Abstract
Introduction
Decision aids (DAs) are helpful instruments used to support shared decision making (SDM). Patients with atrial fibrillation (AF) face complex decisions regarding stroke prevention strategies. While a few DAs have been made for AF stroke prevention, an encounter DA (EDA) and patient DA (PDA) have not been created to be used in conjunction with each other before.
Design
Using iterative user-centered design, we developed 2 DAs for anticoagulation choice and stroke prevention in AF. Prototypes were created, and we elicited feedback from patients and experts via observations of encounters, usability testing, and semistructured interviews.
Results
User testing was done with 33 experts (in AF and SDM) and 51 patients from 6 institutions. The EDA and PDA underwent 1 and 4 major iterations, respectively. Major differences between the DAs included AF pathophysiology and a preparation to meet with the clinician in the PDA as well as different language throughout. Content areas included personalized stroke risk, differences between anticoagulants, and risks of bleeding. Based on user feedback, developers 1) addressed feelings of isolation with AF, 2) improved navigation options, 3) modified content and flow for users new to AF and those experienced with AF, 4) updated stroke risk pictographs, and 5) added structure to the preparation for decision making in the PDA.
Limitations
These DAs focus only on anticoagulation for stroke prevention and are online, which may limit participation for those less comfortable with technology.
Conclusions
Designing complementary DAs for use in tandem or separately is a new method to support SDM between patients and clinicians. Extensive user testing is essential to creating high-quality tools that best meet the needs of those using them.
Highlights
First-time complementary encounter and patient decision aids have been designed to work together or separately.
User feedback led to greater structure and different experiences for patients naïve or experienced with anticoagulants in patient decision aids.
Online tools allow for easier dissemination, use in telehealth visits, and updating as new evidence comes out.
Keywords: atrial fibrillation, shared decision making, patient decision aid, encounter decision aid, stroke prevention
Patient engagement has been an increasingly important area of study to improve health outcomes. One mechanism of increasing patient engagement is via shared decision making (SDM). SDM is a collaborative discussion around complex decisions in health care, and evidence demonstrates it improves adherence and patient satisfaction.1–3 Decision aids (DAs) are instruments used to facilitate and improve SDM. DAs are classified into 2 types: encounter decision aids (EDAs) and patient decisions aids (PDAs). EDAs are used by a clinician and patient collaboratively during a visit, whereas patients typically review PDAs before their visit. DAs present balanced descriptions of all choices for a patient in a situation, including the choice of doing nothing. DAs have shown an improvement in satisfaction with the decision-making process, patient knowledge, communication, and participation in SDM. 4
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia globally, and it increases the risk of cardioembolic stroke approximately 5-fold.5–8 AF-related strokes are particularly likely to cause disability or be fatal in comparison with other types of strokes.9,10 Anticoagulants such as warfarin and direct oral anticoagulants have been shown to be effective in reducing the risk of stroke.11–13 Anticoagulation is recommended based on personalized risk stratification, as intervening to reduce the risk of stroke is not without costs, harms (including fatal and disabling bleeding), and burdens. As the risk for stroke increases, the benefit of reducing this risk becomes increasingly desirable to patients.14,15 However, many patients for whom an anticoagulant is indicated either never start the anticoagulant or discontinue it for several reasons, including risk for bleeding, other perceived adverse effects, and concerns regarding adherence.16–19
Recent guidelines recommend SDM as patients and clinicians decide together on an AF treatment plan.20,21 While a few DAs have been created to aid in SDM related to AF stroke prevention, there has yet to be creation of a complementary EDA and PDA, designed for use separately or together. 22 We sought to develop 2 complementary DAs via an iterative design process and pilot test in preparation for a planned randomized controlled trial (RCT) to understand their effectiveness when used separately and in combination in clinical care.
Methods
Applying user-centered design, we developed 2 DAs to be used for anticoagulation choice and stroke prevention in AF (Figure 1).23,24 Starting with an EDA previously developed by members of our team at the Mayo Clinic for the same purpose, we used an iterative design process to refine the EDA and to design a complementary PDA. 25 User testing included semistructured interviews, observations, and internal testing, the results of which informed improvements to the tools. Interviews took the form of one-on-one journey mapping and exploration of micro scenarios.
Figure 1.
