ABSTRACT
Background
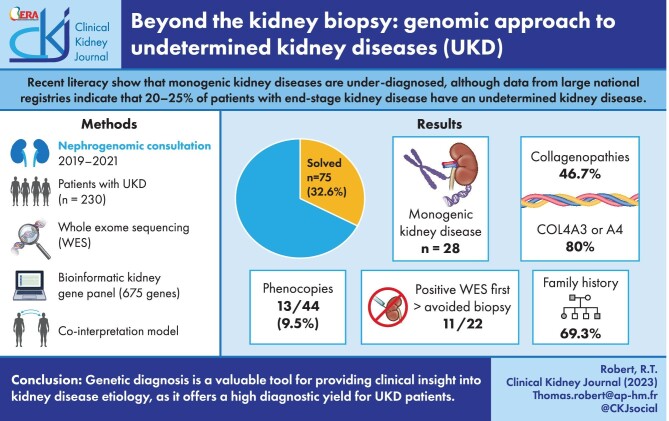
According to data from large national registries, almost 20%–25% of patients with end-stage kidney disease have an undetermined kidney disease (UKD). Recent data have shown that monogenic disease-causing variants are under-diagnosed. We performed exome sequencing (ES) on UKD patients in our center to improve the diagnosis rate.
Methods
ES was proposed in routine practice for patients with UKD including kidney biopsy from January 2019 to December 2021. Mutations were detected using a targeted bioinformatic customized kidney gene panel (675 genes). The pathogenicity was assessed using American College of Medical Genetics guidelines.
Results
We included 230 adult patients, median age 47.5 years. Consanguinity was reported by 25 patients. A family history of kidney disease was documented in 115 patients (50%). Kidney biopsies were either inconclusive in 69 patients (30.1%) or impossible in 71 (30.9%). We detected 28 monogenic renal disorders in 75 (32.6%) patients. Collagenopathies was the most common genetic kidney diagnosis (46.7%), with COL4A3 and COL4A4 accounting for 80% of these diagnoses. Tubulopathies (16%) and ciliopathies (14.7%) yielded, respectively, the second and third genetic kidney diagnosis category and UMOD-associated nephropathy as the main genetic findings for tubulopathies (7/11). Ten of the 22 patients having ES “first” eventually received a positive diagnosis, thereby avoiding 11 biopsies. Among the 44 patients with glomerular, tubulo-interstitial or vascular nephropathy, 13 (29.5%) were phenocopies. The diagnostic yield of ES was higher in female patients (P = .02) and in patients with a family history of kidney disease (P < .0001), reaching 56.8% when the patient had both first- and second-degree family history of renal disease.
Conclusion
Genetic diagnosis has provided new clinical insights by clarifying or reclassifying kidney disease etiology in over a third of UKD patients. Exome “first” may have a significant positive diagnostic yield, thus avoiding invasive kidney biopsy; moreover, the diagnostic yield remains elevated even when biopsy is impossible or inconclusive. ES provides a clinical benefit for routine nephrological healthcare in patients with UKD.
Keywords: chronic kidney disease, exome sequencing, undetermined nephropathy
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Chronic kidney disease (CKD) is a public health problem that is associated with high costs and morbidity. Despite kidney biopsy, different national registries have established that 20%–25% of patients with end-stage kidney disease (ESKD) have an undetermined kidney disease (UKD) [1–3]. In this category, several studies have already suggest an enrichment of monogenic/Mendelian kidney diseases with causative variants [4]. Despite rare Mendelian diseases beginning to appear more frequently than expected in the CKD population, their molecular diagnosis remains challenging, especially given the paucity of the phenotype. In France, as in many developed countries, a national effort to facilitate whole-genome sequencing as part of the routine healthcare process for different disease entities is moving forward. Despite this, few institutions can currently provide exome sequencing (ES) as a first-line clinical diagnostic tool in nephrology practice.
Patients with UKD represent a significant proportion of CKD patients despite there being no clear definition of UKD at present. Since 2012, the national Renal Epidemiology and Information Network (REIN) registry in France has provided an overview of incidental and prevalent patients with ESKD. REIN data show that 20% of registry patients have a UKD, while 13% are still labelled as “other.” A recent Kidney Disease: Improving Global Outcomes (KDIGO) conference suggested that UKD could be an indication for genetic screening. This would be especially beneficial if a kidney biopsy is neither informative nor possible because the patient is at too advanced a stage and there are no indications of an hereditary disease. This approach could reduce costly diagnostic workups for those patients [4, 5]. Genetic screening may even be the only available tool when newly diagnosed ESKD is discovered in adult patients, sometimes resolving atypical cases. And last, but not least, the analysis of a local registry of kidney biopsies (NéphroMIC², NCT03305211) revealed that around 20% of patients still have inconclusive kidney biopsies, thus remaining within the UKD category (data not published). Since 2019, ES has been proposed for all UKD patients in our center.
A small number of studies support the implementation of ES in the diagnostic workup of UKD, particularly if the patient is waiting for kidney transplantation, or if early onset or familial nephropathy is observed. Whether those findings can be extended to the UKD population or, more especially, to sporadic cases, is unclear. Our strategy is based on an in-silico panel analysis of known genes related to kidney diseases (Supplementary data, Table S1). To address the usefulness of ES to resolve UKD in routine care, we reported our experience.
