Conspectus

Hydrogen peroxide (H2O2) for industrial applications is manufactured through an indirect process that relies on the sequential reduction and reoxidation of quinone carriers. While highly effective, production is typically centralized and entails numerous energy-intensive concentration steps. Furthermore, the overhydrogenation of the quinone necessitates periodic replacement, leading to incomplete atom efficiency. These factors, in addition to the presence of propriety stabilizing agents and concerns associated with their separation from product streams, have driven interest in alternative technologies for chemical upgrading. The decoupling of oxidative transformations from commercially synthesized H2O2 may offer significant economic savings and a reduction in greenhouse gas emissions for several industrially relevant processes. Indeed, the production and utilization of the oxidant in situ, from the elements, would represent a positive step toward a more sustainable chemical synthesis sector, offering the potential for total atom efficiency, while avoiding the drawbacks associated with current industrial routes, which are inherently linked to commercial H2O2 production. Such interest is perhaps now more pertinent than ever given the rapidly improving viability of green hydrogen production.
The application of in situ-generated H2O2 has been a long-standing goal in feedstock valorization, with perhaps the most significant interest placed on propylene epoxidation. Until very recently a viable in situ alternative to current industrial oxidative processes has been lacking, with prior approaches typically hindered by low rates of conversion or poor selectivity toward desired products, often resulting from competitive hydrogenation reactions. Based on over 20 years of research, which has led to the development of catalysts for the direct synthesis of H2O2 that offer high synthesis rates and >99% H2 utilization, we have recently turned our attention to a range of oxidative transformations where H2O2 is generated and utilized in situ. Indeed, we have recently demonstrated that it is possible to rival state-of-the-art industrial processes through in situ H2O2 synthesis, establishing the potential for significant process intensification and considerable decarbonization of the chemical synthesis sector.
We have further established the potential of an in situ route to both bulk and fine chemical synthesis through a chemo-catalytic/enzymatic one-pot approach, where H2O2 is synthesized over heterogeneous surfaces and subsequently utilized by a class of unspecific peroxygenase enzymes for C–H bond functionalization. Strikingly, through careful control of the chemo-catalyst, it is possible to ensure that competitive, nonenzymatic pathways are inhibited while also avoiding the regiospecific and selectivity concerns associated with current energy-intensive industrial processes, with further cost savings associated with the operation of the chemo-enzymatic approach at near-ambient temperatures and pressures. Beyond traditional applications of chemo-catalysis, the efficacy of in situ-generated H2O2 (and associated oxygen-based radical species) for the remediation of environmental pollutants has also been a major interest of our laboratory, with such technology offering considerable improvements over conventional disinfection processes.
We hope that this Account, which highlights the key contributions of our laboratory to the field over recent years, demonstrates the chemistries that may be unlocked and improved upon via in situ H2O2 synthesis and it inspires broader interest from the scientific community.
Key References
Freakley S. J.; Kochius S.; van Marwijk J.; Fenner C.; Lewis R. J.; Baldenius K.; Marais S. S.; Opperman D. J.; Harrison S. T. L.; Alcalde M.; Smit M. S.; Hutchings G. J.. A chemo-enzymatic oxidation cascade to activate C-H bonds with in situ generated H2O2. Nat. Commun. 2019, 10( (1), ), 4178. 10.1038/s41467-019-12120-w .1The wide conditions gap that exists between chemo-catalysts and enzymes can be bridged, facilitating efficient C–H bond oxidation.
Richards T.; Harrhy J. H.; Lewis R. J.; Howe A. G. R.; Suldecki G. M.; Folli A.; Morgan D. J.; Davies T. E.; Loveridge E. J.; Crole D. A.; Edwards J. K.; Gaskin P.; Kiely C. J.; He Q.; Murphy D. M.; Maillard J.; Freakley S. J.; Hutchings G. J.. A residue-free approach to water disinfection using catalytic in situ generation of reactive oxygen species. Nat. Catal. 2021, 4( (7), ), 575–585 10.1038/s41929-021-00642-w.2AuPd alloy formation promotes the release of oxygen-based species generated as intermediates during H2O2direct synthesis, with these radicals offering high biocidal efficacy.
Crombie C. M.; Lewis R. J.; Taylor R. L.; Morgan D. J.; Davies T. E.; Folli A.; Murphy D. M.; Edwards J. K.; Qi J.; Jiang H.; Kiely C. J.; Liu X.; Skjøth-Rasmussen M. S.; Hutchings G. J.. Enhanced Selective Oxidation of Benzyl Alcohol via In Situ H2O2 Production over Supported Pd-Based Catalysts. ACS Catal. 2021, 11( (5), ), 2701–2714 10.1021/acscatal.0c04586.3The in situ synthesis of H2O2over Pd coupled with Fenton’s chemistry-mediated radical generation offers improved reactivity in alcohol oxidation.
Lewis R. J.; Ueura K.; Liu X.; Fukuta Y.; Davies T. E.; Morgan D. J.; Chen L.; Qi J.; Singleton J.; Edwards J. K.; Freakley S. J.; Kiely C. J.; Yamamoto Y.; Hutchings G. J.. Highly efficient catalytic production of oximes from ketones using in situ-generated H2O2. Science 2022, 376( (6593), ), 615–620 10.1126/science.abl4822 .4In situ H2O2generation can rival current industrial routes to chemical upgrading and offer improved process efficiencies.
Introduction
The utilization of H2O2 for chemical synthesis typically offers exceptional selectivities, rivaling those achieved by organic peroxides and stoichiometric oxidants (e.g., perchlorate and permanganate) while also avoiding the large quantities of byproducts and subsequent purification costs associated with these conventional reagents. Additionally, H2O2-mediated processes typically allow for lower operating temperatures and improved selectivities compared to alternative aerobic pathways. Currently, the large-scale production of H2O2 is met almost entirely by the highly efficient anthraquinone oxidation (AO) process, where substituted anthraquinones are first hydrogenated and subsequently oxidized, regenerating the quinone carrier and producing an equimolar amount of the oxidant.
A long-standing goal of catalysis has been the synthesis of H2O2 directly from the elements, which would allow for decentralized production and significantly lower capital costs and emission release compared to traditional approaches. However, despite extensive study,5−8 including by our own laboratory9,10 a direct synthesis alternative to the current means of H2O2 production has not yet emerged. In recent years, a significant hindrance to a direct approach, namely, limited catalyst selectivity, has been overcome, with a growing selection of catalyst formulations reported that can achieve near total selectivity toward H2O2.8−11 Importantly, these works have demonstrated that high selective utilization of H2 may be achieved in the absence of the halide and acid stabilizers that had typically been necessary to inhibit competitive H2O2 degradation pathways, particularly over Pd-only catalysts.12 However, in order to rival the AO process it is necessary for concentrations of H2O2 of approximately 5 vol % to be obtained, minimizing costs associated with product separation, prior to shipping. To date, the production of such concentrations has been achieved only through the use of hydrogen/oxygen gas mixtures within the explosive regime, an approach which is clearly not practical.13 As with H2O2 generated ex situ by traditional industrial approaches, that synthesized via the direct combination of H2 and O2 for chemical synthesis would still likely require storage and the dilution of product streams through continual dosing of the oxidant.
Over the past decade, our laboratory has extensively investigated the use of in situ-generated H2O2 for a range of selective oxidative processes and identified the enhanced performance metrics that may be achieved compared to alternative approaches. In this Account, we seek to illustrate the versatility of such chemistry and promote wider interest in the development of novel, more sustainable technologies centered around the in situ production of H2O2. Beyond the realms of traditional heterogeneous catalysis, we further demonstrate that new frontiers in oxidative chemistry are yet to be fully realized, with a particular focus on the application of such technology in chemo/enzymatic cascades and in pollutant remediation.
Challenging Current Industrial Processes
The most pertinent examples of industrial feedstock valorization reliant on preformed H2O2 are perhaps the epoxidation of propylene to propylene oxide (HPPO process) and the ammoximation of cyclohexanone, a key process in the production of the Nylon-6 monomer, ε-caprolactam. In both cases, the utilization of preformed H2O2 has presented significant improvements compared to alternative technologies, primarily associated with lower energy inputs and reduced purification costs. Further interest has been placed on the production of other bulk chemicals, including adipic acid, cyclohexanone, cyclohexanol, phenol, and methanol, with many processes reaching relatively advanced stages of development. However, progression to industrial production has been precluded, at least in part, by financial considerations, with the high cost of preformed H2O2 relative to that of the desired product often prohibiting commercialization. However, through effective in situ production of the oxidant, considerable cost reductions may be achieved, which when coupled with improved environmental credentials improves commercial viability.
