Abstract
Background
Working with patients through meaningful patient engagement (PE) and incorporating patient experience data (PXD) is increasingly important in medicines and medical device development. However, PE in the planning, organization, generation, and interpretation of PXD within regulatory and health technology assessment (HTA) decision-making processes remains challenging. We conducted a global review of the PE and PXD landscape to identify evolving resources by geography to support and highlight the potential of integration of PE and PXD in regulatory assessment and HTA.
Methods
A review of literature/public information was conducted (August 2021–January 2023), led by a multistakeholder group comprising those with lived or professional experience of PE and PXD, to identify relevant regulatory and HTA initiatives and resources reviewed and categorized by geography and focus area.
Results
Overall, 53 relevant initiatives/resources were identified (global, 14; North America, 11; Europe, 11; Asia, nine; UK, six; Latin America, one; Africa, one). Most focused either on PE (49%) or PXD (28%); few (11%) mentioned both PE and PXD (as largely separate activities) or demonstrated an integration of PE and PXD (11%).
Conclusions
Our analysis demonstrates increasing interest in PE, PXD, and guidance on their use individually in decision-making. However, more work is needed to offer guidance on maximizing the value of patient input into decisions by combining both PE and PXD into regulatory and HTA processes; the necessity of integrating PE in the design and interpretation of PXD programs should be highlighted. A co-created framework to achieve this integration is part of a future project.
Supplementary Information
The online version contains supplementary material available at 10.1007/s43441-023-00573-7.
Keywords: Patient experience data, Patient engagement, Health technology assessment, Regulatory assessment, Real-world evidence
Introduction
The goal of healthcare systems and practitioners has always been to deliver better health outcomes for patients. However, despite the best intentions of working to achieve this goal, healthcare has historically made decisions for patients without actively involving them (i.e., without patients co-creating and co-implementing care and research models). This omission was largely because the burden of illness used to be driven by acute diseases that required immediate care by the physician with little or no time for patient input. Twentieth century advances in medical interventions have led to the emergence of chronic diseases as the dominant cause of illness [1], a setting in which the patient has a significant role in both primary prevention and treatment, and therefore has valuable experiences and insights to share. Numerous studies have shown that partnering with patients to address their clinical health needs delivers better outcomes, including satisfaction by both patients and practitioners, and often at reduced cost to society [2–4]. The value of partnering with patients now extends beyond clinical care, into a wider range of healthcare decisions. A recent comprehensive report by the Council for International Organizations of Medical Sciences (CIOMS) highlights the importance of also involving patients systematically and meaningfully throughout medicines lifecycle and development, including in regulatory processes, and provides consolidated evidence for the benefits of patient involvement [5].
Patients and those who look after them (such as family members or friends) have unique and relevant insights into the ways that a condition and its management impact day-to-day life. As such, diverse health stakeholders are increasingly seeking to engage with the patient community for their lived experiences and preferences in terms of treatments, risks, and outcomes. According to the US Food and Drug Administration (FDA) Patient-Focused Drug Development guidance, patient engagement (PE) is defined as “activities that involve patient stakeholders sharing their experiences, perspectives, needs, and priorities that help inform FDA’s public health mission” [6]. The term patient involvement is also widely used, sometimes interchangeably with PE [5, 7] but also with a variable meaning that can encompass more than “engagement”, depending on region and stakeholders. Although the two terms may have slightly different interpretations and understanding [8, 9], they both refer to the concept of bringing patients and their experiences, insights, and perspectives into decision-making processes with the aim of ensuring that decisions are rooted in the needs and experiences of patients. Community engagement is also used as a wider term and encompasses a concept beyond the scope of this work; therefore, we have focused on patient engagement as only one part of community engagement. Regardless of the language used to define patient input, both the engagement of patients in the process (PE) and the consideration of evidence on the patient perspectives and impacts (patient experience data [frequently referred to as ‘PED’ but as ‘PXD’ in this article to differentiate from ‘PE’]) are required. The goal is to inform and drive evidence-based decision-making through the collection and consideration of patient insights provided from patient-involvement practices, alongside evidence and data that capture the real-world experiences, preferences, and needs of patients. In this paper we use the term PE to refer to not only the act of interacting with patients, but also to a more meaningful, active, long-term, two-way collaboration with patients as valued, true partners in the development of medicines and medical technologies throughout the entire product lifecycle. Our definition of patient involvement or PE describes meaningfully working with patients systematically, over a period of time with a focus on delivering better outcomes.
Health technology assessment (HTA) and regulatory practices are deliberative processes that make judgment calls on available evidence; they require different stakeholders to consider the relative importance and impact of the evidence under review. Many HTA and regulatory bodies have long recognized that patient input is needed to provide the patient context in these deliberations, and they are increasingly including patients in decision-making processes that impact medicine and technology development [10]. As such, PE in regulatory practices is not new and has been embedded in some decision-making bodies since the late 1980s, with one early example being the establishment of the FDA patient advisory committee on HIV/AIDS in 1988 [11]. While PE has been used to date, to provide the insights needed to inform regulatory and HTA deliberations, and patient groups contributed to these decisions with their own evidence, decision makers lacked a systematic framework for patient-focused evidence. The need for further well-designed, robust evidence to provide additional clarity on the patient perspective and across a wider group of diverse patients was identified as a critical gap. Moving beyond the ad hoc collection and occasional consideration of patient evidence, the term and concept of PXD as a class of evidence that needs to be considered has emerged. The FDA defines PXD as “information that captures patients’ experiences, needs and priorities related, but not limited to: (1) the symptoms of their condition and its natural history; (2) the impact of the conditions on their functioning and quality of life; (3) their experience with treatments; (4) input on which outcomes are important to them; (5) patient preferences for outcomes and treatments; and (6) the relative importance of any issue as defined by patients” [6]. In this article, which also provides an update of a previous landscape analysis [10], we examine recent developments and evolving PE and PXD resources by geography in regulatory and HTA processes.
Methods
Project Design and Structure
The project took a phased approach, starting with the collection of information/publications on guidance, initiatives, and resources for PE and PXD in regulatory and HTA processes. Approximately 100 contributors and collaborators were approached directly through the “Patient Focused Medicines Development (PFMD) PE and PXD Project” to provide their input and signpost to potentially relevant information. This information (along with results from the literature searches described later) was then compiled into a draft report and landscape analysis (by Daniela Luzuriaga and Gary Finnegan) and disseminated to approximately 25 contributors (who were all members of the PE and PXD Project) for review and consultation. Feedback on the draft report was received from 13 core contributors who further contributed to and refined the landscape analysis. The 13 core contributors represented seven different stakeholder groups: patient representatives/patient advocacy groups, regulatory, HTA, academic research organizations, pharmaceutical industry, consultancy, and PFMD (Supplementary Table 1; Fig. 1).
