Abstract
In healthy individuals, physical exercise improves cardiovascular health and muscle strength, alleviates fatigue and reduces the risk of chronic diseases. Although exercise is suggested as a lifestyle intervention to manage various chronic illnesses, it negatively affects people with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), who suffer from exercise intolerance. We hypothesized that altered extracellular vesicle (EV) signalling in ME/CFS patients after an exercise challenge may contribute to their prolonged and exacerbated negative response to exertion (post‐exertional malaise). EVs were isolated by size exclusion chromatography from the plasma of 18 female ME/CFS patients and 17 age‐ and BMI‐matched female sedentary controls at three time points: before, 15 min, and 24 h after a maximal cardiopulmonary exercise test. EVs were characterized using nanoparticle tracking analysis and their protein cargo was quantified using Tandem Mass Tag‐based (TMT) proteomics. The results show that exercise affects the EV proteome in ME/CFS patients differently than in healthy individuals and that changes in EV proteins after exercise are strongly correlated with symptom severity in ME/CFS. Differentially abundant proteins in ME/CFS patients versus controls were involved in many pathways and systems, including coagulation processes, muscle contraction (both smooth and skeletal muscle), cytoskeletal proteins, the immune system and brain signalling.
Keywords: chronic fatigue syndrome, exercise, extracellular vesicle cargo, ME/CFS, myalgic encephalomyelitis, proteomics
1. INTRODUCTION
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) is a complex and highly disabling chronic illness that affects millions of people worldwide. It can affect individuals of any age, gender or socioeconomic background and is characterized by persistent fatigue that is not relieved by rest, along with a range of other symptoms including muscle and joint pain, cognitive difficulties, sleep disturbances, and headaches (IOM, 2015). While the exact cause of ME/CFS remains unknown, it is believed to involve a complex interplay of genetic, environmental, and immunological factors.
One of the hallmark features of ME/CFS is post‐exertional malaise (PEM), which is an exacerbation of symptoms that occurs after even minimal exertion due to basic activities of daily living, physical activity, cognitive work, or engaging in social interactions. The intensity and duration of PEM is usually unpredictable, making it challenging for individuals with ME/CFS to plan activities or engage in regular daily tasks (IOM, 2015; Stussman et al., 2020). Research suggests that PEM may involve dysregulation of the immune system, impaired energy metabolism, and abnormal responses to exertion at the cellular level (Germain et al., 2022; Glass et al., 2023; Van Booven et al., 2023).
In healthy individuals, physical exercise improves cardiovascular health and muscle strength, alleviates fatigue, anxiety and depression, and reduces the risk of chronic diseases (Nystoriak & Bhatnagar, 2018; Singh et al., 2023). Although exercise is suggested as a lifestyle intervention to manage various chronic illnesses (Li et al., 2023; Puetz & Herring, 2023; Reina‐Gutiérrez et al., 2023; Ross et al., 2023), it negatively affects people with ME/CFS who suffer from exercise intolerance. This intolerance has been extensively documented using a 2‐day cardiopulmonary exercise test (CPET), with the aim to understand and characterize the specific physiological responses and limitations that individuals with ME/CFS experience during and after exercise (Keller et al., 2014; Moore et al., 2023; Stevens et al., 2018).
The health benefits of exercise have been attributed in part to bioactive molecules (metabolites, RNA species and proteins) released into circulation during exercise (Contrepois et al., 2020). These molecules may play a role in the response to exercise by triggering the required adaptations and/or regulating homeostasis and substrate metabolism (Murphy et al., 2020). Such systemic mediators need to be targeted to exert their biological effects, and circulating extracellular vesicles (EVs) can facilitate cell‐to‐cell communication, as well as organ cross‐talk both locally and at distant sites through receptor‐ligand interactions and subsequent release of their cargo (Gurung et al., 2021). EVs are spherical entities protected by a lipid bilayer that range from 30 to 1000 nm in diameter and are detected in most body fluids including blood, urine and saliva. EVs are secreted by skeletal muscle, endothelial, cardiac, hepatic and adipose tissues as well as immune cells, especially platelets (Doyle & Wang, 2019; Haghighitalab et al., 2023; Kehrloesser et al., 2023). The cargo of EVs released after exercise reflects the cellular response to exercise‐induced stress including alterations in metabolism, inflammation, and tissue repair (Whitham et al., 2018). Athletic males exhibit an increase in the concentration of circulating EVs during and immediately after an acute bout of aerobic exercise and analysis of the EV protein cargo showed changes in numerous proteins associated with various signalling pathways including angiogenesis, immune signalling and glycolysis (Frühbeis et al., 2015; Whitham et al., 2018). Regrettably, there is limited research regarding the effects of physical activity on EVs in disease populations, healthy females or sedentary individuals.
To date, few studies on EVs in ME/CFS have been published (Almenar‐Pérez et al., 2020; Bonilla et al., 2022; Castro‐Marrero et al., 2018; Eguchi et al., 2020; Giloteaux et al., 2023, 2020; González‐Cebrián et al., 2022; Tsilioni et al., 2022). In 2018, Castro‐Marrero and colleagues were the first to analyse EVs isolated from ME/CFS patients and they found a higher EV concentration and smaller sized EVs in 10 ME/CFS patients in comparison to five healthy controls (Castro‐Marrero et al., 2018). Almenar‐Pérez et al. replicated these results and found dysregulated neuroimmune pathways via analysis of EV miRNA content in a study with 15 severely affected ME/CFS patients compared to 15 healthy subjects (Almenar‐Pérez et al., 2020). Our group published two studies measuring the cytokine content of EVs isolated from plasma of 35 and 49 ME/CFS patients and matched healthy controls, respectively (Giloteaux et al., 2023, 2020). Although we did not replicate EV size differences between groups, all four studies found significantly higher concentration of EVs in the ME/CFS group. The 35‐subject study revealed few significant differences in cytokines compared to controls but there were significant correlations with patient symptom severity, while significant differences were detected in basal levels of EV cytokines in the 49‐subject study, including IL‐2, TNF‐α, and IL‐1β (Giloteaux et al., 2023). Eguchi et al. also replicated the finding of increased concentration of circulating EVs in a larger cohort of 99 ME/CFS patients compared to 53 controls (Eguchi et al., 2020). However, one study in which EVs were directly quantified without prior isolation showed no difference in EV concentration between 20 ME/CFS patients and 20 controls (Bonilla et al., 2022). Further proteomic analysis of EV cargo in addition to cytokines could help identify a signature biomarker for ME/CFS. Quantitative untargeted proteomics of isolated EVs from three ME/CFS patients and three controls from the larger Eguchi et al. cohort revealed a protein cargo specific to the ME/CFS group including actin network proteins and 14−3‐3 family proteins (Eguchi et al., 2020), but these results need to be validated in a larger study.
We hypothesize that altered EV signalling in ME/CFS patients after an exercise challenge may contribute to the prolonged and exacerbated negative response to exertion in ME/CFS (PEM). It is possible that EVs are carrying aberrant signalling molecules, that signals that should be transmitted in EVs post‐exercise are missing in ME/CFS patients, and/or that the EV response to exercise is temporally dysregulated.
In the present study, we isolated EVs by size exclusion chromatography from the plasma of 18 females diagnosed with ME/CFS and 17 age‐ and Body Mass Index (BMI)‐matched female sedentary controls at three time points: before, 15 min, and 24 h after a maximal exercise challenge. We then characterized the EVs using nanoparticle tracking analysis (NTA) and measured the protein cargo using Tandem Mass Tag‐based (TMT) quantitative proteomics. We demonstrate that exercise affects the EV proteome in ME/CFS patients differently than in healthy individuals and that changes in EV proteins after exercise are strongly correlated with symptom severity in ME/CFS.
2. MATERIALS AND METHODS
2.1. Population characteristics
A cohort of 18 females diagnosed with ME/CFS and 17 female age‐ and BMI‐matched sedentary controls (Table 1) was selected for this study (a subset of the larger cohort, ClinicalTrials.gov Identifier: NCT04026425). All cases met the 2003 Canadian Consensus Criteria for ME/CFS (Carruthers et al., 2003). None of the subjects in our cohort were pregnant or breastfeeding, diabetic, smoked cigarettes, consumed excessive amounts of alcohol, had an orthopaedic limitation preventing them from performing the cardiopulmonary exercise test (CPET), or had any of the following diagnoses: schizophrenia, major depressive disorder, bipolar disorder, or an anxiety disorder. Autoimmune compromised controls were also excluded. Healthy subjects included in the present study were categorized as ‘low‐active’: they had a sit‐down job and had no regular organized physical activity during leisure time such as fitness walking or exercise classes in the past 6 months.
TABLE 1.
Study population characteristics.
| ME/CFS | Controls | p‐value | ||
|---|---|---|---|---|
| n | 18 | 17 | NA | |
| Age (years) | 44.4 ± 7.9 | 44.2 ± 13.4 | 0.87 | |
| BMI | 27.8 ± 5.4 | 29.9 ± 5.8 | 0.26 | |
| Type of onset | Sudden | 83% | NA | NA |
| Gradual | 17% | NA | NA | |
| Bell Disability Scale | Median (IQR) | 30 (17.5) | 100 (10) | p < 0.0001 |
| SF‐36 PCS | Physical component summary | 25.2 ± 6.7 | 56.2 ± 5.0 | p < 0.0001 |
| SF‐36 MCS | Mental component summary | 46.0 ± 8.1 | 54.5 ± 6.9 | p = 0.0001 |
| MFI total | Multi‐dimensional fatigue inventory | 84.4 ± 7.7 | NA | NA |
| CPET parameters (median (IQR)) | VO2peak (mL/kg/min) | 18.45 (3.775) | 19.2 (3.4) | 0.19 |
| VO2@VT (mL/kg/min) | 9.2 (2.6) | 10.5 (2.6) | 0.01 | |
| HRpeak (bpm) | 156 (22.25) | 161 (26) | 0.59 | |
| RERpeak | 1.25 (0.1375) | 1.22 (0.11) | 1 | |
| Work@peak (watts) | 111.5 (25.75) | 127 (26) | 0.08 | |
| Time to peak (s) | 454.5 (94.75) | 529 (100) | 0.02 |
Note: Shown are the mean and standard deviation for demographic parameters in ME/CFS patients and control subjects, except where otherwise indicated. For the Bell disability scale and SF‐36, a higher number corresponds to better health. For the MFI total score, a higher number corresponds to increased fatigue. VT, ventilatory threshold; HR, heart rate; RER, respiratory exchange ratio. The p‐values are from Wilcoxon rank sum tests comparing ME/CFS and control groups. NA, not applicable.
2.2. Exercise testing and blood sample collection
All blood samples were collected prior to March 2020, allowing us to distinguish ME/CFS cases from people with post‐acute sequelae of COVID‐19 (Long COVID). Blood was collected at baseline (0 h), immediately prior to a CPET. All subjects performed a maximal CPET on a stationary bicycle by cycling with an increase in workload of 15 watts per minute of exercise until volitional exhaustion, with a respiratory exchange ratio greater than 1.1 (the threshold for maximum effort). Additional technical details regarding the CPET protocol can be found elsewhere (Stevens et al., 2018). A second blood sample was also collected approximately 15 minutes post‐CPET (15 min) and a third one the following day (24 h). Peripheral blood was drawn in sodium citrate BD Vacutainer™ Cell Preparation Tubes and centrifuged to pellet red blood cells. The resulting plasma samples were stored at −80˚C prior to processing. Baseline health status was assessed using the Short Form 36 Health Survey v2® (SF‐36) (Ware, 1993), the Bell Disability Scale, and the Multidimensional Fatigue Inventory (MFI) (Smets et al., 1995). A modified version of the chronic fatigue syndrome specific symptom severity (SSS) score survey (Baraniuk et al., 2013) was used to assess symptom severity at different time points, including on the days of blood collection. The mean +/‐ standard deviation or median (interquartile range (IQR)) for selected demographic, health status, and CPET parameters are reported in Table 1, along with p‐values from Wilcoxon rank sum tests comparing the control and ME/CFS groups. Written informed consent was obtained from all participants and all protocols were approved by the Ithaca College Institutional Review Board (protocol 1017‐12Dx2), and the Weill Cornell Medical College Institutional Review Board (protocol 1708018518). The research adhered to the tenets of the Declaration of Helsinki.
2.3. Extracellular vesicles purification and characterization
Extracellular vesicles (EVs) were isolated from 500 µL of plasma by size exclusion chromatography using Izon qEVoriginal/70 nm columns (Izon Science) following the manufacturer's instructions. Halt™ Protease Inhibitor Cocktail (1X) (Thermo Scientific™) was added to samples prior to submission for proteomic analysis (see below). Concentration and size distribution of isolated EVs were assayed in all samples using a NanoSight NS300 instrument for nanoparticle tracking analysis (NTA) (Malvern, Worcestershire, UK). Samples were thawed and diluted to 1:2000 in PBS 1X and 1 mL was injected through the laser chamber. Three recordings of 60‐s digital videos of each sample were acquired and analysed by the NanoSight NTA 2.3 software to determine the size and the concentration of EVs. The non‐parametric Wilcoxon rank sum test was used to test the significance of differences (p < 0.05) between cases and controls for the size and EV concentration. The Wilcoxon signed rank test was used to statistically compare the size and concentration at different time points within groups (p < 0.05). EV suspensions were visualized by Transmission Electron Microscopy (TEM). Undiluted samples were applied to copper 300‐mesh Formvar coated carbon stabilized TEM grids (Electron Microscopy Sciences) and were allowed to adsorb to the grid for 5 min. Grids were then washed twice by floating on distilled water for 30 s. Negative staining was performed by placing the grids on a drop of 2% Aqueous Uranyl Acetate for 10 min. The grids were blot dried with Whatman™ paper and imaged with a JEOL JEM 1230 Transmission Electron microscope (JEOL USA, Inc.).
2.4. Proteomics experimental procedures
Detailed information can be found in supplementary materials (Supplementary File S1). A Tandem Mass Tag (TMT) 10‐plex shotgun proteomics analysis was used in this study (Figure S1). Specifically, nine protein‐extracted EV samples from three different subjects at 0 h (baseline), 15 min, and 24 h post‐exercise were included in each of two TMT 10‐plex sets for the ME/CFS group and the control group, respectively. The remaining one channel in each TMT 10‐plex set was used for a mixture (pooled reference) containing equal amounts of proteins from each of the 18 samples to bridge the results between the control and ME/CFS groups. A total of six TMT experiments were conducted generating 12 datasets. Fifteen µg of protein in each sample was reduced, alkylated and digested with 25 µL trypsin at 60 ng/µL in 50 mM Triethylamonium bicarbonate (TEAB) buffer pH 8.5 in a S‐Trap Micro Spin column (Protifi, Huntington, NY). Further labelling of the tryptic digest samples was performed according to Thermo Scientific's TMT Mass Tagging Kits and Reagents protocol. Ten labelled samples in each of TMT 10‐plex sets were pooled together and 80 µg of labelled tryptic peptides were then fractionated using a Pierce™ High pH Reversed‐Phase Peptide Fractionation Kit (Thermo‐Fisher Scientific, San Jose, CA) with a 9‐step isocratic elution. The final three fractions were pooled and subjected to nanoLC‐MS/MS analysis using an UltiMate3000 RSLAnano coupled with Orbitrap Eclipse mass spectrometer (Thermo‐Fisher Scientific, San Jose, CA) operated under the Real Time Research (RTS) SPS MS3 method (Fu et al., 2021). All data was acquired and processed with the Thermo Scientific™ Xcalibur™ software 4.3. Acquired raw MS files were processed and searched using the Sequest HT search engine within the Proteome Discoverer 2.3 software (PD 2.3, Thermo).
The MS/MS spectra were searched against the Homo Sapiens NCBI database downloaded on 3/14/2019 (81,268 sequences). Oxidation of Met (M), deamidation of Asn (N) and Gln (Q), protein N‐terminal acetylation, M‐loss and M‐loss+acetylation were set as variable modifications; carbamidomethyl Cys (C) and TMT 10‐plex (m/z 229.1629) on peptide N‐terminal and Lys (K) residue were specified as a static modification. The TMT quantification workflow within the PD software was used for identity of peptides and to calculate the reporter ion abundances in MS3 spectra corrected for isotopic impurities. Identified peptides were filtered for maximum 1% FDR using the Percolator algorithm in PD 2.3 with peptide confidence set to high and peptide mass accuracy ≤5 ppm. Both unique and razor peptides were used for quantitation. Signal‐to‐noise (S/N) values were used to represent the reporter ion abundance with a co‐isolation threshold of 50% and an average reporter S/N threshold of 10. The S/N values of peptides, which were summed from the S/N values of the peptide‐spectrum matches (PSMs), were summed to represent the abundance of the proteins. For relative ratio between the two groups, normalization on total peptide amount for each sample was applied. Comparison of ratios of each sample versus the pooled reference allows protein abundance across two different TMT sets to be assessed (Unwin et al., 2010).
