Abstract
Here we report preliminary data demonstrating that some patients with myalgic encephalomyelitis/chronic fatiguesyndrome (ME/CFS) may have catalytic autoantibodies that cause the breakdown of myelin basic protein (MBP). We propose that these MBP-degradative antibodies are important to the pathophysiology of ME/CFS, particularly in the occurrence of white matter disease/demyelination. This is supported by magnetic resonance imagining studies that show these findings in patients with ME/CFS and could explain symptoms of nerve pain and muscle weakness. In this work, we performed a series of experiments on patient plasma samples where we isolated and characterized substrate-specific antibodies that digest MBP. We also tested glatiramer acetate (copaxone), an FDA approved immunomodulator to treat multiple sclerosis, and found that it inhibits ME/CFS antibody digestion of MBP. Furthermore, we found that aprotinin, which is a specific serine protease inhibitor, specifically prevents breakdown of MBP while the other classes of protease inhibitors had no effect. This coincides with the published literature describing catalytic antibodies as having serine protease-like activity. Postpandemic research has also provided several reports of demyelination in COVID-19. Because COVID-19 has been described as a trigger for ME/CFS, demyelination could play a bigger role in patient symptoms for those recently diagnosed with ME/CFS. Therefore, by studying proteolytic antibodies in ME/CFS, their target substrates, and inhibitors, a new mechanism of action could lead to better treatment and a possible cure for the disease.
Introduction
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a complex, multisystem disease that is often characterized by postexertional malaise (PEM);1,2 even minor activity can cause the patient to experience a 'crash' in physical and/or mental energy. However, symptoms experienced by these patients fall along a broad spectrum ranging from mild to severe (e.g., bedridden). Therefore, to properly diagnose the patient as having ME/CFS, a clinician refers to a checklist of conditions specific to the disease such as those outlined in the Canadian Consensus Criteria (CCC).3 For example, the CCC considers a patient to have ME/CFS if they present with symptoms of reduced energy levels, PEM, interrupted sleep patterns, muscle pain and weakness, as well as two or more autonomic, neuroendocrine, and immune manifestations.
Since the 1980s, there has been considerable research comparing healthy controls and patients with ME/CFS where several abnormalities of the autonomic and central nervous system (CNS) have been correlated with positron emission tomography studies, revealing vast neuroinflammation.4 Oxidative stress has also been linked to ME/CFS,5 which may negatively impact the metabolic and immune systems, as well as contribute to increased blood–brain barrier (BBB) permeability6 allowing the passage of larger blood components including antibodies (Abs) and other immune cells into the CNS.7
Autoimmunity, which has been reported in people with ME/CFS,8 results from diminished self-tolerance where more self-antigens are seen as foreign substrates. For example, fatigue, which is correlated with PEM in ME/CFS, occurs as a major symptom in other autoimmune, mitochondrial, and infectious diseases;9,10 therefore, PEM in ME/CFS may be the result of poor self-tolerance combined with microbial exposure involving molecular mimicry.11−13
Symptoms of ME/CFS also overlap with those of other autoimmune diseases, in particular, multiple sclerosis (MS).14 MS is an inflammatory disease of the CNS primarily characterized by demyelination, which is the breakdown of the myelin sheath, leading to impaired signal transmission along the axon.15 Here, myelin basic protein (MBP), which maintains the integrity of the myelin sheath in the healthy CNS, dissociates from the membrane during citrullination.16 As a consequence, MBP and its fragments are then free to enter the cytoplasm, general blood supply,17 and cerebral spinal fluid where they can trigger an autoimmune response.
Demyelination may also be a common denominator with ME/CFS where some patients experience symptoms of muscle pain and weakness as a results of diminished nerve function.18 To strengthen this idea, there are several reports of abnormal brain magnetic resonance imagining (MRI) studies in patients with ME/CFS.19−25 Based on images with white/gray matter hyperintensities and cerebral atrophy, Cook et al. found that ME/CFS patients had a higher level of physical impairment (motor and cognitive functions) compared to patients with normal MRI scans.26,27 Furthermore, there is a growing body of literature on COVID-related research postpandemic (e.g., Long-COVID) that makes a strong connection to ME/CFS;28 this suggests that COVID-19 infection may act as a precursor/trigger for ME/CFS. Demyelination in particular, which has been reported numerous times in cases of COVID-19,29−32 may play a larger role in neurological-related symptoms in patients recently diagnosed with ME/CFS.
One contributing factor to demyelination may be catalytic Abs, also called abzymes (antibody + enzyme), that specifically target and digest MBP.33 While abzymes (Abzs) are nearly indistinguishable from the typical Ab structure, they exhibit protease-like activity, which can be naturally or artificially induced. For example, Jencks pioneered an engineered antibody to have catalytic properties through binding to a stable transition-state analogue (TSA),34 and then Schultz and Lerner further developed this research and became leaders in this specialty area of enzymology.35 Catalytic Abs can also be synthesized using the TSA to perform specific functions. In one such case, Landry et al. demonstrated the production of anticocaine Ab conjugates, which exhibit cocaine benzoyl ester hydrolysis to reduce drug addiction.36,37 Furthermore, catalytic Abs have been developed for therapeutic purposes,38 including selective catalytic cleavage by IgM Ab of the HIV coat protein gp120.39,40
Naturally occurring Abzs, on the other hand, were discovered by Paul in 1989.41 Since then, many catalytic Ab types have been identified, including those that target MBP in MS42 and the neuropeptide vasoactive intestinal peptide in pregnant women with asthma.43 In addition to peptides and proteins, Abzs have been shown to hydrolyze other substrates including polysaccharides, DNA, and RNA.44,45 While a low catalytic rate and high substrate affinity have proved a challenge for chemists to design function-specific TSA Abzs, these properties have given clinical researchers the unique perspective of how certain autoimmune pathologies could result from long-term tissue damage as in the case of demyelination. One way catalytic MBP-Abs may cause damage is by directly binding to the neuronal tissue and hydrolyzing basic protein in situ; evidence supporting this has been provided by immunocytochemical studies showing that MBP-specific Abs were localized on the axon during active demyelination.46
In this report, we demonstrate Abs isolated from ME/CFS blood plasma appear to have proteolytic activity that targets the breakdown of MBP. Given the overlap with long COVID/MS neuropathies, catalytic MBP-Abs may contribute to symptoms of muscle weakness and nerve pain in some patients with ME/CFS, which could be the result of demyelination.
Materials and Methods
Patient Samples
ME/CFS patient blood was collected at the Stanford Genome Technology Center (SGTC) in a Vacutainer K2 EDTA tube (BD) and then centrifuged at 1200g. The plasma was aspirated from the top layer, transferred to a 1.2 mL cryovial (Nunc) and flash frozen in liquid nitrogen and stored at −80 °C. MS samples were also provided by the Department of Neurology and Neurological Sciences at Stanford. All ME/CFS patients were diagnosed by licensed clinicians using CCC. We collected total plasma Abs from 19 ME/CFS, 19 HC, and 3 MS patients (male and female), whose ages ranged from 18 to 69 years.
Sample Preparation
Patient plasma samples stored at −80 °C were slowly thawed to room temperature (RT) and then kept at 4 °C during Ab (immunoglobulin (IgG)) collection. Total Abs were isolated by protein-A Sepharose beads, which preferentially capture human IgG1, 2, 4, and IgM/A (Thermo Fisher Scientific, Cat. 101041). Samples were collected with glycine IgG elution buffer (Thermo Fisher Scientific, Cat: 21004); 100 μL of protein-A beads was washed with 25 mM tris-HCL, pH 7.4. The beads were combined with 100 μL of plasma and 300 μL of 25 mM tris-HCL, incubated for 30 min at 4 °C (20 rotations per minute (RPM)), and then washed 5× with 25 mM tris-HCL, pH 7.4; this was followed by 2× collections with 250 μL of elution glycine IgG elution buffer (5 min at 4 °C). Samples were immediately neutralized to ∼pH 7 with 1 M tris, pH 9, followed by 2 buffer exchanges with 25 mM tris-HCL, pH 7.4. Samples were then concentrated using 0.5 mL of 50k molecular weight cutoff (MWCO) Pierce protein concentrators PES (Thermo Fisher Scientific, Cat. 88512). Finally, total Ab yield was determined for each sample with the Nanodrop OneC (Thermo Fisher Scientific) and then normalized to ∼1.5 μg/μL using 25 mM tris-HCL. Some sample aliquots were kept at 4 °C during initial testing, while backup aliquots were stored at −20 °C (in 50% glycerol).
