Abstract
Rationale
Obstructive sleep apnea (OSA) is a common sleep disorder for which the principal treatment option, continuous positive airway pressure, is often poorly tolerated. There is currently no approved pharmacotherapy for OSA. However, recent studies have demonstrated improvement in OSA with combined antimuscarinic and noradrenergic drugs.
Objectives
The aim of this study was to evaluate the efficacy and safety of AD109, a combination of the novel antimuscarinic agent aroxybutynin and the norepinephrine reuptake inhibitor atomoxetine, in the treatment of OSA.
Methods
Phase II randomized, double-blind, placebo-controlled, parallel-group, 4-week trial comparing AD109 2.5/75 mg, AD109 5/75 mg, atomoxetine 75 mg alone, and placebo (www.clinicaltrials.gov identifier NCT 05071612).
Measurements and Main Results
Of 211 randomized patients, 181 were included in the prespecified efficacy analyses. Sleep was assessed by two baseline and two treatment polysomnograms. Apnea–hypopnea index with a 4% desaturation criterion (primary outcome) was reduced from a median (IQR) of 20.5 (12.3–27.2) to 10.8 (5.6–18.5) in the AD109 2.5/75 mg arm (−47.1%), from 19.4 (13.7–26.4) to 9.5 (6.1–19.3) in the AD109 5/75 mg arm (−42.9%; both P < 0.0001 vs. placebo), and from 19.0 (11.8–28.8) to 11.8 (5.5–21.5) with atomoxetine alone (−38.8%; P < 0.01 vs. placebo). Apnea–hypopnea index with a 4% desaturation criterion decreased from 20.1 (11.9–25.9) to 16.3 (11.1–28.9) in the placebo arm. Subjectively, there was improvement in fatigue with AD109 2.5/75 mg (P < 0.05 vs. placebo and atomoxetine). Atomoxetine taken alone decreased total sleep time (P < 0.05 vs. AD109 and placebo). The most common adverse events were dry mouth, insomnia, and urinary hesitancy.
Conclusions
AD109 showed clinically meaningful improvement in OSA, suggesting that further development of the compound is warranted.
Clinical trial registered with www.clinicaltrials.gov (NCT 05071612).
Keywords: obstructive sleep apnea, pharmacology, drug therapy
At a Glance Commentary
Scientific Knowledge on the Subject
Obstructive sleep apnea (OSA) is a common disorder for which the principal treatment, continuous positive airway pressure, is highly efficacious but limited by poor tolerance/adherence. Although there is currently no approved pharmacotherapy for OSA, the combination of noradrenergic and antimuscarinic drugs has recently shown encouraging results in small short-term studies.
What This Study Adds to the Field
In this randomized, placebo-controlled trial, AD109, a combination of aroxybutynin and atomoxetine, demonstrated a clinically significant improvement in OSA over a 4-week period in adults with mild to severe sleep apnea.
Obstructive sleep apnea (OSA) is a common sleep disorder, affecting more than 930 million adults aged 30–69 years globally, with prevalence rates of moderate to severe OSA as high as 23.4% in women and 49.7% in men (1, 2). Untreated OSA is associated with increased incidences of cardiovascular and metabolic disease as well as daytime sleepiness, neurocognitive impairment, and reduced quality of life (3–5). Continuous positive airway pressure (CPAP), the primary therapy for OSA, is highly efficacious, but its real-world effectiveness is limited by poor tolerance and poor adherence in a substantial number of patients (6, 7). The efficacy of alternative treatment options such as oral appliances, upper airway surgeries, and hypoglossal nerve stimulation is patient-dependent and challenging to predict (8, 9).
Past efforts to develop pharmacotherapies to treat OSA have been unsuccessful (10). However, recent developments in the understanding of OSA pathophysiology and the identification of pharmacological targets have led to a more focused approach (11, 12). One such approach is to pharmacologically increase the activity of upper airway dilator muscles, thereby preventing sleep-related loss of muscle activation. Drugs with noradrenergic and antimuscarinic effects have been shown to increase genioglossus muscle activity during sleep in rodents (13–15). Small studies in humans have shown a decrease in OSA severity with the administration of noradrenergic drugs such as atomoxetine or reboxetine in combination with antimuscarinic drugs such as oxybutynin or hyoscine butylbromide (16–19). A larger (N = 60) trial of atomoxetine 80 mg combined with oxybutynin 5 mg also demonstrated a significant decrease in OSA severity after a single night of treatment in patients with moderate pharyngeal collapsibility (20). Atomoxetine has also been studied in combination with aroxybutynin, the R-enantiomer of oxybutynin, which is the stereoisomer responsible for most of the antimuscarinic effects of oxybutynin and potentially may have a better safety profile than its racemic version (21). That study reported a dose–response improvement in OSA severity with a single night of AD109 (aroxybutynin 2.5 mg combined with atomoxetine 37.5 or 75 mg) treatment in patients with mild to moderate OSA. The purpose of the present study was to evaluate the efficacy and safety of AD109 over a longer duration (4 wk) in patients with mild to severe OSA.
Some of the results of this study have been previously reported in the form of abstracts (22, 23).
Methods
Study Design
A phase II randomized, double-blind, placebo-controlled, parallel-arms clinical trial was performed at 25 clinical investigative sites in the United States. The study evaluated the efficacy and safety of two doses of AD109 (aroxybutynin/atomoxetine 2.5/75 mg and 5/75 mg) and atomoxetine 75 mg alone (Ato75) over a 4-week period in adults with mild to severe OSA. The study also evaluated another experimental drug (AD504) in early development, and those results will be reported separately. The study was approved by the institutional review board at each site and performed in accordance with the Declaration of Helsinki.
Participants
Participants were adults with OSA with a mean apnea–hypopnea index using the ⩾4% desaturation criterion for hypopneas (AHI4) of 10–45 based on two screening/baseline polysomnography (PSG) studies, with <25% of events comprising central or mixed apnea. Age requirement was 18–65 years for men and 18–75 for women. Body mass index was required to be ⩽38 kg/m2 for men and ⩽40 kg/m2 for women. Key exclusion criteria were clinically important sleep disorders other than OSA, active cardiac diseases (rhythm disturbance, coronary artery disease, or heart failure), hypertension requiring more than two drugs for control, craniofacial malformation or grade ⩾3 tonsillar hypertrophy, work schedule involving night or shift work, and chronic therapy with monoamine oxidase inhibitors, norepinephrine reuptake inhibitors, α-1 antagonists, tricyclic antidepressants, strong CYP2D6 inhibitors (e.g., fluoxetine, paroxetine, duloxetine), opioids, muscle relaxants, wake-promoting agents, antipsychotic agents, anticholinergic agents, or warfarin. Participants treated with CPAP, oral or nasal devices, or positional devices could enroll as long as the devices had not been used for ⩾2 weeks before the first PSG study and were not used during participation in the study. Participants were recruited from investigators’ clinical populations and databases as well as by advertising. All participants provided written informed consent.
Randomization
Participants who met all enrollment criteria were randomized to one of four treatment arms (AD109 2.5/75 mg, AD109 5/75 mg, Ato75, or placebo) using a randomization ratio of 2:2:3:3. Participants were centrally randomized using an interactive web-based system. Participants, investigators, and study personnel were all blinded to allocation. The study drug was prepared as identical tablets and identical capsules to ensure adequate double-blinding. Participants received a single tablet or a tablet and a capsule. Participants were instructed to take the study drug nightly immediately before bedtime for 4 weeks. The first week of dosing was with a lower-dose run-in (AD109 2.5/37.5 mg or atomoxetine 37.5 mg), followed by the full dose taken in Weeks 2–4. Dose reduction was not permitted; dosing was discontinued if the study drug was not tolerated.
Outcomes
A complete list of the endpoints of the trial is included in Table 1. The primary endpoint was the change from baseline to weeks 3 and 4 in AHI4 for each AD109 dose versus placebo. The secondary endpoint was the change from baseline to weeks 3 and 4 in AHI4 for Ato75 versus placebo. Protocol-specified tertiary and safety outcomes are also listed in Table 1. The schedule of screening, efficacy, and safety assessments is described in Table E1 in the online supplement. Questionnaires and psychometric tests are further described in the online supplement.
Table 1.
