Abstract
Determining pathological complete response (pCR) could be an important step in planning individual treatment, hence improving the prognosis in terms of survival. Achieving breast pCR not only improves survival but is also linked to a disease-free axilla, therefore increasing the likelihood of avoiding axillary surgery safely. The current trend in de-escalating axillary management surgically or in applying radiotherapy to the axilla is dependent primarily on breast cancer (BC) patients achieving pCR. Studies have demonstrated that certain characteristics can predict pCR, even though it is still difficult to identify these elements. A review of the literature was carried out to determine these factors and their clinical applications. A search was carried out in the MEDLINE database using PubMed, Google Scholar, and EMBASE. This yielded 1368 studies, of which 60 satisfied the criteria. The studies were categorized according to the subject they dealt with. These parameters included age, race, subtypes, clinicopathological, immunological, imaging, obesity, Ki-67 status, vitamin D, and genetics. These factors, in combination, can be used for specific subtypes to individualize treatment and monitor response to therapy. The predictors of pCR are diverse and should be utilized to personalize patient treatment, ultimately inducing the best outcomes. These determinants can also be employed for monitoring responses to neoadjuvant therapy, thereby adjusting treatment. The development of standardized markers for the diversity of BC subtypes still needs additional future research. These factors must be applied in concert in order to provide optimal results.
Keywords: Pathological complete response, neoadjuvant therapy, early breast cancer, pCR biomarkers
Key Points
• Achieving pathological complete response (pCR) is the desired end result of using neoadjuvant therapy in responders.
• Identifying factors that determine pCR in breast cancer patients can help guide treatment, hence individualizing it.
• Achieving pCR in the breast correlates well with pCR in the axilla; this can result in the de-escalation of axillary surgery.
• pCR determinants should be used in combination to achieve optimal results. Therefore, standardization of these factors is essential.
Introduction
Locally advanced breast cancer (LABC) presents unique challenges in treatment and management, requiring a multidisciplinary approach that may involve surgery, radiation therapy, chemotherapy, hormone therapy, and targeted therapy. Neoadjuvant therapy is becoming the treatment of choice for responders, helped by the improvements made in effective drugs. Achieving pathological complete response (pCR) is the aim of neoadjuvant therapy. pCR in the breast correlates well with pCR in the axilla. Current research when dealing with axillary surgery focuses on de-escalation. This is especially true when dealing with patients who present with clinically node-negative (cN0) breast cancer and respond well to neoadjuvant breast therapy, achieving pCR. This can also be applied to clinically node-positive axilla (cN+), as seen in certain studies. Therefore, determining the factors that predict pCR is essential. Patients who show these factors can be expected to have improved outcomes and could avoid axillary lymph node dissection (ALND). Determining pCR could be an essential step in planning individual treatment, hence improving the prognosis. This could also help identify patients who could be candidates for the omission of sentinel lymph node biopsy (SLNB). pCR also correlates well with overall survival. Although it remains challenging to determine these factors, studies have shown certain factors to be associated with pCR. The aim of this review was to identify these factors and investigate them extensively in relation to the evidence available in the literature, emphasizing their clinical applications.
Pathological complete response is defined as no residual disease in either the breast or axillary nodes. Locally advanced breast cancer (LABC) that responds to neoadjuvant therapy correlates well with disease-free axilla. The rapid shift now toward de-escalation of treatment for BC, surgically or medically, is gaining acceptance by many authors. With more evidence coming to light, there is a shift in favor of doing more SNLB and avoiding completion of ALND for selected candidates. Moreover, the improvement in neoadjuvant therapy was pivotal in achieving this, as well as improving survival. This de-escalation is further investigated to omit SLNB in clinically node-negative patients (cN0) who achieve pCR (1). These patients are likely to have a lower chance of axillary recurrence, hence avoiding ALND. Omitting ALND not only leads to early recovery but also decreases morbidity and improves quality of life. Furthermore, determining pCR in patients before treatment will help plan and individualize therapy. Post-mastectomy radiotherapy (PMRT) in patients with 1-3 positive lymph nodes who achieve pCR is also a subject of ongoing debate. It is postulated that omitting PMRT in these patients might lead to decreased morbidity, improve quality of life and avoid unnecessary exposure. Factors that determine response to neoadjuvant therapy and hence pCR will help to achieve these goals.
Materials and Methods
The MEDLINE database was searched using PubMed, Google Scholar, and EMBASE up to and including September 2023. The search words included pathological complete response, breast cancer, response to neoadjuvant therapy in breast cancer, and genetic mutations in breast cancer. Inclusion criteria were factors determining pathologic complete response including neoadjuvant therapy, race, age, BC subtypes, genetic mutations, and imaging. Exclusion criteria included case reports, incomplete data, specific treatments, correspondence, papers other than in English, and repetitive topics.