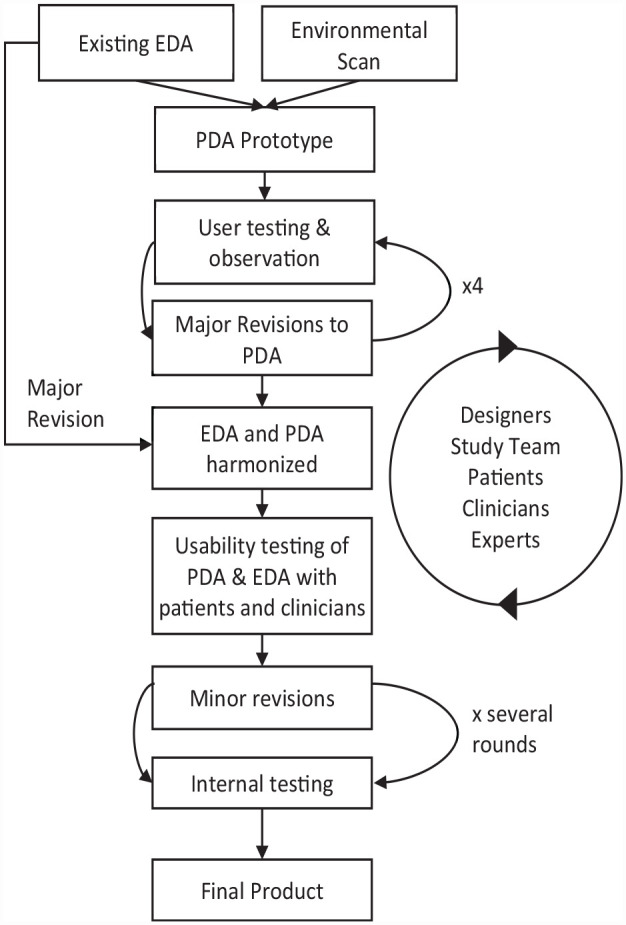
Flow diagram of the user-based iterative design process used to develop complementary encounter and patient decision aids (encounter decision aid [EDA] and patient decisions aid [PDA]).
The development process started with an environmental scan and systematic review of current SDM tools about anticoagulation in AF, which resulted in the identification of 12 DAs for use in SDM conversations about anticoagulation in AF (full methods and results have been previously published 22 ). An expert panel of 10 patients and caregivers, clinicians (physicians, nurse practitioners), experts in SDM, and experts in DA development reviewed the 12 DAs to identify the strengths and weaknesses of each tool. The meeting was recorded, and themes were identified from the transcripts. Notes were taken using an inductive approach by 1 coordinator and then finalized after discussion with the research team.
Prototyping and Refinement
We first created a low-fidelity prototype of the PDA and tested for usability with patients and clinicians. Following the development of the PDA, we further modified the existing EDA to align in final content and style with the complementary PDA. 25 The starting EDA prototype used in this study was based on an interactive and Web-based EDA used by Kunneman et al.25,26 in a previous study. The PDA underwent 4 major iterations while the EDA underwent 1 major update. Further minor adjustments (i.e., button positioning) to both tools occurred over time.
Data Collection
Feedback on the tool was gathered throughout the entire design processes via observations and screen capture of patients using the PDA, semistructured interviews with users, observations of clinical encounters with and without the DAs, and usability testing by a wide variety of clinicians, experts in DAs and SDM, designers, and study team members. The interview guide was developed by the study team, based on perspectives identified by the expert panel. Patients were asked questions about their experiences with AF itself, as well as their interactions with the health care system (i.e., clinicians and procedures) for their AF, especially regarding their initial diagnosis and early experiences with anticoagulation. Providers were asked about the conversations they had with patients and about the usability and acceptability of the DA. Interviews were audio and/or video recorded whenever possible. Interviews were generally one-on-one, but occasionally 2 study coordinators (1 from each site) were present during the interview. Notes were taken during the interviews, and audio/visual recordings were reviewed. Five interviews were analyzed using a journey-mapping approach. For the other interviews, 1 coordinator developed themes using an inductive approach, and these were then discussed with all study coordinators who had conducted interviews to reach a final consensus.