Fig. 1.
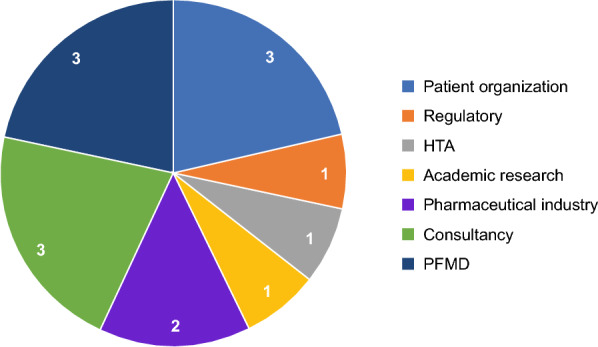
Breakdown of the 13 core contributors by stakeholder group. (Please note that one of the contributors falls under both academic research and patient organization, and so is counted twice). HTA, health technology assessment; PFMD, Patient Focused Medicines Development
Contributing members of the PE and PXD project were identified through PFMD and PFMD member networks, through participation in previous PE projects, and contribution to SYNaPsE—a platform of PE practices and initiatives. Members were invited to participate in the project and contribute to the analysis based on their expertise in PE and/or PXD, prior experience in managing, collating, developing, and using PXD, and/or their lived experience as patients. In line with good PE practices, patients were active participants in the project throughout, alongside industry and other stakeholder-group representatives. The contributors pulled in insights and examples from their networks including Health Technology Assessment International (HTAi), the Centre of Regulatory Excellence, the FDA, and the European Medicines Agency (EMA). Per International Committee of Medical Journal Editors criteria [12], core contributors were also invited to be authors of this paper.
Identification of Global PE and PXD Resources
Google search engine was used to conduct a broad literature search (from August 2021 to January 2023) using keywords around PE and PXD, including “HTA”, “regulators”, “patient engagement”, “patient experience data”, “guidance”, “initiative”, “FDA”, and “EMA”. In addition, a search of “grey” literature (public information available outside of scientific or peer-reviewed journals) was conducted. This search scope included sources such as relevant research, annual reports, public hearings/conference proceedings, webinars, and data updates from the following government health authorities, professional organizations, and regulatory and HTA bodies: CIOMS, EMA, FDA, International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), Medicines and Healthcare products Regulatory Agency (MHRA), Pharmaceuticals and Medical Devices Agency (PMDA), Canadian Agency for Drugs and Technologies in Health (CADTH), Center for Drug Evaluation (CDE), the Japan Agency for Medical Research and Development (AMED), Agency for Care Effectiveness (ACE), HTAi, Institute for Clinical and Economic Review, and Europe Network for Health Technology Assessment (EUnetHTA). Contributing members (see Supplementary Table 1) identified additional relevant resources and comprehensively evaluated the research.
Assessment of the Main Focus Area of Global PE and PXD Resources
We conducted a broad thematic analysis to assess the main focus area of each resource and whether there was systematic and clear integration of PE and PXD in the approach described. Resources were reviewed for alignment with the time period of the current analysis and relevant focus on PE and PXD, and were categorized by region and stakeholder group. Information and descriptions from the title, objectives, and (where available) outputs of the resource were used to categorize its focus area as either primarily PE, primarily PXD, describing both PE and PXD but separately, or describing an integrated PE and PXD approach. Each resource was reviewed separately by a subgroup of the project team comprising four PFMD members with multiple people reviewing different resources. Where the focus area was unclear, this was discussed within the subgroup to reach consensus on thematic interpretation; categorization results were also shared with core contributors for their review and validation.
Results
Global PE and PXD Resources in Regulatory and HTA Processes
The landscape review and analysis provided further evidence for the growing momentum to include PE in the decision-making of global regulatory and HTA bodies with an increase in the number of relevant resources and initiatives identified compared with the previous landscape analyses (53 vs 27) [10]. The current literature search yielded a total of 63 unique PE and PXD resources (comprising initiatives, projects, and papers) in regulatory and HTA decision-making. Of these, 10 were not included in this landscape analysis following review either because they were outside of the time period for the current analysis (five resources) or because they were not relevant for the focus of PE and PXD (five resources). This report focuses on the remaining 53 initiatives and projects, of which 14 were considered global, 11 were from North America, 11 were European in origin or focus, nine were from Asia, six were from the UK, one was from Latin America, and one was from Africa (Table 1). Examples of resources that represent current PE and/or PXD initiatives in different regions are summarized in the following sections and in Table 2 [13–34].
Table 1.
Current global patient engagement (PE) and patient experience data (PXD) resources in regulatory and health technology assessment processes
Table 2.
