Summary
Studies across a diverse group of metazoan embryos indicate that Wnt signaling often activates the transcription factor Sp5, forming a signaling ‘cassette’ that plays critical roles in many developmental processes. This study explores the role of Wnt/Sp5 signaling during the specification and patterning of the primary germ layers during early anterior-posterior axis formation in the deuterostome sea urchin embryo. Our functional analyses show that Sp5 is critical for endomesoderm specification downstream of Wnt/β-catenin in posterior cells as well as anterior neuroectoderm patterning downstream of non-canonical Wnt/JNK signaling in anterior cells. Interestingly, expression and functional data comparisons show that Wnt/Sp5 signaling often plays similar roles in posterior endomesoderm as well as neuroectoderm patterning along the AP axis of several deuterostome embryos, including vertebrates. Thus, our findings provide strong support for the idea that Wnt-Sp5 signaling cassettes were critical for the establishment of early germ layers in the common deuterostome ancestor.
Subject areas: Molecular biology, Evolutionary biology, Embryology
Graphical abstract

Highlights
-
•
A Wnt/β-catenin-Sp5 signaling cassette drives a conserved endoderm regulatory subcircuit
-
•
A Wnt8-JNK-Sp5 signaling cassette patterns the neuroectoderm
-
•
Many deuterostomes use Wnt/Sp5 cassettes to control endoderm and neuroectoderm formation
Molecular biology; Evolutionary biology; Embryology
Introduction
Studies performed in several bilaterian species indicate that high, localized Wnt/β-catenin signaling establishes endomesoderm GRNs around the posterior pole. Conversely, low Wnt signaling around the anterior pole, aided by Wnt signaling antagonists, allows the establishment of ANE GRNs that generate anterior sensory structures.1,2,3 While these studies have established a paradigm for early AP formation, the exact mechanisms involved in these critical processes, and how they evolved, are not fully understood. Sea urchins have long been important models for early germ layer and body axes formation because of their well-characterized germ layer GRNs and phylogenetic position as a sister group to chordates.4,5 Thus, answers to these questions might be found by close examination of this fundamental developmental process in these embryos.
In sea urchins, vegetally localized, maternal intracellular Wnt components (e.g., Disheveled) promote nuclear localization of β-catenin (nβ-catenin) during early cleavage stages (16- to 32-cell stage), initiating primary axis formation.6,7,8 nβ-catenin outcompetes the co-repressor Groucho (a histone deacetylase) in order to interact with the transcription factor (TF) TCF/LEF to activate the endomesoderm GRN (EMGRN) while at the same time repressing ANE GRN gene expression in posterior blastomeres8,9,10,11,12,13 (Figure 1Aa). During these early regulatory stages posterior nβ-catenin activates Wnt1 and Wnt8 expression, and these ligands reinforce posterior nβ-catenin and aspects of the endoderm GRN.9,14,15 These same ligands also initiate the activity of a different, non-canonical Fzd5/8-JNK signaling pathway in adjacent equatorial ectoderm cells by the 60-cell stage (Figure 1Ab). Here, it is important to note that several expression and functional assays from our lab and others indicate that Wnt/β-catenin signaling activity is restricted to posterior blastomeres fated as endomesoderm and does not directly function in anterior ectodermal cells during cleavage and blastula stages.2,9,10,11,13 At these early cleavage stages both wnt1 and wnt8 transcripts are restricted to the endomesoderm9,12,15,16; therefore, we hypothesize that Wnt1 and Wnt8 diffuse into anterior ectodermal blastomeres to activate Fzd5/8-JNK signaling. Once activated, this pathway acts in a progressive posterior-to-anterior signaling wave that downregulates ANE gene expression in ectoderm cells. This Wnt1/Wnt8-Fzd5/8-JNK signaling pathway effectively restricts the ANE GRN to a territory around the anterior pole and establishes a separate equatorial ectodermal domain by the beginning of gastrulation (mesenchyme blastula).2,12,17 wnt8 expression begins to be expressed in equatorial ectodermal cells during early blastula stages, and by the beginning of gastrulation it is primarily expressed in equatorial ectodermal cells.12,15,16 This expression pattern suggests that Wnt8 may be driving the progressive downregulation of the ANE GRN in these cells. As development progresses into late blastula stages, Fzd5/8-JNK signaling activates the secreted Wnt antagonists Dkk1 and sFRP-1 within the ANE territory around the anterior pole. In a classic negative feedback loop, these antagonists block Fzd5/8-JNK signaling within these same cells, preventing ANE GRN downregulation and effectively establishing the ANE territory12,18 (Figure 1Ac). Throughout this progressive process, which we term “ANE restriction”, another broadly active non-canonical Wnt pathway involving Wnt16-Fzd1/2/7-PKC antagonizes both posterior Wnt/β-catenin and anterior Wnt/JNK signaling. This antagonism acts as a “buffer” mechanism that limits the rate of ANE GRN downregulation and together these three pathways form an integrated AP Wnt signaling network that establishes the final positions of the four major germ layer GRNs (endoderm, mesoderm, equatorial ectoderm, and ANE) along the AP axis by gastrulation2,12,19,20,21 (Figure 1A).
Figure 1.
Spatiotemporal expression patterns of sp5 during early germ layer formation
(A) Model summarizing the progressive posterior-to-anterior patterning of early sea urchin germ layer GRNs by the AP Wnt signaling network involving canonical (Wnt/β-catenin) and non-canonical (Wnt1/Wnt8-Fzd5/8-JNK and Fzd1/2/7-PKC) signaling pathways.12,18,19,22 Posterior Wnt/β-catenin and anterior Fzd5/8-JNK signaling pathways are active in distinct territories while Fzd1/2/7-PKC signaling is active throughout the early embryo during this process. See text for details.
(B) Three-color whole mount HCR-FISH for sp5 (magenta), six3 (blue), and foxa (orange). Anterior is top, and posterior is bottom. (Ba, e, i, m) 120-cell stage embryo showing sp5 expression in all germ layers. (Bd, f, j, n) Hatched blastula (Bc, g, k, o) late blastula, and (Bd, h, l, p) mesenchyme blastula stage embryos showing that sp5 expression is downregulated from the ANE where six3 is expressed and restricted to the equatorial ectoderm and the endomesoderm regions overlapping with foxa expression. (Bq-t) Schematic diagrams of the merged expression patterns observed in the HCR RNA-FISH images in Bm-p. six3 (light blue) marks the ANE and later the endomesoderm territory and foxa (orange) marks the endomesoderm. The solid lines in the diagrams demarcate the ANE, equatorial ectoderm (EE) and endomesoderm (EM) GRN territories during early AP patterning. Scale bars: 20 μm.
Our lab has identified several extracellular and intracellular signaling transduction components involved in the interactions among different AP Wnt network pathways,12,18,20,22 but we have yet to determine the transcriptional GRNs activated by each pathway. Here, we analyze the role of the Sp5 transcription factor (TF) in the sea urchin AP Wnt signaling network and show that Sp5 TF is a critical component in two separate, but linked, developmental processes. During endomesoderm specification posterior Wnt/β-catenin signaling activates Sp5 expression, and then this TF becomes integrated into a remarkably conserved endoderm gene regulatory sub-circuit. Our data also indicate that Sp5 is a downstream component of the Wnt1/Wnt8-Fzd5/8-JNK signaling pathway that positions the ANE GRN to a disk of cells around the anterior pole. Finally, we compare several expression and functional studies from several invertebrate and vertebrate deuterostome embryos which strongly suggest that the roles for Wnt/Sp5 signaling cassettes in endoderm specification and neuroectoderm patterning are widely shared by many deuterostomes.
Results
Identification of a putative transcriptional effector of Wnt/β-catenin and Wnt/JNK signaling in the sea urchin AP Wnt signaling network
The initial impetus for study was to identify the TFs that are regulated by the Wnt1/Wnt8-Fzd5/8-JNK signaling pathway that patterns the anterior ectoderm in sea urchins. Thus, we perturbed Fzd5/8-JNK signaling by overexpressing a truncated version of Fzd5/8 lacking the C-terminal domain (ΔFzd5/8) into Strongylocentrotus purpuratus fertilized eggs and performed RNA-Seq on samples collected during ANE restriction (120-cell, blastula and mesenchyme blastula stages). Our attention was drawn to the TF Sp5 because it was one of the most downregulated TFs in these perturbations at the blastulas stage and because it is a TF necessary for endomesoderm specification as well as AP patterning downstream of Wnt signaling in several metazoans23,24,25,26,27,28,29 (Figure S1A). Fzd5/8 signaling also regulates aspects of the endomesoderm GRN;30 therefore, it was important to determine the spatiotemporal expression of sp5 during ANE restriction. We used our recently optimized protocol for whole mount in situ hybridization chain reaction (HCR RNA-FISH) and performed a quantitative spatial co-expression analysis to determine the expression dynamics of sp5 in relation to the expression of the cardinal ANE GRN regulator six3 and the endoderm marker foxa. Corroborating data from a previous study our qPCR analysis showed that sp5 expression was activated around the 60-cell stage (Figure S1A).31 At the 120-cell stage, HCR RNA-FISH showed that sp5 was broadly expressed throughout the embryo, overlapping with six3 (58%) and foxa (87%) expression (Figures 1Ba, e, i, m, q and S3). During the subsequent blastula stages up until the end of the ANE restriction process at the beginning of gastrulation, sp5 transcripts were progressively downregulated in the ANE but remained expressed throughout the equatorial ectoderm (marked by the absence of six3 and foxa expression) and endomesoderm territory (sp5 + foxa co-expression ranged between 80 and 85% during these stages) (Figures 1Bb, f, j, n, r; Bc, g, k, o, s; Bd, h, l, p, t, S1B, and S3). Interestingly, we observed a ventral bias in sp5 expression beginning around the midblastula stages (Figures S1B and S1Ca–h), possibly due to the influence of ventral Nodal signaling which activates dorsal-ventral specification during these developmental stages.
These results demonstrate that the initial activation and spatial expression of sp5 correlates with the spatiotemporal activity of Wnt1-Wnt8-Fzd5/8-JNK signaling in equatorial ectodermal cell during cleavage and blastula stages (Figure 1A),1 being consistent with our differential RNA-Seq data. In addition, the broad expression of sp5 transcripts suggests that Sp5 may contribute to the early regulatory networks governing the ANE and endomesoderm territories.