Endpoints of the Trial
| Endpoint | Description |
|---|---|
| Primary | Change from baseline in AHI4, AD109 vs placebo |
| Secondary | Change from baseline in AHI4, Ato75 vs placebo |
| Tertiary | Change from baseline, cross-arm comparisons:
Proportion of participants with ⩾50% reduction in AHI4 |
| Safety | Physical examination, vital signs, ECG, clinical laboratory assessment Adverse events DSST, PVT, VOLT, CSSA |
Definition of abbreviations: AHI3a = apnea–hypopnea index based on hypopneas with ⩾3% desaturation or arousal; AHI4 = apnea–hypopnea index based on ⩾4% hypopnea desaturation; Ato75 = atomoxetine 75 mg; CSSA = Cocaine Selective Severity Assessment; DSST = Digit Symbol Substitution Test; ECG = electrocardiography; ESS = Epworth Sleepiness Scale; HB = hypoxic burden; ODI4 = oxygen desaturation index based on ⩾4% desaturation; PGI-S = participant global impression–severity; PROMIS = Patient-Reported Outcomes Measurement Information System; PSG = polysomnography; PVT = Psychomotor Vigilance Test; QQ = Quantity and Quality of Work scale; SAQLI = Sleep Apnea Quality of Life Index; T90 = time spent during sleep with oxygen saturation <90%; VOLT = Visual Object Learning Task.
Statistical Analysis
Approximately 200 participants were planned for enrollment, with approximately 40 in each AD109 dose group, 60 in the Ato75 group, and 60 in the placebo group. Sample size was based on the efficacy findings of previous AD109 studies. The present study had >90% power to detect a treatment difference on the primary endpoint (AHI4 for AD109 vs. placebo) at a two-sided 0.05 significance level if the true difference between the active arm and placebo arm on AHI4 was 11 units and the within-subject standard deviation was 11. We present the AD109 doses separately in this article, but we have also included analysis of AHI4 for the combined AD109 doses in Table E2 in the online supplement. Although study eligibility was determined by scoring of PSG findings at the individual study sites, endpoint calculation was based on subsequent scoring by a central scoring laboratory (Sleep Strategies).
Data from the two baseline PSG studies were averaged for analysis, as were data from the two treatment PSG studies (conducted after 3 and 4 weeks of dosing, respectively). For the primary efficacy analysis, a repeated-measures model was used to assess the change in AHI4 from baseline for two doses of AD109, Ato75, and placebo. Percent reduction versus placebo was calculated using an analysis of covariance model with log-transformed data to account for the skewed distribution of values and using baseline as a covariate. Standard descriptive statistics (median [IQR] unless otherwise specified) are presented for the average of two baseline and two treatment PSG parameters. Primary and secondary hypotheses were tested sequentially. Findings were considered significant if the two-sided P value was less than 0.05. Given the exploratory nature of the trial, no adjustment for multiple comparisons was performed.
Hypoxic burden was calculated according to Azarbarzin and colleagues by quantifying the respiratory event–associated area under the desaturation curve for the entire night and dividing by sleep duration to yield a metric expressed in %min per hour of sleep (24). Values were log-transformed before analysis and then back-transformed (geometric mean) for interpretation.
According to a predefined statistical analysis plan, there was no prespecified imputation for missing data. Thus, outcomes were analyzed based on the modified intention-to-treat (mITT) population consisting of all participants who were randomized, took at least one dose of any of the study drugs, and had at least one measurement of the primary endpoint. A post hoc analysis of the primary and secondary outcomes was performed in the ITT population (all individuals who signed the informed consent form and were randomized to any treatment arm) using the baseline observation carried forward to impute missing data. Safety assessments included all participants who received at least one dose of study drug.
Statistical analyses were performed using SAS for Windows, version 9.4 (SAS).
Results
Participant Population
Enrollment began on November 29, 2021 and was completed on August 3, 2022. Of the 764 subjects screened, 211 were randomized to one of the four treatment arms (Figure 1). Of the 209 individuals who were randomized and received at least one dose of treatment (i.e., safety population), 181 had at least one measurement on the primary endpoint (i.e., mITT population) and 176 completed the study. The study diagram in Figure 1 presents a comprehensive overview of the reasons participants discontinued the study. In summary, treatment side effects resulted in one dropout in the placebo arm, 12 in the Ato75 arm, and 5 each in the AD109 2.5/75 mg and AD109 5/75 mg arms. Demographic and clinical characteristics of the safety population at baseline are listed in Table 2.
Figure 1.
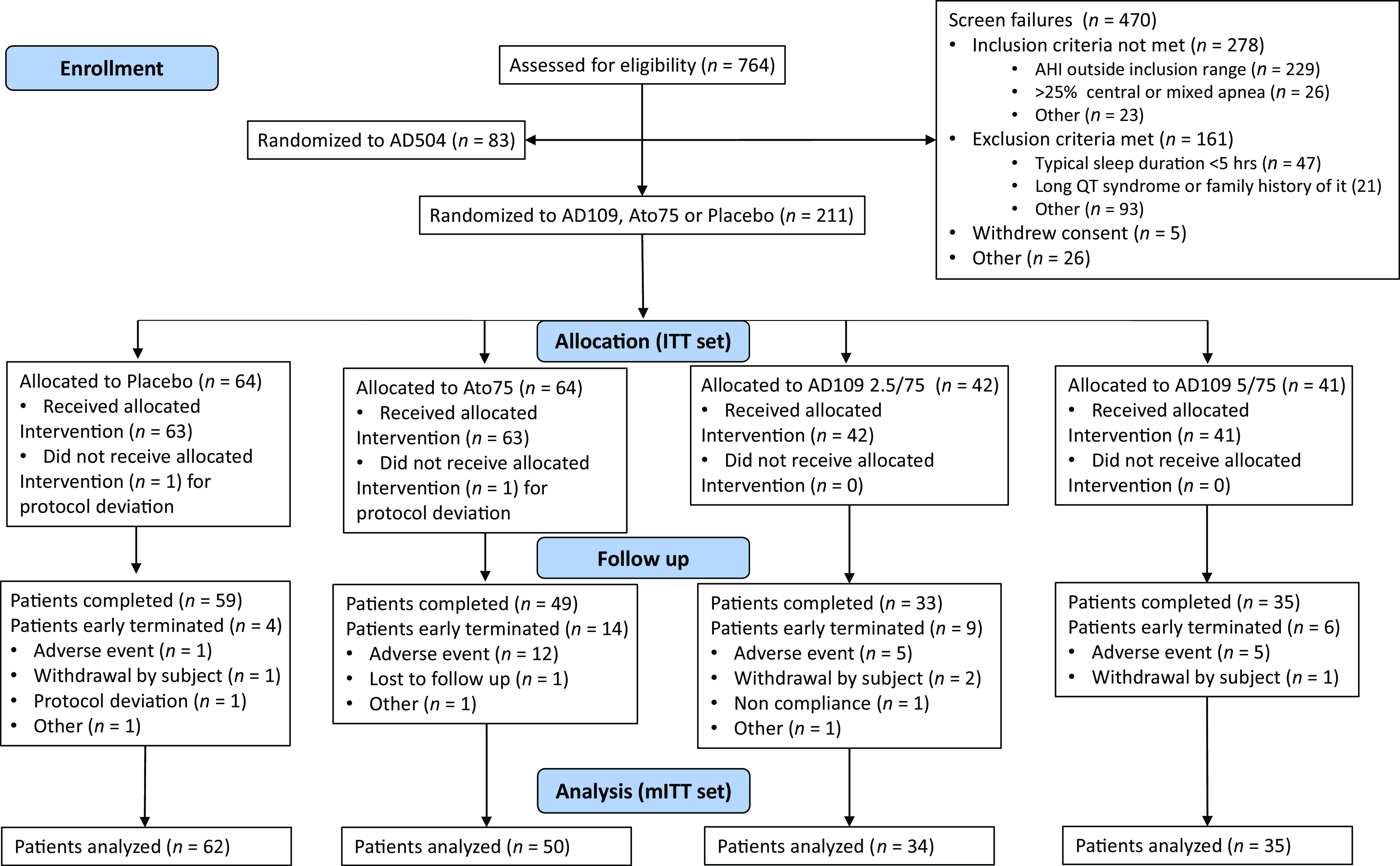
Consolidated Standards of Reporting Trials diagram. Ato75 = atomoxetine 75 mg; ITT = intention-to-treat (all participants who were randomized); mITT = modified intention-to-treat (all participants who were randomized, took at least one dose of any of the study drugs, and had at least one measurement on the primary endpoint).
Table 2.