Digest Assays
For all digest assays, 3 μg of each ME/CFS total Abs was added to 25 mM tris-HCL, pH 7.4, to a final volume of 12.5 μL. We used 1 μg of bovine brain (Thermo Fisher Scientific, Cat. 13228010, lot: 2397969) as the substrate (Note: bovine MBP is 93% homologous with human MBP,47 and only two amino acids differ between the four immunodominant MBP-derived peptides [12–31, 82–98, 110–128 and 144–169]);48 the encephalitogenic peptide region (86–98), however, is conserved between the two species. Bovine brain MBP has been used as a substrate for other catalytic Ab studies49 and for inducing experimental autoimmune encephalitis (EAE) for the study of MS in humans.50 Digest reactions were incubated in a Veriti thermal cycler (96-well) for 24 h at 37 °C and then cooled to 4 °C. Twenty-four hours was chosen as the incubation period based on previous literature studying Abz digest activity.42 All MBP controls were treated under the same conditions as those of the Ab test samples.
High-Performance Liquid Chromatography (HPLC) Analysis, Copaxone Fractionation, and Ab Purification
Size-exclusion HPLC was used to further purify Abs after collection by protein-A Sepharose beads. The key system modules used included: Agilent 1200 Fraction collector (G1364C), Agilent 1100 HiP well plate autosampler (G1367A), Agilent 1100 diode array detector (G1315B), and Chemstation software for sample processing and chromatogram analysis. Abs were fraction-collected using the Superdex-200 Increase 10/300 GL (Cytiva, 28990944) at the flow rate of 0.7 mL/min with running buffer: 50 mM sodium dihydrogen phosphate and 150 mM sodium chloride, pH 7.4. Glatiramer acetate (GA) was also fractionated using size-exclusion chromatography with a YARRA SEC-2000 column (Phenomenex, Cat. 00H-4512-E0). Samples were processed at 1 mL/min with the running buffer: 100 mM potassium phosphate and 200 mM potassium chloride at pH 6.8, RT. For Ab fractionation, we used a protein standard mix as a reference chromatogram with samples ranging in size from 15k to 600k Da (Sigma Aldrich, Cat. 69385). In addition, we used a standard polyclonal Ab as a control (Thermo Fisher Scientific, Cat. PA1–10008) to match the chromatogram peak data with our test Ab samples.
Polyacrylamide Gel Electrophoresis (PAGE)
Postdigest/inhibition assay samples (12.5 μL) for PAGE analysis were mixed with 7.5 μL of Novex tricine SDS sample buffer (2×) (Thermo Fisher Scientific, Cat. No. LC1676) and then loaded into the wells of a Novex 16% tricine gel (Thermo Fisher Scientific, Cat. EC66952BOX); each gel was run in XCell SureLock mini cell (Invitrogen) for 45 V 30 min, 90–128 V 2 h. For gels stained with coomassie blue, we followed the manufacturer’s recommended protocol (Invitrogen, Cat. LC6025). All destained gels (24–48 h in deionized water) and fluorescence images with FITC-labeled MBP (495, 519 nm) were scanned using Go Gel Imaging System with Image Lab Touch (Bio-Rad).
MBP FITC Labeling (Copaxone Study)
Fluorescein isothiocyanate (FITC) (Thermo Fisher Scientific, Cat. F1906) was dissolved in dimethyl sulfoxide to yield 5 μg/μL; 1 μL of FITC (5 μg/μL) was added to 99 μL of MBP solution (2.5 μg/μL) plus 18.8 μL of 0.67 M borate and 31.2 μL of RNase/DNase-free ultrapure water for a total volume of 150 μL and a final pH of 8.5. The light-sensitive solution was added to a black 1.5 mL Eppendorf tube, mixed, and rotated at 20 rpm at RT for 60 min (minute). The reaction was then purified 2× using 7k MWCO Zeba protein spin desalting columns (Thermo Fisher Scientific, Cat. 89882) at 300g at RT using an Eppendorf centrifuge 5420 for 2 min to remove unincorporated FITC.
Miscellaneous Substrate Digest
To test whether Ab-substrate proteolysis was specific for MBP, we compared digest assays using the following substrates: casein (Thermo Fisher Scientific, Cat. AAJ6421422), MBP (Thermo Fisher Scientific, Cat. 13228010), α-lactalbumin (Sigma Aldrich, Cat. L5385), and lysozyme from chicken egg white (Sigma Aldrich, Cat. L6876); 1 μL (1 μg/μL) each was added to 9.5 μL 25 mM Tris-HCL (pH 7.4) and 2 μL (1.5 μg/μL) plasma Ab and incubated at 37 °C for 24 h, and 7.5 μL sample buffer was added and then run on PAGE.
Relative Activity Assays
To measure percent relative activity (dependence of the relative MBP-hydrolyzing activity of ME/CFS Abs based on their concentration), we tested Abs from various ME/CFS patients and healthy controls (HC) at 0, 0.5, 1, 1.5, and 2 μg total Ab with 1 μg MBP; 25 mM Tris-HCL (pH 7.4) was added to each for a final volume of 12.5 μL, incubated for 24 h at 37 °C, then run on 16% tricine SDS-PAGE, and stained with coomassie blue. Gels were imaged using the Go Gel Imaging System, and band intensities were analyzed using the Gel Analyzer software. Only the top bands at ∼18,400 Da were measured for disappearance relative to the control MBP substrate (no Ab addition). All assays were done in duplicate, and their results averaged.
Determination of Ab Purity after Elution from Protein-A Beads
To show purity of Ab mix after collection from protein-A Sepharose beads, we ran Ab preparations (5 μg each) on PAGE under reducing and nonreducing conditions. Here we used Novex 4–12% tris-glycine mini gels (Thermo Fisher Scientific, Cat. XP04120BOX) along with SDS-glycine running buffer and 4× glycine SDS sample buffer (Thermo Fisher Scientific, Cat. LC2672); 10× NuPAGE sample reducing agent was purchased from Invitrogen (NP0009).
Abzyme Inhibition Assays by Glatiramer Acetate (GA) and Its Fractions
GA (pharmaceutical brand name, copaxone) was purchased from Best of Chemical Sciences (molar ratio E(0.14), K(0.34), A(0.43), Y(0.09), average molecular weight (Mw) 9161 Da, Cat. 147245–92–9) and concentrated according to each assay parameter starting at 100 μM in water. Random peptides were also tested in the digest/inhibition assay to determine if GA was specific for inhibiting Abz activity. These included human YY peptide (IKPEAPGEDASPEELNRYYASLRHYLNLVTRQRY-NH2, Sigma Aldrich, Cat. P1306) at 4310 Da, human gastrin releasing peptide (VPLPAGGGTVLTKMYPRGNHWAVGHLM-NH2, Sigma Aldrich, Cat. G8022) at 2860 Da, and human influenza hemagglutinin peptide (YPYDVPDYA-NH2, Sigma Aldrich, Cat. I2149) at 1102 Da. Each was added to the assay at a 30 μM final concentration.
Affinity Purification
CNBr-activated Sepharose 4 Fast Flow (Cytiva 17-0981-01) was used to conjugate bovine brain MBP to the resin (following the manufacturer’s protocol). Total ME/CFS Ab samples were added to the column, incubated for 1 h at RT, and then washed. The supernatant (random Abs that did not bind to MBP on column) was first collected, and then Abs captured on the column were eluted with glycine buffer (pH 3.0); because low pH can potentially damage the Ab function, samples were immediately neutralized to ∼7 by adding 1 M tris-HCL pH 9, normalized to ∼1.5 μg/μL, and then stored at 4 °C for renaturation.