Results
Out of 1368 manuscripts, 60 satisfied the inclusion criteria. Full texts were obtained and analyzed. Factors were identified and grouped individually for discussion (Figure 1). The areas that were most covered and had an abundance of research papers were, subtypes, biomarkers and imaging. Although there were enough studies on most of the subjects to form an opinion, some lacked adequate numbers. This included race, plasma fibrinogen and the use of anti-lipids. In order to be as relevant as possible, the studies used were the most recent.
Figure 1.
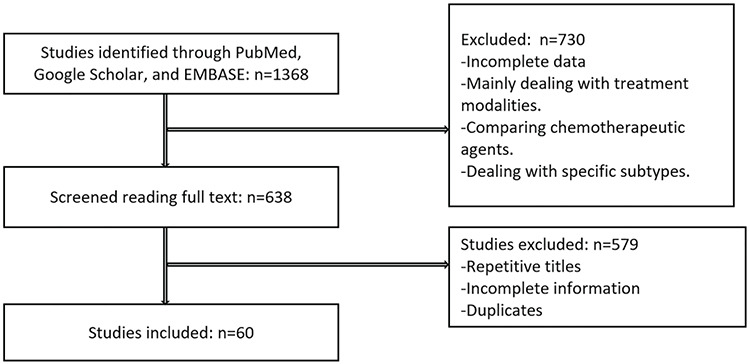
Consort diagram showing the number (n) of studies excluded and included
Although it is challenging to determine the factors that favor pCR, the factors discussed below are well-established and supported by numerous studies. Furthermore, identifying these factors will help improve treatment, hence improving prognosis in terms of disease-free survival and overall survival. However, not all patients achieve pCR due to the biological nature of BC. Therefore, it is essential to identify these patients and improve their response to neoadjuvant therapy. This will also avoid using these cytotoxic drugs in patients who otherwise will not benefit and will require other modes of treatment. The factors that influence pCR are categorized below.
Race
Terman et al. (2) looked at 2196 black and white women treated in Chicago over the past 20 years for early breast cancer. Of the 397 women receiving neoadjuvant chemotherapy (NACT), 47.5% of young white women achieved pCR, compared to 26.8% of young black women. They concluded that black women had a poorer outcome than white women, particularly in the young age group. Hence, the response to NACT and achieving pCR is significantly higher in white women, which might indicate a different pathological process. This racial disparity was also confirmed in another study (3). The disparities and lower pCR achievement were across all subtypes and correlated with poorer survival. Both studies highlighted the need to understand the disease process in black women in order to improve outcome and survival.
These findings call for further research into young black women to understand why this disparity exists and help introduce effective treatment.
Age
Verdial et al. (4) identified 1383 women with stage I-III BC treated with NACT and subsequent surgery. pCR and breast/axillary downstaging rates were assessed and compared across age groups. Younger women were significantly more likely to have ductal histology, poorly differentiated tumors, and BRCA mutations; 35% of tumors were hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR-positive/HER2-negative), 36% were HER2-positive, and 29% were triple-negative breast cancer (TNBC) patients, with similar subtype distribution across age groups. Overall, pCR rates did not differ by age. However, among patients with TNBC tumors, younger women had higher pCR rates (52% vs. 35% among those aged 41–60 years and 29% among those aged ≥61 years). They were more likely to have tumors with high tumor-infiltrating lymphocyte (TIL) concentrations. They concluded that younger women undergoing NACT for axillary downstaging were more likely to avoid ALND across all subtypes. Despite equivalent breast downstaging and breast-conserving surgery (BCS) eligibility rates across age groups, younger women were less likely to undergo BCS.
