Abstract
Springtails (Collembola) inhabit soils from the Arctic to the Antarctic and comprise an estimated ~32% of all terrestrial arthropods on Earth. Here, we present a global, spatially-explicit database on springtail communities that includes 249,912 occurrences from 44,999 samples and 2,990 sites. These data are mainly raw sample-level records at the species level collected predominantly from private archives of the authors that were quality-controlled and taxonomically-standardised. Despite covering all continents, most of the sample-level data come from the European continent (82.5% of all samples) and represent four habitats: woodlands (57.4%), grasslands (14.0%), agrosystems (13.7%) and scrublands (9.0%). We included sampling by soil layers, and across seasons and years, representing temporal and spatial within-site variation in springtail communities. We also provided data use and sharing guidelines and R code to facilitate the use of the database by other researchers. This data paper describes a static version of the database at the publication date, but the database will be further expanded to include underrepresented regions and linked with trait data.
Subject terms: Biodiversity, Community ecology
Background & Summary
Soil biodiversity represents a major fraction of life on Earth1,2. Despite that, globally we know little about the current status and trends of soil life, especially invertebrates. Over the last few years, our knowledge on the global distribution of earthworms3, nematodes4, springtails5, ants6 and other macrofauna7 has advanced, showing trends different from aboveground biodiversity8. This urges us to deliver open and in-depth knowledge on soil animal life for nature conservation and for understanding the functioning of terrestrial ecosystems9. To help with this task, we here present a comprehensive fine-resolution database on the global distribution of springtails (Collembola), based on a compilation of published and unpublished data of researchers worldwide.
With literally worldwide distribution, springtails account for ~32% of the global terrestrial arthropod abundance10 and have global biomass of ~27.5 Megatonn carbon5. They are especially numerous in cold regions, but are also ubiquitous in tropical soils5, and even tropical canopies11. Springtails are central components of the belowground system, affecting litter decomposition, microbial activity, abundance and dispersal, and plant growth, and serving as food for numerous invertebrate predators12. Despite a moderate total diversity (~9500 described species13), springtail communities typically host dozens of species in a few square metres5. Due to their ubiquitous presence, and high abundance and local diversity, springtails represent an ideal model taxon for macroecological studies as well as bioindicators, but so far data limitations have constrained studies to address questions solely at local to regional scales.
In this paper, we describe a novel database mainly compiled from private archives of contributing authors that served as the basis for the recently published global synthesis study on springtail abundance and diversity5. While the site-level summaries of springtail community parameters have been published together with the synthesis5, here we present much more detailed sample-level data that include taxonomic names and 16 additional datasets (1398 new samples). With this effort, we complement the previously published data papers on nematodes14 and earthworms15 in describing the global soil invertebrate diversity. We also take a step further by providing quality-controlled species-level data with standardised taxonomic names at fine-scale resolution, i.e. from individual samples, or even soil layers, within each sampling site. Our dataset allows for both analyses of global and regional patterns of diversity and community composition, species distributions, and within-site variations in abundance and diversity. Below, we first describe how the data were collected, checked, curated, structured, and standardised, then we provide an overview of the data, and finish with some notes on how the data can be used.
Methods
Data sources
The database represents a standardised compilation of available datasets. The data were primarily obtained from individual archives of the contributing authors. To ensure widespread participation, the data collection initiative was announced openly in late summer 2019 through various channels, such as the mailing list of the International Colloquium of Apterygota and social media platforms such as Twitter and ResearchGate. Additionally, colleagues who had expertise in less well investigated regions, such as Africa and South America, were contacted through personal networks established by the initial author group. All individuals who collected, provided and standardised the data were invited to become co-authors of this study, with a defined minimum role in tasks, such as data provision, data cleaning, manuscript editing and approval. Both published16–164 and unpublished data were collected for analysis. Raw data, specifically species counts in samples, were requested whenever possible. Collection methods for the published data can be found in the original publications associated with each sampling event in the database. Furthermore, existing data on springtail communities available from Edaphobase165 were also included. To address the underrepresentation of Africa, South America, Australia, and Southeast Asia in the database, a literature search was conducted in January 2020 using the Web of Science platform with keywords: ‘springtail’ or ‘Collembola’ and ‘density’ or ‘abundance’ or ‘diversity’ along with the region of interest. In 2022–2023, in addition to the data analysed in the synthesis paper5, we included 16 datasets with 1,398 samples from new contributing authors.
The newly reported unpublished data represented 10,616 samples collected from 828 sites (from one to few dozens of samples were collected per site) and years 1975–2022. Springtails from soil and litter were collected using standard soil sampling devices (soil corers, frames). Collection from canopy was done using insecticide fogging, collections from aboveground surfaces were done using pitfall traps, stem eclectors, malaise traps, swipnetting, or vacuum cleaner. Over 90% of these data used different variations of Berlese or Kempson devices for springtail extraction. All springtails were identified under microscopes using regional identification keys (mainly to species, but also high-rank taxa or morphogroups). All sampling information for the entries in the dataset are included in the spreadsheet including the exact places, times, collectors, habitat types, and the collection and identification methods.
Data collection
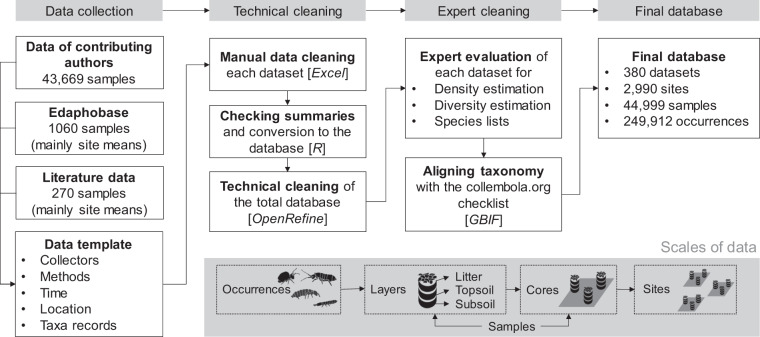
All data were entered into a common Microsoft Excel template (Supplementary materials Data template). The template included 30 columns describing the sampling approach and counts of springtail taxa. The following minimum set of variables was collected: collectors, collection method (including sampling area and depth), extraction method, identification precision and literature, collection date, latitude and longitude, and vegetation type (grassland, scrub, woodland, agriculture and other). Each contributed dataset was checked manually by a trained assistant for technical mistakes and completeness, and were complemented by authors if necessary. Geographical coordinates were checked using Google maps. We additionally performed descriptive statistics to check the consistency of the dataset (number of sites, samples, layers) and converted data in the template into two standard tables: events table (describing samples) and occurrence table (describing taxa counts) in R v. 4.0.2166 with RStudio interface v. 1.4.1103 (RStudio, PBC). The final events table across datasets was then checked for typos, consistency in vocabulary and outliers using OpenRefine v3.3 (https://openrefine.org; Fig. 1).
Fig. 1.
Data collection and evaluation in #GlobalCollembola. Most of the data are raw data collected from archives of the contributing authors of the paper. The data were collected using an Excel template and included in the final database after technical and expert cleaning of each dataset. No data were excluded, instead, expert evaluation is provided for each dataset. Whenever possible, we recorded species occurrences in individual samples (soil cores).
Data evaluation
Every contributed dataset underwent a manual expert evaluation. Our evaluation process involved a board of springtail specialists, each with extensive research experience in specific geographic regions (expert names are listed in the events spreadsheet of the database). The experts individually scored each dataset based on three criteria: reliability of the (1) density, (2) species richness, and (3) the accuracy of the species names provided. The density estimation quality was determined by considering the sampling and extraction method, as well as the density estimation itself for the given ecosystem type. The species richness estimation quality and species names were assessed by considering the identification key used, the experience of the scientist identifying the animals, the species list and the species richness estimation itself for the given ecosystem type. Datasets that were deemed “unreliable” during the evaluation process were still included in the database, but the evaluation results by the experts are provided alongside the data.
Taxonomic alignment
To make taxonomic lists comparable across contributed datasets, we checked all taxonomic names against the global checklist of Collembola (www.collembola.org). We did this using the ‘Species matching’ tool of the Global Biodiversity Information Facility (https://www.gbif.org/tools/species-lookup), which hosts the global checklist of Collembola from 2023. Original names were kept in the database together with the standardised names. For synonyms accepted species names were provided. For morphospecies described taxonomic names of higher ranks (usually genera) were given. Taxonomic hierarchy (genera, families, orders) and other taxonomic information was summarised in an additional spreadsheet. Unfortunately, it was not possible to fully control for factually wrong original identifications, even though the species lists were checked by experts (see above), but most of the records were judged as reliable.
