Key Points
Question
What is the efficacy of fully unsupervised home-use transcranial direct current stimulation (tDCS) combined with either a digital psychological intervention or digital placebo for the treatment of a major depressive episode?
Findings
In this randomized clinical trial including 210 adults with a major depressive episode, no statistically significant differences were detected between home-use tDCS combined with either a digital psychological intervention or digital placebo vs sham in reducing depressive symptoms after 6 weeks.
Meaning
The findings indicate that unsupervised home use tDCS should not be currently recommended in clinical practice.
This randomized clinical trial evaluates the efficacy of home-use transcranial direct current stimulation, combined or not with a digital psychological intervention, vs sham for the treatment of major depression.
Abstract
Importance
Transcranial direct current stimulation (tDCS) is moderately effective for depression when applied by trained staff. It is not known whether self-applied tDCS, combined or not with a digital psychological intervention, is also effective.
Objective
To determine whether fully unsupervised home-use tDCS, combined with a digital psychological intervention or digital placebo, is effective for a major depressive episode.
Design, Setting, and Participants
This was a double-blinded, sham-controlled, randomized clinical trial with 3 arms: (1) home-use tDCS plus a digital psychological intervention (double active); (2) home-use tDCS plus digital placebo (tDCS only), and (3) sham home-use tDCS plus digital placebo (double sham). The study was conducted between April 2021 and October 2022 at participants’ homes and at Instituto de Psiquiatria do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, Brazil. Included participants were aged 18 to 59 years with major depression and a Hamilton Depression Rating Scale, 17-item version (HDRS-17), score above 16, a minimum of 8 years of education, and access to a smartphone and internet at home. Exclusion criteria were other psychiatric disorders, except for anxiety; neurologic or clinical disorders; and tDCS contraindications.
Interventions
tDCS was administered in 2-mA, 30-minute prefrontal sessions for 15 consecutive weekdays (1-mA, 90-second duration for sham) and twice-weekly sessions for 3 weeks. The digital intervention consisted of 46 sessions based on behavioral therapy. Digital placebo was internet browsing.
Main Outcomes and Measures
Change in HDRS-17 score at week 6.
Results
Of 837 volunteers screened, 210 participants were enrolled (180 [86%] female; mean [SD] age, 38.9 [9.3] years) and allocated to double active (n = 64), tDCS only (n = 73), or double sham (n = 73). Of the 210 participants enrolled, 199 finished the trial. Linear mixed-effects models did not reveal statistically significant group differences in treatment by time interactions for HDRS-17 scores, and the estimated effect sizes between groups were as follows: double active vs tDCS only (Cohen d, 0.05; 95% CI, −0.48 to 0.58; P = .86), double active vs double sham (Cohen d, −0.20; 95% CI, −0.73 to 0.34; P = .47), and tDCS only vs double sham (Cohen d, −0.25; 95% CI, −0.76 to 0.27; P = .35). Skin redness and heat or burning sensations were more frequent in the double active and tDCS only groups. One nonfatal suicide attempt occurred in the tDCS only group.
Conclusions and Relevance
Unsupervised home-use tDCS combined with a digital psychological intervention or digital placebo was not found to be superior to sham for treatment of a major depressive episode in this trial.
Trial Registration
ClinicalTrials.gov Identifier: NCT04889976
Introduction
Major depression affects more than 300 million people worldwide.1,2 Treatments include antidepressant drugs, cognitive behavioral therapy, and noninvasive brain stimulation.3,4 However, pharmacotherapy is ineffective for one-third of patients,5 cognitive behavioral therapy might not be readily available,6 transcranial magnetic stimulation is costly and warrants daily visits to clinical facilities, and electroconvulsive therapy presents neurocognitive effects.7
Transcranial direct current stimulation (tDCS) is a noninvasive brain stimulation modality that is moderately effective for major depression.8,9,10 It involves the application of low currents through 2 or more electrodes placed over the scalp, thereby modulating underlying neuronal excitability and neuroplasticity.11 Unlike transcranial magnetic stimulation and electroconvulsive therapy, tDCS is portable, straightforward, inexpensive, and has minimal adverse effects.12,13 However, tDCS needs to be applied daily, and when performed at the clinic, can pose dislocation burdens and is limited by the working hours of the staff.14
Therefore, home-use tDCS could greatly increase scalability and enable its widespread adoption. A small, open-label trial15 showed promising therapeutic results for depression, with satisfactory feasibility and safety profiles. Nonetheless, despite the development and adoption of several portable devices,16 their efficacy has not been confirmed in rigorously controlled clinical studies.
Here, we conducted a randomized, sham-controlled clinical trial to verify the efficacy, acceptability, and tolerability of home-use tDCS combined with either a digital psychological intervention or digital placebo in adults with major depression. We hypothesized that, after 6 weeks of treatment, home-use tDCS combined with a digital psychological intervention (double active) would be superior to either home-use tDCS combined with a digital placebo (tDCS only) or sham home-use tDCS combined with a digital placebo (double sham) and that tDCS only would be superior to double sham in reducing depressive symptoms.
Methods
Overview
The Portable Transcranial Electrical Stimulation and Internet-Based Behavioral Therapy for Major Depression Study (PSYLECT) trial design and rationale have been previously published.17 No major changes occurred from the original protocol, which can be found in Supplement 1. The study was approved by the Comitê de Ética em Pesquisa do Hospital Universitário da USP and Comitê de Ética em Pesquisa do Hospital das Clínicas da Faculdade de Medicina da USP and national ethics committees, is registered in ClinicalTrials.gov (NCT04889976), and abides by the Brazilian (Lei Geral de Proteção de Dados) and international data protection laws. All participants provided written informed consent. This study is reported according to Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.18
PSYLECT was conducted from April 2021 to October 2022 at the Instituto de Psiquiatria do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, Brazil, and in participants’ homes. It used a 3-arm, parallel, double-blinded design, in which 210 patients with major depression were randomly assigned (1:1:1) to double active, tDCS only, or double sham. Randomization was performed upon in-person enrollment at the research center through a remote platform based on a Mersenne Twister algorithm.19 Allocation was concealed via Flow Neuroscience version 2.14.0 (Projeto Psylect) that seamlessly assigned the participants’ groups after online registration. The randomization list was protected by cryptography, and randomization was only broken after the analyses of all a priori hypotheses.
