SUMMARY
Objective:
This study attempted to validate the effects of neonatal estradiol in ameliorating the spasms in the prenatally betamethasone-primed NMDA model of infantile spasms as shown previously in a mouse Arx gene knock-in expansion model of infantile spasms.
Methods:
Neonatal rats prenatally exposed to betamethasone (on day 15 of pregnancy) were treated with 40 ng/g estradiol benzoate (EB) s.c. either between postnatal days (P)3-P10 or P0-P5. A synthetic estrogen analogue, diethylstilbestrol, was used between P0-P5 (2 μg per rat s.c.). On P12, P13 and P15, the rats were subjected to NMDA-triggered spasms, and latency to onset and number of spasms were evaluated. Rats with EB on P3-P10 were tested after spasms in the open field, novel object recognition, and elevated plus maze to determine effects of treatment on behavior. Additional rats with P3-P10 or P0-P5 EB were investigated for GABAergic neurons (GAD67 expression) in the neocortex. As a positive control, a group of rats received either s.c. ACTH (2× 0.3mg/kg on P12 and 3× 0.3 mg/kg on P13 and P14) or vehicle after the first episode of spasms on P12.
Results:
Neither EB treatment nor diethylstilbestrol consistently affected expression of spasms in this model although we found a significant increase in GAD67-immunopositive cells in the neocortex after P3-P10 and P0-P5 EB treatment, consistent with a study in mice. Behavioral tests showed increase in lateralization in male rats treated with P3-P10 EB, a behavioral trait usually associated with female sex. Diethylstilbestrol treatment in males resulted in arrested pubertal descent of testes. ACTH had robust effects in suppressing spasms.
Significance:
Treatment of IS using neonatal EB may be justified in those cases of IS presenting with detectable deficits in GABAergic neurons. In other IS types, the efficacy of neonatal EB and its analogues is not supported.
Keywords: diethylstilbestrol, behavior, ACTH, epileptic spasms, validation
INTRODUCTION
In 2012, Citizens United for Research in Epilepsy (CURE) announced its Infantile Spasms Research Initiative focused on translational and clinical research of infantile spasms (IS). Several centers for translational and clinical research have been established across the USA with the purpose of accelerating development of novel treatments for IS as well as for placing multiple checks on preclinical studies to make the outcome significantly more robust and reliable1.
IS is a devastating epilepsy syndrome occurring between 3–24 months of age with frequency approximately 1 out of 3200 of live births with slight predominance in boys (boy:girl ratio approximately 60:40)2, 3. In affected infants, IS are characterized by clusters of brief spasm seizures (flexion, extension or both), by EEG with irregular, asynchronous, large-amplitude waves occurring in between the spasms (interictal hypsarhythmia), and by mental retardation4. Current treatment of IS relies on hormonal therapy (ACTH, glucocorticoids) and vigabatrin5. None of these substances is completely effective and all have side effects6–8.
Our model of IS utilizes prenatal priming with betamethasone on gestational day 15 in combination with induction of spasms by systemic administration of NMDA between postnatal days (P) 10–159. NMDA-triggered spasms are tightly linked to early development (similar to IS in humans) as the NMDA-induced seizure phenotype changes with maturation10. The spasms are also semiologically similar to human IS, they share similar EEG patterns, and, if triggered in the prenatally corticosteroid- (or stress-) primed brain, the spasms do respond to ACTH, glucocorticoids or vigabatrin treatment11, 12. This model has been independently validated13.
In one of many varieties of IS in humans, mutation in the Aristaless-related homeobox gene (ARX) causes a rare X-linked IS14. The transcription factor encoded by ARX is expressed predominantly in specific subpopulations of interneurons15 and is heavily involved in their tangential migration and maturation. A mouse Arx gene knock-in expansion reproduces the human 7-Ala expansion [Arx(GCG)10+7] and the homozygous females and hemizygous male mutants display frequent spasms between P7 to P1116. In these mutants, early (P3-P10) but not late (P33-P40) administration of estradiol benzoate (EB) led to the rescue of decreased numbers of GABAergic interneurons in the neocortex and cholinergic interneurons in the striatum and this effect was associated with a significant reduction of seizures as well as the number of interictal EEG spikes17.
The main objective of present study was to determine whether the beneficial effects of neonatal EB administration associated with the increase in number of GABAergic neurons in the cortex described in the ARX model of IS17 can be extended to another IS model. Specifically, we were interested if neonatal EB administration improves outcome in our prenatally primed/NMDA-triggered model of IS. Efficacy of neonatal EB treatment in another model of IS would imply that EB may improve condition of IS in general, irrespective of etiology. This finding would be important as there are over 200 different known etiologies of IS in humans18. Thus positive findings in another model and another laboratory would significantly increase robustness of the outcome19. We found that although neonatal estradiol administration does increase the number of GABAergic neurons in the neocortex in prenatally betamethasone-primed rats, this effect is not associated with any improvement in NMDA-triggered spasms. Further, we found that neonatal EB treatment significantly affected male behaviors in the Novel Object Recognition test modifying it to a female phenotype.
METHODS
Animals
All experiments are consistent with the Guide for the Care and Use of Laboratory Animals, Eighth Edition (2011) and have been approved by the IACUC of the New York Medical College. All efforts have been made to minimize pain and keep the numbers of animals low while keeping sufficient statistical power. Timed pregnant Sprague-Dawley rats have been purchased from Taconic Farms (Germantown, NY) on gestational day 8 (G8) and housed in AAALAC-approved animal facility on a regular light-dark cycle (lights on at 07:00; lights off at 19:00). On G15, pregnant rats received two injections of betamethasone (each 0.4 mg/kg body weight diluted in volume of 1 ml/kg of body weight) at 08:00 and 18:0011. The day of delivery was counted as P0 for the offspring. On P0–1, the pups were identified, sexed and weighed, and cross-fostered so that the entire newly formed litter can be injected either with EB or vehicle (peanut oil) to avoid possible transcutaneous hormonal transfer. Four different litters contributed to each experimental or control group. Weights were recorded on P0 or P1 depending on the treatment start time (before treatment) and on each test day at P12, P13, and P15 (before seizure testing).
Neonatal hormonal treatments
In the first cohort, we exactly followed the already published protocol17. Each rat pup received daily s.c. injection of 40 ng/g body weight of EB in peanut oil from P3 to P10 (total of 8 doses). In the second protocol, we adjusted treatment to the critical period (P0-P5) for brain sexual differentiation in the rat20. For injections we used either EB or synthetic estrogen, diethylstilbestrol (DES; in peanut oil) to prevent α-fetoprotein binding20. Thus, the second cohort of neonatal rats either received 40 ng/g of EB s.c. or 2 μg of DES per pup s.c. daily from P0 through P5 (total of 6 doses). Controls for both experiments received peanut oil (vehicle) at appropriate times. Finally a third cohort of rats was a reproduction of our previous results (positive control): The rat pups on P12 were subjected to the first trigger of spasms (between 9:00–10:00) and afterwards randomized into ACTH treatment or saline control group. Thus, ACTH was dosed twice on P12 (14:00 and 21:00), three times on P13 and P14 (07:00, 14:00 and 21:00); each dose was 0.3 mg/kg s.c in saline. Controls received equivalent volumes of saline s.c. at the same times.