Data Records
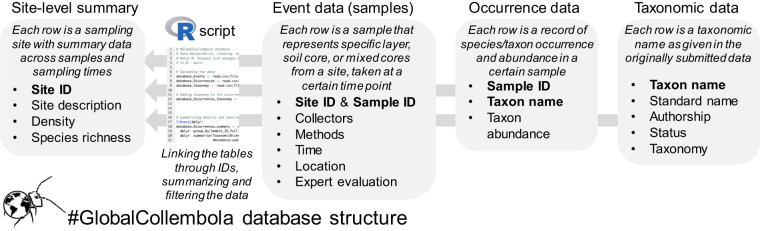
The final dataset included 380 datasets representing 2,990 sites, 44,999 samples and 249,912 occurrences (i.e. observations of taxa in samples). In total, 1,441 taxa including 1,202 species were recorded in the occurrence data. The data were provided on different scales. Most samples represented single layers (i.e. litter, topsoil, deeper soil layers) in a soil core (i.e. soil monolith) or single cores in a sampling site (Fig. 1 ‘scales’). However, some data were available only as averages across samples at the sampling site level (typically an area up to a hundred of metres in diameter). The data were organised in three spreadsheets in the csv format: (1) Events, representing a list of all samples with described methodology, locations, and sampling times; (2) Occurrences, representing a list of all observations of taxa in all samples; and (3) Taxonomy, representing list of unique taxonomic names present in the occurrence data and associated standardised taxonomic names and other taxonomic information. Furthermore, we provided an R script to link the three spreadsheets together, summarise them by soil cores and sites, and filter unreliable data and data out of the scope (Fig. 2). As an example, we also provided a csv spreadsheet with average densities and the total species richness of springtails per site, collected with area-based methods. To facilitate data re-use, we provide a separate Excel spreadsheet with detailed descriptions of all data fields (‘Data description’). All data spreadsheets, R codes and other related information are available from Fighare167.
Fig. 2.
Database structure of #GlobalCollembola. Database consists of three main spreadsheets: (1) Events, (2) Occurrences, and (3) Taxonomy. The spreadsheets can be linked, summarised, and filtered using the associated R script to produce site-level averages.
Technical Validation
Statistical soundness of the database depends on the research question addressed. Below we show representativeness of our data for main types of ecological analysis by showing its spatial and temporal scopes, as well the sampling and identification approaches.
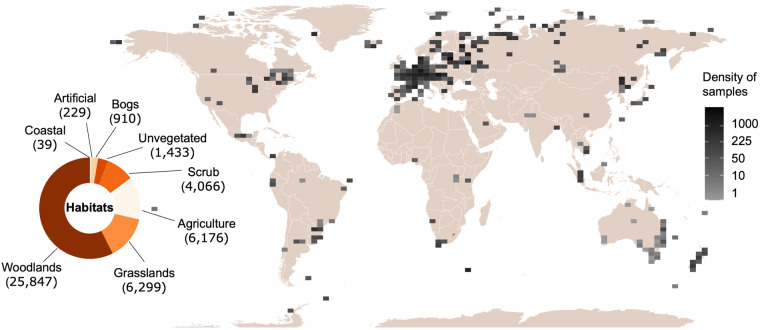
Most macroecological studies require representation of different geographical regions, climates, and ecosystem types4. Since the database is based on an open call for collection of already produced data, there is a clustered spatial distribution of data points in well-explored regions and high variation in collection methodologies. Most collected sample-level data come from Europe (82.5% or 37,137 samples), while other continents were less represented: Asia (5.6% or 2,508 samples), North America (3.4% or 1,528 samples), South America and the Caribbean (3.2% or 1,457 samples), Africa (2.8% or 1,269 samples), Australia (2.1% or 944 samples) and Antarctica (0.3% or 156 samples; Fig. 3). Across habitat types, woodlands are the most represented (57.4% of samples), followed by grasslands (14.0%), agriculture (13.7%), scrub (9.0%) and others (5.9%; Fig. 3). Using bootstrapping of the European data, we were able to do balanced analysis of the data in our synthesis study, and cover global gradients in mean annual temperature, precipitation, aridity, soil organic carbon content, pH, soil texture, vegetation biomass (NDVI), and habitat types (including the effects of agriculture)5. However, regional-scale analyses of the data are possible mainly in Europe, while tropical and subtropical regions, especially in Africa, are represented poorly.
Fig. 3.
Global distribution of the sampling points and habitat types represented in the database. Density of samples per pixel in a global 100 × 100 coordinate grid are shown with grayscale (light – few samples, dark – many samples). Number of collected samples in each habitat type are shown with a doughnut chart; habitat classification follows the European Environmental Agency.
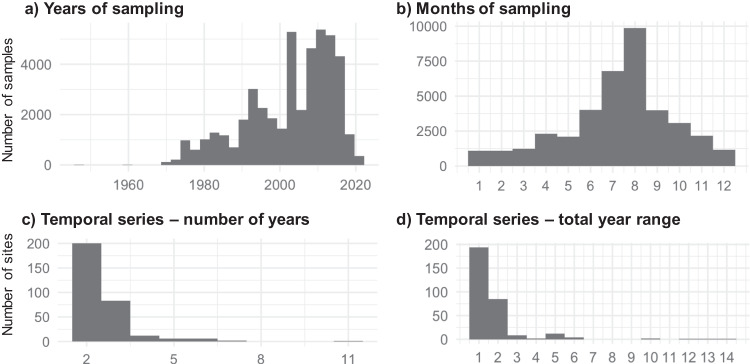
Analyses of temporal variation, especially long-term changes of soil biodiversity9, require time series at different temporal scales. Seasonality is particularly important to consider when addressing macroecological questions, such as latitudinal biodiversity trends and their drivers5. Our database included records from years 1948–2022, with most data collected between 1975 and 2020 (Fig. 4a). Samples were collected throughout the year, with peak data collection in July-August (i.e., assumed peak springtail activity in northern Europe; Fig. 4b). There were 310 sites which were sampled in multiple years. Most of them were sampled only twice (Fig. 4c). However, 36 sites were sampled in 4 or more years and 5 sites were sampled over the range of 10 or more years (Fig. 4c,d). Therefore, it is possible to analyse long-term changes in springtail communities with two approaches: (1) by using available long-term monitoring data from few specific sites; (2) by using regional-scale data across different sites within specific habitat types sampled over decades (representative mainly for Europe, as the most studied region). It is also possible to account for seasonality in the global models because information on the sampling month is available for 86.4% of all sampling events5. However, the sampling is typically done in the periods of high springtail activities in each climate type5 and there is a clear data gap in the global temporal variation in springtail communities which should be addressed in the future data collections.
Fig. 4.
Temporal coverage of the database. Frequency histograms show the number of samples collected in different years (a) and months (b), and the number of sites where samples were collected in multiple years (c) in a certain time range (d).
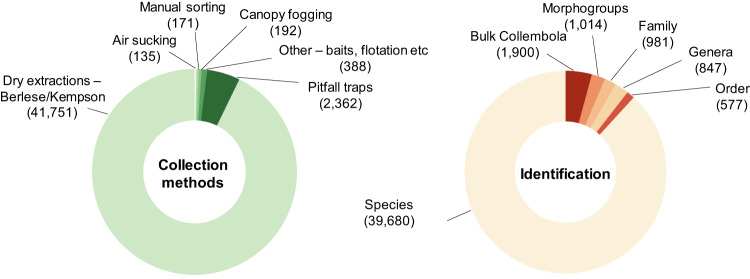
Finally, comparability of different datasets in the database depends on the collection and identification methods. Records in the database represent mainly samples collected using area-based methods such as soil cores and animals extracted with heat (i.e. various modifications of Tullgren, Berlese, Kempson or Macfadyen extractors168,169; 92.8%, Fig. 5). Pitfall traps were the second most represented method (7.2%), and we included a single dataset collected using canopy fogging11. Most of the samples represented ‘soil’ (79.9% or 35,953 samples) and ‘litter’ microhabitats (54.8% or 24,676 samples). In total, 9,058 samples represented individual layers within soil cores, while 1,316 samples represented pooled data across samples within sampling sites. Therefore, data filtering and pooling is necessary to perform quantitative analyses of community metrics. In 88.2% of samples, springtails were identified to species level, while in 2.3% to morphogroups (typically roughly reflecting species-level diversity). For 4.2% of samples, springtails were recorded without further identification (abundance data only), while in the remaining records identification to order, families, or genera are provided (Fig. 5). Since most records in the database are species-level, the database is representative to evaluate global species-richness patterns and analyse species distributions in space and time.
Fig. 5.
Collection methods and identification precision represented in the database. Number of collected samples with different methods and the number of samples where springtails were identified to a certain taxonomic resolution level are shown with doughnut charts.
Usage Notes
Our global fine-resolution data on springtail communities can be openly used to address various (macro)ecological questions in space and time. Although our database is fully open, we encourage other researchers to follow our data usage and sharing guidelines: (1) the data can be openly used if a proper attribution to the data providers is given; (2) carefully evaluate representativeness of the data for your particular question; (3) report any issues you encounter; (4) we are there to support you – get in touch with the #GlobalCollembola expert community whenever you have questions. More detailed guidelines and the issue reporting form are available from Figshare together with the full database167.
For most research questions, different spreadsheets in the database need to be combined and summarised. We suggest that you use our R code for filtering and summarising the data. Please take special care while filtering the database – we kept unreliable records, and included data collected using different methods and with different sampling efforts. For analyses using species-level data, take care for synonymy of the taxa (see ‘canonical name’ and ‘species’ columns in the Taxonomy spreadsheet). As a note of caution, some species names represent complexes with cryptic genetic diversity170,171, or ambiguous (as in most invertebrate taxonomic systems), and thus interpretations about species distributions should be done with care.
The database, as a part of the #GlobalCollembola initiative, will be curated and continue to be expanded with contributions of new data. We also will upload our data to Edaphobase165 and GBIF172 for easier findability and better interoperability. This data paper describes a static version of the database at the publication date, while new updates will be available from other open online sources. We are also working on complementary trait and literature databases on springtails for community use, which will become openly available in upcoming years. This work is currently curated by the core committee of #GlobalCollembola, constituted of 20 volunteer data providers and experts.
