Abstract
Kisspeptin (KP) and neurokinin B (NKB) are neuropeptides that govern the reproductive endocrine axis through regulating hypothalamic gonadotropin-releasing hormone (GnRH) neuronal activity and pulsatile GnRH secretion. Their critical role in reproductive health was first identified after inactivating variants in genes encoding for KP or NKB signaling were shown to result in congenital hypogonadotropic hypogonadism and a failure of pubertal development. Over the past 2 decades since their discovery, a wealth of evidence from both basic and translational research has laid the foundation for potential therapeutic applications. Beyond KP's function in the hypothalamus, it is also expressed in the placenta, liver, pancreas, adipose tissue, bone, and limbic regions, giving rise to several avenues of research for use in the diagnosis and treatment of pregnancy, metabolic, liver, bone, and behavioral disorders.
The role played by NKB in stimulating the hypothalamic thermoregulatory center to mediate menopausal hot flashes has led to the development of medications that antagonize its action as a novel nonsteroidal therapeutic agent for this indication. Furthermore, the ability of NKB antagonism to partially suppress (but not abolish) the reproductive endocrine axis has supported its potential use for the treatment of various reproductive disorders including polycystic ovary syndrome, uterine fibroids, and endometriosis. This review will provide a comprehensive up-to-date overview of the preclinical and clinical data that have paved the way for the development of diagnostic and therapeutic applications of KP and NKB.
Keywords: kisspeptin, neurokinin B, reproduction, metabolism, bone, behavior
Graphical Abstract
Graphical abstract.
Essential Points.
Kisspeptin (KP) and neurokinin B (NKB) stimulate the pulsatile secretion of gonadotropin-releasing hormone and thus are considered key regulators of the reproductive endocrine axis
KP has emerged as a promising diagnostic and therapeutic tool for disorders of puberty, reproduction, pregnancy, metabolism, liver, bone, and behavior
Therapies acting through antagonism of NKB action provide potential therapeutic options for women with menopausal hot flashes, polycystic ovary syndrome, uterine fibroids, and endometriosis.
Kisspeptin (KP) and neurokinin B (NKB) are hypothalamic neuropeptides that play a pivotal role in the regulation of reproductive physiology. In 2003, inactivating variants in the gene encoding for the kisspeptin receptor (KISS1R) was shown to result in congenital hypogonadotropic hypogonadism (CHH) and a failure of pubertal development (1, 2). Following this, inactivating variants of the KISS1 gene were also found to result in normosomic CHH (3). Conversely, in 2008, activating variants in genes encoding for KISS1R resulted in premature activation of the hypothalamic-pituitary-gonadal (HPG) axis and central precocious puberty (CPP) (4). Thus, KP was shown to play a key role in regulating reproductive hormonal secretion and puberty, and it is now established that KP acts to stimulate gonadotropin-releasing hormone (GnRH) neurons in the hypothalamus and the downstream reproductive axis (5-7) (Fig. 1).
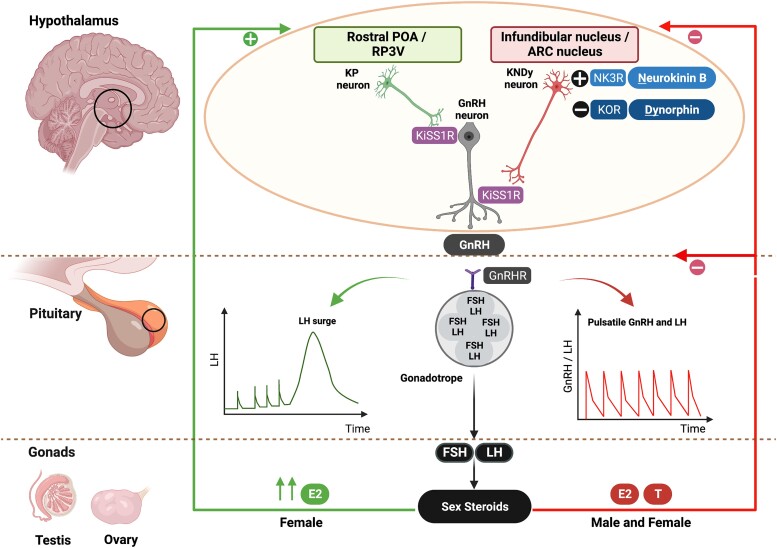
Figure 1.
KP and NKB in the regulation of the HPG axis. KP is released from the POA (equivalent to rostral periventricular area of the third ventricle, RP3V, in nonhumans) and infundibular nucleus (arcuate, ARC, nucleus in nonhumans) of the hypothalamus. The KP neurons in the infundibular nucleus coexpress NKB and dynorphin (known as KNDy neurons) and are involved in the autosynaptic regulation of pulsatile KP secretion via the NKB receptor (NK3R) and kappa opioid peptide receptor (KOR), respectively. Dynorphin inhibits, whereas NKB stimulates, KP release. Following KP's release from the hypothalamus, KP stimulates the hypothalamic GnRH neurons to release GnRH in a pulsatile manner, which stimulates anterior pituitary production of gonadotropins (LH, FSH) and subsequent production of gonadal (testicular/ovarian) sex-steroids (E2, T). The gonadotropins’ effect on the ovary stimulates follicular development, oocyte maturation, and ovulation. The KNDy neurons in the infundibular nucleus mainly receive negative feedback (E2, T) from sex-steroids, whereas KP neurons in the POA receive positive feedback from estrogen in females (high E2), which is involved in the preovulatory LH surge. Sex-steroid communication with the POA has not yet been fully established in males. E2, estrogen; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; HPG, hypothalamic-pituitary-gonadal; KISS1R, kisspeptin receptor; KOR, kappa opioid peptide receptor; KP, kisspeptin; LH, luteinizing hormone; NK3R, neurokinin 3 receptor; NKB, neurokinin B; P, progesterone; POA, preoptic area; RP3V, rostral periventricular area of the third ventricle; T, testosterone. Figure created with BioRender.com.
NKB was also discovered through the study of patients with CHH who were found to have inactivating variants affecting NKB signaling (8). KP colocalizes with NKB and dynorphin (Dyn) in neurons known as “KNDy” neurons in the arcuate nucleus (ARC) of the hypothalamus (equivalent to the infundibular nucleus in humans) (9, 10). These KNDy neurons are now recognized to function as the “GnRH pulse generator,” regulating the pulsatile secretion of GnRH (9, 10). NKB stimulates, whereas Dyn inhibits, the activity of these KNDy neurons, in an auto/paracrine manner to result in the pulsatile release of KP and, in turn, GnRH (11, 12). Pulsatile GnRH secretion subsequently induces the synthesis and secretion of pituitary gonadotropins (ie, luteinizing hormone [LH] and follicle-stimulating hormone [FSH]) (13, 14), which in turn stimulate sex-steroid production (estrogen and testosterone), and gametogenesis within the gonads (oocytes in ovaries and sperm in testes) (15) (see Fig. 1).
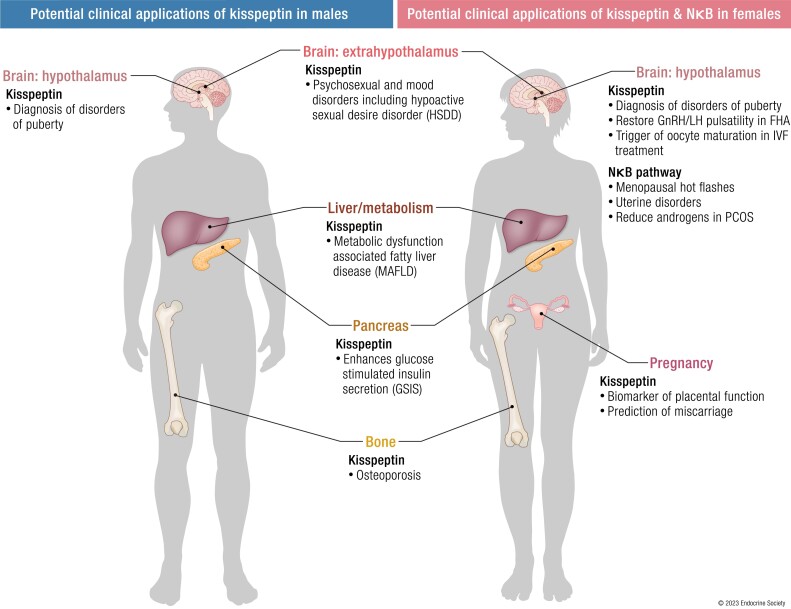
KP neurons integrate sex-steroid and metabolic signals from the periphery, either directly or via interneurons, to affect GnRH secretion and the HPG axis. Several functional reproductive disorders are due to a disturbance in hypothalamic KP neuronal activity, which has sparked interest in the clinical application of KP both for the treatment and diagnosis of pubertal and reproductive disorders. Furthermore, KP is expressed in multiple organs beyond the hypothalamus, including the placenta, liver, pancreas, adipose tissue, bone, and limbic regions, which predicate its use on the diagnosis and treatment of conditions related to pregnancy, metabolism, liver, bone, and behavior (16, 17) (Fig. 2). Discovery of the critical role of NKB in stimulating the hypothalamic thermoregulatory center has resulted in the use of compounds that block NKB action as treatment for menopausal hot flashes (18-20). As these antagonists of NKB action partially suppress (but do not abolish) reproductive hormone secretion, they have also emerged to have utility in the treatment of uterine disorders such as endometriosis and uterine fibroids (21).
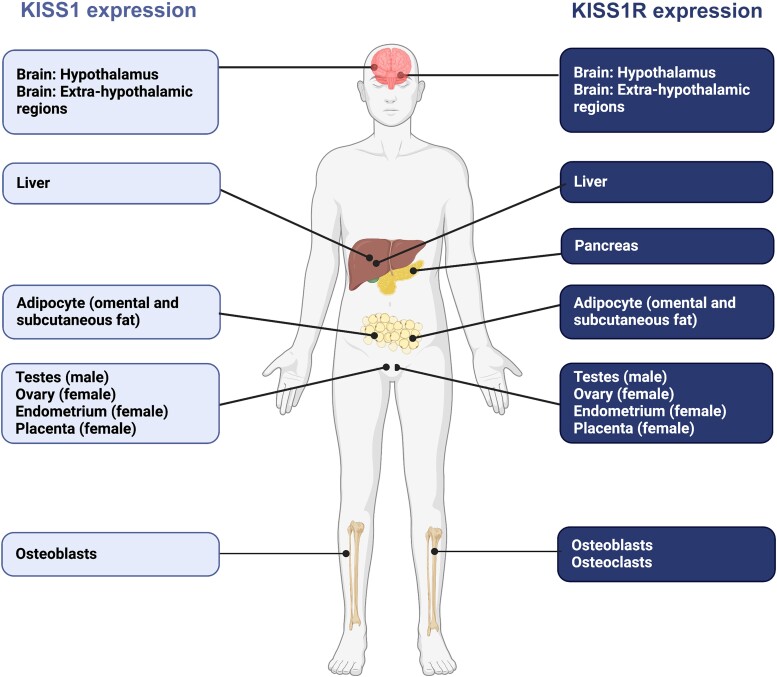
Figure 2.
KISS1 and KISS1R human gene expression in areas where kisspeptin signaling has well-identified roles. Expression is abundant in other areas of the human body, not illustrated in Fig. 2, in which the full role of KP signaling has yet to be elucidated. This widespread distribution of KISS1 and KISS1R reflects the pleiotropic action of KP, beyond reproduction. In humans, the tissue distribution of KISS1 and KISS1R has been identified using RT-PCR methods. KISS1 mRNA is predominantly expressed in the placenta, with the next highest level in the testis, and moderate levels in the pancreas, liver, uterus, gonads, and small intestine. KISS1 mRNA is also strongly expressed in bone, in particular the osteoblasts. KISS1R expression is particularly abundant in the placenta, pituitary, spinal cord, liver, pancreas, and bone (osteoblasts and osteoclasts), but expressed at lower levels in other tissues, such as the stomach, uterus, small intestine, thymus, spleen, lung, gonads, heart, kidney, adrenal gland, bone, and fetal liver. Both KISS1 and KISS1R are also expressed in the brain, and in particular the human hypothalamus, as well as extrahypothalamic regions, such as the amygdala, caudate nucleus, cerebellum, cingulate gyrus, globus pallidus, hippocampus, medial frontal gyrus, nucleus accumbens, parahippocampal gyrus, putamen, spinal cord, striatum, substantia nigra, superior frontal gyrus and thalamus, as localized by RT-PCR. KISS1, kisspeptin gene; KISS1R, kisspeptin receptor gene; KP, kisspeptin; mRNA, messenger RNA; RT-PCR, reverse transcription polymerase chain reaction. Figure created with BioRender.com.
In this review, we provide a comprehensive, up-to-date overview of the relevant preclinical and clinical data that have paved the way for the development of novel diagnostic and therapeutic applications of KP and NKB.
Discovery of Kisspeptin, Neurokinin B, and Their Receptors
Kisspeptin and its Gene
KP was first discovered in 1996 as a tumor-suppressor and initially termed “metastin” due to its antimetastatic action in malignant melanoma cell lines (22). It later acquired the name “kisspeptin” in homage to its discovery in Hershey (Pennsylvania, USA), which is the hometown of the famous chocolate “Hershey's Kisses” (22). The gene for KP in humans is called “KISS1” with the suppressor sequence denoted by “SS.” While KISS1 is used to indicate the gene in humans, Kiss1 is used for nonhuman KP genes (23). In 2003, KP's obligatory role in regulating hypothalamic GnRH neuronal function was first described in 2 landmark reports by de Roux et al and Seminara et al (1, 2).
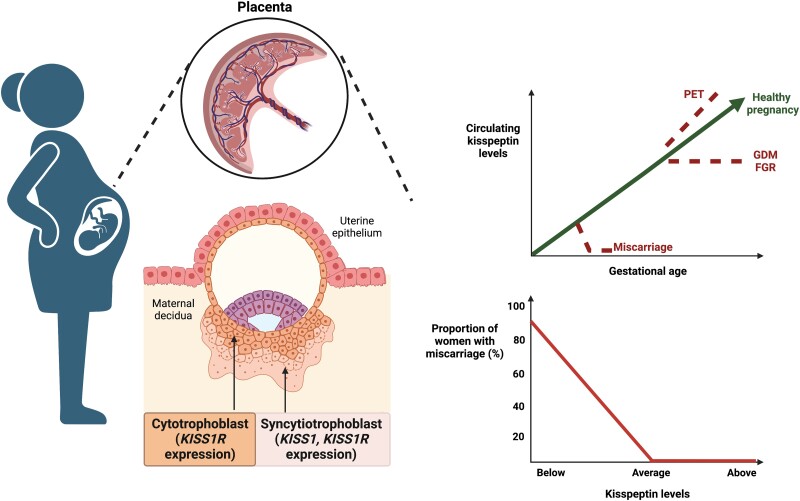
In humans, KP is predominantly expressed in 2 distinct hypothalamic nuclei: the infundibular nucleus (24, 25) (analogous to the ARC in rodents (26)) and the rostral preoptic area (POA) (24, 25) (analogous to the POA, including the anteroventral periventricular area, AVPV, and periventricular nucleus (PeN) in rodents (26)). KP is also expressed within the limbic system (in the amygdala, caudate nucleus, cingulate gyrus, globus pallidus, hippocampus, medial and superior frontal gyrus, nucleus accumbens, parahippocampal gyrus, putamen, striatum, substantia nigra, and thalamus) (16, 17) and has been recognized to play a role in mood and sexual behaviors. Beyond the brain, KISS1 messenger RNA (mRNA) is also highly expressed in the placenta (particularly by syncytiotrophoblasts (27, 28)), gonads (16, 29), adipose tissue (16), pancreas (16, 29), liver (29), small intestine (29), and bone (particularly osteoblasts) (30) (see Fig. 2).
The KISS1 gene is mapped to the long arm of chromosome 1 (1q32-q41) and comprises 4 exons of which only 2 are translated (31). The resultant 145 amino acid prepropeptide is then posttranslationally cleaved into biologically active KP peptides of different amino acid lengths indicated by their suffix, for example, KP-54, -14, -13, and -10 (17, 29, 31). All native KP peptides share a common C-terminal decapeptide sequence, equivalent to KP-10, which includes a terminal RF-amide sequence (Arg-Phe-NH2) (17). This C-terminal amide sequence is important for the binding and activation of the KP receptor. In particular, amidation of the C-terminal is essential for receptor activation, with higher binding affinities observed with KP-10 (Ki = 0.042 nM) and KP-54 (Ki = 0.34 nM) than a C-terminally unamidated form (Ki = 640 nM) (29). KP-10 has a shorter terminal half-life than KP-54 (t1/2 3 vs 28 minutes) (7, 13, 32). Other RF-amide family members such as neuropeptide FF, prolactin (PRL)-releasing peptide, and neuropeptide Y (NPY) do not activate the KP receptor (33).
Kisspeptin Receptor
The KP receptor (encoded by KISS1R) was described in 1999 (23), and was previously known as hOT7T175 (29), AXOR12 (16), or GPR54 (22). The KP receptor is a 398-amino acid peptide encoded by a gene on chromosome 19 (19p13.3) with 5 coding exons interrupted by 4 introns (16). The KP receptor is part of the rhodopsin-like family of G protein–coupled receptors (GPCRs), which is the largest group of GPCRs, and binds its ligand in the binding site within the transmembrane domain (16). KP has a single high-affinity binding site at the human KP receptor (dissociation constant, Kd, 1.9 ± 0.4 nM using 500 nM of 125I-KP10) (17) and induces a biphasic response in downstream signaling, with an acute response (lasting ∼5 minutes) and a prolonged phase (lasting >30 minutes) (34). While KISS1R is expressed in similar areas of the body as KISS1, it is also expressed at low levels in tissues including the stomach, thymus, spleen, lung, gonads, heart, kidney, adrenal gland, bone, and fetal liver (16, 29, 35) (see Fig. 2).
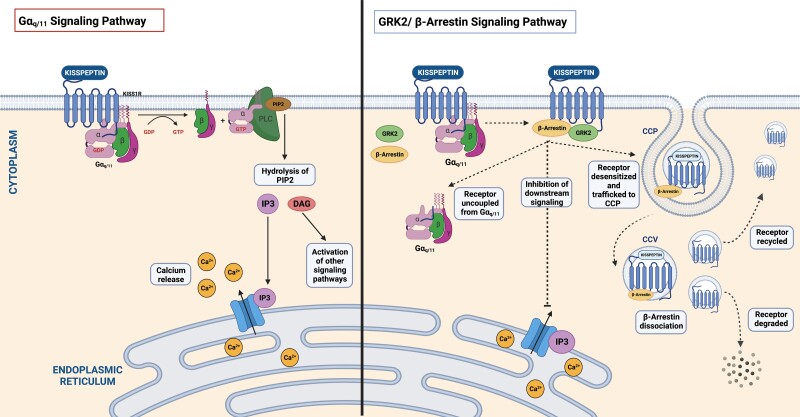
During the basal state (in the absence of KP), the KP receptor couples to Gαq/11 at the cell surface, which triggers KP-independent signaling and downstream activation of phospholipase C (PLC), the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) to inositol triphosphate (IP3) and diacylglycerol (DAG), and intracellular calcium mobilization (36). In the presence of KP, the KP receptor displays increased Gαq/11 signaling through recruitment of GPCR serine/threonine kinases (GRK2) and β-arrestin from the cytosol to the plasma membrane (36). GRK2 phosphorylates the KP receptor (at the intracellular loop and carboxyl terminus) and subsequently facilitates the binding of β-arrestin while preventing further coupling to G proteins (37, 38). β-Arrestin subsequently induces receptor desensitization by uncoupling the KP receptor from Gαq/11 and simultaneously triggers receptor sequestration by trafficking the desensitized KP receptor to the cell surface clathrin-coated pit (36). The sequestered KP receptor (linked to β-arrestin) undergoes β-arrestin–dependent signaling, resulting in receptor internalization and the formation of clathrin-coated vesicles (36). Following this, the KP receptor dissociates from β-arrestin and is either resensitized and recycled back to the cell surface (ready to signal) or targeted for degradation (36) (Fig. 3). Prolonged KP receptor signaling is also dependent on the continuous influx of calcium into the cell as well as maintaining a dynamic pool of receptors at the cell surface including both recycled and nonrecycled receptors (36). While the KP receptor mainly signals via Gαq/11, it can also activate the extracellular signal-regulated kinase 1/2 (ERK1/2) β-arrestin–dependent pathway that also contributes to GnRH secretion (39). Additionally, the KP receptor can form homodimers, heterodimers, or even oligomers with modified actions (40). For instance, the KP receptor heterodimerizes with the G protein estrogen receptor, which reduces its expression at the cell surface and decreases KP receptor signaling (40).
Figure 3.
KP receptor induces differential responses in downstream signaling. KP has a high-affinity binding site for the human KP receptor and induces a biphasic response in downstream signaling, with an acute (lasting ∼5 minutes) and prolonged response (lasting >30 minutes). KISS1R (coupled to Gαq/11) triggers the activation of PLC and subsequent recruitment of secondary intracellular messengers, IP3 and DAG, which in turn mediate intracellular calcium release. DAG additionally activates PKC and induces downstream phosphorylation of ERK 1 and 2. Kisspeptin binding results in the recruitment of β-arrestin and GPCR serine/threonine kinases (GRK2), which leads to desensitization and internalization of the kisspeptin receptor (through uncoupling of Gαq/11). β-Arrestin traffics the desensitized KISS1R to the clathrin-coated pit resulting in sequestration, which results in β-arrestin–dependent signaling. Internalized KISS1R eventually dissociates from β-arrestin and the majority of kisspeptin receptors become resensitized and traffic back to the cell surface, thus maintaining a continuous pool of receptors at the cell surface which are ready to signal while a lesser population of KISS1R are targeted for degradation. DAG; diacylglycerol; ERK, extracellular signal-related kinase; GRK2, GPCR serine/threonine kinases; IP3, inositol triphosphate; KISS1R, kisspeptin receptor gene; KP, kisspeptin; PKC; protein kinase C; PLC, phospholipase C. Figure created with BioRender.com.
The KP receptor is vulnerable to tachyphylaxis, whereby the receptor response is reduced following repeated doses or continuous high doses of KP administration (37). For instance, in agonadal juvenile and adult male monkeys, a 98-hour intravenous (IV) infusion of KP-10 induced a maximal LH response at 3 hours; however, a rapid decline then followed by 12 hours (41, 42). Moreover, an additional bolus of GnRH but not KP-10 resulted in an LH rise, thus indicating that tachyphylaxis is occurring at the level of the KP receptor (41, 42). Likewise, in women with hypothalamic amenorrhea (HA), twice-daily administration of KP-54 resulted in a reduced LH response within a few days (43). Interestingly, KP's responsiveness was maintained with a twice-weekly dosing interval suggesting that chronic stimulation with KP is possible using an appropriate dosing protocol (43). Furthermore, although tachyphylaxis occurs after persistent high-dose exogenous KP, this may not be the case with physiological endogenous KP. Indeed, optogenetic activation of KP neurons in the rostral periventricular area of the third ventricle (RP3V) of female mice can persistently stimulate GnRH neuronal firing (44).
Neurokinin B and its Gene
NKB was first discovered as a central regulator of reproduction in 2009, whereby loss-of-function variants in either NKB or its receptor (NK3R) were identified in 4 of 9 multiplex families affected by hypogonadotropic hypogonadism using genome-wide single-nucleotide polymorphism (now called single-nucleotide variation) analysis (8). In humans, NKB is predominantly expressed in the infundibular nucleus, anterior hypothalamic area septal region, diagonal band of Broca, bed nucleus of the stria terminalis, amygdala, and neocortex (45). The gene encoding NKB (TAC3 in higher primates and Tac2 in rodents) is located on chromosome 12 and is divided into 7 exons, 5 of which are translated to form the preprotachykinin B peptide (46-48). Following proteolytic cleavage, this precursor peptide leads to, first, proneurokinin B, and then NKB (initially contained in exon 5) (46). NKB belongs to the tachykinin family of peptides, which is characterized by a common C-terminal amino-acid sequence (Phe-X-Gly-Leu-Met-NH2) and includes substance P, neurokinin A, and NKB, as well as neuropeptide K, neuropeptide γ, and hemokinin-1 (46, 48).
Neurokinin B Receptor
Three tachykinin receptors have been identified, NK1R, NK2R, and NK3R, with the latter having a longer amino acid sequence (46). The genes encoding the 3 tachykinin receptors are all divided into 5 exons with identical distribution of intronic sequences (46). NKB is an agonist for all 3 receptors; however, it exhibits strong preferential binding for NK3R (encoded by TACR3) (49, 50). Following NKB binding, NK3Rs are activated and result in increased intracellular Ca2+ (through inositol phospholipid hydrolysis) and increased intracellular cyclic adenosine monophosphate levels (through adenylate cyclase activation), and are then internalized (51).
Like NKB, NK3R is also expressed within the central nervous system and spinal cord, although it has also been reported in the uterus, mesenteric vein, gut neurons, and placenta (45). NK3Rs also display species differences and exert differing actions. For instance, while NK3R antagonists have similar potency on NK3Rs in the gerbil, guinea pig, dog, and human, they have lower activity on NK3R in the rat and mouse (52).
Hypothalamic Kisspeptin-Neurokinin B-Dynorphin Neurons and Discrete Kisspeptin Neuronal Populations
KP neuronal bodies are located in 2 discrete hypothalamic nuclei in rodents: the ARC, and the RP3V, which includes the anteroventral periventricular (AVPV) and periventricular (PeN) nuclei (26). The analogous regions in humans are the infundibular nucleus and the rostral POA, respectively (24, 25). Both ARC and RP3V KP neuronal populations innervate GnRH neurons and are responsible for regulating GnRH pulsatility and the mid-cycle LH surge, respectively (53-57) (see Fig. 1). The number and distribution of KP neurons differs between sexes. For instance, while female mice require high hypothalamic Kiss1 expression levels to preserve fertility, male mice need only 5% of Kiss1 expression (58). In rodents and sheep, the proportion of KP neurons in both the AVPV (59) and ARC (26, 60) is greater in females than males. Consistent with this, the number of KP immune-positive cell bodies found in the infundibulum of human brain autopsies is 7-fold higher in women compared to men (24, 25).
Arcuate Kisspeptin Neurons
KP neurons in the ARC nucleus coexpress NKB and Dyn and are hence known as Kisspeptin-Neurokinin-Dynorphin (KNDy) neurons (61). KNDy neurons are regulated in an autocrine/paracrine manner, with NKB stimulating (via NKB receptor—mainly TAC3R) (61) and Dyn inhibiting (via kappa opioid receptor) (25) neuronal activity. This synchronized episodic action results in KP release, which in turn activates distal dendrons of GnRH neurons and leads to the secretion of GnRH pulses (62). Considering KP receptors are highly expressed within GnRH neurons and absent in KNDy neurons, KP's action predominantly occurs via GnRH neurons (62).
ARC-KP neurons are key regulators of GnRH pulsatile secretion and are referred to as the “GnRH pulse generator” (9, 60). Indeed, optogenetic activation of the channel rhodopsin expressing ARC KP-neurons in Kiss1-Cre mice induced pulsatile LH secretion, whereas inhibition suppressed it (63, 64). Likewise, knockout of greater than 90% of ARC Kiss1 neurons resulted in marked suppression of LH pulses in ovariectomized (OVX) female rats (65).
However, a recent report has challenged the KNDy hypothesis suggesting that synchronization within the ARC is dependent on a “glutamate 2-transition” mechanism in male mice (66). In this model, the first transition is dependent on glutamate but gated by Dyn tone to initiate neuron synchronization, and the second transition is dependent on NKB, which potentiates that synchronization (66).
ARC-KP neurons are tightly regulated by intricate feedback mechanisms in response to several modulators, including sex steroids such as estradiol (E2). In the presence of low circulating E2 levels, a negative feedback effect is exerted on ARC-KP neurons. Indeed, a recent RNA sequencing study in mice identified 1583 estrogen-responsive genes in the ARC with the majority of the genes being suppressed in response to a low E2 environment (67). While negative feedback is present continuously in males, in females it occurs during most of the follicular and luteal phases of the menstrual cycle (68). Negative feedback in response to E2 is mediated by the “nonclassic pathway,” whereby the interaction between E2 and its receptor (ERα) results in the recruitment of estrogen response element (ERE)-independent transcriptions factors (69, 70). E2-ERα signaling leads to Kiss1 promoter histone deacetylation, which inhibits chromatin loop formation between the Kiss1 promoter and the Kiss1 gene enhancer, resulting in reduced ARC-specific Kiss1 gene expression.
