Abstract
Primary aldosteronism (PA) is the most common cause of secondary hypertension and is associated with increased morbidity and mortality when compared with blood pressure–matched cases of primary hypertension. Current limitations in patient care stem from delayed recognition of the condition, limited access to key diagnostic procedures, and lack of a definitive therapy option for nonsurgical candidates. However, several recent advances have the potential to address these barriers to optimal care. From a diagnostic perspective, machine-learning algorithms have shown promise in the prediction of PA subtypes, while the development of noninvasive alternatives to adrenal vein sampling (including molecular positron emission tomography imaging) has made accurate localization of functioning adrenal nodules possible. In parallel, more selective approaches to targeting the causative aldosterone-producing adrenal adenoma/nodule (APA/APN) have emerged with the advent of partial adrenalectomy or precision ablation. Additionally, the development of novel pharmacological agents may help to mitigate off-target effects of aldosterone and improve clinical efficacy and outcomes. Here, we consider how each of these innovations might change our approach to the patient with PA, to allow more tailored investigation and treatment plans, with corresponding improvement in clinical outcomes and resource utilization, for this highly prevalent disorder.
Keywords: functional/molecular imaging, adrenal vein sampling, machine learning, metabolomics, ablation, adrenal sparing surgery, partial adrenalectomy
Graphical Abstract
Essential Points.
Despite being a common cause of hypertension, many cases of primary aldosteronism (PA) remain undiagnosed or are only recognized at a late stage when treatment benefits are diminished; challenges in screening and confirmation of diagnosis remain significant hurdles to effective management
Recent advances in metabolomics and machine learning have the potential to increase the diagnosis of PA by identifying patients who should be considered for screening and, at the same time, aiding clinical decision making through prediction of disease subtype
Currently, only a small proportion of patients with PA progress to adrenal surgery, reflecting the challenges inherent in securing a diagnosis of unilateral disease; for others, pharmacotherapy with mineralocorticoid receptor antagonists (MRAs) is advised, but is not always well tolerated or efficacious
Recent advances in diagnostic techniques, including segmental adrenal vein sampling and molecular (functional) imaging (eg, positron emission tomography/computed tomography) have allowed the focus of attention to switch from simple adrenal lateralization to more accurate tumor(s) localization
The ability to localize aldosterone producing adenomas/nodules ultimately paves the way for the use of more focal treatment options (including partial adrenalectomy or adrenal adenoma/nodule ablation), which may in turn open up avenues for intervention in patients with previously deemed inoperable disease (including bilateral PA)
More selective pharmacological agents, including nonsteroidal MRAs, aldosterone synthase inhibitors, and molecules that target calcium signaling are under investigation to permit pharmacotherapy which more effectively alleviates aldosterone excess with fewer adverse effects
The field of research in PA requires high-quality, prospective evidence to progress research advances to clinical care
Introduction: Current Approaches and Challenges in the Diagnosis and Management of Primary Aldosteronism
Primary Aldosteronism: A Common but Under-recognized Cause of Hypertension
Hypertension is the single greatest cause of cardiovascular morbidity and mortality, affecting >1 billion adults worldwide and up to 30% of certain adult populations (1). While primary hypertension (where no clear cause is identified) accounts for the majority (70-85%) of cases, some 15% to 30% of patients have an identifiable endocrine or renal cause (secondary hypertension) (2, 3). Primary aldosteronism (PA) represents the commonest secondary cause of hypertension, with an estimated prevalence between 3.2% and 14% among primary care populations (4-8), increasing to 10% to 20% in the hospital outpatient setting (5, 6, 9). Significant challenges exist in screening and diagnosing PA and therefore current estimates of prevalence may be modest in the overall context of disease (10-12). Despite its commonality as a secondary cause of hypertension, PA has not been a key consideration in the design of over 40 randomized control trials which have investigated the management of hypertension over the past 30 years. Therefore, screening rates for PA remain low, clinical recognition is poor, and current hypertension guidelines do not emphasize the need to investigate for PA in patients presenting with hypertension to their primary care physicians or general internists.
PA arises from excessive, unregulated secretion of the steroid hormone aldosterone. This is most commonly attributable to an aldosterone-producing adenoma (APA), or other neoplasia/hyperplasia of aldosterone-producing cells within the zona glomerulosa (ZG) of the adrenal cortex, with recent consensus reached for nomenclature to describe the underlying histopathological findings (Table 1) (13).
Table 1.
Summary of the HISTALDO (histopathology of primary aldosteronism) consensus nomenclature
| Histopathological finding | Features | Notes |
|---|---|---|
| Aldosterone producing adenoma (APA) | CYP11B2 positive solitary neoplasm ≥10 mm in diameter | Nonfunctioning adenomas may be of similar size/morphology under H&E staining. Hence, differentiation requires a combination of clinical assessment (eg, through the demonstration of ipsilateral aldosterone hypersecretion on adrenal vein sampling) and histologic analysis using CYP11B2 immunostaining with a validated CYP11B2 antibody. APAs exhibit positive CYP11B2 staining, which is either homogenously distributed, or of a diffusely heterogeneous pattern throughout the neoplasm. |
| Aldosterone producing nodule (APN) | CYP11B2 positive lesion <10 mm in diameter | APNs are smaller in size than APAs and are morphologically visible using H&E staining. There is often a polarity of CYP11B2 staining within the nodule, which decreases in intensity from the outer to inner part of the lesion. |
| Aldosterone producing micronodule (APM): formally known as “aldosterone-producing cell cluster” | CYP11B2 positive lesion <10 mm in diameter confined to the outer margin of the subcapsular ZG | APMs do not differ in morphology from surrounding ZG on H&E staining. There is often a polarity of CYP11B2 staining within the nodule, which decreases in intensity from the outer to inner part of the lesion. |
| Multiple aldosterone-producing nodules (MAPNs) or multiple aldosterone-producing micronodules (MAPM): formally known as “micronodular hyperplasia” | Multiple CPY11B2 positive lesions within the same adrenal that coexist with regions of normal ZG | Both MAPN and MAPM can exist within the same adrenal. |
| Aldosterone-producing diffuse hyperplasia (APDH) | The presence of a broad, uninterrupted strip of hyperplastic ZG cells with CPY11B2 positive immunostaining in >50% of cells | The term “aldosterone-producing diffuse hyperplasia” should be applied irrespective of the presence of APN in the same adrenal. |
Table adapted with permission from Williams et al JCEM, 2021; 106(1): 42-54. © The Endocrine Society (13).
Abbreviations: CYP11B2, aldosterone synthase, H&E, hematoxylin & eosin staining; ZG, zona glomerulosa.
Clinically, PA results in stage/grade I to III hypertension in >97% of patients, and spontaneous or drug induced hypokalemia in approximately 50% of cases with an apparent APA (6). PA is associated with higher cardiovascular morbidity than age- and blood pressure (BP)–matched primary hypertension (6, 14-22). This has been demonstrated consistently across several observational studies and confirmed in a recent meta-analysis (15). Individuals with PA demonstrate higher risk of atrial fibrillation (odds ratio 3.52), stroke (2.58), coronary artery disease (1.77), heart failure (2.05), diabetes (1.33), metabolic syndrome (1.53), and left ventricular hypertrophy (2.29) than patients with primary hypertension (15). Elevated aldosterone levels are associated with increased risk of cardiovascular events and mortality, especially in cases where aldosterone levels misalign with renin levels and sodium intake (20, 23-27). In this regard, Hundemer and colleagues have highlighted that renin de-suppression (to within the reference range) in addition to BP control, either by medical therapy or adrenalectomy, is necessary to reduce excess risk to levels seen in those with primary hypertension (28-32). Therefore, it is desirable in PA to target both BP and biochemical outcomes (ie, renin desuppression).
Current challenges in screening
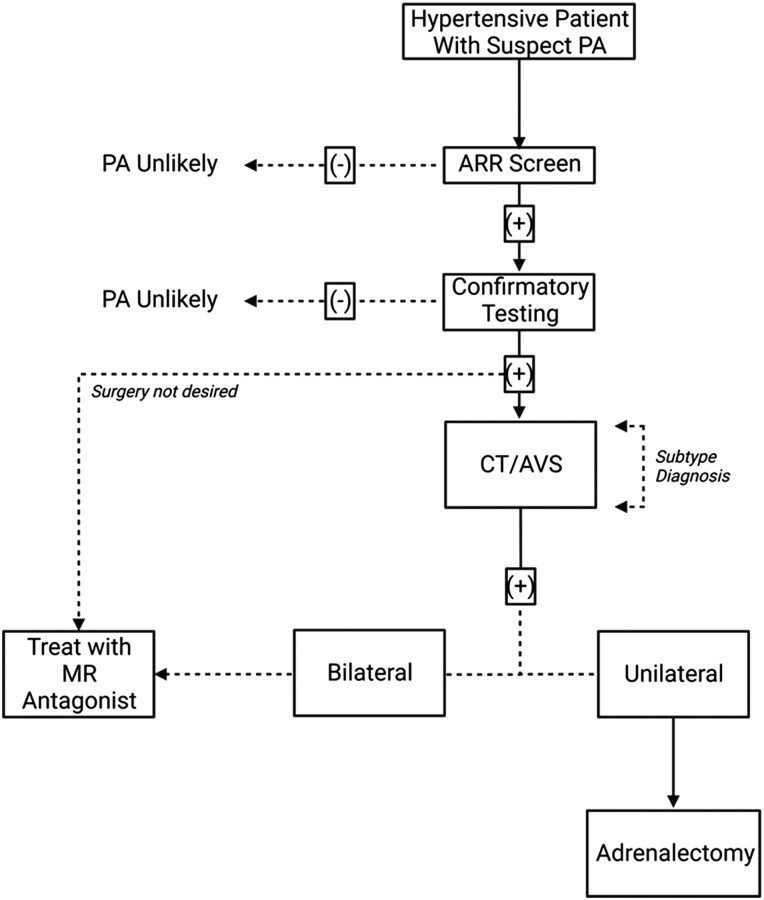
PA is underdiagnosed and undertreated in current clinical practice, where screening and diagnosis are the principal challenges that limit effective management (33-35) (traditional diagnosis/treatment workflow for PA highlighted in Figs. 1 and Figs. 2). Overall, there is no consensus on recommended screening for PA in hypertensive individuals, within either primary or secondary care settings. The Endocrine Society guidelines for the diagnosis and management of PA recommend screening up to 50% of “at risk” patients for PA. Screening under these recommendations should be carried out using the aldosterone–renin ratio (ARR) with particular attention paid to those with (1) severe hypertension; (2) hypertension with spontaneous or diuretic-induced hypokalemia; (3) an adrenal mass; (4) sleep apnea; (5) a family history of early-onset hypertension; (6) stroke at a young age; or (7) a first-degree relative who has PA (36). American Heart Association guidelines for the diagnosis and management of hypertension, align with the Endocrine Society guidelines in terms of recognized risk factors which should trigger screening for PA; in contrast, European Society of Cardiology/European Society of Hypertension guidance do not emphasize the need to screen for PA except in patients with treatment-resistant hypertension (approximately 5% of all patients) (37, 38). Some national guidelines for the management of hypertension, such as those of the National Institute for Health and Care Excellence in the UK, do not specifically reference screening for PA (39, 40).
Figure 1.
The traditional approach to the diagnosis and management of patients with PA: Initial diagnosis is determined by a positive aldosterone–renin ratio (ARR) screen with at least 1 positive confirmatory test (discussed in “Screening, Diagnosis, and the Spectrum of Disease”). Following diagnosis, subtype diagnosis (ie, lateralization) is sought through use of adrenal imaging/adrenal vein sampling (AVS) (discussed in “Current Approach to Lateralization”). Unilateral disease is commonly treated with adrenalectomy of the diseased adrenal whereas in cases of bilateral disease, mineralocorticoid receptor (MR) antagonists are commonly prescribed (discussed in “Advances in Pharmacotherapy”).
Figure 2.
Outline of current “roadblocks” in primary aldosteronism (PA) care, with suggested approaches to addressing these, including: 1. Improved screening for, and diagnosis of PA through application of metabolomics and machine learning; 2. Utilization of advanced lateralization techniques including molecular (functional) imaging to permit precise tumor localization; 3. Use of focal adrenal-sparing interventions (eg, adrenal-sparing surgery or thermal ablation) where appropriate; 4. Continued development of more selective pharmacological agents. Figure created with BioRender.com.
Overall, screening rates for PA are low. In spite of Endocrine Society and American Heart Association guidelines, a population-based retrospective cohort study in Canada reported a screening rate of only 3.9% of all patients with hypokalemia (K+ <3.0 mEq/L) and hypertension, and 1% of patients who were on 4 or more antihypertensive agents. Screening was higher for patients attending a cardiologist, endocrinologist, or nephrologist (41). European data, collected from patient cohorts in Germany and Italy where there is higher awareness of PA, still observed screening rates within a primary care setting that were <8% (36, 42, 43). These findings are important because the diagnosis and management of hypertension is mostly undertaken in primary care or by generalists and it is important therefore to understand the roadblocks to screening among these professionals (Fig. 2).
Typically, low screening rates for PA are matched with less awareness of specific guidelines for its diagnosis and management (such as those of the Endocrine Society (36)), and a greater reliance on more general hypertension guidelines (which are frequently more treat to target oriented). In addition, screening for PA in a primary care setting may be particularly challenging (eg, when there is restricted access to aldosterone and renin assays) (5, 42, 44). Even where available, screening is often not routinely deployed (42, 44). The reasons for this are likely multifactorial and include clinician and/or patient reluctance to countenance withdrawal of potential confounding antihypertensive agents (summarized in Table 2) because of the fear of loss of BP control, coupled with a lack of familiarity with alternative noninterfering medications. These “roadblocks” were highlighted in a qualitative study that probed the experience of 16 general practitioners in screening for PA where knowledge gaps, practical limitations of performing the ARR, and errors in diagnostic reasoning were the main challenges associated with routine PA screening (44).
Table 2.
List of antihypertensive interfering medications
| Medications | Aldosterone levels | Renin Levels | Effect on ARR |
|---|---|---|---|
| Beta-adrenergic blockers | ↓ | ↓↓ | ↑ (FP) |
| Central alpha-2 agonists | ↓ | ↓↓ | ↑ (FP) |
| NSAIDs | ↓ | ↓↓ | ↑ (FP) |
| Dihydropyridines | →↓ | →↑ | ↓ (FN) |
| K+ -wasting diuretics | →↑ | ↑↑ | ↓ (FN) |
| K+ -sparing diuretics | ↑ | ↑↑ | ↓ (FN) |
| ACE inhibitors | ↓ | ↑↑ | ↓ (FN) |
| ARBs | ↓ | ↑↑ | ↓ (FN) |
| Renin inhibitors | ↓ | ↓*↑ | ↑(FP)* OR ↓(FN)* |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II type 1 receptor blocker; ARR, aldosterone–renin ratio; FN, false negative; FP, false positive; K+, potassium; NSAID, nonsteroidal anti-inflammatory drug; ↓, reduces; ↓↓, significantly reduces; ↑, increases; ↑↑, significantly increases; →, no effect; ↓↑, may reduce or increase; → ↑, no effect, or increases.
*Renin inhibitors lower plasma renin activity (PRA) but raise plasmas renin concentrations (PRC). This would be expected to yield false positive ARR levels for renin measured as PRA and false negatives for renin measured as DRC.
Table adapted from Shidlovski et al., 2019 with minor modifications in compliance with the attribution 4.0 international (CC BY 4.0) guidelines (45).
As a result of low screening and a lack of awareness of PA, patients are often hypertensive for many years (eg, 5-20) before BP that is suboptimally controlled on multiple agents triggers the necessary investigations to permit the diagnosis (46). While PA is both a curable and treatable form of hypertension, the effectiveness of targeted intervention may be significantly attenuated by a late diagnosis. Prolonged hypertension induces permanent vascular remodeling and, in turn, persistent, ongoing hypertension following definitive treatment of PA (9, 47-49). Therefore, to improve the outcomes of PA at a population level, greater focus on removing the current “roadblocks” that exist in primary and secondary care is necessary to deliver increased rates of screening and diagnosis (Fig. 2). One such approach is to actively increase awareness of PA in primary care, and among generalists and cardiologists. The potential benefits of this approach have been highlighted in a prospective study within a primary care setting by Libianto and colleagues, where a 30-minute education session was provided to primary care physicians, across 31 practices, to encourage screening for PA in newly diagnosed, treatment naïve cases of hypertension (8). Of 247 treatment naïve patients who were screened for PA, 25% had an elevated ARR, with 14% subsequently confirmed to have PA by saline suppression test (8).
Figure 3.
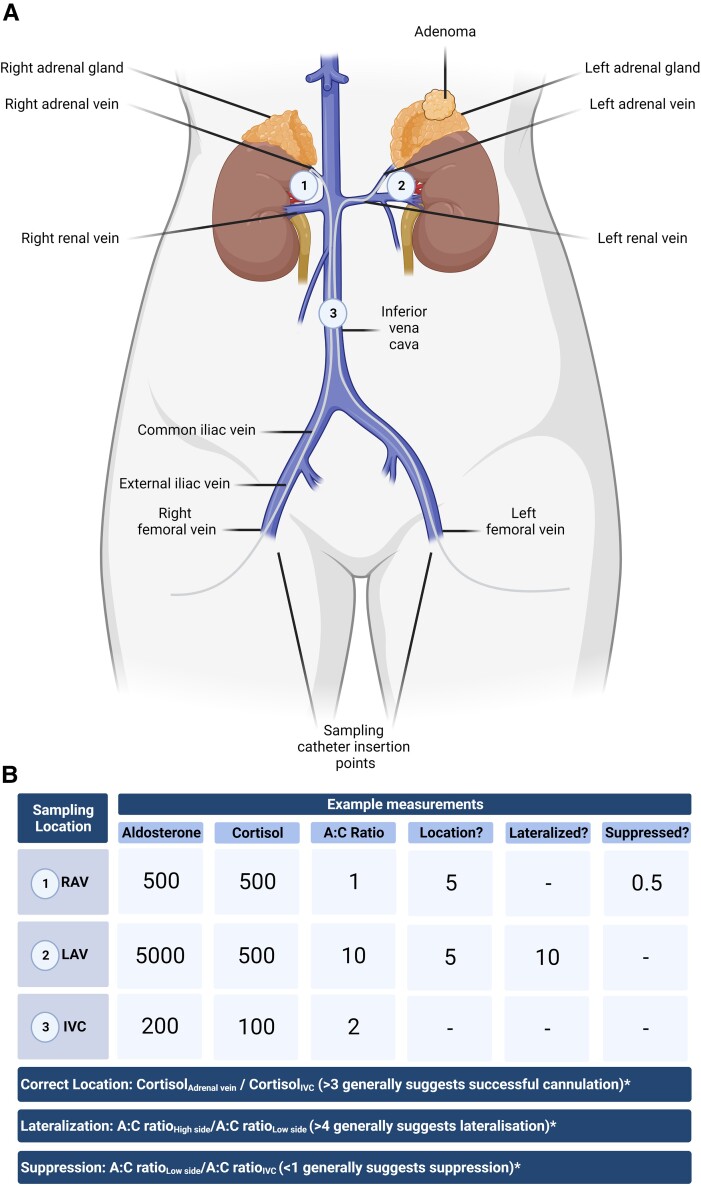
(A) Outline of the adrenal vein sampling (AVS) procedure. A sampling catheter is inserted in either the left or right femoral vein for sampling the left and right adrenal vein in the presence or absence of cosyntropin stimulation. Aldosterone and cortisol are sampled from locations 1-3 and, for each location, the aldosterone to cortisol ratio is calculated. Together, these values are used to determine: (1) Selectivity index: Did sampling occur from the correct location, ie, right adrenal vein (RAV), left adrenal vein (LAV), and inferior vena cava (IVC)? (2) Lateralization: Are 1 or both adrenals the source of aldosterone excess? (3) Is contralateral suppression present? (B) Example measurements demonstrating successful cannulation of both adrenal glands (location) under basal, unstimulated conditions (both locations return a selectivity index >3), with lateralization to the left adrenal and the presence of contralateral suppression. *Note, the cut-offs described in this diagram are arbitrarily defined for illustrative purposes. As is indicated by Quencher and colleagues 2021 there are marked procedural and cut-off heterogeneity between centers (64). Figure created with Biorender.com.
Screening, diagnosis, and the spectrum of disease
Current screening for PA relies on simultaneous measurement of plasma aldosterone concentration and plasma (direct) renin concentration or plasma renin activity, which allow calculation of the ARR. In all but a handful of cases (eg, young age, with marked aldosteronism [hypertension and hypokalemia] and complete renin suppression), a positive ARR screen is followed by confirmatory dynamic testing: (1) saline infusion test (SIT), (2) oral salt suppression test, (3) captopril challenge test, or (4) fludrocortisone suppression test (Fig. 1) (36).
Although this approach is generally advocated by specialists in the field, there remains considerable heterogeneity with respect to the diagnostic thresholds employed for each of these tests. Furthermore, in recent years an “expanding spectrum of primary aldosteronism” has been acknowledged such that PA may not be readily diagnosed or distinguished on the basis of rigid diagnostic cut-offs alone (50, 51). For instance, Vaidya et al, describe a spectrum of disease ranging from clinically overt PA (patients with severe and/or resistant hypertension) that is readily detectable using current thresholds for biochemical confirmation, to unrecognized yet biochemically overt PA (in patients with normotension or mild/moderate hypertension). These findings suggest the existence of “subclinical” or “nonclassical” categories of PA (normotensive patients with autonomous aldosterone secretion) who are likely to go unnoticed because they do not meet current screening indications for PA (50). Expert consensus on a broader classification of PA has yet to be reached, and defining the full spectrum of disease is challenging given the limitations of current clinical testing (discussed further in “Metabolomics and Machine Learning”).
Notwithstanding ongoing work to more comprehensively define this spectrum, current clinical practice still relies on the use of screening and diagnostic thresholds or “cut-offs” to biochemically confirm or exclude the condition (36, 50, 52, 53). However, even here, there can be significant variation between centers reflecting differing background reference populations, laboratory assay architecture (eg, activity vs direct renin measurement; chemiluminescence assay vs liquid chromatography mass spectrometry [LC-MS/MS]; assay performance) (54-56). Reference ranges for aldosterone and renin also differ between the sexes, and in women according to the stage of the reproductive cycle (54, 57-60).
Opinion differs in relation to optimum conditions for ARR screening. Many advocate screening only in the absence of interfering medications (58-60), while others recommend that initial screening be undertaken irrespective of antihypertensive management, and the results interpreted in the context of the expected antihypertensive interference (46, 61). Endocrine Society guidelines recommend (where feasible) that thiazide diuretics, beta-blockers and mineralocorticoid receptor antagonists (MRAs) should be withdrawn prior to screening for PA (36). While there is consensus that all individuals should be normokalemic at the time of ARR determination, there is no clear guidance around salt intake prior to screening for PA. In this regard, dietary sodium intake has been shown to significantly affect plasma renin measurements (62). Postural measurement of the ARR is no longer commonly undertaken and current practice is to sample midmorning, seated for 15 minutes, and following a period of at least 2 hours of ambulation (36, 58).
Irrespective of diagnostic thresholds and/or sampling conditions, interpretation of the ARR is also challenged by the lack of consensus as to whether or not the ARR threshold alone is sufficient to trigger further testing, or if the ARR threshold should be accompanied by a minimum aldosterone cut-off. In this context the performance of the ARR as a screening test varies significantly. For example, in one cohort of patients with hypertension the use of conjunctive standards to define a “positive” screen (ie, ARR >30 ng/dL per ng/ml/h and aldosterone >10 ng/dL) identified 13.9% (232/1672) with possible PA, of whom 99 (ie, 5.9% of the total cohort), were subsequently confirmed to have PA (34). In another cohort of patients with hypertension who were receiving a high dietary sodium intake, the use of an ARR threshold of >20 ng/dL per ng/ml/h, without the requirement to exceed a minimum aldosterone threshold, returned a positive screening rate of 32.7% (79/241), which in turn yielded a higher rate of actual cases (19%, 48/241) following confirmatory testing (62). It is important to note however, that this difference could, at least in part, reflect selection bias whereby only patients considered “at risk” for PA were screened. This differs from other work (34) which carried out universal screening on a hypertensive population. Additional work is therefore still necessary to clarify whether or not ARR threshold alone vs combined aldosterone and ARR thresholds should be employed in PA screening.
Similarly, the gold standard status of confirmatory testing has recently been called in to question in a comprehensive meta-analysis (55 studies; 7357 patients) of the 4 commonly used investigations (SIT, salt-loading test, fludrocortisone suppression test, and captopril challenge test) (63). In analyses of the recumbent SIT (26 studies), seated SIT (4 studies), oral salt-loading test (2 studies), fludrocortisone suppression test (7 studies), and captopril challenge test (25 studies) the authors concluded that overall there was a low standard of evidence to support the use of current confirmatory testing in PA. Specifically, the majority of studies demonstrated significant verification and spectrum bias, which led to overestimation of test accuracy (by 5- to 7-fold) with an excess of missed cases, such that many patients were overlooked for treatment. Of particular concern was the finding that verification of the true diagnosis of PA varied across the study spectrum with inconsistent reference standards. Acknowledging the limitations highlighted within this metanalysis, the fludrocortisone challenge test demonstrated the best performance as a confirmatory test, followed by the recumbent SIT and the captopril challenge test.
In summary, a high-quality, common gold standard to calibrate diagnostic testing is currently absent in the field of PA and this in turn challenges the estimation of PA prevalence in general. It is clearly important that consensus is reached among the expert community as to what constitutes (1) a positive screen to trigger confirmatory testing for PA and (2) a confirmed diagnosis of PA. Ideally validation of a best diagnostic approach would be carried out through multicenter, prospective studies, using an agreed diagnostic standard. However, while a diagnostic standard is more easily established for unilateral PA on the basis of surgical outcomes, deciding upon a comparable standard for bilateral disease is more challenging.
Current approach to lateralization
Lateralization (ie, discrimination between left and right adrenal causes of PA) is commonly assessed as part of the diagnostic work-up of PA (Fig. 1); however, challenges remain in more precisely localizing the site(s) of functioning lesion(s) responsible for unregulated aldosterone secretion. Current guidelines recommend that the invasive procedure adrenal vein sampling (AVS) is performed in the majority of patients who are being considered for surgery, to discriminate unilateral and bilateral causes of PA, and to identify the affected adrenal in unilateral PA (UPA) (AVS procedure outlined in Fig. 3) (36). AVS is therefore considered the gold standard for lateralization, and the use of cross-sectional (anatomical) imaging (eg, computed tomography [CT]) alone is not generally advised (discussed in more detail below). Yet, conventional AVS does not specifically localize disease within the adrenal, but instead lateralizes to the entire gland.
Several studies have compared CT and AVS in the lateralization of PA and the majority of these studies have reported CT to be an inferior lateralization modality. A meta-analysis of studies which compared the performance of cross-sectional imaging (CT/magnetic resonance imaging [MRI]) against an AVS reference standard demonstrated an overall pooled specificity for CT/MRI of 57% in identifying a unilateral APA (65). This meta-analysis included 25 studies, carried out between 2000 and 2020, and involving 4669 patients. In age-related subanalysis of individuals aged <40 years, specificity was 79%. The authors concluded that cross-sectional imaging was not a reliable lateralization modality, even in younger patients, where 21% would have been inappropriately recommended for adrenalectomy. However, there was significant variation in study design, and in the reference standards used for the diagnosis of PA and AVS lateralization across the studies. Additionally, AVS was used as the lateralization reference standard rather than clinical outcomes (eg, response to adrenalectomy). Clinical outcome is difficult to report in PA as this approach usually selects outcomes for UPA only, given that this cohort most commonly undergo adrenalectomy. There is no consensus on a defined clinical outcome, linked to confirmation of disease, for medical therapy in the setting of bilateral PA.