Protease Inhibitor Test
To determine if catalytic Abs in ME/CFS belonged to a specific class of proteases, we compared four protease inhibitors, (1) aprotinin (Sigma Aldrich, Cat. A6106), which is a serine protease inhibitor, (2) bestatin (Sigma Aldrich, Cat. B8385), which is an amino peptidase inhibitor, (3) E-64 (Sigma Aldrich, Cat. 324890), which is a cysteine protease inhibitor, and (4) pepstatin (Sigma Aldrich, Cat. 11359053001), which inhibits aspartic acid proteases; each was added at 0.1 and 0.01 μg/μL final concentration to the digest assays (3 μg of Ab and, 1 μg of MBP in, and 25 mM tris-HCL, pH 7.4) where ME/CFS patient Ab samples previously showed break down of MBP.
Results and Discussion
Given the vast overlap in symptoms with MS14 along with increasing reports of abnormal brain MRIs suggesting white matter disease in ME/CFS,23 muscle weakness and nerve pain as experienced by some patients may be the result of demyelination. With numerous studies of catalytic Abs in MS and other autoimmune diseases, we decided to isolate Abs from ME/CFS plasma to determine whether they too showed proteolytic activity toward MBP.
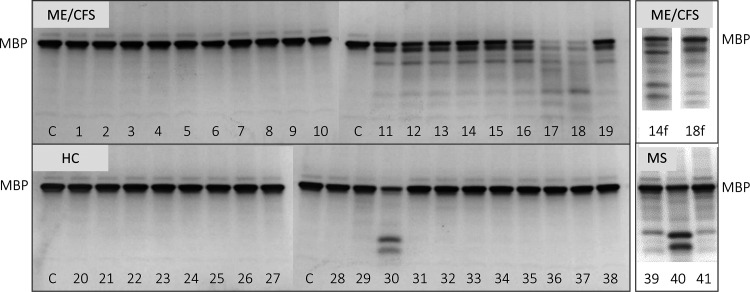
For this study, we compared 19 ME/CFS and 19 healthy control plasma samples. Abs were also collected from MS patients for method development and as a metric for expected digestion of MBP (Figure 1, “MS”, 39–41). Here, we performed digest assays with Abs purified from plasma in addition to MBP as the target substrate (Figure 1). The level of MBP digested (disappearance of the target band “MBP” at ∼18,400 daltons) was compared to the control MBP with no Ab. Within the ME/CFS samples tested for this particular set, 47% showed to digest MBP (e.g., Figure 1, 'ME/CFS' samples 11–19), while in the healthy control group, 5% of the samples also showed digestion of MBP (e.g., Figure 1, 'HC' sample 30). Though other reports have found catalytic Abs in healthy individuals, the extent of activity is believed to be limited or below the threshold of causing pathology.51 In addition, we suggest a few possible clinical explanations for the HC Ab digestion of MBP in this study: HC donor (1) may show digest of MBP only with in vitro conditions, but actual Abz levels, if active in vivo, were below threshold of disease (though, a clinical threshold has not been defined for catalytic Abs), (2) is at the beginning stages of demyelination, or (3) has a viral infection (asymptomatic) or was vaccinated around the time blood was collected (a recent report showed high cross-reactivity between SARS-CoV-2 and human tissue, in particular, MBP52). Furthermore, those ME/CFS patients whose plasma Ab samples did not breakdown MBP (Figure 1, 'ME/CFS' samples 1–10) may have symptoms unrelated to demyelination. For example, they may be experiencing symptoms more related to general malaise and fatigue as might be caused by poor energy levels due to oxidative stress and mitochondrial dysfunction.5 More extensive demographics at the initial patient blood draw including a current list of specific symptoms, their relative severity, and whether they are acute, chronic, or relapse-remitting, would also help with the interpretation of these results.
Figure 1.
PAGE image comparing digestion of MBP by Abs collected from 19 myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), 19 healthy control (HC), and 3 multiple sclerosis (MS) patients. Numbers at the bottom are assigned to each donor sample ('C' is control (only MBP, no Ab)). Samples where Abs were further fractionated using HPLC then tested again in a separate digest assay are denoted with 'f'.
It is interesting to note as well that the MBP digest band patterns between ME/CFS and HC/MS as shown in Figure 1 appear to be different but consistent within each group. Additional studies including sequence analysis of the digest fragments are required to determine the exact substrate cleavage sites. This information could help further validate/characterize Abz properties found in ME/CFS compared to those in healthy controls and MS patient plasma samples.
We next tested three additional substrates to determine if the breakdown of MBP was more substrate-specific or the result of random proteolysis. As shown in Figure 2A, Abs from ME/CFS_11 only digested MBP and not the other substrates tested (casein, α-lactalbumin, and lysozyme). Both casein and MBP are considered unstructured proteins because they do not contain disulfide bridges; as such, the peptide sequences of both substrates are highly exposed in an aqueous medium. Furthermore, casein is a very common substrate used for standard digest assays and is particularly susceptible to hydrolysis by trypsin (serine protease). Though we tested only three substrates that were not digested, there could be others with equal or greater Abz specificity than what was found for MBP in this study. For example, there are reports of substrate-specific Abs in ME/CFS with affinity to human insulin, collagen type IV, γ-interferon, pulmonary elastin, S100 protein, and DNA.53 In addition to MBP, Vojdani and Kharrazian showed that several substrates were also cross-reactive to SARS-CoV-2, including alpha-myosin and actin.52
Figure 2.
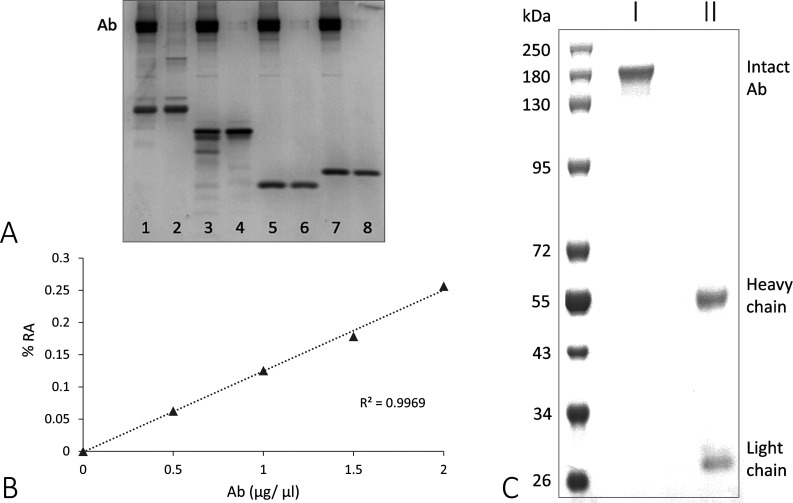
(A) Digest assay comparison between MBP and random substrates: (1) casein, (2) MBP, (3) α-lactalbumin, and (4) lysozyme; each set shows +/− Abs from ME/CFS_11 patient, (B) kinetics of ME/CFS_11 Abs showing correlation/percent relative activity (% RA) of MBP breakdown with increasing Ab concentration, and (C) PAGE image of ME/CFS_11 Ab with (I) nonreducing and (II) reducing conditions after protein-A purification to demonstrate Ab purity; first lane is a protein ladder. Further densitometry analysis of the digest assay (A) comparing the MBP bands +/– Ab showed the target full-length product (∼18,400 Da) for each to be 687 and 1305 (dpi), respectively. All substrates (+/– Ab) were incubated at 37 °C for 24 h.
When we tested ME/CFS_11 for the percent relative digest activity of MBP (% RA) (Figure 2B), we found a strong correlation between catalytic activity and Ab concentration, which is expected in a typical protease kinetics assay. Additionally, Figure 2C demonstrates general ME/CFS, HC, and MS Ab purity following protein-A purification. Under nonreducing conditions, the intact Ab migrates to ∼ 150k Da on PAGE, while under reducing conditions, the same Ab is separated into heavy and light chains (∼55k and 25k Da, respectively). These results show that (1) the Ab structure is maintained during collection and (2) no other bands are visible on the gel that would suggest benon-Abz protease contamination is responsible for the breakdown of MBP during the digest assays.
