Abstract
Biphenyl dioxygenase (BPH dox) oxidizes biphenyl on adjacent carbons to generate 2,3-dihydro-2,3-dihydroxybiphenyl in Comamonas testosteroni B-356 and in Pseudomonas sp. strain LB400. The enzyme comprises a two-subunit (α and β) iron sulfur protein (ISPBPH), a ferredoxin (FERBPH), and a ferredoxin reductase (REDBPH). B-356 BPH dox preferentially catalyzes the oxidation of the double-meta-substituted congener 3,3′-dichlorobiphenyl over the double-para-substituted congener 4,4′-dichlorobiphenyl or the double-ortho-substituted congener 2,2′-dichlorobiphenyl. LB400 BPH dox shows a preference for 2,2′-dichlorobiphenyl, and in addition, unlike B-356 BPH dox, it can catalyze the oxidation of selected chlorobiphenyls such as 2,2′,5,5′-tetrachlorobiphenyl on adjacent meta-para carbons. In this work, we examine the reactivity pattern of BPH dox toward various chlorobiphenyls and its capacity to catalyze the meta-para dioxygenation of chimeric enzymes obtained by exchanging the ISPBPH α or β subunit of strain B-356 for the corresponding subunit of strain LB400. These hybrid enzymes were purified by an affinity chromatography system as His-tagged proteins. Both types, the chimera with the α subunit of ISPBPH of strain LB400 and the β subunit of ISPBPH of strain B-356 (the αLB400βB-356 chimera) and the αB-356βLB400 chimera, were functional. Results with purified enzyme preparations showed for the first time that the ISPBPH β subunit influences BPH dox’s reactivity pattern toward chlorobiphenyls. Thus, if the α subunit were the sole determinant of the enzyme reactivity pattern, the αB-356βLB400 chimera should have behaved like B-356 ISPBPH; instead, its reactivity pattern toward the substrates tested was similar to that of LB400 ISPBPH. On the other hand, the αLB400βB-356 chimera showed features of both B-356 and LB400 ISPBPH where the enzyme was able to metabolize 2,2′- and 3,3′-dichlorobiphenyl and where it was able to catalyze the meta-para oxygenation of 2,2′,5,5′-tetrachlorobiphenyl.
A fraction of the 209 polychlorinated-biphenyl (PCB) congeners can be transformed into chlorobenzoates by the bacterial biphenyl oxidative catabolic pathway. The pathway involves four enzymatic steps (9, 28). The biphenyl dioxygenase (BPH dox) catalyzes the first reaction of this pathway. Aromatic ring dioxygenases catalyze dihydroxylation reactions on two adjacent carbons of the aromatic ring (23). Some of these enzymes can oxygenate a broad range of substrate analogs. For example, naphthalene dioxygenase can catalyze the hydroxylation of several polycyclic aromatic hydrocarbons (18) and BPH dox can oxygenate various PCB analogs (4, 7, 16). Details about the structural features which are responsible for the enzyme’s substrate recognition, binding, and orientation in the direction of the active site will help us to design new enzymes able to use a broader range of substrates than that currently used.
BPH dox has been studied from Comamonas testosteroni B-356 (13, 14) and from Pseudomonas sp. strain LB400 (11, 12). It comprises three components (11, 13, 14). These are the terminal oxygenase, an iron-sulfur protein (ISPBPH) made up of an α subunit (Mr = 51,000) and a β subunit (Mr = 22,000), a ferredoxin (FERBPH; Mr = 12,000), and a ferredoxin reductase (REDBPH; Mr = 43,000). The genes that code for these components in both strain B-356 and strain LB400 are bphA (ISPBPH α subunit), bphE (ISPBPH β subunit), bphF (FERBPH), and bphG (REDBPH) (6, 31). BPH dox hydroxylates adjacent ortho-meta carbons of one of the biphenyl rings to generate 2,3-dihydro-2,3-dihydroxybiphenyl. FERBPH and REDBPH are involved in electron transfer from NADH to ISPBPH (14). One common feature characterizing the terminal oxygenase components of all aromatic ring-hydroxylating dioxygenases is the presence of a [2Fe-2S] Rieske center located on the α subunit (13, 25, 31), which is believed to be involved in electron transfer from the ferredoxin component to a mononuclear Fe2+, which activates molecular oxygen for insertion into the substrate (3, 23). Evidence suggests that the mononuclear Fe2+ is coordinated from a domain of the ISPBPH α subunit (15). Therefore, the α subunit appears to serve a major catalytic function. However, the function of the β subunit in enzyme activity has yet to be elucidated.
Purified active ISPBPH preparations were obtained from Pseudomonas sp. strain LB400 (11) and from C. testosteroni B-356 (14), whereas B-356 REDBPH and FERBPH were purified from Escherichia coli recombinant clones as active His-tagged (H-t) proteins (14). Active preparations of H-t B-356 ISPBPH were also obtained (13).
Pseudomonas sp. strain LB400 is one of the best-performing PCB-degrading gram-negative bacteria. An important feature that distinguishes strain LB400 from other PCB degraders is its capacity to catalyze the oxygenation of adjacent meta-para carbons of congeners such as 2,2′,5,5′-tetrachlorobiphenyl, in which there are no free adjacent ortho-meta carbons (4). Furthermore, strain LB400 metabolizes the double-ortho-substituted congener 2,2′-dichlorobiphenyl very efficiently whereas the double-meta- or -para-substituted congeners such as 3,3′- and 4,4′-dichlorobiphenyl are degraded poorly (4, 7, 10). In spite of the fact that the deduced amino acid sequences of the LB400 and Pseudomonas pseudoalcaligenes KF707 bphA, bphE, bphF, and bphG gene products show 95.5, 99.5, 100, and 100% identity, respectively, KF707 BPH dox is unable to attack 2,2′,5,5′-tetrachlorobiphenyl (7, 10, 17). In addition, it poorly metabolizes the double-ortho-substituted congener and, unlike strain LB400, it degrades the double-para-substituted 4,4′-dichlorobiphenyl more efficiently. Results of recent investigations suggest that a relatively small number of amino acid residues of the carboxy-terminal portion of the ISPBPH α subunit control the substrate selectivity patterns of both strains and their ability to catalyze meta-para dioxygenation (7, 17, 24). However, because the ISPBPH β subunits of the two strains used in this study differed by a single amino acid residue, it was impossible to determine any involvement of the β subunit in substrate selectivity.