Subtypes, Tumor Size, and Grade
The subtypes that have been shown by studies to be favorable to achieving pCR include HER2-positive and TNBC subtypes. The former two are superior for attaining pCR compared to hormone receptor-positive BC. Patients with these subtypes who achieve pCR have a very high chance of avoiding surgery. Those who do not respond tend to have a poor prognosis. pCR achievement is also related to disease-free survival (DFS), particularly for HER2-positive and TNBC (5). Overall, factors related to pCR were nonluminal subtype, high grade, and HER2 overexpression. Residual tumor and nodal stage after NACT significantly correlated with DFS and OS. Similarly, pCR after NACT showed significantly better DFS, particularly for HER2-positive, TNBC, and HER2-positive luminal B profiles (6). Luminal B (HER2-positive) subtype, HER2 overexpression subtype, and TNBC subtype were factors in predicting pCR (7). HER2-low BC patients represent roughly half of the cases treated with neoadjuvant therapy and have poor treatment responses. In the absence of pCR, HER2-low BC patients have a dismal prognosis, especially when their primary tumor hormone receptor status is negative. Therefore, studies are needed to define the biology of these tumors for new therapeutic targets and to incorporate HER2-targeting agents in early-stage treatment (8). Recent studies have reported several subtypes of TNBC, distinguishable by gene expression analysis, that may respond differently to treatment. Furthermore, novel agents, including pertuzumab or T-DM1 for HER2-type BC, bevacizumab or PARP inhibitors for TNBC, or combination regimens with these novel agents, are expected to achieve higher pCR rates and improve patient prognosis (9). Achievement of pCR led to significantly better overall survival in women with HER2-positive tumors and also to significantly better locoregional survival in women treated for TNBC. Predictive factors of pCR were a high pathologic grade, the HER2 molecular subtype, positive estrogenic hormonal receptors, and a positive HER2 receptor (10). Assessing nearly 14,000 women from a contemporary United States database, Haque et al. (11) examined the relationship between response to NACT and molecular subtype. Women with luminal A disease are the least likely to undergo pCR, with the highest rates of HER2 disease. The degree of response is associated with OS, especially in luminal B, HER2, and TNBC patients. Despite the comparatively higher likelihood of achieving pCR in TNBC cases, they found that this subgroup may still experience a survival disadvantage. pCR rate in ER expression also varies. The rate of low ER-positive tumors was similar to that of ER-negative tumors but significantly different from the rate of moderately ER-positive and high ER-positive tumors. Patients with pCR had an excellent prognosis regardless of their ER status. In patients with residual disease (no pCR), the recurrence and death rates were higher in ER-negative and low ER-positive cases compared with moderate and high ER-positive cases (12). When considering HER2-positive/HR-negative and HER2-positive/HR-positive patients, HER2-positive patients achieved more significant benefit from HER2-targeted treatment, although the efficacy of neoadjuvant therapy was relatively poor (13). Patients with TNBC and HER2-positive BC have the highest rates of BCS and pCR after neoadjuvant chemotherapy. Patients with these subtypes are most likely to be candidates for less invasive surgical approaches after chemotherapy (14). Furthermore, tumor size does not impact response to neoadjuvant therapy or pCR rate across all subtypes (15).
Obesity
Obesity is considered a risk factor for BC and is associated in some studies with a low pCR rate; other studies, as discussed below, found no association. There seems to be evidence from the numerous studies of the association of BC with obesity, which warrants further prospective research. Studies have reported that BMI was not found to influence the rate of pCR (16, 17). On the contrary, in other studies, it was found that obesity had a negative impact on pCR. Rasmy and Sorour (18) found that 58.3% of patients who failed to achieve pCR had a BMI above the normal level; they also had higher relapse rates and lower survival rates compared with normal BMI patients. It was observed that obesity was a significant independent prognostic factor that has an adverse effect on pCR (19, 20).
Vitamin D
Numerous studies have explored the relationship between vitamin D and BC incidence, progression, prognosis, and pCR rate. Vitamin D regulates the expression of genes essential in the development and progression of BC. The effect of vitamin D on the pCR rate has been looked at in numerous medical trials. Vitamin D deficiency was defined as <20 ng/mL. Vitamin D deficiency is associated with the inability to reach pCR in patients with BC undergoing NACT (21). Other studies have found no association between vitamin D and pCR (22). However, it is essential to normalize vitamin D pre- and post-therapy to maintain skeletal health. The discrepancies in the role of vitamin D warrant further clinical trials on a larger scale.
Serum Lipids
Serum lipid alteration may play a role in BC progression and achieving pCR. High density lipoprotein (HDL) cholesterol has been linked to a reduced risk of BC incidence, while low density lipoprotein (LDL) cholesterol and triglycerides have shown associations with increased risk. Chemotherapy increased the levels of triglycerides, total cholesterol, and LDL cholesterol but decreased the level of HDL cholesterol. Preoperative dyslipidemia was significantly associated with the axillary pCR rate. Dyslipidemia deteriorated after chemotherapy. Thus, the full-course serum lipid level may serve as a blood marker for predicting BC prognosis (23). The administration of anti-lipids such as simvastatin combined with chemotherapy showed improvements in pathological response in patients with LABC (24).
Plasma Fibrinogen
Elevated levels of plasma fibrinogen have been linked with increased tumor aggressiveness, metastasis, and poor prognosis in various malignancies, including BC. Low plasma fibrinogen pretreatment levels have been associated with higher rates of achieving pCR. This is potentially attributed to reduced tumor cell proliferation and angiogenesis. Low pretreatment plasma fibrinogen (<3.435 g/L) is an independent predictive factor for pCR to NACT in BC patients (25).
Biomarkers
Biomarkers play a pivotal role in predicting pCR in patients undergoing neoadjuvant therapy. While several biomarkers have shown promise, their clinical application requires further specification. Combining two or more biomarkers might be necessary to enhance predictive value. They can be employed to initiate individualized treatment strategies, such as adding targeted therapies for certain subtypes.
