Abstract
Pitolisant, a novel histamine H3‐receptor antagonist, holds significant promise for treating narcolepsy. However, a petition, which highlighted that pitolisant was associated with deaths during clinical trials, has propelled it into the spotlight of widespread societal attention on April 3, 2023. Till now, the clinical safety of pitolisant remains a heatedly debated topic. This study aimed to offer a comprehensive assessment of the safety profile of pitolisant in real‐world clinical settings. Adverse event reports where pitolisant was the primary suspect drug were extracted from the FDA Adverse Event Reporting System database. The clinical characteristics and concomitant drugs of the pitolisant‐associated adverse events were analyzed. The potential adverse event signals of pitolisant were explored using four disproportionality analysis methods. Furthermore, the difference in pitolisant‐associated adverse event signals was investigated concerning sex, age, weight, and dose. A total of 526 reports and 1695 adverse events with pitolisant as the primary suspected drug were identified. The most significant adverse event signals were generally mild and of short duration. The concomitant drugs of pitolisant were highly intricate, mainly included drugs for treating narcolepsy as well as antidepressants. Seven new significant adverse event signals emerged. The safety profile of pitolisant exhibited no significant differences across age and dose groups, although slight variations were observed in relation to sex and weight. The findings from reports of death and life‐threatening outcomes underscore the importance of enhanced monitoring for cardiac and respiratory adverse reactions when utilizing pitolisant. This study provided a broader understanding of the safety profile of pitolisant.
Keywords: adverse event, disproportionality algorithm, FDA Adverse Event Reporting System (FAERS), pharmacovigilance, pitolisant, post‐marketing safety
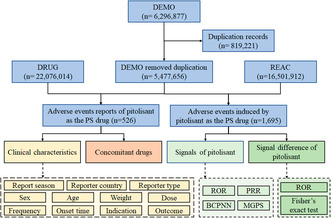
Flow diagram of data collection and analysis of pitolisant‐associated adverse events.
Abbreviations
- BCPNN
Bayesian confidence propagation neural network
- CDER
Center for Drug Evaluation and Research
- EDS
excessive daytime sleepiness
- FAERS
Food and Drug Administration Adverse Event Reporting System
- FDA
Food and Drug Administration
- MGPS
multi‐item gamma Poisson shrinker
- PT
preferred term
- PS
primary suspected
- PRR
proportional reported odds ratio
- ROR
reported odds ratio
- SOC
system organ class
1. INTRODUCTION
Narcolepsy is a rare neurological disorder that affects the brain's ability to regulate sleep–wake cycles. Statistically, type 1 narcolepsy exhibits an estimated prevalence hovering around 25–50 cases per 100 000 individuals, whereas type 2 narcolepsy demonstrates an approximate prevalence rate of 20–34 cases within every 100 000 individuals. 1 People with narcolepsy often struggle with excessive daytime sleepiness (EDS) and may experience uncontrollable episodes of falling asleep during the day. 2 In type 1 narcolepsy, patients sometimes exhibit sudden, brief episodes of muscle weakness leading to falling down, known as “cataplexy”. 3 , 4 The sleep attacks can occur at any time and in various situations, thereby seriously affecting the quality of patients' life. 5 , 6 The pathogenesis of narcolepsy is exceptionally intricate. Current research indicates that it may involve a combination of genetic, immunological, and environmental factors. 7 , 8 , 9 Till now, the precise mechanism of narcolepsy is still not fully understood.
There exist over a dozen medications catering to narcolepsy treatment, such as modafinil and sodium oxybate. 10 Modafinil, positioned as a frontline treatment, primarily targets the brain's dopamine and norepinephrine systems. It can enhance the release of the neurotransmitters, thereby boosting cognitive functions like attention, concentration, and memory with few side effects and a low risk of addiction or withdrawal. 11 Nonetheless, clinical trials reveal that approximately 40% of patients do not respond favorably to modafinil, and it proves ineffective against cataplexy. 12 Sodium oxybate is another representative medication for treating narcolepsy, which can significantly ameliorate both EDS and cataplexy. 13 However, it carries a considerable potential for inducing addiction and brings an exorbitant price tag. Presently, there is still a lack of a medication having the ability to simultaneously treat EDS and cataplexy with minor side effects and non‐addictive properties in clinical practice.
Pitolisant, a novel histamine H3‐receptor antagonist, was approved by the United States Food and Drug Administration (FDA) for the treatment of narcolepsy in 2019. Different from traditional stimulants, pitolisant works by enhancing the release of histamine, promoting wakefulness and reducing excessive sleepiness. 14 The robust efficacy of pitolisant for the reduction in both EDS and cataplexy has been demonstrated. 15 , 16 , 17 , 18 In addition, pitolisant has been found to be well‐tolerated and has a low risk of abuse. Recent real‐world studies have suggested that pitolisant treatment is effective in children with narcolepsy and also is well tolerated. 19 , 20 Overall, the favorable effectiveness and safety profile of pitolisant make it a valuable addition to the treatment options available for patients with narcolepsy.
While pitolisant holds significant promise for treating narcolepsy, a recent petition has propelled it into the spotlight of widespread societal attention. On April 3, 2023, an explosive citizen petition was submitted to the United States FDA. This petition highlighted that during clinical trials, pitolisant was associated with deaths among participants, urging the FDA to promptly revoke its marketing approval and commit to never approving any marketing application for the pitolisant again. On the other hand, details from the pitolisant review report issued by the FDA's Center for Drug Evaluation and Research (CDER) believed the data were insufficient to conclude whether the deaths were related to pitolisant. 21 With respect to the considerable controversial, the clinical safety of pitolisant becomes a heatedly debated topic, which worthy of close attention. Since the sample size during clinical trials is relatively small, further studies are needed to explore the safety of pitolisant through large‐scale post‐market monitoring.
In this study, the clinical safety of pitolisant was investigated based on the FDA Adverse Event Reporting System (FAERS) database. The clinical characteristics and concomitant drugs of the pitolisant‐associated adverse events were analyzed. The potential adverse event signals of pitolisant were explored using four disproportionality analysis methods. Furthermore, the difference in pitolisant‐associated adverse event signals was investigated concerning sex, age, weight, and dose. This study provides comprehensive evaluation for the safety profile of pitolisant in real‐world clinical use.
2. METHODS
2.1. Data source and collection
The data for this retrospective pharmacovigilance study were extracted from the FAERS database covering the period from the fourth quarter of 2019 to the first quarter of 2023. Six datasets were utilized, including patient demographic and administrative information (DEMO), drug information (DRUG), therapy start dates and end dates for reported drugs (THER), coded for the adverse events (REAC), patient outcomes for the event (OUTC), and indications for use/diagnosis (INDI). All raw data were downloaded in ASCII format from the United States FDA website. Cases of pitolisant were identified by searching the DRUG dataset for the generic name (PITOLISANT HYDROCHLORIDE in prod_ai column) and trade name (WAKIX in drugname column). Adverse event reports where pitolisant was the primary suspect (PS) drug were selected based on the role_cod.
2.2. Clinical characteristic analysis
Descriptive analysis was utilized to examine the clinical characteristics of the pitolisant‐associated adverse event reports in detail, including report season, reporter country, reporter type, sex, age, weight, dose, frequency, onset time, indication, and outcome, after removing missing data. Onset time was calculated as the interval between EVENT_DT (event date) in DEMO dataset and START_DT (start date) in THER dataset. Reports with EVENT_DT earlier than START_DT or inaccurate date entries were excluded.
2.3. Concomitant drugs analysis
The concomitant drugs of the pitolisant‐associated adverse event reports were further analyzed. In a report, if the same drug was listed multiple times with different doses or frequencies, it was only recorded once for the purposes of tallying the occurrence of various agents. This ensured that a given drug was not counted more than once per report when summarizing the concomitant medications in the polytherapy approach. The drugs reported as “UNSPECIFIED INGREDIENT” were excluded.
