Abstract
Purpose:
Weight loss surgery is an effective, long-term treatment for severe obesity but individual response to surgery varies widely. The purpose of this study was to test a comprehensive theoretical model of factors that may be correlated with the greatest surgical weight loss at 1 – 3 years following surgery. Such a model would help determine what predictive factors to measure when patients are preparing for surgery that may ensure the best weight outcomes.
Materials and Methods:
The Bariatric Experience Long Term (BELONG) study collected self-reported and medical record-based baseline information as correlates of 1- and 3-year % total weight loss (TWL) in n = 1,341 patients. Multiple linear regression was used to determine the associations between 120 baseline variables and %TWL.
Results:
Participants were 43.4 ± 11.3 years old, Hispanic or Black (52%; n = 699), women (86%; n = 1,149), partnered (72%; n = 965) and had annual incomes of ≥ $51,000 (60%; n = 803). A total of 1,006 (75%) had 3-year follow-up weight. Regression models accounted for 10.1% of the variance in %TWL at 1-year and 13.6% at 3-years. Only bariatric operation accounted for a clinically meaningful difference (~5%) in %TWL at 1-year. At 3-years after surgery, only bariatric operation, Black race, and BMI ≥ 50 kg/m2 were associated with clinically meaningful differences in %TWL.
Conclusions:
Our findings combined with many others support a move away from extensive screening and selection of patients at the time of surgery to a focus on improving access to this treatment.
Introduction
Metabolic and bariatric surgery is the most effective treatment for patients with severe obesity (body mass index [BMI] ≥ 35 kg/m2); compared to conventional weight loss strategies, surgery has resulted in much higher weight loss over a period of 2 – 7 years.[1–4] Despite this clear benefit, there is large variation in weight loss outcomes even within the same bariatric operation ranging from 56% total weight loss (%TWL) to 15% gain up to 7 years after surgery.[5,6] Given this large variation, it is imperative to understand if modifiable factors predict this variability so that we can improve outcomes for all patients.
Metabolic and bariatric surgery programs invest considerable resources in the preparation of patients for surgery based on recommendations from the American Society for Metabolic and Bariatric Surgery (ASMBS).[7] Although guidelines for surgical eligibility were recently updated,[8] there are no universal criteria for who is the best candidate for weight loss surgery. This concept of the best candidate remains controversial, in part because there is not consistent evidence for what factors measured before surgery are related to weight loss after surgery.
To understand correlates of bariatric surgical outcomes, there are two general foci in the literature to date: immutable patient characteristics such as demographics and bariatric operation type that cannot generally be affected by patient, provider, or system level actions;[9–11] and modifiable factors such as health behaviors, weight before surgery, mental health, and social support that could be targeted for change.[12–21] Most of the work on modifiable factors is not grounded in psychosocial theoretical models or theories of health behavior change,[22,23] nor is it informed by the numerous studies on non-surgical weight loss that have provided extensive evidence for what leads to long-term weight loss maintenance.[24,25]
In addition, much of the work done to date on self-reported psychological and behavioral factors measured at the time of surgery has come from the Longitudinal Assessment of Bariatric Surgery (LABS) study, which focused primarily on eating behaviors and psychiatric conditions.[26,27] Patients in this cohort mostly self-identified as White race and with very few sleeve gastrectomy (SG) cases, which is now the most frequently used operation in the U.S.[28]
The Bariatric Experience Long Term (BELONG) prospective cohort study was designed to address the limitations in the literature by applying a comprehensive theoretical model of health behavior change to the collection of data from people preparing to have surgery.[29,30] This cohort of patients is racially diverse and has a large sample of both Roux-en-Y gastric bypass (RYGB) and SG. The purpose of the current study was to test a comprehensive theoretical model (see Figure 1) of factors measured before or at the time of surgery that may be correlated with 1- and 3-year %TWL to address the question of what may be important to measure when patients are preparing for surgery to affect the period when the greatest weight loss occurs (1 – 3 years following surgery).
Figure 1.
