Acute respiratory distress syndrome (ARDS) is a severe and life-threatening complication of coronavirus disease (COVID-19), with significant practice variation seen globally during the pandemic (1). Various interventions have been proposed, and some have been proven to benefit patient-centered outcomes (2–4). Considering the known effects of inhaled nitric oxide (iNO) on improving matching in the setting of lung injury, as well as putative direct antiviral effects of iNO (5,6); Di Fenza and colleagues explored the safety and efficacy of iNO in patients suffering from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia and ARDS (7). The intervention arm (iNO) had a higher mean PaO2/FiO2 (P/F) ratio (mean difference, 39.1 mm Hg) at 48 hours and had a higher proportion of participants (27.7% vs. 17.2%) with a P/F ratio >300 mm Hg at 28 days, but there was no difference in patient-centered outcomes.
To understand the rationale for this study, a discussion of the underlying pathophysiology and respiratory physiology of both COVID-19 ARDS (C-ARDS) and iNO is required. ARDS in COVID-19 is characterized by severe and protracted hypoxemia, with the pathophysiology involving an intense inflammatory response, endothelial and vascular injury, and microvascular dysfunction. This increases pulmonary vascular resistance and mismatch and impairs oxygenation. Although there is an ongoing debate concerning to what extent C-ARDS is distinct from other forms of ARDS, autopsy studies have shown a higher prevalence of microvascular involvement, with endothelial swelling and apoptosis, loss of vasculature, and impaired angiogenesis (8).
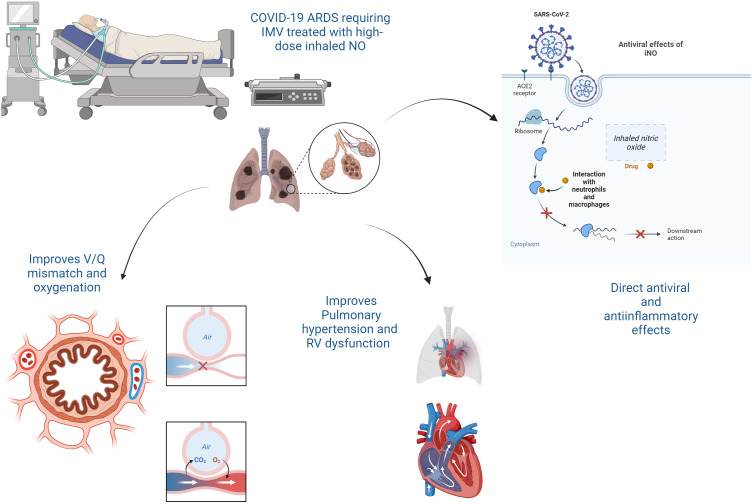
Nitric oxide is a diatomic free radical that diffuses to vascular smooth muscle and directly activates its receptor soluble guanylate cyclase, which generates guanosine 3′,5′-cyclic monophosphate and drives downstream smooth muscle relaxation (9). iNO is a selective pulmonary vasodilator that relaxes the smooth muscle of pulmonary arterioles, reducing pulmonary vascular resistance. iNO helps redirect blood flow to better-ventilated lung regions, improving matching. The overall result is an improvement in oxygenation. It also reduces right-to-left shunting of blood through nonaerated areas of the lung. In addition, iNO exerts antiviral and antiinflammatory properties, including inhibiting neutrophil adhesion and releasing proinflammatory cytokines (10) (Figure 1). The latter observations led the investigators in the current trial to test iNO at a higher dose than used in current clinical practice (80 ppm).
Figure 1.
The mechanisms of action of inhaled nitric oxide (iNO) in coronavirus disease (COVID-19) acute respiratory distress syndrome (ARDS). ACE2 = angiotensin-converting enzyme 2; IMV = invasive mechanical ventilation; RV = right ventricular; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Although iNO clearly improves oxygenation in patients with ARDS during the initial days of therapy, after two to three days of treatment, the improvements in oxygenation in placebo-treated patients are equivalent to iNO-treated patients. Clinical trials have shown no benefit of iNO on patient-centered ARDS outcomes, such as time to extubation, time in the ICU, or mortality before the pandemic (6), and guidelines generally do not recommend its use except in cases of refractory hypoxemia and severe pulmonary hypertension and shock from right ventricular dysfunction (5). The efficacy of iNO in patients with SARS-CoV-2–related ARDS is not known. Di Fenza and colleagues should be commended for this formidable task of putting to the test this physiologically sound hypothesis to generate high-quality, real-world evidence through a well-designed multicenter phase II randomized controlled trial in the middle of the pandemic, published in this issue of the Journal (pp. 1293–1304) (7). The investigators evaluated a unique protocol of short duration (48 h) and high-dose (80 ppm) iNO, in part to evaluate antiviral properties. They enrolled intubated and mechanically ventilated patients with acute hypoxemic respiratory failure in five ICUs (four in the United States and one in Sweden). Appropriately powered sample size and a geographically diverse population add to the scientific rigor and generalizability of their findings, whereas a lack of blinding in the trial is a significant shortcoming. Similar to prior studies in non-COVID ARDS, the study showed an improved P/F ratio in the intervention arm after 48 hours of use of iNO but no difference in patient-centered outcomes. The intervention was generally safe except for an observed nominally higher requirement for renal replacement in the intervention group, which is a concerning observation given the prior associations of iNO and renal dysfunction (11, 12). Exploratory findings of faster decrease in viral load and lower incidence of documented neurologic dysfunction are intriguing and require further study, especially in blinded clinical trials.