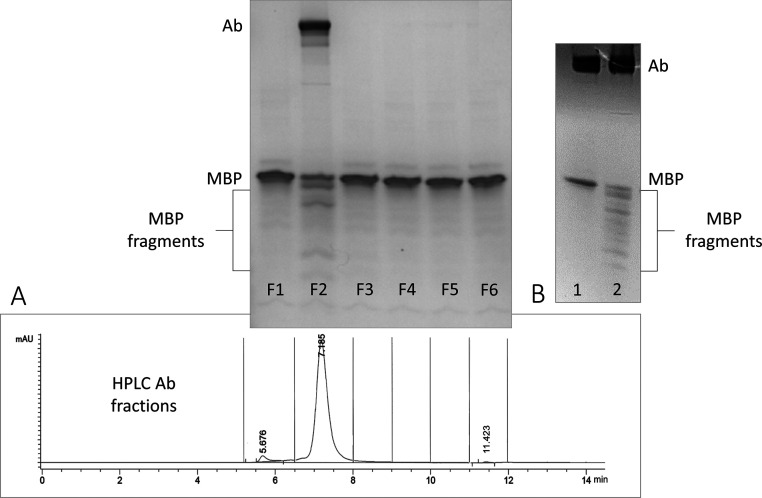
To further purify the Ab samples, we help rule out help rule out possible protease involvement, we ran them through a Superdex-200 column using HPLC size-exclusion chromatography following protein-A purification. For example, in Figure 3A, six fractions were collected from patient ME/CFS_11’s total Ab plasma sample based on time points of a reference chromatogram (corresponding PAGE image above shows the post-digest assay with each fraction). Here, breakdown of MBP was observed only with the intact Ab in fraction 2 (major peak at 7.185 min). If any proteases were present, digest products would have been expected in fractions 4 or 5 (e.g., cysteine, serine, aspartyl, and aminopeptidase proteases range between 23 and 34k Da).
Figure 3.
(A) Digest assay with ME/CFS_11 after HPLC fraction-collection of intact Ab using the Superdex-200 column. After protein-A purification, the sample was processed with HPLC and fraction-collected in six fragments (the chromatogram of Ab sample shows vertical bars separating each fraction corresponding to PAGE image of the postdigest assays above). (B) Digest assay following affinity purification of total Abs collected from ME/CFS_15 plasma sample; lane 1 shows unbound nonspecific Abs from the wash step, and lane 2 shows MBP-specific Abs captured and eluted from the resin conjugated with MBP.
In Figure 3B, MBP was conjugated to CNBr-activated Sepharose 4B resin in a column (affinity precipitation (ppt)), which was used to capture only Abs from ME/CFS_15 plasma that had affinity for MBP. Here, we compared the wash solution (random Abs that did not bind to the resin) with the captured Abs (bound to the resin). It is clear in the PAGE image (Figure 3B, lane 1) that none of the Abs from the wash solution digested MBP. However, MBP-specific Abs from ME/CFS_15 eluted from the resin showed a significant breakdown of MBP (Figure 3B, lane 2).
While Ab binding to the MBP-conjugated resin in our study was expected to be very strong (high affinity/low Kd, which is a characteristic property of Abzs), we first attempted mild elution conditions to recover the Abs/Abz from the resin (e.g., gentle elution (pH 6.8), 1 M KCL, 1 M NaCl); however, only glycine (pH 3) was effective. Recovery yields (nonbinding, MBP-binding Abs) ranged from 4.7, 0.303 to 1.1, 0.147 μg/μL, respectively. For each digest assay, we used 3 μg of nonbinding Ab (from wash step) and 4 μL of eluted Ab and 1 μg MBP (24 h, 37 °C).
Because affinity elution is a very delicate process for standard substrate-specific Ab collection, recovery of Abs with catalytic activity is even more challenging. Abs are very sensitive to extreme pH changes, especially those with enzymatic properties, which could be irreversibly damaged.44 Though Abs/Abzs may be renatured to some degree by dialysis/pH adjustment to ∼7, followed by refrigeration (4 °C), incubation times are sample-dependent.
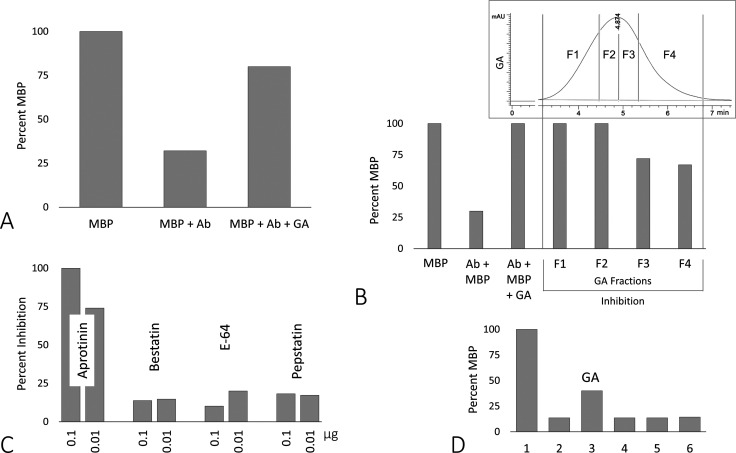
Next, we tested whether glatiramer acetate (GA), which is an immunomodulator drug (pharmaceutical brand name copaxone) used to treat MS patients,54 also inhibited catalytic Abs isolated from ME/CFS plasma that breakdown MBP. In Figure 4A, the first bar in the graph shows the MBP control at 100%; when antibodies from patient sample ME/CFS_11 were added, 68% of MBP was digested (second bar). With the addition of glatiramer acetate, a majority of the MBP remained intact (80%). We believe that peptides from the drug acted as pseudo-MBP substrates that helped block catalytic ME/CFS_11 Abs from digesting MBP. Due to the molecular weight similarities between peptides from GA and MBP and its fragments, MBP was FITC-labeled to distinguish the two during fluorescence imaging. We also processed samples with a HC sample + GA, MBP + GA, and GA only, and the results showed no change in band intensity between HC and MBP, and GA alone was not visible with fluorescence (data not shown). To further validate the specificity of GA to prevent ME/CFS Abz breakdown of MBP, we found that the drug does not inhibit the general proteolytic activity of trypsin (data not shown).
Figure 4.
(A) Digest assay with ME/CFS_11 (Figure 1) before and after addition of whole glatiramer acetate (GA), 30 μM final; (B) GA fractionated using size-exclusion chromatography (top chromatogram with fraction demarcations (vertical lines)) corresponding to degree of inhibition in the bar graph directly below for sample ME/CFS_11; (C) comparison of four class-specific protease inhibitors on preventing ME/CFS_12 Ab digest of MBP; each inhibitor was tested at 0.1 and 0.01 μg (1.22 and 0.122 μM final, respectively); and (D) digest assay comparing inhibition by GA and three random peptides: (1) MBP only, (2) ME/CFS_12 Ab + MBP, (3) GA (30 μM), (4) peptide YY, (5) gastrin releasing peptide, and (6) influenza hemagglutinin peptide (each at 30 μM final concentration with 3 μg Ab and 1 μg MBP).