2.4. Signals detection
The pitolisant‐associated adverse events from the REAC dataset were coded using preferred terms (PTs) derived from the standardized Medical Dictionary for Regulatory Activities (MedDRA) version 25.1, which contains 27 system organ classes (SOCs). All the PTs were classified to the corresponding primary SOC levels.
The adverse event signals of pitolisant were then investigated by describing the frequency and intensity at the SOC and PT levels. Four disproportionality analysis methods were employed, including reporting odds ratio (ROR), proportional reporting ratio (PRR), Bayesian confidence propagation neural network (BCPNN), and the multi‐item gamma Poisson shrinker (MGPS). The fourfold table of disproportionality analysis for pitolisant signal detection is shown in Table S1. The equations and criteria of the four algorithms for pitolisant signal detection are shown in Table S2. In this study, an adverse event signal of pitolisant was detected only when it conformed to all of the four algorithm criteria simultaneously.
2.5. Signal difference detection
Furthermore, the difference of pitolisant‐associated adverse event signals was investigated concerning sex, age, weight, and dose. The ROR algorithm and Fisher's exact test were applied based on a fourfold table, as shown in Table S3. The criteria of ROR and Fisher's exact test for difference detection of pitolisant signals are shown in Table S4. All data processing and statistical analyses were performed using Jupyter Notebook 6.4.12.
3. RESULTS
3.1. Descriptive analysis of clinical characteristics
A total of 6 296 877 adverse event reports were acquired from the DEMO dataset. Following FDA's recommendations, 819221 duplicate reports were identified and removed, resulting in a reduction in the number of adverse event reports to 5 477 656. 526 reports and 1695 adverse events with pitolisant as the PS drug were identified. A flow diagram of data collection and analysis of pitolisant‐associated adverse events is shown in Figure 1.
FIGURE 1.
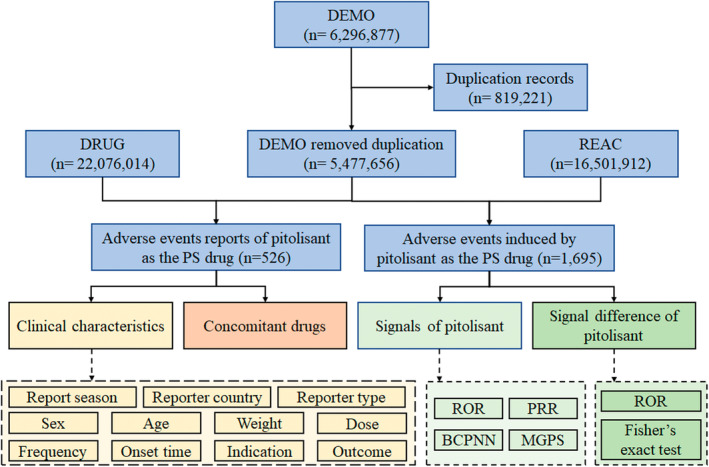
Flow diagram of data collection and analysis of pitolisant‐associated adverse events.
The clinical characteristics of the 526 pitolisant‐associated adverse event reports were examined, as shown in Figure 2. Overall, the number of events remained below 40 per quarter, except for a noticeable increase to 284 in the second quarter of 2022. Regarding reporter country, 97.3% (n = 506) of adverse events originated from the United States. Excluding three unknown reporters, consumers reported the most events at 83.4% (n = 436). Sex data were available for 474 cases. Of these, 70.5% (n = 334) were female and 29.5% (n = 140) were male. Age data were reported for 229 cases, with an average age of 41.7 ± 14.4 years. The majority aged 18–65 years accounted for 92.6% (n = 212) of cases. Weight data were available for 152 patients, with an average weight of 81.3 ± 22.8 kg. Of patients with weight data, 53.3% (n = 81) weighed <80 kg, 27.0% (n = 41) weighed 80–100 kg, and 19.7% (n = 30) weighed >100 kg.
FIGURE 2.
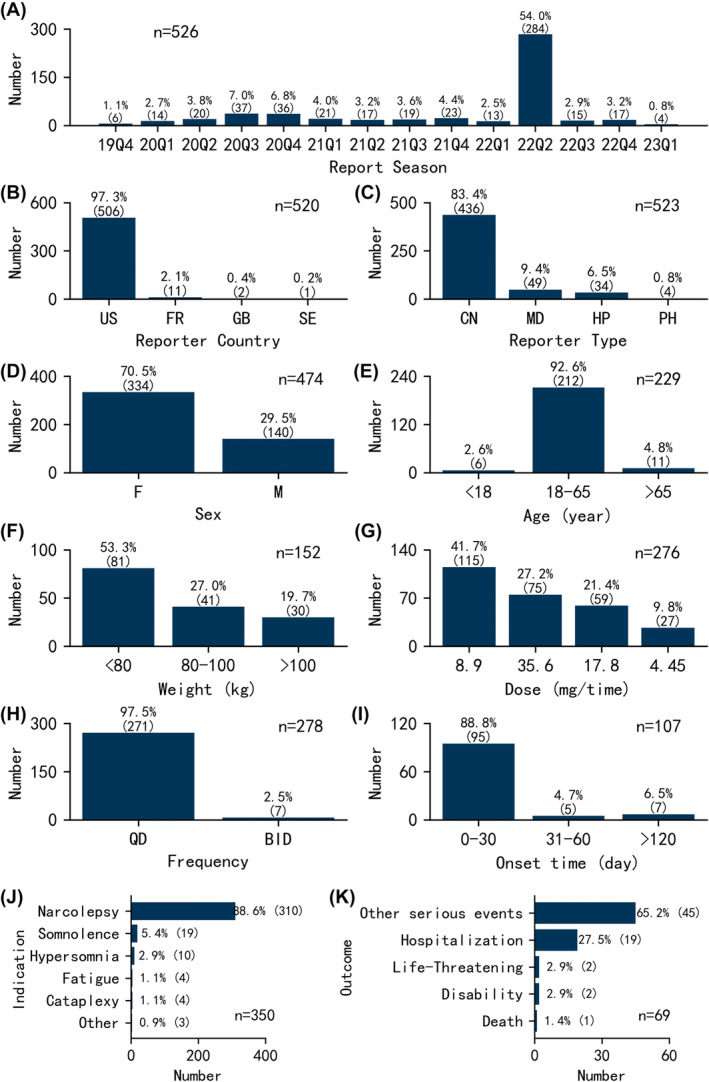
Clinical characteristics of pitolisant‐associated adverse event reports. (A) Report season. (B) Reporter country. (C) Reporter type. (D) Sex. (E) Age. (F) Weight. (G) Dose. (H) Frequency. (I) Onset time. (J) Indication. (K) Outcome.
Pitolisant is available in two strengths, 4.45 and 17.8 mg, both in tablet form. It is taken orally in the morning upon wakening throughout the titration schedule and at the reached maintenance dose. The recommended dosage is 17.8–35.6 mg once daily. The titration schedule starts at a low dose of 8.9 mg once daily in the first week, then gradually increases to 17.8 mg once daily in the second week, finally to the maximal dosage of 35.6 mg once daily in the third week. The dosage can be decreased to 4.45 mg once daily based on the individual patient's clinical response and tolerability. In this study, the reports with doses of 4.5, 9.0, 18.0 and 36.0 mg were approximated as 4.45, 8.9, 17.8 and 35.6 mg, respectively. After excluding missing data, incorrect doses (not divisible by 4.45 mg), and incomparable doses (dose_unit as DF), 276 valid dose records remained. The most common dose was 8.9 mg, accounting for 41.7% (n = 115) of reports, followed by 35.6 mg at 27.2% (n = 75), 17.8 mg at 21.4% (n = 59), and 4.45 mg at 9.8% (n = 27). For dosing frequency, 278 records were available. Of these, 97.5% (n = 271) correctly followed the recommended once‐daily frequency. However, 2.5% (n = 7) incorrectly used a twice‐daily frequency. After excluding false reports and missing data, 107 reports contained valid onset times for pitolisant‐associated adverse events. Of these, 88.8% (n = 95) of adverse events occurred within the first month of administration.