Ki-67
Ki-67 is a nuclear protein used for assessing cell proliferation in BC. High Ki-67 expression is associated with ER negativity and HER2 positivity. The level of Ki-67 expression is a prognostic factor predicting disease-free and overall survival. A high Ki-67 level was significantly associated with breast pCR in BC patients receiving NACT (26). The cut-off of Ki-67 expression has been suggested at greater than 35% (27). Ki-67 expression was found to be a prognostic independent factor across all subtypes, including HR-negative (28). The expression level has also been shown to be associated with pCR of the axilla in HR-positive patients and can guide treatment options. This will improve downstaging of the axilla, leading to the avoidance of axillary surgery, as is the case with HER2-positive and TNBC (29). Although Ki-67 has a significant role in pCR prediction and treatment, it is limited by representative tissue sampling, staining, and interobserver variability. Therefore, it is essential to have standardized guidelines for its clinical application. Ki-67 remains a multifaceted approach to treatment and should be looked at in the context of other biomarkers.
Tumor-Infiltrating Lymphocytes and other Immunological Factors
The tumor microenvironment in BC consists of various immune cell populations, including T lymphocytes (CD4+ and CD8+), B lymphocytes, natural killer cells, and tumor-associated macrophages. These cells play pivotal roles in modulating the immune response of the tumor. TILs are predictive for response to neoadjuvant chemotherapy in HER2-positive and TNBC patients. A pooled analysis of 3771 patients carried out by Denkert et al. (30) found that pCR was consistently higher in higher TIL in luminal-HER2-negative, HER2-positive, and TNBC. TILs were also associated with a survival benefit in HER2-positive BC and TNBC. In contrast, increased TILs were an adverse prognostic factor for survival in luminal HER2-negative BC, suggesting a different biology of the immunological infiltrate in this subtype. Increased levels of TILs were associated with increased rates of response to NACT and an improved prognosis for the molecular subtypes of TNBC and HER2-positive BC but not for patients with HR-positive BC. A threshold of 20% TILs was the most potent outcome prognosticator of pCR (31). The platelet-to-lymphocyte ratio (PLR) has also been found to predict pCR. Low PLR is found to be favorable for achieving pCR (32, 33, 34). The neutrophil-to-lymphocyte ratio has been suggested as a predictive factor for pCR in Luminal B/Her2-negative and postmenopausal subgroups. It was found to be significantly higher in those patients who achieved pCR (35).
MicroRNAs (miRNAs)
MicroRNAs (miRNAs) are small, noncoding RNA molecules that play a crucial role in post-transcriptional gene regulation. Emerging evidence suggests that dysregulation of miRNA expression patterns in BC is associated with treatment response, particularly in achieving pCR following therapy. miRNAs are believed to predict the response to NACT. Therefore, establishing biomarkers that identify responses to NACT is imperative to personalizing treatment strategies. miRNAs, in combination with other biomarkers, hold great promise. A prospective study carried out by Davey et al. (36) found that reduced circulating miRNA was a predictor of pCR.
Circulating Tumor DNA (ctDNA)
Circulating tumor DNA (ctDNA) is a fragmented DNA released into the bloodstream by tumor cells. The noninvasive analysis of ctDNA is emerging as a significant predictor of response to treatment in BC. ctDNA levels correlate with tumor burden, stage, and genetic alteration in BC patients. ctDNA monitoring during and after therapy gives a good indication of the response to therapy. A reduction in ctDNA during treatment may predict a higher likelihood of achieving pCR. Lack of ctDNA clearance was a significant predictor of poor response and metastatic recurrence, while clearance was associated with improved survival even in patients who did not achieve pCR. Personalized monitoring of ctDNA during NACT of high-risk, early BC may aid in real-time assessment of treatment response and help fine-tune pCR as a surrogate endpoint of survival (37). Detection and persistence of ctDNA during therapy may have the potential to negatively predict response to neoadjuvant treatment and identify patients who will not achieve pCR (38). Therefore, integrating ctDNA profiling into the management of LABC patients might improve clinical outcomes (39).
Genetics
BRCA 1 and 2 are genes that are crucial in maintaining genomic stability. Mutations in these genes have been linked with an increased risk of developing BC and TNBC in particular. BRCA mutations have been associated with the likelihood of achieving higher rates of pCR (40). This response to chemotherapy is attributed to various factors, including defective DNA repair mechanisms and increased sensitivity to certain chemotherapeutic agents, such as platinum-based drugs. This fact may guide treatment decisions, leading to more personalized therapeutic strategies for patients with BRCA mutations. TNBC has the highest percentage of BRCA mutations among the BC subtypes. In TNBC patients, platinum-based NACT is associated with significantly increased pCR rates. Platinum-based NACT may be considered an option for TNBC patients (41). Therefore, it is reported that BRCA1/2 mutation status leads to better responses to NACT in BC (42). NACT is not frequently used in ER-positive or HER2-negative BC because around 10% of patients achieve pCR. Since NACT can result in cancer downstaging both in the breast and axilla and prevent morbid surgery, a score to predict pCR in this population will be crucial to identify patients who can benefit from this approach. Oshi et al. (43) looked at the 5-gene score to predict pCR in HR-positive and HER2-patients, and they concluded that the 5-gene score reflects cancer cell proliferation and immune cell infiltration and predicts pCR after NACT in ER-positive and HER2-negative BC.