Rostral Periventricular Area of the Third Ventricle Kisspeptin Neurons
KP neurons in the RP3V, which includes the AVPV and PeN, innervate the soma and proximal dendrites of GnRH neurons to stimulate GnRH secretion (67). This KP neuronal network is mainly regulated by positive feedback from higher levels of E2. In the presence of high E2, RP3V-KP neurons in rodents (rostral POA neurons in humans) continuously produce GnRH leading to an LH surge (71, 72), which occurs during the proestrus phase in rodents and during the late follicular phase (mid-cycle) in women (73). Of note, 222 genes within RP3V-KP neurons are upregulated in response to high E2 levels, demonstrating their importance to facilitating positive feedback (67). The mechanism responsible for positive feedback predominantly involves E2-ERα signaling and recruitment of cofactors to ERE in the “classic pathway” (69, 70). In contrast to the ARC, Kiss1 promoters within the AVPV undergo histone acetylation and subsequent increased AVPV-specific Kiss1 gene expression. The role of these neurons remains uncertain in male mammals that have lower KP expression than female mammals in RP3V-KP neurons (74).
Kisspeptin and Neurokinin B in Healthy Men and Women
Kisspeptin in Healthy Men
In healthy adult men, acute administration of KP-54 induced dose-dependent increases in circulating LH and, to a lesser degree, FSH (13) (Table 1A). In particular, KP-54 (IV infusion 0.24 nmol/kg/h over 90 minutes) increased mean LH levels 2.6-fold higher than placebo (13). Similarly, an IV bolus of KP-10 (0.77 nmol/kg) potently evoked LH secretion from 4.1 to 12.4 ± IU/L and a continuous IV infusion (3.07 nmol/kg/h) of KP-10 led to persistent LH secretion over 22.5 hours (76). The shorter isoform, KP-10, has a briefer half-life and duration of gonadotropin release, with LH levels rising within 30 to 40 minutes after an IV bolus administration (0.3 to 1.0 nmol/kg) (77). In a direct equimolar comparison between KP-54 and KP-10 (hypothalamic stimulation) against GnRH (pituitary stimulation), LH and FSH responses were greater following GnRH, then KP-54, and then KP-10 (32). Although GnRH is more potent than KP, KP is hypothesized to induce the release of GnRH from a limited endogenous pool (126), which could be preferable when stimulating reproductive hormone secretion in a clinical context where there is an unwanted risk of overstimulation.
Table 1.
Clinical trials involving kisspeptin
| Author | Study design | Cohort | Intervention | Results |
|---|---|---|---|---|
| A, KP in healthy men | ||||
| Dhillo et al (2005) (13) | Double-blind placebo-controlled crossover | 6 men | KP54 (IV infusion 4 pmol/kg/min for 90 min) vs vehicle | KP54 increased LH (by 2.6-fold), FSH (by 1.2-fold), and testosterone |
| Chan et al (2011) (75) | Prospective study | 13 men | Baseline sampling (10 min for 6 h) followed by KP10 (IV bolus 0.24 nmol/kg) | KP10 induced immediate LH pulses, regardless of timing of previous endogenous pulse KP10 induced larger amplitude pulses than endogenous pulses (amplitude 5.0 ± 1.0 vs 2.1 ± 0.3 mIU/mL) |
| George et al (2011) (76) | Placebo-controlled | 6 men (acute studies) 4 men (chronic studies) |
KP10 (IV bolus 0.01, 0.03, 0.1, 0.3, 1.0, and 3.0 μg/kg) vs vehicle Baseline sampling (10 min for 9 h) followed by bolus KP10 (IV bolus 3.0 μg/kg) then (IV infusion 1.5 μg/kg/h for 22.5 h) |
KP10 (IV bolus 1 μg/kg) induced max LH response (4.1 ± 0.4 to 12.4 ± 1.7 IU/L) KP10 (IV infusion 1.5 μg/kg/h) increased
|
| Jayasena et al (2011) (77) | Single-blind placebo-controlled | 4 or 5 per group | KP10 (IV bolus at 0.3, 1.0, 3.0, or 10 nmol/kg) vs vehicle | KP10 elevated LH, FSH, and testosterone levels at doses as low as 0.3 and 1.0 nmol/kg, respectively |
| Jayasena et al (2015) (32) | Single-blind placebo-controlled | 5 men | KP10, KP54, GnRH, or vehicle (IV infusion 0.1, 0.3, and 1.0 nmol/kg/h for 3 h) |
Serum LH and FSH ∼3-fold higher during GnRH vs KP10 Serum LH and FSH ∼2-fold higher during GnRH vs KP54 |
| B, KP in healthy premenopausal women | ||||
| Dhillo et al (2007) (14) | Double-blind placebo-controlled | 8 women | KP54 (SC bolus 0.4 nmol/kg) | KP54 increased mean LH ± SEM (IU/L) during follicular (0.12 ± 0.17), preovulatory (20.64 ± 2.91), and luteal (2.17 ± 0.79) phases of menstrual cycle |
| Jayasena et al (2011) (77) | Single blind placebo-controlled | 4 or 5 per group | KP10 (IV bolus 1-10 nmol/kg) (SC bolus 2-32 nmol/kg) (IV infusion 20–720 pmol/kg/min) KP54 (IV bolus 1 nmol/kg) |
KP10 (all doses and routes) did not alter LH and FSH in follicular phase of menstrual cycle KP10 (IV bolus 10 nmol/kg) increased mean AUC LH (30.3 ± 7.7 h·IU/L) and FSH (6.9 ± 0.9 h·IU/L) in preovulatory phase |
| Chan et al (2012) (78) | Prospective study | 3-14 per group | KP 112-121 (IV bolus 0.24, 0.72 nmol/kg) | KP112-121 induced higher LH responses and LH pulses in luteal and preovulatory phases, but not early-mid follicular phase of menstrual cycle |
| George et al (2012) (79) | Prospective study | 10 women | KP10 (IV bolus 0.3 µg/kg) | KP10 increased LH but not FSH during early follicular phase of menstrual cycle |
| Jayasena et al (2013) (80) | Randomized single-blinded placebo-controlled trial | 6 women | KP54 (SC bolus 0.30, 0.60 nmol/kg) vs vehicle | KP54 increased mean LH pulses (KP54; −0·17 ± 0·54, saline; + 2·33 ± 0·56) during follicular phase |
| Jayasena et al (2013) (81) | Prospective single-blinded, placebo-controlled 1-way crossover trial | 5 women | KP54 (SC bolus 6.4 nmol/kg, twice daily, during d 7-14 of menstrual cycle) vs vehicle | KP54 does not cause tachyphylaxis KP54 induced a shorter menstrual cycle length (d 26.8 vs d 28.6), earlier LH peak (d 13 vs d 15.2), and earlier luteal phase vs saline (d 15.8 vs d 18) vs vehicle |
| Narayanaswamy et al (2016) (82) | Prospective single-blinded placebo-controlled trial | 4 women | KP54 (SC infusion 0.3-1.0 nmol/kg/h for 8 h) during early follicular phase of 4 menstrual cycles | KP54 induced mean rise in LH (>8 IU/L) KP54 positively correlated with baseline E2 levels (KP54 dose of 1.0 nmol/kg/h → 100 pmol/L rise in baseline E2 associated with 1.0-IU/L increase in LH) |
| Abbara et al (2020) (83) | Single-blinded randomized controlled trial | 9 women | MVT-602 (SC bolus 0.01, 0.03 nmol/kg) KP-54 (SC bolus 9.6 nmol/kg) during early follicular phase |
MVT-602 and KP54 had similar LH amplitude increases LH peak delayed with MVT-602 vs KP54 (21.4 vs 4.7 hrs) AUC of LH exposure increased with MVT-602 vs KP54 (169 vs 38.5 IU·h/L) MVT-602 induced longer duration of GnRH neuronal firing than KP54 (115 vs 55 min) |
| C, KP in delayed puberty | ||||
| Chan et al (2014) (84) | Longitudinal cohort study, proof of concept | 11 CHH (adult) 1 with reversal of CHH |
KP10 (IV bolus 0.24 nmol/kg) GnRH (IV bolus 75 ng/kg) |
KP10 (unlike GnRH) failed to induce LH response in CHH, but produced LH response in reversal of CHH |
| Lippincott et al (2016) (85) | Single-blinded randomized controlled trial | 4 with reversal of CHH 2 with relapsed CHH |
KP10 (IV bolus 0.24-2.4 nmol/kg) GnRH (IV bolus 75 ng/kg) |
KP10 stimulated LH pulses in reversal of CHH (within 30 min) but not in relapsed CHH |
| Chan et al (2020) (86) | Longitudinal cohort study | 16 with delayed puberty | KP10 (IV bolus 0.313 µg/kg) GnRH (IV bolus 75 ng/kg) |
KP10 increased LH in CDGP (≥0.8 mIU/mL) but not in CHH (≤0.4 mIU/mL) |
| Abbara et al (2021) (87) | Single-blinded randomised controlled trial | 21 CHH 21 controls |
KP54 (IV bolus 6.4 nmol/kg) GnRH (IV 100 mcg) |
KP54 had reduced LH responses in CHH (0.4 IU/L) than controls (12.5 IU/L), and had an AUCROC of 100% (95% CI, 100%-100%) to differentiate CHH from healthy |
| D, KP in precocious puberty | ||||
| Cintra et al (2021) (88) | Systematic review | Systematic review and meta-analysis 316 CPP 251 controls |
KP measurement | KP increased in CPP vs controls (std MD and [95% CI] = 1.53 [0.56-2.51]) KP positively correlated with age and was associated with precocious thelarche |
| Vuralli et al (2023) (89) | Cross-sectional study | 51 CPP 48 PT 42 controls |
KP measurement (ng/mL) | KP increased in CPP (0.43 ± 0.16) vs PT (0.26 ± 0.10) vs controls (0.18 ± 0.07) |
| E: KISSPEPTIN IN HYPOTHALAMIC AMENORRHOEA | ||||
| Podfigurna et al (2020) (90) | Prospective cohort | HA: 58 low LH 13 normal LH |
KP measurement (ng/mL) | KP reduced in HA women with low LH (1.7 ± 0.1) vs normal LH (2.6 ± 0.3) |
| Podfigurna et al (2020) (90) | Prospective cohort | 41 HA 40 controls |
KP measurement (ng/mL) | KP reduced in HA (0.17 ± 0.11) vs controls (0.3 ± 0.36) |
| Hofmann et al (2017) (91) | Prospective cohort | 38 HA (anorexia) | KP measurement | KP negatively correlated with physical activity (r = −0.41) |
| Jayasena et al (2009) (43) | Prospective, randomized, double-blinded | 10 HA | KP54 (SC bolus 6.4 nmol/kg, twice daily for 2 wk) vs vehicle | Acute KP54 (after 4 h) increased LH (to 24 IU/L) and FSH (to 9.1 IU/L) Chronic KP54 (after 2 wk) lowered LH (to 1.5 U/L) and FSH (to 0.5 IU/L) due to tachyphylaxis |
| Jayasena et al (2010) (43) | Randomized, double-blinded, placebo-controlled | 20 HA | KP54 (SC bolus 6.4 nmol/kg, twice weekly for 8 wk) | KP54 (after 1d) increased LH (to 21.5 IU/L) and FSH (to 6.4 IU/L) KP54 (after 2 wk) reduced LH (to 10 IU/L) and FSH (to 2.7 IU/L) KP54 (after 4 wk) maintained LH (9 IU/L) and FSH (2.6 IU/L) KP54 (after 6 wk) maintained LH (8.9 IU/L) and FSH (2.4 IU/L) KP54 (after 8 wk) maintained LH (7.9 IU/L) and FSH (2.7 IU/L) |
| Jayasena et al (2014) (92) | Randomised single-blinded placebo-controlled | 5 HA | KP54 (IV infusion 0.01-0.3 nmol/kg/h, for 8 h; 1.0 nmol/kg/h for 10 h) | Highest dose of KP54 increased LH greater than 10-fold vs placebo (placebo 1.26 ± 0.56, KP54 15.42 ± 3.57 IU/L) Highest dose of KP-54 increased LH pulses by 3-fold (No. of LH pulses over 8 h: placebo 1.6 ± 0.4, KP54 5.0 ± 0.5) |
| Abbara et al (2020) (83) | Single-blinded RCT | 6 HA 9 controls |
MVT-602 (SC bolus 0.03 nmol/kg) | MVT-602 increased LH sooner in HA (6.2 h) vs controls (15.1 h) MVT-602 increased FSH and E2 levels in HA |
| F, KP in PCOS | ||||
| Tang et al (2019) (93) | Systematic literature review | 12 studies | KP measurement | KP increased in PCOS than controls in 9 studies |
| Varikasuvu et al (2019) (94) | Meta-analysis | 23 studies | KP measurement | KP increased in PCOS than controls (std MD and [95% CI] = 0.47 [0.17-0.77]) Diagnostic OR 13.71, AUC 0.835 to differentiate PCOS from controls |
| Ibrahim et al (2020) (95) | Prospective | 60 PCOS 40 controls |
KP measurement (ng/mL) | KP increased in PCOS (1.79 ± 0.98) than controls (1.05 ± 0.86) |
| Akad et al (2022) (96) | Prospective case-control | 37 PCOS 24 controls |
KP measurement (pg/mL) | KP increased in PCOS (130.5) than controls (76.2), 95% CI, 7.55-11.50 |
| Romero-Ruiz et al (2019) (97) | Pilot exploratory cohort | 12 PCOS | KP54 (SC bolus 3.2-12.8 nmol/kg for 21 d) | KP54 increased LH (from 10.8 to 13.4 IU/L) and E2 levels, but did not change FSH |
| Skorupskaite et al (2020) (98) | Single-blinded placebo-controlled trial | 15 PCOS | KP10 (IV infusion 4 μg/kg/h for 7 h) | KP10 increased LH (from 5.2 to 7.8 IU/L) and E2 levels, but did not change FSH |
| Abbara et al (2020) (83) | Single-blinded RCT | 6 PCOS 9 controls |
MVT-602 (SC bolus 0.01–0.03 nmol/kg) | MVT-602 did change LH, FSH, or E2 concentrations in PCOS |
| G, KP in hyperprolactinemia | ||||
| Millar et al (2017) (99) | Prospective exploratory study | 2 women with high PRL | KP10 (IV infusion 1.5 mg/kg/h for 12 h) vs vehicle | KP10 increased LH from 5.3 to 25.4 IU/L and from 1.22 to 5.2 IU/L in each patient |
| Hoskova et al (2022) (100) | Prospective study | 11 high PRL (F) | KP112-121 (IV bolus 0.24 nmol/kg, every h for 11 h) | KP112-121 increased LH pulses from 4.5 ± 0.9 to 7.5 ± 0.5 pulses KP112-121 decreased LH interpulse interval from 2.7 ± 0.5 h to 1.3 ± 0.1 h KP112-121 did not change LH pulse amplitude, FSH, E2, or PRL levels |
| H, KP in IVF | ||||
| Jayasena et al (2014) (101) | Phase 2 randomized | 53 undergoing IVF | KP54 (SC bolus 1.6-12.8 nmol/kg) | ≥1 mature oocyte: 51/53 (96.2%) ≥ 1 fertilized egg: 49/53 (92.5%) Embryo transfer: 49/53 (92.5%) Clinical pregnancy rate per transfer: 12/49 (24.5%) Live birth rate per transfer: 10/49 (20.4%) Moderate to severe OHSS: 0 |
| Abbara et al (2015) (102) | Phase 2, open-label, randomized | 60 with high risk of OHSS undergoing IVF | KP54 (SC bolus 3.2-12.8 nmol/kg) | ≥1 mature oocyte: 57/60 (95.0%) ≥ 1 fertilized egg: 54/60 (90.0%) Embryo transfer: 51/60 (85.0%) Clinical pregnancy rate per transfer: 27/51 (52.9%) Live birth rate per transfer: 23/51 (45.1%) Moderate to severe OHSS: 0 |
| Abbara et al (2017) (103) | Phase 2, placebo-controlled, randomized | 62 with high risk of OHSS undergoing IVF | KP54 (SC bolus 9.6 nmol/kg, 1 dose vs 2 doses) | ≥1 mature oocyte: 61/62 (98.4%) ≥ 1 fertilized egg: 61/62 (98.4%) Embryo transfer: 60/62 (96.8%) Clinical pregnancy rate per transfer: 19/60 (31.7%) Live birth rate per transfer: 18/60 (30.0%) Moderate to severe OHSS: 1/62 (1.6%) |
| I, KP in healthy pregnancy | ||||
| Abbara et al (2021) (104) | Case-control trial | 39 pregnant 10 nonpregnant |
KP measurement (pmol/L) | KP increased linearly with advancing pregnancy |
| J: KISSPEPTIN IN MISCARRIAGE | ||||
| Silva et al (2023) (105) | Systematic review | 7 case-control studies | KP measurement | KP is reduced in miscarriage KP had better discriminatory score than b-hCG to differentiate miscarriage from healthy pregnancy (in 3/7 studies) |
| K, KP in hypertensive disorders of pregnancy | ||||
| Perez-Lopez et al (2021) (106) | Meta-analysis | 7 studies 214 Preeclampsia/gestational hypertension 263 normotensive |
KP measurement | KP is reduced in preeclampsia or gestational hypertension than in normotensive pregnancies (SMD −0.68); I2 = 77% |
| Abbara et al (2022) (107) | Case-control | 265 controls 20 preeclampsia 12 Gestational hypertension |
KP measurement | KP reduced in all hypertensive disorders (at 28-40 wk of gestation) KP increased in late-onset preeclampsia and reduced in early-onset preeclampsia (at 9-13 wk gestation) |
| L, KP in other pregnancy complications | ||||
| i. GDM | ||||
| Cetcovic (2012) (108) | Prospective case-control | 25 controls 20 GDM |
KP measurement (nmol/L) | KP is reduced in GDM (21-25 wk; 4.51, 32-36 wk; 11.64) than controls (21-25 wk; 10.33, 32-36 wk; 20.48) |
| Bowe et al (2019) (109) | Case-control | 62 controls 26 GDM |
KP measurement (pmol/L) | KP is reduced in GDM (889) than controls (1270) at 26-34 wk of gestation |
| Arslan et al (2020) (110) | Cross sectional | 82 controls 76 GDM |
KP measurement (pmol/L) | KP remained unchanged in GDM vs controls at 24-26 wk of gestation |
| Abbara et al (2022) (107) | Case-control | 265 controls 35 GDM |
KP measurement | KP remained unchanged in GDM vs controls in all trimesters |
| ii. Preterm birth | ||||
| Torricelli et al (2008) (111) | Observational | 30 controls 10 preterm |
KP measurement (ng/mL) | KP remained unchanged in preterm birth |
| Abbara (2022) (107) | Case-control | 265 controls 11 preterm |
KP measurement | KP increased in preterm birth than controls in all trimesters |
| iii. FGR | ||||
| Smets et al (2008) (112) | Case-control | 31 controls 31 SGA |
KP measurement (pmol/L) | KP is reduced in SGA (1376) than controls (2035) |
| Armstrong et al (2009) (113) | Retrospective case-control | 317 controls 118 IUGR |
KP measurement (pg/mL) | KP is reduced in IUGR (1164) than controls (1188) |
| Khaled et al (2018) (114) | Case-control | 10 controls 10 PE and IUGR 10 IUGR |
KP measurement (ng/mL) | KP is reduced in IUGR (with PE, 1640; and without PE, 1630) than controls (2900) |
| Abbara et al (2022) (107) | Case-control | 265 controls 17 FGR |
KP measurement | KP is reduced in FGR in all trimesters |
| M, KP in glucose control | ||||
| Izzi-Engbeaya et al (2018) (115) | Randomized, blinded, 2-way crossover | 15 healthy men | KP54 (IV infusion 1 nmol/kg/h for 2 h) vs vehicle | KP induced:
|
| Izzi-Engbeaya et al (2023) (116) | Single-blinded, crossover study | 17 women with overweight or obesity | KP54 (IV infusion 1 nmol/kg/h for 2 h) | KP had no effect on preprandial and postprandial insulin and glucose levels |
| N, KP in appetite regulation and obesity | ||||
| Izzi-Engbeaya et al (2018) (115) | Randomized, blinded, 2-way crossover | 15 healthy men | KP54 (IV infusion 1 nmol/kg/h for 2 h) vs vehicle | KP had no effect on self-reported hunger (assessed by visual analog scores) or objective food intake |
| Yang et al (2021) (117) | Double-blinded, randomized, placebo-controlled, crossover study | 27 healthy men | KP54 (IV infusion 1 nmol/kg/h for 75 min) vs vehicle | KP did not elicit brain responses to visual food stimuli or psychometric parameters |
| Izzi-Engbeaya et al (2023) (116) | Single-blinded, crossover study | 17 women with overweight or obesity | KP54 (IV infusion 1 nmol/kg/h for 2 h) | KP had no effect on self-reported hunger (assessed by visual analog scores) or objective food intake |
| O, KP in MAFLD | ||||
| Guzman et al (2022) (118) | Observational | 31T2DM 34 NAFL 25 NASH 31 healthy men |
KP measurement (pmol/L) | KP increased in NAFL (19.2 ± 2.6) and NASH (18.9 ± 2.4) compared with controls (6.6 ± 0.8) or patients with type 2 diabetes (7.1 ± 0.7) |
| P, KP in bone disorders | ||||
| Comninos et al (2022) (119) | Randomized, placebo-controlled, double-blind, 2-way crossover | 26 healthy men | KP54 (IV infusion 1 nmol/kg/h for 90 min) | KP54 increased osteoblast activity (20.3% increase in osteocalcin, 24.3% increase in carboxylated osteocalcin) |
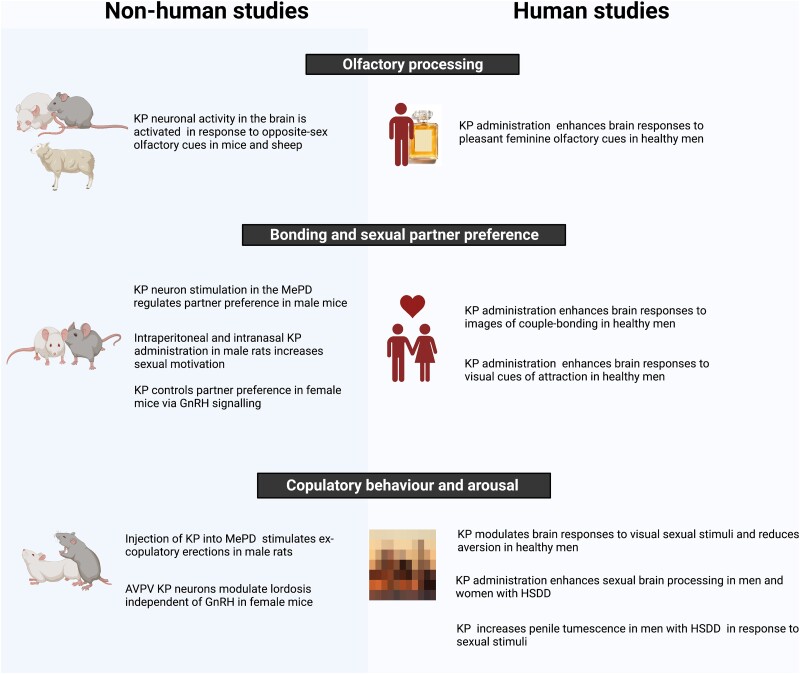
| Q, KP in psychosexual dysfunction | ||||
| Comninos et al (2017) (120) | Randomized, double-blind, 2-way crossover, placebo-controlled, fMRI study | 29 healthy heterosexual men | KP54 (IV infusion 1 nmol/kg/h, for 75 min) vs vehicle | In response to sexual stimuli, KP54 enhanced brain activity in amygdala, globus pallidus, posterior cingulate, putamen and thalamus, compared to placebo. Correlation between baseline reward scores and KP hippocampal enhancement, and change in sexual aversion and KP putamen enhancement |
| Comninos et al (2018) (121) | Randomized, double-blind, 2-way crossover, placebo-controlled, fMRI study | 29 healthy heterosexual men | KP54 (IV infusion 1 nmol/kg/h, for 75 min) vs vehicle | KP's modulation of default mode network correlated with increased limbic activity in response to sexual stimuli. KP's DMN modulation was greater in men with less reward drive and predicted reduced sexual aversion |
| Yang et al (2020) (122) | Randomized, double-blind, 2-way crossover, placebo-controlled, fMRI study | 33 healthy heterosexual men | KP54 (IV infusion 1 nmol/kg/h, for 75 min) vs vehicle | In response to feminine olfactory stimulus, KP54 enhanced brain activity in amygdala, caudate, globus pallidus, putamen, and thalamus, compared to placebo. In response to female faces, KP54 enhanced brain activity in medial prefrontal cortex and superior frontal gyrus, compared to placebo |
| Comninos et al (2021) (123) | Randomized, double-blind, 2-way crossover, placebo-controlled, MR spectroscopy study | 19 healthy heterosexual men | KP54 (IV infusion 1 nmol/kg/h, for 75 min) vs vehicle | Significant decrease (14.1%-15.7%) in total endogenous GABA levels in anterior cingulate cortex during KP, compared to vehicle |
| Thurston et al (2022) (124) | Randomized, double-blind, 2-way crossover, placebo-controlled, fMRI study | 32 eugonadal women with hypoactive sexual desire disorder | KP54 (IV infusion 1 nmol/kg/h, for 75 min) vs vehicle | In response to erotic videos, KP54 deactivated inferior frontal and middle frontal gyri and activated postcentral and supramarginal gyri, compared to placebo. In response to male faces, KP54 deactivated temporoparietal junction, compared to placebo |
| Mills et al (2023) (125) | Randomized, double-blind, 2-way crossover, placebo-controlled, fMRI study | 32 eugonadal men with hypoactive sexual desire disorder | KP54 (IV infusion 1 nmol/kg/h, for 75 min) vs vehicle | In response to erotic videos, KP54 deactivated parahippocampus, precuneus, frontal pole, and posterior cingulate, while activating anterior cingulate, middle frontal gyrus, fusiform gyrus, visual cortex Associated with significant increases in penile tumescence (by 56% more than placebo) and behavioral measures of sexual desire, most notably increased “happiness about sex.” |
Abbreviations: AUC, area under the curve; AUCROC, area under receiver operating characteristic curve; CDGP, constitutional delay of growth and puberty; CHH, congenital hypogonadotropic hypogonadism; CPP, central precocious puberty; E2, estradiol; EP, ectopic pregnancy; F, female; FSH, follicle-stimulating hormone; GA, gestational age; GABA, γ-aminobutyric acid; GDM, gestational diabetes mellitus; fMRI, functional magnetic resonance imaging; GnRH, gonadotropin-releasing hormone; HA, hypothalamic amenorrhea; HCG, human chorionic gonadotropin, IUGR, intrauterine growth restriction; IV, intravenous; IVF, in vitro fertilization; IVGTT-DI, intravenous glucose tolerance test—disposition index; KP, kisspeptin; LH, luteinizing hormone; M, male; NAFL, nonalcoholic fatty liver; MAFLD, metabolic fatty liver disease; NASH, nonalcoholic steatohepatitis; OHSS, ovarian hyperstimulation syndrome; PE, preeclampsia; PRL, prolactin; RCT, randomized controlled trial; SC, subcutaneous, SGA, small for gestational age.
The pulsatile secretion of GnRH is critical for reproductive function. Indeed, KP-10 (IV infusion 3.07 nmol/kg/hour over 22.5 hours) increased LH pulse frequency from 0.7 to 1.0 pulses per 1 hour in men (76). KP has also been shown to reset the “GnRH pulse generator” in healthy men but not women (75). KP-10 (IV bolus 0.24 nmol/kg) resulted in sustained GnRH neuronal activation lasting approximately 17 minutes and immediately induced an LH pulse (irrespective of the timing of the preceding endogenous pulse) and increased the LH pulse amplitude by 2.4-fold (75). Furthermore, the following native pulse was delayed by an interval approximating the usual interpulse interval, indicating that KP-10 had reset the schedule of pulses (75).
Kisspeptin in Healthy Women
In healthy premenopausal women, acute administration of KP-54 (subcutaneous; SC bolus 0.4 nmol/kg) increased circulating LH during all phases of the menstrual cycle, with the highest LH levels being observed during the preovulatory (20.64 ± 2.91 IU/L) compared to the follicular (0.12 ± 0.17 IU/L) or luteal (2.17 ± 0.79 IU/L) phases of the cycle (14) (Table 1B). Similarly, while KP-10 (IV bolus 10 nmol/kg) increased gonadotropins during the preovulatory phase (mean area under the curve; AUC: LH = 30.3 ± 7.7 IU/L, FSH = 6.9 ± 0.9 IU/L), it was least sensitive during the follicular phase (77). However, KP-54 (SC bolus 0.30-0.60 nmol/kg) can still increase LH pulsatility (by 2.33 pulses per 4 hours) during the follicular phase in premenopausal women (80).
The effects of chronic KP administration have also been evaluated in healthy women. For instance, twice-daily KP54 (SC bolus 6.4 nmol/kg) injections for 1 week increased maximal change in LH from baseline on day 7 (8.6 ± 3.4 IU/L), day 11 (8.3 ± 2.4 IU/L), and day 14 (12.7 ± 8.1 IU/L) of the menstrual cycle (81). Furthermore, an infusion of KP54 (SC 0.3-1.0 nmol/kg/hour over 8 hours) induced a mean LH increase (>8 IU/L) during the early-follicular phase (82). KP receptor analogues have been shown to stimulate longer LH responses and are similarly cost-effective to manufacture (83). For example, MVT-602 (formerly known as TAK-448) generated similar LH amplitude responses as KP-54 during the follicular phase, but the peak LH level was later at approximately 21 hours compared to KP-54 (∼5-hours), resulting in a 4-fold increase in the AUC of LH secretion (83).
