Abstract
Medication plays a central role in the treatment of panic disorder, with the goal of eliminating panic attacks and restoring normal function (i.e., achieving full remission). Four drug classes have similar efficacy (tricyclic antidepressants, selective serotonin reuptake inhibitors [SSRIs], benzodiazepines, and monoamine oxidase inhibitors). Nonetheless, benzodiazepines remain the most prescribed medication for panic disorder in the United States. The high-potency benzodiaze-pines alprazolam (available as immediate- and extended-release tablets) and clonazepam (available as tablets and orally disintegrating wafers) are the only benzodiazepines approved by the U.S. Food and Drug Administration for the treatment of panic disorder. High-potency benzodiaze-pines, with their proven efficacy in panic disorder exerted through control of the central nervous system excitability by a selective and potent enhancement of inhibitory γ-aminobutyric acid–mediated neurotransmission, are also a safe and well-tolerated option for potentiation of rapid treatment response when initiating treatment with SSRIs. Judicious use of high-potency benzodiazepines followed by a cautious taper and discontinuation may optimize the benefits and minimize any potential risk associated with this class of drugs.
Panic disorder is a disabling psychiatric condition with a 3.4% prevalence in the general population in the United States.1 Strong lifetime and current comorbidity exists between panic disorder and major depressive disorder, which is associated with greater symptom severity, persistence, role impairment, suicidality, and help seeking.2 The lifetime prevalence in a general practice patient population is at least twice as high compared with the general population, with over half of the subjects with panic disorder having at least 1 additional psychiatric diagnosis.3
NEUROBIOLOGY OF PANIC DISORDER
It is currently hypothesized that patients with panic disorder inherit an especially sensitive fear mechanism involving several brain structures (e.g., the central nucleus of the amygdala, hippocampus, thalamus, and hypothalamus, as well as some brain stem sites).4 Both heritable factors and stressful life events, particularly in early childhood, appear to be associated with the onset of panic disorder.5 Under normal conditions, the central nucleus of the amygdala serves as a relay for sensory information between the higher cortical centers and the brain stem nuclei. In patients with panic disorder and other anxiety disorders, the central nucleus of the amygdala also receives additional information from the higher cortical centers, which represent cortical processing of the initial sensory information.6 Abnormalities in this cognitive processing could lead to the misinterpretation of sensory information (bodily cues) known to be a hallmark of panic disorder.7 It is thus speculated that there is a deficit in the relay and coordination of sensory information originating from the cortex and brain stem, which results in heightened amygdalar activity, with resulting behavioral, autonomic, and neuroendocrine activation typical of the panic attack.4
Gamma-aminobutyric acid (GABA) is quantitatively the most important inhibitory neurotransmitter in the central nervous system (CNS), with approximately one third of all CNS neurons thought to be GABAergic.7 GABAergic neurons are distributed in all regions of the brain and in the spinal cord, but do not exist outside of the CNS.7 The attenuation of the GABAergic system results in arousal, anxiety, restlessness, insomnia, and exaggerated activity, while an overactive GABAergic system is associated with sedation, ataxia, and amnesia. It is thought that GABA controls the excitability in all brain areas by balancing out the excitatory inputs and inhibitory GABAergic activity.