Few large studies have used clinical outcomes to address the question of lateralization using cross-sectional imaging vs AVS for APA lateralization (66, 67). One report retrospectively analyzed 158 patients who underwent AVS (before and after cosyntropin administration) and CT (66). Within this study, CT lateralization agreed with AVS in only 51% of patients, and CT imaging alone was deemed to have significantly overestimated the presence of a unilateral adenoma (CT:114/158 vs AVS:55/158). However, only those who lateralized using AVS (after cosyntropin) underwent surgery (55 patients in total) and therefore, while clinical outcomes are reported in this study, the study design primarily uses AVS lateralization rather than clinical outcomes as the standard for intervention per se. In that regard, the study cannot be described as directly comparing AVS and CT as lateralization modalities, but rather as comparing the performance of CT with that of AVS. Within this study, CT was used to guide partial adrenalectomy of a clearly identified nodule in 3 patients who also lateralized using AVS. However, in 2 of these patients there was persistence of PA due to another lesion in the ipsilateral adrenal, not identified on CT. Additionally, immunohistochemistry with CYP11B2 staining demonstrated that the radiologically identified nodules were not always the culprit lesions for PA, but rather smaller adjacent lesions were responsible, which had not been identified using CT alone. Given the weight of the combined evidence which questioned the performance of CT, the authors justifiably recommended against using CT as a lateralization modality on the basis of these findings (66).
To date, only a single prospective study (SPARTACUS) has directly compared the lateralization of APAs with CT vs AVS, using clinical outcomes as the reference standard in individuals with an adrenal nodule and a confirmed diagnosis of PA (68). In this study the clinical treatment outcomes (intensity of drug treatment to achieve target BP; biochemical outcome in adrenalectomized patients) did not differ after 1 year of follow-up when comparing CT vs AVS findings (68). On the basis of these findings, the authors suggested that lateralization of PA in the presence of an adrenal nodule was equivalent for AVS and CT. However, while prospective, significant concerns have been raised in relation to the trial design, most notably with respect to selection bias, choice of endpoint criteria, and the potential for being underpowered within the adrenalectomy group (69, 70). Therefore, commentary and concern regarding the study design for SPARTACUS has limited the application of its recommendations in the clinical setting. Consequently, CT-guided lateralization of UPA does not currently represent the best standard of practice, with AVS representing the favored gold standard (36). However, in a similar manner to confirmatory testing, the evidence to support AVS as a true gold standard in the lateralization of PA is open to challenge. For instance, while its positive predictive value is well described in the literature, there is a lack of data to describe its negative predictive value; in essence, for patients deemed not suitable for, or who do not lateralize on AVS, a validated, alternative lateralization or localization modality has been notably absent to this point. Consensus is also lacking in relation to the methodological approach and cut-offs for AVS. We discuss the role of AVS and CT, as well as the rise and utility of molecular imaging modalities in the lateralization and localization of PA in further detail within “Advances in Lateralization and Localization.”
Current management and outcomes
With early diagnosis, adrenalectomy is potentially curative in unilateral disease. Current management of bilateral disease relies on medical therapy, with MRAs taking primacy (Fig. 1).
There are 6 consensus outcomes of surgical intervention that are described in the Primary Aldosteronism Surgery Outcome (PASO) study (summarized in Table 3) (49). Using these criteria, adrenalectomy has been reported to deliver complete biochemical success and complete clinical success in 94% and 37% of patients, respectively, ratifying accepted recommendations for adrenalectomy as the first-line therapy in the treatment of UPA (36, 49). Laparoscopic or retroperitoneoscopic adrenalectomy is preferred in most centers, being less invasive than open surgery (36, 71). In particular, duration of anesthesia, recovery period, and length of hospitalization are significantly lower for laparoscopic approaches. Varying degrees of postoperative adrenal insufficiency have been reported in the literature following unilateral adrenalectomy. However, postoperative adrenal insufficiency following treatment of UPA is typically partial and while it has been described in up to 27% of patients, it is almost always subclinical in nature and transient (72-76). Accordingly, patients seldom require regular glucocorticoid replacement but may require cover for intercurrent illness/emergencies as appropriate. Hypoaldosteronism, requiring fludrocortisone replacement therapy has been reported following unilateral adrenalectomy in more severe cases of PA (particularly in cases where there is evidence of significant contralateral gland suppression at AVS) (77-79). Again, it is potentially reversible.
Table 3.
The international standardized outcome criteria following unilateral adrenalectomy described according to the Primary Aldosteronism Surgical Outcome (PASO) study (49)
| Clinical outcome | |
|---|---|
| Complete clinical success | Normal blood pressurea, without the aid of antihypertensive medications. |
| Partial clinical success | The same blood pressure as before surgery, with less antihypertensive medication or a reduction in blood pressure with either the same amount or less antihypertensive medication. |
| Absent clinical success | Unchanged or increased blood pressure, with either the same amount or an increase in antihypertensive medication. |
| Biochemical outcome | |
| Complete biochemical success | Correction of hypokalemiab (if present pre-surgery) and normalization of the aldosterone-to-renin ratioc; in patients with a raised aldosterone to renin ratio postsurgery, aldosterone secretion should be suppressed in a confirmatory test. |
| Partial biochemical success | Correction of hypokalemiab (if present pre-surgery) and a raised aldosterone to renin ratio with 1 or both of the following (compared with presurgery): ≥50% decrease in baseline plasma aldosterone concentration; or abnormal but improved postsurgery confirmatory test result. |
| Absent biochemical success | Persistent hypokalemia (if present presurgery) or persistent raised aldosterone to renin ratio, or both, with failure to suppress aldosterone secretion with a postsurgery confirmatory test. |
Following surgery, outcomes should be assessed: within 3 months, at 6-12 months (the principal timepoint for gauging success), and annually.
Table adapted with permission from Williams et al, 2017 (49).
abc For specific guidance on reference ranges, please refer to Williams et al, 2017.
Recently, a potential role for adrenal surgery in patients with bilateral PA has been considered. Specifically, for a small group of patients who cannot be controlled on medical therapy unilateral adrenalectomy may be performed even when bilateral disease has been confirmed (75, 80-82). In this context, unilateral adrenalectomy does not offer disease cure, but rather it may improve BP in patients uncontrolled on/or intolerant of multiple antihypertensives. Similarly, a potential role for bilateral adrenal surgery (eg, total adrenalectomy on 1 side and partial adrenalectomy on the other side, or bilateral partial adrenalectomies) has been reported (81). However, the decision whether to proceed with adrenal surgery in a patient with bilateral PA remains a very challenging management dilemma (83-85).
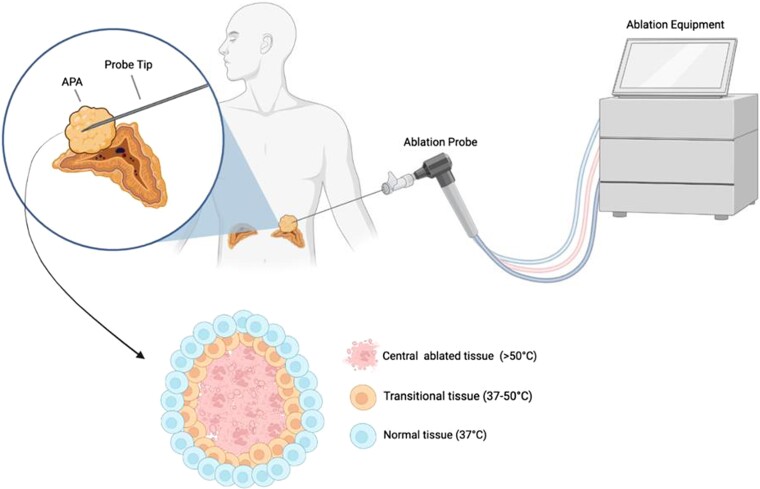
Approaches which can selectively target or remove offending aldosterone-producing lesions might therefore be preferable to unilateral adrenalectomy for bilateral disease. In this regard, localized therapies such as selective adenoma/nodule ablation or adrenal-sparing surgery could have an important role to play in the future management of both unilateral and bilateral PA (discussed in “Partial Adrenalectomy: Targeted Tumor Resection” and “Thermal Ablation: A Targeted Minimally Invasive Therapeutic Approach”). However, before such an approach can be recommended, more reliable and precise localization of culprit APAs and/or APNs is required: (1) to render these therapies feasible; (2) to avoid incorrectly targeting nonfunctioning tumors; (3) to spare as much normal adjacent tissue as possible in cases of bilateral intervention; and (4) to monitor therapeutic success postintervention (74, 75) (Fig. 6). Both APAs and APNs may be more amenable to localized intervention, with APAs occurring in up to one third of PA cases (5, 86). Multiple aldosterone-producing nodules and APDH present a greater challenge for targeted intervention, due to the difficulty in localizing the former, and inability to localize the latter given that hyperplasia normally extends throughout the entire perimeter of the affected gland(s) (13).
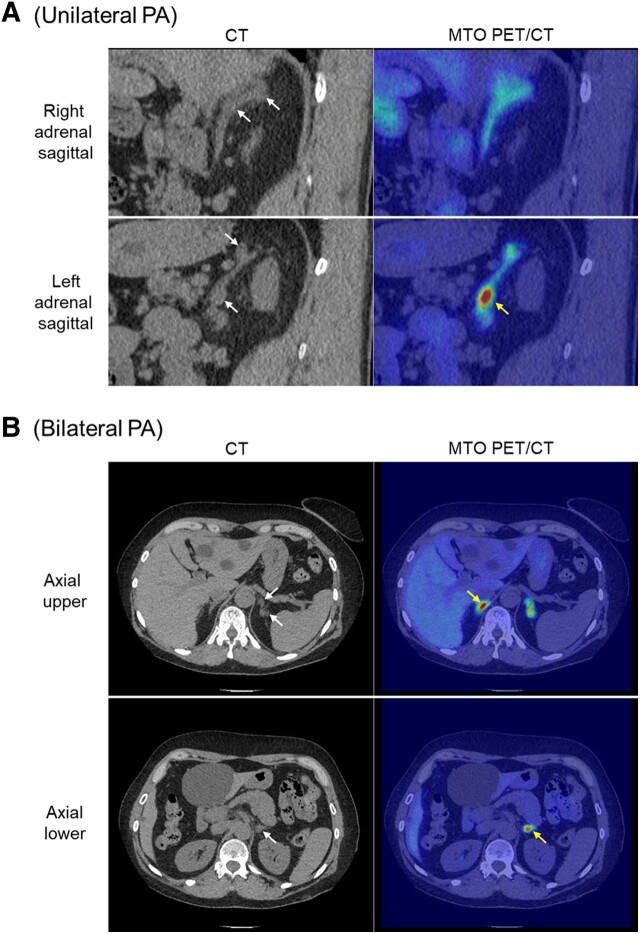
Figure 6.
11C-metomidate PET/CT allows localization of aldosterone-secreting adenomas/nodules in unilateral and bilateral PA. (A) Sagittal CT demonstrating 2 discrete nodules within the left adrenal gland, but with only the inferior 1 showing high focal tracer uptake; the right adrenal gland shows normal background radiotracer uptake despite the initial suspicion of possible nodularity. Following left unilateral adrenalectomy, the patient achieved full clinical and biochemical remission and immunohistochemistry confirmed that the metomidate-avid nodule exhibited strong staining for CYP11B2; in contrast the superior nodule showed only mild CYP11B1 staining. (B) Axial CT and MTO PET/CT in a patient with bilateral PA. Both adrenal glands demonstrate focal high radiotracer uptake; within the left adrenal, the most inferior of 3 discrete nodules shows greatest metomidate uptake. The patient was managed with primary medical therapy. White arrows denote sites of suspected nodules on CT; yellow arrows identify nodules with greatest metomidate avidity.
PA therefore remains an area with significant potential for the rapid translation of clinical, laboratory, radiological, and procedural innovations, which are urgently required to allow more effective management of this common condition. This review focusses on emerging and novel approaches to the diagnosis, localization, and management of PA. In “Improving the Diagnosis of PA,” we examine metabolomics and artificial intelligence approaches in screening and diagnosis of PA, with particular reference to the broader spectrum of disease and how this may be identifiable using newer technologies. In “Advances in Lateralization and Localization,” we examine current approaches to lateralization and discuss how emerging molecular imaging can enable precise localization of disease within the adrenal glands. In “Novel Treatment Approaches,” we discuss the feasibility of localized interventions, both minimally invasive and surgical, in the management of unilateral and bilateral disease. Finally, in “Advances in Pharmacotherapy,” we summarize the clinical trial data and reference emerging pharmacotherapy for the medical management of PA, with particular reference to the management of patients where definitive therapy is not possible.
Improving the Diagnosis of PA
Overcoming Challenges of the Current Diagnostic Approach
Early diagnosis of PA predicts response to therapy (35, 36). Ideally screening should be undertaken in primary care or by generalists (ie, at the time when hypertension is first recognized), using laboratory tests which are readily available and easy to interpret. Current screening for PA typically relies on determining the ARR. Measurement of plasma aldosterone and plasma renin, and interpretation of the ARR, is challenged by (1) assay availability, (2) stability of renin at room temperature following venesection, (3) requirement for specific sampling conditions (midmorning, seated for 15 minutes following 2 hours ambulation), (4) assay interpretation in the face of interfering antihypertensive medications, (5) requirement for confirmatory testing to establish the diagnosis due to low specificity of the ARR (approx. 65%), and (6) the intraindividual variability in screening aldosterone concentrations, renin measurements (plasma renin concentration and plasma renin activity), and their corresponding ARRs (87, 88). Consequently, given these apparent complexities, apprehension among primary care physicians and generalists means that even a 50% screening threshold of “at risk” patients is rarely achieved, let alone the recommendation by some experts that all hypertensive patients should be assessed for PA at the time of diagnosis of hypertension (89).
The finding of a low serum potassium in a patient with hypertension has traditionally been viewed as a key trigger to screen for PA. However, not only does this overlook the significant proportion of patients with PA who do not exhibit hypokalemia, but even when present low serum potassium might not be evident if there are delays in samples reaching the laboratory as may occur with more rural practices, with resultant sample hemolysis (90).
A more pragmatic and practical approach is therefore required to (1) improve recognition of patients who should be prioritized for PA screening, (2) facilitate interpretation of ARR or other screening tests in the face of potential medication interference, and (3) increase access to laboratory assays which do not require specialist sample handling within primary care, and which therefore have greater potential to reveal the presence of PA in hypertensive patients. In this regard, the introduction of laboratory assays/methods that can be more easily performed on samples (blood or urine) collected in a nonspecialist setting should offer the prospect of getting many more patients over the first hurdle and onwards toward successful treatment.
Metabolomics and Machine Learning
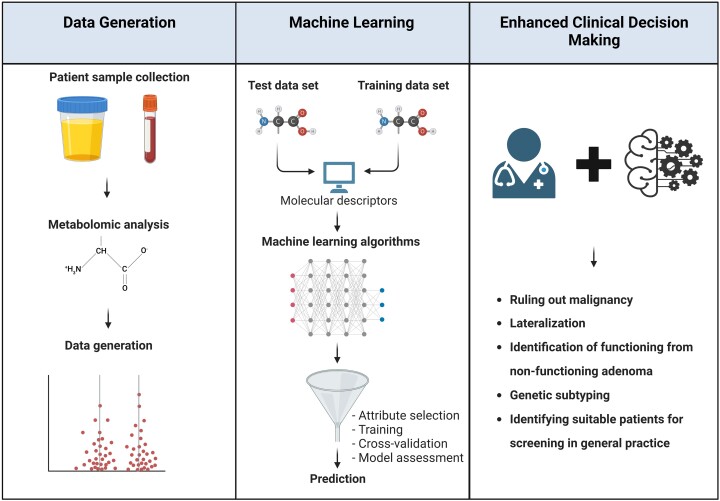
Metabolomics measures several steroids, their metabolites, fatty acids, monoamines, polyamines and other endocrine mediators from a single urine or blood sample. In general, urine samples are preferred because (1) analytes are typically collected over a 24-hour period giving a more complete analysis rather than a single point in time analysis obtained from a blood sample; (2) urine and its metabolite content remain stable at room temperature and can be stored at temperatures between −20 °C and +4 °C for prolonged periods, therefore allowing sampling within environments which are remote to the analyzing laboratory (91). In recent years, metabolomic profiling, with analyses supported by machine-learning algorithms, has demonstrated potential utility in the diagnosis and management of Cushing syndrome and adrenocortical carcinoma (ACC) (92, 93) (Fig. 4). Emerging data in hypertension and PA also points to a possible role in establishing or excluding the diagnosis of PA and in subtype categorization.
Figure 4.
Outline of metabolomics/machine-learning workflow. Following patient sample collection, metabolomic analysis is usually carried out using liquid chromatography tandem mass spectrometry, yielding a large amount of data. Through combination with machine learning, a detailed metabolomic fingerprint can be generated of disease subtypes to streamline clinical decision making. Figure created with BioRender.com.
Several metabolomics studies have investigated hypertensive cohorts (including PA patients) in an effort to distinguish between primary and secondary hypertension, and between unilateral and bilateral PA (Table 4). These studies have identified numerous differences in metabolomic patterns which may enable distinction between different hypertension subtypes. Table 4 summarizes these studies and the performance of metabolomic analyses in diagnosing PA or distinguishing between primary and secondary hypertension. However, as yet, these findings have not translated to the adoption of metabolomics into the diagnostic pathway for PA or secondary hypertension. Consistent with their largely observational and retrospective nature, the study designs have varied significantly, with some reporting untargeted analyses (using “off the shelf” analytical kits), while others have pursued more bespoke targeted analysis (using locally validated analyte panels). Many were designed only to screen for primary hypertension, vs all-cause secondary hypertension, and most were conducted with samples collected across multiple centers without rigid standardization of collection methods, storage, or diagnostic standards. Additionally, interpretation of metabolomic data can be challenging. Prospective studies, with prior assay optimization/validation and standardization of analytical methods to permit accurate test interpretation, will therefore be required before a metabolomics-based approach to the investigation of suspected PA is a feature of routine clinical practice and can be adopted into clinical guidelines.
Table 4.
Recent data utilizing metabolomics and machine learning to identify PA and subtypes
| Study | Cohort | Reference standard for diagnosis | Findings | Notes |
|---|---|---|---|---|
| Constantinescu et al, 2022 (94) This study served to demonstrate the integration of artificial intelligence and plasma steroidomics with laboratory information management systems |
22 patients tested for PA | PA was screened using the ARR, with a cut-off of 31 pmol/mU. PA was confirmed with a positive saline infusion test | Based on a negative ARR screen—18 patients were excluded of PA diagnosis. The other 4 patients had a positive screen The probability of PA in this cohort was predicted using 3 machine-learning models (SVM, RF, and LDA): Probabilities of PA ranged from 89% to 100% (median 99%) in patients with PA and from 2% to 90% (median 21%) in those without PAa aSensitivities/specificities/AUC not defined SVM, support vector machine; RF, random forest; LDA, linear discriminant analysis |
Machine learning specified unilateral PA in all 4 patients (probability range: 73-100% (median 91%), with a consistently high likelihood of KCNJ5 mutations in 3 patients |
| Erlic et al, 2021 (95) Retrospective Targeted metabolomics on plasma samples using LC-MS/MS. Analyzed with classical approach (using a series of univariate and multivariate analyses) or machine learning (random forest) |
Primary hypertension: 282 Secondary hypertension: 223 total, 40 CS, 107 PA, and 76 PPGL |
Not specifically defined: (“The diagnosis (primary hypertension, CS, PA, PPGL) was made according to the current guidelines for screening and management of the specific diseases” with reference to Funder et al, 2016) |
a
AUC
Using metabolites: 80% Using metabolite ratios: 77% bSpecificity for secondary hypertension: Using metabolites: 45% Using metabolite ratios: 37% aCalculated based on the performance of the top 15 metabolites bSensitivity/specificity was reported for differentiation of several forms of secondary hypertension (PA, PPGL, and CS) from primary hypertension |
Classical approach: When comparing primary hypertension and PA, 35 metabolites and 7 metabolite ratios had a significant association with the clinical diagnosis after controlling for sex and age group Machine-learning approach: When comparing primary hypertension and PA, 28 metabolites and 12 ratios were seen as key identifiers |
| Eu Jeong Ku et al, 2021 (96) Prospective multicenter study Targeted metabolomics on serum samples using LC-MS/MS with machine-learning algorithms for differentiation of PA, CS, or NFA (Decision tree, random forest, extreme gradient boost) |
NFA: 73 CS: 30 PA: 40 |
PA was screened using the ARR, with a cut-off value of 30. Positive ARR patients (ARR ≥30) were investigated using 1 or more confirmatory tests including: saline infusion or a high salt loading. PA was defined when aldosterone was not suppressed during confirmatory suppression testing |
Test cohort data:
AUC for PA: a 0.838, b0.933, c0.881 AUC for CS:a0.776, b0.925, 0.911c Sensitivity for PA:a88%, b93%, c95% Sensitivity for CS:a40%, b93%, c93% Sensitivity for NFA:a89%, b99%, c99% Specificity for PA:a92%, b100%, c99% Specificity for CS:a99%, b99%, c99% Specificity for NFA:a69%, b93%, c96% aDecision tree bRandom forest cExtreme gradient boosting |
There were 15 adrenal steroids simultaneously analyzed with each model, with 3 being classified as important discriminator of either NFA, CS or PA: tetrahydrocortisone, 18-hydroxycortisol, and dehydroepiandrosterone No validation cohort was included in the analyses |
| Kaneko et al, 2021 (97) Retrospective cross-sectional study Targeted metabolomics on serum samples using LC-MS/MS, with machine-learning algorithms for differentiation of unilateral PA and bilateral PA (Logistic regression, support vector machines, random forests, and extreme gradient boosting) |
Total PA patients: 229 Unilateral PA: 91 Bilateral PA: 138 Test cohort patient criteria |
For PA diagnosis: Patients were diagnosed with PA according to guidelines of the Japan Endocrine Society (98) and Japanese Society of Hypertension (99) with confirmation of diagnosis with at least 1 confirmatory test (captopril challenge or saline infusion test) For lateralization: AVS with ACTH stimulation (selectivity index >5, lateralization index ≥4). |
For Unilateral PA prediction:
Test cohort data: AUC: a0.948, b0.966, c0.990, d0.976, e0.95 Sensitivity:a83.3, b66.7, c94.4, d72.2, e83.3% Specificity:a92.9, b96.4, c96.4, d96.4, e92.9% Validation cohort data: AUC: a0.877, b0.875, c0.872, d0.848, e0.826 Sensitivity:a69%, b65.5, c69%, d69%, e72.4% Specificity:a94.5%, b95.6, c94.5%, d94.5%, e89.1% aLogistic regression bSupport vector machines cRandom forest dExtreme gradient boosting eOptimized random forest model |
The optimized random forest model was developed with 3 variables: serum potassium, plasma aldosterone concentration, and serum sodium levels |
| Diao et al, 2021 (100) Retrospective Development of a machine-learning model based on up to 79 clinical indicators for subtype diagnosis of either primary aldosteronism, renovascular hypertension, Thyroid dysfunction or aortic stenosis (extreme gradient boosting model) |
Renovascular hypertension: 505 PA: 400 Thyroid dysfunction: 139 Aortic stenosis: 71 |
Not specifically defined. |
For PA prediction
Test cohort data: AUC: 0.961 Sensitivity: 83.6% Specificity: 95.9% Validation cohort data: AUC: 0.965 Sensitivity: 84.4% Specificity: 93.0% |
For the PA model, 21 clinical indicators were used with the top 10 being: Upright ARR, serum potassium, supine ARR, supine plasma aldosterone, upright plasma aldosterone, glycated hemoglobin, nifedipine, albumin to creatinine ratio, 24-hour urinary aldosterone, serum sodium |
| Burrello et al, 2021 (101) Retrospective Development of several machine-learning diagnostic models and a 16-point Primary aldosteronism confirmatory testing (PACT) score using machine-learning and regression analysis to discriminate patients with a confirmed diagnosis of PA |
PA: 1024 | PA was screened using the ARR (developmental cohort, ie, training and internal validation: aldosterone to renin activity or in the external validation cohort: aldosterone to direct renin concentration) with cut-offs of an ARR >30 ng/dL/ng*mL−1*h−1 and aldosterone concentration higher than 10 ng/dL for a positive screen PA was confirmed by either an intravenous saline loading test or a captopril challenge test Subtype diagnosis (lateralization) was determined by CT imaging and AVS |
With a score of ≥8
Training cohort data: AUC: 0.879 Sensitivity: 92.2% Specificity: 71.0% Validation cohort data: AUC: b0.877 Sensitivity:a91.9%, b78.6% Specificity:a73.3%, b83.9% aInternal validation (Turin, Italy) bExternal validation (Munich, Germany) |
The 6 parameters that were selected by regression analysis included: female sex, antihypertensive medications, plasma renin activity at screening, aldosterone at screening, lowest potassium, and organ damage For patients with a score <5, PA diagnosis was excluded without a confirmation test; for patients with a score ≥13, PA diagnosis was confirmed without further tests. |
| Burrello et al, 2020 (102) Retrospective Supervised machine-learning algorithms and regression models were used to develop and validate 2 prediction models (Linear discriminant analysis and random forest), and a CLR score—a 19-point score system to distinguish unilateral PA from bilateral PA in cases of a unilateral successful AVS procedure, with the presence of contralateral suppression of aldosterone secretion |
Patients who successfully underwent AVS: 158 Unilateral PA: 126 Bilateral PA: 32 |
PA was screened using the ARR (aldosterone to plasma renin activity ratio) with a cut-off of 30 ng/dL/ng*mL−1*h for a positive screen PA was confirmed by either an intravenous saline loading test or a captopril challenge test Subtype diagnosis (lateralization) was determined using CT imaging and AVS where an adrenal nodule was reported if a mass of >8 mm was evident AVS was performed both under basal conditions (selectivity index ≥3) or with ACTH stimulation (selectivity index ≥5). Diagnosis of unilateral disease was determined by presence of a lateralization index of at least 4 |
For discrimination of unilateral PA vs bilateral PA
AUC: c 0.971: Generated from the combined cohort (training and external validation n = 158) Training cohort data: Sensitivity:a90.6%, b95.8%, c95.5% Specificity:a99.4%, b94.9%, c100% External validation cohort data: Sensitivity:a84.7%, b83.6%, c94.7% Specificity:a84.2%, b80%, c90% aLinear discriminant analysis bRandom Forest cCLR scoring system with best accuracy (>11) |
In the training cohort, a CLR score greater than 11 displayed the greatest accuracy (96.4%) |
| Eisenhofer et al, 2020 (103) Retrospective Targeted metabolomics of serum samples, with machine-learning analysis (random forest or a nonlinear radial basis function kernel [SVM]) for identification and subtype classification in PA particularly for patients with unilateral adenomas due to pathogenic KCNJ5 sequence variants |
PA: 273 Bilateral: 134 Unilateral: 139, of whom 58 had an APA due to KCNJ5 variant, and 81 did not |
PA was screened for using the ARR (cutoffs used not clearly defined). PA was confirmed using 1 or more of the endocrine society recommended confirmatory tests (oral salt loading test, saline infusion test, fludrocortisone challenge test, Captopril challenge test—specifics not clearly defined) Subtype diagnosis (lateralization) was determined using AVS under basal nonstimulated conditions using a selectivity index of >2 and a lateralization index of >4 or alternatively >3 in the presence of contralateral suppression. |
For PA Prediction
Learning cohort data: a AUC: a0.815, b0.841 aSensitivity:a69%, b70% aSpecificity:a94%, b98% External validation cohort data: aAUC: a0.926, b0.875 aSensitivity:a85%, b78% aSpecificity:a100%, b97% For PA with KCNJ5 prediction Learning cohort data: AUC:a0.714, b0.909 bSensitivity:a46%, b85% bSpecificity:a97%, b97% Validation cohort data: AUC:a0.908, b0.991 bSensitivity:a83%, b100% bSpecificity:a98%, b98% aRandom forest bSVM |
An assortment of 7 steroids + the aldosterone to renin ratio showed improved effectiveness for PA diagnosis over either strategy alone Aldosterone, 18-oxocortisol, and 18-hydroxycortisol had the most discriminatory power although this was model dependent. A random forest model provided optimal performance for the classification of patients with and without PA, whereas an SVM model was optimal for patients with APAs due to KCNJ5 |
Abbreviations: ARR, aldosterone to renin ratio; CS, Cushing syndrome; LC-MS/MS, liquid chromatography–mass spectrometry; NFA, nonfunctioning adenoma; PA, primary aldosteronism; PPGL, pheochromocytoma/paraganglioma.