Strain B-356 BPH dox has unique structural features that distinguish it from strain LB400 BPH dox (the amino acid sequences of the LB400 and B-356 ISPBPH α and β subunits show 76 and 70% identity, respectively) (31). Furthermore, unlike strain LB400, strain B-356 metabolized the double-meta-substituted congener 3,3′-dichlorobiphenyl more efficiently than 2,2′- and 4,4′-dichlorobiphenyl. When tested as a resting-cell preparation in a 1-ml volume, 2.4 mg of the 3.3 mg of 3,3′-dichlorobiphenyl was degraded within 12 h, compared to 0.6 and 0.17 mg of 4,4′- and 2,2′-dichlorobiphenyl, respectively (2). In addition, B-356 was unable to degrade 2,2′,5,5′-tetrachlorobiphenyl (2).
Because the recombinant H-t components of B-356 BPH dox have retained all major biochemical features of the parental proteins, affinity chromatography of tagged protein was proposed as a useful tool for examining features of various purified aryl dioxygenase components (13). It especially offers the possibility of comparing the characteristics of purified reconstituted chimeric ISPBPHs, comprised of α and β subunits derived from distinct parent enzymes. In this work we examine the reactivity pattern of BPH dox toward various PCBs and its capacity to catalyze the meta-para dioxygenation of chimeric enzymes obtained by exchanging the ISPBPH α or β subunit of strain LB400 with the corresponding subunit of strain B-356. Results show that the structure of the β subunit influences both the capacity of the enzyme to catalyze the meta-para oxygenation of the substrate and its substrate reactivity pattern toward PCBs.
MATERIALS AND METHODS
Bacterial strains, culture media, and general protocols.
The bacterial strains used in this study were E. coli M15(pREP4) and SG13009(pREP4) (both from Qiagen, Inc., Chatsworth, Calif.), E. coli SG13009 cured of pREP4 (obtained during this work), C. testosteroni B-356 (1), and Pseudomonas sp. strain LB400 (4) (also referred as Burkholderia sp. strain LB400 or Pseudomonas cepacia LB400 [17]). The media used were Luria-Bertani broth (26), H-plate medium (26), and MM30 (29). The plasmids used were pQE31 and pQE51 (Qiagen, Inc.) and pYH31 (to be described elsewhere), which is a new P15A-based plasmid obtained by introducing the operator and promoter region of pQE31, the six-His fusion gene, and the multiple cloning site of pQE31 into the unique HindIII site of pREP4. pHY31 is compatible with ColE1-based plasmids.
Plasmid DNA from E. coli was obtained and restriction endonuclease reactions, ligations, agarose gel electrophoresis, and transformation of E. coli cells were done according to protocols described by Sambrook et al. (26). PCRs were performed with Pwo DNA polymerase by following the method recommended by Boehringer Mannheim. DNA sequencing was done from subclones of M13mp18 and M13mp19 with a Pharmacia automated laser fluorescence DNA sequencer. Sequence analysis were performed by the DNA sequencing service at the Institut Armand-Frappier, Laval, Québec, Canada.
Previously described procedures (13, 14, 30) were used to obtain purified preparations of each of the following enzymes from recombinant E. coli cells: H-t B-356 FERBPH, H-t B-356 REDBPH, H-t B-356 ISPBPH (which carries the His tag on the α subunit), and H-t B-356 2,3-dihydro-2,3-dihydroxybiphenyl 2,3-dehydrogenase. The only exception was that the E. coli cells harboring the recombinant pQE31 plasmids were induced at 25°C instead of 37°C as previously described (13). This modification also applies to the other enzyme purification protocols described below.
The LB400 ISPBPH component that carries a His tag on the α subunit was expressed in E. coli M15(pREP4), as was done with B-356 H-t ISPBPH (13). The oligonucleotides used to PCR amplify LB400 bphAE from genomic LB400 DNA were based on known DNA nucleotide sequences (6). They were oligonucleotide I (BamHI), 5′-CGGGATCCGATGAGTTCAGCAATCA-3′, and oligonucleotide II (HindIII), 5′-GAGCCAAGCTTGCTAGAAGAACATGCT-3′. The 1.9-kb DNA fragment containing bphAE was cloned into the compatible sites of pQE31. H-t LB400 ISPBPH was purified in the same way as H-t B-356 ISPBPH (13).
Purified H-t LB400 FERBPH was obtained by the protocol described for H-t B-356 FERBPH (14). The oligonucleotides used to amplify LB400 bphF from genomic DNA of strain LB400 were oligonucleotide I (BamHI), 5′-GCGGGATCCGATGAAATTTACCAGAG-3′, and oligonucleotide II (HindIII), 5′-GCGCCAAGCTTGTCATGGCGCCAGATAC-3′.
The ISPBPH chimera with the α subunit of B-356 and the β subunit of LB400 (the αB-356βLB400 chimera) carrying the His tag on the α subunit was expressed in E. coli M15(pREP4) and purified by the protocol described for H-t B-356 ISPBPH (13). In order to construct the pQE31 chimera with bphA from B-356 and bphE from LB400 (pQE31[B-356-bphA/LB400-bphE]), bphE was PCR amplified from LB400 genomic DNA with the following oligonucleotides: oligonucleotide I (KpnI), 5′-CCGGGTACCCATGACAAATCCATCCC-3′, and oligonucleotide II (KpnI), 5′-GGGGTACCCCTAGAAGAACATGCT-3′. The 0.6-kb fragment was cloned at the KpnI site of pQE31[B-356-bphA], which has been described previously (13).
All of our pQE31[LB400-bphA/B-356-bphE] constructs poorly expressed bphE. For this reason, the αLB400βB-356 ISPBPH chimera was obtained by expressing each subunit from separate plasmids inside the same cell. A DNA fragment carrying LB400 bphA was PCR amplified from LB400 genomic DNA with the following oligonucleotides: oligonucleotide I (BamHI), 5′-CGGGATCCGATGAGTTCAGCAATCA-3′, and oligonucleotide II (KpnI), 5′-GCCGGTACCTTCCTGCTCAGGGCTTGAGCGTG-3′. The 1.3-kb DNA fragment was cloned into compatible sites of pYH31 to construct pYH31[LB400-bphA]. B-356 bphE was subcloned from pQE31[bphE], which was described previously (13), into pQE51, which is an expression vector without the six-His fused gene. Both plasmids were transformed in E. coli SG13009 cured of pREP4. Expression and purification of the enzyme were performed by protocols identical to those described previously.