Results
Participants
The development team engaged with several key stakeholders in the development process including patients with AF, clinicians, and experts in SDM. These participants included 33 experts and 51 patients (Table 1) from 6 institutions. Nineteen experts were clinicians (in/outpatient cardiologist and cardiac electrophysiologist MD/NPs, and anticoagulation pharmacists) who helped inform the content of the DAs based on their experience treating patients with AF. The other 14 experts had expertise in SDM, DA development and/or patient communication, health literacy, or were patient advocates. All members of the study team (a subset of the aforementioned clinicians and experts) provided usability feedback regarding the tools. Two clinicians (a cardiac electrophysiologist and a clinical pharmacist) tested the updated version of the EDA during an encounter with a patient who had used the PDA and gave feedback regarding workflow and usability issues. Figure 2 breaks down patient participation in interviews, observed encounters, and usage of the EDA or PDA. Of those who gave feedback on the PDA, 8 participants gave feedback on paper or digital prototypes of the PDA before the Web site version was built. The rest gave feedback on different versions of the PDA as feedback from both patients, clinicians, and study team members was incorporated and the tool was updated.
Table 1.
Patient Participant Demographics at University of Utah and Mayo Clinic
| Utah (n = 17) | Mayo (n = 34) | |
|---|---|---|
| Mean age (standard deviation), y | 66 (12.49) | 62 (11.47) |
| Female | 11.7% | 29.4% |
| Race, White | 94% | 97% |
Figure 2.
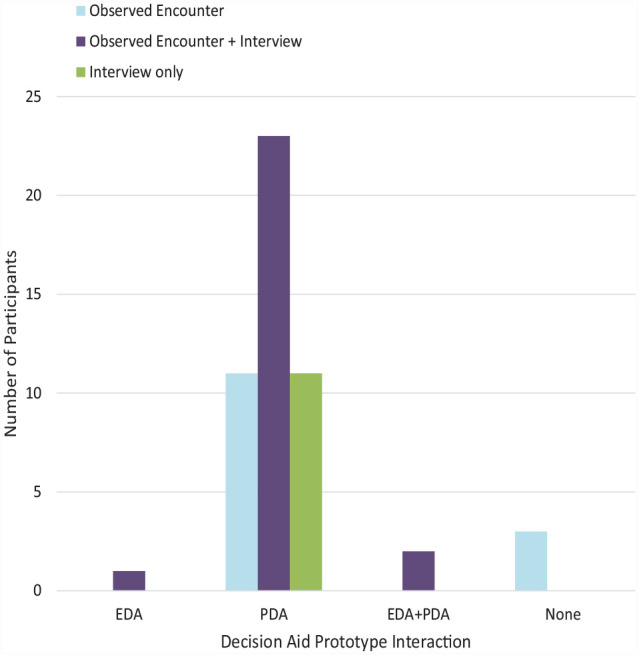
Patient participation in decision aid development feedback. EDA, encounter decision aid; PDA, patient decisions aid.
DA Content
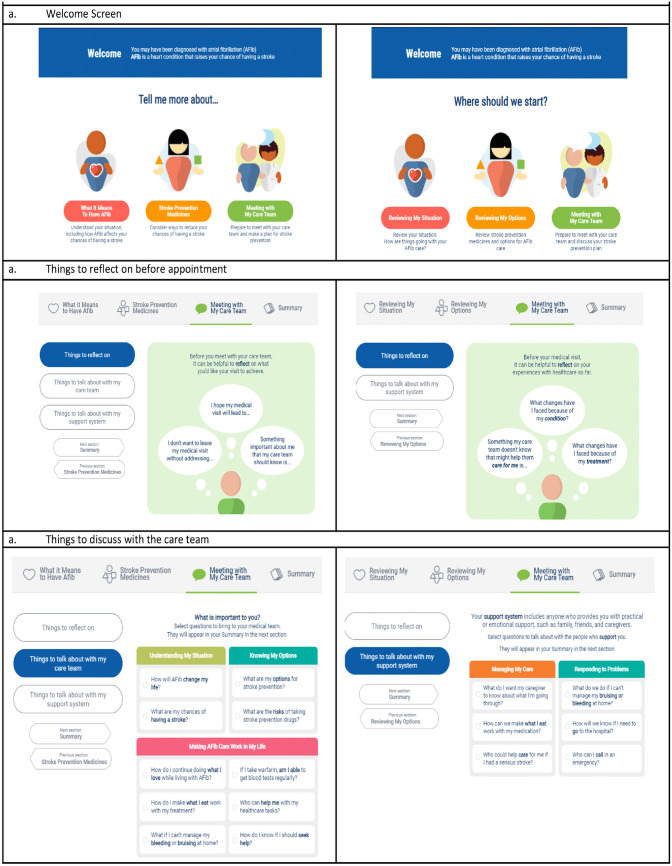
The goal of the EDA was to facilitate provider patient communication during a visit and to help the provider elicit patient values. The PDA’s goal was to prepare the patient for the discussion with their provider by providing education and helping them establish their values before talking with their provider. While many aspects of the 2 DAs are the same, such as an embedded CHA2DS2-VASc calculator and comparisons between different anticoagulants, there were additional sections in the PDA not included in the EDA, including information about the basic pathophysiology of AF and reaffirming language to help the patient feel less alone as they go through the tool without their provider. Patients using the PDA also had a section to prep them for the discussion with their provider, including example questions they could select to bring to their provider. Examples of major content and language differences between the EDA and PDA are shown in Figure 3. Description of the content in the PDA is listed in Table 2.