Neoadjuvant Therapy
The choice, combination, and dose of chemotherapeutic agents play a pivotal role in achieving pCR. The addition of targeted treatment, as in HER2-positive patients, can also increase the rate of achieving pCR. A combination of therapies, including targeted therapy, as in HER2-positive subtypes, has yielded greater results in pCR rates, hence improving the prognosis. Therefore, identifying the right dose and combination for the different subtypes is crucial to achieving these goals. This can be demonstrated as an example in TNBC. Predicted rates of pCR for TNBC treated with sequential taxane/anthracycline regimens range from 35% to 48%. With the addition of a platinum agent, pCR rates of 55% are predicted. Further increases have been observed with the addition of immune checkpoint inhibitors to this standard chemotherapy backbone (44). In the pivotal KEYNOTE-522 clinical trial, pCR rates of 65% and 69% were reported for chemotherapy plus pembrolizumab in the overall and PD-L1-positive subgroups, respectively (45).
Imaging
The use of imaging in predicting pCR is very challenging and may be used in association with other biomarkers. It provides a noninvasive option but is limited to certain subtypes. The most commonly used imaging technique for predicting pCR is magnetic resonance imaging (MRI). Mammography has been employed to look at breast density and its association with pCR. The findings suggest that although mammographic density can be associated with HR positivity and these patients are unlikely to achieve pCR, its role in determining pCR independently is limited (46). However, microcalcification has been reported to be a predictor of poor NACT response and hence a poor rate of pCR (47). The TIL-ultrasonography (US) score determined through characteristic US findings has predictive performance for lymphocyte-predominant breast cancer. TILs-US scores can be used to evaluate the therapeutic effect of NACT and may be used as a noninvasive, convenient, and alternative method to assess stromal TILs in pretreatment biopsies; this is particularly true for HER2-positive and TNBC (48). Choudhery et al. (49) suggested an MRI radiomics by looking at the median volume, median longest axial tumor diameter, and median longest volumetric diameter among tumor subtypes of luminal, HER2-positive, and TNBC, in which there was a significant difference. There was also a significant difference in minimum signal intensity and entropy among the tumor subtypes. Additionally, sphericity in HER2-positive tumors and entropy within luminal tumors were significantly associated with pCR. Multiple features demonstrated a significant association with pCR and these authors suggested that MRI radiomics features are associated with different molecular subtypes of BC and pCR. These features may be noninvasive imaging biomarkers to identify cancer subtypes and predict responses to NACT. Radiomics based on pretreatment staging contrast-enhanced computed tomography has also been developed and validated for individualized prediction of pCR to neoadjuvant therapy in BC, which could assist clinical decision-making and improve patient outcomes (50).
Conclusion
The factors that are well established and supported by ample clinical research include BC subtypes, Ki-67, ctDNA, and TIL, among others. However, despite the suggestions and future potential use of certain factors, they remain in their infancy and require more studies. Such factors include anti-lipids, plasma fibrinogen, and vitamin D. Although race is suggested as a pCR predictor, it has only been looked at in specific populations and has to be applied as such. The predictors of pCR are diverse and should be utilized to personalize patient treatment, ultimately inducing the best outcomes. These determinants can also be employed for monitoring responses to neoadjuvant therapy, thereby adjusting treatment. The development of standardized markers for the diverse subtypes still needs additional future research. These factors must be applied in concert in order to provide optimal results.
Footnotes
Peer-review: Internally peer-reviewed.
Financial Disclosure: The author declared that this study received no financial support.