2.5. Data processing and statistical analysis
A total of 862 proteins were detected in the six TMT experiments. Lipoproteins and keratins were removed prior to analysis as they are known contaminants in EV proteomics studies (Karimi et al., 2018; Théry et al., 2018). Three hundred one proteins remained for analysis after filtering out proteins that were not detected in at least 72 out of 108 samples and 4 out of 6 TMT experiments (two thirds, see Supplementary File S2). For these 301 proteins, missing values were then imputed with random forest (RF, missForest R package, default parameters (Stekhoven & Bühlmann, 2012). All proteins and the following categorical variables with no missing data were included in the RF imputation: experimental group (ME/CFS vs. Control), time point (0 h, 15 min and 24 h) and TMT experiment (sets 1–6).
Original and imputed data for 301 EV proteins were analyzed at all three time points. Due to the experimental setup, ME/CFS and control samples could only be compared within each TMT experiment. Therefore, we used a bootstrapping approach to statistically compare ME/CFS and control samples at each time point. For each protein, the following algorithm was used to generate 10,000 bootstrapped datasets: (1) select 18 values with replacement, 3 per TMT experiment, for ME/CFS and control groups; (2) calculate 18 ME/CFS versus control fold changes from the selected values (all fold changes are matched within experiments) and (3) calculate the median of the fold changes, which is less influenced by outliers than the mean. To compare the ME/CFS to the control group, 95% confidence intervals were calculated for the 10,000 bootstrapped medians. The null hypothesis is that the ratio of the two groups is 1 (no difference). Confidence intervals were adjusted to account for false discovery using the Benjamini and Yekutieli procedure (q < 0.1) (Benjamini & Yekutieli, 2005). Protein levels are considered significantly different between groups if the adjusted confidence interval does not include 1. Those proteins are differentially abundant proteins (DAPs). Therefore, if the confidence interval is below 1, that DAP is significantly lower in ME/CFS compared to controls and if the confidence interval is above 1, then the DAP is significantly higher in the ME/CFS group.
To compare DAPs over time within the ME/CFS and control groups, we calculated the within‐subject fold changes for 15 min versus 0 h, 24 h versus 0 h and 24 h versus 15 min. This analysis did not require a bootstrapping approach because all three samples for every subject were measured within the same TMT experiment. For each protein, the mean fold changes were compared to 1 using a t‐test. Again, the null hypothesis was that the mean was equal to 1 (no difference). The p‐values were adjusted using the Benjamini and Hochberg false discovery rate (BH FDR) correction procedure (q < 0.15). For significantly different proteins, if the mean fold change is above 1, then that protein increased over the two time points compared and if the mean fold change is below 1, then the level of that protein decreased over time.
To assess whether the protein levels was changing differently over time in the ME/CFS versus control groups, we used a similar bootstrapping approach to that described above. For this analysis, we randomly selected 18 within‐subject fold changes with replacement, 3 per TMT experiment, for both the ME/CFS and control groups. Steps 2 and 3 of the algorithms outlined above were the same, and we followed the same statistical procedure. Proteins that are significantly different in this analysis again had adjusted confidence intervals that did not include 1. In this case, the median fold change is simply telling us the difference between the change over time in ME/CFS versus controls, but not the direction of that change within each group.
2.6. Pathway enrichment analyses
Significant DAPs were subjected to functional annotation of Reactome pathway enrichment analysis using the ShinyGO (v0.76) software (Ge et al., 2020). Reactome terms and pathways with adjusted p‐value FDR < 0.05 were considered significantly enriched. FDR is calculated based on nominal p‐value from the hypergeometric test.
Canonical pathway analysis was performed with QIAGEN IPA software (QIAGEN Inc.). For each time point, the input included all 301 proteins and the median fold change of patients versus controls generated from the 10,000 bootstrapped datasets. Two hundred ninety‐five proteins with valid IDs in IPA were preserved for further steps. Because all proteins from the dataset were supplied for each time point, enrichment significance (p‐values) was identical for all time points. The Fisher's Exact Test was used to calculate a statistical significance (p‐value) of overlap of the dataset molecules with various sets of molecules that represent annotations such as canonical pathways. Subsequently, p‐values were adjusted using the BH FDR procedure. The overall activation/inhibition states of canonical pathways are predicted based on a z‐score algorithm using the median fold changes for the proteins in each pathway. The primary purpose of the activation z‐score is to infer the activation states (‘increased’ or ‘decreased’) of implicated biological functions in the two groups being compared (i.e., ME/CFS patients vs. controls). The sign of the calculated z‐score reflects the overall predicted activation state of the biological function (<0: inhibited, >0: activated). An absolute z‐score of ≥2 is considered significant (Krämer et al., 2014). After first sorting by BH FDR corrected p‐values, the top 30 canonical pathways were retained and hierarchically clustered by z‐score where six pathways without a z‐score were eliminated.
2.7. Tissue and cell type enrichment analysis
Tissue and cell type enrichment analysis was performed using the DAPs within each group 15 min post‐exercise (q < 0.15). The 187 DAPs in controls and the 63 DAPs that were significantly increased in ME/CFS patients were compared with the following databases in the tissue enrichment subsection of Enrichr: Human Gene Atlas, ARCHS4 Tissues, and HuBMAP ASCT+ B augmented with RNAseq coexpression (Chen et al., 2013; Xie et al., 2021). Fisher's exact test was used to test whether the proteins that changed after exercise were significantly overlapping with the annotated gene/protein sets, and p‐values were adjusted with the BH FDR correction procedure (significance threshold q < 0.15). We identified the tissue and cell type terms that were uniquely enriched in either the control group or the ME/CFS group, and the terms that were significantly enriched in both groups. Since this analysis allows for overlap (meaning each protein can contribute to the enrichment of several terms), Sankey networks were constructed to show all proteins that contributed to the tissue terms unique to the ME/CFS or control groups (networkD3 package, https://cran.r‐project.org/web/packages/networkD3/index.html). The tissue and cell type terms were grouped in physiologic categories for ease of visualization in the Sankey networks.
2.8. Correlation with clinical parameters
To investigate whether changes in protein abundance after exercise are associated with ME/CFS symptoms, severity metrics, and demographic parameters, Spearman correlation coefficients for the within‐subject fold changes over time for each protein versus a variety of clinical parameters were calculated. Significance was assessed by generating bootstrapped 95% confidence intervals for each correlation coefficient and getting associated p‐values (bootcorci R package) with the null hypothesis that the correlation coefficient equals 0. Similar to the previous bootstrapping analysis, the correlation is significant if the 95% confidence interval does not include 0. For each clinical parameter, p‐values were adjusted using BH FDR correction (q < 0.1). All three time point ratios were evaluated (15 min/0 h, 24 h/0 h and 24 h/15 min). We define significant and strong correlations as an absolute value of Spearman R > 0.7 and q < 0.1.
Demographic parameters included age, BMI, and ME/CFS duration (ME/CFS group only). Survey data that reflects physical function and disease severity were also evaluated including the Bell Disability Scale score, the SF‐36 Physical Component Summary (PCS), and the percentage of waking time spent in a reclined position. The MFI‐20 total score was also used as another metric of general fatigue in the ME/CFS subjects only (range 20–100, higher scores correspond to higher levels of fatigue).
Furthermore, we looked at symptom severity using the specific symptom severity (SSS) scores. Each subject in both the ME/CFS and control groups was asked to rate their symptoms on a scale of 0–10 (0 = not present and 10 = very severe) at three different times: (1) on average over the past month (Initial), (2) on the morning of the CPET (0 h) and (3) 24 h after the CPET (24 h). The ΔSSS are calculated by the score for the symptom at 24 h minus the score at 0 h. Thus, ΔSSS below 0 indicates an improvement in that symptom following exercise and ΔSSS above 0 indicates that the symptom got worse 24 h post‐exercise. The following SSS were included in our analysis: fatigue, impaired memory or concentration, recurrent sore throat, lymph node tenderness, muscle tenderness or pain (myalgia), joint pain (arthralgia), headache and PEM.
2.9. Data visualization
Unless otherwise specified, all plots were generated in R with ggplot2. Volcano plots were made using the EnhancedVolcano package (Blighe et al., 2023).
3. RESULTS
3.1. Study design
A cohort of 17 female sedentary control subjects and 18 females diagnosed with ME/CFS were subjected to a maximal cardiopulmonary exercise test (CPET) on a stationary bicycle. Blood was drawn from all subjects at baseline (0 h), and at 15 min and 24 h after the CPET. Extracellular vesicles (EVs) were isolated from the separated plasma and characterized using Nanoparticle Tracking Analysis (NTA) and Transmission Electron Microscopy (TEM). EV cargo was analysed using nano liquid chromatography tandem mass spectrometry (nanoLC‐MS/MS) untargeted proteomics (Figure 1).
FIGURE 1.
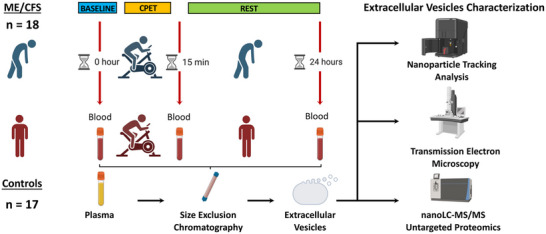
Study design including a cardiopulmonary exercise test (CPET) and blood sample collection at baseline (0 h), 15 min and 24 h post‐exercise, followed by extracellular vesicles (EVs) isolation by size exclusion chromatography and characterization of EVs by means of nanoparticle tracking analysis, transmission electron microscopy and nanoLC‐MS/MS untargeted proteomics.
As shown in Table 1, subjects are age‐ and BMI‐matched. Eighty‐three percent of the ME/CFS patients report an acute, often flu‐like illness that immediately preceded the onset of the disease, while 17% are unaware of an initiating event and consider their onset to be gradual (Table 1). The Bell Disability Scale scores clearly illustrates the condition of ME/CFS subjects versus controls, with a smaller score reflecting the lower functional level of patients compared to the higher score of functional controls. Both the Physical and Mental Component Summaries (PCS and MCS, respectively) derived from the SF‐36 are, as expected, higher in the control group, indicating better health (Table 1).
The ME/CFS and control subjects did not have significantly different performance on the CPET with regard to their peak oxygen consumption and heart rate, and as required, all subjects achieved a peak respiratory exchange ratio >1.1, which indicates maximal effort (Table 1). Nevertheless, the ME/CFS subjects exhibited significantly lower oxygen consumption at ventilatory threshold and time to peak, indicating decreased physiological capacity in the ME/CFS group.
3.2. Characterization of EVs by nanoparticle tracking analysis and transmission electron microscopy
EVs were isolated from plasma by size exclusion chromatography at all three time points. EV size assessed by NTA did not significantly differ between healthy individuals and ME/CFS patients at any of the three time points (Figure S2 and Table S1). For EV concentrations, many differences were observed (Figure 2a and Table S1). At baseline and 15 min post‐exercise, the ME/CFS subjects had a higher concentration of circulating EVs compared to the control group (p = 0.0002 and p = 0.02, respectively). The mean EV concentrations in control subjects significantly increased by 1.4‐fold at 15 min and 2.2‐fold at 24 h compared to baseline (p = 0.03 and p = 0.001 respectively, Figure 2a). The ME/CFS EV concentration was not significantly affected by exercise (Figure 2a).
FIGURE 2.
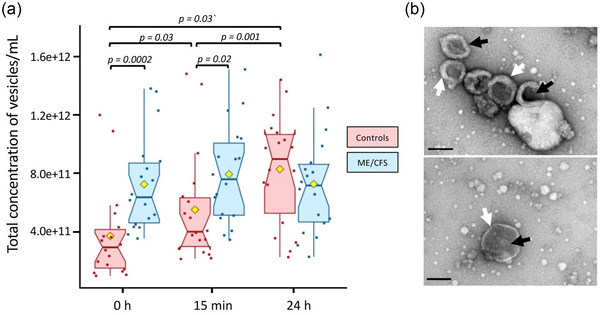
Nanoparticle tracking analysis and transmission electron microscopy. (a) Total concentration of vesicles per mL of plasma in ME/CFS and healthy controls. The yellow dot represents the mean. (b) Representative TEM images obtained by negative staining, showing morphological structures of plasma‐derived extracellular vesicles isolated by size exclusion chromatography. The black arrows highlight a few EV‐like nanoparticles and the white arrows the characteristic lipid bilayer (scale bar: 200 nm). The non‐parametric Wilcoxon signed‐rank test was used to compare time points within the case and control groups and Wilcoxon rank sum test was used to test the significance of differences between cases and controls (p < 0.05).
Representative EVs were further analysed by TEM confirming a population of vesicles with bilayered membranes and ‘saucer shape’ features, which is a typical EV morphology (Figure 2b). The size distribution was consistent with the NTA analysis. However, smaller particles that possibly represent lipoprotein particles and a small number of large particles of 200–350 nm in diameter were also present in the samples analysed.
3.3. Characterization of EV cargo by quantitative proteomics
The EV protein content was further investigated by untargeted nano liquid chromatography tandem‐mass spectrometry (nanoLC‐MS/MS) TMT‐based quantitative proteomics. A total of 862 proteins were detected (full list in Table S2) and 301 were retained for further analysis after filtering out proteins with missing values for more than one third of subjects. One hundred forty‐one proteins had no missing data and the remaining missing values were imputed with random forest (RF, missForest R package, normalized root mean square error of 0.47). RF can handle non‐linear data and outliers with no need for feature scaling and it was found to be the best method for imputing missing values in mass spectrometry data when the reason for the missingness is unknown (missing completely at random; MCAR) (Kokla et al., 2019; Wei et al., 2018). RF also has inherent feature selection, which makes it robust to noisy data (Breiman, 2001).
We cross‐referenced our EV protein dataset with two manually curated EV proteome databases: Vesiclepedia (Kalra et al., 2012) and Exocarta (Keerthikumar et al., 2016). We observed over 94% overlap between our dataset and these databases (Figure 3), and we were able to detect a host of known EV markers such as tetraspanin CD9, vesicle trafficking‐associated protein PDCD6IP (ALIX), heat shock proteins HSP90AA1 and HSPA8, and annexins ANXA3, ANXA5 and ANXA11.
FIGURE 3.
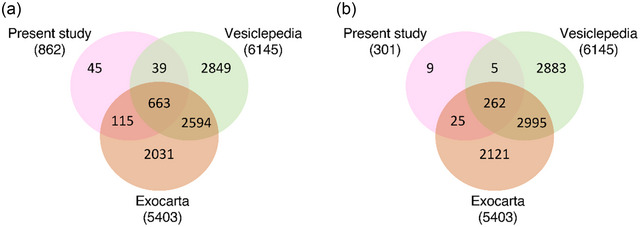
Deep proteomic coverage of the EV proteome. Comparison of proteomic coverage in our dataset versus manually curated vesicle databases Vesiclepedia and Exocarta of (a) all EV proteins identified and (b) after filtering out proteins with more than a third of missing values.
3.4. Comparison of EV protein levels before and after exercise in ME/CFS patients versus controls
Due to the experimental setup outlined in Figure S1, samples could only be compared within each TMT 10‐plex experiment. Differences in EV protein levels between ME/CFS and controls at each time point were analyzed using a bootstrapping strategy (details in Methods). Figure 4a shows the median Log2 fold change of ME/CFS subjects versus controls and both the 95% and FDR‐adjusted confidence intervals from 10,000 bootstrapped datasets. Protein levels are considered significantly different between groups if the adjusted confidence interval does not include 0. Those proteins are differentially abundant proteins (DAPs). Therefore, if the confidence interval is below 0, that DAP is significantly lower in ME/CFS compared to controls and if the confidence interval is above 0, then the DAP is significantly higher in the ME/CFS group.
FIGURE 4.