Figure 3.
Examples of differences between the complementary atrial fibrillation (AF) decision aids. EDA, encounter decision aid; PDA, patient decisions aid.
Table 2.
Content of the Patient Decision Aid for Stroke Prevention in Atrial Fibrillation
| What It Means to Have Afib | Stroke Prevention Medicines | Meeting with My Care Team |
|---|---|---|
| • What’s going on with my heart? ○ Animated figure of the heart showing the pathophysiology of AFib ○ How does AFib affect chances of having a stroke • Enter stroke risk factors into calculator • How will AFib make me feel? ○ Common symptoms and what they mean |
• What are my choices? • Overview of anticoagulants • How would medicines work with my life? ○ Bleeding ○ Medication routine ○ Cost ○ Diet • Stroke prevention and other health care • What else can I do? ○ Prevent stroke ○ Manage symptoms |
• Things to reflect on • Things to talk about with my care team • Things to talk about with my support system |
Feedback and Usability Testing
The expert panel meeting at the beginning of the study provided key guiding themes for the development of the PDA and refinement of the EDA. Themes based on the identified strengths and weaknesses of existing tools included 1) the importance of providing concise information at an appropriate reading level (health literacy), 2) illustrations of basic anatomy, 3) allowing individuals to choose what and how much information they receive, 4) the need for content related to patients’ physical and emotional experience of AF diagnosis, and 5) having opportunities to write down questions for further discussion.
Both patients and clinicians provided feedback on both DAs during semistructured interviews and internal testing. Numerous patients remarked on a feeling of isolation or loneliness when first diagnosed with AF. Patients liked the calming, reassuring language in the PDA such as, “You can live with this,” and “You are not alone.” As a result, in the final version of the PDA landing page, we include the message, “You are not alone,” while presenting the statistic that at least 2.7 million Americans live with AF. Patients valued the cost comparisons for anticoagulation in both tools and felt that the EDA improved their conversation with their provider, specifically through added visuals and the concrete personalized data. Clinicians felt that the bleeding risk information was long, and that there were a few sections that required coaching beforehand so that they could portray it correctly to their patients.
Usability issues included several patients skipping entire portions of the tool and unclear navigation through the tool. Clinicians also had issues navigating early versions of the EDA and felt that information was not always where they expected it to be. Arrows and navigation options were sometimes confusing or not apparent to users. These issues were fixed for the final version. An early iteration of the PDA had more of a forced linear design; for the final version, we allow patients freedom to move nonlinearly through different sections as desired. This feedback underscored the importance of basic user-interface details to usability and effectiveness of a PDA. The final PDA version was optimized for mobile use, allowing patients to view the PDA on their smartphones as well as a tablet or desktop computer. The EDA is intended for use during the clinic visit and is used with desktops or tablets.