Examples of patient engagement (PE) and patient experience data (PXD) initiatives in different regions
| Initiative | Purpose/summary |
|---|---|
| Global | |
| International Coalition of Medicines Regulatory Authorities (ICMRA) Statement |
Identified opportunities for regulatory authorities to collaborate in considering how real-world evidence (RWE) can inform regulatory decision-making Areas recognized were (1) harmonization of terminology to clearly define real-world data and RWE; (2) convergence on guidance and best practice, including common principles on data quality, identification of situations where RWE can appropriately contribute to regulatory decisions, and templates for study protocols and reports that can be used by multiple authorities; (3) rapid creation of international expert groups and collaboration on processes; and (4) definition of common principles for study registration and publication of results in registries and open-source, peer-reviewed journals [13] |
| Proposed International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Guideline |
Proposed ICH Guideline Work to Advance Patient Focused Drug Development identifies areas where including the patient perspective would improve “the quality, relevance, safety and efficiency of drug development and inform regulatory decision-making” Presents opportunities for global harmonization of how the patient perspective is included using a robust methodology that is appropriate for both regulated industry and regulatory authorities [14] |
| Global Patient Experience Data Navigator |
Co-created by Patient Focused Medicines Development (PFMD) as a tool to help navigate the PXD landscape and to add clarity and structure to understanding the generation, use, and interpretation of PXD Includes tools to identify PXD use by different stakeholders throughout the product development cycle and healthcare process, determine what impacts matter most to patients and families, and select appropriate measurement methods of outcomes that matter most to patients and families [15] |
| Europe | |
| European Medicines Agency (EMA) Engagement Framework |
Supporting access to individual patient’s experiences of living with a condition, its management and use of medicines Promoting the generation, collection, and use of evidence-based PXD for benefit-risk decision-making Developed by the Patients and Consumers Working Party (PCWP)—an organization that also informs global guidance provided by ICH and the Council for International Organizations of Medical Sciences (CIOMS) Patients' and Consumers' Working Party (PCWP) members come from patient organizations, industry, regulators, academia, and the World Medical Association and work collectively to highlight pragmatic points around patient involvement strategies [16] |
| Health Technology Assessment International (HTAi) |
360° HTA Patient Involvement Project (supported by HTAi, the European Patient Academy on Therapeutic Innovation [EUPATI] and the European Patients’ Forum [EPF]) Aims to understand how methods and processes for patient involvement in health technology assessment (HTA) processes are perceived, if all stakeholders feel that patients are sufficiently involved, and to provide advice for future directions [17] |
| Innovative Medicines Initiative-PREFER (IMI-PREFER) |
Framework that provides suggestions on how patients’ perspectives could be measured through patient preference studies and then incorporated into regulatory decision processes Framework has three main sections: (1) defining preference study aims and objectives; (2) study planning, design, and conduct; and (3) interpretation and application of study results [18] |
| Europe Network for Health Technology Assessment (EUnetHTA) |
Joint work plan to help build a European network of experts on patient-reported outcomes (PROs), contribute to guideline development and a workshop on PXD for multistakeholders, as well as following up on areas of action identified in the ICH reflection paper on patient-focused drug development [14] The plan will further describe best practice for issues such as compensation for expert participation and, through educating experts on the difference between HTA and regulatory processes, will provide direction on how to incorporate expert input into the regulatory and HTA outputs [19] |
| Medicines and Healthcare products Regulatory Agency (MHRA) Patient Involvement Strategy 2021–2025 |
Sets out objectives to engage the public and patients during all stages of the regulatory process, and to change the internal culture of the agency such that “every member of staff considers the patient and public perspective in their decisions” Sets out objectives for multistakeholder partnerships in acknowledgement that benefits exist from sharing data and avoiding duplication of time and effort [20] |
| Centre for Research in Public Health and Community Care (CRIPACC) | Provides practical advice to health researchers on giving feedback to patient and public contributors, including thanking contributors and providing detailed feedback (such as impact of patient contribution and study progress) to increase motivation, confidence, learning and development, accountability, and transparency [21] |
| National Institute for Health and Care Excellence (NICE) RWE Framework |
RWE framework is primarily aimed at pharmaceutical and health technology companies developing evidence to inform NICE guidance but is also relevant to patients and organizations that gather data and review evidence During development of the framework, NICE sought feedback through workshops and public consultation from numerous bodies, including patients and patient organizations, and revised the framework accordingly A key recommendation is that data should be collected in a patient-centered way that also minimizes the burden on patients and healthcare professionals [22] |
| United States | |
| Patient Focused Drug Development (PFDD) Guidance |
Guidance published in 2020, addressing collection of patient input including sampling methods and target population definition Guidance issued in February 2022, focused on methods used to identify what is important to patients in relation to the burden of their disease and its treatment. It described best practice in conducting qualitative, quantitative, and mixed methods research, as well as considerations for the use of social media Draft guidance issued in June 2022 to help clinical trial sponsors use high quality measures of trial outcomes that are important to patients. Described how to choose, modify, or develop and validate clinical outcome assessments, including PROs, observer-reported outcomes, clinician-reported outcomes, and performance-based outcomes [23–26] The FDA's Center for Biologics Evaluation and Research (CBER) supports the PFDD mission and has a patient engagement program to incorporate patient input in their work. Initiatives include CBER’s Science of Patient Input (SPI) initiative and their Rare Disease program [27] |
| Medical Device Innovation Consortium (MDIC) Patient and Public Involvement (PPI) Clinical Trial Framework |
Describes considerations for regulators wanting to include PPI in clinical trial design Includes leveraging existing opportunities to incorporate PPI into regulatory decision-making; identifying novel endpoints for patient preference studies and aligning these with traditional endpoints; ensuring that the PPI used is relevant to the intended patient population; and using relevant statistical methods for the trial population [28] |
| Patient Insights Database |
Aims to support early inclusion of patients in clinical trial design Generated through patient interviews and testimonials, RWE studies, and health-related quality of life (HRQoL) research Used to further understanding of symptoms and diagnosis, treatment, HRQoL, economic burden, and patients’ hopes for new treatments [29] |
| US Food and Drug Administration’s (FDA) Center for Devices and Radiological Health |
Patient Engagement in the Design and Conduct of Medical Device Clinical Studies—provides guidance on PE in medical device studies and is intended to help sponsors understand how they can use PXD to improve clinical studies for medical devices, to highlight the benefits of early patient engagement, to clarify what are considered relevant engagement activities, and to address questions/misconceptions about PE data collection for medical device design and clinical studies [30] Principles for Selecting, Developing, Modifying, and Adapting Patient-Reported Outcome Instruments for Use in Medical Device Evaluation—presents concepts to consider when using PRO instruments, provides recommendations around ensuring the chosen PRO instruments are sufficient for the task, and describes best practices to develop, modify, or adapt PRO instruments for an optimized outcome [31] |
| Japan, China, and Singapore | |
| Pharmaceuticals and Medical Device Agency (PMDA) in Japan |
Published a report on patient participation in 2021 with the dual aim of gathering patient input and increasing awareness amongst patients of the agency’s work Made a commitment to include PROs in all medicine and device evaluations [32] |
| Center for Drug Evaluation (CDE) in China | Published Guiding Principles for the Application of Patient Reported Outcomes in Drug Clinical Research and Development (Trial) [33] |
| Consumer Engagement and Education (CEE) Agency in Singapore |
Provided a forum for patient and volunteer organizations to publicize their work on patient and public involvement Insights included the value of patient involvement in HTA through the provision of unique first-hand experience; the importance of mutual trust, cooperation, and maintaining a two-way conversation with patients and HTAs; the need to improve patient health literacy and clarity of communication using plain language; and the importance of patient insights for rare diseases to fill data gaps [34] |
Initiatives in Europe
In the European Union, the EMA continues to make patient involvement and the collection of PXD a priority [35], including through the admission of patients as members of the EMA board and the decision to regularly invite patients to attend meetings and public hearings [36]. In addition, the EMA Engagement Framework provides a platform and roadmap for EMA’s engagement with patients and other stakeholders (Table 2) [16, 37–39]. HTAi has established several initiatives to explore optimization of patient ideas in terms of effectively incorporating them into decision-making, and to better understand the impact of patient input in terms of different stakeholder perspectives (Table 2) [17]. The Innovative Medicines Initiative-PREFER (IMI-PREFER) has produced a framework outlining how patient perspectives could be measured through patient preference studies and incorporated into regulatory decision-making [18]. The EMA acknowledged this as an important reference document but noted that there were some limitations in providing a concrete framework due to the limited experience with patient preference studies [40].