GA, which is a complex mixture of peptides (∼1036) that share physical properties with the MBP structure,55 has three proposed mechanisms of action against neuroinflammation in vivo. Peptides from GA may (1) directly compete with fragments of MBP at the junction between the antigen presenting cell’s major histocompatible complex II and the T-cell receptor; this in turn down-regulates T-cell activation and polyclonal expansion of MBP-specific Abs,56 (2) act as a competitive ligand, which inhibits MBP from binding to the αMβ2 integrin,57 and (3) compete with Abzs in proximity to intact axonal MBP.46 It is worth noting here as well, though the BBB is highly substrate-selective with a small number of sequence-specific peptide transporters,58 systemic inflammation, as in MS and ME/CFS, may cause increased BBB permeability allowing leakage of GA; animal studies monitoring radio-labeled peptides of GA after injection showed some fragments actually reached the CNS.59 Furthermore, Nevinsky et al., demonstrated proteolytic activity of MBP-Abs collected from MS plasma and cerebral spinal fluid (CSF) was also inhibited by GA in vitro.44
Next, we fractionated GA into four equal subsets (25% each of the peptide mix) based on HPLC size-exclusion chromatography. We used ME/CFS_11 in a separate digest assay (Figure 4B), which was shown to break down 70% of the MBP substrate (second bar); and when total GA was included, none of the substrate was digested. However, when we added the individual fractions to separate digest assays, fractions 3 and 4 (lower Mw peptides < 9161 Da) were not as potent as fractions 1 and 2 (higher Mw > 9161 Da) in preventing digestion of MBP. As a control, we tested three random peptides with varying Mws (peptide YY, 4310 Da; gastrin releasing peptide, 2860 Da; and influenza hemagglutinin peptide, 1102 Da) and found none had any inhibitory effect (Figure 4D). These results also coincide with those in Figure 4B where the lower Mw peptides appeared to have less potency in protecting MBP from being digested. However, a more extensive study of GA and its fractions might help determine if higher molecular weight peptides in general prevent breakdown of MBP in patients with ME/CFS. We also note here that 30 μM GA in our digest assays (24 h at 37 °C) is significantly more than what is initially administered by injection to a patient (e.g., 20 or 40 mg GA/dose); typically, injections are given every 3 days for an indefinite period of time, and often it can take between 6 and 9 months before the patient reports whether any symptoms have been alleviated.60 From our preliminary results exploring inhibitory effects of GA on the Abz activity, this superdose of the drug in vitro may represent to some degree the cumulative effect of long-term treatment of GA in vivo. Nevertheless, a more extensive look at the pharmacokinetics of GA as an inhibitor to Abz activity is recommended.
We also tested whether catalytic Abs in ME/CFS (i.e., ME/CFS_12) that digested MBP were of a particular class of protease (i.e., serine, cysteine, aspartic acid, or amino peptidase). In Figure 4C, aprotinin (serine protease inhibitor) showed the greatest potency at 0.1 μg (1.22 μM, 100% inhibition) and 0.01 μg (0.122 μM, 74% inhibition), while the other 3 inhibitors averaged 16% inhibition at the same concentrations each. These results coincide with other published results describing catalytic Abs/Abzs as being serine protease-like.61
In many autoimmune disorders, particularly MS, it has been suggested that abzymes may contribute to direct tissue damage.51 Further evidence for this has been provided by immunocytochemical studies showing MBP-specific Abs are localized to the axon during active demyelination.46 Effects of the Abz breakdown of MBP at the neuron, which can take months for disease symptoms to manifest, may be explained by the extremely slow turnover rate of the catalytic Ab. For example, one report showed the catalytic rate of an Abz (4 kcat (S1–)) to be >10-fold slower than trypsin (48–55 (kcat (S1–)).61 Low Abz kcat may be explained by high-affinity binding (low Kd) through the Ab FAB (fragment antigen binding) region and/or increased spacing between the three amino acids that make up a catalytic triad of traditional serine proteases, which includes serine, histidine, and aspartic acid.62 It has also been proposed that only two amino acids in the catalytic site, histidine and serine, are involved in peptide hydrolysis.61 Furthermore, the kcat of Abzs isolated from cerebral spinal fluid (CSF) of MS patients was found to be significantly higher compared to plasma (0.0067 and 0.000123 S1–, respectively).63 Also, binding capacity and degree of catalysis may be contingent on other factors such as the ratio of Abz to Ab in the total Ab population and the conformation/ modification(s) of the substrate.
While natural Abz production in vivo may be the result of random mutation in the Ab FAB region,63 there are many factors that could influence the configuration of MBP in both the healthy and disease states, which can also elicit Abz development. Truscott and Friedrich mentioned that long-lived proteins, in particular MBP, go through posttranslational modifications (PTM) in human adults.64 For example, in the cerebellum, three primary covalent modifications were found: (1) deamidation of asparagine and glutamine, (2) isomerization of aspartic acid, which leads to protein cross-linking and aggregation, and (3) deimination of arginine to citrulline, which is irreversibly catalyzed by the epigenetically controlled peptidylarginine deiminase (PAD);65 this is an especially important modification because citrulline, which has zero charge and is not a natural component of the MBP sequence, causes profound changes to the protein structure. Without the positive charge of arginine to keep it anchored to the phospholipid bilayer in the major dense line of the myelin sheath, MBP becomes unfolded in the cytoplasm making it a prime target for immunogenic attack.66,67 Moreover, anticitrullinated protein autoantibodies have been found to be highly specific in several autoimmune diseases, including rheumatoid arthritis, where inhibition of PAD shows therapeutic potential.65,68
Patients with ME/CFS may also present with fluctuations in severity of the disease akin to MS where symptoms rise and fall through cycles of relapse and remission.69 Onset or exacerbation of ME/CFS symptoms may be triggered as well by factors including (1) infection28 (viral and bacterial),70 (2) diet, (3) hormonal changes,71 (4) age, (5) environment,72 and (6) drug induction.73,74 Molecular mimicry has also been proposed to induce autoimmunity in ME/CFS where viruses present infectious material such as peptides that have similar residue sequences as the host antigen(s); these foreign agents, which may cause an autoimmune response, include, for example, herpes 6, Epstein-Bar, and corona virus 229E.11−13
Conclusions
As this research contributes only one small piece to a much larger, intricate composition of ME/CFS, we have presented evidence that autoantibodies with enzymatic properties (abzymes) may cause demyelination in some patients diagnosed with ME/CFS; and given the broad spectrum of overlapping pathologies with MS and other autoimmune diseases including long COVID, demyelination should be considered a possibility in ME/CFS as well, especially for patients experiencing nerve pain and muscle weakness.
Moving forward with this research, we believe that there are additional steps and considerations worth noting. For example, the MBP substrate used to test proteolytic Ab hydrolysis should closely simulate in vivo conditions; to accomplish this, certain modifications to the substrate could be made in vitro (e.g., methylation/citrullination) or the target substrate can be extracted/isolated from diseased tissue obtained through an ME/CFS patient biobank.75
It is possible too that ME/CFS patient symptoms may be relapsing-remitting as in MS. Therefore, a study should be conducted to examine the catalytic activity of a patient’s plasma Abs over time. In addition to MRI data, this may be a useful diagnostic tool for tracking disease severity with corresponding levels of proteolysis. Also, we have not tested CSF, which may have a higher concentration/more active catalytic Abs (>kcat), as has been shown for MS patient samples in previous reports.42 Further assessment of the CSF Abz content compared with peripheral blood may help generate a more complete profile of the ME/CFS patient’s demographics, which could prove to be an accurate metric in identifying and characterizing disease symptoms and severity.
Finally, there is currently no diagnostic tool or assay that definitively states whether a patient has ME/CFS or is likely to contract the disease; however, there are metrics available that look for commonalities among patients, for example, the use of nanoelectronics in blood-based diagnostics.76 Catalytic Abs have also been used in clinical settings to monitor autoimmune disease progression. For example, Kalinina et al. found that the degree of cardiac myosin (CM) hydrolysis in autoimmune myocarditis (AM) was strongly correlated not only with anti-CM autoantibody serum levels but also with AM disease manifestations and symptom severity.77 In addition to MBP,78,79 there are many substrate-specific Abs (antiprotein and anti-DNA) that have been previously identified,53 which could be of clinical significance if they too cause tissue damage. Therefore, we propose an assay for MBP hydrolysis by catalytic Abs in ME/CFS patients may provide information to better diagnose and treat patients in the early stages of demyelination.
Acknowledgments
First and foremost, we would like to thank all the patients who generously donated to this project. We would also like to thank Whitney Dafoe for raising funds to purchase an HPLC unit for sample processing and analysis performed in this research. We also wish to thank Anna Tomczak, Viktoriya Bourakova, and Drs. Yamuna Joseph, Jeffrey Dunn, and May Han at Stanford’s Department of Neurology and Neurological Sciences for providing MS samples to establish proof of concept in our study of catalytic Abs in ME/CFS. We also would like to thank Angela Chu (SGTC) for reviewing this manuscript and providing comments/edits, as well as discussions regarding our catalytic Ab work. We also would like to thank Dr. Bela Chheda for providing patient samples and Linda Tannenbaum and the Open Medicine Foundation for their support. Finally, the work presented here was also made possible by the generous support of the Khosla Family gift fund.
