Abstract
Background/Objectives
Posterior staphyloma is a hallmark of high myopia and its presence associates to greater degrees of myopic maculopathy. Nonetheless, its development, repercussion on visual function and relationship with maculopathy components, is still unclear. The objective was to analyze the impact of posterior staphyloma on the incidence and severity of myopic maculopathy and its repercussion on visual prognosis.
Subjects/Methods
Cross-sectional study conducted on 473 consecutive eyes of 259 highly myopic patients examined at Puerta de Hierro-Majadahonda University Hospital (Madrid, Spain). All patients underwent complete ophthalmologic examination including best corrected visual acuity (BCVA), axial length (AL), myopic maculopathy classification according to ATN system (atrophic/traction/neovascularization), determined the presence of posterior staphyloma, pathologic myopia (PM) and severe PM. Multimodal imaging were performed including fundus photography, optical coherence tomography (OCT), OCT-angiography, fundus autofluorescence and/ or fluorescein angiography.
Results
Out of the total, 70.65% were female patients (n = 173/259), mean BCVA was 0.41 ± 0.54 logMAR units and mean AL was 29.3 ± 2.6 mm (26–37.6). Posterior staphyloma was present in 69.4% of eyes. Eyes with posterior staphyloma compared to non-staphyloma were older (p < 0.05), had greater AL (p < 0.01), worse BCVA (p < 0.01) and higher stage in ATN components (p < 0.01). Moreover, compound subgroup showed worse BCVA (p < 0.01) and greater stage in each of the ATN components (p < 0.01). Staphylomas with macular involvement presented worse BCVA (p < 0.01), higher AL (p < 0.01), and greater ATN (p < 0.05). The risk of posterior staphyloma presence in eyes with PM and severe PM eyes was 89.8% and 96.7%, respectively. Posterior staphyloma was the best predictor for BCVA in myopic patients (p < 0.01).
Conclusions
Posterior staphyloma’s presence determines high risk of myopic maculopathy and therefore worse visual prognosis, especially those with macular involvement. Posterior staphyloma represented the best predictor for BCVA in highly myopic patients.
Subject terms: Retinal diseases, Risk factors
Introduction
Posterior staphyloma (PS) is a progressive deformation of the posterior ocular wall where a marked change in curvature occurs in a delimited area and must be differentiated from simple scleral backward bowing.
PS is a hallmark of high myopia, nevertheless not pathognomonic as other pathologies such as retinosis pigmentosa, tilted-disc syndrome, osteogenesis imperfecta, oculocutaneous albinism [1–4] among others could also develop this condition. The clinical relevance of this “outpouching of a circumscribed posterior fundus region”, as Spaide defined [5], is associated with more severe myopic related disorders compared with non-staphyloma eyes [6–9].
Nowadays, there are several methods that could be used for the study of PS including stereoscopic fundus examination, ultrasound, optical coherence tomography (OCT), wide-field-OCT and 3D-magnetic resonance imaging (MRI). Curtin [10] was the first to propose a classification using binocular indirect ophthalmoscopy, classifying a total of 10 different primary and compound subtypes. Recently, through the development of different technologies, new classifications have been proposed. Using 3D-MRI, Moriyama et al. [10] analyzed pathologic myopia resulting in four different shapes of eyes: barrel, cylindric, nasally distorted and temporally distorted types. Moreover, Ohno-Matsui [8] classified PS in 6 subtypes based on 3D-MRI and wide-field fundus imaging. This latter classification is based on Curtin’s but renamed according to the distribution and location of the outpouching of the sclera.
Myopic maculopathy was defined by the presence of macular alterations induced by high myopia that leads to a significant decrease in BCVA [11–14]. Ruiz-Medrano et al. [14] developed the ATN classification system to grade myopic maculopathy based on atrophic, tractional and neovascular characteristics with high reproducibility proved [15, 16].
The presence of PS and/or an atrophic myopic maculopathy equal to or more severe than diffuse chorioretinal atrophy define pathologic myopia (PM), as described by Ohno-Matsui et al. [7, 17, 18], severe PM was defined according to ATN grading system [16] as follows: the presence of macular atrophy equal or more severe than patchy chorioretinal atrophy, tractional maculopathy equal or more severe than foveal detachment and/or myopic neovascular maculopathy in active, scar or atrophic stage. Furthermore, optimal cut-off points in terms of axial length (AL) for PM and severe PM have been defined in 28 and 29.5 mm, respectively, with good sensitivity and specificity rates [19].
The aim of the present study was to analyze the impact of posterior staphyloma on incidence and severity of myopic maculopathy and, in addition, the repercussion on patient’s visual prognosis.
Materials/Subjects and methods
This cross-sectional and non-interventional study of 473 consecutive high myopic eyes of 259 patients was carried out at a tertiary referral hospital for vitreoretinal pathology, Puerta de Hierro-Majadahonda University Hospital (Madrid, Spain). The study design adhered to the tenets of the Declaration of Helsinki for research involving humans and design was approved by the corresponding Institution’s Ethics Committee (PI 43/20). All patients signed an informed consent form and were identified through a records search with the study period extending from June 2021 to June 2022.
Inclusion criteria were: presence of high myopia (defined as axial length (AL) ≥ 26 mm), age ≥18 years and availability of good quality ocular images (>45 image quality score on DRI Triton Swept-Source (SS) OCT software [TOPCON Co., Japan]). Examinations were performed in both eyes independently, as long as both fulfilled the inclusion criteria.
Exclusion criteria were previous intraocular surgery (exception for refractive or cataract surgery), coexisting ocular or systemic diseases including optic nerve pathology, multifocal choroiditis, punctate inner choroidopathy, angioid streaks, uveitis, previous ocular trauma, diabetic retinopathy, retinal vein/artery occlusion, connectivopathies such us Marfan syndrome, or idiopathic macular hole.