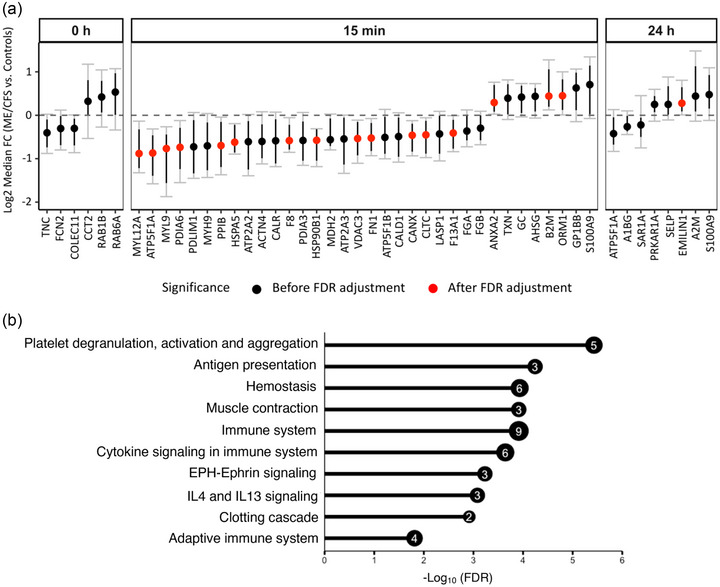
Differences in EV protein levels between ME/CFS and controls at each time point (a) and enrichment analysis (b). (a) The y‐axis shows the Log2 fold change (ME/CFS vs. Controls) of 10,000 bootstrapped datasets at each time point. A median FC of 0 indicates no difference. Black dots show all proteins significant before FDR correction. Black bars show 95% confidence intervals (CI). Gray bars with caps show the false discovery rate adjusted CIs (with q < 0.1). A protein is significant after FDR correction (DAPs, red dots) if the adjusted CI does not include 0. (b) Bar plot showing—Log10(FDR) of the top 10 significant Reactome pathways (FDR < 0.05) enriched for EV proteins that are significantly different (q < 0.1) between ME/CFS and controls 15 min post‐exercise. The number inside the bubble shows the number of EV proteins in each pathway.
At baseline (0 h), none of the six proteins significantly different before FDR correction remained significant after FDR‐adjustment of the 95% confidence intervals (Figure 4a, black dots). 15 min post‐CPET, 13 EV proteins had lower abundances and three had higher abundances in the ME/CFS group (Figure 4a, red dots, q < 0.1). Only one protein (EMILIN1) was significantly increased 24 h post‐exercise in ME/CFS patients (Figure 4a).
To gain functional insight into the proteomic cargo in purified EVs, we performed a Reactome pathway enrichment analysis on the DAPs 15 min post‐exercise using ShinyGO 0.76 (Ge et al., 2020) (Table S3). The most significantly enriched Reactome pathways are illustrated in Figure 4b. Among them, ‘platelet degranulation, activation and aggregation’ was the most enriched pathway including the coagulation factors F8 and F13A1, fibronectin 1 (FN1), and heat shock protein family A member 5 (HSPA5) which were decreased and orosomucoid 1 (ORM1) which was increased (Figure 4a). Other pathways related to blood physiology were enriched such as ‘haemostasis’ and ‘clotting cascade’. The functional enrichment analysis also revealed that EVs at 15 min post‐exercise contained many DAPs that were overrepresented in pathways related to the ‘immune system’ (9 out of the 13 DAPs), ‘cytokine signalling’, ‘adaptive immune system’ and ‘IL4 andIL13 signalling’. Enriched pathways also included ‘muscle contraction’ and ‘EPH‐Ephrin signalling’ which both include members of the myosin family, MYL12A and MYL9, that we found to be decreased in EVs from ME/CFS patients (Figure 4a).
3.5. Ingenuity pathway analysis shows activated or inhibited canonical pathways in ME/CFS cases versus controls
Functional analysis of the median Log2 fold change in ME/CFS patients versus controls from the 10,000 bootstrapped datasets generated for each time point was performed using QIAGEN IPA. Enrichment analysis was performed using the list of 301 proteins analysed in this study and the five most significantly enriched canonical pathways (marked with an asterisk in Figure 5) are ‘Acute phase response signalling’, ‘LXR/RXR activation’, ‘remodelling of epithelial adherens junctions’, ‘actin cytoskeleton signalling’ and ‘integrin signalling’. The enrichment analysis was not time‐point dependent, as the datasets for all three time points include the same 301 proteins.
FIGURE 5.
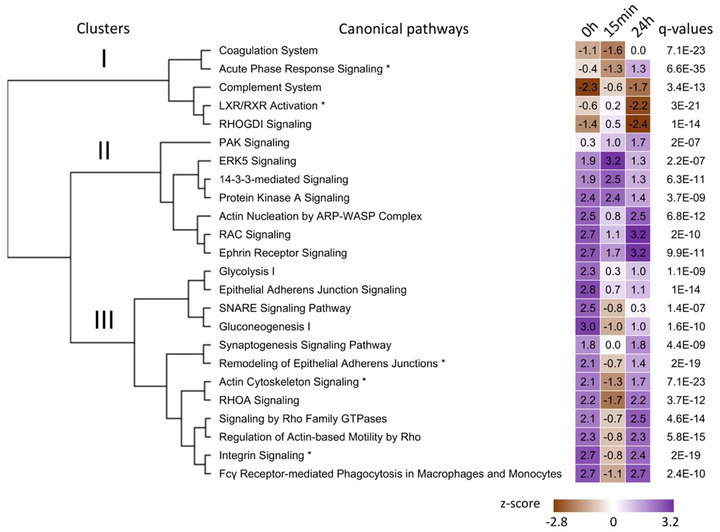
Output for the comparison analysis of the three time points using the QIAGEN IPA software. Top canonical pathways with z‐scores were sorted by hierarchical clustering based on q‐values (Fisher's exact test followed by Benjamini‐Hochberg correction). The top five canonical pathways are identified by an asterisk next to their name. The heatmap visualizes the z‐scores of the canonical pathways at each time point. The sign of the calculated z‐score reflects the overall predicted activation state of the biological function (<0: decreased, >0: increased). An absolute z‐score of ≥2 is considered significant.
A comparison analysis between time points, using the median Log2 fold change in ME/CFS patients versus controls for all 301 proteins, was also performed using QIAGEN IPA (Figure 5). In this analysis, a negative z‐score reflects a predicted inhibition state of the canonical pathway in patients compared to controls, and a positive z‐score indicates the pathway is activated in patients compared to controls. An absolute z‐score of ≥2 indicates a significantly altered pathway in patients versus controls at a specific time point.
Hierarchical clustering of the canonical pathways based on their z‐scores at all three time points yielded three clusters (Figure 5). The five canonical pathways of cluster I were overall inhibited in patients compared to controls at all three time points (brown—negative z‐scores). At baseline, only the ‘complement system’ pathway was significantly inhibited in ME/CFS patients compared to controls (z‐score = −2.3). While none were significantly altered at 15 min post‐exercise, ‘LXR/RXR activation’ and ‘RHOGDI signalling’ were significantly inhibited 24 h post‐exercise (z‐score = −2.2 and −2.4, respectively). The ‘complement system’ and ‘LXR/RXR activation’ are strongly involved in inflammation. The ‘coagulation system’ is a complex process involving platelets and both the formation and degradation of clots requiring a finely balanced system for optimal function. ‘RHOGDI signalling’ is involved in a multitude of biological processes including cell proliferation, apoptosis, cytoskeletal reorganization and membrane trafficking.
Cluster II included seven canonical pathways that remained activated in patients compared to controls throughout the experimental protocol (purple—positive z‐scores in Figure 5). Amongst them, the ‘14‐3‐3‐mediated signalling’ pathway was significantly activated in patients compared to controls at 15 min post‐exercise (z‐score = 2.5) but not 24 h later.
Finally, cluster III included 12 canonical pathways that were initially activated in patients compared to controls and returned to that state 24 h post‐exercise, but were inhibited at 15 min post‐exercise (Figure 5). All but ‘synaptogenesis signalling’ were significantly activated at baseline in patients versus controls (z‐scores > 2). At 24 h post‐exercise, pathways related to the RHO GTPases family, ‘integrin signalling’ and phagocytosis were activated in patients.
Apart from ‘glycolysis I’ and ‘gluconeogenesis I’ which are linked to energy metabolism and were significantly activated in ME/CFS patients versus controls at baseline, most of the other canonical pathways are involved in actin cytoskeleton regulation for cell‐cell communication including neural and immune cells.
3.6. Changes in EV protein levels over time within groups
Changes in EV protein levels over time within the ME/CFS and control groups were assessed by calculating the within‐subject fold changes for 15 min vs. 0 h, 24 h vs. 0 h and 24 h vs. 15 min and the mean fold changes were compared to 1 using a student's t‐test. Amongst all comparisons performed we found significant results only at q < 0.15 for 15 min vs. 0 h and results are displayed as volcano plots in Figure 6a. The mean ratios, p‐values, and q‐values for all 301 proteins are shown in Tables S4 (ME/CFS) and S5 (controls). A total of 63 and 187 DAPs were found in the ME/CFS and control groups, respectively, including 45 common to both (Venn diagram in Figure 6a). All 63 DAPs in the ME/CFS group had higher levels post‐exercise (15 min) compared to baseline (0 h). In the control group, 178 DAPs had higher levels and nine had lower levels 15 min post‐CPET (AGT, C4B, CLU, COLEC11, FCN2, MBL2, ORM2, PON1 and VSIG6). A Reactome pathway enrichment analysis revealed 276 and 298 pathways significantly enriched (q < 0.05) for the ME/CFS and control groups respectively (Tables S6 and S7) with 189 pathways common to both groups.
FIGURE 6.
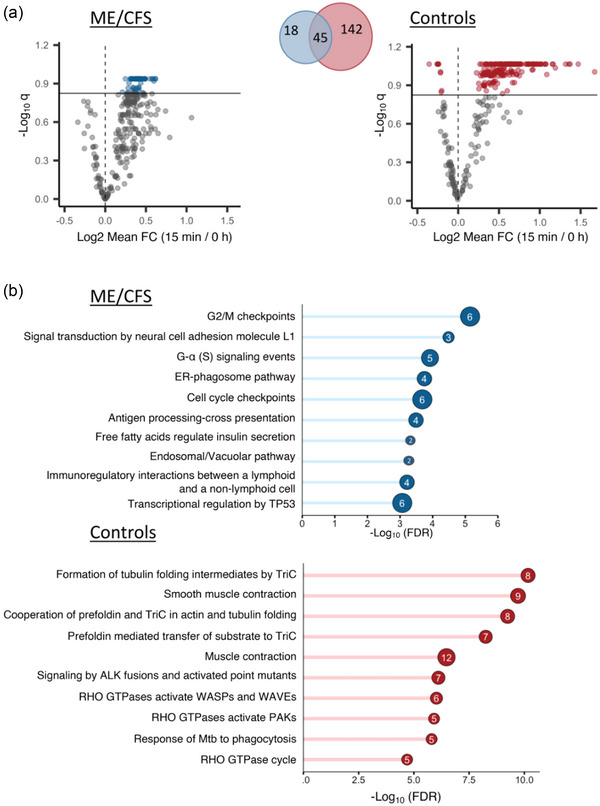
Changes in EV protein levels in ME/CFS and controls at 15 min post‐exercise (a) and enrichment analyses (b). (a) Volcano plots showing significantly different EV protein levels in the ME/CFS and control groups at 15 min post‐exercise compared to baseline. The x‐axis shows the log2 mean fold change (15 min vs. 0 h) and the y‐axis shows the negative log of the q‐value (a higher number represents increasing significance). The horizontal line shows the significance threshold q < 0.15 and coloured dots are EV proteins that are significant at that threshold (grey dots are below the threshold). The Venn diagram is showing the number of significant EV proteins in both groups and their overlap. (b) Bar plots showing ‐Log10(FDR) of the top 10 significant Reactome pathways (FDR < 0.05) uniquely enriched in ME/CFS patients and controls. The number inside the bubble shows the number of EV proteins in each pathway.
We isolated pathways that were uniquely enriched in either the ME/CFS patients or the controls and found 85 enriched pathways unique to ME/CFS and 107 unique to controls (Tables S8 and S9). Figure 6b shows the top 10 unique Reactome pathways for each group. Within the ME/CFS group, the EV proteins with increased abundance include six members of the 14‐3‐3 protein family (YWHAE, YWHAQ, YWHAB, YWHAZ, YWHAG, YWHAH) that were significantly and uniquely associated with pathways related to ‘G2M/checkpoints, cell cycle checkpoints, and transcriptional regulation by TP53’ and three EV proteins were integrins (ITGB1, ITGB3 and ITGA2B) associated with the ‘signal transduction by neural cell adhesion molecule L1’ pathway. In the control group, EV proteins were overrepresented in pathways related to ‘actin and tubulin folding’ (including members of the chaperonin‐containing TCP1 ring complex TRiC (CCT2, CCT5, CCT6A, CCT8, TUBB, TUBB4B, TUBA4A, TUBA8)), ‘muscle contraction’ (MYL6, MYL9, MYL12A, MYLK, TPM4, TMOD3), and ‘Rho GTPases’.
3.7. Tissue and cell type enrichment analysis
We then performed tissue enrichment analysis using Enrichr (Chen et al., 2013) to explore which tissue and cell types typically express the proteins that have changing levels in EVs in ME/CFS patients or controls at 15 min post‐exercise compared to baseline. The 187 DAPs in controls and the 63 DAPs in ME/CFS patients (q < 0.15) were compared to three databases containing reference gene and protein expression for different cell and tissue types (Human Gene Atlas, ARCHS4 Tissues and HuBMAP ASCT+B augmented with RNAseq co‐expression). Significant enrichment was determined using Fisher's exact test and BH FDR correction (significance threshold q < 0.15). While we cannot determine the source of the EVs in the plasma of patients and controls, this analysis links the protein cargo of EVs to tissue and cell types in which they may have originated and reveals how the associated tissues and cell types are different in ME/CFS patients and controls after exercise.
We isolated cell and tissue terms that were uniquely enriched in either ME/CFS patients or controls and grouped them into broader physiological categories (see Tables S10, S11 and S12 for the original terms and to which databases they belong). Sankey networks showing the links between proteins and enriched tissue types for controls and ME/CFS patients are shown in Figure 7a,b. Although there were 45 proteins significantly increased in abundance 15 min post‐exercise in both ME/CFS patients and controls (Venn diagram, Figure 6a), only three of these proteins are contributing to the uniquely enriched tissue terms: TPI1, GNAI2 and PLEK. The broad categories that we found enriched in the controls but not in the patients include myoblasts, smooth muscle, kidney and heart tissue. The only category that we identified to be exclusively enriched in the ME/CFS patients was lung tissue. Most of the 81 proteins in the control network are associated with either myoblasts, smooth muscle or both. Muscle tissue terms were not significantly enriched in the ME/CFS patients, indicating that systemic signalling from muscle tissue through EVs post‐exercise could be disrupted in ME/CFS patients and contributing to their pathophysiological response to exercise.
FIGURE 7.
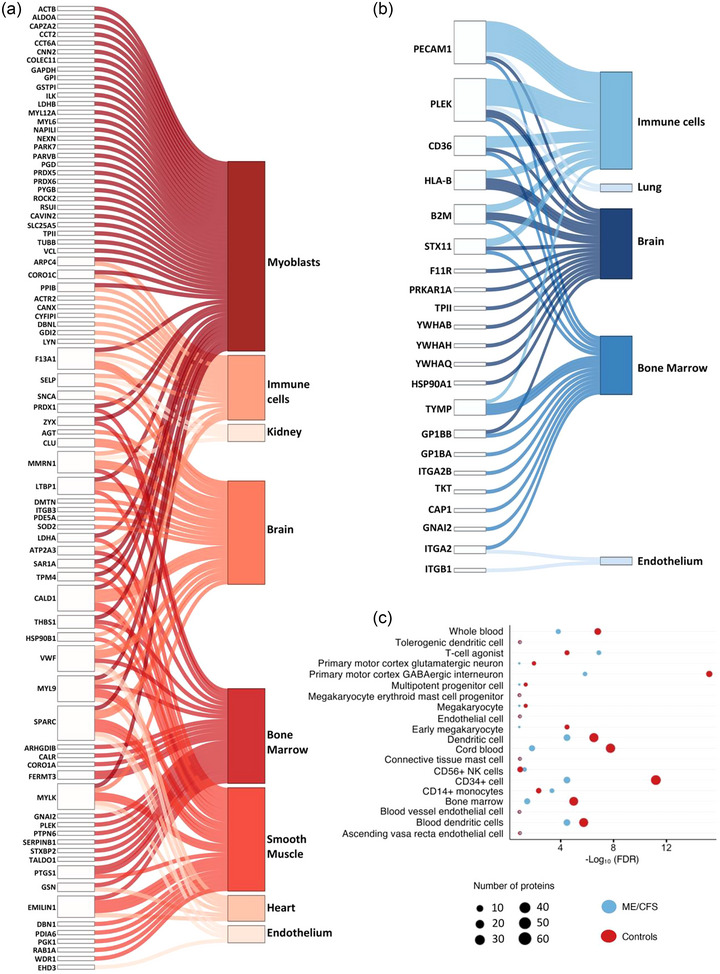
Tissue and cell type enrichment analysis. Sankey network diagrams showing 81 and 22 EV proteins contributing to uniquely enriched tissue type terms in controls (a) and in ME/CFS patients (b), respectively. Each line in the Sankey network shows one connection between a protein and an enriched tissue or cell type term. (c) Bubble plot with terms that are commonly enriched in both ME/CFS patients and controls, as well as their significance (‐Log10 (FDR)) and the number of proteins overlapping with each reference gene/protein set.