Alterations in the GABA system have been linked with the pathophysiology of anxiety disorders.8 Changes in neurotransmitters other than GABA, e.g., increases and decreases of serotonin, have also been implicated in the pathogenesis of panic.6 GABA is known to act on 3 GABA receptor subtypes: GABAA, GABAB, and GABAC. GABAA is a known target for a number of pharmacologic agents, including benzodiazepines, all of which act as modulators of the GABA-mediated inhibition of neuronal overexcitability.8 It has been shown that patients with panic disorder have reduced benzodiazepine binding in various brain regions,9 and some studies show these patients have lower brain levels of GABA than do healthy controls.10 A GABAA-benzodiazepine receptor comprises 5 protein subunits (α1–5) arranged like a rosette around a central channel, crossing the cell membrane, which is permeable to sodium and other anions. While current benzodiazepines are not subunit specific, receptors with the α2 subunit, mostly present in the limbic area, are thought to mediate the anxiolytic effects of benzodiazepines.11
RECOMMENDED TREATMENT OPTIONS FOR PANIC DISORDER
Current treatment guidelines for the treatment of panic disorder, with or without agoraphobia,12 single out 4 drug classes with proven and roughly comparable efficacy: tricyclic antidepressants (TCAs), selective serotonin reup-take inhibitors (SSRIs), monoamine oxidase inhibitors, and benzodiazepines. The guidelines recommend that the decision about which medication to choose for panic disorder should involve considerations of adverse side effects and cost. A similar approach is advocated for primary care patients.13
Tricyclic Antidepressants
Although TCAs show roughly the same efficacy in panic disorder in terms of reduction of the frequency and number of panic attacks14 as do other recommended pharmacologic treatment options, their use as monotherapy is limited due to an unfavorable side effect profile.15 Because of their antidepressant efficacy, they may be useful in patients with comorbid depression.15
Selective Serotonin Reuptake Inhibitors
Paroxetine and sertraline are approved by the U.S. Food and Drug Administration for the treatment of panic disorder, with abundant evidence supporting their short-and long-term efficacy. However, even with a small initial dosage and slow titration some patients are unable to tolerate the initial anxiogenesis associated with initiation of SSRI therapy and delayed onset of efficacy.16
Benzodiazepines
Benzodiazepines remain the mainstay of pharmacotherapy for panic disorder due to rapid onset of action, favorable tolerability, and high patient acceptance.17 In the United States, over half of the benzodiazepines for anxiety disorders (55%) are prescribed by primary care physicians, followed by psychiatrists and physicians in other specialties (18% and 27%, respectively).18 In addition, recently published data19 show that over the past decade benzodiazepines remained the most prescribed medication for panic disorder.
Benzodiazepines act on GABA receptors in a unique way, by binding to the receptor complex to increase the efficiency of GABA, i.e., lowering the quantity of GABA needed to open the channel. Opening of the channel allows sodium flux to hyperpolarize the neuron and, consequently, enables GABAergic neurons to produce a larger inhibitory effect.7 The anatomical distribution of the GABAergic system throughout the CNS and the mechanism of action of benzodiazepines may explain the rapid alleviation of anxiety symptoms occurring after their administration.
The high-potency benzodiazepines alprazolam (available as immediate-release [IR] and extended-release [XR] tablets) and clonazepam (available as tablets and orally disintegrating wafers) are the only benzodiazepines indicated for the treatment of panic disorder in the United States. Alprazolam is the most frequently prescribed psychotropic drug in the United States.18 Other high-potency benzodiazepines such as bromazepam, lorazepam, and triazolam are sometimes used in the treatment of panic disorder, while low- and medium-potency benzodiazepines are not recognized as efficacious for this indication.17 However, use of benzodiazepines is associated with risks for development of dependence or experience of rebound, recurrent, or new withdrawal symptoms upon treatment cessation.17
Psychotherapy
There is consistent evidence that cognitive-behavioral therapy (CBT) is an effective treatment for panic disorder.20 This option should be considered particularly for patients who prefer nonpharmacologic therapy or have refractory symptoms, strong contribution of behavioral patterns, or comorbid conditions.15 CBT can also be effective as adjunctive therapy to psychopharmacotherapy to increase patients' long-term functioning.20
This article reviews the pharmacokinetics, efficacy, and safety of the high-potency benzodiazepines alprazolam and clonazepam, as well as their role in the treatment of panic disorder.
HIGH-POTENCY BENZODIAZEPINES IN PANIC DISORDER
Pharmacokinetic Considerations
Although high-potency benzodiazepines are known to be efficacious and a safe treatment for panic disorder, the short duration of therapeutic effect of the previously available agents and rapid onset of therapeutic action, which may encourage repeated use, have created a negative perception about these compounds.21 The XR formulation of alprazolam was developed with the goal of maintaining efficacy while increasing the duration of therapeutic effect, enhancing compliance, and improving tolerability.22 Alprazolam XR has a time to maximum plasma concentrations (Tmax) of 4 to 12 hours, compared with 1 to 2 hours with the IR formulation. At the same time, the maximum plasma concentration (Cmax) is 50% lower than that of alprazolam IR, resulting in stable plasma concentrations lasting for several hours.23,24 Furthermore, the area under the curve and maximum concentration were similar for alprazolam XR 6 mg q.d. and alprazolam IR 1.5 mg q.i.d. (Figure 1).25
Figure 1.