Demographic data were obtained from the patients’ clinical records. All participants underwent complete ophthalmological examination including: best corrected visual acuity (BCVA) converted into logarithm of the minimum angle of resolution (logMAR), slit-lamp anterior segment examination, optical biometry (IOL Master 500, Carl Zeiss Meditec AG, Jena, Germany), intraocular pressure (Goldman applanation tonometry) and indirect ophthalmoscopy.
Multimodal imaging
Multimodal imaging included color/pseudocolour fundus photography using DRI-OCT Triton plus (Topcon Corporation, Japan), Zeiss Clarus TM 500 (Carl Zeiss Meditec AG, Jena, Germany) or Optos-Optomap Panoramic 200A system (Optos PLC, Dunfermline, United Kingdom). SS-OCT), OCT-angiography and fundus autofluorescence using DRI-OCT Triton-plus (Topcon Corporation, Japan). Structural OCT protocol included radial 12-mm scans centered at the fovea. Fluorescein angiography or OCT-angiography were performed if myopic choroidal neovascularization (CNV) was suspected.
Posterior staphyloma
The presence and type of posterior staphyloma were determined by indirect ophthalmoscopy, fundus photography and OCT by two blinded independent retinal specialists (I.F-M. and M.P.). In those eyes with non-coincident classification, a third blinded senior consultant retinal specialist (JM.R-M.) checked and classified the staphyloma type. Posterior staphylomas were classified according to two classifications: Curtin classification [20] and the recent classification defined by Ohno-Matsui [8]. Both classifications were compared and analyzed in the study. Different posterior staphyloma subtypes are shown in Fig. 1.
Curtin classified posterior staphyloma into 10 types [20]—five primary and five compound forms—based on their funduscopic appearance. Primary staphylomas were classified according to the area involved, as follows: posterior pole of the eye (Type I), macular area (Type II), peripapillary area (Type III), the area nasal to the disk (Type IV) and the area below the disk (Type V). The compound forms combined different posterior ectasias describing staphyloma combining Type I and Type II (Type VI), combination of Type I and Type III (Type VII), multiple steps across the wall of a primary Type I (Type VIII), deep Type I staphyloma associating vertical septum from upper to lower border of the staphyloma or to either side of optic nerve (Type IX), and, ultimately, deep Type I staphyloma divided into different compartments by thin plicae (Type X).
Ohno-Matsui classification differentiated into 6 types [8] of staphylomas renamed according to their location and distribution: the wide macular type, the narrow macular type, the peripapillary type, the nasal type, the inferior type, and others. The first 5 subtypes (wide macular, narrow, peripapillary, nasal and inferior type) coincide with the same subtypes corresponding to Curtin’s classification (from I to V, respectively) and is in the sixth and last subtype named “others” where all the compound forms of staphylomas are grouped.
Fig. 1. Color fundus photography showing different posterior staphyloma subtypes [20].
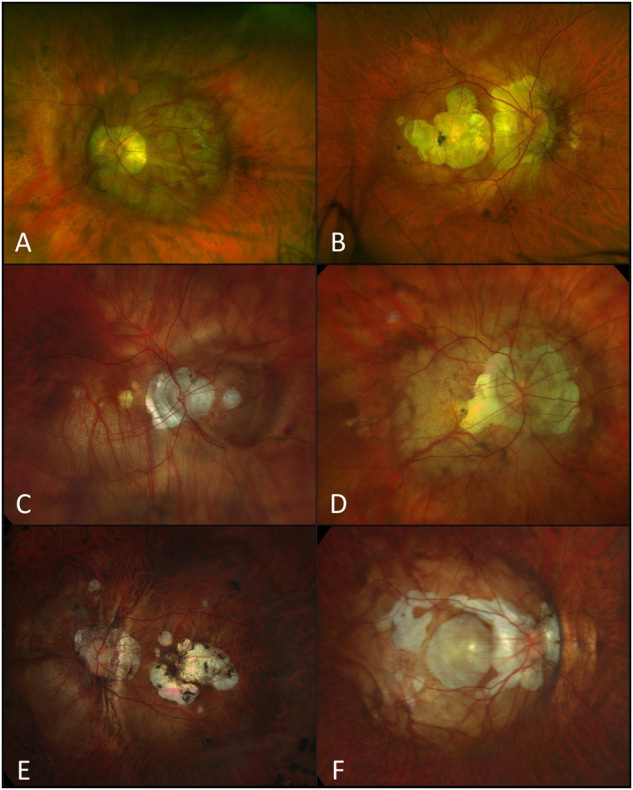
A Left eye wide macular posterior staphyloma (type I); B Right eye presenting a narrow macular posterior staphyloma (type II); C Right eye demonstrating the nasal type (type IV) posterior staphyloma; D Right eye combining peripapillary area and wide macular staphyloma (type VII/Others in Ohno-Matsui classification [8]); E Left eye posterior staphyloma associating a vertical septum from upper to lower border of the staphyloma (Type IX/Others in Ohno-Matsui classification [8]) and F Right eye compound posterior staphyloma type VIII (Others in Ohno-Matsui classification [8]) associating steps across the wall of a primary type I staphyloma.
Moreover, posterior staphylomas were also categorized according to macular region implication. Describing two groups (Curtin classification):
Posterior staphyloma with macular involvement: Type I, Type II, Type VI, Type VII, Type VIII, Type IX and Type X.
Posterior staphyloma without macular involvement: Type III, Type IV and Type V.
