Abstract
The properties of two-dimensional covalent organic frameworks (2D COFs), including porosity, catalytic activity as well as electronic and optical properties, are greatly affected by their interlayer stacking structures. However, the precise control of their interlayer stacking mode, especially in a reversible fashion, is a long-standing and challenging pursuit. Herein, we prepare three 2D copper-organic frameworks, namely JNM-n (n = 7, 8, and 9). Interestingly, the reversible interlayer sliding between eclipsed AA stacking (i.e., JNM-7-AA and JNM-8-AA) and staggered ABC stacking (i.e., JNM-7-ABC and JNM-8-ABC) can be achieved through environmental stimulation, which endows reversible encapsulation and release of lipase. Importantly, JNM-7-AA and JNM-8-AA exhibit a broader light absorption range, higher charge-separation efficiency, and higher photocatalytic activity for sensitizing O2 to 1O2 and O2•− than their ABC stacking isostructures. Consequently, JNM-8-AA deliver significantly enhanced photocatalytic activities for oxidative cross-coupling reactions compared to JNM-8-ABC and other reported homogeneous and heterogeneous catalysts.
Subject terms: Biocatalysis, Synthesis and processing, Polymers
The precise control of interlayer stacking mode of 2D materials is a long-standing pursuit. Here, the authors report two 2D copper-organic frameworks that can undergo the reversible interlayer sliding through environmental stimulation.
Introduction
Two-dimensional (2D) materials, including graphene, transition metal dichalcogenides, MXenes, and graphdiyne, have ignited great interest due to their intriguing optical and electronic properties, as well as quantum size effect. These properties are strongly affected by their interlayer interactions and stacking modes1–3. Thus, the precise modulation of interlayer stacking is a long-standing pursuit but is still hard to achieve. As a class of emerging 2D crystalline materials, 2D covalent organic frameworks (COFs) have been constructed from periodic and extended 2D monolayers that stack together through non-covalent interactions, including π − π interactions, electrostatic attractions, and van der Waals interactions4–9. Owing to their atomically precise and easily tunable structure, 2D COFs offer a promising platform for investigating how altering the stacking of layers affects their properties, such as porosity, crystallinity, chemical stability, and catalytic activities10–12. Therefore, many efforts have been made to modulate the interlayer stacking of 2D COFs. For instance, the interlayer stacking modes of 2D COFs can be adjusted irreversibly via ligand engineering, including steric tuning and functional modification13–17. In addition, Zhao’s group reported guest-triggered (e.g., solvent or gas molecules) reversible interlayer shifting in 2D COFs prepared from the identical monomers, but the resulting quasi-AA or -AB stacking structure cannot preserve in the absence of guests, leading to difficulty in comparing their properties18,19. So far, the preparation of structurally stable 2D COFs with different interlayer stacking modes but with the same components is rarely achieved.
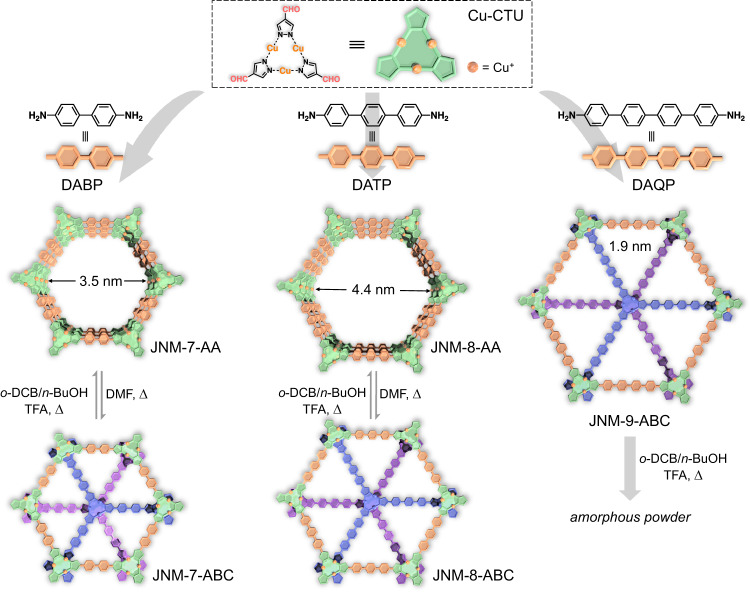
Recently, we synthesized a series of copper cyclic trinuclear unit (CTU)-based 2D copper-organic frameworks (CuOFs) by integrating the chemistry of COFs and MOFs10,20–22. Due to the metallophilic attraction provided by CTUs, JNM-3 (JNM represented Jinan material) with staggered ABC stacking structure can irreversibly transfer to an eclipsed AA stacking mode triggered by the addition of trifluoroacetic acid (TFA)21. Herein, we illustrated the reversible structure transformation between eclipsed AA and staggered ABC stacking, enabling reversible encapsulation and release of enzymes. With a pair of isostructures, the photocatalytic performance of CTU-based 2D CuOFs with different stacking structures was studied and compared. The imine condensation between Cu-CTU and three di-amine linkers produces three CuOFs, denoted to JNM-n (n = 7, 8, and 9) (Fig. 1). Interestingly, the reversible interlayer sliding between AA stacking (i.e., JNM-7-AA and JNM-8-AA) and ABC stacking (i.e., JNM-7-ABC and JNM-8-ABC) configuration can be achieved through environment cue simulation (i.e., solvent, acid, and heat) (Fig. 1). Importantly, their structures remain stable after the removal of solvent and acid, thus allowing the reversible encapsulation and release of lipase. Furthermore, JNM-7-AA and JNM-8-AA exhibit a broader light absorption range, higher charge-separation efficiency, and better photocatalytic activity for sensitizing O2 to 1O2 and O2•− compared to their ABC stacked isomers. Consequently, JNM-8-AA demonstrates remarkably higher photocatalytic activities for oxidative cross-coupling reactions than JNM-8-ABC. We can harness the respective advantages of MOFs and COFs via combination of coordination and dynamic covalent chemistry, allowing us to precisely control the interlayer stacking of 2D materials and understand the structure-property relationship.
Fig. 1. Design of JNMs and the reversible structural transformation triggered by solvent, acid, and heat.
Top, Schematic illustration of the preparation of JNM-7-AA, JNM-8-AA and JNM-9-ABC. Bottom, Schematic representation of reversible interlayer stacking modulation between AA and ABC mode.
Results
Synthesis and characterization
The synthesis of JNM-7-AA, JNM-8-AA, and JNM-9-ABC were conducted under solvothermal conditions (Fig. 1). Typically, a mixture of 1,2-dichlorobenzene (o-DCB), 1-butanol (n-BuOH), and 6 M trifluoroacetic acid (TFA) containing Cu-CTU and di-amine linkers (i.e., 4,4’-diamino-biphenyl (DABP), 4,4’-diamino-p-terphenyl (DATP) or 4,4’-diamino-p-quaterphenyl (DAQP)), was heated at 120 °C for 72 h. Afterward, pale-yellow or dark-brown crystalline powders were obtained with 70% − 97% yields (Fig. 1 and See Method).
