Abstract
We here present a case report of a patient with Stage IV gastric cancer with peritoneal metastasis (P1, CY1) who underwent conversion surgery after a successful response to chemotherapy (S-1 + oxaliplatin + nivolumab). The patient was a woman in her 60 s. Her chief complaint was epigastric pain. Upper gastrointestinal endoscopy showed Type 4 advanced carcinoma on the lesser curvature of the gastric body. Biopsy showed Group 5 (poorly differentiated adenocarcinoma) and HER2 was negative. Staging laparoscopy revealed seeding in the round ligament of the liver (P1) and adenocarcinoma cells in ascites (CY1). Ten courses of chemotherapy (S-1 + oxaliplatin + nivolumab) were administered, after which contrast-enhanced computed tomography showed that the primary tumor had shrunk and seeding was no longer detectable. Upper gastrointestinal endoscopy revealed scar-like changes. A second staging laparoscopy revealed that ascites cytology was negative and a biopsy of the round ligament of the liver showed no malignant cells (P0, CY0). Conversion surgery comprising laparoscopic total gastrectomy with D2 lymph node dissection and resection of the round ligament of the liver was performed. The postoperative course was uneventful. Histopathological examination of the resected specimen revealed no tumor cells in the gastric mesentery or the round ligament of the liver. The pathological diagnosis was gastric cancer [M, U, L, Less, Ant, Post, type4, T3(SS), N0, M0 (H0, P0, CY0), ypStage IIA]. Adjuvant chemotherapy (S-1) was commenced. The patient is still alive 7 months later with no evidence of recurrence.
Keywords: Advanced gastric cancer, Conversion surgery, Immune checkpoint inhibitor, Oxaliplatin, Nivolumab, S-1
Introduction
Gastric cancer (GC) is the fifth most common type of cancer, being responsible for over one million new cases in 2020 and an estimated 769,000 deaths worldwide [1]. The majority of patients with GC present with unresectable or metastatic tumors and have a poor prognosis [2].
Until recently, platinum-based chemotherapy [oxaliplatin + S-1 (SOX)/S-1 + cisplatin] was the most frequently administered first-line therapy for HER2-negative unresectable advanced GC in Japan; however, the overall survival was poor [3–5]. The ATTRACTION-2 trial showed that immune checkpoint inhibitors (ICIs) are useful as third-line therapy for advanced GC [6]. Two randomized controlled trials (RCTs) (ATTRACTION-4, CheckMate 649) have recently reported the usefulness of ICIs as first-line agents [7, 8]. In these trials, nivolumab in combination with chemotherapy (capecitabine + oxaliplatin, folinic acid + fluorouracil + oxaliplatin, or SOX) was more effective than chemotherapy alone. Combination treatment is now a recommended first-line regimen in Japanese gastric cancer treatment guidelines [4].
Conversion surgery (CS) denotes the conversion of a chemotherapeutic treatment strategy to radical surgery [9–12]. Improvements in chemotherapy have given some patients with Stage IV GC the opportunity to undergo CS. Some studies have reported that patients with Stage IV GC who have undergone CS have better survival rates than those who continue on chemotherapy alone [13–15], suggesting that CS is a promising option that may be capable of curing Stage IV GC, although it is still contentious.
We here report a patient with Stage IV GC with peritoneal dissemination who responded well to S-1 + oxaliplatin + nivolumab and underwent CS.