The authors declare no competing financial interest.
References
- Morris G.; Maes M.; Berk M.; Puri B. K. myalgic encephalomyelitis or chronic fatigue syndrome: how could the illness develop?. Metab Brain Dis 2019, 34, 385–415. 10.1007/s11011-019-0388-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G.; Puri B. K.; Walker A. J.; Maes M.; Carvalho A. F.; Walder K.; Mazza C.; Berk M. myalgic encephalomyelitis/chronic fatigue syndrome: From pathophysiological insights to novel therapeutic opportunities. Pharmacol. Res. 2019, 148, 104450 10.1016/j.phrs.2019.104450. [DOI] [PubMed] [Google Scholar]
- Carruthers B. M.; van de Sande M. I.; De Meirleir K. L.; Klimas N. G.; Broderick G.; Mitchell T.; Staines D.; Powles A. C.; Speight N.; Vallings R.; Bateman L.; Baumgarten-Austrheim B.; Bell D. S.; Carlo-Stella N.; Chia J.; Darragh A.; Jo D.; Lewis D.; Light A. R.; Marshall-Gradisnik S.; Mena I.; Mikovits J. A.; Miwa K.; Murovska M.; Pall M. L.; Stevens S. myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. 10.1111/j.1365-2796.2011.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaroff A. L.; Cho T. A. Role of infection and neurologic dysfunction in chronic fatigue syndrome. Semin Neurol 2011, 31, 325–337. 10.1055/s-0031-1287654. [DOI] [PubMed] [Google Scholar]
- Kennedy G.; Spence V. A.; McLaren M.; Hill A.; Underwood C.; Belch J. J. Oxidative stress levels are raised in chronic fatigue syndrome and are associated with clinical symptoms. Free Radic Biol Med 2005, 39, 584–589. 10.1016/j.freeradbiomed.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Bested A. C.; Saunders P. R.; Logan A. C. Chronic fatigue syndrome: neurological findings may be related to blood--brain barrier permeability. Med. Hypotheses 2001, 57, 231–237. 10.1054/mehy.2001.1306. [DOI] [PubMed] [Google Scholar]
- Jain R. W.; Yong V. W. B cells in central nervous system disease: diversity, locations and pathophysiology. Nat Rev Immunol 2022, 22, 513–524. 10.1038/s41577-021-00652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotzny F.; Blanco J.; Capelli E.; Castro-Marrero J.; Steiner S.; Murovska M.; Scheibenbogen C. European Network on, ME/CFS (EUROMENE) myalgic Encephalomyelitis/Chronic Fatigue Syndrome - Evidence for an autoimmune disease. Autoimmun. Rev. 2018, 17, 601–609. 10.1016/j.autrev.2018.01.009. [DOI] [PubMed] [Google Scholar]
- Blomberg J.; Gottfries C. G.; Elfaitouri A.; Rizwan M.; Rosen A. Infection Elicited Autoimmunity and myalgic Encephalomyelitis/Chronic Fatigue Syndrome: An Explanatory Model. Front. Immunol. 2018, 9, 229. 10.3389/fimmu.2018.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth K.; Scheibenbogen C. A Unifying Hypothesis of the Pathophysiology of myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Recognitions from the finding of autoantibodies against ss2-adrenergic receptors. Autoimmun Rev 2020, 19, 102527 10.1016/j.autrev.2020.102527. [DOI] [PubMed] [Google Scholar]
- Schreiner P.; Harrer T.; Scheibenbogen C.; Lamer S.; Schlosser A.; Naviaux R. K.; Prusty B. K. Human Herpesvirus-6 Reactivation, Mitochondrial Fragmentation, and the Coordination of Antiviral and Metabolic Phenotypes in myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Immunohorizons 2020, 4, 201–215. 10.4049/immunohorizons.2000006. [DOI] [PubMed] [Google Scholar]
- Ruiz-Pablos M.; Paiva B.; Montero-Mateo R.; Garcia N.; Zabaleta A. Epstein-Barr Virus and the Origin of myalgic Encephalomyelitis or Chronic Fatigue Syndrome. Front Immunol 2021, 12, 656797 10.3389/fimmu.2021.656797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot P. J.; Paquette J. S.; Ciurli C.; Antel J. P.; Ouellet F. Myelin basic protein and human coronavirus 229E cross-reactive T cells in multiple sclerosis. Ann. Neurol. 1996, 39, 233–240. 10.1002/ana.410390213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G.; Maes M. myalgic encephalomyelitis/chronic fatigue syndrome and encephalomyelitis disseminata/multiple sclerosis show remarkable levels of similarity in phenomenology and neuroimmune characteristics. BMC Med. 2013, 11, 205. 10.1186/1741-7015-11-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson R.; Giovannoni G. Multiple sclerosis - a review. Eur J Neurol 2019, 26, 27–40. 10.1111/ene.13819. [DOI] [PubMed] [Google Scholar]
- Moscarello M. A.; Mastronardi F. G.; Wood D. D. The role of citrullinated proteins suggests a novel mechanism in the pathogenesis of multiple sclerosis. Neurochem. Res. 2007, 32, 251–256. 10.1007/s11064-006-9144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R.; Chepisheva M.; Cronin T.; Seemungal B. M.. Chapter 16 - Diagnostic Approaches Techniques in Concussion/Mild Traumatic Brain Injury: Where are we?, In Neurosensory Disorders in Mild Traumatic Brain Injury (Hoffer M. E., Balaban C. D., Eds.); Academic Press: 2019, 247-277. [Google Scholar]
- Inglese M. Multiple sclerosis: new insights and trends. AJNR Am. J. Neuroradiol. 2006, 27, 954–957. [PMC free article] [PubMed] [Google Scholar]
- Lange G.; DeLuca J.; Maldjian J. A.; Lee H.; Tiersky L. A.; Natelson B. H. Brain MRI abnormalities exist in a subset of patients with chronic fatigue syndrome. J Neurol Sci 1999, 171, 3–7. 10.1016/S0022-510X(99)00243-9. [DOI] [PubMed] [Google Scholar]
- Chen R.; Liang F. X.; Moriya J.; Yamakawa J.; Sumino H.; Kanda T.; Takahashi T. Chronic fatigue syndrome and the central nervous system. J Int Med Res 2008, 36, 867–874. 10.1177/147323000803600501. [DOI] [PubMed] [Google Scholar]
- Barnden L. R.; Shan Z. Y.; Staines D. R.; Marshall-Gradisnik S.; Finegan K.; Ireland T.; Bhuta S. Intra brainstem connectivity is impaired in chronic fatigue syndrome. Neuroimage Clin 2019, 24, 102045 10.1016/j.nicl.2019.102045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y.; Sato N.; Ota M.; Shigemoto Y.; Morimoto E.; Enokizono M.; Matsuda H.; Shin I.; Amano K.; Ono H.; Sato W.; Yamamura T. Brain abnormalities in myalgic encephalomyelitis/chronic fatigue syndrome: Evaluation by diffusional kurtosis imaging and neurite orientation dispersion and density imaging. J Magn Reson Imaging 2019, 49, 818–824. 10.1002/jmri.26247. [DOI] [PubMed] [Google Scholar]
- Shan Z. Y.; Barnden L. R.; Kwiatek R. A.; Bhuta S.; Hermens D. F.; Lagopoulos J. Neuroimaging characteristics of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): a systematic review. J. Transl. Med. 2020, 18, 335. 10.1186/s12967-020-02506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Z. Y.; Kwiatek R.; Burnet R.; Del Fante P.; Staines D. R.; Marshall-Gradisnik S. M.; Barnden L. R. Progressive brain changes in patients with chronic fatigue syndrome: A longitudinal MRI study. J Magn Reson Imaging 2016, 44, 1301–1311. 10.1002/jmri.25283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri B. K.; Jakeman P. M.; Agour M.; Gunatilake K. D.; Fernando K. A.; Gurusinghe A. I.; Treasaden I. H.; Waldman A. D.; Gishen P. Regional grey and white matter volumetric changes in myalgic encephalomyelitis (chronic fatigue syndrome): a voxel-based morphometry 3 T MRI study. Br J Radiol 2012, 85, e270–273. 10.1259/bjr/93889091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D. B.; Lange G.; DeLuca J.; Natelson B. H. Relationship of brain MRI abnormalities and physical functional status in chronic fatigue syndrome. Int J Neurosci 2001, 107, 1–6. 10.3109/00207450109149754. [DOI] [PubMed] [Google Scholar]
