Rheumatology key message.
CD19 CAR-T cell therapy can induce drug-free remission in a patient with autoimmune disease who has previously failed on the treatment with several B-cell depleting antibodies.
Dear Editor, Antisynthetase syndrome (ASS) is a subset of idiopathic inflammatory myopathy (IMM) that affects the lungs, the skin and the joints next to the muscles [1]. The disease is characterized by autoimmunity against aminoacyl transfer ribonucleic acid (RNA) synthetases. The primary treatment goal in ASS is to dampen disease activity and slow down disease progression. While glucocorticoids are used as primary treatment, immune suppressive drugs, such as mycophenolate and B-cell depleting monoclonal antibodies are used in moderate to severe ASS [2]. However, despite these medications, ASS can be refractory and take a life-threatening course, necessitating the development of new treatment strategies [3].
We report on a 44-year-old Caucasian woman (160 cm, 85 kg) with severe refractory ASS. At diagnosis the patient presented with typical manifestations of ASS, including: myositis [elevated creatinine kinase (CK) levels of 29 133 U/l and positive MRI], polyarthritis, holster sign, V-sign and Gottron papules. Serological tests revealed ANA positivity of 1:10 000 titre (speckled pattern). ENA panel showed high titre of anti-Jo-1, Pm-Scl-100 positivity and high RF IgG levels (156 IE/ml). No muscle biopsy was performed due to the typical manifestations of ASS. The patient experienced refractory active myositis over the last 6 years and multiple immunosuppressive drug treatments including glucocorticoids, cyclophosphamide, mycophenolate, tacrolimus, tofacitinib, tocilizumab and intravenous immunoglobulins were ineffective (Fig. 1A). The patient also failed treatment with two CD20-targeting B-cell depleting monoclonal antibodies (rituximab and ocrelizumab). Also, treatment with the cannabinoid receptor type 2 agonist lenabasum in a clinical study yielded only slight improvements [4]. Overall condition of the patient was reduced with severe muscle weakness and pain, shortness of breath, arthritis with 23 tender and eight swollen joints and active skin involvement. Chest CT revealed bullae and signs of inferior ventilation, lung function test showed distribution disorder and incipient diffusion disorder. CK (4298 U/l; normal value <170 U/l), myoglobin (2945 µg/l; normal value <70 µg/l) and CRP (39.9 mg/l; normal value <5 mg/l) were high.
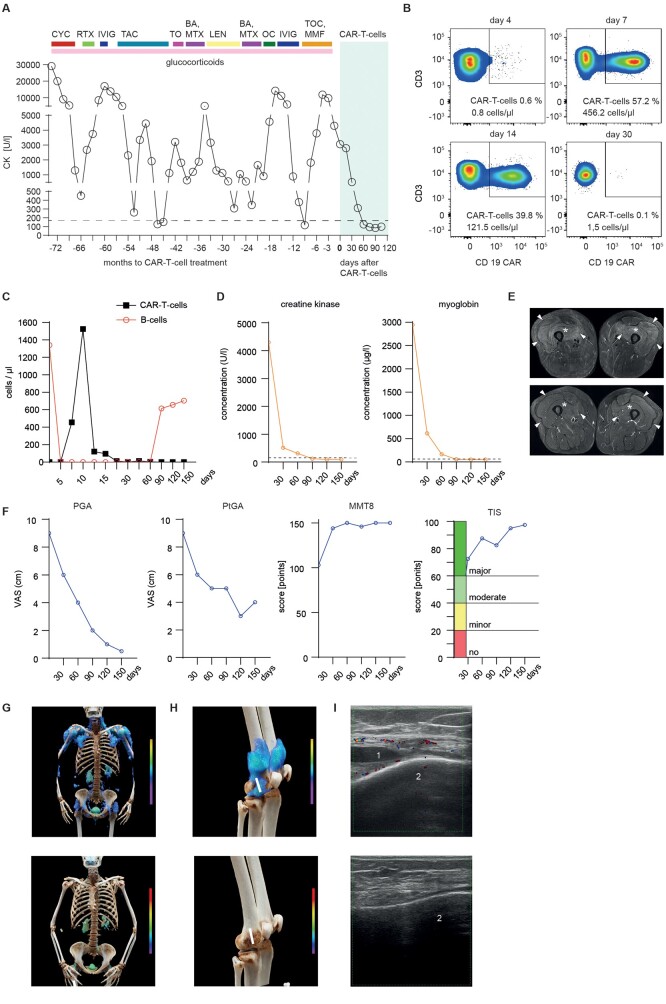
Figure 1.
