Abstract
Rationale
The effects of high-dose inhaled nitric oxide on hypoxemia in coronavirus disease (COVID-19) acute respiratory failure are unknown.
Objectives
The primary outcome was the change in arterial oxygenation (PaO2/FiO2) at 48 hours. The secondary outcomes included: time to reach a PaO2/FiO2.300mmHg for at least 24 hours, the proportion of participants with a PaO2/FiO2.300mmHg at 28 days, and survival at 28 and at 90 days.
Methods
Mechanically ventilated adults with COVID-19 pneumonia were enrolled in a phase II, multicenter, single-blind, randomized controlled parallel-arm trial. Participants in the intervention arm received inhaled nitric oxide at 80 ppm for 48 hours, compared with the control group receiving usual care (without placebo).
Measurements and Main Results
A total of 193 participants were included in the modified intention-to-treat analysis. The mean change in PaO2/FiO2 ratio at 48 hours was 28.3mmHg in the intervention group and 21.4mmHg in the control group (mean difference, 39.1mmHg; 95% credible interval [CrI], 18.1 to 60.3). The mean time to reach a PaO2/FiO2.300mmHg in the interventional group was 8.7 days, compared with 8.4 days for the control group (mean difference, 0.44; 95% CrI, 23.63 to 4.53). At 28 days, the proportion of participants attaining a PaO2/FiO2.300mmHg was 27.7% in the inhaled nitric oxide group and 17.2% in the control subjects (risk ratio, 2.03; 95% CrI, 1.11 to 3.86). Duration of ventilation and mortality at 28 and 90 days did not differ. No serious adverse events were reported.
Conclusions
The use of high-dose inhaled nitric oxide resulted in an improvement of PaO2/FiO2 at 48 hours compared with usual care in adults with acute hypoxemic respiratory failure due to COVID-19.
Keywords: respiration, artificial; pneumonia; critical illness; viremia; COVID-19; nitric oxide
At a Glance Commentary
Scientific Knowledge on the Subject
Prior clinical trials have shown that low-dose, 1–20 ppm of inhaled nitric oxide (NO) leads to short-term improvement in oxygenation in critically ill patients with acute lung injury. During the severe acute respiratory syndrome outbreak of 2003, low-dose inhaled NO was shown to improve oxygenation. Subsequent laboratory studies have demonstrated that NO inhibited the in vitro replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a dose-dependent manner. This phase II, multicenter, single-blind, randomized controlled parallel-arm trial hypothesized that high-dose (up to 80 ppm) NO would inhibit viral replication and cause sustained improvement in oxygenation in patients with coronavirus disease (COVID-19) with acute hypoxemic respiratory failure.
What This Study Adds to the Field
Compared with usual care, inhaled NO improved oxygenation at 48 hours. Administration of inhaled NO did not reduce mortality, length of mechanical ventilation, or duration of hospital stay. Participants treated with NO experienced a faster reduction of viral load in sputum and blood samples and had a reduced rate of sensory and motor neurologic symptoms. Finally, treatment with NO was well tolerated, and no serious adverse events were recorded. Further studies are required to characterize the antiviral properties of high-dose NO and determine the optimal dosage.
Inhaled nitric oxide (NO), a selective pulmonary vasodilator, was first approved by the U.S. Food and Drug Administration in 1999 for the delivery of 20 ppm in newborns with hypoxemic respiratory failure with persistent pulmonary hypertension (1–3). Subsequently, the use of inhaled NO therapy was expanded to critically ill adult patients with hypoxemic respiratory failure and to postoperative cardiac patients (4, 5). The beneficial effects of inhaled NO therapy have been attributed to its ability to reduce intrapulmonary shunting (6), resulting in improved oxygenation for the first 24 hours of inhalation in mechanically ventilated adult patients with severe acute respiratory distress syndrome (ARDS) (7–10). Despite its well-defined physiological effects and excellent safety profile, inhaled NO up to 20 ppm did not demonstrate efficacy in improving clinical outcomes among adults with ARDS in prior randomized trials (7, 10–13).
Numerous in vitro studies have shown that nitric oxide in solution has dose-dependent bactericidal properties (14, 15) and inhibits viral replication (16, 17). Prior studies used low doses of inhaled NO to facilitate pulmonary vasodilation and improve oxygenation (18). Although the antiviral dose of inhaled NO has not been established, early application of high-dose inhaled nitric oxide (up to 300 ppm) has been shown to sustainably improve systemic oxygenation in nonintubated hospitalized adults and decrease the length of hospitalization in pregnant and pediatric patients with viral and bacterial pneumonia (19, 20). However, the role of high antiviral doses of inhaled NO in improving systemic oxygenation has not been assessed in critically ill patients with COVID-19 requiring mechanical ventilation.
Based on mounting evidence (14, 21–28), this study tested the hypothesis that a high concentration of inhaled NO administered early after the onset of infection, beyond what had been previously evaluated, might be beneficial in critically ill patients with acute hypoxemic respiratory failure due to coronavirus disease (COVID-19) pneumonia. This study was designed to evaluate the effect of inhaled NO on systemic oxygenation after 48 hours among critically ill and mechanically ventilated patients with COVID-19 in a phase II, multicenter, single-blind, randomized (1:1) controlled parallel-arm trial.
Methods
Study Design and Participants
This was an investigator-initiated multicenter, single-blind, randomized (1:1) controlled parallel-arm clinical trial conducted at four sites in the United States and one site in Sweden. The study enrolled adult patients with confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (using RT-PCR) admitted to the ICU who were intubated and mechanically ventilated. Detailed information on the study protocol, inclusion and exclusion criteria, randomization, masking, and consent procedures are available in the online supplement. This study was registered on ClinicalTrials.gov as NCT04306393 (Registered on March 12, 2020). Figure 1 describes patient enrollment and follow-up as per Consolidated Standards of Reporting Trials recommendations.
Figure 1.
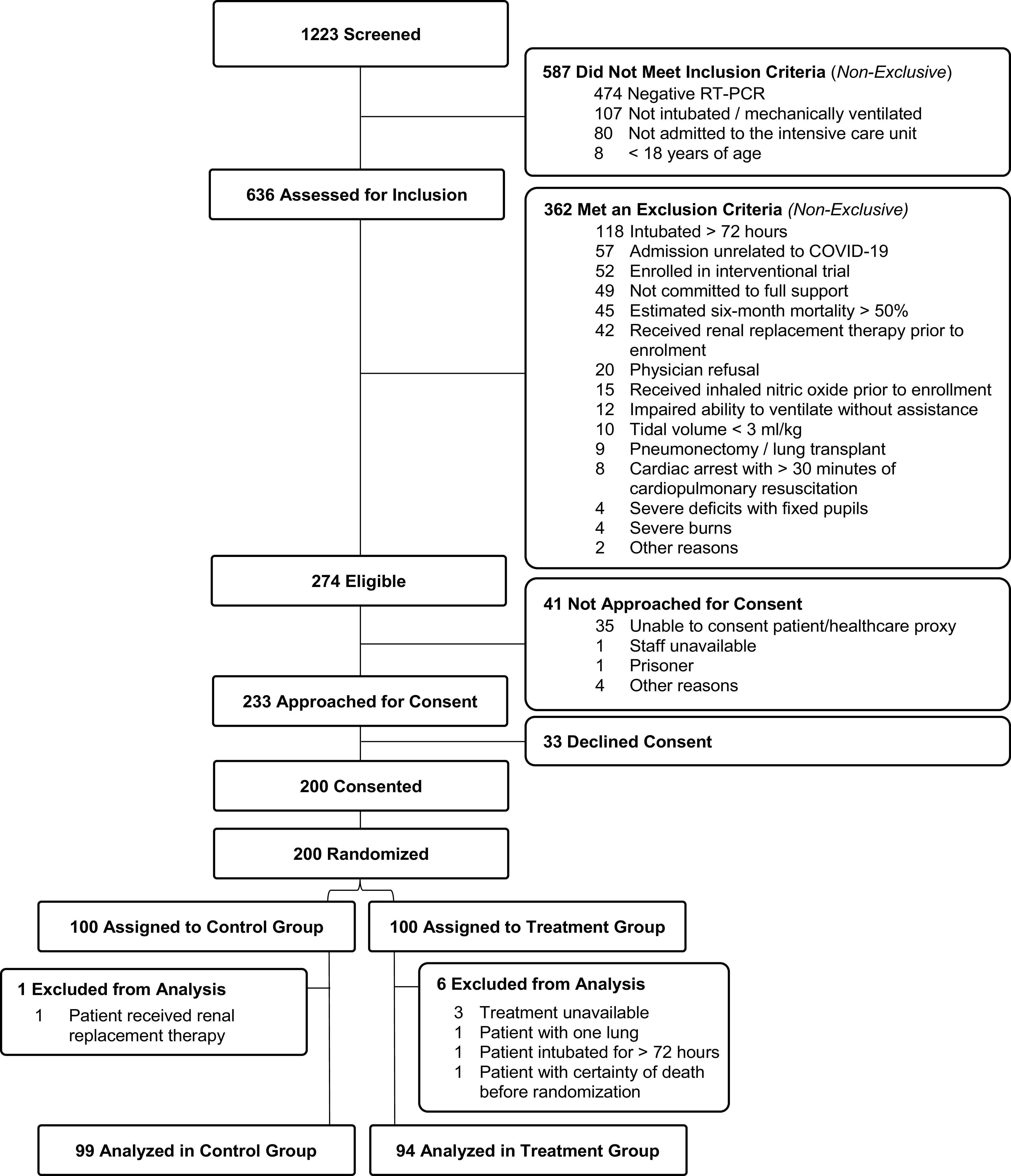
Consolidated Standards of Reporting Trials (CONSORT) diagram. COVID-19 = coronavirus disease.