Refinement
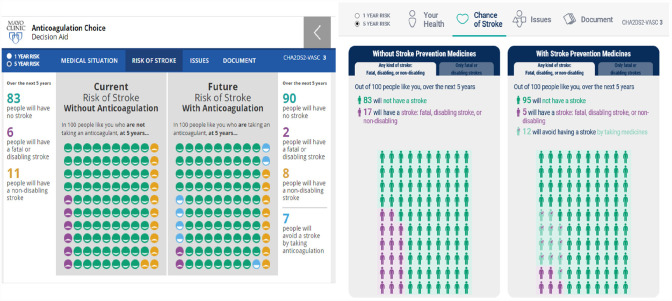
A major update from the initial EDA to the new PDA/EDA was to add the stroke risk calculator. Stroke risk was updated to show the difference between risk for all strokes and then limited to the risk for fatal or disabling stroke. The details and studies used for the stroke risk calculator were described in a previous article about the development of the initial EDA. 25 In addition, the icons in the pictograph were updated from smiley faces to gender-specific icons (based on assigned sex at birth selected in risk factor screen) informed by prior research on the effectiveness of different types of icons 27 (Figure 4).
Figure 4.
Stroke risk calculator in the initial encounter decision aid on the left and the final version on the right.
Initially, the section that helped patients prepare for their visit with the clinician included a free-text box for patients to write notes/questions for their clinicians. Patient feedback indicated that this section was confusing and that they wanted a frequently asked questions (FAQ) section. SDM experts and clinicians also gave feedback that included the concern that patients could enter private health information into the free text thinking it was secure. Thus, the final version has an interactive list of commonly asked questions patients have for their clinicians. Patients were able to select which from this list they would like to discuss with their clinician at their upcoming appointment and then were able to download the personalized list of questions, which eliminated the concern of a breach of privacy.
Early iterations of the PDA had only 1 version of content for both new and experienced patients, but many of the patients who were experienced with AF felt that they did not need all of the information in the tool and perceived it to be too focused toward patients who were new to AF as opposed to those who had lived with it longer. In response, we made a different version of the PDA tool for patients who had a prior history with AF and anticoagulation. Patients now have the decision when they first open the PDA to choose the experienced or the naïve version of the PDA. In the experienced version, we changed the language to take a review approach of the patient’s AF and anticoagulation options as opposed to learning about them for the first time (Figure 5).
Figure 5.
Examples of differences between naïve (left frames) and experienced pathways (right frames) of the patient decision aid. (a) Welcome screen. (b) Things to reflect on before appointment. (c) Things to discuss with the care team.
Discussion
In collaboration with clinicians and patient stakeholders, we created 2 DAs designed to be used primarily by patients (PDA) preappointment and during a clinical appointment collaboratively between clinicians and patients (EDA). These tools are expected to provide decision support for patients with AF and their clinicians when making treatment decisions about beginning, changing, or continuing their anticoagulation medicines. To our knowledge, this is the first effort to design complementary DAs for use in a trial to determine the impact of these tools when used separately and together.
According to the International Patient Decision Aid Society, iterative user-centered methodology with pilot testing is the recommended approach to develop DAs.28–30 Evidence and descriptions of developing DAs and the methodology used have been published for both EDAs and PDAs. 25 However, often DAs are not created using this gold standard practice, or if they are, it is not reported. 22 Compared with most previous DAs for anticoagulation choice in AF, the development process for these tools was more robust and incorporated both patient and clinician needs, review, and testing. Only 2 other DAs for stroke prevention in AF reported a high level of involvement in the development process from multiple sources. 22
High-quality, user-centered design DAs are needed to help patients make decisions regarding their AF treatment plan. While anticoagulation has been shown to significantly decrease the risk of stroke in patients with AF, as few as 50% of patients who may benefit from treatment are actually taking anticoagulation.13,16,31 Our tools are not designed to be persuasive (i.e., to encourage use of anticoagulants) but rather to provide a description of taking each treatment option, including that of not taking any medications, and to help patients decide which option is best for their lives and their current needs, goals, and values. Qualitative studies of clinicians have found worry about bleeding risk and fidelity to the treatment program contribute to lack of anticoagulant uptake. 19 Having a tool that supports conversations about risks and benefits of anticoagulants instead of merely providing information may help clinicians better address these concerns.
These DAs are currently being tested in a randomized controlled trial to evaluate their effect on SDM, knowledge, and decisional conflict. Secondary outcomes include adherence, anticoagulation choice, quality of decision making, encounter length, and clinical events. Enrollment began in December 2020, and patients are being randomized to use either PDA and EDA together, alone, or usual care. Registration of the trial can be found on clinicaltrials.gov (NCT04357288), and a full description of the protocol has been published. 32 In this trial, we aim to extend previous research that has shown that DAs for stroke prevention have improved communication and satisfaction but have not had an impact on long-term adherence to therapy. 26 Based on the findings of an RCT that used the initial version of the EDA and found no effect on adherence, we made critical changes to our study design to minimize the chances that the patients we recruit have no need for a DA.