Demographic and Clinical Characteristics of the Safety Set of Patients at Baseline
| Placebo (n = 63) | Ato75 (n = 63) | AD109 2.5/75 mg (n = 42) | AD109 5/75 mg (n = 41) | |
|---|---|---|---|---|
| Age, yr | 57 (48–62) | 56 (49–60) | 53.5 (44–58) | 55 (49–60) |
| Female sex | 27 (42.9%) | 22 (34.9%) | 17 (40.5%) | 19 (46.3%) |
| BMI, kg/m2 | 32.0 (27.2–35.2) | 31.2 (28.11–34.6) | 34.5 (31.6–36.6) | 31.2 (27.5–34.0) |
| CPAP use | 29 (46.1%) | 30 (47.6%) | 18 (42.9%) | 21 (50.2%) |
| Oral appliance | 5 (7.9%) | 2 (3.2%) | 1 (2.4%) | 0 |
| Race | ||||
| Asian | 5 (7.9%) | 3 (4.8%) | 2 (4.8%) | 2 (4.9%) |
| Black/African American | 9 (14.3%) | 5 (7.9%) | 11 (26.2%) | 6 (14.6%) |
| White | 48 (76.2%) | 52 (82.5%) | 28 (66.7%) | 33 (80.5%) |
| Other | 1 (1.6%) | 2 (3.2%) | 0 | 0 |
| Multiple | 0 | 1 (1.6%) | 1 (2.4%) | 0 |
| Hypertension | 14 (22%) | 8 (13%) | 6 (14%) | 12 (29%) |
| Diabetes | 1 (2%) | 8 (13%) | 3 (7%) | 4 (10%) |
Definition of abbreviations: Ato75 = atomoxetine 75 mg; BMI = body mass index; CPAP = continuous positive airway pressure.
Data presented as median (IQR) where applicable. Percentages are based on the total number of participants in the intention-to-treat population (n) in the respective treatment arm.
Efficacy
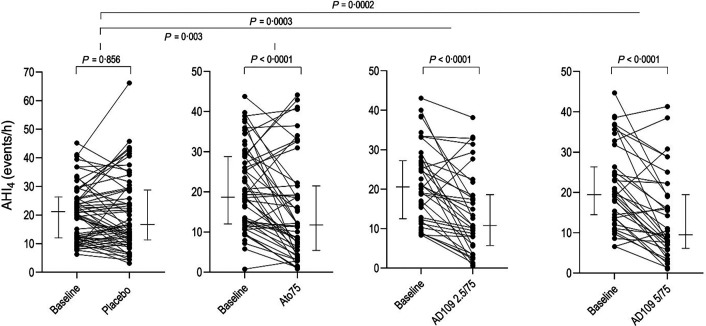
Respiratory parameters obtained from PSG studies are listed in Table 3, and individual data on AHI4 are presented in Figure 2. Both doses of AD109 significantly decreased AHI4 compared with placebo, with mean (95% confidence interval) decreases of −7.16 (−11 to −3.3) events/h for the 2.5/75 mg dose and −7.2 (−10.97 to −3.4) for the 5/75 mg dose (P < 0.001 for both). These decreases represent a placebo-adjusted reduction in AHI4 of 47.1% (27.9–61.2) and 42.9% (22.2–58) for the two doses, respectively. The effects of AD109 on AHI4 were statistically significant in non-REM (NREM) sleep and in the supine and lateral positions. AD109 also decreased the AHI when hypopneas were scored using ⩾3% desaturation or arousal criteria (P < 0.01 for both doses). Although AD109 2.5/75 mg significantly reduced AHI4 during REM sleep versus baseline (P = 0.044), the reduction was not significant when adjusted for placebo (P = 0.116), possibly because limited data were available during REM sleep during treatment. Compared with placebo, hypoxic burden was reduced by −12.7 (−24.3 to −1.14) %min/h (P = 0.03) with AD109 2.5/75 mg and by −16.6 (−28 to −5.2, P = 0.005) with AD109 5/75 mg.
Table 3.
Respiratory Parameters from Polysomnography
| Parameter/Arm | Baseline | Treatment | Placebo-adjusted Change from Baseline |
|---|---|---|---|
| AHI4 events/h | |||
| Placebo | 20.1 (11.9–25.9) | 16.3 (11.1–28.9) | – |
| Ato75 | 19.0 (11.8–28.8) | 11.8 (5.5–21.5) | −5.19 (−8.6 to −1.8)** |
| AD109 2.5/75 mg | 20.5 (12.3–27.2) | 10.8 (5.6–18.5) | −7.16 (−11.0 to −3.3)*** |
| AD109 5/75 mg | 19.4 (13.7–26.4) | 9.5 (6.1–19.3) | −7.20 (−10.97 to −3.4)*** |
| AHI4 supine events/h | |||
| Placebo | 32.7 (19.0–44.9) | 28.4 (17.3–48.0) | – |
| Ato75 | 29.3 (18.3–45.7) | 17.8 (6.6–38.8) | −7.06 (−12.47 to −1.66)* |
| AD109 2.5/75 mg | 29.2 (17.5–46.9) | 18.3 (7.7–26.5) | −8.58 (−14.71 to −2.45)** |
| AD109 5/75 mg | 39.7 (21.6–54.6) | 17.8 (9.5–43.6) | −10.62 (−16.70 to −4.54)*** |
| AHI4 lateral events/h | |||
| Placebo | 10.5 (5.1–10.1) | 9.2 (4.0–16.6) | – |
| Ato75 | 11.1 (3.8–18.4 | 6.0 (2.1–16.2) | −4.31 (−8.97 to 0.35) |
| AD109 2.5/75 mg | 12.4 (4.3–24.6) | 5.7 (3.7–13.7) | −5.89 (−11.02 to −0.76)* |
| AD109 5/75 mg | 14.7 (7.4–25.9) | 7.5 (4.7–14.0) | −6.42 (−11.55 to −1.29)* |
| AHI4 NREM sleep events/h | |||
| Placebo | 19.3 (9.4–27.7) | 16.7 (9.2–27.3) | – |
| Ato75 | 18.4 (9.4–28.6) | 11.4 (4.4–19.4) | −4.22 (−7.81 to −0.63)* |
| AD109 2.5/75 mg | 16.0 (9.6–24.7) | 10.1 (3.5–16.4) | −5.38 (−9.41 to −1.34)** |
| AD109 5/75 mg | 19.0 (10.1–30.7) | 8.3 (5.9–19.5) | −6.45 (−10.44 to −2.46)** |
| AHI4 REM sleep events/h† | |||
| Placebo | 20.9 (9.3–33.9) | 20.3 (6.1–36.1) | – |
| Ato75 | 28.1 (15.7–21.6) | 18.5 (9.1–26.8) | −5.77 (−11.66 to 0.71) |
| AD109 2.5/75 mg | 30.2 (18.1–50.7) | 18.2 (7.6–43.5) | −5.67 (−12.74 to 1.41) |
| AD109 5/75 mg | 22.6 (13.0–34.4) | 13.8 (6.6–31.8) | −3.34 (−10.39 to 3.72) |
| AHI3a events/h | |||
| Placebo | 35.5 (25.6–41.0) | 31.5 (23.0–41.1) | – |
| Ato75 | 33.4 (25.0–45.7) | 26.1 (14.5–42.1) | −3.71 (−8.19 to 0.76) |
| AD109 2.5/75 mg | 37.6 (30.0–47.4) | 28.3 (18.3–39.8) | −7.03 (−12.08 to -1.98)** |
| AD109 5/75 mg | 35.9 (25.5–48.9) | 22.1 (15.9–38.4) | −7.11 (−12.08 to -2.14)** |
| Log10(HB + 1) | |||
| Placebo | 1.51 (1.25–1.75) | 1.48 (1.20–1.73) | – |
| Ato75 | 1.54 (1.36–1.70) | 1.30 (0.95–1.63) | −0.23 (−0.36 to −0.09)** |
| AD109 2.5/75 mg | 1.46 (1.24–1.78) | 1.36 (1.01–1.58) | −0.19 (−0.34 to −0.04)* |
| AD109 5/75 mg | 1.49 (1.34–1.79) | 1.24 (0.97–1.51) | −0.23 (−0.38 to −0.09)*** |
| HB geometric means (95% CI), %min/h | |||
| Placebo | 31.1 (25.8–38.2) | 30.1 (25.0–38.3) | – |
| Ato75 | 31.9 (27.2–40.5) | 18.0 (12.4–26.0) | – |
| AD109 2.5/75 mg | 31.0 (25.1–41.2) | 19.1 (11.3–28.2) | – |
| AD109 5/75 mg | 32.4 (25.6–41.5) | 18.0 (12.0–26.1) | – |
| ODI4 events/h | |||
| Placebo | 26.3 (17.7–35.0) | 23.4 (14.8–35.3) | – |
| Ato75 | 25.8 (17.7–32.8) | 19.0 (11.0–29.6) | −4.82 (−8.16 to −1.49)** |
| AD109 2.5/75 mg | 29.1 (21.9–34.0) | 19.0 (8.3–29.8) | −5.63 (−9.38 to −1.89)** |
| AD109 5/75 mg | 25.5 (18.4–37.5) | 16.9 (9.4–26.3) | −6.01 (−9.71 to −2.30)** |
| T90 minimum | |||
| Placebo | 11.5 (5.5–23.7) | 11.3 (5.0–21.1) | – |
| Ato75 | 14.4 (8.4–31.2) | 9.9 (1.8–24.6) | −5.56 (−12.67 to 1.56) |
| AD109 2.5/75 mg | 9.8 (4.1–25.9) | 7.2 (1.0–13.8) | −3.20 (−11.12 to 4.72) |
| AD109 5/75 mg | 10.4 (3.6–27.3) | 5.8 (1.8–17.8) | −3.68 (−11.50 to 4.15) |
| Proportion (95% CI) of participants with >50% reduction in AHI4 | |||
| Placebo | – | 16.6 (8.4–28.3) | Odds ratio vs placebo |
| Ato75 | – | 39.4 (25.8–54.3) | 3.47 (1.57–7.71)** |
| AD109 2.5/75 mg | – | 43.2 (26.3–61.4) | 4.00 (1.68–9.51)** |
| AD109 5/75 mg | – | 44.3 (27.6–62.0) | 4.23 (1.80–9.97)** |
Definition of abbreviations: Ato75 = atomoxetine 75 mg; AHI4 = apnea–hypopnea index (⩾4% desaturation criterion for hypopneas); AHI3a = apnea–hypopnea index (⩾3% desaturation or arousal definition for hypopneas); CI = confidence interval; HB = hypoxic burden; Log10(HB + 1) = logarithm base 10 of hypoxic burden + 1; NREM = non-REM; ODI4 = oxygen desaturation index using ⩾4% desaturation criterion; T90 = time spent during sleep with oxygen saturation <90%.