Although widely investigated academically, here we raise special mention of the long-standing investigation by Haruta and co-workers,14−16 among many renowned laboratories,17−22 into the production of propylene oxide, and the application of in situ-generated H2O2 for feedstock valorization has faced a number of challenges. These include poor selective H2 utilization, rapid catalyst deactivation, and the formation of complex product mixtures, necessitating extensive purification and the inclusion of promoters. Indeed, in many cases, it is the presence of H2, required to generate H2O2 in situ, that largely promotes the formation of such byproducts.20,23 Such concerns are not limited to alkene epoxidation, with product distributions for a range of transformations influenced by competitive unselective hydrogenation pathways.24,25 It is the overcoming of these challenges that has motivated extensive research from our laboratory, with particular focus placed on the application of Pd-based catalysts for (i) alkane upgrading,26−31 (ii) alcohol oxidation,3,32−34 and recently (iii) the ammoximation of cyclohexanone (and other cyclic ketones) to the corresponding oxime.4,35,36 Regarding alkane oxidation, we direct the reader to our recent Account on methane valorization for an extensive discussion of our contribution to this field.37
Ketone Ammoximation
The development of the titanosilicate TS-1 by EniChem can be considered to be one of the most important innovations in industrial heterogeneous catalysis in recent decades, offering exceptional activities and selectivities for the oxidative transformation of many small molecules, as dictated by the relatively limited pore size of TS-1 (∼5.5 Å), which consists of a 10-membered ring (MFI) framework. In particular, the industrial production of cyclohexanone oxime via the TS-1/H2O2 mediated ammoximation process represents a considerable improvement over conventional approaches, which generate large quantities of low-value byproducts.38 Unlike alternative H2O2-driven chemical transformations, which utilize the oxidant as a source of oxygen-based radicals the ammoximation mechanism is considered to rely on the diffusion of H2O2 to TiIV active sites within the TS-1 framework, forming a Ti-OOH moiety, that is utilized in the formation of hydroxylamine, which subsequently reacts noncatalytically with the ketone to generate the corresponding oxime.39,40 The TS-1/H2O2 approach is highly efficient, avoiding the production of considerable quantities of byproducts associated with conventional approaches that rely on hydroxylamine salts and indeed near total selectivity to the oxime may be achieved, with oxime yields in excess of 98% reported.41 However, given the reaction conditions utilized (high temperature and an elevated pH), an excess of H2O2 is typically utilized to account for the degradation of the oxidant. The inefficient use of H2O2 may be considered to be an additional source of process inefficiency and a challenge that applies to many preformed H2O2-mediated processes.42
Significant process improvements may be achieved by decoupling the commercial ammoximation process from the industrial route to H2O2 production. However, until recently, alternative efforts focused on an integrated process have generated complex and potentially harmful product mixtures, necessitating energy-intensive distillation steps, which would preclude adoption at scale. In particular, the synthesis of H2O2 through the partial oxidation of isopropanol and subsequent utilization in ketone ammoximation has been described but yields considerable levels of byproducts (including acetone as well as phosphoric and acetic acids), in addition to residual isopropanol.43 Such complex product streams are unfavorable, requiring energy-intensive purification steps before application. Notably, the presence of H2O2 and acetone also poses a serious risk through the formation of shock explosives such as diacetone peroxide. By comparison, our one-pot approach to in situ ketone ammoximation avoids the concerns of alternative approaches and utilizes the ability of immobilized Pd-based nanoparticles to synthesize H2O2, which is reacted with ammonia by TS-1 to form a hydroxylamine intermediate, in a manner similar to the current industrial process (Figure 1), with the in situ route offering high selectivity for a range of cyclic oximes (Figure 2A).
Figure 1.
Simplified reaction scheme for the ammoximation of cyclohexanone via in situ H2O2 synthesis. Note that a wide conditions gap exists between the H2O2 direct synthesis and ketone ammoximation reactions, with the former favored by subambient temperatures and acidic conditions while the latter requires elevated reaction temperatures and basic conditions.
Figure 2.
Ketone ammoximation via in situ H2O2 synthesis. (A) Catalytic activity of a 0.66% AuPd/TiO2 catalyst, used in conjunction with TS-1, toward the in situ ammoximation of a range of ketones. (B) Comparison of catalyst support on the activity of AuPd nanoalloys toward the in situ ammoximation of cyclohexanone. Note that with the exception of the TS-1 catalyst, all other formulations were used in conjunction with TS-1 (0.075 g). (C) Effect of the Au:Pd ratio on the catalytic activity of 0.66% PdAu/TS-1 catalysts toward the in situ ammoximation of cyclohexanone. (D) Catalytic activity of 0.66% PdX/TS-1 catalysts toward the in situ ammoximation of cyclohexanone. Note that Pd:X = 1:1 (w/w). Reaction conditions (A, B): Cyclohexanone (2 mmol), NH4HCO3 (4 mmol), 5% H2/N2 (420 psi), 25% O2/N2 (160 psi), catalyst (0.075 g), t-BuOH (5.9 g), H2O (7.5 g), reaction time 3 h, reaction temperature 80 °C, stirring speed 800 rpm. Reaction conditions (C, D): Same as above, but the reaction time is 6 h.
Such systems, utilized either as a physical mixture of two separate catalysts or as a composite material that can catalyze both individual reaction pathways, have been demonstrated to offer exceptional reactivity and selectivity toward a range of oximes, with yields comparable to that achieved by the current state-of-the-art industrial process (Figure 2B). In particular, the formation of AuPd nanoalloys has been found to offer improved performance compared to Pd-only analogues4 or alternative Pd-based formulations (Figure 2C,D)35 despite the enhanced rate of H2O2 synthesis observed over monometallic Pd formulations, under conditions optimized for the stability of the oxidant. Such observations may be attributed to the improved stability of bimetallic AuPd species compared to that of monometallic Pd analogues and the increased selective utilization of H2 over alloyed surfaces.4
In the case of our low-loaded AuPd composite catalyst (0.33% Au-0.33% Pd/TS-1), no clear loss in catalyst stability was detected over 250 h on stream under industrially relevant conditions. Indeed, through-process optimization oxime yields approaching 90% may be obtained (Figure 3A). A subsequent techno-economic analysis revealed that, compared to the current state-of-the-art industrial process, considerable cost reductions (ca. 15%) may be achieved through the in situ approach (Figure 3B). Notably, such calculations are based on material cost alone and do not account for reduced handling and storage costs or attempt to quantify savings associated with reduced GHG emissions. While we have demonstrated the potential for such chemistry to rival the current industrial process and consider that such an approach may be applied to alternative transformations reliant on a combination of H2O2 and TS-1, it is important to note that evaluation at scale and over industrial time scales is still required.
Figure 3.
Industrial viability of the in situ approach to cyclohexanone ammoximation. (A) Cyclohexanone ammoximation via in situ H2O2 synthesis under industrially relevant conditions. (B) Techno-economic analysis comparison of the current commercial and in situ approaches to cyclohexanone ammoximation. Reaction conditions (A): Cyclohexanone (19 wt %): NH3 (aq) (1:1 (mol/mol)), 3.6% H2, 6.4% O2, 90% N2 (580 psi, 20 mL min–1), 0.33% Au 0.33% Pd/TS-1(acetate-O + R):Al2O3 (4:1) (catalyst mass = 0.42 g), t-BuOH: H2O (9:1 (v/v), 0.005–0.10 mL min–1), residence time 75 min at 0.01 mL min–1 liquid flow rate and 150 min at 0.005 mL min–1 liquid flow rate, reaction temperature 80 °C. Note 1: The catalyst was reduced (2 h, 200 °C, H2) following an initial oxidative heat treatment (16 h, 110 °C, static air). Note 2: Reaction conditions between 0 and 1.4 h: As above with liquid flow of 0.1 mL min–1 (purple background). Note 3: Reaction conditions between 1.4 and 12.3 h: As above with liquid flow of 0.01 mL min–1 (green background). Note 4: Reaction conditions between 12.3 and 16 h: As above with liquid flow of 0.005 mL min–1 (orange background). Note 5: The reader is directed to our original work for a complete techno-economic analysis.4
Alcohol Oxidation
Alcohol oxidation utilizing molecular oxygen represents an environmentally friendly route to the synthesis of aldehydes.44 However, while the green credentials of O2-mediated valorization are evident, further improvements in process efficiency may be realized through the use of H2O2, which typically allows for significantly lower operating temperatures and may facilitate improved reaction selectivities. In particular, we have focused our attention on the selective oxidation of benzyl alcohol (Figure 4), with initial studies based on AuPd catalyst formulations32,33 which have been extensively studied for both H2O2 direct synthesis45 and aerobic alcohol oxidation.44,46
Figure 4.