ATN grading system
The ATN grading system for myopic maculopathy was applied in all cases. Two retinal specialists (I.F-M. and M.P.) independently assessed the atrophic (A), tractional (T) and neovascular (N) components based on fundus photography and radial 12-mm scans fovea-centered SS-OCT in a blinded manner. The ATN system grades [14, 15] the A, T, and N components, respectively, as follows:
Atrophic component: A0 (no atrophic retinal lesions), A1 (tessellated fundus), A2 (diffuse chorioretinal atrophy), A3 (patchy chorioretinal atrophy), and A4 (complete macular atrophy).
Tractional component: T0 (no traction), T1 (inner or outer foveoschisis), T2 (inner and outer foveoschisis), T3 (foveal detachment), T4 (full-thickness MH), and T5 (MH + retinal detachment).
Neovascular component: N0 (no myopic CNV), N1 (macular lacquer crack), N2a (active myopic CNV), or N2s (scar/Fuchs spot). N2a and N2s represent dynamic stages, both were considered N2 for statistical analysis.
In patients who had undergone vitreoretinal surgery for tractional maculopathy, the T component considered was the presurgical T score avoiding bias of surgical impact on retinal traction. Furthermore, eyes with myopic CNV or myopic full-thickness MH were included in the study only if diffuse atrophy maculopathy was present in the atrophic maculopathy component to avoid including eyes with CNV or full-thickness MH secondary to other pathologies.
Pathologic myopia and severe pathologic myopia
All eyes were classified into two groups—pathologic myopia (PM) or severe PM—based on ATN grading criteria [PM was defined as ≥A2 and severe PM as ≥A3, ≥T3, and/or N2 (active myopic CNV, scar CNV, or Fuchs spot)] and AL cut-off points defined (considered 28 and 29.5 mm for PM and severe PM, respectively) [16, 19].
Myopic maculopathy
Myopic maculopathy was defined by the presence of macular alterations induced by high myopia that leads to a significant decrease in BCVA, in which an excessive AL and/or posterior staphyloma are the main factors although there are some other factors contributing to myopic maculopathy [11–14]. These macular alterations included not only atrophic changes, but also neovascular and traction-induced alterations. Myopic maculopathy could be classified into three categories: 1) atrophic, 2) tractional and 3) neovascular [14]. Based on the ATN classification and significant BCVA decrease, myopic maculopathy was defined as the presence of ≥A2, ≥T2 and/or ≥N1.
Statistical analysis
All analyses were performed using the IBM-SPSS statistical software program (IBM-SPSS, v. 28.0.1.0, Chicago, IL, USA). A two-tailed p-value < 0.05 was considered statistically significant. Descriptive statistics were provided for normally distributed variables as means with standard deviation (SD) for quantitative variable and n (percentage) for categorical variables. The Kolmogorov-Smirnov test was performed to assess the normality or non-normality of the variables.
Demographic data, BCVA, AL, presence of posterior staphyloma and its subtypes, according to classifications previously described, were compared between groups. Categorical variables were compared using Chi-square test for the normally distributed and Fisher’s exact test for the non-parametric variables, and to compare continuous distributed variables independent t-test was used for normally distributed variables or the non-parametric Mann–Whitney-U test for non-normally distributed variables. Kruskal–Wallis test was used to compare ordinal variables between groups: A, T and N components; posterior staphyloma classification.
Linear regression was performed to assess the impact of age and AL on BCVA and the presence of posterior staphyloma adjusting by age and AL. Furthermore, linear regression was performed to study the principal variables affecting BCVA in PS and non-PS eyes. The coefficient±SD (95% confidence-interval [CI]) and R^2 of each regression was obtained. Moreover, Akaike' and Bayesian’ criterions were applied to compare regressions [21–23].
Ordinal logistic regression was conducted to predict which variable (age, AL or PS) most strongly predicted the increase in the myopic maculopathy component studied (atrophic, tractional or neovascular). Regression coefficients and significance was obtained of each variable. Multivariate logistic regression was performed for posterior staphyloma presence as dependent variable depending on the following independent variables: age and AL. Odds ratio ± SD (95% CI) of each variable was obtained analyzing individual risk of presenting staphyloma for each variable. Area under curve (AUC) and Hosmer–Lemeshow test were calculated to assess the discrimination and calibration of the multivariate logistic regression, respectively.
Positive predictive values for posterior staphyloma were obtained according to AL cut-off points previously established for pathologic myopia and severe pathologic myopia, respectively. Minimum sample size calculation was based on population variance of each given outcome for a 2-sided level of significance (α) at 0.05 and power (β) of 80% to detect significant results. Interobserver agreement (defined as the percentage of agreement among observers) for posterior staphyloma classification and ATN grading system was analyzed. Concordance correlation coefficient was performed, rho coefficient was considered moderate (≥0.4), good (≥0.6), or excellent (≥0.8) [24, 25].
Results
Patient’ demographics
A total of 473 eyes of 259 patients with high myopia were included, being 70.65% female patients (n = 173/259) and 50.5% (n = 239/473) the right eye. Mean age was 62.4 ± 13.6 years-old (18–92). Mean BCVA was 0.41 ± 0.54 logMAR units (0–3.00) equivalent to 0.58 ± 0.33 decimal (0–1). The mean AL was 29.3 ± 2.6 mm (26–37.6); PM was present in 62.2% of eyes (n = 294/473) and, within this group, 39.7% (n = 188/473) were severe PM.
Posterior staphyloma
Out of the total, 69.4% (n = 328/473) of the eyes presented PS; 69.8% belonged to female patients (n = 229/328). According to Curtin’s classification, 73.5% (n = 241/328) were categorized as primary PS. The most frequent subtypes (Table 1) were: peripapillary staphyloma (type III) showed in 20.1% (n = 66/328) (Fig. 2), followed by inferior staphyloma (type V) in 16.8% (n = 55/328) and by narrow morphology (type II) in 16.7% (n = 55/328). Within eyes with PM the most frequent subtypes (Table 1) were: narrow type (type II) present in 18% (n = 46/255), follow by inferior type (type III) (n = 45/255) and by wide macular type (type I) (n = 34/255).