Strengths of our 2 tools are the extensive user testing each have undergone to create a resource that best fits user-identified needs instead of the perceived needs of users by creators. In addition, the DAs are online, which makes it relatively easy to update them as information changes. This allows for easier integration with health care systems or disseminating to patients before a visit. Clinicians can also show the EDA during telehealth visits by sharing their screen, something that is more difficult with a paper tool. Furthermore, the customizable options of the tool allow patients to create a unique experience by showing the personalized risk of stroke based on their specific characteristics and allowing patients to view sections that apply to their personal situation.
A limitation of the tools is that they focus only on anticoagulation for stroke prevention in AF and not on procedural approaches (e.g., left atrial appendage closure device). Another limitation was the lack of diversity in our patient population. This limitation is due both to the location of our recruiting centers (large academic research medical centers where the surrounding population is predominantly White) as well as the nature of AF, which is diagnosed more commonly in those of European descent.33–35 We could not feasibly collect information about insurance type, barriers to accessing health care, income, or health literacy of our patient testers. However, these social risk factors will be assessed in the RCT. Lastly, because the DAs are online, this may limit the participation of patients who are less comfortable with technology or do not have access to the internet and a computer or mobile device at home. For our trial, we will have tablets and research assistants available to assist people who are limited in their digital access or literacy.
Conclusion
Creating complementary DAs to be used together or separately represents a new method to supporting SDM between patients and clinicians regarding stroke prevention in AF and will provide needed evidence regarding the strengths and weaknesses of PDAs compared with EDAs. Extensive user testing and feedback from both patients and clinicians improved the individualization of the PDA and the ability to easily navigate both of the tools. These improvements align patient values and clinical accuracy to improve SDM. An ongoing trial evaluating these tools aims to show the utility of using these DAs and their effect on SDM, knowledge, decisional conflict, as well as adherence and other decision-making outcomes.
Footnotes
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: BAS is supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health (#K23HL143156) and also reports research support from AHA/PCORI, Abbott, Boston Scientific, Cardiva, and AltaThera and consulting to Janssen and AltaThera.
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this article was funded by the American Heart Association (AHA) through a funding collaboration between AHA and the Patient-Centered Outcomes Research Institute (PCORI) grant 18SFRN34110489/ Fagerlin/2018 (STEP-UP) and grant 18SFRN34230142/Ozanne/2018.
ORCID iDs: Aubrey E. Jones  https://orcid.org/0000-0003-1726-702X
https://orcid.org/0000-0003-1726-702X
Kerri L. Cavanaugh  https://orcid.org/0000-0002-4031-1714
https://orcid.org/0000-0002-4031-1714
Benjamin A. Steinberg  https://orcid.org/0000-0002-4729-7820
https://orcid.org/0000-0002-4729-7820
Victor M. Montori  https://orcid.org/0000-0003-0595-2898
https://orcid.org/0000-0003-0595-2898
Elissa M. Ozanne  https://orcid.org/0000-0001-5352-9459
https://orcid.org/0000-0001-5352-9459
Contributor Information
Aubrey E. Jones, College of Pharmacy, Department of Pharmacotherapy, University of Utah, Salt Lake City, UT, USA
Madeleine M. McCarty, School of Medicine, Department of Population Health Sciences, University of Utah, Salt Lake City, UT, USA
Kenzie A. Cameron, Feinberg School of Medicine, Department of Medicine, Division of General Internal Medicine and Geriatrics, Northwestern University, Chicago, IL, USA
Kerri L. Cavanaugh, Department of Medicine, Division of Nephrology and Hypertension, Vanderbilt University Medical Center, Nashville, TN, USA
Benjamin A. Steinberg, School of Medicine, Division of Cardiovascular Medicine, University of Utah, Salt Lake City, UT, USA
Rod Passman, Feinberg School of Medicine, Department of Medicine, Division of Cardiology, Northwestern University, Chicago, IL, USA.
Preeti Kansal, Feinberg School of Medicine, Department of Medicine, Division of Cardiology, Northwestern University, Chicago, IL, USA.
Adriana Guzman, Feinberg School of Medicine, Department of Medicine, Division of General Internal Medicine and Geriatrics, Northwestern University, Chicago, IL, USA.