Sp5 regulates an evolutionarily conserved endoderm GRN kernel
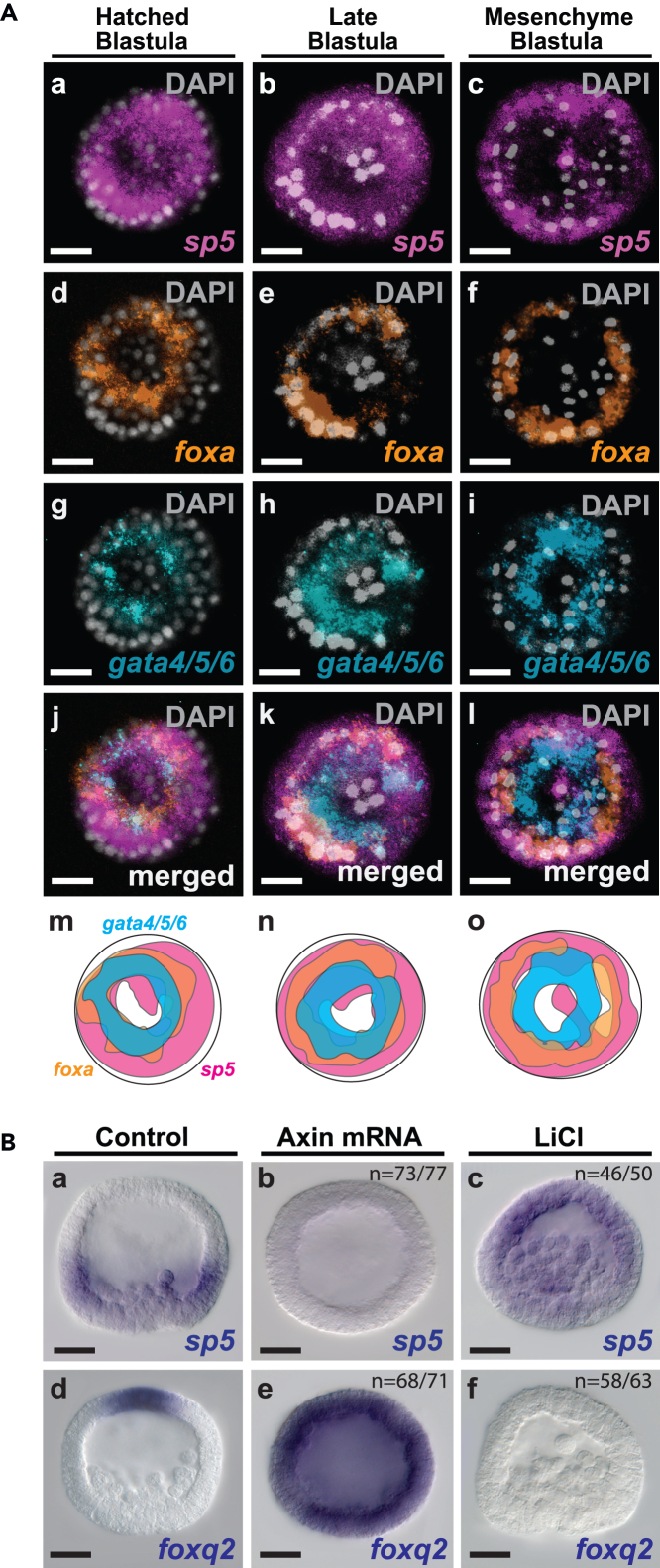
The sea urchin EMGRN is one of the best characterized GRNs of any organism.32,33,34,35,36 Currently, it consists of a hierarchical network of 16 TFs and several signaling components (e.g., Wnt1, Wnt8) that drive the differentiation gene batteries responsible for larval endoderm and mesoderm cells.14,37,38 During late cleavage and early blastula stages many early endoderm GRN TFs are broadly co-expressed throughout the newly specified endoderm territory and excluded from the mesoderm territory around the apex of the posterior/vegetal pole.35,36,37,38,39 Then, the endoderm is progressively segregated into two concentric territories by the beginning of gastrulation: (1) a more posterior domain termed veg2 marked by gata4/5/6 expression (Figure 2Ai, l, o) that contributes to the foregut/midgut, and (2) a more anterior veg1 domain marked by foxa expression (Figure 2Af, l, o) that contributes to the hindgut/midgut. We analyzed the spatiotemporal expression sp5 transcripts from the posterior/vegetal pole in relation to gata4/5/6 and foxA transcripts during this progressive process. During late cleavage and early blastula stages sp5 transcripts were largely co-expressed with both foxa and gata4/5/6 transcripts (sp5 + gata4/5/6 co-expression = ∼90% and sp5 + foxa co-expression = ∼85%) (Figures 2Aa, d, g, j, m, 2Ab, e, h k, n, and S8). As development progressed, sp5 transcript expression progressively decreased around the apex of the posterior/vegetal pole, and its expression overlapped more with anterior/veg1 foxa (71%) than posterior/veg2 gata4/5/6 (62%) expression by mesenchyme blastula (Figures 2Ac, f, i, l, o and S8).
Figure 2.
Wnt/β-catenin signaling activates Sp5 expression in the endomesoderm
(A) Posterior/vegetal views of three-color whole mount HCR-FISH embryos showing sp5 (magenta) expression in relation to foxa (orange) and gata4/5/6 (blue) during blastula stages. sp5, foxa and gata4/5/6 expression overlap in hatched blastula (Aa, d, g, j, m) and late blastula embryos (Ab, e, h, k, n). Mesenchyme blastula embryos showing co-expression of sp5 and foxa in anterior/veg1 and downregulation in posterior/veg2 endoderm cells marked by gata4/5/6 (Ac, f, i, j, o). (Am-o) Schematic diagrams of the merged expression patterns observed in Aj-l. foxa (orange) marks anterior/veg1endoderm, and gata4/5/6 (orange) marks posterior/veg2 endoderm cells by mesenchyme blastula stage (Ao).
(B) Data show colorimetric WMISH images of mesenchyme blastula stage embryos from the same mating pairs. Embryos injected with axin mRNA show downregulation of sp5 transcripts (Ba, b) and broad expression of foxq2 (Bd, e). Ectopic activation of the Wnt/β-catenin pathway by LiCl treatment shows ectopic sp5 (Ba, c) and downregulation of foxq2 (Bd, f). VV, vegetal views. Scale bars: 20 μm.
Based on sp5’s dynamic expression pattern in posterior blastomeres during early endomesoderm formation, we hypothesized that Sp5 is a previously uncharacterized component of the EMGRN. To investigate the possibility that sp5 is a target of Wnt/β-catenin signaling, we first performed phylogenetic footprinting analysis and identified two predicted transcription factor binding sites (TFBSs) for TCF/LEF within a highly conserved and open chromatin region upstream of the 1st exon of sp5 (Figure S2A). Encouraged by these results we next prevented β-catenin nuclearization by injecting axin mRNA12,40 and found that endomesoderm and equatorial ectoderm cells showed downregulation of sp5 transcripts at the mesenchyme blastula stage (Figures 2Ba, b and S6B) while at the same time foxq2 was expressed broadly throughout embryos from the same injected mating pairs as observed in previous studies12,19 (Figure 2Bd, e). LiCl treatment upregulates Wnt/β-catenin signaling in embryos, converting them into endoderm and mesoderm.13,41,42,43 On the converse to the Wnt/β-catenin knockdown experiment, embryos treated with LiCl showed ectopic sp5 expression throughout mesenchyme blastula stage embryos (Figures 2Ba, c and S6B). While these findings do not establish sp5 as a direct target of nβ-catenin/TCF, they indicate that posterior Wnt/β-catenin signaling is essential for sp5 expression in the endomesoderm territory.
Next, we examined the expression of several EMGRN genes in Sp5 knockdown embryos to determine if it is necessary for regulating aspects of the network. During the early stages of endomesoderm specification at the 120-cell stage we found that three genes, gata4/5/6, blimp1, and eve were downregulated while another, otx, was upregulated in embryos injected with either of two different Sp5 morpholinos (Figures 3Aa–j, S3A, S3B, and S3Ca–j). We also assayed for EMGRN gene expression in Sp5 morphants compared to control morpholino injected embryos at the mesenchyme blastula stage when the endoderm and mesoderm GRNs have segregated from one another.14,33,44 We again found downregulated expression of gata4/5/6, blimp1 and eve but also foxa, which was unaffected at the 120-cell stage (Figures 3Ba–d and f–i, S3Da–d, S3Df–I, and S4A). Conversely, Sp5 morphant embryos showed otx upregulation at this stage (Figures 3Be, j, S3De, j, and S4A). Interestingly, the expression of several other EMGRN genes was unaffected at either stage (Figures S4A–S4C), suggesting that Sp5 is not involved in broad regulation of the EMGRN. Based on these functional data we performed phylogenetic footprinting on these genes. We found that each gene contained predicted Sp5 binding sites within highly conserved non-coding regions that also contained open chromatin during the blastula stage (Figures S5A‒S5E). These preliminary data suggest that Sp5 could directly regulate gata4/5/6, blimp1, eve, foxa and otx; however, further cis-regulatory analyses will be needed to confirm this possibility.
Figure 3.
Sp5 is a component of the posterior endomesoderm gene regulatory network
(A) blimp1b, gata4/5/6, eve and foxa expression in 120-cell stage control (Aa-e) and Sp5 morpholino (Af-j) injected embryos (Af-j).
(B) Sp5 morphants at mesenchyme blastula stage showing blimp1b, gata4/5/6, foxa, eve, and otx expression (Bf,g,h,i).
(C) Data show mesenchyme blastula stage embryos injected with Eve morpholinos. (Ca, b) Colorimetric WMISH showing sp5 expression in (Ca) control and (Cb) Eve morpholino injected embryos. (Cc-e) HCR-FISH using probes for sp5 and foxa in the same embryo. (Cc) Control embryo showing co-expression of foxa and sp5. (Cd, e) Separate images of the same Sp5 morphant showing the foxa (Cd) and sp5 (Ce) expression.
(D) Diagram of our new model for the endoderm kernel gene regulatory network. The solid lines indicate previously assessed direct regulation among the transcription factors.45,46,47,48,49,50 Dotted lines show interactions based on the functional evidence in this study. Scale bars: 20 μm. MO, Morpholino. LiCl, Lithium Chloride.
Blimp1, Gata4/5/6, FoxA interact to form a recursive positive feedback loop that is critical for endoderm specification and differentiation in sea urchins.36,37,45,46,51,52,53 While Eve shares few regulatory inputs with these TFs, it has an important role in the specification of anterior-most veg1 endoderm cells as well as posterior equatorial ectoderm cells where sp5 is expressed during later blastula stages.44,47,54 Interestingly, phylogenetic footprinting identified putative TFBS for Eve, Blimp1, GATA4/5/6, FoxA and Otx within a highly conserved putative CRE upstream of sp5’s first exon (Figure S2A). Therefore, we knocked down the function of each gene with previously characterized morpholinos and analyzed the expression of sp5. Surprisingly, only Eve morphants showed downregulated sp5 expression (Figure 3Ca–e), and importantly sp5 was also downregulated in equatorial ectoderm, suggesting that Eve regulates Sp5 in both territories (see Figure 6A). Finally, Sp5 morphants failed to gastrulate, consistent with a regulatory role in the EMGRN (Figure S4Da–d).
Figure 6.
The role of Sp5 in early germ layer GRNs and schematic diagrams depicting similarities among the Wnt-mediated ANE GRN restriction mechanisms in deuterostome embryos
(A) A model of endoderm and equatorial ectoderm GRNs incorporating the data from this study. The solid lines indicate previously assessed direct regulation among the transcription factors.45,46,47,48,49,50,76 Dotted lines show interactions based on the functional evidence in this study.