Clinically, a change in the P/F ratio from 270 to 300 (an improvement of 30 points) may not have the same benefit as a change in the P/F ratio from a lower P/F value associated with more morbidity and mortality, such as a change from 120 to 150, which also reflects an improvement of 30 points. An intervention in a population with higher anticipated mortality may optimize efficacy relative to safety, considering the risks of methemoglobinemia, acute kidney injury, cost, and healthcare resource utilization. The selection of the P/F ratio as their primary outcome and the estimate for oxygenation is also potentially unreliable because of the intrinsic variability of P/F ratio (13). The P/F ratio has also been shown to have a complex relation when mapped against an inspired fraction of oxygen (FiO2), especially when low lung regions, true shunt, and oxygen consumption are all considered. The P/F ratio and its variation with changes in FiO2 depend on many clinical variables and may not be a robust index for determining the true degree of arterial hypoxemia (14).
A careful reading of the supplementary material will show that the use of iNO in a subset of patients confers a more meaningful benefit than the entire cohort, making a case for individualized decision making for its use in moderate to severe ARDS refractory to other interventions, such as recruitment maneuvers, proning, and neuromuscular blockade. Enhancing oxygenation and reducing the severity of hypoxemia, even for short periods, may allow some patients to avoid invasive mechanical ventilation, which is often associated with significant risks and complications. Future trials should consider distinct evaluation of more severely hypoxemic patients or, alternatively, less severe with the inclusion of nonintubated patients on noninvasive ventilation to assess this meaningful endpoint.
Based on the findings of this clinical trial, how do we answer the question posed in the title? As always, the answer is more nuanced than a simple Yes or No. Clinicians should use an individualized approach, integrating the best available evidence with clinical context and patient preference. Similar to non-COVID ARDS, in C-ARDS, without more evidence, the use of iNO should be largely reserved for patients with refractory hypoxemia, severe pulmonary hypertension, and right ventricular failure. Future clinical trials could focus on preventing intubation or rescuing more severely affected patients with higher predicted morbidity and mortality. Direct effects of high-dose iNO on viral replication and inflammation deserve more study.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202310-1823ED on November 7, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Domecq JP, Lal A, Sheldrick CR, Kumar VK, Boman K, Bolesta S, et al. Outcomes of patients with coronavirus disease 2019 receiving organ support therapies: the international Viral Infection and Respiratory Illness Universal Study registry. Crit Care Med . 2021;49:437–448. doi: 10.1097/CCM.0000000000004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Selvaraj V, Finn A, Lal A, Khan MS, Dapaah-Afriyie K, Carino GP. Baricitinib in hospitalised patients with COVID-19: a meta-analysis of randomised controlled trials. EClinicalMedicine . 2022;49:101489. doi: 10.1016/j.eclinm.2022.101489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Selvaraj V, Khan MS, Bavishi C, Dapaah-Afriyie K, Finn A, Lal A, et al. Tocilizumab in hospitalized patients with COVID-19: a meta analysis of randomized controlled trials. Lung . 2021;199:239–248. doi: 10.1007/s00408-021-00451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lal A, Garces JPD, Bansal V, Tekin A, Zec S, Khanna AK, et al. from the Society of Critical Care Medicine Discovery Viral Infection, Respiratory Illness Universal Study (VIRUS): COVID-19 Registry Investigator Group Pre-hospital aspirin use and patient outcomes in COVID-19: results from the international Viral Infection and Respiratory Illness Universal Study (VIRUS) Arch Bronconeumol . 2022;58:746–753. doi: 10.1016/j.arbres.2022.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kamenshchikov NO, Berra L, Carroll RW. Therapeutic effects of inhaled nitric oxide therapy in COVID-19 patients. Biomedicines . 2022;10:369. doi: 10.3390/biomedicines10020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gebistorf F, Karam O, Wetterslev J, Afshari A. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults. Cochrane Database Syst Rev . 2016;2016:CD002787. doi: 10.1002/14651858.CD002787.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Di Fenza R, Shetty NS, Gianni S, Parcha V, Giammatteo V, Safaee Fakhr B, et al. High-dose inhaled nitric oxide in acute hypoxemic respiratory failure due to COVID-19: a multicenter phase 2 trial. Am J Respir Crit Care Med . 2023;208 doi: 10.1164/rccm.202304-0637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med . 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lundberg JO, Gladwin MT, Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Drug Discov . 2015;14:623–641. doi: 10.1038/nrd4623. [DOI] [PubMed] [Google Scholar]
- 10. Pisoschi AM, Pop A, Iordache F, Stanca L, Geicu OI, Bilteanu L, et al. Antioxidant, anti-inflammatory and immunomodulatory roles of vitamins in COVID-19 therapy. Eur J Med Chem . 2022;232:114175. doi: 10.1016/j.ejmech.2022.114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang J, Cong X, Miao M, Yang Y, Zhang J. Inhaled nitric oxide and acute kidney injury risk: a meta-analysis of randomized controlled trials. Ren Fail . 2021;43:281–290. doi: 10.1080/0886022X.2021.1873805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Al Sulaiman K, Korayem GB, Altebainawi AF, Al Harbi S, Alissa A, Alharthi A, et al. Evaluation of inhaled nitric oxide (iNO) treatment for moderate-to-severe ARDS in critically ill patients with COVID-19: a multicenter cohort study. Crit Care . 2022;26:304. doi: 10.1186/s13054-022-04158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gowda MS, Klocke RA. Variability of indices of hypoxemia in adult respiratory distress syndrome. Crit Care Med . 1997;25:41–45. doi: 10.1097/00003246-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 14. Whiteley JP, Gavaghan DJ, Hahn CE. Variation of venous admixture, SF6 shunt, PaO2, and the PaO2/FIO2 ratio with FIO2. Br J Anaesth . 2002;88:771–778. doi: 10.1093/bja/88.6.771. [DOI] [PubMed] [Google Scholar]