Table 1.
Prevalence of posterior staphyloma subtypes within the total sample and in pathologic myopia subgroup according to Curtin’s classification.
| Posterior staphyloma subtype | Total n (%) | Pathologic myopia eyes n (%) |
|---|---|---|
| Type I | 38/328 (11.5%) | 31/255 (12.15%) |
| Type II | 55/328 (16.7%) | 43/255 (16.86%) |
| Type III | 66/328 (20.1%) | 42/255 (16.47%) |
| Type IV | 28/328 (8.5%) | 18/255 (7.06%) |
| Type V | 55/328 (16.7%) | 41/255 (16.08%) |
| Type VI | 3/328 (0.9%) | 3/255 (1.18%) |
| Type VII | 32/328 (9.8%) | 29/255 (11.37%) |
| Type VIII | 9/328 (2.7%) | 9/255 (3.53%) |
| Type IX | 29/328 (8.8%) | 27/255 (10.59%) |
| Type X | 14/328 (4.3%) | 12/255 (4.71%) |
Fig. 2. Right high myopic eye with peripapillary subtype (type III) posterior staphyloma.
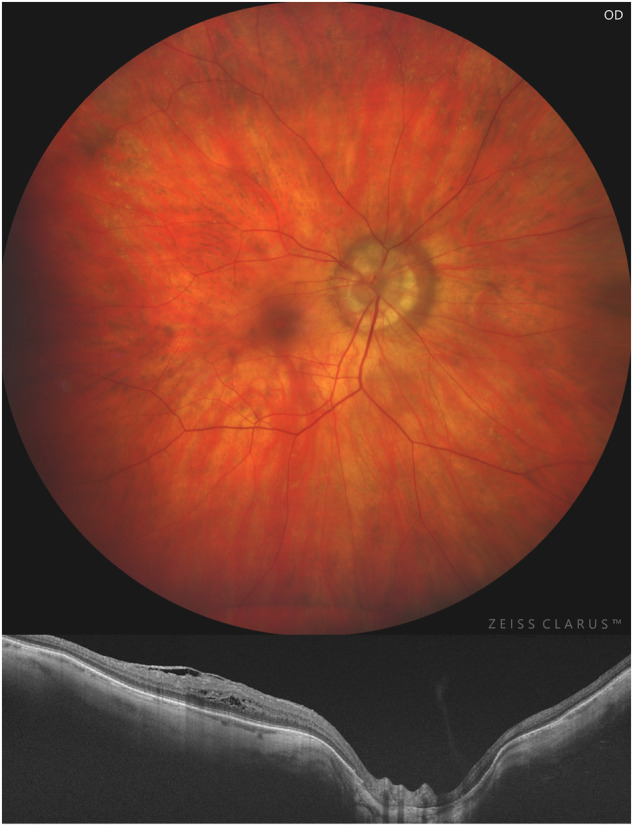
Color fundus photography (up) showing peripapillary staphyloma and corresponding horizontal OCT b-scan (down) demonstrating an inward protrusion of the sclera and choroid thinning at the edge of the staphyloma.
Concordance correlation interobserver was excellent, rho coefficient = 0.987.
ATN grading system
The data of each A, T and N component of eyes with and without PS is outlined in Table 2. Concordance correlation interobservers was excellent, Rho coefficients were as follows; A: 0.98 (p < 0.01); T: 0.98 (p < 0.01); and N: 0.99 (p < 0.01).
Table 2.
ATN classification of posterior staphyloma vs. non-posterior staphyloma eyes.
| A component | T component | N component | ||||||
|---|---|---|---|---|---|---|---|---|
| Posterior staphyloma | Non-posterior staphyloma | Posterior staphyloma | Non-posterior staphyloma | Posterior staphyloma | Non-posterior staphyloma | |||
| A0 | 2 | 37 | T0 | 141 | 107 | N0 | 222 | 128 |
| A1 | 16 | 41 | T1 | 112 | 33 | N1 | 19 | 3 |
| A2 | 138 | 56 | T2 | 51 | 5 | N2 | 87 | 14 |
| A3 | 143 | 9 | T3 | 9 | 0 | |||
| A4 | 29 | 2 | T4 | 13 | 0 | |||
| T5 | 2 | 0 | ||||||
| P < 0.01a | P < 0.01a | P < 0.01a | ||||||
aEyes with posterior staphyloma had worse atrophic, tractional and neovascular component of ATN grading system (p < 0.01).
Comparison of structural and clinical characteristics based on posterior staphyloma presence and subtype
Posterior staphyloma vs. no posterior staphyloma
Eyes with PS showed greater age (p < 0.05), AL (p < 0.01), worse BCVA (p < 0.01), greater stage in A (p < 0.01), T (p < 0.01) and N components (p < 0.01) with statistically significant differences. No differences between gender were found (p > 0.05).
Logistic regression was performed to analyze the influence of age and AL on PS.
The risk of PS increases by 8% (95% CI: 5–12%) every year older (p < 0.01) and 70% (95% CI: 42–106%) for every mm increase in AL (p < 0.01). The discrimination of the logistic regression was 90.2% and Hosmer–Lemeshow test showed excellent calibration when compared to real population data (p < 0.01).
Primary/Compound
Compounds PS showed worse BCVA (p < 0.01), greater stage in A (p < 0.01), T (p < 0.01) and N components (p < 0.01). No statistically differences were found regarding AL, age nor gender (p > 0.05).
Macular involvement
PS with macular involvement (type I, II and compounds) were found in 54.6% (n = 179/328). These subtypes had worse BCVA (p < 0.01), greater AL (p < 0.01) and also, greater stage in A (p < 0.01), T (p < 0.01) and N components (p < 0.05). On the other hand, age and gender were not statistically different (p > 0.05).