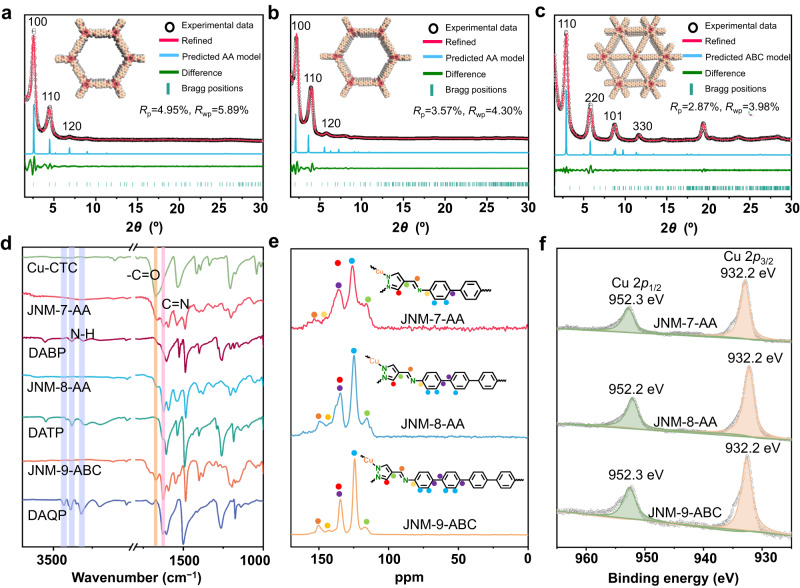
As shown in Fig. 2a, the powder X-ray diffraction (PXRD) patterns of JNM-7-AA feature an intense peak at a low angle of 2.56° attributed to (100) reflection facet along with the minor peaks at 4.52° and 6.92° for (110) and (120) diffractions, respectively. To elucidate the crystal structures of JNMs, three possible configurations, including eclipsed stacking (AA) and staggered stacking (AB and ABC) modes, were simulated by the Materials Studio software. The experimental PXRD patterns of JNM-7-AA matched well with the simulated profiles of AA stacking mode (Fig. 2a and Supplementary Fig. 2). Pawley refinement of JNM-7-AA gave a hexagonal space group P6/m with unit cell parameters of a = b = 41.2260 Å and c = 3.5549 Å and refinement parameters of Rp = 4.95% and Rwp = 5.89%. In addition, the negligible difference plot of JNM-7-AA also suggested a good agreement between the experimental data and the refined PXRD patterns. JNM-8-AA featured an eclipsed configuration and crystalized in the P6/m space group (Fig. 2b and Supplementary Fig. 5). In sharp contrast, the PXRD patterns of JNM-9-ABC displayed six peaks at 2.88°, 5.80°, 8.82°, and 11.58° from the (110), (220), (101), and (330) diffractions in the calculated ABC stacking mode, respectively (Fig. 2c). Pawley refinements of JNM-9-ABC gave a trigonal space group R-3 with an optimized unit cell of a = b = 56.1215 Å and c = 10.2004 Å and refinement parameters of Rp = 2.87% and Rwp = 3.98% (Fig. 2c and Supplementary Fig. 10).
Fig. 2. Characterization of JNM-7-AA, JNM-8-AA and JNM-9-ABC.
Crystal structure of (a) JNM-7-AA, (b) JNM-8-AA, and (c) JNM-9-ABC showing space-filling model (C, light pink; H, white; Cu, red; N, pink). d FT-IR spectra of JNM-7-AA, JNM-8-AA, and JNM-9-ABC, Cu-CTU, and di-amine linkers. e Solid-state 13C CP-MAS NMR spectra (100 MHz, 300 K) of JNM-7-AA, JNM-8-AA and JNM-9-ABC. f XPS spectra of Cu 2p for JNM-7-AA, JNM-8-AA and JNM-9-ABC.
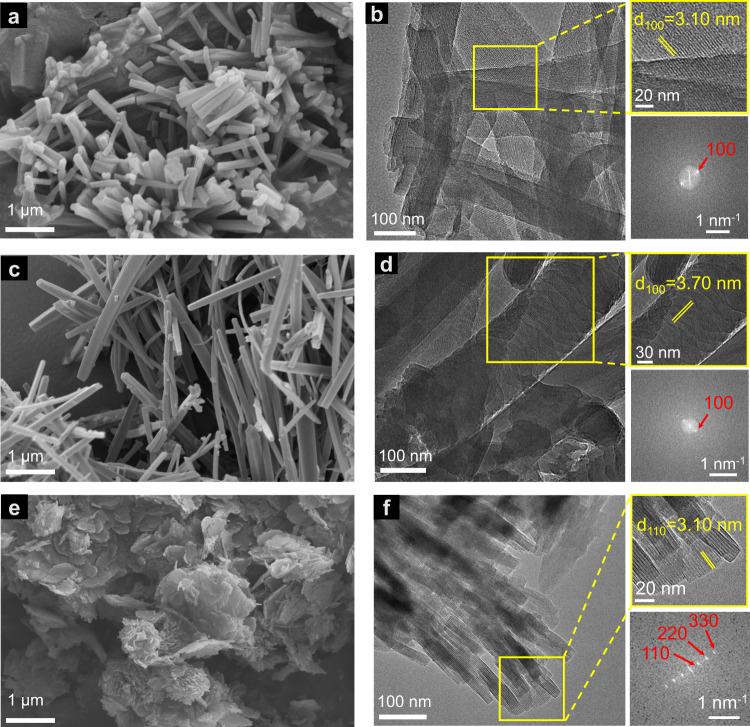
The chemical structure of JNMs was confirmed by Fourier transform infrared (FT-IR) and 13C CP/MAS NMR analysis. The FT-IR spectra of JNMs (Fig. 2d) revealed that the peak at 1670 cm−1 assigned to the C = O stretching vibration in Cu-CTU and the N − H stretching vibrations located at 3210−3432 cm−1 in di-amine linkers vanished. In addition, signals at around 1624−1628 cm−1 attributed to the C = N stretching feature appeared. These results suggested the successful formation of imine bonds, and the Schiff-base condensation entirely proceeded. Furthermore, the 13C CP/MAS NMR spectra of JNMs (Fig. 2e) showed the disappearance of the aldehyde carbon signal at 184 ppm. In comparison, characteristic resonance peaks of the imine carbon at ~153 ppm appeared, further confirming the formation of the imine bond. The X-ray photoelectron spectroscopy (XPS) spectra of JNMs showed that a symmetrical Cu(I) 2p3/2 signal at 932.3 eV without satellite peaks was observed, indicating the oxidation state of copper ions in JNMs was monovalent (Fig. 2f). Scanning electron microscopy (SEM) images of JNM-7-AA, and JNM-8-AA both exhibited needle-like micro-crystals with micrometer size (Fig. 3a, c). In contrast, JNM-9-ABC showed nano-flakes morphologies composed of stick-like micro-crystals with nanometer size (Fig. 3e). The high-resolution transmission electron microscopy (HR-TEM) and fast Fourier transform of JNM-7-AA, JNM-8-AA, and JNM-9-ABC demonstrated that the well-ordered lattice fringe with the d-spacing of 3.10, 3.70, and 3.10 nm, corresponding to the lattice planes of (100), (100) and (110), respectively (Fig. 3b, d, f). This result is in good agreement with their refined PXRD pattern. Energy dispersive X-ray spectroscopy (EDS) of the JNMs revealed a uniform distribution of elements Cu, C, and N within the skeleton (Supplementary Figs. 11−13).
Fig. 3. SEM and TEM imagrs of JNM-7-AA, JNM-8-AA and JNM-9-ABC.
SEM images of (a) JNM-7-AA, (c) JNM-8-AA, and (e) JNM-9-ABC. HR-TEM images of (b) JNM-7-AA, (d) JNM-8-AA, and (f) JNM-9-ABC Top right: enlarged images of a selective area showing well-ordered lattice fringe. Bottom right: fast Fourier transform (FFT) pattern.