Schroeder and colleagues stressed the importance of multistakeholder collaboration in PXD approaches [41] and global health agencies have several ongoing initiatives to promote stakeholder collaboration in the use and generation of PE and PXD. In Europe, the EUnetHTA works toward building collaborations between various HTA agencies, and in September 2021, a 2-year service contract was signed by the European Health and Digital Executive Agency for the “Provision of Joint Health Technology Assessment (HTA) Work Supporting the Continuation of EU Cooperation on HTA”[19, 42]. This joint work plan has identified several areas of focus (including methodological deliverables and guidance for stakeholder interactions), and recognizes that implementation needs to be flexible, to support work towards a legislative framework on European HTA cooperation (Table 2). EUnetHTA has also developed 43 relative effectiveness assessments with an emphasis on patient involvement. The assessments are considered generalizable across countries, aiming to reduce duplication in HTA production [43]. However, it has been noted that timely patient involvement remains to be addressed along with guidance on how to demonstrate visibility of patient contribution [44].
In the UK, the MHRA aims to make consideration of patient perspectives a mandatory part of all clinical trials, and their Patient Involvement Strategy also establishes objectives for multistakeholder partnerships [20]. The UK’s Centre for Research in Public Health and Community Care provides advice to health researchers on giving feedback to patient and public contributors [21]. The National Institute for Health and Care Excellence (NICE) in England and Wales uses real-world evidence (RWE) including patient experiences to inform their guidelines and health technology appraisals, and it recognizes that such data could be used more routinely than currently to address gaps in evidence and speed up patient access to health interventions [22]. NICE therefore launched a framework in June 2022 to identify when RWE can be used to reduce uncertainties and improve NICE guidance, and to describe best practices for organizations to plan, conduct, and report studies (Table 2) [22].
Initiatives in North America and Brazil
In the United States, the FDA has published a series of detailed guidance documents as part of their Patient Focused Drug Development (PFDD) initiatives to help stakeholders collect comprehensive and representative data and answer the question of what matters most to patients [23–26]. In a study conducted by the Medical Device Innovation Consortium, a working group of 17 experts reviewed a series of case studies to help identify the best practice for using patient and public involvement (PPI) to inform clinical trials [45]. The study led to the development of a PPI-Clinical Trial Framework, which identified several considerations for regulators wanting to include PPI in clinical trial design (Table 2). The Patient Insights Database also aims to support early inclusion of patients in clinical trial design and resultant decision-making [29]. Strides in PE have been made in the rare disease sector, where patient populations are small but often highly motivated. Working alongside patients who suffer complex and rare diseases can be crucial to finding treatments and for disease management [46]. Nicod and colleagues have provided recommendations on appropriate use of patient-reported outcomes (PROs) and health state utility values in HTA decisions for rare diseases [47].
Ways to incorporate patient feedback and input into medical technology and devices are also being explored. For example, the FDA’s Center for Devices and Radiological Health released two guidance documents in 2022 on the use of PE in medical device development (Table 2) [30, 31]. Canada’s Drug and Health Technology Agency (CADTH) have included ‘partnerships’ with patient communities (including individual patients, their families and caregivers, and patient representatives) and a pledge to work together ‘to improve and strengthen the quality and significance’ of their work, as a key element of their 2022–2025 strategic plan [48]. In Brazil, a legislative mandate for social participation makes public involvement in HTA processes compulsory. Recommendations have been made for a more systematic approach through “expansion of communication, capacity building, and transparency”, to maximize benefit from such participation [49].
Initiatives in Japan, China, and Singapore
PPI in clinical trials/research in Japan is a relatively new endeavour [50], and until recently, examples of PPI in clinical trials were isolated cases [51, 52]. The starting point of national initiatives was a project led by the Japan Medical Agency and patient groups and supported by the Ministry of Health Labour and Welfare (MHLW) and AMED through a national survey held in 2017. The outcome of this survey, the PPI Guidebook, was published in 2019 in Japanese [53], and in 2022 in English [51]. The Ministry of Health Labour and Welfare has also emphasized the necessity of involving trained patients from the planning phase of clinical trials and in proposing patient-oriented outcomes [54, 55]. In addition to AMED, the PMDA in Japan has published a guidebook for patient centricity and is advancing PE in several sectors [32]; both organizations are beginning to collaborate with other stakeholders. China has also published guidelines around a patient-centred approach to drug development using PROs [33, 56]. With the aim of boosting patient participation in HTAs, Singapore set up the Consumer Engagement and Education agency, and in November 2021 a special dialogue was held for patient and volunteer organizations to publicize their work on PPI (Table 2) [34, 57, 58].
Initiatives in Africa
The African Medicines Agency (AMA)—the second African health agency to help regulate medical products—was recently established to “enhance regulatory oversight and facilitate access to safe and affordable medicines across the continent” [59, 60]. The agency will also focus on harmonizing current regulatory policies/standards and will provide scientific guidelines. Although this is not directly a patient engagement or PXD resource, it does signal a move towards a regulatory system built for and by African people themselves and will promote domestic production and development of medicines within Africa. In turn, this will provide opportunities for direct patient engagement locally, along with initiatives and development of medicines directed towards patients of African descent.
Emerging Global Initiatives
Clinical RWE and the incorporation of PE into the design of RWE studies are areas where PE/PXD is still limited; however, other regulatory authorities (in addition to NICE in the UK) are considering how RWE can inform regulatory decision-making. Following a workshop in June 2022, the International Coalition of Medicines Regulatory Authorities issued a statement identifying opportunities for regulatory authorities to collaborate in this area [13]. PFMD has co-created a Global Patient Experience Data Navigator tool to help navigate the increasingly complex PXD landscape and to add clarity and structure to understanding the “what, how, when, who, and why” of PXD. The Navigator has been designed to be relevant for diverse stakeholders and can be used to help ensure that PXD focuses on impacts that have been identified by patients as most important and meaningful, reviews available PXD measurement tools and methodologies, identifies which stakeholders are using PXD and how it is being used, and understands the impact of PXD use on decision-making [15].