Procedures
Participants in the treatment arm received inhaled NO at 80 ppm for the first 48 hours after enrollment. The gas was started immediately after randomization within the first 72 hours of mechanical ventilation. After the first 48 hours of treatment, the gas was reduced to 40 ppm and maintained at this concentration until severe hypoxemia resolved (PaO2/FiO2 > 300 mm Hg). The procedures for inhaled NO administration and weaning are described in the online supplement.
Outcomes
The primary outcome of this study was the change in arterial oxygenation (PaO2/FiO2) at 48 hours.
The secondary outcomes were all-cause mortality at 28 and 90 days, time to reach PaO2/FiO2 ratio > 300 mm Hg for at least 24 hours, and the proportion of participants attaining a PaO2/FiO2 ratio > 300 mm Hg in the two groups at 28 days. The safety outcomes for this clinical trial included methemoglobinemia defined as methemoglobin (MetHb) > 5%, inhaled nitrogen dioxide > 3 ppm, hemodynamic instability (rebound hypotension) during weaning, the occurrence of acute kidney injury by 28 days, or the initiation of renal replacement therapy by 90 days. Exploratory study outcomes included change in viral load (log10 copies of SARS-CoV-2 RNA per ml) in plasma and sputum, duration of mechanical ventilation, use of venovenous extracorporeal membrane oxygenation, and neurological signs and symptoms (motor and sensory) at 90 days. The 90-day follow-up procedures and the preparation of plasma and sputum samples for measurement are described in the online supplement. To describe oxygenation beyond the PaO2/FiO2 ratio, saturation of oxygen (SaO2), alveolar–arterial oxygenation gradient, and ventilatory ratio were analyzed and presented as exploratory outcomes.
Statistical Analysis
Participants randomized to inhaled NO were hypothesized to have at least 20% greater improvement in PaO2/FiO2 at 48 hours after gas initiation compared with the usual care alone (29). Assuming a two-tailed α of 0.05, the enrollment of 182 participants would provide 90% power to detect an effect size of 38 mm Hg PaO2/FiO2 change based on the effect estimates in a previous investigation in hypoxemic intubated and mechanically ventilated patients. Presuming a 10% dropout, the target sample size was 100 in each group (n = 200 total). The target sample size was 100 in each group (n = 200 total).
The baseline characteristics were summarized as the median and interquartile range (IQR) for continuous data and counts and percentages for categorical data. Standardized mean difference (SMD) is reported to quantify the differences between the two study arms, with values greater than 0.20 suggesting a potential imbalance between groups.
The primary and secondary outcomes analysis was conducted using a Bayesian framework that estimates the treatment effect conditional on prespecified variables defined a priori (age, age2, sex, body mass index, and Acute Physiology and Chronic Health Evaluation [APACHE] II score). In addition, a sensitivity analysis was conducted including the prespecified covariates and variables with SMD > 0.20 (race, study site, hypertension, diabetes, malignancy, and liver disease) (see Table E1 in the online supplement) To assess the primary outcome, the PaO2/FiO2 ratio at 48 hours was regressed on baseline PaO2/FiO2 ratio, randomized group assignment, and additional covariates, as specified above. Time to reach PaO2/FiO2 ratio > 300 mm Hg was evaluated using the Kaplan-Meier method. All study outcomes were analyzed in the modified intention-to-treat population. All statistical analyses were performed using R 4.0.2 (R Core Team) with Bayesian estimation conducted in RStan. The statistical analysis and the detailed statistical analysis plan are described in the online supplement.
Results
Patient Characteristics
The study enrolled 200 participants with respiratory failure due to SARS-CoV-2 between March 2020 and May 2022 (Figure 1). Subject recruitment occurred between March 22, 2020, and May 21, 2021, with the final follow-up on June 15, 2022. The primary modified intention-to-treat analysis included 193 participants who met inclusion criteria and did not meet exclusion criteria. The study cohort had a median age of 62 (IQR, 50–70) years and included 33.7% females; 51.8% identified as White and 29.5% as Hispanic. Baseline clinical and demographic characteristics were balanced between the study arms except for (SMD > 0.20) APACHE II score, hypertension, diabetes, malignancy, liver disease, connective tissue disease, smoking history, race, and creatinine (Table 1). The baseline PaO2/FiO2 ratio was 177 (IQR, 125–241) mm Hg in the treatment arm and 195 (IQR, 120–235) mm Hg in the control arm. Ventilator settings and adjunctive therapies are presented in Table 2 and Table E2, respectively. On December 2, 2020, the data safety monitoring board (DSMB) noted a difference in the primary outcome of the study between the two groups. However, the stopping rule (defined as a significant increase in mortality in the NO group) was not met. Thus, the trial continued to complete enrollment.
Table 1.
Baseline Demographics and Clinical Characteristics of All the Enrolled and Randomized Intubated Patients with COVID-19 Who Were Included in the Modified Intention-to-Treat Analysis
| Treatment Group (n = 94) |
Control Group (n = 99) |
SMD | |
|---|---|---|---|
| Age | 64 (53.0–70.0) | 62 (50.0–69.5) | 0.142 |
| Sex, female | 31 (33.0) | 34 (34.3) | 0.029 |
| Race | 0.282 | ||
| American Indian/Alaska Native | 1 (1.1) | 0 (0.0) | |
| Asian | 6 (6.4) | 7 (7.1) | |
| Black/African American | 23 (24.5) | 20 (20.2) | |
| Other | 14 (14.9) | 11 (11.1) | |
| Unknown | 1 (1.1) | 0 (0.0) | |
| White | 49 (52.1) | 61 (61.6) | |
| Hispanic or Latino ethnicity | 27 (28.7) | 30 (30.3) | 0.035 |
| BMI, kg/m2 | 31.0 (26.9–35.8) | 30.2 (26.8–35.4) | <0.001 |
| Smoking history | 0.202 | ||
| Current smoker | 4 (4.3) | 6 (6.1) | |
| Former smoker | 25 (26.6) | 30 (30.3) | |
| Never smoked | 49 (52.1) | 42 (42.4) | |
| Unknown | 16 (17.0) | 21 (21.2) | |
| Hypertension | 63 (67.0) | 46 (46.5) | 0.424 |
| History of myocardial infarction | 13 (13.8) | 11 (11.1) | 0.082 |
| Diabetes | 39 (41.5) | 29 (29.3) | 0.257 |
| Cerebrovascular disease | 5 (5.3) | 8 (8.1) | 0.111 |
| Chronic kidney disease | 10 (10.6) | 8 (8.1) | 0.088 |
| COPD | 4 (4.3) | 8 (8.1) | 0.160 |
| Connective tissue disease | 6 (6.4) | 1 (1.0) | 0.288 |
| Dementia | 4 (4.3) | 3 (3.0) | 0.065 |
| Hemiplegia | 4 (4.3) | 0 (0.0) | 0.065 |
| Immune deficiency | 5 (5.3) | 3 (3.0) | 0.115 |
| Liver disease | 8 (8.5) | 0 (0.0) | 0.431 |
| History of malignancy | 7 (7.4) | 0 (0.0) | 0.401 |
| History of peptic ulcer | 3 (3.2) | 4 (4.0) | 0.045 |
| ARDS class | |||
| COVID-19, PaO2/FiO2 300–400 mm Hg | 9 (9.6) | 5 (5.1) | 0.170 |
| Mild ARDS, PaO2/FiO2 200–300 mm Hg | 28 (29.8) | 36 (36.4) | 0.140 |
| Moderate ARDS, PaO2/FiO2 100–200 mm Hg | 43 (45.7) | 37 (37.4) | 0.170 |
| Severe ARDS, PaO2/FiO2 < 100 mm Hg | 14 (14.9) | 21 (21.2) | 0.165 |
| APACHE II score, mean (SD) | 24.6 (7.7) | 21.1 (6.5) | 0.499 |
| SOFA score | 8.5 (7–11) | 8 (7–10) | 0.100 |
| Compliance, ml/cm H2O, mean (SD) | 36.7 (18.7) | 36.8 (15.6) | 0.004 |
| PEEP, cm H2O | 12 (10–14) | 12 (10–14) | 0.019 |
| Vt, ml/kg, mean (SD) | 4.7 (1.5) | 4.7 (1.4) | 0.009 |
| PaO2/FiO2 ratio, mm Hg | 177 (125–241) | 195 (120–235) | 0.066 |
| FiO2, mean (SD) | 0.59 (0.21) | 0.61 (0.21) | 0.110 |
| PaCO2, mm Hg, mean (SD) | 42 (37–47) | 43 (39–50) | 0.228 |
| e, L/min | 8.7 (7.3–10.5) | 8.5 (7.2–9.7) | 0.186 |
| Creatinine, mg/dl | 1.08 (0.84–1.96) | 0.99 (0.72–1.42) | 0.261 |
| D-dimer | 2492 (1,414–5,377) | 1,815 (1,014–5,018) | 0.200 |
Definition of abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation; ARDS = acute respiratory distress syndrome; BMI = body mass index; COPD = chronic obstructive pulmonary disease; COVID-19 = coronavirus disease; PEEP = positive end-expiratory pressure; SMD = standardized mean difference; SOA = Sequential Organ Failure Assessment.