MATERIALS AND METHODS
Patient cohort and phenotype characterization
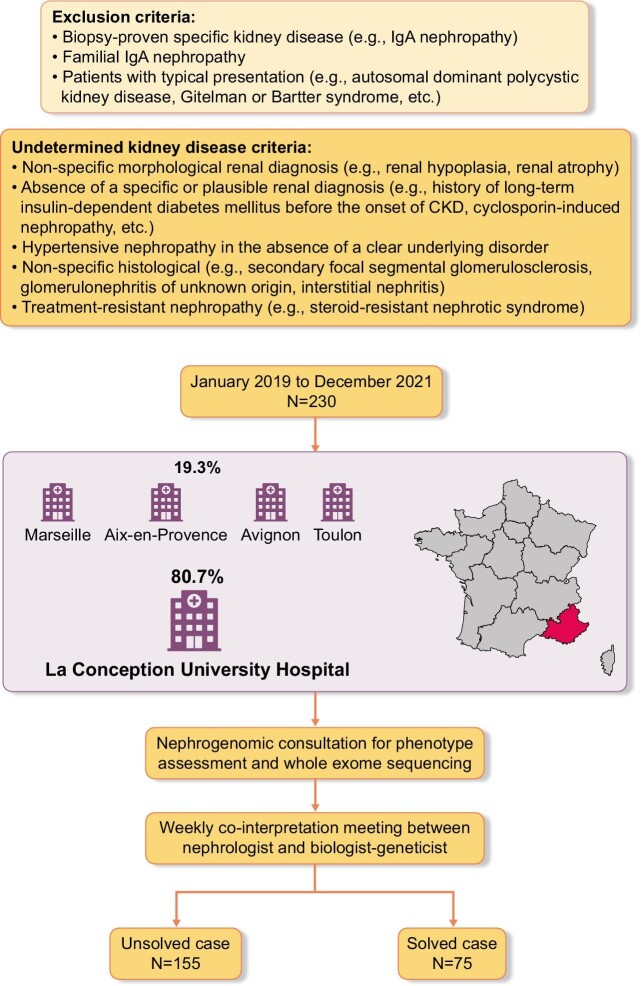
ES was proposed as part of routine clinical care in patients with UKD from January 2019 to December 2021. UKD is defined as the absence of any of the following criteria: biopsy-proven diagnosis [e.g. immunoglobulin A (IgA) nephropathy], absence of a specific morphological renal diagnosis (e.g. polycystic kidney disease suspected to be autosomal/recessive polycystic kidney disease) or absence of a specific or plausible renal diagnosis (such as, history of long-term insulin-dependent diabetes mellitus before the onset of CKD, ciclosporin-induced nephropathy). Patients with kidney disease who underwent ES before kidney biopsy were included in the analysis and considered as the exome “first” approach. Since hypertensive nephropathy is a nonspecific diagnosis, and hypertension is also a very common consequence of CKD, patients with hypertensive nephropathy in the absence of a clear underlying disorder, such as renal artery stenosis, are considered to have unexplained CKD. Patients with renal hypoplasia, renal atrophy and nonspecific histological conditions (such as secondary focal segmental glomerulosclerosis, glomerulonephritis of unknown origin or interstitial nephritis) are also considered to have UKD. We excluded patients with familial IgA nephropathy, patients with typical presentation of Gitelman or Bartter syndrome, or an established kidney-related genetic diagnosis in the family. None of the 230 index cases had a genetic diagnosis and therefore all were considered as unsolved (Fig. 1). If prior genetic analyses were performed, they were at the discretion of the referring clinician. Patients with UKD both from our center and other tertiary care centers were referred for a nephrogenomic consultation with one of our adult nephrologists. Phenotypes were acquired by using a standardized questionnaire and review of medical reports during consultation. Consanguinity was also established during consultation either as reported by the patient or as suspected by the clinician. Patients were assigned to four subgroups of undetermined nephropathy: unclassified nephropathy; undetermined glomerular nephropathy; undetermined tubulointerstitial nephropathy; and undetermined vascular nephropathy. We defined a phenocopy as a patient having a phenotype that corresponded to a specific clinical nephropathy (such as glomerular nephropathy) without detection of the expected genotype (e.g. a glomerular gene such as TRPC6) but with detection of a different genotype (for example, a tubulointerstitial gene such as CLCN5).
Figure 1:
Diagnostic workflow for undetermined kidney diseases selection.
Blood samples were collected after written informed consent from the patients or their legal guardians during the consultation. All patients gave their written informed consent for genetic testing.
Exome sequencing
DNA was extracted from peripheral blood using the QIAsymphony® DSP DNA Mini Kit on a QIAsymphony instrument following the manufacturer's (QIAGEN) guidelines. From 50 ng of fragmented DNA, indexed libraries were prepared and hybridized with a biotinylated probe from Twist Human Core Exome (33 Mb) and, from April 2019, Twist Human Comprehensive Exome (37 Mb). ES was performed on the Illumina NextSeq 500 in paired-end mode (2 × 75 bp reads) then, from March 2021, NextSeq 2000 platform in paired-end mode (2 × 150 bp reads) on FlowCell P3. Raw data (bcl format) were converted to FASTQ format using the Dragen software sequencer (Illumina). Reads were aligned to the human reference genome (UCSC Genome Browser build hg19). Sequences were analyzed according to GATK Broad Institute good practice with two pipelines: Intern pipeline (BWA-MEM, GATK v3.6–44ge7d1cd2) and SeqOne pipeline (v1.2, 2018). Copy number variants (CNVs) calls were performed using the GATK4 CNV calling module and were validated using Multiplex Ligation-dependent Probe Amplification [6].
To identify diagnostic variants, we assessed the pathogenicity of the variant using American College of Medical Genetics (ACMG) guidelines [7]. Variants were filtered according to coverage level (DP > 10), allelic frequency (>20%) and protein effect. Frequency of variants in GnomAd was also considered: for the analysis of de novo, autosomal dominant, autosomal recessive and X-linked variants, only variants with a minor allele frequency (MAF) <1% in the GnomAd database were considered. For the analysis of autosomal recessive and X-linked variants (homozygous, hemizygous or putative compound heterozygous) in unsolved cases, additional research with an MAF up to 3% was considered. In addition, we assessed the APOL1 genotype [G1 (rs73885139 and rs60910145) and G2 (rs71785313)] as Mendelian forms of nephropathy when two copies were present as follows: G1/G1, G1/G2 or G2/G2 [8]. Identified variants were compared with available databases for pathogenic variants such ClinVar, the Human Gene Mutation Database (HGMD) [9] and the Leiden Open Variation Database (LOVD), and databases for pathogenic CNVs such as DECIPHER. Only variants rated as “likely pathogenic” or “pathogenic” according to the ACMG classification, and with a genotype in agreement with the mode of inheritance and the phenotype, led to a positive ES result. Patients with variants classified as benign, likely benign or of unknown significance according to ACMG led to a negative exome result. ES results were communicated to the patients by the same nephrologist with whom they had had the initial nephrogenomic consultation.
Statistical analysis
Baseline characteristics were expressed as frequencies (n, %), means [standard deviations (SDs)] and medians (range). Fisher's exact test was performed for categorical data. Diagnostic yield was calculated based on counts of variants classified as “pathogenic” or “likely pathogenic.” To compare two continuous variables, we used normality tests and then an unpaired t-test or unpaired Mann–Whitney nonparametric test if values were not sampled from Gaussian distribution. P-values <.05 were considered statistically significant. Two-tailed P-values <.05 were regarded as statistically significant. Statistical analyses were performed using Prism 9 (GraphPad Software) software.