Neurokinin B in Healthy Men and Women
Although NKB administration increased LH concentration in male juvenile monkeys (127), no significant changes in circulating LH, FSH, or testosterone concentrations were observed in healthy men during either a 90-minute (doses 0.04-5.12 nmol/kg/hour), a 4-hour (doses 2.56 and 5.12 nmol/kg/hour), or 8-hour (dose 5.12 nmol/kg/hour) IV infusion of NKB (128) (Table 2A). Similarly, no significant differences in either LH pulsatility or mean LH, FSH, or E2 levels have been observed in healthy premenopausal women (128). Interestingly, NKB induced vasoactive effects in healthy men (IV infusion 10.24 nmol/kg/hour) (128) and in 80% of premenopausal healthy women (IV infusion 5.12 nmol/kg/hour) (130) (see Table 2B). These data highlighted the potential of NKB-signaling blockade for the management of vasomotor symptoms (VMS) in postmenopausal women and/or following cancer therapy (eg, breast or prostate cancer). Thereafter, several safe and efficacious NKB receptor (mainly NK3R) antagonists have been investigated for this indication, which are discussed in later sections of this review. Recent in vitro data have also suggested that the NKB receptor, NK1R, may have a role in promoting breast (128) and non–small cell lung cancer (138), hence it is possible that antagonists against NK1R could have a therapeutic role in addition to the relief of VMS.
Table 2.
Clinical trials involving neurokinin B and NKB antagonism
| Author | Study design | Cohort | Intervention | Results |
|---|---|---|---|---|
| A, NKB in healthy males | ||||
| Jayasena et al (2014) (128) | Randomized single-blinded placebo-controlled trial | 23 healthy men | NKB (IV infusion 0.4-5.12 nmol/kg/h over 90 min, 5.12 nmol/kg/h over 4 h) | NKB did not alter LH, FSH, or testosterone levels at all doses |
| Narayanaswamy et al (2016) (129) | Randomized single-blinded placebo-controlled trial | 5 healthy men per group | Naltrexone (oral 50 mg) NKB (IV infusion 2.56 nmol/kg/h over 8 h) KP54 (IV infusion 0.1 nmol/kg/h over 8 h) |
Whereas naltrexone and KP54 increased LH levels, NKB did not alter LH or FSH |
| B, NKB in healthy females | ||||
| Jayasena et al (2014) (128) | Randomized single-blinded placebo-controlled trial | 5-8 premenopausal women per group | NKB (IV infusion 0.32, 0.64, 1.28, 2.56, or 5.12 nmol/kg/h for 3 h) vs vehicle | No change in LH, FSH, and E2 at all doses throughout menstrual cycle |
| Jayasena et al (2015) (130) | Randomized, double-blinded, placebo-controlled, 2-way crossover trial | 10 premenopausal women | NKB (IV infusion 5.12 nmol/kg/h over 30 min) vs vehicle during follicular phase | NKB induced hot flashes in 8/10 women, and elevated heart rate, skin temperature, and thermal imaging |
| C, NK3R antagonism in PCOS | ||||
| George et al (2016) (131) | Double-blind, placebo-controlled, phase 2 trial | 65 PCOS | AZD4901 (oral 20 mg, 40 mg, 80 mg, once daily for 28 d) | Highest dose of AZD4901 reduced:
|
| Skorupskaite et al (2020) (98) | Prospective study | 15 PCOS | MLE4901 (oral 40 mg 2×/d for 7 d) vs vehicle | MLE4901 vs vehicle reduced:
|
| Fraser et al (2021) (132) | Phase 2a, randomized, double-blind, placebo-controlled | 73 PCOS | Fezolinetant (oral 60 mg, 180 mg, 4×/d) | Highest dose of Fezolinetant reduced testosterone by 33%, LH by −10.17 IU/L and FSH by −1.46 IU/L |
| D, NK3R antagonism in menopausal hot flashes | ||||
| Prague et al (2017) (133) | Phase 2, randomized, double-blind, placebo-controlled, crossover | 28 menopausal women | MLE4901(oral 40 mg 2×/d for 4 wk) vs vehicle | MLE4901 reduced hot flash frequency vs vehicle (19.35 vs 49.01 per wk) and decreased hot flash severity vs vehicle (3.27 vs 5.70 per wk) |
| Depypere et al (2019) (134) | Double-blind, randomized, placebo-controlled | 87 menopausal women | Fezolinetant (oral 90 mg 2×/d for 12 wk) vs vehicle | Fezolinetant reduced VMS score vs vehicle (−26.5 vs −12.2) and decreased frequency of moderate/severe VMS by 5 episodes per d |
| Fraser et al (2020) (135) | Phase 2b, double-blind, randomized, placebo-controlled | 287 menopausal women | Fezolinetant (oral 15, 30, 60, 90 mg 2×/d or 30, 60, 120 mg 1×/d for 12 wk) vs vehicle | All doses of fezolinetant, except lowest one, reduced moderate/severe VMS (>2×/d) by 4 and 12 wk |
| Trower et al (2020) (136) | Double-blind, randomized, placebo-controlled | 76 menopausal women | NT-814 (oral 50, 100, 150, 300 mg 1×/d for 14 d) vs vehicle | NT-814 reduced hot flash frequency by 24% (50 mg), 59% (100 mg), 84% (150 mg), and 66% (300 mg) |
| Lederman et al (2023) (137) | Double-blind, randomized, placebo-controlled | 522 menopausal women | Fezolinetant (oral 30 mg or 45 mg 1×/d for 12 wk) vs vehicle followed by 40-wk active treatment extension | Fezolinetant reduced VMS frequency at wk 4 (difference in change in least squares mean –1.87; 30 mg, –2.07; 45 mg) and wk 12 (–2.39; 30 mg, –2.55; 45 mg) Fezolinetant 30 mg or 45 mg 1×/d, reduced severity of VMS at wk 4 (−0.15 to −0.19) and wk 12 (−0.24 to −0.2) |
Abbreviations: AUC, area under the curve; E2, estradiol; FSH, follicle-stimulating hormone; IV, intravenous; KP, kisspeptin; LH, luteinizing hormone; NK3R, neurokinin 3 receptor; NKB, neurokinin B; PCOS, polycystic ovary syndrome; VMS, vasomotor symptoms.
Clinical Applications of Kisspeptin
In Disorders of Puberty
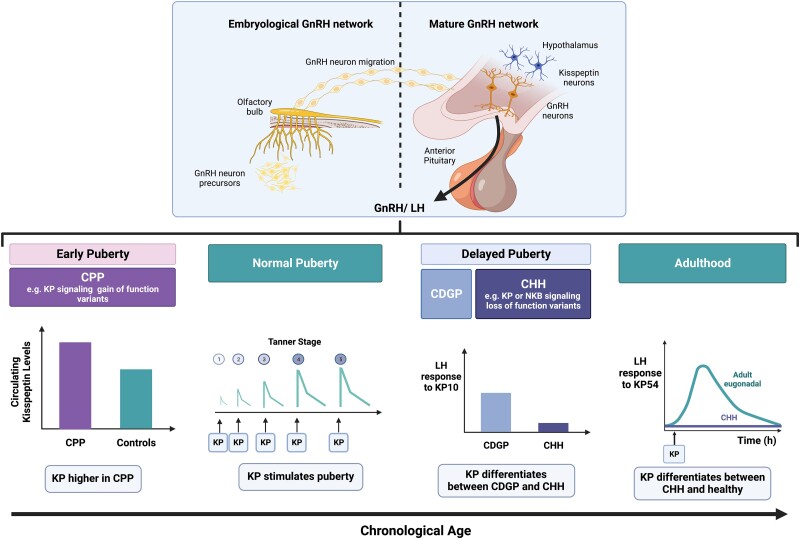
Puberty is characterized by the acquisition of secondary sexual characteristics and reproductive capacity, and the development of important psychosocial behaviors (139). Pubertal onset is dependent on the reawakening of the pulsatile secretion of GnRH and activation of the downstream reproductive endocrine axis (139). During fetal life and infancy, there are 2 periods of transient activations of the HPG axis termed “mini puberty,” followed by a period of relative quiescence until the onset of puberty (139) (Fig. 4).
Figure 4.
Role of KP in disorders of puberty. Puberty is triggered by the pulsatile secretion of GnRH and subsequent downstream activation of the HPG reproductive axis. The pulsatile secretion of GnRH requires adequate development and migration of GnRH neurons from the olfactory bulb to the hypothalamus. The HPG axis is transiently activated at 2 distinct phases: during early developmental life, termed “mini puberty,” and at the onset of puberty. KP stimulates an LH response during the later stages of puberty (Tanner stage 5) thus suggesting KISS1R sensitivity on GnRH neurons develops during the later part of puberty. KISS1 gain-in-function variants can lead to premature activation of the HPG axis resulting in early central precocious puberty (CPP). KP levels are increased in CPP vs age-matched healthy controls and thus KP has potential in aiding in the diagnosis of early puberty. KISS1 loss-of-function variants cause aberrations in GnRH neuronal development or migration and impair GnRH secretion resulting in congenital hypogonadotropic hypogonadism (CHH) and delayed puberty. Constitutional delay of growth and puberty (CDGP) is another common cause of delayed puberty and can be challenging to accurately differentiate from CHH. KP, a potent stimulator of GnRH and LH release, induces differential responses in CDGP (increased LH) and CHH (absent/reduced LH) and thus can aid in the diagnosis of delayed puberty. CDGP, constitutional delay of growth and puberty; CHH, congenital hypogonadotropic hypogonadism; CPP, central precocious puberty; GnRH, gonadotropin-releasing hormone; HPG, hypothalamic-pituitary-gonadal; KP, kisspeptin; LH, luteinizing hormone. Figure created with BioRender.com.
Diagnosing delayed puberty
Delayed puberty is defined as the absence of testicular enlargement (testicular volume <4 mL) in boys, or breast development in girls, at an age that is more than 2 SDs later than the population mean, typically aged 14 years in boys and 13 years in girls (140). The most common cause of delayed puberty is constitutional delay of growth and puberty (CDGP), affecting 60% to 80% of boys and 30% to 55% of girls (141). In CDGP, although puberty is delayed, it is initiated spontaneously without treatment (86, 140). Another important but less common cause of delayed puberty is CHH. CHH affects 10% to 20% of adolescents with delayed puberty and is characterized by failure of GnRH action resulting in absent or incomplete puberty (141). It is caused by genetic variants that either impair developmental GnRH neuronal migration or alter GnRH secretion and/or action (141). The cause of CDGP remains unknown; however, 50% to 75% of patients have a family history of delayed puberty and there is some overlap with genes causing CHH, as well as with nutritional status (141). Accurately diagnosing these conditions is crucial, as although CDGP can be managed conservatively or symptomatically with sex steroids, timely pubertal induction in CHH could safeguard future reproductive, sexual, bone, metabolic, and psychological health (142). Currently, differentiating CDGP and CHH is challenging due to their overlapping clinical presentations, biochemical profiles, and the lack of a “gold-standard” diagnostic test (143).
Animal data
KP is a central regulator of the HPG axis and has a critical role in pubertal initiation and maintenance. Numerous animal studies have investigated KP signaling in the context of delayed or absent puberty. Indeed, Kiss1r-deficient male mice have small testes and female mice have delayed vaginal opening and absent follicular maturation (2). Likewise, targeted disruption of the KP receptor in male and female mice resulted in reduced internal and external reproductive organ size (eg, testicular volume: 0.2 ± 0.04 mL in controls, 0.02 ± 0.01 mL in Kiss1r knockout [KO] mice), altered organ weight/body weight (BW) ratios, and infertility (144). Furthermore, specific KO of Kiss1r only in GnRH neurons led to infertile mice with reduced serum LH and FSH levels (145). External abnormalities, including microphallus and reduced anogenital distance in male mice, and acyclicity in female mice, were also observed (145). Furthermore, intracerebral administration of a KP antagonist (p234) in female rats suppressed markers of puberty including vaginal opening and an increase in uterine weight (146).
Disruption of the Kiss1 gene also results in pubertal failure (147); however, it appears that KO of Kiss1 results in a less severe phenotype (higher gonadal weight and larger vaginal opening) than KO of Kiss1r (148). Interestingly, the degree of disruption of pubertal progression caused by aberrant KP signaling can vary. For instance, female Kiss1 and Kiss1r KO mice can still progress through estrus, suggesting there is some level of retained GnRH activity (78). Likewise, another study showed that female mice with Kiss1 ablation had normal timing of puberty and remained fertile (149). Collectively, these data indicate that pubertal maturation can occur despite impaired KP signaling; however, its development is not entirely normal.
Human data
KP's role in puberty was first identified in humans when loss-of-function variants in KISS1R resulted in failure of pubertal progression and CHH (1, 2). Following this, researchers identified that CHH patients with impaired KISS1R signaling were homozygous for a single variant causing substitution of leucine with proline (150). These patients were still able to respond to exogenous GnRH, suggesting that pituitary function was still intact (150). Likewise, functional KISS1 is also required for normal pubertal development. In a large consanguineous family, members with homozygous (but not heterozygous KISS1 variants) had CHH, thus indicating that one copy of KISS1 is sufficient for functioning of the HPG axis (3).
KP's ability to directly stimulate hypothalamic GnRH release could enable its use as a novel diagnostic tool in identifying patients with CHH. As CHH is predominantly caused by hypothalamic defects, it is expected that the majority of patients with CHH will fail to respond to KP but not to GnRH (84). However, many also fail to respond to an initial dose of exogenous GnRH as they typically have “sleepy” pituitary glands, which have not been primed, resulting in false-negative interpretations (84). To avoid this, researchers used intermittent exogenous GnRH exposure to “prime” pituitary gonadotrophs (84). The first study to evaluate KP as a diagnostic test in adult CHH patients was conducted in 2014 (84) (see Table 1C). Here, while an IV bolus of GnRH induced mild and robust LH responses during the “prepriming” and “postpriming” stages, respectively, no response was observed with KP-10 (IV bolus 0.24 nmol/kg) (84). Some CHH patients can undergo spontaneous activation of their HPG axis and restoration of reproductive function, termed “reversal” (85). KP-10 (IV bolus 0.24-2.4 nmol/kg) induced LH pulses (within 30 minutes) in patients with sustained reversal but not in those who suffered a relapse of CHH, thus confirming KP's ability to assess current GnRH neuronal functional capacity (85).
Another study using KP-54 (IV bolus 6.4 nmol/kg) found that patients with CHH had lower LH responses after KP-54 (0.4 IU/L) than healthy controls (12.5 IU/L) (87). KP-54 had higher discriminatory power than GnRH to accurately differentiate CHH from healthy men with an area under receiver operating characteristic curve (AUCROC) of 1.0 (95% CI, 1.0-1.0) vs 0.88 (95% CI, 0.76-0.99), respectively (87). Additionally, CHH patients with anosmia or those with an identified pathogenic variant in causative genes, such as ANOS1, FGFR1, PROKR2, or SEMA3A, had even lower LH increases following KP-54 than other men with CHH (87).
In patients with delayed puberty, KP-10 has been shown to predict subsequent progression through puberty, which could be used to differentiate CHH from CDGP (86). For instance, “KP responders (LH ≥ 0.8 mIU/mL)” proceeded through puberty spontaneously (ie, CDGP) whereas “KP nonresponders (LH ≤ 0.4 mIU/mL)” did not (ie, CHH) (86). This test had 100% sensitivity and specificity and predicted outcomes more accurately than previously described basal/stimulated hormonal markers and genetic testing (86). These data demonstrate the potential of KP in the context of delayed puberty to differentiate CDGP and CHH.
Diagnosing precocious puberty
Precocious puberty is pubertal development occurring earlier than that which is expected for sex, ethnicity, and race, typically occurring at younger than 9 years in boys and younger than 8 years in girls (151). Precocious puberty can be classified as either a GnRH-dependent or a GnRH-independent process. GnRH-dependent or CPP results from the premature activation of the HPG axis, whereas GnRH-independent or peripheral precocious puberty results from the unregulated gonadal production of sex steroids (151). CPP affects around 1 in 5000 to 10 000 White children and is 10-fold more prevalent in girls than boys (152). As early exposure of high sex steroid concentrations causes premature epiphyseal fusion and reduced final height, as well as psychosocial issues, early diagnosis and treatment of CPP is critical (152). Differentiating CPP from premature thelarche (PT), a condition characterized by isolated breast development with no growth or bone problems, is challenging (152). Although a GnRH stimulation test is often used as a biochemical parameter for diagnosis, it has low sensitivity, thus new markers are required (152).
Animal data
KP has been shown to precociously activate the HPG axis. Indeed, male and female rats persistently express hypothalamic Kiss1 and Kiss1r during postnatal life, with maximum levels expressed at puberty (153). Furthermore, KP induced complete vaginal opening (in 74%) and increased uterine weight (by 3-fold), serum LH (by 10-fold), and serum E2 (by 2-fold) levels in immature female rats compared to controls (154). Likewise, female monkeys with intact ovaries demonstrate increased Kiss1 and Kiss1r (by 3-fold) mRNA levels in the ARC of the hypothalamus during puberty (155). Furthermore, administration of KP-10 to juvenile monkeys has been shown to elicit robust and precocious LH surges (155).
Human data
Activating variants of the KP gene and receptor have been identified in patients with CPP. For instance, an autosomal dominant mutation involving substitution of proline for arginine at codon 386 (Arg386Pro) of KISS1R was discovered in a girl with CPP (4). In vitro studies revealed that this KP receptor variant induced a prolonged response to KP through a reduced rate of degradation (4, 156). Furthermore, 2 KISS1 missense mutations, p.P74S (heterozygous) and p.H90D (homozygous), have also been identified in CPP, with the p.P74S variant displaying higher KP resistance to degradation (157).
Considering gain-of-function variants in the KP gene or receptor result in CPP, KP has been investigated as a potential marker of early pubertal activation and CPP. Indeed, serum KP levels have been shown to be higher in CPP (14.62 ± 10.2 pmol/L) than in age-matched prepubertal controls (8.35 ± 2.98 pmol/L) (158); however, there was some overlap between the groups (see Table 1D). Similarly, a systematic review and meta-analysis (11 studies, CPP n = 316, controls n = 251) demonstrated higher KP levels in CPP vs controls with a bias-corrected SMD of 1.53 (95% CI, 0.56-2.51) (88). Subgroup analyses revealed a positive correlation between serum KP and age in the CPP cohort, and an association between serum KP levels and PT (88). A more recent study demonstrated higher KP levels in age and body mass index (BMI)-matched CPP (0.43 ± 0.16 ng/mL) vs PT (0.26 ± 0.10 ng/mL) and controls (0.18 ± 0.07 ng/mL) (89). While a KP cutoff of 0.41 ng/mL or greater was indicative of CPP, a KP level less than 0.21 ng/mL excluded CPP (AUC = 0.830) (89). KP also positively correlated with increasing bone age, a cardinal feature of CPP (89). Taken together, circulating KP levels may provide a useful adjunct in the diagnosis of CPP especially when the levels are at one end of the spectrum.
In Adult Disorders of Reproductive Function
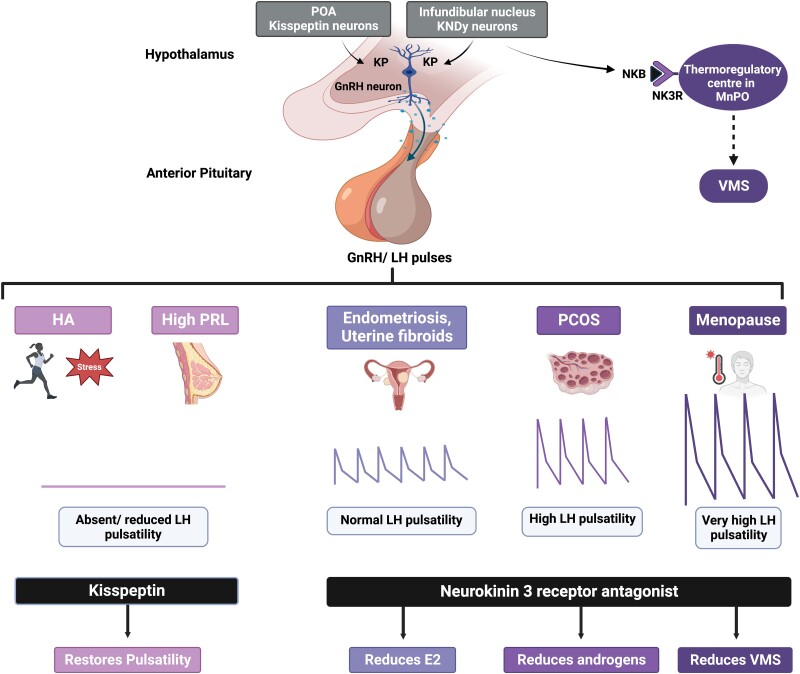
KP’s ability to directly stimulate hypothalamic GnRH release and regulate reproductive hormone secretion can be used to assess hypothalamic function and treat common ovulatory disorders (Fig. 5).
Figure 5.
Therapeutic potential of KP and NKB in female reproductive disorders. Activation of hypothalamic KP neurons directly stimulates GnRH release and regulates reproductive hormone secretion. Absent or reduced GnRH and LH pulses observed in HA and hyperprolactinemia can be restored using exogenous KP. While GnRH/ LH pulsatility is retained in patients with endometriosis/uterine fibroids, patients with PCOS have high pulsatility. During the menopause, increased KNDy neuronal activity results in very high GnRH/LH pulses and induction of vasomotor symptoms through dysregulation of the thermoregulatory center. Considering NKB antagonism partially suppresses (but does not abolish) the reproductive endocrine axis, NK3R antagonists have been developed for the therapeutic potential of these disorders. NK3R antagonism can be used to treat endometriosis/ uterine fibroids (by reducing E2), PCOS (by reducing androgens), and menopausal hot flashes (by reducing vasomotor symptoms). E2, estradiol; GnRH, gonadotropin-releasing hormone; HA, hypothalamic amenorrhea; KP, kisspeptin; LH, luteinizing hormone; NK3R, neurokinin 3 receptor; NKB, neurokinin B; PCOS, polycystic ovary syndrome; KNDy, kisspeptin-neurokinin B-dynorphin. Figure created with BioRender.com.
Diagnosing hypothalamic amenorrhea
HA affects 1% to 4% of women and is characterized by an acquired functional deficiency of hypothalamic function and reduction in GnRH secretion (92). HA is diagnosed by the presence of menstrual disturbance (menstrual cycle length persistently >45 days or amenorrhea >3 months), low BW, excessive exercise, psychological stress, and hypogonadotropic hypoestrogenism (typically <184 pmol/L) (159). Diagnosing HA can be challenging as it requires the exclusion of other causes of amenorrhea before a diagnosis can be made and there can be overlap in features with other common causes of menstrual disturbance (160).
Animal data
Reproductive suppression through food deprivation and/or stress is mediated by hypothalamic KP. For instance, in calorie-restricted sheep models, Kiss1 mRNA expression is reduced in the ARC and the POA of the hypothalamus (161-163). In male castrated sheep with reduced food intake, mean serum LH and hypothalamic ARC Kiss1 mRNA expression were decreased (164). Cows with nonovulatory cycles have a 2-fold reduction in ARC Kiss1 expression compared to controls (165). Stress induced by lipopolysaccharide administration also decreased hypothalamic Kiss1 mRNA expression and serum LH levels in female rats (166). Similarly, central and peripheral activation of the hypothalamic-pituitary-adrenal axis by corticotropin and corticosterone, respectively, reduced ARC KP expression in female mice (166).
Human data
Circulating KP levels are reduced by 13% in HA and are particularly low in women with reduced LH (KP = 1.7 ± 0.1 ng/mL) compared to those with normal LH (KP = 2.6 ± 0.3 ng/mL) levels (90) (see Table 1E). Women with HA with lower KP levels had higher levels of stress hormones such as corticotropin-releasing hormone compared to controls (167). Furthermore, KP levels have been shown to negatively correlate with physical activity (91). While circulating KP levels could be used to diagnose HA, it is important to note that they are challenging to detect accurately at low levels using current methods of measurements, thereby limiting their potential clinical use.
Treating hypothalamic amenorrhea
HA is a chronic endocrine disorder associated with serious negative health consequences including infertility, osteoporosis, and cardiovascular disease (90). Although pulsatile GnRH pump therapy is recommended as the first-line treatment, it has limited availability (92). Estrogen supplementation offers symptom control and only some protection against osteoporosis (92). Furthermore, some women with HA seeking fertility can respond poorly to clomiphene citrate during ovulation induction protocols as E2 is already low (92). Considering KP’s direct potent stimulatory effects on the HPG axis, it has potential for use to restore reproductive function in women with HA.
Animal data
The potential of KP for reinstating reproductive function has been explored in calorie-restricted animal models. For instance, food-deprived prepubertal rats with low hypothalamic Kiss1 and high Kiss1R expression have enhanced LH responses (∼62.5-fold increase) following exogenous KP (168). Although KP did not alter food intake, chronic KP administration induced vaginal opening (in ∼60%) and elicited increases in FSH and E2 (168).
Human data
Women with HA had an earlier increase in LH (6.2 hours) than healthy women (15 hours), and also had increased FSH and E2 levels following administration of the KP receptor agonist (MVT-602) (83) (see Table 1E). In women with HA, KP-54 (SC bolus 6.4 nmol/kg twice daily) induced robust LH increases the first day of treatment (maximum LH increase = 24.0 ± 3.5 IU/L above baseline at 4 hours post injection) (43). However, LH responses were markedly reduced by 2 weeks of treatment (maximum LH increase = 2.5 ± 2.2 IU/L above baseline), consistent with tachyphylaxis at the KP receptor (43). To prevent receptor desensitization and maintain stimulation, the dosing interval can be extended to twice-weekly (43). This dosing protocol maintained stimulation with maximal LH increases of 21.5 ± 10.7 IU/L (at baseline), 10.0 ± 4.3 IU/L (at 2 weeks), 9.0 ± 4.1 IU/L (at 4 weeks), 8.9 ± 3.5 IU/L (at 6 weeks), and 7.9 ± 4.5 IU/L (at 8 weeks) (43). Furthermore, unlike GnRH-based therapies, KP can induce pulsatile secretion of GnRH/LH even when administered in a nonpulsatile manner. For example, women with HA receiving an IV infusion of KP-54 had a 3-fold increase in the number of LH pulses and a 6-fold increase in mean peak LH pulse secretory mass (92). Thus, chronic KP administration could offer a novel approach to restoring physiological LH pulsatility in women with HA.
Diagnosing polycystic ovary syndrome
Polycystic ovary syndrome (PCOS) is a multifactorial condition influenced by genetic and environmental factors, and results in heterogeneous clinical phenotypes including neuroendocrine and metabolic abnormalities (169). PCOS affects 2% to 13% (88) of women of reproductive age and is currently diagnosed by the presence of 2 of the following 3 features: (i) menstrual irregularity, (ii) hyperandrogenism, or (iii) polycystic ovarian morphology on ultrasound (170). A key pathological feature responsible for PCOS is androgen excess (171). Androgens induce PCOS features through a central mechanism via the HPG axis and increase GnRH pulsatility (172). Considering GnRH neurons lack androgen receptors, other intermediate pathways providing afferent inputs to GnRH neurons, such as KP neurons, are crucial to mediating the altered sex steroid feedback found in PCOS (173). Indeed, testosterone exposure upregulates the androgen receptor but downregulates progesterone receptor expression in ARC KP neurons, thus indicating that androgen exposure in PCOS disrupts progesterone-induced negative feedback through a direct action on ARC KP neurons (174). The consequent unrestrained LH secretion stimulates ovarian theca cell androgen production, which in turn reduces sex steroid–mediated negative feedback, thus establishing a vicious cycle (172).
Animal data
Hypothalamic Kiss1 expression differs in various PCOS animal models. For instance, in testosterone and dihydrotestosterone (DHT)-induced PCOS rat models, Kiss1 gene expression is reduced (93). Conversely, ARC KP expression is increased in prenatal androgen models featuring irregular cycles and increased LH and testosterone levels (93). Likewise, prenatal exposure of androgens to sheep and other nonhuman primate models recapitulates many of the cardinal features of PCOS (171). In rodent models of PCOS induced by letrozole, Kiss1 expression is upregulated in the ARC compared to the AVPV, suggesting ARC KP neurons mediate the impaired sex steroid feedback in PCOS (175). Overall, it appears that Kiss1 expression is increased in PCOS phenotypes with higher LH levels and normal BW.