Figure 2J,K displays the indications and outcomes for patients taking pitolisant. The most frequent indication was narcolepsy at 88.6% (n = 310). Regarding outcomes, 69 events were reported for pitolisant‐associated adverse events. Other serious events accounted for 65.2% (n = 45) of outcomes. Crucially, there was one case with death and two cases with life‐threatening outcomes.
3.2. Descriptive analysis of concomitant drugs
The concomitant drugs of 526 pitolisant‐associated adverse event reports were highly intricate, involving 323 different drugs. In summary, the combined drugs of pitolisant primarily involved drugs for treating narcolepsy, antidepressants, and other medications. Figure 3 presents the top 10 ranked combined drugs occurrence in the pitolisant‐associated adverse event reports. Out of 526 reports, sodium oxybate was the most frequently employed agent, accounting for 8.4% (n = 44) of the cases. Other commonly utilized agents encompassed amphetamine at 7.6% (n = 40), vitamins at 5.3% (n = 28), armodafinil at 3.4% (n = 18), modafinil at 3.2% (n = 17), omeprazole at 3.0% (n = 16), venlafaxine at 2.9% (n = 15), and solriamfetol at 2.7% (n = 14). The result suggests that the treatment of narcolepsy typically involved a strategy of combining multiple drugs, primarily using pitolisant in combination with sodium oxybate, amphetamine, armodafinil, modafinil, or solriamfetol. Pitolisant was also frequently combined with various antidepressants of the 526 reports, such as venlafaxine at 2.9% (n = 15), bupropion at 2.3% (n = 12), escitalopram at 2.3% (n = 12), duloxetine at 1.9% (n = 10). The concurrent use of pitolisant with these traditional antidepressants suggests a strategy targeting both narcolepsy and underlying mood disturbances commonly associated with it. In addition, some antidepressant medications also have the effect of treating excessive sleepiness and reversing fainting episodes.
FIGURE 3.
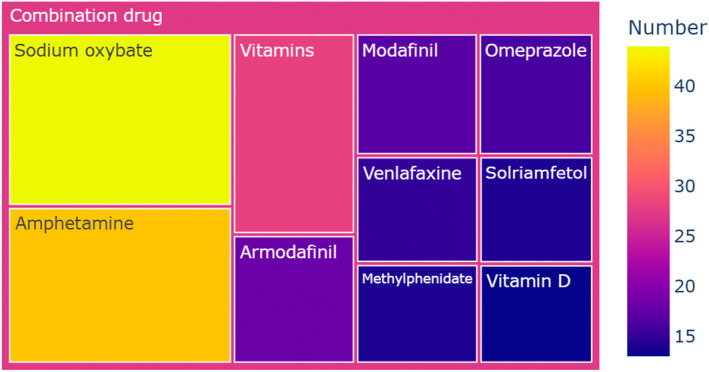
Top 10 ranked combined drugs occurrence in the pitolisant‐associated adverse event reports. Amphetamine represents amphetamine aspartate\amphetamine sulfate\dextroamphetamine saccharate\dextroamphetamine sulfate.
3.3. Signals of pitolisant
The case number and signal strength of pitolisant at the SOC level are described in Table 1. Statistically, the analysis revealed that adverse events associated with pitolisant encompassed 23 SOCs. Among these, psychiatric disorders (SOC: 10037175) emerged as the significant SOC that met all four criteria.
TABLE 1.
Case number and signal strength of pitolisant at the SOC level.
| SOC | Case Number | ROR | Lower limit of 95% CI | PRR | χ 2 | IC | IC025 | EBGM | EBGM05 |
|---|---|---|---|---|---|---|---|---|---|
| Psychiatric disorders (SOC: 10037175) a , b , c , d | 394 | 5.22 | 4.66 | 4.24 | 1030.66 | 2.08 | 1.91 | 4.24 | 3.78 |
| General disorders and administration site conditions (SOC: 10018065) a , c | 346 | 1.20 | 1.07 | 1.16 | 9.17 | 0.21 | 0.04 | 1.16 | 1.03 |
| Nervous system disorders (SOC: 10029205) a , b , c | 252 | 2.24 | 1.96 | 2.05 | 146.52 | 1.04 | 0.84 | 2.05 | 1.79 |
| Gastrointestinal disorders (SOC: 10017947) a , c | 171 | 1.33 | 1.14 | 1.30 | 12.60 | 0.37 | 0.14 | 1.30 | 1.11 |
| Injury, poisoning and procedural complications (SOC: 10022117) | 99 | 0.47 | 0.38 | 0.50 | 55.24 | −0.99 | −1.28 | 0.50 | 0.41 |
| Skin and subcutaneous tissue disorders (SOC: 10040785) | 69 | 0.74 | 0.58 | 0.75 | 5.93 | −0.41 | −0.76 | 0.75 | 0.59 |
| Respiratory, thoracic and mediastinal disorders (SOC: 10038738) | 69 | 0.90 | 0.71 | 0.90 | 0.76 | −0.15 | −0.50 | 0.90 | 0.71 |
| Musculoskeletal and connective tissue disorders (SOC: 10028395) | 53 | 0.59 | 0.45 | 0.60 | 14.59 | −0.73 | −1.12 | 0.60 | 0.46 |
| Investigations (SOC: 10022891) | 47 | 0.46 | 0.34 | 0.47 | 29.47 | −1.08 | −1.49 | 0.47 | 0.35 |
| Infections and infestations (SOC: 10021881) | 46 | 0.47 | 0.35 | 0.49 | 26.11 | −1.03 | −1.45 | 0.49 | 0.36 |
| Cardiac disorders (SOC: 10007541) | 25 | 0.75 | 0.50 | 0.75 | 2.08 | −0.41 | −0.97 | 0.75 | 0.51 |
| Eye disorders (SOC: 10015919) | 23 | 0.72 | 0.48 | 0.73 | 2.40 | −0.46 | −1.04 | 0.73 | 0.48 |
| Immune system disorders (SOC: 10021428) | 23 | 1.20 | 0.80 | 1.20 | 0.77 | 0.26 | −0.34 | 1.20 | 0.80 |
| Metabolism and nutrition disorders (SOC: 10027433) | 22 | 0.69 | 0.45 | 0.69 | 3.13 | −0.54 | −1.12 | 0.69 | 0.45 |
| Vascular disorders (SOC: 10047065) | 16 | 0.50 | 0.31 | 0.51 | 7.79 | −0.98 | −1.64 | 0.51 | 0.31 |
| Surgical and medical procedures (SOC: 10042613) | 11 | 0.47 | 0.26 | 0.47 | 6.68 | −1.09 | −1.86 | 0.47 | 0.26 |
| Renal and urinary disorders (SOC: 10038359) | 8 | 0.24 | 0.12 | 0.24 | 19.83 | −2.07 | −2.90 | 0.24 | 0.12 |
| Hepatobiliary disorders (SOC: 10019805) | 5 | 0.37 | 0.15 | 0.37 | 5.38 | −1.43 | −2.45 | 0.37 | 0.15 |
| Ear and labyrinth disorders (SOC: 10013993) | 5 | 0.73 | 0.30 | 0.73 | 0.49 | −0.45 | −1.56 | 0.73 | 0.30 |
| Reproductive system and breast disorders (SOC: 10038604) | 4 | 0.39 | 0.15 | 0.39 | 3.81 | −1.35 | −2.46 | 0.39 | 0.15 |
| Social circumstances (SOC: 10041244) | 2 | 0.25 | 0.06 | 0.25 | 4.56 | −2.01 | −3.26 | 0.25 | 0.06 |
| Product issues (SOC: 10077536) | 1 | 0.03 | 0.00 | 0.03 | 28.69 | −4.91 | −6.00 | 0.03 | 0.00 |
| Endocrine disorders (SOC: 10014698) | 1 | 0.23 | 0.03 | 0.23 | 2.58 | −2.12 | −3.46 | 0.23 | 0.03 |
Note: The colors of the individual table cells represent the values of each index.