Reversible interlayer structure transformation
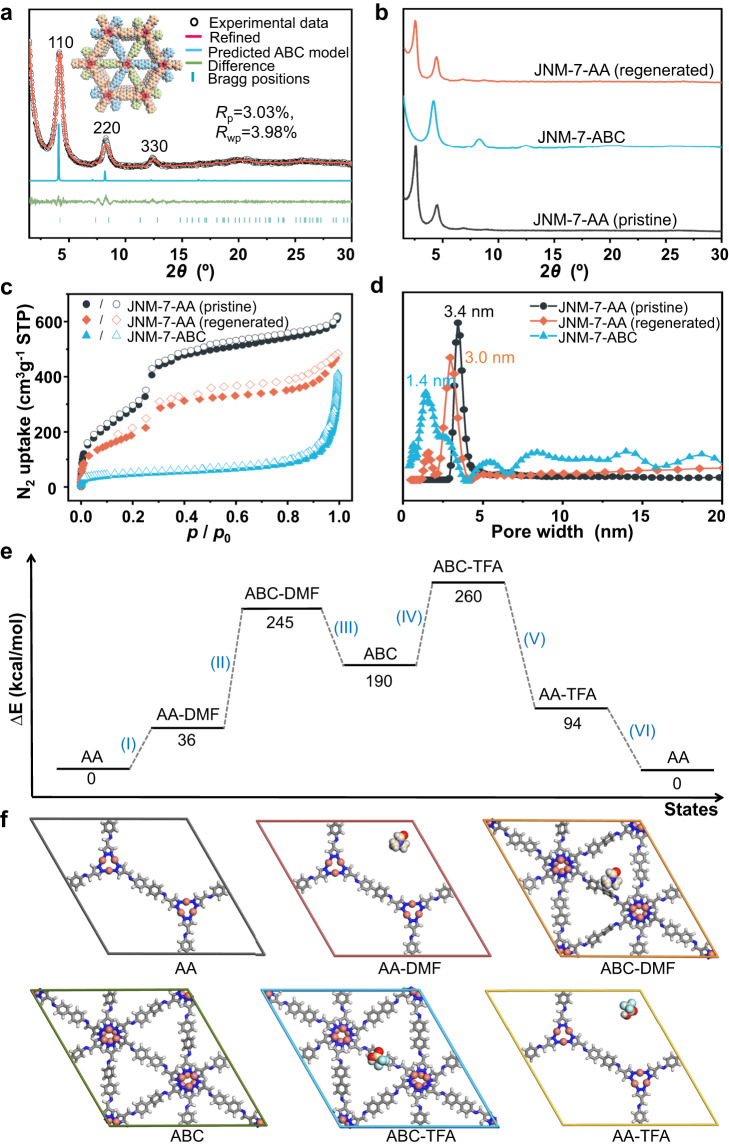
The reversible structure transformation of JNM-7-AA and JNM-8-AA can be triggered by the alteration of solvent and acid. Generally, pale-yellow crystalline powder of JNM-7-ABC or JNM-8-ABC could be obtained when a DMF solution of JNM-7-AA or JNM-8-AA was heated at 80 °C for 10 h (See SI for details). The PXRD patterns of these transformed samples were completely different from their parent sample (Fig. 4a, and Supplementary Fig. 19). Taking JNM-7-ABC as an example, it displayed the PXRD peaks at 4.20°, 8.28° and 12.38° for the (110), (220) and (330) reflection planes, respectively, which matched well with ABC stacking model (Fig. 4a). Pawley refinement of JNM-7-ABC afforded a space group of R-3 with unit cell parameters of a = b = 41.1837 Å and c = 10.2320 Å and refinement parameters of Rp = 3.03% and Rwp = 3.98%. Similarly, JNM-8-ABC showed four observed PXRD peaks at 3.36°, 6.68°, 10.16° and 13.58° assigned to (110), (220), (330), and (440), respectively. This was in good agreement with the simulated ABC stacking model with unit cell parameters of a = b = 48.6710 Å and c = 10.2001 Å and refinement parameters of Rp = 3.32% and Rwp = 4.65% (Supplementary Fig. 19). However, JNM-9-ABC remained intact when immersed in DMF (Supplementary Fig. 20). Interestingly, brown crystalline powders of JNM-7-AA or JNM-8-AA could be regenerated when JNM-7-ABC or JNM-8-ABC was added to a mixed solution of o-DCB, n-BuOH and TFA (v/v/v. 0.5/0.5/0.1) and heated at 80 °C for 10 h. The PXRD patterns of regenerated JNM-7-AA or JNM-8-AA were identical to the as-synthesized ones (Fig. 4b and Supplementary Fig. 21), confirming the reversible structure transformation process.
Fig. 4. Reversible structure transformation of JNM-7.
a crystal structure of JNM-7-ABC showing space-filling model (C, light pink; H, white; Cu, red; N, pink). b PXRD analysis, (c) BET surface analysis, and (d) pore size distribution profiles of JNM-7-AA (pristine), JNM-7-ABC, and JNM-7-AA (regenerated). e DFT-calculated energy landscape and (f) the optimized structures of JNM-7 under different states: (AA) initial AA stacking, (AA-DMF) interaction with DMF in AA stacking, (ABC-DMF) interaction with DMF in ABC stacking, (ABC) dried state in ABC stacking, (ABC-TFA) interaction with TFA in ABC stacking, (AA-TFA) interaction with TFA in AA stacking.
The FT-IR and 13C CP/MAS NMR spectra of JNM-7-ABC or JNM-8-ABC show similar characteristic peaks as JNM-7-AA or JNM-8-AA (Supplementary Figs. 24 and 25), confirming they have identical chemical structures. The SEM images of JNM-7-ABC and JNM-8-ABC show needle-like micro-crystals with much smaller sizes compared to JNM-7-AA and JNM-8-AA (Supplementary Fig. 26). The XPS spectra of JNM-7-ABC and JNM-8-ABC reveal that Cu+ ions remain intact even after interlayer structure transformation (Supplementary Fig. 27). The thermal and chemical stability of the JNMs were investigated by thermogravimetric analysis (TGA) and variable-temperature PXRD. JNM-7-AA, JNM-8-AA, and JNM-9-ABC did not show noticeable weight loss, and their crystallinity remained up to ~300 °C, while JNM-7-ABC and JNM-8-ABC were only stable up to ~200 °C (Supplementary Figs. 28 and 29). All JNMs are stable in common organic solvents, including methanol, acetonitrile, dichloromethane, and chloroform, as well as acid (i.e., 0.1 M HCl) and base (i.e., 0.1 M NaOH) (Supplementary Fig. 30).
Since the interlayer sliding will primarily affect the surface area and pore size, the porosity of JNM-7 and JNM-8 was examined by nitrogen adsorption measurements at 77 K to further verify the reversible structure transformation. The freshly prepared JNM-7-AA and JNM-8-AA demonstrated type IV adsorption curves featuring a mesoporous nature, and the Brunauer–Emmett–Teller (BET) surface areas were calculated to be 1138.36 and 210.70 m2 g−1 (Fig. 4c, and Supplementary Fig. 31), respectively. In addition, Nonlocal density functional theory (NLDFT) suggests a narrow pore-size distribution of JNM-7-AA or JNM-8-AA with an average pore width of about 3.4 or 4.0 nm (Fig. 4d and Supplementary Fig. 31), respectively, which were closed to the simulated values from the eclipsed AA mode (~3.5 and 4.4 nm for JNM-7-AA and JNM-8-AA, respectively). After the addition of DMF, the surface areas of JNM-7-ABC and JNM-8-ABC were remarkably decreased to 157.90 m2 g−1 and 122.49 m2 g−1 (Fig. 4c and Supplementary Fig. 31), respectively. Meanwhile, the pore-size distribution of JNM-7-ABC and JNM-8-ABC also significantly declined to ~1.4 and ~1.5 nm (Fig. 4d, and Supplementary Fig. 31), further supporting the structural transformation from AA to ABC stacking mode. After soaking of JNM-7-ABC and JNM-8-ABC in a TFA solution, the BET surface areas (744.42 and 178.64 m2 g−1) and pore size distribution (3.0 and 3.9 nm) of regenerated JNM-7-AA and JNM-8-AA were close to those of the pristine samples, strongly confirming the reversible structure transformation from the ABC to AA stacking mode (Fig. 4c, d and Supplementary Fig. 31).