Case presentation
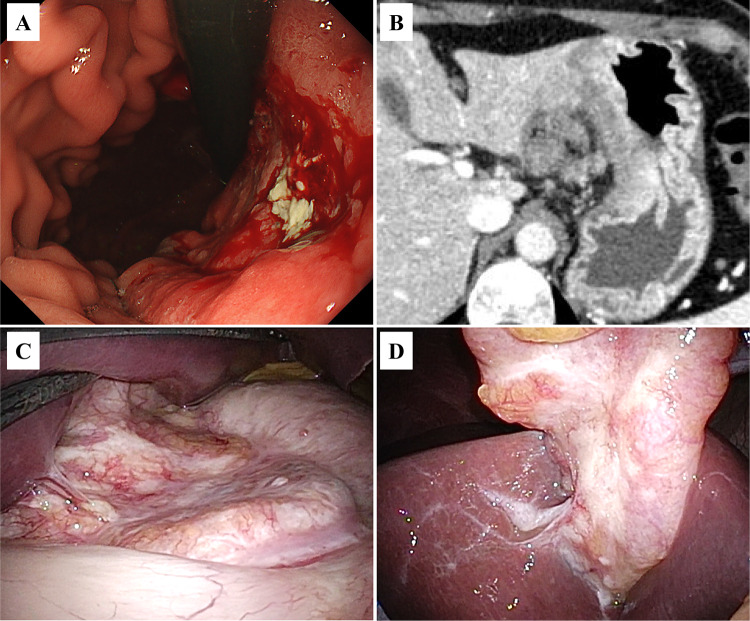
A woman in her 60 s presented with epigastric pain. Upper gastrointestinal endoscopy revealed a type 4 advanced GC extending to the lesser curvature of the gastric body (Fig. 1A). Biopsy showed Group 5 (poorly differentiated adenocarcinoma) and HER2 was negative. The patient was referred to our department for surgery. Laboratory data were as follows: leukocyte count, 4 × 103/μL; hemoglobin, 12.8 g/dL; C-reactive protein, < 0.05 mg/dL; carcinoembryonic antigen, 7.8 ng/mL; carbohydrate antigen 19-9, 137 U/mL; carbohydrate antigen 125, 63 U/mL; and cancer-related antigen 72-4, 31 U/mL. Contrast-enhanced computed tomography showed diffuse gastric wall thickening with a contrast effect on the lesser curvature of the gastric body and a 17 × 10 mm lymph node near the lesser curvature (Fig. 1B).
Fig. 1.
Prechemotherapy studies. A Upper gastrointestinal endoscopy image showing a Type 4 advanced gastric carcinoma extending to the lesser curvature of the gastric body. B Contrast-enhanced computed tomography image showing diffuse gastric wall thickening with contrast effect on the lesser curvature of the gastric body. C Staging laparoscopy photograph showing that the tumor is exposed to the serosa. D Photograph showing dissemination to the round ligament of the liver
Staging laparoscopy revealed that the tumor was mainly located in the gastric body and exposed on the serosa (Fig. 1C). It had disseminated to the round ligament of the liver (P1a) (Fig. 1D). Intraoperative washing cytology showed adenocarcinoma cells in the ascitic fluid (CY1). The patient was diagnosed with GC [M, U, L, Less, Ant, Post, Type 4, T4a, N3a, M1 (H0, P1a, CY1), cStageIV], in accordance with the 15th edition of the Japanese Classification of Gastric Carcinoma.