Our database is useful for analyses of global and regional spatial patterns in springtail abundance and diversity5. The database includes time series data across seasons and years, and data on spatial variation within sites across samples and soil layers, allowing for in-depth analyses of dynamics of springtail communities. We also believe that the database is a valuable resource for species distribution modelling of soil organisms. All records in our database are the ‘event’ type of data, representing communities where all observed species are also recorded. This allows for reconstructing true absences by comparing species lists of different sites across datasets. Overall, we believe that our data will serve to answer multiple long-standing questions in soil ecology and conservation.
Supplementary information
Acknowledgements
Data collection was supported by Deutsche Forschungsgemeinschaft (493345801 to AP and 192626868—SFB 990 to SS) and by the Russian Science Foundation (19-74-00154 to A.P.). Publicaation was funded by the Open Access Publishing Fund of Leipzig University, which is supported by the German Research Foundation within the program Open Access Publication Funding. The following authors acknowledge support of the local funding agencies: NA to Russian Science Foundation, grant number 22-24-00984, BCB to Brazilian National Council for Scientific and Technological Development (CNPq), grant number #309114/2021-7, TWC to German Ministry of Education, Science and Technology (BMBF), LK to VEGA 1/0438/22 (Slovak Scientific Grant Agency), APVV-21-0379 (Slovak Research and Development Agency), JMA to Carl Tryggers Stiftelse för Vetenskaplig Forskning, DBa to CNPq/Brasil, ADB to Marsden Fund Council from Government funding, managed by Royal Society Te Apārangi, MCho to Centre National de la Recherche Scientifique (CNRS), Zone Atelier ‘Arrière-Pays Mediterranéen’, SLC to DST-NRF Centre for Invasion Biology, Australian Research Council SRIEAS Grant SR200100005 Securing Antarctica’s Environmental Future, ATC to US National Science Foundation grants DEB9816001 and DEB0236204 and the American Association of University Women grant., JD to SFB 990-192626868/Deutsche Forschungsgemeinschaft, NE to German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig, funded by the German Research Foundation (FZT 118), OFe to European Research Council, grant no. 677232, German Research Foundation, FZT 118, 202548816, Government of Alberta, Canada (Alberta Environment and Parks), permit no. 19–260, St. John’s University, CF to grant TE, PN-III-P1-1.1-TE-2019-0358, OFr to NWO grant 821.01.015, DW to Ministry of Innovation and Technology of Hungary TKP2021-NKTA-43. MG to National Natural Science Foundation of China, project numbers #41871042, # 42271051, MG to South African National Antarctic Programme Grant, National Research Foundation 17034, ITH to NSERC Discovery Grant RGPIN-2019-07215, MHa to JSPS KAKENHI #22580175, 2645027, 19K06133, CH to Région Île-de-France, TH to JSPS KAKENHI Grant Number #JP19780118, PH to MICINN project INTERCAPA (CGL2014-56739-R) P.H. was supported by a FPI-MICINN grant, MI to Estonian Science Foundation Grant No 9145, BJ to Part financed by the European Social Fund and Martins, TLC Ltd. under the Knowledge Economy Skills Scholarships (KESS2) programme, CJS to SA-France bilateral grant to C.J., SA (NRF)/Russia (RFBR) Joint Science and 750 Technology Research Collaboration project no. 19-516-60002 (FRBR) and no. 118904 (NRF)., MJ to European Research Council, grant no. 677232, German Research Foundation, FZT 118, 202548816; Government of Alberta, Canada (Alberta Environment and Parks), permit no. 19–260; St. John’s University, SJ to These different programs were supported by the French National Agency of Research (ANR) (JASSUR research project; ANR-12-VBDU-0011), by the “Ministère de l’Agriculture et de la Pêche” and the “Ministère de l’Education Nationale de la Recherche et de la Technologie” (ACTA programme), by the “Ministère de l’Aménagement du Territoire et de l’Environnement” (Pnetox programme), by the EU-funded project, ECOGEN QLK5-CT-2002-01666, by the “Agence de l’Environnement et de la Maîtrise de l’Énergie” (BIOINDICATEUR 2, BIOTECHNOSOL), and by ANDRA, BCSJ to Nexus Project grant: MCTI/CNPq Grant 441280/2017-0, EJ to The Latvian Council of Science, Grants #90.108., 93.140., 96.0110., 01.0344., AK to Estonian Science Foundation, Grant No 9258 and R&D project No B02, JZL to Deutsche Forschungsgemeischaft, Projektnummer #316045089, AM to Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) project number 192 626 868, MAM to Massey University Research Fund (2004, 2014, 2017), GIM to We acknowledge the Marsden Fund Council from Government funding, managed by Royal Society Te Apārangi, ROH to Ramón y Cajal program of the MICINN (RYC-2017 22032), by the R&D Project of the Ministry of Science and Innovation PID2019-106004RA-I00 funded by MCIN/AEI/10.13039/501100011033, LCIOF to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the scholarship granted (155778/2018-8), MMP to DFG Priority Program 1374 “Infrastructure-Biodiversity-Exploratories” (SCHE 376/22-3), PQ to MUBIL: Ministry of agriculture; DIANA, Austria Bodenfauna österreichischer Naturwälder - Diversität, Gemeinschaftsmuster, Monitoring; VineDivers Projekt: FWF: I 2042-B25 and EU BiodivERsA/FACCE JPI; Diversität und Wirksamkeit von Nutzarthropoden in Abhängigkeit von der Landschaftsstruktur: FWF Austria P16972; Austrian Climate and Energy Fund: ACRP 6; Austria Academy of Science: Heritage_2020-043_Modeling-Museum, BR to Marsden Fund Council from Government funding, managed by Royal Society Te Apārangi, NR to Slovak Scientific Grant Agency VEGA 1/0438/22 and Slovak Research and Development Agency APVV-21-838 0379, MIR to Higher Education Commission of Pakistan, LR to The Natural Resources Canada EcoEnergy Innovation Initiative (under the Office of Energy Research and Development), the Natural Sciences and Engineering Research Council of Canada, the Canadian Forest Service, the Ontario Ministry of Natural Resources, the Canadian Foundation for Innovation, the regional forest industry (Tembec, Ontario Power Generation), the First Nations (Northeast Superior Regional Chief’s Forum) and the local communities (the Northeast Superior Forest Community)., RAS to RSF 21-74-00126, MSa to French Agence Nationale pour la Recherche #ANR-12-BSV7-0016-01, NS to Deutsche Forschungsgemeinschaft, research Unit FOR918, JS to National Park Hohe Tauern, Grant 7.6.1b-I8-24/16, XS to National Natural Science Foundation of China, Grant/Award Number: 31861133006, 41571052, 41811530086; National Science and Technology Fundamental Resources Investigation Program of ChinaGrant/Award Number: 2018FY100300; the Alexander von Humboldt Foundation, WIS to Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—project number 192626868—SFB 990 in the framework of the collaborative German–Indonesian research project CRC990, AAT to The state assignment of the Institute of Biology of the Komi Science Center of the Ural Branch of the Russian Academy of Sciences (project no.122040600025-2), LST to This project was funded by Nanyang Technological University’s URECA Undergraduate Research Programme., AMT to The work was supported by South African National Research Foundation Grant SNA14071475789, MNT to South African National Antarctic Programme, National Research Foundation Grant 17034, BW to Conselho Nacional de Desenvolvimento Científico eTecnológico CNPq, Brazil (Grant: 141400/2013-7, Grant: 152717/2016-1, Grant: 441280/2017-0, Grant: 151124/2019-1), DZ to CNPq-PQ #301460/2022-1.