RESULTS
Characteristics of the population
Two hundred and thirty-two adult patients were included (133 males) with a median age of 47.5 years, and 173 were self-declared European. Of these, 54 patients were treated with hemodialysis and 38 patients had a kidney transplantation. Consanguinity was reported or suspected in 25 patients, and 126 had kidney disease onset before the age of 35 years. A family history of kidney disease was present in 115 patients and 37 patients had both first and second degree with history of kidney disease. Kidney biopsies were inconclusive in 69 patients and impossible in 71 patients. An ES was performed first to avoid kidney biopsy in 22 patients. A negative genetic testing with gene panel prior to the ES was performed in 34 patients. Finally, 88 patients had unclassified kidney disease (Table 1).
Table 1:
Demographic characteristics of the whole cohort.
| Variablea | Patients (n = 230) |
|---|---|
| Age at ES (years) | 47.5 [36.8–62] |
| Age at kidney disease onset or discovery | 35 [23–50] |
| Male | 133 (57.8) |
| Geographic origin | |
| Europe | 173 (75.2) |
| North Africa | 31 (13.5) |
| Sub-Saharan Africa | 12 (5.2) |
| French Antilles | 7 (3.1) |
| Asia | 2 (0.01) |
| Others | 5 (2.2) |
| Consanguinity | 25 (10.9) |
| Kidney disease onset before 35 years old | 126 (54.8) |
| With familial history | 50 (21.7) |
| Familial history of kidney disease | 115 (50) |
| First degree only | 58 (25.2) |
| Second degree only | 19 (8.3) |
| Both first and second degree | 37 (16.2) |
| Prior negative genetic exploration with gene panel | 34 (14.7) |
| ES first to avoid kidney biopsy | 22 (9.5) |
| Prior inconclusive kidney biopsy | 69 (30.1) |
| Kidney disease discovered at ESKD | 43 (18.7) |
| Impossible kidney biopsy | 71 (30.9) |
| Undetermined clinical nephropathy subgroup | |
| Unclassified | 88 (38.3) |
| Glomerular | 64 (27.8) |
| Tubulointerstitial | 30 (13) |
| Cyst | 32 (13.9) |
| Vascular | 16 (7) |
| Stage of kidney disease at ES | |
| I | 32 (13.9) |
| II | 13 (5.7) |
| III | 39 (17) |
| IV | 32 (13.9) |
| V | 22 (9.5) |
| V (D) | 54 (23.5) |
| V (T) | 38 (16.5) |
| Status of patient transplantation | 80 (34.5) |
| Active in transplant waiting list | 16 (6.9) |
For quantitative variables, values are expressed as median [interquartile ranges]. For qualitative variables, values are expressed as n (%).
Diagnostic yield of the ES
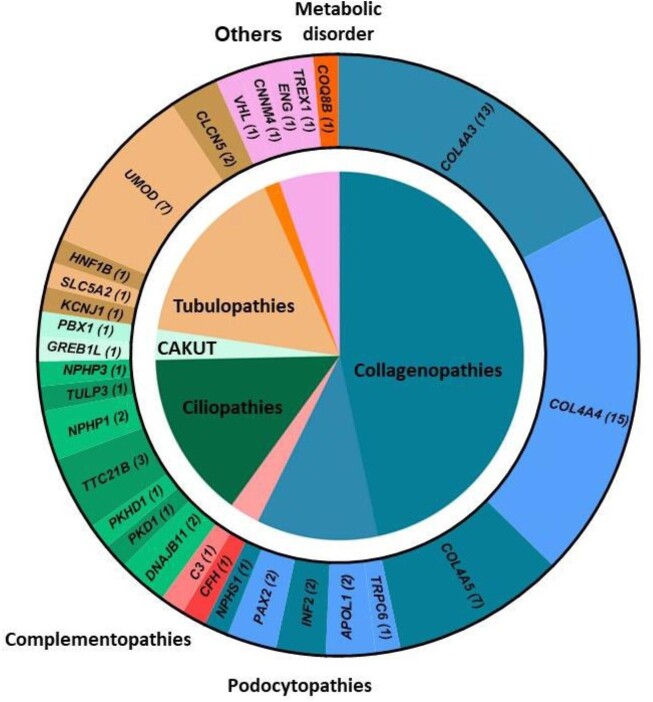
We identified 28 monogenic renal disorders in 75 patients carrying either pathogenic or likely pathogenic variants [single nucleotide variants (SNV)/small indels; n = 70] or CNVs (n = 5) among 230 patients (32.6%) (Supplementary data, Table S2). Collagenopathies were the major genetic kidney disease category with 46.7% (COL4A4 n = 15; COL4A3 n = 13; COL4A5 n = 7), followed by tubulopathies with 16% (UMOD n = 7; CLCN5 n = 2; HNF1B n = 1; KCNJ1 n = 1; SLC5A2 n = 1). Ciliopathies (TTC21B n = 3; NPHP1 n = 2; DNAJB11 n = 2; NPHP3 n = 1; TULP3 n = 1) and podocytopathies (INF2 n = 2; PAX2 n = 2; APOL1 n = 2; TRPC6 n = 1; NPHS1 n = 1) represent, respectively, the third and fourth majors genetic kidney disease category with 14.7% and 10.7% (Fig. 2A). Finally, when ES was performed first to avoid renal biopsy, 11 of the 22 patients (50%) had a positive genetic finding [9 collagenopathies, 1 APOL1 nephropathy and 1 ciliopathy-gene associated disease (TTC21B)] (Table 2).
Figure 2:
Gene-associated kidney disease according to the category of genetic kidney disease. The inner circle represents the categories of genetic kidney disease and in the outer circle, gene reported with a pathogenic variant in patients; numbers in brackets represent the number of patient's carrier. Genes have been clustered according to their associated categories of genetic kidney disease.