Participants
Participants were eligible if they were aged 18 to 59 years, had a diagnosis of major depression, and were in an acute depressive episode of at least moderate severity, defined by a score on the Hamilton Depression Rating Scale, 17-item version (HDRS-17), above 16.20 The diagnosis was confirmed by psychiatrists using the Mini International Neuropsychiatric Interview.21 Additional criteria were access to a smartphone and internet at home and at least 8 years of formal education. We excluded volunteers with major neurologic disorders (eg, epilepsy, traumatic brain injury, and stroke), other psychiatric disorders except for comorbid anxiety, depressive symptoms better explained by other psychiatric or physical conditions, and contraindications to tDCS.
Antidepressant drugs were at a stable dose 6 weeks prior to enrollment and kept unchanged. Benzodiazepines were tolerated at a maximum of 10 mg/d diazepam (or equivalent). Lifetime drug refractoriness could be no more than 3 failed adequate antidepressant trials, per the Antidepressant Treatment History Form.22
Interventions
We used an intervention composed of a home-use tDCS headset (eFigure 1 in Supplement 2), coupled with a low-intensity digital psychological intervention delivered by an application. The application was programmed to initiate and finish the electrical stimulation without user awareness and interference and through the same front-end interface. The system was supplied by Flow Neuroscience (Malmo, Sweden). Each participant received 1 device to be used remotely and in an unsupervised manner and were instructed by the study staff on how to operate them. tDCS sessions were to be performed at least 24 hours apart.
Twenty-one home-use tDCS sessions (2 mA, 30 minutes per day, 5 times per week for the first 3 weeks and then twice a week for the remaining 3 weeks) were delivered through the scalp, via a left (anode) and a right (cathode) electrode (22.9 cm2) positioned over the frontal arc. Their position was fixed (10.5 cm apart and equally distant from the midline), approximately corresponding to the F3 and F4 locations per the international 10-20 electroencephalography system.23 Sponges were premoistened with saline and used only once per session. The stimulation session only started when devices were correctly placed by the participants, who were aided by an augmented-reality tool through the smartphone camera. The electrical stimulation was automatically stopped if the impedance surpassed 9 kOhm (eg, if the device was displaced or removed). The same parameters were used for sham stimulation, except for the current, which was only active at the initial and final 45 seconds of each session and reached a peak of only 1 mA.24
The digital intervention was delivered during the tDCS sessions. Participants were interactively exposed to text- and video-based content derived from behavioral therapy. The intervention had 7 modules, each of which contained approximately 5-minute sessions, including 5 introduction sessions, 5 behavioral activation sessions, 8 mindfulness sessions, 8 physical exercise sessions, 10 healthy diet sessions, 7 sleep hygiene sessions, and 3 future planning sessions. Participants could spend additional time in the application when completing daily diaries. All content was offered in Brazilian Portuguese and curated by our team.17 The sham intervention consisted of free internet browsing delivered through the same interface.
Participants were instructed to perform a maximum of 2 to 3 digital intervention sessions per 30-minute tDCS session. If they finished the digital intervention sessions earlier than the 30-minute electrical stimulation, they were instructed to remain at rest until stimulation completion.
Assessments
Study data were collected and managed using REDCap (Research Electronic Data Capture).25,26 All assessments were performed by board-certified psychiatrists and neuropsychologists, who underwent additional certified training in the application of the scales used in this trial. Except for the first and last assessments, the screening stage and other evaluations were performed remotely. To ensure blinding, we deliberately withheld detailed information about the digital psychological intervention in the informed consent sheet, and participants were asked not to comment on any specific aspects of the interventions during clinical assessments. Research assistants not involved in clinical assessments aided participants if necessary. If clinical evaluators became inadvertently unblinded regarding a specific participant at any time, that participant would be referred to another blinded staff member.
The primary efficacy outcome was the change in HDRS-17 scores over time, assessed via the Structured Interview Guide for the Hamilton Depression Rating Scale,27 which is associated with an intraclass correlation of r = 0.91 for the total HDRS-17 scores between raters.28 tDCS-related adverse events were evaluated per a commonly used tDCS adverse event questionnaire,13 acceptability was assessed by the number of dropouts per group, adherence was calculated dividing the number of minutes of performed stimulation by the total number of minutes in the trial protocol, and usability was assessed according to 5 visual analog scales (eList 1 in Supplement 2).
Secondary outcomes were rates of clinical response (defined as ≥50% reduction in HDRS-17 scores between baseline and end point) and remission (defined as an HDRS-17 score ≤7 at end point). Other outcomes were changes over time indexed by additional scales that assess psychological and psychiatric symptoms (eList 2 in Supplement 2).
Statistical Analyses
For sample size calculations, in the absence of previous trials comparing the effects of tDCS and digital psychological interventions, we estimated the effects of the low-intensity digital intervention based on previous systematic reviews and meta-analyses of clinical trials using these types of interventions29 and methodological articles discussing combined neuromodulation strategies.30 Here, we assumed mean (SD) baseline HDRS-17 scores of 25 (5), and significant improvements of 0.4 (tDCS only) and 0.8 (double active) SDs compared to double sham over the trial period. Dropouts were assumed to increase monotonically (Weibull) and be equally distributed.
The study was powered to detect an effect size (difference in the mean change divided by the SD) of 0.4 or larger for each of the 3 pairwise comparisons. Considering a power (β) of 80% and a Bonferroni-corrected significance level of α = .05/3, we obtained a n = 70 per arm considering a 10% attrition rate. Calculations were performed using the powerlmm in R version 4.2.3 (R Foundation) and are described in the eMethods in Supplement 2.