(B) Embryos are shown at the approximate termination of ANE GRN restriction to territories around the anterior pole during gastrulation. The diagrams for each embryo depict the germ layer territories/sub-territories generated by the end of this developmental process. Dark blue boxes above each embryo indicate the early ANE GRN components conserved among each species. In each of the animals, a posterior-to-anterior Wnt-signaling dependent mechanism restricts initially broadly expressed ANE GRN components to a territory around the anterior pole (see text for details).2 Conserved epistatic relationships among the known Wnt signaling components involved in posterior-to-anterior ANE GRN restriction are indicated. Factors shown in light gray with question marks indicate that the function of the gene has not been assessed. The colored bars next to each embryo indicate the spatial expression domains along the anterior-posterior axis for sp5, wnt8, and fzd5/8 orthologs. In hemichordate embryos, the expression of sp5 has not been characterized (shaded gray bar). Expression and functional data were taken from.2,23,25,26,77,78,79,80,81
In one of the first direct species comparisons of GRNs controlling similar developmental processes, Hinman et al., 2003 showed that orthologs of Blimp1, Gata4/5/6, FoxA and Otx also form recursive regulatory interactions critical for endoderm specification in sea star embryos which diverged from sea urchins ∼550 mya.32,51 While the exact regulatory circuitry among sea star Blimp1, Gata4/5/6, FoxA and Otx are not identical to those in sea urchins, the overall maintenance of the positive regulatory feedback interactions among them is remarkably similar. These observations led the authors to propose that this regulatory subcircuit represents an evolutionarily conserved GRN kernel necessary for endoderm fate in echinoderms.32,51,55,56 Our results show that Sp5 is an essential, and previously uncharacterized, component of this remarkably conserved endoderm kernel in the sea urchin that acts downstream of posterior nβ-catenin to lock down endoderm specification and differentiation (Figure 3D). It would be interesting in the future to assess Sp5’s function in the sea star embryos to determine whether it is also a critical component of this conserved endoderm regulatory kernel.
Sp5 downregulates the ANE GRN downstream of non-canonical Wnt/JNK signaling
Our perturbation of early Wnt/β-catenin signaling also resulted in sp5 downregulation in the equatorial ectoderm territory (Figure 2Ba, b). This result is consistent with our hypothesis that Wnt1/Wnt8-Fzd5/8-JNK signaling activates sp5 expression in these cells since posterior Wnt/β-catenin signaling is required for Wnt1 and Wnt8 expression. To further examine this idea, we perturbed the function of each known member of the Wnt1/Wnt8-Fzd5/8-JNK signaling pathway by injecting fertilized eggs with either morpholinos for Wnt1, Wnt8, JNK or mRNA encoding ΔFzd5/8. In each of these knockdown experiments, sp5 was severely downregulated throughout the embryo (Figures 4Aa–e and S6C), and overexpression of either Wnt1 or Wnt8 caused ectopic anterior expression of sp5 transcripts (Figure 4Ba–c). As an internal control, we examined the spatial expression of foxq2 in the same batches of perturbed embryos for each molecule (Figure S6Aa–e). In previous studies, we showed that the Wnt/β-catenin and Wnt/JNK signaling pathways are upregulated in the absence of negative inputs from Wnt signaling antagonists12,18 causing an anterior shift of the endomesoderm and equatorial ectoderm territories and the elimination of the ANE GRN. We perturbed the function of two antagonists of the Wnt1/Wnt8-Fzd5/8-JNK pathways, Fzd1/2/7 and Dkk1 (see Figure 1A for model) and assayed for sp5 expression to add more evidence that Sp5 expression was regulated by the AP Wnt network. As expected, the sp5 expression territory shifted toward the anterior pole (Figure 4Ba, d, e). Together, these results demonstrate that the Wnt1/Wnt8-Fzd5/8-JNK signaling pathway activates Sp5 in equatorial ectoderm cells; however, we also unexpectedly found that these perturbations also cause sp5 downregulation in the endomesodermal territory. As mentioned above, Wnt1 and Wnt8 are critical components of the endomesodermal network. In addition, a non-canonical Fzd5/8 signaling pathway also regulates aspects of the endomesoderm GRN during late blastula/early gastrula stages resulting in a gastrulation defect as observed in Sp5 knockdown embryos.30 These observations suggest that outside of its role in ANE restriction non-canonical Fzd5/8 signaling may also be involved in later maintenance of Sp5 expression in the endoderm GRN.
Figure 4.
Sp5 signaling is necessary for Wnt1/Wnt8-Fzd5/8-JNK mediated ANE restriction mechanism in anterior blastomeres
(A) Wnt1, Wnt8, Fzd5/8, and JNK knockdown embryos show downregulated sp5 expression (compare Aa to Ab-e).
(B) Embryos treated with wnt1 mRNA (Bb), Wnt8 mRNA (Bd), Fzd1/2/7 MO (Bd), and Dkk1 MO (Be) show ectopic sp5 expression compared control (Ba).
(C) Injection of Sp5 morpholino 1 causes posterior expansion of foxq2 expression (compare Ca, b, c), and ectopic sp5 mRNA expression downregulates foxq2 expression (compare Ca, d). The angle α shown in Ca-c was measured with ImageJ. Volume = 0.5(1-cos α/2) was used to calculate the percentage of the surface area (±sem) occupied by foxq2 expression in control and Sp5 knockdowns.
(D) Sp5 MO2 rescue experiment. (Db) Sp5 MO2 morphant showing foxq2 expansion. (Dc) A representative sp5 mRNA-injected embryo with downregulated foxq2 expression. (Da, d, e) foxq2 expression rescued by double injection of Sp5MO2 and sp5 mRNA in a majority of embryos (Ed, e).
(E) Model based on the data from this figure for the Wnt8-Fzd5/8-JNK-Sp5 ANE restriction mechanism in equatorial ectoderm cells from late cleavage to mesenchyme blastula. All embryos in this figure are mesenchyme blastulae. MO, Morpholino. Δ, Dominant-negative. Scale bars: 20 μm.
The Wnt8-Fzd5/8-JNK activation of sp5 expression in the equatorial ectoderm strengthens our hypothesis that Sp5 acts as a transcriptional repressor of ANE genes. To test this idea, we injected fertilized eggs with either of the two morpholinos targeting sp5 and assayed for six3 and foxq2 expression, the two cardinal regulators of the ANE GRN.2,57 We found that six3 and foxq2 expression expanded posteriorly in Sp5 morphants (Figures 4Ca–c, S3E, and S3F). Importantly, these results phenocopied the posteriorly expanded foxq2 expression phenotypes observed in Wnt1 and Wnt8 knockdown embryos which typically show slightly less posterior foxq2 expansion than Fzl5/8 and JNK knockdown embryos2,12,18,19 (Figure S6Aa–e). Conversely, when we overexpressed sp5 mRNA, foxq2 expression was eliminated from mesenchyme blastula staged embryos (Figure 4Cd) consistent with a role for Sp5 as a transcriptional repressor of ANE genes. Importantly, we were able to rescue the foxq2 expression in embryos injected with both sp5 mRNA and Sp5 MO2 and (Figure 4Da–e), indicating that Sp5 MO phenotypes are not due to the morpholino binding to off target mRNA transcripts.
Wnt8 and Sp5 feedback interactions position the ANE GRN around the anterior pole
As discussed above, early ANE GRN regulatory factors (e.g., foxq2 and six3) are progressively downregulated from equatorial ectoderm by Wnt/JNK signaling. wnt1 expression is restricted to the endomesoderm territory throughout the ANE GRN restriction process, suggesting that it is primarily responsible for the initial activation of Wnt/JNK signaling in the equatorial ectoderm12,14,16 (Figure 5Ab). Thus, we have assumed that Wnt8, not Wnt1, is likely the primary driver of ANE GRN downregulation because it is progressively upregulated at the same time that the ANE GRN is downregulated in these cells2,12,18,19 (Figure 5Af). However, we have yet to determine the mechanism driving progressive Wnt8 expression in the equatorial ectoderm. Based on several studies in vertebrates, as well as in hydra, that show that Wnt/Sp5 signaling cassettes are often involved in positive and/or negative feedback loops24,29,58,59,60 we explored the possibility that Sp5 and Wnt8 may work in a positive feedback loop during ANE restriction. First, we examined sp5’s spatial expression in more detail by comparing it to wnt8 expression in mesenchyme blastula embryos. sp5 and wnt8 transcripts were largely co-expressed in the endoderm and equatorial ectoderm (89%) (Figure 5Ae–h and S3). Interestingly, we observed sp5 transcripts in approximately two cells anterior to wnt8 transcripts (avg. 1.83 cells; n = 12), suggesting that Wnt8 may diffuse anteriorly and activate sp5 expression in these cells. Also, wnt8 had a ventral bias like sp5 (Figures S1Ca–h and S1Da–h). Together, our expression and functional data suggest that the Wnt8 ligand may diffuse several cells further toward the anterior to activate sp5. Next, we performed phylogenetic footprinting to assess whether Sp5 may regulate wnt8 expression and identified a highly conserved predicted Sp5 TF binding site in a putative wnt8 CRE (Figure S2B). Based on these lines of evidence we perturbed the function of the Fzd5/8 and Sp5 pathway by injecting either ΔFzd5/8 mRNA or Sp5 morpholinos into fertilized eggs. Both perturbations resulted in the downregulation of wnt8 expression at the mesenchyme blastula stage (Figure 5Ba–c) and, conversely, sp5 mRNA overexpression resulted in robust wnt8 expression throughout the embryo (Figure 5Ba, d) indicating that Sp5 can drive wnt8 expression. Finally, we identified a putative sp5 CRE with a highly conserved predicted Sp5 TFBS (Figure S2A), suggesting that Sp5 may also regulate its own expression. Thus, we knocked down Sp5 expression, and found that these morphants showed ectopic sp5 expression in anterior ectoderm cells (Figure 5Ca, b) similar to the Fzd1/2/7 and Dkk1 morphant phenotypes in Figure 4Bd, e.
Figure 5.
Regulatory feedback loops among Wnt8, Fzd5/8, and Sp5 in the equatorial ectoderm
(A) HCR RNA-FISH in situ images of sp5 (magenta), fzd5/8 (orange), wnt1 and wnt8 (both turquoise) transcripts during in mesenchyme blastula stage embryos. sp5 (Aa and e) is co-expressed with wnt1 (Ab) and fzd5/8 (Ac, g) in the endoderm and with wnt8 (Af) in the equatorial ectoderm.
(B) wnt8 expression is downregulated in ΔFzd5/8 injected embryos (compare Ba to Bb). Sp5 morphants show downregulated wnt8 expression (compare Ba with Bc) and embryos injected with sp5 mRNA show ectopic wnt8 expression (compare Ba to Bd).
(C) Sp5 morphants express sp5 transcripts ectopically at the mesenchyme blastula stage.
(D) Model for the Wnt8-Fzd5/8-JNK-Sp5 ANE restriction mechanism in equatorial ectoderm cells based on the data represented in this figure. MO, Morpholino. Δ, Dominant-negative. Scale bars: 20 μm.
While these functional experiments do not prove that Sp5 directly regulates either wnt8 or its own expression, the discovery of the Wnt8-Sp5 positive feedback circuit downstream of the Fzd5/8-JNK signaling pathway fills a gap in our knowledge of how the Wnt8-Fzd5/8-JNK-Sp5 signaling cassette drives ANE GRN downregulation in equatorial ectoderm cells. In addition, the data showing that Sp5 negatively regulates its own expression suggesting another mechanism by which the AP Wnt signaling network maintains precise spatiotemporal balance among its signaling components to pattern early germ layer GRNs.