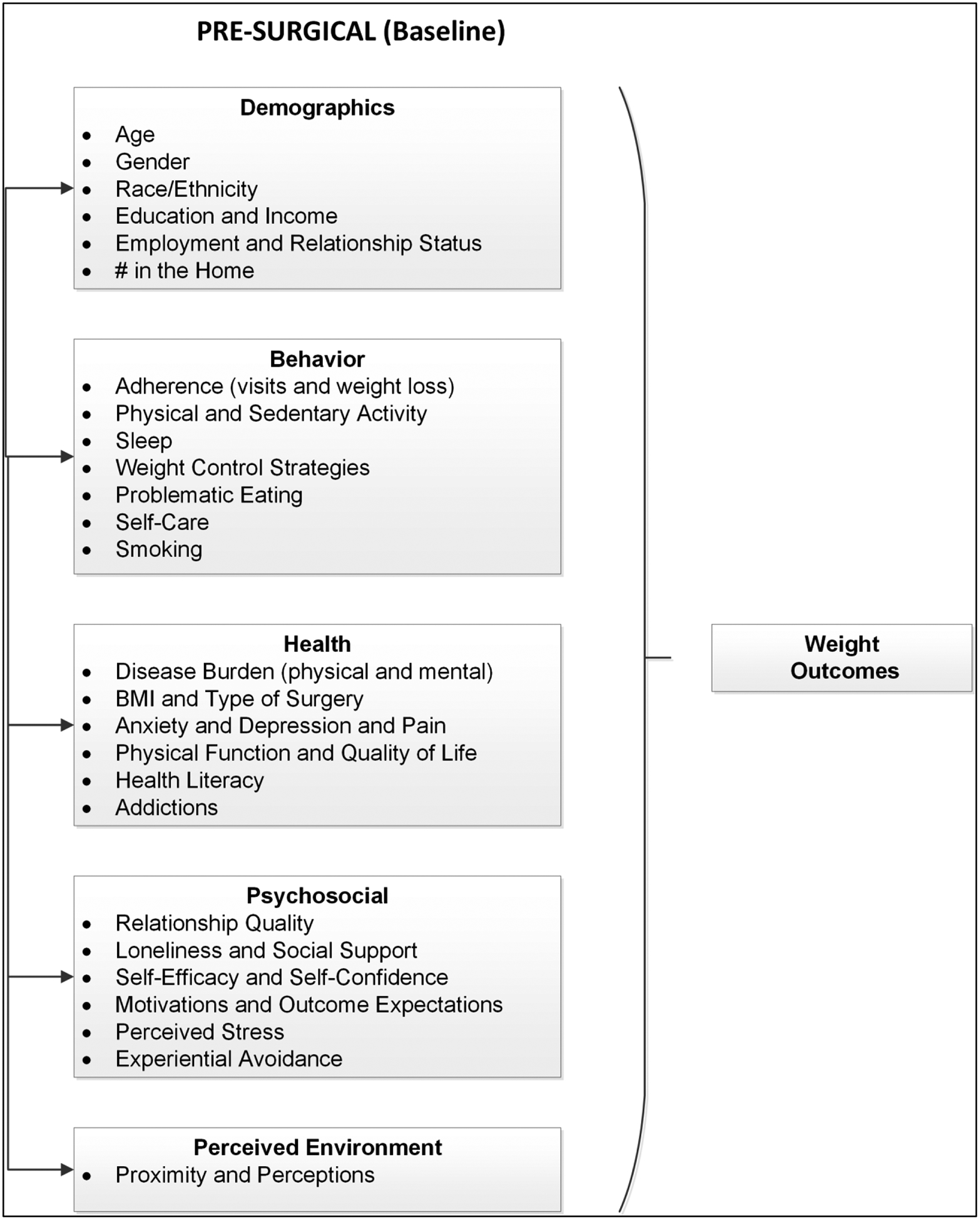
Theoretical model used to test baseline factors correlated with 1- and 3-year % Total Weight Loss (%TWL). All 120 survey variables chosen to operationalize the domains of interest (demographics, behavior, health, psychosocial, and environment) are summarized in Table S1 in the Online Supplement. Details for the selection of these variables and their scale reliabilities have been described previously.[29,30]
METHODS
Participants
The BELONG cohort has been described in detail elsewhere.[29,30] Eligibility criteria for inclusion in the BELONG study were: 1) member of the healthcare system at the time of recruitment; 2) enrolled in a 12-week bariatric surgery preparation course; 3) planning to have a first bariatric surgery within 6 months of the baseline survey; 4) adult 18 years of age and older; and 5) meeting general eligibility criteria for weight loss surgery at the time of enrollment.[31] Recruitment for the survey began in February 2016 and ended in May 2017. A total of n = 1,975 patients were surveyed at baseline (42% response rate). Further exclusions were made (please see Online Supplement for details) that resulted in n = 1,338 patients available for analyses.