Mean Steady-State Plasma Concentrations After the Administration of Alprazolam XR 6 mg q.d. and Alprazolam IR 1.5 mg q.i.d.a
The result of these characteristics of the XR formulation is a reduction in peaks and troughs in alprazolam plasma concentrations that in turn reduces the occurrence of side effects. The bioavailability and pharmacokinetics of alprazolam XR are similar to those of alprazolam IR tablets, with the exception of a prolonged absorption time.26
Clonazepam reaches peak plasma concentration at 1 to 4 hours after oral dosing, with an elimination half-life of 30 to 40 hours.27 Clonazepam pharmacokinetics are dose-independent throughout the dosing range. The orally disintegrating formulation of clonazepam enhances ease of administration without altering the drug's pharmacology.28
Efficacy and Tolerability of High-Potency Benzodiazepines in Panic Disorder
Alprazolam and clonazepam are approved for the treatment of panic disorder by the U.S. Food and Drug Administration and were found to be effective in double-blind, placebo-controlled studies.
Alprazolam has been studied extensively in the treatment of panic disorder, with 3 placebo-controlled studies of up to 10 weeks' duration.29–31 Alprazolam is the only high-potency benzodiazepine developed in an XR formulation.
The efficacy of alprazolam XR 1 to 10 mg using a flexible-dose study design once daily in the treatment of panic disorder was established in two 6-week placebo-controlled studies.23,32 The studies had similar designs, with a placebo run-in, 3-week titration, and 3-week maintenance period, and they achieved a mean daily dosage of less than 5 mg. The studies also included a complex discontinuation design to examine and compare various aspects of withdrawal-related symptoms. Two additional 8-week fixed-dose placebo-controlled studies24,33 involving final doses of 4 mg24 and 6 mg33 once daily did not show a benefit for either dose. These findings suggest the importance of flexible dosing to meet the patient's personal needs.
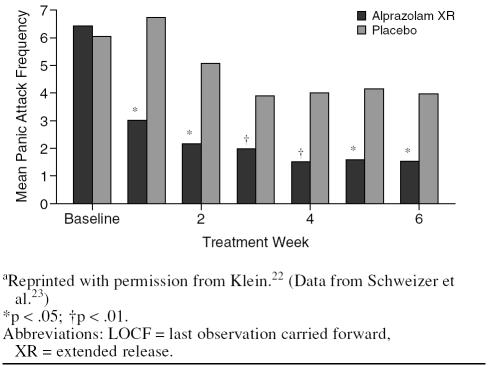
In the Pecknold et al. study,32 alprazolam XR and IR were equally efficacious and superior to placebo on the Hamilton Rating Scale for Anxiety phobia and work disability measures. In the Schweizer et al. study,23 the mean weekly frequency of panic attacks decreased significantly and abruptly from week 1 onward throughout the whole study period (Figure 2).
Figure 2.
Mean Frequency of Panic Attacks at Endpoint (LOCF) Analysis Treated With Alprazolam XR Compared With Placeboa
Sedation was the most frequently registered adverse event of alprazolam IR, alprazolam XR, and placebo in the placebo-controlled trials (57.0% with IR and 45.2% with XR vs. 22.6% with placebo), followed by somnolence/drowsiness (41.0% with IR and 23.0% with XR vs. 6.0% with placebo), memory impairment (18.0% with IR and 15.4% XR vs. 6.9% with placebo), fatigue/tiredness (18.4% with IR and 13.9% with XR vs. 9.2% with placebo), dysarthria (18.0% with IR and 10.9% with XR vs. 2.6% with placebo), mental impairment (13.7% with IR and 7.2% with XR vs. 5.7% with placebo), and ataxia (7.1% with XR vs. 3.2% with placebo).26
A crossover study compared alprazolam IR tablets (1 and 2 mg) with alprazolam XR (2 and 3 mg) and placebo in 14 healthy men with a history of sedative abuse.34 The subjects received the drugs in a random order, and assessments were made 30 minutes before administration of each drug and 30 minutes and 1, 3, 5, 7, 9, 12, and 24 hours after administration. Assessments included measures of psychomotor-cognitive performance (Digit Symbol Substitution Test), motor coordination (circular lights and balance), and memory (digit entry and recall). Compared with placebo, alprazolam XR 2 mg did not affect any measures of psychomotor and cognitive performance, motor coordination, and memory, while alprazolam IR 2 mg affected all measures. The 3-mg dose of alprazolam XR affected only 1 measure (motor coordination as assessed by a circular lights test).34
Clonazepam was found to be superior to placebo in 2 placebo-controlled studies.35,36 In a 9-week study,35 74% of patients treated with 1 mg/day of clonazepam (administered b.i.d. after up-titration during 3 days) and 56% of placebo-treated patients were completely free of panic attacks at the study endpoint. In another 6-week, flexible-dose comparison,36 62% of clonazepam-treated patients (mean dose = 2.3 mg/day) and 37% of placebo-treated patients were free of panic attacks at the study endpoint. The most frequent adverse events of clonazepam in the panic disorder placebo-controlled trials were somnolence (37% vs. 10% with placebo), depression (7% vs. 1% with placebo), abnormal coordination (6% vs. 0% with placebo) and ataxia (5% vs. 0% with placebo).