SOCs met the criteria of ROR algorithm.
SOCs met the criteria of PRR algorithm.
SOCs met the criteria of BCPNN algorithm.
SOCs met the criteria of MGPS algorithm.
There were 102, 76, 70, and 111 signals of pitolisant‐induced adverse events that met the criteria of ROR, PRR, BCPNN, and MGPS algorithms, respectively. A total of 51 signals were detected after conforming to the four algorithms simultaneously. The Venn plots for signal detection of pitolisant‐associated adverse events by four algorithms are shown in Figure 4. Since all the PTs were collected by FAERS, it was found that there were signals of unevaluable event (PT: 10062355), drug interaction (PT: 10013710), and labeled drug–drug interaction medication error (PT: 10064373). In addition, the signals of somnolence (PT: 10041349), cataplexy (PT: 10007737), hypersomnia (PT: 10020765), narcolepsy (PT: 10028713) were consistent with indications in the INDI dataset, which might be considered to be caused by disease progression or efficacy reduction. The case number and signal strength of 7 pitolisant‐unrelated signals at the PT level are listed in Table S5.
FIGURE 4.
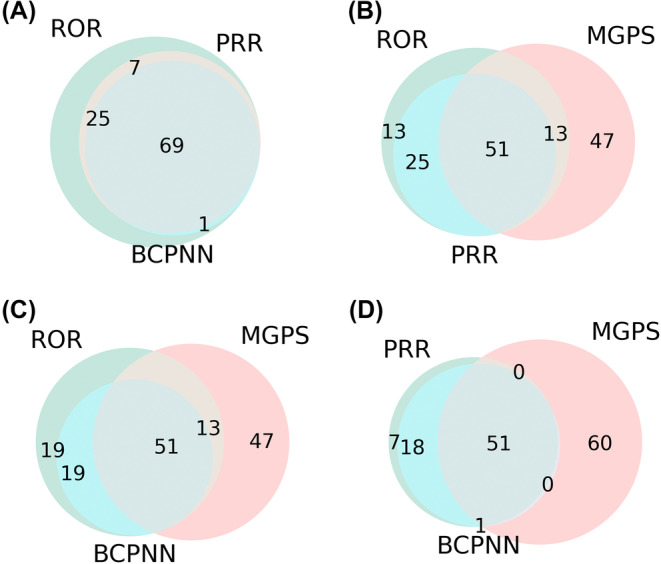
Venn plots for signal detection of pitolisant‐associated adverse events by four algorithms.
After excluding the 7 pitolisant‐unrelated signals, 44 significant disproportionality signals were detected at the PT level. The case number and signal strength of 44 significant disproportionality signals are shown in Table 2. In this study, the most common adverse reactions of pitolisant were insomnia, headache, nausea, anxiety, and depression, which aligned with the safety data from label. Interestingly, seven new significant adverse event signals uncovering in the label emerged, including somnambulism (PT: 10041347), sleep terror (PT: 10041010), sensation of foreign body (PT: 10061549), palpitations (PT: 10033557), rhinorrhoea (PT: 10039101), nasal congestion (PT: 10028735), and sneezing (PT: 10041232).
TABLE 2.
Case number and signal strength of pitolisant at the PT level.
| PT | Case Number | ROR | Lower limit of 95% CI | PRR | χ 2 | IC | IC025 | EBGM | EBGM05 |
|---|---|---|---|---|---|---|---|---|---|
| Psychiatric disorders (SOC: 10037175) | |||||||||
| Insomnia (PT: 10022437) | 85 | 15.03 | 12.09 | 14.33 | 1056.20 | 3.84 | 3.31 | 14.31 | 11.50 |
| Anxiety (PT: 10002855) | 49 | 6.59 | 4.96 | 6.43 | 225.67 | 2.68 | 2.12 | 6.43 | 4.84 |
| Depression (PT: 10012378) | 19 | 3.85 | 2.45 | 3.82 | 39.58 | 1.93 | 1.09 | 3.81 | 2.43 |
| Middle insomnia (PT: 10027590) | 17 | 40.79 | 25.27 | 40.39 | 650.56 | 5.33 | 2.98 | 40.23 | 24.92 |
| Irritability (PT: 10022998) | 16 | 15.22 | 9.30 | 15.09 | 210.28 | 3.91 | 2.34 | 15.07 | 9.21 |
| Depressed mood (PT: 10012374) | 15 | 10.83 | 6.51 | 10.74 | 132.47 | 3.42 | 2.01 | 10.73 | 6.45 |
| Sleep disorder (PT: 10040984) | 13 | 6.57 | 3.81 | 6.53 | 60.88 | 2.71 | 1.45 | 6.52 | 3.78 |
| Abnormal dreams (PT: 10000125) | 13 | 34.08 | 19.73 | 33.83 | 412.81 | 5.08 | 2.56 | 33.71 | 19.52 |
| Nightmare (PT: 10029412) | 10 | 15.34 | 8.24 | 15.26 | 133.08 | 3.93 | 1.86 | 15.24 | 8.18 |
| Poor quality sleep (PT: 10062519) | 10 | 17.69 | 9.50 | 17.59 | 156.28 | 4.13 | 1.94 | 17.56 | 9.43 |
| Initial insomnia (PT: 10022035) | 9 | 44.23 | 22.94 | 44.00 | 376.58 | 5.45 | 2.13 | 43.81 | 22.72 |
| Hallucination (PT: 10019063) | 8 | 4.17 | 2.08 | 4.16 | 19.19 | 2.05 | 0.66 | 4.15 | 2.07 |
| Mood altered (PT: 10027940) | 6 | 10.88 | 4.88 | 10.84 | 53.58 | 3.44 | 1.08 | 10.83 | 4.86 |
| Mania (PT: 10026749) | 6 | 20.35 | 9.12 | 20.28 | 109.77 | 4.34 | 1.34 | 20.24 | 9.07 |
| Panic attack (PT: 10033664) | 6 | 7.64 | 3.43 | 7.62 | 34.50 | 2.93 | 0.88 | 7.62 | 3.42 |
| Emotional disorder (PT: 10014551) | 5 | 7.46 | 3.10 | 7.44 | 27.88 | 2.90 | 0.66 | 7.44 | 3.09 |
| Decreased interest (PT: 10011971) | 4 | 35.32 | 13.22 | 35.24 | 132.60 | 5.13 | 0.87 | 35.12 | 13.14 |
| Mood swings (PT: 10027951) | 4 | 6.71 | 2.52 | 6.70 | 19.38 | 2.74 | 0.35 | 6.69 | 2.51 |
| Anger (PT: 10002368) | 4 | 6.04 | 2.26 | 6.03 | 16.78 | 2.59 | 0.29 | 6.03 | 2.26 |
| Somnambulism (PT: 10041347) a | 4 | 35.84 | 13.41 | 35.76 | 134.65 | 5.15 | 0.87 | 35.63 | 13.33 |
| Abnormal behavior (PT: 10061422) | 4 | 7.09 | 2.66 | 7.07 | 20.85 | 2.82 | 0.38 | 7.07 | 2.65 |
| Psychotic disorder (PT: 10061920) | 4 | 6.95 | 2.60 | 6.93 | 20.29 | 2.79 | 0.37 | 6.93 | 2.60 |
| Bipolar disorder (PT: 10057667) | 4 | 17.61 | 6.60 | 17.57 | 62.41 | 4.13 | 0.73 | 17.54 | 6.57 |
| Apathy (PT: 10002942) | 3 | 9.85 | 3.17 | 9.83 | 23.77 | 3.30 | 0.17 | 9.82 | 3.16 |
| Sleep terror (PT: 10041010) a | 3 | 34.26 | 11.01 | 34.20 | 96.34 | 5.09 | 0.43 | 34.08 | 10.96 |
| Nervous system disorders (SOC: 10029205) | |||||||||
| Headache (PT: 10019211) | 85 | 5.65 | 4.54 | 5.41 | 308.46 | 2.44 | 2.04 | 5.41 | 4.35 |