Author contributions
J.M.A., D.A., F.A., A.B.B., I.B., C.R.D.M.B., D.Ba., M.B., M.P.B., V.B., S.B., A.I.B., T.B., M.B., R.A.B., G.C.M., M.Cha., T.W.C., M.Cho., S.L.C., A.T.C., J.C., P., E.C.A.D.L., L.E.D., E.D.M., J.D., N.E., O.Fe., A.S.F., S.S.D.F., C.F., J.F., O.Fr., S.F., M.G., B.G.B., C.G., M.G., S.H.K., I.T.H., M.Ha., C.H., T.H., M.Ho., P.H., T.T.H., M.I., B.J., C.J.S., S.J., B.C.S.J., E.J., E.M.K., K., E.J.K., A.K., N.K., W.N.L., D.L., Z.L., W.P.A.L., J.Z.L., M.J.L., M.T.M., A.M., M.A.M.C., M.A.M., G.I.M., D.M., T.N., I.N., R.O.H., L.C.I.O.F., J.M.P.V., M.M.P., J.F.P., A.M.P., M.B.P., P.Q., B.R., N.R., M.I.R., L.J.R.L., A.S.R., G.M.R., L.R., D.J.R., R.A.S., S.Sa., M.Sa., A.K.S., E.J.S., N.S., J.S., Y.B.S., S.St., X.S., A.A.T, L.S.T., M.P.T., A.M.T., M.T., M.N.T., A.V.U., L.A.W., B.W., D.Wi., D.Wu., Z.X., R.Y., R.A.Z., D.Z., B.Z., M.Z. collected and extracted springtails for the raw data production. D.A., J.A., T.A., F.A., A.B.B., I.B., C.R.D.M.B., D.Ba., B.C.B., M.B., M.P.B., V.B., S.B., A.I.B., T.B., R.A.B., M.Cha., T.W.C., M.Cho., Y.C., J.C., P., E.C.A.D.L., L.E.D., E.D.M., A.S.F., S.S.D.F., C.F., J.F., O.Fr., S.F., E.G.K., M.G., B.G.B., C.G., S.H.K., M.Ha., C.H., T.H., P.H., B.J., C.J.S., S.J., B.C.S.J., E.J., E.M.K., K., E.J.K., P.H.K., A.K., N.K., W.N.L., D.L., W.P.A.L., M.J.L., M.T.M., A.M., M.A.M.C., M.A.M., G.I.M., T.N., I.N., U.N.N., L.C.I.O.F., J.M.P.V., M.M.P., J.F.P., A.M.P., M.B.P., P.Q., N.R., M.I.R., L.J.R.L., G.M.R., L.R., D.J.R., R.A.S., S.Sa., M.Sa., A.K.S., N.S., C.S., P.S., Y.B.S., S.St., M.St., X.S., W.I.S., A.A.T., L.S.T., M.P.T., A.M.T., M.T., M.N.T., A.V.U., L.A.W., B.W., D.Wi., D.Wu., Z.X., R.Y., D.Z., B.Z., M.Z. identified springtails for the raw data production. J.M.A., D.A., J.A., T.A., F.A., A.B.B., I.B., C.R.D.M.B., D.Ba., M.P.B., S.B., T.B., G.C.M., M.Cha., T.W.C., M.Cho., S.L.C., A.T.C., J.C., P.,E.C.A.D.L., L.E.D., E.D.M., J.D., O.Fe., C.F., J.F., O.Fr., S.F., E.G.K., M.G., B.G.B., M.G., M.Ha., T.H., M.Ho., P.H., T.T.H., M.I., B.J., C.J.S., M.J., S.J., B.C.S.J., E.J., E.M.K., K., E.J.K., P.H.K., A.K., N.K., W.N.L., D.L., Z.L., W.P.A.L., J.Z.L., M.J.L., M.T.M., A.M., M.A.M.C., M.A.M., T.N., I.N., R.O.H., M.M.P., J.F.P., A.M.P., M.B.P., P.Q., B.R., M.I.R., L.J.R.L., G.M.R., L.R., R.A.S., S.Sa., M.Sa., A.K.S., E.J.S., N.S., C.S., J.S., Y.B.S., M.St., A.V.S., X.S., W.I.S., A.A.T., L.S.T., M.P.T., M.T., M.N.T., A.V.U., L.A.W., B.W., D.Wi., D.Wu., Z.X., R.Y. cleaned and standardized the data according to the template. D.A., J.A., F.A., I.B., C.R.D.M.B., D.Ba., A.D.B., M.B., M.P.B., A.I.B., M.B., R.A.B., D.Bu., G.C.M., T.W.C., S.L.C., A.T.C., J.C., E.C.A.D.L., E.D.M., J.D., N.E., J.E., O.Fe., C.F., O.Fr., E.G.K., B.G.B., C.G., M.G., S.H.K., I.T.H., M.Ha.,. T.H., M.Ho., T.T.H., M.I., C.J.S., S.J., B.C.S.J., E.J., E.M.K., K., E.J.K., P.H.K., A.K., N.K., W.N.L., D.L., Z.L., W.P.A.L., J.Z.L., M.J.L., M.T.M., A.M., M.A.M.C., D.M., U.N.N., R.O.H., J.F.P., A.M.P., M.B.P., P.Q., M.I.R., G.M.R., D.J.R., S.Sa., M.Sa., A.K.S., E.J.S., S.Sc., J.S., Y.B.S., E.M.S., M.St., X.S., A.A.T., A.M.T., A.V.U., L.A.V., R.W., B.W., D.Wu., Z.X., R.Y., D.Z., O.K.F. closely supervised the process of data collection, identification, and standardization. A.B.B., B.C.B., T.W.C., L.E.D., K., N.K., J.F.P., A.M.P., M.B.P., A.V.S. curated and/or evaluated the contributed datasets in the common database. A.M.P. coordinated the database creation and drafted the manuscript. All authors edited and commented on the final draft.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Code availability
Programming R code is openly available together with the database from Figshare167.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41597-023-02784-x.
References
- 1.Decaëns T, Jiménez JJ, Gioia C, Measey GJ, Lavelle P. The values of soil animals for conservation biology. Eur. J. Soil Biol. 2006;42:S23–S38. doi: 10.1016/j.ejsobi.2006.07.001. [DOI] [Google Scholar]
- 2.Food and Agriculture Organization of the United Nations, Global Soil Biodiversity Initiative, Secretariat of the Convention of Biological, European Commission & Intergovernmental Technical Panel on Soils. State of knowledge of soil biodiversity - Status, challenges and potentialities: Report 2020. (Food & Agriculture Org., 2020).
- 3.Phillips HRP, et al. Global distribution of earthworm diversity. Science. 2019;366:480–485. doi: 10.1126/science.aax4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Hoogen J, et al. Soil nematode abundance and functional group composition at a global scale. Nature. 2019;572:194–198. doi: 10.1038/s41586-019-1418-6. [DOI] [PubMed] [Google Scholar]
- 5.Potapov AM, et al. Globally invariant metabolism but density-diversity mismatch in springtails. Nat. Commun. 2023;14:674. doi: 10.1038/s41467-023-36216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schultheiss P, et al. The abundance, biomass, and distribution of ants on Earth. Proc. Natl. Acad. Sci. USA. 2022;119:e2201550119. doi: 10.1073/pnas.2201550119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavelle P, et al. Soil macroinvertebrate communities: A world‐wide assessment. Glob. Ecol. Biogeogr. 2022;31:1261–1276. doi: 10.1111/geb.13492. [DOI] [Google Scholar]
- 8.Cameron EK, et al. Global mismatches in aboveground and belowground biodiversity. Conserv. Biol. 2019;33:1187–1192. doi: 10.1111/cobi.13311. [DOI] [PubMed] [Google Scholar]
- 9.Guerra CA, et al. Tracking, targeting, and conserving soil biodiversity. Science. 2021;371:239–241. doi: 10.1126/science.abd7926. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg Y, et al. The global biomass and number of terrestrial arthropods. Sci Adv. 2023;9:eabq4049. doi: 10.1126/sciadv.abq4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mawan A, et al. Response of arboreal Collembola communities to the conversion of lowland rainforest into rubber and oil palm plantations. BMC Ecol Evol. 2022;22:144. doi: 10.1186/s12862-022-02095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Potapov A, 2020. Towards a global synthesis of Collembola knowledge – challenges and potential solutions. [DOI]
- 13.Bellinger PF, Christiansen KA, Janssens F. Checklist of the Collembola of the World. In O. Bánki, et al., Catalogue of Life Checklist (Apr 2023). 2023 doi: 10.48580/dfs6-4kh. [DOI] [Google Scholar]
- 14.van den Hoogen J, et al. A global database of soil nematode abundance and functional group composition. Sci Data. 2020;7:103. doi: 10.1038/s41597-020-0437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips HRP, et al. Global data on earthworm abundance, biomass, diversity and corresponding environmental properties. Sci Data. 2021;8:136. doi: 10.1038/s41597-021-00912-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Susanti WI, et al. Conversion of rainforest into oil palm and rubber plantations affects the functional composition of litter and soil Collembola. Ecol Evol. 2021;11:10686–10708. doi: 10.1002/ece3.7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alatalo JM, Jägerbrand AK, Čuchta P. Collembola at three alpine subarctic sites resistant to twenty years of experimental warming. Scientific Reports. 2015;5:18161. doi: 10.1038/srep18161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arbea JI, Blasco-Zumeta J. Ecología de los Colémbolos (Hexapoda, Collembola) en los Monegros (Zaragoza, España) Boletín de la Soc Entom Arag (S.E.A.) 2001;28:35–48. [Google Scholar]
- 19.Arbea JI, Martínez-Monteagudo A. Los colémbolos (Hexapoda, Collembola) asociados a plantas aromáticas (Labiatae) silvestres y cultivadas de la comarca valenciana de la Serranía. Boletín de la Asoc esp de Entom. 2006;30:59–71. [Google Scholar]
- 20.Arbea JI, Ariza E. Dinámica estacional y características de las comunidades de Collembola en playas de la Costa Brava (Girona, España) Boletín de la Soc Entom Arag (S.E.A.) 2012;51:203–210. [Google Scholar]
- 21.Ashwood, F. et al. Earthworms and soil mesofauna as early bioindicators for landfill restoration. Soil Research Online Early (2022).