Discontinuation of High-Potency Benzodiazepine Therapy
About 40% to 80% of patients treated with benzodiazepines for ≥ 4 months experience a discontinuation or withdrawal syndrome, and discontinuation symptoms may appear even after relatively short administration of benzodiazepines.37 The benzodiazepine dose, half-life, and potency, as well as treatment duration, rate of benzodiazepine withdrawal, and severity of underlying anxiety disorder, were associated with the severity of withdrawal symptoms. In addition, a recently published study demonstrated that psychological and personality factors also play a role in the severity of withdrawal symptoms.38 However, because withdrawal symptoms of variable severity may appear in parallel with rebound symptoms of a panic disorder, usually peaking toward the end of the taper period, it usually takes 2 to 4 benzodiazepine-free weeks to discern the nature of the emerging symptoms.39 Thus, symptoms upon tapering or cessation of therapy may represent a discontinuation syndrome or reemergence of the anxiety disorder.
The recommended length of taper depends on the duration of benzodiazepine treatment, the drug's half-life, and the patient's willingness to discontinue benzodiazepines.39 Tapering should be gradual, with the first half of the taper taking much less time than the second half. The recommended length of the taper is up to 2 days after 2 weeks of benzodiazepine treatment, up to 2 weeks after 4 weeks of treatment, and 2 to 3 weeks after 8 weeks of treatment.39
Results of several studies suggest alprazolam XR may be associated with fewer difficulties during discontinuation than are other high-potency benzodiazepines. In a double-blind trial of patients with panic disorder, the occurrence of panic attacks was no greater following discontinuation of alprazolam XR than placebo.40 In another double-blind study in generalized anxiety disorder patients, more alprazolam XR–treated patients were able to discontinue medication without any difficulties compared with patients taking the short-acting benzodiazepine bromazepam (60% vs. 46%, respectively).41
The previously mentioned crossover study by Mumford et al.34 also compared the drug reinforcement effects of alprazolam IR tablets (1 and 2 mg) with alprazolam XR (2 and 3 mg) and placebo in 14 healthy men with a history of sedative abuse. Drug reinforcement effects were assessed by the Next Day Questionnaire, which assessed the overall drug experience of the previous day (i.e., strength, good and bad effects, liking, and pharmacologic class liking). A direct measure of drug reinforcement was obtained by completing a drug-versus-money version of Multiple-Choice Procedure. The forms contained 9 drug choices for the drug received the previous day and 9 monetary values arranged on an exponential scale. Alprazolam IR tablets were associated with significantly increased strength and sedating ratings compared with placebo. Although subject ratings of desire to take the drug again were higher for both alprazolam formulations compared with placebo, subjects' ratings of their willingness to pay for the drug were only increased for 2-mg alprazolam IR tablets. Furthermore, compared with placebo, neither dose of alprazolam XR increased the maximum value at which the subjects chose the drug over money. By contrast, both doses of alprazolam IR tablets significantly increased this value. These results indicate that the XR formulation of alprazolam may have less abuse liability than alprazolam IR tablets, but further data are needed concerning higher dosage amounts.
Patients with panic disorder appear to have greater difficulties withdrawing from benzodiazepines than do patients with generalized anxiety disorder, possibly due to the triggering of panic attacks by withdrawal symptoms and consequent relapse to the full clinical syndrome, in particular during too-aggressive down-tapering.42 Although alprazolam-treated patients who have achieved a reduction in their baseline symptoms at the end of the study period may show a slight increase in symptoms after a 4-week taper, this is followed by a further reduction in symptom scores at the drug-free follow-up.40,42,43
As withdrawal symptoms may also appear in some patients treated with alprazolam XR, these patients should be instructed not to stop medication abruptly or without supervision. Although there are no systematically collected data to support a specific discontinuation schedule with alprazolam XR, based on expert opinion, it is recommended to reduce the daily dosage by 0.5 mg every 3 days under supervision.26 However, some patients may require an even slower taper.