Discussion
Our data demonstrate that Sp5 is a key component of two different interconnected, but spatially distinct, Wnt signaling pathways (Wnt/β-catenin and Wnt/JNK) in the sea urchin AP Wnt signaling network (Figures 1A and 6). These results reinforce striking parallels among the Wnt signaling mechanisms used by sea urchins and other metazoan embryos to specify and pattern early germ layer GRNs along the AP axis.1,2,3,33,61 For instance, Wnt/β-catenin-Sp5 signaling has been implicated in endomesoderm specification and patterning in several chordates.25,27,29,59,62 In zebrafish embryos Wnt/β-catenin signaling directly activates Sp5 which subsequently patterns mesoderm and in mice Wnt/β-catenin-Sp5 signaling cassettes are necessary for paraxial mesoderm gene activation as well as hindgut formation.62,63,64 Similarly, a study in the invertebrate chordate Amphioxus indicates that Wnt/β-catenin activates sp5 expression in early endomesodermal cells, suggesting a broader function for Wnt-Sp5 signaling in the specification of this germ layer outside of vertebrates.23 It is unclear whether chordate Wnt/β-catenin-Sp5 signaling activates similar endomesoderm genes as those in the sea urchin (Figure 6A), but a recent study showed that Sp5 interacts with nβ-catenin and TCF/LEF at ∼ 45% of CREs regulating Wnt/β-catenin signaling target genes in mouse embryonic stem cells and definitive endoderm.59 Thus, it is interesting that vertebrate orthologs of the echinoderm endoderm kernel genes described in this study are also involved in endomesoderm specification. These include Gata 4, 5 and 6 in Xenopus, Gata5 and 6 in zebrafish, Gata4 in mice as well as Gata6 in human pluripotent stem cells.65,66,67,68,69,70 Similarly, FoxA2 has been shown to also be necessary for definitive endoderm specification in mice71,72 and Blimp1/PRDM1 is involved in endoderm specification in Xenopus73,74 as well as endomesoderm gene repression in zebrafish embryos.75 The extent that GATA4/5/6, FoxA, and Blimp1/PRDM1 orthologs interact as an endomesoderm GRN in any vertebrate embryo remains to be determined, but a recent mouse study showed that FoxA directly activates gata4 expression in mouse definitive endoderm cells.70 Based on these studies and our data, we propose that the Wnt/β-catenin-Sp5 signaling cassette’s role in endomesoderm specification evolved in early deuterostomes before the split between chordates and ambulacrarians.
The other main implication of this study is that we established Sp5 as a critical downstream component of the Wnt8-Fzd5/8-JNK mediated ANE GRN restriction mechanism that positions the ANE territory around the anterior pole in sea urchin embryos. It is important to note that early ANE GRNs are remarkably similar among deuterostome embryos2,82,83 (Figure 6B). In fact, a recent study by Fueda and Peter (2022) showed that various orthologues of 28 of the 31 identified sea urchin ANE GRN TFs were also expressed in the ANEs of either mice, zebrafish, Xenopus, Drosophila and Caenorhabditis elegans (20 of 31 were expressed in the ANEs of all species).83 Adding to the similarities among species, ANE factors are initially expressed broadly in equatorial territories in many invertebrate deuterostome embryos (sea urchins, sea stars, hemichordates, and cephalochordates) and the posterior neural plate in vertebrate embryos during early AP patterning. Then, ANE gene expression is restricted around the anterior pole in each species by a mechanism that depends on posterior-to-anterior gradient of Wnt signaling1,2,3,12,17,84,85,86,87(Figure 6B). Remarkably, functional experiments show that ANE GRN restriction in vertebrates depends on orthologs of many of the same factors involved in the sea urchin ANE restriction mechanism (Wnt1, Wnt8, Fzd5, Fzd8 as well as the anterior Wnt antagonists like Dkk1 and sFRPs)84,85,88,89,90,91,92,93,94 (Figure 6B). These parallels between the vertebrate and sea urchin ANE restriction, as well as expression and functional data from amphioxus and hemichordate embryos (Figure 6B), led us to previously propose that aspects of this mechanism existed in the common deuterostome ancestor.2,12 Work in zebrafish and the basal chordate Amphioxus shows that Wnt-Sp5 signaling is involved in positioning the ANE GRNs to anterior neuroectoderm territories in both species. Zebrafish embryos use a strikingly similar Wnt8-Fzd8-Sp5-like signaling pathway to restrict its ANE GRN to the anterior neural plate (Sp5-like is an orthologue of sea urchin Sp5).25,91,93,94,95 The functional data in Amphioxus is limited, but a recent study showed that spatiotemporal expression pattern of sp5 is similar to both sea urchins and vertebrate embryos during ANE restriction and that Wnt/β-catenin signal overactivation caused ectopic sp5 expression like in these embroyos.23 Thus, the data in this study lend further support to our notion of a deep homology among the early Wnt dependent AP patterning mechanisms used by deuterostome embryos.
In diploblastic cnidarian embryos Wnt/β-catenin specifies the endoderm around the oral pole, which many consider to be analogous to the posterior pole in bilaterians.96,97,98,99,100 Then, a Wnt/β-catenin dependent mechanism progressively restricts the broadly expressed aboral GRN, which remarkably contains many sea urchin ANE GRN components like Six3/6, FoxQ2 and Fzd5/8 to a territory around the aboral pole.100,101 A recent study shows that localized oral Wnt/β-catenin signaling activates Sp6-9 in a more aborally localized equatorial mid-body domain, like Sp5’s equatorial ectoderm expression in sea urchin embryos. Subsequently, Sp6-9 drives progressive Six3/6 and Fzd5/8 downregulation within this territory, resulting in the positioning of the aboral GRN around the aboral pole.99 Interestingly, a study by Schaeper et al. (2010) that investigated the evolution of Sp transcription factors shows that Sp6-9 is part of a closely related group of transcription factors that includes Sp5 which they defined as an ancestral metazoan Sp5-9 subgroup.102 Cnidarians also possess an Sp5 orthologue,102 and while it has not been implicated in this process to date, it is tempting to speculate that the role of Sp transcription factors downstream of Wnt signaling during early primary axis specification may be an ancient metazoan early patterning mechanism. Despite these remarkable similarities among echinoderms, hemichordates, chordates and cnidarians, there are several known, and likely many undiscovered, differences among the early primary axis patterning mechanisms mediated by Wnt signaling in the animals. One of the most conspicuous differences is the role of JNK12 (Figure 6B), possibly because this aspect of the mechanism has not been explored in other organisms thus far. To our knowledge this study is the first to show a role for Sp5 downstream of a Wnt/JNK signaling pathway in any context. Thus, it will be important to examine to what extent Wnt-JNK-Sp5 signaling cassettes are conserved in early AP axis formation as well as other developmental contexts.
Limitations of the study
The expression and functional assays in this study demonstrate that Sp5 is involved in critical gene regulatory interactions downstream of both canonical Wnt/β-catenin and non-canonical Wnt/JNK signaling pathways. However, we do not definitively show that these interactions are direct. In the future, it will be important to perform in depth cis-regulatory analyses to verify these interactions to solidify our understanding of the GRNs governing early AP axis specification and patterning in sea urchin embryos.
Another caveat to this study is our statement that Sp5 is a critical component of an evolutionary conserved regulatory circuit necessary for endoderm specification in echinoderm sea urchin and sea star embryos, which are separated by 500 million years of evolution. In sea star embryos there is currently no evidence for a role for Sp5 in endoderm specification. Therefore, it will be important in the future to perform functional studies on Sp5 in these embryos to determine if it is involved in this endoderm regulatory sub-circuit.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-Digoxigenin-AP, Fab fragments | Roche | 11093274910 |
| Meso1 | Gifted by McClay Lab | Generated by McClay Lab |
| 1D5 | Gifted by Angerer Lab | Generated by Angerer Lab |
| Serotonin | Sigma | AB125 |
| Alexa Flour-coupled 555 goat anti mouse IgG | ThermoFisher | A-21422 |
| Alexa Flour-coupled 488 goat anti mouse IgM | ThermoFisher | A-11001 |
| Critical commercial assays | ||
| RNeasy Plus Mini Kit protocol | Qiagen | 74034 |
| SuperScript IV First-Strand Synthesis System | Invitrogen | 11483188001 |
| pGEMT®-easy | Promega | A137A |
| SYBER green qPCR mix | BioRad | 1725121 |
| Sp6 and T7 RNA Polymerase | New England Biolabs | M0207S and M0251S |
| Tyramide Signaling Amp Kit | Perkin Elmer | Discontinued product |
| DIG RNA Labeling Mix | Roche | 11277073910 |
| Fluorescein RNA Labeling Mix | Roche | 11685619910 |
| Not1Restriction Enzyme | New England Biolabs | R0189S |
| mMessage Machine kit | Ambion | AM1344 |
| Gibson gene assembly | New England Biolabs | E5510S |
| Experimental models: Organisms/strains | ||
| Strongylocentrotus purpuratus | Point Loma Marine Invertebrate Lab, Lakeside CA, Marinus, Garden Grove, CA, and Monterey Abalone Company, Monterey, CA. | N/A |
| Oligonucleotides | ||
| Full length gene sp5 Forward: 5’ AGCATGCC GAAAGGTGAAGCC-3’ |
Cloning | N/A |
| Full length gene sp5 Reverse: 5’-CAGTTAGTC AGATGTCACATC-3’. |
Cloning | N/A |
| Overhang sp5 Forward: 5’-ATAGTGAAGTAGAAA TTGTTGATGTGACATCTGACTAAGGCCTCTC-GAGCCTCTAGAAC-3’ |
Cloning | N/A |
| Overhang sp5 Reverse: 5’-TAATACGACTCACTATA GTTCTAGAGGCTCGAG-AGGCCTTAGTCAGAT GTCACATCAAC-3’ |
Cloning | N/A |
| pCS2+ Forward: 5’-ATAGTGAAGATGAAAT-TGTTG ATGTGACATCTGACTAAGGCCTCTCG AGCCTCTAGAAC-3 |
Cloning | N/A |
| pCS2+ Reverse: 5’-AGCT-CTAGGAATCTTGCTAAG GCTTCACCTTTCGGCATTTGAATTCGA ATCGATGGGATC-3’. |
Cloning | N/A |
| Sp5_UTR_Forward: 5’-TAGAATACAAGCTACTTG TTCTTTTTGCAGGATCCACCAATAC-GCA CCCATTGCCAACCCT-3’ |
Cloning | N/A |
| Sp5_UTR_Reverse: 5’-GGCACCACGCCGGTAAACAGTT-CTTCGCCTTTGCTCATGCTTTATCCTCTCCAACTGA-3’ | Cloning | N/A |
| Venus_pCS2+_Forward: 5’- AG-CTGATTGGCTAAGA GGGTTGGCAATGGGTGCGTATTGGTGGATCCT GCAAAAAGAA-CA-3’ |
Cloning | N/A |
| Venus_pCS2+_Reverse: 5’-AGCTGATTGGCTAAGAG GGTTGGCAATGGGTGCGTA-TTGGTGGATCCT GCAAAAAGAACA-3’ |
Cloning | N/A |
| QPCR primer set presented in Table S2 | QPCR | N/A |
| Recombinant DNA | ||
| pCS2+ vector | Gifted by Angerer Lab | N/A |
| Venus pCS2+ vector | Gifted by Angerer Lab | N/A |
| Software and algorithms | ||
| Fiji (ImageJ) | NIH | https://fiji.sc/ |
| VISTA | Genomics Division of Lawrence Berkeley National Laboratory | https://genome.lbl.gov/vista/index.shtml |
| HISAT2 | UT Southwestern and Johns Hopkins center for computational Biology | http://daehwankimlab.github.io/hisat2/manual/ |
| Integrative Genomics Viewer (IGV) | NCI | https://igv.org/doc/desktop/ |
| NEBuilder assembly tool | New England Biolabs | https://nebuilder.neb.com/#!/ |
| Other | ||
| RNA-HCR-FISH Buffer Set | Molecular Instruments | N/A |
| RNA-HCR-FISH Probe Sets | Molecular Instruments | N/A |
| RNA-HCR-FISH Amplifier Sets in Alexa 488, 594, 647 | Molecular Instruments | N/A |
| Sp5 MO1: 5’-TTGCTAAGGCTTCACCTTTCGGCAT-3’ | GeneTools | N/A |
| Sp5 MO2: 5’-AATGTACTAATGTCCAGATACACGT-3’ | GeneTools | N/A |
| Fzl1/2/7 MO: 5′-CATCTTCTAACCGTATATCTTCTGC-3′ | GeneTools | N/A |
| Dkk1 MO: 5′-GCGTCTAAATCCTAAATTCCTTCCT-3′ | GeneTools | N/A |
| Wnt1 MO: 5′-ATCCTCATCAAAACTAACTCCAAGA-3′ | GeneTools | N/A |
| Wnt8 MO: 5′-GTAAAGTGTTTTTCTTACCTTGGAT-3′ | GeneTools | N/A |
| JNK MO: 5′-CCTCATCGTTCTAGACTCACCGTTC-3′ | GeneTools | N/A |
| Eve MO: 5’- CAGAAACCACTCGATCAATGTTTGC-3’ | GeneTools | N/A |
| control morpholino: 5’-CCTCTTACCTCAGTTACA A TTTATA-3’ |
GeneTools | N/A |
Resource availability
Lead contact
Resource and reagent requests and any further information regarding this manuscript should be directed to Ryan Range (range@auburn.edu).
