cell Transplant vs Single Transplant on Event-Free Survival in Patients With High-Risk Neuroblastoma: A Randomized Clinical Trial. JAMA. 2019;322: 746–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.London WB, Bagatell R, Weigel BJ, et al. Historical time to disease progression and progression-free survival in patients with recurrent/refractory neuroblastoma treated in the modern era on Children’s Oncology Group early-phase trials. Cancer. 2017;123: 4914–4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.London WB, Castel V, Monclair T, et al. Clinical and biologic features predictive of survival after relapse of neuroblastoma: a report from the International Neuroblastoma Risk Group project. J Clin Oncol. 2011;29: 3286–3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreno L, Rubie H, Varo A, et al. Outcome of children with relapsed or refractory neuroblastoma: A meta-analysis of ITCC/SIOPEN European phase II clinical trials. Pediatr Blood Cancer. 2017;64: 25–31. [DOI] [PubMed] [Google Scholar]
- 10.Zhou MJ, Doral MY, DuBois SG, Villablanca JG, Yanik GA, Matthay KK. Different outcomes for relapsed versus refractory neuroblastoma after therapy with (131)I-metaiodobenzylguanidine ((131)I-MIBG). Eur J Cancer. 2015;51: 2465–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinto N, Naranjo A, Hibbitts E, et al. Predictors of differential response to induction therapy in high-risk neuroblastoma: A report from the Children’s Oncology Group (COG). Eur J Cancer. 2019;112: 66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanik GA, Parisi MT, Shulkin BL, et al. Semiquantitative mIBG Scoring as a Prognostic Indicator in Patients with Stage 4 Neuroblastoma: A Report from the Children’s Oncology Group. J Nucl Med. 2013;54: 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ladenstein R, Lambert B, Potschger U, et al. Validation of the mIBG skeletal SIOPEN scoring method in two independent high-risk neuroblastoma populations: the SIOPEN/HR-NBL1 and COG-A3973 trials. Eur J Nucl Med Mol Imaging. 2018;45: 292–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mody R, Naranjo A, Van Ryn C, et al. Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): an open-label, randomised, phase 2 trial. Lancet Oncol. 2017;18: 946–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mody R, Yu AL, Naranjo A, et al. Irinotecan, Temozolomide, and Dinutuximab With GM-CSF in Children With Refractory or Relapsed Neuroblastoma: A Report From the Children’s Oncology Group. J Clin Oncol. 2020;38: 2160–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DuBois SG, Matthay KK. 131I-Metaiodobenzylguanidine therapy in children with advanced neuroblastoma. Q J Nucl Med Mol Imaging. 2013;57: 53–65. [PubMed] [Google Scholar]
- 17.London WB, Frantz CN, Campbell LA, et al. Phase II randomized comparison of topotecan plus cyclophosphamide versus topotecan alone in children with recurrent or refractory neuroblastoma: a Children’s Oncology Group study. J Clin Oncol. 2010;28: 3808–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bagatell R, London WB, Wagner LM, et al. Phase II study of irinotecan and temozolomide in children with relapsed or refractory neuroblastoma: a Children’s Oncology Group study. J Clin Oncol. 2011;29: 208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369: 2106–2120. [DOI] [PubMed] [Google Scholar]
- 20.Park JR, Bagatell R, Cohn SL, et al. Revisions to the International Neuroblastoma Response Criteria: A Consensus Statement From the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2017;35: 2580–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharp SE, Gelfand MJ, Shulkin BL. Pediatrics: diagnosis of neuroblastoma. Semin Nucl Med. 2011;41: 345–353. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958;53: 457–481. [Google Scholar]