Procedures
All data were collected at baseline before surgery. Half of survey respondents completed the baseline survey using a self-guided study website; the remainder completed a survey by phone with a trained surveyor. The following data were also abstracted from the EHR at the time of surgery: diagnoses and pharmacy records to determine disease burden (including mental health-related), adherence to scheduled visits for routine medical care in the year before surgery, weight and height to determine both BMI and %TWL in the year before surgery, and date of birth to calculate age. Height and weight were collected by clinical staff as part of routine clinical care.
Measures
All 120 survey variables chosen to operationalize the domains of interest (demographics, behavior, health, psychosocial, and environment) in the BELONG theoretical model (see Figure 1) are summarized in Table S1 in the Online Supplement. Details for the selection of these variables and their scale reliabilities have been described previously.[29,30]
Outcome
Our weight loss outcome was %TWL calculated as [((weight [kg] at surgery - weight [kg])/weight at surgery [kg]) x100], at 1- and 3-years of follow-up. Using this formula resulted in positive values for weight loss. Weight was objectively measured by clinic staff.
Analyses
Imputation.
The linear regression analysis used to test the contributions of all 120 variables in the BELONG theoretical model to %TWL required that each participant have a value for every variable in the model. Although the rates of missingness were low for each individual variable (< 3%), when all variables were combined in linear regressions, 783 participants were excluded (leaving n = 555 with complete data). To mitigate the loss of participants’ data, we chose to use the MICE (Multivariate Imputation by Chain Equations) R package to impute the missing values for baseline survey data.[32] MICE generated 5 imputed data sets and provided a pooled linear regression model using the imputed data sets. The pooled linear regression analysis was used for estimation and inferences for the relationships between selected correlates and %TWL at year-1 and year-3.
Regressions.
Three separate linear regressions were conducted for each of the 1- and 3-year weight loss outcomes using the imputed baseline correlates sample: a basic model using a set of factors that have been shown to affect weight loss after surgery (age, gender, race/ethnicity, socioeconomic status, type of surgery, having a BMI ≥ 50 kg/m2, comorbidity burden, presence of diabetes and/or hypertension at surgery, and %TWL in the 12 months before surgery); a full model with all 120 variables to help us determine which variables to select for the final regression. This final parsimonious regression included all significant variables (p <.05) from the full model (second regression). Results are shown for the basic and final models.
Sensitivity analyses.
Table S1 in the Online Supplement provides descriptive statistics for each variable in our theoretical model for the full sample with imputation (n = 1,338) and the restricted sample without imputation (n = 555). These two samples were compared to each other for each variable in the model to determine if the sample with imputation differed significantly from those without imputation. Significance level was adjusted for multiple comparisons using a Bonferroni correction, to control Type I Error inflation (p ≤ .0004). In addition, we conducted 1-year regressions using only the n = 555 participants who had complete responses for all survey items (see Table S2 in the Online Supplement) to determine if imputation affected the findings. Analyses were conducted in R Version 4.1.1 which was downloaded from the R CRAN website.[33]
RESULTS
Participants
Participant demographic characteristics for the analytic cohort (n = 1,338) are shown in Table S1 in the Online Supplement. All participants had measured weight at year-1 (100% follow-up) and n = 1,006 had a 3-year follow-up measured weight (75% follow-up rate). Participants were 43.4 ± 11.3 years old, self-identified as Hispanic or Black race (52%; n = 699), women (86%; n = 1,149), and partnered (72%; n = 965). Over half of all participants (60%; n = 803) had annual incomes of ≥ $51,000.