Data were analyzed using R in the intention-to-treat sample. For continuous outcomes, we used 2-level linear mixed-effects regression models, assuming a linear relationship over time and an unstructured covariance structure. The linear mixed-effects regression models included a discrete factor for the treatment group (3 levels: double active, tDCS only, and double sham), a continuous time factor over 5 time points (baseline and weeks 2, 3, 4, and 6), and the interaction of group and time as fixed effects; and patient as random effect, with random slope and random intercept. The interaction of group and time was tested as the relevant criterion for treatment efficacy. We reported the effect size for the between-group difference in symptom improvement as Cohen d, which was computed using the emmeans::eff_size() function.
Frequency and severity of adverse events, blinding integrity, and usability were compared between groups with the χ2-test or Fisher exact test. Negative binomial regression was used to compare the number of adverse events and to evaluate if tolerability was similar between the groups.
Results
Participants
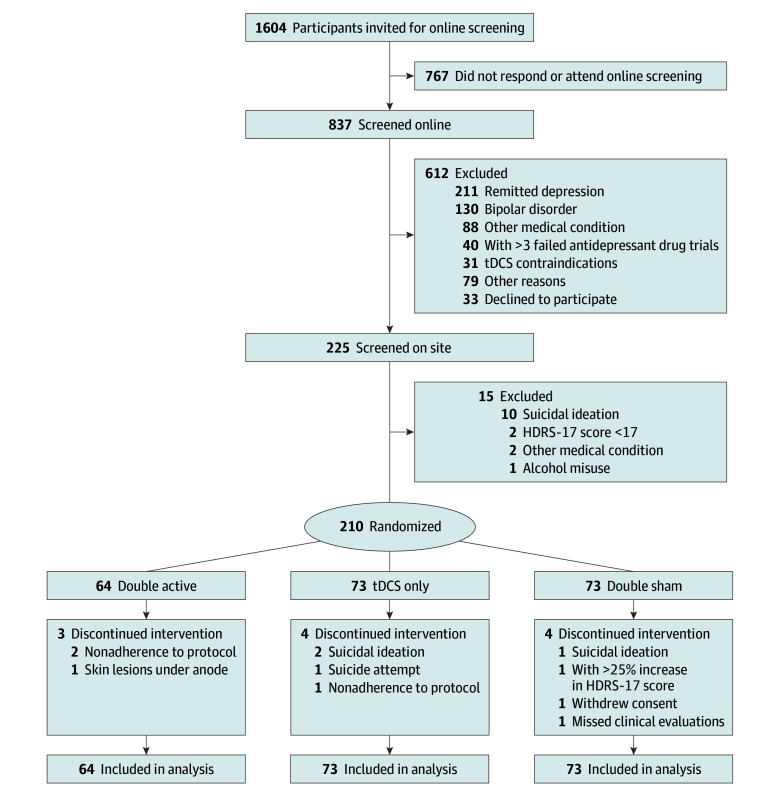
Of 1604 prescreened and 837 individually screened volunteers, 210 participants were enrolled (180 [86%] female; mean [SD] age, 38.9 [9.3] years; 7 [3%] Asian, 13 [6%] Black, 152 [72%] White, 33 [16%] multiple races, and 5 [3%] not declared), and 199 (95%) completed the study. A total of 119 participants (57%) declared a personal monthly wage of $1300 or less (equivalent in Brazilian reais), and 29 (14%) were unemployed (Figure 1 and Table 131,32; eTable 1 in Supplement 2). Participants were distributed over a wide geographical area in the greater city of São Paulo, Brazil, with a larger concentration of participants near the research center (eFigure 2 in Supplement 2).
Figure 1. Study Flow Diagram.
Table 1. Demographic and Clinical Characteristics of Participants at Baseline.
| No. (%) | Double active (n = 64) | tDCS only (n = 73) | Double sham (n = 73) |
|---|---|---|---|
| Demographic characteristics | |||
| Sex | |||
| Male | 10 (16) | 5 (7) | 15 (21) |
| Female | 54 (84) | 68 (93) | 58 (79) |
| Age, mean (SD), y | 38.7 (10.1) | 38.8 (9.1) | 39.2 (8.8) |
| Racea | |||
| Asian | 0 | 4 (5) | 3 (4) |
| Black | 2 (3) | 8 (11) | 3 (4) |
| White | 48 (75) | 54 (74) | 50 (68) |
| Multiple races | 14 (22) | 5 (7) | 14 (19) |
| Not declared | 0 | 2 (3) | 3 (4) |
| Completed graduate school | 40 (63) | 57 (78) | 51 (70) |
| ≤$1300 (equivalent in Brazilian reais) | 33 (52) | 43 (59) | 43 (59) |
| Currently working | 36 (56) | 47 (64) | 58 (79) |
| Sedentary lifestyle | 44 (69) | 51 (70) | 54 (74) |
| Clinical characteristics | |||
| Age at onset of major depression, mean (SD), y | 24.5 (10.6) | 24.2 (8.6) [n = 72]b | 25.6 (8.3) [n = 72]b |
| No. of previous depressive episodes, median (IQR) | 4 (2-5) | 3 (2-5) [n = 71]b | 3 (2-5) [n = 71]b |
| Duration of current episode, mean (SD), mo | 24.0 (39.9) | 34.5 (58.6) [n = 72]b | 25.0 (22.9) [n = 72]b |
| No. of failed AD trials, median (IQR) | 1 (0-2) | 1 (0-2) | 1 (0-2) |
| Treatment resistance in current episodec | |||
| ≥1 Failed AD trial | 16 (25) | 21 (29) | 21 (29) |
| ≥2 Failed AD trials | 6 (9) | 3 (4) | 3 (4) |
| Type of depression | |||
| Recurrentd | 51 (80) | 62 (85) | 66 (90) |
| Chronic (>2-y episode) | 19 (30) | 30 (41) | 32 (44) |
| Melancholic | 22 (34) | 26 (36) | 25 (34) |
| Comorbid anxiety disorderse | 43 (67) | 56 (77) | 53 (73) |
| Family history of mental disorderf | 54 (84) | 63 (86) | 66 (90) |
| Treatments in current depressive episode | |||
| SSRIs | 22 (34) | 23 (32) | 26 (36) |
| SNRIs | 16 (25) | 20 (27) | 19 (26) |
| Bupropion | 6 (9) | 11 (15) | 10 (14) |
| Other antidepressants | 13 (20) | 10 (14) | 12 (16) |
| No antidepressants | 23 (36) | 27 (37) | 22 (30) |
| Benzodiazepines | 17 (27) | 12 (16) | 14 (19) |
| Concurrent psychotherapy | 19 (30) | 31 (42) | 37 (51) |
| Depression scale scores, mean (SD) | |||
| HDRS-17g | 21.0 (3.3) | 20.8 (3.5) | 20.8 (3.3) |
| MADRSh | 29.9 (5.7) | 30.3 (6.1) | 29.9 (5.7) |
| BDI-IIi | 36.5 (7.4) | 36.1 (8.7) | 36.1 (8.8) [n = 72]c |
Abbreviations: AD, antidepressant; BDI-II, Beck Depression Inventory-II; BRL$, Brazilian reais; HDRS-17, Hamilton Scale of Depressive Symptoms, 17-item version; MADRS, Montgomery Åsberg Depression Rating Scale; SNRI, serotonin and norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; tDCS, transcranial direct current stimulation.