Materials availability
In situ probes and primary antibodies are available upon request to the lead contact.
Data and code availability
-
•
Data produced in this paper is available upon request to the lead contact.
-
•
This paper does not report any original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available upon request to the lead contact.
Experimental model and study participant details
Sea urchins
Adult male and female Strongylocentrotus purpuratus were obtained from Point Loma Marine Invertebrate Lab, Lakeside CA, Marinus, Garden Grove, CA, and Monterey Abalone Company, Monterey, CA. Fertilized embryos of both sexes were cultured in artificial seawater (ASW) at 15°C-17°C. For lithium chloride (LiCl) treatment 8-cell stage embryos were exposed to 30 mmol/L LiCl in artificial sea water (ASW). Embryo cultures were grown in LiCl-ASW until embryos developed to mesenchyme blastula stage. Then, LiCl was replaced with ASW and embryos were fixed in 4% paraformaldehyde in ASW for in situ hybridization. These experiments were repeated with embryos from at least three mating pairs.
Method details
Preparation of cDNA clones
Total RNA was extracted from the mesenchyme blastula stage (24 hours post-fertilization [hpf]) using RNeasy Plus Mini Kit protocol (Qiagen) to amplify the full-length clone for sp5. cDNA was synthesized from purified RNA using SuperScript IV First-Strand Synthesis System (Invitrogen). The following primers were used to obtain the full-length sp5 gene and insert sp5 into pGEMT®-easy (Promega):
Full length gene sp5 Forward: 5’ AGCATGCCGAAAGGTGAAGCC-3’ and.
Full length gene sp5 Reverse: 5’-CAGTTAGTCAGATGTCACATC-3’.
sp5 was assembled into the pCS2+ expression vector using the Gibson gene assembly kit from New England Biolabs (NEB). Gene and vector segments were assembled using the following primers with overhangs:
sp5 overhang Forward: 5’-ATAGTGAAGTAGAAATTGTTGATGTGACATCTGACTAAGGCCTCTC-GAGCCTCTAGAAC-3’; sp5 overhang Reverse: 5’-TAATACGACTCACTATAGTTCTAGAGGCTCGAG-AGGCCTTAGTCAGATGTCACATCAAC-3’; pCS2+ Forward: 5’-ATAGTGAAGATGAAAT-TGTTGATGTGACATCTGACTAAGGCCTCTCGAGCCTCTAGAAC-3’, Reverse: 5’-AGCT-CTAGGAATCTTGCTAAGGCTTCACCTTTCGGCATTTGAATTCGAATCGATGGGATC-3’. The overhangs were designed using the NEBuilder assembly tool (https://nebuilder.neb.com/#!/).
The 5’ UTR of sp5 was assembled into the Venus pCS2+ vector using the Gibson assembly protocol from NEB. Gene and vector segments were amplified using the following primers with overhangs:
Sp5_UTR_Forward: 5’-TAGAATACAAGCTACTTGTTCTTTTTGCAGGATCCACCAATAC-GCACCCATTGCCAACCCT-3’; Sp5_UTR_Reverse: 5’-GGCACCACGCCGGTAAACAGTT-CTTCGCCTTTGCTCATGCTTTATCCTCTCCAACTGA-3’; Venus_pCS2+_Forward: 5’- AG-CTGATTGGCTAAGAGGGTTGGCAATGGGTGCGTATTGGTGGATCCTGCAAAAAGAA-CA-3’Venus_pCS2+_Reverse: 5’-AGCTGATTGGCTAAGAGGGTTGGCAATGGGTGCGTA-TTGGTGGATCCTGCAAAAAGAACA-3’.
Quantitative polymerase chain reaction
Quantitative PCR was performed as described previously57 using SYBER green qPCR mix (BioRad). Each set of experiments was repeated at least three times with different mating pairs. For developmental expression analysis the estimated number of sp5 transcripts per embryo was calculated based on the Ct value of the z12 transcript.1,103 To calculate the changes in gene expression between control and perturbed embryos, mitochondrial 12s rRNA ΔCt values were used to normalize relative target gene expression levels. Differences of 2-fold or higher change in expression level was considered significant. Primer set information is presented in Table S2.
Colorimetric and fluorescent whole-mount in situ hybridization
Antisense RNA probes complementary to the mRNA of each analyzed gene were synthesized from PCR products from cDNA as well as from clones in linearized pGEM®-T Easy or pCS2+ plasmids using T7 or SP6 polymerase enzyme. Alkaline phosphatase and two-color fluorescent in situ hybridization were carried out as previously described.21,57,104 Embryos were fixed in 4% paraformaldehyde in ASW for 1 hour at room temperature. Probes, labeled with Digoxigenin, were hybridized for 5-7 days. Colorimetric in situs were carried out using BCIP/NBT. For two-color fluorescent in situ hybridization sp5 was labeled with Digoxigenin and detected with Digoxigenin-TSA while wnt8 and nkx2.2 were labeled with fluorescein and detected with Cy3-TSA.
Whole-mount fluorescence in situ using hybridization chain reaction
Probe sets for six3, sp5, foxa and gata4/5/6 were designed and purchased from Molecular Instruments (www.molecularinstruments.com). Fluorescence in situ hybridization chain reaction (HCR RNA-FISH) experiments were conducted using a protocol modified from Choi et al 2016.105 Embryos were fixed in 4% paraformaldehyde in ASW for 1 hour at room temperature. Samples were incubated at a probe concentration of 0.4 pmol in hybridization buffer and samples were incubated between 18-24 hours at 37°C. Probes were removed with probe wash buffer (Molecular Instruments) and all other wash steps used 5X saline-sodium citrate 0.1% Tween (SSCT) and 1X phosphate buffered saline 0.1% Tween (PBST). Amplifier Fluorescent hairpins (Molecular Instruments) were added at a concentration of 6 pmol per hairpin and incubated at room temperature in the dark for 18-24 hours. Embryos were stained with DAPI, mounted in 30% glycerol, and imaged immediately to prevent fluorescent signal degradation.
Immunohistochemistry
Embryos were fixed in 4% paraformaldehyde in ASW at room temperature for 20 min and washed for 5 times with 1X phosphate-buffered saline containing 0.1% Tween 20 (PBST). Then, embryos were incubated at 4C overnight with primary antibodies against Meso1 (1:50), 1D5 (1:25) and Serotonin (1:1000) in 4% Normal goat serum in PBST. Primary antibodies were detected by incubating embryos for 1 hour at RT with 1:1000 Alexa Flour-coupled 555 goat anti mouse IgG (for Meso1) 1:1000 Alexa Flour-coupled 488 goat anti mouse IgM (for 1D5) and 1:1000 Alexa Flour-coupled 488 goat anti rabbit IgG (for serotonin) (ThermoFisher Scientific).
mRNA and morpholino injections
pCS2 constructs containing each analyzed gene were linearized with Not1. mRNA was synthesized with mMessage Machine kit (Ambion), purified by LiCl precipitation and injected at the following concentrations: ΔFzl5/8 mRNA = 1.0 μg/μl;1,106 wnt1 mRNA = 0.05 μg/μl;1 wnt8 mRNA = 0.80 μg/μl;20 axin mRNA = 1.0 μg/μl,41 sp5 mRNA = 2.0 μg/μl.; sp5 UTR-GFP mRNA = 0.05 μg/μl. S. purpuratus genome, transcriptome and EST sequences for Sp5 were used to generate the translation-blocking oligonucleotides produced by Gene Tools LLC (Eugene, OR). The morpholinos used in this study are as follows:
Sp5 MO1: 5’-TTGCTAAGGCTTCACCTTTCGGCAT-3’(1.5-2.25 mM); Sp5 MO2: 5’-AATGTACTAATGTCCAGATACACGT-3’(1.5-2.25 mM); Fzl1/2/7 MO: 5′-CATCTTCTAACCGTATATCTTCTGC-3′ (1.3 mM), from;1 Dkk1 MO: 5′-GCGTCTAAATCCTAAATTCCTTCCT-3′ (1.5 mM), from;1 Wnt1 MO: 5′-ATCCTCATCAAAACTAACTCCAAGA-3′ (0.4 mM), from;1 Wnt8 MO: 5′-GTAAAGTGTTTTTCTTACCTTGGAT-3′ (0.7 mM), from;11 JNK MO: 5′-CCTCATCGTTCTAGACTCACCGTTC-3′ (1.25 mM), from;1 Eve MO: 5’- CAGAAACCACTCGATCAATGTTTGC-3’, from.54
Embryos from at least three different mating pairs were injected immediately after fertilization with solutions containing 15% Fluorescein isothiocyanate, 20% glycerol and mRNA and/or morpholino oligonucleotides and cultured at 15-18°C. 50 – 300 embryos were microinjected for each experiment unless otherwise stated. As a control for morphological and developmental defects related to injections, we used a standard control morpholino: 5’-CCTCTTACCTCAGTTACAA TTTATA-3’ from Gene Tools LLC, Eugene, OR. Standard control and experimental morpholinos were injected at the same concentrations. Experiments were scored only if a change in phenotype or marker expression was seen in at least 80% of the manipulated embryos.