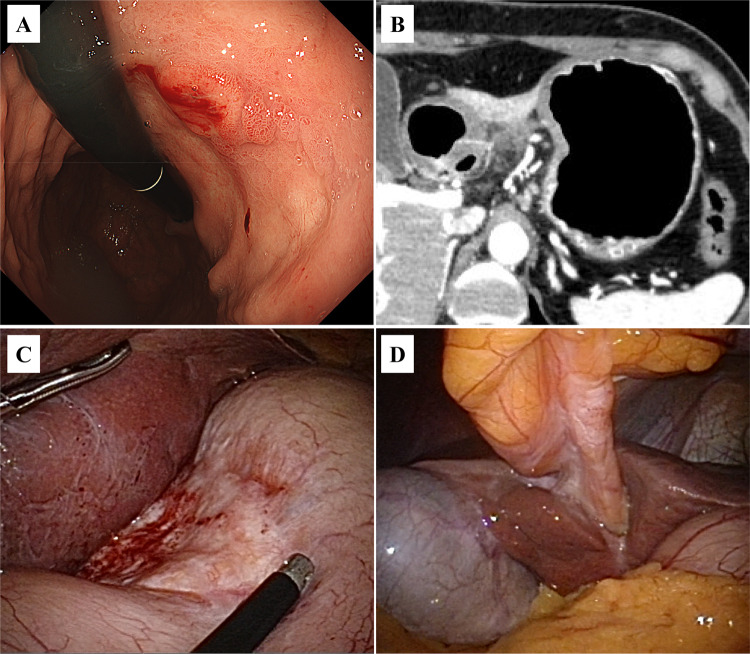
We administered 10 courses of chemotherapy (S-1 + oxaliplatin + nivolumab). The patient didn’t have any immune-related adverse events (irAEs). Tumor markers decreased as follows: CEA, 2.8 ng/mL; CA 19–9, 30 U/mL; CA125, 9 U/mL; and CA 72-4, 1.8 U/mL. Upper gastrointestinal endoscopy revealed scar-like changes and a 15 mm residual tumor on the lesser curvature of the gastric body (Fig. 2A). Computed tomography showed that the primary tumor had shrunk (Fig. 2B). Fluorodeoxyglucose-position emission tomography showed no abnormal accumulation in the primary tumor, lymph nodes, or round ligament of liver. A second staging laparoscopy revealed a whitish area on the lesser curvature of the gastric body (Fig. 2C), scar-like changes on the round ligament of the liver, and no evidence of dissemination in the peritoneal cavity (Fig. 2D). Intraoperative washing cytology was negative (CY0), as were two biopsies of the round ligament of the liver. Considering the above findings, the diagnosis after chemotherapy was GC (M, U, L, Less, Ant, Post, Type4, T4a, N1, M0 [H0, P0, CY0], ycStageIII), and CS was scheduled.
Fig. 2.
Post-chemotherapy studies. A Upper gastrointestinal endoscopy image revealing scar-like changes and a 15-mm residual tumor on the lesser curvature of the gastric body. B Computed tomography image showing that the primary tumor has shrunk. C Staging laparoscopy photograph showing a residual whitish area on the lesser curvature side of the stomach that is confined to the gastric mesentery. D Intraoperative photograph showing scar-like change in the round ligament of the liver and no evidence of dissemination in the peritoneal cavity
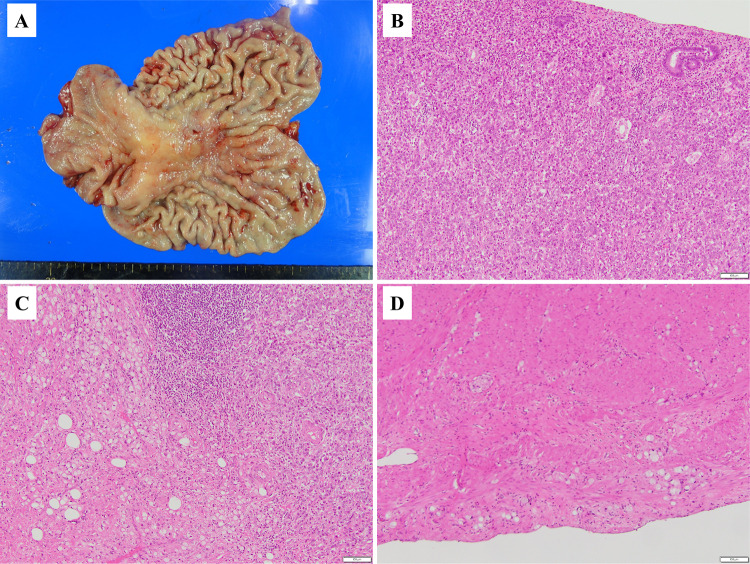
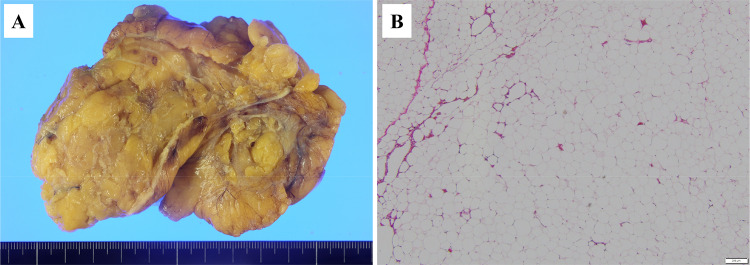
The patient underwent laparoscopic total gastrectomy with D2 lymph node dissection and resection of the round ligament of the liver. The operation time was 354 min and the blood loss 10 mL. The postoperative course was uneventful. Gross examination of the resected specimen showed that the primary tumor was mainly located on the lesser curvature of the gastric body (Fig. 3A). Pathological examination showed that the center of the tumor was composed of poorly differentiated adenocarcinoma (por1) (Fig. 3B) surrounded by signet ring cell carcinoma (sig) (Fig. 3C). The primary tumor was infiltrated by a small to moderate number of lymphocytes; however, there was little phagocytosis by neutrophils or macrophages. Small amounts of fibrosis were also evident (Fig. 3B, C). The depth of invasion was T3(SS) (Fig. 3D). The round ligament of the liver showed no seeding