Race data were based on self-classification from a number of options defined by the investigators. This information was collected to assess the diversity, representativeness, and generalizability of the study.
Different denominators owing to missing data.
Treatment resistance was defined as 1 or more treatment failure with an antidepressant at adequate doses and over an appropriate period in the current episode.
Recurrent depression is defined as the occurrence of more than 1 depressive episode during a lifetime.
Anxiety disorders are generalized anxiety disorder, social anxiety disorder, and panic disorder.
Family history of mental disorder includes depression, bipolar disorder, schizophrenia, alcohol and substance use disorder, anxiety disorders, obsessive-compulsive disorder, and dementia.
HDRS-1720 scores range from 0 to 52, with higher scores indicating greater depression severity.
MADRS31 scores range from 0 to 60, with higher scores indicating greater depression severity.
BDI-II32 scores range from 0 to 63, with higher scores indicating greater depression severity.
Primary Outcome
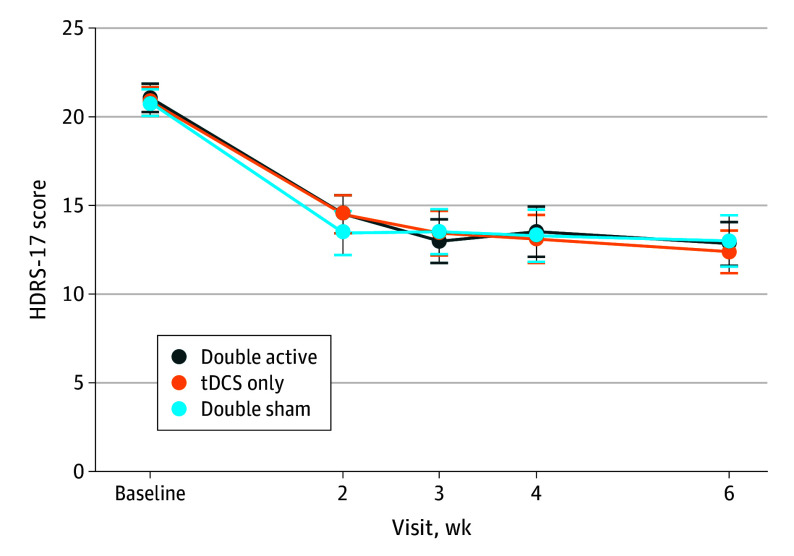
The mean (SD) change in HDRS-17 scores from baseline was 8.2 (5.6) in double active, 8.5 (5.1) in tDCS only, and 7.7 (6.0) in double sham. Linear mixed-effects models did not reveal statistically significant group differences in treatment by time interactions for HDRS-17 (Figure 2 and Table 2).
Figure 2. Change in Depression Scores Over Time.
Table 2. Primary Outcomea.
| Double active | tDCS only | Double sham | |
|---|---|---|---|
| HDRS-17 scores at various time points, mean (SD)b | |||
| Baseline | 21.0 (3.3) | 20.8 (3.5) | 20.8 (3.3) |
| Week 2 | 14.5 (4.4) | 14.5 (4.7) | 13.4 (5.4) |
| Week 3 | 13.0 (5.0) | 13.4 (5.3) | 13.5 (5.5) |
| Week 4 | 13.5 (5.8) | 13.1 (5.7) | 13.3 (6.4) |
| Week 6 | 12.8 (5.0) | 12.4 (5.1) | 13.0 (6.2) |
| Response/remission rates at various time points, No. (%)c | |||
| Baseline | NA | NA | NA |
| Week 2 | 12 (19)/5 (8) | 13 (18)/6 (8) | 20 (28)/12 (17) |
| Week 3 | 21 (33)/10 (16) | 21 (30)/9 (13) | 23 (32)/9 (12) |
| Week 4 | 24 (38)/8 (13) | 19 (28)/10 (14) | 24 (33)/15 (21) |
| Week 6 | 20 (31)/9 (14) | 26 (36)/13 (18) | 28 (38)/15 (21) |
| Change in HDRS-17 score per wk, β (95% CI)d | −1.30 (−1.55 to −1.04) | −1.33 (−1.57 to −1.09) | −1.17 (−1.41 to −0.93) |
Abbreviations: HDRS-17, Hamilton Depression Rating Scale 17-item version; tDCS, transcranial direct current stimulation.