Analyses between PS vs. non-PS eyes, primary vs. compound and macular vs. non-macular involvement staphyloma is summarized in Table 3.
Table 3.
Analysis of clinical differences presented between posterior staphyloma vs non-posterior staphyloma eyes, primary vs. compound subgroups and macular involvement vs. no macular involvement posterior staphyloma.
| Posterior staphyloma | n (%) | Age (years-old) Mean ± SD | p | BCVA (logMAR/ decimal) Mean ± SD | p | AL (mm) Mean ± SD | p | A (p) | T (p) | N (p) |
|---|---|---|---|---|---|---|---|---|---|---|
| N total | N = 473 | |||||||||
| Presence | 328 (69.4%) | 66.1 ± 11.6 | <0.05 | 0.51 ± 0.59/0.5 ± 0.32 | <0.01 | 30.14 ± 2.57 | <0.01 | <0.01 | <0.01 | <0.01 |
| Absent | 145 (30.6%) | 53.9 ± 13.9 | 0.19 ± 0.3/0.75 ± 0.27 | 27.48 ± 1.38 | ||||||
| Type of staphyloma | N = 328 | |||||||||
| Primary | 240 (73.4%) | 65.53 ± 11 | >0.05 | 0.44 ± 0.53/0.54 ± 0.32 | <0.01 | 29.32 ± 2.19 | >0.05 | <0.01 | <0.01 | <0.01 |
| Compound | 87 (26.6%) | 67.63 ± 12.9 | 0.72 ± 0.72/0.40 ± 0.32 | 32.31 ± 2.23 | ||||||
| Macular involvement | N = 328 | |||||||||
| Yes | 179 (54.6%) | 66.6 ± 12.1 | >0.05 | 0.62 ± 0.67/0.44 ± 0.33 | <0.01 | 31.40 ± 2.41 | <0.01 | <0.01 | <0.01 | <0.05 |
| No | 149 (45.4%) | 65.4 ± 10.8 | 0.38 ± 0.47/0.57 ± 0.31 | 28.62 ± 1.83 | ||||||
AL axial length, BCVA best corrected visual acuity, SD standard deviation.
Analysis of posterior staphyloma in pathologic myopia and severe PM
Among eyes that met criteria for PM and severe PM, 86.7% (n = 255/294) and 94.1% (n = 177/188) presented posterior staphyloma, respectively. The positive predictive value of PM for the presence of staphyloma was 89.8% (95%CI: 86.1–93.5), which means that PM eyes had 89.8% probability of presenting staphyloma. Moreover, the positive predictive value for severe PM was 96.7% (95% CI: 93.6–99.8), therefore eyes classified as severe PM presented a 96.7% probability of having posterior staphyloma.
Different analyses between subgroups were performed:
PM vs. severe PM: The latter showed higher incidence of PS (p < 0.05). No differences were found between primary/compound nor macular involvement (p > 0.05).
Within PM group (excluding severe PM), staphyloma (n = 78/106) vs. non-staphyloma eyes (n = 28/106): Eyes with staphyloma presented worse BCVA, higher AL and were older (p < 0.01). Regarding ATN, PS eyes showed higher A (p < 0.01) and T component (p < 0.05).
Within severe PM group, staphyloma (n = 177/188) vs. non-staphyloma eyes (n = 11/188): Eyes with PS had worse BCVA, higher AL and were older (p < 0.01). Nevertheless, no differences were obtained in ATN components (p > 0.05).
Influence of posterior staphyloma and AL on visual function and myopic maculopathy
The prevalence of myopic maculopathy (due to atrophic, tractional and/or neovascular components) in posterior staphyloma, PM and severe PM groups. Considering eyes with myopic maculopathy, posterior staphyloma was present in 81.7% of eyes that presented atrophic myopic maculopathy, in 93.8% of eyes with tractional myopic maculopathy and in the 84.5% with neovascular myopic maculopathy.
An ordinal regression was performed to predict the probability of higher A, T and N components depending on age, AL and presence of PS. It was found with statistically significant differences (p < 0.01) that the presence of posterior staphyloma is the variable that is more likely to increase the risk of higher A, T and N component. Followed in the three analyses as well by age (p < 0.01) and lastly by AL (p < 0.01).
Linear regression was performed to study the influence of age, AL and PS on BCVA. According to Akaike’s and Bayesian’s criteria [21–23], the presence of posterior staphyloma was a better predictor of BCVA according to this analysis.
The results showed that:
Age explained 5.1% of the variability of visual function and for every additional year of age there was a loss in BCVA of 0.006 decimal (p < 0.01).
AL explained 10.85% of the variability of visual function and for every mm increase in AL there was a loss in BCVA corresponding to 0.04 decimal (p < 0.01).
Posterior staphyloma explained 12.1% of visual function variability and its presence associated a decrease in BCVA of 0.25 decimal (p < 0.01).
Moreover, it was analyzed separately the combination of variables in PS and non-PS group that influence the visual function.
In PS group, the variable that influenced most the visual acuity was the atrophic component (p < 0.01) associating a mean loss of 0.18 decimal of BCVA per each increase of A component. Followed by those PS that affected the macular region (p < 0.01) and compound PS (p < 0.01) with a loss of visual acuity of 0.15 and 0.14 decimal, respectively. Finally, neovascular component (p < 0.01), tractional component (p < 0.01) and AL (p < 0.01) led to a mean BCVA loss of 0.08, 0.05 and 0.02 decimal per mm increase; in this order.