While the categories immune cells, brain, bone marrow, and endothelium appear in both networks, the terms within those categories are unique to either patients or controls. Within immune cells, BDCA4+ Dendritic Cells and Tissue Resident Mucosal Type Mast Cells (id:605) were enriched only in controls. Despite a much smaller set of proteins changing significantly after exercise, the increased DAPs found in ME/CFS patients are significantly and uniquely associated with substantially more immune cell types, including Mast Cell (id:394), CD4 T Cell Memory (id:90), Non‐classical Monocyte (id:475), Interstitial Macrophage (id: 348), Macrophage‐Resident (id:388), Basophil (id:66) and multiple types of dendritic cells (Table S10).
Figure 7c shows the terms that are commonly enriched in both ME/CFS patients and controls, as well as their significance and the number of proteins overlapping with each reference gene/protein set. Within immune cells, the proteins changing in response to exercise in both patients and controls are associated with CD14+ Monocytes, CD56+ NK cells, Dendritic Cells, T‐cell agonist (id:580), Connective Tissue Mast Cell (id: 133) and Tolerogenic Dendritic Cells (Tdc) (id:606). Many of the common terms are related to blood, bone marrow, and endothelium.
Within the brain category, only ME/CFS patients have changes in EV proteins that are expressed in the Subthalamic Nucleus and Amygdala which are both part of the basal ganglia of the brain (Chaudhuri & Behan, 2000). Since EVs can cross the blood‐brain barrier, it is possible that EVs carrying these proteins originated in these parts of the brain. However, while these proteins are enriched in brain tissue, they are also expressed in other tissues (Thul & Lindskog, 2018), and we do not have information about which proteins are co‐localized in specific EVs.
3.8. EV proteins are changing differently over time in ME/CFS patients versus controls in response to exercise
We used a similar bootstrapping approach to that described previously to compare the within‐subject protein fold changes over time in ME/CFS patients versus controls. Because the median fold change represents the difference between the change over time in ME/CFS versus controls, but not the direction of that change within each group (red dots in Figure 8), we are providing the box plots at all three time point comparisons (15 min/0 h, 24 h/0 h and 24 h/15 min, Figure S3).
FIGURE 8.
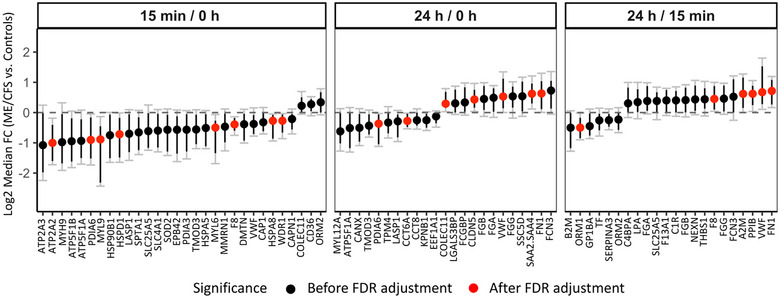
Changes in EV protein levels between ME/CFS and controls over time. The y‐axis shows the median Log2 fold change (ME/CFS vs. Controls) of 10,000 bootstrapped datasets for each time point comparison. A median FC of 0 indicates no difference. Black dots show all proteins significant before FDR correction. Black bars show 95% confidence intervals (CI). Grey bars with caps show the false discovery rate adjusted CIs (with q < 0.1). A protein ratio is significant after FDR correction (red dots) if the adjusted CI does not include 0.
After FDR correction several EV proteins were found to be changing significantly differently in ME/CFS patients versus controls in response to exercise (red dots, Figure 8). Eight EV proteins had lower 15 min/0 h ratios in ME/CFS patients versus controls including coagulation factor VIII (F8). Four of these proteins (ATP2A2, MYL6, MYL9 and WDR1) are related to muscle physiology. ATP2A2 is an enzyme that catalyses the hydrolysis of ATP and is involved in the regulation of the contraction/relaxation cycle of skeletal muscle. The myosin light‐chain elements, MYL6 and MYL9, regulate the mechano‐enzymatic function of myosin, that is, the force produced during muscular cross‐bridge cycles. The WD‐repeat protein 1 (WDR1) promotes actin filament disassembly and is involved in adaptive sarcomere reorganization. Finally, PDIA6, HSPD1 and HSPA8 are chaperones implicated in a wide variety of functions including protection of the proteome from stress, proteolysis, folding, and transport of newly synthesized polypeptides, and play a pivotal role in the protein quality control system.
When comparing the change from baseline to 24 h post‐CPET (red dots, Figure 8), chaperone PDIA6 again had lower 24 h/0 h ratios in ME/CFS patients versus controls. The 24 h/0 h ratio for CCT6A, a component of the chaperonin‐containing TCP1 ring complex TRiC, was also lower while collecting COLEC11, claudin CLDN5, von Willebrand factor (VWF), the acute phase reactant serum amyloid read through (SAA2.SAA4), and fibronectin (FN1) ratios were higher in ME/CFS patients. Finally, when comparing 24 h to 15 min post‐exercise, only one protein had a lower 24 h/15 min ratio in ME/CFS patients (orosomucoid ORM1) and four were higher in patients including coagulation factor F8, alpha‐macroglobulin A2M, PPIB, VWF and FN1.
3.9. Changes in EV protein levels post‐exercise strongly correlate with ME/CFS symptoms and disease severity metrics
For this analysis, Spearman's correlation coefficients for the within‐subject fold changes of the 301 EV proteins and selected clinical parameters were calculated. Bootstrapped 95% confidence intervals around the correlation coefficients from 2000 bootstrapped datasets and associated p‐values (null hypothesis that the correlation coefficient is 0) were calculated using the bootcorci R package, and q‐values were obtained using the BH‐FDR correction procedure. We define significant and strong correlations as those with |R| > 0.7 and q < 0.1. For the specific symptom severity (SSS) scores, each subject in both the ME/CFS and control groups was asked to rate their symptoms on a scale of 0–10 (0 = not present and 10 = very severe) at three different times: (1) on average over the past month (Initial), (2) on the morning of the CPET (0 h) and (3) 24 h after the CPET (24 h).
The change in EV protein levels from 0 to 24 h in ME/CFS patients had the greatest number of significant correlations at q < 0.1 (878 significant correlations; 648 R < 0 and 230 R > 0, Table 2, Table S13). Sixty nine of the negative correlations and 15 of the positive correlations also had |R| > 0.7. We found 72 significant correlations of clinical data with the proteins’ 15 min/0 h fold changes and 115 significant correlations for the 24 h/15 min fold changes (q < 0.1, Table 2).
TABLE 2.
Number of significant correlations with clinical parameters (q < 0.1).
| R > 0 (>0.7) | R < 0 (<−0.7) | Total | |
|---|---|---|---|
| Protein Fold Change 15 min/0 h | 43 (8) | 29 (8) | 72 |
| 24 h/0 h | 230 (15) | 648 (69) | 878 |
| 24 h/15 min | 8 (2) | 107 (8) | 115 |
Note: In parentheses are the number of correlations with |R| > 0.7.
Two out of the eight SSS scores, the initial survey of muscle tenderness and pain (myalgia) and the initial survey of joint pain (arthralgia), had significant and strong correlations with the change in several EV protein levels from baseline to 24 h. Figure 9a shows volcano plots of all correlation coefficients for those two symptoms. The proteins shown in dark blue on the volcano plots are those with Spearman |R| > 0.7 and q < 0.1 (32 and 34 proteins for myalgia and arthralgia, respectively, with 11 common to both symptoms, Figure 9a, Venn diagram). For both symptoms, only clusterin (CLU) had a strong positive correlation.
FIGURE 9.
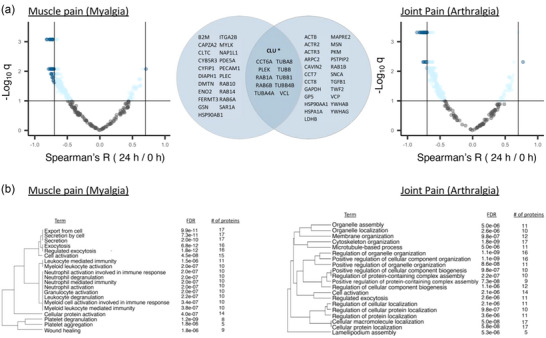
Associations between changes in the post‐exercise EV proteome and average severity of ME/CFS symptoms myalgia and arthralgia. (a) Volcano plots showing correlations between the initial specific symptom severity (SSS) scores of muscle tenderness and pain (myalgia) and joint pain (arthralgia) and within‐subject 24 h/0 h ratios for each protein. For initial SSS scores, subjects were asked to rate their average symptom severity over the last month. The x‐axis shows the Spearman's correlation coefficient R, with vertical solid lines indicating R = ‐ 0.7 and R = 0.7. The y‐axis shows the negative log of the q‐value, with horizontal solid line indicating q < 0.1. The dark blue dots show proteins with |R| > 0.7 and q < 0.1, and light blue dots show the remaining proteins with q < 0.1. The Venn diagram shows the 32 and 34 proteins correlating at |R| > 0.7 and q < 0.1 with myalgia and arthralgia, respectively, with 11 being common to both. CLU* is the only protein with a positive correlation. (b) Pathway enrichment analysis of the 32 and 34 proteins displayed as hierarchical clustering dendrograms with the top 20 enriched pathways. Pathways with many shared proteins are clustered together. FDR and number of proteins belonging to each term are shown.
Pathway enrichment analysis was performed on these 32 and 34 strongly correlated proteins (|R| > 0.7 and q < 0.1) and displayed as hierarchical clustering dendrograms in Figure 9b. For the 32 proteins correlating with myalgia, there was an enrichment of pathways related to cell export, ‘leukocyte mediated immunity’, neutrophil activities, and platelet degranulation and aggregation (Figure 9b). For the 34 proteins correlating with arthralgia, pathways related to ‘cytoskeleton organization’, cellular component assembly and biogenesis, and ‘regulation of cellular protein localization’ were the most significantly enriched (Figure 9b). Several of these proteins also contributed to tissue enrichment terms that were unique in ME/CFS patients (Figure 7b), including B2M, ITGA2B, PECAM1 and PLEK for myalgia and PLEK and YWHAB for arthralgia.
For all SSS scores on the days that blood was collected, we calculated the change in symptom severity post‐exercise as the difference in SSS from 0 to 24 h post‐exercise (ΔSSS). Thus, a positive ΔSSS indicates a worsening of that symptom 24 h post‐exercise and a negative ΔSSS indicates that symptom improved 24 h post‐exercise. The 24 h/0 h ratios for seven proteins had strong and significant positive correlations to the change in myalgia after exercise in ME/CFS patients (Figure 10a), such that when the protein level increased from 0 to 24 h (Log2FC > 0), myalgia also increased (Δ > 0), and when the protein level decreased post‐exercise, myalgia was also decreased. Amongst them, developmentally regulated brain protein (Drebrin‐1, DBN1) is a cytoplasmic actin‐binding protein primarily expressed in the nervous system that plays a role in processes related to learning, memory, and neuronal plasticity. Glutathione S‐Transferase Pi 1 (GSTP1) is an enzyme involved in the detoxification of various xenobiotics and the metabolism of endogenous compounds. Levels of HSPB1, a member of the heat shock protein family, also strongly positively correlated with myalgia. HSPB1 acts as a chaperone, has anti‐apoptotic effects, organizes neurofilaments, and is involved in protecting muscle cells from damage during exercise and periods of stress. Thrombospondin‐1 (THBS1) is an extracellular matrix glycoprotein involved in platelet aggregation, angiogenesis, inflammation, wound healing, and tissue repair. Finally, tropomodulin (TMOD3), tropomyosin (TPM4), and capping actin protein of muscle z‐line subunit beta (CAPZB) are all involved in muscle contraction. Additionally, the change in CAPZB levels from 0 h to 15 min post‐exercise also positively correlated with the change in myalgia from baseline to 24 h post‐exercise (Figure 10b). All of these correlations were absent in controls.
FIGURE 10.
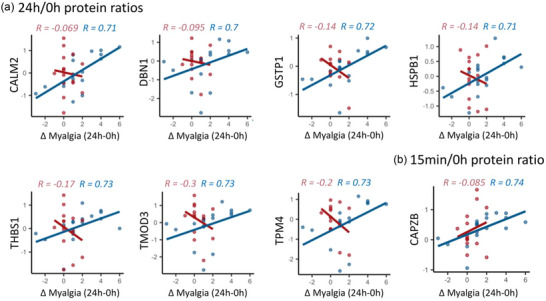
Correlations between the change in myalgia from baseline to 24 h post‐exercise (Δ Myalgia) and the within‐subject 24 h/0 h ratios (a) or within‐subject 15 min/0 h ratios (b). Each dot is one subject. The lines are linear regression lines for each group, ME/CFS or control. Spearman's R is shown on each plot for controls (red) and ME/CFS patients (blue). All correlations presented are significant in ME/CFS patients (q < 0.1).
Two core symptoms of ME/CFS are post‐exertional malaise (PEM) and fatigue. The SSS scores for PEM correlated with the change in several proteins post‐exercise. In particular, the 15 min/0 h ratio of the two fibrinogen chain proteins FGA and FGB positively correlated with PEM that subjects were experiencing 24 h post‐exercise (Figure 11a). The 24 h/0 h ratio of nexilin f actin binding protein (NEXN) levels in ME/CFS patients correlated with ΔPEM such that an increase in NEXN correlated with a larger increase in PEM after exercise (Figure 11a). We found the 15 min/0 h ratios of two proteins, fibrinogen gamma chain (FGG) and clusterin (CLU), positively correlated with worse fatigue on the morning of the CPET (Figure 11b).
FIGURE 11.
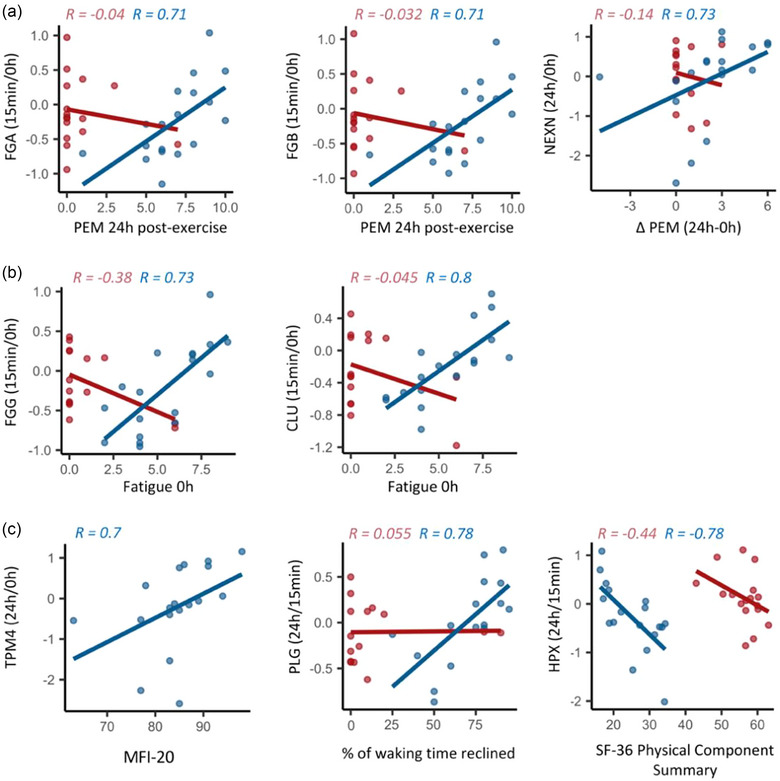
Associations between changes in the post‐exercise EV proteome and (a) post‐exertional malaise severity, (b) fatigue severity the day of exercise and (c) metrics of overall disease severity, including the MFI‐20, the percentage of waking time spent reclined and the SF‐36 Physical Component Summary. Each dot is one subject. The lines are linear regression lines and Spearman's R are shown on each plot for controls (red) and ME/CFS patients (blue). All correlations presented are significant in ME/CFS patients (q < 0.1).
In addition to the SSS survey data, we also looked at correlations to survey data that are indicators of overall disease severity (Figure 11c). We found that the 24 h/0 h ratio of TPM4 positively correlated with the multidimensional fatigue inventory 20 (MFI‐20) total score. For the MFI‐20, a higher score corresponds to worse fatigue. The 24 h/15 min ratio of plasminogen (PLG) positively correlated with the percentage of waking time spent reclined or lying down (Figure 11c). A higher percentage of waking time spent reclined indicates worse overall ME/CFS severity. The 24 h/15 min ratio of hemopexin (HPX) negatively correlated with the SF‐36 physical component summary (PCS) (Figure 11c), where higher scores indicate better health.