Data are presented as median (interquartile range) or n (%) unless otherwise noted. Vt has been calculated for the ideal body weight (ml/kg). Patients with COVID-19 pneumonia and impaired PaO2/FiO2 but with PaO2/FiO2 between 300 and 400 mm Hg are listed as “COVID-19 with PaO2/FiO2 300–400 mm Hg.” Patients with ARDS are classified as mild PaO2/FiO2 200–300 mm Hg, moderate PaO2/FiO2 100–200 mm Hg, and severe PaO2/FiO2 < 100 mm Hg according to the Berlin Definition.
Table 2.
Ventilator Settings at Baseline, 24 Hours, and 48 Hours
| Variables | Baseline |
24 Hours |
48 Hours |
|||
|---|---|---|---|---|---|---|
| Treatment Group | Control Group | Treatment Group | Control Group | Treatment Group | Control Group | |
| PaO2/FiO2 ratio, mm Hg | 177 (125–241) | 195 (120–235) | 196 (150–252) | 188 (130–263) | 200 (157–239) | 183 (122–235) |
| FiO2, % | 51.5 (40–70) | 60 (42.5–75) | 45 (36–57) | 50 (40–60) | 45 (38–60) | 50 (40–60) |
| PEEP, cm H2O | 12 (10–14) | 12 (10–14) | 12 (10–14) | 12 (10–14) | 12 (10–13) | 12 (10–14) |
| Plateau pressure, cm H2O | 24 (21–28) | 23 (21–27) | 24 (21–26) | 23 (20–26) | 24 (21–26) | 24 (20–26) |
| Respiratory system compliance, ml/cm H2O | 31.7 (24.1–37.5) | 32.0 (27.0–40.0) | 32.0 (25.5–41.9) | 32.0 (27.0–41.0) | 31.0 (25.0–38.0) | 35.0 (27.0–42.0) |
| Respiratory rate, breaths per min | 22 (20–25) | 22 (18–25) | 22 (20–25) | 23 (19–26) | 22 (18–26) | 24 (20–27) |
| Vt/IBW, ml/kg | 6.0 (6–6.8) | 6.0 (5.6–6.7) | 6.1 (5.6–6.6) | 6.0 (5.4–6.5) | 6.0 (5.5–6.5) | 5.9 (5.5–6.5) |
| e, L/min | 8.8 (7.1–10.1) | 8.4 (7.2–9.9) | 8.4 (7.5–10.1) | 8.7 (7.3–9.9) | 8.6 (7.0–10.2) | 8.7 (7.4–10.4) |
| Use of neuromuscular blockade | 53 (56) | 45 (45) | 46 (49) | 39 (39) | 46 (49) | 30 (30) |
| Lifting sedation | 0 (0) | 4 (4) | 0 (0) | 3 (3) | 1 (1) | 3 (3) |
Definition of abbreviations: IBW = ideal body weight; PEEP = positive end-expiratory pressure.
Data are presented as median (interquartile range) or n (%).
Primary Outcome
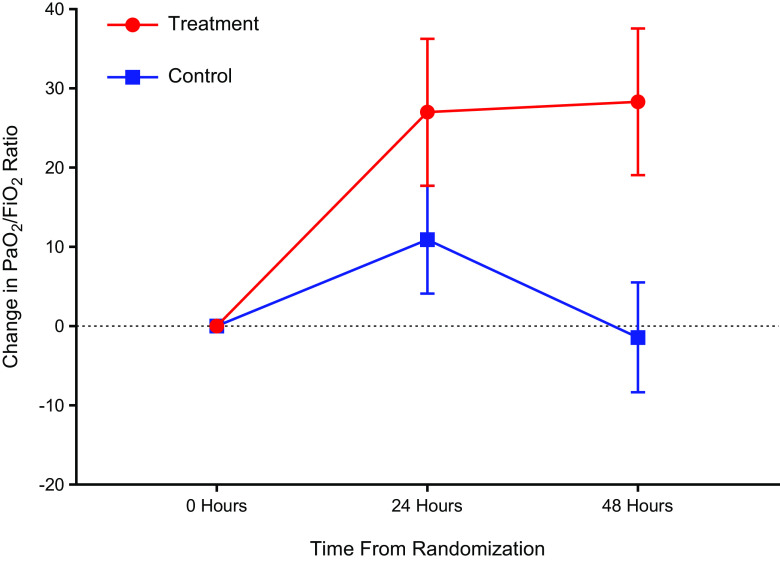
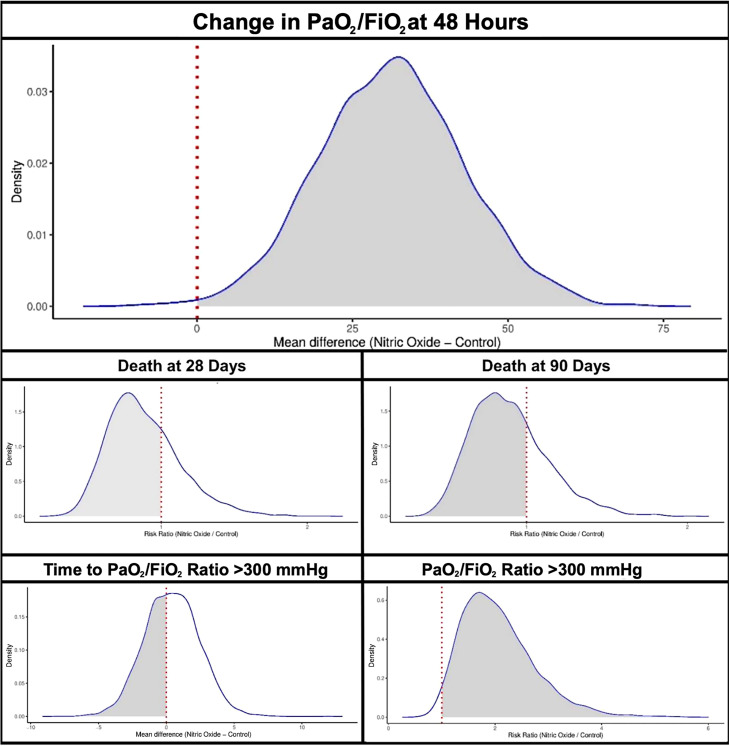
The mean change in PaO2/FiO2 at 48 hours in the inhaled NO arm was 28.3 (89.3) mm Hg and −1.4 (68.9) mm Hg in the usual care arm. The change in PaO2/FiO2 from baseline to 48 hours was 39.1 (95% credible interval [CrI], 18.1 to 60.3) higher in the treatment arm compared with the usual care arm (Table 3 and Figure 2). Inhaled NO therapy had a 99.5% probability of increasing the PaO2/FiO2 at 48 hours (Figure 3). The probability of inhaled NO therapy improving the PaO2/FiO2 at 48 hours using various thresholds is described in Table E3.