Emily Chen, Department of Internal Medicine, University of Michigan, Ann Arbor, MI, USA.
Lingzi Zhong, School of Medicine, Department of Population Health Sciences, University of Utah, Salt Lake City, UT, USA.
Angela Fagerlin, School of Medicine, Department of Population Health Sciences, University of Utah, Salt Lake City, UT, USA; Salt Lake City VA Informatics Decision-Enhancement and Analytic Sciences (IDEAS) Center for Innovation, Salt Lake City, UT, USA.
Ian Hargraves, Department of Cardiovascular Diseases, Mayo Clinic, Rochester, MN, USA.
Victor M. Montori, Department of Cardiovascular Diseases, Mayo Clinic, Rochester, MN, USA
Juan P. Brito, Department of Cardiovascular Diseases, Mayo Clinic, Rochester, MN, USA
Peter A. Noseworthy, Department of Cardiovascular Diseases, Mayo Clinic, Rochester, MN, USA
Elissa M. Ozanne, School of Medicine, Department of Population Health Sciences, University of Utah, Salt Lake City, UT, USA.
References
- 1. Moyer A, Salovey P. Patient participation in treatment decision making and the psychological consequences of breast cancer surgery. Womens Health. 1998;4:103–16. [PubMed] [Google Scholar]
- 2. Haynes RB, McKibbon KA, Kanani R. Systematic review of randomised trials of interventions to assist patients to follow prescriptions for medications. Lancet. 1996;348:383–6. DOI: 10.1016/s0140-6736(96)01073-2 [DOI] [PubMed] [Google Scholar]
- 3. Street RL, Jr, Voigt B. Patient participation in deciding breast cancer treatment and subsequent quality of life. Med Decis Making. 1997;17:298–306. DOI: 10.1177/0272989X9701700306 [DOI] [PubMed] [Google Scholar]
- 4. Stacey D, Legare F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014:CD001431. DOI: 10.1002/14651858.CD001431.pub4 [DOI] [PubMed] [Google Scholar]
- 5. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA. 2001;285:2370–5. [DOI] [PubMed] [Google Scholar]
- 6. Kim MH, Johnston SS, Chu B-C, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–20. [DOI] [PubMed] [Google Scholar]
- 7. Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham heart study. Circulation. 2004;110:1042–6. [DOI] [PubMed] [Google Scholar]
- 8. Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104:1534–9. [DOI] [PubMed] [Google Scholar]
- 9. Lin H-J, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation: the Framingham study. Stroke. 1996;27:1760–4. [DOI] [PubMed] [Google Scholar]
- 10. Tu HT, Campbell BC, Christensen S, et al. Pathophysiological determinants of worse stroke outcome in atrial fibrillation. Cerebrovasc Dis. 2010;30:389–95. [DOI] [PubMed] [Google Scholar]
- 11. Go AS, Hylek EM, Chang Y, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA. 2003;290:2685–92. [DOI] [PubMed] [Google Scholar]
- 12. Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999;131:492–501. [DOI] [PubMed] [Google Scholar]
- 13. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–67. [DOI] [PubMed] [Google Scholar]
- 14. Agarwal S, Hachamovitch R, Menon V. Current trial-associated outcomes with warfarin in prevention of stroke in patients with nonvalvular atrial fibrillation: a meta-analysis. Arch Intern Med. 2012;172:623–31. DOI: 10.1001/archinternmed.2012.121 [DOI] [PubMed] [Google Scholar]
- 15. Gómez-Outes A, Terleira-Fernández AI, Calvo-Rojas G, Suárez-Gea ML, Vargas-Castrillón E. Dabigatran, rivaroxaban, or apixaban versus warfarin in patients with nonvalvular atrial fibrillation: a systematic review and meta-analysis of subgroups. Thrombosis. 2013;2013:640723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123:638–645.e634. DOI: 10.1016/j.amjmed.2009.11.025 [DOI] [PubMed] [Google Scholar]
- 17. Friberg L, Hammar N, Ringh M, Pettersson H, Rosenqvist M. Stroke prophylaxis in atrial fibrillation: who gets it and who does not? Report from the Stockholm Cohort-study on Atrial Fibrillation (SCAF-study). Eur Heart J. 2006;27:1954–64. DOI: 10.1093/eurheartj/ehl146 [DOI] [PubMed] [Google Scholar]