Proposed reaction scheme for the oxidation of benzyl alcohol via in situ H2O2 synthesis.
The oxidation of benzyl alcohol is an often-used model transformation for alcohol upgrading due to the limited number of products and the well-understood mechanisms involved in their formation. Importantly, the primary product, benzaldehyde, also finds wide-scale applications in perfumery and in the agrochemical sector. The catalytic generation of oxygen-based radicals is known to be key in the oxidative mechanism, catalyzing proton abstraction from the alcohol moiety,47 with the presence of radical quenchers shown to effectively suppress catalytic performance.33 Notably, it is crucial to ensure a continual supply of H2O2 (and therefore oxygen-based radicals) in order to maintain high selectivity toward benzaldehyde. Indeed, when limited by H2O2 availability, Pd-catalyzed disproportionation pathways are favored, leading to the production of toluene,33 and as such, it is also crucial that competitive H2O2 degradation pathways are inhibited in order to achieve high process efficiency. Likewise, the continual supply of stabilizer-free H2O2 via an in situ approach avoids the dilution of product streams associated with the use of commercial H2O2 and the need for the separation of proprietary stabilizing agents while also offering high efficacy toward benzyl alcohol valorization compared to that offered by the preformed oxidant. Indeed, the in situ approach also greatly outperforms the purely aerobic pathway, which can be attributed to the requirement for relatively high operation temperatures when utilizing oxygen as the terminal oxidant (Figure 5A).
Figure 5.
Benzyl alcohol oxidation via in situ H2O2 synthesis. (A) Comparative performance of in situ H2O2 synthesis toward the oxidation of benzyl alcohol using a 1% Pd/TiO2 catalyst. (B) Comparison of the catalytic activity of 1% Pd/TiO2, 1% PdAu/TiO2, and 1% PdFe/TiO2 catalysts over sequential reactions. (C) Effect of reaction solvent on the performance of a 5% AuPd/TiO2 catalyst. (D) Experimental (black) and simulated (red) EPR spectra of DMPO-radical adducts formed during the oxidation reaction. Reactions were conducted in the presence of DMPO and (i) 1% Pd/TiO2 (0.083 h), (ii) 1% Pd/TiO2 (0.5 h), (iii) 1% PdAu/TiO2 (0.083 h), (iv) 1% PdAu/TiO2 (0.5 h), (v) 1% PdFe/TiO2 (0.083 h), and (vi) 1% PdFe/TiO2 (0.5 h). Reaction conditions: Catalyst (0.01 g), benzyl alcohol (1.04 g, 9.62 mmol), solvent (7.1 g), 5% H2/CO2 (420 psi), 25% O2/CO2 (160 psi), 0.5 h, 50 °C, 1200 rpm. Note 1: In A, the concentration of commercial H2O2 used is comparable to that produced if all H2 in a standard in situ reaction is converted to H2O2. H2O2 was not continually introduced into the reactor. N2 in parentheses is indicative of a gaseous atmosphere (580 psi). For experiments carried out using H2 or O2 only, a gaseous mixture of 5%H2/CO2 (420 psi) or 25%O2/CO2 (160 psi) was used, with the total pressure being maintained at 580 psi using N2. Note 2: In A–D, the solvent used was MeOH.
The electronic and structural modifications that result from the formation of AuPd nanoalloys are known to promote catalyst efficacy toward alcohol oxidation, and in part, this is achieved through the control of the Pd oxidation state, a key parameter given the presence of H2 and the enhanced selectivity of Pd2+ (i.e., PdO) species toward H2O2, compared to Pd0 analogues.3 However, it is likely that structural effects also contribute to the improved performance of alloyed species, compared to the Pd-only analogue.48 Our observation of relatively large concentrations of residual H2O2 in benzyl alcohol oxidation product streams,32 as a result of improved catalytic selectivity toward H2O2, combined with the general acceptance of radical-mediated pathways prompted us to explore catalyst formulations alternative to those centered around AuPd. In particular, motivated by concurrent studies into pollutant remediation, we have focused on the development of bifunctional catalysts that are able to synthesize H2O2 in situ and subsequently catalyze the production of oxygen-based radicals. The alloying of Pd with Fenton’s active metals, in particular Fe, was found to be highly effective, with optimal catalyst formulations achieving product yields double that of the AuPd analogue, with high selectivity toward benzaldehyde observed (Figure 5B).3
The presence of low concentrations of benzyl alcohol is well known to promote selectivity toward benzaldehyde and prevent overoxidation to benzoic acid.49 This is achieved by intercepting the benzoylperoxy radical species which catalyze competing reaction pathways, resulting in the formation of perbenzoic acid, and ultimately benzoic acid, via a non-Baeyer–Villiger-type oxidation process.49 Interestingly, the presence of an aliphatic alcohol solvent, in this case methanol, is also able to promote process efficiency, improving both the rate of benzyl alcohol conversion and selectivity toward the aldehyde (Figure 5C).3,33 While this may be attributed to the improved solubility of gaseous reagents, particularly H2, and a subsequent improvement in H2O2 synthesis rates, we have also observed the noninnocent nature of the solvent via electron paramagnetic resonance (EPR) spectroscopy (Figure 5.D).3 These studies revealed the presence of O-centered methoxy radicals (CH3O·), which result from the scavenging of hydroperoxyl and hydroxyl radicals by the solvent. Notably, the primary radical species (·OOH, ·OH, ·O2–), were observed only in the presence of AuPd and PdFe catalysts and were absent over the Pd-only analogue, suggesting a possible surface-mediated mechanism over the latter formulation, which aligns well with investigations into Pd and AuPd nanoparticles for H2O2 synthesis.50 As may have been expected based on product yields, the concentration of radical species was found to be significantly greater over the PdFe formulation compared to the AuPd analogue. However, the presence of low concentrations of residual H2O2 over the PdFe formulation suggests that further improvements may yet be achieved through further catalyst design, particularly if the in situ approach is to compete with the exceptional yields which may be achieved by high-temperature aerobic oxidation.
Chemo-Enzymatic Cascades
The selective oxidative valorization of hydrocarbon C–H bonds represents a longstanding challenge for heterogeneous catalysis, with overoxidation and low regioselectivity typically inhibiting process efficiency and necessitating limited conversion rates in order to prevent the formation of undesirable byproducts. Alternatively, a number of H2O2-mediated enzymatic approaches have been developed, including those centered around chloroperoxidases (CPOs) and the closely related class of unspecific peroxygenases (UPOs). Although highly selective, such enzymatic approaches suffer from low sensitivity to even moderate concentrations of H2O2. In order to avoid these concerns and the continual dilution of product streams that would result from the utilization of commercial H2O2, numerous groups have sought to generate H2O2 in situ, with photo- and electro-catalytic methodologies reported. However, the scale-up of such approaches would be challenging, and concerns around enzyme lifetime remain. Accordingly, a number of coenzymatic systems have been developed to directly supply H2O2 to peroxygenases for C–H bond activation and include the use of glucose oxidase (GOx), formate oxidase (FOx), and choline oxidase (ChOx). However, such multienzymatic cascades have suffered from the need for continual pH maintenance, poor atom efficiency, and the formation of large quantities of byproducts, which represent a source of enzyme deactivation if not removed from the reactor system.
Alternatively, we have recently developed a tandem chemo-catalytic/enzymatic approach centered around the direct synthesis of H2O2 over Pd-based heterogeneous catalysts coupled with the unspecific oxygenase PaDa-I (Figure 6), bridging the wide conditions gap that exists between the two processes and achieving product yields several orders of magnitude greater than that achieved over an in situ approach purely reliant on chemo-catalysis.28,29
Figure 6.
Proposed reaction pathways associated with the chemo-catalytic/enzymatic valorization of cyclohexane via in situ H2O2 synthesis.
In particular, we have demonstrated the efficacy of the chemo-catalytic/enzymatic system over a wide range of substrates, achieving total turnover numbers rivaling the most efficient approaches reported in the literature1 while also maintaining the high stereoselectivity one may expect of enzymatically mediated transformations (Table 1). Indeed, through control of nanoparticle composition, it is further possible to modulate reaction pathways, controlling H2O2-mediated enzymatic oxidation and chemo-catalyzed hydrogenation and, in the case of our exemplar study, allowing for the production of either primary or secondary alcohols from terminal alkenes.51
Table 1. Substrate Scope of the Chemo-Enzymatic Cascade towards C–H Bond Hydroxylationa.