nodules (Fig. 4A). Pathological examination showed that the tumor was composed mainly of adipose tissues with some lymphocytes, but no malignant cells (Fig. 4B). Thus, the pathological diagnosis was GC [M, U, L, Less, Ant, Post, type4, T3(SS), N0, M0 (H0, P0, CY0), ypStageIIA]. The efficacy of chemotherapy was grade 1a. Our patient is currently undergoing postoperative adjuvant chemotherapy (S-1) and has survived 7 months with no evidence of recurrence.
Fig. 3.
Surgical specimen (stomach). A Photograph showing the tumor is mainly located on the lesser curvature of the gastric body. B Photomicrograph with H&E staining showing the center of the tumor is composed of poorly differentiated adenocarcinoma (por1). C Photomicrograph with H&E showing signet ring cell carcinoma (sig) surrounds the center of the tumor. D Photomicrograph with H&E showing the deepest part of the lesion extends close to the serosa
Fig. 4.
Surgical specimen (round ligament of the liver). A Photograph showing no visible seeding on the round ligament of the liver. B Photomicrograph with H&E showing abundant adipose tissues and some lymphocytes, but no tumor cells
Discussion
In recent years, cancer treatment, including for GC, has changed dramatically with the development of ICIs targeting immune checkpoints such as programmed death 1 (PD-1) and programmed death-ligand 1 (PD-L1). Nivolumab monotherapy is reportedly more effective than placebo or standard chemotherapy for GC, non-small cell lung cancer, renal cell carcinoma, and squamous cell carcinoma of the head and neck [6, 16–19].
The CheckMate 649 trial, a phase 3 RCT, evaluated first-line PD-1 inhibitor-based therapies (nivolumab + chemotherapy) for untreated, HER2-negative, unresectable advanced gastric cancer. In this study, nivolumab + chemotherapy achieved significantly better overall and progression-free survival than chemotherapy in patients with PD-L1 combined positive score ≥ 5. In addition, subgroup analysis suggested a benefit in patients with PD-L1 combined positive score ≥ 1 [7]. Another phase 2–3 RCT (ATTRACTION-4) found that nivolumab combined with oxaliplatin-based chemotherapy significantly improved progression-free survival in Asian patients with stage IV GC [8]. These findings have resulted in nivolumab in combination with chemotherapy becoming the first line regimen for stage IV GC [4]. In the current case, the patient received 10 courses of chemotherapy (S-1 + oxaliplatin + nivolumab). Fortunately, the patient didn’t have any irAEs. Since irAEs can occur even after completion of ICIs administration, it is necessary to monitor irAEs over the long term during follow-up.