Quantification and statistical analysis
Genomic sequences comparison and transcription factor (TF) prediction
The reference genome sequences of S.purpuratus (Spur_5.0) and L. variegatus (Lvar_3.0) were downloaded from the NCBI database GCA_000002235.4 and GCA_018143015.1, respectively. The mVISTA comparative genomics tool (https://genome.lbl.gov/vista/index.shtml) was then used to compare the conserved sequences between two species (Window size:50-100 bp; conservation identity: 75%). The conserved aligned sequences from mVISTA were used as an input for JASAPR to identify the conserved predictive binding sites of TFs of interest as described in JASPER database.107 We included TF binding site prediction with relative matrix scores of more than 0.80 for all genes except Sp5 binding prediction score in the putative wnt8 CRE is 0.77. For the ATAC-seq analysis 18 hpf ATAC-seq raw data was downloaded from the NCBI (GEO: GSE95651). Quality control was implemented by FASTP for clean data. ATAC-seq alignments were filtered for S. purpuratus genome (GCF_000002235.5) by HISAT2. MACS2 call peak function were used to call peaks from filtered alignments. The peaks were visualized using the Integrative Genomics Viewer (IGV).
Quantitative spatial analysis for gene co-expression
Spatial overlap of HCR RNA-FISH gene expression images was measured using Fiji.106 Each merged HCR RNA-FISH image was imported into Fiji, and the fluorescent channels were split by color into separate images. Regions of interest (ROI) were generated by Fiji for each image, representing the region of the embryo in which a gene is expressed. The area of each ROI was measured for each gene, and a total area of expression for each gene was generated in relation to the entire embryo. Measurements were performed on the sp5 ROI overlap with the ROI of co-expressed genes of interest (six3/6, foxa, gata4/5/6, wnt1, or wnt8), and a new ROI was generated from only the areas of overlap between sp5 and the gene of interest. The area of this new overlap ROI was then measured. The percentage of sp5’s gene expression overlap with each gene of interest was calculated by dividing the overlap ROI by the ROI of the gene of interest. This process was then repeated for a minimum of 10 images for each representative image presented in the main figures.
Statical analysis of experimental phenotypes
For each perturbation experiment performed the numbers of embryos exhibiting a phenotype that deviated from the wildtype is reported in Figure S8. Student’s T-tests were conducted to measure if the numbers of perturbed embryos observed in each experiment were significantly different from the control groups. To account for multiple test errors a Bonferroni correction was applied and reported in Figure S8. Any experiments whose T-test p-values fell under the corrected alpha value were represented as significant by an asterisk in the bar graphs presented in Figure S8.
Acknowledgments
The NIH R15HD088272-03 and the Department of Biological Sciences at Auburn University, provided support to RCR for this project. We thank Ruth Ann Range for her help with copy editing the manuscript. We also thank Cheikhouna Ka and Dr. Marina Martinez-Bartolome for many fruitful discussions that led to several experiments in this study.
Author contributions
Conceptualization: S.G., J.L.F., and R.C.R.
Methodology: S.G., J.L.F., B.W., and R.C.R.
Investigation: S.G., J.L.F., B.W., and R.C.R.
Visualization: S.G., J.L.F., B.W., and R.C.R.
Funding acquisition: R.C.R.
Writing—original draft: S.G., J.L.F., B.W., and R.C.R.
Writing—review and editing: S.G., J.L.F., and R.C.R.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in their field of research or within their geographical location. One or more of the authors of this paper self-identifies as a gender minority in their field of research. One or more of the authors of this paper self-identifies as a member of the LGBTQIA+ community. We support inclusive, diverse, and equitable conduct of research.
Published: December 2, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.108616.
Supplemental information
References
- 1.Niehrs C. On growth and form: a Cartesian coordinate system of Wnt and BMP signaling specifies bilaterian body axes. Development. 2010;137:845–857. doi: 10.1242/dev.039651. [DOI] [PubMed] [Google Scholar]
- 2.Range R. Specification and positioning of the anterior neuroectoderm in deuterostome embryos: Wnts and Anterior Neuroectoderm Positioning. Genesis. 2014;52:222–234. doi: 10.1002/dvg.22759. [DOI] [PubMed] [Google Scholar]
- 3.Petersen C.P., Reddien P.W. Wnt Signaling and the Polarity of the Primary Body Axis. Cell. 2009;139:1056–1068. doi: 10.1016/j.cell.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 4.McClay D.R. Evolutionary crossroads in developmental biology: sea urchins. Development. 2011;138:2639–2648. doi: 10.1242/dev.048967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martik M.L., Lyons D.C., McClay D.R. Developmental gene regulatory networks in sea urchins and what we can learn from them. F1000Res. 2016;5 doi: 10.12688/f1000research.7381.1. F1000 Faculty Rev-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weitzel H.E., Illies M.R., Byrum C.A., Xu R., Wikramanayake A.H., Ettensohn C.A. Differential stability of -catenin along the animal-vegetal axis of the sea urchin embryo mediated by dishevelled. Development. 2004;131:2947–2956. doi: 10.1242/dev.01152. [DOI] [PubMed] [Google Scholar]
- 7.Leonard J.D., Ettensohn C.A. Analysis of Dishevelled Localization and Function in the Early Sea Urchin Embryo. Dev. Biol. 2007;306:50–65. doi: 10.1016/j.ydbio.2007.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng C.J., Wikramanayake A.H. Differential Regulation of Disheveled in a Novel Vegetal Cortical Domain in Sea Urchin Eggs and Embryos: Implications for the Localized Activation of Canonical Wnt Signaling. PLoS One. 2013;8 doi: 10.1371/journal.pone.0080693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wikramanayake A.H., Peterson R., Chen J., Huang L., Bince J.M., McClay D.R., Klein W.H. Nuclear beta-catenin-dependent Wnt8 signaling in vegetal cells of the early sea urchin embryo regulates gastrulation and differentiation of endoderm and mesodermal cell lineages. Genesis. 2004;39:194–205. doi: 10.1002/gene.20045. [DOI] [PubMed] [Google Scholar]
- 10.Logan C.Y., Miller J.R., Ferkowicz M.J., McClay D.R. Nuclear beta-catenin is required to specify vegetal cell fates in the sea urchin embryo. Development. 1999;126:345–357. doi: 10.1242/dev.126.2.345. [DOI] [PubMed] [Google Scholar]
- 11.Range R.C., Venuti J.M., McClay D.R. LvGroucho and nuclear β-catenin functionally compete for Tcf binding to influence activation of the endomesoderm gene regulatory network in the sea urchin embryo. Dev. Biol. 2005;279:252–267. doi: 10.1016/j.ydbio.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 12.Range R.C., Angerer R.C., Angerer L.M. Integration of Canonical and Noncanonical Wnt Signaling Pathways Patterns the Neuroectoderm Along the Anterior–Posterior Axis of Sea Urchin Embryos. PLoS Biol. 2013;11 doi: 10.1371/journal.pbio.1001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vonica A., Weng W., Gumbiner B.M., Venuti J.M. TCF Is the Nuclear Effector of the β-Catenin Signal That Patterns the Sea Urchin Animal–Vegetal Axis. Dev. Biol. 2000;217:230–243. doi: 10.1006/dbio.1999.9551. [DOI] [PubMed] [Google Scholar]
- 14.Sethi A.J., Wikramanayake R.M., Angerer R.C., Range R.C., Angerer L.M. Sequential Signaling Crosstalk Regulates Endomesoderm Segregation in Sea Urchin Embryos. Science. 2012;335:590–593. doi: 10.1126/science.1212867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui M., Siriwon N., Li E., Davidson E.H., Peter I.S. Specific functions of the Wnt signaling system in gene regulatory networks throughout the early sea urchin embryo. Proc. Natl. Acad. Sci. USA. 2014;111:E5029–E5038. doi: 10.1073/pnas.1419141111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robert N., Lhomond G., Schubert M., Croce J.C. A comprehensive survey of wnt and frizzled expression in the sea urchin Paracentrotus lividus. Genesis. 2014;52:235–250. doi: 10.1002/dvg.22754. [DOI] [PubMed] [Google Scholar]
- 17.Yaguchi S., Yaguchi J., Angerer R.C., Angerer L.M. A Wnt-FoxQ2-Nodal Pathway Links Primary and Secondary Axis Specification in Sea Urchin Embryos. Dev. Cell. 2008;14:97–107. doi: 10.1016/j.devcel.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Khadka A., Martínez-Bartolomé M., Burr S.D., Range R.C. A novel gene’s role in an ancient mechanism: secreted Frizzled-related protein 1 is a critical component in the anterior–posterior Wnt signaling network that governs the establishment of the anterior neuroectoderm in sea urchin embryos. EvoDevo. 2018;9:1. doi: 10.1186/s13227-017-0089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Range R.C. Canonical and non-canonical Wnt signaling pathways define the expression domains of Frizzled 5/8 and Frizzled 1/2/7 along the early anterior-posterior axis in sea urchin embryos. Dev. Biol. 2018;444:83–92. doi: 10.1016/j.ydbio.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martínez-Bartolomé M., Range R.C. A biphasic role of non-canonical Wnt16 signaling during early anterior-posterior patterning and morphogenesis of the sea urchin embryo. Development. 2019;146:dev168799. doi: 10.1242/dev.168799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ka C., Gautam S., Marshall S.R., Tice L.P., Martinez-Bartolome M., Fenner J.L., Range R.C. Receptor Tyrosine Kinases ror1/2 and ryk Are Co-expressed with Multiple Wnt Signaling Components During Early Development of Sea Urchin Embryos. Biol. Bull. 2021;241:140–157. doi: 10.1086/715237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Range R.C., Wei Z. An anterior signaling center patterns and sizes the anterior neuroectoderm of the sea urchin embryo. Development. 2016;143:1523–1533. doi: 10.1242/dev.128165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dailey S.C., Kozmikova I., Somorjai I.M.L. Amphioxus Sp5 is a member of a conserved Specificity Protein complement and is modulated by Wnt/β-catenin signalling. Int. J. Dev. Biol. 2017;61:723–732. doi: 10.1387/ijdb.170205is. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vogg M.C., Beccari L., Iglesias Ollé L., Rampon C., Vriz S., Perruchoud C., Wenger Y., Galliot B. An evolutionarily-conserved Wnt3/β-catenin/Sp5 feedback loop restricts head organizer activity in Hydra. Nat. Commun. 2019;10:312. doi: 10.1038/s41467-018-08242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weidinger G., Thorpe C.J., Wuennenberg-Stapleton K., Ngai J., Moon R.T. The Sp1-Related Transcription Factors sp5 and sp5-like Act Downstream of Wnt/β-Catenin Signaling in Mesoderm and Neuroectoderm Patterning. Curr. Biol. 2005;15:489–500. doi: 10.1016/j.cub.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 26.Thorpe C.J., Weidinger G., Moon R.T. Wnt/β-catenin regulation of the Sp1-related transcription factor sp5l promotes tail development in zebrafish. Development. 2005;132:1763–1772. doi: 10.1242/dev.01733. [DOI] [PubMed] [Google Scholar]