- 22.Bendjaballah M, et al. Annotated checklist of the springtails (Hexapoda: Collembola) of the Collo massif, northeastern Algeria. Zoosystema. 2018;40:389–414. doi: 10.5252/zoosystema2018v40a16. [DOI] [PubMed] [Google Scholar]
- 23.Bokhorst S, Berg MP, Wardle DA. Micro-arthropod community responses to ecosystem retrogression in boreal forest. Soil Biol Biochem. 2017;110:79–86. doi: 10.1016/j.soilbio.2017.03.009. [DOI] [Google Scholar]
- 24.Bokhorst S, et al. Dwarf shrub and grass vegetation resistant to long-term experimental warming while microarthropod abundance declines on the Falkland Islands. Austral Ecology. 2017;42:984–994. doi: 10.1111/aec.12527. [DOI] [Google Scholar]
- 25.Bokhorst S, et al. Climate change effects on soil arthropod communities from the Falkland Islands and the Maritime Antarctic. Soil Biol Biochem. 2008;40:1547–1556. doi: 10.1016/j.soilbio.2008.01.017. [DOI] [Google Scholar]
- 26.Bokhorst S, et al. Responses of communities of soil organisms and plants to soil aging at two contrasting long-term chronosequences. Soil Biol Biochem. 2017;106:69–79. doi: 10.1016/j.soilbio.2016.12.014. [DOI] [Google Scholar]
- 27.Bokhorst S, Metcalfe DB, Wardle DA. Reduction in snow depth negatively affects decomposers but impact on decomposition rates is substrate dependent. Soil Biol Biochem. 2013;62:157–164. doi: 10.1016/j.soilbio.2013.03.016. [DOI] [Google Scholar]
- 28.Bokhorst S, et al. Extreme winter warming events more negatively impact small rather than large soil fauna: shift in community composition explained by traits not taxa. Global Change Biology. 2012;18:1152–1162. doi: 10.1111/j.1365-2486.2011.02565.x. [DOI] [Google Scholar]
- 29.Bokhorst S, et al. Contrasting responses of springtails and mites to elevation and vegetation type in the sub-Arctic. Pedobiologia. 2018;67:57–64. doi: 10.1016/j.pedobi.2018.02.004. [DOI] [Google Scholar]
- 30.Bokhorst S, et al. Impact of understory mosses and dwarf shrubs on soil micro-arthropods in a boreal forest chronosequence. Plant and Soil. 2014;379:121–133. doi: 10.1007/s11104-014-2055-3. [DOI] [Google Scholar]
- 31.Bokhorst S, Wardle DA. Snow fungi as a food source for micro-arthropods. Europ Jour Soil Biol. 2014;60:77–80. doi: 10.1016/j.ejsobi.2013.11.006. [DOI] [Google Scholar]
- 32.Bolger T, Curry JP. Effects of cattle slurry on soil arthropods in grassland. Pedobiologia. 1980;20:246–253. doi: 10.1016/S0031-4056(23)03537-0. [DOI] [Google Scholar]
- 33.Bolger T, Curry JP. Influences of pig slurry on soil microarthropods in grassland. Rev d’Ecolog Biolog Sol. 1984;21:269–281. [Google Scholar]
- 34.Raymond-Leonard L, et al. Dead wood provides habitat for springtails across a latitudinal gradient of forests in Quebec, Canada. Forest Ecol Manag. 2020;472:118237. doi: 10.1016/j.foreco.2020.118237. [DOI] [Google Scholar]
- 35.Gomez-Anaya JA, Castaño-Meneses G, Palacios-Vargas JG. Land use at St. Marta Range, Los Tuxtlas, Veracruz, Mexico-how does it affect the Collembola community? Appl Ecol Envir Res. 2018;16:4357–4373. doi: 10.15666/aeer/1604_43574373. [DOI] [Google Scholar]
- 36.Chauvat M, et al. Changes in soil faunal assemblages during conversion from pure to mixed forest stands. For Ecol Manag. 2011;262:317–324. doi: 10.1016/j.foreco.2011.03.037. [DOI] [Google Scholar]
- 37.Chomel M, et al. Secondary metabolites of Pinus halepensis alter decomposer organisms and litter decomposition during afforestation of abandoned agricultural zones. Journal of Ecology. 2014;102:411–424. doi: 10.1111/1365-2745.12205. [DOI] [Google Scholar]
- 38.Liu WPA, Janion C, Chown SL. Collembola diversity in the critically endangered Cape Flats Sand Fynbos and adjacent pine plantations. Pedobiologia. 2012;55:203–209. doi: 10.1016/j.pedobi.2012.03.002. [DOI] [Google Scholar]
- 39.Janion-Scheepers C, et al. High spatial turnover of springtails in the Cape Floristic Region. J Biogeogr. 2020;47:1007–1018. doi: 10.1111/jbi.13801. [DOI] [Google Scholar]
- 40.Treasure AM, et al. Species-energy relationships of indigenous and invasive species may arise in different ways – a demonstration using springtails. Scientific Reports. 2019;9:13799. doi: 10.1038/s41598-019-48871-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Classen AT, et al. Impacts of herbivorous insects on decomposer communities during the early stages of primary succession in a semi-arid woodland. Soil Biol Biochem. 2006;38:972–982. doi: 10.1016/j.soilbio.2005.08.009. [DOI] [Google Scholar]
- 42.Classen AT, et al. Season mediates herbivore effects on litter and soil microbial abundance and activity in a semi-arid woodland. Plant Soil. 2007;295:217–227. doi: 10.1007/s11104-007-9277-6. [DOI] [Google Scholar]
- 43.Cebron A, et al. Biological functioning of PAH-polluted and thermal desorption-treated soils assessed by fauna and microbial bioindicators. Res Microbiol. 2011;162:896–907. doi: 10.1016/j.resmic.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Cluzeau D, et al. Intégration de la biodiversité des sols dans les reseaux de surveillance de la qualité des sols: exemple du programme-pilote à l’échelle régionale, le RMQS BioDiv. Etude Gest Sols. 2009;16:187–201. [Google Scholar]
- 45.Cluzeau D, et al. Integration of biodiversity in soil quality monitoring: baselines for microbial and soil fauna parameters for different land-use types. Eur J Soil Biol. 2012;49:63–72. doi: 10.1016/j.ejsobi.2011.11.003. [DOI] [Google Scholar]
- 46.Cortet J, et al. Evaluation of effects of transgenic Bt maize on microarthropods in a European multi-site experiment. Pedobiologia. 2007;51:207–218. doi: 10.1016/j.pedobi.2007.04.001. [DOI] [Google Scholar]
- 47.Cortet J, et al. Impacts of different agricultural practices on the biodiversity of microarthropod communities in arable crop systems. Eur J Soil Biol. 2002;38:239–244. doi: 10.1016/S1164-5563(02)01152-4. [DOI] [Google Scholar]
- 48.El Zemrany H, et al. Field survival of the phytostimulator Azospirillum lipoferum CRT1 and functional impact on maize crop, biodegradation of crop residues, and soil faunal indicators in a context of decreasing nitrogen fertilisation. Soil Biol Biochem. 2006;38:1712–1726. doi: 10.1016/j.soilbio.2005.11.025. [DOI] [Google Scholar]
- 49.Huot H, et al. Diversity and activity of soil fauna in an industrial settling pond managed by natural attenuation. Appl Soil Ecol. 2018;132:34–44. doi: 10.1016/j.apsoil.2018.08.020. [DOI] [Google Scholar]
- 50.Joimel S, et al. Contrasting homogenization patterns of plant and collembolan communities in urban vegetable gardens. Urban Ecosystems. 2019;22:553–556. doi: 10.1007/s11252-019-00843-z. [DOI] [Google Scholar]
- 51.Joimel S, et al. Functional and Taxonomic Diversity of Collembola as Complementary Tools to Assess Land Use Effects on Soils Biodiversity. Frontiers Ecol Evol. 2021;9:630919. doi: 10.3389/fevo.2021.630919. [DOI] [Google Scholar]
- 52.Renaud A, Poinsot-Balaguer N, Cortet J. & Le Petit, J. Influence of four soil maintenance practices on Collembola communities in a Mediterranean vineyard. Pedobiologia. 2004;48:623–630. doi: 10.1016/j.pedobi.2004.07.002. [DOI] [Google Scholar]
- 53.Santorufo L, et al. Early colonization of constructed Technosol by microarthropods. Ecol Engin. 2021;162:106174. doi: 10.1016/j.ecoleng.2021.106174. [DOI] [Google Scholar]
- 54.Doblas-Miranda E, Espelta JM, Pino J. Connectivity affects species turnover in soil microarthropod communities during Mediterranean forest establishment. Ecosphere. 2021;12:e03865. doi: 10.1002/ecs2.3865. [DOI] [Google Scholar]
- 55.Ferreira AS, Bellini BC, Vasconcellos A. Temporal variations of Collembola (Arthropoda: Hexapoda) in the semiarid Caatinga in northeastern Brazil. Zoologia. 2013;30:639–644. doi: 10.1590/S1984-46702013005000009. [DOI] [Google Scholar]
- 56.Franken O, et al. A Common Yardstick to Measure the Effects of Different Extreme Climatic Events on Soil Arthropod Community Composition Using Time-Series Data. Frontiers Ecol Evol. 2018;6:195. doi: 10.3389/fevo.2018.00195. [DOI] [Google Scholar]
- 57.Gao MX, et al. Distinct patterns suggest that assembly processes differ for dominant arthropods in above-ground and below-ground ecosystems. Pedobiologia. 2018;69:17–28. doi: 10.1016/j.pedobi.2018.06.003. [DOI] [Google Scholar]
- 58.Cassagne N, et al. Changes in humus properties and collembolan communities following the replanting of beech forests with spruce. Pedobiologia. 2004;48:267–276. doi: 10.1016/j.pedobi.2004.01.004. [DOI] [Google Scholar]
- 59.Hasegawa M, et al. Effects of roads on collembolan community structure in subtropicalevergreen forests on Okinawa Island, southwestern Japan. Pedobiologia. 2015;58:13–21. doi: 10.1016/j.pedobi.2014.11.002. [DOI] [Google Scholar]
- 60.Hasegawa M, et al. The effects of mixed broad-leaved trees on the collembolan community in larch plantations of central Japan. Appl Soil Ecol. 2014;83:125–132. doi: 10.1016/j.apsoil.2013.06.005. [DOI] [Google Scholar]
- 61.Heiniger C, et al. Effect of habitat spatiotemporal structure on collembolan diversity. Pedobilogia. 2014;57:103–117. doi: 10.1016/j.pedobi.2014.01.006. [DOI] [Google Scholar]
- 62.Hishi, T. et al. Topography is more important than forest type as a determinant for functional trait composition of Collembola community. Pedobiologia90, 150776