Reaction conditions: Catalyst (0.001 g), substrate (10 mM), PaDa-I (15 U mLRM–1), phosphate buffer (100 mM, 10 mL, pH 6.0), using a gas mixture of 80% H2/air H2 (23 psi) and air (6 psi), 2 h, 20 °C, 250 rpm. Note: The yield is reported as that of the primary hydroxylated product.
Although the combination of PaDa-I with AuPd chemo-catalyst formulations is highly effective for the hydroxylation of unactivated sp3 carbons, significantly outperforming the activity of coenzymatic systems (Figure 7A), further process efficiencies may be obtained through (1) the improved selective utilization of H2 (indeed, in the case of cyclohexane hydroxylation selectivity based on H2 in the AuPd/PaDa-I system is as low as 10%),1 (2) the inhibition of purely enzymatic and chemo-catalyzed over oxidation pathways (Figure 7B–D),52 and (3) improving H2O2 synthesis rates (Figure 7B)53 in order to ensure a continual supply of desirable concentrations of the oxidant.
Figure 7.
Chemo/enzymatic C–H bond oxidation, via in situ H2O2synthesis. (A) Comparison of the chemo-catalytic/enzymatic system (where H2O2 is synthesized over a 5% AuPd/TiO2 catalyst) with the coenzymatic approach using glucose oxidase (GOx, 0.2 UmLRM–1) and 200 mM glucose under an oxidative atmosphere (air, 6 psi). (B) Product selectivity as a function of catalyst formulation when used in conjunction with PaDa-I. (C) Chemo-catalytic and (D) enzymatic overoxidation of cyclohexanol. Reaction conditions: Catalyst (0.001 g), substrate (10 mM), PaDa-I (15 U mLRM–1), phosphate buffer (100 mM, 10 mL, pH 6.0), using a gas mixture of 80% H2/air H2 (23 psi) and air (6 psi), X h, 20 °C, 250 rpm. Note 1: In B–D, the catalyst formulation is 1% PdX/TiO2 (Pd:X = 1:1 (w/w)).
However, it is important to note that due to the sensitivity of the UPO enzyme to H2O2 there is a requirement for the reaction to remain limited by H2O2 availability, rather than its subsequent utilization in oxidative valorization, to promote enzyme stability. Indeed, such concerns exist for all approaches which seek to continually supply H2O2 for enzymatic utilization.
While the potential of this technology is particularly exciting, given the high product selectivities and yields achieved, it is important to note that several hurdles must first be overcome if it is to rival industrially operated processes. From a safety perspective, it is imperative that explosive mixtures of H2/O2 are avoided. To date, our studies have utilized gaseous reagent mixtures above the upper explosive limit to ensure that the process is not limited by H2 availability. However, it would be beneficial to operate under H2-lean conditions (i.e., under the lower explosive limit), particularly if concerns around gaseous substrate availability can be minimized and indeed in doing so one may expect potential competitive hydrogenation pathways to be minimized. With this in mind, we consider recent reports by Hollmann and co-workers, which have demonstrated the improved stability of unspecific peroxygenases in the presence of a range of organic cosolvents to be particularly noteworthy, given the improved reagent solubility which may result from the use of such mixed-solvent systems.54 Despite the improvements in enzyme stability that can be obtained through modulation of the solvent composition, it is clear that the stability of the free enzyme, under large-scale operation, as well as issues with separation from product streams, would lead to additional complexity. The use of an immobilized enzyme would, in particular, overcome purification concerns and would allow for operation in a continuous/semicontinuous mode, and indeed enzyme immobilization has been reported to lead to improved lifetimes. As such, it is recommended that enzyme immobilization is treated as a priority in future studies, particularly with a focus on the development of composite materials that consist of the chemo-catalytic and enzymatic components.
While H2O2 concentration is a major contributor to enzyme deactivation, we have also observed a degree of deactivation associated with the presence of both the organic substrate and major reaction products (in this case cyclohexane and cyclohexanol, respectively) (Figure 8A). Furthermore, while our chemo-catalytic formulations have proven to be stable over our chosen time frame (up to 8 h), we have identified the contribution of leached metal species (Au, Pd, and Pt), as well as those common ions typically found in water (Mg, Na, and Cl), to enzyme deactivation (Figure 8B). Such observations highlight the need for a holistic understanding of any chemo-enzymatic process, particularly given the relative costs associated with enzyme synthesis.53
Figure 8.
Sources of enzyme deactivation in the chemo-enzymatic approach to cyclohexane oxidation. (A) Effect of key reaction parameters and (B) homogeneous metal species on enzyme deactivation, as determined by ABTS assay. ABTS assay reaction conditions for (A) and (B): 100 μL of the reaction solution was added to 900 μL of ABTS solution (100 mM sodium citrate–phosphate buffer, pH 4.4 with 0.3 mM ABTS and 2 mM H2O2), and substrate conversion was followed by measuring the absorption at 418 nm (ε 418 = 36 000 M–1 cm–1) at 30 °C. Note for A: In the case of the blank experiment, the UPO was stirred under ambient conditions and the pressure of the buffer only. In the case of the cyclohexanol experiment, the concentration of cyclohexanol was equivalent to that present at 30% conversion of cyclohexane. The blank cyclohexane oxidation reaction conditions for (B): Metal salt ((5.1–9.4) × 10–9 M), cyclohexane (10 mM), PaDa (15 U mLRM–1), and phosphate buffer (100 mM, 10 mL, pH 6.0), using a gas mixture of 80% H2 (23 psi) in air (6 psi), 2 h, 250 rpm.
Water Treatment
The bioremediation of contaminated water bodies and degradation of low levels of bioactive compounds (such as exogenous hormones and pharmaceuticals) and organic pollutants released from agricultural, industrial, and urban activities into groundwater represents a growing challenge to aquatic and human health. These concerns are compounded by the limited degradation efficacy of conventional chemical treatments, such as chlorination, to recalcitrant remediation and the growing health concerns associated with the chemical residues that result from traditional disinfection agents.55 As such, growing interest has been placed on the application of advanced oxidation processes (AOPs) that utilize the high reactivity of oxygen-based radicals (·OH, ·OOH, and ·O2–) for water disinfection, with the combination of H2O2 and O-zone (H2O2/O3) or UV irradiation (H2O2/UV), well studied. However, while effective, the costs associated with the use of oxidizing reagents (H2O2 and O3) and practical limitations of high-energy light sources as well as the presence of H2O2 stabilizing agents will likely preclude their application at scale and have led our laboratory to investigate the generation of these reactive oxygen species in situ through the combination of H2 and O2.
Utilizing dilute streams of H2 (at levels comparable to those generated via water splitting) and air to synthesize stabilizer-free H2O2 and short-lived reactive oxygen species over AuPd nanoalloys, we have established that our chemo-catalytic approach can offer excellent bactericidal and virucidal efficacy.2 The presence of Au is crucial in the release of intermediate oxygen-based species from catalytic surfaces, as identified through extensive EPR spectroscopy (Figure 9A). Indeed, our technology achieves an 8.1 log10 reduction in E. coli levels (Figure 9B) and an 8.0 log10 reduction in the viability of the nonenveloped virus MS2, a surrogate for the polio virus, representing a 99.999999% reduction in pathogen viability after a contact time of 30 s, which is several orders of magnitude more effective than that achieved through the use of preformed H2O2 or through chlorination, with these alternative approaches requiring relatively long contact times to offer appreciable levels of efficacy. Notably, in addition to oxygen-based radicals, low concentrations of residual H2O2, which offers broad spectrum bactericidal efficacy,2 are also synthesized, which may prolong the potable lifetime of the treated water and have been shown to aid in the prevention of biofilm formation, which represents a crucial challenge to water decontamination, again largely due to the poor efficacy of chlorination technologies.2
Figure 9.
Bioremediation via in situ H2O2 synthesis. (A) Proposed reaction scheme for the in situ remediation of E. coli. K12 JM109 by reactive oxygen species generated over AuPd surfaces, summarizing our observations of catalytic performance and O-centered radical speciation. (B) Comparison of microbicidal activity using aqueous NaOCl, preformed H2O2, and in situ-synthesized H2O2. Reaction conditions: Catalyst (0.12 g), 2% H2, 20% O2, 78% N2, (145 psi, 42 mL min–1), reaction solution (H2O and E. coli K12 JM109 (2 × 108 c.f.u. mL–1) 0.2 mL min–1 liquid flow), 0.5 h, 2 °C.