Improvements in chemotherapy have enabled them to undergo CS. In patients with Stage IV GC, CS has been reported to achieve a better overall survival than is achieved in patients who do not undergo resection. In particular, the R0 group had a better prognosis than the R1/2 resection group [15, 20]. In addition, CS reportedly achieves a better prognosis in patients who have progressed to P0CY0 with chemotherapy than in patients who undergo chemotherapy alone [21]. The CONVO-GC-1 and AIO-FLOT5 trials are currently evaluating the efficacy of CS in Stage IV GC [22]. A new classification of Stage IV GC has been proposed to clarify the indications for CS [23]. In this classification, Categories 3 and 4 are defined as advanced GC with macroscopic peritoneal dissemination. Chemotherapy ± molecular targeted therapy, including ICI ± intraperitoneal chemotherapy, is recommended for patients with Category 3 and 4 disease. CS may be indicated in patients with partial or complete responses in whom it is considered that achieving CY0 is possible.
In our institution, the current indications for CS are as follows: (1) normalization of tumor markers; (2) achievement of a partial response by chemotherapy ± ICI; and (3) absence of factors indicating that the tumor is not resectable. The current patient was initially classified as having Category 4 disease. This is considered the most advanced of the Stage IV categories and was allocated because of metastasis to the round ligament of the liver and positive peritoneal washing cytology. After chemotherapy, her tumor markers were within the normal range, two biopsies of the round ligament of the liver were negative, and the primary tumor had shrunk. There being no indicators of unresectability, we decided to perform CS. Regarding the surgical approach, we performed laparoscopic total gastrectomy with D2 lymph node dissection because we have standardized the surgical procedure for minimally invasive surgery, including robotic and laparoscopic surgery, for advanced GC [24]. However, the safety and feasibility of using a minimally invasive approach for CS have not yet been established. The indications for CS, approach (open, laparoscopic, robotic), and extent of lymph node dissection are still controversial. In this case, we did not perform splenectomy and omentectomy. Omentectomy is often performed for advanced GC. However, the survival superiority of omentectomy has not been established even in now [25]. As for splenectomy, the tumor did not invade to the greater curvature and No.10 lymph nodes were not swelled in both prechemotherapeutic and post-chemotherapeutic state. In total gastrectomy for proximal gastric cancer that does not invade the greater curvature, splenectomy should be avoided as it increases operative morbidity without improving survival [26]. Furthermore, the benefits of omentectomy and splenectomy in conversion surgery were unclear. Therefore, we did not perform omentectomy and splenectomy.
Regarding adjuvant chemotherapy, S-1, capecitabine + oxaliplatin, and S-1 + docetaxel regimens are standard treatment regimens for Stage II or III GC [27–30]. However, no definitive evidence is available for postoperative chemotherapy after CS. Some studies have reported the benefit of postoperative chemotherapy for patients with Stage IV GC who have undergone resection [31, 32]. In our patient's case, although her ypStage was IIA, her disease had progressed prior to chemotherapy. We, therefore, decided to administer postoperative adjuvant chemotherapy with a single agent (S-1) for 1 year.
In conclusion, we here report successfully performing conversion gastrectomy using a minimally invasive approach for a patient with Stage IV GC in whom peritoneal dissemination and metastases had apparently been eradicated by S-1 + oxaliplatin + nivolumab. Our findings may justify CS for Stage IV GC after a good response to an ICI combined with chemotherapy.