Table 2:
Comparison of characteristics between exome-solved and -unsolved cases.
| Variablea | Unsolved (n = 155) | Solved (n = 75) | P-value |
|---|---|---|---|
| Age at ES (years) | 46 [36–62] | 50 [40–63] | .25 |
| Age at kidney disease onset or discovery | 37 [24–50] | 32 [20–49] | .26 |
| Male | 98 (63.2) | 35 (46.7) | .02 |
| Geographic origin | .2 | ||
| Europe | 116 (74.8) | 57 (76) | |
| North Africa | 17 (11) | 14 (18.6) | |
| Sub-Saharan Africa | 10 (6.5) | 2 (2.7) | |
| French Antilles | 5 (3.2) | 2 (2.7) | |
| Asia | 2 (1.3) | 0 | |
| Others | 5 (3.2) | 0 | |
| Consanguinity | 13 (8.5) | 12 (16) | .1 |
| Kidney disease onset before 35 years old | 81 (52.3) | 45 (60) | .3 |
| With familial history | 26 (16.8) | 24 (32) | .01 |
| Familial history of kidney disease | 63 (40.6) | 52 (69.3) | .0001 |
| First degree only | 33 (21.3) | 25 (33.3) | .05 |
| Second degree only | 13 (8.4) | 6 (8) | .9 |
| Both first and second degree | 16 (10.3) | 21 (28) | .001 |
| Prior negative genetic exploration with gene panel | 22 (14.2) | 12 (16) | .7 |
| ES first to avoid kidney biopsy | 11 (7.1) | 11 (14.7) | .09 |
| Prior inconclusive kidney biopsy | 45 (29.2) | 24 (32) | .7 |
| Impossible kidney biopsy | 48 (31) | 23 (30.7) | 1 |
| Undetermined clinical nephropathy subgroup | |||
| Unclassified | 57 (36.8) | 31 (41.4) | |
| Glomerular | 39 (25.2) | 25 (33.3) | |
| Tubulointerstitial | 23 (14.8) | 7 (9.3) | .3 |
| Cyst | 23 (14.8) | 9 (12) | |
| Vascular | 13 (8.4) | 3 (4) | |
| Stage of kidney disease at ES | |||
| I | 22 (14.2) | 10 (13.3) | |
| II | 10 (6.5) | 3 (4) | |
| III | 28 (18.1) | 11 (14.7) | |
| IV | 20 (12.9) | 12 (16) | .3 |
| V | 16 (10.3) | 6 (8) | |
| V (D) | 39 (25.2) | 15 (20) | |
| V (T) | 20 (12.9) | 18 (24) |
Relationship between genotype and nephropathy category in ES solved cases
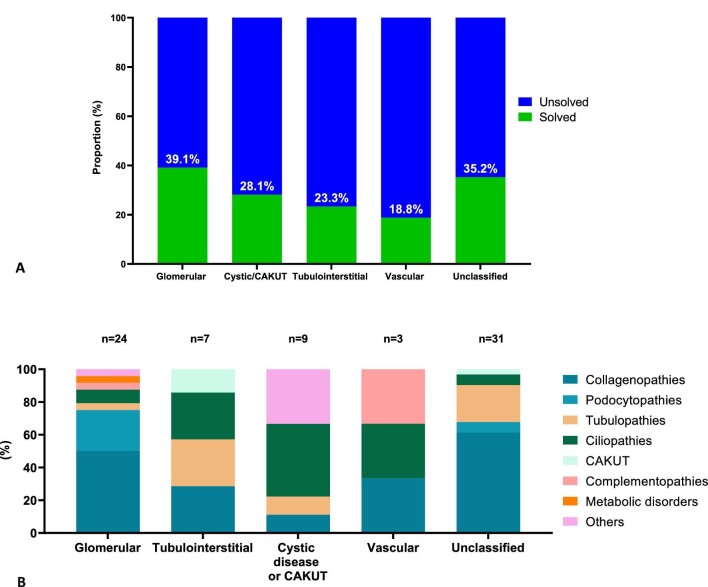
Glomerular and unclassified nephropathy had the highest ES-solved case rates in, respectively, 39.1% and 35.2%, whilst tubulointerstitial and vascular nephropathy had the lowest ES-solved case rates (respectively, 23.3% and 18.8%) (Fig. 3A). Among the 44 patients who had their nephropathy categorized, 13 (29.5%) were phenocopies (Fig. 3B and Supplementary data, Fig. 1). Vascular (2/3) and tubulointerstitial nephropathy (4/7) had the highest phenocopy rates, at 66.3% and 42.9%, respectively. Among glomerular nephropathy, 16% (4/24) were phenocopies. Cystic kidney disease and congenital anomalies of the kidneys and urinary tract (CAKUT) had a phenocopy rate of 22.2% (2/9) (Fig. 3B). Collagenopathies (COL4A3 and COl4A4), ciliopathy category (TTC21B, TULP3) and tubulopathies (UMOD, CLCN5) show the highest phenocopy rates (Supplementary data, Fig. S2). Of the unclassified nephropathies, the largest proportions were due to collagenopathies (61.3%) and tubulopathies (22.6%) (Fig. 3B and Supplementary data, Fig. 3).
Figure 3:
Genetic findings according to nephropathy category. (A) The diagnostic yielded according to the clinical nephropathy category. (B) The distribution of the genetic kidney disease category according to the clinical nephropathy category.
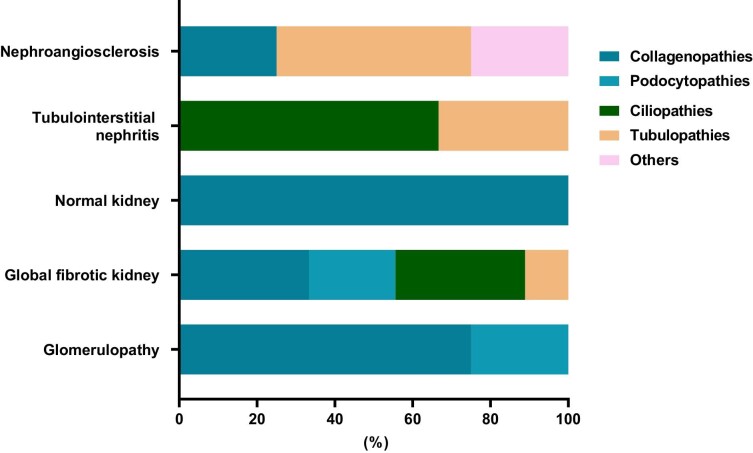
In biopsy-associated UKD patients, the pathologist's conclusion about the main histological lesion was consistent with the genetic findings for all glomerular and tubulo-interstitial diseases. In patients whose pathologist concluded that nephroangiosclerosis was the primary histological lesion, ES revealed two tubulopathies—one collagenopathy and one vasculopathy associated with the TREX1 gene (Fig. 4). The most frequent causes of global fibrosis in the kidneys were found to be either a collagenopathy (33.3%) or a ciliopathy (33.3%) (Fig. 4).