The estimated effect sizes between groups were as follows: double active vs tDCS only (Cohen d, 0.05; 95% CI, −0.48 to 0.58; P = .86), double active vs double sham (Cohen d,−0.20; 95% CI, −0.73 to 0.34; P = .47), and tDCS only vs double sham (Cohen d, −0.25; 95% CI, −0.76 to 0.27; P = .35). Group comparisons represent estimated differences in HDRS-17 change per week between groups.
HDRS-1720 scores range from 0-52; minimally significant score, 0-7; remission, ≤7.
Response was defined as a ≥50% decrease in baseline HDRS-17 score and remission as an HDRS-17 score ≤7.
β Values are model estimations of changes in HDRS-17 scores for 1 week.
Secondary Outcomes
For the double active, tDCS only, and double sham groups, 20 (31%), 26 (36%), and 28 (38%) participants, respectively, presented response, and 9 (14%), 13 (18%), and 15 (21%) participants presented remission. No significant differences among treatment groups were found (Table 2; eTable 2 in Supplement 2). Likewise, other secondary outcomes were not significant (eTables 3 to 13 in Supplement 2).
Safety, Adherence and Acceptability, Usability, and Integrity of Blinding
At the study end point, all groups considered the interventions to be very easy to use (eTable 14 in Supplement 2). We could not identify group differences in the frequency, severity, and the number of reported adverse events (eTables 15-17 in Supplement 2). Local skin redness was more frequently reported in the double active and the tDCS only groups, and heat or burning sensations were more frequent in the double active group than in the double sham group. One nonfatal suicide attempt occurred in the tDCS only group. No manic or hypomanic episodes occurred.
The overall rate of adherence to home-use tDCS was 97.5% in double active, 97% in tDCS only, and 96.3% in double sham (eFigure 3 in Supplement 2). Regarding adherence to the digital psychological intervention sessions, 87.5% of participants completed more than 75% of sessions. Dropouts were balanced between groups (eTable 18 in Supplement 2). Participants and raters could not correctly guess to which group participants had been allocated (Table 3).
Table 3. Blinding Integrity Assessments.
| Group | No. (%) | Total, No. | P valuea | |
|---|---|---|---|---|
| Correct guesses | Wrong guesses | |||
| Participants | ||||
| Double active | 35 (57) | 26 (43) | 61 | .31 |
| tDCS only | 22 (32) | 47 (68) | 69 | .004 |
| Double sham | 25 (36) | 44 (64) | 69 | .03 |
| Total | 82 (41) | 117 (59) | 199 | .02 |
| Raters | ||||
| Double active | 26 (43) | 35 (57) | 61 | .31 |
| tDCS only | 12 (17) | 57 (83) | 69 | <.001 |
| Double sham | 29 (42) | 40 (58) | 69 | .23 |
| Total | 67 (34) | 132 (66) | 199 | <.001 |
P Values were computed using a χ2 1-sample proportion test.
Discussion
In this randomized clinical trial involving participants with major depression, we were unable to show that fully unsupervised home-use tDCS combined with either a digital psychological intervention or digital placebo was superior to sham.
These findings cannot be explained by either low adherence or poor usability of the home-use device, which have been shortcomings in most previous digital trials29 but were not observed in our study. Dropout rates were equally distributed among the treatment arms and were lower compared to on-site tDCS trials.33,34 Moreover, blinding was effective. Taken together, no major threats to internal validity were observed.
Notwithstanding, certain clinical characteristics of our sample might have affected the outcomes. For instance, most participants were in a stable pharmacotherapy regimen or undergoing concurrent psychotherapy. The antidepressant effects of tDCS are larger when it is used without concomitant pharmacotherapies,8 while certain medications, like benzodiazepines and mood stabilizers, have been implicated in decreasing tDCS effects.35 Moreover, compared to previous trials,33,34 here we had more participants with recurrent depression and longer depressive episodes, which have been associated with greater treatment resistance.36 In this regard, tDCS has indeed displayed lower antidepressant effects in more treatment-resistant patients.37,38,39,40 Also, our sample was representative of a middle-income country, with relatively low educational levels and a vulnerable socioeconomic status—conditions in which the effectiveness of digital technologies needs to be better elucidated.41
Although the home-based setting could theoretically have diminished the placebo response, as patients did not visit the clinical center on a daily basis, our results were similar to recent on-site tDCS trials in depression.38,42,43 Interestingly, in a recent consensus statement, specialists did not agree on whether the placebo response would be larger in digital trials.16 Also, this trial was executed during the initial years of the COVID-19 pandemic. Receiving remote care in such a scenario might have further contributed to the placebo response.
The home-use tDCS devices used a fixed electrode position, which allowed unsupervised and self-applied sessions. However, using the same tDCS montage for all patients regardless of head size likely induced distinct patterns of electric field density in the brain, which might have further influenced clinical effects.44,45 In contrast, in a randomized clinical trial evaluating supervised home-use tDCS for attention-deficit/hyperactivity disorder, the position of the electrodes was individually adjusted before home use; there, tDCS was effective.46 With respect to the home-use tDCS protocol, the interval between sessions had a minimal but not maximum time span, unlike in on-site tDCS studies, where stimulation sessions are performed in approximately regular intervals. This could have also influenced the clinical effects, as cortical excitability might vary according to the between-session intervals,47 and should be regarded as a limitation of this study.