- Sbardella E.; Petsas N.; Tona F.; Prosperini L.; Raz E.; Pace G.; Pozzilli C.; Pantano P. Assessing the correlation between grey and white matter damage with motor and cognitive impairment in multiple sclerosis patients. PLoS One 2013, 8, e63250 10.1371/journal.pone.0063250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaroff A. L.; Lipkin W. I. ME/CFS and Long COVID share similar symptoms and biological abnormalities: road map to the literature. Front. Med. (Lausanne) 2023, 10, 1187163 10.3389/fmed.2023.1187163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodanovich M. Y.; Kamaeva D. A.; Naumova A. V. Role of Demyelination in the Persistence of Neurological and Mental Impairments after COVID-19. Int J Mol Sci 2022, 23, 11291. 10.3390/ijms231911291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabani Z. Demyelination as a result of an immune response in patients with COVID-19. Acta Neurol Belg 2021, 121, 859–866. 10.1007/s13760-021-01691-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khair A. M.; Nikam R.; Husain S.; Ortiz M.; Kaur G. Para and Post-COVID-19 CNS Acute Demyelinating Disorders in Children: A Case Series on Expanding the Spectrum of Clinical and Radiological Characteristics. Cureus 2022, 14, e23405 10.7759/cureus.23405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara S.; Candelore T.; Youssef P.; Nedd K. Evidence of Post-COVID-19 Transverse Myelitis Demyelination. Cureus 2021, 13, e19087 10.7759/cureus.19087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokat K. M.; Schultz P. G. Catalytic antibodies. Annu. Rev. Immunol. 1990, 8, 335–363. 10.1146/annurev.iy.08.040190.002003. [DOI] [PubMed] [Google Scholar]
- Jencks W. P.Catalysis in Chemistry and Enzymology; Dover: New York, NY, 1969, 836. [Google Scholar]
- Schultz P; Lerner R. A. Antibody catalysis of difficult chemical transformations. Acc. Chem. Res. 1993, 26, 391–395. 10.1021/ar00032a001. [DOI] [Google Scholar]
- Landry D. W.; Zhao K.; Yang G. X.; Glickman M.; Georgiadis T. M. Antibody-catalyzed degradation of cocaine. Science 1993, 259, 1899–1901. 10.1126/science.8456315. [DOI] [PubMed] [Google Scholar]
- Landry D. W.; Yang G. X. Anti-cocaine catalytic antibodies--a novel approach to the problem of addiction. J Addict Dis 1997, 16, 1–17. 10.1300/J069v16n03_01. [DOI] [PubMed] [Google Scholar]
- Mahendra A.; Sharma M.; Rao D. N.; Peyron I.; Planchais C.; Dimitrov J. D.; Kaveri S. V.; Lacroix-Desmazes S. Antibody-mediated catalysis: induction and therapeutic relevance. Autoimmun Rev 2013, 12, 648–652. 10.1016/j.autrev.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Paul S.; Karle S.; Planque S.; Taguchi H.; Salas M.; Nishiyama Y.; Handy B.; Hunter R.; Edmundson A.; Hanson C. Naturally occurring proteolytic antibodies: selective immunoglobulin M-catalyzed hydrolysis of HIV gp120. J. Biol. Chem. 2004, 279, 39611–39619. 10.1074/jbc.M406719200. [DOI] [PubMed] [Google Scholar]
- Planque S.; Nishiyama Y.; Taguchi H.; Salas M.; Hanson C.; Paul S. Catalytic antibodies to HIV: physiological role and potential clinical utility. Autoimmun Rev 2008, 7, 473–479. 10.1016/j.autrev.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S.; Volle D. J.; Beach C. M.; Johnson D. R.; Powell M. J.; Massey R. J. Catalytic hydrolysis of vasoactive intestinal peptide by human autoantibody. Science 1989, 244, 1158–1162. 10.1126/science.2727702. [DOI] [PubMed] [Google Scholar]
- Ponomarenko N. A.; Durova O. M.; Vorobiev I. I.; Belogurov A. A. Jr.; Kurkova I. N.; Petrenko A. G.; Telegin G. B.; Suchkov S. V.; Kiselev S. L.; Lagarkova M. A.; Govorun V. M.; Serebryakova M. V.; Avalle B.; Tornatore P.; Karavanov A.; Morse H. C. 3rd; Thomas D.; Friboulet A.; Gabibov A. G. Autoantibodies to myelin basic protein catalyze site-specific degradation of their antigen. Proc Natl Acad Sci U S A 2006, 103, 281–286. 10.1073/pnas.0509849103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olopade C. O.; Yu J.; Abubaker J.; Mensah E.; Paul S. Catalytic hydrolysis of VIP in pregnant women with asthma. J Asthma 2006, 43, 429–432. 10.1080/02770900600710730. [DOI] [PubMed] [Google Scholar]
- Nevinsky G. A.; Buneva V. N. Human catalytic RNA- and DNA-hydrolyzing antibodies. J Immunol Methods 2002, 269, 235–249. 10.1016/S0022-1759(02)00234-X. [DOI] [PubMed] [Google Scholar]
- Nevinsky G. A.; Buneva V. N. Natural catalytic antibodies in norm, autoimmune, viral, and bacterial diseases. ScientificWorldJournal 2010, 10, 1203–1233. 10.1100/tsw.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genain C. P.; Cannella B.; Hauser S. L.; Raine C. S. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med 1999, 5, 170–175. 10.1038/5532. [DOI] [PubMed] [Google Scholar]
- Eylar E. H. Amino acid sequence of the basic protein of the myelin membrane. Proc Natl Acad Sci U S A 1970, 67, 1425–1431. 10.1073/pnas.67.3.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarenko N. A.; Durova O. M.; Vorobiev I. I.; Belogurov A. A.; Telegin G. B.; Suchkov S. V.; Misikov V. K.; Morse H. C. 3rd; Gabibov A. G. Catalytic activity of autoantibodies toward myelin basic protein correlates with the scores on the multiple sclerosis expanded disability status scale. Immunol. Lett. 2006, 103, 45–50. 10.1016/j.imlet.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Eylar E. H.; Salk J.; Beveridge G. C.; Brown L. V. Experimental allergic encephalomyelitis. An encephalitogenic basic protein from bovine myelin. Arch. Biochem. Biophys. 1969, 132, 34–48. 10.1016/0003-9861(69)90336-1. [DOI] [PubMed] [Google Scholar]
- Hedegaard C. J.; Chen N.; Sellebjerg F.; Sorensen P. S.; Leslie R. G.; Bendtzen K.; Nielsen C. H. Autoantibodies to myelin basic protein (MBP) in healthy individuals and in patients with multiple sclerosis: a role in regulating cytokine responses to MBP. Immunology 2009, 128, e451–461. 10.1111/j.1365-2567.2008.02999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gronow M; Pizzo S. V. Relevance of Catalytic Autoantibodies to Myelin Basic Protein (MBP) in Autoimmune Disorders. J Neurol Neuromedicine 2018, 3 (4), 75–78. 10.29245/2572.942X/2018/4.1199. [DOI] [Google Scholar]