- 63.Bonfanti J, et al. Communities of Collembola show functional resilience in a long-term field experiment simulating climate change. Pedobiologia. 2022;90:10. doi: 10.1016/j.pedobi.2022.150789. [DOI] [Google Scholar]
- 64.Holmstrup M, et al. Functional diversity of Collembola is reduced in soils subjected to short-term, but not long-term, geothermal warming. Funct Ecol. 2018;32:1304–1316. doi: 10.1111/1365-2435.13058. [DOI] [Google Scholar]
- 65.Holmstrup M, et al. Soil microarthropods are only weakly impacted after 13 years of repeated drought treatment in wet and dry heathland soils. Soil Biol Biochem. 2013;66:110–118. doi: 10.1016/j.soilbio.2013.06.023. [DOI] [Google Scholar]
- 66.Homet P, et al. Soil fauna modulates the effect of experimental drought on litter decomposition in forests invaded by an exotic pathogen. Journal of Ecology. 2021;109:2963–2980. doi: 10.1111/1365-2745.13711. [DOI] [Google Scholar]
- 67.Ivask M, et al. Springtails of flooded meadows along Matsalu Bay and the Kasari River, Estonia. Pedobiologia. 2018;66:1–10. doi: 10.1016/j.pedobi.2017.12.001. [DOI] [Google Scholar]
- 68.Jacques RG, et al. Earthworm-Collembola interactions affecting water-soluble nutrients, fauna and physiochemistry in a mesocosm manure-straw composting experiment. Waste Management. 2021;134:57–66. doi: 10.1016/j.wasman.2021.08.008. [DOI] [PubMed] [Google Scholar]
- 69.Ouvrard S, et al. In situ assessment of phytotechnologies for multicontaminated soil management. Int J Phytoremed. 2011;13:245–263. doi: 10.1080/15226514.2011.568546. [DOI] [PubMed] [Google Scholar]
- 70.Jorge BCS, et al. Effects of defoliation frequencies on above- and belowground biodiversity and ecosystem processes in subtropical grasslands of southern Brazil. Pedobiologia. 2022;90:150786. doi: 10.1016/j.pedobi.2021.150786. [DOI] [Google Scholar]
- 71.Jorge BCS, et al. Grassland afforestation with Eucalyptus affect Collembola communities and soil functions in southern Brazil. Biodivers Conserv. 2022;32:275–295. doi: 10.1007/s10531-022-02501-x. [DOI] [Google Scholar]
- 72.Jucevica E, Melecis V. Global warming affect Collembola community: A long-term study. Pedobiologia. 2006;50:177–184. doi: 10.1016/j.pedobi.2005.10.006. [DOI] [Google Scholar]
- 73.Juceviča E, Melecis V. Long-term dynamics of Collembola in a pine forest ecosystem. Pedobiologia. 2002;46:365–372. doi: 10.1078/0031-4056-00144. [DOI] [Google Scholar]
- 74.Kapinga EM, et al. Collembola Communities, 20 Years After the Establishment of Distinct Revegetation Treatments in a Severely Eroded Area in South Iceland. Studia Ecolog Bioethic. 2022;20:37–50. doi: 10.21697/seb.2022.28. [DOI] [Google Scholar]
- 75.Kováč, et al. Soil Oribatida and Collembola communities across a land depression in an arable field. Eur J Soil Biol. 2001;37:285–289. doi: 10.1016/S1164-5563(01)01106-2. [DOI] [Google Scholar]
- 76.Kováč Ľ, et al. Comparison of collembolan assemblages (Hexapoda, Collembola) of thermophilous oak wood and Pinus nigra plantation in the Slovak Karst (Slovakia) Pedobiologia. 2005;49:29–40. doi: 10.1016/j.pedobi.2004.07.009. [DOI] [Google Scholar]
- 77.Krab EJ, et al. Turning northern peatlands upside down: disentangling microclimate and substrate quality effects on vertical distribution of Collembola. Functional Ecology. 2010;24:1362–1369. doi: 10.1111/j.1365-2435.2010.01754.x. [DOI] [Google Scholar]
- 78.Krab EJ, et al. Plant expansion drives bacteria and collembola communities under winter climate change in frost-affected tundra. Soil Biol Biochem. 2019;138:107569. doi: 10.1016/j.soilbio.2019.107569. [DOI] [Google Scholar]
- 79.Kuznetsova NA, Sterzynska M. Effects of single trees on the community structure of soil-dwelling Collembola in urban and non-urban environments. Fragmenta faunistica. 1995;37:413–426. doi: 10.3161/00159301FF1995.37.18.413. [DOI] [Google Scholar]
- 80.Sterzynska M, Kuznetsova N. The faunal complex of Collembola in lowland subcontinental pine forests (Peucedano-Pinetum) of Poland, Byelorussia, Lithuania and Russia. Fragmenta faunistica. 1995;38:145–153. doi: 10.3161/00159301FF1995.38.4.145. [DOI] [Google Scholar]
- 81.Krest’yaninova AI, Kuznetsova NA. Dynamics of collembolan (Hexapoda, Collembola) association in the soil of an urban boulevard. Entomological Review. 1996;76:1220–1230. [Google Scholar]
- 82.Kuznetsova NA, Potapov MB. Changes in structure of communities of soil springtails (Hexapoda: Collembola) under industrial pollution of the south-taiga bilberry pine forests. Russian. J Ecology. 1997;28:386–392. [Google Scholar]
- 83.Sterzynska M, Kuznetsova N. Comparative analysis of dominant species in springtail communities (Hexapoda: Collembola) of urban greens in Moscow and Warsaw. Fragmenta faunistica. 1997;40:15–26. doi: 10.3161/00159301FF1997.40.2.015. [DOI] [Google Scholar]
- 84.Chernova NM, Kuznetsova NA. Collembolan community organization and its temporal predictability. Pedobiologia. 2000;44:451–466. doi: 10.1078/S0031-4056(04)70063-3. [DOI] [Google Scholar]
- 85.Kuznetsova NA, Krest’yaninova AI. Long-term dynamics of collembolan communities (Hexapoda: Collembola) in hydrological series of pine forests in southern taiga. Entomological Review. 1998;78:969–981. [Google Scholar]
- 86.Kuznetsova NA. Classification of collembolan communities in the East-European taiga. Pedobiologia. 2002;46:373–384. [Google Scholar]
- 87.Kuznetsova NA. Biotopic Groups of Collembolans in the Mixed Forest Subzone of Eastern Europe. Entomological Review. 2002;82:1047–1057. [Google Scholar]
- 88.Kuznetsova NA. Humidity and Distribution of Springtails. Entomological Review. 2003;83:230–238. [Google Scholar]
- 89.Kuznetsova NA. Long-term dynamics of Collembola in two contrast ecosystems. Pedobiologia. 2006;50:157–164. doi: 10.1016/j.pedobi.2005.12.004. [DOI] [Google Scholar]
- 90.Kuznetsova NA. Long-term Dynamics of Collembolan population in Forest and Meadow Ecosystems. Entomological Review. 2007;87:11–24. doi: 10.1134/S0013873807010022. [DOI] [Google Scholar]
- 91.Kuznetsova NA. Soil-Dwelling Collembola in Coniferous Forests along the Gradient of Pollution with Emissions from the Middle Ural Copper Smelter. Russian J Ecology. 2009;40:415–423. doi: 10.1134/S106741360906006X. [DOI] [Google Scholar]
- 92.Chernov AV, Kuznetsova NA, Potapov MB. Springtail communities (Collembola) of Eastern European broad-leaf forests. Entomological Review. 2010;90:556–570. doi: 10.1134/S0013873810050039. [DOI] [Google Scholar]
- 93.Saraeva AK, Potapov MB, Kuznetsova NA. Different-Scale Distribution of Collembola in Uniform Ground Cover: Sphagnum Moss. Entomological Review. 2015;95:557–577. doi: 10.1134/S0013873815050012. [DOI] [Google Scholar]
- 94.Saraeva AK, Potapov MB, Kuznetsova NA. Different-Scale Distribution of Collembola in Uniform Ground Cover: stability of parameters in space and time. Entomological Review. 2015;95:699–713. doi: 10.1134/S0013873815060032. [DOI] [Google Scholar]
- 95.Kuznetsova NA, Saraeva AK. Beta-diversity partitioning approach in soil zoology: A case of Collembola in pine forests. Geoderma. 2018;332:142–152. doi: 10.1016/j.geoderma.2017.09.030. [DOI] [Google Scholar]
- 96.Kuznetsova N, Gomina A, Smirnova O, Potapov M. Soil mesofauna and diversity of vegetation: Collembola in pristine taiga forests (Pechora-Ilych Biosphere Reserve, Russia) Eur J Forest Res. 2018;137:659–674. doi: 10.1007/s10342-018-1132-1. [DOI] [Google Scholar]
- 97.Kuznetsova NA, Bokova AI, Saraeva AK, Shveenkova YB. Communities of Collembola in the Forests of Southern Primorye as a Benchmark of High Diversity and Organization Complexity. Biology Bulletin. 2019;46:483–491. doi: 10.1134/S1062359019050066. [DOI] [Google Scholar]
- 98.Kuznetsova NA, Bokova AI, Saraeva AK, Shveenkova YB. Structure of the Species Diversity of Soil Springtails (Hexapoda, Collembola) in Pine Forests of the Caucasus and the Russian Plain: a Multi-Scale Approach. Entomological Review. 2019;99:1–15. doi: 10.1134/S0013873819020027. [DOI] [Google Scholar]
- 99.Kuznetsova N, Ivanova N. Diversity of Collembola under various types of anthropogenic load on ecosystems of European part of Russia. Biodiv Data J. 2020;8:e58951. doi: 10.3897/BDJ.8.e58951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kuznetsova N, et al. The extremely high diversity of Collembola in relict forests of Primorskii Krai of Russia. Biodiv Data J. 2021;9:e76007. doi: 10.3897/BDJ.9.e76007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vasenkova NV, Kuznetsova NA. A multiscale approach to evaluating the diversity structure of Collembola in boreo-nemoral forests of the Russian Plane. Nature Cons Res. 2022;7:38–51. [Google Scholar]
- 102.Potapov MB, et al. Organic farming and moderate tillage change the dominance and spatial structure of soil Collembola communities but have little effects on bulk abundance and species richness. Soil Organisms. 2022;94:99–110. [Google Scholar]
- 103.Striuchkova A, Malykh I, Potapov M, Kuznetsova N. Sympatry of genetic lineages of Parisotoma notabilis s. l. (Collembola, Isotomidae) in the East European Plain. ZooKeys. 2022;1137:1–15. doi: 10.3897/zookeys.1137.95769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lu J-Z, Scheu S. 2022. RTG 2300 - Soil microarthropods (Collembola, Insecta) in current and future forest stands of Central Europe. Pangaea. [DOI]