Human data
A recent meta-analysis (of 23 studies) reported that circulating KP levels were raised in PCOS (SMD = 0.47 and 95% CI, 0.17-0.77) and had a diagnostic OR of 13.71 and an AUC of 0.835 to differentiate PCOS from controls in BMI-matched women (94) (see Table 1F). Additionally, 2 further studies have also observed higher KP levels in PCOS than controls: 1.79 ng/mL vs 1.05 ng/mL (176) and 0.131 ng/mL vs 0.076 ng/mL (96). As KP is a potent stimulator of GnRH and LH release, one would expect a positive correlation between KP and the high serum LH levels observed in PCOS. However, while oligomenorrheic PCOS women have loss of temporal coupling of KP and LH pulses, coupling is preserved in PCOS women with eumenorrhea (177).
Treating polycystic ovary syndrome
PCOS treatments are currently directed toward a specific symptom of PCOS, such as ovulation induction for infertility, rather than aiming to treat the underlying pathophysiological process. Approximately 40% of women with PCOS have increased LH pulse frequency (22-24 vs 16 pulses per 24 hours), with PCOS often being described as a state of relative FSH deficiency (178). Considering KP administration induces a greater LH than FSH response, KP could exacerbate the relative FSH deficiency, potentially limiting its use as an agent to restore folliculogenesis in PCOS (172). Furthermore, KP can evoke differential gonadotropin and ovulation responses in different PCOS phenotypes, thus indicating the need for individualized management of women with PCOS.
Animal data
KP-54 (SC bolus 100 μg/kg) increased both LH and FSH levels in prenatal, neonatal, and postweaning androgenized PCOS-like rat models (97). In anovulatory rats with neonatal androgen exposure, KP induced marked LH and FSH responses, increased follicle growth, and rescued ovulation (increased number of corpora lutea) (97). However, in postweaning androgenized rats with persistently increased androgen levels, KP had blunted LH responses and failed to induce ovulation (97). These data indicate that KP responses are more robust in PCOS phenotypes linked to early androgenization, without marked elevation of circulating androgens.
Human data
Like animal data, women with PCOS also have increased LH and FSH responses following administration of the KP receptor agonist, MVT-602 (SC bolus 0.01-0.03 nmol/kg) (83) (see Table 1F). However, in women with PCOS receiving KP-54 (SC bolus 3.2 and 12.8 nmol/kg twice daily for 21 days), LH (from 10.8 to 13.4 IU/L) but not FSH (from 3.9 to 3.5 IU/L) levels were raised (97). Similarly, KP-10 (IV infusion 4 µg/kg/h for 7 hours) increased LH (from 5.2 to 7.8 IU/L) and E2 concentrations but did not increase FSH secretion in women with PCOS (98). However, pretreatment with an NK3R antagonist increased the FSH increase following KP (98). Thus, the relative FSH deficiency observed in women with PCOS could be exacerbated by KP and limit its use as a sole agent to restore healthy folliculogenesis. In 2 women with PCOS and amenorrhea but no biochemical hyperandrogenism, KP (SC bolus 9.6 nmol/kg twice daily over 3 weeks) stimulated follicle growth and ovulation, and these effects continued even after KP administration ceased (97). Consistent with animal data, KP is more effective in PCOS phenotypes linked to anovulation without marked elevation of circulating androgen levels.
Treating hyperprolactinemia
Hyperprolactinemia has an annual incidence of 23.9 per 100 000 person-years and is a major cause of anovulatory infertility in women of reproductive age (179). Elevated prolactin (PRL) levels suppress GnRH release and result in reduced LH pulse frequency and amplitude and hypogonadotropic hypogonadism (180). Dopamine agonists (eg, cabergoline, bromocriptine) are the first-line treatment for hyperprolactinemia as they effectively normalize PRL levels and restore gonadal function. However, up to 30% of patients have drug resistance and others cease therapy due to intolerable side effects such as impulse-control disorders (181).
Animal data
The mechanism of PRL action on GnRH neurons has remained elusive. However, as most GnRH neurons do not express PRL receptors, PRL inhibitory action is thought to be mediated indirectly through PRL-sensitive afferent pathways such as KP neurons (182). Indeed, PRL-induced anovulatory female mice have reduced Kiss1 and GnRH expression levels (183). KP neurons within the ARC of the hypothalamus regulate PRL-mediated LH suppression. For instance, lactating female rats with elevated PRL levels have a 58% reduction in ARC KP neuron immunoreactivity vs nonlactating rats (184). Furthermore, PRL induces greater inhibitory signal transduction responses in ARC KP neurons (70.6% ± 5.9%) vs KP neurons of the RP3V (38.5% ± 6.7%) (185). Additionally, KP administration restored cyclicity and ovulation rate (number of corpora lutea following KP: 7.8 ± 0.6, controls: 7.5 ± 0.6) in female mice with hyperprolactinemia (183). Consistent with this, specific KO of the PRL receptor within ARC KP neurons prevents PRL-induced suppression of LH secretion (184).
Tuberoinfundibular dopamine (TIDA) neurons within the ARC, which are essential for maintaining PRL homeostasis, can be modulated by Dyn action (182, 186). As ARC KP neurons coexpress Dyn (and NKB) and Dyn cells project onto TIDA neurons, KP neurons may be directly involved in regulating PRL secretion (182, 186). Furthermore, KP has been shown to regulate PRL release through suppression of TIDA neuronal activity (187).
Human data
Considering PRL exerts its effects on fertility through suppression of KP inputs to GnRH neurons, KP could have the potential to be used for treatment of hyperprolactinemia. In women with PRL-induced chronic amenorrhea, KP-10 (IV infusion 1.5 mg/kg/h over 12 hrs) increased LH pulse frequency, serum LH, FSH, and ovarian hormones (E2, inhibin B, and testosterone) levels (99) (see Table 1G). Similarly, an IV bolus of KP-10 (0.24 nmol/kg) given every hour for 10 hours increased LH pulse frequency from 4.5 ± 0.9 to 7.5 ± 0.5 per 10 hours and elevated mean LH levels from 3.32 ± 0.60 IU/L to 5.91 ± 0.65 IU/L in women with hyperprolactinemia (100).
In vitro fertilization
Infertility is the inability to conceive after 12 months or more of regular unprotected sexual intercourse and affects 1 in 6 couples (188). In vitro fertilization (IVF) is the main treatment offered and has resulted in more than 8 million live births worldwide over the past 40 years (189, 190). In brief, IVF involves the use of supraphysiological doses of FSH to induce follicle development, followed by human chorionic gonadotropin (hCG) or a GnRH agonist to provide LH-like exposure and induce oocyte maturation (191). The half-life of exogenous hCG is double that of the endogenous physiological LH surge and hence exogenous hCG may persist in the circulation for up to 7 days (192). A serious life-threatening complication of hCG treatment is severe ovarian hyperstimulation syndrome (OHSS), affecting 2% to 6% of women (193), and women with PCOS are at higher risk (172). In this condition, excessive ovarian stimulation causes aberrant release of vascular endothelial growth factor (194), which results in increased vascular permeability and third spacing of fluids, ultimately leading to the development of ascites, pleural effusions, and hemoconcentration (103). Thus, treatments that effectively trigger an LH surge to induce oocyte maturation, while avoiding overstimulation and OHSS. are of clinical value.
Animal data
KP induces the LH surge necessary for ovulation and oocyte maturation. Indeed, approximately 30% of KP neurons in the RP3V are activated during the LH surge (195). Transgenic mice null of Kiss1 or its receptor lack the LH surge and GnRH neuronal activity (195), and treatment of the hypothalamic POA with a neutralizing monoclonal antibody inhibits ovulation in female rodents (72). Notably, KP administration generated an LH-surge inducing ovulation to a similar degree as hCG in gonadotropin-pretreated rats (196). KP also stimulated ovulation in other mammals, including ewes (197) and musk shrews (196), suggesting that hypothalamic KP signaling is requisite for physiological ovulation.
Human data
In humans, KP induces a more similar LH rise to that observed after the physiological mid-cycle LH surge than either GnRH agonist or hCG, and therefore could be a promising ovulation induction agent in IVF (see Table 1H). For instance, the physiological midcycle LH surge has a mean amplitude of 56.5 IU/L (SD 23.4; range, 25-144 IU/L) (198), which is similar to the LH increase at 4 to 6 hours following KP (LH ∼45 IU/L) (101) whereas that induced by GnRH agonists is supraphysiological (LH 140.4 IU/L) (199). In 2014, KP-54 (SC bolus 1.6, 12.8 nmol/kg) was administered during a GnRH antagonist cotreated IVF cycle to 53 women with subfertility (101). KP-54 resulted in the retrieval of at least 1 mature oocyte in 51 of 53 women, 1 embryo for implantation in 49 of 53 women, and the birth of 12 healthy babies (8 singleton, 2 twin pregnancies) (101).
KP has also been proposed to suppress vascular endothelial growth factor levels through a direct action at the ovary and potentially reduce the risk of OHSS, making it a safe and attractive therapeutic agent for IVF (102). In women with high risk of OHSS, 95% had oocyte maturation (highest oocyte yield = 121%) and 90% formed embryos following KP-54 (SC bolus 3.2-12.8 nmol/kg) (102). The rates of biochemical pregnancy, clinical pregnancy, and live births per transfer were 85%, 77%, and 62%, respectively, following a dose of 9.6 nmol/kg of KP-54 (102). Importantly, none of the women developed moderate, severe, or critical OHSS (102). To determine whether the duration of the physiological LH surge (24-28 hours) is crucial for IVF treatment, KP-54 (SC bolus 9.6 nmol/kg) was administered as either a single dose or 2 doses (10 hours apart), to women at high risk of OHSS (103). Women receiving 2 doses of KP-54 had higher oocyte yields (71% vs 45%), implantation rates (37% vs 23%), and live birth rates (39% vs 19%) compared to those receiving a single dose (103). Critically, 2 doses of KP-54 still did not result in OHSS despite extending the duration of LH exposure (103). The KP analogue, MVT-602, induced a similar amplitude of LH surge as KP-54 (83) but a longer duration of LH increase, and therefore also has potential as a trigger for oocyte maturation.
In a retrospective, single-center comparison, the risk of OHSS was greater following hCG (OR 33.6; CI, 12.6-89.5) and GnRH agonist treatment (OR 3.6; CI 1.8-7.1) than KP-54 (190). Ovarian volumes were larger by 20-fold with hCG, 8-fold with GnRH agonist, and 5-fold with KP-54, compared to baseline prestimulation ovarian volumes (190). Similarly, mean ascitic volumes were greatest following hCG (62 ± 84 mL) than GnRH agonist (9 ± 44 mL) or KP-54 (5 ± 8 mL) (190). Collectively, these data highlight KP's use as a safe and efficacious agent for oocyte maturation in IVF protocols.
In disorders of pregnancy
KP is a putative regulator of trophoblast invasion (200) and placentation (201) in pregnancy. The Kiss1 gene is abundantly expressed in syncytiotrophoblasts, whereas its receptor is expressed both in cytotrophoblasts and syncytiotrophoblasts (27, 28). KP levels increase linearly during healthy pregnancy from less than 8 pmol/L (nonpregnant levels) to 1230 pmol/L in the first trimester, and 9590 pmol/L in the third trimester (104) (see Table 1I). While high circulating KP levels are associated with advanced maternal age, lower KP levels are associated with Afro-Caribbean ethnicity, smoking, and high BMI (104). Importantly, KP has emerged as a promising biomarker to predict several adverse pregnancy complications (Fig. 6).
Figure 6.
The utility of KP in the prediction of pregnancy complications. KP regulates trophoblast invasion and placentation during pregnancy and has emerged as a promising biomarker to predict several adverse pregnancy complications. The KISS1 gene is abundantly expressed in syncytiotrophoblasts, whereas its receptor (KISS1R) is expressed in both cytotrophoblasts and syncytiotrophoblasts. Circulating KP levels increase linearly in healthy pregnancy but are reduced in miscarriage during early pregnancy. KP can accurately predict the risk of miscarriage with average/above average levels of KP being associated with a less than 1% risk of miscarriage. KP levels are reduced in FGR and GDM and raised in PET during the later stages of pregnancy. FGR, fetal growth restriction; GDM, gestational diabetes mellitus; KISS1, kisspeptin gene; KISS1R, kisspeptin receptor; KP, kisspeptin; PET, preeclampsia. Figure created with BioRender.com.
Miscarriage
Miscarriage is the spontaneous loss of an intrauterine pregnancy before 24 weeks of gestation and affects 20% of pregnancies (202). Miscarriage can be difficult to diagnose as a pregnancy can be failing for a period before miscarriage is conclusively confirmed. Therefore, biomarkers that could aid in the evaluation of miscarriage, such as KP, are valuable.
Human data
KP levels adjusted for gestation are reduced (by 79%) in women with miscarriage compared to healthy pregnancy (104, 203-206) and are particularly low in complete vs incomplete (retained products of conception) or missed (empty gestational sac with absent heartbeat) miscarriage (see Table 1J). Unlike β-hCG, KP has been shown to maintain a high diagnostic performance throughout the first trimester (104, 203). Indeed, a combined KP and β-hCG measurement had the highest diagnostic accuracy to predict miscarriage at all gestations with an AUCROC of 0.92 (0.89-0.95) (104).
Hypertensive disorders of pregnancy
Pregnancy-induced hypertension and preeclampsia are defined as new-onset hypertension (blood pressure ≥140/90 mm Hg) following 20 weeks’ gestation. Preeclampsia also includes the presence of proteinuria (>3 g per 24 hours), neurological complications, and a high risk of significant end-organ dysfunction (207).
Human data
KP levels vary according to preeclampsia subtype, severity, and time of disease onset. KP concentrations are generally reduced in preeclampsia, especially during the first and second trimesters (108, 113, 208-212), and levels decline further with increasing disease severity (211, 212) (see Table 1K). In contrast, KP levels were found to be increased during the third trimester of pregnancy in keeping with placental KP expression data (107).
Ectopic pregnancy
Ectopic pregnancy (EP) occurs when a fertilized ovum implants outside of the uterine cavity and affects 2% of pregnancies (213). Its current diagnostic methods (serial β-hCG measurements and laparoscopy) have low sensitivity and specificity and are associated with high morbidity (213).
Human data
While some studies have reported low levels of KP in EP (206, 214), others did not find any significant differences after adjusting for confounding variables (107) (see Table 1L). These differing results are likely due to the early gestational age (GA) at presentation of EP.
Fetal growth restriction and preterm birth
Fetal growth restriction (FGR) encompasses intrauterine growth restriction (IUGR; fetal weight <10th percentile for GA with abnormal Doppler artery results (215)) and small for gestational age (SGA; weight at delivery <10th percentile for gestational age) (107, 112-114).
Human data
KP levels are consistently reduced in IUGR (113, 114) and pregnancies with SGA (107, 112) and therefore KP could aid in the assessment of these conditions (see Table 1L). In contrast, in preterm birth (delivery prior to 37 weeks’ gestation (216)), circulating KP levels were increased during the first trimester but were unaltered in the third trimester (107).
Gestational diabetes mellitus
Gestational diabetes mellitus (GDM) affects up to 20% of pregnancies worldwide (217) and develops when pancreatic β cells fail to respond to the physiological increase in insulin resistance that occurs during pregnancy (218, 219). In vitro and in vivo studies suggest that KP could potentiate glucose-stimulated insulin secretion (GSIS) (220-223) and thus improve glucose tolerance (109) (further discussed in the later section on metabolism in this review).
Human data
In studies involving women with GDM, KP concentrations were either decreased (108, 109) or not significantly different (107, 110) (see Table 1L).
Although evidence to date is convincing regarding KP's utility for diagnosing miscarriage, further larger studies with sufficiently sized control cohorts, and adjustments for gestation, BMI, comorbidities, and disease severity, are required to assess KP's potential as a biomarker in other pregnancy complications.
In Disorders of Metabolism
Glucose homeostasis
Glucose regulation is dependent on the meticulous control of blood glucose concentrations by several hormones released from central and peripheral tissues (224). KP and its receptor are expressed in murine and human pancreatic β cells, liver, and adipose tissue (16, 29, 220, 225), suggesting that it could have a putative role in glucose regulation.
In vitro data
The effect of KP on GSIS is conflicted within the literature. Using isolated islets and/or perfused pancreata from mice (226, 227) and rats (228), KP induced an inhibitory effect on insulin secretion. In contrast, studies employing static incubation and/or periperfusion experiments using islets from mice (220-223), rats (223), and pigs (223) found that KP potentiated insulin secretion. These differing results may be due to the differences in experimental protocols used. Indeed, human islets incubated with glucose (3- and 17-mM) and KP (0, 2.7, and 1000 nM) demonstrated that KP stimulates GSIS in a dose-dependent manner in the presence of high (but not low) glucose levels (115). Consistent with this, KP stimulates insulin secretion at higher ambient glucose concentrations (20 mM) vs lower concentrations (2 mM) in human islet cells (220, 222). Taken together, these findings suggest that KP stimulates insulin release at high ambient glucose concentrations.
Animal data
Female but not male mice null of Kiss1r have higher fasted basal glucose levels, impaired glucose tolerance, and increased BW (229, 230), which suggests that KP signaling may influence glucose homeostasis in a sexually dimorphic manner. As global Kiss1r KO animals are also profoundly hypogonadal and lack gonadal sex steroids, this could influence the effect on glucose tolerance (231). To account for this, Kiss1r KO mice with selective reintroduction of Kiss1r only in GnRH cells were generated, thus preserving gonadal function (230). Using this approach, females with preserved gonadal function still displayed perturbed glucose tolerance, albeit with a milder phenotype (230). In pregnant mice, specific KO of Kiss1r in pancreatic β cells caused glucose intolerance (109). These changes were not observed in the nonpregnant state, which suggests that KP has an adaptive role in compensating for gestational insulin resistance through regulation of β-cell function (109).
In adult male rats, peripheral (IV) rather than central (by intracerebroventricular injection [ICV]) KP administration induced rapid increases in plasma insulin levels (4-fold), suggesting that KP's effects are peripherally mediated (222). Likewise, peripheral injections (intraperitoneal) of KP-10 resulted in a 3-fold increase in plasma insulin concentrations (232). Furthermore, KP administration significantly heightened GSIS both in fed and fasted monkeys (233). However, despite increases in insulin secretion, no changes in glucose tolerance have been observed following short-term administration of KP (232).
Human data
The first study evaluating the effects of KP on GSIS in humans (n = 15) was conducted in 2018 (115) (see Table 1M). Here, an IV infusion of KP-54 (1 nmol/kg/hour) increased both insulin secretion and the deposition index (an assessment of β-cell function) by 35% compared to placebo (115). This effect was only observed in response to an intravenous glucose tolerance test and not a mixed-meal tolerance test (115), thus suggesting that KP increases insulin only in the presence of high glucose levels. On the contrary, an IV infusion of KP-54 (1 nmol/kg/hour) did not influence preprandial and postprandial glucose and insulin levels in women with overweight or obesity (116). These data indicate that KP could have a potential role in glucose metabolism, especially during pregnancy, a state of insulin resistance.
Appetite regulation and obesity
Appetite is intricately regulated by hypothalamic ARC neurons including proopiomelanocortin (POMC), agouti-related peptide (AgRP) and neuropeptide Y (NPY) neurons (234). While POMC neurons are anorexigenic (appetite-suppressing) (235), NPY and AgRP neurons are orexigenic (appetite-stimulating) (236, 237). Considering KP has a critical role in reproduction, and that adequate reproductive function is dependent on sufficient energy stores, studies have investigated the anatomical and functional reciprocal connections between KP, POMC and NPY/AgRP neurons (234).
Appetite regulation
Animal data
Evidence of the interactions between KP, POMC, and NPY neurons is controversial. While KP has been shown to stimulate POMC and AgRP (238), and inhibit NPY neurons (239) (overall reduced food intake), other studies have reported the opposite (163, 240). Notably, toxin-induced silencing of ARC Kiss1 neurons altered circadian food intake (less food eaten during the dark phase) but not total food intake (241). Likewise, global KO of Kiss1r in mice resulted in reduced food intake both in dark and light phases (229, 242), suggesting that KP has appetite-suppressive effects. Interestingly, like glucose homeostasis, appetite regulation also displays sexual dimorphism. For instance, while female Kiss1r null mice have reduced food intake, their male counterparts have either similar or only mildly reduced food intake compared with controls (229). However, this effect is lost in Kiss1r KO male mice with preserved gonadal function (230), thus indicating that the effects of KP on food intake is mediated by changes in gonadal sex steroids in males.
KP's effect on appetite regulation varies within the literature and differences can occur according to the species type involved. For instance, central (ICV) administration of KP-10 reduced food intake in fasted adult male mice (243) and female jerboas (244) but had no effect in fasted prepubertal (168) or adult male rats (5). However, higher doses (4.6 nmol) of ICV KP-10 markedly reduced food intake in rats (245). Similarly, peripheral (intraperitoneal) injections of KP-10 have been shown to decrease food intake in mice in some (232) but not all studies (243, 246). In contrast, chicks had increased food intake following administration of ICV KP-10 (115). These species differences are likely due to alterations in experimental methodology (eg, food intake being measured in light vs dark phases, KP administration to fed vs fasted animals) between studies, but could be due to the presence of different appetite circuits between species.
Human data
In fasted healthy men, KP-54 (IV infusion 1 nmol/kg/h over 2 hours) had no effect on self-reported hunger or objective food intake (115). Furthermore, in healthy men, an IV bolus of KP-54 did not alter brain signal responses (limbic and hypothalamic) to visual food stimuli (117) (see Table 1N).
Obesity
Animal data
KO of the KP receptor in adult female mice display increased adiposity and leptin levels from as early as age 6 weeks followed by a dramatic rise in BW of 30% (229). Although increased BW did not correlate with increased food intake, it was associated with lower respiratory rates, energy expenditure, and locomotor activity (229). The E2-deficient state following Kiss1r KO could have also contributed to the changes in BW observed. However, a higher BW was observed in OVX vs gonadal intact in Kiss1r KO mice (229), thus suggesting KP's effect on energy homeostasis is likely to be mediated in both a direct (via energy expenditure) and indirect (via sex steroid hormones) manner (229). Once again, sexual dimorphism was exhibited as male Kiss1r null mice had normal BW (229).
Brown adipose tissue (BAT), a marker of energy expenditure, regulates thermogenesis and metabolic rate. Interestingly, selective Kiss1r KO from BAT (BAT-Kiss1r KO) reduced BW and increased energy expenditure, locomotor activity, body temperature, and BAT gene expression (specifically Cox8b) in female mice (247). Collectively, these data indicate that the obesity and decreased metabolism in global Kiss1r KOs reflect impaired KP signaling in non-BAT tissues and that BAT-specific KP induction could be a potential target for obesity treatment (247). More research elucidating the specific tissues and cell types where KP signaling influences metabolic and thermogenic parameters is required.
Human data
The first human study to evaluate the acute effects of KP in obesity was conducted in 2023.
Here, an IV infusion of KP-54 (1 nmol/kg/h over 2 hours) administered to women with overweight or obesity had no effect on self-reported appetite or objective food intake (116). Thus, the appetite regulatory effects of KP appear to be species specific, with no changes being observed in humans.
Metabolic fatty liver disease
Metabolic fatty liver disease (MAFLD) is highly prevalent with global rates reaching 25% and is a leading cause of liver transplantation in the United Kingdom (248). It encompasses a spectrum of disease from excessive liver fat/steatosis (“nonalcoholic fatty liver” [NAFL]), necroinflammation and fibrosis (“nonalcoholic steatohepatitis” [NASH]), to NASH-cirrhosis and ultimately hepatocellular carcinoma (249, 250). MAFLD is associated with significant comorbidities including central obesity, type 2 diabetes mellitus, dyslipidemia, and metabolic syndrome (251). From a therapeutic perspective, there are currently no approved pharmacotherapeutic options for the treatment of MAFLD.
Diagnosing metabolic fatty liver disease
Animal data
To study the effects of KP signaling in MAFLD, mouse models have been generated in which wild-type mice are administered high-fat diets over several weeks (Fig. 7). In MAFLD mice, hepatic Kiss1 and Kiss1r mRNA expression is enhanced and circulating KP levels are 50% higher than controls (118). This could indicate that KP increases as a compensatory response to liver damage from MAFLD/NASH.
Figure 7.
Effects of liver-specific Kiss1r KO and enhanced KP signaling. Liver-specific Kiss1r KO mice model placed on a high-fat diet exhibited increased lipogenesis, TG synthesis, and reduced mitochondrial β oxidation compared to controls. This resulted in increased TG levels, serum ALT levels (indicating hepatocellular injury), and hepatic steatosis. Increased body weight and reduced energy expenditure were observed. Higher fasting glucose and basal insulin levels, indicating glucose intolerance and insulin resistance, were also observed. Moreover, markers of inflammation and early stages of fibrosis were upregulated. Effects of enhanced KP signaling: wild-type mice were placed on a high-fat diet for 6 weeks prior to administration of MVT-602, a KP receptor agonist, for 5 weeks on a high-fat diet. MVT-602 alleviated hepatic steatosis and metabolic deterioration through improvements in insulin sensitivity, lower basal insulin levels, reduced TGs, and ALT levels. MVT-602–treated mice had slightly lower body weight compared to controls with increased energy expenditure in the light phase. Mechanistically MVT-602 treatment under high-fat diet conditions significantly reduced TG synthesis, increased lipolysis, and mitochondrial β oxidation compared to controls. Markers of inflammation and early stages of fibrosis were downregulated. ALT, alanine transaminase; Kiss1r, kisspeptin receptor; KO, knockout; KP, kisspeptin; TGs, triglycerides. Figure created with BioRender.com.
Human data
Liver biopsies from men with MAFLD and NASH have increased expression of both KISS1 and KISS1R (mRNA and protein levels) and have 3-fold higher plasma KP levels, compared with healthy controls (118) and thus could have potential as a marker for grading MAFLD severity (see Table 1O).
Treating metabolic fatty liver disease
Animal data
In MAFLD mice, specific deletion of Kiss1r has been shown to worsen hepatic steatosis, impair glucose tolerance and upregulate markers of inflammation (such as macrophage inflammatory protein-2 and chemokines interferon-γ–induced protein 10) and fibrosis (such as collagen, smooth muscle actin, and matrix metalloproteinases) (118) (see Fig. 7). Conversely, enhanced stimulation of Kiss1r, through administration of a KP receptor agonist (MVT-602), alleviated hepatic steatosis and metabolic deterioration in MAFLD mice and prevented liver fibrosis in NASH mice (118). The mechanism by which KP exerts these protective effects is via activation of hepatic adenosine 5′-triphosphate (AMPK) with resultant inhibition of triglyceride accumulation. However, KP failed to protect against NAFLD livers deplete of AMPK or Kiss1r (118). Thus, KP receptor signaling plays an important role in the suppression of MAFLD/NASH disease progression by reducing hepatic lipogenesis, and therefore could have potential as future treatment targets for these conditions.
In Disorders of Bone
Direct effects of kisspeptin in bone with potential to treat osteoporosis
From an evolutionary perspective, during the physiological response to starvation, energy-demanding processes such as skeletal integrity and reproduction may be relinquished (252). Therefore, it is unsurprising that an established relationship between bone and reproductive hormones exists, with hormones from all levels of the HPG axis implicated in the growth and maintenance of the mammalian skeleton (reviewed recently and extensively in (253)).
The importance of the interaction between reproductive hormones and bone is clearly illustrated by reproductive disorders, which contribute to the clinical burden of low bone mineral density, such as primary ovarian insufficiency, HA, CHH, and hyperprolactinemia. In addition, postmenopausal bone loss is a central risk factor for developing osteoporosis (254, 255), with higher risk and prevalence of fractures resulting in disability, poor quality of life, and increased mortality (256). Taken together, this stresses the need to better understand bone physiology and the pathogenesis of bone loss, to identify new safe and effective therapeutic targets.
In vitro studies
Bone mass is maintained by a tight balance between osteoclastic bone resorption and osteoblastic bone formation (253). KP receptor expression has been detected on osteoclast cell lines differentiated in vitro from CD14-selected monocytes (257). Moreover, both KISS1 mRNA and protein are strongly expressed in the normal human osteoblast cell line hFOB1.19 (30). This compares with KISS1 mRNA and protein expression, which are moderate, weak, and almost lost in the human osteosarcoma cell lines U-2 OS, Saos-2, and MG-63, respectively (30). Interestingly, the cell invasion ability of these cell lines reveals a gradually increasing aggressive phenomenon in U-2 OS, Saos-2, and MG-63, suggesting that lower KISS1 expression might be associated with a stronger invasive capability (30). Regarding the kisspeptin receptor, Kiss1r mRNA and protein have been observed in normal canine osteoblasts (258), as well as high expression of KISS1R protein on MG-63 osteoblast-like osteosarcoma cells (259). KISS1R expression has also been reported on osteoblast precursors, including primary human mesenchymal stem cells and osteoprogenitor cells (260).
Rodent data reveal that KP enhances osteoblast differentiation (osteoblastogenesis). In C3H10T/2 mouse mesenchymal stem cells, incubation with KP increases the expression of osteogenic marker genes, including distal-less homeobox 5 (Dlx5), runt-related transcription factor 2 (Runx2), and alkaline phosphatase (ALP) (261). Of note, the growth factor bone morphogenetic protein 2 (BMP2) stimulates bone formation by activating these osteogenic genes (262, 263). It is therefore pertinent that KP has been documented to stimulate osteoblast differentiation by increasing the expression and activation of BMP2 in C3H10T/2 cells (via the transcriptional factor NFATc4), whereas in Kiss1r null cells, osteoblast differentiation was suppressed (261). Collectively, this reveals that in C3H10T/2 cells, KP (acting via Kiss1r) stimulates osteoblastogenesis through NFATc4-mediated BMP2 expression and activation (261).