Baseline and treatment data are shown as median (IQR) unless otherwise specified. Placebo-adjusted change from baseline data are shown as least-square mean (LSmean) (95% CI).
P < 0.05, **P < 0.01, ***P < 0.001 vs. placebo.
Sleep studies with ⩾10 minutes of REM sleep were included in this analysis.
Figure 2.
Individual data of AHI4. Lines on the sides indicate medians (IQR). Note that the scale for the placebo arm is larger (to 70 events per hour) than for the other arms. AHI4 = apnea–hypopnea index (⩾4% desaturation criterion for hypopneas); Ato75 = atomoxetine 75 mg.
Ato75 alone reduced AHI4 by −5.19 (−8.6 to −1.8) events/h, or by 38.8% (19.5–53.6) compared with placebo (P = 0.003). Effects of Ato75 on AHI4 were present in NREM sleep and in the supine position, but not during REM or in the lateral position. Ato75 reduced hypoxic burden by −10.8 (−21.0 to −0.5) %min/h versus placebo (P = 0.04) but did not significantly decrease AHI when hypopneas were scored using ⩾3% desaturation or arousal criteria. Ato75 did not differ from either dose of AD109 in any respiratory parameter.
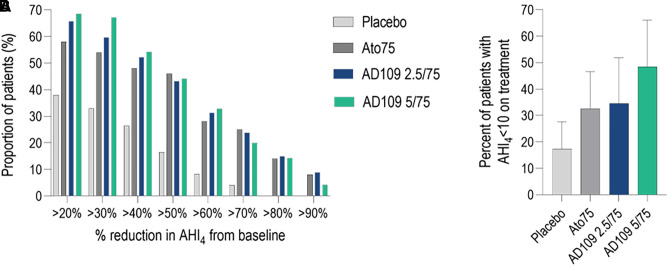
The proportions of patients with a >50% decrease from baseline in AHI4 and with AHI <10 during treatment is reported in Figure 3.
Figure 3.
Proportion of patients with a ⩾20% reduction in AHI4 from baseline (A) and with apnea–hypopnea index (⩾4% desaturation criterion for hypopneas) of fewer than 10 events per hour during treatment (B) in the different treatment arms. Error bars in B indicate 95% confidence intervals. AHI4 = apnea–hypopnea index (⩾4% desaturation criterion for hypopneas); Ato75 = atomoxetine 75 mg.
Sleep parameters obtained from PSG studies are listed in Table 4. There was no significant difference in the arousal index with either dose of AD109 or Ato75 compared with placebo. However, the respiratory arousal index was significantly reduced with AD109 5/75 mg (P = 0.01) and demonstrated a trend for reduction with AD109 2.5/75 mg (P = 0.059). Total sleep time (TST) did not change with AD109 compared with placebo. However, Ato75 reduced TST by 21.5 (8.6–34.3) minutes compared with placebo (P = 0.001) and by 23.93 (8.89–38.98) and 16.09 (1.24–30.94) minutes compared with AD109 2.5/75 mg and 5/75 mg, respectively (P = 0.002 and P = 0.03). AD109 and Ato75 increased NREM stages 1 and 2 and reduced REM sleep compared with placebo (P < 0.001 for all).
Table 4.
Sleep Parameters from Polysomnography
| Parameter/Arm | Baseline | Treatment | Placebo-adjusted Change from Baseline |
|---|---|---|---|
| TST, min | |||
| Placebo | 386.4 (362.3–428.0) | 399.4 (358.5–426.5) | – |
| Ato75 | 396.4 (364.0–427.0) | 372.2 (340.5–406.0) | −21.46 (−34.3 to −8.59)** |
| AD109 2.5/75 mg | 387.5 (352.8–412.9) | 385.5 (364.8–416.5) | 2.48 (−12.01 to 16.96)†† |
| AD109 5/75 mg | 387.9 (360.9–425.8) | 392.6 (355.5–421.8) | −5.37 (−19.65 to 8.91)† |
| Total arousal index, events/h | |||
| Placebo | 26.9 (21.5–34.7) | 23.7 (18.5–34.1) | – |
| Ato75 | 27.2 (19.5-34.6) | 26.5 (18.3–36.2) | 1.38 (−1.51 to 4.27) |
| AD109 2.5/75 mg | 28.5 (21.5–36.2) | 24.2 (18.5–34.3) | −1.19 (−4.43 to 2.06) |
| AD109 5/75 mg | 29.4 (21.0–37.9) | 24.9 (17.2–31.3) | −2.92 (−6.13, 0.29)† |
| Respiratory arousal index, events/h | |||
| Placebo | 21.6 (15.0 – 28.3) | 18.7 (12.6–26.4) | – |
| Ato75 | 21.6 (13.8–28.4) | 15.1 (7.6–26.7) | −0.92 (−4.00 to 2.16) |
| AD109 2.5/75 mg | 22.0 (16.8–29.8) | 15.6 (11.3–21.8) | −3.08 (−6.54 to 0.38) |
| AD109 5/75 mg | 22.5 (15.4–29.3) | 14.0 (8.4–25.1) | −4.66 (−8.08 to −1.24)* |
| WASO, min | |||
| Placebo | 65.1 (41.5–97.8) | 58.9 (44.5–101.8) | – |
| Ato75 | 70.1 (44.3–98.8) | 92.9 (61–124.3) | 20.32 (8.07 to 32.58)** |
| AD109 2.5/75 mg | 75.6 (56.8–104.3) | 78.9 (56.8–98.3) | 0.11 (−13.69 to 13.91) |
| AD109 5/75 mg | 73.3 (43.3–93.5) | 77.0 (48.3–106.3) | 8.21 (−5.37 to 21.79) |
| Sleep latency, min | |||
| Placebo | 10.6 (6.2–16.7) | 9.8 (5.5–16.9) | – |
| Ato75 | 10.4 (5.2–17.5) | 9.8 (3.8–14.2) | −0.03 (−4.26 to 4.21) |
| AD109 2.5/75 mg | 8.4 (4.7–21.0) | 7.8 (4.8–15.0) | −2.77 (−7.53 to 1.98) |
| AD109 5/75 mg | 10.3 (5.4–25.4) | 10.7 (5.1–18.9) | 0.68 (−4.06 to 5.42) |
| N1, %TST | |||
| Placebo | 13.4 (11.8–19.6) | 14.1 (10.3–18.6) | – |
| Ato75 | 15.5 (12.1–21.8) | 18.7 (15.3–25.1) | 4.21 (2.20 to 6.22)*** |
| AD109 2.5/75 mg | 15.9 (11.8–19.6) | 18.4 (12.8–25.0) | 3.98 (1.73 to 6.24)*** |
| AD109 5/75 mg | 15.2 (10.7–19.8) | 19.6 (15.0–25.3) | 4.18 (1.95 to 6.41)*** |
| N2, %TST | |||
| Placebo | 57.5 (50.6–62.5) | 58.9 (50.6–66.4) | – |
| Ato75 | 53.5 (50.5–61.8) | 61.1 (55.2–70.1) | 4.73 (2.10 to 7.37)*** |
| AD109 2.5/75 mg | 56.1 (49.1–60.5) | 63.7 (57.0–71.2) | 7.40 (4.43 to 10.36)*** |
| AD109 5/75 mg | 55.5 (51.5–61.3) | 63.7 (57.9–71.1) | 6.54 (3.61 to 9.46)*** |
| N3, %TST | |||
| Placebo | 7.9 (3.0–15.1) | 8.3 (2.7–13.3) | – |
| Ato75 | 9.8 (6.5–15.8) | 9.9 (5.1–14.6) | −0.09 (−1.78 to 1.61) |
| AD109 2.5/75 mg | 8.8 (2.7–12.3) | 7.0 (4.3–11.1) | −1.09 (−2.98 to 0.79) |
| AD109 5/75 mg | 8.3 (5.8–16.4) | 9.3 (3.9–16.3) | −0.18 (−2.06 to 1.69) |
| REM sleep, %TST | |||
| Placebo | 17.9 (14.7–20.1) | 17.2 (13.6–20.8) | – |
| Ato75 | 17.8 (13.7–19.0) | 6.8 (3.5–11.3) | −9.32 (−11.14 to −7.50)*** |
| AD109 2.5/75 mg | 18.6 (14.3–23.0) | 7.5 (3.7–12.7) | −9.74 (−11.78 to −7.69)*** |
| AD109 5/75 mg | 17.3 (13.5–20.1) | 5.6 (2.5–10.2) | −10.79 (−12.79 to −8.78)*** |
Definition of abbreviations: Ato75 = atomoxetine 75 mg; N1/N2/N3 = non-REM sleep stage 1/2/3; SOL = sleep onset latency; TST = total sleep time; WASO = wake after sleep onset.