Figure 4:
Distribution of genetic kidney disease categories based on kidney biopsy findings.
Clinical predictors of positive exome diagnosis for kidney disease
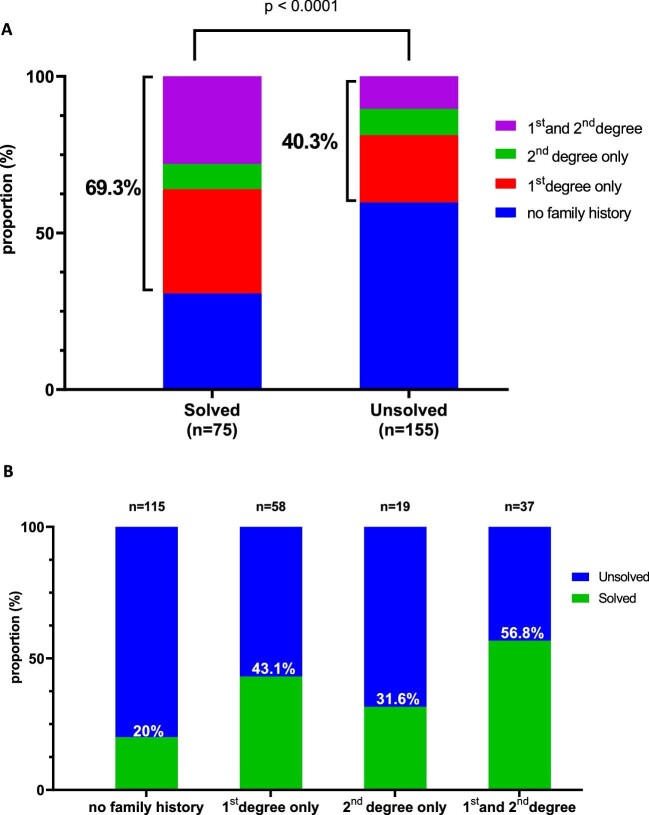
The ES diagnostic yield was higher in female patients (54.5% vs 47%, P = .01) and those with a familial history of kidney disease (69.2% vs 40.4%, P < .0001) (Table 2 and Fig. 5A). Out of patients with a family history of kidney disease in both first and second degree, 56.8% had a positive ES (Fig. 5B). Younger age at onset of the disease (<35 years) was not significantly associated with a positive ES (P = .3) except for those with positive family history of kidney disease (32% vs 16.8%, P = .01) (Table 2).
Figure 5:
ES yielded according family history of kidney disease. (A) Comparison of the prevalence of kidney disease in relatives of positive and negative ES patients; panel (B) shows in positive ES patients the proportion of familial history of kidney disease by degree in the relatives.
DISCUSSION
In the present UKD cohort, implementation of ES in routine healthcare allowed us to resolve diagnosis in one-third of patients (32.6%). Previous studies provided a diagnostic yield of genetic testing in patients with CKD, in the absence of a specific suspicion of monogenic kidney disease, of between 10% and 30% in adults [4, 5, 10] and between 30% and 50% for pediatric cohorts [11–14]. Our study illustrates the underestimation of the prevalence of inherited case. CNV accounts for 6.7% of genetic findings, suggesting that a combination of SNV and CNV detection is suitable for genomic analysis prior to concluding that ES is negative and that whole-genome sequencing is necessary [6]. Interestingly, the Groopman study was unable to detect CNVs, suggesting that the proportion of inherited kidney disease among its participants is likely greater than was reported [4]. CKD affects 11%–13% of the world population [15], but UKD accounts for approximately 20% of CKD patients in developed countries. The application of ES to nephrology, and especially to UKD, has the potential to revolutionize the way kidney diseases are classified, taking into account not only clinical features and kidney biopsy, but also the underlying pathogenesis of the disease. This could lead to a much more accurate and comprehensive reclassification and ontology of kidney diseases.
In selecting a panel of genes for testing, geneticists traditionally take a phenotypically driven approach. This is referred to as the “candidate gene genetics” method and is responsible for most of the human gene–disease associations that have so far been discovered with penetrant phenotypes. In recent years, and with the ease of access to NGS, a gene-oriented approach has emerged which consists of beginning with the variant in a gene and looking back to the patient's phenotype. This method is called “reverse phenotyping” and seems particularly interesting in the context of UKD patients. This approach is especially valuable in genetically related kidney diseases that may remain completely undiagnosed until ESKD [16].
Glomerular diseases, most particularly collagenopathies, are the major genetic findings in our cohort, which differs from the pediatric and young adult population where CAKUT constitutes the major clinical diagnostic group (50% of cases) [17]. In our elderly population (most of whom were over 30 years old), nearly half of the genetic findings were a collagen type IV nephropathy, demonstrating wide variability in phenotype among patients with mutations affecting type IV collagen. Interestingly, mutations in the COL4A3 and COL4A4 genes accounted for 80% of the collagenopathies, which is much higher than previously reported [18]. This suggests that collagen IV nephropathy associated with these two genes is underdiagnosed. Of the patients with a pathogenic variation in either COL4A3 or COL4A4, 78.6% had a stage of chronic kidney disease below stage 3 chronic kidney disease and may have been classified as having “thin basement membrane disease,” which does not accurately reflect this mild condition. This finding supports the recent consensus report which proposed that all individuals with mutations in either the COL4A3 or COL4A4 gene should be diagnosed as having Alport Syndrome [19]. This suggests that the accurate clinical diagnosis of monogenic kidney diseases in elderly CKD patients is challenging due to their high phenotypic overlap, extensive genetic heterogeneity and the presence of comorbidities. This result also confirms that collagenopathies are the major causative genes of monogenic CKD in a UKD population [4]. Tubulopathies was the second genetic kidney disease category with UMOD as the next main genetic finding in our study and ciliopathy accounted for the third genetic kidney disease category. This confirms that this locus is one of those most strongly associated with CKD [20], representing one of the most common monogenic kidney diseases with an overall prevalence of ∼2% in patients with renal failure [21, 22].