A key challenge in the roll out of metabolomics for screening and diagnosing disease has been the generation of large quantities of data and its subsequent handling, analysis and interpretation (95). While, on the one hand, this presents an opportunity to better understand the pathophysiology of PA and to develop more sensitive and specific tools for diagnosis, on the other hand, it also represents a significant challenge in terms of the accuracy of interpretation and subsequent categorization of disease. This challenge is being increasingly addressed using machine learning to analyze and interpret data generated from metabolomics studies with greater efficiency, consistency, and accuracy.
Machine learning requires careful design, training and validation. Appropriate algorithms must be chosen and optimized to best match the data and the analysis. Ideally the performance of machine learning–based analysis should be tested against, and compared with, human expert analysis. In turn, the quality of the outputs provided by machine learning–based analysis is reliant upon (1) robust study design at the outset, (2) the input of high-quality data that has been collected appropriately, (3) the reference standards against which the machine learns, (4) the availability of sufficient numbers of samples in training, and (5) appropriately selected/recruited verification cohorts. Prior to mainstream clinical use, properly carried out prospective validation studies, in accordance with the Standards for Reporting of Diagnostic Accuracy Studies (STARD) guidance, are required (104). In the context of the screening and diagnosis of PA, the current diagnostic tests and reference standards, against which metabolomic datasets are trained for interpretation by machine learning, are not yet supported by high quality evidence as previously discussed in “Screening, Diagnosis, and the Spectrum of Disease.” Secondly, the performance of traditional diagnostic testing is variable and diagnostic thresholds do not generally recognize the likely full spectrum of disease represented by PA.
Steroid metabolomic profiling
Steroid metabolomic profiling has been used clinically for many years to aid in the diagnosis of disorders of adrenal biosynthesis and metabolism (105). It has also gained widespread clinical use in the analysis and detection of patterns of steroid metabolite excretion which reveal the use of performance-enhancing drugs in sport (106, 107). The more mainstream options for steroid metabolomic assays are gas chromatography mass spectrometry (GC-MS/MS) or liquid chromatography tandem mass spectrometry (LC-MS/MS). The former is a labor-intensive and expensive technique, requiring significant expertise and set-up, and which is limited to a handful of international centers. In contrast, LC-MS/MS, a high-throughput technique which is more widely available, provides precise, well-validated, clinical laboratory–based assays for large steroid metabolite panels in both urine and blood. Detailed steroid metabolomic profiling of functioning adrenal tumors, supported by machine-learning analysis has advanced significantly over the past decade. Arlt et al, first used this approach to define the metabolomic signature of ACC when compared with benign functioning adrenocortical adenomas, including PA (92). This was followed by a prospective, multicenter Evaluation of Urine Steroid Metabolomics for the Differential Diagnosis of Adrenocortical Tumours (EURINE-ACT) which combined spectrometry-based urinary steroid metabolite profiling and machine learning–based data analysis for detection of malignant lesions (108). The optimum diagnostic performance for the detection of malignant ACC in this study was superior to radiologic characterization combined with traditional biochemistry. The combined criteria: tumor diameter >4 cm, unenhanced CT tumor attenuation greater than 20 HU, and machine-learning analysis of urine steroid metabolomics achieved a positive predictive value of 76.4% and a negative predictive value of 99.7%. This study was a prospective validation of a prior study which compared the performance of a wider GC-MS panel when compared with a smaller, high-throughput LC-MS/MS steroid metabolite panel and, as such, provides a useful model for future studies of metabolomics in PA and hypertension. A generalized linear model was used to determine an overall score for ACC diagnosis rather than individualized interpretation of each steroid metabolite. The findings of this study preceded an evaluation of the ability of metabolomic profiling to predict the recurrence of ACC, in the presence of corticosteroid replacement and mitotane therapy (which interfere with the interpretation of blood-based assay of cortisol and cortisol metabolism) (93). A smaller number of patients with ACC had longitudinal, 24-hour urine collections for urine steroid metabolomics from prerecurrence and postrecurrence states. Blinded analysis of steroid metabolomics alone by 3 clinical experts detected 69% to 92% of recurrence by the time of radiological diagnosis, where preoperative urine samples were available. There was significant variability in the sensitivity of recurrence diagnosis when interpreted by clinical experts. However, machine learning was consistent in picking up 75% of recurrence, without the variability of sensitivity when challenged with the same data on multiple occasions. In this study, metabolomics outperformed imaging in 22% of patients where recurrence was detected by metabolomics 2 months in advance of radiological recurrence. The study was small and the data availability was incomplete and subject to selection bias often seen in early observational studies which evaluate the follow-up and outcomes of rare diseases. The findings of this study therefore need to be tested in a prospective, longitudinal manner in larger patient cohorts.
The importance of these studies is that they illustrate the potential of steroid metabolomics to accurately diagnose and distinguish with high specificity, between endocrinopathies which are driven by steroid-producing adrenal tumors. They also show the potential for machine learning to overcome observer bias in this context and to provide more consistent sensitivity and specificity for diagnosis, when compared with human interpretation. They demonstrate the opportunity for longitudinal monitoring of disease cure, recurrence, or progression in patients with an endocrinopathy of steroid hormone overproduction. They importantly highlight that steroid metabolomics, supported by machine learning, can be successfully used to overcome the difficulty of data interpretation in the face of interfering medications. But what about metabolomics in the context of hypertension and PA?
Metabolomic profiling of PA and hypertension
Investigation of metabolomic profiling in hypertension and PA is still at an early stage, with several retrospective studies examining 24-hour urinary steroid profiles, plasma steroid profiles, and unselected metabolomic panels. Arlt et al have also investigated the urinary steroid metabolite/metabolomic signature of PA and its subtypes. This study involved (1) a large exploratory cohort of patients with PA (103 UPA, 74 bilateral disease) and nonaldosterone producing adrenocortical adenomas (NAPACA) across subclinical Cushing (47 patients), overt adrenal Cushing syndrome (104 patients), nonfunctioning adrenal adenomas (56 patients) and healthy controls (162 individuals), and (2) a prospective validation cohort with PA (46 patients) (109). The GC-MS assay used detected higher levels of aldosterone and aldosterone metabolites in the urine of patients with a confirmed diagnosis of PA. Within this study, metabolomic profiling also demonstrated phenotypes of PA not previously recognized, whereby some degree of glucocorticoid cosecretion could be inferred from an excess of cortisol and total glucocorticoid metabolites in addition to the expected mineralocorticoid metabolite profile. In patients diagnosed with PA, the percentage difference in total glucocorticoid excretion relative to healthy controls was +25.0% (109). The authors coined the term “Connshing's syndrome” in order to describe this phenomenon. “Connshing's syndrome” was not associated with overt hypercortisolism/features of overt Cushing syndrome and patients demonstrating this metabolomic profile neither failed screening for Cushing syndrome with the overnight dexamethasone suppression test, nor did they demonstrate the typically suppressed adrenocorticotropin (ACTH) level associated with adrenal Cushing syndrome. Higher glucocorticoid metabolite levels in the urine of patients with PA was associated with higher CYP11B1 expression on immunohistochemistry and higher overall urinary cortisol and glucocorticoid metabolite levels than those seen in patients with subclinical Cushing. Higher glucocorticoid metabolites in the urine in PA was also accompanied by production of higher rather than lower androgen metabolites, in contrast to the typical androgen suppression associated with benign adrenal Cushing syndrome or mild autonomous cortisol secretion (110, 111). Post hoc correlation analysis across the total cohort identified an association between high glucocorticoid metabolite excretion and several markers of an adverse metabolic profile. The authors suggested that MRA alone within this subgroup of PA patients were sufficient to control hypertension and desuppress renin, but may not be sufficient to control a disease phenotype driven by aldosterone and cortisol. Resolution of the metabolomic abnormality for mineralocorticoid and glucocorticoid metabolites was demonstrated following adrenalectomy in patients with UPA.
This pattern of steroid secretome/metabolome was not expected within the PA cohort and suggested that mild steroid cosecretion occurs at a frequency not previously anticipated. The study was appropriately designed and powered to distinguish between PA, other adrenal endocrinopathies and healthy controls. A well-validated GC-MS metabolomic assay system was used within an internationally recognized center. There was however a degree of selection bias in the recruited subjects, which favored patients with UPA and overt Cushing syndrome when compared to the typical prevalence of UPA within the spectrum of PA, and the prevalence of endocrinopathy in the setting of an adrenal nodule. The confirmatory cohort all had a diagnosis of PA. The study did not directly examine the potential effects of medication interference on the diagnosis of PA using steroid metabolomics. Finally, the study was not designed nor powered to address the clinical significance of the glucocorticoid metabolite cosecretion in patients with PA, and as such assigning the name Connshing syndrome may be premature until further prospective studies are carried out to add clarity to the true clinical significance of what is, in essence, a biochemical entity. Nonetheless, this study has provoked discussion relating to the greater PA disease spectrum and the possibility of greater disease diversity of PA which may explain metabolic disease, cardiovascular outcomes and sleep apnea in this population (109).
While Arlt and colleagues have demonstrated high mineralocorticoid metabolite levels in the urine of patients with PA, other authors have described challenges with this technique. In general, GC-MS rather than LC-MS/MS is used for the detection of mineralocorticoid and aldosterone metabolites in urine. Due to their low concentration in the urine, these can fall below the lower limits of assay detection using standard analysis in many commercial laboratories. As a consequence, many patients with PA may demonstrate a normal profile and are indistinguishable from primary hypertension at baseline, and in the absence of salt loading. This highlights the reliance upon machine sensitivity and the specific technical expertise required to run these assays which makes their current availability more challenging.
Urine analysis offers the convenience of noninvasive sampling and sample stability for screening (or diagnostic) samples which have been collected in locations remote from the analyzing laboratory. It also provides measurements reflective of a longer period of time. However, urine sampling requires adherence to a sampling procedure over 24 hours and is not always convenient for, or properly collected by, patients. In this regard, a more detailed steroid analysis of a single blood sample collected at the time of ARR screening offers the potential to conveniently and accurately diagnose PA. In a study using targeted plasma steroid metabolomics (LC-MS/MS), analyzed using machine learning, Eisenhofer et al, (103) demonstrated the utility of a multianalyte steroid profile drawn at the time of ARR measurement: (1) to distinguish between primary hypertension and PA with greater sensitivity and specificity than the ARR alone and (2) to identify patients with unilateral adenomas, driven by KCNJ5 mutations vs KCNJ5 wildtype. A score derived from a total combination of 7 steroids was useful in stratifying PA subtypes. The top 3 ranking steroids were consistently identified as aldosterone, 18-oxocortisol, and 18-hydroxycortisol. Patients with KCNJ5 mutations demonstrated considerably higher levels of all 3 steroids than other PA subtypes. While this steroid combination had previously been identified as distinguishing between PA and primary hypertension, this study differed from others in using machine learning (random forest model) to interpret the results of the plasma steroid profile combined with the ARR. The area under the receiver operated curve was higher using the machine-learning analysis when than ARR alone for distinguishing PA from primary hypertension (0.92 [0.899-0.946] vs 0.89 [0.856-0.916]) and considerably higher for identifying UPA driven by KCNJ5 mutations vs primary hypertension and all other PA (area under the curve: 0.95 [0.922-0.969] vs 0.817 [0.758-0.863]) (Table 4). Albeit retrospective, this was a well-executed study which was designed and powered from the outset to address the ability of steroid profiling to identify and classify subtypes of PA. The machine-learning approach was carefully developed and the validation cohort yielded similar results to the initial test cohorts. Within the overall participant patient group however, there was a selection bias towards UPA (60%), probably reflective of the centers from which patients were recruited. The study was retrospective, with analysis performed on biobanked samples. Traditional standards of diagnosis for PA across multiple recruitment-sites were used (see “Screening, Diagnosis, and the Spectrum of Disease”) and the machine-learning algorithm was trained against these data, with consequent unavoidable heterogeneity. However, the results of the study were validated against surgical outcome data, which were available for a large number of patients with UPA (157/304 PA patients) who had undergone adrenalectomy. The authors concluded that their findings highlight the potential for the metabolomic model, supported by machine learning, to identify patients who would benefit most from surgical intervention (103). They also contended that biochemical distinction between unilateral and bilateral forms of PA, particularly in identifying KCNJ5 mutations may facilitate improved confidence in the interpretation of adrenal CT as a lateralization modality.
In another study by the same researchers, plasma metabolomic steroid profiling was combined with plasma metanephrines and tumor size on CT in an effort to distinguish between the underlying pathologies of different types of adrenal incidentaloma including ACC, pheochromocytoma, and APA (112). While this was again a retrospective study, it demonstrated in a similar manner to EURINE-ACT, how the combination of radiology and steroid metabolomics offers the potential for development as a powerful tool to diagnose and distinguish between different pathologies of adrenal tumors. In this study APA was again characterized by elevated aldosterone, 18-oxocortisol and 18-hydroxycortisol. ACC was distinguishable by 11-deoxycortisol, 11-dexoycorticosterone, 17-hydroxyprogesterone, androstenedione, and dehydroepiandrosterone sulfate. When combined with cortisol, corticosterone, dehydroepiandrosterone sulfate, and plasma metanephrines, this metabolomic panel plus tumor size demonstrated high sensitivity and specificity in distinguishing between pheochromocytoma, PA, and other adrenal tumor types. Overall, subtype classification of adrenal incidentalomas demonstrated optimized sensitivities for ACC, PA, and pheochromocytoma of 83.3% (66.1-100%), 90.8% (83.7%-97.8%), and 94.8% (89.8%-99.8%), respectively, with specificities of 98.0% (96.9%-99.2%), 92.0% (89.6%-94.3%), and 98.6% (97.6%-99.6%), respectively (Table 4) (112). Acknowledging that this study was again retrospective, the data are compelling when taken in context of other studies such as EURINE-ACT and earlier studies by Eisenhofer et al, that plasma and/or urine steroid metabolomic panels offer greater sensitivity and specificity in diagnosing APA with a higher degree of accuracy than traditional approaches. In this study plasma profiling was used which, as highlighted previously, does not offer the ease of sample collection or analyte stability upon storage when compared with urinary analysis (91). It may also be argued that the current diagnostic pathway is sufficient for distinguishing between ACC, APA, and pheochromocytoma with a high degree of accuracy, using readily available and relatively cheap tests combined with clinical judgment. The challenge with the diagnosis of PA does not usually arise from distinguishing between it and other types of adrenal incidentalomas, but rather in differentiating between primary hypertension and PA.
In this regard, Erlic et al, tested plasma metabolomic analysis using a large “off the shelf” LC-MS/MS–based assay system to distinguish between primary and endocrine hypertension. The principal aim of the study was to identify a prescreening panel which could be measured in a single plasma sample and which would identify patients suitable for further more specific screening/investigation for endocrine hypertension, including PA, Cushing (ACTH-dependent and -independent), and pheochromocytoma/paraganglioma. This investigation used targeted metabolomics in an unselected and exploratory manner rather than using steroid metabolites or other analytes specific to the underlying pathology of different types of endocrine hypertension. The metabolomic panel used an unselected set of 188 metabolites (157 of which were detectable and included in the analysis) across acylcarnitines, biogenic amines, and glycerophospholipids. A classical statistical approach, using regression analysis was compared with a machine-learning, random forest approach, with similar results. Physician interpretation of the results was not undertaken (95). When comparing primary hypertension and PA, the machine-learning model selected 28 metabolites and 12 metabolite ratios which could differentiate between the 2 (Table 4). However, there was considerable overlap between metabolites across the various forms of endocrine hypertension and distinction between different types of endocrine hypertension could not be made. Overall, the best performance of this analysis compared primary hypertension against the composite outcome of all endocrine hypertension (PA, Cushing syndrome, and pheochromocytoma/paraganglioma inclusive). The analysis selected distinguishing metabolites and built a receiver operating characteristic curve using machine learning and classical statistical analyses, which provided respective areas under the curve (AUC) values of 0.83 and 0.86 (95% CI 0.806-0.907) for the differentiation of primary from composite endocrine hypertension. While the study identified differences between the endocrine and primary hypertension groups, the results are exploratory and preliminary, and need to be prospectively validated. The overall study cohort was small and probably underpowered to detect true differences between the study groups, given (1) the number of analytes investigated, (2) the number of study groups (4 in total), and (3) the need for training and validation cohorts within the machine-learning group. The cohort also demonstrated a selection bias towards endocrine hypertension, reflective of the clinical centers from which patients were recruited. There was a sex bias within certain groups, which may have affected the findings of the study. Diet and smoking were not taken into account in the analysis, which could be considered a weakness given the metabolites which were measured. Finally, as a prescreening approach, the analysis required an LC-MS/MS analysis on a plasma sample, which was expensive, difficult to analyze and which would not overcome the multiple sample processing challenges which currently limit screening in primary care, such as sample storage and sample stability at room temperature.
When taken together, the cumulative findings of the studies presented here and in Table 4 suggest that a combined approach which employs metabolomics, tumor size, and character on CT, and analyzed using machine learning offers the prospect of improving diagnostic efficiency for PA and distinguishing primary and endocrine hypertension. This combination approach may also offer the prospect of better informing intervention or even personalizing therapy (medical or interventional) for PA (as well as other forms of hypertension) to achieve the best individualized patient outcome. In the context of urinary collection, there is a clear benefit in patients providing samples with relative ease, which reflect a 24-hour or overnight time-course and which remain analytically stable, such that they can be collected (with very little processing) in locations remote from the analyzing laboratory, such as primary care. However, while encouraging, these studies must be interpreted with caution. The majority are retrospective and exploratory. There is therefore a significant need to test these diagnostic approaches prospectively in large multicenter studies, which are informed by clinical outcomes. Prior to further investigation there is also a pressing need for consensus to standardize the current diagnostic approach for PA, such that future diagnostic studies using a combined approach can be compared to a robust gold standard (discussed previously in “Screening, Diagnosis, and the Spectrum of Disease”).
Machine learning to interpret traditional parameters
The use of machine learning to interpret clinical and laboratory parameters that are routinely collected during the initial assessment and diagnostic work-up of hypertension could help practitioners in primary and nonspecialist care to identify patients who should be offered screening for PA and referral for specialist care and/or confirmatory testing. This may improve patient outcomes and direct resources more appropriately. However, any use of machine learning must be prospectively analyzed and must show clear and consistent benefit over clinical decision-making alone. Additionally, any machine-learning approach must be clearly interpretable and designed to simplify the diagnostic pathway, rather than adding a layer of complexity.
In a multicenter study across 2 sites (Torino and Munich), Buffolo et al compared a clinical prediction score with a machine-learning algorithm based on existing data in a retrospective study of 4059 patients with hypertension, all of whom had completed the diagnostic workup for PA. The authors created a clinical prediction rule/score from 6 predictive clinical variables (male sex, systolic BP, antihypertensive treatment, body mass index, lowest potassium, and organ damage) to distinguish primary hypertension from PA, and to distinguish UPA from bilateral PA, which they named, a Score To Predict Primary Aldosteronism (SToP-PA) (113). In parallel with the SToP-PA score, the authors tested 4 machine-learning algorithms and chose a machine-learning model (random forest regressor) that was based on the same 6 variables. The training (3045 patients) and validation (1014 patients) cohorts for both models were recruited at University of Torino and both models were then tested against an external validation cohort of 584 from Munich. The SToP-PA score ranges from 0 to 21.5 points, where optimized cut-offs for prediction of a positive screening result for PA, or UPA diagnosis were 7.0, 7.5, and 8.0 points, achieving sensitivities of 92.0%, 90.7%, and 92.3% respectively at validation, with higher cut-offs (13.5, 14.0, and 15.0 points) necessary to achieve optimum specificities (91.8%, 96.4%, and 98.1% at validation). The machine-learning model achieved optimum sensitivity for (1) positive screening results, (2) PA, or (3) UPA diagnosis of (1) 95.5%, (2) 96.6%, and (3) 100.0% (at validation) with coefficient cut-offs of 0.23, 0.24, and 0.35, respectively. Again, higher cut-offs of 0.70, 0.76, and 0.81 were required to achieve optimum sensitivity of 94.6%, 96.2%, and 96.9% (at validation), respectively. Ultimately, both models successfully identified all patients with UPA and additionally circumvented the necessity for screening in 32.7% of patients with non-PA hypertension, while simultaneously better selecting those who would benefit from screening. Both the SToP-PA score and machine-learning models have been made available as open-source software by the authors. However, they have not yet been validated in a large prospective study. Surprisingly, there are no data testing and validating these resources among retrospective cohorts in other centers where the work-up and diagnosis of patients with PA is routinely undertaken.
Intercenter considerations for machine learning
While machine learning offers great promise to overcome many of the diagnostic challenges associated with PA, it is important to balance these potential gains with the risks associated with overinterpreting machine-learning data. Firstly, data derived from individuals with an established diagnosis (using the conventional gold standard must be deployed to train the machine-learning algorithm with an appropriate balance of positive/negative datapoints. As discussed previously, the current gold standards and reference standards for the diagnosis of PA demonstrate significant heterogeneity and almost certainly do not reflect the overall spectrum of disease. When validation datasets are subsequently used to test the accuracy of the algorithm, a significant reduction in its performance may be observed. This is particularly true for data and samples collected across different centers, where center-specific data characteristics have been shown to significantly impact algorithm performance. For instance, in the study of Burrello and colleagues (Table 4) a disparity in validation performance was observed between the internal (Turin) and external (Munich) cohorts (101). This speaks to the importance of training and validating datasets across multiple centers. Finally, external validation of machine-learning algorithms is often carried out on retrospectively collected data. In the context of the diagnosis of PA, machine learning presents a powerful tool for the interpretation of metabolomics and the improvement of diagnostic performance of existing tests. Prior to clinical application of these algorithms, there remains an overall need for prospective, multicenter evaluation of their performance, compared with expert-led data interpretation and diagnostic performance.
Advances in Lateralization and Localization
Lateralization and Localization: A Fundamental Requirement for Optimal Clinical Management
Adrenal vein sampling
As previously discussed in “Current Approach to Lateralization,” following confirmation of a diagnosis of PA, cross-sectional imaging of the adrenal glands (CT or MRI) is typically undertaken to investigate for the presence of 1 or more nodules and exclude ACC (Fig. 1). Depending on local practice, it may also be used to derive information regarding the adrenal vasculature. However, as highlighted in an earlier section (“Current Approach to Lateralization”), anatomical imaging alone is generally not considered sufficiently sensitive or specific to be the sole mode of lateralization in most patients. This reflects the findings of several studies which have reported unsatisfactory performance in distinguishing UPA and bilateral disease to guide unilateral adrenalectomy (114-116). Although a single prospective study (SPARTACUS) challenged previously published retrospective data and suggested that AVS and adrenal CT were of equivalence as lateralization modalities for UPA, significant concerns have been raised regarding the study's design, which, in turn, has led several experts to question the validity of its findings (68).
Other workers have postulated that anatomical imaging alone may be sufficient to allow a patient to proceed direct to surgery without further lateralization when certain stringent conditions pertain: (1) severe PA phenotype with clearcut unilateral lesion >1.5 cm in diameter (70, 117), (2) young patients (<35 years) with spontaneous hypokalemia, marked aldosterone excess, and unilateral adrenal lesion with radiological features consistent with a cortical adenoma on adrenal CT scan (36). However, careful clinical decision making is still necessary in these cases given that discordance between CT and AVS has been reported in younger patients (36, 65, 118, 119). Additionally, as discussed in “Current Approach to Lateralization,” even in cases where CT and AVS appear “concordant” prior to surgery (ie, identification of a prominent nodule on CT, with concordant ipsilateral AVS) the prominent CT identifiable nodule is not always the source of aldosterone secretion (66). As such, the use of AVS in tandem with CT imaging may not increase the lateralization power of CT to the degree that might be expected, and thereby question the validity of proceeding directly to adrenalectomy without assessing whether a CT identifiable nodule is functioning or not.
AVS has remained the so-called gold standard lateralization modality for UPA for decades as it is deemed to provide accurate functional information and is associated with minimal complications when performed by an expert interventional radiologist (120-122). Nonetheless, AVS is challenged by a number of factors (123). In the first instance, the procedure is technically demanding, time consuming, associated with significant radiation exposure and demonstrates considerable center to center and operator to operator variability in terms of success (124, 125). Successful cannulation of both adrenal veins is not achieved in up to 50% of patients and operator skill must be maintained with a critical number of procedures yearly (125, 126). Historically, there was a lack of consensus on the methodology for AVS as outlined in the Adrenal Vein Sampling International Study (AVIS) that noted marked variation in technique and interpretation of results between centers—prompting the need for more definitive AVS guidelines (127). This knowledge gap was subsequently addressed by a panel of experts who published a consensus statement on the use of AVS (128). Despite this, there was considerable flexibility in the recommendations for procedural activity within this consensus statement and there still exists variable adherence to the overall recommendations. Common methodological differences exist between centers relating to the use of periprocedural and intraprocedural ACTH stimulation, simultaneous vs sequential adrenal vein cannulation, and ratio calculation using peripheral vs inferior vena cava blood samples (129). Furthermore, differing cut-off values for the selectivity index and lateralization index have an effect on reporting of the rate of bilateral cannulation success and identification of unilateral PA, while tumors cosecreting cortisol have the potential to influence nonstimulated AVS result interpretation (130-132). There is not consistency across expert centers on the use of a contralateral suppressibility index (<1.0) to guide lateralization where a gradient exists. Blood samples drawn during the procedure must be processed and analyzed quickly, and ratios calculated from the results. This is resource heavy and increases the time from diagnostic procedure to interpretation of results. Therefore, availability of high quality AVS is limited to specialist centers. Additionally, standard AVS lateralizes PA to 1 adrenal but cannot specifically localize the culprit lesion within the gland. Therefore, if a nonfunctioning macroadenoma existed in 1 part of the gland, and a less visible functioning microadenoma was located distally, it is possible that in conjunction with CT imaging, the macroadenoma would be mistakenly identified as the source of aldosterone hypersecretion as previously described by Nanba and colleague (66, 133). Consequently, complete adrenalectomy, rather than adrenal-sparing surgery is preferred following standard AVS.
Advancing to localization using segmental AVS
Segmental adrenal vein sampling (sAVS) represents a more advanced technique that has emerged in some specialist centers. This highly skilled procedure can localize functioning adrenal tumors within and between glands (133-137). Under cosyntropin stimulation, multiple branches of the adrenal vein are typically cannulated to calculate the aldosterone:cortisol ratio, distinguishing between healthy tissue and a localized source of excess aldosterone (Fig. 5). However, sensitivity and specificity for this procedure have not yet been reported within the currently available literature. While offering a modality for localization of aldosterone-secreting tumors within either adrenal gland, the feasibility of this procedure is offset by the requirement for a highly skilled operator and hence its limited availability.
Figure 5.
Schematic diagram outlining an AVS or segmental AVS (sAVS) procedure as described by Satani et al, 2016 (137). During a conventional AVS procedure, both central adrenal veins of the left and right adrenal are sampled to lateralize the affected adrenal. sAVS involves sampling from several adrenal tributaries to localize a section of adrenal as the source of aldosterone hypersecretion. Commonly, 3 main tributaries converge into 1 central vein. However, in some patients ≥3 tributaries may be present. Figure created with BioRender.com.
Overall, AVS remains the key imaging modality informing the surgical vs medical management of PA. Notwithstanding, the invasive and technically challenging nature of the AVS procedure, coupled with the usual need for medication withdrawal (particularly MRAs) for several weeks beforehand, can contribute to further delay in diagnosing PA (69, 127, 138). Therefore, several groups have turned their attention to the development of more accessible and technically less challenging approaches which permit not only lateralization, but also localization of a causative APA/APN.
Noninvasive Lateralization
Recent advances in molecular (functional) imaging [especially positron emission tomography (PET)], now permit detection of small (even subcentimeter) functioning adrenal tumors. The use of hybrid imaging modalities (combining functional with anatomical imaging, eg, PET/CT or PET/MR) offers the potential to not only lateralize but also precisely localize the source of aldosterone excess in the affected adrenal. This is an attractive approach for the management of PA given that it is (1) noninvasive, (2) offers an alternative in situations where the technical challenges of AVS cannot be overcome (75, 139), and (3) has potential to be more widely accessible than AVS. Importantly, however, this will be dependent on the availability of radiotracers which can be distributed to centers from a remote production site (in the same way that 18F-fluoro-deoxyglucose is used in routine clinical oncology practice).