Impact of Patient Contributions on Decision-Making
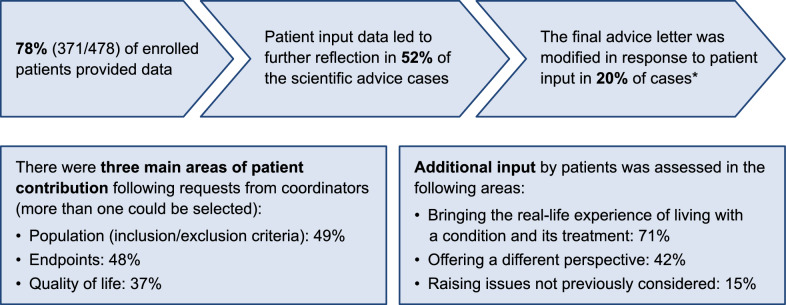
While there are many examples globally of patient contribution to regulatory decision-making, there is still limited documentation of the impact of such contributions [61, 62]. However, there are some insights; for example, in Europe, patient input is an established part of the medicine development process for all scientific advice provided by the EMA. A recent case study examined the extent and value of this input over a 4-year period [63]. The study quantified the number of patients involved and in which areas they contributed (Fig. 2). Importantly, patient input was shown to directly impact 52% of the cases, leading to further reflection, and in 20% of the cases, PE led to modification of the final advice letter, indicating a direct and tangible impact. The EMA also quantified the contribution of patients and healthcare professionals to their work during 2020–2021; it found that patient contributions outnumbered those of healthcare professionals across committee consultations and review of documents [64].
Fig. 2.
Case study of patient contributions to scientific advice provided by the EMA. The number of patient contributions and how they contributed to various aspects of scientific advice provided by the EMA was assessed as part of a 4-year case study. *In most cases where patient input did not make a change, most of the patients agreed with the development plan set out. EMA, European Medicines Agency
In their guidance for providing patient input to inform reimbursement reviews, CADTH describes the development of patient input summaries and how the patient contribution is used by their clinical and economic teams, in the CADTH clinical report, and to inform development of the review protocol [65]. The Scottish Medicines Consortium encourages patient input through its patient group submission process and has established Patient and Clinician Engagement (PACE) meetings that can be used where more detailed input is valuable, such as in the rare disease setting [66]. A summary of patient input is made publicly available, and the PACE report is incorporated in documents for committee deliberations [62, 66]. Similarly, at NICE in England, patient advocates and expert patients are able to both submit written responses and provide testimony in the deliberative committee meeting, this too is made publicly available in the HTA reports [62]. In the US since 2017, the FDA have required incorporation of a table (the “PXD Table”) within the assessment report, tabulating the patient experience data included in the submission [67]. These examples indicate that while there is increasing acknowledgment of patient input, this is currently not consistently or systematically reported, and the impact of the contribution is rarely articulated. It is likely that clearer documentation of the impact of patient contribution, with feedback to patient groups submitting their input, would demonstrate transparency in the process and also provide contributors with practical examples of what inputs are most informative [62].
Assessment of the Main Focus Area of Global PE and PXD Resources
The collective value of PE alongside PXD is being increasingly recognized by diverse health stakeholders [41, 68–71]. There has been a call for multistakeholder collaboration and a more aligned approach to the generation, collection, and use of PXD that incorporates established elements of good practice in PE [41, 72]. The aim is to ensure that resulting insights reflect patients’ needs and priorities, meet the collective needs of diverse stakeholder groups, and minimize duplication of effort as well as burden on patients being asked for their insights [41]. We assessed whether this integrated approach is apparent in identified PE/PXD resources and found that the vast majority of resources focused either on PE or PXD; only very few had a joint focus on PE and PXD, or demonstrated an integration of PE and PXD. Of 53 resources included in this landscape review, the majority (26 [49%]) were categorized as focusing primarily on PE and 15 (28%) as focusing primarily on PXD. Overall, six (11%) resources were categorized as focusing on PE and PXD but not in an integrated approach, rather in separate sections of the resource. Only six (11%) resources were categorized as demonstrating a focus on an integrated and combined approach to PE and PXD that reflects the interdependency between the two.
Discussion
This landscape review of recent initiatives, papers, and publications confirms and extends previous accounts of increased attention to reporting both PE and PXD by regulatory and HTA organizations. Overall, we found that this attention continues to expand globally and also to evolve from interest and openness toward guidance and expectations. The guidance and expectations that are increasingly expressed by regulatory and HTA organizations around the world pertain to both PE and PXD, albeit to varying degrees and proportions. This observation raises the question of the relationship between, and the value of, both concepts.
The FDA initiative to hold PFDD meetings [73] was in many ways a milestone in highlighting the uniqueness, value, and need for PXD as part of the regulatory review process and to complement and augment insights gained from scientific and medical data. The success of the PFDD meetings in raising awareness of the importance of PXD was in no small part due to meetings being based on quality engagement with patients testifying as an essential and early step in the process, thus providing a strong example of the value of integrating PXD and PE. Our landscape review indicates that much effort has been invested in PE, which is the focus of many initiatives. Much effort has also been invested in clarifying the format, quality, and use of PXD. However, these are largely separate approaches where the patient often still is simply the data “source” for PXD; they lack the critical integration of PE into the design, generation, collection, interpretation and use of PXD. By focusing preferentially or almost exclusively on the “data” in PXD, there is the risk of repeating the historic omission of the “patient” in PXD, and thus potentially jeopardizing the quality, value, and usefulness of the resulting PXD. Quality PE is necessary for the generation of quality PXD, and in fact well beyond that stage. PE also has an important role in the interpretation and understanding of PXD (it can uniquely help to answer the “why” question and thus explain the relevance of the observed PXD) and in helping all stakeholders understand the evidence and agree on a course of action. PXD and PE are interdependent, and both are essential for better regulatory and HTA decision-making. However, our landscape analysis highlights that there are few resources or initiatives where this interdependence is highlighted and where both are optimally integrated or even recommended.
Health stakeholders are calling on regulators and HTA bodies to give definitive guidance on how and when to conduct PE and PXD, but these agencies cannot provide robust guidance without first gaining their own experience on how PXD insights can be combined with PE to improve the quality of their deliberative processes. While collaborative global efforts such as PFMD’s Global Patient Experience Data Navigator [15] aim to provide clarity and direction around PXD, there is still a long way to go toward robust guidance.
The collective value of PXD to diverse health stakeholders has been described [41]. This indicates that development of a collaborative framework to accelerate the learnings from ongoing PE/PXD initiatives would be beneficial, as proposed at a multistakeholder forum in 2022 [74]; for example, it could provide advice and best practice examples on the generation of PXD with PE and the use of PXD and PE in decision-making. We acknowledge that there may be some limitations to this landscape review in terms of researching and providing an overview of all relevant global initiatives. However, as there is not one global database to search for such initiatives, in order to find relevant programs, in addition to relevant literature searches, we drew on expertise globally through a diverse group of stakeholders whose collective aim is to widen the reach of their connections to maximise communication and patient engagement. So, to the best of our knowledge, we have included all available resources at the time of writing but would also like to emphasize that the onus is on everyone to connect, join larger networks and to share learnings and best practice, to prevent duplication of efforts and to maximise outcomes for patients. We appreciate there is also value in systematic monitoring of the rapidly evolving and maturing PE and PXD landscape to identify and share emerging trends and positive initiatives, resources, and tools. As such, the authors recommend and plan a regular evaluation and update of this PE/PXD landscape review and invite all stakeholders globally to join this collaborative effort and to share their resources and insights. At the same time, we recognize that major barriers stand in the way of a true global collaboration and that while we extend this invite, we recognize that an active approach to seeking out more diverse collaborators is needed and will collectively be working towards ways to address this.