Effects of CD19 CAR-T cells therapy on the manifestations of antisynthetase syndrome (ASS). (A) Serum creatinine kinase (CK) levels during previous treatment regimens and after treatment with CD19 CAR-T cells. The horizontal dotted line indicates the upper limit of normal range (<170 U/l). (B) Dot plots showing circulating CD3 T cells (upper two quadrants) and CAR-T cells (upper right quadrant), quantified by fluorescence-activated cell sorting on days 4, 7, 14 and 30 after infusion. (C) Concentration of circulating CD19 CAR T cells and CD19+ B cells over time. (D) Concentrations of serum creatinine kinase (CK; left) and myoglobin (right). Dotted line indicates normal level (CK <170 U/l; myoglobin: <70 µg/l); (E) MRI showing treatment effects over the course of 3 months. Top shows axial T2-weighted fat suppressed MRI at baseline with marked oedematous symmetrical intramuscular signal alterations in the quadriceps muscles group on both sides consistent with active myositis, including the M. vastus lateralis (arrowheads), M. vastus medialis (arrow) and M. vastus intermedius (asterisks). Bottom shows follow-up MRI with complete resolution of signal changes and normalization of muscle signal in the same locations. (F) Disease activity assessed by physician global (PGA; left), patient global (PtGA; middle) and manual muscle testing score 8 (MMT8; right). Measurement of response as American College of Rheumatology/European League Against Rheumatism total improvement score (TIS) with cut-offs for major, moderate, minor or no response. (G) Cinematic rendering reformatted upper body FAPI-PET-CT scans showing significant mesenchymal activity in the muscles at baseline that is reduced after CAR-T cells treatment (bottom). (H) Cinematic rendering reformatted FAPI-PET-CT of knees showing a significant reduction in mesenchymal activity in the joints. White line demonstrates ultrasound device positioning. (I) Ultrasound examination of the knee joint showing effusion (1) related to lateral recessus bursitis at the distal femoral condyle (2; top) and its resolution after treatment (bottom). BA: baricitinib; CAR: chimeric antigen receptor; LEN: lenabasum; OC: ocrelizumab; RTX: rituximab; TAC: tacrolimus; TO: tofacitinib; TOC: tocilizumab
Due to the severe course with multiple treatment failures, CAR-T cell treatment was initiated. Glucocorticoids were tapered to 10 mg/day at time of leukapheresis (day – 13) and mycophenolate was stopped 4 weeks before the leukapheresis to ascertain functionality of T cells. Enriched T cells were transfected by a CD19 CAR lentiviral vector (Miltenyi Biotech) and expanded over 12 days [5, 6]. After standard conditioning treatment (1 g/m2 cyclophosphamide and 3 × 25 mg fludarabine) 1 × 106 CAR-T cells/kg body weight were administered by a single i.v. infusion. After transfer, CAR-T cells massively (>1000 fold) expanded (day 4: 0.8 cells/µl; day 7: 456.2 cells/µl, day 10: 1524.2 cells/µl) (Fig. 1B). CAR-T expansion preceded the complete depletion of circulating B cells over a period of 58 days (Fig. 1C). CK dropped from 4298 U/l at baseline to 99 U/l at day 150, myoglobin from 2945 µg/l to 53 µg/l (Fig. 1D) and alanine aminotransferase (ALT) levels from 317 U/l to 37 U/l (norm <35 U/l). MRI of the thighs showed complete resolution of myositis (Fig. 1E). Patient showed remarkable improvement in physical function in all International Myositis Assessment & Clinical Studies Group (IMACS) core set measures and regained muscle strength with a manual muscle test score of 103/150 at baseline to full strength (150/150) at the latest follow-up. This improvement was paralleled by regained muscle endurance. Thus, holding a filled water bottle of 1.3 kg with one outstretched arm improved from 10 s (baseline) to 61 s (day +150), number of repetitions in the 30-s Sit-to-Stand test increased from 0 to 13 repetitions, maximum walking distance increased from 50 m to 2000 m. Overall, the patient showed major improvement according to the 2016 ACR/EULAR total improvement score (TIS) at day +90 (TIS 82.5) and day +150 (TIS 97.5) (Fig. 1F) [7]. At the latest follow-up (+150 days), the patient was still in drug-free (including glucocorticoid-free) remission. Cinematic rendering reformatted FAPI-PET-CT scans at baseline and follow-up showed significant reduction in mesenchymal tissue activation in the muscles and joints. Ultrasound examination of the knee joints showed resolution of arthritis (Fig. 1G–I). With respect to safety, the patient developed mild cytokine release syndrome (Grade I), which resolved after a single infusion of the anti-IL-6R antibody tocilizumab. One week after CAR-T cell administration the patient developed mild dizziness, which was interpreted as potential grade I immune-related effector cell neurotoxicity syndrome (ICANS). Symptoms quickly resolved after a short course of dexamethasone.
These data show that a single infusion of CAR-T cells leads to resolution of muscle, joint and lung inflammation in ASS. Together with the first described case of CAR-T cell therapy in ASS [8], these data suggest that CD19 CAR-T cell therapy provides a possibility to induce sustained drug-free remission of ASS. Remarkably, CAR-T cell therapy rescued this patient despite previous failure of two B-cell depleting antibodies, suggesting that depth of B-cell depletion is far higher with CAR-T cell based than antibody-based B-cell depletion. This notion is particularly supported by the massive proliferation of CD19 CAR-T cells in vivo, indicating recognition of large amounts of B cells in a patient that has received previous ‘depletion’ of B cells with rituximab and ocrelizumab.