To further elucidate the interlayer shifting processes, the density functional theory (DFT) calculations were conducted on the JNM-7 system using the DMol3 molecular dynamics module (Fig. 4e, f). Six processes were considered in the calculation: (I) the interaction between DMF molecules and JNM-7-AA; (II) the interaction between DMF molecules and JNM-7-ABC; (III) the removal of DMF from JNM-7-ABC; (IV) the interaction between TFA molecules and JNM-7-ABC; (V) the interaction between TFA molecule and JNM-7-AA; (VI) the removal of TFA from JNM-7-AA. As shown in Fig. 4e, the energy of JNM-7-ABC in DMF was much higher than that of JNM-7-AA, suggesting ABC stacking was thermodynamics unfavored state in DMF and the energy input was required (i.e., heating) to trigger the structure transformation. These results are consistent with the experimental observations, in which JNM-7-AA in DMF cannot transfer to JNM-7-ABC without heating. In addition, the energy of JNM-7-AA in TFA is much smaller than that of JNM-7-ABC, suggesting AA stacking is thermodynamics preferred.
Reversible encapsulation and release of lipase
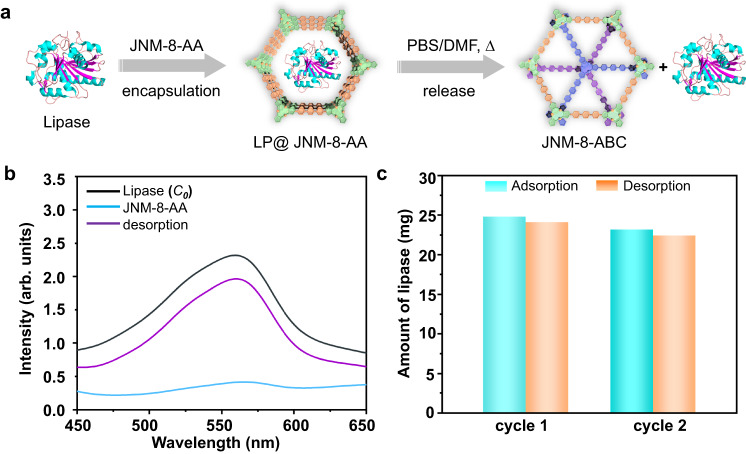
The adsorption of enzymes by porous material is an important research field for developing advanced composite materials with advanced functions. However, achieving reversible adsorption and desorption of enzymes using COFs triggered by environmental stimulation is still hard23–26. Since lipase (from thermophilic bacteria) has a size of 3.5 nm × 3.0 nm × 4.3 nm, which is smaller than the pore size of JNM-8-AA (4.0 nm) but more significant than that of JNM-8-ABC (1.5 nm), we attempt to demonstrate the reversible encapsulation and release of lipase via reversible structure transformation process of JNM-8. To a phosphate buffer solution (PBS) of lipase (30 mg mL−1, pH = 7.0), JNM-8-AA (15 mg) was added, and the resulting mixture was shaken at 175 rpm. After centrifugation and filtration, Ultraviolet−visible (UV−vis) spectroscopy of collected filtrate was recorded to monitor the change of concentration of lipase using the n-butyl cyanoacrylate (BCA) method (Fig. 5a)27–29. JNM-8-AA can absorb 24.80 mg lipase, denoted to LP@JNM-8-AA, within 6 h, showing an adsorption efficiency of 82.7% (Fig. 5b, c). In addition, the installed lipase can be released via the interlayer structure transformation. Specifically, after centrifugation, the collected powder of LP@JNM-8-AA was added to a solution of PBS and DMF. The resulting mixture was heated at 80 °C for 6 h. After then, lipase (24.14 mg) was released, and the desorption efficiency was estimated to be 97.3%, as confirmed by the UV−vis analysis (Fig. 5b, c). The PXRD experiment of the resulting powder suggested that JNM-8-AA ultimately transferred to JNM-8-ABC (Supplementary Fig. 33). Such reversible adsorption and desorption processes can be repeated at least twice with a slight decrease in efficiency in the second cycle (i.e., adsorption and desorption efficiency of 77.3% and 96.7%, respectively) (Supplementary Fig. 34).
Fig. 5. Reversible encapsulation and release of lipase.
a Schematic illustration of reversible encapsulation and release of lipase. b UV−vis absorption spectra of the solution of lipase. black line: original solution containing 30 mg of lipase; light blue line: after treatment with JNM-8-AA for 6 h; purple line: LP@JNM-8-AA after treatment with DMF for 6 h. c Reversibility test of JNM-8-AA for adsorption (cyan) and desorption (orange) of lipase.
Optical and electronic properties
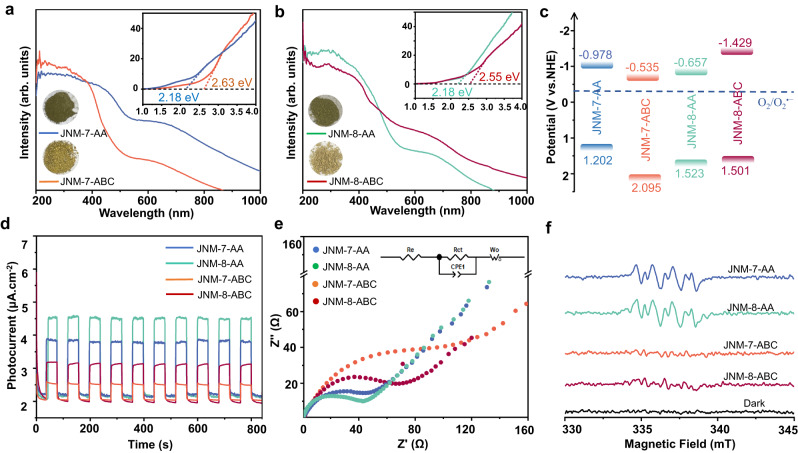
Besides the tailoring porosity, the alteration of interlayer stacking of JNM-7 and JNM-8 greatly affected their optical and electronic properties. As shown in Fig. 6, the solid-state UV-Vis diffuse reflectance spectroscopy of JNM-7-AA exhibited a strong absorption band range from 200 nm to 450 nm. In contrast, the absorption edge of JNM-7-ABC was located at 380 nm, indicating the AA stacking model delivered broader light absorption range than that of ABC stacking mode. Consequently, the optical bandgap (Eg) of JNM-7-AA was estimated to be 2.18 eV by the Tauc plot, which was much narrower than that of JNM-7-ABC (2.63 eV) (Fig. 6a). Similar to JNM-7, JNM-8-AA possessed a wider absorption range (200−450 nm) and a narrower Eg of 2.18 eV than those of JNM-8-ABC (200−400 nm and 2.55 eV) (Fig. 6b). In addition, the flat band potentials of JNM-7-AA, JNM-7-ABC, JNM-8-AA, and JNM-8-ABC were determined to be −1.178, −0.735, −0.857, and −1.620 eV vs. Ag/AgCl, respectively (Supplementary Fig. 35), which were equal to their conduction band (CB) potentials, by the Mott−Schottky experiments. Afterward, their valence band (VB) potentials could be calculated to be 1.202, 2.095, 1.523, and 1.501 eV vs. NHE, respectively (Fig. 6c), by a combination of the Mott−Schottky experiments and its optical bandgap data. These results suggest that the AA stacking model has a broader light absorption and narrower Eg than the corresponding ABC stacking model. This could be attributed to an eclipsed AA stacking model has the stronger π-π and Cu-Cu interactions between layers compared to the corresponding ABC stacking model. Moreover, to further evaluate the photo-electrochemical properties of JNM-7 and JNM-8, the transient photocurrent measurements and the electrochemical impedance spectroscopy (EIS) were conducted. Their transient photocurrent intensity follows the order of JNM-8-AA > JNM-7-AA > JNM-8-ABC > JNM-7-ABC. Importantly, the photocurrent intensity of JNM-8-AA and JNM-7-AA are ~1 time larger than those of JNM-8-ABC and JNM-7-ABC (Fig. 6d), respectively, suggesting the spatial separation of photogenerated charge carriers in AA stacking model is more effective compared to the corresponding ABC stacking model. Furthermore, JNM-8-AA and JNM-7-AA delivered similar charge transfer resistances, which were much lower than those of JNM-8-ABC and JNM-7-ABC (Fig. 6e), indicating the higher charge-separation efficiency in the AA stacking model compared to ABC isomer.