We also acknowledge that the review will not directly advance scientific approaches, methods, and hypotheses suitable for concretely advancing the scientific field of patient involvement, as this falls beyond the scope of a landscape review. However, by providing a comprehensive list of resources we have created a de facto global database that lays the foundation for further analysis of each resource. Specific methodologies to advance patient involvement is also the focus of ongoing work, research, and development at many of the key organisations mentioned that continue to develop resources and guidance in this area. Hence, we recommend a call to action for existing organizations and new organisations to work together to develop suitable and credible guidance. It is also important to note that whilst patient engagement is a form of community engagement, it was beyond the scope of this review to specifically address all such community initiatives, including those associated with health equity and diverse populations.
Conclusions
Both PE and PXD are central to achieving an approach to healthcare that reflects and addresses needs, priorities, and outcomes that matter most to patients, in addition to clinical outcomes. The combination and integration of PE and PXD is needed to generate meaningful data that captures the patient experience and accurately reflects the patient perspective, and also to contextualise its interpretation within a deliberative process such as regulatory and HTA—ultimately leading to better quality (value-based) decisions. However, our landscape review demonstrates that the tendency has been to offer guidance/policies on either PE or PXD in isolation, and that the majority of approaches are not (yet) thinking about the benefits of combining the two. Although there are a few examples where PE and PXD have been integrated, such integration needs to be expanded and normalized. We are currently in a transition stage, where the supporting guidance has not kept pace and therefore, a key step forward will be developing robust guidance and evolving dynamic processes that can be adopted universally and in different contexts. For example, to recommend how and when to involve patients in the design, generation, collection, and application of PXD, and there are emerging tools that can support this integrated approach. To this end, multistakeholder co-creation of a framework for integration of PE and PXD is under way as part of PFMD’s PE and PXD project. Without this and other initiatives towards integration, we will lose key engagement opportunities and important patient influence on decision-making and slow progression towards building patient-centred healthcare systems. In addition, the PXD used by regulators and HTA are at risk of being of lower quality when collected (and interpreted) without patient engagement.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all the members of the PFMD Patient Engagement and Patient Experience Data Fusion project for their insights and contribution to the project. We also thank Neil McAuslane (Director, Centre for Innovation in Regulatory Science), for his feedback on the draft manuscript, and Gary Finnegan for his help in compiling the draft report and landscape analysis.
Author Contributions
All authors contributed to the landscape analysis, review of identified PE and PXD resources through collaborative meetings, and conceptualization and development of the manuscript. DL and HC coordinated assessment of the focus area of resources. LD and NB drafted the abstract. IS drafted the manuscript. All authors reviewed and provided substantial and comprehensive feedback on each draft of the manuscript. All authors read and approved the final manuscript for submission. HC and DL agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
Development of this manuscript was funded by PFMD. Medical writing support was provided by Ify Sargeant and Mary Greenacre on behalf of Twist Medical, USA, in accordance with Good Publication Practice (GPP) 2022 guidelines and funded by PFMD.
Data availability
Not applicable. All information is available in the references cited.
Declarations
Conflict of interest
RV is an employee of Roche Pharmaceuticals. All other authors have not declared a potential source of conflict of interest. The views and opinions by the authors here do not reflect the opinions of their respective organizations; they reflect personal accounts from the varied expertise and perspectives.
Footnotes
All authors were also members of the PFMD Patient Engagement and Patient Experience Data project at the time of manuscript development.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Holman H, Lorig K. Patients as partners in managing chronic disease: partnership is a prerequisite for effective and efficient health care. BMJ. 2000;320:526–527. doi: 10.1136/bmj.320.7234.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doyle C, Lennox L, Bell D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open. 2013;3:e001570. doi: 10.1136/bmjopen-2012-001570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laurance J, Henderson S, Howitt PJ, et al. Patient engagement: four case studies that highlight the potential for improved health outcomes and reduced costs. Health Aff. 2014;33:1627–1634. doi: 10.1377/hlthaff.2014.0375. [DOI] [PubMed] [Google Scholar]
- 4.Bergerum C, Engström AK, Thor J, et al. Patient involvement in quality improvement—a ‘tug of war’ or a dialogue in a learning process to improve healthcare? BMC Health Serv Res. 2020;20:1–3. doi: 10.1186/s12913-020-05970-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Council for International Organizations of Medical Sciences (CIOMS). Patient involvement in the development, regulation and safe use of medicines. https://cioms.ch/publications/product/patient-involvement/.
- 6.US Food and Drug Administration. Patient focused drug development glossary. https://www.fda.gov/drugs/development-approval-process-drugs/patient-focused-drug-development-glossary.
- 7.Pushparajah DS. Making patient engagement a reality Patient. 2018;11:1–8. doi: 10.1007/s40271-017-0264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Facey K, Hansen HP, Single A. Patient involvement in health technology assessment. Singapore: ADIS; 2017. [Google Scholar]
- 9.Barello S, Graffigna G, Vegni E, et al. The challenges of conceptualizing patient engagement in health care: a lexicographic literature review. J Particip Med. 2014;6:259–267. [Google Scholar]
- 10.Patient Focused Medicines Development (PFMD). Highlighting recent trends in the fast-evolving patient engagement & patient experience data landscape. https://patientengagement.synapseconnect.org/resources/highlighting-recent-trends-in-the-fast-evolving-patient-engagement-patient-experience-data-landscape
- 11.US Food and Drug Administration. Evolution of patient engagement at the FDA. https://www.fda.gov/patients/learn-about-fda-patient-engagement/evolution-patient-engagement-fda-text-description.
- 12.International Committee of Medical Journal Editors. https://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html
- 13.International Coalition of Medicines Regulatory Authorities. ICMRA statement on international collaboration to enable real-world evidence (RWE) for regulatory decision-making. https://icmra.info/drupal/sites/default/files/2022-07/icmra_statement_on_rwe.pdf.