11C-metomidate imaging
Among the PET radiotracers which have been trialed for adrenocortical imaging in PA, 11C-metomidate (MTO) alone represents a noninferior lateralization modality to AVS. It is also a useful adjunct to CT and AVS in identifying the causative lesion(s) in PA (74, 75) (Fig. 6). Metomidate, an imidazole-based methyl ester derivative of the anesthetic agent etomidate, binds with high affinity to the adrenal steroidogenic enzymes 11β-hydroxylase (encoded by CYP11B1) and aldosterone synthase (encoded by CYP11B2), which are rate-limiting steps in the glucocorticoid and mineralocorticoid synthesis pathways (140). When radiolabeled with 11C, which releases a positron as it decays, it can be used as a PET radiotracer to visualize adrenocortical lesions (74, 141, 142). Importantly, when deployed to detect APAs/APNs in PA, dexamethasone pretreatment (for 72 hours) appears an essential prerequisite to achieve selectivity for CYP11B2-expressing lesions (through suppression of CYP11B1 expression) (74, 75). The importance of this key step has been highlighted in a recent study in which the absence of routine pretreatment with dexamethasone was associated with reduced sensitivity to lateralize unilateral causes of PA (143) (and outlined in Table 5).
Table 5.
Overview of clinically tested adrenocortical PET radiotracers
| Tracer | Author | Patient number | Sensitivity/Specificity | Notes |
|---|---|---|---|---|
| 11C-metomidate (MTO) | With dexamethasone pretreatment | |||
| Prospective Wu et al, 2023 (144) |
143 PA | Hierarchical primary outcomes based on PASO criteria: using the first outcome (complete or partial biochemical success): True positive (sensitivity): MTO 74.3% vs AVS 64.9% False positive (1 – specificity): MTO 4.8% vs AVS 4.8% Accuracies (at predicting biochemical and clinical success following adrenalectomy): MTO 72.7% and 65.4% vs AVS 63.6% and 61.5%, respectively |
In addition to establishing MTO as a noninvasive alternative to AVS for diagnosing surgically curable PA, this study also confirmed that while some patients will be shown to have unilateral disease on both modalities, for others only 1 or the other will reveal the unilateral nature of their PA. All 4 PASO measures of success were as likely to be achieved whether patients were diagnosed by 1 or both investigations The study also assessed a number of secondary endpoints including: prediction of subsequent surgical outcomes based on a trial of spironolactone; effect of spironolactone treatment on MTO lateralization; influence of age, sex, and ethnicity on clinical outcomes; roles of differing somatic driver mutations; utility of hybrid steroid measurements in predicting favorable outcomes |
|
| Retrospective Lu et al, 2022 (145) |
17 PA | Not reported | MTO-PET findings were investigated in patients with PA who were managed according to international guidelines.a Correlation with the findings of other lateralization modalities and outcome prediction methods were also explored. Patients with PA under medical treatment showed significantly lower tracer uptake in responders than nonresponders with respect to biochemical or clinical outcomes. Concordance of MTO and AVS was 33% (4/12 patients) aNote: clinical decision making was not guided by MTO |
|
| Prospective Puar et al, 2022 (139) |
25 PA |
a
Sensitivity: 81.9% aSpecificity: 100% aFor secondary outcome: accuracy of MTO for subtype diagnosis |
Concordance of MTO and AVS was 60% (15/25 patients). Post-surgical outcomes did not differ between PA patients identified by either AVS or MTO. MTO identified additional surgical candidates who were not considered to have unilateral disease by AVS alone | |
| Retrospective audit/clinical service evaluation O'Shea et al, 2019 (75) |
15 PA | Not reported | MTO was used in parallel to AVS to guide clinical decision making. Concordance of MTO and AVS was 75% (6/8 patients). Irrespectively, 11C-Metomidate PET/CT informed the decision to proceed to adrenalectomy in 4 patients who would not otherwise have been offered surgery | |
| Prospective Burton et al, 2011 (74) |
39 PA 5 NFA |
a
Sensitivity: 76% aSpecificity: 87% aWith an SUV max ratio of 1.25 : 1 |
Dexamethasone pretreatment for 3-days prior to imaging increased tumor to normal adrenal SUV (max) ratio by 25.6 ± 5.0% (P < .01) Note: Firstly, a preliminary study involving 6 patients investigated whether dexamethasone pretreatment reduced normal adrenal 11C-metomidate uptake. Subsequent patients received 0.5 mg dexamethasone 4 times daily for 72 hours prior to imaging |
|
| Without dexamethasone pretreatment | ||||
| Retrospective Isojärvi et al, 2022 (146) |
44 PA | Not reported | Outcomes of adrenalectomy based on clinically significant lateralization in 11C-MTO-PET alone corresponded to those based on 11C-MTO-PET with concordant AVS lateralization. Similar immunohistochemical profiles and cure rates were seen after MTO alone or AVS based surgical resection. | |
| Prospective Soinio et al, 2020 (143) |
58 PA |
Sensitivity: 55% Specificity: 44% |
Concordance of MTO and AVS for unilateral and bilateral disease were 55% and 44% respectively. MTO diagnostic performance did not outperform adrenal CT | |
| 18F-CETO | Prospective Sillin et al, 2022 (147) |
5 PA 5 HV 3 AC 5 NFA |
Not reported | High adrenal uptake combined with a low unspecific liver uptake point toward 18FCETO as a suitable tracer for adrenal imaging with longer T1/2 than MTO. Subsequent studies will determine sensitivity/specificity for distinguishing unilateral and bilateral PA |
| CXCR4 | Prospective Gao et al, 2022 (148) |
50 PA 10 NFA |
Sensitivity: 93% Specificity: 84.6% |
The optimum SUVmax cut-off for the identification of functional nodules was 8.95 (AUC 0.914 [0.828-1.000], P < .001). Diagnostic efficiency was greater for tumors >1 cm in size (sensitivity up to 97%) |
| Prospective Ding et al, 2020 (149) |
25 APA 4 IAH 10 NFA |
a
Sensitivity: 100% aSpecificity: 78.6% aFor identification of APA |
The SUVmax of APA (21.34 ± 9.41, n = 25) was significantly higher than that of non-APA lesions (6.29 ± 2.10, n = 14, P < .0001) while an optimal threshold of SUVmax = 11.18 was determined for predicting APA with a sensitivity of 88.0%, specificity of 100% | |
| Prospective/Retrospective Heinze et al, 2018 (150) |
9 PA |
a
Sensitivity: 88.9% aSpecificity: 87.2% aWith a cut-off SUVMAX = 4.9 for diagnosing PA |
68Ga-pentixafor uptake in the adrenal nodule of patients with PA was observed with median SUVmax = 8.6 whereas in the contralateral gland SUVmax was significantly lower = 4.0 (2.3-6.5; P < .001) | |
The dexamethasone pretreatment regimen included administration of 0.5 mg of dexamethasone 4 times daily for 72 hours prior to imaging unless otherwise specified.
Abbreviations: AC, adrenal Cushing; APA, aldosterone-producing adenoma; AVS, adrenal vein sampling; HV, healthy volunteer; IHA, idiopathic adrenal hyperplasia; NFA, nonfunctioning adenoma; PA, primary aldosteronism; PASO, primary aldosteronism surgical outcome.
In the earlier study of Burton and colleagues, which reported 25 patients with UPA, 10 with bilateral PA (as defined by AVS), and 5 patients with nonfunctioning adrenal incidentalomas, 11C-metomidate PET/CT (MTO-PET) was found to be a sensitive and specific noninvasive alternative to AVS in the management of PA (74). On receiver operating characteristic analysis a maximum standardized uptake value (SUVmax) ratio of 1.25:1 (comparing uptake over an adenoma vs background normal adrenal uptake) yielded a sensitivity of 76% and specificity of 87%, with specificity rising to 100% in tumors with an absolute SUVmax >17) (74). Subsequently, O'Shea and colleagues showed that in selected patients deemed unsuitable for AVS, or in whom cannulation of both adrenal veins could not be achieved, MTO-PET enabled accurate lateralization, permitting adrenalectomy to proceed confidently (75).
Consistent with these findings, in a recent small prospective clinical trial comparing MTO-PET with AVS for subtype diagnosis of PA, Puar and colleagues demonstrated that MTO performed comparably to AVS (139). Twenty-five patients with confirmed PA underwent MTO imaging (with dexamethasone pretreatment) and AVS (with 100% successful cannulation rate). Twenty-two patients were determined to have unilateral and 2 bilateral disease, while 1 patient was deemed to have indeterminate findings due to discordant lateralization. Of the unilateral cases, 20 underwent surgery with 100% complete biochemical success, and 75% complete/partial clinical success. Twelve of 20 (60%) patients had concordant PET and AVS lateralization, 4 were lateralized on PET only (20%), 3 were lateralized on AVS only (15%), and 1 patient did not lateralize on PET or AVS. Postsurgery outcomes did not differ between patients identified by either test.
In the largest prospective trial to date of molecular imaging in PA, dexamethasone-suppressed MTO-PET was compared with AVS in 143 unselected patients with PA who underwent both imaging modalities (144). Each investigation was independently scored by a clinical multidisciplinary team as showing high, intermediate or low probability of a unilateral cause of PA using established thresholds for defining lateralization. Findings on MTO-PET were scored independent, and prior to, review of AVS results. Patients proceeded to surgery if either result was scored as high probability, with the exception of 2 patients in whom discordant lateralization was observed. The primary outcome of the study was to compare the accuracy of the noninvasive test (MTO-PET) with AVS in predicting biochemical resolution of PA and the resolution of hypertension after surgery. A total of 128 patients reached the 6- to 9-month follow-up, with 61% proceeding to surgery, and 39% managed medically. Importantly, despite a larger proportion of the cohort undergoing surgery (n = 78) than might have originally been anticipated based on historical unselected cohorts, all but 1 patient achieved 1 or more PASO criterion for surgical success. Specifically, the accuracies of MTO and AVS in predicting biochemical success following surgery were 72.7% and 65.4%, respectively, and accuracies in predicting clinical success were 65.4% and 61.5%, respectively. Although, MTO-PET was not significantly superior to AVS, coprimary outcomes judged against each of the 4 PASO criteria favored MTO-PET over AVS (144). Importantly, the design of the study also allowed several key secondary outcomes to be examined. Young age, female sex, and a lower starting BP predicted patients who achieved complete clinical success following subsequent unilateral adrenalectomy; in contrast, absence of clinical success was observed in 13 patients, the majority of whom were Black and treated with a calcium channel blocker. A trial of spironolactone therapy prior to surgery demonstrated that patients whose systolic BP fell to ≤135 mmHg always achieved clinical success (partial or complete), with 67% of these patients able to discontinue all antihypertensive medications. Clinical outcomes were also found to be associated with underlying tumor somatic genotype, with all those harboring KCNJ5 mutations achieving clinical success (78% complete success), whereas those found to have CACNA1D mutations rarely achieved complete success (16%). Detection of raised levels of the hybrid steroid 18-hydroxycortisol in patients harboring KCNJ5 mutant APAs also suggests that patient stratification—both with respect to choice of lateralizing/localizing investigation and strength of recommendation for surgery—may be just around the corner (144).
A key limitation of MTO-PET is the short half-life (T1/2 20 minutes) of the radiotracer such that scanning is only available at sites with an on-site cyclotron facility (74). However, development of more stable isotopes, such as 18F-CETO (para-chloro-2-(18F)-fluoroethyl-etomidate) with a longer half-life (T1/2 110 minutes), is likely to significantly increase accessibility to molecular imaging using this family of radiotracers (151).
18F-CETO imaging
Preclinical testing showed 18F-CETO to be a specific adrenal tracer and led to a first in human evaluation in patients with various functioning and nonfunctioning adrenal tumors (n = 15) and healthy volunteers (n = 5) (147, 151). Notably, high adrenal uptake was observed which, when combined with low nonspecific liver uptake, suggests that 18F-CETO is likely to be a suitable alternative to MTO, with potential advantages in terms of adrenal selectivity and with respect to more widespread geographical availability. Two studies are currently examining the ability of 18F-CETO (including dexamethasone suppressed CETO-PET) to distinguish unilateral and bilateral causes of PA, including in an extension to the recently published MATCH trial which will allow a direct comparison with MTO-PET and AVS (ClinicalTrials.gov Identifiers: NCT04529018; NCT02945904).
18F-AldoView imaging
Several other 18F-labelled radiotracers have also been developed with a view to selectively targeting CYP11B1 (eg, 18F-FAMTO) or more specifically CYP11B2 (eg, 18F-CDP2230), with preclinical studies, suggesting potential application in PA, although clinical trials are still awaited (152, 153). In recent years, Merck and Co have developed more specific CYP11B2 ligands, which are derived from benzimidazole (154). In subsequent work, Sander and colleagues selected the benchmark benzimidazole derivative and labelled it with fluorine-18 to generate a highly selective radiotracer known as 18F-AldoView (CYP11B2 IC50 4.7 nM vs 435 nM for CYP11B1) (155). The selectivity of this radiotracer was investigated by quantitative phosphor imaging in human adrenal tissue sections from 5 patients with PA who had undergone adrenalectomy and demonstrated a high degree of concordance between immunohistochemistry and 18F-AldoView binding patterns in each case. The specific tracer binding in CYP11B2 positive regions ranged from 8.6 to 19.1 kBq/cm2 and in APAs from 2.5 to 19.7 kBq/cm2 (n = 6). Promisingly, CYP11B2 negative regions did not show significant tracer uptake, with low tracer binding in both control subjects (2.6 ± 1.8 kBq/cm2; n = 3) and in CYP11B2-negative areas in samples from patients with PA (3.2 ± 1.1 kBq/cm2; n = 4). The authors also demonstrated a favorable pharmacokinetic profile in studies in mice that included rapid distribution and clearance of the tracer. Clinical studies are awaited.
CXCR4 imaging
As an alternative to targeting CYP11B2, Heinze and colleagues, based on the observation that high levels of the CXC chemokine receptor type 4 (CXCR4) are expressed in APAs (with strong correlation with sites of CYP11B2 expression), explored the use of the CXCR4 ligand 68Ga-pentixafor in imaging 9 patients with APAs (150). Reference values for 68Ga-pentixafor uptake on PET/CT were determined by retrospective analysis of studies of normal adrenals in patients with multiple myeloma (n = 20), pleural mesothelioma (n = 6), and gastrointestinal tumors (n = 18) who had undergone imaging for other clinical indications. In these controls, SUVmax values ranged from 1.0 to 5.8 (mean SUVmax: left adrenal gland, 3.4; right adrenal gland, 3.0). Patients with an APA had a median SUVmax = 8.6 (4.7-18.3), while in the contralateral adrenal, uptake was significantly lower: SUVmax = 4.0 (2.3-6.5; P < .001). Receiver operating characteristic analysis in APA patients revealed that at 100% specificity (SUVmax cut-off 7.3), the sensitivity was 77.8% and at 100% sensitivity (SUVmax cut-off 4.7), specificity was 83.7% with an AUC of 0.964 (150). In a prospective study by Ding and colleagues in 36 patients suspected to have PA, 68Ga-pentixafor PET/CT was reported to show comparable sensitivity and specificity. In this study, 39 adrenal lesions in 36 patients were identified (25 APA, 4 idiopathic adrenal hyperplasia, and 10 nonfunctioning adenomas based on histology and clinical assessment). In APAs, the SUVmax (21.34 ± 9.41) was significantly higher than that of non-APA lesions (6.29 ± 2.10, n = 14, P < .0001), while an optimal threshold of SUVmax = 11.18 achieved sensitivity and specificity of 88% and 100% respectively with an AUC of 0.98% (95% CI 0.94-1.01) (149). Both studies were of limited sample size, and the ubiquity of expression of CXCR4 may challenge the specificity of this approach in larger studies. Furthermore, increased CXCR4 expression has been reported in cortisol producing adenomas, while increased 68Ga-pentixafor uptake is also observed in patients with Cushing syndrome (150, 156).
In another study, Hu and colleagues prospectively evaluated 68Ga-pentixafor PET-CT for the lateralization of PA in 100 patients. While surgical outcomes were reported in just under a third of cases (n = 31), the primary reference standard against which 68Ga-pentixafor was compared for accuracy of lateralization was AVS (sequential cannulation, without ACTH-stimulation). The SUVmax was measured at 10 and 40 minutes, and lateralization indices (LI = [SUVmax of dominant side]/[SUVmax of nondominant side] between dominant and nondominant sides were calculated and evaluated. 68Ga-pentixafor PET/CT scans were independently analyzed by 2 nuclear medicine experts who were blinded to AVS results (157). The authors reported that the 10-minute timepoint was superior to 40 minutes. When comparing against AVS, a 10-minute LI of 1.65 achieved sensitivity of 0.77 and specificity of 1.00. An LI of 1.57 achieved the best combined sensitivity (0.86) and specificity (0.91) Using criteria for maximum specificity, no bilateral disease was misclassified as having UPA. 68Ga-pentixafor PET-CT successfully diagnosed all AVS confirmed unilateral PA cases when a nodule of >10 mm in diameter was present. However, 44.4% of those who had micronodular pathology (4 of 9 patients) were missed by 68Ga pentixafor PET/CT, suggesting the sensitivity of this method might not be ideal for detecting smaller lesions. Surgical outcomes were reported as a secondary analysis for 31 patients all of whom lateralized by AVS, of whom 27 also lateralized by PET/CT. The smallest nodule detected by PET/CT was 8 mm in diameter.
Importantly, the study was prospective in nature and adds to the body of evidence supporting an emerging role for molecular imaging in the investigation of PA. However, unlike the MATCH trial comparing MTO-PET with AVS, the study of Hu and colleagues lacks important surgical endpoint data for true validation of the imaging findings, as only 31 patients progressed to surgery, reflecting the fact that where adrenalectomy was performed, the decision was informed by AVS alone. In addition, the tracer appears to be less sensitive in detecting micronodular disease, which could mean that an important subgroup of UPA cases go unrecognized. However, performance of 68Ga-pentixafor was superior to CT scan alone—which in this prospective study had a concordance rate of only 54% with AVS. The study did not include a control population with nonfunctioning adrenocortical nodules, pheochromocytoma, or adrenal Cushing. Interestingly, patients with suspected Cushing or subclinical Cushing were excluded from the study on the basis of previously reported high CXCR4 expression in cortisol secreting adrenal adenomas. This is also relevant, because a criticism which has been levelled when using 11C-Metomidate as a PET tracer has been its lack of specificity for CYP11B2, and hence the essential requirement for preteatment with dexamethasone to achieve this specificity. It now appears that the specificity of 68Ga-pentixafor may also be limited in this regard. However, the key question in relation to the performance of any of these tracers will be their ability to distinguish between functioning and nonfunctioning nodules.
An upcoming 2-step randomized control trial (CASTUS) will assess (1) The accuracy of 68Ga-pentixafor PET/CT by determining the concordance between 68Ga-Pentixafor PET/CT and AVS. If found to meet the predefined concordance threshold, the study will progress to assess (2) the clinical outcome in a randomized controlled trial of 68Ga-Pentixafor PET/CT vs AVS with the primary outcome being the daily defined doses of antihypertensive drugs for BP regulation after 1 year, and secondary outcomes being quality of life, biochemical, and clinical cure (according to the PASO criteria) and costs (158). While current data highlight promising lateralization ability of 68Ga-Pentixafor PET/CT, this tracer is not widely available for routine clinical use for patients with PA at this time. Furthermore, evaluation of 68Ga-Pentixafor PET/CT vs AVS based on clinical outcomes in the CASTUS study will prove essential to determine future clinical utility of this tracer.
Radiomics
Radiomics represents a quantitative approach to medical imaging, assisted by mathematical models and artificial intelligence, which can identify disease specific patterns in biomedical images that may not be visible to the human eye (159). In the context of the adrenal, several studies have examined the ability of radiomics to differentiate adrenal tumor subtypes, including adenoma, metastases, pheochromocytoma, ACC, and cortisol-secreting adenomas (systematically reviewed by Stanzione et al, 2022) (160-165).
To date, however, only a small number have focused on the differentiation of functioning and nonfunctioning adenomas. Most recently, Chen and colleagues investigated the use of radiomics to distinguish nonfunctioning adenoma in essential hypertension and functioning adenomas in PA (166). Data from unenhanced and venous phase CT images of 60 patients with nonfunctioning adenoma, and 91 patients with surgically proven APA were retrospectively interrogated using least absolute shrinkage and selection operator (LASSO) logistic regression for feature selection. The model was capable of differentiating nonfunctioning adrenal nodules, from APAs with sensitivity, specificity, and accuracy of 83.3%, 78.9% and 80.6% (AUC = 0.91 [0.72, 0.97]) on unenhanced CT and 81.2%, 100%, and 87.5% (AUC = 0.98 [0.77, 1.00]) on venous phase CT, respectively. The model was also capable of predicting biochemical (unenhanced/venous CT: AUC = 0.67 [0.52, 0.79]/0.62 [0.46, 0.76]) and clinical success (unenhanced/venous CT: AUC = 0.59 [0.47, 0.70]/0.64 [0.51, 0.74]) following surgery.
In a separate study, He et al, 2021 retrospectively combined radiomic signatures with common clinical features for prediction of APA (167). Here, radiomic features from unenhanced CTs of 90 patients with confirmed unilateral APA (as confirmed by AVS), were analyzed using LASSO logistic regression for radiomic feature selection, with subsequent generation of a Radscore (radiomic score) for each individual patient using multiple logistic regression. A clinical-radiomic nomogram model was then developed, which comprised the Radscore, age, sex, serum potassium level, and ARR, and performance characteristics were evaluated using calibration plots. The model demonstrated good discrimination in both the training, and validation cohort achieving an AUC of 0.900 (95% CI 0.807-0.993) and 0.912(95% CI 0.761-1.000), respectively. Ultimately, both studies point toward the potential utility of radiomics and machine learning in aiding clinical decision-making, as a noninvasive means of identifying APAs.
Although promising, it is important to bear in mind that the application of radiomics in clinical practice is still in its infancy and, as such, interstudy variability in methodology has highlighted significant problems with reproducibility (159). Indeed, as was noted in the study of Stanzione et al, 2022 where a radiomics quality score was used to assess the crucial steps in radiomic pipelines (including but not limited to imaging protocol, features extraction and handling, clinical relevance, and data openness), the quality of radiomic studies in adrenal disorders was deemed unsatisfactory, with a median radiomics quality score of 6% (IQR 0-22%) across 25 studies reported between 2016 and 2022 (163). The authors pointed toward 3 potential reasons for the low radiomics quality score: a lack of prospectively designed studies, an absence of proper validation, and poor openness of data. Indeed, all studies currently reporting radiomics findings from adrenal imaging are retrospective in nature, thereby emphasizing the requirement for future prospective studies to understand the true effectiveness of radiomics in aiding clinical decision making in the management of PA.
Future perspective of PA lateralization
Several advances have occurred in the research and clinical research space that point to improved methodologies for lateralization and localization of APA, with the potential to significantly reduce, and perhaps even supersede, our reliance on AVS. If we are to fully capitalize on these developments it is crucial that further high-quality evidence is generated in the fields of molecular imaging and radiomics to facilitate this transition.
For now, it seems that CT imaging alone, as currently performed and analyzed (and without the application of radiomics), is an inferior alternative to AVS, except in a small number of clinical contexts. In contrast, emerging data from studies with several different PET radiotracers suggests that molecular imaging offers the possibility of delivering a step-change in the management of PA, even potentially replacing AVS as the lateralization standard. This will not happen immediately and any change in practice will require sufficient prospective evidence to justify such change. Nonetheless, the fact that molecular imaging is a noninvasive modality, which is faster and more easily interpretable, which can precisely localize lesions within the gland, and which can be performed without the technical challenges associated with AVS, confers significant advantages. Initially, we envisage that with greater availability of molecular imaging, PET CT may become the first line lateralizing procedure—perhaps even performed as the initial radiological investigation (ie, without prior CT) because of its ability to provide both anatomical and functional information. AVS might then be reserved for the small number of cases where unilateral disease is strongly suspected but molecular imaging does not clearly localize a lesion. Such a scenario might occur when both adrenal glands appear morphologically normal on CT (ie, harbor lesions that are beyond the resolution of even modern PET CT). Further work is also needed to determine what should be done if molecular imaging and AVS are both performed, but suggest discordant lateralization. No clear evidence exists from the very small number of cases reported to date (139, 144), and further comparative studies will be required. Ideally, such studies should also include comparison of unstimulated and stimulated AVS with molecular imaging, given a previous report of a single patient in whom discordant lateralization was observed with stimulated AVS, but concordant lateralization when subsequent unstimulated AVS was performed (139). Understanding why such discordance occurs and which investigation is likely to be “correct” in a given clinical context will be crucial to ensuring clinicians can confidently recommend surgery or other targeted intervention.
For all PET radiotracers, their sensitivity and performance is likely to be enhanced in patients with more severe disease phenotypes. Therefore, further prospective studies (including in comparison with AVS) will need to evaluate their accuracy in more subtle disease phenotypes. Linked to this, at a more basic level, the selection of novel candidate radiotracers for molecular imaging in PA is likely to be informed by further improvements in our understanding of the pathophysiology of the different subtypes of PA.
Finally, in the emerging world of multi-omics, and in the context of the rich metabolomic potential of PA (“Improving the Diagnosis of PA”), the prospect of using molecular imaging in the context of radiomics, radiometabolomics or radiogenomics to identify and lateralize functioning aldosterone producing lesions offers an exciting glimpse of what might be possible in the not too distant future.
Novel Treatment Approaches
Partial Adrenalectomy: Targeted Tumor Resection
Limitations of adrenalectomy
Complete unilateral adrenalectomy is often the treatment of choice for UPA (Fig. 1). In general, it is a well-tolerated, safe, and effective procedure for the treatment of unilateral disease. With laparoscopic and retroperitoneoscopic approaches, it is also a quick and relatively uncomplicated procedure, usually requiring only a short hospital stay. However, there are some drawbacks to complete adrenalectomy. Firstly, adrenalectomy has traditionally been considered in lateralized cases with an apparent APA, amounting to approximately 30% of PA patients, with the remaining 70% of patients with bilateral disease reliant on pharmacological intervention (34, 168). Many patients with cardiorenal complications of PA often represent poor operative candidates and patients with bilateral disease (even where localizable) do not normally undergo bilateral adrenalectomy due to inevitable and unacceptable, postprocedural adrenocortical insufficiency. Additionally, as previously discussed, up to 30% of patients undergoing unilateral adrenalectomy for PA demonstrate partial (often subclinical) or, less commonly, complete postprocedural glucocorticoid insufficiency, albeit a temporary phenomenon in most instances (75, 77, 109). It is not clear whether this is due to loss of adrenal volume or the presence of co-secretion of glucocorticoids from APAs. Nakada et al, interestingly, have previously demonstrated a blunted response to stress in adrenalectomized patients, when compared to control subjects (169). In addition, a small but important group of patients experience mineralocorticoid insufficiency (170).
Partial adrenalectomy/adrenal sparing nodulectomy
Kitamoto et al, in 2020, explored the effectiveness of partial adrenalectomy (PAY), retrospectively reporting their experience over a 7-year period (135). The authors evaluated the diagnostic accuracy of sAVS (see “Advancing to Localization using Segmental AVS” for procedure overview) and the outcome of PAY according to PASO criteria in both unilateral and bilateral forms of PA in 278 patients. Segmental AVS facilitated the creation of an intra-adrenal aldosterone activity map (IAMap) which was used with CT to categorize patients with either unilateral or bilateral disease (120 and 158 patients, respectively). These patients were further categorized as suitable or unsuitable surgical candidates, dependent on the confirmation of a sole source of aldosterone secretion. Of 128 unilateral cases, 68 were deemed suitable for PAY, with postoperative evaluation reporting complete biochemical success in all patients. This was comparable to the outcome of complete adrenalectomy with partial adrenalectomy possessing the added benefit of shorter surgical time (PAY = 93.7 ± 31.1 minute vs total adrenalectomy = 114.1 ± 28.1 minute). The performance of partial adrenalectomy in bilateral disease was not as encouraging. Following partial adrenalectomy in the 25/158 bilateral cases, the complete biochemical success rate was 28%, the partial biochemical success rate was 72% and the complete clinical success rate was 36%. Therefore, there was considerable residual disease in those undergoing partial adrenalectomy.