Table 3.
Primary and Secondary Outcomes in the Final Analysis Population
| Treatment Group (n = 94) |
Control Group (n = 99) |
Difference or RR (95% CrI) |
|
|---|---|---|---|
| Primary endpoint: change in PaO2/FiO2 ratio at 48 h, mm Hg | |||
| Overall population | 28.3 (89.3) | −1.4 (68.9) | 39.1 (18.1 to 60.3) |
| Stratified by baseline PaO2/FiO2 ratio | |||
| <100 mm Hg | 85.9 (72.1) | 31.0 (44.6) | 50.6 (5.1 to 95.6) |
| 100–200 mm Hg | 34.6 (74.1) | 10.2 (53.2) | 32.5 (1.9 to 63.1) |
| ⩾200 mm Hg | −0.6 (101.9) | −28.5 (80.9) | 27.6 (−16.5 to 72.3) |
| Secondary endpoints | |||
| Mortality within 28 d, n (%) | 27 (28.7) | 27 (27.3) | RR, 0.85 (0.50 to 1.46) |
| Mortality within 90 d, n (%) | 32 (34.0) | 32 (32.3) | RR, 0.87 (0.52 to 1.43) |
| Time to PaO2/FiO2 ratio > 300 mm Hg, d* | 8.7 (5.0) | 8.4 (6.5) | 0.44 (−3.63 to 4.53) |
| Patients reaching PaO2/FiO2 ratio > 300 mm Hg, n (%)* | 33 (35.1) | 21 (21.2) | RR, 2.03 (1.11 to 3.86) |
Definition of abbreviations: CrI = credible interval; RR = risk ratio.
Data are presented as mean (SD) unless otherwise noted.
Measured over 28 days after randomization in survivors with baseline PaO2/FiO2 < 300 mm Hg, as prespecified (67 patients in the treatment group and 72 in the control group).
Figure 2.
Systemic oxygenation at baseline, 24 hours, and 48 hours. This figure depicts the mean change in the PaO2/FiO2 ratio from baseline to 24 and 48 hours from the time of randomization. The treatment group (n = 94) and the control group (n = 99) have been depicted in red and blue, respectively. The data are represented as mean (point) and SEM (error bars).
Figure 3.
Posterior probability curves for the association of study outcomes with inhaled nitric oxide therapy.
Secondary Outcomes
The mean time to reach a sustained PaO2/FiO2 ratio > 300 mm Hg in survivors was 8.7 (5.0) days in the treatment group versus 8.4 (6.5) days in the usual care arm. Among survivors, the probability that inhaled NO therapy would decrease the time to PaO2/FiO2 ratio > 300 mm Hg was 42.9% compared with the usual care arm (mean difference, 0.44; 95% CrI, −3.63 to 4.53). At 28 days, the overall proportion of participants with a PaO2/FiO2 ratio > 300 mm Hg was 27.7% in the inhaled NO group and 17.2% in the control subjects, respectively. There was a 98.1% probability that inhaled NO therapy would increase the chance of attaining a PaO2/FiO2 ratio > 300 mm Hg (risk ratio [RR], 2.03; 95% CrI, 1.11 to 3.86; Table 3 and Figure E1). In the inhaled NO arm, the proportion of deaths within 28 days and 90 days was 28.7% and 34.0%, respectively. In the usual care arm, the proportion of deaths within 28 days and 90 days was 27.3% and 32.3%, respectively. Participants randomized to the inhaled NO group had a 71.9% and 71.4% probability of having a lower risk of death at 28 days and 90 days, respectively, compared with usual care alone (RR for 28-day mortality, 0.85; 95% CrI, 0.50 to 1.46; RR for 90-day mortality, 0.87; 95% CrI, 0.52 to 1.43).
The posterior probability curves of the secondary outcomes with inhaled NO therapy have been depicted in Figure 3.
Safety and Adverse Outcomes
High-dose inhaled NO therapy was well tolerated, with no serious adverse events related to inhaled NO reported (Table 4). The median duration of inhaled NO therapy of ⩾20 ppm was 10.8 days (IQR, 5.1–16.3 days). MetHb exceeded the threshold of 5% eight times during the 1,282 inhaled NO–treatment days. In five of these events, a dose reduction of inhaled NO by 50% was required to achieve an appropriate reduction in MetHb under 5%. The inhaled nitrogen dioxide reached 3 ppm on one occasion and rapidly decreased upon reduction of inhaled NO from 80 ppm to 40 ppm. No events of hemodynamic instability or rebound pulmonary hypertension were reported during the inhaled NO treatment and subsequent weaning of inhaled NO. NO did not increase the risk for acute kidney injury (RR, 0.82; 95% CrI, 0.39–1.70) or the need for renal replacement therapy (RR, 1.65; 95% CrI, 0.78–3.56).
Table 4.
Safety Outcomes in the Final Analysis Population Assigned to Treatment
| Treatment Group (n = 94) |
Control Group (n = 99) |
Difference or RR (95% CrI) | |
|---|---|---|---|
| Safety outcomes | |||
| Acute kidney injury | 65 (69.1) | 69 (69.7) | RR, 0.82 (0.39–1.70) |
| Class 1 | 17 (18.1) | 20 (20.2) | |
| Class 2 | 11 (11.7) | 22 (22.2) | |
| Class 3 | 37 (39.3) | 27 (27.2) | |
| RRT | 33 (35.1) | 22 (22.2) | RR, 1.65 (0.78–3.56) |
| Hemodynamic instability during weaning | 0 (0) | ||
| MetHb > 5% | |||
| Events/treatment days overall | 8/1,282 | ||
| Events/treatment days at 80 ppm | 7/292 | ||
| Events requiring dose reduction | 5 | ||
| MetHb highest daily level, % | |||
| Overall | 1.4 (0.7–1.5) | ||
| At 80 ppm | 2.2 (1.5–3.0) | ||
| NO2 > 3 ppm | |||
| Events/treatment days overall | 1/1,282 | ||
| Events/treatment days at 80 ppm | 1/292 | ||
| Events requiring dose reduction | 1 | ||
| NO2 highest daily level, ppm | |||
| Overall | 0.8 (0.0–1.0) | ||
| At 80 ppm | 1.0 (1.0–1.8) | ||
Definition of abbreviations: CrI = credible interval; MtHb = methemoglobin; RR = risk ratio; RRT = renal replacement therapy.
Data are presented as median (interquartile range), n, or n (%).
Exploratory Outcomes
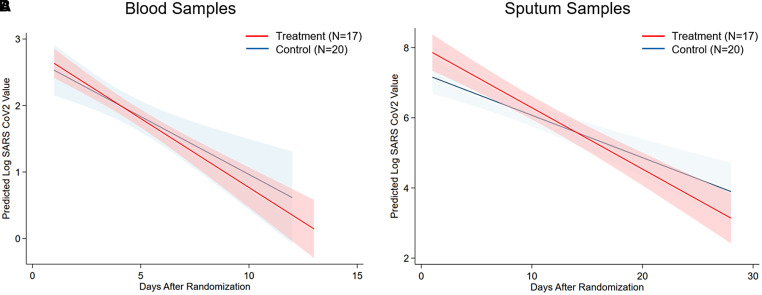
For quantitative SARS-CoV-2 viral load testing, plasma was collected serially for 2 weeks from 37 patients (17 in the treatment group and 20 in the control group), for a total of 145 samples (68 from participants enrolled in the treatment group and 77 from participants enrolled in the control group). Patient characteristics are listed in Table E4. Sputum was collected for up to 7 weeks from 37 participants (17 in the treatment group and 20 in the control group), for a total of 82 samples (38 from participants in the treatment group and 44 from participants in the control group). The median viral loads in the first plasma samples obtained after randomization did not differ between study arms: 2.6 log10 RNA copies/ml (IQR, 2.2 to 3.4 log10 RNA copies/ml) in the treatment group versus 2.8 log10 RNA copies/ml (IQR, 1.8 to 3.7 log10 RNA copies/ml) in the control group. Similarly, the median viral loads when comparing the first sputum samples obtained after randomization were similar in the study groups: 7.6 log10 RNA copies/ml (IQR, 6.0 to 9.0 log10 RNA copies/ml) in the treatment group versus 6.9 log10 RNA copies/ml (IQR, 5.9 to 8.0 log10 RNA copies/ml) in the control group. Over time, there was a steeper decline in plasma viral load (change per unit time, −0.21; 95% CrI, −0.25 to −0.17; group differences, −0.30; 95% CrI, −1.00 to 0.42) in patients enrolled in the inhaled NO arm compared with those in the control arm (time × group estimate, −0.04; 95% CrI, −0.12 to 0.04; Figure 4). Similarly, among the subset of patients from whom sputum samples were taken, there was a greater decline in viral load over time (change per unit time, −0.13; 95% CrI, −0.16 to −0.11; group differences, 0.29; 95% CrI, −0.87 to 1.44) in the treatment arm compared with the control arm (time × group estimate, −0.04; 95% CrI, −0.09 to 0.01).