- 18. Lip GYH, Pan X, Kamble S, et al. Discontinuation risk comparison among ‘real-world’ newly anticoagulated atrial fibrillation patients: apixaban, warfarin, dabigatran, or rivaroxaban. PLoS One. 2018;13:e0195950. DOI: 10.1371/journal.pone.0195950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yao C, Jones AE, Slager S, Fagerlin A, Witt DM. Exploring clinician perspectives on patients with atrial fibrillation who are not prescribed anticoagulation therapy. PEC Innov. 2022;1:100062. DOI: 10.1016/j.pecinn.2022.100062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74:104–32. DOI: 10.1016/j.jacc.2019.01.011 [DOI] [PubMed] [Google Scholar]
- 21. Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2020;42:373–498. DOI: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 22. Torres Roldan VD, Brand-McCarthy SR, Ponce OJ, et al. Shared decision making tools for people facing stroke prevention strategies in atrial fibrillation: a systematic review and environmental scan. Med Decis Making. 2021;41:540–9. DOI: 10.1177/0272989x211005655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elwyn G, O’Connor A, Stacey D, et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ. 2006;333:417. DOI: 10.1136/bmj.38926.629329.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gould JD, Lewis C. Designing for usability: key principles and what designers think. Commun ACM. 1985;28:300–11. DOI: 10.1145/3166.3170 [DOI] [Google Scholar]
- 25. Zeballos-Palacios CL, Hargraves IG, Noseworthy PA, et al. Developing a conversation aid to support shared decision making: reflections on designing anticoagulation choice. Mayo Clin Proc. 2019;94:686–96. DOI: 10.1016/j.mayocp.2018.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kunneman M, Branda ME, Hargraves IG, et al. Assessment of shared decision-making for stroke prevention in patients with atrial fibrillation: a randomized clinical trial. JAMA Intern Med. 2020. DOI: 10.1001/jamainternmed.2020.2908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zikmund-Fisher BJ, Witteman HO, Dickson M, et al. Blocks, ovals, or people? Icon type affects risk perceptions and recall of pictographs. Med Decis Making. 2014;34:443–53. DOI: 10.1177/0272989X13511706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Coulter A, Kryworuchko J, Mullen P, et al. Using a systematic development process. In: Volk R, Llewellyn-Thomas H, eds. 2012 Update of the International Patient Decision Aids Standards (IPDAS) Collaboration’s Background Document. Chapter A. 2012. http://ipdas.ohri.ca/resources.html [Google Scholar]
- 29. Coulter A, Stilwell D, Kryworuchko J, Mullen PD, Ng CJ, van der Weijden T. A systematic development process for patient decision aids. BMC Med Inform Decis Mak. 2013;13(suppl 2):S2. DOI: 10.1186/1472-6947-13-S2-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vaisson G, Provencher T, Dugas M, et al. User involvement in the design and development of patient decision aids and other personal health tools: a systematic review. Med Decis Making. 2021;41:261–74. DOI: 10.1177/0272989X20984134 [DOI] [PubMed] [Google Scholar]
- 31. Johansson C, Hägg L, Johansson L, Jansson JH. Characterization of patients with atrial fibrillation not treated with oral anticoagulants. Scand J Prim Health Care. 2014;32:226–31. DOI: 10.3109/02813432.2014.984952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jones AE, McCarty MM, Brito JP, et al. Randomized evaluation of decision support interventions for atrial fibrillation: rationale and design of the RED-AF study. Am Heart J. 2022;248:42–52. DOI: 10.1016/j.ahj.2022.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kornej J, Börschel CS, Benjamin EJ, Schnabel RB. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res. 2020;127:4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sivly A, Gorr HS, Gravholt D, et al. Enrolling people of color to evaluate a practice intervention: lessons from the Shared Decision-Making for Atrial Fibrillation (SDM4AFib) trial. BMC Health Serv Res. 2022;22:1032. DOI: 10.1186/s12913-022-08399-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heckbert SR, Austin TR, Jensen PN, et al. Differences by race/ethnicity in the prevalence of clinically detected and monitor-detected atrial fibrillation. Circ Arrhythm Electrophysiol. 2020;13:e007698. DOI: 10.1161/CIRCEP.119.007698 [DOI] [PMC free article] [PubMed] [Google Scholar]