1-Year Weight Loss
Table 1 provides findings for variables associated with %TWL at 1-year. The basic model accounted for 7.0% of the variance in weight loss at 1-year. Adding variables increased the variance accounted for to 10.1%. In the final model, two immutable factors (having RYGB; higher annual income) and one modifiable factor (indicator of food addiction) were significantly associated with greater %TWL at 1-year. Conversely, three immutable (Black or Hispanic race; lower employment density for retail, entertainment, and educational uses in a neighborhood; older age) and four modifiable (BMI ≥ 50 kg/m2; having adequate health literacy; better sleep efficiency; fewer days of strength training) factors were significantly associated with lower %TWL at 1-year. Detailed model effects are provided in the Online Supplement. Only having RYGB (3.63% more TWL) and identifying as Black race (3.63% less TWL) approached clinically meaningful effects (~5% TWL).
Table 1.
Linear regression results for basic (adjusted R2 = 7.0%) and final (adjusted R2 = 10.1%) models of baseline factors that were related to 1-year % total weight loss (%TWL) in the Bariatric Experience Long Term (BELONG) study cohort. Regressions use the full analytic sample (n = 1,338) which contains some imputation of survey variables.
| Basic Model | Final Model | |||||
|---|---|---|---|---|---|---|
| Variable | Est | SE | p | Est | SE | p |
| Intercept | 32.62 | 1.67 | <.001 | 33.76 | 1.83 | <.001 |
| Type of Surgery (% Gastric Bypass) | 3.58* | 0.53 | <.001 | 3.63 | 0.53 | <.001 |
| Age (years) | −0.11** | 0.03 | <.001 | −0.12 | 0.03 | <.001 |
| Race (compared to Caucasian/White) | ||||||
| Hispanic/Latino | −1.80 | 0.60 | <.001 | −1.74 | 0.61 | <.001 |
| African American/Black | −4.26 | 0.77 | <.001 | −3.63 | 0.80 | <.001 |
| Other | −2.28 | 1.11 | .04 | −1.77 | 1.10 | .11 |
| Mixed | −1.71 | 0.88 | .05 | −1.59 | 0.88 | .07 |
| Gender (% Women) | 0.46 | 0.69 | .51 | 0.08 | 0.69 | .91 |
| Socioeconomic Status (range 8 – 67) | 0.01 | 0.02 | .59 | 0.01 | 0.02 | .46 |
| Comorbidity Burden | −0.09 | 0.24 | .71 | −0.12 | 0.23 | .60 |
| Hypertension (% yes) | −0.10 | 0.59 | .86 | −0.25 | 0.58 | .67 |
| Type 2 Diabetes Mellitus (% yes) | −1.26 | 0.66 | .06 | −1.21 | 0.65 | .07 |
| %TWL 1 Year Before Surgery | 0.06 | 0.05 | .22 | 0.03 | 0.05 | .63 |
| BMI ≥ 50 kg/m2 (% yes) | −1.79 | 0.71 | .01 | −1.73 | 0.70 | .01 |
| Food Addiction: More Time to Obtain (% yes) | --- | --- | --- | 1.67 | 0.54 | <.001 |
| Annual Income ≥ $51,000 (% yes) | --- | --- | --- | 1.03 | 0.52 | .05 |
| Neighborhood Deprivation Index | --- | --- | --- | 0.47 | 0.28 | .09 |
| Dog Walking (min/day) | --- | --- | --- | 0.004 | 0.002 | .07 |
| Outcome Expectations for %TWL: Disappointed | --- | --- | --- | −0.01 | 0.01 | .50 |
| Proportion of Retail Space | --- | --- | --- | −0.31 | 0.12 | .01 |
| Strength Training (days/week) | --- | --- | --- | −0.58 | 0.15 | <.001 |
| Adequate Health Literacy (% yes) | --- | --- | --- | −1.53 | 0.59 | .01 |
| Sleep Efficiency Rating (% better/somewhat better) | --- | --- | --- | −1.57 | 0.63 | .01 |
A positive estimate means that as the value of the variable in the model increased, there was more %TWL.
A negative variable estimate means that as the value of the variable in the model increased, there was less %TWL.