References
- 1.Alamoodi M, Wazir U, Mokbel K, Patani N, Varghese J, Mokbel K. Omitting Sentinel Lymph Node Biopsy after Neoadjuvant Systemic Therapy for Clinically Node Negative HER2 Positive and Triple Negative Breast Cancer: A Pooled Analysis. Cancers (Basel) 2023;15:3325. doi: 10.3390/cancers15133325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terman E, Sheade J, Zhao F, Howard FM, Jaskowiak N, Tseng J, et al. The impact of race and age on response to neoadjuvant therapy and long-term outcomes in Black and White women with early-stage breast cancer. Breast Cancer Res Treat. 2023;200:75–83. doi: 10.1007/s10549-023-06943-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao F, Miyashita M, Hattori M, Yoshimatsu T, Howard F, Kaneva K, et al. Racial disparities in pathological complete response among patients receiving neoadjuvant chemotherapy for early-stage breast cancer. JAMA Netw Open. 2023;6:e233329. doi: 10.1001/jamanetworkopen.2023.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verdial FC, Mamtani A, Pawloski KR, Sevilimedu V, D’Alfonso TM, Zhang H, et al. The effect of age on outcomes after neoadjuvant chemotherapy for breast cancer. Ann Surg Oncol. 2022;29:3810–3819. doi: 10.1245/s10434-022-11367-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orsaria P, Grasso A, Ippolito E, Pantano F, Sammarra M, Altomare C, et al. Clinical Outcomes Among Major Breast Cancer Subtypes After Neoadjuvant Chemotherapy: Impact on Breast Cancer Recurrence and Survival. Anticancer Res. 2021;41:2697–2709. doi: 10.21873/anticanres.15051. [DOI] [PubMed] [Google Scholar]
- 6.Orsaria P, Grasso A, Ippolito E, Pantano F, Sammarra M, Altomare C, et al. Clinical Outcomes Among Major Breast Cancer Subtypes After Neoadjuvant Chemotherapy: Impact on Breast Cancer Recurrence and Survival. Anticancer Res. 2021;41:2697–2709. doi: 10.21873/anticanres.15051. [DOI] [PubMed] [Google Scholar]
- 7.Chou HH, Kuo WL, Yu CC, Tsai HP, Shen SC, Chu CH, et al. Impact of age on pathological complete response and locoregional recurrence in locally advanced breast cancer after neoadjuvant chemotherapy. Biomed J. 2019;42:66–74. doi: 10.1016/j.bj.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Cosimo S, La Rocca E, Ljevar S, De Santis MC, Bini M, Cappelletti V, et al. Moving HER2-low breast cancer predictive and prognostic data from clinical trials into the real world. Front Mol Biosci. 2022;9:996434. doi: 10.3389/fmolb.2022.996434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashi N, Iwase M, Ochi T, Seki A, Matsuda N, Yamauchi H. [The Role of Preoperative Chemotherapy Depending on Breast Cancer Subtype] Gan To Kagaku Ryoho. 2016;43:1149–1156. [PubMed] [Google Scholar]
- 10.Cirier J, Body G, Jourdan ML, Bedouet L, Fleurier C, Pilloy J, et al. Impact de la réponse histologique complète à la chimiothérapie néoadjuvante pour cancer du sein selon le sous-type moléculaire [Impact of pathological complete response to neo-adjuvant chemotherapy in invasive breast cancer according to molecular subtype] Gynecol Obstet Fertil Senol. 2017;45:535–544. doi: 10.1016/j.gofs.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Haque W, Verma V, Hatch S, Suzanne Klimberg V, Brian Butler E, Teh BS. Response rates and pathologic complete response by breast cancer molecular subtype following neoadjuvant chemotherapy. Breast Cancer Res Treat: 2018;170:559–567. doi: 10.1007/s10549-018-4801-3. [DOI] [PubMed] [Google Scholar]
- 12.Landmann A, Farrugia DJ, Zhu L, Diego EJ, Johnson RR, Soran A, et al. Low Estrogen Receptor (ER)-Positive Breast Cancer and Neoadjuvant Systemic Chemotherapy: Is Response Similar to Typical ER-Positive or ER-Negative Disease? Am J Clin Pathol. 2018;150:34–42. doi: 10.1093/ajcp/aqy028. [DOI] [PubMed] [Google Scholar]
- 13.Zhao B, Zhao H, Zhao J. Impact of hormone receptor status on the efficacy of HER2-targeted treatment. Endocr Relat Cancer. 2018;25:687–697. doi: 10.1530/ERC-18-0029. [DOI] [PubMed] [Google Scholar]
- 14.Boughey JC, McCall LM, Ballman KV, Mittendorf EA, Ahrendt GM, Wilke LG, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg. 2014;2260:608–616. doi: 10.1097/SLA.0000000000000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baron P, Beitsch P, Boselli D, Symanowski J, Pellicane JV, Beatty J, et al. Impact of tumor size on probability of pathologic complete response after neoadjuvant chemotherapy. Ann Surg Oncol. 2016;23:1522–1529. doi: 10.1245/s10434-015-5030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erbes T, Stickeler E, Rücker G, Buroh S, Asberger J, Dany N, et al. BMI and Pathologic Complete Response to Neoadjuvant Chemotherapy in Breast Cancer: A Study and Meta-Analysis. Clin Breast Cancer. 2016;16:119–132. doi: 10.1016/j.clbc.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Aguiar D, Ros L, Pérez D, Croissier L, Mori M, Hernández M, et al. Impact of body mass index on pathological complete response and survival of breast cancer patients receiving neoadjuvant chemotherapy. Breast Dis. 2022;41:351–361. doi: 10.3233/BD-210071. [DOI] [PubMed] [Google Scholar]