- 105.Ochoa-Hueso R, et al. Simulated nitrogen deposition affects soil fauna from a semiarid Mediterranean ecosystem in central Spain. Biol Fertil Soil. 2014;50:191–196. doi: 10.1007/s00374-013-0838-y. [DOI] [Google Scholar]
- 106.Marx MT, et al. Responses and adaptations of collembolan communities (Hexapoda: Collembola) to flooding and hypoxic conditions. Pesq Agropec Brasil. 2009;44:1002–1010. doi: 10.1590/S0100-204X2009000800032. [DOI] [Google Scholar]
- 107.Marx MT, Weber D. Cave Collembola from Southwestern Germany. Soil Organisms. 2015;87:201–208. [Google Scholar]
- 108.Lessel T, Marx MT, Eisenbeis G. Effects of ecological flooding on the temporal and spatial dynamics of carabid beetles (Coleoptera, Carabidae) and springtails (Collembola) in a polder habitat. ZooKeys. 2011;100:421–446. doi: 10.3897/zookeys.100.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McCary MA, Wise DH. Plant invader alters soil food web via changes to fungal resources. Oecologia. 2019;191:587–599. doi: 10.1007/s00442-019-04510-0. [DOI] [PubMed] [Google Scholar]
- 110.Minor M, Babenko A, Ermilov S. Oribatid mites (Acari: Oribatida) and springtails (Collembola) in alpine habitats of southern New Zealand. NZ J Zoology. 2017;44:65–85. doi: 10.1080/03014223.2016.1251950. [DOI] [Google Scholar]
- 111.Nakamori T, et al. Collembolan fauna in arable land, including the first record of Mesaphorura silvicola (Folsom) from Japan. Edaphologia. 2009;84:5–9. [Google Scholar]
- 112.Negri I. Spatial distribution of Collembola in presence and absence of a predator. Pedobiologia. 2004;48:585–588. doi: 10.1016/j.pedobi.2004.07.004. [DOI] [Google Scholar]
- 113.Frati F, et al. Ultrastructural and molecular identification of a new Rickettsia endosymbiont in the springtail Onychiurus sinensis (Hexapoda, Collembola) J Invert Path. 2006;93:150–156. doi: 10.1016/j.jip.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 114.Frati F, et al. High levels of genetic differentiation between Wolbachia-infected and non-infected populations of Folsomia candida (Collembola, Isotomidae) Pedobiologia. 2004;48:461–468. doi: 10.1016/j.pedobi.2004.04.004. [DOI] [Google Scholar]
- 115.Mazzoglio PJ, et al. Pedofaunistic and soil investigation in Scots pine forests in the Aosta Valley and Piedmont (northwest Italy) Rev Vald d’Hist Nat. 2011;65:153–170. [Google Scholar]
- 116.Machado JS, et al. Morphological diversity of springtails (Hexapoda: Collembola) as soil quality bioindicators in land use systems. Biota Neotropica. 2019;19:e20180618. doi: 10.1590/1676-0611-bn-2018-0618. [DOI] [Google Scholar]
- 117.Ortiz DC, et al. Diversity of springtails (Collembola) in agricultural and forest systems in Southern Santa Catarina. Biota Neotropica. 2019;19:e20180720. doi: 10.1590/1676-0611-bn-2018-0720. [DOI] [Google Scholar]
- 118.Santos MAB, et al. Morphological Diversity of Springtails in Land Use Systems. Rev Brasil Ciên Solo. 2018;41:e0170277. [Google Scholar]
- 119.Pollierer MM, Scheu S. Driving factors and temporal fluctuation of Collembola communities and reproductive mode across forest types and regions. Ecol Evol. 2017;7:4390–4403. doi: 10.1002/ece3.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Querner P, Bruckner A. Combining pitfall traps and soil cores to collect Collembola for site scale biodiversity assessments. Appl Soil Ecol. 2010;45:293–297. doi: 10.1016/j.apsoil.2010.05.005. [DOI] [Google Scholar]
- 121.Querner P, et al. Effects of site and landscape parameters on Collembola diversity in 29 winter oilseed rape fields. Agr Ecos Env. 2013;164:145–154. doi: 10.1016/j.agee.2012.09.016. [DOI] [Google Scholar]
- 122.Winkler M, et al. Side by side? Vascular plant, invertebrate and microorganism distribution patterns along an alpine to nival elevation gradient. AAAR. 2018;50:e1475951. [Google Scholar]
- 123.Buchholz J, et al. Soil biota in vineyards are more influenced by plants than by tillage intensity, site parameters or the surrounding landscape. Scentific Reports. 2017;7:17445. doi: 10.1038/s41598-017-17601-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bruckner A, et al. No indication of methodological biases in tullgren and macfadyen extraction of edaphic microarthropods. Eur J Soil Biol. 2023;115:103464. doi: 10.1016/j.ejsobi.2022.103464. [DOI] [Google Scholar]
- 125.Kováč Ľ, Raschmanová N, Miklisová D. Comparison of collembolan assemblages (Hexapoda, Collembola) of thermophilous oak wood and Pinus nigra plantation in the Slovak Karst (Slovakia) Pedobiologia. 2005;49:29–40. doi: 10.1016/j.pedobi.2004.07.009. [DOI] [Google Scholar]
- 126.Raschmanová N, Kováč Ľ, Miklisová D. The effect of mesoclimate on the Collembola diversity in the Zádiel Valley, Slovak Karst (Slovakia) Eur J Soil Biol. 2008;44:463–472. doi: 10.1016/j.ejsobi.2008.07.005. [DOI] [Google Scholar]
- 127.Raschmanová N, Miklisová D, Kováč Ľ. A unique small-scale microclimatic gradient in a temperate karst harbours exceptionally high diversity of soil Collembola. Int J Speleol. 2018;47:247–262. doi: 10.5038/1827-806X.47.2.2194. [DOI] [Google Scholar]
- 128.Rashid MI, et al. Production-ecological modelling explains the difference between potential soil N mineralisation and actual herbage N uptake. Appl Soil Ecol. 2014;84:83–92. doi: 10.1016/j.apsoil.2014.07.002. [DOI] [Google Scholar]
- 129.Raymond-Léonard LJ, et al. Springtail community structure is influenced by functional traits but not biogeographic origin of leaf litter in soils of novel forest ecosystems. Proc Roy Soc B. 2018;285:20180647. doi: 10.1098/rspb.2018.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Raymond-Léonard LJ, Bouchard M, Handa IT. Dead wood provides habitat for springtails across a latitudinal gradient of forests in Quebec. Canada. For Ecol Manag. 2020;472:118237. [Google Scholar]
- 131.Rousseau L, et al. Long-term effects of biomass removal on soil mesofaunal communities in northeastern Ontario (Canada) jack pine (Pinus banksiana) stands. For Ecol Manag. 2018;421:72–83. doi: 10.1016/j.foreco.2018.02.017. [DOI] [Google Scholar]
- 132.Rousseau L, et al. Forest floor mesofauna communities respond to a gradient of biomass removal and soil disturbance in a boreal jack pine (Pinus banksiana) stand of northeastern Ontario (Canada) For Ecol Manag. 2018;407:155–165. doi: 10.1016/j.foreco.2017.08.054. [DOI] [Google Scholar]
- 133.Saifutdinov RA, Gongalsky KB, Zaitsev AS. Evidence of a trait-specific response to burning in springtails (Hexapoda: Collembola) in the boreal forests of European Russia. Geoderma. 2018;332:173–179. doi: 10.1016/j.geoderma.2017.07.021. [DOI] [Google Scholar]
- 134.Saifutdinov RA, Gongalsky KB, Zaitsev AS. Springtail (Hexapoda: Collembola) fauna in the burnt boreal forests of European Russia. Invert. Zoology. 2018;15:115–130. [Google Scholar]
- 135.Zaitsev AS, et al. Reduced functionality of soil food webs in burnt boreal forests: a case study in Central Russia. Contemp Probl Ecol. 2017;10:277–285. doi: 10.1134/S199542551703012X. [DOI] [Google Scholar]
- 136.Sayer EJ, et al. Arthropod abundance and diversity in the forest floor of a lowland tropical forest: the role of habitat space vs. nutrient concentrations. Biotropica. 2010;42:194–200. doi: 10.1111/j.1744-7429.2009.00576.x. [DOI] [Google Scholar]
- 137.Sayer EJ, Tanner EVJ, Lacey AL. Litter quantity affects early-stage decomposition and meso-arthropod abundance in a moist tropical forest. For Ecol Manag. 2006;229:285–293. doi: 10.1016/j.foreco.2006.04.007. [DOI] [Google Scholar]
- 138.Laird-Hopkins BC, Brechet LM, Sayer EJ. Tree functional diversity affects litter decomposition and arthropod community composition in a tropical forest. Biotropica. 2017;49:903–911. doi: 10.1111/btp.12477. [DOI] [Google Scholar]
- 139.Scheunemann N, et al. The role of shoot residues vs. crop species for soil arthropod diversity and abundance of arable systems. Soil Biol Biochem. 2015;81:81–88. doi: 10.1016/j.soilbio.2014.11.006. [DOI] [Google Scholar]
- 140.Seeber J, et al. Soil invertebrate diversity across steep high elevation snowmelt gradients in the European Alps. Arct Antar Alpine Res. 2021;53:288–299. doi: 10.1080/15230430.2021.1982665. [DOI] [Google Scholar]
- 141.Sterzynska M, et al. Urban species richness decreases with increasing air pollution. Ecological Indicators. 2018;94:328–335. doi: 10.1016/j.ecolind.2018.06.063. [DOI] [Google Scholar]
- 142.Rzeszowski K, Sterzyńska M. Changes through time in soil Collembola communities exposed to urbanization. Urban Ecosys. 2015;19:143–158. doi: 10.1007/s11252-015-0478-0. [DOI] [Google Scholar]
- 143.Xie Z, et al. Drivers of Collembola assemblages along an altitudinal gradient in northeast China. Ecol Evol. 2022;12:e8559. doi: 10.1002/ece3.8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sun X, et al. Response of Collembola to the addition of nutrients along an altitudinal gradient of tropical montane rainforests. Appl Soil Ecol. 2020;147:103382. doi: 10.1016/j.apsoil.2019.103382. [DOI] [Google Scholar]
- 145.Taskaeva, A. A. et al. Diversity of soil invertebrates in ecosystems near the Padimeyskie lakes in the Bolshezemelskaya tundra region of Russia. Euroas Entomol J14, 480–488 [in Russian] (2015).