All constructions were such that the His tail added the same 13 amino acids (MRGSHHHHHHTDP) to the protein at the N-terminal portion. The DNA sequences of the constructions obtained by PCR amplification were analyzed to make certain that no mutations were introduced in the amplified DNA.
Protein characterization.
Sodium dodecyl sulfate (SDS)-polyacrylamide gels were developed according to the method of Laemmli (20). Proteins were stained with Coomassie brillant blue (26). Protein concentrations were estimated by the method of Lowry et al. (21) with bovine serum albumin as the standard. The concentrations of H-t ISPBPH and FERBPH preparations were also determined spectrophotometrically. The published ɛ450 of 10,100 M−1 cm−1 (11) was used to determine the concentration of H-t LB400 ISPBPH, and the published ɛ455 of 8,300 M−1 cm−1 (13) was used to determine the concentrations of H-t B-356 ISPBPH preparations. The concentrations of both LB400 and B-356 H-t FERBPH preparations were determined with the ɛ460 of 7,455 M−1 cm−1 established for the Rieske center of B-356 FERBPH (14). The concentrations of αLB400βB-356 and αB-356βLB400 chimeras were determined with an ɛ455 of 8,300 M−1 cm−1. The Mr of native protein was determine by high-performance liquid chromatography (HPLC) as described previously (13).
Monitoring of the enzymes’ activities and identification of metabolites.
Enzyme assays for BPH dox reactions were performed as described previously (13). A reaction was initiated by adding 100 nmol of biphenyl or of one of the following chlorobiphenyls: 2,2′-, 3,3′-, or 4,4′-dichlorobiphenyl; 2,5-dichlorobiphenyl; and 2,2′,5,5′-tetrachlorobiphenyl (all from ULTRAScientific, Kingstown, R.I.) (added in 2 μl of acetone). The reconstituted LB400 H-t BPH dox comprised H-t LB400 ISPBPH plus H-t LB400 FERBPH and H-t B-356 REDBPH; the reconstituted B-356 BPH dox comprised H-t B-356 ISPBPH plus H-t B-356 FERBPH and H-t B-356 REDBPH. Catalytic oxygenation was evaluated by monitoring substrate depletion by HPLC analysis as described previously (13), except that the UV detector of the Hewlett-Packard series 1050 HPLC was set at 203 nm. The Km and maximal rate of metabolism (Vmax) for biphenyl was obtained as described previously (13). The catalytic oxygenation of PCBs was evaluated by monitoring substrate depletion 5 min after initiation of the reaction when 100 nmol of substrate was added to initiate the reaction. The metabolites were detected with a Perkin-Elmer LC95 UV- and visible-light detector set at the maximal wavelength established by Haddock et al. (12). Purification of metabolites was as previously described (13, 30). The 2,3-dihydro-2,3-dihydroxybiphenyl 2,3-dehydrogenase assay was performed as previously described (30). The 3,4-dihydroxybiphenyl used as the standard was from ULTRAScientific. Metabolites were identified by gas chromatographic-mass spectrometric (GC-MS) analysis of their trimethylsilyl (TMS) or butylboronate derivatives, using protocols described previously (14, 27). All values reported in the present study are averages of the results of triplicate experiments for at least two distinct enzyme preparations.
Site-directed mutagenesis at position Thr-375 of the strain B-356 ISPBPH α subunit.
Site-directed mutagenesis was carried out with Pharmacia Biotech’s unique site elimination mutagenesis kit, according to the protocol described by Wang and Sul (32). The original sequence of the strain B-356 ISPBPH α subunit, 5′-GC TTG CAG AAG ATC CGC ACC TTT AAC GCC GGC GGC-3′, was modified to 5′-GC TTG CAG AAG ATC CGC AAC TTT AAC GCC GGC GGC-3′ by replacing Thr-375 with Asn-375 (boldface). Successful mutations were identified by DNA sequencing. Mutant genes were cloned in pQE31. The mutant enzyme was expressed in E. coli M15 and purified as fused H-t protein according to protocols described previously (13).
RESULTS
Characterization of H-t LB400 ISPBPH and LB400-B-356 ISPBPH chimeras.
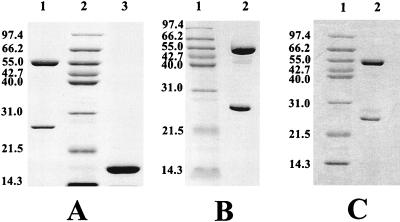
Purified H-t LB400 ISPBPH was obtained as described in Materials and Methods. SDS-polyacrylamide gel electrophoresis (PAGE) of the purified preparation showed two single peptide bands with Mrs corresponding to those of the H-t α and β subunits of the LB400 ISPBPH component (Fig. 1A). SDS-PAGE of H-t LB400 FERBPH preparations showed a single band of the expected Mr (Fig. 1A).
FIG. 1.
SDS-PAGE of purified preparations of BPH dox components. (A) Lane 1, preparation of H-t LB400 ISPBPH (3 μg); lane 2, Mr markers; lane 3, preparation of H-t LB400 FERBPH (5 μg); (B) lane 1, Mr markers; lane 2, preparation of the H-t αB-356βLB400 ISPBPH hybrid (2.5 μg); (C) lane 1, Mr markers; lane 2, preparation of the H-t αLB400βB-356 ISPBPH hybrid (3 μg). Molecular weights (in thousands) are noted at the left sides of the gels.
The recombinant chimeric H-t ISPBPH preparations used in this study were produced in vivo in E. coli clones. H-t αB-356 βLB400 ISPBPH was produced in an E. coli clone carrying chimeric pQE31[B-356-bphA/LB400-bphE], whereas H-t αLB400βB-356 ISPBPH was produced in an E. coli clone carrying pYH31[LB400-bphA] and pQE51[B-356-bphE] together. For the latter chimeric protein, SDS-PAGE analysis of the protein eluted from the Ni-nitrilotriacetic acid resin at a low concentration of imidazole combined with densitometric measurement of bands showed that negligible amounts of the ISPBPH β subunit produced inside the cell did not assemble with the α subunit (not shown). Therefore, in spite of the fact that both subunits of H-t αLB400βB-356 ISPBPH were expressed from genes located on separate plasmids, the enzyme was effectively reconstituted inside the cell. Purified preparations of both chimeras showed two major bands corresponding to the H-t α and the β subunits (Fig. 1B and C).