Moving from rodents, recent work provides the first evidence for direct effects of KP on human bone metabolism. Using the human cell line hMSCs, exposure to KP for 7 days induced a 41.1% increase in ALP activity, signifying enhanced osteoblastogenesis (119). It is notable that KP administration had no effect on ALP activity in either osteoblast monoculture or cocultures, indicating that KP does not modulate mature osteoblast activity but instead has a predominant effect on osteoblastogenesis, at least in vitro. In terms of human osteoclasts, KISS1R mRNA was identified throughout the 10-day process of osteoclastogenesis (ie, from CD14+ to mature human osteoclast). Indeed, KP administration exerted a potent and dose-dependent antiresorptive effect on osteoclast activity both in monocultures and osteoclast/osteoblast cocultures. In cocultures, this inhibitory effect ranged from 26.2% (0.01 nM kisspeptin) to 53.4% (10 nM kisspeptin) (119). Taken together, these in vitro data reveal that in humans KP enhances osteoblastogenesis and potently inhibits osteoclast activity.
In vivo nonhuman studies
Using a combination of genetic models and stereotaxic surgery, recent pivotal work has identified a neuroskeletal axis, whereby deleting ERα-signaling in the ARC promotes significant increases in bone mass without affecting food intake (264). This skeletal phenotype was sex specific (occurring in female but not male mice) with a remarkable increase in trabecular bone mass of approximately 700%, an average 80% increase in bone volume over total volume, as well as increases in trabecular number and thickness and overall mechanical strength of long bones (264). These changes were accompanied by a significant increase in bone formation rate and mineralized surface (indicating enhanced osteoblastic functions) and upregulation of BMP signaling and osteoblast differentiation on transcriptional profiling (264). Notably, acute ablation of ARC ERα after ovariectomy resulted in a 50% increase in bone density, demonstrating that even in the absence of gonadal hormones, the brain circuit remains intact (264). Finally, loss of ERα specifically in KP-expressing ARC recapitulated this bone phenotype, defining central KP signaling as a key node in the ER-neuroskeletal circuit regulating sex-dependent bone remodeling in females (264).
In vivo human studies
Translating the preclinical evidence into humans, a recent clinical study investigated the acute effects of KP administration on bone turnover markers in humans for the first time (119) (see Table 1P). Involving 26 healthy eugonadal young men, an acute 90-minute infusion of KP elicited a 20.3% maximal increase in total osteocalcin (an established marker of bone formation) and 24.3% maximal increase in carboxylated osteocalcin (which predominates in bone remodeling) but had no acute effects on circulating P1NP levels (a further bone formation marker) in this short time course. Interestingly, a comparable magnitude of increase in osteocalcin along with bone-forming effects has been observed with short-term teriparatide administration (a recombinant parathyroid hormone used for the treatment of osteoporosis) (265). Moreover, during the acute experimental time-course, kisspeptin administration had no significant effects on the bone resorption marker CTx (which may require a longer experimental duration to detect changes) or on downstream testosterone levels (119). Collectively, these data highlight that KP administration acutely increases the bone formation marker osteocalcin in healthy men, independently of downstream sex steroid levels.
Taken together, across a series of experimental models, an emerging and favorable link between KP and bone metabolism has been identified. Importantly, human evidence demonstrates that KP enhances osteoblastogenesis and potently inhibits osteoclast activity in vitro, while also acutely increasing the bone formation marker osteocalcin in healthy men. Therefore, these findings suggest that KP administration may beneficially uncouple bone turnover in humans, which warrants further investigation in chronic KP administration studies and in patients with disorders of bone metabolism to examine KP's clinical therapeutic potential.
In Disorders of Sexual Behavior
Reproductive behaviors are complex strategies related to the ultimate production of offspring. They include the identification of suitable mating partners (principally using olfactory and auditory signals), as well as copulatory and sexual behaviors (266). Furthermore, advanced species (including humans) have evolved to gain reward and satisfaction from sex itself and its precursors (sexual desire and arousal) (267). A persistent disturbance with any stage of normal sexual activity can result in sexual dysfunction (ie, sexual desire, arousal, and orgasmic disorders).
Along these lines, it is therefore pertinent that beyond the hypothalamus, KP and its receptor have been localized to numerous limbic brain structures in rodents (22) and humans (18) that are areas implicated in the neurocircuitry regulating sexual and emotional behaviors (268). Consistent with this, a wealth of literature implicates KP signaling in the neuroendocrine control of all aspects of reproductive behavior across a range of species as discussed next (Fig. 8).
Figure 8.
The effects of KP signaling on key reproductive behaviors in rodents, sheep, and humans, including olfactory processing, sexual partner preference, copulatory behavior, and arousal and bonding. KP is widely expressed in limbic and paralimbic regions of the brain, which are involved in reproductive behaviors. Olfaction: KP expression and activity increased in several brain regions in response to opposite sex olfactory cues in male mice and female mice and ewes. In humans, KP administration enhanced limbic brain activity when men were exposed to a pleasant feminine scent. Sexual partner preference and bonding: Studies showed that the KP MePD neurons regulate partner preference in male mice, whereas intraperitoneal and intranasal administration of KP to male rats increases sexual motivation. When peripheral KP was administered to Kiss KO female mice, normal male-directed sexual preference was restored. In healthy heterosexual men, peripheral KP administration increased brain activity in aesthetic brain regions in response to viewing female faces. Copulatory behavior and arousal: KP stimulation in the MePD resulted in erections in male rats, whereas both peripheral and central KP administration to female mice robustly stimulated lordosis. In healthy heterosexual men, peripheral KP administration enhanced limbic brain activity when exposed to visual sexual stimuli. KP administration to males with HSDD deactivated brain regions involved in self- monitoring and introspection, and increased brain activity in sexual arousal centers, in response to watching erotic videos in the fMRI scanner. KP administration also led to increases in penile tumescence. KP administration to pre-menopausal women with HSDD, deactivated brain regions involved in inhibitory control and activated areas known to be activated in the context of sexual arousal in response to erotic visual cues. AVPV; anteroventral periventricular nucleus; fMRI, functional magnetic resonance imaging; GnRH, gonadotropin-releasing hormone; HSDD, hypoactive sexual desire disorder; KO, knockout; KP, kisspeptin; MePD, posterodorsal subnucleus of medial amygdala. Figure created with BioRender.com.
Male reproductive behavior
In vivo nonhuman studies. Olfactory processing. In adult male rats, reciprocal connectivity between the accessory olfactory bulb and amygdala KP neurons has been visualized (269). Given the established role for the accessory olfactory bulb in relaying pheromonal signals (270), this suggests that amygdala KP neurons are targeted directly by pheromonal pathways. Moreover, amygdala KP neurons project to GnRH neurons in the hypothalamic POA, with approximately 15% receiving inputs from this amygdala KP population (269). Collectively, these neuroanatomical data define a physiological framework for how KP signaling serves as a relay between olfactory signals and the HPG axis.
To provide biological significance for the neuroanatomical connections, rodent models have investigated whether sex-related olfactory signals can modulate central KP expression. In male mice, exposure to female urine (as a pheromone stimulus) for 30 minutes has been observed to increase the number of KP-neurons coexpressing c-Fos in the medial amygdala (MeA) by 2-fold, with a concomitant rise in LH release within 15 minutes (271). Notably, no changes in AVPV or ARC KP activity was observed (271). Building on these findings, the acute effects of olfactory signals in male rats has been recently examined (272). In this study, within 5 minutes of exposure to a female rat, KP expression was significantly enhanced in the AVPV and PeN, resulting in significant increases in LH and testosterone levels, followed by increased male sexual behavior (272). In contrast, exposure to solely female-soiled bedding failed to increase KP expression in the AVPV/PeN or testosterone levels, suggesting that a physical stimulus animal is required to induce AVPV/PeN KP expression in male rats (272). In contrast to the earlier discussed mouse study (271), neither exposure to a female rat nor female-soiled bedding affected KP expression in the MeA (or ARC), which may be accounted for by species differences, or the experimental model.
Sexual partner preference. Gonad-intact Kiss1r KO male mice display no partner preference for either male or female stimulus animals (273). Specifically, despite normosmia (determined using a “hidden cookie test’), they spend an equal investigatory duration with male and female stimulus animals (48% vs 52%, respectively), whereas wild-type male mice spend more than 70% with females (273). Notably, this behavioral deficit is not rescued by testosterone replacement (273), suggesting that the KP receptor is indispensable for regulating sexual partner preference in male mice. Along similar lines, using a chemogenetic approach, DREADDs stimulation of KP neurons in the posterodorsal MeA (MePD) has been reported to double the time male mice spend investigating an estrous female over another gonadally intact male (274). Furthermore, to define direct KP effects, a recent study investigated sexual motivation in male rats following 3 interventions: intranasal administration of a GnRH analogue, intraperitoneal KP, or intranasal KP (275). Using this experimental paradigm, intranasal GnRH augmented circulating testosterone levels but did not affect sexual motivation, whereas intraperitoneal KP increased both testosterone and sexual motivation (275). Importantly, despite not affecting testosterone levels, intranasal KP increased sexual motivation (275), highlighting KP is a GnRH/testosterone-independent regulator of sexual motivation in male rats.
Sexual and copulatory behaviors. Direct infusion of KP into the MePD of male rats dose-dependently results in multiple ex-copula erections, an effect that is blocked by pretreatment with a KP receptor antagonist (peptide-234) (276). Comparatively, when KP is infused into the lateral cerebroventricle, despite a similar increase in circulating LH, no erections are observed (276), indicating GnRH/LH independence and site specificity of the MePD for KP's erectile response in rodents.
Given the previous data highlighting that testosterone replacement fails to restore sexual partner preference in Kiss1r KO male mice (273), it is interesting to consider whether this happens with other reproductive behaviors. When paired with a hormone-primed receptive female for 45 minutes, Kiss1r KO male mice display an absence of all normal male-like sexual parameters (mounts, thrusts, intromissions, and ejaculation) (273). In contrast, castration followed by testosterone-replacement elicits a robust increase in mounts and thrusts at a ratio with that of testosterone-treated wild-type males (273). This is highly congruent with evidence in Kiss1 KO male rats (277) and mice (278), whereby testosterone-supplemented males show mounting behavior, but not ejaculation (which may be attributable to incomplete penile development) in mating trials. Taken together, these findings indicate that restoration of testosterone levels partly rescues some but not all sexual behaviors (especially mounting) both in Kiss1r and Kiss1 KO male rodents.
Moving from rodents to male domestic animals, recent data provide evidence for the relationship between circulating KP levels and sexual behavior in buffalo bulls (279). In this study, it was observed that KP levels were significantly lower in bulls with longer reaction times (ie, time from exposure to mounting the female). Moreover, on approach to the female, males displaying characteristic aggressive behaviors (ie, uncontrollable, and extremely eager to mount and approach with full vigor) had significantly higher KP levels, compared to dull males (ie, proceeding with a dull expression and longer time to mount). In keeping with earlier rodent evidence (273, 276), males with lower KP levels also exhibited incomplete penile erection and protrusion (279). Hence, these findings suggest that circulating KP levels may offer a novel biomarker for sexual behavior in male domestic animals.
In vivo human studies
The application of functional neuroimaging (including functional MRI [fMRI] and proton magnetic resonance spectroscopy) has been indispensable to facilitate the noninvasive study of sexual brain processing in humans by mapping activated areas of the brain (280). To date, clinical studies have been undertaken both in healthy men and patients with low sexual desire to investigate the effects of KP across a range of behavioral domains as detailed next (see Table 1Q).
Resting brain activity. KP's effects on resting brain activity has been explored using 2 established neuroimaging techniques. First, using fMRI in healthy heterosexual men, peripheral KP administration has been shown to modulate resting brain connectivity (121), which is an important element of human behavior, frequently disrupted in psychosexual and emotional disorders (281). Specifically, KP modulated the default mode network (the most defined resting state (282)), which correlated with enhanced limbic brain activity later in response to visual sexual images (121). Additionally, KP's modulation of this network was greater in men with less reward drive and correlated with reduced sexual aversion (121). In a further study, proton magnetic resonance spectroscopy was employed to examine the in vivo effects of KP administration on central levels of the key inhibitory neurotransmitter γ-aminobutyric acid (GABA) in the human brain (123). Using this approach, peripheral KP administration significantly decreased endogenous GABA by 15% in the anterior cingulate cortex of healthy men (123). Of note, a similar magnitude of GABA change has previously been reported in psychological studies with functional impact (283, 284).
Olfactory processing. In healthy heterosexual men, peripheral KP administration has been observed to enhance limbic brain activity when men are exposed to an established feminine olfactory stimulus, “Chanel No. 5” (122). Specifically, brain activation was demonstrated in limbic regions implicated in olfactory processing, hedonic valuation of olfactory stimuli, and sexual arousal, including the amygdala, hippocampus, and insula (285). Comparatively, KP did not affect brain activity in the motor cortex (which was employed as a control region), highlighting the specificity of KP's effects in olfactory and limbic circuits regulating sexual behavior on exposure to a feminine olfactory stimulus (122).
Sexual partner preference. Attraction is an important initiating step in human sexual behavior, involving numerous aesthetic brain regions, including the medial prefrontal cortex (286-288) and superior frontal gyrus (289). In healthy heterosexual men, peripheral KP administration increases brain activity in both regions in response to viewing female faces (122). From a functional perspective, significant correlations were observed between KP-enhanced brain activity and important psychometric parameters. For example, the effects of KP in the anterior cingulate cortex and insula were more pronounced in men with lower baseline reward and sexual quality of life, which is relevant given these areas are implicated in sexual arousal (290), facial attraction (289) and motivation toward reward (291, 292). It is interesting to speculate about the biological significance of this differential effect. From an evolutionary perspective, KP's enhancement of these brain regions may serve to strengthen feelings of reward, attraction, and motivation in individuals with lower sexual quality of life, to promote sexual attraction and ultimately encourage reproduction at a population level.
Sexual behavior. In healthy heterosexual men, peripheral KP administration enhances limbic brain activity when men are exposed to visual sexual stimuli (but not other stimuli, such as negative, neutral, happy, or fearful-themed images), including in the anterior and posterior cingulate and amygdala (120). Additionally, the more KP was observed to enhance brain activity in key limbic structures involved in sexual arousal (such as the putamen, anterior cingulate, and globus pallidus), the less aversion to sex healthy men displayed (120). Thus, given that desire for sexual stimulation is a fundamental component of the human sexual response (293), these findings laid the foundation for potential clinical application of KP for the treatment of patients with psychosexual dysfunction. Along these lines, in a recently published work, the clinical and mechanistic effects of KP administration were investigated in men with distressing low sexual desire due to hypoactive sexual desire disorder (HSDD) (125). This condition is characterized by increased activity of higher cortical and cognitive brain regions, which inhibits lower limbic and emotional regions, thus interfering with sexual desire (294). It is therefore significant that in response to watching erotic videos in the fMRI scanner, KP administration was observed to significantly deactivate brain regions involved in self-monitoring and introspection (such as the parahippocampus, frontal pole, and precuneus), while increasing brain activity in sexual arousal centers (such as the anterior cingulate) in this cohort of men with psychosexual dysfunction (125). Indeed, in response to KP's restoration of sexual brain processing, significant increases in penile tumescence (by 56% more than placebo) and behavioral measures of sexual desire (including increased “happiness about sex”) were observed, providing functional and behavioral relevance (125).
In this collection of neuroimaging studies (120–125), an identical administration protocol with peripheral KP-54 was employed. Although different KP isoforms display different degrees of blood-brain barrier penetrance, it is well established that peripheral KP-54 can activate GnRH neuron dendritic terminals before the blood-brain barrier (295), as well as cross the blood-brain barrier to directly access deeper brain structures expressing KP receptors (120). In all the highlighted clinical studies, KP largely modulated brain regions matching KP receptor expression in humans (16, 17, 29), which could suggest direct actions of KP on its receptor. In addition, the administration protocol was selected to ensure steady-state levels of KP during the data collection period (brain imaging and behavioral testing), while avoiding downstream testosterone increases that occur later (77). Finally, across this series of studies, KP modulated brain activity in relation to sexual and emotional tasks. Indeed, of note, recent data reveal that using the same administration protocol, KP does not affect brain responses to visual food stimuli in healthy young men (117), highlighting that KP's effects on limbic brain regions are specific to sexual and emotional stimuli.
Female reproductive behavior
In vivo nonhuman studies. Olfactory processing. In seasonally anestrous ewes, the introduction of a novel male sheep has been observed to result in 9-fold and 3-fold increases in KP c-Fos activity in the rostral and mid ARC, respectively (296). This was associated with increases in LH pulse amplitude and pulse frequency, an effect that was abolished by central infusion of a KP antagonist (peptide-271) (296). Turning to rodents, in female mice exposure to opposite-sex (but not same-sex) urinary pheromones induces KP c-Fos activity by almost 40% in the AVPV (297). This is in close agreement with data from OVX female rats (implanted with preovulatory levels of E2), whereby exposure to male-soiled bedding (but not clean or female-soiled bedding) significantly activated AVPV (but not ARC) KP neurons, as well as inducing cell activation in key limbic regions (including the MeA, bed nucleus of the stria terminalis, and cortical amygdala) (298). Importantly, concomitant LH surges were also evident in those female rats exposed to male-soiled bedding, with maximal LH stimulation within 1 to 2 hours of the onset of bedding exposure (298).
Auditory processing. Certain male species, such as rodents, emit song-like ultrasonic vocalizations (USVs) to communicate their motivational state, facilitate female approach behavior, and ultimately promote reproduction (299). Evidence reveals that these USVs promote fertility in female mice by activating hypothalamic KP neurons (300). As part of these experiments, females were housed in a soundproof chamber and exposed to a sound file consisting of either male mice USVs or background noise (as a control sound) repeatedly for 20 minutes. This significantly increased the number of KP neurons expressing pCREB (an indicator of neural activation) in the ARC (but not the AVPV) after exposure to male USVs, compared with background noise. To provide functional relevance for the enhanced neuronal activity, it was observed that a positive correlation existed between ARC KP neuronal activity and the duration of female searching behavior, suggesting that the female's approaching behavior toward USVs of male mice relates to the activation of KP neurons (300). Collectively, these data suggest KP's key involvement in the mechanism by which USVs of male mice promote copulation in female mice by activating their approaching behavior.
Sexual partner preference. OVX and hormone-primed Kiss1 KO female mice do not display male-directed preference (301). In fact, an equivalent perturbation is observed following selective viral ablation of AVPV KP neurons (301). Notably, in both experimental paradigms, normal male-directed sexual preference is rescued following a single peripheral injection of KP (301), highlighting site specificity of AVPV KP neurons in the control of mate preference in female mice. Regarding the downstream pathways, exploiting a transgenic GnRH-deficient mouse model (which progressively loses GnRH expression during adulthood) results in female mice displaying female rather than male-directed preference. Functionally, this behavioral deficit normalizes following a single peripheral injection of GnRH (but not KP as downstream GnRH is lacking) (301), indicating that KP signals through GnRH to regulate sexual partner preference.
Copulatory and sexual behavior. In a sexually receptive female rodent, fertile copulation involves the adoption of a posture that facilitates intravaginal ejaculation to occur, termed lordosis (302). Regarding this key reproductive behavior, both peripheral and central KP administration to female mice robustly stimulates lordosis (301). Interestingly, when OVX Kiss1r KO female mice are hormone-primed, they display normal lordosis (273), suggesting that the KP receptor may not be essential for lordosis (given it is rescued by gonadal sex hormone replacement). In contrast, even when hormone-primed, OVX Kiss1 KO female mice fail to display lordosis behavior, whereas this deficit normalizes following a single peripheral injection of KP (301). In terms of the neurocircuitry controlling lordosis, acute ablation of AVPV KP neurons results in a profound deficit in lordosis behavior in OVX and hormone-primed female mice, whereas optogenetic stimulation enhances lordosis (301). Using mutant female mice that lack GnRH secretion in adulthood reveals that unlike male-directed preference (which is abolished), lordosis behavior is not affected (301), indicating that lordosis is independent of GnRH signaling.
Viral tracing studies reveal that AVPV KP neurons communicate with 2 populations of neurons that express nitric oxide synthase (nNOS) in the ventrolateral part of the ventromedial hypothalamus (VMHvL) (301) and the paraventricular nucleus (303). This is pertinent given that female mice deficient in nNOS display a strong decrease in lordosis and whereas an injection of KP or GnRH fails to stimulate lordosis, a nitric oxide donor (SNAP + BAY) restores lordosis (301). Moreover, administration of SNAP + BAY to Kiss1 KO female mice also restores lordosis, confirming that nitric oxide acts downstream of KP neurons to mediate lordosis (301). Recent experiments have sought to elucidate which neuronal population expressing nNOS are the target of AVPV KP-signaling. In these studies, central administration of KP or a nitric oxide donor (SNAP + BAY) into the VMHvL significantly increased lordosis, whereas administration of an nNOS inhibitor (I-NAME) decreased lordosis (304). Moreover, central administration of KP into the paraventricular nucleus had no effect on lordosis, indicating that KP modulates lordosis behavior through nNOS neurons in the VMHvL (304).
In vivo human studies. Unlike the aforementioned functional neuroimaging studies in healthy men investigating the effects of KP on sexual and emotional brain activity, there are currently no published studies in healthy women. However, a recent study examined KP's effects on sexual and attraction brain processing in premenopausal women with low sexual desire due to HSDD (124) (see Table 1Q). In response to erotic videos, KP administration was observed to deactivate the inferior frontal and middle frontal gyri (regions involved in inhibitory control (305, 306)) and activate the postcentral and supramarginal gyri (areas known to be activated in the context of sexual arousal (307-309)). It is well established that women with HSDD are characterized by specific alterations in the motivational component of men's perception (310). It is therefore pertinent that in this patient cohort of women with HSDD, KP administration deactivated the temporoparietal junction (an area whose deactivation is linked with reducing negative perception of others and reducing self-consciousness (311)) in response to viewing male faces (124). Of note, KP's enhancement of posterior cingulate activity in response to male faces was observed to correlate with reduced sexual aversion, providing behavioral and functional significance (124). To what extent KP influences sexual brain processing and associated physiological and behavioral measures of sexual desire and arousal in postmenopausal women with HSDD is currently unknown but would be a fruitful area for study given its high prevalence (312).
Taken together, an explosion of experimental evidence reveals important neuromodulatory roles for KP-signaling in all aspects of reproductive behavior from regulating sexual partner preference and sexual motivation to copulatory and sexual behaviors. In addition, clinical studies in men and patients with both normal and abnormal sexual function illustrate the emerging influence of KP in human sexual and emotional brain processing. Given these exciting data, future studies in broader patient cohorts (such as different sexual identities and orientations) and other forms of sexual dysfunction (such as erectile dysfunction) are much warranted to provide further evidence for clinical applications of KP-based therapies in patients with common reproductive and psychosexual disorders.
Clinical Applications of Neurokinin B Antagonism
Treating Polycystic Ovary Syndrome
PCOS is a heterogeneous condition affecting 2% to 13% (88) of women of reproductive age and is currently diagnosed by the Rotterdam criteria (170). PCOS is associated with adverse endocrine, reproductive, metabolic (insulin resistance, dyslipidemia), and psychological features (170). Despite its high prevalence and substantial clinical burden, current treatment strategies for PCOS are suboptimal as they rely on treatment of symptoms rather than the underlying pathophysiological process. The lack of mechanism-based treatments is attributable to the complex and unclear etiology of PCOS, and hence defining the causative factors driving PCOS pathogenesis has been of interest.
A cardinal feature of PCOS is androgen excess driven by increased GnRH and LH) pulsatility (313. As hypothalamic ARC KP-neurokinin B-dynorphin (KNDy) neurons regulate GnRH pulse generation and express androgen receptors, KNDy neurons have been implicated in mediating the androgenic effects of PCOS (Fig. 5). Indeed, NKB and KP gene expression are increased in some PCOS-like animal models, thus suggesting that overactivity of KNDy neurons is responsible for the increased GnRH pulsatility observed in PCOS (35, 173). Additionally, patients with PCOS with inactivating variants in the NKB gene (TAC3) or NKB receptor (TACR3) have low baseline LH secretion and low LH pulse frequency (8). However, women with functionally null TAC3 can still conceive and mice lacking NKB (gene or receptor) can generate LH pulses, thus indicating that GnRH impairment is reduced rather than abolished (8, 314). This diminished action that NKB inhibition has on GnRH pulsatility is of therapeutic benefit as it enables GnRH pulsatile secretion to be normalized rather than terminated. Thus, there has been great interest in the use of NKB signaling blockade as a therapeutic agent in targeting the central pathophysiology of LH hypersecretion and hyperandrogenism in PCOS. Considering NK3Rs have a high binding affinity for NKB and are highly expressed in humans, antagonists of NK3R have been the preferential developmental agents for PCOS treatment (8).
Animal data
In peripubertal DHT-induced PCOS mice, NK3R antagonism (MLE4901) improved several metabolic parameters (eg, adiposity, adipocyte hypertrophy, glucose tolerance) but failed to ameliorate reproductive phenotypes (eg, ovarian acyclicity) (315). NK3R antagonist treatment reduced adipocyte area without affecting food intake, energy expenditure, or locomotor activity, but altered metabolic status by using carbohydrate as the predominant fuel source (315). In parallel, NK3R antagonism also reduced circulating leptin levels (315). Although NK3R blockade did not alter fasting glucose levels, NK3R antagonism reduced the effects of DHT-induced hyperglycemia (315). The lack of a reproductive phenotype may be due to KNDy neurons not being hyperactive in this model of PCOS (chronic DHT), as other models of androgenization (eg, prenatal) do recapitulate KNDy neuronal overactivity. Alternatively, the dose of the NK3R antagonist may have been inadequate and was unable to overcome the elevated androgens observed in this chronic DHT model.
Human data
In a randomized, multicenter clinical trial, women with PCOS received the NK3R antagonist MLE4901 (also known as AZD4901) at doses of either 20 mg/day, 40 mg/day, or 80 mg/day, or placebo for 28 days (131) (see Table 2C). Women receiving 80 mg/day of MLE4901 demonstrated a 52% baseline-adjusted reduction in the AUC of LH, a 79% reduction in basal LH secretion, and an LH pulse decrease of 3.6 pulses/8 hours, compared to placebo (131). Similarly, total testosterone and free testosterone levels were reduced by 29% and 19%, respectively (131). These effects were marked following 7 days of treatment and continued to be effective until the end of treatment (28 days) in women who did not ovulate during the study (131). A more recent study using a similar dose of MLE4901 (40 mg orally twice a day for 7 days) demonstrated a reduction in LH secretion (from 6.5 to 4.0 IU/L), LH pulse frequency (from 0.8 to 0.5 pulses/hour) and FSH levels (2.5 to 2 IU/L) compared to placebo in women with PCOS (98).
Another NK3R antagonist, fezolinetant (60 mg daily or 180 mg daily for 12 weeks), reduced the LH:FSH ratio and suppressed hyperandrogenism in women with PCOS (132). While both doses reduced LH and FSH throughout the study, only fezolinetant 180 mg daily reduced testosterone levels at all time points, thus indicating a dose-dependent response (132). Overall, fezolinetant 180 mg/day reduced testosterone by 33%, LH by −10.17 IU/L, and FSH by −1.46 IU/L, while fezolinetant 60 mg/day reduced testosterone by 17% nmol/L, LH by −8.21 IU/L, and FSH by −0.92 IU/L (132). No changes were observed in E2 and progesterone levels, endometrial thickness, follicle development, or menstrual cycle irregularity over the 12-week study (132). The lack of ovulation may have been due to the increased suppressive effects of fezolinetant on NK3R signaling. To avoid this, a different dose or shorter duration of therapy of fezolinetant may be more successful in restoring ovulation. Overall, manipulation of neuroendocrine signaling with NK3R antagonism may provide novel therapeutic approaches to treat specific phenotypic features of PCOS.
Treating Uterine Disorders
Uterine fibroids and endometriosis are common disorders of the reproductive system affecting up to 80% and 15% of women of reproductive age, respectively (316, 317). Uterine fibroids are benign, smooth muscle tumors of the uterus, whereas endometriosis is the presence of endometrial glands or stroma-like lesions outside the uterine cavity (316, 317). Both conditions cause severe symptoms including abnormal uterine bleeding, chronic pelvic pain, and infertility (316, 317). Women with early-age menarche and short menstrual cycle length are at high risk of developing these conditions, which suggests that continuous exposure of the endometrium and myometrium to estrogen is a key pathological driver of the disease (316, 317). Thus, suppressing E2 levels through downregulation of the HPG axis using GnRH modulators (agonists and antagonists) is a clinically validated therapeutic approach for the treatment of these disorders (318, 319). However, the approved duration of GnRH therapy is restricted due to its castrating effects and consequent menopausal-like symptoms, including bone loss and vasomotor hot flashes (320, 321).