Alternatively, if combined with Fenton-active species, this residual H2O2 may act as a secondary source of oxygen-based radicals, in a manner similar to that observed for alkane56−58 and alcohol valorization.3,33 Inspired by these studies into radical-based selective oxidation, we subsequently turned our attention to the oxidative degradation of chemical pollutants. As with alcohol oxidation, the combination of Pd with Fe was found to be highly effective compared to monometallic Pd analogues or bimetallic formulations consisting of Pd with alternative Fenton-active metals (Cu, Mn, and Co) or Au.59,60 The poor performance of PdCu formulations in particular may be surprising, given the high efficacy of Cu-based catalysts in the oxidative valorization of alkanes61 when used in conjunction with preformed H2O2. However, it is important to note that theoretical studies have revealed the thermodynamic instability of intermediate hydroperoxyl species and in turn H2O2 over Cu-containing catalytic surfaces,62 with these studies aligning well with our experimental observations.63,64 Interestingly, we have recently established that the incorporation of dopant concentrations of Cu (<0.2% of total metal loading) into AuPd nanoalloys results in a considerable improvement in catalytic performance toward H2O2 direct synthesis, with these formulations offering comparable activities to those of better-studied AuPdPt systems.65 As such, these formulations may be considered to be attractive candidates for future use in water remediation or other in situ processes.66,67
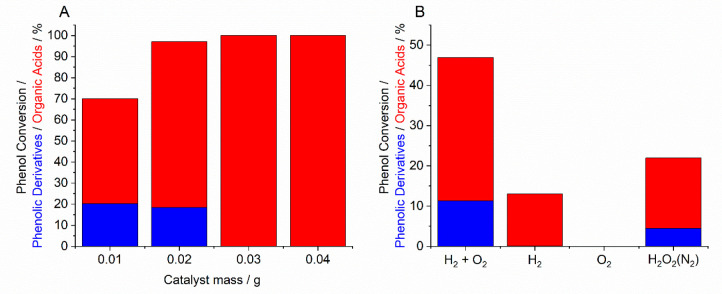
Clearly for any approach to chemical pollutant degradation to be effective there is a need to drive product distribution down the oxidative pathway, toward H2O and CO2. To date, our studies have focused on the total oxidation of phenol, an often-used proxy for pharmaceuticals and agrochemicals. In particular, phenol degradation represents a major challenge given the increased toxicity of partial oxidation products (catechol and benzoquinone and a range of diacids) and the propensity for such species to promote the leaching of catalytically active species, with organic acids in particular known to be strong chelating agents. Early catalyst formulations, prepared by wet coimpregnation techniques and utilizing common oxide supports (TiO2 and SiO2), were found to be somewhat effective (Figure 10A,B) but were unable to fully oxidize the phenolic derivatives while also suffering from considerable leaching of the Fe component. While the loss of active metal species is a concern for catalyst stability, it is important to note that homogeneous contributions toward the observed catalysis were found to be negligible. Subsequent studies, again focusing on PdFe formulations, demonstrated that considerable improvements in both activity and stability may be obtained through the incorporation of Fe into the zeolitic framework of ZSM-5 (Figure 10C,D).68
Figure 10.
Oxidative degradation of phenol via in situ H2O2 synthesis. Comparison of catalytic activity using Pd-based catalysts (A) 1% Pd/TiO2, (B) 0.25% Pd-0.27% Fe/TiO2, and (C) 0.5% Pd/3% Fe-ZSM-5. (D) Performance of the 0.5% Pd/3% Fe-ZSM-5 catalyst over sequential reactions. Reaction conditions: Catalyst (0.01 g), phenol (1000 ppm, 8.5 g), 5% H2/CO2 (420 psi), 25% O2/CO2 (160 psi), 2 h, 30 °C, 2 h.
Importantly, through the manipulation of the catalyst:substrate ratio, the optimal Pd/Fe-ZSM-5 formulation was also able to offer the total degradation of phenol, with complete selectivity toward the highly oxidized organic acids, representing a significant development compared to previous catalyst iterations (Figure 11A).68 While such technology is extremely promising, offering considerable improvements over the use of commercial H2O2 (Figure 11B) and demonstrating the potential role of catalysis in water treatment, further improvements in process efficacy are still required. With extended contact times currently required to achieve reasonable rates of phenol degradation, a focus should be placed on improving catalytic activity, particularly given the likely requirement for flow, rather than batch regimes to be utilized at scale. Additionally, there is a need to ensure high catalytic performance against a broad range of chemical pollutants, microorganisms, and a combination thereof, particularly using real-world water solutions. However, results to date are promising, and when used in combination with other emerging technologies, including biofiltration, we consider that the application of in situ-generated H2O2 and associated radical species may play a key role in safeguarding clean drinking water.
Figure 11.
(A) Efficacy of the 0.5% Pd/3% Fe-ZSM-5 catalyst toward the oxidative degradation of phenol via in situ H2O2 synthesis as a function of the catalyst:phenol ratio. (B) Comparative performance of in situ H2O2 synthesis toward phenol degradation over a 0.5% Pd/0.5% Fe-ZSM-5 catalyst (0.0 1 g). Reaction conditions: Catalyst (X g), phenol (1000 ppm, 8.5 g), 5% H2/CO2 (420 psi), 25% O2/CO2 (160 psi), 2 h, 30 °C, 2 h. Note: In B, the concentration of commercial H2O2 used is comparable to that produced if all H2 in a standard in situ reaction is converted to H2O2. H2O2 was not continually introduced into the reactor. N2 in parentheses is indicative of the gaseous atmosphere (580 psi). For experiments carried out using H2 or O2 only, a gaseous mixture of 5% H2/CO2 (420 psi) or 25% O2/CO2 (160 psi) was used, with the balance consisting of N2.
Conclusions and Future Perspectives
Minimizing the use of finite resources and preventing the formation of pollutants must be a major focus if industrial chemical production is to be fit for the 21st century. Although still in its infancy, the effective generation and utilization of H2O2 (and its intermediates) in situ represents a potential sea-change in oxidative chemistry, allowing for considerable process intensification and moving the chemical synthesis sector closer toward a clean growth strategy.
Clearly, for many tandem chemical transformations that utilize H2O2, it is imperative that the rate of oxidant synthesis does not exceed the capacity of the secondary component to utilize the active species in selective oxidation, while in the case of radical-mediated transformations, there is a need to ensure effective utilization of the radical flux and minimize competitive termination reactions. Such transformations may be promoted through the careful selection of both catalyst formulation and reaction solvents, which can aid in radical propagation. There is an additional concern associated with the unselective utilization of H2, primarily through H2O2 decomposition but also via competitive substrate hydrogenation pathways, ensuring that high H2 selectivity is a major hurdle which must be overcome in order to achieve high process efficiency. While such considerations may direct future attention toward the development of catalyst formulations that are highly selective toward H2O2, it is also important to consider that in many cases there is rapid capture and utilization of H2O2 by the secondary active site. As such, we recommend that future studies focus on the development of catalyst formulations that are highly active toward H2O2 synthesis, with the caveat that the H2O2 production rate does not exceed that of its subsequent utilization.
We consider that the contributions to the field from our laboratory reported within this Account may offer considerable benefits to the chemical synthesis sector and beyond. However, in order to truly capitalize on such technology there is a clear need to progress beyond research-scale catalysts and toward technical-grade materials. Doing so will require a multidisciplinary collaborative effort involving specialists in characterization and material synthesis, in addition to theoreticians and chemical engineers, as well as necessitating improved dialogue between industry and academia and balancing the often competing interests and demands of these partners. It is only in adopting such a unified approach that we foresee development beyond the realm of academia and the true realization of the potential of this chemistry.
Acknowledgments
The authors gratefully acknowledge the Max Planck Centre for Fundamental Heterogeneous Catalysis (FUNCAT) for financial support and thank UBE Corporation, Haldor Topsøe and Welsh Water for their respective funding which supported major parts of the work discussed.
Biographies
Richard J. Lewis obtained his Ph.D. from Cardiff University, where he continues his research into the design and synthesis of heterogeneous catalysis, with a particular interest in oxidative chemistry.
Graham J. Hutchings is the Regius Professor of Chemistry at Cardiff University with broad interests in heterogeneous catalysis.
The authors declare no competing financial interest.