3-Year Weight Loss
Table 2 provides findings for variables associated with %TWL at 3-years. The basic model accounted for 8.1% of the variance in weight loss at 3-years, and adding variables increased the variance accounted for to 13.6%. In the final model, two immutable factors (having RYGB, perception of neighborhood having more four-way intersections – an indicator of more walkability) and four modifiable factors (indicator of food addiction, greater self-care, higher comorbidity burden, greater % TWL in 12 months before surgery) were significantly associated with greater %TWL at 3-years. Conversely, four immutable (Black or Hispanic race, perception that the neighborhood had less heavy traffic – an indicator of more walkability; lower employment density for retail, entertainment, and educational uses in a participant’s neighborhood; older age) and five modifiable (BMI ≥ 50 kg/m2; better sleep efficiency; more days of strength training; having hypertension and/or diabetes) factors were associated with lower %TWL at 3-years. Detailed model effects are provided in the Online Supplement. Only having RYGB (4.61% more TWL) and identifying as Black race (4.24% less TWL) approached clinically meaningful effects (~5% TWL).
Table 2.
Linear regression results for basic (adjusted R2 = 8.1%) and final (adjusted R2 = 13.6%) models of baseline factors that were related to 3-year % total weight loss (%TWL) in the Bariatric Experience Long Term (BELONG) study cohort. Regressions use those participants from the full analytic sample (n = 1,338) which contains some imputation of survey variables who also had a weight measure at year 3 (n = 1,006).
| Basic Model | Final Model | |||||
|---|---|---|---|---|---|---|
| Variable | Est | SE | p | Est | SE | p |
| Intercept | 29.39 | 2.41 | <.001 | 25.06 | 3.17 | <.001 |
| Type of Surgery (% Gastric Bypass) | 4.48* | 0.71 | <.001 | 4.61 | 0.69 | <.001 |
| Age (years) | −0.08** | 0.03 | .02 | −0.10 | 0.03 | .01 |
| Race (compared to Caucasian/White) | ||||||
| Hispanic/Latino | −1.98 | 0.79 | .01 | −1.86 | 0.79 | .02 |
| African American/Black | −4.49 | 1.01 | <.001 | −4.24 | 1.01 | <.001 |
| Other | −1.66 | 1.49 | .26 | −1.22 | 1.46 | .40 |
| Mixed | −0.58 | 1.17 | .62 | −0.27 | 1.14 | .82 |
| Gender (% Women) | −0.34 | 0.94 | .72 | −0.53 | 0.92 | .57 |
| Socioeconomic Status (range 8 – 67) | 0.04 | 0.05 | .41 | 0.06 | 0.05 | .23 |
| Comorbidity Burden | 0.69 | 0.31 | .03 | 0.70 | 0.30 | .02 |
| Hypertension (% yes) | −1.62 | 0.77 | .04 | −1.64 | 0.75 | .03 |
| Type 2 Diabetes Mellitus (% yes) | −2.39 | 0.88 | .01 | −2.36 | 0.86 | .01 |
| %TWL 1 Year Before Surgery | 0.24 | 0.07 | <.001 | 0.20 | 0.07 | <.001 |
| BMI ≥ 50 kg/m2 (% yes) | −3.19 | 0.94 | <.001 | −2.93 | 0.92 | <.001 |
| Perception that Neighborhood Has Four Way Intersections (% yes) | --- | --- | --- | 2.70 | 0.66 | <.001 |
| Perception that Neighborhood Does Not Have Heavy Traffic (% yes) | --- | --- | --- | −1.79 | 0.68 | .01 |
| Food Addiction: More Time to Obtain (% yes) | --- | --- | --- | 2.58 | 0.70 | <.001 |
| Self-Care (range 4 – 20) | --- | --- | --- | 0.18 | 0.07 | .02 |
| Experiential Avoidance (range 15 – 75) | --- | --- | --- | 0.06 | 0.04 | .12 |
| Dog Walking (min/day) | --- | --- | --- | 0.01 | 0.00 | .08 |
| Proportion of Retail Space | --- | --- | --- | −0.53 | 0.16 | <.001 |
| Strength Training (days/week) | --- | --- | --- | −0.68 | 0.20 | <.001 |
| Sleep Efficiency Rating (% better/somewhat better) | --- | --- | --- | −1.73 | 0.80 | .03 |
A positive estimate means that as the value of the variable in the model increased, there was more %TWL.