| Migraine (PT: 10027599) | 12 | 4.64 | 2.63 | 4.61 | 33.98 | 2.20 | 1.05 | 4.61 | 2.61 |
| Lethargy (PT: 10024264) | 6 | 5.02 | 2.25 | 5.00 | 19.23 | 2.32 | 0.58 | 5.00 | 2.24 |
| Sleep paralysis (PT: 10041002) | 3 | 90.29 | 28.94 | 90.14 | 262.02 | 6.48 | 0.50 | 89.32 | 28.63 |
| Gastrointestinal disorders (SOC: 10017947) | |||||||||
| Nausea (PT: 10028813) | 62 | 3.34 | 2.59 | 3.25 | 97.88 | 1.70 | 1.28 | 3.25 | 2.52 |
| Dry mouth (PT: 10013781) | 10 | 5.64 | 3.03 | 5.61 | 37.93 | 2.49 | 1.11 | 5.61 | 3.01 |
| Dyspepsia (PT: 10013946) | 9 | 3.93 | 2.04 | 3.92 | 19.57 | 1.97 | 0.68 | 3.92 | 2.03 |
| General disorders and administration site conditions (SOC: 10018065) | |||||||||
| Therapeutic product effect decreased (PT: 10082201) | 16 | 5.92 | 3.62 | 5.87 | 64.75 | 2.55 | 1.49 | 5.87 | 3.59 |
| Therapeutic product effect delayed (PT: 10082202) | 7 | 28.36 | 13.49 | 28.25 | 183.50 | 4.82 | 1.66 | 28.17 | 13.40 |
| Crying (PT: 10011469) | 4 | 5.92 | 2.22 | 5.91 | 16.32 | 2.56 | 0.28 | 5.91 | 2.21 |
| Drug effect less than expected (PT: 10083365) | 3 | 7.11 | 2.29 | 7.10 | 15.71 | 2.83 | 0.05 | 7.09 | 2.28 |
| Energy increased (PT: 10048779) | 3 | 21.43 | 6.90 | 21.39 | 58.20 | 4.42 | 0.36 | 21.35 | 6.87 |
| Sensation of foreign body (PT: 10061549) a | 3 | 13.05 | 4.20 | 13.03 | 33.29 | 3.70 | 0.25 | 13.02 | 4.19 |
| Cardiac disorders (SOC: 10007541) | |||||||||
| Palpitations (PT: 10033557) a | 12 | 4.56 | 2.59 | 4.54 | 33.15 | 2.18 | 1.03 | 4.54 | 2.57 |
| Respiratory, thoracic and mediastinal disorders (SOC: 10038738) | |||||||||
| Rhinorrhoea (PT: 10039101) a | 9 | 4.97 | 2.58 | 4.95 | 28.36 | 2.31 | 0.91 | 4.94 | 2.57 |
| Nasal congestion (PT: 10028735) a | 7 | 4.26 | 2.03 | 4.25 | 17.41 | 2.09 | 0.57 | 4.25 | 2.02 |
| Sneezing (PT: 10041232) a | 6 | 10.44 | 4.68 | 10.41 | 51.01 | 3.38 | 1.06 | 10.40 | 4.66 |
| Immune system disorders (SOC: 10021428) | |||||||||
| Seasonal allergy (PT: 10048908) | 7 | 15.22 | 7.24 | 15.16 | 92.48 | 3.92 | 1.43 | 15.14 | 7.20 |
| Metabolism and nutrition disorders (SOC: 10027433) | |||||||||
| Increased appetite (PT: 10021654) | 3 | 8.27 | 2.66 | 8.26 | 19.12 | 3.04 | 0.11 | 8.25 | 2.66 |
Note: The colors of the individual table cells represent the values of each index.
Emerging findings of pitolisant‐induced adverse events.
3.4. Signal difference of pitolisant
The ROR algorithm and Fisher's exact test were applied to investigating the difference of pitolisant signals concerning sex, age, weight, and dose. The volcano plots for difference detection of pitolisant signals are shown in Figure 5. As illustrated in Figure 5B, no significant age difference between patients with age 18–65 years and >65 years in pitolisant‐related signals was found. Figure 5D further proves that the safety profile of pitolisant had no significant difference between doses of 35.6 mg (the maximal dose) and 8.9 mg (the most common dose) groups. Notably, slight variations were observed in relation to sex (Figure 5A) and weight (Figure 5C), with females more likely to develop anxiety (PT: 10002855), and patients with weight >100 kg more prone to lead to lethargy (PT: 10024264).
FIGURE 5.
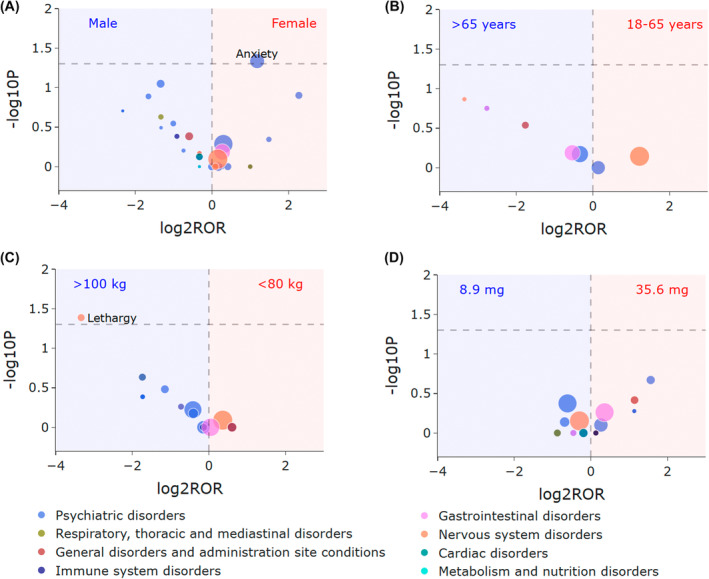
Volcano plots for difference detection of pitolisant signals. (A) Signal difference between females and males. (B) Signal difference between patients with age 18–65 and >65 years. (C) Signal difference between patients with weight <80 and >100 kg. (D) Signal difference between patients taking dose of 35.6 and 8.9 mg. The x‐axis is the logarithm of the ROR value (log2ROR) based on ROR algorithm, and the y‐axis is the negative logarithm of the p‐value calculated using Fisher's exact test (−log10P). The colors of each point represent different SOCs. The sizes of each point represent the case numbers of each PT induced by pitolisant. The larger values in y‐direction represented a strongly significant difference and the bigger size represented a high frequency of each signal at PT level. In this volcano plot, signals within 44 significant disproportionality PTs of pitolisant are shown.
3.5. Focus on death and life‐threatening reports
Special attention was paid on three reports involving death or life‐threatening outcomes. The death case involved a 70‐year‐old male with narcolepsy who was taking pitolisant at a dosage of 8.9 mg once daily. The report date of adverse events and start date of using pitolisant were both July 26, 2022, indicating death occurred on the first day after taking pitolisant. The reported adverse reactions in this case were myocardial infarction and dizziness, with myocardial infarction considered as the most probable cause of death. In addition, this patient also took venlafaxine, methylphenidate, pramipexole, adderall, and Chinese herbal medicines concomitantly.