Fig. 6. Optical and electronic properties of JNMs.
The solid-state UV-Vis diffuse reflectance spectroscopy of (a) JNM-7-AA and JNM-7-ABC. b JNM-8-AA and JNM-8-ABC. Inset, the photographs of powder samples of JNMs. c Energy level diagrams of JNMs. d Photocurrent response curves of JNMs. e Nyquist plots of EIS of JNMs. Inset, equivalent circuit diagram. f the EPR spectra of JNMs (5 mg) in 3 mL of CH3CN with DMPO under air atmosphere in the dark or upon white LED light irradiation for 5 min.
The thermodynamically suitable Eg of JNM-7 and JNM-8 for photocatalytic reduction of O2 to O2•− (Fig. 6c)30–33 encouraged us to investigate their photogenerated 1O2 and O2•− capability. To accomplish this, electron paramagnetic resonance (EPR) measurements were conducted. By addition of a radical trapping reagent, 5,5-dimethyl-1-pyrroline N-oxide (DMPO), the intense signals of O2•− appeared upon the light irradiation of JNM-7-AA and JNM-8-AA in air (Fig. 6f). In addition, as shown in Supplementary Fig. 37, in the presence of 2,2,6,6-tetramethyl-4-piperidone (TEMP), a 1O2 trapping reagent, the characteristic signals of TEMPO were observed under photo-irradiation of JNM-7-AA and JNM-8-AA in air. In sharp contrast, only weak signals of O2•− or TEMPO were observed using JNM-7-ABC and JNM-8-ABC as photosensitizers under the same conditions (Fig. 6f). These results illustrated that JNM-7 and JNM-8 in AA stacking model have much higher photocatalytic activity for sensitizing O2 to 1O2 and O2•− than their ABC isomers.
Heterogeneous photocatalysis
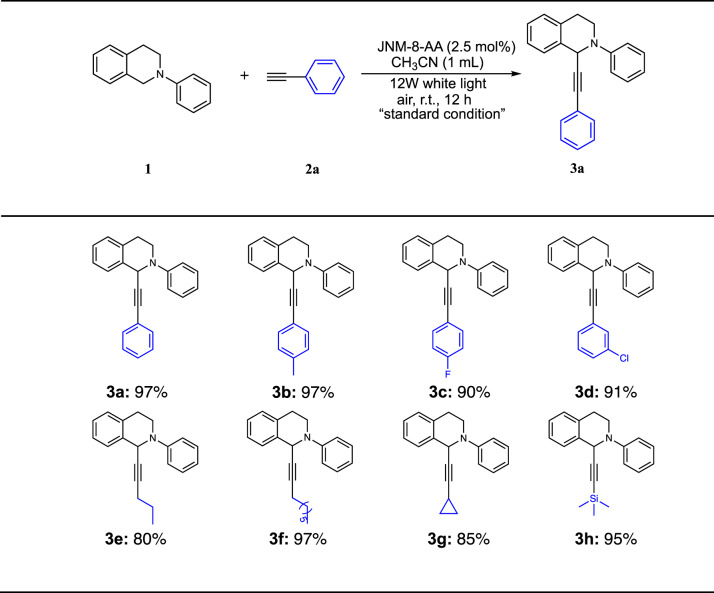
Due to their favorable optical and electronic properties, JNM-7 and JNM-8 are potential photocatalysts for oxidative cross-coupling reactions. We initially tried the photo-induced cross-dehydrocoupling (CDC) reaction of N-phenyl-tetrahydroisoquinoline (1) and phenylacetylene (2a), and C-1 substituted tetrahydroisoquinoline derivatives, which were important drug motifs and exhibited a variety of biological activities34–39, can be synthesized. To our delight, the coupling product (3a) can be obtained in 97% isolated yield using JNM-8-AA as a photocatalyst (Table 1, entry 1). Subsequently, we optimized the reaction conditions (Table 1, entries 2−16). Specifically, the mixture of 1 (0.1 mmol), 2a (0.1 mmol), and JNM-8-AA (2.5 mol%) as a catalyst in CH3CN at room temperature (rt) under photo-irradiation with 12 W white LED for 12 h, affording 3a in a > 99% conversion confirmed by GC-MS spectra (Table 1, entry 1). JNM-7-AA gave a slightly lower yield of 87%, while JNM-7-ABC and JNM-8-ABC delivered remarkably lower yields of <1% and 20%, respectively, due to their much lower capacity for sensitizing O2 to 1O2 and O2•− (Table 1, entries 2−4).
Table 1.
Screen reaction conditions for photoinduced CDC reaction
 | ||
|---|---|---|
| Entry | Change from the “standard condition” | Yield (%)a |
| 1 | none | 99 (97)b |
| 2 | JNM-7-AA | 87 |
| 3 | JNM-7-ABC | <1 |
| 4 | JNM-8-ABC | 20 |
| 5 | no JNMs | 0 |
| 6 | no hv | 0 |
| 7 | under N2, instead of air | <1 |
| 8 | CHCl3, instead of CH3CN | 45 |
| 9 | DMF, instead of CH3CN | 1 |
| 10 | toluene, instead of CH3CN | 7 |
| 11 | 1 mol% JNM-8-AA | 60 |
| 12 | linker DABP, instead of JNM-8-AA | 0 |
| 13 | linker DATP, instead of JNM-8-AA | 0 |
| 14 | Cu-CTU, instead of JNM-8-AA | 85 |
| 15 | CuO, instead of JNM-8-AA | 19 |
| 16 | CuI, instead of JNM-8-AA | 13 |
| 17 | addition of 1,4-benzoquinonec | <1 |
| 18 | addition of TEMPO | 90 |
| 19c | 1 g scale | 60 |
Reaction conditions: 1a (0.1 mmol), 2a (0.1 mmol), JNM-8-AA (2.5 mol%), CH3CN (1 mL), room temperature (rt), white LED (12 W), air.
aDetermined by GC-MS analysis.
bIsolated yields.
cJNM-8-AA (6.9 × 10−3 mmol) was used instead of 2.5 mol%.
The reaction cannot proceed in the absence of JNMs, light irradiation, and O2 (Table 1, entries 5-7). Changing the solvent to CHCl3, DMF, and toluene also remarkably reduced the yields to 45%, 1%, and 7%, respectively (Table 1, entries 8−10). Reduction of JNM-8-AA loading to 1 mol% will also reduce the yield to 60% (Table 1, entry 11). No product was observed when linker DABP or DATP was employed as a catalyst instead of JNM-8-AA (Table 1, entries 12−13). Various copper-based catalysts were employed, a yield of 85%, 19%, and 13% were obtained, implying the Cu-CTU is crucial for the photocatalytic CDC reactions (Table 1, entries 14−16). A large-scale reaction with 1 g of 1 and low loading of JNM-8-AA was further performed, and then 0.92 g of 3a was obtained with a yield of 60%. The turnover frequency (TOF) is estimated to be ~36 h−1, which is faster compared to reported representative catalysts (Supplementary Table 13). To further study the reusability of catalyst, JNM-8-AA was collected after the completion of the CDC reaction and reused for the next catalytic cycle with the addition of a fresh reaction solution. Interestingly, catalytic performance of JNM-8-AA did not show noticeable decrease after three catalytic cycles (Supplementary Fig. 40). More importantly, the crystallinity of JNM-8-AA remained and Cu(I) ions in JNM-8-AA were unchanged after three catalytic cycles confirmed by PXRD and XPS analysis (Supplementary Figs. 40 and 41).