4. DISCUSSION
Overall, this study characterized changes in the EV proteome of healthy sedentary control females in response to a single bout of exercise, while also demonstrating highly disrupted EV signalling post‐exercise in ME/CFS patients. This is evidenced by: (1) fewer differentially abundant proteins (DAPs) in EVs 15 min post‐exercise in patients compared to controls (Figure 4a); (2) 63 EV proteins increasing due to exercise in ME/CFS patients whereas 178 EV proteins increased in controls (only 45 overlapping) (Figure 6a); (3) a delayed increase in abundance of several EV proteins in response to exercise in ME/CFS patients compared to controls (Figure 8, Figure S3). Thus, ME/CFS patients exhibit altered EV signalling dynamics and failure to mount an adequate response to exercise at the molecular level.
Multiple enrichment analyses were performed to enhance our understanding of the impact of this dysregulation of the EV proteome post‐exercise on protein pathways and what tissues and cell types express these proteins. Taken together, these results show that the DAPs are involved in many pathways and systems, including platelet functions, muscle contraction (both smooth and skeletal muscle), cytoskeletal protein functions, the immune system, and brain signalling.
Several phenomena may contribute to this dysregulated signalling: (1) the tissue and cellular sources of the EVs may differ; (2) the EV cargo may differ between cases and controls, even when released from the same source; (3) cases and controls may differ in EV targets and/or uptake by destination cells and tissues.
Finally, we found strong and significant correlations between the change in EV protein levels post‐exercise in ME/CFS patients and clinical metrics (myalgia, arthralgia, post‐exertional malaise (PEM), fatigue, % of waking time spent reclined, and SF‐36 physical component summary) (Figures 9, 10, 11). This suggests that the profound disruption of EV signalling post‐exercise may contribute to the inability of ME/CFS patients to recover from exertion.
4.1. EV concentration is differently affected by exercise in ME/CFS patients and controls
Plasma EVs were isolated from ME/CFS subjects and healthy controls and quantified by NTA. Our study revealed a significantly higher concentration of circulating EVs in individuals with ME/CFS compared to healthy controls at baseline and 15 min post‐CPET (Figure 2), which supports recent findings previously reported in ME/CFS (Almenar‐Pérez et al., 2020; Castro‐Marrero et al., 2018; Eguchi et al., 2020; Giloteaux et al., 2023, 2020). Similar observations have also been documented in other conditions, including Alzheimer's disease (Rajendran et al., 2006) and cerebrovascular diseases (Jung et al., 2009).
In the control cohort, there was a significant increase in the total concentration of EVs 15 min and 24 h post‐exercise as compared to baseline, while no changes were detected in the ME/CFS group. Multiple studies conducted in both humans and rodents have documented a rapid rise in EVs following a single bout of physical exercise, leading to changes in EV plasma concentrations, markers, and contents (Bei et al., 2017; Brahmer et al., 2019; Oliveira Jr et al., 2018; Whitham et al., 2018). While our results show an increased concentration of EVs 24 h post‐exercise as compared to baseline in the controls, multiple studies have reported that circulating EVs returned to baseline levels 2–6 h post‐exercise and remained stable for the next 24 h (Kirk et al., 2014; Rakobowchuk et al., 2017; Sossdorf et al., 2011; Wilhelm et al., 2016), remained unchanged (Möbius‐Winkler et al., 2009) or even decreased post‐exercise (Wahl et al., 2014). However, most of these studies focused on platelet‐derived EVs and their participants were mainly healthy, recreationally active or athletic males. The study with the most similar subject profile to our control group is consistent with our results—a single session of acute high‐intensity continuous exercise increased circulating endothelial microparticles measured on the morning following exercise in seven overweight/obese females (Durrer et al., 2015), which suggests that results in athletic males should not be generalized to sedentary or female populations.
In a study with 17 healthy and 15 prediabetic men performing an acute cycling bout, a significant increase of the total concentration of vesicles in plasma was found exclusively in the healthy group during exercise from baseline to 30 min; however, in the prediabetic group, the total concentration of vesicles in the plasma was not affected by exercise (Warnier et al., 2022). Thus, elevated EV concentration relative to healthy controls at baseline and post‐exercise may be explained by the inflammatory state characteristic of the disease. Indeed, another study which specifically measured cytokines in EVs found elevated levels of classic pro‐inflammatory cytokines including IL‐1β, IL‐2, and TNF‐α at baseline in 49 ME/CFS subjects compared to healthy controls (Giloteaux et al., 2023).
4.2. Large‐scale increases in EV protein levels in healthy sedentary females post‐exercise
We found 62% of proteins analysed to be differentially abundant at 15 min post‐exercise in healthy control females, with the vast majority increased, while none were significantly altered 24 h post‐exercise. This is consistent with a study characterizing the EV proteome of healthy males in response to a single bout of cycling exercise where differences were observed immediately post‐exercise in 322 out of 1199 proteins analysed, with most of them increased and only three DAPs 4 h post‐exercise (Whitham et al., 2018). It seems that in both females and males, the increase in EV protein abundance post‐exercise is a transient phenomenon. The DAPs in EVs post‐exercise in healthy sedentary females are expressed by many cell and tissue types, indicating a systemic, coordinated response to exertion by smooth and skeletal muscle tissue, the immune system, the brain, the kidney, as well as circulating cells and blood vessel endothelial cells (Figure 7a,c).
4.3. Dysregulated coagulation processes in ME/CFS post‐exercise
Throughout our analyses, we found many DAPs between ME/CFS patients and controls involved in coagulation processes. The coagulation cascade proteins factor VIII (F8), factor XIII A1 (F13A1) and fibronectin (FN1), were significantly decreased 15 min post‐exercise in ME/CFS versus controls (Figure 4a,b). F8 and FN1 also changed significantly over time in ME/CFS patients versus controls (F8 at 15 min/0 h and 24 h/15 min; FN1 at 24 h/0 h and 24 h/15 min; Figure 8). Von Willebrand factor (VWF) had a similar expression pattern as FN1 (Figure 8, Figure S3).
Changes from baseline to 15 min post‐exercise in the fibrinogen chain proteins, FGA, FGB and FGG, which are also part of the coagulation cascade, positively correlate with the ME/CFS symptoms fatigue and PEM post‐exercise (Figure 11a,b). Additionally, the pathway analysis of the 32 proteins strongly correlating with general myalgia severity included ‘platelet degranulation’ (8 proteins), ‘platelet aggregation’ (5 proteins) and ‘wound healing’ (9 proteins) (Figure 9b). Finally, the change in plasminogen (PLG) from 15 min post‐exercise to 24 h post‐exercise (recovery) strongly correlated with the percentage of waking time spent reclining or lying down (a measure of overall disease severity), where a higher percentage of waking time spent reclining corresponds to more severe ME/CFS (Figure 11c).
Blood coagulation is enhanced in response to acute exercise in healthy individuals (Menzel & Hilberg, 2011) and in those with cardiovascular diseases (Lowe & Rumley, 2014). This elevated coagulation potential is usually accompanied with increased fibrinolytic activity (Womack et al., 2003) to maintain haemostatic balance. Exercise‐induced increases in F8 activity have been reported (Arai et al., 1990; Davis et al., 1976), and align with our results of increased F8 levels in control EVs 15 min post‐exercise. Furthermore, muscle FN1 abundance was found to increase after moderate exercise (Mavropalias et al., 2023). Specific to EVs, Kobayashi and colleagues examined the EV proteome before and 30 min after high‐intensity interval cycling in three healthy males and found significant changes in 20/558 proteins analysed including increased abundance of FN1, VWF, FGA, FGB and FGG (Kobayashi et al., 2021). Post‐exercise increases in F8, FGA, FGB and FGG were also found in 11 healthy males (Whitham et al., 2018). Here, we found significant increases 15 min post‐exercise for VWF and F8 in healthy females, but no significant changes in FN1, FGA, FGG or FGB (Figure 6a). Also, increases in FGA and FGB in patients 15 min post‐exercise correlated with worse PEM 24 h post‐exercise, and increases in FGG post‐exercise correlated with worse fatigue the morning of the exercise test (Figure 11a,b).
EVs play a dual role in blood coagulation by promoting clot formation and initiating the conversion of plasminogen to plasmin, leading to fibrinolysis. We found that a larger increase in plasminogen abundance in EVs from 15 min post‐exercise to 24 h post‐exercise was associated with worse overall ME/CFS severity (Figure 11c). This indicates a balance shift toward fibrinolysis during recovery which could be detrimental. EVs also have a transient hypercoagulable function and may play a role in the early phase of haemostasis after injury in trauma patients, with an increased concentration in the first 3 h after injury (Rognes et al., 2021). The altered temporal profiles of clotting cascade factors in ME/CFS EVs post‐exercise reveals a disruption in the haemostatic balance between clot formation and fibrinolysis.
Levels of coagulation‐related EV proteins are also altered in other disease states. For instance, FGA, FGB, FGG, VWF and F13 all had increased abundance in EVs of patients with idiopathic inflammatory myopathies compared to healthy controls (Meng et al., 2022). A recent proteomic analysis revealed F13A1, FGA and FGB were all enriched in EVs from COVID‐19 patients (Setua et al., 2022). FGB was also highly abundant in EVs of non‐critical COVID‐19 patients (Sur et al., 2021), while another study conducted on critical COVID‐19 patients requiring mechanical ventilation found decreased EV fibrinogen components (Barberis et al., 2021).
Taken together, our results show that an altered haemostatic balance between coagulation and fibrinolysis may be contributing to PEM and exercise intolerance in ME/CFS patients. Other recent reports corroborate the importance of coagulation processes in ME/CFS. Dysregulation in platelet gene expression was found at 24 h following maximal exercise (Ahmed et al., 2022). Likewise, an approximately 10‐fold greater occurrence of fibrinaloid microclots was discovered in people with ME/CFS compared to healthy controls, which is clear evidence of hypercoagulability in ME/CFS even in the absence of an exercise challenge (Nunes et al., 2022). Considering the importance of EVs in maintaining haemostatic balance, future studies exploring the complex interplay between EVs, platelets, and the endothelium in ME/CFS patients are warranted.
4.4. EV signalling relating to muscle contraction is impaired in ME/CFS
Our analyses also reveal impaired EV signalling in proteins involved in muscle contraction in ME/CFS patients, and some of these proteins were significantly associated with the symptoms myalgia (Figure 10) and fatigue (Figure 11c). Muscle contraction was in the top 10 altered pathways at 15 min post‐exercise in ME/CFS patients versus controls (Figure 4b). The DAPs in controls 15 min post‐exercise were associated with muscle and smooth muscle contraction pathways (Figure 6b), as well as myoblast and smooth muscle cell and tissue types (Figure 7a), while no muscle related tissue or protein pathway enrichment was found in patients. Myosin light chains MYL9 and MYL12A had decreased abundance in ME/CFS patients versus controls 15 min post‐exercise (Figure 4a). MYL9 and myosin light chain MYL6 were significantly increased post‐exercise in EVs in controls but not in patients (Figure 6a) and this change over time was significantly different between groups (Figure 8). Indeed, in healthy controls, the protein with the largest mean increase 15 min post‐exercise was MYL9 (3.2‐fold) and MYL12A and MYL6 both showed greater than 2‐fold increases (Table S5). MYL12A, MYL6 and TPM4 were also found to be increased in EVs post‐exercise in healthy males (Whitham et al., 2018).
Increases in EV levels of tropomyosin TPM4, tropomodulin TMOD3, and calmodulin CALM2 from baseline to 24 h post‐exercise were strongly correlated with higher levels of myalgia after exercise in the ME/CFS subjects (Figure 10). The 24 h/0 h ratio of TPM4 was also positively correlated with the MFI‐20, which measures fatigue (Figure 11). Finally, the 24 h/0 h ratio of myosin light chain kinase (MYLK) was one of the many strong negative correlations with the average muscle pain rated by the patients over the last month (Figure 9). During exercise, myosin, tropomodulin, and tropomyosin all play crucial roles in muscle contraction and the regulation of muscle function in both smooth and skeletal muscle (Kishi et al., 1998).
The contribution of skeletal‐muscle derived EVs to the post‐exercise EV circulatory pool is not completely understood. Whitham et al. identified candidate myokines coming from skeletal muscle EVs by isolating EVs from both the femoral artery and femoral vein (Whitham et al., 2018). There is also strong in vitro evidence that myoblasts, myotubes and skeletal muscle tissue explants release EVs (Estrada et al., 2022; Forterre et al., 2014). However, another study examining the surface markers of exercise‐induced EVs in male athletes found no change in skeletal muscle markers and instead found increases in markers associated with lymphocytes, monocytes, platelets, endothelial cells and antigen presenting cells (Brahmer et al., 2019). More recently, Estrada et al., using immunocapture of plasma EVs from transgenic mice expressing eGFP only in skeletal muscle, showed that ∼ 5% of CD9+ and CD81+ EVs were derived from skeletal muscle (Estrada et al., 2022).
The smooth muscle protein elastin microfibrillar interface protein 1 (EMILIN1) had increased abundance in ME/CFS patients versus controls 24 h post‐CPET (Figure 4a). EMILIN1 is an ECM component that is abundantly expressed in elastin‐rich tissues such as the blood vessels, skin, heart and lung (Colombatti et al., 2000). EMILIN1 produced by vascular smooth muscle cells is a major regulator of resting blood pressure levels (Litteri et al., 2012). Interestingly, EMILIN1 is proteolyzed and secreted in small EVs as a mechanism to reduce its intracellular level (Amor López et al., 2021). Smooth muscle cells in the vasculature undergo a number of changes in response to acute exercise (Newcomer et al., 2011), secrete EVs in vitro and in vivo (Kapustin & Shanahan, 2016), and are responsive to EV signalling from vascular endothelial cells in vitro (Boyer et al., 2020). However, exercise‐induced changes in EVs derived from smooth muscle cells have not been studied.
As of now, we are unable to determine the origin of the measured circulating EVs in this study. However, our analysis shows that a transient post‐exercise increase in EV muscle‐related proteins is part of the healthy response to exercise which is reduced in ME/CFS patients. The delayed increase in some of these EV proteins at 24 h post‐exercise in ME/CFS patients is associated with worse skeletal muscle tenderness and pain at the same time point (Figure 10).
4.5. Changes in cytoskeletal proteins correlate with ME/CFS symptoms
We observed many correlations between changes in EV cytoskeletal proteins and ME/CFS symptom severity. Pathway analysis of the proteins correlating with arthralgia shows that these proteins are involved in cytoskeleton organization (17 proteins) and microtubule‐based processes (11 proteins) (Figure 9). The 24 h/0 h ratios of tubulin proteins (TUBB, TUBA4A, TUBA8, TUBB1, TUBB4B) are negatively correlating with the general severity of both myalgia and arthralgia, so higher levels of the proteins 24 h post‐exercise compared to baseline correspond to lower levels of pain (Figure 9a). TUBA1B/A/C measured in plasma was recently included in a set of 20 proteins (15 measured in plasma and 5 measured in EVs) used to classify ME/CFS patients versus controls with 86% accuracy (Giloteaux et al., 2023).
We observed an upregulation of tubulins (TUBB, TUBB4B, TUBA4A, TUBA8) and members of the TRiC complex (CCT2/5/6A/8) in EVs from controls 15 min post‐exercise (Figure 6b). TUBB and CCT2 are both contributing to the enrichment of the myoblast cell type in controls (Figure 7). The 24 h/0 h ratio of CCT6A was significantly different between patients and controls (Figure 8, Figure S3) and is negatively correlated with both myalgia and arthralgia in ME/CFS patients (Figure 9a). Microtubules are one of the principal components of the cytoskeletal system and are involved in muscle development and contraction, but they are also involved in maintenance of neuronal morphology and the formation of axonal and dendritic processes (Baas et al., 2016; Becker et al., 2020).
Changes 24 h post‐exercise in proteins related to actin polymerization, including actin beta (ACTB), actin‐related protein 2 (ACTR2), and actin‐related protein 3 (ACTR3) are negatively correlating with arthralgia in ME/CFS patients (Figure 9a). Also, the 24 h/0 h ratio for Nexilin F‐actin binding protein NEXN is strongly correlated with the change in PEM in ME/CFS patients such that an increase in NEXN corresponds to a larger increase in PEM 24 h post‐exercise (Figure 11a). We observed significant increases 15 min post‐exercise in actin proteins in the controls, with ACTB, CAPZA2 and NEXN contributing to enrichment of the myoblast tissue term, and ACTR2 contributing to the immune cell category (Figure 7a). The rapid increase in actin cytoskeletal proteins in circulating EVs after exercise is part of the healthy response to exercise in the controls and may be related to myoblast functions to repair skeletal muscle damaged during exercise. Eguchi et al. found regulation of actin cytoskeleton to be the most significantly altered pathway in a proteomic analysis of plasma EVs isolated from four ME/CFS patients compared to patients with idiopathic chronic fatigue (ICF) or depression at baseline (Eguchi et al., 2020), which is further evidence that disruption of actin signaling in EVs is involved in ME/CFS pathophysiology.