References
- Freakley S. J.; Kochius S.; van Marwijk J.; Fenner C.; Lewis R. J.; Baldenius K.; Marais S. S.; Opperman D. J.; Harrison S. T. L.; Alcalde M.; Smit M. S.; Hutchings G. J. A chemo-enzymatic oxidation cascade to activate C-H bonds with in situ generated H2O2. Nat. Commun. 2019, 10 (1), 4178. 10.1038/s41467-019-12120-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards T.; Harrhy J. H.; Lewis R. J.; Howe A. G. R.; Suldecki G. M.; Folli A.; Morgan D. J.; Davies T. E.; Loveridge E. J.; Crole D. A.; Edwards J. K.; Gaskin P.; Kiely C. J.; He Q.; Murphy D. M.; Maillard J.; Freakley S. J.; Hutchings G. J. A residue-free approach to water disinfection using catalytic in situ generation of reactive oxygen species. Nat. Catal. 2021, 4 (7), 575–585. 10.1038/s41929-021-00642-w. [DOI] [Google Scholar]
- Crombie C. M.; Lewis R. J.; Taylor R. L.; Morgan D. J.; Davies T. E.; Folli A.; Murphy D. M.; Edwards J. K.; Qi J.; Jiang H.; Kiely C. J.; Liu X.; Skjøth-Rasmussen M. S.; Hutchings G. J. Enhanced Selective Oxidation of Benzyl Alcohol via In Situ H2O2 Production over Supported Pd-Based Catalysts. ACS Catal. 2021, 11 (5), 2701–2714. 10.1021/acscatal.0c04586. [DOI] [Google Scholar]
- Lewis R. J.; Ueura K.; Liu X.; Fukuta Y.; Davies T. E.; Morgan D. J.; Chen L.; Qi J.; Singleton J.; Edwards J. K.; Freakley S. J.; Kiely C. J.; Yamamoto Y.; Hutchings G. J. Highly efficient catalytic production of oximes from ketones using in situ generated H2O2. Science 2022, 376 (6593), 615–620. 10.1126/science.abl4822. [DOI] [PubMed] [Google Scholar]
- Staykov A.; Kamachi T.; Ishihara T.; Yoshizawa K. Theoretical study of the direct synthesis of H2O2 on Pd and Pd/Au surfaces. J. Phys. Chem. C 2008, 112, 19501–19505. 10.1021/jp803021n. [DOI] [Google Scholar]
- Xu H.; Cheng D.; Gao Y. Design of High-Performance Pd-Based Alloy Nanocatalysts for Direct Synthesis of H2O2. ACS Catal. 2017, 7 (3), 2164–2170. 10.1021/acscatal.6b02871. [DOI] [Google Scholar]
- Wilson N. M.; Priyadarshini P.; Kunz S.; Flaherty D. W. Direct synthesis of H2O2 on Pd and AuxPd1 clusters: Understanding the effects of alloying Pd with Au. J. Catal. 2018, 357, 163–175. 10.1016/j.jcat.2017.10.028. [DOI] [Google Scholar]
- Li H.; Wan Q.; Du C.; Zhao J.; Li F.; Zhang Y.; Zheng Y.; Chen M.; Zhang K. H. L.; Huang J.; Fu G.; Lin S.; Huang X.; Xiong H. Layered Pd oxide on PdSn nanowires for boosting direct H2O2 synthesis. Nat. Commun. 2022, 13 (1), 6072. 10.1038/s41467-022-33757-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. K.; Solsona B.; Ntainjua E. N.; Carley A. F.; Herzing A. A.; Kiely C. J.; Hutchings G. J. Switching Off Hydrogen Peroxide Hydrogenation in the Direct Synthesis Process. Science 2009, 323, 1037–1041. 10.1126/science.1168980. [DOI] [PubMed] [Google Scholar]
- Freakley S. J.; He Q.; Harrhy J. H.; Lu L.; Crole D. A.; Morgan D. J.; Ntainjua E. N.; Edwards J. K.; Carley A. F.; Borisevich A. Y.; Kiely C. J.; Hutchings G. J. Palladium-tin catalysts for the direct synthesis of H2O2 with high selectivity. Science 2016, 351, 965–968. 10.1126/science.aad5705. [DOI] [PubMed] [Google Scholar]
- Yu S.; Cheng X.; Wang Y.; Xiao B.; Xing Y.; Ren J.; Lu Y.; Li H.; Zhuang C.; Chen G. High activity and selectivity of single palladium atom for oxygen hydrogenation to H2O2. Nat. Commun. 2022, 13 (1), 4737. 10.1038/s41467-022-32450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyadarshini P.; Ricciardulli T.; Adams J. S.; Yun Y. S.; Flaherty D. W. Effects of bromide adsorption on the direct synthesis of H2O2 on Pd nanoparticles: Formation rates, selectivities, and apparent barriers at steady-state. J. Catal. 2021, 399, 24–40. 10.1016/j.jcat.2021.04.020. [DOI] [Google Scholar]
- Gosser L. W.EIDP Inc., EP0132294B1, 1987.
- Hayashi T.; Tanaka K.; Haruta M. Selective Vapor-Phase Epoxidation of Propylene over Au/TiO2 Catalysts in the Presence of Oxygen and Hydrogen. J. Catal. 1998, 178 (2), 566–575. 10.1006/jcat.1998.2157. [DOI] [Google Scholar]
- Uphade B. S.; Akita T.; Nakamura T.; Haruta M. Vapor-Phase Epoxidation of Propene Using H2 and O2 over Au/Ti-MCM-48. J. Catal. 2002, 209 (2), 331–340. 10.1006/jcat.2002.3642. [DOI] [Google Scholar]
- Huang J.; Lima E.; Akita T.; Guzmán A.; Qi C.; Takei T.; Haruta M. Propene epoxidation with O2 and H2: Identification of the most active gold clusters. J. Catal. 2011, 278 (1), 8–15. 10.1016/j.jcat.2010.11.012. [DOI] [Google Scholar]
- de Oliveira A. L.; Wolf A.; Schüth F. Highly selective propene epoxidation with hydrogen/oxygen mixtures over titania-supported silver catalysts. Catal. Lett. 2001, 73 (2), 157–160. 10.1023/A:1016641708074. [DOI] [Google Scholar]
- Kanungo S.; Perez Ferrandez D. M.; Neira d’Angelo F.; Schouten J. C.; Nijhuis T. A. Kinetic study of propene oxide and water formation in hydro-epoxidation of propene on Au/Ti-SiO2 catalyst. J. Catal. 2016, 338, 284–294. 10.1016/j.jcat.2016.03.019. [DOI] [Google Scholar]
- Kertalli E.; Neira d’Angelo M. F.; Schouten J. C.; Nijhuis T. A. Design and optimization of a catalytic membrane reactor for the direct synthesis of propylene oxide. Chem. Eng. Sci. 2015, 138, 465–472. 10.1016/j.ces.2015.08.034. [DOI] [Google Scholar]
- Wang G.; Du W.; Duan X.; Cao Y.; Zhang Z.; Xu J.; Chen W.; Qian G.; Yuan W.; Zhou X.; Chen D. Mechanism-guided elaboration of ternary Au-Ti-Si sites to boost propylene oxide formation. Chem. Catal. 2021, 1 (4), 885–895. 10.1016/j.checat.2021.06.006. [DOI] [Google Scholar]
- Yap N.; Andres R. P.; Delgass W. N. Reactivity and stability of Au in and on TS-1 for epoxidation of propylene with H2 and O2. J. Catal. 2004, 226 (1), 156–170. 10.1016/j.jcat.2004.05.016. [DOI] [Google Scholar]