Acknowledgements
We thank Dr Trish Reynolds, MBBS, FRACP, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Abbreviations
- CS
Conversion surgery
- GC
Gastric cancer
- ICI
Immune checkpoint inhibitor
- irAEs
Immune-related adverse events
- RCT
Randomized controlled trial
- PD-1
Programmed death 1
- PD-L1
Programmed death-ligand 1
- SOX
Oxaliplatin + S-1
Funding
None.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249 [DOI] [PubMed]
- 2.Field K, Michael M, Leong T. Locally advanced and metastatic gastric cancer: current management and new treatment developments. Drugs. 2008;68(3):299–317. doi: 10.2165/00003495-200868030-00004. [DOI] [PubMed] [Google Scholar]
- 3.Koizumi W, Takiuchi H, Yamada Y, Boku N, Fuse N, Muro K, Komatsu Y, Tsuburaya A. Phase II study of oxaliplatin plus S-1 as first-line treatment for advanced gastric cancer (G-SOX study) Ann Oncol. 2010;21(5):1001–1005. doi: 10.1093/annonc/mdp464. [DOI] [PubMed] [Google Scholar]
- 4.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2021 (6th edition) Gastric Cancer. 2023;26(1):1–25. doi: 10.1007/s10120-022-01331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9(3):215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 6.Kang Y-K, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 7.Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40. doi: 10.1016/S0140-6736(21)00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, Chung IJ, Yamaguchi K, Kato K, Sym SJ, Kadowaki S, Tsuji K, Chen JS, Bai LY, Oh SY, Choda Y, Yasui H, Takeuchi K, Hirashima Y, Hagihara S, Boku N. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-esophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(2):234–247. doi: 10.1016/S1470-2045(21)00692-6. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida K, Yamaguchi K, Okumura N, Osada S, Takahashi T, Tanaka Y, et al. The roles of surgical oncologists in the new era—minimally invasive surgery for early gastric cancer and adjuvant surgery for metastatic gastric cancer. Pathobiology. 2011;78:343–352. doi: 10.1159/000328197. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki T, Tanabe K, Taomoto J, Yamamoto H, Tokumoto N, Yoshida K, et al. Preliminary trial of adjuvant surgery for advanced gastric cancer. Oncol Lett. 2010;1:743–747. doi: 10.3892/ol_00000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oshiro Y, Takahashi K, Sasaki R, Kondo T, Sakashita S, Ohkohchi N. Adjuvant surgery for advanced extrahepatic cholangiocarcinoma. World J Gastroenterol. 2013;19:6934–6938. doi: 10.3748/wjg.v19.i40.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones RP, Hamann S, Malik HZ, Fenwick SW, Poston GJ, Folprecht G. Defined criteria for resectability improves rates of secondary resection after systemic therapy for liver limited metastatic colorectal cancer. Eur J Cancer. 2014;50:1590–1601. doi: 10.1016/j.ejca.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 13.Ito S, Oki E, Nakashima Y, Ando K, Hiyoshi Y, Ohgaki K, Saeki H, Morita M, Sakaguchi Y, Maehara Y. Clinical significance of adjuvant surgery following chemotherapy for patients with initially unresectable stage IV gastric cancer. Anticancer Res. 2015;35(1):401–406. [PubMed] [Google Scholar]
- 14.Arigami T, Matsushita D, Okubo K, Sasaki K, Noda M, Kita Y, Mori S, Kurahara H, Yanagita S, Uenosono Y, Ishigami S, Ohtsuka T, Natsugoe S. Clinical significance of conversion surgery for gastric cancer with peritoneal dissemination: a retrospective study. Oncology. 2020;98(11):798–806. doi: 10.1159/000509530. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi K, Yoshida K, Tanahashi T, Takahashi T, Matsuhashi N, Tanaka Y, Tanabe K, Ohdan H. The long-term survival of stage IV gastric cancer patients with conversion therapy. Gastric Cancer. 2018;21(2):315–323. doi: 10.1007/s10120-017-0738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du R, Hu P, Liu Q, Zhang J. Conversion surgery for unresectable advanced gastric cancer: a systematic review and meta-analysis. Cancer Invest. 2019;37(1):16–28. doi: 10.1080/07357907.2018.1551898. [DOI] [PubMed] [Google Scholar]