Expression of GAD67 in the neocortex
Separate cohort of neonatally EB-treated rats (EB P3-P10) as well as another cohort treated with EB between P0-P5 was deeply anesthetized with ketamine/xylazine mixture (70 mg/kg/10 mg/kg) on P15 and transcardially perfused with saline and ice-cold paraformaldehyde solution. It should be emphasized that these rats were not subjected to any spasms. Brains were removed and after cryoprotection in ascending sucrose concentrations (10, 20, and 30%) were frozen. For free-floating immunohistochemical staining, 40 μm thick sagittal sections were cut using a cryostat (Leica 1850 CM). GAD67 is an isozyme of the GABA synthesizing enzyme glutamic acid decarboxylase that tags almost the entire population of GABAergic neurons. We used anti-GAD67 primary antibody (1:4000; Millipore, Temecula, CA). The immunostaining was visualized using the chromogenic detection of avidin-biotin horseradish peroxidase (Vectastain AB kit, Vector Laboratories, Burlingame, CA) using 3,3’-Diaminobenzidine (DAB, Thermo Scientific)21, 22. After processing, the brain sections were mounted on microscopy slides. Section images were digitally captured and calibrated for distance. Counts were performed in the neocortex. We selected the neocortex to collect data comparable with the previous study17. We used the following protocol: In each animal, three position-matched sections (80 μm apart) were selected (Fig. 1D). Three non-adjacent, 150 μm wide rectangles spanning the entire thickness of the cortex were placed on the section marking the area of interest. All immunopositive neurons in the areas of interest were counted23, 24. We also counted separately numbers of immunopositive cells in layers V-VI. Counts from three sections were normalized per 100,000 μm2, averaged for each rat (one rat=one subject), and this average entered statistical evaluation. The figures are showing relative difference from oil-injected controls.
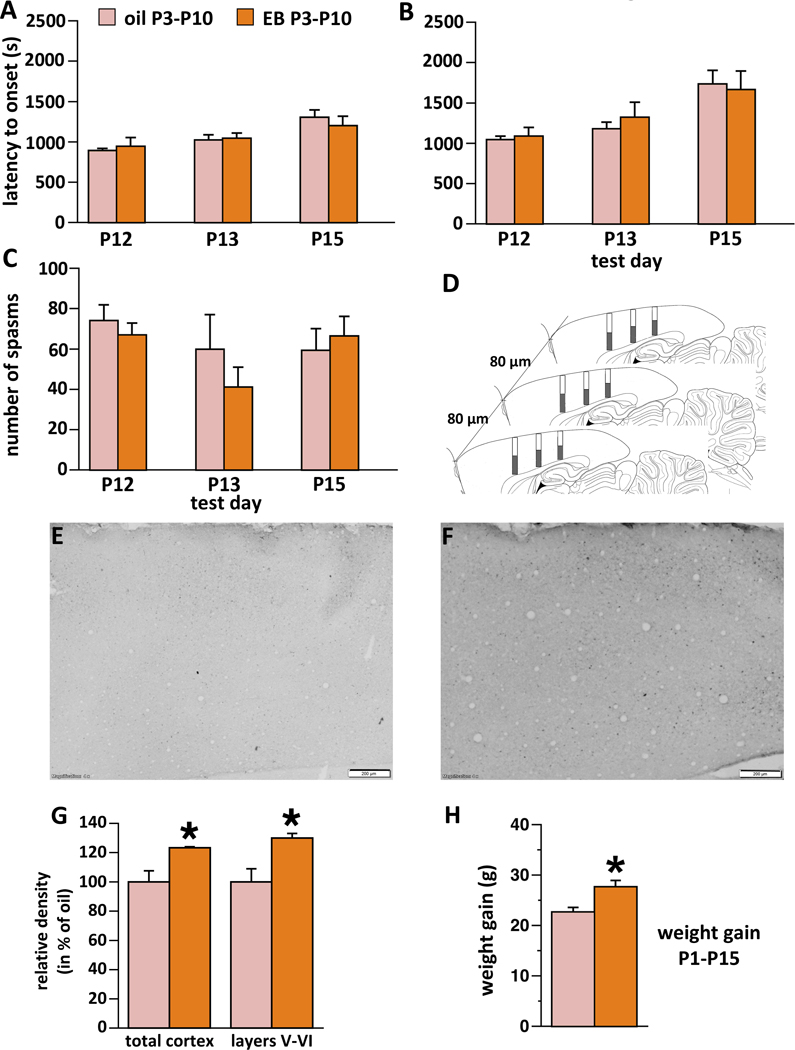
Figure 1.
Effects of neonatal (P3-P10) treatment with estradiol benzoate (EB) on the expression of spasms, number of GAD67 immunopositive cells in the neocortex, and body weight gain
Controls were injected with oil between P3-P10, EB rats received 40 ng/g body weight of EB daily on P3-P1017. There was never main effect of sex and no interaction of sex with treatment; therefore the factor of sex was removed from all analyses. All values refer to mean±SEM.
(A) Latency to onset of the first occurrence of spasm on P12, P13, and P15 day of spasms. No significant effect was found.
(B) Latency to onset of the fully developed spasm on P12, P13, and P15. No significant effect was found.
(C) Number of spasms per observation period on P12, P13, and P15. No significant effect was found.
(D) Scheme for counting neurons in the neocortex. Three brain sections at matching positions were selected from each animal (80 μm apart; not to scale). Three rectangles (150 μm wide) were placed on the neocortex in each section spanning its entire depth. Images were calibrated for distance so the height of each column was measured. All neurons within columns were counted and their density averaged per animal. The average entered statistical evaluation (each animal was a unit). Shaded parts of rectangles represent counting layers V-VI.
(E) Example of the neocortical cross-section with GAD67 immunopositive cells in the rat treated with oil between P3-P10. Note few immunopositive cells in the section. Lens magnification 4x. Scale bar is 200 μm.
(F) Example of the neocortical cross-section with GAD67 immunopositive cells in the rat treated with EB between P3-P10. Note numerous immunopositive cells in the section. Lens magnification 4x. Scale bar is 200 μm.
(G) Number of GAD67 immunopositive cells in the sampled area of sensorimotor cortex. N of subjects in the EB group = 6; n of subjects in the oil group = 6. Left, counting neurons throughout the cortex, EB induced significant increase compared to oil, *p=0.01. Right, counts within layers V-VI, again there was a significant increase after EB compared to oil, *p=0.01. None of these animals were subjected to spasms.
(H) Body weight gain between P1 and P15. EB-treated rats gained significantly less weight compared to oil-injected, *p=0.005.
Induction of spasms
On P12, all cohorts of pups received 7.5 mg/kg of NMDA in normal saline i.p., On P13, the rats were injected with 12 mg/kg of NMDA i.p. and on P15, the dose of NMDA was 15 mg/kg i.p11. Immediately after the NMDA injections, the rats were observed for 90 minutes for the occurrence of individual symptoms of the NMDA-triggered syndrome: tail twisting, arching, and flexion spasms. If tail twisting was present within the usual time frame after the NMDA injection, we considered the animal as properly injected with NMDA and the data on spasms entered further statistical evaluation. We determined latency to onset of the first spasm, fully developed spasm, as well as number of flexion spasms per observation period.