HPLC analysis showed that the native conformation of both H-t ISPBPH chimeras was α3β3, as was shown previously for LB400 and B-356 ISPBPH (11, 13). Although we previously reported that types of subunit associations other than that of the α3β3 conformation were detected in some purified preparations of B-356 H-t ISPBPH (13), in the course of the present study we found that when the concentrations of the purified recombinant enzyme preparations were above 100 μM, the α3β3 structure remained intact for all H-t ISPBPH preparations.
As with the parental ISPBPH preparations, the spectral features of both chimeric enzyme preparations were typical of a [2Fe-2S] Rieske-type protein showing maxima at 323 and 455 nm and a shoulder at about 575 nm (not shown). The enzymes were active, and they both catalyzed the oxygenation of biphenyl in the reconstituted BPH dox system.
A Km value of 103 ± 17 μM and a Vmax of 1 ± 0.05 nmol of substrate converted per min per μg of ISPBPH (means ± standard deviations) were obtained when H-t LB400 BPH dox composed of H-t LB400 ISPBPH, H-t LB400 FERBPH, and H-t B-356 REDBPH was used to catalyze the dioxygenation of biphenyl. These values are close to those reported for H-t B-356 BPH dox (13). Kinetic parameters of the reconstituted H-t BPH dox comprised of H-t LB400 ISPBPH with H-t LB400 FERBPH were identical to those of the reconstituted enzyme when H-t LB400 FERBPH was replaced by H-t B-356 FERBPH. Similar results were obtained when B-356 ISPBPH was used in combination with H-t LB400 FERBPH or H-t B-356 FERBPH. Therefore, both FERBPHs can be interchanged with no effect on activity. Similar Km (66 ± 9 μM) and Vmax (0.6 ± 0.02 nmol/min/μg of ISPBPH) values were obtained for the H-t chimeric BPH dox composed of H-t αB-356βLB400 ISPBPH, H-t B-356 FERBPH, and H-t B-356 REDBPH. The reconstituted H-t chimeric αLB400βB-356 ISPBPH was also functional, but its Km value towards biphenyl was slightly higher (Km = 370 ± 65 μM; Vmax = 0.9 ± 0.04 nmol/min/μg of ISPBPH).
Reactivities of reconstituted purified H-t LB400 dox and H-t B-356 BPH dox and of H-t LB400–B-356 BPH dox chimeras toward selected PCBs.
In a previous study, when biphenyl was used as the substrate, the TMS-derived diol products of the B-356 BPH dox reaction were resolved into two distinct peaks by GC-MS analysis. These two peaks were postulated to be 2,3-dihydro-2,3-dihydroxybiphenyl and 3,4-dihydro-3,4-dihydroxybiphenyl, respectively (14). However, it was later found that when n-butylboronate was used for chemical derivatization of biphenyl metabolites, a single GC-MS peak was obtained. Moreover, the biphenyl-derived dihydrodiol product of the B-356 BPH dox reaction eluted as a single HPLC peak (30). The TMS-derived dihydrodiol obtained from this HPLC peak was resolved as two GC-MS peaks (results not shown). However, when the HPLC-purified 2,3-dihydro-2,3-dihydroxybiphenyl was used as the substrate for the 2,3-dihydro-2,3-dihydroxybiphenyl 2,3-dehydrogenase reaction, both of these TMS-derived dihydrodiol peaks disappeared from the reaction medium and 2,3-dihydroxybiphenyl was the unique metabolite generated in this reaction (results not shown). 3,4-Dihydroxybiphenyl was not produced. Therefore, the resolution of the TMS-derived dihydrodiol into two GC-MS peaks is an artifact. Although at this time we cannot provide an explanation for this artifact, the data presented above clearly show that our former assumption that B-356 BPH dox can catalyze a meta-para hydroxylation of biphenyl was erroneous.
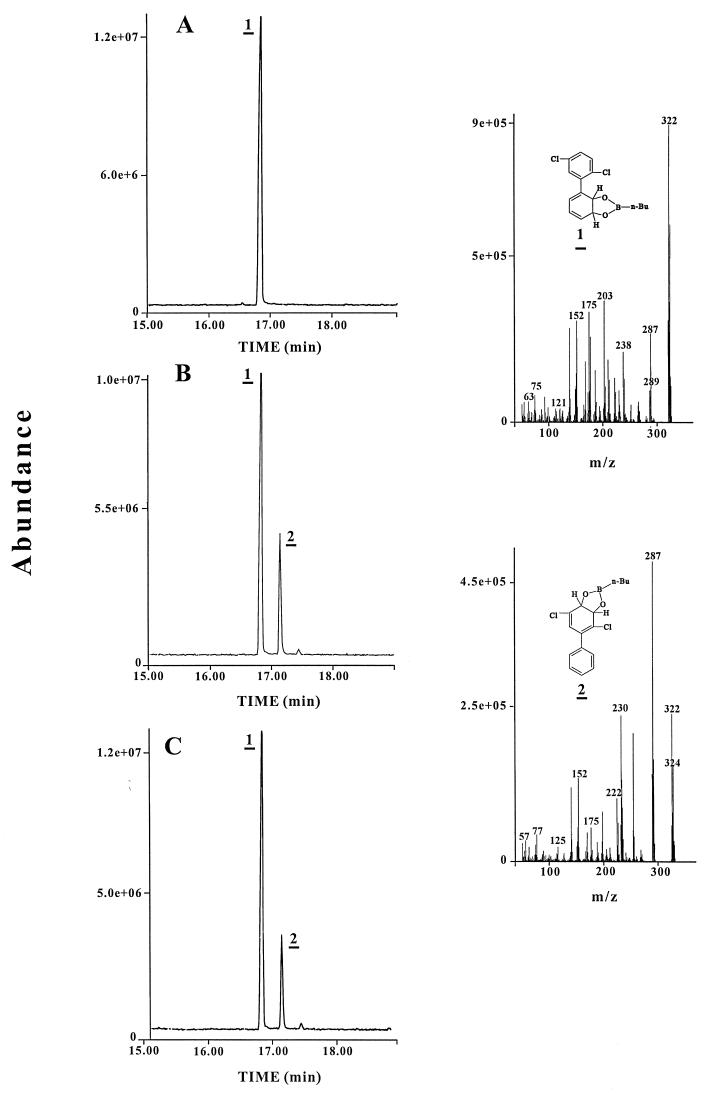
Two other observations supported the inability of B-356 BPH dox to catalyze the meta-para hydroxylation reaction. When n-butylboronate was used for chemical derivatization of the metabolites obtained from 2,5-dichlorobiphenyl, a single dihydrodiol was detected by GC-MS from the B-356 BPH dox reaction whereas two n-butylboronate-derived dihydrodiol metabolites were detected from LB400 BPH dox reaction media. The retention time and mass spectral features of the single dihydrodiol metabolite produced by B-356 BPH dox were identical to those of the major metabolite produced by LB400 BPH dox (Fig. 2). This metabolite must be the 2′,3′-dihydro-2′,3′-dihydroxy-2,5-dichlorobiphenyl which has previously been identified by nuclear magnetic resonance as the major metabolite produced from 2,5-dichlorobiphenyl by LB400 BPH dox (12). The minor metabolite produced by LB400 BPH dox had tentatively been identified as the product resulting from the meta-para oxygenation of the molecule (12), and this metabolite was not produced in the B-356 BPH dox reaction mixture (Fig. 2).