Figure 4.
Blood and sputum viral count. (A and B) Predicted log10 of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral load by PCR over time in blood (A) and sputum (B). (A) Treatment group (n = 17 patients; 68 samples) in red, and control group (n = 20 patients; 77 samples) in blue. (B) Treatment group (n = 17 patients; 38 samples) in red, and control group (n = 20 patients; 44 samples) in blue.
In the exploratory analysis, although the duration of mechanical ventilation and the use of venovenous extracorporeal membrane oxygenation were not different between the two groups, the frequency of neurological signs and symptoms in the inhaled NO group at 90 days was lower compared with the usual care group (4.2% and 17.2%, respectively; RR, 0.17; 95% CrI, 0.04–0.62; Table 5). Compared with usual care, participants in the treatment group with inhaled NO demonstrated fewer sensory symptoms (0% vs. 14.1%; RR, 0.01; 95% CrI, 0.00–0.12; Table 5). Detailed motor and sensory findings from notes by a physician caring for the patient are listed in Table E5.
Table 5.
Exploratory Outcomes in the Final Analysis Population
| Exploratory Outcomes | Treatment Group (n = 94) |
Control Group (n = 99) |
Difference or RR (95% CrI) |
|---|---|---|---|
| Requirement for VV-ECMO | 4 (4.2) | 5 (5.0) | RR, 0.70 (0.14 to 3.39) |
| Neurological signs and symptoms (Day 90)* | 4 (4.2) | 17 (17.2) | RR, 0.17 (0.04 to 0.62) |
| Motor | 4 (4.2) | 12 (12.1) | RR, 0.36 (0.08 to 1.42) |
| Sensory | 0 (0.0) | 14 (14.1) | RR, 0.01 (0.00 to 0.12) |
| Ventilator time, h, mean (SD) | 447.1 (225.4) | 448.6 (962.4) | 33.73 (−187.52 to 254.18) |
| Mean Difference (95% CrI) | |||
|---|---|---|---|
| Change in A–a gradient at 48 h | −28.8 (−59.8 to 1.0) | ||
| Change in SaO2 at 48 h | 0.12 (0.00 to 0.24) | ||
| Change in ventilatory ratio at 48 h | −0.10 (−0.30 to 0.09) |
Definition of abbreviations: CrI = credible interval; RR = risk ratio; VV-ECMO = veno-venous extracorporeal membrane oxygenation.
Data are presented as n (%) unless otherwise noted.
Measured at 90 days after randomization in survivors: 62 patients in the treatment group and 67 in the control group.
Change in the SaO2, alveolar–arterial oxygenation gradient, and ventilatory index at 48 hours with inhaled NO therapy are presented in Table 5, and a subgroup analysis stratified for PaO2/FiO2 is presented in Table E6.
Discussion
This investigator-initiated, phase II, multicenter, single-blind, randomized controlled parallel-arm trial showed that high-dose inhaled NO improved systemic oxygenation in mechanically ventilated critically ill participants with acute hypoxemic respiratory failure due to COVID-19 pneumonia. The median PaO2/FiO2 ratio increased from 177 (IQR, 125–241) mm Hg to 200 (IQR, 157–239) mm Hg in the treatment arm but decreased from 195 (IQR, 120–235) mm Hg to 183 (IQR, 122–235) mm Hg in the control arm. Compared with the usual care group, a larger proportion of participants in the inhaled NO group reached PaO2/FiO2 > 300 mm Hg for at least 24 hours at 28 days, but the time to attain the level of oxygenation was similar. Furthermore, although there was no difference in mortality or other exploratory clinical outcomes, participants who received inhaled NO had a lower occurrence of sensory symptoms than those who received usual care alone 90 days after randomization.
Prior evidence from a meta-analysis combining four randomized controlled trials demonstrated that inhaled NO therapy in patients with ARDS was associated with an increased risk of acute kidney injury (AKI) (30). However, this large contemporary randomized controlled trial of critically ill mechanically ventilated patients with ARDS showed that the incidence of AKI was high, but similar, in both arms of the study. Thus, we cannot conclude whether NO reduces or increases the risk of AKI and reduces or increases the need for kidney replacement therapy. The high incidence of AKI may be secondary to ARDS and COVID-19 infection. The increased risk of AKI due to COVID-19 has been attributed to direct cytotoxicity, microvascular thrombosis, and endothelial dysfunction (31). Inhaled NO therapy was not associated with an increased risk of any adverse events, including AKI and the need for renal replacement therapy. However, the present trial does not eliminate the possibility that NO therapy could be potentially nephrotoxic because of the relatively small number of participants. Future larger trials are needed to evaluate the renal toxicity of high doses of early administration of inhaled NO. All participants in the treatment group tolerated the administration and weaning of inhaled NO. Although there were eight events of MetHb > 5% and one with inhaled nitrogen dioxide > 3 ppm, reduction of inhaled NO led to the resolution of these abnormalities.
In multiple randomized clinical trials conducted more than 2 decades ago (7, 8, 10–13), inhaled NO between 0.01 and 20 ppm was shown to improve systemic oxygenation in adult patients with ARDS, presumably because of decreased intrapulmonary shunting (3). However, the previous studies showed that oxygenation improved at 24 hours but not at 48–72 hours after initiation of inhaled NO therapy. In contrast, in the current trial, a sustained improvement in systemic oxygenation was noted in the NO group at least up to 28 days after initiation of inhaled NO in patients with respiratory failure due to COVID-19 pneumonia. The reasons for this observed discordance may include the implementation of protective lung ventilation in this trial, the depletion of NO synthesis because of widespread injury of the endothelium caused by the viral infection, and the antiviral effects of high-dose NO. Furthermore, a homogenous population of patients with acute hypoxic respiratory failure due to COVID-19 was included in the current investigation instead of the numerous heterogeneous etiologies of ARDS in prior investigations.
To date, randomized trials on inhaled NO preceded the implementation of the 2000 ARDS Network (ARDSnet) ventilatory strategies for acute hypoxemic respiratory failure (32) and used high Vt and high airway pressure, which likely induced lung injury. A large randomized trial demonstrated that such a ventilatory approach itself results in lung injury leading to death (32). In contrast, patients enrolled in this trial received low Vt and low airway pressure ventilation according to the ARDSnet tables for mechanical ventilation. The avoidance of injurious ventilation in this trial may have unmasked beneficial effects of inhaled NO and markedly prolonged the improvement in oxygenation compared with the pre-ARDSnet NO trials. This is reminiscent of the trial results on prone positioning (33–35) and ECMO (36, 37) in patients with respiratory failure.
At the pathophysiological level, COVID-19 pneumonia is characterized by severe endothelial injury with widespread thrombosis and microangiopathy of the pulmonary vessels (37), resulting in profound perfusion abnormalities seen in dual-energy computed tomography imaging studies (38). In an autopsy study, Villalba and colleagues compared histological parenchymal and vascular alterations of patients deceased for respiratory failure due to COVID-19 pneumonia to those for other etiologies (38). Lungs of patients with COVID-19 showed increased pulmonary congestion and aberrant alveolar-septal congestion (38). Administration of inhaled NO might replete the NO deficiency observed in patients with COVID-19 (39). Bypassing the dysfunctional endothelium, inhaled NO may directly alleviate intrapulmonary shunting and improve pulmonary blood flow, resulting in sustained improvements in oxygenation. Moreover, the observed improved ventilatory ratio indicates a reduction of alveolar dead space, possibly due to the antiplatelet or antileukocyte adhesion properties of NO (39, 40).