Baseline and treatment data are shown as median (IQR) unless otherwise specified. Placebo-adjusted change from baseline data are shown as LSmean (95% confidence interval).
P < 0.01, **P < 0.05 and ***P < 0.001 vs. placebo.
P < 0.05 and ††P < 0.01 vs. Ato75.
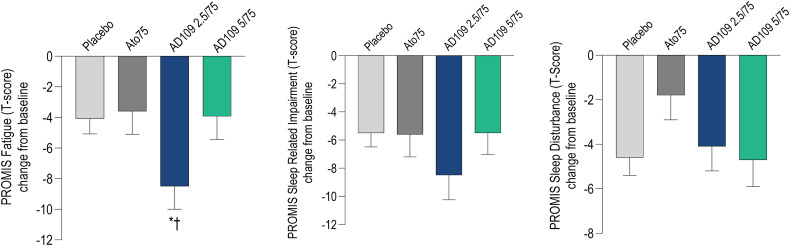
There was a significant improvement of Patient-Reported Outcome Measurement Information System (PROMIS) fatigue scores with AD109 2.5/75 mg compared with placebo (−3.56 [−6.77 to −0.35]; P < 0.05) and Ato75 (−3.49 [−6.84 to −0.13]; P < 0.05; Figure 4 and Table 5). There was no significant improvement in subjective outcomes with Ato75 versus placebo. However, the PROMIS sleep disturbance scale showed a trend toward increasing (i.e., worsening) with Ato75 compared with placebo (P = 0.056).
Figure 4.
LSmean (standard error of the mean) reduction from baseline in Patient-Reported Outcome Measurement Information System scales (t-score). Higher bars represent greater improvement. Ato75 = atomoxetine 75 mg; PROMIS = Patient-Reported Outcome Measurement Information System. *P < 0.05 versus placebo, †P < 0.05 versus Ato75.
Table 5.
Patient-reported Outcomes
| Outcome/Arm | Baseline | Treatment | Placebo-adjusted Change from Baseline |
|---|---|---|---|
| PROMIS fatigue, adjusted T-score | |||
| Placebo | 55.7 (50.5–61.3) | 51.4 (44.3–55.8) | – |
| Ato75 | 53.1 (49.1–58.2) | 49.2 (45.7–53.1) | −0.07 (−2.93 to 2.79) |
| AD109 2.5/75 mg | 56.2 (51.6–62.4) | 49.4 (41.1–53.6) | −3.56 (−6.77 to −0.35)*† |
| AD109 5/75 mg | 55.9 (48.1–62.3) | 50.5 (44.4–56.9) | 0.10 (−3.02 to 3.21) |
| PROMIS sleep Impairment, adjusted t-score | |||
| Placebo | 56.9 (51.3–61.2) | 52.2 (44.0–55.8) | – |
| Ato75 | 55.8 (51.4–61.9) | 52.8 (43.6–56.6) | −0.02 (−3.28 to 3.24) |
| AD109 2.5/75 mg | 56.9 (55.0–63.2) | 51.5 (42.5–55.3) | −1.99 (−5.67 to 1.69) |
| AD109 5/75 mg | 57.6 (52.6–62.1) | 50.9 (42.6–56.6) | −0.35 (−3.91 to 3.21) |
| PROMIS sleep disturbance, adjusted t-score | |||
| Placebo | 54.6 (51.4–60.0) | 50.3 (45.2–55.4) | – |
| Ato75 | 54.8 (50.4–59) | 51.5 (48.4–58.3) | 2.40 (−0.07 to 4.87) |
| AD109 2.5/75 mg | 56.1 (52.1–61.8) | 53.1 (47.9–56.0) | 1.12 (−1.66 to 3.89) |
| AD109 5/75 mg | 56.8 (51.3–61.4) | 50.9 (45.6–57.1) | 0.21 (−2.48 to 2.90) |
| Modified short SAQLI | |||
| Placebo | 34.5 (21.5–43.5) | 19.5 (12.0–30.0) | – |
| Ato75 | 35.5 (20.8–43.3) | 19.5 (14.0–32.3) | 0.54 (−4.29 to 5.37) |
| AD109 2.5/75 mg | 41.0 (22.8–52.3) | 19.5 (13.0–30.3) | −2.28 (−7.73 to 3.17) |
| AD109 5/75 mg | 35.0 (22.0–42.0) | 23.0 (11.0–37.0) | 2.02 (−3.25 to 7.29) |
| ESS score | |||
| Placebo | 10.5 (6.0–14.8) | 8.0 (4.0–11.0) | – |
| Ato75 | 10.0 (7.8–12.0) | 7.0 (5.0–9.0) | 0.30 (−1.15 to 1.74) |
| AD109 2.5/75 mg | 12.0 (9.0–14.3) | 7.0 (4.0–11.3) | −0.23 (−1.85 to 1.39) |
| AD109 5/75 mg | 9.0 (6.0–12.0) | 7.0 (5.0–11.0) | 0.64 (−0.94 to 2.22) |
| PGI-S | |||
| Placebo | 3.0 (2.5–3.5) | 2.0 (1.0–3.0) | – |
| Ato75 | 3.0 (2.0–3.0) | 2.0 (2.0–3.0) | −0.25 (−0.73 to 0.23) |
| AD109 2.5/75 mg | 3.0 (2.4–3.0) | 2.0 (1.8–3.0) | −0.16 (−0.72 to 0.41) |
| AD109 5/75 mg | 3.0 (2.0–3.0) | 2.0 (1.0–3.0) | −0.19 (−0.74 to 0.36) |
| Placebo | 17.0 (12.0–18.0) | 18.0 (12.3–20.0) | – |
| Ato75 | 16.0 (13.0–18.0) | 18.0 (13.8–20.0) | 0.44 (−1.2 to 2.12) |
| AD109 2.5/75 mg | 14.5 (11.8–16.3) | 13.5 (12.0–17.3) | −0.26 (−2.14 to 1.63) |
| AD109 5/75 mg | 16 (13.0–18.0) | 16 (12.0–18.0) | −1.01 (−2.85 to 0.83) |
Definition of abbreviations: Ato75 = atomoxetine 75 mg; ESS = Epworth Sleepiness Scale; PGI-S = Patient Global Impression–Severity; PROMIS = Patient-Reported Outcome Measurement Information System; QQ = Quality and Quantity of Work; SAQLI = Sleep Apnea Quality of Life Index.