FIG. 2.
GC-MS spectra of the butylboronate-derived metabolites obtained from 2,5-dichlorobiphenyl when the substrate was oxygenated with B-356 H-t BPH dox (A), LB400 H-t BPH dox (B), or H-t αB-356βLB400 BPH dox (C). n-Bu, n-butylboronate.
Furthermore, no dihydrodiol metabolite was detected by GC-MS and by HPLC analyses when 2,2′,5,5′-tetrachlorobiphenyl was supplied as the substrate for the H-t B-356 BPH dox reaction. Conversely, as expected from a previous report (12), the corresponding 3,4-dihydrodiol metabolite was produced in a large amount when this reaction was catalyzed by H-t LB400 BPH dox.
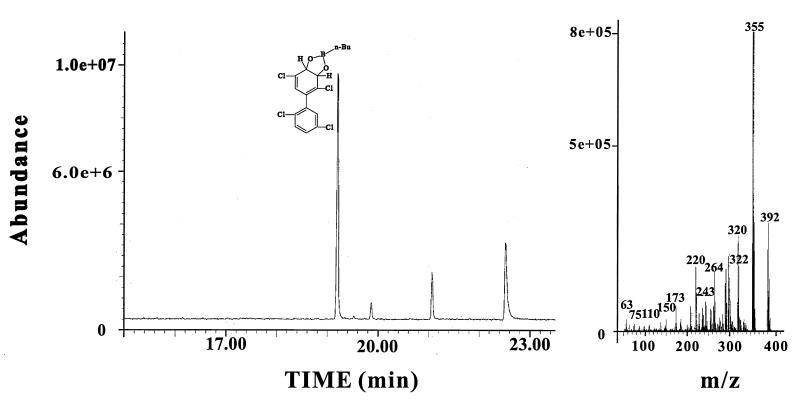
It is noteworthy that the metabolites produced from 2,5-dichlorobiphenyl when H-t αB-356βLB400 ISPBPH was used to reconstitute BPH dox were the same as those generated by LB400 H-t ISPBPH (Fig. 2). Furthermore, 2,2′,5,5′-tetrachlorobiphenyl was metabolized to the 3,4-dihydro-3,4-dihydroxy-2,2′,5,5′-tetrachlorobiphenyl (Fig. 3) by this chimeric enzyme. 3,4-Dihydro-3,4,-dihydroxy-2,2′,5,5′-tetrachlorobiphenyl was also produced from 2,2′,5,5′-tetrachlorobiphenyl by the H-t αLB400βB-356 ISPBPH chimera (not shown). However, as seen below, the rate of transformation was not as high as for the H-t αB-356βLB400 ISPBPH hybrid. If the α subunit were the sole determinant controlling the capacity of the enzyme to catalyze a meta-para oxygenation, αB-356βLB400 ISPBPH should not have metabolized 2,2′,5,5′-tetrachlorobiphenyl. Thus, considered together, these data show that the capacity of the enzyme to catalyze a meta-para hydroxylation of selected congeners is largely determined by the overall enzyme structure imposed by the association between the α and β subunits.
FIG. 3.
GC-MS spectra of the dihydrodiol metabolite produced from 2,2′,5,5′-tetrachlorobiphenyl when H-t αB-356βLB400 ISPBPH was used to reconstitute BPH dox. n-Bu, n-butylboronate.
In previous investigations (4, 7, 10) LB400 BPH dox was found to metabolize efficiently 2,2′-dichlorobiphenyl but to metabolize poorly 3,3′- and 4,4′-dichlorobiphenyl whereas strain B-356 was found to preferentially metabolize 3,3′-dichlorobiphenyl. It was thus convenient to use these three dichlorinated congeners to compare the substrate preferences of the parental and chimeric enzymes.
It was virtually impossible to obtain statistically significant values to determine the kinetic parameters of these substrates because their water solubility is very low (5, 8, 22), they are degraded very slowly by the enzyme, and some of their enzyme components lose their activity within a few minutes after initiation of the reaction. Instead, we have determined the activities of the various enzyme preparations toward PCB congeners by evaluating their degradation over a period of 5 min.
Data shown in Table 1 confirmed the preference of LB400 BPH dox for 2,2′-dichlorobiphenyl (24). Product analysis showed that 3,3′- and 4,4′-dichlorobiphenyl were metabolized to dihydrodiol derivatives. However, the rate of transformation was extremely low. In fact, less than 5 nmol of these substrates was depleted from the reaction medium when the reaction was prolonged for 10 min. On the other hand, under the same conditions B-356 BPH dox metabolized 3,3′-dichlorobiphenyl much faster than 2,2′- and 4,4′-dichlorobiphenyl, which also confirmed previously reported results (2). It is noteworthy that when the reaction was catalyzed by the H-t αB-356βLB400 ISPBPH hybrid, the reactivity pattern was similar to that of H-t LB400 BPH dox. The H-t BPH dox αLB400βB-356 chimera has acquired features of both parents. It transformed both 2,2′- and 3,3′-dichlorobiphenyl at comparable rates (Table 1). Similar to the observation made when 2,2′,5,5′-tetrachlorobiphenyl was used as a substrate, data showed that the α subunit is not the sole determinant of the enzyme’s reactivity pattern toward the dichlorinated congeners. Thus, the substrate metabolization pattern of αB-356βLB400 ISPBPH is similar to that of LB400 ISPBPH instead of B-356 ISPBPH. On the other hand, the αLB400βB-356 chimera transformed 2,2′,5,5′-tetrachlorobiphenyl less efficiently than the αB-356βLB400 chimera.