In a subset of participants with daily sputum and plasma sampling for quantitative SARS-CoV-2 viral load estimation, the use of inhaled NO was associated with faster clearance of viremia and a more rapid viral load reduction in the sputum. This antiviral property of NO may have contributed to the sustained improvement in systemic oxygenation observed in this trial. Because SARS-CoV-2 viral load is associated with increased disease severity and mortality (41, 42), faster reduction of viral load by inhaled NO is expected to decrease the disease severity of pneumonia and improve oxygenation. Previous in vitro studies showed that the antiviral or antibacterial effects of NO are dose dependent. For example, laboratory studies showed that NO directly inhibits SARS-CoV-2 replication by nitrosating viral membrane proteins and hindering SARS-CoV-2 viral protease in a dose–response manner (16). A recent phase III randomized trial showed that, compared with a placebo, repeated NO nasal spray administrations reduced SARS-CoV-2 viral load from the nasal cavity (43). Antiviral activity of NO has also been demonstrated against influenza, Coxsackie, and SARS-CoV-1 (17, 23, 44). Although the concentrations of inhaled NO that exert antimicrobial effects are unknown, studies have shown that high-dose inhaled NO (up to 300 ppm) is well tolerated and improves respiratory function in hospitalized adults and decreases the length of hospitalization in pregnant patients and pediatric patients with viral and bacterial pneumonia (19–22, 45).
Experimental evidence in animals and recent human studies suggests that SARS-CoV-2 infection causes neuroinflammation and neuronal damage (46, 47). Furthermore, accumulating evidence points to an increased risk of long-term neurologic disorders in people who had COVID-19 (48). In the current study, participants receiving inhaled NO had reduced rates of sensory findings at 90 days. Inhaled NO has been shown to elicit systemic antiinflammatory and antithrombotic responses, which may explain our findings (49). Further studies are needed to investigate the mechanisms and effects of inhaled NO on neurological outcomes, as persistent neurological deficits are a major driver of healthcare burden in survivors of ARDS and severe COVID-19 infection (50, 51).
This study presents some limitations that warrant discussion. First, this study was a relatively small phase II trial that was not powered to test whether NO exposure reduces mortality. Nevertheless, the positive findings and the pragmatic design of this multicenter study pave the way for larger and more extensive phase III clinical trials evaluating the effects of high-dose inhaled NO on mortality. Second, healthcare providers were not blind, and the control (usual care) group lacked a placebo intervention. This was done to protect healthcare workers from an increased risk of COVID-19 exposure. The trial started in March 2020, when no vaccines were available and disconnection of respiratory tubing from the ventilator could expose healthcare workers to contaminated respiratory equipment and aerosolization. Thus, in agreement with the investigational review board at our institutions, the trial was designed without a placebo and with an absence of blinding. Similarly, baseline levels of right heart dysfunction measured by transthoracic echocardiography were not obtained, to minimize healthcare workers’ exposure to COVID-19. A third limitation of this study is that the trial enrolled exclusively critically ill participants with COVID-19 pneumonia, limiting the generalizability of the results to other causes of acute hypoxemic respiratory failure. Differently from most critical care trials in respiratory failure and, specifically, from prior inhaled NO trials, this investigation included participants with a singular etiology of hypoxemic respiratory failure (i.e., COVID-19 pneumonia). Enrolling such a well-defined population allowed us to avoid heterogeneity from other mechanisms of respiratory failure. It enabled the characterization of the effects of inhaled NO in this specific patient population. Future studies are required to evaluate the benefits of inhaled NO therapy in other patient populations. Fourth, the time from the onset of first symptoms of COVID-19 to the time of intubation and the use (and duration) of noninvasive ventilation and high flow were not recorded, and the protocol of the study allowed intensivists to implement the local guideline recommendations on COVID-19 ARDS to care for the patients enrolled in the study. Hence, the trial protocol did not mandate the optimization of positive end-expiratory pressure before enrollment or the use of recruitment maneuvers. Fifth, the formation of MetHb during inhaled nitric oxide treatment might decrease the oxygen-carrying capacity, which may offset the improvement in the PaO2/FiO2 ratio (52). To address this concern, this study also measured changes in SaO2 from the arterial blood samples at 48 hours, which was similar to usual care. Sixth, this study did not investigate a concentration >80 ppm of inhaled NO. Other studies have shown that up to 300 ppm of inhaled NO is safe and decreases the length of stay in patients with viral pneumonia. This was observed in both COVID-19 (19) and respiratory syncytial virus pneumonia (20). The role of high-dose NO as a therapeutic for respiratory infections needs further investigation. Finally, the impact of inhaled NO on the length of ICU and hospital stay could not be accurately evaluated in this study. During the pandemic, ICUs and hospital floors underwent major modifications. Many regular hospital floors were transformed into ICUs to allow caring for intubated and mechanically ventilated patients. Patients were discharged directly from the ICU to their homes, whereas others were discharged to improvised facilities where patients were allowed to recover until they tested negative for COVID-19. The above conditions made it impossible to compare ICU and hospital stay between groups.
Conclusions
In mechanically ventilated critically ill participants with acute hypoxemic respiratory failure due to COVID-19 pneumonia, high-dose inhaled NO at 80 ppm for the first 48 hours of mechanical ventilation improved PaO2/FiO2 compared with the use of usual care alone. The treatment with inhaled NO did not reduce mortality or duration of mechanical ventilation, but exploratory results suggest that participants with inhaled NO had a steeper reduction in plasma viral load and reduced rates of sensory neurologic symptoms and signs at 90 days. Finally, treatment with inhaled NO was well tolerated, and no serious adverse events related to the intervention were reported. Overall, the findings highlight the importance of planning future dose–response investigations into the antimicrobial and clinical properties of high-dose inhaled NO therapy in adults with acute hypoxemic respiratory failure.
Acknowledgments
Acknowledgment
The authors thank the participants, site staff, site investigators, and the entire Nitric Oxide Study Team. This work is dedicated to the recently deceased Prof. Warren M. Zapol, M.D., Prof. Robert M. Kacmarek, Ph.D., and Prof. Goran Hedenstierna, M.D., members of the initial steering committee of the present trial and instrumental in the design of the trial itself. The authors thank Prof. Zapol, Director of the Anesthesia Center for Critical Care Research, who pioneered the work on inhaled nitric oxide more than 30 years ago at the Massachusetts General Hospital in Boston. Prof. Zapol and colleagues presented the first physiological results on inhaled nitric oxide in newborns with persistent pulmonary hypertension in 1992 (Roberts et al. Inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 1992;239:818–819). Over the past decades, Prof. Zapol taught and discovered groundbreaking therapeutic implications of inhaled nitric oxide and provided intellectual insight during the first phases of the present work. The authors thank Prof. Kacmarek, Director of the Respiratory Care Department at Massachusetts General Hospital. Prof. Kacmarek was pivotal in performing the first studies on inhaled nitric oxide, teaching our respiratory care personnel, and creating and coordinating the nitric oxide delivery in the present work. The authors thank Prof. Hedenstierna, Director of the Laboratory of Clinical Physiology at Uppsala University in Sweden. Prof. Hedenstierna and colleagues first documented the benefits of high-dose inhaled nitric oxide in a series of patients during the SARS pandemic in China and performed in vitro studies demonstrating dose-dependent antiviral properties of nitric oxide donors.
Additional Nitric Oxide Investigators include: Caio C. Araujo Morais1,7, Lauren E. Gibson1,7, Takamitsu Ikeda1,7, Eizo Marutani1,7, Yusuke Miyazaki1,7, Anna Fischbach1,7, Lisa Traeger1,7, Martin I. Capriles1, Eduardo Diaz Delgado1, Grant M. Larson1, Roberta Ribeiro De Santis Santiago1,7, Carolyn La Vita2, Binglan Yu1,7, Maurizio F. Cereda1,7, Nattaly Greene3, Paula Restrepo4, James P. Flynn8, James Regan8, Riccardo Pinciroli7,9, Elizabeth I. Caskey10, Kimberley Hutchinson10, N. Stuart Harris5, Josanna Rodriguez-Lopez6,7, Marvin G. Chang1,7, Jacob Wideaus11,12, Matilda Widaeus12, Kambiz Shahgaldi11,12, Karl Hagman12,13, Garima Arora14, Robert Johnson15
1Department of Anesthesia, Critical Care, and Pain Medicine, 2Respiratory Care Services, 3Orthopedic Surgery, 4Nursing Services, 5Division of Wilderness Medicine, Department of Emergency Medicine, and 6Pulmonary and Critical Care Medicine, Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts; 7Harvard Medical School, Boston, Massachusetts; 8Division of Infectious Diseases, Brigham and Women’s Hospital, Boston, Massachusetts; 9Department of Anesthesia, Critical Care, and Pain Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts; 10Critical Care Medicine, Department of Medicine, Louisiana State University Health Shreveport, Shreveport, Louisiana; 11Department of Cardiology and Clinical Physiology and 12Department of Clinical Sciences, Danderyd Hospital, and 13Department of Clinical Science and Education, Sodersxjukhuset, Karolinska Institutet, Stockholm, Sweden; and 14Division of Cardiovascular Disease and 15Department of Respiratory Therapy, University of Alabama at Birmingham, Birmingham, Alabama
A complete list of the Nitric Oxide Investigators may be found before the beginning of the References.