- 27.Harrison S.M., Houzelstein D., Dunwoodie S.L., Beddington R.S. Sp5, a new member of the Sp1 family, is dynamically expressed during development and genetically interacts with Brachyury. Dev. Biol. 2000;227:358–372. doi: 10.1006/dbio.2000.9878. [DOI] [PubMed] [Google Scholar]
- 28.Tewari A.G., Owen J.H., Petersen C.P., Wagner D.E., Reddien P.W. A small set of conserved genes, including sp5 and Hox, are activated by Wnt signaling in the posterior of planarians and acoels. PLoS Genet. 2019;15 doi: 10.1371/journal.pgen.1008401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morley R.H., Lachani K., Keefe D., Gilchrist M.J., Flicek P., Smith J.C., Wardle F.C. A gene regulatory network directed by zebrafish No tail accounts for its roles in mesoderm formation. Proc. Natl. Acad. Sci. USA. 2009;106:3829–3834. doi: 10.1073/pnas.0808382106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Croce J., Duloquin L., Lhomond G., McClay D.R., Gache C. Frizzled5/8 is required in secondary mesenchyme cells to initiate archenteron invagination during sea urchin development. Development. 2006;133:547–557. doi: 10.1242/dev.02218. [DOI] [PubMed] [Google Scholar]
- 31.Materna S.C., Nam J., Davidson E.H. High accuracy, high-resolution prevalence measurement for the majority of locally expressed regulatory genes in early sea urchin development. Gene Expr. Patterns. 2010;10:177–184. doi: 10.1016/j.gep.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cary G.A., McCauley B.S., Zueva O., Pattinato J., Longabaugh W., Hinman V.F. Systematic comparison of sea urchin and sea star developmental gene regulatory networks explains how novelty is incorporated in early development. Nat. Commun. 2020;11:6235. doi: 10.1038/s41467-020-20023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McClay D.R., Croce J.C., Warner J.F. Conditional specification of endomesoderm. Cells Dev. 2021;167 doi: 10.1016/j.cdev.2021.203716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erkenbrack E.M., Davidson E.H., Peter I.S. Conserved regulatory state expression controlled by divergent developmental gene regulatory networks in echinoids. Development. 2018;145:dev167288. doi: 10.1242/dev.167288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine M., Davidson E.H. Gene regulatory networks for development. Proc. Natl. Acad. Sci. USA. 2005;102:4936–4942. doi: 10.1073/pnas.0408031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peter I.S., Davidson E.H. A gene regulatory network controlling the embryonic specification of endoderm. Nature. 2011;474:635–639. doi: 10.1038/nature10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peter I.S., Davidson E.H. In: Pattern Formation in Morphogenesis Springer Proceedings in Mathematics. Capasso V., Gromov M., Harel-Bellan A., Morozova N., Pritchard L.L., editors. Springer; 2013. Pattern Formation in Sea Urchin Endomesoderm as Instructed by Gene Regulatory Network Topologies; pp. 75–92. [Google Scholar]
- 38.Smith J., Davidson E.H. Gene regulatory network subcircuit controlling a dynamic spatial pattern of signaling in the sea urchin embryo. Proc. Natl. Acad. Sci. USA. 2008;105:20089–20094. doi: 10.1073/pnas.0806442105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peter I.S. In: Methods in Cell Biology Echinoderms. Part B., Hamdoun A., Foltz K.R., editors. Academic Press; 2019. Chapter 2 - Methods for the experimental and computational analysis of gene regulatory networks in sea urchins; pp. 89–113. [DOI] [PubMed] [Google Scholar]
- 40.Sun H., Peng C.F.J., Wang L., Feng H., Wikramanayake A.H. An early global role for Axin is required for correct patterning of the anterior-posterior axis in the sea urchin embryo. Development. 2021;148:dev191197. doi: 10.1242/dev.191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poustka A.J., Kühn A., Groth D., Weise V., Yaguchi S., Burke R.D., Herwig R., Lehrach H., Panopoulou G. A global view of gene expression in lithium and zinc treated sea urchin embryos: new components of gene regulatory networks. Genome Biol. 2007;8:R85. doi: 10.1186/gb-2007-8-5-r85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molina M.D., Quirin M., Haillot E., De Crozé N., Range R., Rouel M., Jimenez F., Amrouche R., Chessel A., Lepage T. MAPK and GSK3/ß-TRCP-mediated degradation of the maternal Ets domain transcriptional repressor Yan/Tel controls the spatial expression of nodal in the sea urchin embryo. PLoS Genet. 2018;14 doi: 10.1371/journal.pgen.1007621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Emily-Fenouil F., Ghiglione C., Lhomond G., Lepage T., Gache C. GSK3beta/shaggy mediates patterning along the animal-vegetal axis of the sea urchin embryo. Development. 1998;125:2489–2498. doi: 10.1242/dev.125.13.2489. [DOI] [PubMed] [Google Scholar]
- 44.Sethi A.J., Angerer R.C., Angerer L.M. Gene Regulatory Network Interactions in Sea Urchin Endomesoderm Induction. PLoS Biol. 2009;7 doi: 10.1371/journal.pbio.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee P.Y., Nam J., Davidson E.H. Exclusive Developmental Functions of gatae cis-Regulatory Modules in the Strongylocentrorus purpuratus Embryo. Dev. Biol. 2007;307:434–445. doi: 10.1016/j.ydbio.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de-Leon S.B.-T., Davidson E.H. Information processing at the foxa node of the sea urchin endomesoderm specification network. Proc. Natl. Acad. Sci. USA. 2010;107:10103–10108. doi: 10.1073/pnas.1004824107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith J., Kraemer E., Liu H., Theodoris C., Davidson E. A spatially dynamic cohort of regulatory genes in the endomesodermal gene network of the sea urchin embryo. Dev. Biol. 2008;313:863–875. doi: 10.1016/j.ydbio.2007.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuh C.-H., Dorman E.R., Howard M.L., Davidson E.H. An otx cis-regulatory module: a key node in the sea urchin endomesoderm gene regulatory network. Dev. Biol. 2004;269:536–551. doi: 10.1016/j.ydbio.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 49.Davidson E.H., Rast J.P., Oliveri P., Ransick A., Calestani C., Yuh C.-H., Minokawa T., Amore G., Hinman V., Arenas-Mena C., et al. A provisional regulatory gene network for specification of endomesoderm in the sea urchin embryo. Dev. Biol. 2002;246:162–190. doi: 10.1006/dbio.2002.0635. [DOI] [PubMed] [Google Scholar]
- 50.Davidson E.H., Rast J.P., Oliveri P., Ransick A., Calestani C., Yuh C.-H., Minokawa T., Amore G., Hinman V., Arenas-Mena C., et al. A genomic regulatory network for development. Science. 2002;295:1669–1678. doi: 10.1126/science.1069883. [DOI] [PubMed] [Google Scholar]
- 51.Hinman V.F., Nguyen A.T., Cameron R.A., Davidson E.H. Developmental gene regulatory network architecture across 500 million years of echinoderm evolution. Proc. Natl. Acad. Sci. USA. 2003;100:13356–13361. doi: 10.1073/pnas.2235868100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oliveri P., Walton K.D., Davidson E.H., McClay D.R. Repression of mesodermal fate by foxa, a key endoderm regulator of the sea urchin embryo. Development. 2006;133:4173–4181. doi: 10.1242/dev.02577. [DOI] [PubMed] [Google Scholar]
- 53.Livi C.B., Davidson E.H. Expression and function of blimp1/krox, an alternatively transcribed regulatory gene of the sea urchin endomesoderm network. Dev. Biol. 2006;293:513–525. doi: 10.1016/j.ydbio.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 54.Peter I.S., Davidson E.H. The endoderm gene regulatory network in sea urchin embryos up to mid-blastula stage. Dev. Biol. 2010;340:188–199. doi: 10.1016/j.ydbio.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erkenbrack E.M., Davidson E.H. Evolutionary rewiring of gene regulatory network linkages at divergence of the echinoid subclasses. Proc. Natl. Acad. Sci. USA. 2015;112:E4075–E4084. doi: 10.1073/pnas.1509845112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hinman V.F., Nguyen A., Davidson E.H. Caught in the evolutionary act: Precise cis-regulatory basis of difference in the organization of gene networks of sea stars and sea urchins. Dev. Biol. 2007;312:584–595. doi: 10.1016/j.ydbio.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 57.Wei Z., Yaguchi J., Yaguchi S., Angerer R.C., Angerer L.M. The sea urchin animal pole domain is a Six3-dependent neurogenic patterning center. Development. 2009;136:1179–1189. doi: 10.1242/dev.032300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huggins I.J., Bos T., Gaylord O., Jessen C., Lonquich B., Puranen A., Richter J., Rossdam C., Brafman D., Gaasterland T., Willert K. The WNT target SP5 negatively regulates WNT transcriptional programs in human pluripotent stem cells. Nat. Commun. 2017;8:1034. doi: 10.1038/s41467-017-01203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kennedy M.W., Chalamalasetty R.B., Thomas S., Garriock R.J., Jailwala P., Yamaguchi T.P. Sp5 and Sp8 recruit β-catenin and Tcf1-Lef1 to select enhancers to activate Wnt target gene transcription. Proc. Natl. Acad. Sci. USA. 2016;113:3545–3550. doi: 10.1073/pnas.1519994113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Azambuja A.P., Simoes-Costa M. A regulatory sub-circuit downstream of Wnt signaling controls developmental transitions in neural crest formation. PLoS Genet. 2021;17 doi: 10.1371/journal.pgen.1009296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shi D.-L. Wnt/planar cell polarity signaling controls morphogenetic movements of gastrulation and neural tube closure. Cell. Mol. Life Sci. 2022;79:586. doi: 10.1007/s00018-022-04620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dunty W.C., Kennedy M.W.L., Chalamalasetty R.B., Campbell K., Yamaguchi T.P. Transcriptional Profiling of Wnt3a Mutants Identifies Sp Transcription Factors as Essential Effectors of the Wnt/β-catenin Pathway in Neuromesodermal Stem Cells. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garriock R.J., Chalamalasetty R.B., Zhu J., Kennedy M.W., Kumar A., Mackem S., Yamaguchi T.P. A dorsal-ventral gradient of Wnt3a/β-catenin signals controls mouse hindgut extension and colon formation. Development. 2020;147:dev185108. doi: 10.1242/dev.185108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye S., Zhang D., Cheng F., Wilson D., Mackay J., He K., Ban Q., Lv F., Huang S., Liu D., Ying Q.L. Wnt/β-catenin and LIF–Stat3 signaling pathways converge on Sp5 to promote mouse embryonic stem cell self-renewal. J. Cell Sci. 2016;129:269–276. doi: 10.1242/jcs.177675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weber H., Symes C.E., Walmsley M.E., Rodaway A.R., Patient R.K. A role for GATA5 in Xenopus endoderm specification. Development. 2000;127:4345–4360. doi: 10.1242/dev.127.20.4345. [DOI] [PubMed] [Google Scholar]