TABLE 1.
Amounts of substrate depleted 5 min after initiation of reactions with the indicated enzymesa
| Substrate | nmol of substrate depleted/0.6 nmol of enzyme:
|
|||
|---|---|---|---|---|
| αB-356βB-356 ISPBPH | αLB400βLB400 ISPBPH | αB-356βLB400 ISPBPH | αLB400βB-356 ISPBPH | |
| 2,2′-Dichlorobiphenyl | <10 | 50 | 50 | 30 |
| 3,3′-Dichlorobiphenyl | 50 | <10 | <10 | 30 |
| 4,4′-Dichlorobiphenyl | T | T | T | T |
| 2,5-Dichlorobiphenyl | 50 | 60 | 30 | <10 |
| 2,2′,5,5′-Tetrachlorobiphenyl | − | 35 | 40 | 20 |
BPH dox reactions were carried out as described in Materials and Methods. Substrate (100 nmol) was added to initiate each reaction. Numbers refer to the amounts of substrate consumed 5 min after initiation of the reactions. The values are averages of results from three separate experiments with two different enzyme preparations. The variance was less than 10% in all cases. −, no degradation; T, trace amounts of metabolite produced.
Site-directed mutagenesis at position Thr-375 of the strain B-356 ISPBPH α subunit.
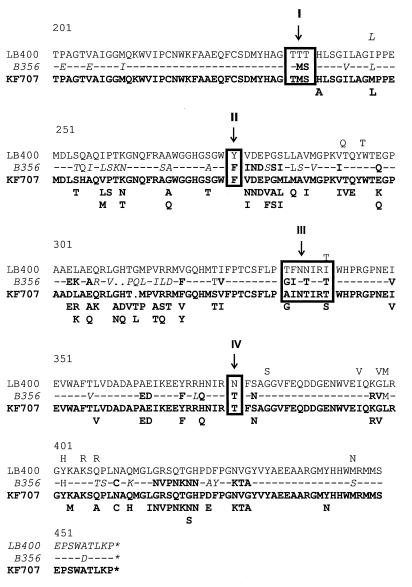
Mondello et al. (24) have categorized 15 strains into two groups based on their ability to degrade PCB congeners. One group (LB400 type) showed a broad range of congener specificity, including the capacity to degrade 2,2′,5,5′-tetrachlorobiphenyl, whereas the second group (KF707 type) showed a much narrower range of PCB congener specificity. Sequence comparison between the ISPBPH α subunits of these two groups identified four regions of the protein C-terminal portion in which specific sequences were consistently associated with either broad or narrow substrate specificity (24). Strain B-356 fits in with the KF707-type strains. It shows a narrow congener substrate specificity. It poorly degrades 2,2′-dichlorobiphenyl, and it is unable to catalyze the meta-para hydroxylation of 2,2′,5,5′-tetrachlorobiphenyl. Furthermore, the amino acid residues of all four regions identified by Mondello et al. (24) as being involved in determining substrate specificity are identical to the residues found in the KF707-type strains (Fig. 4). Moreover, among the residues that are not conserved in all the sequences shown in Fig. 4, 42 residues found in the B-356 ISPBPH α subunit are also found in the KF707-type ISPBPH, compared with only 4 residues found in the LB400 type.
FIG. 4.
Sequence comparison between the C-terminal portion of the B-356 ISPBPH α subunit with those of KF707- and LB400-type ISPBPHs. Regions I, II, III, and IV are those designated by Mondello et al. (24). Residues above the LB400 sequence are, according to the work of Mondello et al. (24), residues found at these positions in other LB400-type ISPBPHs; residues below the KF707 sequence are those found at these positions in other KF707-type ISPBPHs. Dashes represent residues that are conserved in all three sequences. Residues of B-356 ISPBPH in boldface type are found in KF707-type ISPBPHs; those in lightface type are found in LB400-type ISPBPHs. Characters in italics are either found in both types or differ from both types.
In a previous investigation, site-directed mutagenesis of the KF707 ISPBPH α subunit at Thr-376 (KF707) (region IV) to Asn-376 (as in LB400) (17) resulted in the expansion of the range of biodegradable PCB congeners, including those requiring a meta-para dioxygenation of the molecule. Furthermore, a combination of mutations at regions III and IV of the LB400 ISPBPH α subunit showed that replacing Asn-377 by Thr-377 (as in KF707) strongly hindered the capacity of the enzyme to catalyze the oxygenation of 2,2′,5,5′-tetrachlorobiphenyl when the amino acid sequence of region III was identical to the one found in the KF707 ISPBPH α subunit. Region III of the B-356 ISPBPH α subunit is very similar to that of KF707, except that Ala-336 of KF707 is replaced in B-356 by Gly-335, which is of similar hydrophobicity (19, 33). Therefore, it was interesting to evaluate the effect of changing Thr-375 of the B-356 ISPBPH α subunit to Asn-375 as in LB400. Based on the investigations cited above, the mutant enzyme was expected to catalyze meta-para hydroxylations. However, when the purified H-t mutant enzyme was assayed with 2,5-dichlorobiphenyl as the substrate, HPLC and GC-MS analyses of the reaction product showed that 2′,3′-dihydro-2′,3′-dihydroxy-2,5-dichlorobiphenyl was the unique metabolite of this reaction (not shown). Furthermore, this mutant was unable to transform 2,2′,5,5′-tetrachlorobiphenyl. Therefore, in spite of the strong structural similarity of designated regions between KF707 and B-356 ISPBPH α subunits, the structural features of the ISPBPH α subunit C-terminal portion that were found to strongly influence KF707 and LB400 BPH dox activity did not influence the substrate reactivity pattern of B-356 BPH dox. Altogether, our data show that other structural features, some of which are associated with the β subunit, also influence enzyme activity toward PCB congeners.
DISCUSSION
In this study we have engineered novel ISPBPH hybrids by replacing the B-356 ISPBPH α or β subunit with the corresponding polypeptide recruited from strain LB400 BPH dox. The ISPBPH chimeras representing the two associations (the αLB400βB-356 and αB-356βLB400 chimeras) were functional.