Baseline and treatment data are shown as median (IQR). Medians (IQR) were calculated from mITT set for the subset of patients with an assessment of patient-reported outcomes at baseline and on treatment (placebo, n = 57/62; ato75, n = 48/50; AD109 2.5/75, n = 32/34; AD109, 5/75 n = 35/35). Placebo-adjusted change from baseline data are shown as LSmean (95% confidence interval).
P < 0.05 vs placebo.
P < 0.05 vs Ato75.
Post hoc analysis of the primary and secondary outcomes using the ITT population and considering zero change from baseline for missing data showed results similar to those in the mITT population. Compared with placebo, AHI4 decreased by −5.9 (−9.4 to −2.4) events/h (P = 0.001) with AD109 2.5/75 mg, by −6.3 (−9.8 to –2.8, P < 0.001) with AD109 5/75 mg, and by −4.3 (−7.5 to −1.2, P = 0.007) with Ato75.
Post hoc analyses showed no statistically significant differences in AHI4 reduction between subgroups based on age, sex, obesity, or baseline OSA severity (Tables E4–E7 in the online supplement). However, a higher proportion of participants with a baseline AHI4 in the mild to moderate range (AHI4 10–30 events/h) experienced a resolution of OSA (defined as AHI4 <10 events/h) compared with those with a baseline AHI4 in the severe range (AHI4 ⩾30; 52.2% vs. 7%; P = 0.004; Figure E2 in the online supplement).
Safety
No serious adverse events were reported. A total of 373 adverse events were reported in 104 (49.8%) participants in the safety population: 26 (61.9%) individuals treated with AD109 2.5/75 mg, 32 (78.0%) treated with AD109 5/75 mg, 51 (81.0%) treated with Ato75, and 25 (39.7%) treated with placebo. Table 6 provides a list of the most common adverse events. Twenty-three (11%) individuals discontinued study participation because of adverse events: 5 (12%) in each of the AD109 groups, 12 (19%) in the atomoxetine group, and 1 (2%) in the placebo group. Events leading to study discontinuation in at least three participants were insomnia, nausea, and dry mouth. The most common adverse events were dry mouth, urinary hesitancy, and insomnia (Table 6). Most events were rated as mild. Two events classified as serious (nausea and migraine) led to study discontinuation in two participants in the Ato75 group.
Table 6.
Adverse Events
| Placebo (n = 63) | Ato75 (n = 63) | AD109 2.5/75 mg (n = 42) | AD109 5/75 mg (n = 41) | |
|---|---|---|---|---|
| Any adverse event | 25 (39.7%) | 51 (81%) | 26 (61.9%) | 32 (78%) |
| Adverse event leading to discontinuation | 1 (2%) | 12 (19%) | 5 (12%) | 5 (12%) |
| Most common adverse events* | ||||
| Dry mouth | 3 (5%) | 17 (27%) | 10 (24%) | 24 (59%) |
| Insomnia | 2 (3%) | 23 (37%) | 11 (26%) | 9 (22%) |
| Urinary hesitation/flow decrease | 0 | 14 (22%) | 3 (7%) | 9 (22%) |
| Constipation | 2 (3%) | 2 (3%) | 0 | 5 (12%) |
| Nausea | 2 (3%) | 4 (6%) | 5 (12%) | 4 (10%) |
| Decreased appetite | 1 (2%) | 5 (8%) | 2 (5%) | 4 (10%) |
| Feeling jittery | 1 (2%) | 2 (3%) | 2 (5%) | 3 (7%) |
| Somnolence | 1 (2%) | 0 | 1 (2%) | 3 (7%) |
Definition of abbreviation: Ato75 = atomoxetine 75 mg.
Most common adverse events are those reported in at least three participants in any treatment group.
Placebo-adjusted mean (95% confidence interval) change from baseline in overnight heart rate was slightly higher in all treatment groups: 7.7 (5.7–9.6), 5.1 (2.9–7.4), and 5.5 (3.3–7.1) beats/min for Ato75, Ad109 2.5/75 mg, and AD109 5/75 mg, respectively (Table 7). The lack of baseline measurements performed at the same time of day during the screening PSG studies makes it difficult to compare evening (before dosing) and morning heart rate and blood pressure between treatments. However, compared with placebo, mean heart rate was slightly higher for all treatment groups in the morning: 7.0 (3.3–10.6) beats/min for Ato75, 5.5 (1.4–9.6) beats/min for AD109 2.5/75 mg, and 7.4 (3.4–11.4) beats/min for AD109 5/75 mg (Table 7). Diastolic blood pressure was slightly higher for the AD109 2.5/75 mg group in the evening and in the morning, at 4.7 (1.1–8.3) and 4.2 (0.5–7.9) mm Hg, respectively (Table 7).
Table 7.
Difference in Heart Rate and Blood Pressure between Placebo and Active Treatment Arms at Different Time Points
| Arm | HR, beats/min |
SBP, mm Hg |
DBP, mm Hg |
||||
|---|---|---|---|---|---|---|---|
| Evening | Overnight* | Morning | Evening | Morning | Evening | Morning | |
| Ato75 | 3.7 (−0.5 to 7.8) | 7.7 (5.7 to 9.6) | 7.0 (3.3 to 10.6) | −2.7 (−8.9 to 3.6) | −0.8 (−5.3 to 3.8) | 1.5 (−1.7 to 4.7) | 1.2 (−2.1 to 4.5) |
| AD109 2.5/75 mg | 2.0 (−2.8 to 6.7) | 5.1 (2.9 to 7.4) | 5.5 (1.4 to 9.6) | 3.4 (−3.6 to 10.3) | 1.9 (−3.2 to 7.0) | 4.7 (1.1 to 8.3) | 4.2 (0.5 to 7.9) |
| AD109 5/75 mg | 1.5 (−3.1 to 6.1) | 5.5 (3.3 to 7.7) | 7.4 (3.4 to 11.4) | −3.3 (−10.1 to 3.5) | −4.0 (−9.0 to 1.1) | 0.5 (−3.0 to 4.0) | −0.8 (−4.4 to 2.8) |
Definition of abbreviations: Ato75 = atomoxetine 75 mg, HR = heart rate, DBP = diastolic blood pressure, SBP = systolic blood pressure.
Data show mean (95% confidence interval) difference from placebo derived from the average of the two treatment polysomnography nights. Evening values were obtained before dosing.
HR overnight data are additionally adjusted for baseline.
There was no reduction in weight across all treatment arms. Results of the psychometric tests are reported in the online supplement (see Figure E1 and Table E3) and were not different compared with placebo across all treatment arms.
Discussion
In this phase II randomized, double-blind, placebo-controlled study, both doses of AD109 showed clinically meaningful improvement in OSA over a 1-month treatment period in patients with mild to severe OSA. The primary endpoint, AHI4, was reduced by 45% with AD109 compared with placebo. Forty-four percent of participants who received AD109 had a >50% reduction in AHI4. Furthermore, AHI4 decreased to fewer than 10 events per hour in 42% of patients who received AD109. In addition, the 2.5/75 mg dose demonstrated improvement on the PROMIS fatigue scale. AD109 was generally safe and well tolerated, with no impairment in psychomotor performance, attention, or memory. The most common side effects (dry mouth, urinary hesitancy, and insomnia) are consistent with the known side-effect profiles of the individual drugs. There were mild increases in heart rate.
Improvement in AHI4 and other respiratory parameters with AD109 occurred during NREM and REM sleep, although the latter did not reach statistical significance versus placebo, as well as during supine and lateral sleep. In the mITT population, a significant reduction in AHI4 occurred in patients with a baseline AHI4 as high as 45 events per hour, although fewer patients with severe OSA at baseline (AHI4 ⩾30) had an AHI4 <10 during treatment (7%) compared with those with mild (AHI4 10–15, 77%) or moderate (AHI4 15–30, 42%) OSA. A prior study of atomoxetine plus oxybutynin indicated that patients with a less collapsible upper airway were more likely to have an optimal response (25). It is unclear why some patients with a severely collapsible airway have an excellent response, although the degree of impairment in pharyngeal dilator responsiveness or other endotypes may be a factor.