- Meiers R.; Hölderich W. F. Epoxidation of propylene and direct synthesis of hydrogen peroxide by hydrogen and oxygen. Catal. Lett. 1999, 59 (2), 161–163. 10.1023/A:1019024705869. [DOI] [Google Scholar]
- Chen Q.; Beckman E. J. One-pot green synthesis of propylene oxide using in situ generated hydrogen peroxide in carbon dioxide. Green Chem. 2008, 10 (9), 934–938. 10.1039/b803847c. [DOI] [Google Scholar]
- Lyu J.; Niu L.; Shen F.; Wei J.; Xiang Y.; Yu Z.; Zhang G.; Ding C.; Huang Y.; Li X. In Situ Hydrogen Peroxide Production for Selective Oxidation of Benzyl Alcohol over a Pd@Hierarchical Titanium Silicalite Catalyst. ACS Omega 2020, 5 (27), 16865–16874. 10.1021/acsomega.0c02065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S.; Hamakawa S.; Tanaka S.; Sato K.; Esashi M.; Mizukami F. A one-step conversion of benzene to phenol using MEMS-based Pd membrane microreactors. Chem. Eng. J. 2009, 155 (3), 829–837. 10.1016/j.cej.2009.09.007. [DOI] [Google Scholar]
- Ab Rahim M. H.; Forde M. M.; Jenkins R. L.; Hammond C.; He Q.; Dimitratos N.; Lopez-Sanchez J. A.; Carley A. F.; Taylor S. H.; Willock D. J.; Murphy D. M.; Kiely C. J.; Hutchings G. J. Oxidation of Methane to Methanol with Hydrogen Peroxide Using Supported Gold-Palladium Alloy Nanoparticles. Angew. Chem., Int. Ed. 2013, 52 (4), 1280–1284. 10.1002/anie.201207717. [DOI] [PubMed] [Google Scholar]
- Ni F.; Richards T.; Smith L. R.; Morgan D. J.; Davies T. E.; Lewis R. J.; Hutchings G. J. Selective Oxidation of Methane to Methanol via In Situ H2O2 Synthesis. ACS Org. Inorg. Au 2023, 3 (4), 177–183. 10.1021/acsorginorgau.3c00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombie C. M.; Lewis R. J.; Kovačič D.; Morgan D. J.; Davies T. E.; Edwards J. K.; Skjøth-Rasmussen M. S.; Hutchings G. J. The Influence of Reaction Conditions on the Oxidation of Cyclohexane via the In-Situ Production of H2O2. Catal. Lett. 2021, 151 (1), 164–171. 10.1007/s10562-020-03281-1. [DOI] [Google Scholar]
- Crombie C. M.; Lewis R. J.; Kovačič D.; Morgan D. J.; Slater T. J. A.; Davies T. E.; Edwards J. K.; Skjøth-Rasmussen M. S.; Hutchings G. J. The Selective Oxidation of Cyclohexane via In-situ H2O2 Production Over Supported Pd-based Catalysts. Catal. Lett. 2021, 151 (9), 2762–2774. 10.1007/s10562-020-03511-6. [DOI] [Google Scholar]
- Carter J. H.; Lewis R. J.; Demetriou N.; Williams C.; Davies T. E.; Qin T.; Dummer N. F.; Morgan D. J.; Willock D. J.; Liu X.; Taylor S. H.; Hutchings G. J. The selective oxidation of methane to methanol using in situ generated H2O2 over palladium-based bimetallic catalysts. Catal. Sci. Technol. 2023, 13, 5848–5858. 10.1039/D3CY00116D. [DOI] [Google Scholar]
- Li G.; Edwards J.; Carley A. F.; Hutchings G. J. Direct synthesis of hydrogen peroxide from H2 and O2 and in situ oxidation using zeolite-supported catalysts. Catal. Commun. 2007, 8 (3), 247–250. 10.1016/j.catcom.2006.06.021. [DOI] [Google Scholar]
- Moreno I.; Dummer N. F.; Edwards J. K.; Alhumaimess M.; Sankar M.; Sanz R.; Pizarro P.; Serrano D. P.; Hutchings G. J. Selective oxidation of benzyl alcohol using in-situ generated H2O2 over hierarchical Au-Pd titanium silicalite catalysts. Catal. Sci. Technol. 2013, 3 (9), 2425–2434. 10.1039/c3cy00493g. [DOI] [Google Scholar]
- Santonastaso M.; Freakley S. J.; Miedziak P. J.; Brett G. L.; Edwards J. K.; Hutchings G. J. Oxidation of benzyl alcohol using in situ generated hydrogen peroxide. Org. Process Res. Dev. 2014, 18 (11), 1455–1460. 10.1021/op500195e. [DOI] [Google Scholar]
- Underhill R.; Douthwaite M.; Lewis R. J.; Miedziak P. J.; Armstrong R. D.; Morgan D. J.; Freakley S. J.; Davies T.; Folli A.; Murphy D. M.; He Q.; Akdim O.; Edwards J. K.; Hutchings G. J. Ambient base-free glycerol oxidation over bimetallic PdFe/SiO2 by in situ generated active oxygen species. Res. Chem. Intermed. 2021, 47 (1), 303–324. 10.1007/s11164-020-04333-2. [DOI] [Google Scholar]
- Lewis R. J.; Ueura K.; Liu X.; Fukuta Y.; Qin T.; Davies T. E.; Morgan D. J.; Stenner A.; Singleton J.; Edwards J. K.; Freakley S. J.; Kiely C. J.; Chen L.; Yamamoto Y.; Hutchings G. J. Selective Ammoximation of Ketones via In Situ H2O2 Synthesis. ACS Catal. 2023, 13 (3), 1934–1945. 10.1021/acscatal.2c05799. [DOI] [Google Scholar]
- Lewis R. J.; Ueura K.; Fukuta Y.; Davies T. E.; Morgan D. J.; Paris C. B.; Singleton J.; Edwards J. K.; Freakley S. J.; Yamamoto Y.; Hutchings G. J. Cyclohexanone ammoximation via in situ H2O2 production using TS-1 supported catalysts. Green Chem. 2022, 24, 9496–9507. 10.1039/D2GC02689A. [DOI] [Google Scholar]
- Freakley S. J.; Dimitratos N.; Willock D. J.; Taylor S. H.; Kiely C. J.; Hutchings G. J. Methane Oxidation to Methanol in Water. Acc. Chem. Res. 2021, 54 (11), 2614–2623. 10.1021/acs.accounts.1c00129. [DOI] [PubMed] [Google Scholar]
- Mokaya R.; Poliakoff M. A cleaner way to nylon?. Nature 2005, 437 (7063), 1243–1244. 10.1038/4371243a. [DOI] [PubMed] [Google Scholar]
- Wang L.; Xiong G.; Su J.; Li P.; Guo H. In Situ UV Raman Spectroscopic Study on the Reaction Intermediates for Propylene Epoxidation on TS-1. J. Phys. Chem. C 2012, 116 (16), 9122–9131. 10.1021/jp3017425. [DOI] [Google Scholar]
- Chu C. Q.; Zhao H. T.; Qi Y. Y.; Xin F. Density functional theory studies on hydroxylamine mechanism of cyclohexanone ammoximation on titanium silicalite-1 catalyst. J. Mol. Model. 2013, 19 (6), 2217–2224. 10.1007/s00894-013-1768-1. [DOI] [PubMed] [Google Scholar]
- Fukao M.; Oikawa M.. Sumitomo Chemical Company Ltd., US 2006/0252962 A1, 2006.