To understand the reaction mechanism, control experiments were conducted. CDC product of 3a did not observed after adding an O2•− quench reagent (i.e., 1,4-benzoquinone (BQ)). However, a 90% yield was obtained upon the introduction of TEMPO, a quencher of 1O2 (Supplementary Fig. 42). These results suggested the photo-induced CDC reaction is driven by O2•− rather than 1O2, which is similar to reported examples40,41. Thus, a reaction mechanism is proposed as shown in Supplementary Fig. 43. We further screened the substrate scope of substituted terminal alkyne under standard catalytic conditions (Table 2). As shown in Table 2, electron-donating and electron-withdraw substituents (2a-2d), aliphatic alkynes (2e-2g), and trimethylsilyl groups (2 h) can be tolerant and give good yields of CDC products from 80% to 97%, suggesting JNM-8-AA a promising photocatalyst for CDC reactions.
Table 2.
Substrate Scope for CDC reaction photocatalyzed with JNM-8-AA.a
aIsolated yields
Discussion
In summary, we have successfully synthesized three Cu-CTU-based CuOFs, denoted to JNM-n (n = 7, 8, and 9), through imine condensation between Cu-CTU and three diamine linkers. Upon environmental stimulation, JNM-7-AA and JNM-8-AA can reversibly transfer to JNM-7-ABC and JNM-8-ABC, respectively, enabling the reversible adsorption and desorption of lipase. Due to the control of interlayer interactions and stacking mode, JNM-7-AA and JNM-8-AA exhibit a broader light absorption range, higher charge-separation efficiency, and better photocatalytic activity for sensitizing O2 to 1O2 and O2•− than their ABC stacking isostructure. In addition, JNM-8-AA exhibits much higher photocatalytic activities for oxidative cross-coupling reactions than JNM-8-ABC. Moreover, JNM-8-AA delivers good reusability and catalytic activity with a TOF of about 36 h−1 for CDC reaction, much faster than many other reported homogeneous and heterogeneous catalysts.
Methods
General procedure
PXRD data was collected on a Rigaku Ultima IV diffractometer (40 kV, 30 mA). The parameters are as follows: scan speed (0.5° per min), step size (0.02°), and scan range (1.5°−30°). Thermogravimetric analysis was performed on a Mettler-Toledo (TGA/DSC1) thermal analyzer. The SEM images and EDS were acquired on a JEOLJSM7600F microscope with an acceleration voltage of 5 kV. TEM analysis was conducted on an FEI Titan 80−300 S/TEM operated at 200 kV. A Nicolet Avatar 360 FT-IR spectrophotometer was used for conducting FT-IR spectroscopy. XPS were measured utilizing a Thermo ESCALAB 250XI system. GC-MS analysis was carried out on an Agilent 7890B GC analyzer. The 1H and 13C NMR spectra were recorded on Bruker Biospin Avance (400 MHz) equipment. Solid-state NMR experiments were conducted on a Bruker WB Avance II 400 MHz NMR spectrometer. Gas sorption analyses were preformed on an ASAP 2020 PLUS Analyzer (Micromeritics). Surface areas and pore size distribution profiles were determined using Brunauer-Emmett-Teller methods and the DFT method, respectively. All chemicals were used without further purification and purchased from commercial sources.
Synthesis of JNMs
General procedure
Di-amine linkers (i.e., DABP, DATP or DAQP, 0.075 mmol), Cu-CTU (23.7 mg, 0.05 mmol)20, a mixed solvent of 1,2-dichlorobenzene and 1-butanol (1 mL, v- v = 7: 3, 1: 1 or 3: 7), and TFA (0.1 mL, 6 M) were charged to a Schlenk tube (10 mL). Afterward, the mixture was frozen and degassed with three freeze-pump-thaw cycles using liquid nitrogen. Upon warming to rt, the mixture was heated at 120 °C for 3 days. The crystalline powder was collected by filtration, and then, the crystalline solid was washed with methanol, ethanol and dichloromethane. The resulting solid was further dried at 100 °C under vacuum for 8 h to give JNMs.
JNM-7-AA
According to general procedure, p-4,4’-diaminobiphenyl (DABP) (13.8 mg, 0.075 mmol), 1 mL mixed solution of 1,2-dichlorobenzene and 1-butanol (v: v = 7: 3) was used and JNM-7-AA was obtained as brown powders. Yield: 21.5 mg (70.6% based on Cu). IR (KBr): ν = 1668 (m), 1625 (s), 1592 (m), 1541 (m), 1488 (s), 1403 (w), 1376 (w), 1315 (w), 1201 (m), 1139 (w), 1062 cm−1 (w). Elemental analysis for C60H42N18Cu6·2C6H4Cl2·11H2O, calcd (%): C 45.79, H 3.84, N 13.35; Found (%): C 42.04, H 2.80, N 13.82.
JNM-8-AA
According to general procedure, p-4,4’-diamino-p-terphenyl (DATP) (19.5 mg, 0.075 mmol), 1 mL mixed solution of 1,2-dichlorobenzene and 1-butanol (v: v = 1: 1) was used and JNM-8-AA was obtained as brown powders. Yield: 35.0 mg (97% based on Cu). IR (KBr): ν = 1666 (m), 1625 (s), 1589 (m), 1545 (m), 1483 (s), 1393 (w), 1375 (w), 1316 (w), 1207 (m), 1171 (m), 1058 (m), 1002 cm−1 (m). Elemental analysis for C78H54N18Cu6·3C6H4Cl2·10H2O, calcd (%): C 51.34, H 3.86, N 11.23; Found (%): C 48.67, H 2.12, N 11.13.
JNM-9-ABC
According to general procedure, 4,4’-diamino-p-quaterphenyl (DAQP) (25.2 mg, 0.075 mmol), 1 mL mixed solution of 1,2-dichlorobenzene and 1-butanol (v: v = 3: 7) was used and JNM-9-ABC was obtained as pale-yellow powders. Yield: 38.5 mg (92% based on Cu). IR (KBr): ν = 1670 (m), 1623 (s), 1589 (m), 1551 (m), 1487 (s), 1404 (w), 1323 (w), 1256 (w), 1193 (m), 1045 cm−1 (w). Elemental analysis for C96H66N18Cu6·C6H4Cl2·2H2O, calcd (%): C 60.17, H 3.66, N 12.38; Found (%): C 60.37, H 3.39, N 11.81.
Reversible structure transformation
Structure transformation from AA to ABC
To a 10 mL Schlenk tube, a pristine sample of JNM-7-AA (50 mg) or JNM-8-AA (50 mg) and 5 mL DMF was added. The resulting mixture was stirred at 80 °C for 10 h, and then the solid was isolated by filtration, washed, and solvent exchanged with EtOH and acetone several times. The resulting powder was dried under vacuum at room temperature for 3 h. Finally, the pale-yellow powders JNM-7-ABC or JNM-8-ABC were obtained. JNM-7-ABC IR (KBr): ν = 1669 (m), 1624 (s), 1594 (m), 1543 (m), 1489 (s), 1406 (w), 1376 (w), 1306 (w), 1241 (m), 1202 (s), 1241 (m), 1056 cm−1 (m). JNM-8-ABC IR (KBr): ν = 1669 (m), 1625 (s), 1589 (m), 1532 (m), 1484 (s), 1404 (w), 1376 (w), 1320 (w), 1201 (s), 1175 (w), 1056 cm−1 (m).
Structure transformation from ABC to AA
JNM-7-ABC (20 mg) or JNM-8-ABC (20 mg), 0.5 mL of 1,2-dichlorobenzene, 0.5 mL of 1-butanol, and 0.1 mL of TFA (6 M) were added a 10 mL Schlenk tube. The resulting mixture was stirred at 80 °C for 10 h, and then the solid was isolated by filtration, washed, and solvent exchanged with EtOH and acetone several times. The resulting powder was dried under vacuum at room temperature for 3 h. Finally, the brown powder JNM-7-AA or JNM-8-AA with similar diffraction peaks to the AA simulation was obtained.