AD109 had no effect on TST or sleep latency, but did reduce REM sleep, consistent with prior studies of AD109, atomoxetine combined with racemic oxybutynin, and atomoxetine alone (16, 20, 21). The decrease in REM sleep did not appear as marked as the decrease noted in single-night studies of these drugs, suggesting that the effect on REM may ameliorate over time. There was no correlation between the decrease in REM sleep and the decrease in AHI4. REM and NREM sleep showed similar reductions in AHI4.
AD109 did not differ significantly from atomoxetine alone (Ato75) for most respiratory parameters, suggesting that the noradrenergic activity of atomoxetine is primarily responsible for the effect of AD109 on upper airway obstruction. However, Ato75 was more likely to disturb sleep than AD109 based on objective and subjective measurements. After 3–4 weeks of nightly treatment, Ato75 demonstrated a decrease in TST of 16–24 minutes compared with placebo and both doses of AD109. Insomnia was also the most frequently reported adverse event with Ato75 and led to study discontinuation in six individuals (6.9%). In addition, the PROMIS sleep disturbance scale suggested worsening of sleep during treatment with Ato75 alone. Moreover, adverse events leading to study discontinuation were more common with Ato75 than with AD109. Thus, Ato75 alone, despite improvements in sleep-disordered breathing, was not sufficiently well tolerated to be a standalone treatment for OSA.
Dry mouth was a common side effect with all three drug regimens, but was much more common with AD109 5/75 mg, suggesting a dose-dependent increase with aroxybutynin, an effect also reported in a recent 1-month study of atomoxetine combined with racemic oxybutynin (26). Symptoms were generally mild, like those of most adverse events reported.
Small increases in heart rate were in the range reported in short- and long-term studies of atomoxetine in the treatment of attention deficit/hyperactivity disorder in children and adults, and were deemed not clinically significant in that patient population (27). We note that the increase in heart rate in the present study was less than the increase reported with atomoxetine 80 mg plus oxybutynin 5 mg in the 1-month study by Aishah and colleagues, possibly suggesting a better safety profile of AD109 (26). However, because there is an increased risk for cardiovascular disease in the OSA population, small increases in heart rate may potentially be more clinically important. On the contrary, AD109 has been shown to significantly decrease hypoxic burden, a metric shown to predict adverse cardiovascular outcomes (24, 28). For comparison, the heart rate increase noted with AD109 is similar to that reported with solriamfetol, a drug prescribed to improve wakefulness in patients with OSA, and for which prolonged use has demonstrated no significant increase in serious adverse events (29, 30).
The interpretation of the blood pressure data poses challenges due to the lack of baseline measurements performed at the same time of the day during the screening visits. The present trial data do not provide a definitive conclusion or understanding of the AD109 pressor effect in OSA. A different pattern was observed among participants who received the higher AD109 dose (5/75 mg) compared with the lower dose (2.5/75 mg). Participants in the higher-dose group exhibited lower systolic and diastolic blood pressure than the placebo group, whereas those in the lower-dose group showed slightly higher values than the placebo group. Because there is no physiological explanation for why the two AD109 doses would have the opposite impact on blood pressure and the magnitude of the differences is small, we assume that the trends observed are simply due to random variability.
Further investigation to assess the impact of AD109 on cardiovascular risk in people with OSA is ongoing in a larger and longer phase III study (www.clinicaltrials.gov identifier NCT 05811247) that includes patients with stable cardiovascular conditions at baseline. This study includes further evaluation of heart rate and blood pressure, employing 24-hour monitoring.
Improvement in the PROMIS fatigue scale was noted with AD109 2.5/75 mg compared with placebo and Ato75. We do not believe this improvement is solely attributable to chance because PROMIS sleep impairment rating and Sleep Apnea Quality of Life Index performance also suggest improvement relative to the other cohorts. It is unclear why we did not see improvement with the higher dose of AD109, although higher rates of daytime symptoms such as dry mouth, urinary hesitation, constipation, and somnolence were reported with the higher dose of aroxybutynin, suggesting more tolerability issues with the AD109 5/75 mg dose.
Although the change from baseline in Epworth Sleepiness Scale score did not differ versus placebo for any drug regimen, the median change from baseline was similar to the clinically important difference of 2–3 points in CPAP-treated patients (31, 32). The lack of significant improvement compared with placebo on most of the patient-reported outcomes is likely related to the strong placebo effect that is common in such subjective measures (33). Indeed, most measures showed significant change from baseline in all treatment groups, including placebo. The results may also have been affected by the generally mild symptoms reported by our patient population at baseline. Longer use of the study drug may also be required to demonstrate changes in quality-of-life measures.
These data extend the findings of a cross-over single-night exposure study of AD109 in patients with mild to moderate OSA (21). The present study included patients with mild to severe OSA studied over a 1-month duration. These data are also consistent with single-night studies of atomoxetine plus racemic oxybutynin (16, 20). The data from the present study differ from those from a recent 1-month study of atomoxetine plus oxybutynin (doses of 80/5, 40/5, and 40/2.5 mg) that reported no significant difference versus placebo in AHI4 (26). However, those results were likely affected by the much lower baseline AHI4 in the placebo group compared with the drug groups, along with small numbers of participants per group (n = 9–10). Examination of individual data in the 80/5 mg dose group in that study shows decreases in AHI4 and hypoxic burden consistent with the present study.
As shown in Figure 2 and Table 3, AD109 did not completely eliminate sleep-disordered breathing, as is often the case with the use of CPAP. Nevertheless, the effectiveness of CPAP is reduced when there is low adherence and patients use the device for only a portion of the night. This reduction in effectiveness may result in overall disease alleviation similar to that seen with AD109.
The mechanism by which AD109 promotes improvement in OSA severity is likely enhanced upper airway muscle activity from noradrenergic stimulation by atomoxetine (25). The present study and the study of Rosenberg and colleagues suggest that the optimal dosage for AD109 may be 2.5/75 mg (21). A lower dose of atomoxetine appears to be less effective in decreasing OSA severity, and a higher dose of aroxybutynin increases antimuscarinic side effects. Aroxybutynin provides an important function in ameliorating the sleep-disruptive effect of atomoxetine and may further improve ventilation.
Strengths of the present study include the randomized parallel-group study design, the 1-month study duration, and the inclusion of patients with mild to severe OSA. Limitations include the randomization of patients based on PSG scoring at individual sites that resulted in the inclusion of 29 patients with a baseline AHI4 <10 after central scoring. These individuals were included in statistical analysis but not in the calculation of the percentage of patients with an AHI4 <10 during treatment. Second, analysis of evening and morning heart rate and blood pressure data may have been affected by the lack of vital sign measurements in the evening and morning of the baseline PSG examinations. Thus, the baseline characteristics of the patients may have affected the findings. For instance, patients in the AD109 2.5/75 mg arm had a significantly higher body mass index at baseline (Table 2), which may partly explain the higher blood pressure in that group. Finally, we have no objective measure of medication adherence and thus cannot comment on how frequently the medication was taken.
In conclusion, in this study of adults with mild to severe OSA, AD109 demonstrated clinically meaningful improvement in OSA and was generally well tolerated over a 1-month treatment period. OSA is a common disorder that is associated with considerable morbidity and mortality. Many patients are untreated or inadequately treated because of poor tolerance and/or adherence to CPAP. These data suggest that AD109 may be an effective treatment option for some patients. However, further evaluation of efficacy and safety in larger populations for longer durations of time is ongoing, along with identification of phenotypic and endotypic traits that predict response to treatment.
Acknowledgments
Acknowledgment
The authors thank the study participants, study investigators, and study staff for their contributions to this research. The complete list of study sites can be found at https://clinicaltrials.gov/study/NCT05071612?cond=osa&term=mariposa&rank=1.
Footnotes
Supported by Apnimed Inc.
Author Contributions: P.K.S. wrote the original draft of the manuscript with input from other authors, enrolled participants, and verified underlying data. L.T.-M. participated in trial design and data analysis and verified underlying data. J.M.O., S.G.T., C.L.D., R.R., B.C., B.A., R.B.S., and J.M. enrolled participants and participated in manuscript revision. All authors contributed to interpretation of results and revision of the manuscript. All authors approved the final manuscript and shared in the decision to submit the manuscript for publication.