Among patients with unclassified nephropathy, collagenopathies and tubulopathies are the major findings, highlighting the clinical heterogeneity and variable expression of gene-associated phenotype. Our result confirms Groopman's findings, with 45% and 29.4% of glomerular and TI-gene associated disease, respectively, in patients with nephropathy of unknown origin. The former should be more accurately termed unclassified nephropathy as Groopman et al. classified patients by clinical diagnosis subgroup (glomerulopathy, congenital or cystic kidney disease, hypertensive nephropathy, diabetic nephropathy, tubulointerstitial disease and nephropathy of unknown origin) [4]. As recommended by KDIGO, there is an urgent need to clarify UKD definition, which is referred to using different terms: unexplained kidney disease, undetermined kidney disease, nephropathy of unknown origin. We propose that UKD is a clinical diagnostic group that should be based on clinical findings, renal morphology, biological parameters and, if available, renal histology. Undetermined kidney disease can thus be classified as being glomerular, tubulointerstitial, vascular, cystic or related to a renal development abnormality. If there is not a strong enough argument bundle, the undetermined kidney disease remains unclassifiable.
Our study reveals that nearly one-quarter of the classifiable UKDs are phenocopies with a wide range of clinical presentations. This suggests that whole-exome sequencing is more suitable than gene panels in UKD [23]. However, the pathologist's conclusion can be used to help select the most suitable gene panel when kidney biopsy is possible. Ottlewski et al. observed a 12% genetic diagnostic yield using a 200-gene panel in a UKD cohort [5]. In comparison, Schrezenmeier and colleagues reported a higher yield of 20% using a larger 600-gene panel [24]. All these results suggest that, given the regular discovery of new genes associated with UKD and the need for rapid and cost-effective sequencing, whole-exome sequencing with a bioinformatic gene panel is advantageous. In our center, we follow the ACMG guidelines in reanalyzing ES data for each newly discovered gene-associated kidney disease. To this end, we conduct periodically a literature review to identify newly published genes and incorporate them into our bioinformatics panel and we then reanalyze all unsolved ES. As an example, index case 240 displaying TULP3 variants was initially classified as negative. Upon the discovery of TULP3 as a ciliopathy-associated disease gene, we were able to reanalyze all sample displaying rare variants in TULP3 with their phenotype, leading to the reclassification of the original case as positive for ciliopathy [25, 26]. The TULP3 gene was incorporated into our custom kidney gene panel. We have included APOL1 in our gene panel but the current clinical use of APOL1 genotyping has been widely discussed, and there is still no clear agreement among clinicians. Identifying the triggers of APOL1 kidney disease can be challenging. Possible triggers include infection, interferon activation, environmental kidney damage, superimposed lupus, diabetes and arterial hypertension. However, determining the direction of causality between APOL1 kidney disease and diabetes or arterial hypertension is particularly difficult. Given that many biotechnology and academic groups are now looking into potential therapies for APOL1 kidney disease, we have chosen to consider APOL1-related nephropathy as resolved in our study and as suggested by a recent review paper [27].
In our experience, ES avoided renal biopsy in 12 of 23 selected patients (52.2% diagnostic yield rate) at high suspicion of hereditary kidney disease. Alport disease represented the major finding. Renal biopsy is the traditional diagnostic approach, but it may either reveal nonspecific findings or be completely contraindicated. In addition, unlike in the USA or UK, electron microscopy is not readily available in routine care in France, and its use is highly restricted to a few indications. ES represents a non-invasive new tool that should be included in the diagnostic workup of nephropathy, either when biopsy features are nonspecific or when hereditary kidney disease is suspected and renal biopsy has not been helpful [28]. ES could replace renal biopsy as the gold standard in challenging situations such as pregnancy, in patients with atrophic kidney, or in the case of a high suspicion of inherited kidney disease (glomerular presentation and familial history, for example). With expanding indications of genetic tests, registries will be essential for improving our understanding of genetic kidney disease [29].
We observed that among patients with classified nephropathy, almost one-third were phenocopies, with collagenopathies, tubulopathies and ciliopathies being the most common contributors among all nephropathy categories. This proportion is slightly higher than that reported by Riedhammer et al. [30]. ES in patient known as vascular or hypertensive nephropathy based on histological finding could lead to recategorization of the kidney disease and suggest that a diagnosis of unclassifiable UKD would be more accurate before ES. Patients with suspected vascular or hypertensive nephropathy, who are young and have few comorbidities, a family history of kidney disease and/or extrarenal features such as hearing impairment, should be classified as vascular kidney-NOS, a condition similar to autosomal dominant tubulointerstitial kidney disease not otherwise specified (ADTKD-NOS) [31].
The literature reports a positive family history of CKD in 25%–44% of cases in patients with CKD, and familial clustering is a common phenomenon in patients with ESKD, confirming that inherited kidney disease is a common cause of CKD [3, 32–35]. In our study, the segregation of kidney disease in a family was the main predictive factor associated with a high rate of ES resolution in patients with UKD, demonstrating that inherited kidney disease should not be overlooked in the adult population. In clinical nephrology practice, the systematic investigation of family history should be a standard element of the consultation, especially when the renal disease is of undetermined origin. History of kidney disease in a relative can be defined as requiring renal replacement therapy, either with dialysis or kidney transplant, or kidney disease that warrants repeated outpatient follow-up with a nephrology service. Depending on the patient, this may require a thorough survey of both the patient's history and that of their relatives prior to the nephrology consultation. Consanguinity was also associated with a slightly higher likelihood of ES-resolved case rates, confirming what is already known in the literature.
Female sex was also reportedly associated with a positive family history of kidney disease. Being female has a protective effect on the progression of chronic renal disease [36]. Nowadays, X-linked kidney disorders, including Alport syndrome, Fabry disease, nephrogenic diabetes insipidus, X-linked hypophosphatemic rickets, Dent disease, oral facial digital syndrome and Lowe syndrome, are widely recognized [37]. Males have single X chromosome whereas females have two X chromosomes, and thus 100 Mb more DNA than males (X: 155 Mb, Y: 55 Mb), and this leads to a dosage imbalance between males and females for the 1000 X-linked genes. Most of these diseases predominantly affect males and have variable penetrance in female carriers. In our cohort, only two X-linked inherited kidney diseases (COL4A5 and CLCN5) are present and do not explain this difference. X inactivation may contribute to the difference by cellular mosaicism because most of the kidney is formed after the onset of X inactivation. The distribution of the two mosaic populations is expected to be random, following a bell-shaped curve around a mean of 50%. It is possible that women with inherited kidney disease may have very skewed patterns of X inactivation, leading to major expression of the maternal autosomal chromosome.