A negative variable estimate means that as the value of the variable in the model increased, there was less %TWL.
Sensitivity Analyses
Table S2 in the Online Supplement provides the results for 1-year %TWL using only the n = 555 participants who did not have any scale items imputed. Similar to the model using imputation (Table 1), the final model accounted for 14.0% of the variance in 1-year %TWL. Like the model using imputation, the final model also found associations for operation type, race, age, strength training, health literacy, and sleep (although this was latency not efficiency). However, socioeconomic status, BMI ≥ 50 kg/m2, adherence rate to outpatient visits in the year before surgery, and weight loss self-efficacy were also significantly associated with %TWL at 1-year.
DISCUSSION
The BELONG cohort is one of the largest, most diverse and comprehensive assessments of factors theoretically related to weight loss following metabolic and bariatric surgery. We found that 120 variables accounted for 10.1% of the variance in %TWL at 1-year and 13.6% of the variance in %TWL at 3-years. After accounting for all variables in the theoretical model, the type of bariatric operation accounted for the greatest difference in %TWL at both 1- and 3-years, with RYGB having 4.6% more TWL than SG at 3-years. In addition, at 3-years after surgery, participants who identified as Black had 4.24% less TWL than those who identified as White and participants with a BMI ≥ 50 kg/m2 had 2.93% less TWL than those with < 50 kg/m2. Other variables were statistically significant in multivariate models, however, the effect was small at 1%−3% TWL and likely not clinically meaningful. These findings have been reported in numerous other studies,[34–39] where demographic, physical health and behavioral factors are often the only variables related to weight loss at 2 – 5 years after surgery. A comprehensive meta-analysis found no consistent evidence for the role of any mental health condition in post-operative weight loss.[40]
Some of our findings were in the opposite direction than hypothesized if they were used in univariate analyses, such as spending more and more time obtaining food (an indicator of food addiction) resulting in 2.58% greater TWL 3-years following surgery. The LABS study also reported counter-intuitive findings for baseline factors affecting weight loss after RYGB surgery.[41] For example, their patients with the highest rates of disordered eating at baseline had the greatest %TWL 3-years following surgery.[42] Very few studies report variance accounted for, however, a recent study by Chang and colleagues found that age and type of operation explained 17.5% of the variance in weight loss at 3- and 14.6% of the variability at 5-years.[43]
Our study had several important limitations. We had a low survey response rate of 42% limiting our generalizability. Because of the limitations in the analytic strategy for survey item non-response, findings were based on some imputation of survey summary scores for over half the sample. We conducted two analyses to understand the impact of missing data on our findings, univariate comparisons for variables in each model between those participants with (n = 1,338) and without (n = 555) imputation and regressions with only the 555 participants for whom there was no imputation (see Table S1 and S2 in the Online Supplement). Although some of the correlates differed and their magnitude was reduced, the variance accounted for was similar to the full sample (14% for the final model) supporting the overall conclusion. Finally, even though this healthcare system included 23 bariatric surgeons across 9 practices, our findings may not apply to other bariatric practices and thus should be replicated more systematically in other settings.
Another limitation was that we did not include every possible factor that could affect weight loss after surgery. For example, a recent publication found that impulsivity was the strongest predictor of outcomes from metabolic and bariatric surgery.[44] However, we carefully considered the existing evidence for both surgical and non-surgical weight loss when building our theoretical model (see Figure 1),[29] and feel confident that our findings are some of the most comprehensive in the literature. Finally, we only looked at 3-year weight outcomes. More studies are needed to determine if baseline psychosocial, behavioral, and health factors are related to very long-term outcomes (5 – 10 years).