A period of taper with 0.25-mg dose decrements every 3 days was incorporated in both placebo-controlled studies of clonazapam.35,36 Although there was no evidence of rebound in comparison with baseline in either study, patients experienced an increased number of panic attacks compared with the last week of treatment. The most frequently reported adverse events were headache and insomnia. Based on the above results, a taper schedule based on 0.125-mg b.i.d. decrements every 3 days is recommended for clonazepam.27
An improved understanding of mechanisms of dependence, as well as the development of appropriate and clearer dosing and taper regimens, will undoubtedly help in optimizing the benefits and minimizing the unwanted effects of benzodiazepines.
Concomitant Use of Benzodiazepines and SSRIs
Because many patients with panic disorder are very sensitive to antidepressant medications at treatment initiation, it is recommended to begin with doses of approximately half the usual amount for panic disorder.18 The dose is then increased to a full therapeutic dose over subsequent days and as tolerated by the patient. However, because SSRI antidepressants recommended in the treatment of the panic disorder generally take 4 to 6 weeks to become effective for panic disorder, many patients become increasingly anxious during the first few weeks of treatment and stop taking the antidepressants before any beneficial effects are achieved. For this reason, it may be clinically appropriate to use a combination of high-potency benzodiazepines and SSRIs.12
Efficacy of the early coadministration of SSRIs and the high-potency benzodiazepine clonazepam was fully examined in 2 double-blind studies.44,45 In the Goddard et al. study,44 50 patients with DSM-IV panic disorder receiving open-label treatment with sertraline (target dose = 100 mg/day) for 12 weeks were randomly assigned to double-blind treatment with clonazepam 0.5 mg or placebo 3 times daily during the first 4 weeks of the trial. The clonazepam dose was then tapered over 3 weeks and discontinued. Dropout rates were similar in both groups (38% with placebo vs. 25% with clonazepam; p = .5). The combination treatment group had a significantly higher percentage of responders than the sertraline/placebo group at the end of week 1 (41% vs. 4%, respectively; p = .003) and week 3 (63% vs. 32%; p = .05), but not at other assessment points. These data indicate that rapid stabilization of panic symptoms occurs with a combination of sertraline and a high-potency benzodiazepine and supports the clinical utility of such a regimen.
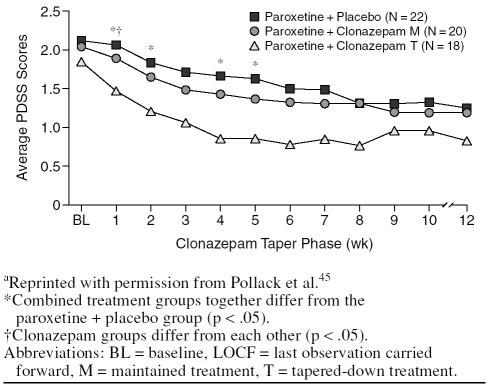
Similar results were obtained in the study comparing a combination of paroxetine and clonazepam followed by a tapered benzodiazepine discontinuation phase, ongoing combination treatment, and paroxetine and placebo in 60 patients with panic disorder.45 Dropout rates were higher in the paroxetine + placebo group (50%) than in the maintained combination treatment (45%) or tapered treatment (33%) groups. A significant advantage for both combination therapy groups was found early in the treatment (Figure 3).
Figure 3.
Change in Panic Disorder Severity Scale (PDSS) Score During 12 Weeks of Treatment by Treatment Group (LOCF)a
In both studies,44,45 coadministration of high-potency benzodiazepines was associated with fewer dropouts and a more rapid response than were SSRIs alone. The treatment gains achieved during the coadministration of benzodiazepines remained present after benzodiazepine dose taper. These results suggest that high-potency benzodiazepines may play a significant role in the early phases of the psychopharmacologic treatment of panic disorder by potentiation of response early on in treatment, which is maintained after benzodiazepine taper and discontinuation.