Indeed, several studies have reported comparable levels of clinical and biochemical success for complete adrenalectomy and PAY (Table 6) (135, 171-173). However, PAY is suitable only for a subgroup of patients, although increased access to molecular imaging could mitigate its current dependence on sAVS which is available in only a handful of centers. PAY is also limited by the increased risk of hemorrhage from residual, nonresected ipsilateral adrenal tissue, as was reported in the study of Bin Fu et al, 2011, where mean intraoperative blood loss was 15.1+/−6 mL (in the total adrenalectomy group) vs 35.3+/−10 mL (in the partial adrenalectomy group), P = 0.01 (72, 171). Additionally, preservation of adrenal blood supply may be necessary for residual adrenal function, and therefore resection of an adrenal body tumor is likely to require complete rather than partial adrenalectomy (136, 174). However, perhaps the most important limitation relates to the potential presence of multiple nodules, both contralateral and ipsilateral, which can render PAY futile if residual functioning tumors are left behind. Several histological studies have demonstrated that MAPNs are found in as many as 21% of complete adrenalectomy specimens (83, 132, 135, 171, 175-178). Given the nature of PAY and the likely formation of scar tissue following the procedure, it seems likely that any future surgical intervention following incomplete removal of abnormal functioning tissue would face added complexity.
Table 6.
Outcomes of partial adrenalectomy in the treatment of primary aldosteronism. Clinical outcome is described in accordance with PASO criteria (Williams 2017) as either complete (C), partial (P) or absent (A) clinical (CCS, PCS, ACS) and/or biochemical success (CBS, PBS, ABS)
| Study type/author and year | Surgical approach | Tumor type/identification method | Unilateral (UL)/bilateral (BL) | Patient no. | Clinical outcome at follow-up: | Complications and procedural challenges | Follow-up (months) | Length of hospitalization |
|---|---|---|---|---|---|---|---|---|
| Case report Chen et al, 2022 (132) |
Laparoscopic right partial nephrectomy for a renal mass, a right partial adrenalectomy, and a left total adrenalectomy | Right adrenal: CYP11B2 IHC positive nodule 1.7 × 0.8 × 0.7 cm in size (CYP11B1 negative) Left adrenal: CYP11B1 IHC positive nodule (CYP11B2 negative) Note: Bilateral CYP11B2 positive MAPM staining was present |
BL | 1 | Normotensive, with complete biochemical success. Note: The patient also presented with autonomous cortisol secretion, which was resolved at 12 month follow-up |
None reported | 12 | Not defined |
| Retrospective, Multicenter Anceschi et al, 2020 (172) |
Laparoscopic, PAY and TAY | Single adenoma <3 cm identified with CT or MRI or AVS | UL | UL, PAY = 29 UL, TAY = 61 |
PAY:
CCS = 72.4% PCS = 6.2% ACS = 20.7% TAY: CCS = 54% PCS = 23% ACS = 23% |
No major complications | 42 | 3 days vs 4 days in total adrenalectomy cohort (P = .038) |
| Retrospective, multicenter Kitamto et al, 2020 (135) |
Laparoscopic PAY and TAY |
Single adenoma confirmed with CT and sAVS | UL and BL | UL, PAY = 68 BL, PAY = 25 |
PAY:
UL, CCS = 63.2% UL PCC = 36.8% UL, CBS = 100% BL, CCS = 36% BL, PCS = 72% BL, CBS = 28% BL, PBS = 72% aOutcomes comparable vs TAY (P > .05) |
Difficulty canulating segmental adrenal veins during screening | 12 | Not defined |
| Prospective, multicenter Simone et al, 2018 (179) |
Robot Assisted, Laparoscopic PAY | Single adenoma <3 cm identified with CT or MRI and AVS | UL | UL PAY = 10 |
PAY:
CBS = 100% CCS = 90% |
1 patient; postoperative fever requiring antibiotic treatment | 12 | 3 days |
| Case study, Morimoto et al, 2015 (136) |
Laparoscopic PAY (right adrenal) (TAY left adrenal) | Bilateral, adenoma <3 cm in both the left and right adrenal, confirmed with CT and sAVS (right = 10 mm and left = 12 mm in diameter) | BL | 1 | 100% PCS (1 antihypertensive required) and 100% CBS achieved | Left adrenal underwent TA as it was determined unsuitable for PAY based on the central location of the tumor | 8.3 | Not defined |
| Prospective Bin Fu et al, 2011 (171) |
Laparoscopic, PAY and TAY | Single adenoma, confirmed with CT and MRI and AVS | UL | UL, PAY = 104 UL, TAY = 108 |
PAY:
CCS = 72.1% CBS = 100% aOutcomes comparable vs TAY (P > .05) |
Significantly higher intraoperative blood loss in PAY vs TAY (P < .05) | 96 | 3.9 days |
| Retrospective Toutounchi et al, 2020 (180) |
Laparoscopic PAY | Single adenoma Confirmed with imaging, and AVS aImaging method not defined |
UL | UL, PAY = 8 | Cure of PA was achieved in all patients, and sustained at 1 year follow-upa aNo clinical/biochemical information provided |
No major complications | 12 | Not defined |
| Case study Yetişir et al 2013 (181) |
Laparoscopic, PAY | Single adenoma, confirmed with MRI and CT | UL | UL, PAY = 1a aRight kidney + adrenal had been previously removed prior to presentation |
CCS = 100% CBS = 100% |
No major complications | 3 | 4 days |
| Retrospective, single center Ko et al 2019 (182) |
Laparoscopic, PAY and TAY | Single adenoma confirmed with CT or MRI aPatients who had other adrenal tumors have been excluded from this table |
UL and BL | UL, PAY = 8 BL, PAY = 3 UL, TAY = 16 |
PAY
CCS = 73% PCS = 27% CBS = 100% aOutcomes comparable vs TAY (P > .05) |
Significantly higher intraoperative blood loss in PAY vs TAY (P < .05) | 49.2 | 6 days |
| Retrospective, Single center aJeschke et al, 2003 (174) |
Laparoscopic, PAY | Single adenoma <3 cm, confirmed with CT | UL | UL, PAY = 13 | CCS = 100% CBS = 100% |
No major complications | 39 | 4.3 days |
| Retrospective Single center Liao et al, 2009 (183) |
Laparoscopic, Bilateral PAY | Bilateral, single adenoma <3 cm confirmed with CT or MRI and adrenal scan with 131I-6β- iodomethyl-norcholesterol (NP-59) under dexamethasone suppression to confirm functioning tumors | BL | BL, PAY = 5 | CCS = 80% PCS = 20%a |
No major complications | 44 | 5.1 days |
| Prospective, Single Center Kok et al 2002 (184) |
Laparoscopic, PAY | Single adenoma, confirmed with CT | UL | 8 | CCS = 87.5% PCS = 12.5% CBS = 100% |
One tumor was located posteriorly, requiring an alternative method of resection due to fear of increased blood loss. Regardless, the tumor was removed, but patient required continued (albeit lower dosage) antihypertensive medications | 25 | 3.8 days |
Abbreviations: IHC, immunohistochemistry; PAY, partial adrenalectomy; sAVS, segmented adrenal vein sampling; TAY, total adrenalectomy.
a The underlined studies did not formally use PASO outcome criteria during their analysis. However, for consistency of comparison within this review, PASO criteria were applied to each study where outcome measures were available.
Thermal Ablation: A Targeted Minimally Invasive Therapeutic Approach
Thermal ablation can potentially target the source of aldosterone hypersecretion using a minimally invasive percutaneous approach, with a short procedure time, without the need for overnight hospitalization and usually without the need for general anesthetic (185, 186). Ablation involves localized delivery of nonionizing energy with a minimally invasive applicator placed under imaging guidance, typically CT or ultrasound, with the goal of delivering sufficient heating (>∼50 °C) to achieve coagulative necrosis (187) (Fig. 7). A variety of energy sources are available for thermal ablation, of these, radiofrequency ablation (RFA) and microwave ablation (MWA) have received the most attention for treatment of adrenal disease in recent decades, with demonstrated preclinical and clinical efficacy (188). Notably, use of MWA has potential for treatment of localized bilateral adrenal nodules with minimal risk of adrenocortical insufficiency, as was reported in an in vivo porcine model with a MWA applicator designed for precision therapy (86), while a recent meta-analysis reports that RFA offers comparable efficacy to laparoscopic adrenalectomy (188-190). In this analysis, patients who underwent RFA had shorter operative time (standard mean difference: −1.98), less intraoperative blood loss (standard mean difference: −0.61), and shorter hospital stay (weight mean difference: −1.40) while no significant differences were found in the complication rate vs those who underwent laparoscopic adrenalectomy (189). However, the use of ablation for adrenal tumors is in its infancy and bespoke probe designs may be necessary with ablation patterns designed to minimize thermal damage to normal adrenal tissue.
Figure 7.
Outline of adrenal ablation. During the procedure, energy in the form of radiofrequency or microwave is transferred from the ablation probe to the tumor producing lethal hyperthermia (≥50 °C) that kills cells within the targeted lesion. Outside of the core ablation zone, the transitional zone experiences various levels of sublethal hyperthermia that have differential effects on cellular viability. Figure created with BioRender.com.
Clinically, most ablations of the adrenal gland have been carried out using CT-guided, retroperitoneoscopic RFA (Table 7). RFA has been most widely used for inoperable malignancies/metastases of the liver or kidney, with recent application in bone, lung and primary breast cancers (191, 192).
Table 7.
Outcomes of ablation in the treatment of primary aldosteronism.
| Study type/author and year | Ablation modality | Tumor size Unilateral (UL)/bilateral (BL) |
Patient no. | Outcome at latest follow-up: | Complications | Latest follow-up | Length of hospitalization |
|---|---|---|---|---|---|---|---|
| Prospective, multicenter study assessing: Safety and feasibility of radiofrequency ablation using bipolar electrodes for aldosterone Oguro et al, 2022 (193) |
Percutaneous CT-guided RFA using bipolar electrodes | 14.8 ± 3.8 mm UL |
Enrolled for study: 40 37 ablated |
Normalized hormone activity:
In cases with technical successa: 31/35 cases In cases with incomplete ablationb: 1/2 cases = 86.5% reached normalized excretion of aldosterone on day 84 Number/types of antihypertensive drugs day 0 = 1.6 ± 1.3 reduced to 0.5 ± 0.7 on 84 days after the procedure (P < .001) Normalized hormone activity = plasma aldosterone concentration < 15 ng/dL or aldosterone-renin ratio (ARR) < 30 aTechnical success = Ablated tumor volume = 100% as determined by postprocedural dynamic contrast-enhanced CT 7 days post first ablation session bIncomplete ablation = <100% ablated tumor volume |
Intraprocedural hypertensiona: 16 of 38 sessions (42.1%) Thoracic bleeding with hemorrhagic shock due to intercostal arterial injury: 1/38 (2.6%) aSystolic blood pressure >180 mm Hg |
84 days | Not defined |
| Retrospective, cohort analysis of laparoscopic adrenalectomy vs radiofrequency ablation Valderrama et al, 2022 (194) aSame RFA cohort used in Valderrama et al, 2021. See below… |
RFA | Not defined UL |
Ablation: 10 Laparoscopic adrenalectomy: 24 |
Ablation:
%Patients cured: a11.1, b0 %Patients improved: a66.7, %Patients unchanged: a22.2, Laparoscopic adrenalectomy (LA): %Patients cured: a26.1, b29.2 %Patients improved: a56.5, %Patients unchanged: a17.5, aResults at 3 months follow-up. bResults at latest follow-up “Cured” = patients that were not taking any antihypertensive medications and had controlled blood pressure at follow-up. “Improved” = patients who were taking fewer hypertension drugs and/or had controlled or improved blood pressure when compared with preintervention |
Hypertensive crisis was seen more often after RFA (1 patient, 4.2% in the LA group vs 7 patients, 70.0% in the RFA group, P < .001). Although no patient suffered any complication deriving from the crisis aThe authors did not provide a reference range of systolic/diastolic blood pressure to define hypertensive crisis |
Minimum 3 months Median latest follow-up for ablation (months): 94.7 Median latest follow-up for LA (Months): 26.9 |
Median length of stay for ablation: 1 day Median length of stay for LA: 2 days P < .001 |
| Prospective, randomized medication-controlled Zhao et al, 2021 (195) |
Catheter based ablation Medical group = Spironolactone (20-60 mg daily) |
Not defined UL |
Ablation: 26 Medication: 25 |
Ablation:
Outcomes described according to PASO criteria CCS: 26.9% PCS: 53.9% CBS: 57.7% Medication: Similar improvements in blood pressure control compared w/ablation, but no reduction in plasma aldosterone was observed |
Ablation: N/A Medication: 52% patients experienced spironolactone-related adverse events |
6 months | Not defined |
| Prospective, bicentric, pilot study Bouhanick et al, 2021 (196) |
Percutaneous CT guided RFA | <4 cm UL |
30 |
Clinical
Mean blood pressure pre- vs post-procedure = 143.9 ± 18.5/95.4 ± 14.8 vs 131 ± 14/87 ± 10 Mean no. of hypertensive medications pre vs postprocedure = 2.5 ± 1.2 vs 1.4 ± 1.6a a(12/28 = 43%) received no antihypertensive treatment of which (7/28 = 25%) had normal daytime BP Biochemical Serum K+ normalization = 70% ARR normalization = 90.5% |
(3/30) Pneumothorax (1/30) Backpain post procedurally (1/30) Lesion of polar adrenal artery |
6 months | 1-3 days |
| Retrospective Valderrama et al, 2021 (194) |
Percutaneous CT guided RFA | UL | 10 |
Clinical
“Controlled blood pressure” = 50% a aConsidered controlled if BP was lower than 140/90 mmHg Mean no. of hypertensive medications pre- vs post-procedure = 4 vs 3 Biochemical Serum K+ normalization = 70% ARR normalization = 25% |
(7/10) Intra-procedural Hypertensive crisisa aThe authors did not provide a reference range of systolic/diastolic blood pressure to define hypertensive crisis |
46.2 months | 1-2 days |
| Retrospective Lo et al, 2020 (197) |
Percutaneous CT guided RFA | <3 cm 19 mm ± 6 (Mean) UL |
8 |
Clinical
Mean blood pressure pre- vs post-procedure = 162 ± 19/96 ± 13 vs 125 ± 16/69 ± 6 (P = .0273/.0277) Mean no. of hypertensive medications pre- vs post-procedure = 3.33 ± 0.82 vs 1.33 ± 1.21 (P = .023) Biochemical Mean serum K + Levels pre- vs post-procedure = 2.16 ± 0.22 vs 4.34 meq/L ± 0.54 (P = .0431) Mean ARR pre- vs post-procedure = 100.73 ± 124.44 vs 28.73 ng/dL per ng/mL/h ± 30.74, (P = .138) |
(1/8) Intra-procedural hypertensive crisis (SBP = 301/108 mmHg) (1/8) Mild retroperitoneal hematoma |
80.4 ± 25.2a Months aOnly 5 patients had long-term follow-up data |
Not defined |
| Retrospective Liu et al, 2016 (198) |
Percutaneous CT guided RFA | <3 cm 15.5 mm ± 5.0 UL |
36 |
Clinical
Mean blood pressure pre- vs post-procedure = 158 ± 18/94 ± 12 vs 128 ± 10/76 ± 9 (P = < .01/.01) Reduced hypertensive medications = 55.6% Biochemical Hypokalemia resolved = 100% ARR within normal range = 92% |
(3/36) Intraprocedural hypertensive crisis (SBP >180 mm Hg) (3/36) Retroperitoneal Hematomas (3/36) Pneumothorax (1/36) infected retroperitoneal hematoma |
74.4 ± 30 months | 2.4 ± 1.0 days |
| Retrospective Sarwar et al, 2016 (185) |
Percutaneous CT guided RFA | <4 cm 10-30 mm UL |
12 |
Clinical
Mean blood pressure pre- vs post-procedure = 145 ± 19/94 ± 13 vs 129 ± 11/81 ± 11 (P = .02/.001) Reduced hypertensive medications = 58% Biochemical Hypokalemia resolved = 100% Mean ARR pre- vs post-procedure = Not reported |
(8/12) Intraprocedural hypertensive crisis (SBP >180 mm Hg) | 9-1408 days | 0.6 ± 0.8 days |
| Retrospective Yang et al, 2014 (186) |
Retroperitoneoscopic, laparoscopy and ultrasound guided cool-tip radiofrequency ablation (RCRFA) | <4 cm 26.7 + 5.4 mm |
12 |
Clinical
Mean blood pressure pre- vs post-procedure = 173 ± 12/98 ± 8 vs 138 ± 16/81 ± 9 (P < .05/.05) Reduced hypertensive medications = 91.7% Biochemical Hypokalemia resolved = 100% Mean ARR pre- vs post-procedure = 54.33 ± 24.90 vs 5.50 ± 3.30 |
(1/12) Post-operative Perinephric hematoma (1/12) Postoperative paresthesias |
49.2 ± 15.6 Months | 2.3 ± 0.65 days |
| Case study Nunes et al, 2013 (199) |
Percutaneous CT-guided RFA | 22 cm UL |
1 |
Clinical
Blood pressure pre- vs post-procedure = 150/100 vs 120/80 Reduced hypertensive medications = yes, from 4 to 2 Biochemical Hypokalemia resolved = 100% ARR pre- vs post-procedure = 94.16 vs 4.2 |
N/A | 6-month intervals | Not defined |
Abbreviations: ARR, aldosterone to renin ratio; DBP, diastolic blood pressure; RFA, radiofrequency ablation; SBP, systolic blood pressure.
To the authors’ knowledge, only 2 prospective studies using RFA in the treatment of PA have been published to date, 1 of which was a pilot study involving 30 patients (Table 7) (196). At 6-month follow-up, 47% of patients met the primary endpoint criterion (BP reduction to less than 135/85 mmHg without antihypertensive medications or a reduction of at least 20 mmHg for systolic or 10 mmHg for diastolic BP) (196). Oguro and colleagues carried out a prospective multicenter study using RFA in 37 patients. Technical success was defined as 100% ablated tumor volume, determined by independent analysis of a postprocedural dynamic contrast-enhanced CT, performed 7 days post-treatment. The technical success rate was 94.6% (35/37) with 1 patient requiring a second ablation. The primary endpoint was defined as the normalization of hormone activity (defined as either plasma aldosterone concentration <15 ng/dL (<416 pmol/L) or ARR <30) on day 84 postablation. 86.5% achieved the primary endpoint criterion of normalized aldosterone secretion. Notably, the mean number of antihypertensive medications was reduced from 1.6 ± 1.3 to 0.5 ± 0.7 on day 84 after the procedure (P < .001).
Indeed, several retrospective studies summarized in Table 7 demonstrate favorable outcomes of RFA. The main limitation of the cumulative retrospective analyses is the variation in outcome criteria and the inconsistent use of preprocedural AVS (186, 197, 198).
Other ablation modalities include catheter-based ethanol ablation, which has previously been used in cases of PA without apparent APAs (200). This minimally invasive approach involves selective infusion of high concentration ethanol into adrenal arteries that supply the lateralized adrenal gland or APA resulting in tissue necrosis. The technique can be technically challenging with limited control of the ethanol distribution profile, and as such its application has been limited. However, it does provide an alternative option for patients who refuse surgery or in those for whom surgery is considered high risk (201). In a recent prospective, medication-controlled study, the authors observed complete or partial hypertension remission as well as biochemical improvement in most patients following catheter-based adrenal ablation at a comparable level to those undergoing adrenalectomy. This was applied in unilateral disease only (195, 202).
Drawbacks of thermal ablation
A number of important considerations have been highlighted in relation to RFA of adrenal lesions. These include studies reporting an intraprocedural hypertensive crisis (presumed consequent to medullary degranulation), which suggests the requirement for preprocedural and periprocedural administration of pharmacological alpha-blockade (185, 194, 197, 198). However, the prevalence of this phenomenon is difficult to gauge and may be subject to reporting bias. Larger tumors can require multiple treatment cycles to achieve complete destruction of the tumor (193). Tumors that are located close to structures such as the inferior vena cava, lung, kidney, splenic artery, or liver are often not suited for ablation, given the difficulty in electrode placement and/or risk of organ injury (203), although this can be partially mitigated using hydrodissection (186, 198). Tumors near blood vessels are also subject to a “heat-sink” effect where heat from the RF applicator dissipates through the circulating blood, reducing the effectiveness of the ablation procedure (186). Probe placement can also prove problematic, as seen in the case of Oguro and colleagues where intercostal arterial injury occurred during insertion of the needle (193).
As an alternative to RFA, MWA may offer some benefits. In general, MWA is less susceptible to heat-sink, while also being capable of producing larger ablation zones in a shorter timeframe, using continuous rather than pulsed energy application. MWA technology development, and subsequent application in the clinical setting, was motivated by the challenges with achieving large volume ablation zones with RFA (204). Controlled delivery of MWA has also been challenging, although the development of MWA applicators with angular control of radiation pattern may provide a means to target tumors without penetrating the target with the applicator probe (205).
Furthermore, RFA and MWA do not discriminate between the targeted lesion and normal tissue, with the threshold thermal dose for ablative effect similar across most tissue types. As such, localizing ablation zones to the target tissues requires design of devices with energy delivery profiles matched to the target tissue, and careful selection of energy delivery profiles. Nanoparticle-based approaches offer the opportunity for high precision therapy by coupling an energy source that has minimal effect on tissue in the absence of exogenous materials that absorb the delivered energy; precisely delivering nanoparticles to the target tissue offers a means for highly localized therapy. Photothermal therapy with gold nanoparticles or carbon nanotubes has been explored for treatment of disease in the prostate, liver, and other sites (206). Due to the limited penetration of light in the body, photothermal therapy would require a minimally invasive applicator to target adrenal lesions. The use of alternating magnetic fields to heat magnetic nanoparticles offers the potential for noninvasive thermal therapy of adrenal nodules, though it is technically challenging to deliver adequate magnetic field strengths at depth while limiting off-target heating due to Eddy currents (207). A challenge with nanoparticle-based approaches is achieving adequate accumulation of the nanoparticles within the targeted tissue, while limiting toxicity and uptake in nontargeted tissues. Advances in these technologies may offer the possibility for more precise thermal targeting of adrenal tumors in the future.
Future perspectives of adrenal thermal ablation
Overall, to capitalize on the potential of selective ablation in the treatment of PA, identification of suitable patients is paramount. It is particularly important to be able to distinguish functioning from nonfunctioning lesions, which speaks to the need for accurate tumor localization rather than lateralization alone, as discussed previously (13, 186, 197, 198). There is also a clear need for larger prospective studies that select suitable patients based on appropriate localization of functioning lesions. In this respect, results from upcoming clinical trials including “Radiofrequency Endoscopic Ablation With Ultrasound Guidance: a Non-surgical Treatment for Aldosterone-producing Adenomas (FABULAS: ClinicalTrials.gov Identifier: NCT03405025) and “A Prospective Randomised Trial Comparing Radiofrequency Ablation With Laparoscopic Adrenalectomy as an alternatiVE Treatment for Unilateral Asymmetric Primary Aldosteronism (WAVE: ClinicalTrials.gov Identifier: NCT05405101)” will enhance our understanding of the true efficacy of thermal ablation in the treatment of PA.
Future studies should also focus on generation of a consensus for the optimal preprocedural, intraprocedural, and postprocedural planning of adrenal ablation. Areas for consideration include how to appropriately measure temperature application during the ablation procedures. Current methodologies using ultrasound are unreliable, due to the generation of steam. MR thermometry is expensive and has not been tested within the clinical environment. It is also important to be able to confirm ablation success following the procedure. This is normally carried out through comparison of preintervention and postintervention CT scans, including use of contrast to identify extent of the ablation zone (199). However, recent data from liver tumor ablations suggests that visual comparison of preintervention and postintervention CT scans alone may not be a suitable method to confirm successful ablation, and would benefit from development of image fusion and deformable registration techniques to facilitate more accurate assessment of treatment effect (208). Other uncertainties include whether it is necessary to perform imaging with or without contrast, at what timepoint should postprocedural imaging be carried out, and the added complexity of regular CT scans not being suitable to discriminate functioning and nonfunctioning lesions. As such, postprocedural evaluation of ablation success has most widely been assessed through post procedural biochemical workup, although the interstudy reporting criteria vary significantly (Table 7).
It is possible that, with wider application, adrenal molecular imaging (like PET-CT previously discussed) may have a role to play in judging the efficacy of selective APA/APN ablation, alongside clinical and biochemical parameters. Finally, future studies should consider reporting outcomes according to common criteria such as those provided by PASO, to provide a clearer, comparative picture of the efficacy of ablation in the treatment of PA.
Advances in Pharmacotherapy
Following a confirmed PA diagnosis and subtype categorization, if a patient is (1) deemed a nonsurgical candidate or (2) opts against surgical treatment, pharmacotherapy is the preferred treatment option (Fig. 1). The steroidal MRAs spironolactone and eplerenone remain the mainstay pharmacological management of PA, except in glucocorticoid-remediable, familial hyperaldosteronism type I where glucocorticoids are preferred (209-212). Spironolactone is generally the first line MRA for PA. However due to off-target effects, gynecomastia, erectile dysfunction, and reduced libido in men, and mastodynia in women may complicate treatment. Eplerenone, is a more selective MRA approved for the management of heart failure and hypertension. It provides an alternative to spironolactone with fewer off-target side-effects but requires twice daily dosing and is metabolized by CYP3A4. It has approximately 50% the potency of spironolactone (36, 213, 214). Second-line therapies include potassium-sparing diuretics (eg, amiloride and triamterene) but the antihypertensive effect of these is counterbalanced by a compensatory but nonantagonized increase in aldosterone which may compromise endothelial function, although this has not been verified by clinical studies (215-217).
As such, there has been a drive to develop pharmacotherapy of greater potency and selectivity than traditional MRAs to manage PA and primary hypertension. These predominantly include nonsteroidal MRAs and aldosterone synthase inhibitors. There are many third-generation compounds in various stages of clinical trials, with some gaining market approval.
Nonsteroidal Mineralocorticoid Receptor Antagonists
Esaxerenone (CS-3150) is a nonsteroidal MRA which at low concentrations inhibits aldosterone-binding to the MR without glucocorticoid, androgen or progesterone activity (218, 219). It gained first global approval for management of primary hypertension in Japan in 2019 (220). It has high bioavailability and a longer half-life (t1/2) when compared with first-generation MRAs (221). Dosing at 2.5 mg/day demonstrated noninferiority to eplerenone 50 mg/day in its antihypertensive effects, whereas a higher 5 mg/day demonstrated superiority (222). Improvements in microalbuminuria have also been demonstrated in patients with type 2 diabetes alone and in combination with other antihypertensives (223, 224).
The first in class dihydropyridine-based compound finerenone is a bulky passive antagonist that has a high selectivity for the MR. This agent received FDA approval in July 2021 for the management of chronic kidney disease associated with type 2 diabetes mellitus (225-227). In vitro studies have also demonstrated broader activity for finerenone at the MR compared to spironolactone or eplerenone, blocking both the wild-type MR as well as the rare gain of function S810L MR mutant, responsible for severe glucocorticoid and progesterone activated hypertension (228). This mutant is paradoxically activated by steroidal MR antagonists (228). There are currently no human trials registered with the primary outcome of investigating the antihypertensive properties of finerenone in PA. Interestingly, 1 study which assessed its impact on BP compared with spironolactone or placebo as a secondary outcome in patients with heart failure with reduced ejection fraction associated with mild or moderate chronic kidney disease demonstrated that systolic BP change with finerenone was comparable to treatment with placebo, despite finerenone leading to lower incidence of worsening renal function and decreased biomarkers of hemodynamic stress than the latter (229). This reinforces the need for clinical trials specifically for PA, given its significant potential in this cohort of patients. Several other compounds in various stages of development are detailed in Table 8.