Behavioral testing
Behavior of the rats treated with EB between P3–10 (first cohort) was evaluated after experimental IS. Three behavioral tests were used sequentially: the open field test (P21–23), novel object recognition test (P22–24) and the elevated plus maze (P31–32). We selected prepubertal testing because in our mixed male-female cohort, pubertal or even postpubertal testing might capture an additional behavioral confounder of female estrous cycle.
Open field test:
Open field provides information about spontaneous behavior, velocity and anxiety as during normal conditions, the rats prefer staying in relatively safer environment close to the walls (thigmotaxis) compared to the open central area25. Animals were tested in an open field arena with dark walls with dimensions of 45.5 × 45.5 cm. The monitoring system consisted of three pairs of IR diode arrays and receivers capable of monitoring the motion of animals in 3D using the Activities 5® software (Med Associates, St. Albans, VT). Animal was placed into the center of the open field and activity was monitored for 5 minutes.
Novel Object Recognition test:
This test provides information about cognitive function26 and also about the animal’s lateralization27, 28. The animals were tested in the same open area used for open field testing (45.5 × 45.5 cm), but this time the walls were transparent and the behavior was videotracked by AnyMaze® software (Stoelting, Wood Dale, IL). Each test consisted of five sessions separated by one hour (see Fig. 2A for timing and arena set-up). During the first three sessions, the animals were exposed to the same objects A1 (left) and A2 (right). For the fourth test, the A1 object was kept to the left position and a novel object was placed to the right position (B). For the fifth test, a new, yet identical in shape and color, “A” object was placed to the left position (A3) and another novel object to the right (C). We determined latency to onset of exploration of the object, duration of exploration and nose pokes of the object (contacts with the objects). Indices were calculated to determine preference for novel objects as well as sides (lateralization) and curiosity according to the equations in Supplemental Material.
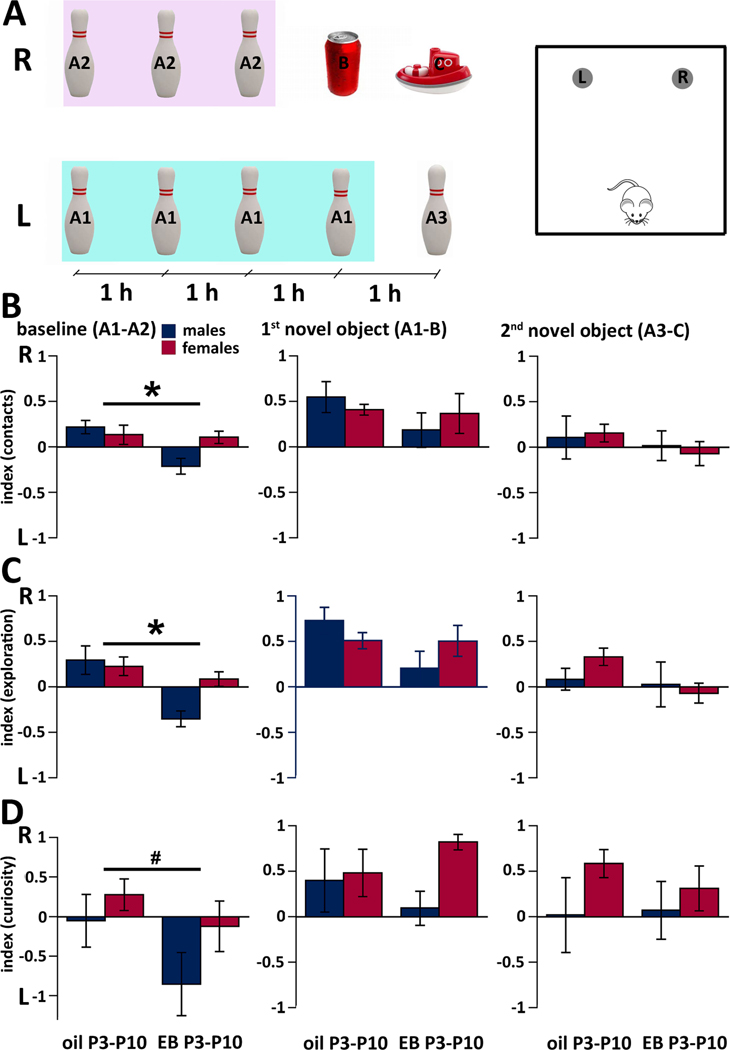
Figure 2.
Performance of the P22-P24 rats in the Novel Object Recognition test after neonatal (P3-P10) treatment with EB and three bouts of spasms on P12, P13, and P15. All values refer to mean±SEM. Group sizes are oil-male n=5; oil-female n=6; EB male n=5 EB female n=6.
(A) Scheme of the experiment (left-timing; right-arena). For each animal, there were 5 test sessions separated with 1 hour. First three sessions used objects A1 on the left (L) and A2 on the right (R, these were always the same bowling pins). Data from these sessions were averaged for baseline indices. Note that negative numbers in the indices indicate preference for the left side object, positive numbers for the right side objects. The fourth session used objects A1 (L) and a novel object B (R). Finally, in the fifth session, the pin was replaced for a different pin from the same set A3 (L) and another novel object C was placed on the position R. Scheme of the arena for the experiment. We used rectangular arena of size 45.5 × 45.5 cm with 30 cm high transparent walls. L and R indicate positions of the objects shown on the left. The cartoon rat represents starting position for each trial.
(B) Preference index for contacts with objects (number of contacts). Baseline represents mean values from three separate trials with identical objects A1 and A2. In the graph “1st novel object” depicts the preference for the novel object (B) always placed in the right position, while object A1 was kept on the left. The graph “2nd novel object” shows the preference for another novel object (C, right) in comparison to the object A3 (left), which is similar to A1/A2 objects. There was an effect of treatment on baseline performance; *p=0.02.
(C) Preference index for exploration of objects (time spent exploring the objects). There was an effect of treatment in the baseline performance *p=0.002. Other details as above.
(D) Curiosity index (composed of the latency to onset of exploration and duration of exploration of the particular object). There was a trend in the effect of treatment during baseline testing #p=0.08. Other details as above.
Elevated plus maze test:
Elevated plus maze uses innate fear of rats to open raised areas29, 30. The rat should spend more time in the closed arms (perceived as relatively safe environment) compared to open arms (perceived as relatively dangerous). Elevated plus maze consisted of two open arms and two closed arms connected in a central area of 10 × 10 cm. The animal was placed on the central square facing the open arm and activity was video-tracked using a camera and Any-Maze software for 3 minutes.