4.6. Increased clusterin abundance post‐exercise positively correlates with pain and fatigue
We found that the clusterin (CLU) 15 min/0 h ratio positively correlated with fatigue the morning of exercise (Figure 11b) and it was the only protein in which increased abundance 24 h post‐exercise positively correlated with both average severity of myalgia and arthralgia (Figure 9a). CLU was also one of the few proteins with decreased abundance 15 min post‐exercise in controls (Figure 6a). CLU is a multifunctional molecular chaperone protein involved in a variety of physiological and pathologic processes, including clearance of cell debris, apoptosis, and assisting in the folding and conformational maturation of newly synthesized proteins as well as stress‐denatured proteins (Poon et al., 2002).
Increased levels of clusterin were found in blood and brain tissues of patients with neurodegenerative diseases (Grewal et al., 1999; Ingram et al., 2014; Shepherd et al., 2020). CLU concentration in cerebrospinal fluid positively correlated with cognitive impairment severity, indicating a direct link with synaptic degeneration (Wang et al., 2020). Serum CLU has been associated with pain and inflammation and was increased in idiopathic inflammatory myopathy and early rheumatoid arthritis (Kropáčková et al., 2021, 2020). CLU levels in EVs were also elevated in myelodysplastic syndrome (Pecankova et al., 2022) and in multiple neurodegenerative diseases (Jiang et al., 2020). In our study, elevated CLU in EVs post‐exercise is associated with worse myalgia, arthralgia, and fatigue indicating that this protein may have an important role in ME/CFS pathophysiology.
4.7. Dysfunctional immune signalling in EVs in ME/CFS post‐exercise
We found increased ANXA2, B2M and ORM1 in ME/CFS versus controls 15 min post‐exercise. These three proteins are members of the immune system protein pathways ‘antigen presentation’, ‘cytokine signaling’ and ‘adaptive immune system’ (Figure 4b).
Annexin II (ANXA2) is a calcium‐dependent phospholipid‐binding protein that orchestrates membrane repair, vesicle fusion and cytoskeletal organization during the inflammatory response or tissue injury (Lim & Hajjar, 2021). ANXA2 is known to be involved in the biosynthesis of EVs and found in their cargo (Luo & Hajjar, 2013) and it has been shown that exosomal‐ANXA2 promotes angiogenesis (Maji et al., 2017). ANXA2 in serum can increase inflammation by mediating macrophage M2 to M1 phenotypic change (Lin & Hu, 2017). Single‐cell transcriptomics analysis has recently implicated enhanced macrophage differentiation from monocytes in ME/CFS pathophysiology (Ahmed et al., 2022).
Beta‐2‐microglobulin (B2M) is necessary for the cell surface expression and structural stability of the major histocompatibility complex (MHC‐I) which plays key roles in antigen presentation and processing, inflammation, the complement cascade, and stress response (Trowsdale & Knight, 2013). In our study, the B2M 24 h/0 h ratio was also negatively correlated with myalgia general severity (Figure 9a). B2M is contributing to uniquely enriched tissue terms in ME/CFS patients relating to immune cells, brain and bone marrow (Figure 7b). In serum, a meta‐analysis found significant associations between higher B2M levels and increased risks of cardiovascular diseases outcomes (Shi et al., 2021) and that higher B2M levels were positively associated with inflammatory markers (Juraschek et al., 2013). Alternatively, B2M could be released in the systemic circulation as a result of inadequate blood supply (ischemia), which has been observed during exercise in patients with periphery artery disease (PAD) and suffering from intermittent pain, cramping or fatigue in the muscles. In an extensive proteomic profiling study, increased levels of plasma B2M were observed in patients with PAD and correlated with disease severity. In the CNS, B2M can also negatively affect hippocampal neurogenesis and synaptic plasticity and has been linked to clinical depression (Lamers et al., 2016). While B2M dysregulation has not been previously identified in ME/CFS patients, this finding is consistent with dysfunctional adaptive immune cells in ME/CFS (Maya, 2023).
Orosomucoid (ORM1) is an acute phase protein associated with fatigue. In our study, ORM1 had a significantly lower 24 h/15 min ratio in ME/CFS patients versus controls, due to opposing trends in controls versus patients (Figure 8, Figure S3). A previous study found that the change in muscle soreness scores in healthy individuals positively correlated with serum ORM1 levels 24 h post‐exercise, suggesting that ORM1 is associated with the extent of exercise‐induced damage and inflammation (Tékus et al., 2017). However, ORM1 is also upregulated in response to fatigue, leading to increased muscle glycogen levels and improved endurance. This positive feedback loop helps combat fatigue and maintain physiological homeostasis (Lei et al., 2016; Qin et al., 2016). Serum ORM levels correlated with fatigue severity in long‐COVID patients, a disease with many overlapping symptoms with ME/CFS (Zavori et al., 2023). Elevated serum total ORM (Sun et al., 2016) and ORM2 in cerebrospinal fluid have been found previously in ME/CFS patients versus controls (Baraniuk et al., 2005). This upregulation of serum and CSF ORM at baseline may reflect the body's attempt to mitigate the everyday fatigue that occurs in ME/CFS, while the altered dynamics of ORM1 in EVs post‐exercise may indicate disruption of the muscle fatigue—ORM1 feedback loop.
4.8. Members of the 14‐3‐3 protein family are increased in ME/CFS patients 15 min post‐exercise
Increased DAPs 15 min post‐exercise within the ME/CFS group were mainly associated with the apoptosis and cell cycle check points pathways, which include members of the 14‐3‐3 protein family (YWHAQ, YWHAG, YWHAB, YWHAZ) (Figure 6b). The Ingenuity pathway analysis showed that the 14‐3‐3 signalling pathway was significantly activated (z‐score 2.5) in patients versus controls at 15 min post‐exercise (Figure 5). Three of these proteins (YWHAB, YWHAH and YWHAQ) are expressed by the amygdala (Figure 7).
The 14‐3‐3 proteins are molecular chaperones widely expressed in all eukaryotic cells and especially in the central nervous system (CNS), accounting for about 1% of the total soluble brain protein (Berg et al., 2003). In the CNS, 14‐3‐3 proteins are usually induced in response to stresses, such as nerve damage and oxidation (Satoh et al., 2006). They play a key role in both development and neurodegeneration (Colucci et al., 2004; Morales et al., 2012; Shiga et al., 2006; Yacoubian et al., 2010). Interestingly, overexpression of YWHAQ enhanced the anti‐apoptotic and anti‐inflammatory effects of neural stem cell‐derived EVs, which improved recovery after spinal cord injury (Rong et al., 2019). Furthermore, Eguchi et al. found YWHAH and YWHAQ were increased in abundance in the EVs of three ME/CFS patients compared to three controls (Eguchi et al., 2020). Pooled EVs from five ME/CFS patients after an exercise challenge stimulated increased IL‐1β release from cultured human microglial cells compared to baseline, which suggests a potential mechanism for EV cargo to influence neuroinflammation post‐exercise (Tsilioni et al., 2022). Higher levels of 14‐3‐3 protein family members in EVs in ME/CFS patients could be an attempt by the CNS to combat exertion‐induced inflammatory signalling, which in our study was also increased in patients versus controls.
4.9. Limitations
The main limitation of this study is the small number of subjects, particularly for correlation analyses in which correlation coefficients are less stable with fewer samples. For that reason, we only presented correlations with |R| > 0.7. However, our study has more subjects than similar studies of EVs in a single group (Kobayashi et al., 2021; Whitham et al., 2018). Another key limitation is that we only included females. While the majority of ME/CFS patients are female, there are also male patients and it cannot be assumed that these results would apply to males as well, as many sex differences have been documented (Friedberg et al., 2023; Germain et al., 2022). A third limitation is that this study is limited to patients that were physically able to complete the exercise protocol, so the most severe and disabled ME/CFS patients are not represented in our study population. However, we did include mild and moderate ME/CFS patients with a wide range of symptom severity both on average and in response to exercise, which allowed us to find correlations between the EV proteome and symptom severity. The exercise test allowed us to synchronize the patients’ and sedentary controls’ molecular response to exertion, which enabled us to compare healthy controls’ and ME/CFS patients’ EV protein cargo dynamics post‐exercise.
Regarding analysis of EVs, one limitation is that we did not isolate EVs from platelet‐free plasma. While size exclusion chromatography removes platelets and larger microparticles from analysis, there is a possibility that platelets continued to release EVs ex vivo (Karimi et al., 2022) which can affect EV protein markers after exercise (McIlvenna et al., 2023). However, because we isolated plasma at three time points from the same individuals, we are still able to detect a specific effect of exercise. We also used the same methods for all samples and all subjects which allowed us to compare changes due to exercise across clinical groups.
5. CONCLUSIONS
EV protein cargo changed differently in response to exercise in ME/CFS cases and sedentary controls. The changes in abundance post‐exercise of several EV proteins in ME/CFS cases strongly correlate with the severity of certain symptoms. Dysregulated release of EVs and the presence of aberrant cargo relative to controls following exercise may play a role in the PEM experienced in females with ME/CFS.
AUTHOR CONTRIBUTIONS
Ludovic Giloteaux: Conceptualization (lead); formal analysis (lead); investigation (lead); methodology (lead); visualization (lead); writing—original draft (lead); writing—review and editing (lead). Katherine A. Glass: Conceptualization (lead); formal analysis (lead); investigation (lead); methodology (lead); visualization (lead); writing—original draft (lead); writing—review and editing (lead). Arnaud Germain: Formal analysis (supporting); visualization (supporting); writing—original draft (supporting); writing—review and editing (supporting). Arnaud Germain: Formal analysis (supporting); visualization (supporting); writing—original draft (supporting); writing—review and editing (supporting). Sheng Zhang: Methodology (supporting); resources (supporting); software (supporting); writing—review and editing (supporting). Carl J. Franconi: Methodology (supporting); resources (supporting); software; writing—review and editing (supporting). Maureen R. Hanson: Conceptualization (lead); funding acquisition; project administration; resources; supervision; writing—original draft; writing—review and editing.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflict of interest.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
We are grateful to the individuals who participated in our strenuous exercise protocol. This research was supported by NIH NINDS/OD/NIDA/NHLBI/NHGRI through U54NS105541 and by NIH NIAID U54AI178855 to the Cornell Center for Enervating Neuroimmune Disease and by a private donor. We thank Stephen Parry and Lynn Johnson at the Cornell Statistical Consulting Unit for their guidance on our statistical analysis. This work utilized equipment at the Cornell NanoScale Science & Technology Facility (CNF), a member of the National Nanotechnology Coordinated Infrastructure NNCI, which is supported by the National Science Foundation (Grant NNCI‐2025233). The REDCap database and blood processing at the Weill Cornell Medicine Clinical and Translational Science Center (CTSC) was supported by UL1 TR 002384 from the National Center for Advancing Translational Sciences of the NIH. We thank the following individuals who participated in participant screening, and/or CPET, and/or blood collection: Victoria Birdsall, John Chia, Patricia Doty, Tiffany Ong, Betsy Keller, Susan Levine, Xiangling Mao, Geoffrey Moore, Maria Russell, Dikoma Shungu, Jared Stevens, Kristin Treat, and David Wang. We acknowledge the Cornell Proteomics and Metabolomics Facility, especially Ruchika Bhawal, Elizabeth T. Anderson, and Qin Fu, for technical assistance with the nano‐LC MS/MS and preprocessing of the proteomics data.
Giloteaux, L. , Glass, K. A. , Germain, A. , Franconi, C. J. , Zhang, S. , & Hanson, M. R. (2024). Dysregulation of extracellular vesicle protein cargo in female myalgic encephalomyelitis/chronic fatigue syndrome cases and sedentary controls in response to maximal exercise. Journal of Extracellular Vesicles, 13, e12403. 10.1002/jev2.12403
Ludovic Giloteaux and Katherine A. Glass contributed equally to this study.
DATA AVAILABILITY STATEMENT
The protein abundance data for each protein and subject and matching phenotype data is available on mapMECFS, an interactive portal that provides access to research results from studies focused on ME/CFS at https://www.mapmecfs.org. Additional data from our analyses is available in the supplementary tables.