Table 8.
Nonsteroidal MR antagonist and aldosterone synthase inhibitor compounds in completed phase 2 and 3 clinical trials involving cardiovascular and chronic kidney disease outcomes.
| Compound/drug name | Pharmacology | Identifier, Investigative phase + completion date | Condition/disease | Intervention | Primary outcome | Results/adverse effects |
|---|---|---|---|---|---|---|
| Nonsteroidal MRAs | ||||||
| Apararenone |
|
NCT02517320 Phase 2 January 2017 (231) |
T2DM + eGFR ≥30 mL/min/1.73 m2 + median UACR ≥50 mg/g and <300 mg/g |
|
Percentage change in UACR from baseline at 24 weeks (in the dose–response part of study) | UACR decreased significantly in all groups but not in the placebo group (P < .001) |
| Esaxerenone |
|
NCT02885662 JapicCTI-163349 Phase 3 July 2017 (220) |
Diagnosed with PA in past 5 years, based on a screening and confirmatory test, SBP ≥140 < 180 mmHg + DBP ≥90 < 110 mmHg, no antihypertensives ≥4 weeks prior or taking a stable dose of 1 basic antihypertensive agent (eg, CCB) |
|
Change in sitting SBP and DBP from baseline to the end of esaxerenone treatment (12 weeks) |
|
|
NCT02890173 Phase 3 July 2017 (222) |
Primary hypertension, with mean sitting SBP 140-179, DBP 90-109 mmHg during the washout period |
|
Change in sitting SBP + DBP from baseline until end of treatment (mean BP at Weeks 10 and 12) | Esaxerenone 2.5 mg was noninferior to Eplerenone 50 mg, with esaxerenone 5 mg superior to both | ||
|
NCT02808026 JapicCTI-163287 Phase 3 February 2017 (unpublished results) |
Severe hypertension (SBP ≥ 180 mmHg or DBP ≥ 110 mmHg), not on any antihypertensive drugs (except for potassium-sparing diuretics) during run-in period | Esaxerenone titrated from 2.5 to 5 mg | Change in seated blood pressure after 8 weeks | Both SBP and DBP significantly decreased relative to baseline blood pressure (paired t-test, both P < .0001). | ||
| JapicCTI-173695 Phase 3 April 2019 (233) |
Both hypertension and T2DM. All had received RAS inhibitor treatment for ≥12 weeks, had urinary albumin-to-creatinine ratio (UACR) of 45-299 mg/g creatinine on >1 occasion + eGFR >30 mL/min/1.73 m2 |
|
UACR <30 mg/g creatinine and a ≥30% reduction in UACR from baseline |
>30% reduction in UACR in approx. 70% of patients with T2DM, returned to <30 mg/g creatinine in approx. 22% of patients | ||
|
NCT02807974 Phase 3 March 2017 (234) |
Both hypertension (on an ARB or ACEi) and T2DM with albuminuria | Esaxerenone 1.25 titrated to 2.5, 5 mg | Change from baseline in sitting SBP and DBP | Not published (on clinical trials.gov) | ||
|
NCT02848170 JapicCTI-163324 Phase 3 April 2017 (235) |
Primary hypertension
|
|
Change from baseline to Week 12 in 24 hour blood pressure | Not published (on clinical trials.gov) | ||
|
NCT02722265 Phase 3 July 2017 (224) |
Primary hypertension and did not receive any antihypertensive agents or were treated with agents other than a RAS inhibitor or CCB |
|
Change from baseline in SBP and DBP over weeks 12, 28, and 52 | Associated with sustained and stable antihypertensive effects whether as a monotherapy or in combination | ||
| Finerenone |
NCT02540993 Phase 3 April 2020 (225) |
T2DM, CKD, albuminuria and on maximum tolerable dose of a RAS inhibitor, with serum K+ ≤ 4.8 mmol/L |
|
First occurrence of the composite endpoint of kidney failure, a sustained decrease of eGFR ≥40% from baseline over at least 4 weeks or renal death Average follow-up 32 months |
Finerenone resulted in lower risks of CKD progression and CV events than placebo | |
|
NCT02545049 Phase 3 February 2021 (225) |
T2DM, CKD, albuminuria and on maximum tolerable dose of a RAS inhibitor, with serum K+ ≤4.8 mmol/L |
|
Time to first occurrence of the composite endpoint of cardiovascular death and nonfatal cardiovascular events (myocardial infarction, stroke, or hospitalization for heart failure) Follow-up 53 months |
Among patients with T2DM and stage 2-4 CKD with moderately elevated albuminuria or stage 1-2 CKD with severely elevated albuminuria, finerenone improved CV outcomes compared with placebo | ||
| LY2623091 |
|
NCT01427972 Phase 2 March 2013 (239) |
Chronic kidney disease with PCR ≥400 mg/g, on a RAS inhibitor for at least 3 months, SBP ≤ 160 mmHg + DBP ≤100 mmHg, serum K+ ≤5.0 mEq/L) |
|
Change in proteinuria based on 24-hours pooled urine, from baseline to Day 21 | Not published (on clinical trials.gov) |
|
NCT02194465 Phase 2 March 2015 (240) |
Primary hypertension |
|
Change in SBP from baseline to 4 weeks | Not published (on clinical trials.gov) | ||
| KBP-5074 |
|
NCT03574363 Phase 2b August 2020 (242) |
Participants with both stage 3b/4 CKD+ uncontrolled hypertension (SBP ≥140 ≤ 179 mm Hg) |
|
Change in SBP from baseline to Day 84. | Effectively lowers blood pressure, compared to placebo. No reported cases of severe hyperkalemia |
| Aldosterone Synthase Inhibitors | ||||||
| Baxdrostat (RO6836191) |
|
NCT04519658 Phase 2 June 2022 (243) |
Exclusion criteria:
|
|
Change in mean seated SBP from baseline to the end of 12 weeks |
|
| Osilodrostat (LCI699) |
|
NCT00732771 Phase 2 May 2009 (245) |
Diagnosis of PA within past 3 years (with or without an aldosterone producing adenoma) Hypertension at screening |
|
Change in SBP over a 7-week forced titration treatment period |
|
Aldosterone Synthase Inhibitors
Aldosterone synthase is mainly expressed in the zona glomerulosa, where it catalyzes the final stages of aldosterone synthesis from cholesterol (246-248). Inhibiting aldosterone synthase (CYP11B2) for a therapeutic benefit was first investigated by inhibiting steroidogenesis using fadrozole, a fungicide derivative (249). Selective inhibition of CYP11B2 while leaving 11β-hydroxylase (CYP11B1) uninhibited is challenging given the homology between both enzymes” amino acidic sequences (250).
Several CYP11B2 inhibitors have been investigated, with Osilodrostat (LCI699) being 1 of the first agents of its class to reach the early phase of clinical trials. This agent was effective at lowering BP in PA and in those with essential or resistant hypertension (245, 251). For instance, in patients with primary hypertension, 1 mg of LCI699 lowered BP by 7.1 mm Hg (P = .0012) vs placebo (−2.6 mm Hg). This compared to eplerenone 50 mg twice daily (−7.9 mm Hg; P < .0001). However, 20.8% of patients (5 of 24) who received LCI699 0.5 mg twice daily and 21.4% (6 of 28) who received LCI699 1.0 mg once daily exhibited an attenuated cortisol response on ACTH stimulation (609 ± 108 and 604 ± 125 nmol/L respectively), vs 802 ± 134 and 823 ± 116 nmol/L for eplerenone and placebo (251). This nonselectivity and consequent off-target effects at CYP11B1 (with inhibition of cortisol synthesis) meant that this agent has not progressed further for the treatment of PA, but instead has recently received approval by the FDA for treatment of Cushing syndrome (252).
In contrast, baxdrostat (RO6836191) was found to be a selective and potent inhibitor of CYP11B2, demonstrating a Ki value in human studies of 13 ± 2.2 nmol/L towards CYP11B2 vs 1310 ± 533 nmol/L for CYP11B1 (253). In healthy subjects, single doses of baxdrostat across a 360-fold dose range, reduced plasma and urine aldosterone levels, with the maximum effect seen at 10 mg. Importantly, cortisol secretion following ACTH stimulation was unchanged. Histopathological analysis from preclinical studies demonstrated increased hypertrophy, proliferation, and apoptosis of ZG cells. If a similar process occurs in human aldosterone-producing tumors then baxdrostat could potentially not only reduce aldosterone secretion but also promote tumor involution (leading to a more effective reduction in plasma aldosterone levels, and contrasting with MRAs, which typically increase renin-driven aldosterone secretion) (253).
A phase 2 clinical safety and efficacy study of baxdrostat in 274 subjects taking at least 3 antihypertensive medications (1 of which was a diuretic), and with a mean automated in-office seated BP ≥130/80 mmHg, has recently been reported (254). Subjects underwent a single-blind run-in to ensure antihypertensive adherence of at least 70% (based on pill counting), with discontinuation of potassium sparing diuretics or MRAs 4 weeks prior to randomization. Subjects were then randomized to the following arms: baxdrostat 0.5 mg, 1 mg, 2 mg, and placebo. Baxdrostat was associated with dose-dependent reductions in mean (±SE) systolic BPs of −20.3 ± 2.1 mmHg, −17.5 ± 2.0 mmHg and −12.1 ± 1.9 mmHg at 2 mg, 1 mg, and 0.5 mg doses, respectively. This compared to a reduction of −9.4 mmHg in the placebo group, with a significant difference in BP reduction found between the placebo arm and the 1 mg (P = .003) and 2 mg (P < .001) groups, respectively. Blood pressure reduction was associated with decreased plasma aldosterone levels and compensatory increased plasma renin activity, with no reduction in cortisol levels. Although this study did not directly assess subjects with PA, the findings have clear relevance given the drug's mechanism of action and the over-representation of patients with PA in cohorts of patients with resistant hypertension.
As already discussed, CYP11B2 inhibitors also offer the potential to be adapted for the development of radiotracer molecules (such as 18F-AldoView) to aid in the localization and subtyping of PA, which transforms not only the diagnostic approach, but which could also open the therapeutic options for selective and definitive management of PA (255).
Potential Personalized Pharmacotherapy for PA: Calcium Signaling
In a recent analysis, up to 85% of APAs were found to harbor mutations in so-called “aldosterone driver genes”. Of these mutations, 95% are sporadic with the remaining 5% comprising familial forms (22). Therefore, there exists the potential to target pharmacotherapy to mutations. This approach is however challenged by the fact that most mutations driving PA are somatic and therefore not easily identifiable without tissue biopsy. Sporadic somatic mutations most commonly occur in APAs and aldosterone-producing diffuse hyperplasia. A substantial proportion of these mutations affect genes encoding ion channels such as the potassium channel KCNJ5, the calcium channels CACNA1D/CACNA1H, the chloride channel CLCN1 and ATPases ATP1A1 and ATP2B3 (256-259). There are also several familial forms of PA, driven by autosomal dominant germline mutations (260). Familial form I (FH-I) is associated with CYP11B1 and CYP11B2 recombination (261). FH-II comprises individuals whom have a germline gain of function mutation in CLCN2 resulting in a higher probability that the channel will exist in the open state at the glomerulosa resting potential (262). FH-III includes patients who have a heterozygous germline mutation in the KCNJ5 gene, appearing to cause extreme Na+ influx, membrane depolarization and activation of voltage gated Ca2+ channels (263, 264). While FH-IV denotes patients who have heterozygous gain of function germline mutations in the CACNA1H gene causing increased calcium influx and therefore aldosterone production (265). Lastly, there is a rare form referred to as PASNA syndrome; primary aldosteronism, seizures, and neurologic abnormalities that result from a de novo gain of function mutation in the CACNA1D gene (266-268).
Although the precise mechanism through which each mutation contributes to the pathogenesis of PA may differ, many mutations ultimately increase intracellular calcium signaling resulting in amplification of CYP11B2 expression and enhanced secretion of aldosterone (256, 257, 269-274). In this regard, calcium channel blockers may demonstrate particular efficacy in the management of PA driven by somatic mutations in channels like CACNA1D or CACNA1H (35). Furthermore, GIRK4, a G protein-activated inward rectifier potassium channel, is a potential target for those with KCNJ5 mutations, given that mutations in this channel result in altered channel selectivity, causing cell membrane depolarization, increased calcium channel signaling, and increased aldosterone production (35, 257, 275). In vitro studies have shown that macrolide antibiotics (eg, roxithromycin) selectively inhibit mutated KCNJ5 channels (276).
Novel Methods of Drug Delivery
Newer drug delivery systems offer the opportunity to overcome many obstacles associated with conventional drug delivery systems, optimizing drug targeting, half-lives, reducing toxicities, and improving drug efficacies. Due to nonadherence and partial adherence of antihypertensive medication in patients with apparent treatment-resistant hypertension, including in those with PA who are pharmacologically managed, BP control remains poor (277, 278). Adherence and resultant BP control could be improved by reducing pill burden and frequency (279). The use of a subcutaneous PEGylated thermogeling system for spironolactone in animal models has been studied, prolonging its release to nearly 2 months (280, 281). Nanostructured dense collagen–polyester composite hydrogels have also shown promise in drug loading and the drug release profile of spironolactone, with nanostructured lipid carriers demonstrating a greater dissolution rate (282, 283). Nanosuspension forms of spironolactone have also been developed preclinically to enhance its bioavailability, with future potential to reduce the variability in its pharmacokinetics (284).
Future outlooks should also be focused on the use of novel drug delivery systems in greater receptor targeting and avoidance of off-target effects associated with MRAs as well as the use of nanotechnology in the direct treatment of PA (eg, thermal therapy) (285).
Future Perspectives of PA Clinical Care
The field of PA research is progressing rapidly and is yielding significant advances that are already being transitioned into the clinical arena, raising the hope that many more patients will soon benefit from earlier diagnosis, more effective clinical, laboratory and radiological phenotyping, and the development of a personalized management strategy. The past decade in particular, has established PA as the major, potentially reversible, secondary cause of hypertension, provided novel insights in to the underlying pathogenesis of this heterogeneous disorder, delivered improved and more robust assays for measurement of plasma renin and aldosterone, yielded major advances in molecular imaging that permit not just lateralization but precise localization of culprit lesions, and provided insights (albeit in limited circumstances) for adrenal sparing interventions. In parallel, the arrival of more effective medical therapies should allow more effective prevention and mitigation of the adverse effects associated with hyperaldosteronism in those patients for whom other interventions are not feasible or preferred.
However, it would be remiss to suggest that the considerable challenges presented by PA are all in hand. In spite of these advances, on the ground care delivery in the setting of PA has not appreciably advanced in over 30 years. Screening still relies largely on determining the ARR, followed by a confirmatory suppression test, followed in turn by lateralization using AVS. Standard of treatment remains unilateral adrenalectomy for lateralized disease and/or mineralocorticoid antagonists for bilateral disease or in those unsuitable for (or unwilling to) undergo adrenalectomy (Fig. 1). Unfortunately, this lack of progress in the delivery of routine clinical care is at least partly attributable to a paucity of well-designed, large-scale, multicenter prospective clinical trials. To this end, it is critical that future clinical studies address the following questions: (1) What truly constitutes a positive screening test for PA? (2) what reference standards should be used for screening and diagnosis of PA? (3) How should AVS be performed and what thresholds should be adopted to help ensure workers in the field are comparing like with like cohorts? While experienced clinical practitioners often work comfortably within this space, they are simultaneously working around retrospective evidence. The lack of standardization within the field and the over-reliance on retrospective data will compromise the ability to move forward into the field of AI and multi-omics with the necessary validity to facilitate a change in clinical practice. To gather prospective data is neither easy, nor easily affordable. However, in the context of the commonality of PA among patients with hypertension, it is a matter of accurate study design that consideration of the diagnosis of PA is built into, and nested within, future clinical trials and randomized controlled trials in the area of hypertension.
Notwithstanding, the future of the diagnosis and management of PA is exciting. Metabolomics, supported by machine learning offers the opportunity to screen and diagnose from a single urine or blood sample, which may be drawn in primary care practices remote from the analyzing laboratory. The prospect of being able to identify unilateral from bilateral disease with confidence based on biochemical testing alone is an enticing prospect, and in turn may inform the approach to lateralization. Future studies should also seek to validate these approaches in the context of (1) antihypertensive medication interference, (2) stage of the menstrual cycle in women, and (3) reflect the spectrum, progression and severity of disease. Lateralization techniques using molecular imaging are already entering the clinical arena, with some of the first prospective studies demonstrating equivalence to AVS, when measured against clinical outcomes. Encouragingly, lateralization using molecular imaging also offers the prospect of intraglandular localization of disease, which in turn could inform adrenal sparing interventions. Future use of these adrenal-sparing methodologies should be carried out prospectively within a clinical trial-based environment, where patients are recruited without selection bias. Additionally, current thermal ablation devices are not designed to target adrenocortical lesions. Consequently, ablation antennae and other minimally invasive technologies require bespoke design, coupled with the use of appropriate treatment planning technologies in order to accurately target pathological APAs while simultaneously attempting to spare normally functioning adrenal tissue, and minimizing damage to any adjacent organs or major structures/vessels.
To conclude, the past decade has taught us a lot about PA, its prevalence and its causes. New technology offers the prospect of capitalizing on this knowledge to address a serious and common cause of patient morbidity and mortality. The provision of well-designed, prospective research data are however critical to advance the field clinically, such that patients see the benefits of research advances.
Acknowledgments
The authors would like to acknowledge Kevin Mc Donell BSc, MSc, PhD, for his guidance and interpretations of machine-learning data.
Abbreviations
- ACC
adrenocortical carcinoma
- ACTH
adrenocorticotropin
- APA
aldosterone-producing adenoma
- APN
aldosterone-producing nodule
- ARR
aldosterone–renin ratio
- AUC
area under the curve
- BP
blood pressure
- FH
familial form
- GC-MS
gas chromatography mass spectrometry
- LC
liquid chromatography
- LI
lateralization index
- MRA
mineralocorticoid receptor antagonist
- MS
mass spectrometry
- MTO
11C-metomidate
- MWA
microwave ablation
- PA
primary aldosteronism
- PAY
partial adrenalectomy
- PET
positron emission tomography
- RFA
radiofrequency ablation
- sAVS
segmental adrenal vein sampling
- SIT
saline infusion test
- SUV
standardized uptake value
- UPA
unilateral PA
- ZG
zona glomerulosa
Contributor Information
Nathan Mullen, The Discipline of Pharmacology and Therapeutics, School of Medicine, University of Galway, Galway H91V4AY, Ireland.
James Curneen, The Discipline of Pharmacology and Therapeutics, School of Medicine, University of Galway, Galway H91V4AY, Ireland.
Padraig T Donlon, The Discipline of Pharmacology and Therapeutics, School of Medicine, University of Galway, Galway H91V4AY, Ireland.
Punit Prakash, Department of Electrical and Computer Engineering, Kansas State University, Manhattan, KS 66506, USA.
Irina Bancos, Division of Endocrinology, Diabetes, Metabolism, and Nutrition, Department of Internal Medicine, Mayo Clinic, Rochester, MN 55905, USA.
Mark Gurnell, Wellcome-MRC Institute of Metabolic Science, University of Cambridge and NIHR Cambridge Biomedical Research Centre, Addenbrooke's Hospital, Cambridge Biomedical Campus, Cambridge CB2 0QQ, UK.
Michael C Dennedy, The Discipline of Pharmacology and Therapeutics, School of Medicine, University of Galway, Galway H91V4AY, Ireland.
Funding
This publication has emanated from research supported in part by a grant from Science Foundation Ireland under Grant number [20/US/ 3676], the National Institutes of Health Grant number [R01EB028848] and NIHR Cambridge Biomedical Research Centre grant number [NIHR203312]. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Author Contributions
M.C.D. N.M., and J.C. designed the paper. N.M. and J.C. wrote the first draft. P.D. produced the figures. M.C.D., M.G., P.P., I.B. provided critical revision of the manuscript.
Disclosures
N.M., P.T.D, J.C, M.C.D. and M.G report no relevant disclosures for this body of work. I.B. reports consulting, advisory board participation, or data monitoring safety board participation (fees to institution) with Diurnal, Neurocrine, Spruce, Adrenas, Corcept, Sparrow, Recordati, HRA Pharma, outside this work. I.B. reports research funding (to institution) from Recordati for investigator-initiated project, outside this work. P.P. receives royalties from KSU Research Foundation (institutional technology transfer office) for patents on microwave ablation technology, outside of this work.
References
- 1. Forouzanfar MH, Alexander L, Anderson HR, et al. GBD 2013 Risk factors collaborators global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;386(10010):2287‐2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sukor N. Endocrine hypertension—current understanding and comprehensive management review. Eur J Intern Med. 2011;22(5):433‐440. [DOI] [PubMed] [Google Scholar]
- 3. Carretero OA, Oparil S. Essential hypertension: part I: definition and etiology. Circulation. 2000;101(3):329‐335. [DOI] [PubMed] [Google Scholar]
- 4. Käyser SC, Dekkers T, Groenewoud HJ, et al. Study heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and meta-regression analysis. J Clin Endocrinol Metab. 2016;101(7):2826‐2835. [DOI] [PubMed] [Google Scholar]
- 5. Buffolo F, Monticone S, Tetti M, Mulatero P. Primary aldosteronism in the primary care setting. Curr Opin Endocrinol Diabetes Obes. 2018;25(3):155‐159. [DOI] [PubMed] [Google Scholar]
- 6. Rossi GP, Bernini G, Caliumi C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48(11):2293‐2300. [DOI] [PubMed] [Google Scholar]
- 7. Funder JW, Carey RM, Fardella C, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(9):3266‐3281. [DOI] [PubMed] [Google Scholar]
- 8. Libianto R, Russell GM, Stowasser M, et al. Detecting primary aldosteronism in Australian primary care: a prospective study. Med J Aust. 2022;216(8):408‐412. [DOI] [PubMed] [Google Scholar]
- 9. Stowasser M, Gordon RD. Primary aldosteronism: changing definitions and new concepts of physiology and pathophysiology both inside and outside the kidney. Physiol Rev. 2016;96(4):1327‐1384. [DOI] [PubMed] [Google Scholar]
- 10. Funder J. Primary aldosteronism: treatment of the disease, and new therapeutic approaches. Best Pract Res Clin Endocrinol Metab. 2020;34(2):101368. [DOI] [PubMed] [Google Scholar]
- 11. Libianto R, Fuller PJ, Young MJ, Yang J. Primary aldosteronism is a public health issue: challenges and opportunities. J Hum Hypertens. 2020;34(7):478‐486. [DOI] [PubMed] [Google Scholar]
- 12. Brown JM, Siddiqui M, Calhoun DA, et al. The unrecognized prevalence of primary aldosteronism: a cross-sectional study. Ann Intern Med. 2020;173(1):10‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Williams TA, Gomez-Sanchez CE, Rainey WE, et al. International histopathology consensus for unilateral primary aldosteronism. J Clin Endocrinol Metab. 2021;106(1):42‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Milliez P, Girerd X, Plouin PF, et al. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45(8):1243‐1248. [DOI] [PubMed] [Google Scholar]
- 15. Monticone S, D'Ascenzo F, Moretti C, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6(1):41‐50. [DOI] [PubMed] [Google Scholar]
- 16. Stowasser M, Sharman J, Leano R, et al. Evidence for abnormal left ventricular structure and function in normotensive individuals with familial hyperaldosteronism type I. J Clin Endocrinol Metab. 2005;90(9):5070‐5076. [DOI] [PubMed] [Google Scholar]
- 17. Iwakura Y, Morimoto R, Kudo M, et al. Predictors of decreasing glomerular filtration rate and prevalence of chronic kidney disease after treatment of primary aldosteronism: renal outcome of 213 cases. J Clin Endocrinol Metab. 2014;99(5):1593‐1598. [DOI] [PubMed] [Google Scholar]
- 18. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Renal outcomes in medically and surgically treated primary aldosteronism. Hypertension. 2018;72(3):658‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hundemer GL, Vaidya A. Primary aldosteronism diagnosis and management: a clinical approach. Endocrinol Metab Clin North Am. 2019;48(4):681‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ohno Y, Sone M, Inagaki N, et al. Prevalence of cardiovascular disease and its risk factors in primary aldosteronism: a multicenter study in Japan. Hypertension. 2018;71(3):530‐537. [DOI] [PubMed] [Google Scholar]
- 21. Quinkler M, Born-Frontsberg E, Fourkiotis V. Comorbidities in primary aldosteronism. Hormone Metab Res. 2010;42(6):429‐434. [DOI] [PubMed] [Google Scholar]
- 22. Williams TA, Reincke M. Pathophysiology and histopathology of primary aldosteronism. Trends Endocrinol Metab. 2022;33(1):36‐49. [DOI] [PubMed] [Google Scholar]
- 23. Hannemann A, Wallaschofski H, Lüdemann J, et al. Plasma aldosterone levels and aldosterone-to-renin ratios are associated with endothelial dysfunction in young to middle-aged subjects. Atherosclerosis. 2011;219(2):875‐879. [DOI] [PubMed] [Google Scholar]
- 24. Beygui F, Collet JP, Benoliel JJ, et al. High plasma aldosterone levels on admission are associated with death in patients presenting with acute ST-elevation myocardial infarction. Circulation. 2006;114(24):2604‐2610. [DOI] [PubMed] [Google Scholar]
- 25. Briet M, Barhoumi T, Mian MOR, et al. Aldosterone-induced vascular remodeling and endothelial dysfunction require functional angiotensin type 1a receptors. Hypertension. 2016;67(5):897‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ivanes F, Susen S, Mouquet F, et al. Aldosterone, mortality, and acute ischaemic events in coronary artery disease patients outside the setting of acute myocardial infarction or heart failure. Eur Heart J. 2012;33(2):191‐202. [DOI] [PubMed] [Google Scholar]
- 27. Buffolo F, Tetti M, Mulatero P, Monticone S. Aldosterone as a mediator of cardiovascular damage. Hypertension. 2022;79(9):1899‐1911. [DOI] [PubMed] [Google Scholar]
- 28. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6(1):51‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Incidence of atrial fibrillation and mineralocorticoid receptor activity in patients with medically and surgically treated primary aldosteronism. JAMA Cardiol. 2018;3(8):768‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Born-Frontsberg E, Reincke M, Rump LC, et al. Cardiovascular and cerebrovascular comorbidities of hypokalemic and normokalemic primary aldosteronism: results of the German Conn's Registry. J Clin Endocrinol Metab. 2009;94(4):1125‐1130. [DOI] [PubMed] [Google Scholar]
- 31. Reincke M, Meisinger C, Holle R, et al. Is primary aldosteronism associated with diabetes mellitus? Results of the German Conn's Registry. Horm Metab Res. 2010;42(6):435‐439. [DOI] [PubMed] [Google Scholar]
- 32. Reincke M, Rump LC, Quinkler M, et al. Risk factors associated with a low glomerular filtration rate in primary aldosteronism. J Clin Endocrinol Metab. 2009;94(3):869‐875. [DOI] [PubMed] [Google Scholar]
- 33. Rossi GP. Prevalence and diagnosis of primary aldosteronism. Curr Hypertens Rep. 2010;12(5):342‐348. [DOI] [PubMed] [Google Scholar]
- 34. Monticone S, Burrello J, Tizzani D, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017;69(14):1811‐1820. [DOI] [PubMed] [Google Scholar]
- 35. Zennaro M-C, Boulkroun S, Fernandes-Rosa FL. Pathogenesis and treatment of primary aldosteronism. Nat Rev Endocrinol. 2020;16(10):578‐589. [DOI] [PubMed] [Google Scholar]
- 36. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889‐1916. [DOI] [PubMed] [Google Scholar]
- 37. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127‐e248. [DOI] [PubMed] [Google Scholar]
- 38. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J. 2018;39(33):3021‐3104. [DOI] [PubMed] [Google Scholar]
- 39. National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management. 2019; NICE Guideline 136]. Available from: https://www.nice.org.uk/guidance/ng136.