- 146.Babenko AB, Potapov MB, Taskaeva AA. The Collembola fauna of the East-European tundra. Rus Entomol J. 2017;26:1–30. doi: 10.15298/rusentj.26.1.01. [DOI] [Google Scholar]
- 147.Konakova, T. N. et al. Diversity of soil invertebrates in ecosystems of the Chernaya river basin, the Bolshezemelskaya tundra, Nenetskii Autonomnyi Okrug, Russia. Euroas Entomol J16, 88–91 [in Russian] (2017).
- 148.Taskaeva, A. A. & Nakul, G. L. Collembola from the Korotaikha river valley of Bolshezemelskaya tundra, Nenetskii Autonomnyi Okrug of Russia. Euroas Entomol J16, 57–59 [in Russian] (2017).
- 149.Taskaeva AA, et al. Characteristics of the Microarthropod Communities in Postagrogenic and Tundra Soils of the European Northeast of Russia. Euras Soil Sci. 2019;52:661–670. doi: 10.1134/S1064229319060127. [DOI] [Google Scholar]
- 150.Konakova TN, Kolesnikova AA, Taskaeva AA. Soil invertebrate occurrences in European North-East of Russia. Biodiv Data J. 2020;8:e58836. doi: 10.3897/BDJ.8.e58836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Taskaeva AA, Kolesnikova AA, Nakul GL. Springtails (Hexapoda, Collembola) of some plant communities of the Pechora Delta. Rus Entomol J. 2020;29:343–349. doi: 10.15298/rusentj.29.4.01. [DOI] [Google Scholar]
- 152.2020. Taskaeva, Collembola of Kolguev, Malozemelskaya tundra and Delta Pechora. GBIF. [DOI]
- 153.Taskaeva A. 2019. Collembola of the Chernaya river basin. GBIF. [DOI]
- 154.Taskaeva A. 2018. Collembola of Padimeiskie lakes territory on the Bolshezemelskaya tundra (European North-East Russia) GBIF. [DOI]
- 155.Konakova T, Kolesnikova A, Taskaeva A. 2020. Soil invertebrates occurrences in European North-East of Russia. GBIF. [DOI] [PMC free article] [PubMed]
- 156.Thakur MP, Berg MP, Eisenhauer N, Van Langevelde F. Disturbance-diversity relation is explained by the community biomass of soil fauna in salt marsh. Soil Biol Biochem. 2014;78:30–37. doi: 10.1016/j.soilbio.2014.06.021. [DOI] [Google Scholar]
- 157.Tsiafouli MA, et al. Responses of soil microarthropods to experimental short-term manipulations of soil moisture. Appl Soil Ecol. 2005;29:17–26. doi: 10.1016/j.apsoil.2004.10.002. [DOI] [Google Scholar]
- 158.Widenfalk LW, et al. Small-scale Collembola community composition in a pine forest soil - Overdispersion in functional traits indicate the importance of species interactions. Soil Biol Biochem. 2016;103:52–62. doi: 10.1016/j.soilbio.2016.08.006. [DOI] [Google Scholar]
- 159.Winkler D, et al. Long-term ecological effects of the red mud disaster in Hungary: Regeneration of red mud flooded areas in a contaminated industrial region. Sci Tot Env. 2018;644:1292–1303. doi: 10.1016/j.scitotenv.2018.07.059. [DOI] [PubMed] [Google Scholar]
- 160.Harta I, et al. Collembola communities and soil conditions in forest plantations established in an intensively managed agricultural area. J For Res. 2021;32:1819–1832. doi: 10.1007/s11676-020-01238-z. [DOI] [Google Scholar]
- 161.Winkler D, Tóth V. Effects of afforestation with pines on Collembola diversity in the limestone hills of Szárhalom (West Hungary) Acta Silv Lign Hung. 2012;8:9–20. doi: 10.2478/v10303-012-0001-8. [DOI] [Google Scholar]
- 162.Winkler D, Traser GN. Eco-faunistic study on the Collembola fauna in the Vasvár-Nagymákfa area (Western Hungary) Natura Somogyiensis. 2012;22:39–52. doi: 10.24394/NatSom.2012.22.39. [DOI] [Google Scholar]
- 163.Szigeti N, et al. Soil mesofauna and herbaceous vegetation patterns in an agroforestry landscape. Agroforestry Systems. 2022;96:773–786. doi: 10.1007/s10457-022-00739-6. [DOI] [Google Scholar]
- 164.Ni Z, et al. Habitat preferences rather than morphological traits affect the recovery process of Collembola (Arthropoda, Hexapoda) on a bare saline–alkaline land. PeerJ. 2020;8:e9519. doi: 10.7717/peerj.9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Burkhardt U, et al. The Edaphobase project of GBIF-Germany—A new online soil-zoological data warehouse. Appl. Soil Ecol. 2014;83:3–12. doi: 10.1016/j.apsoil.2014.03.021. [DOI] [Google Scholar]
- 166.R Core Team, 2023. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- 167.Potapov A, 2023. #GlobalCollembola - full sample-level database. Figshare. [DOI]
- 168.Macfadyen A. Improved funnel-type extractors for soil arthropods. J. Anim. Ecol. 1961;30:171. doi: 10.2307/2120. [DOI] [Google Scholar]
- 169.Edwards CA. The assessment of populations of soil-inhabiting invertebrates. Agric. Ecosyst. Environ. 1991;34:145–176. doi: 10.1016/0167-8809(91)90102-4. [DOI] [Google Scholar]
- 170.Zhang B, Chen T-W, Mateos E, Scheu S, Schaefer I. Cryptic species in Lepidocyrtus lanuginosus (Collembola: Entomobryidae) are sorted by habitat type. Pedobiologia. 2018;68:12–19. doi: 10.1016/j.pedobi.2018.03.001. [DOI] [Google Scholar]
- 171.Porco D, et al. Challenging species delimitation in Collembola: Cryptic diversity among common springtails unveiled by DNA barcoding. Invertebrate Systematics. 2012;26:470–477. doi: 10.1071/IS12026. [DOI] [Google Scholar]
- 172.Heberling, J. M., Miller, J. T., Noesgaard, D., Weingart, S. B. & Schigel, D. Data integration enables global biodiversity synthesis. Proc. Natl. Acad. Sci. USA118, (2021). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Potapov A, 2020. Towards a global synthesis of Collembola knowledge – challenges and potential solutions. [DOI]
- Lu J-Z, Scheu S. 2022. RTG 2300 - Soil microarthropods (Collembola, Insecta) in current and future forest stands of Central Europe. Pangaea. [DOI]
- 2020. Taskaeva, Collembola of Kolguev, Malozemelskaya tundra and Delta Pechora. GBIF. [DOI]
- Taskaeva A. 2019. Collembola of the Chernaya river basin. GBIF. [DOI]
- Taskaeva A. 2018. Collembola of Padimeiskie lakes territory on the Bolshezemelskaya tundra (European North-East Russia) GBIF. [DOI]
- Konakova T, Kolesnikova A, Taskaeva A. 2020. Soil invertebrates occurrences in European North-East of Russia. GBIF. [DOI] [PMC free article] [PubMed]
- Potapov A, 2023. #GlobalCollembola - full sample-level database. Figshare. [DOI]
Supplementary Materials
Data Availability Statement
Programming R code is openly available together with the database from Figshare167.