CAR-T cell treatment was performed as named patient use. The collection and analysis of data is covered by the ethics vote of the local Ethics Committee of the University of Erlangen (334_18 B). Informed consent was provided for the publication of this manuscript.
Acknowledgements
We thank S. Miltenyi for providing the lentiviral vector, D. Werner and J. Hofer for assistance. For cinematic rendering we thank Dr Klaus Engel, Siemens Healthineers, Erlangen.
Contributor Information
Jule Taubmann, Department of Internal Medicine 3, Rheumatology and Immunology, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen, Erlangen, Germany; Deutsches Zentrum Immuntherapie, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen, Erlangen, Germany.
Johannes Knitza, Department of Internal Medicine 3, Rheumatology and Immunology, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen, Erlangen, Germany; Deutsches Zentrum Immuntherapie, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen, Erlangen, Germany.
Fabian Müller, Deutsches Zentrum Immuntherapie, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen, Erlangen, Germany; Department of Internal Medicine 5- Hematology and Oncology, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen, Erlangen, Germany.
Simon Völkl, Department of Internal Medicine 5- Hematology and Oncology, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen, Erlangen, Germany.
Michael Aigner, Department of Internal Medicine 5- Hematology and Oncology, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen, Erlangen, Germany.
Arnd Kleyer, Department of Internal Medicine 3, Rheumatology and Immunology, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen, Erlangen, Germany; Deutsches Zentrum Immuntherapie, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen, Erlangen, Germany.
Regina Gary, Department of Internal Medicine 5- Hematology and Oncology, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen, Erlangen, Germany.
Sascha Kretschmann, Department of Internal Medicine 5- Hematology and Oncology, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen, Erlangen, Germany.
Sebastian Boeltz, Department of Internal Medicine 3, Rheumatology and Immunology, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen, Erlangen, Germany; Deutsches Zentrum Immuntherapie, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen, Erlangen, Germany.
Armin Atzinger, Department Nuclear Medicine, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen, Erlangen, Germany.
Torsten Kuwert, Department Nuclear Medicine, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen, Erlangen, Germany.
Frank Roemer, Institute of Radiology, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen, Erlangen, Germany.
Michael Uder, Institute of Radiology, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen, Erlangen, Germany.
Andreas Mackensen, Deutsches Zentrum Immuntherapie, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen, Erlangen, Germany; Department of Internal Medicine 5- Hematology and Oncology, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen, Erlangen, Germany.
Georg Schett, Department of Internal Medicine 3, Rheumatology and Immunology, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen, Erlangen, Germany; Deutsches Zentrum Immuntherapie, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen, Erlangen, Germany.
Data availability
Data will be available upon request (georg.schett@uk-erlangen.de).
Funding
The study was supported by the Deutsche Forschungsgemeinschaft (FOR2886, CRC1181 and TRR221), the Bundesministerium für Bildung und Forschung (BMBF; MASCARA), the European Union (ERC Synergy grant 4D Nanoscope, ERC Consolidator grant INSPIRE) and the IMI funded project RTCure.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Lundberg IE, Fujimoto M, Vencovsky J. et al. Idiopathic inflammatory myopathies. Nat Rev Dis Primers 2021;7:86. [DOI] [PubMed] [Google Scholar]
- 2. Oddis CV, Aggarwal R.. Treatment in myositis. Nat Rev Rheumatol 2018;14:279–89. [DOI] [PubMed] [Google Scholar]
- 3. Cavagna L, Trallero-Araguás E, Meloni F. et al. Influence of antisynthetase antibodies specificities on antisynthetase syndrome clinical spectrum time course. J Clin Med 2019;8:2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Werth VP, Hejazi E, Pena SM. et al. Safety and efficacy of lenabasum, a cannabinoid receptor type 2 agonist, in patients with dermatomyositis with refractory skin disease: a randomized clinical trial. J Invest Dermatol 2022;142:2651–9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mackensen A, Müller F, Mougiakakos D. et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat Med 2022;28:2124–32. [DOI] [PubMed] [Google Scholar]
- 6. Mougiakakos D, Krönke G, Völkl S. et al. CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N Engl J Med 2021;385:567–9. [DOI] [PubMed] [Google Scholar]
- 7. Saygin D, Kim H, Douglas C. et al. Performance of the 2016 ACR-EULAR myositis response criteria in adult dermatomyositis/polymyositis therapeutic trials and consensus profiles. Rheumatology (Oxford) 2023;kead110. https://10.1093/rheumatology/kead110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bergmann C, Müller F, Jörg D. et al. Treatment of a patient with severe systemic sclerosis (SSc) using CD19-targeted CAR T cells. Ann Rheum Dis 2023;82:1621.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available upon request (georg.schett@uk-erlangen.de).