Furthermore, the choice of a fixed current intensity, while common practice in tDCS research, does not address the issue of the optimal individual intensity to elicit effective electrical fields in brain areas of interest, amounting to the variability of our outcomes.48,49,50 Moreover, our shorter trial duration of 6 weeks, while more likely to diminish attrition rates and favor the completion of a home-based protocol, might have been insufficient to allow for the differentiation between active and sham tDCS, and should be regarded as an additional limitation of the current study. For instance, in our previous noninferiority randomized trial comparing on-site tDCS with escitalopram, treatment with tDCS was superior to sham only after the sixth week of treatment.33 Also, a recent trial that investigated the additive effects of on-site tDCS in patients with depression receiving stable pharmacotherapy showed no difference between active and sham tDCS, but also lasted only 6 weeks.42
Our standardized digital approach, in which all modules were offered to participants regardless of their specific symptoms, might have contained mixed interventions with different efficacies.51 Furthermore, participants used the digital modules on demand, without a fixed order. Therefore, we could not disentangle the particular behavioral effects that each module could have elicited. Also, digital interventions might be more effective in patients with less severe depressive symptoms.29,52 A recent clinical trial that investigated the efficacy of supervised tDCS combined with in-group cognitive behavioral therapy also yielded negative results, leading the authors to speculate if the less directed and more multifaceted group intervention could have increased outcome variability.43
Limitations
This study has limitations. A 6-week treatment course might have been too short to induce clinical effects. This time frame was also used in 2 large-scale tDCS randomized clinical trials that yielded negative results,42,43 while significant tDCS effects were observed in a study that spanned 22 tDCS sessions over 10 weeks.33 Also, a recent meta-analysis53 suggested that tDCS might display larger antidepressant effects over longer treatment courses. Moreover, while we used a validated and structured interview for the primary outcome applied by board-certified psychiatrists and neuropsychologists, our interrater reliability was not formally assessed. Other limitations include the lack of precision on electrode positioning and the heterogeneous effects of the modules composing the digital psychological intervention.
Conclusions
In a randomized and sham-controlled clinical trial evaluating the efficacy of fully unsupervised, remotely administered, and self-applied home-use tDCS combined with either a digital psychological intervention or digital placebo, we could not show that the active interventions differed from sham in improving depressive symptoms. These findings indicate that unsupervised home use should not currently be recommended as a tDCS modality in clinical practice.
Trial protocol
eAppendix. eFigure 1. Transcranial direct current stimulation (tDCS) headset used in the Psylect trial
eList 1. Usability VAS-Scales [0 (completely disagree) to 100 (completely agree)]
eList 2. Secondary scales
eMethods. Power calculations used for the Psylect trial
eTable 1. Baseline characteristics of the sample (complementary material)
eFigure 2. Participant distribution density heat map
eTable 2. Response and remission rates at endpoint
eTable 3. Secondary outcomes
eTable 4. MADRS at various timepoints
eTable 5. BDI-II at various timepoints
eTable 6. CGI-S at baseline and endpoint
eTable 7. CGI-I at endpoint
eTable 8. HAM-A at various timepoints
eTable 9. YMRS at various timepoints
eTable 10. PANAS negative at various timepoints
eTable 11. PANAS positive at various timepoints
eTable 12. STAI-S at various timepoints
eTable 13. STAI-T at various timepoints
eTable 14. Average usability scores at various time-points
eTable 15. Adverse events and serious adverse events at week 6
eTable 16. Individual adverse events at week 6
eTable 17. Adverse events per week
eFigure 3. tDCS stimulation time (average minutes per week per participant)
eTable 18. Reasons for dropout
eReferences
Data sharing statement
References
- 1.GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204-1222. doi: 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herrman H, Patel V, Kieling C, et al. Time for united action on depression: a Lancet-World Psychiatric Association Commission. Lancet. 2022;399(10328):957-1022. doi: 10.1016/S0140-6736(21)02141-3 [DOI] [PubMed] [Google Scholar]
- 3.Malhi GS, Mann JJ. Depression. Lancet. 2018;392(10161):2299-2312. doi: 10.1016/S0140-6736(18)31948-2 [DOI] [PubMed] [Google Scholar]
- 4.Otte C, Gold SM, Penninx BW, et al. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065. doi: 10.1038/nrdp.2016.65 [DOI] [PubMed] [Google Scholar]
- 5.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917. doi: 10.1176/ajp.2006.163.11.1905 [DOI] [PubMed] [Google Scholar]
- 6.Kuo C. 5 - Cognitive behavioral therapy around the globe. In: Stein DJ, Bass JK, Hofmann SG, eds. Global Mental Health and Psychotherapy. Academic Press; 2019:87-126. doi: 10.1016/B978-0-12-814932-4.00005-7 [DOI] [Google Scholar]
- 7.McClintock SM, Choi J, Deng ZD, Appelbaum LG, Krystal AD, Lisanby SH. Multifactorial determinants of the neurocognitive effects of electroconvulsive therapy. J ECT. 2014;30(2):165-176. doi: 10.1097/YCT.0000000000000137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Razza LB, Palumbo P, Moffa AH, et al. A systematic review and meta-analysis on the effects of transcranial direct current stimulation in depressive episodes. Depress Anxiety. 2020;37(7):594-608. doi: 10.1002/da.23004 [DOI] [PubMed] [Google Scholar]
- 9.Moffa AH, Martin D, Alonzo A, et al. Efficacy and acceptability of transcranial direct current stimulation (tDCS) for major depressive disorder: an individual patient data meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2020;99:109836. doi: 10.1016/j.pnpbp.2019.109836 [DOI] [PubMed] [Google Scholar]