- 21.Molfino S, Ballarini Z, Gheza F, Portolani N, Baiocchi GL. Is there a role for treatment-oriented surgery in stage IV gastric cancer? A systematic review. Updates Surg. 2019;71(1):21–27. doi: 10.1007/s13304-018-0571-z. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida K, Yasufuku I, Terashima M, Young Rha S, Moon Bae J, Li G, Katai H, Watanabe M, Seto Y, Hoon Noh S, Kwang Yang H, Ji J, Baba H, Kitagawa Y, Morita S, Nishiyama M, Kodera Y, CONVO‐GC‐1 Study Group. Federation of Asian Clinical Oncology (FACO) International retrospective cohort study of conversion therapy for stage IV gastric cancer 1 (CONVO-GC-1) Ann Gastroenterol Surg. 2021;6(2):227–240. doi: 10.1002/ags3.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida K, Yamaguchi K, Okumura N, Tanahashi T, Kodera Y. Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer. 2016;19(2):329–338. doi: 10.1007/s10120-015-0575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishi M, Shimada M, Yoshikawa K, Takasu C, Wada Y, Tokunaga T, Nakao T, Kashihara H, Yoshimoto T, Yamashita S. Propensity score-matched analysis of the short- and long-term outcomes of robotic versus laparoscopic gastrectomy for gastric cancer. Ann Surg Oncol. 2022;29(6):3887–3895. doi: 10.1245/s10434-021-11203-7. [DOI] [PubMed] [Google Scholar]
- 25.Ri M, Nunobe S, Honda M, Akimoto E, Kinoshita T, Hori S, Aizawa M, Yabusaki H, Isobe Y, Kawakubo H, Abe T. Gastrectomy with or without omentectomy for cT3-4 gastric cancer: a multicentre cohort study. Br J Surg. 2020;107(12):1640–1647. doi: 10.1002/bjs.11702. [DOI] [PubMed] [Google Scholar]
- 26.Sano T, Sasako M, Mizusawa J, Yamamoto S, Katai H, Yoshikawa T, Nashimoto A, Ito S, Kaji M, Imamura H, Fukushima N, Fujitani K, Stomach Cancer Study Group of the Japan Clinical Oncology Group Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma. Ann Surg. 2017;265(2):277–283. doi: 10.1097/SLA.0000000000001814. [DOI] [PubMed] [Google Scholar]
- 27.Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, Higashino M, Yamamura Y, Kurita A, Arai K, ACTS-GC Group Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810–1820. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 28.Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29(33):4387–4393. doi: 10.1200/JCO.2011.36.5908. [DOI] [PubMed] [Google Scholar]
- 29.Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, Mok YJ, Kim YH, Ji J, Yeh TS, Button P, Sirzén F, Noh SH, CLASSIC Trial Investigators Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379(9813):315–321. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 30.Kakeji Y, Yoshida K, Kodera Y, Kochi M, Sano T, Ichikawa W, Lee SW, Shibahara K, Shikano T, Kataoka M, Ishiguro A, Ojima H, Sakai Y, Musha N, Takase T, Kimura T, Takeuchi M, Fujii M. Three-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 plus docetaxel versus S-1 alone in stage III gastric cancer: JACCRO GC-07. Gastric Cancer. 2022;25(1):188–196. doi: 10.1007/s10120-021-01224-2. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi T, Takashima A, Nagashima K, Makuuchi R, Aizawa M, Ohashi M, Tashiro K, Yamada T, Kinoshita T, Hata H, Kawachi Y, Kawabata R, Tsuji T, Hihara J, Sakamoto T, Fukagawa T, Katai H, Higuchi K, Boku N. Efficacy of postoperative chemotherapy after resection that leaves no macroscopically visible disease of gastric cancer with positive peritoneal lavage cytology (CY1) or localized peritoneum metastasis (P1a): a multicenter retrospective study. Ann Surg Oncol. 2020;27(1):284–292. doi: 10.1245/s10434-019-07697-x. [DOI] [PubMed] [Google Scholar]
- 32.Tiberio GA, Ministrini S, Gardini A, Marrelli D, Marchet A, Cipollari C, Graziosi L, Pedrazzani C, Baiocchi GL, La Barba G, Roviello F, Donini A, de Manzoni G. Factors influencing survival after hepatectomy for metastases from gastric cancer. Eur J Surg Oncol. 2016;42(8):1229–1235. doi: 10.1016/j.ejso.2016.03.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.