- 66.Aronson B.E., Stapleton K.A., Krasinski S.D. Role of GATA factors in development, differentiation, and homeostasis of the small intestinal epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 2014;306:G474–G490. doi: 10.1152/ajpgi.00119.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tseng W.-F., Jang T.-H., Huang C.-B., Yuh C.-H. An evolutionarily conserved kernel of gata5, gata6, otx2 and prdm1a operates in the formation of endoderm in zebrafish. Dev. Biol. 2011;357:541–557. doi: 10.1016/j.ydbio.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 68.Chan T.-M., Longabaugh W., Bolouri H., Chen H.-L., Tseng W.-F., Chao C.-H., Jang T.-H., Lin Y.-I., Hung S.-C., Wang H.-D., Yuh C.H. Developmental gene regulatory networks in the zebrafish embryo. Biochim. Biophys. Acta. 2009;1789:279–298. doi: 10.1016/j.bbagrm.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 69.Soudais C., Bielinska M., Heikinheimo M., MacArthur C.A., Narita N., Saffitz J.E., Simon M.C., Leiden J.M., Wilson D.B. Targeted mutagenesis of the transcription factor GATA-4 gene in mouse embryonic stem cells disrupts visceral endoderm differentiation in vitro. Development. 1995;121:3877–3888. doi: 10.1242/dev.121.11.3877. [DOI] [PubMed] [Google Scholar]
- 70.Rojas A., Schachterle W., Xu S.-M., Black B.L. An endoderm-specific transcriptional enhancer from the mouse Gata4 gene requires GATA and homeodomain protein–binding sites for function in vivo. Dev. Dyn. 2009;238:2588–2598. doi: 10.1002/dvdy.22091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cirillo L.A., Lin F.R., Cuesta I., Friedman D., Jarnik M., Zaret K.S. Opening of Compacted Chromatin by Early Developmental Transcription Factors HNF3 (FoxA) and GATA-4. Mol. Cell. 2002;9:279–289. doi: 10.1016/s1097-2765(02)00459-8. [DOI] [PubMed] [Google Scholar]
- 72.McKnight K.D., Hou J., Hoodless P.A. Foxh1 and Foxa2 are not required for formation of the midgut and hindgut definitive endoderm. Dev. Biol. 2010;337:471–481. doi: 10.1016/j.ydbio.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 73.Bikoff E.K., Morgan M.A., Robertson E.J. An expanding job description for Blimp-1/PRDM1. Curr. Opin. Genet. Dev. 2009;19:379–385. doi: 10.1016/j.gde.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 74.Hohenauer T., Moore A.W. The Prdm family: expanding roles in stem cells and development. Development. 2012;139:2267–2282. doi: 10.1242/dev.070110. [DOI] [PubMed] [Google Scholar]
- 75.Wilm T.P., Solnica-Krezel L. Essential roles of a zebrafish prdm1/blimp1 homolog in embryo patterning and organogenesis. Development. 2005;132:393–404. doi: 10.1242/dev.01572. [DOI] [PubMed] [Google Scholar]
- 76.Echinobase Home. https://www.echinobase.org/echinobase/
- 77.Zhao J., Cao Y., Zhao C., Postlethwait J., Meng A. An SP1-like transcription factor Spr2 acts downstream of Fgf signaling to mediate mesoderm induction. EMBO J. 2003;22:6078–6088. doi: 10.1093/emboj/cdg593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao C., Meng A. Sp1-like transcription factors are regulators of embryonic development in vertebrates. Dev. Growth Differ. 2005;47:201–211. doi: 10.1111/j.1440-169X.2005.00797.x. [DOI] [PubMed] [Google Scholar]
- 79.Darras S., Fritzenwanker J.H., Uhlinger K.R., Farrelly E., Pani A.M., Hurley I.A., Norris R.P., Osovitz M., Terasaki M., Wu M., et al. Anteroposterior axis patterning by early canonical Wnt signaling during hemichordate development. PLoS Biol. 2018;16 doi: 10.1371/journal.pbio.2003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gray J., Fritzenwanker J.H., Cunningham D.D., Lowe C.J. In: Current Topics in Developmental Biology Emerging Model Systems in Developmental Biology. Goldstein B., Srivastava M., editors. Academic Press; 2022. Chapter Nineteen - Saccoglossus kowalevskii: Evo-devo insights from the mud; pp. 545–562. [DOI] [PubMed] [Google Scholar]
- 81.Yu J.-K., Satou Y., Holland N.D., Shin-I T., Kohara Y., Satoh N., Bronner-Fraser M., Holland L.Z. Axial patterning in cephalochordates and the evolution of the organizer. Nature. 2007;445:613–617. doi: 10.1038/nature05472. [DOI] [PubMed] [Google Scholar]
- 82.Angerer L.M., Yaguchi S., Angerer R.C., Burke R.D. The evolution of nervous system patterning: insights from sea urchin development. Development. 2011;138:3613–3623. doi: 10.1242/dev.058172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feuda R., Peter I.S. Homologous gene regulatory networks control development of apical organs and brains in Bilateria. Sci. Adv. 2022;8 doi: 10.1126/sciadv.abo2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Varga M., Maegawa S., Weinberg E.S. Correct anteroposterior patterning of the zebrafish neurectoderm in the absence of the early dorsal organizer. BMC Dev. Biol. 2011;11:26. doi: 10.1186/1471-213X-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shinya M., Eschbach C., Clark M., Lehrach H., Furutani-Seiki M. Zebrafish Dkk1, induced by the pre-MBT Wnt signaling, is secreted from the prechordal plate and patterns the anterior neural plate. Mech. Dev. 2000;98:3–17. doi: 10.1016/s0925-4773(00)00433-0. [DOI] [PubMed] [Google Scholar]
- 86.Lagutin O.V., Zhu C.C., Kobayashi D., Topczewski J., Shimamura K., Puelles L., Russell H.R.C., McKinnon P.J., Solnica-Krezel L., Oliver G. Six3 repression of Wnt signaling in the anterior neuroectoderm is essential for vertebrate forebrain development. Genes Dev. 2003;17:368–379. doi: 10.1101/gad.1059403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Steinmetz P.R., Urbach R., Posnien N., Eriksson J., Kostyuchenko R.P., Brena C., Guy K., Akam M., Bucher G., Arendt D. Six3 demarcates the anterior-most developing brain region in bilaterian animals. EvoDevo. 2010;1:14. doi: 10.1186/2041-9139-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kiecker C., Niehrs C. A morphogen gradient of Wnt/β-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development. 2001;128:4189–4201. doi: 10.1242/dev.128.21.4189. [DOI] [PubMed] [Google Scholar]
- 89.Miao N., Bian S., Lee T., Mubarak T., Huang S., Wen Z., Hussain G., Sun T. Opposite Roles of Wnt7a and Sfrp1 in Modulating Proper Development of Neural Progenitors in the Mouse Cerebral Cortex. Front. Mol. Neurosci. 2018;11:247. doi: 10.3389/fnmol.2018.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Onai T., Takai A., Setiamarga D.H.E., Holland L.Z. Essential role of Dkk3 for head formation by inhibiting Wnt/β-catenin and Nodal/Vg1 signaling pathways in the basal chordate amphioxus. Evol. Dev. 2012;14:338–350. doi: 10.1111/j.1525-142X.2012.00552.x. [DOI] [PubMed] [Google Scholar]
- 91.Nikaido M., Law E.W.P., Kelsh R.N. A Systematic Survey of Expression and Function of Zebrafish frizzled Genes. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sumanas S., Kim H.J., Hermanson S., Ekker S.C. Zebrafish frizzled-2 morphant displays defects in body axis elongation. Genesis. 2001;30:114–118. doi: 10.1002/gene.1043. [DOI] [PubMed] [Google Scholar]
- 93.Lekven A.C., Thorpe C.J., Waxman J.S., Moon R.T. Zebrafish wnt8 Encodes Two Wnt8 Proteins on a Bicistronic Transcript and Is Required for Mesoderm and Neurectoderm Patterning. Dev. Cell. 2001;1:103–114. doi: 10.1016/s1534-5807(01)00007-7. [DOI] [PubMed] [Google Scholar]
- 94.Kim S.-H., Shin J., Park H.-C., Yeo S.-Y., Hong S.-K., Han S., Rhee M., Kim C.-H., Chitnis A.B., Huh T.-L. Specification of an anterior neuroectoderm patterning by Frizzled8a-mediated Wnt8b signalling during late gastrulation in zebrafish. Development. 2002;129:4443–4455. doi: 10.1242/dev.129.19.4443. [DOI] [PubMed] [Google Scholar]
- 95.Kim S.-H., Park H.-C., Yeo S.-Y., Hong S.-K., Choi J.-W., Kim C.-H., Weinstein B.M., Huh T.-L. Characterization of two frizzled8 homologues expressed in the embryonic shield and prechordal plate of zebrafish embryos1The entire nucleotide sequences for Zfz8a and Zfz8b cDNA were deposited to the GenBank database under the Accession numbers AF060697 and AF060696, respectively.1. Mech. Dev. 1998;78:193–198. doi: 10.1016/s0925-4773(98)00137-3. [DOI] [PubMed] [Google Scholar]
- 96.Technau U., Steele R.E. Evolutionary crossroads in developmental biology: Cnidaria. Development. 2011;138:1447–1458. doi: 10.1242/dev.048959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lebedeva T., Aman A.J., Graf T., Niedermoser I., Zimmermann B., Kraus Y., Schatka M., Demilly A., Technau U., Genikhovich G. Cnidarian-bilaterian comparison reveals the ancestral regulatory logic of the β-catenin dependent axial patterning. Nat. commun. 2021;12:4032. doi: 10.1038/s41467-021-24346-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Holstein T.W. The role of cnidarian developmental biology in unraveling axis formation and Wnt signaling. Dev. Biol. 2022;487:74–98. doi: 10.1016/j.ydbio.2022.04.005. [DOI] [PubMed] [Google Scholar]
- 99.Lebedeva T., Aman A.J., Graf T., Niedermoser I., Zimmermann B., Kraus Y., Schatka M., Demilly A., Technau U., Genikhovich G. Cnidarian-bilaterian comparison reveals the ancestral regulatory logic of the β-catenin dependent axial patterning. Nat. Commun. 2021;12:4032. doi: 10.1038/s41467-021-24346-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Leclère L., Bause M., Sinigaglia C., Steger J., Rentzsch F. Development of the aboral domain in Nematostella requires β-catenin and the opposing activities of Six3/6 and Frizzled5/8. Development. 2016;143:1766–1777. doi: 10.1242/dev.120931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Duffy D.J., Plickert G., Kuenzel T., Tilmann W., Frank U. Wnt signaling promotes oral but suppresses aboral structures in Hydractinia metamorphosis and regeneration. Development. 2010;137:3057–3066. doi: 10.1242/dev.046631. [DOI] [PubMed] [Google Scholar]
- 102.Schaeper N.D., Prpic N.-M., Wimmer E.A. A clustered set of three Sp-family genes is ancestral in the Metazoa: evidence from sequence analysis, protein domain structure, developmental expression patterns and chromosomal location. BMC Evol. Biol. 2010;10:88. doi: 10.1186/1471-2148-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang D.G., Kirchhamer C.V., Britten R.J., Davidson E.H. SpZ12-1, a negative regulator required for spatial control of the territory-specific CyIIIa gene in the sea urchin embryo. Development. 1995;121:1111–1122. doi: 10.1242/dev.121.4.1111. [DOI] [PubMed] [Google Scholar]
- 104.Erkenbrack E.M., Croce J.C., Miranda E., Gautam S., Martinez-Bartolome M., Yaguchi S., Range R.C. Whole mount in situ hybridization techniques for analysis of the spatial distribution of mRNAs in sea urchin embryos and early larvae. Methods Cell Biol. 2019;151:177–196. doi: 10.1016/bs.mcb.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 105.Choi H.M.T., Calvert C.R., Husain N., Huss D., Barsi J.C., Deverman B.E., Hunter R.C., Kato M., Lee S.M., Abelin A.C.T., et al. Mapping a multiplexed zoo of mRNA expression. Development. 2016;143:3632–3637. doi: 10.1242/dev.140137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Castro-Mondragon J.A., Riudavets-Puig R., Rauluseviciute I., Lemma R.B., Turchi L., Blanc-Mathieu R., Lucas J., Boddie P., Khan A., Manosalva Pérez N., et al. JASPAR 2022: the 9th release of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2022;50:D165–D173. doi: 10.1093/nar/gkab1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Data produced in this paper is available upon request to the lead contact.
-
•
This paper does not report any original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available upon request to the lead contact.