Reversible encapsulation and release of lipase
Encapsulation of lipase
In a typical adsorption experiment, 15 mg of JNM-8-AA was added to 1 mL of an aqueous phosphate buffer solution of lipase (pH = 7.0, C0 = 30 mg/mL), and the mixture was shaken at 175 rpm and 25 °C for 6 h. Afterward, the resultants were centrifuged, and the solid was collected to give LP@JNM-8-AA. The supernatant was further analyzed by UV-Vis spectroscopy using the n-butyl cyanoacrylate (BCA) method to provide the concentration of lipase (Cat)27,28.
The adsorption efficiency of lipase was calculated as following equation (1):
Adsorption efficiency (%) = (C0 − Cat) / C0 × 100 (1)
C0 and Cat are the lipase concentrations at the initial condition and in the filtrate after adsorption, respectively.
Release of lipase
The LP@JNM-8-AA obtained in the above adsorption process was added to an aqueous phosphate buffer (0.5 mL), and DMF mixture solution (0.5 mL), and then the mixture was heated at 80 °C for 6 h, followed by cooling down to room temperature. Afterward, the resultants were centrifuged and filtrated, and the solid was collected to give JNM-8-ABC. The filtrate was further analyzed by UV-Vis spectroscopy using the n-butyl cyanoacrylate (BCA) method to provide the concentration of lipase (Cdt).
The desorption efficiency of lipase was calculated as following equation (2):
Desorption efficiency (%) = Cdt / (C0 − Cat) × 100 (2)
C0, Cat, and Cdt are the lipase concentration at the initial condition, in the filtrate after the adsorption process, and in the filtrate after the desorption process, respectively.
Recyclability test
After one run of adsorption and desorption, JNM-8-ABC obtained from the above desorption process was treated with TFA. The solid was isolated by filtration and washed with ultra-pure water and methanol. The resultant solid was dried under vacuum at 80 °C for 6 h to give regenerated JNM-8-AA, which was used for another adsorption and desorption experiment.
DFT calculations
All calculations were performed by using the density functional method conducted out by the DMol3 molecular dynamics module embedded in Materials Studio 2018 (MS 2018). Generalized gradient approximation (GGA) and Perdew–Burke–Ernzerhof (PBE) were used. The total energy difference and maximum residual force converged within 10−4 Ha and 0.05 Ha/Å during optimization. In all calculations, we used periodic boundary conditions and a supercell large enough to present the full coordination environments of JNM-7. The simulated AA-stacked and ABC-stacked JNM-7 structures were optimized first; solvent molecules were introduced to the channel pore.
General procedure for the CDC reaction
The JNM-8-AA were activated in a vacuum at 120 °C for 8 h prior to use for the catalytic experiment. A mixture of 1 (19.03 µL, 0.1 mmol), alkynes (2a-h) (0.1 mmol), JNM-8-AA (1.82 mg, 0.0025 mmol) in CH3CN (1 mL) was stirred at rt, and the resulting mixture was irradiated for 12 h utilizing 12 W white LED. Afterward, the supernatant was analyzed by GC–MS, and the reaction conversion was estimated based on the alkynes.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
G.H.N. is thankful for the financial support from Guangdong Basic and Applied Basic Research Foundation (no. 2019B151502024) and Guangzhou Science and Technology Project (202201020038). This study was supported financially by the National Natural Science Foundation of China (nos. 22371091, 21975104, 22150004, and 21731002), the Guangdong Major Project of Basic and Applied Research (no. 2019B030302009). Q.G. is thankful for the financial support from Special Project for Peak Carbon Dioxide Emissions-Carbon Neutrality (21DZ1206900) from the Shanghai Municipal Science and Technology Commission. The authors acknowledge Prof. Daqiang Yuan for the density functional theory (DFT) calculations. The authors acknowledge Dr. Heng Zeng for the dissucssion of BET analysis and Dr. Zhongxin Chen for the English editing of the manuscript.
Author contributions
G.H.N and D.L. designed the research; P.Y.Y. and K.M.M. conducted the experiments and data analysis; Y.M.W., X.C.L., K.M.M., J.T.L. and R.J.W. contributed to material analysis; M.X. contributed to DFT calculation; Q.G. contributed to the BET analysis; P.Y.Y., G.H.N. and D.L. co-wrote the manuscript. All authors read and commented on the manuscript.
Peer review
Peer review information
Nature Communications thanks Shengqian Ma and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Data availability
The data that support the findings of this study are available within the paper and its supplementary information files or are available from the corresponding authors upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Guo-Hong Ning, Email: guohongning@jnu.edu.cn.
Dan Li, Email: danli@jnu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-44552-w.
References
- 1.Yoffe AD. Low-dimensional systems: quantum size effects and electronic properties of semiconductor microcrystallites (zero-dimensional systems) and some quasi-two-dimensional systems. Adv. Phys. 2002;51:799–890. doi: 10.1080/00018730110117451. [DOI] [Google Scholar]
- 2.Bernardi M, Ataca C, Palummo M, Grossman JC. Optical and electronic properties of two-dimensional layered materials. Nanophotonics. 2017;6:479–493. doi: 10.1515/nanoph-2015-0030. [DOI] [Google Scholar]
- 3.He F, Li Y. Advances on theory and experiments of the energy applications in graphdiyne. CCS Chem. 2023;5:72–94. doi: 10.31635/ccschem.022.202202328. [DOI] [Google Scholar]
- 4.Geng K, et al. Covalent organic frameworks: design, synthesis, and functions. Chem. Rev. 2020;120:8814–8933. doi: 10.1021/acs.chemrev.9b00550. [DOI] [PubMed] [Google Scholar]
- 5.Bisbey RP, Dichtel WR. Covalent organic frameworks as a platform for multidimensional polymerization. Acs. Cent. Sci. 2017;3:533–543. doi: 10.1021/acscentsci.7b00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuc A, et al. Proximity effect in crystalline framework materials: stacking-induced functionality in MOFs and COFs. Adv. Funct. Mater. 2020;30:1908004. doi: 10.1002/adfm.201908004. [DOI] [Google Scholar]
- 7.Pütz AM, et al. Total scattering reveals the hidden stacking disorder in a 2D covalent organic framework. Chem. Sci. 2020;11:12647–12654. doi: 10.1039/D0SC03048A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cote AP, et al. Porous, crystalline, covalent organic frameworks. Science. 2005;310:1166–1170. doi: 10.1126/science.1120411. [DOI] [PubMed] [Google Scholar]
- 9.Huang N, Wang P, Jiang D. Covalent organic frameworks: a materials platform for structural and functional designs. Nat. Rev. Mater. 2016;1:1–19. doi: 10.1038/natrevmats.2016.68. [DOI] [Google Scholar]
- 10.Feng X, Ding X, Jiang D. Covalent organic frameworks. Chem. Soc. Rev. 2012;41:6010–6022. doi: 10.1039/c2cs35157a. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Chen W, Xing G, Jiang D, Chen L. New synthetic strategies toward covalent organic frameworks. Chem. Soc. Rev. 2020;49:2852–2868. doi: 10.1039/D0CS00199F. [DOI] [PubMed] [Google Scholar]