On the other hand, in those eyes without PS the variable that most affected BCVA was neovascular component (p < 0.01) followed by atrophic component (p < 0.01), associating a mean loss of 0.11and 0.09 decimal for each increase N and A component, respectively. Every mm higher of AL and every year higher of age, led to a loss of 0.05 and 0.003 decimal (p < 0.01). Tractional component repercussion on visual acuity were not significant (p > 0.05).
Discussion
Posterior staphyloma in high myopia represents the best predictor for BCVA and has a significant impact on myopic maculopathy severity. From our results, it is possible to affirm that compound staphylomas and those staphylomas with macular involvement present higher degrees of atrophic, tractional and neovascular myopic maculopathy and, therefore, worse visual acuity.
Out of all PS of this series, 73.4% were categorized as primary. The most frequent subtype was the peripapillary (type III), followed by the inferior (type V), narrow-macular (type II) and wide-macular staphyloma (type I): 20.1%, 16.8%, 16.5% and 11.6%, respectively. These results differ from previous reports which found wide-macular (type I) the most frequent in 76% [20], 41.9% [26] and 44.4% [27] followed by the narrow-macular (type II) in 11.5% [20], 41.2% [26], 43.3% [27]. Zheng et al. [9], differing from studies described above, obtained the following frequencies: 38.3% narrow-macular staphyloma (type II), followed by 25.5% wide-macular staphyloma (type I), 21.3% parapapillary (type III), 12.8% of inferior staphylomas and 2.1% type VII staphyloma. These differences may respond to ethnicity, most of our cohort were Caucasian-Mediterranean and there was not an AL difference between studies that could be a bias. From our results, it can be considered that PS classifications are useful from an anatomic point of view; although for clinical prognosis, regarding BCVA and myopic maculopathy, the most important statement would be the presence of staphyloma and whether it affects the macular area.
Being older, having a high myopic spherical equivalent and increased AL have been described as risk factors for PS [9, 17]. In our sample, older age and longer AL were also risk factors for the development of staphyloma; but when considering primary vs. compound staphyloma, AL and age there were no different. A genetic factor is assumed that should be considered in the development of staphyloma subtype.
It has been widely studied that the increase of severity of myopic maculopathy is associated with growth of the ocular globe [12, 28–32]. Prospective follow-up studies demonstrated that high myopic eyes without concomitant disorders could increase AL, develop PS and myopic maculopathy [12, 33]. Nevertheless, the range of AL associated with myopic maculopathy and staphyloma subtypes is highly variable. As previously suggested by several authors [7, 8, 20, 34] and according to our results, AL might not be the best indicator to characterize pathologic myopia.
Myopic maculopathy is the main conditioning factor for visual acuity in myopic eyes. Nowadays there is still no treatment for many myopic maculopathy disorders, but some are susceptible to treatment, requiring a strict follow-up. PS is critically important for myopic maculopathy and its severity, particularly in compound and/or macular involvement subtypes. As shown in this study, maculopathy was practically constant when PS was present, associating 81.7% of them atrophic myopic maculopathy, 93.8% tractional myopic maculopathy and 84.5% neovascular myopic maculopathy. In agreement with previous studies, eyes with PS had worse visual prognosis and anatomic changes and was the main predictive variable of a greater atrophic, tractional and neovascular component [11, 30, 31, 33].
Several factors have an impact on visual acuity in high myopic patients [7, 8, 11, 12, 30, 32]. According to our results, the best predictor for BCVA in myopic patients was the presence of PS justifying 12.1% of BCVA variability and leading to a decrease of 0.24 logMAR units. Moreover, the increase in AL was associated with a decrease in BCVA explaining 10.85% of its variability, for every 1 mm increase in AL there was a decrease in BCVA of 0.052 logMAR units. Thus, an increase of 4.6 mm of AL would be necessary to produce a reduction in BCVA similar to the one caused by the presence of PS. Nevertheless, as shown previously, the main condition that affected visual acuity in patients with PS was the atrophic component of myopic maculopathy, followed by macular involvement due to PS. On the other hand, in those eyes without PS, the conditions that most affected the visual acuity were the neovascular and atrophic component in this order.
Considering PM and severe PM, as part of their definition, severe PM presented higher degrees of myopic maculopathy, but also higher incidence of PS. Regarding the PM group, eyes with PS had worse BCVA, higher AL, were older and more advanced stage of maculopathy. Studying severe PM, results were similar in terms of BCVA, AL and age within the PS group, although there were no differences in myopic maculopathy as both groups had late stages of maculopathy. Furthermore, both groups showed a very high positive predictive value for the presence of PS, indicating that eyes with PM or severe PM will be at risk of having subsequent PS of 89.8% and 96.7%, respectively. Suggesting that its presence is practically constant in most of PM and, even more frequently, severe PM eyes. Hence, accurate diagnosis of pathologic myopia and its severity would be essential for correct follow-up.
Limitations
The limitations of our study were: 1) Nowadays there is not a uniform and standardized method to measure the AL in eyes with PS. The current biometers available may provide an inaccurate measurement depending on whether the staphyloma affects the macular region or not. Therefore, it might be recommended to use the term of axial length as "foveal axial length", as a standard among all studies in high myopia. The potential bias measuring AL might be greater in those PS without macular involvement as they are not centered in the most concave part of the eyeball. Despite this fact, as shown in the study’s results, PS’s eyes without macular involvement showed less clinical repercussion. 2) Ultra-wide-field OCT was not employed to classify and study our sample, so the depth of the staphyloma was not studied and could be an important factor that could influence on myopic maculopathy. Nonetheless with actual technology available with a depth-scan between 5 and 6 mm, very deep staphylomas cannot be completely visualized and measured. 3) Campimetry was not performed on all patients, thus some incipient nerve alterations could be misdiagnosed. 4) The race of our series was mainly Caucasian-Mediterranean. A study including different races would enhance the results. 5) Due to cross-sectional study design the development and evolution of PS and myopic maculopathy were not studied. 6) The sample studied was from a tertiary high-myopia hospital and non-random sampling, these factors may have been a bias so general population of high myopic may not be represented affecting the generalizability of the results. 7) Selection bias: the inclusion criteria may have introduced selection bias as only patients with high image quality score were included. Due to this, patients with more severe pathology could have been excluded. 8) Limited sample size that may not be large enough to draw firm conclusions, especially when subgroup analyses were performed which makes the sample size smaller. 9) Lack of a control group: a control group of non-high myopic eyes was not included, which complicates the comparison of the results with the general population. 10) Variability in measurement: although the classification of posterior staphyloma had been performed by two retina specialists and, in those cases of discrepancy, confirmed with a third blinded senior consultant, there may still be variability in the measurements.