Statistics
We always tested datasets for normal distribution (using Kolmogorov-Smirnov test vs. a dataset with known Gaussian distribution as provided by our software; Statview 5.1) and for approximately equal variance. If these conditions were satisfied, we used parametric statistics, ANOVA for multiple groups or Student’s t-test for 2 groups. ANOVA was followed by post-hoc pairwise tests (Fisher’s PLSD) with significance level adjusted for multiple comparisons. We always tested for sex differences first. If no main effect of sex and no interaction of sex with other main factors were found, the factor of sex was removed (males and females were pooled). If there was no Gaussian distribution of the dataset we used nonparametric statistics, Kruskal-Wallis test for multiple groups or Mann-Whitney U-test for two groups. Level of significance was always preset to p=0.05.
RESULTS
Neonatal EB does not affect expression of spasms yet increases the number of GAD67-immunopositive neurons
Neonatal s.c. administration of 40 ng/g body weight of EB on P3-P10 did not have any effect on the latency to onset of the initial spasm (Fig. 1A), on the latency to onset of the fully developed spasms (Fig. 1B) or on the total number of spasms (Fig. 1C) during the entire period of observation on any test day (P12, P13, P15). None of the tests showed effects of sex or interaction of sex with treatment. Number of subjects was: on P12 (n-oil=13; n-EB=11), on P13 (n-oil=11; n-EB=11), and on P15 (n-oil=10; n-EB=7). Two oil-injected rats did not survive P12 spasms and one P13 spasms. Finally, two EB-treated males and two EB-treated females did not develop any signs of NMDA activity on P15 (indicative of either ineffective i.p. injection such as inside intestine or, less likely, of cure). Because those rats were used in behavioral tests (total n-EB=11), we additionally recalculated the data omitting those animals.
In a different cohort of rats (without spasms), we determined number of GAD67-immunopositive cells in the matching samples of the neocortex (Fig. 1D). Here we found that neonatal EB (n=6) significantly increased (Fig. 1F; by 23.3%) relative density of GAD67-immunopositive cells (per 100,000 μm2) compared to oil controls (Fig. 1E; n=6; Student t-test; *p=0.01; Fig. 1G). We also determined relative density of GAD67-immunopositive cells in layers V-VI of the neocortex (see the original study17). Consistently, we found a larger contrast between neonatal EB and oil (30.0% increase after EB; Student t-test; p=0.01). Note that we did not attempt to determine differences in total number of GAD67-positive cells; rather we compared relative density of cells per matched neocortical samples between EB-treated and control rats. There was no main effect of sex and no interaction between sex and treatment on the number of GAD65-immunopositive cells in the neocortex.
Further, EB significantly increased body weight gain between P1 and P15 in the rats treated with EB compared to oil (ANOVA F(1,22)=9.795; *p=0.005; Fig. 1F). There was no main effect of sex and no interaction between sex and neonatal treatment.
Neonatal EB alters basal exploration pattern in the novel object recognition
We calculated three indices (for object contacts, for duration of object exploration, and for curiosity; see Methods for details). The first three sessions out of total five were performed with identical objects and the averaged data represented baseline performance. Additionally, since there may be sex differences in the NOR performance, we tested for those and separately evaluated males and females.
For the index of contacts (with the object; Fig 2B) for the baseline performance we found differences between neonatal EB treatment (n=11) and oil injection (n=10; ANOVA F(1,17)=6.672; *p=0.02). Although there was no difference in the baseline recognition of objects between males (n=9) and females (n=12; ANOVA F(1,17)=1.741; p=0.2), there was a significant interaction between the factors of sex and neonatal treatment (ANOVA F(1,17)=5.202; p=0.04) indicating that there was a significant shift in lateralization in males. When the first novel object was introduced to the position on the right, the index for both oil and EB shifted from around zero to positive values indicating an increased number of contacts with the object on the right (novel object). There was no effect of neonatal treatment or sex or interaction between these two factors on this index after the first novel object was introduced. Once the second novel object was introduced, the index returned back to almost zero values indicating approximately equal interest for the novel object and one of the original objects. Again, there was no effect of neonatal treatment or sex, or interaction between these two factors on this index.
The index for exploration (Fig. 2C) reflects total time the rat spent exploring the object. We found significant effect of treatment on baseline exploration of identical objects (ANOVA F(1,17)=14.163; *p=0.002; Fig. 2B), additionally we saw a trend in the effect of sex (ANOVA F(1,17)=3.1635; p=0.09) and there was an interaction between both main factors (ANOVA F(1,17)=5.915; p=0.03). Adding novel objects did not reveal any differences between the groups (no effect of neonatal treatment or sex) and there was no interaction between the main factors.
Finally, the curiosity index (Fig. 2D) was created as proportional to the time spent with the object with an inverse proportion to the onset of exploration of this object. This index during baseline revealed only a trend in the effects of neonatal treatment (ANOVA F(1,17)=3.556; #p=0.08) with no effect of sex and interaction. Adding novel objects to the right position (see positive values of the index showing preference for the right side) did not reveal any differences based on the neonatal treatment or sex or any interaction between the factors.
The notable finding after neonatal EB treatment in baseline performance is that only one sex (males) is responsible for the difference: During their baseline training both male and female oil-injected rats had almost equal interest in both objects. Males after neonatal EB significantly shifted their interest to the object on the left. Once novel objects were introduced, interest of all rats irrespective of sex and treatment shifted to that novel object. Recalculation of data omitting those four animals without any symptoms of NMDA action on P15 did not change either significance or pattern of the outcome in any of the indices.
Neonatal EB does not affect anxiety traits measured in the elevated plus-maze or open field behavior
In the elevated plus maze, we initially tested all parameters for the effects of sex but we did not find any effect and there was no interaction with the treatment. Thus, for all further tests the factor of sex was removed. We did not find any significant effect of neonatal EB treatment (n=11) on the time spent on the open arms, in the central area or in the closed arms compared to oil (n=10). There was no effect of neonatal treatment on the index composed from time spent in the distal portions of open and closed arms (Fig. 3A). Recalculation of data omitting those four animals without any symptoms of NMDA action on P15 did not change either significance or pattern of the outcome.
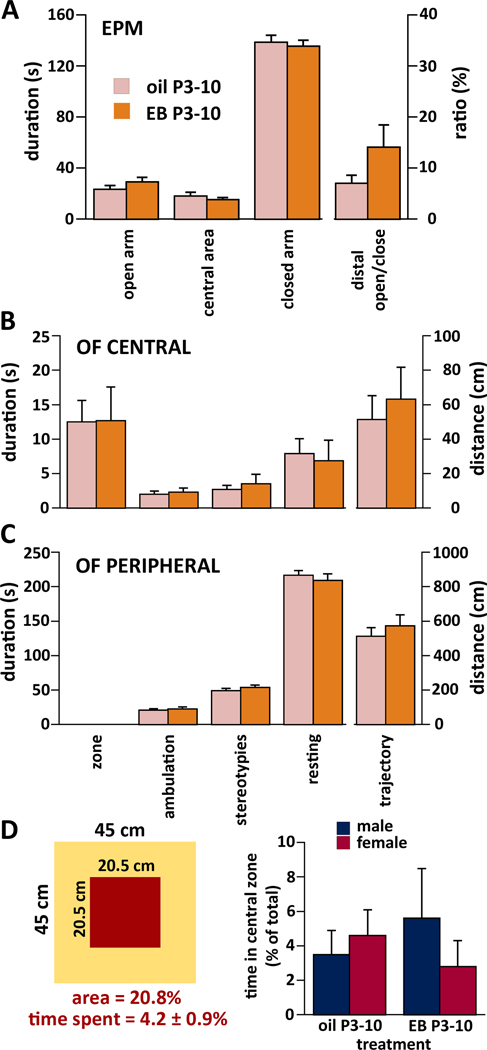
Figure 3.