Early-onset CKD is frequently associated with inherited kidney disease and may even be the leading cause of CKD in children. Interestingly, in our older cohort, early-onset disease was not the characteristic criterion associated with cases resolved by ES, as classically described. Our results indicate that late-onset disease or late age of end-stage renal failure should not negate the value of a genetic test such as ES.
Combining SNV and CNV using an exome-wide CNV detection pipeline raises the diagnostic yield of Mendelian kidney disease in our experience (5/75 patients, 6.7%) without adding any direct laboratory cost. It confirmed the result of previous studies [6]. This requires a bioinformatics team to set up CNV analysis in exome sequencing data-analysis pipelines with method validation by control samples. A careful analysis with updated CNV calling should be considered before performed a whole-genome sequencing in patients with high suspicion of inherited kidney disease.
An important limitation of our study is the analysis of variable number tandem repeat of the MUC1 gene (GC-rich sequence), which is not really feasible despite it being the dominant gene associated with tubulointerstitial disease. In the future, long-read single molecule technology could directly assess many difficult or even previously unsequenceable regions of the genome by ES, such as repetitive elements of the MUC1 gene, non-targeted structural variant breakpoints at base-pair resolution or discrimination of pseudogenes, and this would increase the diagnostic yield in patients with genetic CKD. However, variant interpretation remains a challenge with both short- and long-read technologies.
Most studies of genetic testing in CKD are limited to a research setting. Our study highlighted the importance of opening up genetic testing in routine nephrological care to address the problem of UKD, which accounts for nearly 20% of the CKD population. In addition, the clinical impact is significant because of 73 actionable genes proposed by the ACMG, many of which are associated with phenotypes relevant to nephrology. Furthermore, the most recent KDIGO conference, which centered on the controversies surrounding the topic of genetics in CKD, named additional kidney-specific actionable genes, selected on the basis of the availability of interventions, which could prevent renal morbidity [29].
CONCLUSION
Genetic diagnosis has provided new clinical insights in UKD patients by clarifying or reclassifying kidney disease etiology or informing prognosis, treatment or transplant decisions. The exome first approach can have a high diagnostic yield, avoiding an invasive procedure such as renal biopsy or in cases where a biopsy is not possible. ES appears to be a compelling tool in a routine healthcare setting for patients with UKD.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank CHU de Marseille for its investment in the genomic diagnosis process. The authors thank the affected individuals and their families for agreeing to participate in this study. The authors would also like to thank all nephrologists who referred samples from patients.
Contributor Information
Thomas Robert, Centre of Nephrology and Renal Transplantation, Hôpital de la Conception, CHU de Marseille, Marseille, France; Marseille Medical Genetics, Bioinformatics & Genetics, INSERM U1251, Aix-Marseille Université, Marseille, France.
Laure Raymond, Genetics Department, Laboratoire Eurofins Biomnis, Lyon, France.
Marine Dancer, Genetics Department, Laboratoire Eurofins Biomnis, Lyon, France.
Julia Torrents, Department of Renal Pathology, CHU Timone, AP-HM, Marseille, France.
Noémie Jourde-Chiche, Centre of Nephrology and Renal Transplantation, Hôpital de la Conception, CHU de Marseille, Marseille, France; Aix-Marseille Univ, INSERM, INRAE, C2VN, Marseille, France.
Stéphane Burtey, Centre of Nephrology and Renal Transplantation, Hôpital de la Conception, CHU de Marseille, Marseille, France; Aix-Marseille Univ, INSERM, INRAE, C2VN, Marseille, France.
Christophe Béroud, Marseille Medical Genetics, Bioinformatics & Genetics, INSERM U1251, Aix-Marseille Université, Marseille, France.
Laurent Mesnard, Urgences Néphrologiques et Transplantation Rénale, Sorbonne Université, APHP, Hôpital Tenon, Paris, France.
CONFLICT OF INTEREST STATEMENT
All the authors declared no competing interests.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
(See related article by Torra et al. Replacing a kidney biopsy by exome sequencing in undetermined kidney diseases—not yet ready for prime time! Clin Kidney J (2024) 17: sfad250.)
REFERENCES
- 1. Titze S, Schmid M, Köttgen Aet al. . Disease burden and risk profile in referred patients with moderate chronic kidney disease: composition of the German Chronic Kidney Disease (GCKD) cohort. Nephrol Dial Transplant 2015;30:441–51. 10.1093/ndt/gfu294 [DOI] [PubMed] [Google Scholar]
- 2. Johansen KL, Chertow GM, Foley RNet al. . US Renal Data System 2020 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2021;77:A7–8. 10.1053/j.ajkd.2021.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Connaughton DM, Kennedy C, Shril Set al. . Monogenic causes of chronic kidney disease in adults. Kidney Int 2019;95:914–28. 10.1016/j.kint.2018.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Groopman EE, Marasa M, Cameron-Christie Set al. . Diagnostic utility of exome sequencing for kidney disease. N Engl J Med 2019;380:142–51. 10.1056/NEJMoa1806891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ottlewski I, Münch J, Wagner Tet al. . Value of renal gene panel diagnostics in adults waiting for kidney transplantation due to undetermined end-stage renal disease. Kidney Int 2019;96:222–30. 10.1016/j.kint.2019.01.038 [DOI] [PubMed] [Google Scholar]
- 6. Testard Q, Vanhoye X, Yauy Ket al. . Exome sequencing as a first-tier test for copy number variant detection: retrospective evaluation and prospective screening in 2418 cases. J Med Genet 2022;59:1234–40. 10.1136/jmg-2022-108439 [DOI] [PubMed] [Google Scholar]