Further analysis was conducted on the details of the two reports with life‐threatening outcomes. In the first report, a 24‐year‐old male with narcolepsy was prescribed pitolisant at a dosage of 17.8 mg twice daily. The reported adverse reaction was myocarditis. In addition, the patient was taking concomitant medications including amphetamine, lisdexamfetamine dimesylate, sodium oxybate, escitalopram oxalate, and desloratadine. The second life‐threatening report involved a 62‐year‐old female patient who was taking pitolisant at a dosage of 4.5 mg once daily. The reported adverse reaction in this case was pulmonary embolism. Importantly, the patient was concurrently using fluvoxamine, aripiprazole, and diazepam.
3.6. Focus on second quarter of 2022
A significantly high number (n = 284) of reported pitolisant‐associated adverse events was observed in the second quarter of 2022, as shown in Figure 2A. Therefore, special attention was given to the reports in this quarter. The report dates for the 284 cases in the second quarter of 2022 are shown in Figure 6B. Notably, 94.4% (n = 268) of events were reported on May 13, 2022. The event years and report times for the 268 events were further examined, as shown in Figure 6C,D. The majority of 268 pitolisant‐associated adverse events occurred in 2020 and 2019, accounting for 58.2% (n = 156) and 24.3% (n = 65), respectively. None of the 268 events were reported for the first time. As seen in Figure 6D, 68.3% (n = 183) were reported for the second time, followed by 19.8% (n = 53) being reported for the third time, 7.8% (n = 21) being reported for the fourth time.
FIGURE 6.
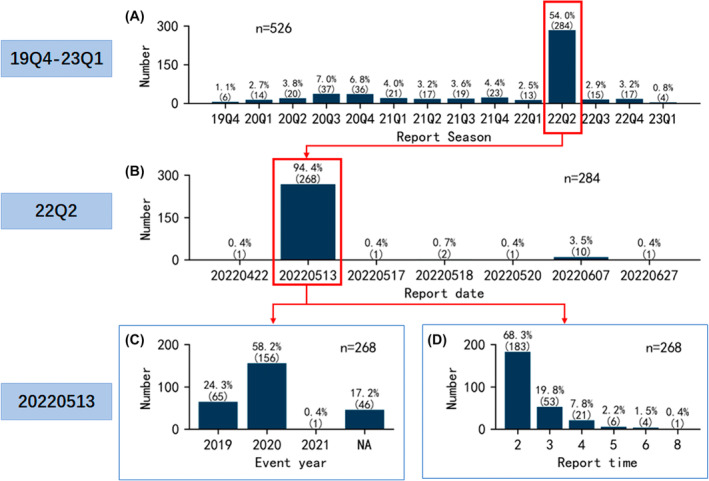
Characteristics of pitolisant‐associated adverse events focusing on the second quarter of 2022. (A) Report seasons of events from the fourth quarter of 2019 to the first quarter of 2023. (B) Report dates of events in the second quarter of 2022. (C) Event years of events reported on May 13, 2022. (D) Report times of events reported on May 13, 2022.
4. DISSCUSSION
Being the first and only medication for narcolepsy not regulated by the United States Drug Enforcement Administration, the question of pitolisant currently under dispute is: Is pitolisant safe for clinical use? As revealed by the pitolisant review report issued by the FDA's CDER, several deaths occurred in the pitolisant development program. However, details from the report showed that the instances of deaths associated with pitolisant were predominantly observed within clinical trials focusing on obstructive sleep apnea (OSA). 21 In two independent, multicenter, double‐blind, randomized, placebo‐controlled, parallel‐design trials for patients with OSA, the adverse events of pitolisant were mainly headache, insomnia, nausea, and vertigo, with no cardiovascular or other significant safety concerns. 22 , 23 A meta‐analysis also proved the security of pitolisant for EDS in OSA. 24 In this study, the indications of pitolisant in all reports were in accordance with the specification, and no report for OSA was detected. As a consequence, the clinical safety of pitolisant for OSA treatment still need to be unveiled by further investigations. Since narcolepsy is currently the only approved indication for pitolisant, the clinical safety of pitolisant for treating narcolepsy is the foremost concern of this study.
Spontaneous reporting systems for suspected adverse drug reactions remain a cornerstone of pharmacovigilance. 25 In the current landscape, signal detection within spontaneous reporting system databases predominantly relies on the application of disproportionality analysis methods. These methods can be broadly categorized into two main groups: frequency count methods and Bayesian methods. 26 The former category primarily encompasses measures such as the ROR, the PRR, and the medicines and healthcare products regulatory agency (MHRA), while the latter category mainly includes the BCPNN and the MGPS. Although some studies have employed individual algorithms to identify meaningful signals, 27 , 28 each algorithm has its own set of limitations. To mitigate potential bias in data analysis, most recent studies have adopted a combination of multiple algorithms. For instance, the combination of ROR and BCPNN has been employed to detect potential adverse events linked to ceftriaxone. 29 In another case, a combination of ROR, MHRA, BCPNN, and MGPS was applied to quantify signals associated with emurafenib. 26 Additionally, the ROR, PRR, BCPNN, and MGPS algorithms have been utilized for data mining in order to quantify signals associated with drugs like secukinumab 30 and osimertinib. 31 Based on the literatures, this study finally chosen two frequency count methods (ROR and PRR) and two predominant Bayesian methods (BCPNN and MGPS) for the exploration of potential associations between adverse event signals and the use of pitolisant.
In this study, death did not emerge as a significant disproportionality signal linked to pitolisant. A multicenter retrospective observational study, encompassing 55 narcolepsy patients from three international narcolepsy centers (Germany, France, and Italy), indicated that insomnia was the most frequently reported adverse event, 19 which aligns with the findings of our study. Notably, the majority (88.8%) of adverse events occurred within the first month of administration in our study. The long‐term safety of pitolisant was also confirmed by a Harmony III Study, which involved 68 patients with 12‐month treatment. 32 Both our study and previous research have consistently demonstrated that significant adverse event signals associated with pitolisant were generally mild and of short duration, underscoring the robust safety profile of the medication.
This study unveiled that there was no notable disparity in the safety profile of pitolisant between the elderly and adults. Recently, a double‐blind, randomized, placebo‐controlled, multisite study recruited 110 patients aged 6–17 years with narcolepsy in 11 sleep centers in five countries (Italy, France, Netherlands, Russia, and Finland). The safety profile of pitolisant for treating narcolepsy symptoms in children was similar to that in adults. 20 Furthermore, there was no significant variance in the safety profile of pitolisant among different dose groups, although slight variations were observed concerning sex and weight. The results illustrated the overall safety of pitolisant across different sex, age, weight, and dose groups.
Seven new significant adverse event signals were detected as related to pitolisant. Notably, a new cardiac disorder signal (palpitations) emerged. Palpitations refer to the perception of an accelerated, irregular, or forceful heartbeat. Although medication‐induced palpitations are generally mild and transient, in some cases, they may trigger severe arrhythmias. Additionally, the result of concomitant drugs suggested a multiple pharmacologic strategy was commonly employed for treating narcolepsy. Some concomitant drugs such as amphetamine, armodafinil, modafinil, and solriamfetol may increase the risk of cardiac disorder. The result emphasized that heart‐related tests should be enhanced during the clinical use of pitolisant, especially when pitolisant is used in combination with drugs that may potentially cause cardiac disorder. In SOC of respiratory, thoracic, and mediastinal disorders, three new signals (rhinorrhoea, nasal congestion, and sneezing) emerged. It is essential to be vigilant about the potential respiratory adverse events, especially when prescribing pitolisant to patients with pre‐existing respiratory conditions.