- 10.Fregni F, El-Hagrassy MM, Pacheco-Barrios K, et al. ; Neuromodulation Center Working Group . Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation in neurological and psychiatric disorders. Int J Neuropsychopharmacol. 2021;24(4):256-313. doi: 10.1093/ijnp/pyaa051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lefaucheur JP, Wendling F. Mechanisms of action of tDCS: a brief and practical overview. Neurophysiol Clin. 2019;49(4):269-275. doi: 10.1016/j.neucli.2019.07.013 [DOI] [PubMed] [Google Scholar]
- 12.Brunoni AR, Sampaio-Junior B, Moffa AH, et al. Noninvasive brain stimulation in psychiatric disorders: a primer. Braz J Psychiatry. 2019;41(1):70-81. doi: 10.1590/1516-4446-2017-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aparício LVM, Guarienti F, Razza LB, Carvalho AF, Fregni F, Brunoni AR. A systematic review on the acceptability and tolerability of transcranial direct current stimulation treatment in neuropsychiatry trials. Brain Stimul. 2016;9(5):671-681. doi: 10.1016/j.brs.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 14.Charvet LE, Shaw MT, Bikson M, Woods AJ, Knotkova H. Supervised transcranial direct current stimulation (tDCS) at home: a guide for clinical research and practice. Brain Stimul. 2020;13(3):686-693. doi: 10.1016/j.brs.2020.02.011 [DOI] [PubMed] [Google Scholar]
- 15.Alonzo A, Fong J, Ball N, Martin D, Chand N, Loo C. Pilot trial of home-administered transcranial direct current stimulation for the treatment of depression. J Affect Disord. 2019;252:475-483. doi: 10.1016/j.jad.2019.04.041 [DOI] [PubMed] [Google Scholar]
- 16.Brunoni AR, Ekhtiari H, Antal A, et al. Digitalized transcranial electrical stimulation: a consensus statement. Clin Neurophysiol. 2022;143:154-165. doi: 10.1016/j.clinph.2022.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borrione L, Cirillo PC, Aparicio LV, et al. A study protocol for an ongoing multi-arm, randomized, double-blind, sham-controlled clinical trial with digital features, using portable transcranial electrical stimulation and internet-based behavioral therapy for major depression disorders: the PSYLECT study. Expert Rev Neurother. 2022;22(6):513-523. doi: 10.1080/14737175.2022.2083959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boutron I, Altman DG, Moher D, Schulz KF, Ravaud P; CONSORT NPT Group . CONSORT statement for randomized trials of nonpharmacologic treatments: a 2017 update and a CONSORT extension for nonpharmacologic trial abstracts. Ann Intern Med. 2017;167(1):40-47. doi: 10.7326/M17-0046 [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto M, Nishimura T. Mersenne twister: a 623-dimensionally equidistributed uniform pseudo-random number generator. ACM Trans Model Comput Simul. 1998;8(1):3-30. doi: 10.1145/272991.272995 [DOI] [Google Scholar]
- 20.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. doi: 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The validity of Mini International Neuropsychiatric Interview (MINI). the development and validation of a structured diagnostic interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:211-232. [PubMed] [Google Scholar]
- 22.Sackeim HA, Aaronson ST, Bunker MT, et al. The assessment of resistance to antidepressant treatment: rationale for the Antidepressant Treatment History Form: Short Form (ATHF-SF). J Psychiatr Res. 2019;113:125-136. doi: 10.1016/j.jpsychires.2019.03.021 [DOI] [PubMed] [Google Scholar]
- 23.Borrione L, Suen PJC, Razza LB, Santos LAD, Sudbrack-Oliveira P, Brunoni AR. The Flow brain stimulation headset for the treatment of depression: overview of its safety, efficacy and portable design. Expert Rev Med Devices. 2020;17(9):867-878. doi: 10.1080/17434440.2020.1813565 [DOI] [PubMed] [Google Scholar]
- 24.De Smet S, Nikolin S, Moffa A, et al. Determinants of sham response in tDCS depression trials: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2021;109:110261. doi: 10.1016/j.pnpbp.2021.110261 [DOI] [PubMed] [Google Scholar]
- 25.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium . The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45(8):742-747. doi: 10.1001/archpsyc.1988.01800320058007 [DOI] [PubMed] [Google Scholar]
- 28.Moberg PJ, Lazarus LW, Mesholam RI, et al. Comparison of the standard and structured interview guide for the Hamilton Depression Rating Scale in depressed geriatric inpatients. Am J Geriatr Psychiatry. 2001;9(1):35-40. doi: 10.1097/00019442-200102000-00006 [DOI] [PubMed] [Google Scholar]
- 29.Firth J, Torous J, Nicholas J, et al. The efficacy of smartphone-based mental health interventions for depressive symptoms: a meta-analysis of randomized controlled trials. World Psychiatry. 2017;16(3):287-298. doi: 10.1002/wps.20472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunoni AR, Valim C, Fregni F. Combination of noninvasive brain stimulation with pharmacotherapy. Expert Rev Med Devices. 2011;8(1):31-39. doi: 10.1586/erd.10.62 [DOI] [PubMed] [Google Scholar]
- 31.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389. doi: 10.1192/bjp.134.4.382 [DOI] [PubMed] [Google Scholar]
- 32.Beck AT, Steer RA, Brown GK. BDI-II, Beck Depression Inventory: Manual. Psychological Corporation; 1996. [Google Scholar]
- 33.Brunoni AR, Moffa AH, Sampaio-Junior B, et al. ; ELECT-TDCS Investigators . Trial of electrical direct-current therapy versus escitalopram for depression. N Engl J Med. 2017;376(26):2523-2533. doi: 10.1056/NEJMoa1612999 [DOI] [PubMed] [Google Scholar]
- 34.Brunoni AR, Valiengo L, Baccaro A, et al. The sertraline vs. electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry. 2013;70(4):383-391. doi: 10.1001/2013.jamapsychiatry.32 [DOI] [PubMed] [Google Scholar]