- 7. Richards S, Aziz N, Bale Set al. . Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–24. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kopp JB, Nelson GW, Sampath Ket al. . APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 2011;22:2129–37. 10.1681/ASN.2011040388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stenson PD, Mort M, Ball EVet al. . The Human Gene Mutation Database: towards a comprehensive repository of inherited mutation data for medical research, genetic diagnosis and next-generation sequencing studies. Hum Genet 2017;136:665–77. 10.1007/s00439-017-1779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yao T, Udwan K, John Ret al. . Integration of genetic testing and pathology for the diagnosis of adults with FSGS. Clin J Am Soc Nephrol 2019;14:213–23. 10.2215/CJN.08750718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van der Ven AT, Connaughton DM, Ityel Het al. . Whole-exome sequencing identifies causative mutations in families with congenital anomalies of the kidney and urinary tract. J Am Soc Nephrol 2018;29:2348–61. 10.1681/ASN.2017121265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Domingo-Gallego A, Pybus M, Bullich Get al. . Clinical utility of genetic testing in early-onset kidney disease: seven genes are the main players. Nephrol Dial Transplant 2022;37:687–96. 10.1093/ndt/gfab019 [DOI] [PubMed] [Google Scholar]
- 13. Bullich G, Domingo-Gallego A, Vargas Iet al. . A kidney-disease gene panel allows a comprehensive genetic diagnosis of cystic and glomerular inherited kidney diseases. Kidney Int 2018;94:363–71. 10.1016/j.kint.2018.02.027 [DOI] [PubMed] [Google Scholar]
- 14. Mann N, Braun DA, Amann Ket al. . Whole-exome sequencing enables a precision medicine approach for kidney transplant recipients. J Am Soc Nephrol 2019;30:201–15. 10.1681/ASN.2018060575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hill NR, Fatoba ST, Oke JLet al. . Global Prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS One 2016;11:e0158765. 10.1371/journal.pone.0158765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dryja TP. Gene-based approach to human gene-phenotype correlations. Proc Natl Acad Sci USA 1997;94:12117–21. 10.1073/pnas.94.22.12117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith JM, Stablein DM, Munoz Ret al. . Contributions of the transplant registry: the 2006 annual report of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS). Pediatr Transplant 2007;11:366–73. 10.1111/j.1399-3046.2007.00704.x [DOI] [PubMed] [Google Scholar]
- 18. Quinlan C, Rheault MN. Genetic basis of type IV collagen disorders of the kidney. Clin J Am Soc Nephrol 2021;16:1101–9. 10.2215/CJN.19171220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kashtan CE, Ding J, Garosi Get al. . Alport syndrome: a unified classification of genetic disorders of collagen IV α345: a position paper of the Alport Syndrome Classification Working Group. Kidney Int 2018;93:1045–51. 10.1016/j.kint.2017.12.018 [DOI] [PubMed] [Google Scholar]
- 20. Köttgen A, Glazer NL, Dehghan Aet al. . Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet 2009;41:712–7. 10.1038/ng.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Olinger E, Schaeffer C, Kidd Ket al. . An intermediate-effect size variant in. Proc Natl Acad Sci USA 2022;119:e2114734119. 10.1073/pnas.2114734119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gast C, Marinaki A, Arenas-Hernandez Met al. . Autosomal dominant tubulointerstitial kidney disease-UMOD is the most frequent non polycystic genetic kidney disease. BMC Nephrol 2018;19:301. 10.1186/s12882-018-1107-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahram DF, Aggarwal VS, Sanna-Cherchi S, Phenocopies, phenotypic expansion, and coincidental diagnoses: time to abandon targeted gene panels? Am J Kidney Dis 2020;76:451–3. 10.1053/j.ajkd.2020.07.003 [DOI] [PubMed] [Google Scholar]
- 24. Schrezenmeier E, Kremerskothen E, Halleck Fet al. . The underestimated burden of monogenic kidney disease in adults waitlisted for kidney transplantation. Genet Med 2021;23:1219–24. 10.1038/s41436-021-01127-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robert T, Savenkoff B, Legris Tet al. . Exome sequencing reanalysis solved case in undetermined nephropathy with detection of TULP3-truncating variant. Nephrol Dial Transplant 2023;38:1057–60. 10.1093/ndt/gfac319 [DOI] [PubMed] [Google Scholar]
- 26. Devane J, Ott E, Olinger EGet al. . Progressive liver, kidney, and heart degeneration in children and adults affected by TULP3 mutations. Am J Hum Genet 2022;109:928–43. 10.1016/j.ajhg.2022.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Friedman DJ, Pollak MR. APOL1 nephropathy: from genetics to clinical applications. Clin J Am Soc Nephrol 2021;16:294–303. 10.2215/CJN.15161219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kalatharan V, Lemaire M, Lanktree MB. Opportunities and challenges for genetic studies of end-stage renal disease in Canada. Can J Kidney Health Dis 2018;5:205435811878936. 10.1177/2054358118789368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.KDIGO Conference Participants. Genetics in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int 2022;101:1126–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Riedhammer KM, Braunisch MC, Günthner Ret al. . Exome sequencing and identification of phenocopies in patients with clinically presumed hereditary nephropathies. Am J Kidney Dis 2020;76:460–70. 10.1053/j.ajkd.2019.12.008 [DOI] [PubMed] [Google Scholar]
- 31. Eckardt KU, Alper SL, Antignac Cet al. . Autosomal dominant tubulointerstitial kidney disease: diagnosis, classification, and management—A KDIGO consensus report. Kidney Int 2015;88:676–83. 10.1038/ki.2015.28 [DOI] [PubMed] [Google Scholar]
- 32. Shang N, Khan A, Polubriaginof Fet al. . Medical records-based chronic kidney disease phenotype for clinical care and “big data” observational and genetic studies. NPJ Digit Med 2021;4:70. 10.1038/s41746-021-00428-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Akrawi DS, Li X, Sundquist Jet al. . Familial risks of kidney failure in Sweden: a nationwide family study. PLoS One 2014;9:e113353. 10.1371/journal.pone.0113353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Skrunes R, Svarstad E, Reisæter AV. et al. Familial clustering of ESRD in the Norwegian population. Clin J Am Soc Nephrol 2014;9:1692–700. 10.2215/CJN.01680214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Freedman BI, Robinson TW. Risk factors: familial aggregation of ESRD in Europeans-is it in the genes? Nat Rev Nephrol 2014;10:677–8. 10.1038/nrneph.2014.181 [DOI] [PubMed] [Google Scholar]
- 36. Connaughton DM, Bukhari S, Conlon Pet al. . The Irish kidney gene project—prevalence of family history in patients with kidney disease in Ireland. Nephron 2015;130:293–301. 10.1159/000436983 [DOI] [PubMed] [Google Scholar]
- 37. Quinlan C, Rheault MN. X-linked kidney disorders in women. Semin Nephrol 2022;42:114–21. 10.1016/j.semnephrol.2022.04.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.