Regarding the three reported cases of death or life‐threatening outcomes, the most probable adverse reactions were myocardial infarction, myocarditis, and pulmonary embolism. However, according to the signal mining results, none of these adverse reactions were significant disproportionality signals associated with pitolisant. In the REAC dataset, myocardial infarction was reported once, myocarditis was reported once, and pulmonary embolism was reported twice, each accounting for less than 0.2% of the total 1695 adverse events. Despite the low incidence, the occurrence of the serious outcomes highlights the importance of carefully considering cardiac disorder (myocardial infarction, myocarditis) and respiratory, thoracic, and mediastinal disorders (pulmonary embolism). To minimize the potential risk of severe adverse events, it is recommended to enhance monitoring of cardiac and respiratory symptoms in patients using pitolisant.
The concomitant drugs involved in the pitolisant‐associated adverse event reports were highly complex. Taking the second case of a life‐threatening outcome as an example, the patient was concurrently using fluvoxamine, aripiprazole, and diazepam. Pitolisant is primarily metabolized by CYP2D6 and to a lesser extent by CYP3A4, and it also exhibits borderline/weak induction of CYP3A4. However, fluvoxamine acts as an inhibitor of both CYP2D6 and CYP3A4, which can increase the exposure of pitolisant. Additionally, CYP3A4 is involved in the metabolism of aripiprazole. Therefore, the interactions among these medications were quite complex. Furthermore, the results regarding concomitant drugs indicated that pitolisant was frequently used in combination with antidepressants. Some antidepressants are potent inhibitors of CYP2D6 and CYP3A4, which may affect the metabolism of pitolisant. This finding emphasizes the importance of enhanced surveillance for adverse reactions when pitolisant is used in combination with drugs that act as inhibitors of CYP2D6 and CYP3A4 or their substrates.
It was noted that in the first case of a life‐threatening outcome, pitolisant was administered at an incorrect frequency of twice daily, which is higher than the recommended once‐daily frequency. This finding emphasized the importance of the rational use of pitolisant, with particular attention to the dose and frequency of administration.
Interestingly, this study observed a significant increase in the number of adverse events reported for pitolisant during the second quarter of 2022. Following FDA's recommendations, the duplicate reports were identified and removed by selecting the most recent FDA_DT when CASEIDs matched, and choosing the higher PRIMARYID when both CASEID and FDA_DT were identical. The reasons behind the abrupt repeat reporting during the second quarter of 2022 (particularly on May 13, 2022) remain unclear. However, the significant uptick in reports does not necessarily correlate with a proportionate increase in the actual occurrence of adverse events.
There are some limitations that warrant discussion in this study. A notable limitation is that FAERS is an adverse event reporting system based on voluntary reports. The data may be subject to errors, inconsistencies, and duplications due to variations in the quality of reporting sources. Furthermore, the FAERS database lacks detailed medical histories and essential contextual information that can help provide a more comprehensive understanding of the circumstances surrounding an adverse event. Addressing confounding factors such as comorbidities that may impact the occurrence of adverse events makes it difficult to establish direct causal relationships between a specific drug and an adverse event. Finally, the FAERS database typically lacks control groups for comparison. As a consequence, the current findings still need to be confirmed by further empirical investigations. Despite these limitations, this study provides a straightforward and comprehensive evidence of the safety profile of pitolisant in real‐world clinical use.
5. CONCLUSIONS
This study provided a broader understanding of the safety profile of pitolisant. The significant adverse event signals of pitolisant were generally mild and of short duration, indicating the robust safety of pitolisant. However, the complicated interactions of concomitant drugs highlight the need of enhanced surveillance for severe adverse reactions when pitolisant is used in combination with drugs that act as inhibitors of CYP2D6 and CYP3A4 or their substrates. This study further underscore the importance of increased monitoring for cardiac and respiratory adverse reactions when utilizing pitolisant.
AUTHOR CONTRIBUTIONS
Conceptualization: Junxian Zheng and Hongwei Wang. Formal analysis: Cheng Jiang and Jiancheng Qian. Writing—original draft preparation: Cheng Jiang, Jiancheng Qian, Xin Jiang and Shuohan Zhang. Writing, reviewing and editing: Junxian Zheng and Hongwei Wang. All authors have read and agreed to the published version of the manuscript.
FUNDING INFORMATION
This study was supported by the National Natural Science Foundation of China (82174132), Public Service Technology Research Project of Zhejiang Province (LGF22H300010), Medical Health Science and Technology Project of Zhejiang Province (2023RC141), State Administration of Traditional Chinese Medicine Science and Technology Department‐Zhejiang Provincial Administration of Traditional Chinese Medicine Co‐construction of Key Laboratory of Research on Prevention and Treatment for Depression Syndrome (GZY‐ZJ‐SY‐2402), and Special Scientific Research Project of Pharmaceutical Management in County‐level Medical Community of Zhejiang Pharmaceutical Association (2020ZYYJC04).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Approval from an institutional review board is not applicable.
Supporting information
Table S1.
Table S2.
Table S3.
Table S4.
Table S5.
ACKNOWLEDGEMENTS
The authors are grateful to all those who reported the pitolisant‐associated adverse events in FAERS database.
Jiang C, Qian J, Jiang X, Zhang S, Zheng J, Wang H. Is pitolisant safe for clinical use? A retrospective pharmacovigilance study focus on the post‐marketing safety. Pharmacol Res Perspect. 2024;12:e1161. doi: 10.1002/prp2.1161
Cheng Jiang and Jiancheng Qian have contributed equally to this work.
Contributor Information
Junxian Zheng, Email: zjx203337@sina.com.
Hongwei Wang, Email: wanghw1022@163.com.