Data sharing: Data sharing requests will be considered from research groups that submit a research proposal and an appropriate statistical analysis and dissemination plan. Data will be shared via a secure data access system. Requests should be directed to ltaranto@apnimed.com.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202306-1036OC on October 9, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med . 2019;7:687–698. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med . 2015;3:310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69:841–858. doi: 10.1016/j.jacc.2016.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Drager LF, Lopes HF, Maki-Nunes C, Trombetta IC, Toschi-Dias E, Alves MJ, et al. The impact of obstructive sleep apnea on metabolic and inflammatory markers in consecutive patients with metabolic syndrome. PLoS One . 2010;5:e12065. doi: 10.1371/journal.pone.0012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Antic NA, Catcheside P, Buchan C, Hensley M, Naughton MT, Rowland S, et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep (Basel) . 2011;34:111–119. doi: 10.1093/sleep/34.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weaver TE. Best predictors of continuous positive airway pressure adherence. Sleep Med Clin . 2022;17:587–595. doi: 10.1016/j.jsmc.2022.07.005. [DOI] [PubMed] [Google Scholar]
- 7. Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg . 2016;45:43. doi: 10.1186/s40463-016-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Verbraecken J, Dieltjens M, Op de Beeck S, Vroegop A, Braem M, Vanderveken O, et al. Non-CPAP therapy for obstructive sleep apnoea. Breathe (Sheff) . 2022;18:220164. doi: 10.1183/20734735.0164-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: a review. JAMA . 2020;323:1389–1400. doi: 10.1001/jama.2020.3514. [DOI] [PubMed] [Google Scholar]
- 10. Gaisl T, Haile SR, Thiel S, Osswald M, Kohler M. Efficacy of pharmacotherapy for OSA in adults: a systematic review and network meta-analysis. Sleep Med Rev . 2019;46:74–86. doi: 10.1016/j.smrv.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 11. Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med . 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horner RL, Grace KP, Wellman A. A resource of potential drug targets and strategic decision-making for obstructive sleep apnoea pharmacotherapy. Respirology . 2017;22:861–873. doi: 10.1111/resp.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grace KP, Hughes SW, Horner RL. Identification of a pharmacological target for genioglossus reactivation throughout sleep. Sleep (Basel) . 2014;37:41–50. doi: 10.5665/sleep.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chan E, Steenland HW, Liu H, Horner RL. Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states. Am J Respir Crit Care Med . 2006;174:1264–1273. doi: 10.1164/rccm.200605-597OC. [DOI] [PubMed] [Google Scholar]
- 15. Grace KP, Hughes SW, Horner RL. Identification of the mechanism mediating genioglossus muscle suppression in REM sleep. Am J Respir Crit Care Med . 2013;187:311–319. doi: 10.1164/rccm.201209-1654OC. [DOI] [PubMed] [Google Scholar]
- 16. Taranto-Montemurro L, Messineo L, Sands SA, Azarbarzin A, Marques M, Edwards BA, et al. The combination of atomoxetine and oxybutynin greatly reduces obstructive sleep apnea severity. A randomized, placebo-controlled double-blind crossover trial. Am J Respir Crit Care Med . 2019;199:1267–1276. doi: 10.1164/rccm.201808-1493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perger E, Taranto Montemurro L, Rosa D, Vicini S, Marconi M, Zanotti L, et al. Reboxetine plus oxybutynin for OSA treatment: a 1-week randomized, placebo-controlled, double-blind crossover trial. Chest . 2022;161:237–247. doi: 10.1016/j.chest.2021.08.080. [DOI] [PubMed] [Google Scholar]
- 18. Lim R, Messineo L, Grunstein RR, Carberry JC, Eckert DJ. The noradrenergic agent reboxetine plus the antimuscarinic hyoscine butylbromide reduces sleep apnoea severity: a double-blind, placebo-controlled, randomised crossover trial. J Physiol . 2021;599:4183–4195. doi: 10.1113/JP281912. [DOI] [PubMed] [Google Scholar]
- 19. Altree TJ, Aishah A, Loffler KA, Grunstein RR, Eckert DJ. The norepinephrine reuptake inhibitor reboxetine alone reduces obstructive sleep apnea severity: a double-blind, placebo-controlled, randomized crossover trial. J Clin Sleep Med . 2023;19:85–96. doi: 10.5664/jcsm.10256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schweitzer PK, Maynard JP, Wylie PE, Emsellem HA, Sands SA. Efficacy of atomoxetine plus oxybutynin in the treatment of obstructive sleep apnea with moderate pharyngeal collapsibility. Sleep Breath . 2023;27:495–503. doi: 10.1007/s11325-022-02634-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosenberg R, Abaluck B, Thein S. Combination of atomoxetine with the novel antimuscarinic aroxybutynin improves mild to moderate OSA. J Clin Sleep Med . 2022;18:2837–2844. doi: 10.5664/jcsm.10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schweitzer P, Ojile J, Thein S, Drake C, Rosenberg R, Corser B, et al. The oral agent AD109 improves objective and subjective outcomes in obstructive sleep apnea patients. Results from the Mariposa study, a randomized, controlled clinical trial [abstract] Am J Respir Crit Care Med . 2023;207:A1052. doi: 10.1164/rccm.202306-1036OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ojile J, Schweitzer P, Thein S, Drake C, Rosenberg R, Corser B, et al. MARIPOSA: oral therapy AD109 improves objective & patient reported outcomes in OSA. Randomized, placebo controlled clinical trial [abstract] Sleep (Basel) . 2023;46:A237. [Google Scholar]
- 24. Azarbarzin A, Sands SA, Taranto-Montemurro L, Vena D, Sofer T, Kim SW, et al. The sleep apnea-specific hypoxic burden predicts incident heart failure. Chest . 2020;158:739–750. doi: 10.1016/j.chest.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taranto-Montemurro L, Messineo L, Azarbarzin A, Vena D, Hess LB, Calianese NA, et al. Effects of the combination of atomoxetine and oxybutynin on OSA endotypic traits. Chest . 2020;157:1626–1636. doi: 10.1016/j.chest.2020.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aishah A, Loffler KA, Toson B, Mukherjee S, Adams RJ, Altree TJ, et al. One month dosing of atomoxetine plus oxybutynin in obstructive sleep apnea: a randomized, placebo-controlled trial. Ann Am Thorac Soc . 2023;20:584–595. doi: 10.1513/AnnalsATS.202206-492OC. [DOI] [PubMed] [Google Scholar]
- 27. Wernicke JF, Faries D, Girod D, Brown J, Gao H, Kelsey D, et al. Cardiovascular effects of atomoxetine in children, adolescents, and adults. Drug Saf . 2003;26:729–740. doi: 10.2165/00002018-200326100-00006. [DOI] [PubMed] [Google Scholar]
- 28. Azarbarzin A, Sands SA, Stone KL, Taranto-Montemurro L, Messineo L, Terrill PI, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J . 2019;40:1149–1157. doi: 10.1093/eurheartj/ehy624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang J, Yang S, Li X, Wang T, Xu Z, Xu X, et al. Efficacy and safety of solriamfetol for excessive sleepiness in narcolepsy and obstructive sleep apnea: findings from randomized controlled trials. Sleep Med . 2021;79:40–47. doi: 10.1016/j.sleep.2020.12.039. [DOI] [PubMed] [Google Scholar]
- 30. Malhotra A, Shapiro C, Pepin JL, Hedner J, Ahmed M, Foldvary-Schaefer N, et al. Long-term study of the safety and maintenance of efficacy of solriamfetol (JZP-110) in the treatment of excessive sleepiness in participants with narcolepsy or obstructive sleep apnea. Sleep (Basel) . 2020;43:zsz220. doi: 10.1093/sleep/zsz220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patel S, Kon SSC, Nolan CM, Barker RE, Simonds AK, Morrell MJ, et al. The Epworth Sleepiness Scale: minimum clinically important difference in obstructive sleep apnea. Am J Respir Crit Care Med . 2018;197:961–963. doi: 10.1164/rccm.201704-0672LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Crook S, Sievi NA, Bloch KE, Stradling JR, Frei A, Puhan MA, et al. Minimum important difference of the Epworth Sleepiness Scale in obstructive sleep apnoea: estimation from three randomised controlled trials. Thorax . 2019;74:390–396. doi: 10.1136/thoraxjnl-2018-211959. [DOI] [PubMed] [Google Scholar]
- 33. Labarca G, Montenegro R, Oscullo G, Henriquez-Beltran M, Uribe JP, Gómez-Olivas JD, et al. Placebo response in objective and subjective measures of hypersomnia in randomized clinical trials on obstructive sleep apnea. A systematic review and meta-analysis. Sleep Med Rev . 2023;67:101720. doi: 10.1016/j.smrv.2022.101720. [DOI] [PubMed] [Google Scholar]