An ideal therapy would be one that offers a more refined modulation of the HPG axis and lowers estrogenic drive to endometriosis and fibroid cell growth without causing the adverse events that are associated with current treatments. Indeed, lowering E2 levels to a range between 110 and 184 pmol/L has been recommended to be effective in reducing the symptoms of uterine fibroids and endometriosis (322, 323). One such novel therapeutic approach is to use NKB receptor antagonists to reduce LH while preserving FSH secretion (see Fig. 5).
Animal data
In OVX ewes, NK3R antagonism (MRK-08) decreased LH pulse frequency while maintaining FSH concentrations (324). Likewise, in castrated nonhuman primates (Macaca fascicularis), repeated daily dosing of the NK3R antagonist (ESN364) decreased plasma LH levels, inhibited the LH surge, but did not change FSH concentrations (325). NK3R blockade also lowered E2 levels in a dose-dependent manner, although nadir levels of E2 were maintained well above menopausal levels (325).
Human data
Several NK3R antagonists have also shown similar patterns of gonadotropin secretion (reduced LH with preserved FSH) in healthy women (see Table 2). For instance, AZD4901 (also known as MLE4901, formerly AZD2624) reduced E2 levels, endometrial thickness and folliculogenesis (326) during the follicular phase. In the early mid-follicular phase, AZD4901 resulted in reduced basal LH levels and a delayed LH-surge (by 7 days), without altering LH pulse frequency (327). Another NKB antagonist, fezolinetant (ESN364), led to a dose-dependent (doses 40-120 mg once daily for 21 days) reduction in LH but not FSH, and reduced endometrial thickness. The dual NK1,3R antagonist elinzanetant (40, 80, and 120 mg once daily) administered orally over a full menstrual cycle safely reduced serum LH in a dose-dependent manner, although in a nonsignificant trend (21). Progesterone levels consistent with ovulation were reduced, especially during the luteal phase of the cycle (21). Moreover, the highest dose of 120 mg of elinzanetant once a day lowered E2 to a level ideal for treating uterine fibroids and endometriosis, and lengthened menstrual cycles from 27 to 34 days (21). Thus, NKB antagonism is a promising treatment option and studies are now required to evaluate their use in women with uterine disorders.
Treating Menopausal Hot Flashes
Menopause is the complete cessation of menstruation due to ovarian insufficiency and occurs between ages 45 and 55 years (328). Hot flashes and sweats, collectively known as VMS, are the most debilitating symptom described by more than 80% of women during the menopausal transition (20). On average, symptoms last for 7 years, but they can persist, with 1 in 10 women experiencing symptoms for up to 12 years (328). Although hormone replacement therapy or menopausal hormone therapy is an effective treatment for VMS, it is contraindicated in women at high risk of breast and endometrial cancer as well as thromboembolic disease (20). Therefore, alternative treatments that can safely and effectively alleviate VMS are desired.
The median preoptic nucleus (MnPO) of the hypothalamus is the control center for body temperature regulation and downstream thermoregulatory pathways (19). This thermoregulatory center is dysregulated during menopause and results in the activation of inappropriate heat dissipation responses including VMS (19). As ARC KNDy neurons project onto both NK3R expressing neurons in the MnPO and GnRH neurons in the median eminence, they have been implicated in the pathogenesis of menopausal VMS (see Fig. 5) (19).
Animal data
E2 deficiency increases LH pulsatility and hot flashes, and this close temporal relationship between temperature and reproduction is mediated by KNDy neuronal activity (19). Indeed, while ovariectomy (E2-deficient state) increased ARC KNDy gene expression and neuronal hypertrophy, E2 supplementation reversed it (24, 329, 330), suggesting that E2 withdrawal leads to increased KNDy expression in rodents. Furthermore, tract tracing studies revealed that KNDy neurons project to the MnPO (the thermoregulatory center) and GnRH axons in the median eminence of the hypothalamus (331). The MnPO, which is altered by E2 and temperature, also expresses NK3R mRNA and protein (332), thus indicating KNDy neurons influence heat dissipation responses through projections to NK3R-expressing neurons in the MnPO. Notably, direct activation of NK3R in the MnPO by an NKB agonist (senktide) reduced core body temperature and activated heat dissipation effectors (tail skin vasodilatation) (333). Likewise, NKB agonist administration increased tail skin vasodilatation in OVX mice, however, this effect was lost following E2 replacement, suggesting that E2 lowers the sensitivity of the thermoregulatory center to NKB/NK3R signaling (334). Furthermore, selective toxin ablation of ARC KNDy neurons reduced both cutaneous vasodilatation and LH secretion in female mice (335), thus supporting the role of KNDy neurons in mediating temperature and reproduction regulation. Additionally, while E2 replacement restored body temperature regulation in OVX rats with intact KNDy neurons, this was not observed in KNDy-ablated OVX rats (335). These studies strongly support NKB and NK3R signaling as important mediators of postmenopausal flushing, and therefore this pathway could be targeted for future therapies.
Human data
KNDy neurons in the infundibular nucleus of the hypothalamus are hypertrophied and overexpressed during E2-deficient states such as menopause (336). Furthermore, genome-wide association studies revealed that menopausal women with VMS had single-nucleotide variations in the TACR3 locus, the gene that encodes NK3R (337). Additionally, NKB has been shown to induce hot flashes in healthy women to a similar degree as those experienced by women in menopause (130). These data indicate that antagonism of NKB/NK3R signaling could provide a novel, nonhormone-based approach for the management of menopausal hot flashes.
The NK3R antagonist, MLE4901 (oral pavinetant), was the first drug to demonstrate a reduction in the number (by 45%) and severity of weekly hot flashes experienced by menopausal women (133) (see Table 2D). Another NK3R antagonist, fezolinetant (ESN364, oral 90 mg twice daily for 12 weeks), reduced VMS scores (fezolinetant: −26.5 vs placebo: −12.2) and improved VMS severity and quality-of-life measures (134). Furthermore, all doses of fezolinetant (30 mg once daily to 90 mg twice daily), except the lowest one, reduced moderate/severe VMS (>2 per day) by 4 and 12 weeks (135). A more recent phase 3 trial involving fezolinetant 30 mg or 45 mg once daily reduced the severity of VMS at week 4 (−0.15 to −0.19) and week 12 (−0.24 to −0.2). Furthermore, the improvements in VMS frequency and severity were sustained over 52 weeks (137). The dual NK1R/NK3R antagonist NT-814 (elinzanetant, dose 150 mg once daily for 2 weeks) also reduced hot flashes (−84%) vs placebo (37%) in menopausal women (136). While NK1R antagonism alone is ineffectual in attenuating VMS, its antiemetic and anxiolytic effects may benefit the poor sleep quality that women experience during menopause (338). Indeed, nocturnal awakening due to night sweats in menopause was reduced following NT-814 (−81%) compared to placebo (32%) (136).
NK3R antagonists display distinct side-effect profiles. For instance, MLE4901 was discontinued following its association with transient increases in liver enzymes. Although ESN364 and NT-814 have been associated with headaches, gastrointestinal disturbance, and fatigue, no clinically significant effects on liver enzymes have been reported. Furthermore, E2 levels (134) and endometrial thickness or hyperplasia (135) remain unaffected, indicating that NK3R action is independent of effects on ovarian hormones (134). These data demonstrate that NK3R antagonists provide a safe and efficacious treatment option for managing menopausal women with VMS.
Conclusion
KP and upstream NKB govern the reproductive endocrine axis through their critical role in regulating GnRH neuronal activity and stimulating GnRH pulsatile secretion. Their fundamental role in reproductive hormone secretion has opened several avenues for their use in diagnosing and treating several pubertal, reproductive, metabolic, bone, and behavioral disorders.
For instance, KP induces lower LH increases in patients with CHH than in those with CDGP or in healthy controls. Additionally, higher circulating KP levels are observed in CPP, thus highlighting KP's utility in diagnosing puberty-related disorders.
KP levels rise linearly with advancing pregnancy, and therefore it could be developed as a promising marker for predicting pregnancy complications. In particular, the reduced KP levels associated with miscarriage and IUGR could enable its use in risk-stratifying women presenting with possible complications during pregnancy.
MAFLD/NASH is associated with upregulated hepatic-KP signaling and increased circulating KP concentrations; therefore, KP measurements could potentially be used to discriminate patients with MAFLD/NASH from healthy controls. Thus, assessing gonadotropin responses to KP or measuring circulating KP levels directly could aid in the diagnosis of common disorders. However, further studies to validate KP's diagnostic accuracy are necessary.
KP-based therapies have been extensively explored over the past decade. In hypogonadal disorders such as HA, hyperprolactinemia, and diabetes-induced hypogonadism, KP induces gonadotropin increases that could restore reproductive function. KP and KP receptor agonists also mirror the physiological ovulatory mid-cycle LH surge and thus could be used therapeutically to induce oocyte maturation during IVF protocols in women seeking fertility. Further studies evaluating KP's safety and efficacy in comparison to current agents, especially in women at high risk of OHSS, are warranted. The intricate connections between KP neurons and hypothalamic neurons involved in appetite regulation have implicated a potential role for KP in obesity-related disorders. Although absence of KP has been associated with increased BW, KP's effects on appetite in animals and humans remain unclear.
KP receptor agonism has also been shown to alleviate hepatic steatosis and fibrosis and thus could play an important role in suppressing the progression of hepatic lipogenesis in patients with MAFLD. With regard to bone metabolism, KP enhances osteoblastogenesis and inhibits osteoclast activity in vitro, and therefore could be used as a complementary treatment for osteoporosis. KP also has potential as a therapy for men and women with psychosexual dysfunction, as it has been shown to enhance sexual brain processing and associated physiological and behavioral measures of sexual function in patients with distressing low sexual desire.
NKB antagonism, in particular potent NK3 receptor antagonists, have emerged as an advantageous therapeutic tool for treating PCOS, uterine fibroids, and endometriosis through their unique ability to partially suppress (and not abolish) the reproductive endocrine axis. Additionally, the critical interaction between NKB and the hypothalamic thermoregulatory center has resulted in the development of NKB antagonists as efficacious nonhormonal treatment options for women with menopausal VMS.
Since the pivotal discoveries of KP and NKB's role in reproduction in 2003 and 2009, respectively, there has been an abundance of basic science and translational studies demonstrating their function in the pathophysiology of several disorders including reproduction, metabolism, bone, and behavior. The wealth of evidence accumulated over the past 2 decades, alongside the development of potent KP and NKB antagonist-based therapies, has provided the opportunity for these peptide hormones to be investigated as promising diagnostic and management tools in the coming years.
Acknowledgments
This article represents independent research funded by the Medical Research Council (MRC), the National Institute for Health Research (NIHR), Imperial Biomedical Research Centre (BRC), and NIHR Clinical Research Facility. The views expressed are those of the authors and not necessarily those of the MRC, the NIHR, or the Department of Health.
Abbreviations
- AgRP
agouti-related peptide
- ALP
alkaline phosphatase
- ARC
arcuate nucleus
- AVPV
anteroventral periventricular area
- BAT
brown adipose tissue
- BMI
body mass index
- BW
body weight
- CDGP
constitutional delay of growth and puberty
- CHH
congenital hypogonadotropic hypogonadism
- CPP
central precocious puberty
- DAG
diacylglycerol
- DHT
dihydrotestosterone
- Dyn
dynorphin
- E2
estradiol
- EP
ectopic pregnancy
- ER-α
estrogen receptor α
- ERE
estrogen response element
- ERK
extracellular signal-related kinase
- FSH
follicle-stimulating hormone
- GA
gestational age
- GABA
γ-aminobutyric acid
- GnRH
gonadotropin-releasing hormone
- GPCR
G protein–coupled receptor
- GRK
GPCR serine/threonine kinases
- GSIS
glucose-stimulated insulin secretion
- HA
hypothalamic amenorrhea
- hCG
human chorionic gonadotropin
- HPG
hypothalamic-pituitary-gonadal
- HSDD
hypoactive sexual desire disorder
- IP3
inositol triphosphate
- IUGR
intrauterine growth restriction
- IVF
in vitro fertilization
- KISS1R
gene encoding for kisspeptin
- KNDy
kisspeptin-neurokinin B-dynorphin
- KO
knockout
- KP
kisspeptin
- LH
luteinizing hormone
- MAFLD/NASH
metabolic fatty liver disease/nonalcoholic steatohepatitis
- MeA
medial amygdala
- MePD
posterodorsal MeA
- MnPO
median preoptic nucleus
- mRNA
messenger RNA
- NK3R
neurokinin 3 receptor
- NKB
neurokinin B
- nNOS
nitric oxide synthase
- NPY
neuropeptide Y
- OHSS
ovarian hyperstimulation syndrome
- OVX
ovariectomized
- PCOS
polycystic ovary syndrome
- PeN
periventricular nucleus
- POA
preoptic area
- PKC
protein kinase C
- PLC
phospholipase C
- POMC
proopiomelanocortin
- PRL
prolactin
- PT
premature thelarche
- RP3V
rostral periventricular area of the third ventricle
- SC
subcutaneous
- TAC3R
gene encoding NKB3 receptor
- TIDA
tuberoinfundibular dopamine
- USV
ultrasonic vocalization
- VMHvL
ventromedial hypothalamus
- VMS
vasomotor symptoms
Contributor Information
Bijal Patel, Section of Investigative Medicine, Department of Metabolism, Digestion and Reproduction, Imperial College School of Medicine, Imperial College London, London, W12 0NN, UK.
Kanyada Koysombat, Section of Investigative Medicine, Department of Metabolism, Digestion and Reproduction, Imperial College School of Medicine, Imperial College London, London, W12 0NN, UK; Department of Diabetes and Endocrinology, Imperial College Healthcare NHS Trust, 72 Du Cane Rd, London, W12 0HS, UK.
Edouard G Mills, Section of Investigative Medicine, Department of Metabolism, Digestion and Reproduction, Imperial College School of Medicine, Imperial College London, London, W12 0NN, UK; Department of Diabetes and Endocrinology, Imperial College Healthcare NHS Trust, 72 Du Cane Rd, London, W12 0HS, UK.
Jovanna Tsoutsouki, Section of Investigative Medicine, Department of Metabolism, Digestion and Reproduction, Imperial College School of Medicine, Imperial College London, London, W12 0NN, UK.
Alexander N Comninos, Section of Investigative Medicine, Department of Metabolism, Digestion and Reproduction, Imperial College School of Medicine, Imperial College London, London, W12 0NN, UK; Department of Diabetes and Endocrinology, Imperial College Healthcare NHS Trust, 72 Du Cane Rd, London, W12 0HS, UK.
Ali Abbara, Section of Investigative Medicine, Department of Metabolism, Digestion and Reproduction, Imperial College School of Medicine, Imperial College London, London, W12 0NN, UK; Department of Diabetes and Endocrinology, Imperial College Healthcare NHS Trust, 72 Du Cane Rd, London, W12 0HS, UK.
Waljit S Dhillo, Section of Investigative Medicine, Department of Metabolism, Digestion and Reproduction, Imperial College School of Medicine, Imperial College London, London, W12 0NN, UK; Department of Diabetes and Endocrinology, Imperial College Healthcare NHS Trust, 72 Du Cane Rd, London, W12 0HS, UK.
Funding
B.P. is supported by an MRC Clinical Research Training Fellowship (MR/W024144/1). K.K. is supported by NIHR Academic Clinical Fellowship Award ACF-2021-21-001. E.G.M. is supported by an NIHR Academic Clinical Lectureship in Endocrinology. J.T. is supported by the NIHR Biomedical Research Centre Funding Scheme. A.N.C. is supported by the NHS. A.A. is supported by an NIHR Clinician Scientist award (CS-2018-18-ST2-002). W.S.D. is funded by an NIHR Research Professorship (NIHR No. RP-2014-05-001). All authors acknowledge infrastructure support for this research from the BRC.
Disclosures
B.P., K.K., E.G.M., J.T., and A.N.C. have nothing to disclose. A.A. and W.S.D. have undertaken consultancy work for Myovant Sciences Ltd. W.S.D. has conducted consultancy work for KaNDy Therapeutics.
References
- 1. De Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100(19):10972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614‐1627. [DOI] [PubMed] [Google Scholar]
- 3. Topaloglu AK, Tello JA, Kotan LD, et al. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med. 2012;366(7):629‐635. [DOI] [PubMed] [Google Scholar]
- 4. Teles MG, Bianco SDC, Brito VN, et al. A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med. 2008;358(7):709‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thomson EL, Patterson M, Murphy KG, et al. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16(10):850‐858. [DOI] [PubMed] [Google Scholar]
- 6. Messager S, Chatzidaki EE, Ma D, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102(5):1761‐1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Tassigny XDA, Jayasena C, Murphy KG, Dhillo WS, Colledge WH. Mechanistic insights into the more potent effect of KP-54 compared to KP-10 in vivo. PLoS One. 2017;13(1):e0192014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Topaloglu AK, Reimann F, Guclu M, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goodman RL, Lehman MN, Smith JT, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148(12):5752‐5760. [DOI] [PubMed] [Google Scholar]
- 10. Lehman MN, Hileman SM, Goodman RL. Neuroanatomy of the kisspeptin signaling system in mammals: comparative and developmental aspects. Adv Exp Med Biol. 2013;784:27‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mostari MP, Ieda N, Deura C, et al. Dynorphin-kappa opioid receptor signaling partly mediates estrogen negative feedback effect on LH pulses in female rats. J Reprod Dev. 2013;59(3):266‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dellovade TL, Merchenthaler I. Estrogen regulation of neurokinin B gene expression in the mouse arcuate nucleus is mediated by estrogen receptor α. Endocrinology. 2004;145(2):736‐742. [DOI] [PubMed] [Google Scholar]
- 13. Dhillo WS, Chaudhri OB, Patterson M, et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90(12):6609‐6615. [DOI] [PubMed] [Google Scholar]
- 14. Dhillo WS, Chaudhri OB, Thompson EL, et al. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab. 2007;92(10):3958‐3966. [DOI] [PubMed] [Google Scholar]
- 15. George JT, Seminara SB. Kisspeptin and the hypothalamic control of reproduction: lessons from the human. Endocrinology. 2012;153(11):5130‐5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Muir AI, Chamberlain L, Elshourbagy NA, et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276(31):28969‐28975. [DOI] [PubMed] [Google Scholar]
- 17. Kotani M, Detheux M, Vandenbogaerde A, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. Journal of Biological Chemistry. 2001;276(37):34631‐34636. [DOI] [PubMed] [Google Scholar]
- 18. Rance NE. Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides (N.Y.). 2009;30(1):111‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34(3):211‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patel B, Dhillo WS. Menopause review: emerging treatments for menopausal symptoms. Best Pract Res Clin Obstet Gynaecol. 2022;81:134‐144. [DOI] [PubMed] [Google Scholar]
- 21. Pawsey S, Mills EG, Ballantyne E, et al. Elinzanetant (NT-814), a neurokinin 1,3 receptor antagonist, reduces estradiol and progesterone in healthy women. J Clin Endocrinol Metab. 2021;106(8):e3221‐e3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee JH, Miele ME, Hicks DJ, et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88(23):1731‐1737. [DOI] [PubMed] [Google Scholar]
- 23. Gottsch ML, Clifton DK, Steiner RA. From KISS1 to kisspeptins: an historical perspective and suggested nomenclature. Peptides. 2009;30(1):4‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92(7):2744‐2750. [DOI] [PubMed] [Google Scholar]
- 25. Hrabovszky E, Ciofi P, Vida B, et al. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neurosci. 2010;31(11):1984‐1998. [DOI] [PubMed] [Google Scholar]
- 26. Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147(12):5817‐5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu S, Zhang H, Tian J, Liu L, Dong Y, Mao T. Expression of kisspeptin/GPR54 and PIBF/PR in the first trimester trophoblast and decidua of women with recurrent spontaneous abortion. Pathol Res Pract. 2014;210(1):47‐54. [DOI] [PubMed] [Google Scholar]
- 28. Bilban M, Ghaffari-Tabrizi N, Hintermann E, et al. Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. J Cell Sci. 2004;117(8):1319‐1328. [DOI] [PubMed] [Google Scholar]
- 29. Ohtaki T, Shintani Y, Honda S, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G protein-coupled receptor. Nature. 2001;411(6837):613‐617. [DOI] [PubMed] [Google Scholar]
- 30. Wang FS, Chen H, Wu ZY, Lin JH. KISS1 expression in osteosarcoma: high in Chinese clinical cases, but lower in cell lines. Asian Pac J Cancer Prev. 2011;12(12):3229‐3234. [PubMed] [Google Scholar]
- 31. West A, Vojta PJ, Welch DR, Weissman BE. Chromosome localization and genomic structure of the KiSS-1 metastasis suppressor gene (KISS1). Genomics. 1998;54(1):145‐148. [DOI] [PubMed] [Google Scholar]
- 32. Jayasena CN, Abbara A, Narayanaswamy S, et al. Direct comparison of the effects of intravenous kisspeptin-10, kisspeptin-54 and GnRH on gonadotrophin secretion in healthy men. Hum Reprod. 2015;30(8):1934‐1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Orsini MJ., Klein MA, Beavers MP, Connolly PJ, Middleton SA, Mayo KH. Metastin (KiSS-1) mimetics identified from peptide structure-activity relationship-derived pharmacophores and directed small molecule database screening. J Med Chem. 2007;50(3):462‐471. [DOI] [PubMed] [Google Scholar]
- 34. Min L, Soltis K, Reis ACS, et al. Dynamic kisspeptin receptor trafficking modulates kisspeptin-mediated calcium signaling. Mol Endocrinol. 2014;28(1):16‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Osuka S, Iwase A, Nakahara T, et al. Kisspeptin in the hypothalamus of 2 rat models of polycystic ovary syndrome. Endocrinology. 2017;158(2):367‐377. [DOI] [PubMed] [Google Scholar]
- 36. Millar RP, Babwah AV. KISS1R: hallmarks of an effective regulator of the neuroendocrine axis. Neuroendocrinology. 2015;101(3):193‐210. [DOI] [PubMed] [Google Scholar]
- 37. Pampillo M, Camuso N, Taylor JE, et al. Regulation of GPR54 signaling by GRK2 and β-arrestin. Mol Endocrinol. 2009;23(12):2060‐2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Szereszewski JM, Pampillo M, Ahow MR, Offermanns S, Bhattacharya M, Babwah AV. GPR54 regulates ERK1/2 activity and hypothalamic gene expression in a Gαq/11 and β-arrestin-dependent manner. PLoS One. 2010;5(9):e12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ahow M, Min L, Pampillo M, et al. KISS1R signals independently of Gαq/11 and triggers LH secretion via the β-arrestin pathway in the male mouse. Endocrinology. 2014;155(11):4433‐4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ke R, Lok SIS, Singh K, Chow BKC, Janovjak H, Lee LTO. Formation of Kiss1R/GPER heterocomplexes negatively regulates Kiss1R-mediated signalling through limiting receptor cell surface expression. J Mol Biol. 2021;433(7):166843. [DOI] [PubMed] [Google Scholar]
- 41. Seminara SB, DiPietro MJ, Ramaswamy S, Crowley WF, Plant TM. Continuous human metastin 45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology. 2006;147(5):2122‐2126. [DOI] [PubMed] [Google Scholar]
- 42. Ramaswamy S, Seminara SB, Pohl CR, DiPietro MJ, Crowley WF, Plant TM. Effect of continuous intravenous administration of human metastin 45–54 on the neuroendocrine activity of the hypothalamic-pituitary-testicular axis in the adult male rhesus monkey (Macaca mulatta). Endocrinology. 2007;148(7):3364‐3370. [DOI] [PubMed] [Google Scholar]
- 43. Jayasena CN, Nijher GMK, Abbara A, et al. Twice-weekly administration of kisspeptin-54 for 8 weeks stimulates release of reproductive hormones in women with hypothalamic amenorrhea. Clin Pharmacol Ther. 2009;88(6):840‐847. [DOI] [PubMed] [Google Scholar]
- 44. Piet R, Kalil B, McLennan T, Porteous R, Czieselsky K, Herbison AE. Dominant neuropeptide cotransmission in kisspeptin-GABA regulation of GnRH neuron firing driving ovulation. J Neurosci. 2018;38(28):6310‐6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Navarro VM. Interactions between kisspeptins and neurokinin B. Adv Exp Med Biol. 2013;784:325‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Almeida TA, Rojo J, Nieto PM, et al. Tachykinins and tachykinin receptors: structure and activity relationships. Curr Med Chem. 2012;11(15):2045‐2081. [DOI] [PubMed] [Google Scholar]
- 47. Bonner TI, Affolter HU, Young AC, Young WS. A cDNA encoding the precursor of the rat neuropeptide, neurokinin B. Mol Brain Res. 1987;2(3):243‐249. [DOI] [PubMed] [Google Scholar]
- 48. Page NM, Morrish DW, Weston-Bell NJ. Differential mRNA splicing and precursor processing of neurokinin B in neuroendocrine tissues. Peptides. 2009;30(8):1508‐1513. [DOI] [PubMed] [Google Scholar]
- 49. Mussap CJ, Geraghty DP, Burcher E. Tachykinin receptors: a radioligand binding perspective. J Neurochem. 1993;60(6):1987‐2009. [DOI] [PubMed] [Google Scholar]
- 50. Seabrook GR, Bowery BJ, Hill RG. Pharmacology of tachykinin receptors on neurones in the ventral tegmental area of rat brain slices. Eur J Pharmacol. 1995;273(1-2):113‐119. [DOI] [PubMed] [Google Scholar]
- 51. Satake H, Kawada T. Overview of the primary structure, tissue-distribution, and functions of tachykinins and their receptors. Curr Drug Targets. 2006;7(8):963‐974. [DOI] [PubMed] [Google Scholar]
- 52. Leffler A, Ahlstedt I, Engberg S, et al. Characterization of species-related differences in the pharmacology of tachykinin NK receptors 1, 2 and 3. Biochem Pharmacol. 2009;77(9):1522‐1530. [DOI] [PubMed] [Google Scholar]
- 53. Gottsch ML, Cunningham MJ, Smith JT, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073‐4077. [DOI] [PubMed] [Google Scholar]
- 54. Kalló I, Vida B, Deli L, et al. Co-localisation of kisspeptin with galanin or neurokinin B in afferents to mouse GnRH neurones. J Neuroendocrinol. 2012;24(3):464‐476. [DOI] [PubMed] [Google Scholar]
- 55. Mikkelsen JD, Simonneaux V. The neuroanatomy of the kisspeptin system in the mammalian brain. Peptides. 2009;30(1):26‐33. [DOI] [PubMed] [Google Scholar]
- 56. Clarkson J, d’Anglemont de Tassigny X, Colledge WH, Caraty A, Herbison AE. Distribution of kisspeptin neurones in the adult female mouse brain. J Neuroendocrinol. 2009;21(8):673‐682. [DOI] [PubMed] [Google Scholar]
- 57. Wang L, Guo W, Shen X, et al. Different dendritic domains of the GnRH neuron underlie the pulse and surge modes of GnRH secretion in female mice. Elife. 2020;9:e53945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Popa SM, Moriyama RM, Caligioni CS, et al. Redundancy in Kiss1 expression safeguards reproduction in the mouse. Endocrinology. 2013;154(8):2784‐2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kauffman AS, Gottsch ML, Roa J, et al. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148(4):1774‐1783. [DOI] [PubMed] [Google Scholar]
- 60. Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology. 2010;151(1):301‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Skorupskaite K, George JT, Anderson RA. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum Reprod Update. 2014;20(4):485‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Herbison AE. The gonadotropin-releasing hormone pulse generator. Endocrinology. 2018;159(11):3723‐3736. [DOI] [PubMed] [Google Scholar]
- 63. Clarkson J, Han SY, Piet R, et al. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci U S A. 2017;114(47):E10216‐E10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Han SK, Gottsch ML, Lee KJ, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25(49):11349‐11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nagae M, Uenoyama Y, Okamoto S, et al. Direct evidence that KNDy neurons maintain gonadotropin pulses and folliculogenesis as the GnRH pulse generator. Proc Natl Acad Sci U S A. 2021;118(5):e2009156118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Han SY, Morris PG, Kim JC, et al. Mechanism of kisspeptin neuron synchronization for pulsatile hormone secretion in male mice. Cell Rep. 2023;42(1):111914. [DOI] [PubMed] [Google Scholar]