- 12.Sick T, et al. Switching on and off interlayer correlations and porosity in 2D covalent organic frameworks. J. Am. Chem. Soc. 2019;141:12570–12581. doi: 10.1021/jacs.9b02800. [DOI] [PubMed] [Google Scholar]
- 13.Wu X, Han X, Liu Y, Liu Y, Cui Y. Control interlayer stacking and chemical stability of two-dimensional covalent organic frameworks via steric tuning. J. Am. Chem. Soc. 2018;140:16124–16133. doi: 10.1021/jacs.8b08452. [DOI] [PubMed] [Google Scholar]
- 14.Haase F, et al. Tuning the stacking behaviour of a 2D covalent organic framework through non-covalent interactions. J. Mater. Sci. 2017;1:1354–1361. [Google Scholar]
- 15.Du Y, et al. Kinetic and mechanistic study of COF-1 phase change from a staggered to eclipsed model upon partial removal of mesitylene. J. Phys. Chem. C. 2014;118:399–407. doi: 10.1021/jp4097293. [DOI] [Google Scholar]
- 16.Chen X, Addicoat M, Irle S, Nagai A, Jiang D. Control of crystallinity and porosity of covalent organic frameworks by managing interlayer interactions based on self-complementary π-electronic force. J. Am. Chem. Soc. 2013;135:546–549. doi: 10.1021/ja3100319. [DOI] [PubMed] [Google Scholar]
- 17.Yang S, et al. Modulating the stacking model of covalent organic framework isomers with different generation efficiencies of reactive oxygen species. ACS Appl. Mater. Interfaces. 2021;13:29471–29481. doi: 10.1021/acsami.1c03170. [DOI] [PubMed] [Google Scholar]
- 18.Kang C, et al. Interlayer shifting in two-dimensional covalent organic frameworks. J. Am. Chem. Soc. 2020;142:12995–13002. doi: 10.1021/jacs.0c03691. [DOI] [PubMed] [Google Scholar]
- 19.Kang C, et al. Tunable interlayer shifting in two-dimensional covalent organic frameworks triggered by CO2 sorption. J. Am. Chem. Soc. 2022;144:20363–20371. doi: 10.1021/jacs.2c08214. [DOI] [PubMed] [Google Scholar]
- 20.Wei R-J, Zhou H-G, Zhang Z-Y, Ning G-H, Li D. Copper (I)–organic frameworks for catalysis: networking metal clusters with dynamic covalent chemistry. CCS Chem. 2021;3:2045–2053. doi: 10.31635/ccschem.020.202000401. [DOI] [Google Scholar]
- 21.Zhou H-G, et al. Acid-triggered interlayer sliding of two-dimensional copper(i)–organic frameworks: more metal sites for catalysis. Chem. Sci. 2021;12:6280–6286. doi: 10.1039/D1SC00924A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei R-J, et al. Ultrathin metal–organic framework nanosheets exhibiting exceptional catalytic activity. J. Am. Chem. Soc. 2022;144:17487–17495. doi: 10.1021/jacs.2c06312. [DOI] [PubMed] [Google Scholar]
- 23.Doonan C, Riccò R, Liang K, Bradshaw D, Falcaro P. Metal–organic frameworks at the biointerface: synthetic strategies and applications. Acc. Chem. Res. 2017;50:1423–1432. doi: 10.1021/acs.accounts.7b00090. [DOI] [PubMed] [Google Scholar]
- 24.Lee C-H, Lin T-S, Mou C-Y. Mesoporous materials for encapsulating enzymes. Nano Today. 2009;4:165–179. doi: 10.1016/j.nantod.2009.02.001. [DOI] [Google Scholar]
- 25.Lei C, Shin Y, Liu J, Ackerman EJ. Entrapping enzyme in a functionalized nanoporous support. J. Am. Chem. Soc. 2002;124:11242–11243. doi: 10.1021/ja026855o. [DOI] [PubMed] [Google Scholar]
- 26.Sun Q, et al. Pore environment control and enhanced performance of enzymes infiltrated in covalent organic frameworks. J. Am. Chem. Soc. 2018;140:984–992. doi: 10.1021/jacs.7b10642. [DOI] [PubMed] [Google Scholar]
- 27.Huang T, Long M, Huo B. Competitive binding to cuprous ions of protein and BCA in the bicinchoninic acid protein assay. Open Biomed. Eng. J. 2010;4:271–278. doi: 10.2174/1874120701004010271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desjardins P., Hansen J. B., Allen M. Microvolume protein concentration determination using the nanodrop 2000c Spectrophotometer. J. Vis. Exp.33, e1610 (2009). [DOI] [PMC free article] [PubMed]
- 29.Smith PE, et al. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 30.Srivastava V, Singh PK, Singh PP. Recent advances of visible-light photocatalysis in the functionalization of organic compounds. J. Photoch. Photobio. C. 2022;50:100488. doi: 10.1016/j.jphotochemrev.2022.100488. [DOI] [Google Scholar]
- 31.Ghogare AA, Greer A. Using singlet oxygen to synthesize natural products and drugs. Chem. Rev. 2016;116:9994–10034. doi: 10.1021/acs.chemrev.5b00726. [DOI] [PubMed] [Google Scholar]
- 32.Qian Y, Li D, Han Y, Jiang H-L. Photocatalytic molecular oxygen activation by regulating excitonic effects in covalent organic frameworks. J. Am. Chem. Soc. 2020;142:20763–20771. doi: 10.1021/jacs.0c09727. [DOI] [PubMed] [Google Scholar]
- 33.Li M, Sun J, Chen G, Wang S, Yao S. Inducing photocarrier separation via 3D porous faveolate cross-linked carbon to enhance photothermal/pyroelectric property. Adv. Powder Mater. 2022;1:100032. doi: 10.1016/j.apmate.2022.01.005. [DOI] [Google Scholar]
- 34.Welsch ME, Snyder SA, Stockwell BR. Privileged scaffolds for library design and drug discovery. Curr. Opin. Chem. Biol. 2010;14:347–361. doi: 10.1016/j.cbpa.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grunewald GL, Dahanukar VH, Caldwell TM, Criscione KR. Examination of the Role of the Acidic Hydrogen in Imparting Selectivity of 7-(Aminosulfonyl)-1,2,3,4-tetrahydroisoquinoline (SK&F 29661) Toward Inhibition of Phenylethanolamine N-Methyltransferase vs the α2-Adrenoceptor1a. J. Med. Chem. 1997;40:3997–4005. doi: 10.1021/jm960235e. [DOI] [PubMed] [Google Scholar]
- 36.Dohle W, et al. Synthesis, Antitubulin, and Antiproliferative SAR of C3/C1-Substituted Tetrahydroisoquinolines. ChemMedChem. 2014;9:350–370. doi: 10.1002/cmdc.201300412. [DOI] [PubMed] [Google Scholar]
- 37.Renaud J, et al. Estrogen receptor modulators: Identification and structure− activity relationships of potent ERα-selective tetrahydroisoquinoline ligands. J. Med. Chem. 2003;46:2945–2957. doi: 10.1021/jm030086h. [DOI] [PubMed] [Google Scholar]
- 38.Chelopo MP, et al. Anticancer activity of ruthenium(II) arene complexes bearing 1,2,3,4-tetrahydroisoquinoline amino alcohol ligands. Eur. J. Med. Chem. 2013;66:407–414. doi: 10.1016/j.ejmech.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 39.Liu W, Liu S, Jin R, Guo H, Zhao J. Novel strategies for catalytic asymmetric synthesis of C1-chiral 1,2,3,4-tetrahydroisoquinolines and 3,4-dihydrotetrahydroisoquinolines. Org. Chem. Front. 2015;2:288–299. doi: 10.1039/C4QO00294F. [DOI] [Google Scholar]
- 40.Kumar G, Verma S, Ansari A, Noor-ul HK, Kureshy RI. Enantioselective cross dehydrogenative coupling reaction catalyzed by Rose Bengal incorporated-Cu (I)-dimeric chiral complexes. Catal. Commun. 2017;99:94–99. doi: 10.1016/j.catcom.2017.05.026. [DOI] [Google Scholar]
- 41.Zhi Y, et al. Construction of donor-acceptor type conjugated microporous polymers: a fascinating strategy for the development of efficient heterogeneous photocatalysts in organic synthesis. Appl. Catal. B Environ. 2019;244:36–44. doi: 10.1016/j.apcatb.2018.11.032. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available within the paper and its supplementary information files or are available from the corresponding authors upon request.