Conclusions
The results of this study demonstrate the importance of posterior staphyloma in high myopic patients, especially when involving the macular region. Its presence determines high risk of myopic maculopathy and therefore worse visual prognosis, representing the best predictor for BCVA. Posterior staphyloma should be considered practically as a constant hallmark of PM and severe PM determining the follow-up and prognosis of these patients.
Summary
What was known before
Posterior staphyloma is a hallmark of high myopia and its presence associate higher degrees of myopic maculopathy, nevertheless it is still unclear its development, repercussion on visual function and relationship with maculopathy components.
What this study adds
The results of this study demonstrate the importance of posterior staphyloma in high myopic patients, especially when involving the macular region.
Posterior staphyloma determines higher risk of myopic maculopathy and therefore worse visual prognosis, representing the best predictor for BCVA.
Posterior staphyloma should be considered practically as a constant hallmark of PM and severe PM determining the follow-up and prognosis of these patients.
The study' results highlight the importance of assessing the posterior staphyloma in patients with high myopia.
Author contributions
IFM and MP were involved in research design, data acquisition, analysis, and interpretation; and drafted the final manuscript. IFM, MP, JRM, EAA, MGZ and JMRM made substantial contributions to the conception of the study, approved the final version and have agreed to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work.
Funding
Prof. José M. Ruiz-Moreno disclosed receipt of the following financial support for the research, authorship, and/or publication of this article from: Topcon, Co. The sponsor had no role in the design or conduct of this research.
Data availability
Most of the data analyzed during the study that support the findings are included in this published article. Further data are not publicly available due to privacy reasons but are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ignacio Flores-Moreno, Mariluz Puertas.
References
- 1.Maruko I, Iida T, Sugano Y, Oyamada H, Sekiryu T. Morphologic choroidal and scleral changes at the macula in tilted disc syndrome with staphyloma using optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:8763–8. doi: 10.1167/iovs.11-8195. [DOI] [PubMed] [Google Scholar]
- 2.Xu X, Fang Y, Yokoi T, Shinohara K, Hirakata A, Iwata T, et al. Posterior staphylomas in eyes with retinitis pigmentosa without high myopia. Retina. 2019;39:1299–304. doi: 10.1097/IAE.0000000000002180. [DOI] [PubMed] [Google Scholar]
- 3.Scott A, Kashani S, Towler HMA. Progressive myopia due to posterior staphyloma in type I Osteogenesis Imperfecta. Int Ophthalmol. 2005;26:167–9. doi: 10.1007/s10792-006-9012-y. [DOI] [PubMed] [Google Scholar]
- 4.Lee S, Schimmenti LA, King RA, Brilliant M. Posterior staphyloma in oculocutaneous albinism: antoher possible cause of reduced visual acuity. J AAPOS. 2015;19:562–4. doi: 10.1016/j.jaapos.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spaide RF. Staphyloma: Part I. In: Pathologic myopia. New York, NY: Springer; 2014. pp. 167–76.
- 6.Oie Y, Ikuno Y, Fujikado T, Tano Y. Relation of posterior staphyloma in highly myopic eyes with macular hole and retinal detachment. Jpn J Ophthalmol. 2005;49:530–2. doi: 10.1007/s10384-005-0249-1. [DOI] [PubMed] [Google Scholar]
- 7.Ohno-Matsui K, Lai TYY, Lai CC, Cheung CMG. Updates of pathologic myopia. Prog Retin Eye Res. 2016;52:156–87. doi: 10.1016/j.preteyeres.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Ohno-Matsui K. Proposed classification of posterior staphylomas based on analyses of eye shape by three-dimensional magnetic resonance imaging and wide-field fundus imaging. Ophthalmology. 2014;121:1798–809. doi: 10.1016/j.ophtha.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 9.Zheng F, Wong CW, Sabanayagam C, Cheung YB, Matsumura S, Chua J, et al. Prevalence, risk factors and impact of posterior staphyloma diagnosed from wide-field optical coherence tomography in Singapore adults with high myopia. Acta Ophthalmol. 2021;99:e144–e153. doi: 10.1111/aos.14527. [DOI] [PubMed] [Google Scholar]
- 10.Moriyama M, Ohno-Matsui K, Hayashi K, Shimada N, Yoshida T, Tokoro T, et al. Topographic analyses of shape of eyes with pathologic myopia by high-resolution three-dimensional magnetic resonance imaging. Ophthalmology. 2011;118:1626–37. doi: 10.1016/j.ophtha.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 11.Vongphanit J, Mitchell P, Wang JJ. Prevalence and progression of myopic retinopathy in an older population. Ophthalmology. 2002;109:704–11. doi: 10.1016/S0161-6420(01)01024-7. [DOI] [PubMed] [Google Scholar]