- 14.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). ICH reflection paper: proposed ICH guideline work to advance patient focused drug development. https://admin.ich.org/sites/default/files/2021-06/ICH_ReflectionPaper_PFDD_FinalRevisedPostConsultation_2021_0602.pdf.
- 15.Patient Focused Medicines Development (PFMD). Global patient experience data navigator. https://pemsuite.org/ped-navigator/.
- 16.European Medicines Agency. Engagement framework: EMA and patients, consumers and their organisations. https://www.ema.europa.eu/en/documents/other/engagement-framework-european-medicines-agency-patients-consumers-their-organisations_en.pdf.
- 17.360° HTA Patient Involvement Project Information. https://www.eu-patient.eu/Projects/ongoing-projects/360-hta-patient-involvement/.
- 18.Innovative Medicines Initiative-PREFER (IMI-PREFER). The PREFER framework. https://www.imi-prefer.eu/public-consultation/the-prefer-framework/.
- 19.Europe Network for Health Technology Assessment (EUNetHTA). European collaboration between regulators and health technology assessment bodies: joint work plan (2021–2023) between EMA and European HTA bodies facilitated through EUnetHTA21. https://www.ema.europa.eu/en/documents/work-programme/european-collaboration-between-regulators-health-technology-assessment-bodies-joint-work-plan-2021_en.pdf.
- 20.Medicines and Healthcare products Regulatory Agency. Patient involvement strategy 2021–25. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1022370/Patient_involvement_strategy.pdf.
- 21.Centre for Research in Public Health and Community Care. Guidance for researchers: feedback. Patient and public involvement (PPI): feedback from researchers to PPI contributors. https://www.nihr.ac.uk/documents/briefing-notes-for-researchers-public-involvement-in-nhs-health-and-social-care-research/27371.
- 22.National Institute for Health and Care Excellence. NICE real-world evidence framework. Corporate document (ECD9). June 2022. https://www.nice.org.uk/corporate/ecd9/chapter/overview.
- 23.US Food and Drug Administration. FDA patient-focused drug development guidance series for enhancing the incorporation of the patient’s voice in medical product development and regulatory decision making. https://www.fda.gov/drugs/development-approval-process-drugs/fda-patient-focused-drug-development-guidance-series-enhancing-incorporation-patients-voice-medical.
- 24.US Food and Drug Administration. Patient-focused drug development: collecting comprehensive and representative input. https://www.fda.gov/media/139088/download.
- 25.US Food and Drug Administration. Patient-focused drug development: methods to identify what is important to patients. https://www.fda.gov/media/131230/download.
- 26.US Food and Drug Administration. Patient-focused drug development: selecting, developing, or modifying fit-for-purpose clinical outcome assessments. https://www.fda.gov/media/159500/download.
- 27.US Food and Drug Administration. Center for biologics evaluation and research patient engagement program. https://www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/center-biologics-evaluation-and-research-patient-engagement-program.
- 28.Medical Device Innovation Consortium. Using patient preference information in the design of clinical trials framework. https://mdic.org/resource/using-patient-preference-information-in-the-design-of-clinical-trials-framework/.
- 29.Patel A, Fiebig D, Muszka J. The utility of patient engagement in drug research and development. Pharmaceut Med. 2021;35:157–162. doi: 10.1007/s40290-021-00388-7. [DOI] [PubMed] [Google Scholar]
- 30.US Food and Drug Administration. Patient engagement in the design and conduct of medical device clinical studies. https://www.fda.gov/media/130917/download.
- 31.US Food and Drug Administration. Principles for selecting, developing, modifying, and adapting patient-reported outcome instruments for use in medical device evaluation. https://www.fda.gov/media/141565/download.
- 32.Japan Pharmaceuticals and Medical Devices Agency. Patient centricity guidebook. https://www.pmda.go.jp/files/000243407.pdf.
- 33.Center for Drug Evaluation (CDE). Guiding principles for the application of patient reported outcomes in drug clinical research and development (trial). https://min.news/en/health/c82b12d465a506a814a78d4a27062cba.html.
- 34.Agency for Care Effectiveness (ACE). Opportunities for patient involvement. https://www.ace-hta.gov.sg/Patients-And-Community/opportunities-for-patient-involvement.
- 35.European Medicines Agency. Patient experience data in EU medicines development and regulatory decision-making. https://www.ema.europa.eu/en/documents/other/executive-summary-patient-experience-data-eu-medicines-development-regulatory-decision-making_en.pdf.
- 36.European Medicines Agency. Public hearings. https://www.ema.europa.eu/en/about-us/how-we-work/public-hearings.
- 37.European Medicines Agency. PWCP mandate. https://www.ema.europa.eu/en/documents/other/mandate-objectives-composition-patients-consumers-working-party-pcwp_en.pdf
- 38.The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). https://www.ich.org.
- 39.The Council for International Organizations of Medical Sciences (CIOMS). https://cioms.ch/.
- 40.European Medicines Agency. Qualification opinion on IMI-PREFER framework for patient preference studies. https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/qualification-opinion-imi-prefer_en.pdf.
- 41.Schroeder K, Bertelsen N, Scott J, et al. Building from patient experiences to deliver patient-focused healthcare systems in collaboration with patients: a call to action. Ther Innov Regul Sci. 2022;56:848–858. doi: 10.1007/s43441-022-00432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.EUnetHTA21. https://www.eunethta.eu/eunethta-21/.
- 43.Willemsen A, Ettinger S, Helmink C, et al. EUnetHTA relative effectiveness assessments: efforts to increase usability, transparency and inclusiveness. Int J Technol Assess Health Care. 2022;38(e41):1–8. doi: 10.1017/S0266462322000058. [DOI] [PubMed] [Google Scholar]
- 44.Elvsaas I, Ettinger S, Willemsen A, et al. Patient involvement in relative effectiveness assessments in the European Network for Health Technology Assessment. Int J Technol Assess Health Care. 2021;37:E24. doi: 10.1017/S0266462320002226. [DOI] [PubMed] [Google Scholar]
- 45.Rincon-Gonzalez L, Selig WKD, Hauber B, et al. Leveraging patient preference information in medical device clinical trial design. Ther Innov Regul Sci. 2023;57:152–159. doi: 10.1007/s43441-022-00450-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patient Focused Drug Development (PFDD). Guide to patient involvement in rare disease therapy development. A publication of the rare disease PFDD compendium workshop series. https://everylifefoundation.org/wp-content/uploads/2022/01/Guide-to-Patient-Involvement-FINAL-COMPLETE-GUIDE-Rev.pdf.