When the catalytic activities towards various PCBs of the novel chimeric enzymes were compared to those of the parent enzymes, results showed that the structure of the ISPBPH β subunit influences the reactivity pattern of the enzymes as well as their capacity to catalyze the oxygenation of the meta-para carbons. At first glance, these results appear to contradict those obtained by Mondello et al. (24) and Kimura et al. (17), who found that only a small number of amino acid residues located in the carboxy-terminal halves of the KF707 and LB400 ISPBPH α subunits control the enzymes’ reactivities toward PCB congeners. However, it is noteworthy that these studies were carried out with two very closely related dioxygenases. The sequences of the β subunits for LB400 and KF707 ISPBPH differ by a single amino acid residue (7). In the present study, by using two more distantly related BPH doxes, it was possible to show an influence of the structure of the β subunit on the enzyme reactivity pattern. Furthermore, data obtained with the LB400–B-356 chimeric enzymes show that the amino acid residues of the LB400 and KF707 α subunits, which were found in previous investigations to greatly influence the substrate reactivity patterns of LB400 and KF707 BPH dox, did not appear to influence the reactivity patterns of B-356 BPH dox and of LB400–B-356 BPH dox chimeras.
By changing amino acids 335 to 341 (Thr-Phe-Asn-Asn-Ile-Arg-Ile [region III]) of the Pseudomonas sp. strain LB400 ISPBPH α subunit to the sequence Ala-Ile-Asn-Thr-Ile-Arg-Thr as in the P. pseudoalcaligenes KF707 ISPBPH α subunit, Erickson and Mondello (7) obtained mutants which were able to efficiently metabolize the otherwise poorly degraded substrate 4,4′-dichlorobiphenyl, thus broadening the enzyme’s substrate reactivity.
As noticed above, the region III amino acid sequence of the strain B-356 ISPBPH α subunit differs from that of strain KF707 by a single amino acid residue (Fig. 4). Yet, unlike the enzyme of strain KF707, both strain B-356 BPH dox and the chimeric enzyme αB-356βLB400 ISPBPH poorly metabolize 4,4′-dichlorobiphenyl.
Similarly, changing simultaneously Asn-377 and Phe-336 of the LB400 ISPBPH α subunit to Thr-377 and Ile-336 (as in the KF707 α subunit) drastically reduced the capacity of the mutant to catalyze the meta-para oxygenation of the molecule (24). Changing Thr-376 of the KF707 ISPBPH α subunit to Asn-376, as in LB400, resulted in an expansion of the range of biodegradable congeners, including those requiring a meta-para attack. However, although the corresponding position is occupied by a Thr in the strain B-356 α subunit, αB-356βLB400 ISPBPH, unlike KF707 ISPBPH, can transform 2,2′,5,5′-tetrachlorobiphenyl into the 3,4-dihydro-3,4-dihydroxychlorobiphenyl derivative. Furthermore, changing Thr-375 of the B-356 ISPBPH α subunit to Asn-375, as in LB400, did not confer to the mutant the capacity to oxygenate 2,5-dichlorobiphenyl onto meta-para carbons.
Finally, the fact that the substrate selectivity pattern of the αLB400βB-356 chimera differs from that of LB400 and of B-356 BPH dox is further evidence that the structures of both subunits influence the substrate selectivity of the enzyme.
Our data are insufficient to precisely determine the function of the β subunit in BPH dox activity. However, for the first time, we provide clear evidence with purified enzyme preparations that both the α and β subunits of the aryl-hydroxylating dioxygenases influence the enzyme-substrate interaction. The amino acid residues of the α subunit that affect enzyme reactivity in one type of α-β arrangements, that of the αLB400βLB400 arrangement, have no effect on other types of α-β arrangements, such as that of the αB-356βLB400 chimera. To explain these results, it is likely that a catalytic poach is created by the association between the α and β subunits. Structural features of both subunits would then influence the dimension and shape of the catalytic poach to determine which PCBs can be oxygenated as well as the orientations of the adjacent reactive carbons toward the active site. This observation is important in terms of engineering enzymes to increase the range of the catalytic activities toward PCBs. Current investigation in our laboratory aims at identifying the residues of both the large and small ISPBPH subunits which are involved subunit association as well as substrate binding and orientation in the direction of the enzyme’s active site.
ACKNOWLEDGMENT
This work was supported by grant STP0193182 from the Natural Sciences and Engineering Research Council of Canada.
REFERENCES
- 1.Ahmad D, Massé R, Sylvestre M. Cloning and expression of genes involved in 4-chlorobiphenyl transformation by Pseudomonas testosteroni strain B-356: homology to polychlorobiphenyl-degrading genes in other bacteria. Gene. 1990;86:53–61. doi: 10.1016/0378-1119(90)90113-6. [DOI] [PubMed] [Google Scholar]
- 2.Barriault D, Pelletier C, Hurtubise Y, Sylvestre M. Substrate-selectivity pattern of Comamonas testosteroni B-356 towards dichlorobiphenyls. Int Biodeterior Biodegrad. 1997;39:311–316. [Google Scholar]
- 3.Batie C J, Ballou D P, Correll C J. Phatalte dioxygenase reductase and related flavin-iron-sulfur containing electron transferases. In: Müller F, editor. Chemistry and biochemistry of flavoenzymes. Boca Raton, Fla: CRC Press; 1991. pp. 544–554. [Google Scholar]
- 4.Bedard D L, Unterman R, Bopp L H, Brennan M J, Haberl M L, Johnson C. Rapid assay for screening and characterizing microorganisms for the ability to degrade polychlorinated biphenyls. Appl Environ Microbiol. 1986;51:761–768. doi: 10.1128/aem.51.4.761-768.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dulfer W J, Grovers H A J. Solubility and micell-water partitioning of polychlorinated biphenyls in solutions of bile salt micelles. Chemosphere. 1995;30:293–306. doi: 10.1016/0045-6535(94)00390-g. [DOI] [PubMed] [Google Scholar]