Performance of the rats in the Elevated Plus-Maze test (EPM; P31-P32) and in the Open Field test (OF; P21–22) after neonatal (P3-P10) treatment with EB and three bouts of spasms on P12, P13, and P15.
Since sex as a factor had no significant effect on performance and there was no interaction with treatment, males and females were pooled. All values refer to mean±SEM.
(A) Elevated Plus Maze. Left side – duration of exploration of the open arm, central area and the closed arm. Right side – ratio between time spent in the distal half of the open arm over time spent in the distal half of the closed arm. No effect of treatment was found.
(B) Central zone of the open field (OF CENTRAL): From left – total time spent in the central zone out of 300 s test time, time spent ambulating, time spent with stereotypies, time spent in resting (all in seconds). Right – total distance traveled in the central zone (in cm). No effect of treatment was found.
(C) Peripheral zone of the open field (OF PERIPHERAL): From left, total time spent in the zone is omitted, otherwise details as above.
(D) Left, arrangement of the zones in the open field with the representation of the central area (20.75% out of the total area) and time spent in the central area as a proportion of total test time (4.2%; i.e., five times less than the area proportion out of total open field is). Right, time spent in the central zone as a proportion of total test time in the open field broken down by treatment and sex.
In the open field test, we determined duration (time) of being present in the central zone of the open field (out of total 300 s test duration). Further, we determined separately for both peripheral and central zones of the open field the duration of ambulation, stereotypies (grooming) and resting time. Additionally, we determined distance of the path (trajectory) traveled in each zone. There was no difference in any of these parameters between neonatally EB-treated (n=10) and oil-injected (n=10) rats (Fig. 3B,C). For illustration, Fig. 3D shows the total and central area of the open field. While the central area represents 20.75% of the open field, the rats spent only 4.2% of their test time there. There was no effect of treatment (ANOVA F(1,16)=0.007; p=0.94) or sex (ANOVA F(1,16)=0.186; p=0.67) and no interaction between the main factors (Fig. 3D). Recalculation of the data omitting the four rats without any sign of NMDA activity on P15 did not change either significance or pattern of the outcome.
Inconsistent effects of early neonatal steroid treatments on expression of spasms and arrested descent of testes in male rats by neonatal DES
Since rats may have different critical period for effects of sex steroids than mice, in this experiment we adjusted treatment period to early neonatal critical periods between P0-P5. Additionally, as rats may have more α-fetoprotein for binding of EB, for one cohort we used treatment with diethylstilbestrol (DES), a synthetic estrogen analogue, which does not bind to α-fetoprotein. The group of rats in this part of the experiment received six treatment (or vehicle) injections between P0-P5 and the spasms were triggered on P12, P13, and P15. No effects were found for sex or interaction of sex with treatment. During P12 testing, we found a transient effect of neonatal DES (n=13) expressed as the delay in the onset of spasms (first spasm) compared to both EB treatment (n=10) and oil controls (n=25; ANOVA F(2,44)=3.579; *p=0.04; Fig. 4A). Similarly, there was also a corresponding trend in the decreased number of spasms on P12. However, this variable did not follow Gaussian distribution, and therefore we used non-parametric Kruskal-Wallis test (DF=2; number of groups=3, ties=7; H corrected for ties=5.986; #p=0.05; Fig. 4C). We found one additional effect of treatment on spasms; EB treatment delayed onset of fully developed spasms on P15 (ANOVA F(2,26)=4.162; *p=0.03; Fig. 4B).
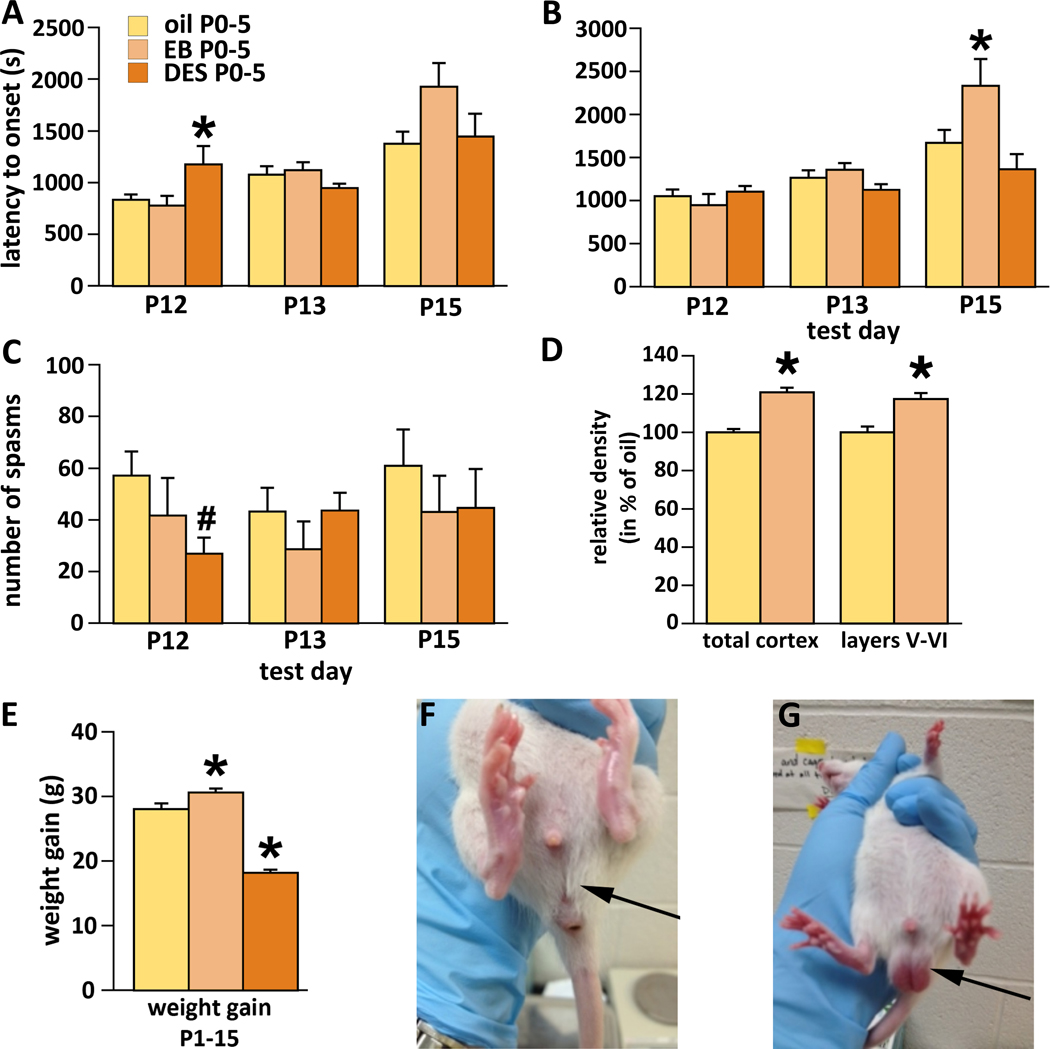
Figure 4.