THE ROLE OF HIGH-POTENCY BENZODIAZEPINES IN THE TREATMENT OF PANIC DISORDER
The currently recommended treatment strategies for panic disorder are based on a substantial body of evidence demonstrating its neurobiological basis combined with hereditary and environmental factors.5,6
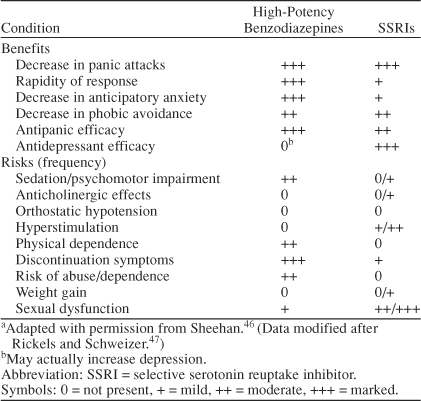
Currently advised comprehensive treatment strategies are based on the use of medication, CBT, psychosocial therapies, and patient education. Medication has a central role through controlling the biological basis of the panic disorder, i.e., elimination of panic attacks through stabilization of GABAergic and serotonergic neurotransmitter systems, thereby reducing the magnitude of phobic avoidance to a level that can be treated by CBT. Thus, high-potency benzodiazepines appear to be effective in enhancing the early treatment of patients with panic disorder. An overview of the comparative risks and benefits of high-potency benzodiazepines and SSRIs in panic disorder is given in Table 1.
Table 1.
Comparative Risks and Benefits of High-Potency Benzodiazepines and SSRIs in Panic Disordera
CONCLUSION
The potential of benzodiazepines to cause dependence and withdrawal after chronic use has led to a control of their use in many countries. However, this control has resulted in a significant number of patients being denied a therapeutic option that could be appropriate and effective.47 Recently, there has been an effort to reassess the place of this class of drugs and provide a more balanced view of their relative risks and benefits.48 The recent data show that high-potency benzodiazepines, with their proven efficacy in panic disorder exerted through control of excitability of the CNS by a selective and potent enhancement of inhibitory GABAergic neurotransmission, appear to be a safe and well-tolerated option for potentiation of initial treatment response when used in combination with SSRIs.
In conclusion, high-potency benzodiazepines continue to play an important role in the treatment of panic disorder. Judicious use of appropriate formulations during initial treatment with SSRIs, followed by a cautious taper and discontinuation, may optimize the benefits and minimize any potential risk associated with this class of drugs.
Drug names: alprazolam (Xanax and others), clonazepam (Klonopin and others), lorazepam (Ativan and others), paroxetine (Paxil and others), sertraline (Zoloft), triazolam (Halcion and others).
Footnotes
Dr. Susman has been a consultant for, received honoraria from, or conducted clinical research supported by Pfizer, Wyeth-Ayerst, Organon, and Forest.
REFERENCES
- Eaton WW, Kessler RC, and Wittchen HU. et al. Panic and panic disorder in the United States. Am J Psychiatry. 1994 151:413–420. [DOI] [PubMed] [Google Scholar]
- Roy-Byrne PP, Stang P, and Wittchen HU. et al. Lifetime panic-depression comorbidity in the National Comorbidity Survey: association with symptoms, impairment, course and help-seeking. Br J Psychiatry. 2000 176:229–235. [DOI] [PubMed] [Google Scholar]
- Birchall H, Brandon S, Taub N. Panic in a general practice population: prevalence, psychiatric comorbidity and associated disability. Soc Psychiatry Psychiatr Epidemiol. 2000;35:235–241. doi: 10.1007/s001270050233. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Kent JM, and Sullivan GM. et al. Neuroanatomical hypothesis of panic disorder, revised. Am J Psychiatry. 2000 157:493–505. [DOI] [PubMed] [Google Scholar]
- Horesh N, Amir M, and Kedem P. et al. Life events in childhood, adolescence and adulthood and the relationship to panic disorder. Acta Psychiatr Scand. 1997 96:373–378. [DOI] [PubMed] [Google Scholar]
- Nutt D, Feeney A, and Argyropoulos S. Anxiety Disorders Comorbid With Depression: Panic Disorder and Agoraphobia. London, UK: Martin Dunitz Ltd. 2002 [Google Scholar]