Footnotes
Supported by funding from each institution for costs related to the trial. At Massachusetts General Hospital, the study was supported by internal funds from the Department of Anesthesia, Critical Care, and Pain Medicine and by a grant provided by iNO Therapeutics, LLC, a subsidiary of Mallinckrodt Pharmaceuticals. This research was also supported in part by the Massachusetts Consortium for Pathogen Readiness (J.Z.L.) and the Foundation for the NIH UM1AI069412 award (J.Z.L.) for virology studies. At the University of Alabama at Birmingham, the study was supported by internal funds from the Department of Medicine. At the Beth Israel Deaconess Medical Center, the study was supported by funding from the Department of Anesthesia, Critical Care, and Pain Medicine, and the gas was provided by iNO Therapeutics, LLC, a subsidiary of Mallinckrodt Pharmaceuticals. At Louisiana State University Shreveport, the study was supported by internal funds from the Department of Medicine. The part of the study conducted at Danderyd Hospital in Stockholm, Sweden, was supported by Linde plc, who provided the nitric oxide and its delivery system, the INOmax. The funding source at each site had no role in the design, analysis, or decision to publish.
Author Contributions: Study conception and design: Steering Committee: P.A., L.B., E.A.B., R.W.C., Robert Kacmarek, and Warren M. Zapol. Acquisition, analysis, or interpretation of the data: P.A., L.B., E.A.B., R.W.C., R.D.F., V.G., T.T.H., A.L.M., V.P., and N.S.S. had full access to the study data and take responsibility for the integrity of the data. Drafting the manuscript: P.A., L.B., R.D.F., V.G., V.P., and N.S.S. Revised article for important intellectual content: S.G., B.S.F., D.T., O.W., P.S.L., J.Z.L., S.P., S.B., L.K.S., T.T.H., A.L.M., O.A., F.I., and M.H. Approved of the final version for publication: R.D.F., V.P., N.S.S., S.G., V.G., B.S.F., D.T., O.W., P.S.L., J.Z.L., A.L.M., T.T.H., S.B., L.K.S., O.A., E.A.B., R.W.C., F.I., M.H., P.A., and L.B.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202304-0637OC on September 29, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
on behalf of the Nitric Oxide Investigators:
Caio C. Araujo Morais, Lauren E. Gibson, Takamitsu Ikeda, Eizo Marutani, Yusuke Miyazaki, Anna Fischbach, Lisa Traeger, Martin I. Capriles, Eduardo Diaz Delgado, Grant M. Larson, Roberta Ribeiro De Santis Santiago, Carolyn La Vita, Binglan Yu, Maurizio F. Cereda, Nattaly Greene, Paula Restrepo, James P. Flynn, James Regan, Riccardo Pinciroli, Elizabeth I. Caskey, Kimberley Hutchinson, N. Stuart Harris, Josanna Rodriguez-Lopez, Marvin G. Chang, Jacob Wideaus, Matilda Widaeus, Kambiz Shahgaldi, Karl Hagman, Garima Arora, and Robert Johnson
References
- 1.Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/nda/99/20845_INOmax.cfm
- 2. Frostell C, Fratacci MD, Wain JC, Jones R, Zapol WM. Inhaled nitric oxide: a selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation . 1991;83:2038–2047. doi: 10.1161/01.cir.83.6.2038. [DOI] [PubMed] [Google Scholar]
- 3. Roberts JD, Polaner DM, Lang P, Zapol WM. Inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Lancet . 1992;340:818–819. doi: 10.1016/0140-6736(92)92686-a. [DOI] [PubMed] [Google Scholar]
- 4.European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/inomax#overview-section
- 5.Japan Pharmaceuticals and Medical Devices Agency. 2015. https://www.pmda.go.jp/files/000229077.pdf
- 6. Rossaint R, Falke KJ, López F, Slama K, Pison U, Zapol WM. Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med . 1993;328:399–405. doi: 10.1056/NEJM199302113280605. [DOI] [PubMed] [Google Scholar]
- 7. Taylor RW, Zimmerman JL, Dellinger RP, Straube RC, Criner GJ, Davis K, Jr, et al. Inhaled Nitric Oxide in ARDS Study Group Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA . 2004;291:1603–1609. doi: 10.1001/jama.291.13.1603. [DOI] [PubMed] [Google Scholar]
- 8. Gerlach H, Keh D, Semmerow A, Busch T, Lewandowski K, Pappert DM, et al. Dose-response characteristics during long-term inhalation of nitric oxide in patients with severe acute respiratory distress syndrome: a prospective, randomized, controlled study. Am J Respir Crit Care Med . 2003;167:1008–1015. doi: 10.1164/rccm.2108121. [DOI] [PubMed] [Google Scholar]
- 9. Gerlach H, Rossaint R, Pappert D, Falke KJ. Time-course and dose-response of nitric oxide inhalation for systemic oxygenation and pulmonary hypertension in patients with adult respiratory distress syndrome. Eur J Clin Invest . 1993;23:499–502. doi: 10.1111/j.1365-2362.1993.tb00797.x. [DOI] [PubMed] [Google Scholar]
- 10. Dellinger RP, Zimmerman JL, Taylor RW, Straube RC, Hauser DL, Criner GJ, et al. Inhaled Nitric Oxide in ARDS Study Group Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Crit Care Med . 1998;26:15–23. doi: 10.1097/00003246-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 11. Troncy E, Collet JP, Shapiro S, Guimond JG, Blair L, Ducruet T, et al. Inhaled nitric oxide in acute respiratory distress syndrome: a pilot randomized controlled study. Am J Respir Crit Care Med . 1998;157:1483–1488. doi: 10.1164/ajrccm.157.5.9707090. [DOI] [PubMed] [Google Scholar]
- 12. Lundin S, Mang H, Smithies M, Stenqvist O, Frostell C, The European Study Group of Inhaled Nitric Oxide Inhalation of nitric oxide in acute lung injury: results of a European multicentre study. Intensive Care Med . 1999;25:911–919. doi: 10.1007/s001340050982. [DOI] [PubMed] [Google Scholar]
- 13. Michael JR, Barton RG, Saffle JR, Mone M, Markewitz BA, Hillier K, et al. Inhaled nitric oxide versus conventional therapy: effect on oxygenation in ARDS. Am J Respir Crit Care Med . 1998;157:1372–1380. doi: 10.1164/ajrccm.157.5.96-10089. [DOI] [PubMed] [Google Scholar]
- 14. Wiegand SB, Traeger L, Nguyen HK, Rouillard KR, Fischbach A, Zadek F, et al. Antimicrobial effects of nitric oxide in murine models of Klebsiella pneumonia. Redox Biol . 2021;39:101826. doi: 10.1016/j.redox.2020.101826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fang FC. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest . 1997;99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Akaberi D, Krambrich J, Ling J, Luni C, Hedenstierna G, Järhult JD, et al. Mitigation of the replication of SARS-CoV-2 by nitric oxide in vitro. Redox Biol . 2020;37:101734. doi: 10.1016/j.redox.2020.101734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Keyaerts E, Vijgen L, Chen L, Maes P, Hedenstierna G, Van Ranst M. Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int J Infect Dis . 2004;8:223–226. doi: 10.1016/j.ijid.2004.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Adhikari NK, Dellinger RP, Lundin S, Payen D, Vallet B, Gerlach H, et al. Inhaled nitric oxide does not reduce mortality in patients with acute respiratory distress syndrome regardless of severity: systematic review and meta-analysis. Crit Care Med . 2014;42:404–412. doi: 10.1097/CCM.0b013e3182a27909. [DOI] [PubMed] [Google Scholar]
- 19. Valsecchi C, Winterton D, Safaee Fakhr B, Collier AY, Nozari A, Ortoleva J, et al. DELiverly oF iNO (DELFiNO) Network Collaborators High-dose inhaled nitric oxide for the treatment of spontaneously breathing pregnant patients with severe coronavirus disease 2019 (COVID-19) pneumonia. Obstet Gynecol . 2022;140:195–203. doi: 10.1097/AOG.0000000000004847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goldbart A, Lavie M, Lubetzky R, Pillar G, Landau D, Schlesinger Y, et al. Inhaled nitric oxide for the treatment of acute bronchiolitis: a multicenter randomized controlled clinical trial to evaluate dose response. Ann Am Thorac Soc . 2023;20:236–244. doi: 10.1513/AnnalsATS.202103-348OC. [DOI] [PubMed] [Google Scholar]