Effects of early neonatal (P0-P5) treatment with EB or diethylstilbestrol (DES) We never detected main effect of sex or interaction of sex with the factor of treatment. Therefore, male and female data were pooled. Number of subjects in each subgroup was between 7 and 25. All values refer to mean±SEM.
(A) Latency to onset of the first occurrence of spasms on P12, P13, and P15 test days. DES delayed onset of the first spasm during P12 testing; *p=0.04.
(B) Latency to onset of the fully developed spasms on P12, P13, and P15. EB delayed latency to onset of fully developed spasms on P15; *p=0.03.
(C) Number of spasms per observation period on P12, P13, and P15. There was a trend to decreased number of spasms on P12 after DES treatment; *p=0.05 (Kruskal-Wallis test corrected for ties).
(D) Relative numbers of GAD67-immunopositive cells in the neocortex. Early neonatal (P0-P5) EB increased number of GAD67 cells by 21.0% compared to oil throughout neocortex (*p=0.002). Counts only in layers V and VI revealed smaller, yet significant (*p=0.02) increase by 17.3% compared to oil.
(E) Body weight gain between P1 and P15. Overall significance; ANOVA p<0.0011. Effect of EB treatment vs. oil; *p=0.04. Effect of DES treatment vs. oil; *p<0.001.
(F) P31 male (time of puberty onset) neonatally injected with DES. Note that the testes still remain in the abdominal cavity (no descent). The scrotum is not formed (arrow).
(G) P31 male neonatally injected with oil. Descended testes in the scrotum are clearly visible (arrow) compared to (C).
A separate cohort of rats treated between P0-P5 with EB was evaluated for number of GAD-67 immunopositive neurons in the neocortex (Fig. 4D). We found that EB between P0-P5 also relatively increased density of GAD67-immunopositive cells throughout neocortex compared to oil controls (Student’s t-test, *p=0.002). This increase by 21.0% was comparable to the increase associated with P3-P10 EB treatment (23.3%, see above). Separate counts of layers V and VI did not reveal larger contrast as above, the increase was only 17.3% after EB, yet significant (*p=0.02).
When we investigated weight gain as in the previous experiment, we found significant effects of both EB and DES treatments (ANOVA F(2, 38)=68.399; *p<0.0001; Fig. 4E). The effect of EB was consistent with the previous experiment. EB induced significant increase in body weight gain compared to oil-injected controls. On the other hand, DES induced significant decrease in body weight gain compared to both controls and EB treatment.
We found that neonatal DES treatment prevented normal testicular descent in male rats, thus causing cryptorchidism. In oil controls, testes descended by P31 marking the onset of puberty, but in DES-treated animals at P31, the testes remained in the abdominal cavity (Fig. 4F,G).
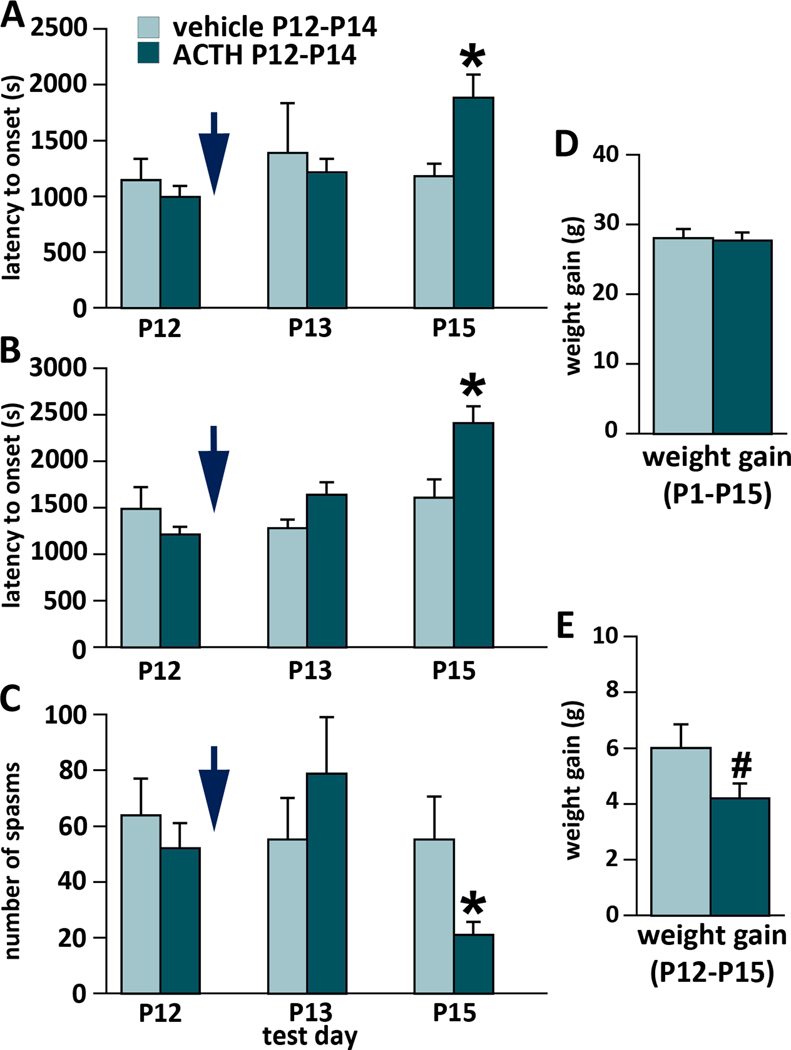
Chronic ACTH treatment suppresses the occurrence of spasms
As a positive control, we ran a prospective randomized trial on ACTH. Rats were randomized to ACTH and vehicle groups after the first trigger of spasms on P12. Efficacy of randomization is shown in the P12 columns of Fig. 5A-C. Treatment with ACTH was completed on P14 and the rats were subjected to the final trigger of spasms on P15. ACTH (n=8) significantly delayed onset of the first spasm compared to vehicle (n=6; Fig. 5A; t-Value=2.684; *p=0.02). ACTH also significantly delayed onset of the fully developed spasms (Fig. 5B; t-Value=3.015; *p=0.01) and suppressed number of spasms per subject (Fig. 5C; t-Value=2.565; *p=0.02). We never found any sex difference or interaction of sex with treatment. There was no effect of ACTH on body weight gain in the P1-P15 interval (Fig. 5D). However, if we followed body weight gain just through the treatment period of P12-P15, there was a trend to decreased weight gain after ACTH (t-Value=1.898; #p=0.08; Fig. 5E).
Figure 5.