- Nutt DJ, Malizia AL. New insights into the role of the GABA(A)-benzodiazepine receptor in psychiatric disorder. Br J Psychiatry. 2001;179:390–396. doi: 10.1192/bjp.179.5.390. [DOI] [PubMed] [Google Scholar]
- Lydiard RB. The role of GABA in anxiety disorder. J Clin Psychiatry. 2003 64suppl 3. 21–27. [PubMed] [Google Scholar]
- Malizia AL, Cunningham VJ, and Bell CJ. et al. Decreased brain GABA(A)-benzodiazepine receptor binding in panic disorder: preliminary results from a quantitative PET study. Arch Gen Psychiatry. 1998 55:715–720. [DOI] [PubMed] [Google Scholar]
- Goddard AW, Mason GF, and Almai A. et al. Reduction in occipital cortex GABA levels in panic disorder detected with 1H-magnetic resonance spectroscopy. Arch Gen Psychiatry. 2001 58:556–561. [DOI] [PubMed] [Google Scholar]
- Rudolf U, Crestani F, and Benke D. et al. Benzodiazepine actions mediated by specific gamma-aminobutyric acidA receptor subtypes. Nature. 1999 401:796–800. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Practice Guideline for the Treatment of Patients With Panic Disorder. Am J Psychiatry. 1998 155suppl 5. 1–34. [PubMed] [Google Scholar]
- Saeed SA, Bruce TJ. Panic disorder: effective treatment options. Am Fam Physician. 1998;57:2405–2412. [PubMed] [Google Scholar]
- Rickels K, Schweizer E. Panic disorder: long-term pharmacotherapy and discontinuation. J Clin Psychopharmacol. 1998 18suppl 2. 12S–18S. [DOI] [PubMed] [Google Scholar]
- Culpepper L. Identifying and treating panic disorder in primary care. J Clin Psychiatry. 2004 65suppl 5. 19–23. [PubMed] [Google Scholar]
- Doyle A, Pollack MH. Long-term management of panic disorder. J Clin Psychiatry. 2004 65suppl 5. 24–28. [PubMed] [Google Scholar]
- Chouinard G. Issues in the clinical use of benzodiazepines: potency, withdrawal, and rebound. J Clin Psychiatry. 2004 65suppl 5. 7–12. [PubMed] [Google Scholar]
- IMS Health. National Prescription Audit. NAT. Available at http://www.imshealth.com. Accessed August 2003. [Google Scholar]
- Bruce SE, Vasile RG, and Goisman RM. et al. Are benzodiazepines still the medication of choice for patients with panic disorder with or without agoraphobia? Am J Psychiatry. 2003 160:1432–1438. [DOI] [PubMed] [Google Scholar]
- Otto MW, Smits JA, and Reese HE. Cognitive-behavioral therapy for the treatment of anxiety disorders. J Clin Psychiatry. 2004 65suppl 5. 34–41. [PubMed] [Google Scholar]
- Uhlenhuth EH, DeWit H, and Balter MB. et al. Risks and benefits of long-term benzodiazepine use. J Clin Psychopharmacol. 1988 8:161–167. [PubMed] [Google Scholar]
- Klein E. The role of extended-release benzodiazepines in the treatment of anxiety: a risk-benefit evaluation with a focus on extended-release alprazolam. J Clin Psychiatry. 2002 63suppl 14. 27–33. [PubMed] [Google Scholar]
- Schweizer E, Patterson W, and Rickels K. et al. Double-blind, placebo-controlled study of a once-a-day, sustained-release preparation of alprazolam for the treatment of panic disorder. Am J Psychiatry. 1993 150:1210–1215. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Alprazolam XR: dosage considerations. Psychiatr Ann. 1993 23suppl 10. 27–31. [Google Scholar]
- Wright CE. Clinical pharmacokinetics of alprazolam extended release: a summary. Curr Ther Res. 1995;56:947–956. [Google Scholar]
- Xanax XR. [package insert]. New York, NY: USPI, Pfizer Inc. 2003. [Google Scholar]
- Klonopin Tablets and Wafers [package insert]. Basel, Switzerland: USPI, Roche. 2001. [Google Scholar]
- Moroz G. High-potency benzodiazepines: recent clinical results. J Clin Psychiatry. 2004 65suppl 5. 13–18. [PubMed] [Google Scholar]