- Vojdani A.; Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol 2020, 217, 108480 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilenko O. V.; Gavrilova N. Y.; Churilov L. P. Chronic Fatigue Exhibits Heterogeneous Autoimmunity Characteristics Which Reflect Etiology. Pathophysiology 2022, 29, 187–199. 10.3390/pathophysiology29020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalive P. H.; Neuhaus O.; Benkhoucha M.; Burger D.; Hohlfeld R.; Zamvil S. S.; Weber M. S. Glatiramer acetate in the treatment of multiple sclerosis: emerging concepts regarding its mechanism of action. CNS Drugs 2011, 25, 401–414. 10.2165/11588120-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemssen T.; Schrempf W. Glatiramer acetate: mechanisms of action in multiple sclerosis. Int Rev Neurobiol 2007, 79, 537–570. 10.1016/S0074-7742(07)79024-4. [DOI] [PubMed] [Google Scholar]
- Johnson K. P. Glatiramer acetate and the glatiramoid class of immunomodulator drugs in multiple sclerosis: an update. Expert Opin Drug Metab Toxicol 2010, 6, 643–660. 10.1517/17425251003752715. [DOI] [PubMed] [Google Scholar]
- Stapulionis R.; Pinto Oliveira C. L.; Gjelstrup M. C.; Pedersen J. S.; Hokland M. E.; Hoffmann S. V.; Poulsen K.; Jacobsen C.; Vorup-Jensen T. Structural Insight into the Function of Myelin Basic Protein as a Ligand for Integrin αMβ21. The Journal of Immunology 2008, 180, 3946–3956. 10.4049/jimmunol.180.6.3946. [DOI] [PubMed] [Google Scholar]
- Banks W. A. Peptides and the blood-brain barrier. Peptides 2015, 72, 16–19. 10.1016/j.peptides.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prod’homme T.; Zamvil S. S. The Evolving Mechanisms of Action of Glatiramer Acetate. Cold Spring Harb. Perspect. Med. 2019, 9, 29249. 10.1101/cshperspect.a029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporro M.; Disanto G.; Gobbi C.; Zecca C. Two decades of subcutaneous Glatiramer acetate injection: current role of the standard dose, and new high-dose low-frequency Glatiramer acetate in relapsing-remitting multiple sclerosis treatment. Patient Prefer Adherence 2014, 8, 1123–1134. 10.2147/PPA.S68698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G. W.; Guo J.; Huang W.; Fletterick R. J.; Scanlan T. S. Crystal structure of a catalytic antibody with a serine protease active site. Science 1994, 265, 1059–1064. 10.1126/science.8066444. [DOI] [PubMed] [Google Scholar]
- Dimitrov J. D.; Lacroix-Desmazes S. Noncanonical Functions of Antibodies. Trends Immunol 2020, 41, 379–393. 10.1016/j.it.2020.03.006. [DOI] [PubMed] [Google Scholar]
- Doronin V. B.; Parkhomenko T. A.; Castellazzi M.; Padroni M.; Pastore M.; Buneva V. N.; Granieri E.; Nevinsky G. A. Comparison of antibodies hydrolyzing myelin basic protein from the cerebrospinal fluid and serum of patients with multiple sclerosis. PLoS One 2014, 9, e107807 10.1371/journal.pone.0107807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truscott R. J. W.; Friedrich M. G. Can the Fact That Myelin Proteins Are Old and Break down Explain the Origin of Multiple Sclerosis in Some People?. J. Clin. Med. 2018, 7, 7090281 10.3390/jcm7090281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Magid A. F. Great Therapeutic Potential of Peptidylarginine deiminase 4 (PAD4) Inhibitors: Treatment of Rheumatoid Arthritis, Epigenetic Tools, Regulation of Pluripotency in Stem Cells, and More. ACS Med Chem Lett 2017, 8, 19–21. 10.1021/acsmedchemlett.6b00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesielski O.; Biesiekierska M.; Panthu B.; Soszynski M.; Pirola L.; Balcerczyk A. citrullination in the pathology of inflammatory and autoimmune disorders: recent advances and future perspectives. Cell. Mol. Life Sci. 2022, 79, 94. 10.1007/s00018-022-04126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolarz B.; Ciesla M.; Dryglewska M.; Majdan M. Peptidyl Arginine deiminase Type 4 Gene Promoter Hypo-Methylation in Rheumatoid Arthritis. J. Clin. Med. 2020, 9, 72049. 10.3390/jcm9072049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliko A.; Kaminska M.; Falkowski K.; Bielecka E.; Benedyk-Machaczka M.; Malicki S.; Koziel J.; Wong A.; Bryzek D.; Kantyka T.; Mydel P Discovery of Novel Potential Reversible Peptidyl Arginine deiminase Inhibitor. Int. J. Mol. Sci. 2019, 20, 92174. 10.3390/ijms20092174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M.; Bar-Or A.; Piehl F.; Preziosa P.; Solari A.; Vukusic S.; Rocca M. A. Multiple sclerosis. Nat. Rev. Dis. Primers 2018, 4, 43. 10.1038/s41572-018-0041-4. [DOI] [PubMed] [Google Scholar]
- Gross C. M.; Baumgartner A.; Rauer S.; Stich O. Multiple sclerosis rebound following herpes zoster infection and suspension of fingolimod. Neurology 2012, 79, 2006–2007. 10.1212/WNL.0b013e3182735d24. [DOI] [PubMed] [Google Scholar]
- Ysrraelit M. C.; Correale J. Impact of sex hormones on immune function and multiple sclerosis development. Immunology 2019, 156, 9–22. 10.1111/imm.13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux J.; Bard D.; Le Pabic E.; Segala C.; Reis J.; Ongagna J. C.; de Seze J.; Leray E. Air pollution by particulate matter PM10 may trigger multiple sclerosis relapses. Environ Res 2017, 156, 404–410. 10.1016/j.envres.2017.03.049. [DOI] [PubMed] [Google Scholar]
- Dedeoglu F. Drug-induced autoimmunity. Curr Opin Rheumatol 2009, 21, 547–551. 10.1097/BOR.0b013e32832f13db. [DOI] [PubMed] [Google Scholar]
- Niklas K.; Niklas A. A.; Majewski D.; Puszczewicz M. Rheumatic diseases induced by drugs and environmental factors: the state-of-the-art - part one. Reumatologia 2016, 54, 122–127. 10.5114/reum.2016.61212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacerda E. M.; Mudie K.; Kingdon C. C.; Butterworth J. D.; O’Boyle S.; Nacul L. The UK ME/CFS biobank: A Disease-Specific biobank for Advancing Clinical Research Into myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Front. Neurol. 2018, 9, 1026. 10.3389/fneur.2018.01026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfandyarpour R.; Kashi A.; Nemat-Gorgani M.; Wilhelmy J.; Davis R. W. A nanoelectronics-blood-based diagnostic biomarker for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Proc Natl Acad Sci U S A 2019, 116, 10250–10257. 10.1073/pnas.1901274116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinina E. V.; Ponomarenko N. A.; Durova O. M.; Paleev F. N.; Vorob’ev I. I.; Kekenadze N. N.; Shogenov Z. S.; Zemtsova M. E.; Gnuchev N. V.; Gabibov A. G. Catalytic autoantibodies in autoimmune myocarditis: clinical and pathogenetic implications. Ter. Arkh. 2005, 77, 65–70. [PubMed] [Google Scholar]
- Belogurov A. A. Jr.; Kurkova I. N.; Friboulet A.; Thomas D.; Misikov V. K.; Zakharova M. Y.; Suchkov S. V.; Kotov S. V.; Alehin A. I.; Avalle B.; Souslova E. A.; Morse H. C. 3rd; Gabibov A. G.; Ponomarenko N. A. Recognition and degradation of myelin basic protein peptides by serum autoantibodies: novel biomarker for multiple sclerosis. J Immunol 2008, 180, 1258–1267. 10.4049/jimmunol.180.2.1258. [DOI] [PubMed] [Google Scholar]
- Gabibov A. G.; Ponomarenko N. A.; Tretyak E. B.; Paltsev M. A.; Suchkov S. V. Catalytic autoantibodies in clinical autoimmunity and modern medicine. Autoimmun Rev 2006, 5, 324–330. 10.1016/j.autrev.2006.01.004. [DOI] [PubMed] [Google Scholar]