- 12.Yan YN, Wang YX, Yang Y, Xu L, Xu J, Wang Q, et al. Ten-year progression of myopic maculopathy: the Beijing eye study 2001–2011. Ophthalmology. 2018;125:1253–63. doi: 10.1016/j.ophtha.2018.01.035. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi K, Ohno-Matsui K, Shimada N, Moriyama M, Kojima A, Hayashi W, et al. Long-term pattern of progression of myopic maculopathy: a natural history study. Ophthalmology. 2010;117:1595–1611. doi: 10.1016/j.ophtha.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz-Medrano J, Montero JA, Flores-Moreno I, Arias L, García-Layana A, Ruiz-Moreno JM. Myopic maculopathy: current status and proposal for a new classification and grading system (ATN) Prog Retin Eye Res. 2019;69:80–115. doi: 10.1016/j.preteyeres.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz-Medrano J, Flores-Moreno I, Ohno-Matsui K, Cheung CMG, Silva R, Ruiz-Moreno JM. Validation of the recently developed ATN classification and grading system for myopic maculopathy. Retina. 2020;40:2113–8. doi: 10.1097/IAE.0000000000002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruiz-Medrano J, Flores-Moreno I, Ohno-Matsui K, Cheung CMG, Silva R, Ruiz-Moreno JM. Correlation between ATN grade for myopic maculopathy and clinical severity. Retina. 2021;41:1867–73. doi: 10.1097/IAE.0000000000003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohno-Matsui K, Jonas JB. Posterior staphyloma in pathologic myopia. Prog Retin Eye Res. 2019;70:99–109. doi: 10.1016/j.preteyeres.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Ohno-Matsui K, Kawasaki R, Jonas JB, Cheung CMG, Saw S-M, Verhoeven VJM, et al. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol. 2015;159:877–883.e7. doi: 10.1016/j.ajo.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 19.Flores-Moreno I, Puertas M, Almazán-Alonso E, Ruiz-Medrano J, García-Zamora M, Vega-González R, et al. Pathologic myopia and severe pathologic myopia: correlation with axial length. Graefe’s Arch Clin Exp Ophthalmol. 2022;260:133–40. doi: 10.1007/s00417-021-05372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curtin BJ. The posterior staphyloma of pathologic myopia. Trans Am Ophthalmol Soc. 1978;75:67–86. [PMC free article] [PubMed] [Google Scholar]
- 21.Dziak JJ, Coffman DL, Lanza ST, Li R, Jermiin LS. Sensitivity and specificity of information criteria. Brief Bioinform. 2020;21:553–65. doi: 10.1093/bib/bbz016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, Nyholt DR. Marker selection by akaike information criterion and Bayesian information criterion. Genet Epidemiol. 2001;21:S272–S277.. doi: 10.1002/gepi.2001.21.s1.s272. [DOI] [PubMed] [Google Scholar]
- 23.Vrieze SI. Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC) Psychol Methods. 2012;17:228–43. doi: 10.1037/a0027127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gisev N, Bell JS, Chen TF. Interrater agreement and interrater reliability: Key concepts, approaches, and applications. Res Soc Adm Pharm. 2013;9:330–8. doi: 10.1016/j.sapharm.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Landis J, Koch G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 26.Igarashi-Yokoi T, Shinohara K, Fang Y, Ogata S, Yoshida T, Imanaka T, et al. Prognostic factors for axial length elongation and posterior staphyloma in adults with high myopia: a Japanese observational study. Am J Ophthalmol. 2021;225:76–85. doi: 10.1016/j.ajo.2020.11.023. [DOI] [PubMed] [Google Scholar]
- 27.Frisina R, Baldi A, Cesana BM, Semeraro F, Parolini B. Morphological and clinical characteristics of myopic posterior staphyloma in Caucasians. Graefe’s Arch Clin Exp Ophthalmol. 2016;254:2119–29. doi: 10.1007/s00417-016-3359-1. [DOI] [PubMed] [Google Scholar]
- 28.Du R, Xie S, Igarashi-Yokoi T, Watanabe T, Uramoto K, Takahashi H, et al. Continued increase of axial length and its risk factors in adults with high myopia. JAMA Ophthalmol. 2021;139:1096–103. doi: 10.1001/jamaophthalmol.2021.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asakuma T, Yasuda M, Ninomiya T, Noda Y, Arakawa S, Hashimoto S, et al. Prevalence and risk factors for myopic retinopathy in a japanese population: the Hisayama study. Ophthalmology. 2012;119:1760–5. doi: 10.1016/j.ophtha.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 30.Mo Y, Wang MF, Zhou LL. Risk factor analysis of 167 patients with high myopia. Int J Ophthalmol. 2010;3:80–82. doi: 10.3980/j.issn.2222-3959.2010.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen SJ, Cheng CY, Li AF, Peng KL, Chou P, Chiou SH, et al. Prevalence and associated risk factors of myopic maculopathy in elderly Chinese: the Shihpai eye study. Invest Ophthalmol Vis Sci. 2012;53:4868–73. doi: 10.1167/iovs.12-9919. [DOI] [PubMed] [Google Scholar]
- 32.Verkicharla PK, Ohno-Matsui K, Saw SM. Current and predicted demographics of high myopia and an update of its associated pathological changes. Ophthalmic Physiol Opt. 2015;35:465–75. doi: 10.1111/opo.12238. [DOI] [PubMed] [Google Scholar]
- 33.Shih YF, Ho TC, Hsiao CK, Lin LLK. Visual outcomes for high myopic patients with or without myopic maculopathy: a 10 year follow up study. Br J Ophthalmol. 2006;90:546–50. doi: 10.1136/bjo.2005.081992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curtin BJ. Ocular findings and complications. In: The myopias: basic science and clinical management. New York, NY: Harper and Row; 1985. pp. 277–382.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Most of the data analyzed during the study that support the findings are included in this published article. Further data are not publicly available due to privacy reasons but are available from the corresponding author upon reasonable request.