- Chouinard G, Annable L, and Fontaine R. et al. Alprazolam in the treatment of generalized anxiety and panic disorders: a double-blind placebo-controlled study. Psychopharmacology (Berl). 1982 77:229–233. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Burrows GD, and DuPont RL Jr. et al. Alprazolam in panic disorder and agoraphobia: results from a multicenter trial, 1: efficacy in short-term treatment. Arch Gen Psychiatry. 1988 45:413–422. [DOI] [PubMed] [Google Scholar]
- Munjack DJ, Crocker B, and Cabe D. et al. Alprazolam, propranolol, and placebo in the treatment of panic disorder and agoraphobia with panic attacks. J Clin Psychopharmacol. 1989 9:22–27. [PubMed] [Google Scholar]
- Pecknold J, Luthe L, and Munjack D. et al. A double-blind, placebo-controlled, multicenter study with alprazolam and extended-release alprazolam in the treatment of panic disorder. J Clin Psychopharmacol. 1994 14:314–321. [PubMed] [Google Scholar]
- Alexander PR. Alprazolam-XR in the treatment of panic disorder: results of a multicenter study. Psychiatr Ann. 1993 23suppl 10. 14–19. [Google Scholar]
- Mumford GK, Evans SM, and Fleishaker JC. et al. Alprazolam absorption kinetics affects abuse liability. Clin Pharmacol Ther. 1995 57:356–365. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JF, Moroz G, Bowden CL. Clonazepam in the treatment of panic disorder with or without agoraphobia: a dose-response study of efficacy, safety, and discontinuance. Clonazepam Panic Disorder Dose-Response Study Group. J Clin Psychopharmacol. 1997;17:390–400. doi: 10.1097/00004714-199710000-00008. [DOI] [PubMed] [Google Scholar]
- Tesar GE, Rosenbaum JF, and Pollack MH. et al. Double-blind, placebo-controlled comparison of clonazepam and alprazolam for panic disorder. J Clin Psychiatry. 1991 52:69–76. [PubMed] [Google Scholar]
- Rickels K, Rynn M. Pharmacology of generalized anxiety disorder. J Clin Psychiatry. 2002 63suppl 14. 9–16. [PubMed] [Google Scholar]
- Roy-Byrne P, Russo J, and Pollack M. et al. Personality and symptom sensitivity predictors of alprazolam withdrawal in panic disorder. Psychol Med. 2003 33:511–518. [DOI] [PubMed] [Google Scholar]
- Rickels K, DeMartinis N, and Rynn M. et al. Pharmacologic strategies for discontinuing benzodiazepines treatment. J Clin Psychopharmacol. 1999 19suppl 2. 12S–16S. [DOI] [PubMed] [Google Scholar]
- Pecknold J, Alexander PE, and Munjack D. Alprazolam XR in the management of anxiety: discontinuations. Psychiatr Ann. 1993 23(suppl). 38–44. [Google Scholar]
- Figueira ML. Alprazolam extended release in the management of anxiety. Curr Ther Res. 1995;56:957–965. [Google Scholar]
- Klein E, Colin V, and Stolk J. et al. Alprazolam withdrawal in patients with panic disorder and generalized anxiety disorder: vulnerability and effect of carbamazepine. Am J Psychiatry. 1994 151:1760–1766. [DOI] [PubMed] [Google Scholar]
- Klein E. Discontinuation of benzodiazepines in patients with anxiety disorder: a focus on alprazolam and alprazolam extended release. Curr Ther Res. 1995;56:969–974. [Google Scholar]
- Goddard AW, Brouette T, and Almai A. et al. Early coadministration of clonazepam with sertraline for panic disorder. Arch Gen Psychiatry. 2001 58:681–686. [DOI] [PubMed] [Google Scholar]
- Pollack MH, Simon NM, and Worthington JJ. et al. Combined paroxetine and clonazepam treatment strategies compared to paroxetine monotherapy for panic disorder. J Psychopharmacol. 2003 17:276–282. [DOI] [PubMed] [Google Scholar]
- Sheehan DV. The management of panic disorder. J Clin Psychiatry. 2002 63suppl 14. 17–21. [PubMed] [Google Scholar]
- Rickels K, Schweizer E. Panic disorder: long-term psychotherapy and discontinuation. J Clin Psychopharmacology. 1998 59suppl 8. 30–36. [DOI] [PubMed] [Google Scholar]
- Williams DD, McBride A. Benzodiazepines: time for reassessment. Br J Psychiatry. 1998;173:361–362. doi: 10.1192/bjp.173.5.361. [DOI] [PubMed] [Google Scholar]