- 47.Nicod E, Lloyd AJ, Morel T, et al. Improving interpretation of evidence relating to quality of life in health technology assessments of rare disease treatments. Patient. 2023;16:7–17. doi: 10.1007/s40271-022-00598-4. [DOI] [PubMed] [Google Scholar]
- 48.Canada’s Drug and Health Technology Agency (CADTH). Ahead of the curve: shaping future-ready health systems 2022–2025 strategic plan. https://strategicplan.cadth.ca/
- 49.Silva A, Facey K, Bryan S, et al. A framework for action to improve patient and public involvement in health technology assessment. Int J Technol Assess Health Care. 2022;38:E8. doi: 10.1017/S0266462321000647. [DOI] [PubMed] [Google Scholar]
- 50.Tanemura N, Sasaki T, Sato J, et al. Real world survey of patient engagement status in clinical research: the first input from Japan. Patient. 2020;13:623–632. doi: 10.1007/s40271-020-00436-5. [DOI] [PubMed] [Google Scholar]
- 51.Japan Agency for Medical Research and Development. Patient and public involvement (PPI) guide book, 2022 (in English). https://www.amed.go.jp/content/000097557.pdf.
- 52.Patient Engagement Synapse. The road to treating chronic active Epstein-Barr virus infection in collaboration with citizens. https://patientengagement.synapseconnect.org/resources/the-road-to-treating-chronic-active-epstein-barr-virus-infection-in-collaboration-with-citizens?_gl=1%2A168cksl%2A_ga%2AMTMxOTY4MzEzNi4xNjU3MDE2OTAw%2A_ga_8DS3Y1MR34%2AMTY1NzEyMTc0OC41LjEuMTY1NzEyMTc2MS4w.
- 53.Japan Agency for Medical Research and Development. Patient and public involvement (PPI) guide book, 2019 (in Japanese). https://www.amed.go.jp/ppi/guidebook.html.
- 54.Ministry of Health Labour and Welfare. Third-term basic plan to promote cancer control programs, March 2018. https://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/0000196975.pdf.
- 55.Nagashima F, Furuse J. Treatments for elderly cancer patients and reforms to social security systems in Japan. Int J Clin Oncol. 2022;27:310–315. doi: 10.1007/s10147-021-02099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Center for Drug Evaluation (CDE). Summary of PRO guidance. https://www.signanthealth.com/blog/ecoa/chinas-center-for-drug-evaluation-cde-draft-pro-guidance/.
- 57.Agency for Care and Effectiveness (ACE). Consumer engagement and education. https://www.ace-hta.gov.sg/docs/default-source/educational-resources/helping-patients-become-involved-in-healthcare-decision-making-(21-feb-2022).pdf.
- 58.Agency for Care and Effectiveness (ACE). Event report: the future of patient and public involvement in health technology in Singapore; 18 November 2021. https://www.duke-nus.edu.sg/docs/librariesprovider5/reports/2021_r001_core_the-future-of-patient-and-public-involvement-in-hta-in-singapore56ecfafbbf654a32ba928a740dbd0fde.pdf?sfvrsn=e35d2e93_0.
- 59.African Medicines Agency. https://www.nepad.org/microsite/african-medicines-agency-ama.
- 60.Sidibé M, Dieng A, Buse K. Advance the African Medicines Agency to benefit health and economic development. BMJ. 2023;380:386. doi: 10.1136/bmj.p386. [DOI] [PubMed] [Google Scholar]
- 61.Campbell B, Sedrakyan A. Patient involvement in regulation: an unvalued imperative. Lancet. 2021;397:2147–2148. doi: 10.1016/S0140-6736(21)00977-6. [DOI] [PubMed] [Google Scholar]
- 62.Wale JL, Sullivan M. Exploration of the visibility of patient input in final recommendation documentation for three health technology assessment bodies. Int J Technol Assess Health Care. 2020;36:197–203. doi: 10.1017/S0266462320000240. [DOI] [PubMed] [Google Scholar]
- 63.Murphy A, Bere N, Vamvakas S, et al. The added value of patient engagement in early dialogue at EMA: scientific advice as a case study. Front Med (Lausanne) 2022;8:811855. doi: 10.3389/fmed.2021.811855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.European Medicines Agency. Stakeholder engagement biennial report 2021–2021. https://www.ema.europa.eu/en/documents/report/stakeholder-engagement-report-2020-2021_en.pdf.
- 65.Canadian Agency for Drugs and Technologies in Health (CADTH). https://www.cadth.ca/sites/default/files/Drug_Review_Process/patient_input_guidance.pdf. [PubMed]
- 66.Scottish Medicines Consortium. https://www.scottishmedicines.org.uk/media/2771/guidance-on-summary-information-for-patient-groups.pdf.
- 67.Schultz-Knudsen K, Sabaliauskaite U, Hellsten J, et al. New drug and biologics approvals in 2019: a systematic analysis of patient experience data in FDA drug approval packages and product labels. Ther Innov Regul Sci. 2021;55:503–513. doi: 10.1007/s43441-020-00244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Santana MJ, Ahmed S, Lorenzetti D, et al. Measuring patient-centred system performance: a scoping review of patient-centred care quality indicators. BMJ Open. 2019;9:e023596. doi: 10.1136/bmjopen-2018-023596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Santana MJ, Manalili K, Jolley RJ, et al. How to practice person-centred care: a conceptual framework. Health Expect. 2018;21:429–440. doi: 10.1111/hex.12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gregg A, Getz N, Benger J, et al. A novel collaborative approach to building better clinical trials: new insights from a patient engagement workshop to propel patient-centricity forward. Ther Innov Regul Sci. 2020;54:485–491. doi: 10.1007/s43441-019-00080-8. [DOI] [PubMed] [Google Scholar]
- 71.Sandbæk A, Møller MCR, Bro F, et al. Involving patients in medicines optimisation in general practice: a development study of the "PREparing patients for active involvement in medication review" (PREPAIR) tool. BMC Prim Care. 2022;23:122. doi: 10.1186/s12875-022-01733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deane K, Delbecque L, Gorbenko O, et al. Co-creation of patient engagement quality guidance for medicines development: an international multistakeholder initiative. BMJ Innov. 2019;5:43–55. doi: 10.1136/bmjinnov-2018-000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.FDA. FDA-led patient-focused drug development (PFDD) public meetings. https://www.fda.gov/industry/prescription-drug-user-fee-amendments/fda-led-patient-focused-drug-development-pfdd-public-meetings.
- 74.Patient Engagement Open Forum. The fusion of patient engagement & patient experience data: strengthening global focus and stakeholder convergence, 12 April 2022. https://patientengagement.synapseconnect.org/events/the-fusion-of-patient-engagement-patient-experience-data-strengthening-global-focus-and-stakeholder-convergence.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable. All information is available in the references cited.