Effects of ACTH in the randomized prospective trial on the expression of spasms
First bout of spasms was triggered on P12 and afterwards the rats were randomized to the treatment (ACTH) or vehicle groups (efficacy of randomization is shown in the P12 column in A, B, C; blue arrow marks randomization). Afterwards they received ACTH 0.3 mg/kg per dose s.c. twice on P12 and three times on P13, and P14. Spasms were triggered on P13 and again on P15 (12–14 hours after the last ACTH dose). There were consistent effects of ACTH on the expression of spasms despite smaller n of subjects (5–10 in each subgroup).
(A) Latency to onset of the first occurrence of spasms on P12, P13, and P15 test days. There was an effect of ACTH in delaying onset of the first spasm during P15 testing; *p=0.02.
(B) Latency to onset of the fully developed spasms on P12, P13, and P15. We found an effect of ACTH again in delaying latency to onset of fully developed spasms on P15; *p=0.01.
(C) Number of spasms per observation period on P12, P13, and P15. There was a significant decrease in number of spasms on P15 after ACTH treatment; *p=0.02.
(D) Body weight gain between P1 and P15. There was no effect of ACTH treatment.
(E) Body weight gain during the ACTH treatment period P12-P15. There was a trend to decreased body weight gain after the ACTH treatment; #p=0.08.
DISCUSSION
Our study showed that neonatal estrogen administration increased number of GAD67-immunopositive neurons in the neocortex. These changes were comparable or even exceeded those reported previously in mice17. Our study expands these previous findings to neonatal rats and further supports the findings of EB effects on neuronal migration originally determined in songbirds31. Further, we have shown that in our model of cryptogenic infantile spasms, an increase in cortical GABAergic neurons does not affect the expression of spasms. In fact, none of the estrogen-related treatments delivered neonatally either very early (P0-P5) or later (P3-P10) suppressed the spasms consistently in our model11. Additionally, we demonstrated that in males, neonatal exposure to EB causes feminizing features in behavior (lateralization preference) and the neonatal DES treatment, while not very impressive in suppression of spasms, arrests descent of testes inducing a condition similar to human cryptorchidism32. Results of standard treatment with repeated doses of ACTH confirmed significant protection against the spasm in the model.
We found evidence that neonatal EB administration (P3-P10 as well as P0-P5) resulted in significant increase of GAD67-immunopositive (presumably GABAergic) neurons in the neocortex of infant rats (without exposure to spasms). This feature of EB (promoting migration of GABAergic neurons) has been shown in songbirds31 and recently also in mice with triplet repeat expansion of the Arx gene [Arx(GCG)10+7]17. The primary deficit in [Arx(GCG)10+7] mice is missing interneurons that is corrected by EB. EB also suppresses spasms that occur in these mice16, 17. Consistent with previous and current findings on neonatal estrogen effects, only neonatal EB increases GABA-mediated currents in the hippocampus33. It is reasonable to argue that if there was no deficit in GABAergic interneurons in our model of infantile spasms in the first place, there would be no corrective effect of neonatal EB; or, in other words the overexpression was not providing any additional benefit. Further our results indicate that early neonatal (P0-P5) EB may be responsible for increase in GAD67 immunopositive neurons throughout neocortex, while later EB treatment (after P5) contributes more to increased numbers of GAD67 immunopositive neurons in layers V and VI. Decreased number of neocortical GABAergic interneurons has been identified postmortem in the cerebral cortex of a patient with Arx gene mutation34, which has been reproduced by the two Arx gene-related mutation models in mice16, 35. However, association of decreased number in cortical GABAergic interneurons and IS with other origin than Arx gene mutation has not been consistently studied and little is known about GABAergic interneuron deficits in other published models of IS.
In rats, there is sex-specific side preference in behavioral tests (female lateralization)27, 36. However, we did not observe this effect as it was likely eliminated by prenatal betamethasone exposure. Similar dissipation of female lateralization can be accomplished by prenatal stress28, 37. Yet in rats after prenatal exposure to betamethasone and serial spasms in infancy, we do observe lateralization in those males treated with EB. This finding strongly suggests feminization of their behavioral approach to the task.
In males, DES also significantly affected testicular descent, as shown, the situation was of resemblance to human cryptorchism. This feature of DES has been well documented before38. This side effect does not appear to be overweighed by inconsistent and minor effects of DES on spasms (counteracting occurrence of spasms only on P12). Similarly, EB administration can induce cryptorchism yet its effect is dose-dependent (appearing after high doses of EB)39. Nevertheless, EB did not have any consistent effects on the spasms in our model.
Finally, we reconfirmed substantial and significant effects of ACTH (in prospective randomized trial) on the expression of spasms in this model of infantile spasms. ACTH significantly suppressed occurrence of spasms consistent with previous studies11, 13 and with its effects in human IS5, 40.
In conclusion, enhancing GABAergic inhibition using neonatal EB administration was effective in controlling seizures in the ARX gene mutation model14, 15 associated with detectable deficits in GABAergic neurons. However in models without detectable GABAergic deficits, the efficacy of neonatal estrogen administration is debatable and serious side effects may prevail over any perceivable benefits.
Supplementary Material
KEY POINT BOX.
These experiments were designed to assess whether neonatal estradiol suppresses spasms in an animal model of symptomatic infantile spasms, as previously shown in a mouse Arx gene expansion model
We used a model of prenatal priming with corticosteroids and repeated postnatal trigger of spasms with NMDA on postnatal days 12, 13 and 15
Two paradigms of neonatal estradiol or neonatal diethylstilbestrol did not have any protective effects against spasms in contrast to positive results with ACTH
Neonatal injections of estradiol increased number of GABAergic neurons in the neocortex
ACKNOWLEDGMENTS
Supported by CURE Infantile Spasms Research Initiative, by NIH grants NS072966 (LV); NS056093 (JV); and Epilepsy Foundation of America (JV).
LV has received grant support from Neurocrine Biosciences, Inc. not related to this study.