REFERENCES
- Ahmed, F. , Vu, L. T. , Zhu, H. , Iu, D. S. H. , Fogarty, E. A. , Kwak, Y. , Chen, W. , Franconi, C. J. , Munn, P. R. , Levine, S. M. , Stevens, J. , Mao, X. , Shungu, D. C. , Moore, G. E. , Keller, B. A. , Hanson, M. R. , Grenier, J. , & Grimson, A. (2022). Single‐cell transcriptomics of the immune system in ME/CFS at baseline and following symptom provocation. BioRxiv, 2022, 2010. 2013.512091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almenar‐Pérez, E. , Sarría, L. , Nathanson, L. , & Oltra, E. (2020). Assessing diagnostic value of microRNAs from peripheral blood mononuclear cells and extracellular vesicles in myalgic encephalomyelitis/chronic fatigue syndrome. Scientific Reports, 10(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor López, A. , Mazariegos, M. S. , Capuano, A. , Ximénez‐Embún, P. , Hergueta‐Redondo, M. , Recio, J. Á. , Muñoz, E. , Al‐Shahrour, F. , Muñoz, J. , & Megías, D. (2021). Inactivation of EMILIN‐1 by proteolysis and secretion in small extracellular vesicles favors melanoma progression and metastasis. International Journal of Molecular Sciences, 22(14), 7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai, M. , Yorifuji, H. , Ikematsu, S. , Nagasawa, H. , Fujimaki, M. , Fukutake, K. , Katsumura, T. , Ishii, T. , & Iwane, H. (1990). Influences of strenuous exercise (triathlon) on blood coagulation and fibrinolytic system. Thrombosis Research, 57(3), 465–471. [DOI] [PubMed] [Google Scholar]
- Baas, P. W. , Rao, A. N. , Matamoros, A. J. , & Leo, L. (2016). Stability properties of neuronal microtubules. Cytoskeleton, 73(9), 442–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraniuk, J. N. , Adewuyi, O. , Merck, S. J. , Ali, M. , Ravindran, M. K. , Timbol, C. R. , Rayhan, R. , Zheng, Y. , Le, U. , & Esteitie, R. (2013). A chronic fatigue syndrome (CFS) severity score based on case designation criteria. American Journal of Translational Research, 5(1), 53. [PMC free article] [PubMed] [Google Scholar]
- Baraniuk, J. N. , Casado, B. , Maibach, H. , Clauw, D. J. , Pannell, L. K. , & Hess, S. S. (2005). A chronic fatigue syndrome–related proteome in human cerebrospinal fluid. BMC Neurology, 5(1), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis, E. , Vanella, V. V. , Falasca, M. , Caneapero, V. , Cappellano, G. , Raineri, D. , Ghirimoldi, M. , De Giorgis, V. , Puricelli, C. , & Vaschetto, R. (2021). Circulating exosomes are strongly involved in SARS‐CoV‐2 infection. Frontiers in Molecular Biosciences, 8, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, R. , Leone, M. , & Engel, F. B. (2020). Microtubule organization in striated muscle cells. Cells, 9(6), 1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei, Y. , Xu, T. , Lv, D. , Yu, P. , Xu, J. , Che, L. , Das, A. , Tigges, J. , Toxavidis, V. , & Ghiran, I. (2017). Exercise‐induced circulating extracellular vesicles protect against cardiac ischemia–reperfusion injury. Basic Research in Cardiology, 112, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini, Y. , & Yekutieli, D. (2005). False discovery rate–adjusted multiple confidence intervals for selected parameters. Journal of the American Statistical Association, 100(469), 71–81. [Google Scholar]
- Berg, D. , Holzmann, C. , & Riess, O. (2003). 14‐3‐3 proteins in the nervous system. Nature Reviews Neuroscience, 4(9), 752–762. [DOI] [PubMed] [Google Scholar]
- Blighe, K. , Rana, S. , & Lewis, M. (2023). EnhancedVolcano: Publication‐Ready Volcano Plots with Enhanced Colouring and Labeling. EnhancedVolcano. Available online: https://github.com/kevinblighe
- Bonilla, H. , Hampton, D. , Marques de Menezes, E. G. , Deng, X. , Montoya, J. G. , Anderson, J. , & Norris, P. J. (2022). Comparative analysis of extracellular vesicles in patients with severe and mild myalgic encephalomyelitis/chronic fatigue syndrome. Frontiers in Immunology, 13, 841910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer, M. J. , Kimura, Y. , Akiyama, T. , Baggett, A. Y. , Preston, K. J. , Scalia, R. , Eguchi, S. , & Rizzo, V. (2020). Endothelial cell‐derived extracellular vesicles alter vascular smooth muscle cell phenotype through high‐mobility group box proteins. Journal of Extracellular Vesicles, 9(1), 1781427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer, A. , Neuberger, E. , Esch‐Heisser, L. , Haller, N. , Jorgensen, M. M. , Baek, R. , Möbius, W. , Simon, P. , & Krämer‐Albers, E.‐M. (2019). Platelets, endothelial cells and leukocytes contribute to the exercise‐triggered release of extracellular vesicles into the circulation. Journal of Extracellular Vesicles, 8(1), 1615820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman, L. (2001). Random forests. Machine Learning, 45, 5–32. [Google Scholar]
- Carruthers, B. M. , Jain, A. K. , De Meirleir, K. L. , Peterson, D. L. , Klimas, N. G. , Lerner, A. M. , Bested, A. C. , Flor‐Henry, P. , Joshi, P. , & Powles, A. P. (2003). Myalgic encephalomyelitis/chronic fatigue syndrome: Clinical working case definition, diagnostic and treatment protocols. Journal of Chronic Fatigue Syndrome, 11(1), 7–115. [Google Scholar]
- Castro‐Marrero, J. , Serrano‐Pertierra, E. , Oliveira‐Rodríguez, M. , Zaragozá, M. C. , Martínez‐Martínez, A. , Blanco‐López, M. d. C. , & Alegre, J. (2018). Circulating extracellular vesicles as potential biomarkers in chronic fatigue syndrome/myalgic encephalomyelitis: An exploratory pilot study. Journal of Extracellular Vesicles, 7(1), 1453730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri, A. , & Behan, P. O. (2000). Fatigue and basal ganglia. Journal of the Neurological Sciences, 179(1‐2), 34–42. [DOI] [PubMed] [Google Scholar]
- Chen, E. Y. , Tan, C. M. , Kou, Y. , Duan, Q. , Wang, Z. , Meirelles, G. V. , Clark, N. R. , & Ma'ayan, A. (2013). Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics [Electronic Resource], 14(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombatti, A. , Doliana, R. , Bot, S. , Canton, A. , Mongiat, M. , Mungiguerra, G. , Paron‐Cilli, S. , & Spessotto, P. (2000). The EMILIN protein family. Matrix Biology, 19(4), 289–301. [DOI] [PubMed] [Google Scholar]
- Colucci, M. , Roccatagliata, L. , Capello, E. , Narciso, E. , Latronico, N. , Tabaton, M. , & Mancardi, G. (2004). The 14‐3‐3 protein in multiple sclerosis: A marker of disease severity. Multiple Sclerosis Journal, 10(5), 477–481. [DOI] [PubMed] [Google Scholar]
- Contrepois, K. , Wu, S. , Moneghetti, K. J. , Hornburg, D. , Ahadi, S. , Tsai, M.‐S. , Metwally, A. A. , Wei, E. , Lee‐McMullen, B. , & Quijada, J. V. (2020). Molecular choreography of acute exercise. Cell, 181(5), 1112–1130. e1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, G. , Abildgaard, C. , Bernauer, E. , & Britton, M. (1976). Fibrinolytic and hemostatic changes during and after maximal exercise in males. Journal of Applied Physiology, 40(3), 287–292. [DOI] [PubMed] [Google Scholar]
- Doyle, L. M. , & Wang, M. Z. (2019). Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells, 8(7), 727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrer, C. , Robinson, E. , Wan, Z. , Martinez, N. , Hummel, M. L. , Jenkins, N. T. , Kilpatrick, M. W. , & Little, J. P. (2015). Differential impact of acute high‐intensity exercise on circulating endothelial microparticles and insulin resistance between overweight/obese males and females. PLoS ONE, 10(2), e0115860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi, A. , Fukuda, S. , Kuratsune, H. , Nojima, J. , Nakatomi, Y. , Watanabe, Y. , & Feldstein, A. E. (2020). Identification of actin network proteins, talin‐1 and filamin‐A, in circulating extracellular vesicles as blood biomarkers for human myalgic encephalomyelitis/chronic fatigue syndrome. Brain, Behavior, and Immunity, 84, 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada, A. L. , Valenti, Z. J. , Hehn, G. , Amorese, A. J. , Williams, N. S. , Balestrieri, N. P. , Deighan, C. , Allen, C. P. , Spangenburg, E. E. , & Kruh‐Garcia, N. A. (2022). Extracellular vesicle secretion is tissue‐dependent ex vivo and skeletal muscle myofiber extracellular vesicles reach the circulation in vivo. American Journal of Physiology‐Cell Physiology, 322(2), C246–C259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forterre, A. , Jalabert, A. , Berger, E. , Baudet, M. , Chikh, K. , Errazuriz, E. , De Larichaudy, J. , Chanon, S. , Weiss‐Gayet, M. , & Hesse, A.‐M. (2014). Proteomic analysis of C2C12 myoblast and myotube exosome‐like vesicles: A new paradigm for myoblast‐myotube cross talk? PLoS ONE, 9(1), e84153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg, F. , Adamowicz, J. L. , Bruckenthal, P. , Milazzo, M. , Ramjan, S. , Zhang, X. , & Yang, J. (2023). Sex differences in post‐exercise fatigue and function in myalgic encephalomyelitis/chronic fatigue syndrome. Scientific Reports, 13(1), 5442. 10.1038/s41598-023-32581-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frühbeis, C. , Helmig, S. , Tug, S. , Simon, P. , & Krämer‐Albers, E.‐M. (2015). Physical exercise induces rapid release of small extracellular vesicles into the circulation. Journal of Extracellular Vesicles, 4(1), 28239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, Q. , Liu, Z. , Bhawal, R. , Anderson, E. T. , Sherwood, R. W. , Yang, Y. , Thannhauser, T. , Schroyen, M. , Tang, X. , & Zhang, H. (2021). Comparison of MS 2, synchronous precursor selection MS 3, and real‐time search MS 3 methodologies for lung proteomes of hydrogen sulfide treated swine. Analytical and Bioanalytical Chemistry, 413, 419–429. [DOI] [PubMed] [Google Scholar]
- Ge, S. X. , Jung, D. , & Yao, R. (2020). ShinyGO: A graphical gene‐set enrichment tool for animals and plants. Bioinformatics, 36(8), 2628–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain, A. , Giloteaux, L. , Moore, G. E. , Levine, S. M. , Chia, J. K. , Keller, B. A. , Stevens, J. , Franconi, C. J. , Mao, X. , Shungu, D. C. , Grimson, A. , & Hanson, M. R. (2022). Plasma metabolomics reveals disrupted response and recovery following maximal exercise in myalgic encephalomyelitis/chronic fatigue syndrome. JCI Insight, 7(9), e157621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giloteaux, L. , Li, J. , Hornig, M. , Lipkin, W. I. , Ruppert, D. , & Hanson, M. R. (2023). Proteomics and cytokine analyses distinguish myalgic encephalomyelitis/chronic fatigue syndrome cases from controls. Journal of Translational Medicine, 21(1), 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giloteaux, L. , O'Neal, A. , Castro‐Marrero, J. , Levine, S. M. , & Hanson, M. R. (2020). Cytokine profiling of extracellular vesicles isolated from plasma in myalgic encephalomyelitis/chronic fatigue syndrome: A pilot study. Journal of Translational Medicine, 18(1), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass, K. A. , Germain, A. , Huang, Y. V. , & Hanson, M. R. (2023). Urine metabolomics exposes anomalous recovery after maximal exertion in female ME/CFS patients. International Journal of Molecular Sciences, 24(4), 3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González‐Cebrián, A. , Almenar‐Pérez, E. , Xu, J. , Yu, T. , Huang, W. E. , Giménez‐Orenga, K. , Hutchinson, S. , Lodge, T. , Nathanson, L. , & Morten, K. J. (2022). Diagnosis of myalgic encephalomyelitis/chronic fatigue syndrome with partial least squares discriminant analysis: Relevance of Blood extracellular vesicles. Frontiers in Medicine, 9, 842991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal, R. P. , Morgan, T. E. , & Finch, C. E. (1999). C1qB and clusterin mRNA increase in association with neurodegeneration in sporadic amyotrophic lateral sclerosis. Neuroscience Letters, 271(1), 65–67. [DOI] [PubMed] [Google Scholar]
- Gurung, S. , Perocheau, D. , Touramanidou, L. , & Baruteau, J. (2021). The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Communication and Signaling, 19(1), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighitalab, A. , Dominici, M. , Matin, M. M. , Shekari, F. , Ebrahimi Warkiani, M. , Lim, R. , Ahmadiankia, N. , Mirahmadi, M. , Bahrami, A. R. , & Bidkhori, H. R. (2023). Extracellular vesicles and their cells of origin: Open issues in autoimmune diseases. Frontiers in Immunology, 14, 1090416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram, G. , Loveless, S. , Howell, O. W. , Hakobyan, S. , Dancey, B. , Harris, C. L. , Robertson, N. P. , Neal, J. W. , & Morgan, B. P. (2014). Complement activation in multiple sclerosis plaques: An immunohistochemical analysis. Acta Neuropathologica Communications, 2(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome . (2015). Beyond myalgic encephalomyelitis/chronic fatigue syndrome: Redefining an illness. National Academies Press. 10.17226/19012 [DOI] [Google Scholar]
- Jiang, C. , Hopfner, F. , Katsikoudi, A. , Hein, R. , Catli, C. , Evetts, S. , Huang, Y. , Wang, H. , Ryder, J. W. , & Kuhlenbaeumer, G. (2020). Serum neuronal exosomes predict and differentiate Parkinson's disease from atypical parkinsonism. Journal of Neurology, Neurosurgery & Psychiatry, 91(7), 720–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, K. H. , Chu, K. , Lee, S. T. , Park, H. K. , Bahn, J. J. , Kim, D. H. , Kim, J. H. , Kim, M. , Kun Lee, S. , & Roh, J. K. (2009). Circulating endothelial microparticles as a marker of cerebrovascular disease. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 66(2), 191–199. [DOI] [PubMed] [Google Scholar]
- Juraschek, S. P. , Coresh, J. , Inker, L. A. , Levey, A. S. , Köttgen, A. , Foster, M. C. , Astor, B. C. , Eckfeldt, J. H. , & Selvin, E. (2013). Comparison of serum concentrations of β‐trace protein, β2‐microglobulin, cystatin C, and creatinine in the US population. Clinical Journal of the American Society of Nephrology, 8(4), 584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra, H. , Simpson, R. J. , Ji, H. , Aikawa, E. , Altevogt, P. , Askenase, P. , Bond, V. C. , Borràs, F. E. , Breakefield, X. , & Budnik, V. (2012). Vesiclepedia: A compendium for extracellular vesicles with continuous community annotation. PLoS Biology, 10(12), e1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapustin, A. , & Shanahan, C. (2016). Emerging roles for vascular smooth muscle cell exosomes in calcification and coagulation. The Journal of Physiology, 594(11), 2905–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi, N. , Cvjetkovic, A. , Jang, S. C. , Crescitelli, R. , Hosseinpour Feizi, M. A. , Nieuwland, R. , Lötvall, J. , & Lässer, C. (2018). Detailed analysis of the plasma extracellular vesicle proteome after separation from lipoproteins. Cellular and Molecular Life Sciences, 75, 2873–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi, N. , Dalirfardouei, R. , Dias, T. , Lötvall, J. , & Lässer, C. (2022). Tetraspanins distinguish separate extracellular vesicle subpopulations in human serum and plasma–Contributions of platelet extracellular vesicles in plasma samples. Journal of Extracellular Vesicles, 11(5), e12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keerthikumar, S. , Chisanga, D. , Ariyaratne, D. , Al Saffar, H. , Anand, S. , Zhao, K. , Samuel, M. , Pathan, M. , Jois, M. , & Chilamkurti, N. (2016). ExoCarta: A web‐based compendium of exosomal cargo. Journal of Molecular Biology, 428(4), 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrloesser, S. , Cast, O. , Elliott, T. S. , Ernst, R. J. , Machel, A. C. , Chen, J.‐X. , Chin, J. W. , & Miller, M. L. (2023). Cell‐of‐origin–specific proteomics of extracellular vesicles. PNAS Nexus, 2(4), pgad107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, B. A. , Pryor, J. L. , & Giloteaux, L. (2014). Inability of myalgic encephalomyelitis/chronic fatigue syndrome patients to reproduce VO2peak indicates functional impairment. Journal of Translational Medicine, 12(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk, R. J. , Peart, D. J. , Madden, L. A. , & Vince, R. V. (2014). Repeated supra‐maximal sprint cycling with and without sodium bicarbonate supplementation induces endothelial microparticle release. European Journal of Sport Science, 14(4), 345–352. [DOI] [PubMed] [Google Scholar]
- Kishi, H. , Ye, L.‐H. , Nakamura, A. , Okagaki, T. , Iwata, A. , Tanaka, T. , & Kohama, K. (1998). Structure and Function of Smooth Muscle Myosin Light Chain Kinase. In: Sugi, H. , Pollack, G. H. (eds.), Mechanisms of Work Production and Work Absorption in Muscle. Advances in Experimental Medicine and Biology, vol 453 (pp. 229–234). Springer, Boston, MA. 10.1007/978-1-4684-6039-1_26 [DOI] [PubMed] [Google Scholar]
- Kobayashi, Y. , Eguchi, A. , Tamai, Y. , Fukuda, S. , Tempaku, M. , Izuoka, K. , Iwasa, M. , Takei, Y. , & Togashi, K. (2021). Protein composition of circulating extracellular vesicles immediately changed by particular short time of high‐intensity interval training exercise. Frontiers in Physiology, 12, 693007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokla, M. , Virtanen, J. , Kolehmainen, M. , Paananen, J. , & Hanhineva, K. (2019). Random forest‐based imputation outperforms other methods for imputing LC‐MS metabolomics data: A comparative study. BMC Bioinformatics [Electronic Resource], 20(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer, A. , Green, J. , Pollard, Jr, J. , & Tugendreich, S. (2014). Causal analysis approaches in ingenuity pathway analysis. Bioinformatics, 30(4), 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropáčková, T. , Mann, H. , Růžičková, O. , Šléglová, O. , Vernerová, L. , Horváthová, V. , Tomčík, M. , Pavelka, K. , Vencovský, J. , & Šenolt, L. (2021). Clusterin serum levels are elevated in patients with early rheumatoid arthritis and predict disease activity and treatment response. Scientific Reports, 11(1), 11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropáčková, T. , Vernerová, L. , Štorkánová, H. , Horváthová, V. , Vokurková, M. , Klein, M. , Oreská, S. , Špiritović, M. , Heřmánková, B. , & Kubínová, K. (2020). Clusterin is upregulated in serum and muscle tissue in idiopathic inflammatory myopathies and associates with clinical disease activity and cytokine profile. Clinical and Experimental Rheumatology, 39(5), 1021–1032. [DOI] [PubMed] [Google Scholar]