- 40. McCormack T, Boffa RJ, Jones NR, Carville S, McManus RJ. The 2018 ESC/ESH hypertension guideline and the 2019 NICE hypertension guideline, how and why they differ. Eur Heart J. 2019;40(42):3456‐3458. [DOI] [PubMed] [Google Scholar]
- 41. Hundemer GL, Imsirovic H, Vaidya A, et al. Screening rates for primary aldosteronism among individuals with hypertension plus hypokalemia: a population-based retrospective cohort study. Hypertension. 2022;79(1):178‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mulatero P, Monticone S, Burrello J, et al. Guidelines for primary aldosteronism: uptake by primary care physicians in Europe. J Hypertens. 2016;34(11):2253‐2257. [DOI] [PubMed] [Google Scholar]
- 43. Asbach E, Kellnar A, Bekeran M, et al. Prevalence of primary aldosteronism in newly diagnosed hypertensive patients in primary care. Exp Clin Endocrinol Diabetes. 2022;130(12):801‐805. [DOI] [PubMed] [Google Scholar]
- 44. Nainani AK, Yang J, Peters S, Russell G. ‘I can’t understand why others don’t screen more’: a qualitative study exploring why Australian general practitioners screen for primary aldosteronism. BMJ Open. 2022;12(6):e061671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shidlovskyi VO, Shidlovskyi OV, Tovkai OA, et al. Topical diagnosis and determination of the primary hyperaldosteronism variant. J Med Life. 2019;12(4):322‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Young W Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285(2):126‐148. [DOI] [PubMed] [Google Scholar]
- 47. Logan AG. Hypertension in aging patients. Expert Rev Cardiovasc Ther. 2011;9(1):113‐120. [DOI] [PubMed] [Google Scholar]
- 48. Hacini I, De Sousa K, Boulkroun S, et al. Somatic mutations in adrenals from patients with primary aldosteronism not cured after adrenalectomy suggest common pathogenic mechanisms between unilateral and bilateral disease. Eur J Endocrinol. 2021;185(3):405‐412. [DOI] [PubMed] [Google Scholar]
- 49. Williams TA, Lenders JWM, Mulatero P, et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017;5(9):689‐699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vaidya A, Mulatero P, Baudrand R, Adler GK. The expanding spectrum of primary aldosteronism: implications for diagnosis, pathogenesis, and treatment. Endocr Rev. 2018;39(6):1057‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brown JM, Robinson-Cohen C, Luque-Fernandez MA, et al. The spectrum of subclinical primary aldosteronism and incident hypertension: a cohort study. Ann Intern Med. 2017;167(9):630‐641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Turcu AF, Yang J, Vaidya A. Primary aldosteronism—a multidimensional syndrome. Nat Rev Endocrinol. 2022;18(11):665‐682. [DOI] [PubMed] [Google Scholar]
- 53. Vaidya A, Hundemer GL, Nanba K, Parksook WW, Brown JM. Primary aldosteronism: state-of-the-art review. Am J Hypertens. 2022;35(12):967‐988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. O'Shea PM, Griffin TP, Denieffe S, Fitzgibbon MC. The aldosterone to renin ratio in the diagnosis of primary aldosteronism: promises and challenges. Int J Clin Pract. 2019;73(7):e13353. [DOI] [PubMed] [Google Scholar]
- 55. Yozamp N, Hundemer GL, Moussa M, et al. Intraindividual variability of aldosterone concentrations in primary aldosteronism: implications for case detection. Hypertension. 2021;77(3):891‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf). 2007;66(5):607‐618. [DOI] [PubMed] [Google Scholar]
- 57. Ahmed AH, Gordon RD, Taylor PJ, et al. Are women more at risk of false-positive primary aldosteronism screening and unnecessary suppression testing than men? J Clin Endocrinol Metab. 2011;96(2):E340‐E346. [DOI] [PubMed] [Google Scholar]
- 58. Stowasser M, Ahmed A, Pimenta E, et al. Factors affecting the aldosterone/renin ratio. Hormone Metab Res. 2012;44(3):170‐176. [DOI] [PubMed] [Google Scholar]
- 59. Browne GA, Griffin TP, O' et al. Β-blocker withdrawal is preferable for accurate interpretation of the aldosterone-renin ratio in chronically treated hypertension. Clin Endocrinol (Oxf). 2016;84(3):325‐331. [DOI] [PubMed] [Google Scholar]
- 60. Griffin TP, Browne GA, Wall D, Dennedy MC, O'Shea PM. A cross-sectional study of the effects of β-blocker therapy on the interpretation of the aldosterone/renin ratio: can dosing regimen predict effect? J Hypertens. 2016;34(2):307‐315. [DOI] [PubMed] [Google Scholar]
- 61. Stowasser M, Taylor PJ, Pimenta E, Ahmed AH, Gordon RD. Laboratory investigation of primary aldosteronism. Clin Biochem Rev. 2010;31(2):39‐56. [PMC free article] [PubMed] [Google Scholar]
- 62. Baudrand R, Guarda FJ, Torrey J, Williams G, Vaidya A. Dietary sodium restriction increases the risk of misinterpreting mild cases of primary aldosteronism. J Clin Endocrinol Metab. 2016;101(11):3989‐3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Leung AA, Symonds CJ, Hundemer GL, et al. Performance of confirmatory tests for diagnosing primary aldosteronism: a systematic review and meta-analysis. Hypertension. 2022;79(8):1835‐1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Quencer KB. Adrenal vein sampling: technique and protocol, a systematic review. CVIR Endovascular. 2021;4(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhou Y, Wang D, Jiang L, et al. Diagnostic accuracy of adrenal imaging for subtype diagnosis in primary aldosteronism: systematic review and meta-analysis. BMJ Open. 2020;10(12):e038489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nanba AT, Nanba K, Byrd JB, et al. Discordance between imaging and immunohistochemistry in unilateral primary aldosteronism. Clin Endocrinol (Oxf). 2017;87(6):665‐672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Aono D, Kometani M, Karashima S, et al. Primary aldosteronism subtype discordance between computed tomography and adrenal venous sampling. Hypertens Res. 2019;42(12):1942‐1950. [DOI] [PubMed] [Google Scholar]
- 68. Dekkers T, Prejbisz A, Kool LJS, et al. Adrenal vein sampling versus CT scan to determine treatment in primary aldosteronism: an outcome-based randomised diagnostic trial. Lancet Diabetes Endocrinol. 2016;4(9):739‐746. [DOI] [PubMed] [Google Scholar]
- 69. Beuschlein F, Mulatero P, Asbach E, et al. The SPARTACUS trial: controversies and unresolved issues. Hormone Metab Res. 2017;49(12):936‐942. [DOI] [PubMed] [Google Scholar]
- 70. Rossi GP, Funder JW. Adrenal venous sampling versus computed tomographic scan to determine treatment in primary aldosteronism (the SPARTACUS trial). Hypertension. 2017;69(3):396‐397. [DOI] [PubMed] [Google Scholar]
- 71. Reincke M, Bancos I, Mulatero P, et al. Diagnosis and treatment of primary aldosteronism. Lancet Diabetes Endocrinol. 2021;9(12):876‐892. [DOI] [PubMed] [Google Scholar]
- 72. Kwak J, Lee KE. Minimally invasive adrenal surgery. Endocrinol Metab. 2020;35(4):774‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Alemanno G, Bergamini C, Prosperi P, Valeri A. Adrenalectomy: indications and options for treatment. Updates Surg. 2017;69(2):119‐125. [DOI] [PubMed] [Google Scholar]
- 74. Burton TJ, Mackenzie IS, Balan K, et al. Evaluation of the sensitivity and specificity of 11C-metomidate positron emission tomography (PET)-CT for lateralizing aldosterone secretion by Conn's adenomas. J Clin Endocrinol Metab. 2012;97(1):100‐109. [DOI] [PubMed] [Google Scholar]
- 75. O'Shea PM, O’Donoghue D, et al. 11C-Metomidate PET/CT is a useful adjunct for lateralization of primary aldosteronism in routine clinical practice. Clin Endocrinol (Oxf). 2019;90(5):670‐679. [DOI] [PubMed] [Google Scholar]
- 76. Huyghe E, Crenn G, Duly-Bouhanick B, et al. Retroperitoneoscopic adrenalectomy: comparison of retrograde and antegrade approach among a series of 279 cases. Urology. 2013;81(1):85‐92. [DOI] [PubMed] [Google Scholar]
- 77. Heinrich DA, Adolf C, Holler F, et al. Adrenal insufficiency after unilateral adrenalectomy in primary aldosteronism: long-term outcome and clinical impact. J Clin Endocrinol Metab. 2019;104(11):5658‐5664. [DOI] [PubMed] [Google Scholar]
- 78. Yorke E, Stafford S, Holmes D, Sheth S, Melck A. Aldosterone deficiency after unilateral adrenalectomy for Conn's syndrome: a case report and literature review. Int J Surg Case Rep. 2015;7:141‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shariq OA, Bancos I, Cronin PA, et al. Contralateral suppression of aldosterone at adrenal venous sampling predicts hyperkalemia following adrenalectomy for primary aldosteronism. Surgery. 2018;163(1):183‐190. [DOI] [PubMed] [Google Scholar]
- 80. Sukor N, Gordon RD, Ku YK, Jones M, Stowasser M. Role of unilateral adrenalectomy in bilateral primary aldosteronism: a 22-year single center experience. J Clin Endocrinol Metab. 2009;94(7):2437‐2445. [DOI] [PubMed] [Google Scholar]
- 81. Williams TA, Gong S, Tsurutani Y, et al. Adrenal surgery for bilateral primary aldosteronism: an international retrospective cohort study. Lancet Diabetes Endocrinol. 2022;10(11):769‐771. [DOI] [PubMed] [Google Scholar]
- 82. Szabo Yamashita T, Shariq OA, Foster TR, et al. Unilateral adrenalectomy for primary aldosteronism due to bilateral adrenal disease can result in resolution of hypokalemia and amelioration of hypertension. World J Surg. 2023;47(2):314‐318. [DOI] [PubMed] [Google Scholar]
- 83. Perysinakis I, Aggeli CH, Kaltsas GR, Zografos GN. Adrenal-sparing surgery: current concepts on a theme from the past. Hormones. 2020;19(3):317‐327. [DOI] [PubMed] [Google Scholar]
- 84. Seyam R, Khalil MI, Kamel MH, et al. Organ-sparing procedures in GU cancer: part 1—organ-sparing procedures in renal and adrenal tumors: a systematic review. Int Urol Nephrol. 2019;51(3):377‐393. [DOI] [PubMed] [Google Scholar]
- 85. Hundemer GL, Vaidya A. Management of endocrine disease: the role of surgical adrenalectomy in primary aldosteronism. Eur J Endocrinol. 2020;183(6):R185‐R196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Donlon PT, Fallahi H, Beard WL, et al. Using microwave thermal ablation to develop a subtotal, cortical-sparing approach to the management of primary aldosteronism. Int J Hyperthermia. 2019;36(1):904‐913. [DOI] [PubMed] [Google Scholar]
- 87. Schwartz GL, Chapman AB, Boerwinkle E, Kisabeth RM, Turner ST. Screening for primary aldosteronism: implications of an increased plasma aldosterone/renin ratio. Clin Chem. 2002;48(11):1919‐1923. [PubMed] [Google Scholar]
- 88. Yozamp N, Hundemer GL, Moussa M, et al. Intraindividual variability of aldosterone concentrations in primary aldosteronism. Hypertension. 2021;77(3):891‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Vaidya A, Carey RM. Evolution of the primary aldosteronism syndrome: updating the approach. J Clin Endocrinol Metab. 2020;105(12):3771‐3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Asirvatham JR, Moses V, Bjornson L. Errors in potassium measurement: a laboratory perspective for the clinician. N Am J Med Sci. 2013;5(4):255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. González-Domínguez R, González-Domínguez Á, Sayago A, Fernández-Recamales Á. Recommendations and best practices for standardizing the pre-analytical processing of blood and urine samples in metabolomics. Metabolites. 2020;10(6):229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Arlt W, Biehl M, Taylor AE, et al. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. J Clin Endocrinol Metab. 2011;96(12):3775‐3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chortis V, Bancos I, Nijman T, et al. Urine steroid metabolomics as a novel tool for detection of recurrent adrenocortical carcinoma. J Clin Endocrinol Metab. 2020;105(3):e307‐e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Constantinescu G, Schulze M, Peitzsch M, et al. Integration of artificial intelligence and plasma steroidomics with laboratory information management systems: application to primary aldosteronism. Clin Chem Lab Med. 2022;60(12):1929‐1937. [DOI] [PubMed] [Google Scholar]
- 95. Erlic Z, Reel P, Reel S, et al. Targeted metabolomics as a tool in discriminating endocrine from primary hypertension. J Clin Endocrinol Metab. 2021;106(4):e1111‐e1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ku EJ, Lee C, Shim J, et al. Metabolic subtyping of adrenal tumors: prospective multi-center cohort study in Korea. Endocrinol Metab. 2021;36(5):1131‐1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kaneko H, Umakoshi H, Ogata M, et al. Machine learning based models for prediction of subtype diagnosis of primary aldosteronism using blood test. Sci Rep. 2021;11(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nishikawa T, Omura M, Satoh F, et al. Guidelines for the diagnosis and treatment of primary aldosteronism-the Japan endocrine society 2009. Endocr J. 2011;58(9):711‐721. [DOI] [PubMed] [Google Scholar]
- 99. Shimamoto K, Ando K, Fujita T, et al. The Japanese society of hypertension guidelines for the management of hypertension (JSH 2014). Hypertens Res. 2014;37(4):253‐390. [DOI] [PubMed] [Google Scholar]
- 100. Diao X, Huo Y, Yan Z, et al. An application of machine learning to etiological diagnosis of secondary hypertension: retrospective study using electronic medical records. JMIR Med Inform. 2021;9(1):e19739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Burrello J, Amongero M, Buffolo F, et al. Development of a prediction score to avoid confirmatory testing in patients with suspected primary aldosteronism. J Clin Endocrinol Metab. 2021;106(4):e1708‐e1716. [DOI] [PubMed] [Google Scholar]
- 102. Burrello J, Burrello A, Pieroni J, et al. Prediction of hyperaldosteronism subtypes when adrenal vein sampling is unilaterally successful. Eur J Endocrinol. 2020;183(6):657‐667. [DOI] [PubMed] [Google Scholar]
- 103. Eisenhofer G, Durán C, Cannistraci CV, et al. Use of steroid profiling combined with machine learning for identification and subtype classification in primary aldosteronism. JAMA Network Open. 2020;3(9):e2016209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cohen JF, Korevaar DA, Altman DG, et al. STARD 2015 Guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open. 2016;6(11):e012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Storbeck K-H, Schiffer L, Baranowski ES, et al. Steroid metabolome analysis in disorders of adrenal steroid biosynthesis and metabolism. Endocr Rev. 2019;40(6):1605‐1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Shackleton C, Pozo OJ, Marcos J. GC/MS in recent years has defined the normal and clinically disordered steroidome: will it soon be surpassed by LC/tandem MS in this role? J Endocr Soc. 2018;2(8):974‐996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Thevis M, Schänzer W. Mass spectrometry in sports drug testing: structure characterization and analytical assays. Mass Spectrom Rev. 2007;26(1):79‐107. [DOI] [PubMed] [Google Scholar]
- 108. Bancos I, Taylor AE, Chortis V, et al. Urine steroid metabolomics for the differential diagnosis of adrenal incidentalomas in the EURINE-ACT study: a prospective test validation study. Lancet Diabetes Endocrinol. 2020;8(9):773‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Arlt W, Lang K, Sitch AJ, et al. Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism. JCI Insight. 2017;2(8):e93136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Carafone LE, Zhang CD, Li D, et al. Diagnostic accuracy of dehydroepiandrosterone sulfate and corticotropin in autonomous cortisol secretion. Biomedicines. 2021;9(7):741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Dennedy MC, Annamalai AK, Prankerd Smith O, et al. Low DHEAS: a sensitive and specific test for the detection of subclinical hypercortisolism in adrenal incidentalomas. J Clin Endocrinol Metab. 2017;102(3):786‐792. [DOI] [PubMed] [Google Scholar]
- 112. Berke K, Constantinescu G, Masjkur J, et al. Plasma steroid profiling in patients with adrenal incidentaloma. J Clin Endocrinol Metab. 2021;107(3):e1181‐e1192. [DOI] [PubMed] [Google Scholar]
- 113. Buffolo F, Burrello J, Burrello A, et al. Clinical score and machine learning-based model to predict diagnosis of primary aldosteronism in arterial hypertension. Hypertension. 2021;78(5):1595‐1604. [DOI] [PubMed] [Google Scholar]
- 114. Wada N, Shibayama Y, Yoneda T, et al. Lateralizing asymmetry of adrenal imaging and adrenal vein sampling in patients with primary aldosteronism. J Endocr Soc. 2019;3(7):1393‐1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhu L, Zhang Y, Zhang H, et al. Comparison between adrenal venous sampling and computed tomography in the diagnosis of primary aldosteronism and in the guidance of adrenalectomy. Medicine (Baltimore). 2016;95(39):e4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Magill SB, Raff H, Shaker JL, et al. Comparison of adrenal vein sampling and computed tomography in the differentiation of primary aldosteronism. J Clin Endocrinol Metab. 2001;86(3):1066‐1071. [DOI] [PubMed] [Google Scholar]
- 117. Umakoshi H, Ogasawara T, Takeda Y, et al. Accuracy of adrenal computed tomography in predicting the unilateral subtype in young patients with hypokalaemia and elevation of aldosterone in primary aldosteronism. Clin Endocrinol (Oxf). 2018;88(5):645‐651. [DOI] [PubMed] [Google Scholar]
- 118. Gkaniatsa E, Sakinis A, Palmér M, et al. Adrenal venous sampling in young patients with primary aldosteronism. Extravagance or irreplaceable? J Clin Endocrinol Metab. 2021;106(5):e2087‐e2095. [DOI] [PubMed] [Google Scholar]
- 119. Kaur R, Young S. Discordant imaging-adrenal vein sampling in almost half of patients with primary aldosteronism and a unilateral adrenal adenoma. Intern Med J. Published online March 23, 2022. Doi: 10.1111/imj.15752 [DOI] [PubMed] [Google Scholar]
- 120. Rossi GP, Maiolino G, Seccia TM. Adrenal venous sampling: where do we stand? Endocrinol Metab Clin North Am. 2019;48(4):843‐858. [DOI] [PubMed] [Google Scholar]
- 121. Kline G, Holmes DT. Adrenal venous sampling for primary aldosteronism: laboratory medicine best practice. J Clin Pathol. 2017;70(11):911‐916. [DOI] [PubMed] [Google Scholar]
- 122. Rossi GP, Funder JW. Adrenal vein sampling is the preferred method to select patients with primary aldosteronism for adrenalectomy: pro side of the argument. Hypertension. 2018;71(1):5‐9. [DOI] [PubMed] [Google Scholar]
- 123. Turcu AF, Auchus R. Approach to the patient with primary aldosteronism: utility and limitations of adrenal vein sampling. J Clin Endocrinol Metab. 2021;106(4):1195‐1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Fuss CT, Treitl M, Rayes N, et al. Radiation exposure of adrenal vein sampling: a German multicenter study. Eur J Endocrinol. 2018;179(4):261‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lin Y, Luo J, Liu S, Jiang Q, Hu C. Learning curve of adrenal vein sampling. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2021;46(9):996‐1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Araujo-Castro M, Paja Fano M, González Boillos M, et al. Adrenal venous sampling in primary aldosteronism: experience of a Spanish multicentric study (results from the SPAIN-ALDO register). Endocrine. 2022;78(2):363‐372. [DOI] [PubMed] [Google Scholar]
- 127. Rossi GP, Barisa M, Allolio B, et al. The adrenal vein sampling international study (AVIS) for identifying the major subtypes of primary aldosteronism. J Clin Endocrinol. 2012;97(5):1606‐1614. [DOI] [PubMed] [Google Scholar]
- 128. Rossi GP, Auchus RJ, Brown M, et al. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension. 2014;63(1):151‐160. [DOI] [PubMed] [Google Scholar]
- 129. Deinum J, Prejbisz A, Lenders JWM, van der Wilt GJ. Adrenal vein sampling is the preferred method to select patients with primary aldosteronism for adrenalectomy: con side of the argument. Hypertension. 2018;71(1):10‐14. [DOI] [PubMed] [Google Scholar]
- 130. Rossitto G, Amar L, Azizi M, et al. Subtyping of primary aldosteronism in the AVIS-2 study: assessment of selectivity and lateralization. J Clin Endocrinol Metab. 2020;105(6):2042‐2052. [DOI] [PubMed] [Google Scholar]
- 131. Heinrich DA, Quinkler M, Adolf C, et al. Influence of cortisol cosecretion on non-ACTH-stimulated adrenal venous sampling in primary aldosteronism: a retrospective cohort study. Eur J Endocrinol. 2022;187(5):637‐650. [DOI] [PubMed] [Google Scholar]
- 132. Chen Y-J, Peng K-Y, Chueh JS, et al. Case report: primary aldosteronism due to bilateral aldosterone-producing micronodules with HISTALDO classical and contralateral non-classical pathology. Front Endocrinol (Lausanne). 2022;13:816754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Satoh F, Morimoto R, Seiji K, et al. Is there a role for segmental adrenal venous sampling and adrenal sparing surgery in patients with primary aldosteronism? Eur J Endocrinol. 2015;173(4):465‐477. [DOI] [PubMed] [Google Scholar]
- 134. Makita K, Nishimoto K, Kiriyama-Kitamoto K, et al. A novel method: super-selective adrenal venous sampling. J Vis Exp. 2017;127:55716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kitamoto T, Kitamoto KK, Omura M, et al. Precise mapping of intra-adrenal aldosterone activities provides a novel surgical strategy for primary aldosteronism. Hypertension. 2020;76(3):976‐984. [DOI] [PubMed] [Google Scholar]
- 136. Morimoto R, Satani N, Iwakura Y, et al. A case of bilateral aldosterone-producing adenomas differentiated by segmental adrenal venous sampling for bilateral adrenal sparing surgery. J Hum Hypertens. 2016;30(6):379‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Satani N, Ota H, Seiji K, et al. Intra-adrenal aldosterone secretion: segmental adrenal venous sampling for localization. Radiology. 2016;278(1):265‐274. [DOI] [PubMed] [Google Scholar]
- 138. Stewart PM, Allolio B. Adrenal vein sampling for primary aldosteronism: time for a reality check. Clin Endocrinol (Oxf). 2010;72(2):146‐148. [DOI] [PubMed] [Google Scholar]
- 139. Puar TH, Khoo CM, Tan CJ, et al. 11C-Metomidate PET-CT versus adrenal vein sampling to subtype primary aldosteronism: a prospective clinical trial. J Hypertens. 2022;40(6):1179‐1188. [DOI] [PubMed] [Google Scholar]
- 140. Hahner S, Stuermer A, Kreissl M, et al. [123I] Iodometomidate for molecular imaging of adrenocortical cytochrome P450 family 11B enzymes. J Clin Endocrinol Metab. 2008;93(6):2358‐2365. [DOI] [PubMed] [Google Scholar]
- 141. Hahner S, Sundin A. Metomidate-based imaging of adrenal masses. Horm Cancer. 2011;2(6):348‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Chen Cardenas SM, Santhanam P. 11C-metomidate PET in the diagnosis of adrenal masses and primary aldosteronism: a review of the literature. Endocrine. 2020;70(3):479‐487. [DOI] [PubMed] [Google Scholar]
- 143. Soinio M, Luukkonen A-K, Seppänen M, et al. Functional imaging with 11C-metomidate PET for subtype diagnosis in primary aldosteronism. Eur J Endocrinol. 2020;183(6):539‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Wu X, Senanayake R, Goodchild E, et al. [11C]metomidate PET-CT versus adrenal vein sampling for diagnosing surgically curable primary aldosteronism: a prospective, within-patient trial. Nat Med. 2023;29(1):190‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Lu C-C, Chen C-J, Peng K-Y, et al. Predicting treatment response in primary aldosteronism using 11C-metomidate positron emission tomography. Clin Nucl Med. 2022;47(11):936‐942. [DOI] [PubMed] [Google Scholar]
- 146. Isojärvi J, Viukari M, Pörsti I, et al. Lateralization in 11C-metomidate PET and outcome of adrenalectomy in primary aldosteronism. Endocrinol Diabetes Metab. 2022;5(6):e368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Silins I, Sundin A, Lubberink M, et al. First-in-human evaluation of [18F] CETO: a novel tracer for adrenocortical tumours. Eur J Nucl Med Mol Imaging. 2022;50(2):398‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Gao Y, Ding J, Cui Y, et al. Functional nodules in primary aldosteronism: identification of CXCR4 expression with 68Ga-pentixafor PET/CT. Eur Radiol. 2022;33(2):996‐1003. [DOI] [PubMed] [Google Scholar]
- 149. Ding J, Zhang Y, Wen J, et al. Imaging CXCR4 expression in patients with suspected primary hyperaldosteronism. Eur J Nucl Med Mol Imaging. 2020;47(11):2656‐2665. [DOI] [PubMed] [Google Scholar]
- 150. Heinze B, Fuss CT, Mulatero P, et al. Targeting CXCR4 (CXC chemokine receptor type 4) for molecular imaging of aldosterone-producing adenoma. Hypertension. 2018;71(2):317‐325. [DOI] [PubMed] [Google Scholar]
- 151. Silins I, Sundin A, Nordeman P, et al. Para-chloro-2-[18F] fluoroethyl-etomidate: a promising new PET radiotracer for adrenocortical imaging. Int J Med Sci. 2021;18(10):2187‐2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Abe T, Naruse M, Young WF, et al. A novel CYP11B2-specific imaging agent for detection of unilateral subtypes of primary aldosteronism. J Clin Endocrinol Metab. 2016;101(3):1008‐1015. [DOI] [PubMed] [Google Scholar]
- 153. Bongarzone S, Basagni F, Sementa T, et al. Development of [18F] FAMTO: a novel fluorine-18 labelled positron emission tomography (PET) radiotracer for imaging CYP11B1 and CYP11B2 enzymes in adrenal glands. Nucl Med Biol. 2019;68:14‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Hoyt SB, Park MK, London C, et al. Discovery of benzimidazole CYP11B2 inhibitors with in vivo activity in rhesus monkeys. ACS Med Chem Lett. 2015;6(5):573‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Sander K, Gendron T, Cybulska KA, et al. Development of [18F] AldoView as the first highly selective aldosterone synthase PET tracer for imaging of primary hyperaldosteronism. J Med Chem. 2021;64(13):9321‐9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Ding J, Tong A, Hacker M, et al. Usefulness of 68Ga-pentixafor PET/CT on diagnosis and management of Cushing syndrome. Clin Nucl Med. 2022;47(8):669‐676. [DOI] [PubMed] [Google Scholar]
- 157. Hu J, Xu T, Shen H, et al. Accuracy of gallium-68 pentixafor positron emission tomography-computed tomography for subtyping diagnosis of primary aldosteronism. JAMA Network Open. 2023;6(2):e2255609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Chaman Baz AH, van de Wiel E, Groenewoud H, et al. Protocol: CXCR4-directed [68Ga] Ga-PentixaFor PET/CT versus adrenal vein sampling performance: a study protocol for a randomised two-step controlled diagnoStic Trial Ultimately comparing hypertenSion outcome in primary aldosteronism (CASTUS). BMJ Open. 2022;12(8):e060779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Van Timmeren JE, Cester D, Tanadini-Lang S, Alkadhi H, Baessler B. Radiomics in medical imaging—“how-to” guide and critical reflection. Insights Imaging. 2020;11(1):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Yi X, Guan X, Zhang Y, et al. Radiomics improves efficiency for differentiating subclinical pheochromocytoma from lipid-poor adenoma: a predictive, preventive and personalized medical approach in adrenal incidentalomas. EPMA J. 2018;9(4):421‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Winkelmann MT, Gassenmaier S, Walter SS, et al. Differentiation of adrenal adenomas from adrenal metastases in single-phased staging dual-energy CT and radiomics. Diagn Interv Radiol. 2022;28(3):208‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Torresan F, Crimì F, Ceccato F, et al. Radiomics: a new tool to differentiate adrenocortical adenoma from carcinoma. BJS Open. 2021;5(1):zraa061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Stanzione A, Galatola R, Cuocolo R, et al. Radiomics in cross-sectional adrenal imaging: a systematic review and quality assessment study. Diagnostics. 2022;12(3):578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Maggio R, Messina F, D’Arrigo B, et al. Machine learning-based texture analysis in the characterization of cortisol secreting vs. Non-secreting adrenocortical incidentalomas in CT scan. Front Endocrinol (Lausanne). 2022;13:873189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Piskin FC, Akkus G, Yucel SP, et al. A machine learning approach to distinguishing between non-functioning and autonomous cortisol secreting adrenal incidentaloma on magnetic resonance imaging using texture analysis. Ir J Med Sci. 2022;192(3):1155‐1161. [DOI] [PubMed] [Google Scholar]