- 6.Erickson B D, Mondello F J. Nucleotide sequencing and transcriptional mapping of the genes encoding biphenyl dioxygenase, a multicomponent polychlorinated-biphenyl-degrading enzyme in Pseudomonas strain LB400. J Bacteriol. 1992;174:2903–2912. doi: 10.1128/jb.174.9.2903-2912.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erickson B D, Mondello F J. Enhanced biodegradation of polychlorinated biphenyl after site-directed mutagenesis of a biphenyl dioxygenase gene. Appl Environ Microbiol. 1993;59:3858–3862. doi: 10.1128/aem.59.11.3858-3862.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erickson D E. Analytical chemistry of PCB’s. Boston, Mass: Ann Arbor Science Book, Butterworth; 1986. [Google Scholar]
- 9.Furukawa K, Hayashida S, Taira K. Biochemical and genetic basis for the degradation of polychlorinated biphenyls in soil bacteria. In: Galli E, Silver S, Witholt B, editors. Pseudomonas molecular biology and biotechnology. Washington, D.C: American Society for Microbiology; 1992. pp. 257–267. [Google Scholar]
- 10.Gibson D T, Cruden D L, Haddock J D, Zylstra G J, Brand J M. Oxidation of polychlorinated biphenyls by Pseudomonas sp. strain LB400 and Pseudomonas pseudoalcaligenes KF707. J Bacteriol. 1993;175:4561–4564. doi: 10.1128/jb.175.14.4561-4564.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haddock J D, Gibson D T. Purification and characterization of the oxygenase component of biphenyl 2,3-dioxygenase from Pseudomonas sp. strain LB400. J Bacteriol. 1995;177:5834–5839. doi: 10.1128/jb.177.20.5834-5839.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haddock J D, Horton J R, Gibson D T. Dihydroxylation and dechlorination of chlorinated biphenyls by purified biphenyl 2,3-dioxygenase from Pseudomonas sp. strain LB400. J Bacteriol. 1995;177:20–26. doi: 10.1128/jb.177.1.20-26.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurtubise Y, Barriault D, Sylvestre M. Characterization of active recombinant His-tagged oxygenase component of Comamonas testosteroni B-356 biphenyl dioxygenase. J Biol Chem. 1996;271:8152–8156. doi: 10.1074/jbc.271.14.8152. [DOI] [PubMed] [Google Scholar]
- 14.Hurtubise Y, Barriault D, Powlowski J, Sylvestre M. Purification and characterization of the Comamonas testosteroni B-356 biphenyl dioxygenase components. J Bacteriol. 1995;177:6610–6618. doi: 10.1128/jb.177.22.6610-6618.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang H, Parales R E, Lynch N A, Gibson D T. Site-directed mutagenesis of conserved amino acids in the alpha subunit of toluene dioxygenase: potential mononuclear non-heme iron coordination sites. J Bacteriol. 1996;178:3133–3139. doi: 10.1128/jb.178.11.3133-3139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura N, Kato H, Akito N, Furukawa K. Analysis of substrate range of biphenyl-catabolic enzymes. Biosci Biotechnol Biochem. 1996;60:220–223. doi: 10.1271/bbb.60.220. [DOI] [PubMed] [Google Scholar]
- 17.Kimura N, Nishi A, Goto M, Furukawa K. Functional analysis of a variety of chimeric dioxygenases constructed from two biphenyl dioxygenases that are similar structurally but different functionally. J Bacteriol. 1997;179:3936–3943. doi: 10.1128/jb.179.12.3936-3943.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiyohara H, Torigoe S, Kaida N, Asaki T, Iida T, Hayashi H, Takizawa N. Cloning and characterization of a chromosomal gene cluster, pah, that encodes the upper pathway for phenanthrene and naphthalene utilization by Pseudomonas putida OUS82. J Bacteriol. 1994;176:2439–2443. doi: 10.1128/jb.176.8.2439-2443.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyte J, Doolite R. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Lowry O H, Rosebrough N J, Far A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 22.MacKay D, Mascarenhas P, Shiu W Y. Aqueous solubility of polychlorinated biphenyls. Chemosphere. 1980;9:257–264. [Google Scholar]
- 23.Mason J R, Cammack R. The electron-transport proteins of hydroxylating bacterial dioxygenases. Annu Rev Microbiol. 1992;46:277–305. doi: 10.1146/annurev.mi.46.100192.001425. [DOI] [PubMed] [Google Scholar]
- 24.Mondello F J, Turcich M P, Lobos J H, Erickson B D. Identification and modification of biphenyl dioxygenase sequences that determine the specificity of polychorinated biphenyl degradation. Appl Environ Microbiol. 1997;63:3096–3103. doi: 10.1128/aem.63.8.3096-3103.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neidle E L, Hartnett C, Ornston N, Bairoch A, Rekik M, Harayama S. Nucleotide sequences of the Acinetobacter calcoaceticus benABC genes for benzoate 1,2-dioxygenase reveal evolutionary relationships among multicomponent oxygenases. J Bacteriol. 1991;173:5385–5395. doi: 10.1128/jb.173.17.5385-5395.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 27.Sondossi M, Sylvestre M, Ahmad D. Effects of chlorobenzoate transformation of the Pseudomonas testosteroni biphenyl and chlorobiphenyl degradation pathway. Appl Environ Microbiol. 1992;58:485–495. doi: 10.1128/aem.58.2.485-495.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sylvestre M. Biphenyl/chlorobiphenyls catabolic pathway of Comamonas testosteroni B-356: prospect for use in bioremediation. Int Biodeterior Biodegrad. 1995;34:189–211. [Google Scholar]
- 29.Sylvestre M, Fauteux J. A new facultative anaerobe capable of growth on chlorobiphenyls. J Gen Appl Microbiol. 1982;28:61–72. [Google Scholar]
- 30.Sylvestre M, Hurtubise Y, Barriault D, Bergeron J, Ahmad D. Characterization of active recombinant 2,3-dihydro-2,3-dihydroxybiphenyl dehydrogenase from Comamonas testosteroni B-356 sequence of the encoding gene (bphB) Appl Environ Microbiol. 1996;62:2710–2715. doi: 10.1128/aem.62.8.2710-2715.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sylvestre M, Sirois M, Hurtubise Y, Bergeron J, Ahmad D, Shareck F, Larose A, Barriault D, Guillemette I, Juteau J M. Sequencing of Comamonas testosteroni strain B-356-biphenyl/chlorobiphenyl dioxygenase genes: evolutionary relationships among Gram-negative biphenyl dioxygenases. Gene. 1996;174:195–202. doi: 10.1016/0378-1119(96)00039-x. [DOI] [PubMed] [Google Scholar]
- 32.Wang D, Sul H S. Site-directed mutagenesis for large insertions by oligonucleotide primers in optimized molar ratios. BioTechniques. 1996;22:70–72. doi: 10.2144/97221bm14. [DOI] [PubMed] [Google Scholar]
- 33.Wolfenden R, Andersson L, Cullis P, Southgate C. Affinities of amino acid side chains for solvent water. Biochemistry. 1981;20:849–855. doi: 10.1021/bi00507a030. [DOI] [PubMed] [Google Scholar]