- 18.Rasmy A, Sorour Y. Effect of Obesity on Neoadjuvant Systemic Therapy Outcomes in Patients with Early Breast Cancer: A Retrospective Institutional Study. Asian Pac J Cancer Prev. 2020;21:683–691. doi: 10.31557/APJCP.2020.21.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karatas F, Erdem GU, Sahin S, Aytekin A, Yuce D, Sever AR, et al. Obesity is an independent prognostic factor of decreased pathological complete response to neoadjuvant chemotherapy in breast cancer patients. Breast. 2017;32:237–244. doi: 10.1016/j.breast.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Emirzeoglu L, Arici S, Sahin AB, Ocak B, Ak N, Ay S, et al. The predictive importance of body mass index on response to neoadjuvant chemotherapy in patients with breast cancer. Breast Care (Basel) 2023;18:42–48. doi: 10.1159/000526732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viala M, Chiba A, Thezenas S, Delmond L, Lamy PJ, Mott SL, et al. Impact of vitamin D on pathological complete response and survival following neoadjuvant chemotherapy for breast cancer: a retrospective study. BMC Cancer. 2018;18:770. doi: 10.1186/s12885-018-4686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JS, Haule CC, Kim JH, Lim SM, Yoon KH, Kim JY, et al. Association between changes in serum 25-hydroxyvitamin D levels and survival in patients with breast cancer receiving neoadjuvant chemotherapy. J Breast Cancer. 2018;21:134–141. doi: 10.4048/jbc.2018.21.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma Y, Lv M, Yuan P, Chen X, Liu Z. Dyslipidemia is associated with a poor prognosis of breast cancer in patients receiving neoadjuvant chemotherapy. BMC Cancer. 2023;23:208. doi: 10.1186/s12885-023-10683-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yulian ED, Siregar NC. Combination of Simvastatin and FAC Improves Response to Neoadjuvant Chemotherapy in Locally Advanced Breast Cancer. Cancer Res Treat. 2021;53:1072–1083. doi: 10.4143/crt.2020.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Wang Y, Chen R, Tang Z, Peng Y, Jin Y, et al. Plasma fibrinogen acts as a predictive factor for pathological complete response to neoadjuvant chemotherapy in breast cancer: a retrospective study of 1004 Chinese breast cancer patients. BMC Cancer. 2021;21:542. doi: 10.1186/s12885-021-08284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ekinci F, Uzun M, Demir B, Unek IT, Erdogan AP. Factors Predicting Response in Breast Cancer Receiving Neoadjuvant Therapy and the Role of Ki67 Labeling Index. J Coll Physicians Surg Pak. 2023;33:872–878. doi: 10.29271/jcpsp.2023.08.872. [DOI] [PubMed] [Google Scholar]
- 27.Jain P, Doval DC, Batra U, Goyal P, Bothra SJ, Agarwal C, et al. Ki-67 labeling index as a predictor of response to neoadjuvant chemotherapy in breast cancer. Jpn J Clin Oncol. 2019;49:329–338. doi: 10.1093/jjco/hyz012. [DOI] [PubMed] [Google Scholar]
- 28.Tan QX, Qin QH, Yang WP, Mo QG, Wei CY. Prognostic value of Ki67 expression in HR-negative breast cancer before and after neoadjuvant chemotherapy. Int J Clin Exp Pathol. 2014;7:6862–6870. [PMC free article] [PubMed] [Google Scholar]
- 29.Boughey JC, Hoskin TL, Goetz MP. Neoadjuvant Chemotherapy and Nodal Response Rates in Luminal Breast Cancer: Effects of Age and Tumor Ki67. Ann Surg Oncol. 2022;29:5747–5756. doi: 10.1245/s10434-022-11871-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40–50. doi: 10.1016/S1470-2045(17)30904-X. [DOI] [PubMed] [Google Scholar]
- 31.Li S, Zhang Y, Zhang P, Xue S, Chen Y, Sun L, et al. Predictive and prognostic values of tumor infiltrating lymphocytes in breast cancers treated with neoadjuvant chemotherapy: A meta-analysis. Breast. 2022;66:97–109. doi: 10.1016/j.breast.2022.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma R, Wei W, Ye H, Dang C, Li K, Yuan D. A nomogram based on platelet-to-lymphocyte ratio for predicting pathological complete response of breast cancer after neoadjuvant chemotherapy. BMC Cancer. 2023;23:245. doi: 10.1186/s12885-023-10703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Acikgoz O, Yildiz A, Bilici A, Olmez OF, Basim P, Cakir A. Pretreatment platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio as a predictor of pathological complete response to neoadjuvant chemotherapy in patients with breast cancer: single center experience from Turkey. Anticancer Drugs. 2022;33:1150–1155. doi: 10.1097/CAD.0000000000001389. [DOI] [PubMed] [Google Scholar]