DATA AVAILABILITY STATEMENT
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
REFERENCES
- 1. Partinen M, Kornum BR, Plazzi G, Jennum P, Julkunen I, Vaarala O. Narcolepsy as an autoimmune disease: the role of H1N1 infection and vaccination. Lancet Neurol. 2014;13(6):600‐613. doi: 10.1016/S1474-4422(14)70075-4 [DOI] [PubMed] [Google Scholar]
- 2. Vanini G. Narcolepsy: mending a broken neural circuit that controls arousal. Curr Biol. 2023;33(8):R316‐R318. doi: 10.1016/j.cub.2023.02.053 [DOI] [PubMed] [Google Scholar]
- 3. Kumar S, Sagili H. Etiopathogenesis and neurobiology of narcolepsy: a review. J Clin Diagn Res. 2014;8(2):190‐195. doi: 10.7860/JCDR/2014/7295.4057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gool JK, van Heese EM, Schinkelshoek MS, et al. The therapeutic potential of opioids in narcolepsy type 1: a systematic literature review and questionnaire study. Sleep Med. 2023;109:118‐127. doi: 10.1016/j.sleep.2023.06.008 [DOI] [PubMed] [Google Scholar]
- 5. Davidson RD, Biddle K, Nassan M, Scammell TE, Zhou ES. The impact of narcolepsy on social relationships in young adults. J Clin Sleep Med. 2022;18(12):2751‐2761. doi: 10.5664/jcsm.10212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abenza Abildúa MJ, Suárez Gisbert E, Pérez Villena A, Lores Gutiérrez V, Pérez‐López C. Labor impact on patients with narcolepsy. Sleep Med. 2023;103:98‐99. doi: 10.1016/j.sleep.2023.01.018 [DOI] [PubMed] [Google Scholar]
- 7. Hansen BH, Andresen HN, Gjesvik J, Thorsby PM, Naerland T, Knudsen‐Heier S. Associations between psychiatric comorbid disorders and executive dysfunctions in hypocretin‐1 deficient pediatric narcolepsy type1. Sleep Med. 2023;109:149‐157. doi: 10.1016/j.sleep.2023.06.021 [DOI] [PubMed] [Google Scholar]
- 8. Liblau RS, Latorre D, Kornum BR, Dauvilliers Y, Mignot EJ. The immunopathogenesis of narcolepsy type 1. Nat Rev Immunol. 2023. doi: 10.1038/s41577-023-00902-9 [DOI] [PubMed] [Google Scholar]
- 9. Hirano K, Morishita Y, Minami M, Nomura H. The impact of pitolisant, an H(3) receptor antagonist/inverse agonist, on perirhinal cortex activity in individual neuron and neuronal population levels. Sci Rep‐UK. 2022;12(1):7015. doi: 10.1038/s41598-022-11032-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abad VC. Calcium, magnesium, potassium, and sodium oxybates oral solution for cataplexy or excessive daytime sleepiness associated with narcolepsy. Expert Opin Pharmacother. 2023;24(8):875‐885. doi: 10.1080/14656566.2023.2204187 [DOI] [PubMed] [Google Scholar]
- 11. Simon P, Hémet C, Ramassamy C, Costentin J. Non‐amphetaminic mechanism of stimulant locomotor effect of modafinil in mice. Eur Neuropsychopharmacol. 1995;5(4):509‐514. doi: 10.1016/0924-977X(95)80011-P [DOI] [PubMed] [Google Scholar]
- 12. Randomized trial of modafinil as a treatment for the excessive daytime somnolence of narcolepsy. Neurology. 2000;54(5):1166‐1175. doi: 10.1212/WNL.54.5.1166 [DOI] [PubMed] [Google Scholar]
- 13. Bae CJ, Zee PC, Leary EB, et al. Effectiveness and tolerability in people with narcolepsy transitioning from sodium oxybate to low‐sodium oxybate: data from the real‐world TENOR study. Sleep Med. 2023;109:65‐74. doi: 10.1016/j.sleep.2023.05.023 [DOI] [PubMed] [Google Scholar]
- 14. Krief S, Berrebi‐Bertrand I, Nagmar I, et al. Pitolisant, a wake‐promoting agent devoid of psychostimulant properties: preclinical comparison with amphetamine, modafinil, and solriamfetol. Pharmacol Res Perspect. 2021;9(5):e855. doi: 10.1002/prp2.855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meskill GJ, Davis CW, Zarycranski D, Doliba M, Schwartz JC, Dayno JM. Clinical impact of pitolisant on excessive daytime sleepiness and cataplexy in adults with narcolepsy: an analysis of randomized placebo‐controlled trials. CNS Drugs. 2022;36(1):61‐69. doi: 10.1007/s40263-021-00886-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sarfraz N, Okuampa D, Hansen H, et al. Pitolisant, a novel histamine‐3 receptor competitive antagonist, and inverse agonist, in the treatment of excessive daytime sleepiness in adult patients with narcolepsy. Health Psychol Res. 2022;10(3):34222. doi: 10.52965/001c.34222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mutti C, Brunetti V, Figorilli M, et al. Clinical characteristics of a large cohort of patients with narcolepsy candidate for pitolisant: a cross‐sectional study from the Italian PASS Wakix® Cohort. Neurol Sci. 2022;43(9):5563‐5574. doi: 10.1007/s10072-022-06210-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Watson NF, Davis CW, Zarycranski D, et al. Time to onset of response to pitolisant for the treatment of excessive daytime sleepiness and cataplexy in patients with narcolepsy: an analysis of randomized, placebo‐controlled trials. CNS Drugs. 2021;35(12):1303‐1315. doi: 10.1007/s40263-021-00866-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Triller A, Pizza F, Lecendreux M, et al. Real‐world treatment of pediatric narcolepsy with pitolisant: a retrospective, multicenter study. Sleep Med. 2023;103:62‐68. doi: 10.1016/j.sleep.2023.01.015 [DOI] [PubMed] [Google Scholar]
- 20. Dauvilliers Y, Lecendreux M, Lammers GJ, et al. Safety and efficacy of pitolisant in children aged 6 years or older with narcolepsy with or without cataplexy: a double‐blind, randomised, placebo‐controlled trial. Lancet Neurol. 2023;22(4):303‐311. doi: 10.1016/S1474-4422(23)00036-4 [DOI] [PubMed] [Google Scholar]
- 21. Center for Drug Evaluation and Research . Application number: 211150Orig2s000, Clinical Reviews(S). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/211150Orig2s000MedR.pdf
- 22. Pepin JL, Georgiev O, Tiholov R, et al. Pitolisant for residual excessive daytime sleepiness in OSA patients adhering to CPAP: a randomized trial. Chest. 2021;159(4):1598‐1609. doi: 10.1016/j.chest.2020.09.281 [DOI] [PubMed] [Google Scholar]
- 23. Dauvilliers Y, Verbraecken J, Partinen M, et al. Pitolisant for daytime sleepiness in patients with obstructive sleep apnea who refuse continuous positive airway pressure treatment. A randomized trial. Am J Resp Crit Care. 2020;201(9):1135‐1145. doi: 10.1164/rccm.201907-1284OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang J, Li X, Yang S, et al. Pitolisant versus placebo for excessive daytime sleepiness in narcolepsy and obstructive sleep apnea: a meta‐analysis from randomized controlled trials. Pharmacol Res. 2021;167:105522. doi: 10.1016/j.phrs.2021.105522 [DOI] [PubMed] [Google Scholar]
- 25. Egberts AC, Meyboom RH, van Puijenbroek EP. Use of measures of disproportionality in pharmacovigilance: three Dutch examples. Drug Saf. 2002;25(6):453‐458. doi: 10.2165/00002018-200225060-00010 [DOI] [PubMed] [Google Scholar]
- 26. Liu Y, Dong C, He X, Shu Y, Wu P, Zou J. Post‐marketing safety of vemurafenib: a real‐world pharmacovigilance study of the FDA Adverse Event Reporting System. J Pharm Pharm Sci. 2022;25:377‐390. doi: 10.18433/jpps33020 [DOI] [PubMed] [Google Scholar]
- 27. Shu Y, He X, Wu P, Liu Y, Ding Y, Zhang Q. Gastrointestinal adverse events associated with semaglutide: a pharmacovigilance study based on FDA Adverse Event Reporting System. Front Public Health. 2022;10:996179. doi: 10.3389/fpubh.2022.996179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kong W, Mao W, Zhang L, Wu Y. Disproportionality analysis of quinolone safety in children using data from the FDA Adverse Event Reporting System (FAERS). Front Pediatr. 2022;10:1069504. doi: 10.3389/fped.2022.1069504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu X, Xu Z, Ma J, et al. Hepatobiliary calculi associated with ceftriaxone treatment: an analysis of FAERS data from 2004 to 2021. J Infect Chemother. 2023;29(2):136‐142. doi: 10.1016/j.jiac.2022.10.006 [DOI] [PubMed] [Google Scholar]
- 30. Shu Y, Ding Y, Liu Y, Wu P, He X, Zhang Q. Post‐marketing safety concerns with secukinumab: a disproportionality analysis of the FDA Adverse Event Reporting System. Front Pharmacol. 2022;13:862508. doi: 10.3389/fphar.2022.862508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yin Y, Shu Y, Zhu J, Li F, Li J. A real‐world pharmacovigilance study of FDA Adverse Event Reporting System (FAERS) events for osimertinib. Sci Rep‐UK. 2022;12(1):19555. doi: 10.1038/s41598-022-23834-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dauvilliers Y, Arnulf I, Szakacs Z, et al. Long‐term use of pitolisant to treat patients with narcolepsy: Harmony III Study. Sleep. 2019;42(11). doi: 10.1093/sleep/zsz174 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Table S2.
Table S3.
Table S4.
Table S5.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.