- Lamers, F. , Bot, M. , Jansen, R. , Chan, M. , Cooper, J. , Bahn, S. , & Penninx, B. (2016). Serum proteomic profiles of depressive subtypes. Translational Psychiatry, 6(7), e851–e851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, H. , Sun, Y. , Luo, Z. , Yourek, G. , Gui, H. , Yang, Y. , Su, D.‐F. , & Liu, X. (2016). Fatigue‐induced orosomucoid 1 acts on CC chemokine receptor type 5 to enhance muscle endurance. Scientific Reports, 6(1), 18839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, G. , You, Q. , Hou, X. , Zhang, S. , Du, L. , Lv, Y. , & Yu, L. (2023). The effect of exercise on cognitive function in people with multiple sclerosis: A systematic review and meta‐analysis of randomized controlled trials. Journal of Neurology, 270(6), 2908–2923. [DOI] [PubMed] [Google Scholar]
- Lim, H. I. , & Hajjar, K. A. (2021). Annexin A2 in fibrinolysis, inflammation and fibrosis. International Journal of Molecular Sciences, 22(13), 6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, L. , & Hu, K. (2017). Tissue‐type plasminogen activator modulates macrophage M2 to M1 phenotypic change through annexin A2‐mediated NF‐κB pathway. Oncotarget, 8(50), 88094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litteri, G. , Carnevale, D. , D'Urso, A. , Cifelli, G. , Braghetta, P. , Damato, A. , Bizzotto, D. , Landolfi, A. , Ros, F. D. , & Sabatelli, P. (2012). Vascular smooth muscle Emilin‐1 is a regulator of arteriolar myogenic response and blood pressure. Arteriosclerosis, Thrombosis, and Vascular Biology, 32(9), 2178–2184. [DOI] [PubMed] [Google Scholar]
- Lowe, G. , & Rumley, A. (2014). The relevance of coagulation in cardiovascular disease: what do the biomarkers tell us? Thrombosis and Haemostasis, 112(5), 860–867. [DOI] [PubMed] [Google Scholar]
- Luo, M. , & Hajjar, K. A. (2013). Annexin A2 system in human biology: Cell surface and beyond. Seminars in thrombosis and hemostasis. Seminars in Thrombosis and Hemostasis, 39(4), 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maji, S. , Chaudhary, P. , Akopova, I. , Nguyen, P. M. , Hare, R. J. , Gryczynski, I. , & Vishwanatha, J. K. (2017). Exosomal annexin II promotes angiogenesis and breast cancer MetastasisExosomal Anx II in angiogenesis and metastasis. Molecular Cancer Research, 15(1), 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavropalias, G. , Boppart, M. , Usher, K. M. , Grounds, M. D. , Nosaka, K. , & Blazevich, A. J. (2023). Exercise builds the scaffold of life: Muscle extracellular matrix biomarker responses to physical activity, inactivity, and aging. Biological Reviews, 98(2), 481–519. [DOI] [PubMed] [Google Scholar]
- Maya, J. (2023). Surveying the metabolic and dysfunctional profiles of T cells and NK cells in myalgic encephalomyelitis/chronic fatigue syndrome. International Journal of Molecular Sciences, 24(15), 11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlvenna, L. C. , Parker, H. J. , Seabright, A. P. , Sale, B. , Anghileri, G. , Weaver, S. R. , Lucas, S. J. , & Whitham, M. (2023). Single vesicle analysis reveals the release of tetraspanin positive extracellular vesicles into circulation with high intensity intermittent exercise. The Journal of Physiology, 601(22), 5093–5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, S. , Wang, T. , Zhao, Q. , Hu, Q. , Chen, Y. , Li, H. , Liu, C. , Liu, D. , & Hong, X. (2022). Proteomics analysis of plasma‐derived exosomes unveils the aberrant complement and coagulation cascades in dermatomyositis/polymyositis. Journal of Proteome Research, 22(1), 123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel, K. , & Hilberg, T. (2011). Blood coagulation and fibrinolysis in healthy, untrained subjects: Effects of different exercise intensities controlled by individual anaerobic threshold. European Journal of Applied Physiology, 111, 253–260. [DOI] [PubMed] [Google Scholar]
- Möbius‐Winkler, S. , Hilberg, T. , Menzel, K. , Golla, E. , Burman, A. , Schuler, G. , & Adams, V. (2009). Time‐dependent mobilization of circulating progenitor cells during strenuous exercise in healthy individuals. Journal of Applied Physiology, 107(6), 1943–1950. [DOI] [PubMed] [Google Scholar]
- Moore, G. E. , Keller, B. A. , Stevens, J. , Mao, X. , Stevens, S. R. , Chia, J. K. , Levine, S. M. , Franconi, C. J. , & Hanson, M. R. (2023). Recovery from exercise in persons with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Medicina, 59(3), 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales, D. , Skoulakis, E. C. , & Acevedo, S. F. (2012). 14‐3‐3s are potential biomarkers for HIV‐related neurodegeneration. Journal of Neurovirology, 18, 341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, R. M. , Watt, M. J. , & Febbraio, M. A. (2020). Metabolic communication during exercise. Nature Metabolism, 2(9), 805–816. [DOI] [PubMed] [Google Scholar]
- Newcomer, S. C. , Thijssen, D. H. , & Green, D. J. (2011). Effects of exercise on endothelium and endothelium/smooth muscle cross talk: Role of exercise‐induced hemodynamics. Journal of Applied Physiology, 111(1), 311–320. [DOI] [PubMed] [Google Scholar]
- Nunes, J. M. , Kruger, A. , Proal, A. , Kell, D. B. , & Pretorius, E. (2022). The occurrence of hyperactivated platelets and fibrinaloid microclots in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Pharmaceuticals, 15(8), 931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystoriak, M. A. , & Bhatnagar, A. (2018). Cardiovascular effects and benefits of exercise. Frontiers in Cardiovascular Medicine, 5, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira, Jr, G. P. , Porto, W. F. , Palu, C. C. , Pereira, L. M. , Petriz, B. , Almeida, J. A. , Viana, J. , Filho, N. N. , Franco, O. L. , & Pereira, R. W. (2018). Effects of acute aerobic exercise on rats serum extracellular vesicles diameter, concentration and small RNAs content. Frontiers in Physiology, 9, 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecankova, K. , Pecherkova, P. , Gasova, Z. , Sovova, Z. , Riedel, T. , Jäger, E. , Cermak, J. , & Majek, P. (2022). Proteome changes of plasma‐derived extracellular vesicles in patients with myelodysplastic syndrome. PLoS ONE, 17(1), e0262484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon, S. , Treweek, T. M. , Wilson, M. R. , Easterbrook‐Smith, S. B. , & Carver, J. A. (2002). Clusterin is an extracellular chaperone that specifically interacts with slowly aggregating proteins on their off‐folding pathway. FEBS Letters, 513(2‐3), 259–266. [DOI] [PubMed] [Google Scholar]
- Puetz, T. W. , & Herring, M. P. (2023). Physical activity and feelings of fatigue. In Routledge handbook of physical activity and mental health (pp. 422–439). Routledge. [Google Scholar]
- Qin, Z. , Wan, J.‐J. , Sun, Y. , Wang, P.‐Y. , Su, D.‐F. , Lei, H. , & Liu, X. (2016). ORM promotes skeletal muscle glycogen accumulation via CCR5‐activated AMPK pathway in mice. Frontiers in Pharmacology, 7, 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran, L. , Honsho, M. , Zahn, T. R. , Keller, P. , Geiger, K. D. , Verkade, P. , & Simons, K. (2006). Alzheimer's disease β‐amyloid peptides are released in association with exosomes. Proceedings of the National Academy of Sciences, 103(30), 11172–11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakobowchuk, M. , Ritter, O. , Wilhelm, E. N. , Isacco, L. , Bouhaddi, M. , Degano, B. , Tordi, N. , & Mourot, L. (2017). Divergent endothelial function but similar platelet microvesicle responses following eccentric and concentric cycling at a similar aerobic power output. Journal of Applied Physiology, 122(4), 1031–1039. [DOI] [PubMed] [Google Scholar]
- Reina‐Gutiérrez, S. , Meseguer‐Henarejos, A. B. , Torres‐Costoso, A. , Álvarez‐Bueno, C. , Cavero‐Redondo, I. , Núñez de Arenas‐Arroyo, S. , Guzmán‐Pavón, M. J. , Sánchez‐López, M. , & Martínez‐Vizcaíno, V. (2023). Effect of different types of exercise on fitness in people with multiple sclerosis: A network meta‐analysis. Scandinavian Journal of Medicine & Science in Sports, 33(10), 1916–1928. [DOI] [PubMed] [Google Scholar]
- Rognes, I. N. , Hellum, M. , Ottestad, W. , Bache, K. G. , Eken, T. , & Henriksson, C. E. (2021). Extracellular vesicle–associated procoagulant activity is highest the first 3 hours after trauma and thereafter declines substantially: A prospective observational pilot study. The Journal of Trauma and Acute Care Surgery, 91(4), 681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong, Y. , Liu, W. , Lv, C. , Wang, J. , Luo, Y. , Jiang, D. , Li, L. , Zhou, Z. , Zhou, W. , & Li, Q. (2019). Neural stem cell small extracellular vesicle‐based delivery of 14‐3‐3t reduces apoptosis and neuroinflammation following traumatic spinal cord injury by enhancing autophagy by targeting Beclin‐1. Aging (Albany NY), 11(18), 7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, R. E. , VanDerwerker, C. J. , Saladin, M. E. , & Gregory, C. M. (2023). The role of exercise in the treatment of depression: Biological underpinnings and clinical outcomes. Molecular Psychiatry, 28(1), 298–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh, K. , Shirabe, S. , Eguchi, H. , Tsujino, A. , Eguchi, K. , Satoh, A. , Tsujihata, M. , Niwa, M. , Katamine, S. , & Kurihara, S. (2006). 14‐3‐3 protein, total tau and phosphorylated tau in cerebrospinal fluid of patients with Creutzfeldt‐Jakob disease and neurodegenerative disease in Japan. Cellular and Molecular Neurobiology, 26, 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setua, S. , Thangaraju, K. , Dzieciatkowska, M. , Wilkerson, R. B. , Nemkov, T. , Lamb, D. R. , Tagaya, Y. , Boyer, T. , Rowden, T. , & Doctor, A. (2022). Coagulation potential and the integrated omics of extracellular vesicles from COVID‐19 positive patient plasma. Scientific Reports, 12(1), 22191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd, C. E. , Affleck, A. J. , Bahar, A. Y. , Carew‐Jones, F. , & Halliday, G. M. (2020). Intracellular and secreted forms of clusterin are elevated early in Alzheimer's disease and associate with both Aβ and tau pathology. Neurobiology of Aging, 89, 129–131. [DOI] [PubMed] [Google Scholar]
- Shi, F. , Sun, L. , & Kaptoge, S. (2021). Association of beta‐2‐microglobulin and cardiovascular events and mortality: A systematic review and meta‐analysis. Atherosclerosis, 320, 70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiga, Y. , Wakabayashi, H. , Miyazawa, K. , Kido, H. , & Itoyama, Y. (2006). 14‐3‐3 protein levels and isoform patterns in the cerebrospinal fluid of Creutzfeldt‐Jakob disease patients in the progressive and terminal stages. Journal of Clinical Neuroscience, 13(6), 661–665. [DOI] [PubMed] [Google Scholar]
- Singh, B. , Olds, T. , Curtis, R. , Dumuid, D. , Virgara, R. , Watson, A. , Szeto, K. , O'Connor, E. , Ferguson, T. , & Eglitis, E. (2023). Effectiveness of physical activity interventions for improving depression, anxiety and distress: An overview of systematic reviews. British Journal of Sports Medicine, 57(18), 1203–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets, E. , Garssen, B. , Bonke, B. d. , & De Haes, J. (1995). The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. Journal of Psychosomatic Research, 39(3), 315–325. [DOI] [PubMed] [Google Scholar]
- Sossdorf, M. , Otto, G. P. , Claus, R. A. , Gabriel, H. H. , & Lösche, W. (2011). Cell‐derived microparticles promote coagulation after moderate exercise. Medicine and Science in Sports and Exercise, 43(7), 1169–1176. [DOI] [PubMed] [Google Scholar]
- Stekhoven, D. J. , & Bühlmann, P. (2012). MissForest—non‐parametric missing value imputation for mixed‐type data. Bioinformatics, 28(1), 112–118. [DOI] [PubMed] [Google Scholar]
- Stevens, S. , Snell, C. , Stevens, J. , Keller, B. , & VanNess, J. M. (2018). Cardiopulmonary exercise test methodology for assessing exertion intolerance in myalgic encephalomyelitis/chronic fatigue syndrome. Frontiers in Pediatrics, 6, 386825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stussman, B. , Williams, A. , Snow, J. , Gavin, A. , Scott, R. , Nath, A. , & Walitt, B. (2020). Characterization of post–exertional malaise in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Frontiers in Neurology, 11, 1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Y. , Zhang, Z. X. , & Liu, X. (2016). Orosomucoid (ORM) as a potential biomarker for the diagnosis of chronic fatigue syndrome (CFS). CNS Neuroscience & Therapeutics, 22(3), 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur, S. , Khatun, M. , Steele, R. , Isbell, T. S. , Ray, R. , & Ray, R. B. (2021). Exosomes from COVID‐19 patients carry tenascin‐C and fibrinogen‐β in triggering inflammatory signals in cells of distant organ. International Journal of Molecular Sciences, 22(6), 3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tékus, É. , Váczi, M. , Horváth‐Szalai, Z. , Ludány, A. , Kőszegi, T. , & Wilhelm, M. (2017). Plasma actin, gelsolin and orosomucoid levels after eccentric exercise. Journal of Human Kinetics, 56(1), 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry, C. , Witwer, K. W. , Aikawa, E. , Alcaraz, M. J. , Anderson, J. D. , Andriantsitohaina, R. , Antoniou, A. , Arab, T. , Archer, F. , & Atkin‐Smith, G. K. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles, 7(1), 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thul, P. J. , & Lindskog, C. (2018). The human protein atlas: A spatial map of the human proteome. Protein Science, 27(1), 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowsdale, J. , & Knight, J. C. (2013). Major histocompatibility complex genomics and human disease. Annual Review of Genomics and Human Genetics, 14, 301–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilioni, I. , Natelson, B. , & Theoharides, T. C. (2022). Exosome‐associated mitochondrial DNA from patients with myalgic encephalomyelitis/chronic fatigue syndrome stimulates human microglia to release IL‐1β. European Journal of Neuroscience, 56, 5784–5794. [DOI] [PubMed] [Google Scholar]
- Unwin, R. D. , Griffiths, J. R. , & Whetton, A. D. (2010). Simultaneous analysis of relative protein expression levels across multiple samples using iTRAQ isobaric tags with 2D nano LC–MS/MS. Nature Protocols, 5(9), 1574–1582. [DOI] [PubMed] [Google Scholar]
- Van Booven, D. J. , Gamer, J. , Joseph, A. , Perez, M. , Zarnowski, O. , Pandya, M. , Collado, F. , Klimas, N. , Oltra, E. , & Nathanson, L. (2023). Stress‐induced transcriptomic changes in females with myalgic encephalomyelitis/chronic fatigue syndrome reveal disrupted immune signatures. International Journal of Molecular Sciences, 24(3), 2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl, P. , Jansen, F. , Achtzehn, S. , Schmitz, T. , Bloch, W. , Mester, J. , & Werner, N. (2014). Effects of high intensity training and high volume training on endothelial microparticles and angiogenic growth factors. PLoS ONE, 9(4), e96024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Zhang, X. , Zhu, B. , Fu, P. , & Initiative, A. s. D. N. (2020). Association of clusterin levels in cerebrospinal fluid with synaptic degeneration across the Alzheimer's disease continuum. Neuropsychiatric Disease and Treatment, 16, 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware, J. E. (1993). SF‐36 health survey. Manual and interpretation guide. The Health Institute, 6(1–6), 22. [Google Scholar]
- Warnier, G. , De Groote, E. , Britto, F. A. , Delcorte, O. , Nederveen, J. P. , Nilsson, M. I. , Pierreux, C. E. , Tarnopolsky, M. A. , & Deldicque, L. (2022). Effects of an acute exercise bout in hypoxia on extracellular vesicle release in healthy and prediabetic subjects. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 322(2), 112–122. [DOI] [PubMed] [Google Scholar]
- Wei, R. , Wang, J. , Su, M. , Jia, E. , Chen, S. , Chen, T. , & Ni, Y. (2018). Missing value imputation approach for mass spectrometry‐based metabolomics data. Scientific Reports, 8(1), 663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham, M. , Parker, B. L. , Friedrichsen, M. , Hingst, J. R. , Hjorth, M. , Hughes, W. E. , Egan, C. L. , Cron, L. , Watt, K. I. , & Kuchel, R. P. (2018). Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metabolism, 27(1), 237–251. e234. [DOI] [PubMed] [Google Scholar]
- Wilhelm, E. N. , González‐Alonso, J. , Parris, C. , & Rakobowchuk, M. (2016). Exercise intensity modulates the appearance of circulating microvesicles with proangiogenic potential upon endothelial cells. American Journal of Physiology‐Heart and Circulatory Physiology, 311(5), H1297–H1310. [DOI] [PubMed] [Google Scholar]
- Womack, C. J. , Nagelkirk, P. R. , & Coughlin, A. M. (2003). Exercise‐induced changes in coagulation and fibrinolysis in healthy populations and patients with cardiovascular disease. Sports Medicine, 33, 795–807. [DOI] [PubMed] [Google Scholar]
- Xie, Z. , Bailey, A. , Kuleshov, M. V. , Clarke, D. J. , Evangelista, J. E. , Jenkins, S. L. , Lachmann, A. , Wojciechowicz, M. L. , Kropiwnicki, E. , & Jagodnik, K. M. (2021). Gene set knowledge discovery with Enrichr. Current Protocols, 1(3), e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoubian, T. A. , Slone, S. R. , Harrington, A. J. , Hamamichi, S. , Schieltz, J. M. , Caldwell, K. A. , Caldwell, G. A. , & Standaert, D. G. (2010). Differential neuroprotective effects of 14‐3‐3 proteins in models of Parkinson's disease. Cell Death & Disease, 1(1), e2–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavori, L. , Molnar, T. , Varnai, R. , Kanizsai, A. , Nagy, L. , Vadkerti, B. , Szirmay, B. , Schwarcz, A. , & Csecsei, P. (2023). Cystatin‐c may indicate subclinical renal involvement, while orosomucoid is associated with fatigue in patients with long‐COVID syndrome. Journal of Personalized Medicine, 13(2), 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Data Availability Statement
The protein abundance data for each protein and subject and matching phenotype data is available on mapMECFS, an interactive portal that provides access to research results from studies focused on ME/CFS at https://www.mapmecfs.org. Additional data from our analyses is available in the supplementary tables.