- 34.Cuello-López J, Fidalgo-Zapata A, López-Agudelo L, Vásquez-Trespalacios E. Platelet-to-lymphocyte ratio as a predictive factor of complete pathologic response to neoadjuvant chemotherapy in breast cancer. PLoS One. 2018;13:e0207224. doi: 10.1371/journal.pone.0207224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Au A, Shencoru S, Uhlmann L, Mayer L, Michel L, Wallwiener M, et al. Predictive value of neutrophil-to-lymphocyte-ratio in neoadjuvanttreated patients with breast cancer. Arch Gynecol Obstet. 2022;307:1105–1113. doi: 10.1007/s00404-022-06726-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davey MG, Casey MC, McGuire A, Waldron RM, Paganga M, Holian E, et al. Evaluating the Role of Circulating MicroRNAs to Aid Therapeutic Decision Making for Neoadjuvant Chemotherapy in Breast Cancer: A Prospective, Multicenter Clinical Trial. Ann Surg. 2022;276:905–912. doi: 10.1097/SLA.0000000000005613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Magbanua MJM, Swigart LB, Wu HT, Hirst GL, Yau C, Wolf DM, et al. Circulating tumor DNA in neoadjuvant-treated breast cancer reflects response and survival. Ann Oncol. 2021;32:229–239. doi: 10.1016/j.annonc.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Q, Gampenrieder SP, Frantal S, Rinnerthaler G, Singer CF, Egle D, et al. Persistence of ctDNA in Patients with Breast Cancer During Neoadjuvant Treatment Is a Significant Predictor of Poor Tumor Response. Clin Cancer Res. 2022;28:697–707. doi: 10.1158/1078-0432.CCR-21-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Y, Xu Y, Wang C, Gong Y, Zhang Y, Yao R, et al. Serial circulating tumor DNA identification associated with the efficacy and prognosis of neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat. 2021;188:661–673. doi: 10.1007/s10549-021-06247-y. [DOI] [PubMed] [Google Scholar]
- 40.Arun B, Bayraktar S, Liu DD, Gutierrez Barrera AM, Atchley D, Pusztai L, et al. Response to Neoadjuvant Systemic Therapy for Breast Cancer in BRCA Mutation Carriers and Noncarriers: A Single-Institution Experience. J Clin Oncol. 2011;29:3739–3746. doi: 10.1200/JCO.2011.35.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poggio F, Bruzzone M, Ceppi M, Pondé NF, La Valle G, Del Mastro L, et al. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis. Ann Oncol. 2018;29:1497–1508. doi: 10.1093/annonc/mdy127. [DOI] [PubMed] [Google Scholar]
- 42.Wunderle M, Gass P, Häberle L, Flesch VM, Rauh C, Bani MR, et al. BRCA mutations and their influence on pathological complete response and prognosis in a clinical cohort of neoadjuvantly treated breast cancer patients. Breast Cancer Res Treat. 2018;171:85–94. doi: 10.1007/s10549-018-4797-8. [DOI] [PubMed] [Google Scholar]
- 43.Oshi M, Gandhi S, Angarita FA, Kim TH, Tokumaru Y, Yan L, et al. A novel five-gene score to predict complete pathological response to neoadjuvant chemotherapy in ER-positive/HER2-negative breast cancer. Am J Cancer Res. 2021;11:3611–3627. [PMC free article] [PubMed] [Google Scholar]
- 44.Lucas MW, Kelly CM. Optimal Choice of Neoadjuvant Chemotherapy for HER2-Negative Breast Cancer: Clinical Insights. Cancer Manag Res. 2022;14:2493–2506. doi: 10.2147/CMAR.S341466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med. 2020;382:810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 46.Cullinane C, Brien AO, Shrestha A, Hanlon EO, Walshe J, Geraghty J, et al. The association between breast density and breast cancer pathological response to neoadjuvant chemotherapy. Breast Cancer Res Treat. 2022;194:385–392. doi: 10.1007/s10549-022-06616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu Y, Mao L, Wang M, Li Z, Li M, Wang C, et al. New insights into breast microcalcification for poor prognosis: NACT cohort and bone metastasis evaluation cohort. J Cancer Res Clin Oncol. 2023;149:7285–7297. doi: 10.1007/s00432-023-04668-4. [DOI] [PubMed] [Google Scholar]
- 48.Kimura Y, Masumoto N, Kanou A, Fukui K, Sasada S, Emi A, et al. The TILs-US score on ultrasonography can predict the pathological response to neoadjuvant chemotherapy for human epidermal growth factor receptor 2-positive and triple-negative breast cancer. Surg Oncol. 2022;41:101725. doi: 10.1016/j.suronc.2022.101725. [DOI] [PubMed] [Google Scholar]
- 49.Choudhery S, Gomez-Cardona D, Favazza CP, Hoskin TL, Haddad TC, Goetz MP, et al. MRI Radiomics for Assessment of Molecular Subtype, Pathological Complete Response, and Residual Cancer Burden in Breast Cancer Patients Treated With Neoadjuvant Chemotherapy. Acad Radiol. 2020;29(Suppl 1):145–154. doi: 10.1016/j.acra.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang X, Mai J, Huang Y, He L, Chen X, Wu X, et al. Radiomic nomogram for pretreatment prediction of pathologic complete response to neoadjuvant therapy in breast cancer: predictive value of staging contrast-enhanced CT. Clin Breast Cancer. 2021;21:388–401. doi: 10.1016/j.clbc.2020.12.004. [DOI] [PubMed] [Google Scholar]