- 166. Chen P-T, Chang D, Liu K-L, et al. Radiomics utilization to differentiate nonfunctional adenoma in essential hypertension and functional adenoma in primary aldosteronism. Sci Rep. 2022;12(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. He K, Zhang Z-T, Wang Z-H, et al. A clinical-radiomic nomogram based on unenhanced computed tomography for predicting the risk of aldosterone-producing adenoma. Front Oncol. 2021;11:634879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Mulatero P, Stowasser M, Loh K-C, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89(3):1045‐1050. [DOI] [PubMed] [Google Scholar]
- 169. Nakada T, Kubota Y, Sasagawa I, et al. Therapeutic outcome of primary aldosteronism: adrenalectomy versus enucleation of aldosterone-producing adenoma. J Urol. 1995;153(6):1775‐1780. [DOI] [PubMed] [Google Scholar]
- 170. Fischer E, Hanslik G, Pallauf A, et al. Prolonged zona glomerulosa insufficiency causing hyperkalemia in primary aldosteronism after adrenalectomy. J Clin Endocrinol Metab. 2012;97(11):3965‐3973. [DOI] [PubMed] [Google Scholar]
- 171. Fu B, Zhang X, Wang G-X, et al. Long-term results of a prospective, randomized trial comparing retroperitoneoscopic partial versus total adrenalectomy for aldosterone producing adenoma. J Urol. 2011;185(5):1578‐1582. [DOI] [PubMed] [Google Scholar]
- 172. Anceschi U, Tuderti G, Fiori C, et al. Minimally invasive partial versus total adrenalectomy for the treatment of primary aldosteronism: results of a multicenter series according to the PASO criteria. Eur Urol Focus. 2021;7(6):1418‐1423. [DOI] [PubMed] [Google Scholar]
- 173. Flammia RS, Anceschi U, Tufano A, et al. Minimally invasive partial vs. total adrenalectomy for the treatment of unilateral primary aldosteronism: a systematic review and meta-analysis. J Clin Med. 2022;11(5):1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Jeschke K, Janetschek G, Peschel R, et al. Laparoscopic partial adrenalectomy in patients with aldosterone-producing adenomas: indications, technique, and results. Urology. 2003;61(1):69‐72. [DOI] [PubMed] [Google Scholar]
- 175. Quillo AR, Grant CS, Thompson GB, et al. Primary aldosteronism: results of adrenalectomy for nonsingle adenoma. J Am Coll Surg. 2011;213(1):106‐112. [DOI] [PubMed] [Google Scholar]
- 176. van de Wiel EC, Küsters B, Mann R, et al. Partial adrenalectomy carries a considerable risk of incomplete cure in primary aldosteronism. J Urol. 2021;206(2):219‐228. [DOI] [PubMed] [Google Scholar]
- 177. Monticone S, Castellano I, Versace K, et al. Immunohistochemical, genetic and clinical characterization of sporadic aldosterone-producing adenomas. Mol Cell Endocrinol. 2015;411:146‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Dekkers T, ter Meer M, Lenders JWM, et al. Adrenal nodularity and somatic mutations in primary aldosteronism: one node is the culprit? J Clin Endocrinol Metab. 2014;99(7):E1341‐E1351. [DOI] [PubMed] [Google Scholar]
- 179. Simone G, Anceschi U, Tuderti G, et al. Robot-assisted partial adrenalectomy for the treatment of Conn's syndrome: surgical technique, and perioperative and functional outcomes. Eur Urol. 2019;75(5):811‐816. [DOI] [PubMed] [Google Scholar]
- 180. Toutounchi S, Pogorzelski R, Wołoszko T, et al. Adrenal-sparing surgery for a hormonally active tumour—a single-centre experience. Endokrynol Pol. 2020;71(5):388‐391. [DOI] [PubMed] [Google Scholar]
- 181. Yetişir F, Salman AE, Ozkardes A, et al. Cortex sparing laparoscopic adrenalectomy in a patient with Conn's syndrome. Ulus Cerrahi Derg. 2013;29(1):38‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Ko OS, Kim JY, Kim HJ, Jeong YB. Laparoscopic partial adrenalectomy: surgical technique and outcome. Korean J Urol Oncol. 2019;17(2):103‐109. [Google Scholar]
- 183. Liao CH, Chung S-D, Lai M-K, Yu H-J, Chueh S-C. Laparoscopic simultaneous bilateral partial and total adrenalectomy: a longer follow-up. BJU Int. 2009;104(9):1269‐1273. [DOI] [PubMed] [Google Scholar]
- 184. Kok K, Yapp S. Laparoscopic adrenal-sparing surgery for primary hyperaldosteronism due to aldosterone-producing adenoma. Surg Endosc. 2002;16(1):108‐111. [DOI] [PubMed] [Google Scholar]
- 185. Sarwar A, Brook OR, Vaidya A, et al. Clinical outcomes following percutaneous radiofrequency ablation of unilateral aldosterone-producing adenoma: comparison with adrenalectomy. J Vasc Interv Radiol. 2016;27(7):961‐967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Yang R, Xu L, Lian H, Gan W, Guo H. Retroperitoneoscopic-guided cool-tip radiofrequency ablation of adrenocortical aldosteronoma. J Endourol. 2014;28(10):1208‐1214. [DOI] [PubMed] [Google Scholar]
- 187. Ahmed M, Brace CL, Lee FT, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology. 2011;258(2):351‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Donlon P, Dennedy MC. Thermal ablation in adrenal disorders: a discussion of the technology, the clinical evidence and the future. Curr Opin Endocrinol Diabetes Obes. 2021;28(3):291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Guo R-Q, Li Y-M, Li X-G. Comparison of the radiofrequency ablation versus laparoscopic adrenalectomy for aldosterone-producing adenoma: a meta-analysis of perioperative outcomes and safety. Updates Surg. 2021;73(4):1477‐1485. [DOI] [PubMed] [Google Scholar]
- 190. Chen J, Wu J, Zhu R, Lu L, Ma X-J. Ablation versus laparoscopic adrenalectomy for the treatment of aldosterone–producing adenoma: a meta-analysis. Abdom Radiol (NY). 2021;46(6):2795‐2804. [DOI] [PubMed] [Google Scholar]
- 191. Li Y-C, Wang Y, Ma T, et al. Clinical outcomes of surgical resection versus radiofrequency ablation in very-early-stage hepatocellular carcinoma: a propensity score matching analysis. BMC Gastroenterol. 2021;21(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Wang L, Xu J, Yu J, Liang P. Review of clinical tumor ablation advance in Asia. Int J Hyperthermia. 2021;38(1):1639‐1649. [DOI] [PubMed] [Google Scholar]
- 193. Oguro S, Morimoto R, Seiji K, et al. Safety and feasibility of radiofrequency ablation using bipolar electrodes for aldosterone-producing adenoma: a multicentric prospective clinical study. Sci Rep. 2022;12(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Cano-Valderrama O, González-Nieto J, Abad-Cardiel M, et al. Laparoscopic adrenalectomy vs. Radiofrequency ablation for the treatment of primary aldosteronism. A single center retrospective cohort analysis adjusted with propensity score. Surg Endosc. 2021;36(3):1970‐11978. [DOI] [PubMed] [Google Scholar]
- 195. Zhao Z, Liu X, Zhang H, et al. Catheter-Based adrenal ablation remits primary aldosteronism: a randomized medication-controlled trial. Circulation. 2021;144(7):580‐582. [DOI] [PubMed] [Google Scholar]
- 196. Bouhanick B, Delchier MC, Lagarde S, et al. Radiofrequency ablation for adenoma in patients with primary aldosteronism and hypertension: ADERADHTA, a pilot study. J Hypertens. 2021;39(4):759‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Lo C-H, Tyan Y-S, Ueng K-C. Immediate results and long-term outcomes following percutaneous radiofrequency ablation of unilateral aldosterone-producing adenoma. Acta Cardiologica Sinica. 2020;36(2):160‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Liu SYW, Chu CCM, Tsui TKC, et al. Aldosterone-producing adenoma in primary aldosteronism: CT-guided radiofrequency ablation—long-term results and recurrence rate. Radiology. 2016;281(2):625‐634. [DOI] [PubMed] [Google Scholar]
- 199. Nunes TF, Szejnfeld D, Xavier ACW, Goldman SM. Percutaneous ablation of functioning adenoma in a patient with a single adrenal gland. BMJ Case Rep. 2013;2013:bcr2013009692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Zhang H, Li Q, Liu X, et al. Adrenal artery ablation for primary aldosteronism without apparent aldosteronoma: an efficacy and safety, proof-of-principle trial. J Clin Hypertens (Greenwich). 2020;22(9):1618‐1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Zhou Y, Wang D, Liu Q, Hou J, Wang P. Case report: percutaneous adrenal arterial embolization cures resistant hypertension. Front Cardiovasc Med. 2022;9:1013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Fowler AM, Burda JF, Kim SK. Adrenal artery embolization: anatomy, indications, and technical considerations. Am J Roentgenol. 2013;201(1):190‐201. [DOI] [PubMed] [Google Scholar]
- 203. Sebek J, Cappiello G, Rahmani G, et al. Image-based computer modeling assessment of microwave ablation for treatment of adrenal tumors. Int J Hyperthermia. 2022;39(1):1264‐1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol. 2009;38(3):135‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Pfannenstiel A, Sebek J, Fallahi H, et al. Directional microwave ablation: experimental evaluation of a 2.45-GHz applicator in ex vivo and in vivo liver. J Vasc Interv Radiol. 2020;31(7):1170‐1177.e2. [DOI] [PubMed] [Google Scholar]
- 206. Ren Y, Yan Y, Qi H. Photothermal conversion and transfer in photothermal therapy: from macroscale to nanoscale. Adv Colloid Interface Sci. 2022;308:102753. [DOI] [PubMed] [Google Scholar]
- 207. Sharma A, Cressman Erik, Attaluri A, Kraitchman DL, Ivkov R. Current challenges in image-guided magnetic hyperthermia therapy for liver cancer. Nanomaterials. 2022;12(16):2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Laimer G, Schullian P, Putzer D, et al. Can accurate evaluation of the treatment success after radiofrequency ablation of liver tumors be achieved by visual inspection alone? Results of a blinded assessment with 38 interventional oncologists. Int J Hyperthermia. 2020;37(1):1362‐1367. [DOI] [PubMed] [Google Scholar]
- 209. Byrd JB, Turcu AF, Auchus RJ. Primary aldosteronism: practical approach to diagnosis and management. Circulation. 2018;138(8):823‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Miyake Y, Tanaka K, Nishikawa T, et al. Prognosis of primary aldosteronism in Japan: results from a nationwide epidemiological study. Endocr J. 2013;61(1):35‐40. [DOI] [PubMed] [Google Scholar]
- 211. Štrauch B, Petrak O, Zelinka T, et al. Adrenalectomy improves arterial stiffness in primary aldosteronism. Am J Hypertens. 2008;21(10):1086‐1092. [DOI] [PubMed] [Google Scholar]
- 212. Vaidya A, Hamrahian AH, Auchus RJ. Genetics of primary aldosteronism. Endocr Pract. 2015;21(4):400‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Parthasarathy HK, Ménard J, White WB, et al. A double-blind, randomized study comparing the antihypertensive effect of eplerenone and spironolactone in patients with hypertension and evidence of primary aldosteronism. J Hypertens. 2011;29(5):980‐990. [DOI] [PubMed] [Google Scholar]
- 214. Brown NJ. Eplerenone: cardiovascular protection. Circulation. 2003;107(19):2512‐2518. [DOI] [PubMed] [Google Scholar]
- 215. Pratt JH, Eckert GJ, Newman S, Ambrosius WT. Blood pressure responses to small doses of amiloride and spironolactone in normotensive subjects. Hypertension. 2001;38(5):1124‐1129. [DOI] [PubMed] [Google Scholar]
- 216. Gritting GT, Cole AG, Aurecchia SA, et al. Amiloride in primary hyperaldosteronism. Clin Pharmacol Ther. 1982;31(1):56‐61. [DOI] [PubMed] [Google Scholar]
- 217. Farquharson CAJ, Struthers A. Increasing plasma potassium with amiloride shortens the QT interval and reduces ventricular extrasystoles but does not change endothelial function or heart rate variability in chronic heart failure. Heart. 2002;88(5):475‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Arai K, Homma T, Morikawa Y, et al. Pharmacological profile of CS-3150, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist. Eur J Pharmacol. 2015;761:226‐234. [DOI] [PubMed] [Google Scholar]
- 219. Kolkhof P, Bärfacker L. 30 Years of the mineralocorticoid receptor: mineralocorticoid receptor antagonists: 60 years of research and development. J Endocrinol. 2017;234(1):T125‐T140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Satoh F, Ito S, Itoh H, et al. Efficacy and safety of esaxerenone (CS-3150), a newly available nonsteroidal mineralocorticoid receptor blocker, in hypertensive patients with primary aldosteronism. Hypertens Res. 2021;44(4):464‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Yamada M, Takei M, Suzuki E, et al. Pharmacokinetics, distribution, and disposition of esaxerenone, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist, in rats and monkeys. Xenobiotica. 2017;47(12):1090‐1103. [DOI] [PubMed] [Google Scholar]
- 222. Ito S, Itoh H, Rakugi H, et al. Double-blind randomized phase 3 study comparing esaxerenone (CS-3150) and eplerenone in patients with essential hypertension (ESAX-HTN study). Hypertension. 2020;75(1):51‐58. [DOI] [PubMed] [Google Scholar]
- 223. Ito S, Shikata K, Nangaku M, Okuda Y, Sawanobori T. Efficacy and safety of esaxerenone (CS-3150) for the treatment of type 2 diabetes with microalbuminuria: a randomized, double-blind, placebo-controlled, phase II trial. Clin J Am Soc Nephrol. 2019;14(8):1161‐1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Rakugi H, Ito S, Itoh H, Okuda Y, Yamakawa S. Long-term phase 3 study of esaxerenone as mono or combination therapy with other antihypertensive drugs in patients with essential hypertension. Hypertens Res. 2019;42(12):1932‐1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219‐2229. [DOI] [PubMed] [Google Scholar]
- 226. Pitt B, Filippatos G, Agarwal R, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021;385(24):2252‐2263. [DOI] [PubMed] [Google Scholar]
- 227. Bramlage P, Swift SL, Thoenes M, et al. Non-steroidal mineralocorticoid receptor antagonism for the treatment of cardiovascular and renal disease. Eur J Heart Fail. 2016;18(1):28‐37. [DOI] [PubMed] [Google Scholar]
- 228. Amazit L, Le Billan F, Kolkhof P, et al. Finerenone impedes aldosterone-dependent nuclear import of the mineralocorticoid receptor and prevents genomic recruitment of steroid receptor coactivator-1. J Biol Chem. 2015;290(36):21876‐21889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Pitt B, Kober L, Ponikowski P, et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J. 2013;34(31):2453‐2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Nakamura T, Kawaguchi A. Phase 1 studies to define the safety, tolerability, and pharmacokinetic and pharmacodynamic profiles of the nonsteroidal mineralocorticoid receptor antagonist apararenone in healthy volunteers. Clin Pharmacol Drug Dev. 2021;10(4):353‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Wada T, Inagaki M, Yoshinari T, et al. Apararenone in patients with diabetic nephropathy: results of a randomized, double-blind, placebo-controlled phase 2 dose-response study and open-label extension study. Clin Exp Nephrol. 2021;25(2):120‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Yamada M, Mendell J, Takakusa H, Shimizu T, Ando O. Pharmacokinetics, metabolism, and excretion of [14C] esaxerenone, a novel mineralocorticoid receptor blocker in humans. Drug Metab Dispos. 2019;47(3):340‐349. [DOI] [PubMed] [Google Scholar]
- 233. Ito S, Kashihara N, Shikata K, et al. Esaxerenone (CS-3150) in patients with type 2 diabetes and microalbuminuria (ESAX-DN): phase 3 randomized controlled clinical trial. Clin J Am Soc Nephrol. 2020;15(12):1715‐1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Study of CS-3150 in Hypertensive Patients With Type 2 Diabetes and Albuminuria. Accessed April 20, 2023. https://ClinicalTrials.gov/show/NCT02807974
- 235. Study of CS-3150 Compared to Olmesartan in Patients With Essential Hypertension. Accessed April 20, 2023. https://ClinicalTrials.gov/show/NCT02848170
- 236. Lentini S, Heinig R, Kimmeskamp-Kirschbaum N, Wensing G. Pharmacokinetics, safety and tolerability of the novel, selective mineralocorticoid receptor antagonist finerenone-results from first-in-man and relative bioavailability studies. Fundam Clin Pharmacol. 2016;30(2):172‐184. [DOI] [PubMed] [Google Scholar]
- 237. Heinig R, Gerisch M, Engelen A, Nagelschmitz J, Loewen S. Pharmacokinetics of the novel, selective, non-steroidal mineralocorticoid receptor antagonist finerenone in healthy volunteers: results from an absolute bioavailability study and drug–drug interaction studies in vitro and in vivo. Eur J Drug Metab Pharmacokinet. 2018;43(6):715‐727. [DOI] [PubMed] [Google Scholar]
- 238. Wang EB, Chaudhary A, Waterhouse TH, Dickinson GL. Population pharmacokinetics of LY2623091 in patients with hypertension and chronic kidney disease. J Clin Pharmacol. 2017;57(6):739‐746. [DOI] [PubMed] [Google Scholar]
- 239. A Study of LY2623091 in Male and Females With Chronic Kidney Disease. Accessed April 20, 2023. https://ClinicalTrials.gov/show/NCT01427972
- 240. A Study of LY2623091 in Participants With High Blood Pressure. Accessed April 20, 2023. https://ClinicalTrials.gov/show/NCT02194465
- 241. Pitt B, Jaisser F, Bakris G. An evaluation of KBP-5074 in advanced chronic kidney disease with uncontrolled hypertension. Expert Opin Invest Drugs. 2021;30(10):1017‐1023. [DOI] [PubMed] [Google Scholar]
- 242. Bakris G, Pergola PE, Delgado B, et al. Effect of KBP-5074 on blood pressure in advanced chronic kidney disease: results of the BLOCK-CKD study. Hypertension. 2021;78(1):74‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. A Study of CIN-107 in Adults With Treatment-Resistant Hypertension (rHTN). Accessed April 20, 2023. https://ClinicalTrials.gov/show/NCT04519658
- 244. Armani S, Ting L, Sauter N, et al. Drug interaction potential of osilodrostat (LCI699) based on its effect on the pharmacokinetics of probe drugs of cytochrome P450 enzymes in healthy adults. Clin Drug Investig. 2017;37(5):465‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Amar L, Azizi M, Menard J, et al. Aldosterone synthase inhibition with LCI699: a proof-of-concept study in patients with primary aldosteronism. Hypertension. 2010;56(5):831‐838. [DOI] [PubMed] [Google Scholar]
- 246. Ogishima T, Suzuki H, Hata J, Mitani F, Ishimura Y. Zone-specific expression of aldosterone synthase cytochrome P-450 and cytochrome P-45011 beta in rat adrenal cortex: histochemical basis for the functional zonation. Endocrinology. 1992;130(5):2971‐2977. [DOI] [PubMed] [Google Scholar]
- 247. Domalik LJ, Chaplin DD, Kirkman MS, et al. Different isozymes of mouse 11/ss-hydroxylase produce mineralocorticoids and glucocorticoids. Mol Endocrinol. 1991;5(12):1853‐1861. [DOI] [PubMed] [Google Scholar]
- 248. Weldon SM, Brown NF. Inhibitors of aldosterone synthase. Vitam Horm. 2019;109:211‐239. [DOI] [PubMed] [Google Scholar]
- 249. Trunet P, Mueller P, Girard F, et al. The effects of fadrozole hydrochloride on aldosterone secretion in healthy male subjects. J Clin Endocrinol Metab. 1992;74(3):571‐576. [DOI] [PubMed] [Google Scholar]
- 250. Mornet E, Dupont J, Vitek A, White PC. Characterization of two genes encoding human steroid 11β-hydroxylase (P-45011β). J Biol Chem. 1989;264(35):20961‐20967. [PubMed] [Google Scholar]
- 251. Calhoun DA, White WB, Krum H, et al. Effects of a novel aldosterone synthase inhibitor for treatment of primary hypertension: results of a randomized, double-blind, placebo-and active-controlled phase 2 trial. Circulation. 2011;124(18):1945‐1955. [DOI] [PubMed] [Google Scholar]
- 252. Dougherty JA, Desai DS, Herrera JB. Osilodrostat: a novel steroidogenesis inhibitor to treat Cushing's disease. Ann Pharmacother. 2021;55(8):1050‐1060. [DOI] [PubMed] [Google Scholar]
- 253. Bogman K, Schwab D, Delporte M-L, et al. Preclinical and early clinical profile of a highly selective and potent oral inhibitor of aldosterone synthase (CYP11B2). Hypertension. 2017;69(1):189‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Freeman MW, Halvorsen Y-D, Marshall W, et al. Phase 2 trial of baxdrostat for treatment-resistant hypertension. N Engl J Med. 2023;388(5):395‐405. [DOI] [PubMed] [Google Scholar]
- 255. Lenzini L, Zanotti G, Bonchio M, Rossi GP. Aldosterone synthase inhibitors for cardiovascular diseases: a comprehensive review of preclinical, clinical and in silico data. Pharmacol Res. 2021;163:105332. [DOI] [PubMed] [Google Scholar]
- 256. Beuschlein F, Boulkroun S, Osswald A, et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. 2013;45(4):440‐444. [DOI] [PubMed] [Google Scholar]
- 257. Choi M, Scholl UI, Yue P, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331(6018):768‐772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Nanba K, Blinder AR, Rege J, et al. Somatic CACNA1H mutation as a cause of aldosterone-producing adenoma. Hypertension. 2020;75(3):645‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Dutta RK, Arnesen T, Heie A, et al. A somatic mutation in CLCN2 identified in a sporadic aldosterone-producing adenoma. Eur J Endocrinol. 2019;181(5):K37‐K41. [DOI] [PubMed] [Google Scholar]
- 260. Scholl UI. Genetics of primary aldosteronism. Hypertension. 2022;79(5):887‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. LIfton RP, Dluhy RG, Powers M, et al. A chimaeric llβ-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature. 1992;355(6357):262‐265. [DOI] [PubMed] [Google Scholar]
- 262. Scholl UI, Stölting G, Schewe J, et al. CLCN2 Chloride channel mutations in familial hyperaldosteronism type II. Nat Genet. 2018;50(3):349‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Geller DS, Zhang J, Wisgerhof MV, et al. A novel form of human mendelian hypertension featuring nonglucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab. 2008;93(8):3117‐3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Mulatero P, Tauber P, Zennaro M-C, et al. KCNJ5 Mutations in European families with nonglucocorticoid remediable familial hyperaldosteronism. Hypertension. 2012;59(2):235‐240. [DOI] [PubMed] [Google Scholar]
- 265. Scholl UI, Stölting G, Nelson-Williams C, et al. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. elife. 2015;4:e06315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Ortner NJ, Kaserer T, Copeland JN, Striessnig J. De novo CACAN1D Ca2+ channelopathies: clinical phenotypes and molecular mechanism. Pflügers Arch. 2020;472(7):755‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Semenova N, Ryzhkova OR, Strokova TV, Taran NN. The third case report a patient with primary aldosteronism, seizures, and neurologic abnormalities (PASNA) syndrome de novo variant mutations in the CACNA1D gene. Zh Nevrol Psikhiatr Im S S Korsakova. 2018;118(12):49‐52. [DOI] [PubMed] [Google Scholar]
- 268. Flanagan S, Vairo F, Johnson MB, et al. A CACNA1D mutation in a patient with persistent hyperinsulinaemic hypoglycaemia, heart defects, and severe hypotonia. Pediatr Diabetes. 2017;18(4):320‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Omata K, Satoh F, Morimoto R, et al. Cellular and genetic causes of idiopathic hyperaldosteronism. Hypertension. 2018;72(4):874‐880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Seidel E, Schewe J, Scholl UI. Genetic causes of primary aldosteronism. Exp Mol Med. 2019;51(11):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Pauzi FA, Azizan EA. Functional characteristic and significance of aldosterone-producing cell clusters in primary aldosteronism and age-related hypertension. Front Endocrinol (Lausanne). 2021;12:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Scholl UI, Goh G, Stölting G, et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet. 2013;45(9):1050‐1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Azizan EA, Poulsen H, Tuluc P, et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet. 2013;45(9):1055‐1060. [DOI] [PubMed] [Google Scholar]
- 274. Stindl J, Tauber P, Sterner C, et al. Pathogenesis of adrenal aldosterone-producing adenomas carrying mutations of the Na+/K+-ATPase. Endocrinology. 2015;156(12):4582‐4591. [DOI] [PubMed] [Google Scholar]
- 275. Mulatero P, Monticone S, Rainey WE, Veglio F, Williams TA. Role of KCNJ5 in familial and sporadic primary aldosteronism. Nat Rev Endocrinol. 2013;9(2):104‐112. [DOI] [PubMed] [Google Scholar]
- 276. Scholl UI, Abriola L, Zhang C, et al. Macrolides selectively inhibit mutant KCNJ5 potassium channels that cause aldosterone-producing adenoma. J Clin Invest. 2017;127(7):2739‐2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Pandey A, Raza F, Velasco A, et al. Comparison of Morisky medication adherence scale with therapeutic drug monitoring in apparent treatment–resistant hypertension. J Am Soc Hypertens. 2015;9(6):420‐426.e2. [DOI] [PubMed] [Google Scholar]
- 278. Curneen JM, Rabbitt L, Browne D, et al. Major disparities in patient-reported adherence compared to objective assessment of adherence using mass spectrometry: a prospective study in a tertiary-referral hypertension clinic. Br J Clin Pharmacol. 2022;89(7):1948‐1955. [DOI] [PubMed] [Google Scholar]
- 279. Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296‐1310. [DOI] [PubMed] [Google Scholar]
- 280. Bikram M, West JL. Thermo-responsive systems for controlled drug delivery. Expert Opin Drug Delivery. 2008;5(10):1077‐1091. [DOI] [PubMed] [Google Scholar]
- 281. Yu L, Chang GT, Zhang H, Ding JD. Injectable block copolymer hydrogels for sustained release of a PEGylated drug. Int J Pharm. 2008;348(1-2):95‐106. [DOI] [PubMed] [Google Scholar]
- 282. Wang X, Ronsin O, Gravez B, et al. Nanostructured dense collagen-polyester composite hydrogels as amphiphilic platforms for drug delivery. Adv Sci (Weinh). 2021;8(7):2004213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Kelidari H, Saeedi M, Akbari J, et al. Development and optimisation of spironolactone nanoparticles for enhanced dissolution rates and stability. AAPS PharmSciTech. 2017;18(5):1469‐1474. [DOI] [PubMed] [Google Scholar]
- 284. Langguth P, Hanafy A, Frenzel D, et al. Nanosuspension formulations for low-soluble drugs: pharmacokinetic evaluation using spironolactone as model compound. Drug Dev Ind Pharm. 2005;31(3):319‐329. [DOI] [PubMed] [Google Scholar]
- 285. Wang H, Shrestha TB, Basel MT, et al. Magnetic-Fe/Fe3O4-nanoparticle-bound SN38 as carboxylesterase-cleavable prodrug for the delivery to tumors within monocytes/macrophages. Beilstein J Nanotechnol. 2012;3(1):444‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]