- 67. Göcz B, Takács S, Skrapits K, et al. Estrogen differentially regulates transcriptional landscapes of preoptic and arcuate kisspeptin neuron populations. Front Endocrinol (Lausanne). 2022;13:960769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stevenson H, Bartram S, Charalambides MM, et al. Kisspeptin-neuron control of LH pulsatility and ovulation. Front Endocrinol (Lausanne). 2022;13:951938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yaşar P, Ayaz G, User SD, Güpür G, Muyan M. Molecular mechanism of estrogen-estrogen receptor signaling. Reprod Med Biol. 2016;16(1):4‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tomikawa J, Uenoyama Y, Ozawa M, et al. Epigenetic regulation of kiss1 gene expression mediating estrogen-positive feedback action in the mouse brain. Proc Natl Acad Sci U S A. 2012;109(20):E1294‐E1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Adachi S, Yamada S, Takatsu Y, et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53(2):367‐378. [DOI] [PubMed] [Google Scholar]
- 72. Kinoshita M, Tsukamura H, Adachi S, et al. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146(10):4431‐4436. [DOI] [PubMed] [Google Scholar]
- 73. Herbison AE. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat Rev Endocrinol. 2016;12(8):452‐466. [DOI] [PubMed] [Google Scholar]
- 74. Lee EB, Dilower I, Marsh CA, et al. Sexual dimorphism in kisspeptin signaling. Cells. 2022;11(7):1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chan YM, Butler JP, Pinnell NE, et al. Kisspeptin resets the hypothalamic GnRH clock in men. J Clin Endocrinol Metab. 2011;96(6):E908‐E915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. George JT, Veldhuis JD, Roseweir AK, et al. Kisspeptin-10 is a potent stimulator of LH and increases pulse frequency in men. J Clin Endocrinol Metab. 2011;96(8):E1228‐E1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jayasena CN, Nijher GMK, Comninos AN, et al. The effects of kisspeptin-10 on reproductive hormone release show sexual dimorphism in humans. J Clin Endocrinol Metab. 2011;96(12):E1963‐E1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chan YM, Butler JP, Sidhoum VF, Pinnell NE, Seminara SB. Kisspeptin administration to women: a window into endogenous kisspeptin secretion and GnRH responsiveness across the menstrual cycle. J Clin Endocrinol Metab. 2012;97(8):E1458‐E1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. George JT, Anderson RA, Millar RP. Kisspeptin-10 stimulation of gonadotrophin secretion in women is modulated by sex steroid feedback. Hum Reprod. 2012;27(12):3552‐3559. [DOI] [PubMed] [Google Scholar]
- 80. Jayasena CN, Comninos AN, Veldhuis JD, et al. A single injection of kisspeptin-54 temporarily increases luteinizing hormone pulsatility in healthy women. Clin Endocrinol (Oxf). 2013;79(4):558‐563. [DOI] [PubMed] [Google Scholar]
- 81. Jayasena CN, Comninos AN, Nijher GMK, et al. Twice-daily subcutaneous injection of kisspeptin-54 does not abolish menstrual cyclicity in healthy female volunteers. J Clin Endocrinol Metab. 2013;98(11):4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Narayanaswamy S, Jayasena CN, Ng N, et al. Subcutaneous infusion of kisspeptin-54 stimulates gonadotrophin release in women and the response correlates with basal oestradiol levels. Clin Endocrinol (Oxf). 2016;84(6):939‐945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Abbara A, Eng PC, Phylactou M, et al. Kisspeptin receptor agonist has therapeutic potential for female reproductive disorders. J Clin Invest. 2020;130(12):6739‐6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chan YM, Lippincott MF, Butler JP, et al. Exogenous kisspeptin administration as a probe of GnRH neuronal function in patients with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2014;99(12):E2762‐E2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lippincott MF, Chan YM, Delaney A, Rivera-Morales D, Butler JP, Seminara SB. Kisspeptin responsiveness signals emergence of reproductive endocrine activity: implications for human puberty. J Clin Endocrinol Metab. 2016;101(8):3061‐3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chan YM, Lippincott MF, Sales BP, et al. Using kisspeptin to predict pubertal outcomes for youth with pubertal delay. J Clin Endocrinol Metab. 2020;105(8):e2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Abbara A, Eng PC, Phylactou M, et al. Kisspeptin-54 accurately identifies hypothalamic gonadotropin-releasing hormone neuronal dysfunction in men with congenital hypogonadotropic hypogonadism. Neuroendocrinology. 2021;111(12):1176‐1186. [DOI] [PubMed] [Google Scholar]
- 88. Cintra RG, Wajnsztejn R, Trevisan CM, et al. Kisspeptin levels in girls with precocious puberty: a systematic review and meta-analysis. Horm Res Paediatr. 2020;93(11-12):589‐598. [DOI] [PubMed] [Google Scholar]
- 89. Vuralli D, Ciftci N, Demirbilek H. Serum kisspeptin, neurokinin B and inhibin B levels can be used as alternative parameters to distinguish idiopathic CPP from premature thelarche in the early stages of puberty. Clin Endocrinol (Oxf). 2023;98:788‐795. [DOI] [PubMed] [Google Scholar]
- 90. Podfigurna A, Maciejewska-Jeske M, Meczekalski B, Genazzani AD. Kisspeptin and LH pulsatility in patients with functional hypothalamic amenorrhea. Endocrine. 2020;70(3):635‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hofmann T, Elbelt U, Haas V, et al. Plasma kisspeptin and ghrelin levels are independently correlated with physical activity in patients with anorexia nervosa. Appetite. 2017;108:141‐150. [DOI] [PubMed] [Google Scholar]
- 92. Jayasena CN, Abbara A, Veldhuis JD, et al. Increasing LH pulsatility in women with hypothalamic amenorrhoea using intravenous infusion of kisspeptin-54. J Clin Endocrinol Metab. 2014;99(6):953‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tang R, Ding X, Zhu J. Kisspeptin and polycystic ovary syndrome. Front Endocrinol (Lausanne). 2019;10:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Varikasuvu SR, Prasad VS, Vamshika VC, Satyanarayana MV, Panga JR. Circulatory metastin/kisspeptin-1 in polycystic ovary syndrome: a systematic review and meta-analysis with diagnostic test accuracy. Reprod Biomed Online. 2019;39(4):685‐697. [DOI] [PubMed] [Google Scholar]
- 95. Ibrahim RO, Omer SH, Fattah CN. The correlation between hormonal disturbance in PCOS women and serum level of kisspeptin. Int J Endocrinol. 2020;2020:6237141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Akad M, Socolov R, Furnică C, et al. Kisspeptin variations in patients with polycystic ovary syndrome—a prospective case control study. Medicina (Kaunas). 2022;58(6):776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Romero-Ruiz A, Skorupskaite K, Gaytan F, et al. Kisspeptin treatment induces gonadotropic responses and rescues ovulation in a subset of preclinical models and women with polycystic ovary syndrome. Hum Reprod. 2019;34(12):2495‐2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Skorupskaite K, George JT, Veldhuis JD, Millar RP, Anderson RA. Kisspeptin and neurokinin B interactions in modulating gonadotropin secretion in women with polycystic ovary syndrome. Hum Reprod. 2020;35(6):1421‐1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Millar RP, Sonigo C, Anderson RA, et al. Hypothalamic-pituitary-ovarian axis reactivation by kisspeptin-10 in hyperprolactinemic women with chronic amenorrhea. J Endocr Soc. 2017;1(11):1362‐1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hoskova K, Kayton BN, Chen ME, et al. Kisspeptin overcomes GnRH neuronal suppression secondary to hyperprolactinemia in humans. J Clin Endocrinol Metab. 2022;107(8):e3515‐e3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Jayasena CN, Abbara A, Comninos AN, et al. Kisspeptin-54 triggers egg maturation in women undergoing in vitro fertilization. J Clin Invest. 2014;124(8):3667‐3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Abbara A, Jayasena CN, Christopoulos G, et al. Efficacy of kisspeptin-54 to trigger oocyte maturation in women at high risk of ovarian hyperstimulation syndrome (OHSS) during in vitro fertilization (IVF) therapy. J Clin Endocrinol Metab. 2015;100(9):3322‐3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Abbara A, Clarke S, Islam R, et al. A second dose of kisspeptin-54 improves oocyte maturation in women at high risk of ovarian hyperstimulation syndrome: a phase 2 randomized controlled trial. Hum Reprod. 2017;32(9):1915‐1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Abbara A, Al-Memar M, Phylactou M, et al. Performance of plasma kisspeptin as a biomarker for miscarriage improves with gestational age during the first trimester. Fertil Steril. 2021;116(3):809‐819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Silva PHAD, Romão LGM, Freitas NPA, et al. Kisspeptin as a predictor of miscarriage: a systematic review. J Matern Fetal Neonatal Med. 2023;36(1):2197097. [DOI] [PubMed] [Google Scholar]
- 106. Pérez-López FR., López-Baena MT, Varikasuvu SR, Ruiz-Román R, Fuentes-Carrasco M, Savirón-Cornudella R. Preeclampsia and gestational hypertension are associated to low maternal circulating kisspeptin levels: a systematic review and meta-analysis. Gynecol Endocrinol. 2021;37(12):1055‐1062. [DOI] [PubMed] [Google Scholar]
- 107. Abbara A, Al-Memar M, Phylactou M, et al. Changes in circulating kisspeptin levels during each trimester in women with antenatal complications. J Clin Endocrinol Metab. 2022;107(1):e71‐e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ćetković A, Miljic D, Ljubić A, et al. Plasma kisspeptin levels in pregnancies with diabetes and hypertensive disease as a potential marker of placental dysfunction and adverse perinatal outcome. Endocr Res. 2012;37(2):78‐88. [DOI] [PubMed] [Google Scholar]
- 109. Bowe JE, Hill TG, Hunt KF, et al. A role for placental kisspeptin in β cell adaptation to pregnancy. JCI Insight. 2019;4(20):e124540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Arslan E, Gorkem U, Togrul C. Is there an association between kisspeptin levels and gestational diabetes mellitus? Gynecol Obstet Reprod Med. 2020;26(3):179‐183. [Google Scholar]
- 111. Torricelli M, Galleri L, Voltolini C, et al. Changes of placental kiss-1 mRNA expression and maternal/cord kisspeptin levels at preterm delivery. Reprod Sci. 2008;15(8):779‐784. [DOI] [PubMed] [Google Scholar]
- 112. Smets EML, Deurloo KL, Go ATJI, van Vugt JMG, Blankenstein MA, Oudejans CBM. Decreased plasma levels of metastin in early pregnancy are associated with small for gestational age neonates. Prenat Diagn. 2008;28(4):299‐303. [DOI] [PubMed] [Google Scholar]
- 113. Armstrong AR, Reynolds RM, Leask R, Shearing CH, Calder AA, Riley SC. Decreased serum levels of kisspeptin in early pregnancy are associated with intra-uterine growth restriction and pre-eclampsia. Prenat Diagn. 2009;29(10):982‐985. [DOI] [PubMed] [Google Scholar]
- 114. Khalil SS, Abulfadle KA, Elnagar WM. Serum kisspeptin-10 levels in pregnant women complicated with intrauterine growth restriction with or without preeclampsia. Med J Cairo Univ. 2018;86(6):1975‐1982. [Google Scholar]
- 115. Izzi-Engbeaya C, Comninos AN, Clarke SA, et al. The effects of kisspeptin on β-cell function, serum metabolites and appetite in humans. Diabetes Obes Metab 2018;20(12):2800‐2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Izzi-Engbeaya C, Choudhury MM, Patel B, et al. The effects of kisspeptin on food intake in women with overweight or obesity. Diabetes Obes Metab. 2023;25(8):2393‐2397. [DOI] [PubMed] [Google Scholar]
- 117. Yang L, Demetriou L, Wall MB, et al. The effects of kisspeptin on brain response to food images and psychometric parameters of appetite in healthy men. J Clin Endocrinol Metab. 2021;106(4):1837‐1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Guzman S, Dragan M, Kwon H, et al. Targeting hepatic kisspeptin receptor ameliorates nonalcoholic fatty liver disease in a mouse model. J Clin Invest. 2022;132(10):e145889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Comninos AN, Hansen MS, Courtney A, et al. Acute effects of kisspeptin administration on bone metabolism in healthy men. J Clin Endocrinol Metab. 2022;107:1529‐1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Comninos AN, Wall MB, Demetriou L, et al. Kisspeptin modulates sexual and emotional brain processing in humans. J Clin Invest. 2017;127(2):709‐719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Comninos AN, Demetriou L, Wall MB, et al. Modulations of human resting brain connectivity by kisspeptin enhance sexual and emotional functions. JCI Insight. 2018;3(20):e121958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Yang L, Demetriou L, Wall MB, et al. Kisspeptin enhances brain responses to olfactory and visual cues of attraction in men. JCI Insight. 2020;5(3):e133633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Comninos AN, Yang L, O’Callaghan J, et al. Kisspeptin modulates gamma-aminobutyric acid levels in the human brain. Psychoneuroendocrinology. 2021;129:105244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Thurston L, Hunjan T, Ertl N, et al. Effects of kisspeptin administration in women with hypoactive sexual desire disorder: a randomized clinical trial. JAMA Netw Open. 2022;5(10):e2236131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Mills EG, Ertl N, Wall MB, et al. Effects of kisspeptin on sexual brain processing and penile tumescence in men with hypoactive sexual desire disorder: a randomized clinical trial. JAMA Netw Open. 2023;6(2):e2254313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Tovar S, Vázquez MJ, Navarro V, et al. Effects of single or repeated intravenous administration of kisspeptin upon dynamic LH secretion in conscious male rats. Endocrinology. 2006;147(6):2696‐2704. [DOI] [PubMed] [Google Scholar]
- 127. Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151(9):4494‐4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Jayasena CN, Comninos AN, De Silva A, et al. Effects of neurokinin B administration on reproductive hormone secretion in healthy men and women. J Clin Endocrinol Metab. 2014;99(1):E19‐E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Narayanaswamy S, Prague JK, Jayasena CN, et al. Investigating the KNDy hypothesis in humans by coadministration of kisspeptin, neurokinin B, and naltrexone in men. J Clin Endocrinol Metab. 2016;101(9):3429‐3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Jayasena CN, Comninos AN, Stefanopoulou E, et al. Neurokinin B administration induces hot flushes in women. Sci Rep. 2015;5:8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. George JT, Kakkar R, Marshall J, et al. Neurokinin B receptor antagonism in women with polycystic ovary syndrome: a randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2016;101(11):4313‐4321. [DOI] [PubMed] [Google Scholar]
- 132. Fraser GL, Obermayer-Pietsch B, Laven J, et al. Randomized controlled trial of neurokinin 3 receptor antagonist fezolinetant for treatment of polycystic ovary syndrome. J Clin Endocrinol Metab. 2021;106(9):e3519‐e3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Prague JK, Roberts RE, Comninos AN, et al. Neurokinin 3 receptor antagonism as a novel treatment for menopausal hot flushes: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389(10081):1809‐1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Depypere H, Timmerman D, Donders G, et al. Treatment of menopausal vasomotor symptoms with fezolinetant, a neurokinin 3 receptor antagonist: a phase 2a trial. J Clin Endocrinol Metab. 2019;104(12):5893‐5905. [DOI] [PubMed] [Google Scholar]
- 135. Fraser GL, Lederman S, Waldbaum A, et al. A phase 2b, randomized, placebo-controlled, double-blind, dose-ranging study of the neurokinin 3 receptor antagonist fezolinetant for vasomotor symptoms associated with menopause. Menopause. 2020;27(4):382‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Trower M, Anderson RA, Ballantyne E, Joffe H, Kerr M, Pawsey S. Effects of NT-814, a dual neurokinin 1 and 3 receptor antagonist, on vasomotor symptoms in postmenopausal women: a placebo-controlled, randomized trial. Menopause. 2020;27(5):498‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401(10382):1091‐1102. [DOI] [PubMed] [Google Scholar]
- 138. Zhang XW, Li L, Hu WQ, et al. Neurokinin-1 receptor promotes non-small cell lung cancer progression through transactivation of EGFR. Cell Death Dis. 2022;13(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Young J, Xu C, Papadakis GE, et al. Clinical management of congenital hypogonadotropic hypogonadism. Endocr Rev. 2019;40(2):669‐710. [DOI] [PubMed] [Google Scholar]
- 140. Palmert MR, Dunkel L. Delayed puberty. N Engl J Med. 2012;366(5):443‐453. [DOI] [PubMed] [Google Scholar]
- 141. Harrington J, Palmert MR. An approach to the patient with delayed puberty. J Clin Endocrinol Metab. 2022;107(6):1739‐1750. [DOI] [PubMed] [Google Scholar]
- 142. Boehm U, Bouloux PM, Dattani MT, et al. Expert consensus document: European consensus statement on congenital hypogonadotropic hypogonadism-pathogenesis, diagnosis and treatment. Nat Rev Endocrinol. 2015;11(9):547‐564. [DOI] [PubMed] [Google Scholar]
- 143. Harrington J, Palmert MR. Distinguishing constitutional delay of growth and puberty from isolated hypogonadotropic hypogonadism: critical appraisal of available diagnostic tests. J Clin Endocrinol Metab. 2012;97(9):3056‐3067. [DOI] [PubMed] [Google Scholar]
- 144. Funes S, Hedrick JA, Vassileva G, et al. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312(4):1357‐1363. [DOI] [PubMed] [Google Scholar]
- 145. Novaira HJ, Sonko ML, Hoffman G, et al. Disrupted kisspeptin signaling in GnRH neurons leads to hypogonadotrophic hypogonadism. Mol Endocrinol. 2014;28(2):225‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Pineda R, Garcia-Galiano D, Roseweir A, et al. Critical roles of kisspeptins in female puberty and preovulatory gonadotropin surges as revealed by a novel antagonist. Endocrinology. 2010;151(2):722‐730. [DOI] [PubMed] [Google Scholar]
- 147. De Tassigny XDA, Fagg LA, Dixon JPC, et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci U S A. 2007;104(25):10714‐10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Lapatto R, Pallais JC, Zhang D, et al. Kiss1–/– mice exhibit more variable hypogonadism than Gpr54–/– mice. Endocrinology. 2007;148(10):4927‐4936. [DOI] [PubMed] [Google Scholar]
- 149. Mayer C, Boehm U. Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat Neurosci. 2011;14(6):704‐710. [DOI] [PubMed] [Google Scholar]
- 150. Tenenbaum-Rakover Y, Commenges-Ducos M, Iovane A, Aumas C, Admoni O, de Roux N. Neuroendocrine phenotype analysis in five patients with isolated hypogonadotropic hypogonadism due to a L102P inactivating mutation of GPR54. J Clin Endocrinol Metab. 2007;92(3):1137‐1144. [DOI] [PubMed] [Google Scholar]
- 151. Fuqua JS. Treatment and outcomes of precocious puberty: an update. J Clin Endocrinol Metab. 2013;98(6):2198‐2207. [DOI] [PubMed] [Google Scholar]
- 152. Maione L, Bouvattier C, Kaiser UB. Central precocious puberty: recent advances in understanding the aetiology and in the clinical approach. Clin Endocrinol (Oxf). 2021;95(4):542‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Navarro VM, Castellano JM, Fernández-Fernández R, et al. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145(10):4565‐4574. [DOI] [PubMed] [Google Scholar]
- 154. Navarro VM, Fernández-Fernández R, Castellano JM, et al. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004;561(2):379‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A. 2005;102(6):2129‐2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Bianco SDC, Vandepas L, Correa-Medina M, et al. KISS1R intracellular trafficking and degradation: effect of the Arg386Pro disease-associated mutation. Endocrinology. 2011;152(4):1616‐1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Silveira LG, Noel SD, Silveira-Neto AP, et al. Mutations of the KISS1 gene in disorders of puberty. J Clin Endocrinol Metab. 2010;95(5):2276‐2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. de Vries L, Shtaif B, Phillip M, Gat-Yablonski G. Kisspeptin serum levels in girls with central precocious puberty. Clin Endocrinol (Oxf). 2009;71(4):524‐528. [DOI] [PubMed] [Google Scholar]
- 159. Gordon CM, Ackerman KE, Berga SL, et al. Functional hypothalamic amenorrhea: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(5):1413‐1439. [DOI] [PubMed] [Google Scholar]
- 160. Phylactou M, Clarke SA, Patel B, et al. Clinical and biochemical discriminants between functional hypothalamic amenorrhoea (FHA) and polycystic ovary syndrome (PCOS). Clin Endocrinol (Oxf). 2021;95(2):239‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Dudek M, Ziarniak K, Sliwowska JH. Kisspeptin and metabolism: the brain and beyond. Front Endocrinol (Lausanne). 2018;9:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Wolfe A, Hussain MA. The emerging role(s) for kisspeptin in metabolism in mammals. Front Endocrinol (Lausanne). 2018;9:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Backholer K, Smith JT, Rao A, et al. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology. 2010;151(5):2233‐2243. [DOI] [PubMed] [Google Scholar]
- 164. Merkley CM, Renwick AN, Shuping SL, Harlow K, Sommer JR, Nestor CC. Undernutrition reduces kisspeptin and neurokinin B expression in castrated male sheep. Reprod Fertil. 2020;1(1):21‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Clarke IJ, Reed CB, Burke CR, Li Q, Meier S. Kiss1 expression in the hypothalamic arcuate nucleus is lower in dairy cows of reduced fertility. Biol Reprod. 2022;106(4):802‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Iwasa T, Matsuzaki T, Murakami M, et al. Decreased expression of kisspeptin mediates acute immune/inflammatory stress-induced suppression of gonadotropin secretion in female rat. J Endocrinol Invest. 2008;31(7):656‐659. [DOI] [PubMed] [Google Scholar]
- 167. Podfigurna A, Szeliga A, Meczekalski B. Serum kisspeptin and corticotropin-releasing hormone levels in patients with functional hypothalamic amenorrhea. GREM Gynecological and Reproductive Endocrinology & Metabolism. 2020:37‐42. doi: 10.53260/grem.201017 [DOI] [Google Scholar]
- 168. Castellano JM, et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146(9):3917‐3925. [DOI] [PubMed] [Google Scholar]
- 169. Balen A. The pathophysiology of polycystic ovary syndrome: trying to understand PCOS and its endocrinology. Best Pract Res Clin Obstet Gynaecol. 2004;18(5):685‐706. [DOI] [PubMed] [Google Scholar]
- 170. Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33(9):1602‐1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Xita N, Tsatsoulis A. Review: fetal programming of polycystic ovary syndrome by androgen excess: evidence from experimental, clinical, and genetic association studies. J Clin Endocrinol Metab. 2006;91(5):1660‐1666. [DOI] [PubMed] [Google Scholar]
- 172. Garg A, Patel B, Abbara A, Dhillo WS. Treatments targeting neuroendocrine dysfunction in polycystic ovary syndrome (PCOS). Clin Endocrinol (Oxf). 2022;97(2):156‐164. [DOI] [PubMed] [Google Scholar]
- 173. McCartney CR, Campbell RE. Abnormal GnRH pulsatility in polycystic ovary syndrome: recent insights. Curr Opin Endocr Metab Res. 2020;12:78‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Moore AM, Lohr DB, Coolen LM, Lehman MN. Prenatal androgen exposure alters KNDy neurons and their afferent network in a model of polycystic ovarian syndrome. Endocrinology. 2021;162(11):bqab158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Aliabadi E, Namavar MR, Mortezaee K, et al. Kisspeptin expression features in the arcuate and anteroventral periventricular nuclei of hypothalamus of letrozole-induced polycystic ovarian syndrome in rats. Arch Gynecol Obstet. 2017;296(5):957‐963. [DOI] [PubMed] [Google Scholar]
- 176. Ahmed MI, Duleba AJ, El Shahat O, Ibrahim ME, Salem A. Naltrexone treatment in clomiphene resistant women with polycystic ovary syndrome. Hum Reprod. 2008;23(11):2564‐2569. [DOI] [PubMed] [Google Scholar]
- 177. Katulski K, Podfigurna A, Czyzyk A, Meczekalski B, Genazzani AD. Kisspeptin and LH pulsatile temporal coupling in PCOS patients. Endocrine. 2018;61(1):149‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Morales AJ, Laughlin GA, Bützow T, Maheshwari H, Baumann G, Yen SS. Insulin, somatotropic, and luteinizing hormone axes in lean and obese women with polycystic ovary syndrome: common and distinct features. J Clin Endocrinol Metab. 1996;81(8):2854‐2864. [DOI] [PubMed] [Google Scholar]
- 179. Soto-Pedre E, Newey PJ, Bevan JS, Leese GP. Morbidity and mortality in patients with hyperprolactinaemia: the PROLEARS study. Endocr Connect. 2017;6(8):580‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Melmed S, Casanueva FF, Hoffman AR, et al. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(2):273‐288. [DOI] [PubMed] [Google Scholar]
- 181. Maiter D. Management of dopamine agonist-resistant prolactinoma. Neuroendocrinology. 2019;109(1):42‐50. [DOI] [PubMed] [Google Scholar]
- 182. Kokay IC, Petersen SL, Grattan DR. Identification of prolactin-sensitive GABA and kisspeptin neurons in regions of the rat hypothalamus involved in the control of fertility. Endocrinology. 2011;152(2):526‐535. [DOI] [PubMed] [Google Scholar]
- 183. Sonigo C, Bouilly J, Carré N, et al. Hyperprolactinemia-induced ovarian acyclicity is reversed by kisspeptin administration. J Clin Invest. 2012;122(10):3791‐3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Araujo-Lopes R, Crampton JR, Aquino NSS, et al. Prolactin regulates kisspeptin neurons in the arcuate nucleus to suppress LH secretion in female rats. Endocrinology. 2014;155(3):1010‐1020. [DOI] [PubMed] [Google Scholar]
- 185. Brown RSE, Khant AZ, Phillipps HR, et al. Acute suppression of LH secretion by prolactin in female mice is mediated by kisspeptin neurons in the arcuate nucleus. Endocrinology. 2019;160(5):1323‐1332. [DOI] [PubMed] [Google Scholar]
- 186. Fitzsimmons MD, Olschowka JA, Wiegand SJ, Hoffman GE. Interaction of opioid peptide-containing terminals with dopaminergic perikarya in the rat hypothalamus. Brain Res. 1992;581(1):10‐18. [DOI] [PubMed] [Google Scholar]
- 187. Szawka RE, Ribeiro AB, Leite CM, et al. Kisspeptin regulates prolactin release through hypothalamic dopaminergic neurons. J Clin Endocrinol Metab. 2010;95(6):3073‐3073. [DOI] [PubMed] [Google Scholar]
- 188. Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22(6):1506‐1512. [DOI] [PubMed] [Google Scholar]
- 189. Fauser BC. Towards the global coverage of a unified registry of IVF outcomes. Reprod Biomed Online. 2019;38(2):133‐137. [DOI] [PubMed] [Google Scholar]
- 190. Abbara A, Islam R, Clarke SA, et al. Clinical parameters of ovarian hyperstimulation syndrome following different hormonal triggers of oocyte maturation in IVF treatment. Clin Endocrinol (Oxf). 2018;88(6):920‐927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Nel-Themaat L, Nagy ZP. A review of the promises and pitfalls of oocyte and embryo metabolomics. Placenta. 2011;32(Suppl 3):S257‐S263. [DOI] [PubMed] [Google Scholar]
- 192. Damewood MD, Shen W, Zacur HA, Schlaff WD, Rock JA, Wallach EE. Disappearance of exogenously administered human chorionic gonadotropin. Fertil Steril. 1989;52(3):398‐400. [DOI] [PubMed] [Google Scholar]
- 193. Delvinge A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Hum Reprod Update. 2002;8(6):559‐577. [DOI] [PubMed] [Google Scholar]
- 194. Humaidan P, Nelson SM, Devroey P, et al. Ovarian hyperstimulation syndrome: review and new classification criteria for reporting in clinical trials. Hum Reprod. 2016;31(9):1997‐2004. [DOI] [PubMed] [Google Scholar]
- 195. Clarkson J, d’Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28(35):8691‐8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;320(2):383‐388. [DOI] [PubMed] [Google Scholar]
- 197. Caraty A, Smith JT, Lomet D, et al. Kisspeptin synchronizes preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology. 2007;148(11):5258‐5267. [DOI] [PubMed] [Google Scholar]
- 198. Cohlen BJ, te Velde ER, Scheffer G, van Kooij RJ, de Brouwer CPM, van Zonneveld P. The pattern of the luteinizing hormone surge in spontaneous cycles is related to the probability of conception. Fertil Steril. 1993;60(3):413‐417. [DOI] [PubMed] [Google Scholar]
- 199. Abbara A, Hunjan T, Ho VNA, et al. Endocrine requirements for oocyte maturation following hCG, GnRH agonist, and kisspeptin during IVF treatment. Front Endocrinol (Lausanne). 2020;11:537205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Hiden U, Bilban M, Knöfler M, Desoye G. Kisspeptins and the placenta: regulation of trophoblast invasion. Rev Endocr Metab Disord. 2007;8(1):31‐39. [DOI] [PubMed] [Google Scholar]