- 23.Peto R, Peto J. Asymptotically Efficient Rank Invariant Test Procedures. Journal of the Royal Statistical Society. Series A (General). 1972;135: 185–207. [Google Scholar]
- 24.London WB, Castleberry RP, Matthay KK, et al. Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children’s Oncology Group. J Clin Oncol. 2005;23: 6459–6465. [DOI] [PubMed] [Google Scholar]
- 25.Cohn SL, Tweddle DA. MYCN amplification remains prognostically strong 20 years after its “clinical debut”. Eur J Cancer. 2004;40: 2639–2642. [DOI] [PubMed] [Google Scholar]
- 26.DuBois SG, Granger MM, Groshen S, et al. Randomized Phase II Trial of MIBG Versus MIBG, Vincristine, and Irinotecan Versus MIBG and Vorinostat for Patients With Relapsed or Refractory Neuroblastoma: A Report From NANT Consortium. J Clin Oncol. 2021: JCO2100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DuBois SG, Marachelian A, Fox E, et al. Phase I Study of the Aurora A Kinase Inhibitor Alisertib in Combination With Irinotecan and Temozolomide for Patients With Relapsed or Refractory Neuroblastoma: A NANT (New Approaches to Neuroblastoma Therapy) Trial. J Clin Oncol. 2016;34: 1368–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Allmen D, Davidoff AM, London WB, et al. Impact of Extent of Resection on Local Control and Survival in Patients From the COG A3973 Study With High-Risk Neuroblastoma. J Clin Oncol. 2017;35: 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon T, Haberle B, Hero B, von Schweinitz D, Berthold F. Role of surgery in the treatment of patients with stage 4 neuroblastoma age 18 months or older at diagnosis. J Clin Oncol. 2013;31: 752–758. [DOI] [PubMed] [Google Scholar]
- 30.Holmes K, Potschger U, Pearson ADJ, et al. Influence of Surgical Excision on the Survival of Patients With Stage 4 High-Risk Neuroblastoma: A Report From the HR-NBL1/SIOPEN Study. J Clin Oncol. 2020;38: 2902–2915. [DOI] [PubMed] [Google Scholar]
- 31.Ryan AL, Akinkuotu A, Pierro A, Morgenstern DA, Irwin MS. The Role of Surgery in High-risk Neuroblastoma. J Pediatr Hematol Oncol. 2020;42: 1–7. [DOI] [PubMed] [Google Scholar]
- 32.Mora J, Castaneda A, Gorostegui M, et al. Naxitamab combined with granulocyte-macrophage colony-stimulating factor as consolidation for high-risk neuroblastoma patients in complete remission. Pediatr Blood Cancer. 2021;68: e29121. [DOI] [PubMed] [Google Scholar]
- 33.Haghiri S, Fayech C, Mansouri I, et al. Long-term follow-up of high-risk neuroblastoma survivors treated with high-dose chemotherapy and stem cell transplantation rescue. Bone Marrow Transplant. 2021;56: 1984–1997. [DOI] [PubMed] [Google Scholar]
- 34.Jodele S, Dandoy CE, Myers K, et al. High-dose Carboplatin/Etoposide/Melphalan increases risk of thrombotic microangiopathy and organ injury after autologous stem cell transplantation in patients with neuroblastoma. Bone Marrow Transplant. 2018;53: 1311–1318. [DOI] [PubMed] [Google Scholar]
- 35.Garaventa A, Luksch R, Biasotti S, et al. A phase II study of topotecan with vincristine and doxorubicin in children with recurrent/refractory neuroblastoma. Cancer. 2003;98: 2488–2494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figure 1: Dot plot graph showing the distribution of bridge and post-induction regimens Cohort 2 (n=51) and 3 patients (n=27) respectively received between 2008–2019. *** = padj <0.001, * = padj <0.05
Supporting Figure 2: Dot plot graph showing the distribution of Curie Scores at diagnosis and end-induction for Cohort 1 (n=42) and Cohort 2 (n=37) patients with end-induction metastatic disease and available scores at both time points. Cohort 1 and 2 Curie scores were not statistically different at diagnosis (p=0.87). At end-induction, the Curie scores of patients in Cohort 2 Curie scores were significantly higher than patients in Cohort 1 (p<0.001). ns=not significant, *=p<0.001