Footnotes
DISCLOSURE OF CONFLICT OF INTEREST
All other authors have no conflict of interest.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.Ioannidis JP. How to make more published research true. PLoS Med. 2014;11:e1001747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dulac O, Soufflet C, Chiron C, et al. What is West syndrome? Int Rev Neurobiol. 2002;49:1–22. [DOI] [PubMed] [Google Scholar]
- 3.Riikonen R. Epidemiological data of West syndrome in Finland. Brain Dev. 2001;23:539–41. [DOI] [PubMed] [Google Scholar]
- 4.Widjaja E, Go C, McCoy B, et al. Neurodevelopmental outcome of infantile spasms: A systematic review and meta-analysis. Epilepsy Res. 2015;109:155–62. [DOI] [PubMed] [Google Scholar]
- 5.Go CY, Mackay MT, Weiss SK, et al. Evidence-based guideline update: medical treatment of infantile spasms. Report of the Guideline Development Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2012;78:1974–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lux AL, Edwards SW, Hancock E, et al. The United Kingdom Infantile Spasms Study (UKISS) comparing hormone treatment with vigabatrin on developmental and epilepsy outcomes to age 14 months: a multicentre randomised trial. Lancet Neurol. 2005;4:712–7. [DOI] [PubMed] [Google Scholar]
- 7.Riikonen R. ACTH therapy of West syndrome: Finnish views. Brain Dev. 2001;23:642–6. [DOI] [PubMed] [Google Scholar]
- 8.Westall CA, Wright T, Cortese F, et al. Vigabatrin retinal toxicity in children with infantile spasms: An observational cohort study. Neurology. 2014;83:2262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Velíšek L, Jehle K, Asche S, et al. Model of infantile spasms induced by N-methyl-D-aspartic acid in prenatally impaired brain. Ann Neurol. 2007;61:109–19. [DOI] [PubMed] [Google Scholar]
- 10.Mareš P, Velíšek L. N-methyl-D-aspartate (NMDA)-induced seizures in developing rats. Brain Res Dev Brain Res. 1992;65:185–9. [DOI] [PubMed] [Google Scholar]
- 11.Chachua T, Yum M-S, Velíšková J, et al. Validation of the rat model of cryptogenic infantile spasms. Epilepsia. 2011;52:1666–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yum MS, Chachua T, Velíšková J, et al. Prenatal stress promotes development of spasms in infant rats. Epilepsia. 2012;53:e46–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuji M, Takahashi Y, Watabe AM, et al. Enhanced long-term potentiation in mature rats in a model of epileptic spasms with betamethasone-priming and postnatal N-methyl-d-aspartate administration. Epilepsia. 2016;57:495–505. [DOI] [PubMed] [Google Scholar]
- 14.Stromme P, Mangelsdorf ME, Shaw MA, et al. Mutations in the human ortholog of Aristaless cause X-linked mental retardation and epilepsy. Nat Genet. 2002;30:441–5. [DOI] [PubMed] [Google Scholar]
- 15.Poirier K, Van Esch H, Friocourt G, et al. Neuroanatomical distribution of ARX in brain and its localisation in GABAergic neurons. Brain Res Mol Brain Res. 2004;122:35–46. [DOI] [PubMed] [Google Scholar]
- 16.Price MG, Yoo JW, Burgess DL, et al. A triplet repeat expansion genetic mouse model of infantile spasms syndrome, Arx(GCG)10+7, with interneuronopathy, spasms in infancy, persistent seizures, and adult cognitive and behavioral impairment. J Neurosci. 2009;29:8752–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olivetti PR, Maheshwari A, Noebels JL. Neonatal estradiol stimulation prevents epilepsy in Arx model of X-linked infantile spasms syndrome. Sci Transl Med. 2014;6:220ra12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunson KL, Eghbal-Ahmadi M, Baram TZ. How do the many etiologies of West syndrome lead to excitability and seizures? The corticotropin releasing hormone excess hypothesis. Brain Dev. 2001;23:533–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iqbal SA, Wallach JD, Khoury MJ, et al. Reproducible Research Practices and Transparency across the Biomedical Literature. PLoS Biol. 2016;14:e1002333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–303. [DOI] [PubMed] [Google Scholar]
- 21.Velíšková J, Velíšek L. Beta-estradiol increases dentate gyrus inhibition in female rats via augmentation of hilar neuropeptide Y. J Neurosci. 2007;27:6054–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Velíšek L, Shang E, Velíšková J, et al. GABAergic neuron deficit as an idiopathic generalized epilepsy mechanism: the role of BRD2 haploinsufficiency in juvenile myoclonic epilepsy. PLoS One. 2011;6:e23656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rieux C, Carney R, Lupi D, et al. Analysis of immunohistochemical label of Fos protein in the suprachiasmatic nucleus: comparison of different methods of quantification. J Biol Rhythms. 2002;17:121–36. [DOI] [PubMed] [Google Scholar]
- 24.Ravizza T, Friedman LK, Moshé SL, et al. Sex differences in GABA(A)ergic system in rat substantia nigra pars reticulata. Int J Dev Neurosci. 2003;21:245–54. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein LE, Fisher AM, Tagge CA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4:134ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathiasen JR, DiCamillo A. Novel object recognition in the rat: a facile assay for cognitive function. Curr Protoc Pharmacol. 2010;Chapter 5:Unit 5.59. [DOI] [PubMed] [Google Scholar]
- 27.Castellano MA, Diaz-Palarea MD, Barroso J, et al. Behavioral lateralization in rats and dopaminergic system: individual and population laterality. Behav Neurosci. 1989;103:46–53. [DOI] [PubMed] [Google Scholar]
- 28.Alonso J, Castellano MA, Rodriguez M. Behavioral lateralization in rats: prenatal stress effects on sex differences. Brain Res. 1991;539:45–50. [DOI] [PubMed] [Google Scholar]
- 29.Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. [DOI] [PubMed] [Google Scholar]
- 30.Korte SM, De Boer SF. A robust animal model of state anxiety: fear-potentiated behaviour in the elevated plus-maze. Eur J Pharmacol. 2003;463:163–75. [DOI] [PubMed] [Google Scholar]
- 31.Williams S, Leventhal C, Lemmon V, et al. Estrogen promotes the initial migration and inception of NgCAM-dependent calcium-signaling by new neurons of the adult songbird brain. Mol Cell Neurosci. 1999;13:41–55. [DOI] [PubMed] [Google Scholar]
- 32.Kollin C, Ritzen EM. Cryptorchidism: a clinical perspective. Pediatr Endocrinol Rev. 2014;11 Suppl 2:240–50. [PubMed] [Google Scholar]
- 33.Wojtowicz T, Lebida K, Mozrzymas JW. 17beta-estradiol affects GABAergic transmission in developing hippocampus. Brain Res. 2008;1241:7–17. [DOI] [PubMed] [Google Scholar]
- 34.Okazaki S, Ohsawa M, Kuki I, et al. Aristaless-related homeobox gene disruption leads to abnormal distribution of GABAergic interneurons in human neocortex: evidence based on a case of X-linked lissencephaly with abnormal genitalia (XLAG). Acta Neuropathol. 2008;116:453–62. [DOI] [PubMed] [Google Scholar]
- 35.Marsh E, Fulp C, Gomez E, et al. Targeted loss of Arx results in a developmental epilepsy mouse model and recapitulates the human phenotype in heterozygous females. Brain. 2009;132:1563–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ross DA, Glick SD, Meibach RC. Sexually dimorphic brain and behavioral asymmetries in the neonatal rat. Proc Natl Acad Sci U S A. 1981;78:1958–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fride E, Weinstock M. Alterations in behavioral and striatal dopamine asymmetries induced by prenatal stress. Pharmacol Biochem Behav. 1989;32:425–30. [DOI] [PubMed] [Google Scholar]
- 38.McKinnell C, Atanassova N, Williams K, et al. Suppression of androgen action and the induction of gross abnormalities of the reproductive tract in male rats treated neonatally with diethylstilbestrol. J Androl. 2001;22:323–38. [PubMed] [Google Scholar]
- 39.Zadina JE, Dunlap JL, Gerall AA. Modifications induced by neonatal steroids in reproductive organs and behavior of male rats. J Comp Physiol Psychol. 1979;93:314–22. [DOI] [PubMed] [Google Scholar]
- 40.Mackay MT, Weiss SK, Adams-Webber T, et al. Practice parameter: medical treatment of infantile spasms: report of the American Academy of Neurology and the Child Neurology Society. Neurology. 2004;62:1668–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.