- 35.Brunoni AR, Ferrucci R, Bortolomasi M, et al. Interactions between transcranial direct current stimulation (tDCS) and pharmacological interventions in the major depressive episode: findings from a naturalistic study. Eur Psychiatry. 2013;28(6):356-361. doi: 10.1016/j.eurpsy.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 36.Rybak YE, Lai KSP, Ramasubbu R, et al. Treatment-resistant major depressive disorder: Canadian expert consensus on definition and assessment. Depress Anxiety. 2021;38(4):456-467. doi: 10.1002/da.23135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blumberger DM, Tran LC, Fitzgerald PB, Hoy KE, Daskalakis ZJ. A randomized double-blind sham-controlled study of transcranial direct current stimulation for treatment-resistant major depression. Front Psychiatry. 2012;3:74. doi: 10.3389/fpsyt.2012.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loo CK, Husain MM, McDonald WM, et al. ; International Consortium of Research in tDCS (ICRT) . International randomized-controlled trial of transcranial direct current stimulation in depression. Brain Stimul. 2018;11(1):125-133. doi: 10.1016/j.brs.2017.10.011 [DOI] [PubMed] [Google Scholar]
- 39.Bennabi D, Nicolier M, Monnin J, et al. Pilot study of feasibility of the effect of treatment with tDCS in patients suffering from treatment-resistant depression treated with escitalopram. Clin Neurophysiol. 2015;126(6):1185-1189. doi: 10.1016/j.clinph.2014.09.026 [DOI] [PubMed] [Google Scholar]
- 40.Woodham R, Rimmer RM, Mutz J, Fu CHY. Is tDCS a potential first line treatment for major depression? Int Rev Psychiatry. 2021;33(3):250-265. doi: 10.1080/09540261.2021.1879030 [DOI] [PubMed] [Google Scholar]
- 41.Naslund JA, Aschbrenner KA, Araya R, et al. Digital technology for treating and preventing mental disorders in low-income and middle-income countries: a narrative review of the literature. Lancet Psychiatry. 2017;4(6):486-500. doi: 10.1016/S2215-0366(17)30096-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burkhardt G, Kumpf U, Crispin A, et al. Transcranial direct current stimulation as an additional treatment to selective serotonin reuptake inhibitors in adults with major depressive disorder in Germany (DepressionDC): a triple-blind, randomised, sham-controlled, multicentre trial. Lancet. 2023;402(10401):545-554. doi: 10.1016/S0140-6736(23)00640-2 [DOI] [PubMed] [Google Scholar]
- 43.Aust S, Brakemeier EL, Spies J, et al. Efficacy of augmentation of cognitive behavioral therapy with transcranial direct current stimulation for depression: a randomized clinical trial. JAMA Psychiatry. 2022;79(6):528-537. doi: 10.1001/jamapsychiatry.2022.0696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung H, Im C, Seo H, Jun SC. Is electric field strength deterministic in cortical neurons’ response to transcranial electrical stimulation? Annu Int Conf IEEE Eng Med Biol Soc. 2021;2021:6025-6028. doi: 10.1109/EMBC46164.2021.9629873 [DOI] [PubMed] [Google Scholar]
- 45.Nandi T, Puonti O, Clarke WT, et al. tDCS induced GABA change is associated with the simulated electric field in M1, an effect mediated by grey matter volume in the MRS voxel. Brain Stimul. 2022;15(5):1153-1162. doi: 10.1016/j.brs.2022.07.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi: 10.1001/jamapsychiatry.2022.2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monte-Silva K, Kuo MF, Hessenthaler S, et al. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul. 2013;6(3):424-432. doi: 10.1016/j.brs.2012.04.011 [DOI] [PubMed] [Google Scholar]
- 48.Lisanby SH. Noninvasive brain stimulation for depression—the devil is in the dosing. N Engl J Med. 2017;376(26):2593-2594. doi: 10.1056/NEJMe1702492 [DOI] [PubMed] [Google Scholar]
- 49.Esmaeilpour Z, Marangolo P, Hampstead BM, et al. Incomplete evidence that increasing current intensity of tDCS boosts outcomes. Brain Stimul. 2018;11(2):310-321. doi: 10.1016/j.brs.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Voineskos D, Blumberger DM. Transcranial direct current stimulation as a treatment for major depressive disorder. Lancet. 2023;402(10401):506-507. doi: 10.1016/S0140-6736(23)00822-X [DOI] [PubMed] [Google Scholar]
- 51.Furukawa TA, Suganuma A, Ostinelli EG, et al. Dismantling, optimising, and personalising internet cognitive behavioural therapy for depression: a systematic review and component network meta-analysis using individual participant data. Lancet Psychiatry. 2021;8(6):500-511. doi: 10.1016/S2215-0366(21)00077-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moshe I, Terhorst Y, Philippi P, et al. Digital interventions for the treatment of depression: a meta-analytic review. Psychol Bull. 2021;147(8):749-786. doi: 10.1037/bul0000334 [DOI] [PubMed] [Google Scholar]
- 53.Nikolin S, Moffa A, Razza L, et al. Time-course of the tDCS antidepressant effect: an individual participant data meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2023;125:110752. doi: 10.1016/j.pnpbp.2023.110752 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eAppendix. eFigure 1. Transcranial direct current stimulation (tDCS) headset used in the Psylect trial
eList 1. Usability VAS-Scales [0 (completely disagree) to 100 (completely agree)]
eList 2. Secondary scales
eMethods. Power calculations used for the Psylect trial
eTable 1. Baseline characteristics of the sample (complementary material)
eFigure 2. Participant distribution density heat map
eTable 2. Response and remission rates at endpoint
eTable 3. Secondary outcomes
eTable 4. MADRS at various timepoints
eTable 5. BDI-II at various timepoints
eTable 6. CGI-S at baseline and endpoint
eTable 7. CGI-I at endpoint
eTable 8. HAM-A at various timepoints
eTable 9. YMRS at various timepoints
eTable 10. PANAS negative at various timepoints
eTable 11. PANAS positive at various timepoints
eTable 12. STAI-S at various timepoints
eTable 13. STAI-T at various timepoints
eTable 14. Average usability scores at various time-points
eTable 15. Adverse events and serious adverse events at week 6
eTable 16. Individual adverse events at week 6
eTable 17. Adverse events per week
eFigure 3. tDCS stimulation time (average minutes per week per participant)
eTable 18. Reasons for dropout
eReferences
Data sharing statement