- 201. Hu KL, Chang H-M, Zhao H-C, Yu Y, Li R, Qiao J. Potential roles for the kisspeptin/kisspeptin receptor system in implantation and placentation. Hum Reprod Update. 2019;25(3):326‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Quenby S, Gallos ID, Dhillon-Smith RK, et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. 2021;397(10285):1658‐1667. [DOI] [PubMed] [Google Scholar]
- 203. Jayasena CN, Abbara A, Izzi-Engbeaya C, et al. Reduced levels of plasma kisspeptin during the antenatal booking visit are associated with increased risk of miscarriage. J Clin Endocrinol Metab. 2014;99(12):E2652‐E2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Mumtaz A, Khalid A, Jamil Z, Fatima SS, Arif S, Rehman R. Kisspeptin: a potential factor for unexplained infertility and impaired embryo implantation. Int J Fertil Steril. 2017;11(2):99‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Sullivan-Pyke C, Haisenleder DJ, Senapati S, et al. Kisspeptin as a new serum biomarker to discriminate miscarriage from viable intrauterine pregnancy. Fertil Steril. 2018;109(1)137‐141.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Yuksel S, Ketenci Gencer F. Serum kisspeptin, to discriminate between ectopic pregnancy, miscarriage and first trimester pregnancy. J Obstet Gynaecol. 2022;42(6):2095‐2099. [DOI] [PubMed] [Google Scholar]
- 207. Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 208. Matjila M, Millar R, Van Der Spuy Z, Katz A. Elevated placental expression at the maternal-fetal interface but diminished maternal circulatory kisspeptin in preeclamptic pregnancies. Pregnancy Hypertens. 2016;6(1):79‐87. [DOI] [PubMed] [Google Scholar]
- 209. Kucur M, Madazli R, Bulut B, Tuten A, Aydin B. First-trimester maternal serum metastin, placental growth factor and chitotriosidase levels in pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 2012;164(2):146‐149. [DOI] [PubMed] [Google Scholar]
- 210. Logie JJ, Denison FC, Riley SC, et al. Evaluation of kisspeptin levels in obese pregnancy as a biomarker for pre-eclampsia. Clin Endocrinol (Oxf). 2012;76(6):887‐893. [DOI] [PubMed] [Google Scholar]
- 211. Adali E, Kurdoglu Z, Kurdoglu M, Kamaci M, Kolusari A, Yildizhan R. Metastin levels in pregnancies complicated by pre-eclampsia and their relation with disease severity. J Matern Fetal Neonatal Med. 2012;25(12):2671‐2675. [DOI] [PubMed] [Google Scholar]
- 212. Ziyaraa MA, Hamdan FB, Mousa LR. Correlation of kisspeptin-10 level and fetal well-being in preeclamptic patients. Taiwan J Obstet Gynecol. 2016;55(6):840‐846. [DOI] [PubMed] [Google Scholar]
- 213. Petrini A, Spandorfer S. Recurrent ectopic pregnancy: current perspectives. Int J Womens Health. 2020;12:597‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Romero-Ruiz A, Avendaño MS, Dominguez F, et al. Deregulation of miR-324/KISS1/kisspeptin in early ectopic pregnancy: mechanistic findings with clinical and diagnostic implications. Am J Obstet Gynecol. 2019;220(5):480.e1-480.e17. [DOI] [PubMed] [Google Scholar]
- 215. Unterscheider J, Daly S, Geary MP, et al. Optimizing the definition of intrauterine growth restriction: the multicenter prospective PORTO study. Am J Obstet Gynecol. 2013;208(4):290.e1-290.e6. [DOI] [PubMed] [Google Scholar]
- 216. Vogel JP, Chawanpaiboon S, Moller AB, Watananirun K, Bonet M, Lumbiganon P. The global epidemiology of preterm birth. Best Pract Res Clin Obstet Gynaecol. 2018;52:3‐12. [DOI] [PubMed] [Google Scholar]
- 217. Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract. 2014;103(2):176‐185. [DOI] [PubMed] [Google Scholar]
- 218. Ryan EA, Enns L. Role of gestational hormones in the induction of insulin resistance. J Clin Endocrinol Metab. 1988;67(2):341‐347. [DOI] [PubMed] [Google Scholar]
- 219. Buchanan TA. Pancreatic B-cell defects in gestational diabetes: implications for the pathogenesis and prevention of type 2 diabetes. J Clin Endocrinol Metab. 2001;86(3):989‐993. [DOI] [PubMed] [Google Scholar]
- 220. Hauge-Evans AC, Richardson CC, Milne HM, Christie MR, Persaud SJ, Jones PM. A role for kisspeptin in islet function. Diabetologia. 2006;49(9):2131‐2135. [DOI] [PubMed] [Google Scholar]
- 221. Schwetz TA, Reissaus CA, Piston DW. Differential stimulation of insulin secretion by GLP-1 and kisspeptin-10. PLoS One. 2014;9(11):113020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Bowe JE, King AJ, Kinsey-Jones JS, et al. Kisspeptin stimulation of insulin secretion: mechanisms of action in mouse islets and rats. Diabetologia. 2009;52(5):855‐862. [DOI] [PubMed] [Google Scholar]
- 223. Bowe JE, Foot VL, Amiel SA, et al. GPR54 peptide agonists stimulate insulin secretion from murine, porcine and human islets. Islets. 2012;4(1):20‐23. [DOI] [PubMed] [Google Scholar]
- 224. Röder PV, Wu B, Liu Y, Han W. Pancreatic regulation of glucose homeostasis. Exp Mol Med. 2016;48(3):e219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Brown RE, Imran SA, Ur E, Wilkinson M. KiSS-1 mRNA in adipose tissue is regulated by sex hormones and food intake. Mol Cell Endocrinol. 2008;281(1-2):64‐72. [DOI] [PubMed] [Google Scholar]
- 226. Vikman J, Ahrén B. Inhibitory effect of kisspeptins on insulin secretion from isolated mouse islets. Diabetes Obes Metab. 2009;11(Suppl 4):197‐201. [DOI] [PubMed] [Google Scholar]
- 227. Song WJ, Mondal P, Wolfe A, et al. Glucagon regulates hepatic kisspeptin to impair insulin secretion. Cell Metab. 2014;19(4):667‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Silvestre RA, Egido EM, Hernández R, Marco J. Kisspeptin-13 inhibits insulin secretion without affecting glucagon or somatostatin release: study in the perfused rat pancreas. J Endocrinol. 2008;196(2):283‐290. [DOI] [PubMed] [Google Scholar]
- 229. Tolson KP, Garcia C, Yen S, et al. Impaired kisspeptin signaling decreases metabolism and promotes glucose intolerance and obesity. J Clin Invest. 2014;124(7):3075‐3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Velasco I, León S, Barroso A, et al. Gonadal hormone-dependent vs. -independent effects of kisspeptin signaling in the control of body weight and metabolic homeostasis. Metab Clin Exp. 2019;98:84‐94. [DOI] [PubMed] [Google Scholar]
- 231. Tolson KP, Marooki N, Wolfe A, Smith JT, Kauffman AS. Cre/lox generation of a novel whole-body Kiss1r KO mouse line recapitulates a hypogonadal, obese, and metabolically-impaired phenotype HHS public access. Mol Cell Endocrinol. 2019;498:110559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Dong TS, Vu JP, Oh S, et al. Intraperitoneal treatment of kisspeptin suppresses appetite and energy expenditure and alters gastrointestinal hormones in mice. Dig Dis Sci. 2020;65(8):2254‐2263. [DOI] [PubMed] [Google Scholar]
- 233. Wahab F, Riaz T, Shahab M. Study on the effect of peripheral kisspeptin administration on basal and glucose-induced insulin secretion under fed and fasting conditions in the adult male rhesus monkey (Macaca mulatta). Horm Metab Res. 2011;43(1):37‐42. [DOI] [PubMed] [Google Scholar]
- 234. Izzi-Engbeaya C, Dhillo WS. Emerging roles for kisspeptin in metabolism. J Physiol. 2022;600(5):1079‐1088. [DOI] [PubMed] [Google Scholar]
- 235. Boston BA. Pro-opiomelanocortin and weight regulation: from mice to men. J Pediatr Endocrinol Metab. 2001;14(Suppl 6):1409‐1416. [PubMed] [Google Scholar]
- 236. Wang Q, Bing C, Al-Barazanji K, et al. Interactions between leptin and hypothalamic neuropeptide Y neurons in the control of food intake and energy homeostasis in the rat. Diabetes. 1997;46(3):335‐341. [DOI] [PubMed] [Google Scholar]
- 237. Ilnytska O, Argyropoulos G. The role of the agouti-related protein in energy balance regulation. Cell Mol Life Sci. 2008;65(17):2721‐2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Nestor CC, Qiu J, Padilla SL, et al. Optogenetic stimulation of arcuate nucleus Kiss1 neurons reveals a steroid-dependent glutamatergic input to POMC and AgRP neurons in male mice. Mol Endocrinol. 2016;30(6):630‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Fu LY, van den Pol AN. Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J Neurosci. 2010;30(30):10205‐10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Orlando G, Leone S, Ferrante C, et al. Effects of kisspeptin-10 on hypothalamic neuropeptides and neurotransmitters involved in appetite control. Molecules. 2018;23(12):3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Padilla SL, Perez JG, Ben-Hamo M, et al. Kisspeptin neurons in the arcuate nucleus of the hypothalamus orchestrate circadian rhythms and metabolism. Curr Biol. 2019;29(4):592‐604.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Tolson KP, Garcia C, Delgado I, Marooki N, Kauffman AS. Metabolism and energy expenditure, but not feeding or glucose tolerance, are impaired in young Kiss1r KO female mice. Endocrinology. 2016;157(11):4192‐4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Stengel A, Wang L, Goebel-Stengel M, Taché Y. Centrally injected kisspeptin reduces food intake by increasing meal intervals in mice. Neuroreport. 2011;22(5):253‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Talbi R, Laran-Chich MP, Magoul R, El Ouezzani S, Simonneaux V. Kisspeptin and RFRP-3 differentially regulate food intake and metabolic neuropeptides in the female desert jerboa. Sci Rep. 2016;6:36057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Saito R, Tanaka K, Nishimura H, et al. Centrally administered kisspeptin suppresses feeding via nesfatin-1 and oxytocin in male rats. Peptides. 2019;112:114‐124. [DOI] [PubMed] [Google Scholar]
- 246. Thompson EL, Murphy KG, Patterson M, et al. Chronic subcutaneous administration of kisspeptin-54 causes testicular degeneration in adult male rats. Am J Physiol Endocrinol Metab. 2006;291(5):E1074‐E1082. [DOI] [PubMed] [Google Scholar]
- 247. Tolson KP, Marooki N, De Bond JAP, et al. Conditional knockout of kisspeptin signaling in brown adipose tissue increases metabolic rate and body temperature and lowers body weight. FASEB J. 2020;34(1):107‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73‐84. [DOI] [PubMed] [Google Scholar]
- 249. Petroni ML, Brodosi L, Bugianesi E, Marchesini G. Management of non-alcoholic fatty liver disease. BMJ. 2021;372:m4747. [DOI] [PubMed] [Google Scholar]
- 250. Pouwels S, Sakran N, Graham Y, et al. Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr Disord. 2022;22(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Godoy-Matos AF, Silva Júnior WS, Valerio CM. NAFLD as a continuum: from obesity to metabolic syndrome and diabetes. Diabetol Metab Syndr. 2020;12:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Dwyer DS, Horton RY, Aamodt EJ. Role of the evolutionarily conserved starvation response in anorexia nervosa. Mol Psychiatry. 2011;16(6):595‐603. [DOI] [PubMed] [Google Scholar]
- 253. Mills EG, Yang L, Nielsen MF, Kassem M, Dhillo WS, Comninos AN. The relationship between bone and reproductive hormones beyond estrogens and androgens. Endocr Rev. 2021;42(6):691‐719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Tu KN, Lie JD, Wan CKV, et al. Osteoporosis: a review of treatment options. P T. 2018;43(2):92‐104. [PMC free article] [PubMed] [Google Scholar]
- 255. Eastell R, O’Neill TW, Hofbauer LC, et al. Postmenopausal osteoporosis. Nat Rev Dis Primers. 2016;2(1):16069. [DOI] [PubMed] [Google Scholar]
- 256. Sattui SE, Saag KG. Fracture mortality: associations with epidemiology and osteoporosis treatment. Nat Rev Endocrinol. 2014;10(10):592‐602. [DOI] [PubMed] [Google Scholar]
- 257. Knowles HJ, Cleton-Jansen AM, Korsching E, Athanasou NA. Hypoxia-inducible factor regulates osteoclast-mediated bone resorption: role of angiopoietin-like 4. FASEB J. 2010;24:4648‐4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Weinman MA, Fischer JA, Jacobs DC, Goodall CP, Bracha S, Chappell PE. Autocrine production of reproductive axis neuropeptides affects proliferation of canine osteosarcoma in vitro. BMC Cancer. 2019;19(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Olbrich T, Ziegler E, Türk G, Schubert A, Emons G, Gründker C. Kisspeptin-10 inhibits bone-directed migration of GPR54-positive breast cancer cells: evidence for a dose-window effect. Gynecol Oncol. 2010;119(3):571‐578. [DOI] [PubMed] [Google Scholar]
- 260. Dotterweich J, Tower RJ, Brandl A, et al. The KISS1 receptor as an in vivo microenvironment imaging biomarker of multiple myeloma bone disease. PLoS One. 2016;11(5):e0155087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Son HE, Kim KM, Kim EJ, Jang WG. Kisspeptin-10 (KP-10) stimulates osteoblast differentiation through GPR54-mediated regulation of BMP2 expression and activation. Sci Rep. 2018;8(1):2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Javed A, Bae JS, Afzal F, et al. Structural coupling of Smad and Runx2 for execution of the BMP2 osteogenic signal. J Biol Chem. 2008;283(13):8412‐8422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Jang WG, Kim EJ, Lee KN, Son HJ, Koh JT. AMP-activated protein kinase (AMPK) positively regulates osteoblast differentiation via induction of Dlx5-dependent Runx2 expression in MC3T3E1 cells. Biochem Biophys Res Commun. 2011;404(4):1004‐1009. [DOI] [PubMed] [Google Scholar]
- 264. Herber CB, Krause WC, Wang L, et al. Estrogen signaling in arcuate Kiss1 neurons suppresses a sex-dependent female circuit promoting dense strong bones. Nat Commun. 2019;10(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. de Sousa IO, Diniz ET, Marques TF, Griz L, Coutinho MdAP, Bandeira F. Short-term bone marker responses to teriparatide and strontium ranelate in patients with osteoporosis previously treated with bisphosphonates. Arq Bras Endocrinol Metabol. 2010;54(2):244‐249. [DOI] [PubMed] [Google Scholar]
- 266. Snoeren EMS. Female reproductive behavior. Curr Top Behav Neurosci. 2019;43:1‐44. [DOI] [PubMed] [Google Scholar]
- 267. Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl). 2008;199(3):457‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Yang L, Comninos AN, Dhillo WS. Intrinsic links among sex, emotion, and reproduction. Cell Mol Life Sci. 2018;75(12):2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Pineda R, Plaisier F, Millar RP, Ludwig M. Amygdala kisspeptin neurons: putative mediators of olfactory control of the gonadotropic axis. Neuroendocrinology. 2017;104(3):223‐228. [DOI] [PubMed] [Google Scholar]
- 270. Dulac C, Torello AT. Molecular detection of pheromone signals in mammals: from genes to behaviour. Nat Rev Neurosci. 2003;4(7):551‐562. [DOI] [PubMed] [Google Scholar]
- 271. Aggarwal S, Tang C, Sing K, Kim HW, Millar RP, Tello JA. Medial amygdala Kiss1 neurons mediate female pheromone stimulation of luteinizing hormone in male mice. Neuroendocrinology. 2019;108(3):172‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Watanabe Y, Ikegami K, Nakamura S, et al. Mating-induced increase in Kiss1 mRNA expression in the anteroventral periventricular nucleus prior to an increase in LH and testosterone release in male rats. J Reprod Dev. 2020;66(6):579‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Kauffman AS, Park JH, McPhie-Lalmansingh AA, et al. The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J Neurosci. 2007;27(33):8826‐8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Adekunbi DA, Li XF, Lass G, et al. Kisspeptin neurones in the posterodorsal medial amygdala modulate sexual partner preference and anxiety in male mice. J Neuroendocrinol. 2018;30(3):e12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Magarramova LA, Tissen IY, Blazhenko AA, Lebedev AA, Loskutov SI, Proshin SN. Kisspeptin is testosterone independent regulator of sexual motivation in male rats. J Exp Biol Agric Sci. 2022;10(1):131‐134. [Google Scholar]
- 276. Gresham R, Li S, Adekunbi DA, Hu M, Li XF, O’Byrne KT. Kisspeptin in the medial amygdala and sexual behavior in male rats. Neurosci Lett. 2016;627:13‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Nakamura S, Uenoyama Y, Ikegami K, et al. Neonatal kisspeptin is steroid-independently required for defeminisation and peripubertal kisspeptin-induced testosterone is required for masculinisation of the brain: a behavioural study using Kiss1 knockout rats. J Neuroendocrinol. 2016;28(10):1‐11. [DOI] [PubMed] [Google Scholar]
- 278. Goto T, Hirabayashi M, Watanabe Y, et al. Testosterone supplementation rescues spermatogenesis and in vitro fertilizing ability of sperm in Kiss1 knockout mice. Endocrinology. 2020;161(9):bqaa092. [DOI] [PubMed] [Google Scholar]
- 279. Bhardwaj S, Kumar P, Jerome A, et al. Serum kisspeptin: new possible biomarker for sexual behaviour and sperm concentration in Buffalo bulls. Reprod Domest Anim. 2020;55(9):1190‐1201. [DOI] [PubMed] [Google Scholar]
- 280. Georgiadis JR, Kringelbach ML. The human sexual response cycle: brain imaging evidence linking sex to other pleasures. Prog Neurobiol. 2012;98(1):49‐81. [DOI] [PubMed] [Google Scholar]
- 281. Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700‐711. [DOI] [PubMed] [Google Scholar]
- 282. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard Debra A, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Cleve M, Gussew A, Reichenbach JR. In vivo detection of acute pain-induced changes of GABA+ and Glx in the human brain by using functional 1H MEGA-PRESS MR spectroscopy. Neuroimage. 2015;105:67‐75. [DOI] [PubMed] [Google Scholar]
- 284. Bollmann S, Ghisleni C, Poil SS, et al. Developmental changes in gamma-aminobutyric acid levels in attention-deficit/hyperactivity disorder. Transl Psychiatry. 2015;5(6):e589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Fjaeldstad A, Fernandes HM, Van Hartevelt TJ, et al. Brain fingerprints of olfaction: a novel structural method for assessing olfactory cortical networks in health and disease. Sci Rep. 2017;7(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Aharon I, Etcoff N, Ariely D, Chabris CF, O’Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32(3):537‐551. [DOI] [PubMed] [Google Scholar]
- 287. O’Doherty J, Winston J, Critchley H, Perrett D, Burt DM, Dolan RJ. Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41(2):147‐155. [DOI] [PubMed] [Google Scholar]
- 288. Cela-Conde CJMarty G, Maestú F, et al. Activation of the prefrontal cortex in the human visual aesthetic perception. Proc Natl Acad Sci U S A. 2004;101(16):6321‐6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Winston JS, O’Doherty J, Kilner JM, Perrett DI, Dolan RJ. Brain systems for assessing facial attractiveness. Neuropsychologia. 2007;45(1):195‐206. [DOI] [PubMed] [Google Scholar]
- 290. Arnow BA, Desmond JE, Banner LL, et al. Brain activation and sexual arousal in healthy, heterosexual males. Brain. 2002;125(Pt 5):1014‐1023. [DOI] [PubMed] [Google Scholar]
- 291. Holroyd CB, Yeung N. Motivation of extended behaviors by anterior cingulate cortex. Trends Cogn Sci. 2012;16(2):122‐128. [DOI] [PubMed] [Google Scholar]
- 292. Murty VP, LaBar KS, Adcock RA. Distinct medial temporal networks encode surprise during motivation by reward versus punishment. Neurobiol Learn Mem. 2016;134(Pt A):55‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Kaplan HS. The New Sex Therapy: Active Treatment of Sexual Dysfunctions. Psychology Press; 1974. [Google Scholar]
- 294. Cacioppo S. Neuroimaging of female sexual desire and hypoactive sexual desire disorder. Sex Med Rev. 2017;5(4):434‐444. [DOI] [PubMed] [Google Scholar]
- 295. Mills EG, Izzi-Engbeaya C, Abbara A, Comninos AN, Dhillo WS. Functions of galanin, spexin and kisspeptin in metabolism, mood and behaviour. Nat Rev Endocrinol. 2021;17(2):97‐113. [DOI] [PubMed] [Google Scholar]
- 296. de Bond JAP, Li Q, Millar RP, Clarke IJ, Smith JT. Kisspeptin signaling is required for the luteinizing hormone response in anestrous ewes following the introduction of males. PLoS One. 2013;8(2):e57972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Bakker J, Pierman S, González-Martínez D. Effects of aromatase mutation (ArKO) on the sexual differentiation of kisspeptin neuronal numbers and their activation by same versus opposite sex urinary pheromones. Horm Behav. 2010;57(4-5):390‐395. [DOI] [PubMed] [Google Scholar]
- 298. Watanabe Y, Ikegami K., Ishigaki R, et al. Enhancement of the luteinising hormone surge by male olfactory signals is associated with anteroventral periventricular Kiss1 cell activation in female rats. J Neuroendocrinol. 2017;29(8):e12505. [DOI] [PubMed] [Google Scholar]
- 299. Dombret C, Capela D, Poissenot K, et al. Neural mechanisms underlying the disruption of male courtship behavior by adult exposure to di(2-ethylhexyl) phthalate in mice. Environ Health Perspect. 2017;125(9):097001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Asaba A, Osakada T, Touhara K, Kato M, Mogi K, Kikusui T. Male mice ultrasonic vocalizations enhance female sexual approach and hypothalamic kisspeptin neuron activity. Horm Behav. 2017;94:53‐60. [DOI] [PubMed] [Google Scholar]
- 301. Hellier V, Brock O, Candlish M, et al. Female sexual behavior in mice is controlled by kisspeptin neurons. Nat Commun. 2018;9(1):400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Tsukahara S, Kanaya M, Yamanouchi K. Neuroanatomy and sex differences of the lordosis-inhibiting system in the lateral septum. Front Neurosci. 2014;8:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Hellier V, Brock O, Bakker J. The role of kisspeptin in sexual behavior. Semin Reprod Med. 2019;37(2):84‐92. [DOI] [PubMed] [Google Scholar]
- 304. Bentefour Y, Bakker J. Kisspeptin signaling and nNOS neurons in the VMHvl modulate lordosis behavior but not mate preference in female mice. Neuropharmacology. 2021;198:108762. [DOI] [PubMed] [Google Scholar]
- 305. Swick D, Ashley V, Turken AU. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 2008;9:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Sharp DJ, Bonnelle V, De Boissezon X, et al. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc Natl Acad Sci U S A. 2010;107(13):6106‐6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Gillath O, Canterberry M. Neural correlates of exposure to subliminal and supraliminal sexual cues. Soc Cogn Affect Neurosci. 2012;7(8):924‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Stoléru S, Fonteille V, Cornélis C, Joyal C, Moulier V. Functional neuroimaging studies of sexual arousal and orgasm in healthy men and women: a review and meta-analysis. Neurosci Biobehav Rev. 2012;36(6):1481‐1509. [DOI] [PubMed] [Google Scholar]
- 309. Parada M, Gérard M, Larcher K, Dagher A, Binik YM. Neural representation of subjective sexual arousal in men and women. J Sex Med. 2016;13(10):1508‐1522. [DOI] [PubMed] [Google Scholar]
- 310. Ferdenzi C, Delplanque S, Vorontsova-Wenger O, Pool EVA, Bianchi-Demicheli F, Sander D. Perception of men's beauty and attractiveness by women with low sexual desire. J Sex Med. 2015;12(4):946‐955. [DOI] [PubMed] [Google Scholar]
- 311. Bretas RV, Taoka M, Suzuki H, Iriki A. Secondary somatosensory cortex of primates: beyond body maps, toward conscious self-in-the-world maps. Exp Brain Res. 2020;238(2):259‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Leiblum SR, Koochaki PE, Rodenberg CA, Barton IP, Rosen RC. Hypoactive sexual desire disorder in postmenopausal women: US results from the Women's International Study of Health and Sexuality (WISHeS). Menopause. 2006;13(1):46‐56. [DOI] [PubMed] [Google Scholar]
- 313. Caldwell ASL, Edwards MC, Desai R, et al. Neuroendocrine androgen action is a key extraovarian mediator in the development of polycystic ovary syndrome. Proc Natl Acad Sci U S A. 2017;114(16):E3334‐E3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Talbi R, Ferrari K, Choi JH, et al. Characterization of the action of tachykinin signaling on pulsatile LH secretion in male mice. Endocrinology. 2021;162(8):bqab074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Sucquart IE, Nagarkar R, Edwards MC, et al. Neurokinin 3 receptor antagonism ameliorates key metabolic features in a hyperandrogenic PCOS mouse model. Endocrinology. 2021;162(5):bqab020. [DOI] [PubMed] [Google Scholar]
- 316. Giuliani E, As-Sanie S, Marsh EE. Epidemiology and management of uterine fibroids. Int J Gynecol Obstet. 2020;149(1):3‐9. [DOI] [PubMed] [Google Scholar]
- 317. Parasar P, Ozcan P, Terry KL. Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep. 2017;6(1):34‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Bedaiwy MA, Allaire C, Alfaraj S. Long-term medical management of endometriosis with dienogest and with a gonadotropin-releasing hormone agonist and add-back hormone therapy. Fertil Steril. 2017;107(3):537‐548. [DOI] [PubMed] [Google Scholar]
- 319. Lethaby A, Vollenhoven B, Sowter MC. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2001(2):CD000547. [DOI] [PubMed] [Google Scholar]
- 320. Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis results from two extension studies. Obstet Gynecol. 2018;132(1):147‐160. [DOI] [PubMed] [Google Scholar]
- 321. Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135(6)1313‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166(2):740‐745. [DOI] [PubMed] [Google Scholar]
- 323. Friedman AJ, Lobel SM, Rein MS, Barbieri RL. Efficacy and safety considerations in women with uterine leiomyomas treated with gonadotropin-releasing hormone agonists: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1990;163(4):1114‐1119. [DOI] [PubMed] [Google Scholar]
- 324. Li Q, Millar RP, Clarke IJ, Smith JT. Evidence that neurokinin B controls basal gonadotropin-releasing hormone secretion but is not critical for estrogen-positive feedback in sheep. Neuroendocrinology. 2015;101(2):161‐174. [DOI] [PubMed] [Google Scholar]
- 325. Fraser GL, Hoveyda HR, Clarke IJ, et al. The NK3 receptor antagonist ESN364 interrupts pulsatile LH secretion and moderates levels of ovarian hormones throughout the menstrual cycle. Endocrinology. 2015;156(11):4214‐4225. [DOI] [PubMed] [Google Scholar]
- 326. Skorupskaite K, George J, Anderson RA. Role of a neurokinin B receptor antagonist in the regulation of ovarian function in healthy women. Lancet. 2015;385(Suppl 1):S92. [DOI] [PubMed] [Google Scholar]
- 327. Skorupskaite K, George JT, Veldhuis JD, Anderson RA. Neurokinin B regulates gonadotropin secretion, ovarian follicle growth, and the timing of ovulation in healthy women. J Clin Endocrinol Metab. 2018;103(1):95‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. National Institute for Health and Care Excellence (NICE) . Menopause: Diagnosis and Management. National Institute for Health and Care Excellence (NICE); 2015. [Google Scholar]
- 329. Abel TW, Voytko ML, Rance NE. The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause. J Clin Endocrinol Metab. 1999;84(6):2111‐2118. [DOI] [PubMed] [Google Scholar]
- 330. Sandoval-Guzmán T, Stalcup ST, Krajewski SJ, Voytko ML, Rance NE. Effects of ovariectomy on the neuroendocrine axes regulating reproduction and energy balance in young cynomolgus macaques. J Neuroendocrinol. 2004;16(2):146‐153. [DOI] [PubMed] [Google Scholar]
- 331. Krajewski SJ, Burke MC, Anderson MJ, Mcmullen NT, Rance NE. Forebrain projections of arcuate neurokinin b neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience. 2010;166(2):680‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Shughrue PJ, Lane MV, Merchenthaler I. In situ hybridization analysis of the distribution of neurokinin-3 mRNA in the rat central nervous system. J Comp Neurol. 1996;372(3):395‐414. [DOI] [PubMed] [Google Scholar]
- 333. Dacks PA, Krajewski SJ, Rance NE. Activation of neurokinin 3 receptors in the median preoptic nucleus decreases core temperature in the rat. Endocrinology. 2011;152(12):4894‐4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Krajewski-Hall SJ, Blackmore EM, McMinn JR, Rance NE. Estradiol alters body temperature regulation in the female mouse. Temperature. 2018;5(1):56‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, McMullen NT, Rance NE. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci U S A. 2012;109(48):19846‐19851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Rance NE, Young WS. Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology. 1991;128(5):2239‐2247. [DOI] [PubMed] [Google Scholar]
- 337. Crandall CJ, Manson JE, Hohensee C, et al. Association of genetic variation in the tachykinin receptor 3 locus with hot flashes and night sweats in the Women's Health Initiative Study. Menopause. 2017;24(3):252‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Abbara A, Phylactou M, Dhillo WS. Commentary on “pharmacodynamic activity of the novel neurokinin-3 receptor antagonist SJX-653 in healthy men.” J Clin Endocrinol Metab. 2021;106(2):e1028‐e1030. [DOI] [PubMed] [Google Scholar]