- Yip A. C. K.; Hu X. Formulation of Reaction Kinetics for Cyclohexanone Ammoximation Catalyzed by a Clay-Based Titanium Silicalite-1 Composite in a Semibatch Process. Ind. Eng. Chem. Res. 2011, 50 (24), 13703–13710. 10.1021/ie201467u. [DOI] [Google Scholar]
- Liang X.; Mi Z.; Wang Y.; Wang L.; Zhang X.; Liu T. An Integrated Process of H2O2 Production through Isopropanol Oxidation and Cyclohexanone Ammoximation. Chem. Eng. Technol. 2004, 27 (2), 176–180. 10.1002/ceat.200401862. [DOI] [Google Scholar]
- Enache D. I.; Edwards J. K.; Landon P.; Solsona-Espriu B.; Carley A. F.; Herzing A. A.; Watanabe M.; Kiely C. J.; Knight D. W.; Hutchings G. J. Solvent-free Oxidation of Primary Alcohols to Aldehydes using Au-Pd/TiO2 Catalyst. Science 2006, 311, 362–365. 10.1126/science.1120560. [DOI] [PubMed] [Google Scholar]
- Edwards J. K.; Solsona B. E.; Landon P.; Carley A. F.; Herzing A.; Kiely C. J.; Hutchings G. J. Direct synthesis of hydrogen peroxide from H2 and O2 using TiO2-supported Au-Pd catalysts. J. Catal. 2005, 236, 69–79. 10.1016/j.jcat.2005.09.015. [DOI] [Google Scholar]
- He Q.; Miedziak P. J.; Kesavan L.; Dimitratos N.; Sankar M.; Lopez-Sanchez J. A.; Forde M. M.; Edwards J. K.; Knight D. W.; Taylor S. H.; Kiely C. J.; Hutchings G. J. Switching-off toluene formation in the solvent-free oxidation of benzyl alcohol using supported trimetallic Au-Pd-Pt nanoparticles. Faraday Discuss. 2013, 162, 365–378. 10.1039/c2fd20153d. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Xiao S.; Chen R.; Chen F. Promotional effect of short-chain saturated alcohols on Fe3O4-catalyzed decomposition of H2O2 and its application in selective oxidation of benzyl alcohol. J. Chem. Technol. Biotechnol. 2019, 94 (5), 1613–1621. 10.1002/jctb.5929. [DOI] [Google Scholar]
- Tiruvalam R. C.; Pritchard J. C.; Dimitratos N.; Lopez-Sanchez J. A.; Edwards J. K.; Carley A. F.; Hutchings G. J.; Kiely C. J. Aberration Corrected Analytical Electron Microscopy Studies of Sol-Immobilized Au + Pd, Au{Pd} and Pd{Au} Catalysts Used for Benzyl Alcohol Oxidation and Hydrogen Peroxide Production. Faraday Discuss. 2011, 152, 63–86. 10.1039/c1fd00020a. [DOI] [PubMed] [Google Scholar]
- Sankar M.; Nowicka E.; Carter E.; Murphy D. M.; Knight D. W.; Bethell D.; Hutchings G. J. The benzaldehyde oxidation paradox explained by the interception of peroxy radical by benzyl alcohol. Nat. Commun. 2014, 5 (1), 3332. 10.1038/ncomms4332. [DOI] [PubMed] [Google Scholar]
- Li J.; Ishihara T.; Yoshizawa K. Theoretical Revisit of the Direct Synthesis of H2O2 on Pd and Au@Pd Surfaces: A Comprehensive Mechanistic Study. J. Phys. Chem. C 2011, 115, 25359–25367. 10.1021/jp208118e. [DOI] [Google Scholar]
- Wilbers D.; Brehm J.; Lewis R. J.; van Marwijk J.; Davies T. E.; Morgan D. J.; Opperman D. J.; Smit M. S.; Alcalde M.; Kotsiopoulos A.; Harrison S. T. L.; Hutchings G. J.; Freakley S. J. Controlling product selectivity with nanoparticle composition in tandem chemo-biocatalytic styrene. Green Chem. 2021, 23 (11), 4170–4180. 10.1039/D0GC04320F. [DOI] [Google Scholar]
- Brehm J.; Lewis R. J.; Richards T.; Qin T.; Morgan D. J.; Davies T. E.; Chen L.; Liu X.; Hutchings G. J. Enhancing the Chemo-Enzymatic One-Pot Oxidation of Cyclohexane via In Situ H2O2 Production over Supported Pd-Based Catalysts. ACS Catal. 2022, 12, 11776–11789. 10.1021/acscatal.2c03051. [DOI] [Google Scholar]
- Stenner A.; Lewis R. J.; Brehm J.; Qin T.; Lopez-Martin A.; Morgan D. J.; Davies T. E.; Chen L.; Liu X.; Hutchings G. J. Chemo-Enzymatic One-Pot Oxidation of Cyclohexane via in-situ H2O2 Production over Supported AuPdPt Catalysts. ChemCatChem. 2023, 15 (10), e202300162 10.1002/cctc.202300162. [DOI] [Google Scholar]
- Hilberath T.; van Troost A.; Alcalde M.; Hollmann F.. Assessing Peroxygenase-Mediated Oxidations in the Presence of High Concentrations of Water-Miscible Co-Solvents. Front. Catal. 2022, 2, 10.3389/fctls.2022.882992. [DOI] [Google Scholar]
- Nieuwenhuijsen M. J.; Toledano M. B.; Eaton N. E.; Fawell J.; Elliott P. Chlorination disinfection byproducts in water and their association with adverse reproductive outcomes: a review. Occup. Environ. Med. 2000, 57 (2), 73–85. 10.1136/oem.57.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C.; Jenkins R. L.; Dimitratos N.; Lopez-Sanchez J. A.; ab Rahim M. H.; Forde M. M.; Thetford A.; Murphy D. M.; Hagen H.; Stangland E. E.; Moulijn J. M.; Taylor S. H.; Willock D. J.; Hutchings G. J. Catalytic and Mechanistic Insights of the Low-Temperature Selective Oxidation of Methane over Cu-Promoted Fe-ZSM-5. Chem. - Eur. J. 2012, 18 (49), 15735–15745. 10.1002/chem.201202802. [DOI] [PubMed] [Google Scholar]
- Forde M. M.; Armstrong R. D.; Hammond C.; He Q.; Jenkins R. L.; Kondrat S. A.; Dimitratos N.; Lopez-Sanchez J. A.; Taylor S. H.; Willock D.; Kiely C. J.; Hutchings G. J. Partial Oxidation of Ethane to Oxygenates Using Fe- and Cu-Containing ZSM-5. J. Am. Chem. Soc. 2013, 135 (30), 11087–11099. 10.1021/ja403060n. [DOI] [PubMed] [Google Scholar]
- Al-Shihri S.; Richard C. J.; Al-Megren H.; Chadwick D. Insights into the direct selective oxidation of methane to methanol over ZSM-5 zeolytes in aqueous hydrogen peroxide. Catal. Today 2020, 353, 269–278. 10.1016/j.cattod.2018.03.031. [DOI] [Google Scholar]
- Underhill R.; Lewis R. J.; Freakley S. J.; Douthwaite M.; Miedziak P. J.; Edwards J. K.; Akdim O.; Hutchings G. J. Oxidative Degradation of phenol using in-situ generated H2O2 combined with Fenton’s process. Johnson Matthey Technol. Rev. 2018, 62, 417–425. 10.1595/205651318X15302623075041. [DOI] [Google Scholar]
- Santos A.; Lewis R. J.; Morgan D. J.; Davies T. E.; Hampton E.; Gaskin P.; Hutchings G. J. The degradation of phenol via in situ H2O2 production over supported Pd-based catalysts. Catal. Sci. Technol. 2021, 11 (24), 7866–7874. 10.1039/D1CY01897C. [DOI] [Google Scholar]
- Xu J.; Armstrong R. D.; Shaw G.; Dummer N. F.; Freakley S. J.; Taylor S. H.; Hutchings G. J. Continuous selective oxidation of methane to methanol over Cu- and Fe-modified ZSM-5 catalysts in a flow reactor. Catal. Today 2016, 270, 93–100. 10.1016/j.cattod.2015.09.011. [DOI] [Google Scholar]
- Joshi A. M.; Delgass W. N.; Thomson K. T. Investigation of Gold–Silver, Gold–Copper, and Gold–Palladium Dimers and Trimers for Hydrogen Peroxide Formation from H2 and O2. J. Phys. Chem. C 2007, 111 (20), 7384–7395. 10.1021/jp066828a. [DOI] [Google Scholar]
- Ab Rahim M. H.; Armstrong R. D.; Hammond C.; Dimitratos N.; Freakley S. J.; Forde M. M.; Morgan D. J.; Lalev G.; Jenkins R. L.; Lopez-Sanchez J. A.; Taylor S. H.; Hutchings G. J. Low temperature selective oxidation of methane to methanol using titania supported gold palladium copper catalysts. Catal. Sci. Technol. 2016, 6 (10), 3410–3418. 10.1039/C5CY01586C. [DOI] [Google Scholar]
- Alotaibi F.; Al-Mayman S.; Alotaibi M.; Edwards J. K.; Lewis R. J.; Alotaibi R.; Hutchings G. J. Direct Synthesis of Hydrogen Peroxide Using Cs-Containing Heteropolyacid-Supported Palladium-Copper Catalysts. Catal. Lett. 2019, 149 (4), 998–1006. 10.1007/s10562-019-02680-3. [DOI] [Google Scholar]
- Gong X.; Lewis R. J.; Zhou S.; Morgan D. J.; Davies T. E.; Liu X.; Kiely C. J.; Zong B.; Hutchings G. J. Enhanced catalyst selectivity in the direct synthesis of H2O2 through Pt incorporation into TiO2 supported AuPd catalysts. Catal. Sci. Technol. 2020, 10, 4635–4644. 10.1039/D0CY01079K. [DOI] [Google Scholar]
- Barnes A.; Lewis R. J.; Morgan D. J.; Davies T. E.; Hutchings G. J. Enhancing catalytic performance of AuPd catalysts towards the direct synthesis of H2O2 through incorporation of base metals. Catal. Sci. Technol. 2022, 12 (6), 1986–1995. 10.1039/D1CY01962G. [DOI] [Google Scholar]
- Barnes A.; Lewis R. J.; Morgan D. J.; Davies T. E.; Hutchings G. J. Improving Catalytic Activity towards the Direct Synthesis of H2O2 through Cu Incorporation into AuPd Catalysts. Catalysts 2022, 12 (11), 1396. 10.3390/catal12111396. [DOI] [Google Scholar]
- Santos A.; Lewis R. J.; Morgan D. J.; Davies T. E.; Hampton E.; Gaskin P.; Hutchings G. J. The oxidative degradation of phenol via in situ H2O2 synthesis using Pd supported Fe-modified ZSM-5 catalysts. Catal. Sci. Technol. 2022, 12 (9), 2943–2953. 10.1039/D2CY00283C. [DOI] [Google Scholar]