- 21. Bartley BL, Gardner KJ, Spina S, Hurley BP, Campeau D, Berra L, et al. High-dose inhaled nitric oxide as adjunct therapy in cystic fibrosis targeting Burkholderia multivorans. Case Rep Pediatr . 2020;2020:1536714. doi: 10.1155/2020/1536714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen L, Liu P, Gao H, Sun B, Chao D, Wang F, et al. Inhalation of nitric oxide in the treatment of severe acute respiratory syndrome: a rescue trial in Beijing. Clin Infect Dis . 2004;39:1531–1535. doi: 10.1086/425357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Regev-Shoshani G, Vimalanathan S, McMullin B, Road J, Av-Gay Y, Miller C. Gaseous nitric oxide reduces influenza infectivity in vitro. Nitric Oxide . 2013;31:48–53. doi: 10.1016/j.niox.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deppisch C, Herrmann G, Graepler-Mainka U, Wirtz H, Heyder S, Engel C, et al. Gaseous nitric oxide to treat antibiotic resistant bacterial and fungal lung infections in patients with cystic fibrosis: a phase I clinical study. Infection . 2016;44:513–520. doi: 10.1007/s15010-016-0879-x. [DOI] [PubMed] [Google Scholar]
- 25.Miller CC, Hergott CA, Rohan M, Arsenault-Mehta K, Döring G, Mehta S. Inhaled nitric oxide decreases the bacterial load in a rat model of Pseudomonas aeruginosa pneumonia. J Cyst Fibros. 2013;12:817–820. doi: 10.1016/j.jcf.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 26. Jean D, Maître B, Tankovic J, Meignan M, Adnot S, Brun-Buisson C, et al. Beneficial effects of nitric oxide inhalation on pulmonary bacterial clearance. Crit Care Med . 2002;30:442–447. doi: 10.1097/00003246-200202000-00029. [DOI] [PubMed] [Google Scholar]
- 27.Miller C, McMullin B, Ghaffari A, Stenzler A, Pick N, Roscoe D, et al. Gaseous nitric oxide bactericidal activity retained during intermittent high-dose short duration exposure. Nitric Oxide. 2009;20:16–23. doi: 10.1016/j.niox.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 28. McMullin BB, Chittock DR, Roscoe DL, Garcha H, Wang L, Miller CC. The antimicrobial effect of nitric oxide on the bacteria that cause nosocomial pneumonia in mechanically ventilated patients in the intensive care unit. Respir Care . 2005;50:1451–1456. [PubMed] [Google Scholar]
- 29. Talmor D, Sarge T, Malhotra A, O’Donnell CR, Ritz R, Lisbon A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med . 2008;359:2095–2104. doi: 10.1056/NEJMoa0708638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang J, Cong X, Miao M, Yang Y, Zhang J. Inhaled nitric oxide and acute kidney injury risk: a meta-analysis of randomized controlled trials. Ren Fail . 2021;43:281–290. doi: 10.1080/0886022X.2021.1873805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Legrand M, Bell S, Forni L, Joannidis M, Koyner JL, Liu K, et al. Pathophysiology of COVID-19-associated acute kidney injury. Nat Rev Nephrol . 2021;17:751–764. doi: 10.1038/s41581-021-00452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A, Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med . 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 33. Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al. PROSEVA Study Group Prone positioning in severe acute respiratory distress syndrome. N Engl J Med . 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 34. Guerin C, Gaillard S, Lemasson S, Ayzac L, Girard R, Beuret P, et al. Effects of systematic prone positioning in hypoxemic acute respiratory failure: a randomized controlled trial. JAMA . 2004;292:2379–2387. doi: 10.1001/jama.292.19.2379. [DOI] [PubMed] [Google Scholar]
- 35. Gattinoni L, Tognoni G, Pesenti A, Taccone P, Mascheroni D, Labarta V, et al. Prone-Supine Study Group Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med . 2001;345:568–573. doi: 10.1056/NEJMoa010043. [DOI] [PubMed] [Google Scholar]
- 36. Zapol WM, Snider MT, Hill JD, Fallat RJ, Bartlett RH, Edmunds LH, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure: a randomized prospective study. JAMA . 1979;242:2193–2196. doi: 10.1001/jama.242.20.2193. [DOI] [PubMed] [Google Scholar]
- 37. Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. CESAR trial collaboration Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet . 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 38. Villalba JA, Hilburn CF, Garlin MA, Elliott GA, Li Y, Kunitoki K, et al. Vasculopathy and increased vascular congestion in fatal COVID-19 and acute respiratory distress syndrome. Am J Respir Crit Care Med . 2022;206:857–873. doi: 10.1164/rccm.202109-2150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sinha P, Calfee CS, Beitler JR, Soni N, Ho K, Matthay MA, et al. Physiologic analysis and clinical performance of the ventilatory ratio in acute respiratory distress syndrome. Am J Respir Crit Care Med . 2019;199:333–341. doi: 10.1164/rccm.201804-0692OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gkaliagkousi E, Ritter J, Ferro A. Platelet-derived nitric oxide signaling and regulation. Circ Res . 2007;101:654–662. doi: 10.1161/CIRCRESAHA.107.158410. [DOI] [PubMed] [Google Scholar]
- 41. Fajnzylber J, Regan J, Coxen K, Corry H, Wong C, Rosenthal A, et al. Massachusetts Consortium for Pathogen Readiness SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun . 2020;11:5493. doi: 10.1038/s41467-020-19057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li Y, Schneider AM, Mehta A, Sade-Feldman M, Kays KR, Gentili M, et al. SARS-CoV-2 viremia is associated with distinct proteomic pathways and predicts COVID-19 outcomes. J Clin Invest . 2021;131:e148635. doi: 10.1172/JCI148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tandon M, Wu W, Moore K, Winchester S, Tu YP, Miller C, et al. Study Group SARS-CoV-2 accelerated clearance using a novel nitric oxide nasal spray (NONS) treatment: a randomized trial. Lancet Reg Health Southeast Asia . 2022;3:100036. doi: 10.1016/j.lansea.2022.100036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Akerström S, Gunalan V, Keng CT, Tan YJ, Mirazimi A. Dual effect of nitric oxide on SARS-CoV replication: viral RNA production and palmitoylation of the S protein are affected. Virology . 2009;395:1–9. doi: 10.1016/j.virol.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Safaee Fakhr B, Di Fenza R, Gianni S, Wiegand SB, Miyazaki Y, Araujo Morais CC, et al. Nitric Oxide Study Investigators Inhaled high dose nitric oxide is a safe and effective respiratory treatment in spontaneous breathing hospitalized patients with COVID-19 pneumonia. Nitric Oxide . 2021;116:7–13. doi: 10.1016/j.niox.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Beckman D, Bonillas A, Diniz GB, Ott S, Roh JW, Elizaldi SR, et al. SARS-CoV-2 infects neurons and induces neuroinflammation in a non-human primate model of COVID-19. Cell Rep . 2022;41:111573. doi: 10.1016/j.celrep.2022.111573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fernández-Castañeda A, Lu P, Geraghty AC, Song E, Lee MH, Wood J, et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell . 2022;185:2452–2468.e16. doi: 10.1016/j.cell.2022.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xu E, Xie Y, Al-Aly Z. Long-term neurologic outcomes of COVID-19. Nat Med . 2022;28:2406–2415. doi: 10.1038/s41591-022-02001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ichinose F, Roberts JD, Jr, Zapol WM. Inhaled nitric oxide: a selective pulmonary vasodilator: current uses and therapeutic potential. Circulation . 2004;109:3106–3111. doi: 10.1161/01.CIR.0000134595.80170.62. [DOI] [PubMed] [Google Scholar]
- 50. Graham EL, Clark JR, Orban ZS, Lim PH, Szymanski AL, Taylor C, et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Ann Clin Transl Neurol . 2021;8:1073–1085. doi: 10.1002/acn3.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson BT, Bellamy SL, et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med . 2012;185:1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Iotti GA, Olivei MC, Palo A, Galbusera C, Veronesi R, Braschi A. Acute effects of inhaled nitric oxide in adult respiratory distress syndrome. Eur Respir J . 1998;12:1164–1171. doi: 10.1183/09031936.98.12051164. [DOI] [PubMed] [Google Scholar]