CONCLUSION
Our findings combined with many other studies on baseline factors related to weight loss following metabolic and bariatric surgery suggest that pre-surgical characteristics, especially those that are modifiable, may only predict small variations (1% – 3%) in weight loss. Based on this work, we feel that the field should move away from extensive screening and selection of patients at the time of surgery to a focus on improving access to this treatment, especially for people who suffer disproportionately from severe obesity such as Black and Hispanic patients. Many studies have shown a marked improvement in mortality rates for those patients who have metabolic and bariatric surgery,[45,46] clearly indicating that this is a life-saving approach to treating severe obesity. Other life-saving operations for conditions related to obesity, such as coronary artery revascularization, do not require such extensive screening and selection for patients.[47] Research and clinical care resources should be moved to the post-operative period to see if modifiable post-operative factors such as physical activity, social isolation, weight control strategies, and weight loss expectations, can help us understand why some patients may not experience the same benefits from metabolic and bariatric surgery than others.
Supplementary Material
Key Points.
120 baseline variables accounted for only 13.6% of weight loss at 3-years
Only operation type, Black race, and BMI ≥ 50 kg/m2 were clinically meaningful
Baseline modifiable characteristics predict small weight loss variations (1% – 3%)
This supports less pre-selection of patients and a focus on improving access
Funding Sources:
This work was funded by an award from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) #R01DK108522 and #R01MD013874 from the National Institute on Minority Health and Health Disparities (NIMHD).
Footnotes
CONFLICTS OF INTEREST: The authors declare that they have no conflict of interest.
ETHICAL APPROVAL: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
CONSENT: All participants gave verbal consent at the time of recruitment.
REFERENCES
- 1.Ribaric G, Buchwald JN, McGlennon TW. Diabetes and weight in comparative studies of bariatric surgery vs conventional medical therapy: a systematic review and meta-analysis. Obes Surg. 2014; 24:437–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arterburn DE, Telem DA, Kushner RF, Courcoulas AP. Benefits and Risks of Bariatric Surgery in Adults: A Review. JAMA. 2020; 324:879–887. [DOI] [PubMed] [Google Scholar]
- 3.Wölnerhanssen BK, Peterli R, Hurme S, Bueter M, Helmiö M, Juuti A, Meyer-Gerspach AC, Slawik M, Peromaa-Haavisto P, Nuutila P, Salminen P. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy: 5-year outcomes of merged data from two randomized clinical trials (SLEEVEPASS and SM-BOSS). Br J Surg. 2021; 108:49–57. [DOI] [PubMed] [Google Scholar]
- 4.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, Kashyap SR; STAMPEDE Investigators. Bariatric Surgery versus Intensive Medical Therapy for Diabetes – 5-Year Outcomes. N Engl J Med. 2017; 376:641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Courcoulas AP, King WC, Belle SH, Berk P, Flum DR, Garcia L, Gourash W, Horlick M, Mitchell JE, Pomp A, Pories WJ, Purnell JQ, Singh A, Spaniolas K, Thirlby R, Wolfe BM, Yanovski SZ. Seven-Year Weight Trajectories and Health Outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018; 153:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arterburn D, Wellman R, Emiliano A, Smith SR, Odegaard AO, Murali S, Williams N, Coleman KJ, Courcoulas A, Coley RY, Anau J, Pardee R, Toh S, Janning C, Cook A, Sturtevant J, Horgan C, McTigue KM; PCORnet Bariatric Study Collaborative. Comparative Effectiveness and Safety of Bariatric Procedures for Weight Loss: A PCORnet Cohort Study. Ann Intern Med. 2018; 169:741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter J, Chang J, Birriel TJ, Moustarah F, Sogg S, Goodpaster K, Benson-Davies S, Chapmon K, Eisenberg D. ASMBS position statement on preoperative patient optimization before metabolic and bariatric surgery. Surg Obes Relat Dis. 2021; 17:1956–1976. [DOI] [PubMed] [Google Scholar]
- 8.Eisenberg D, Shikora SA, Aarts E, Aminian A, Angrisani L, Cohen RV, de Luca M, Faria SL, Goodpaster KPS, Haddad A, Himpens JM, Kow L, Kurian M, Loi K, Mahawar K, Nimeri A, O’Kane M, Papasavas PK, Ponce J, Pratt JSA, Rogers AM, Steele KE, Suter M, Kothari SN. 2022 American Society of Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) Indications for Metabolic and Bariatric Surgery. Obes Surg. 2023; 33:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao J, Samaan JS, Abboud Y, Samakar K. Racial disparities in bariatric surgery postoperative weight loss and co-morbidity resolution: a systematic review. Surg Obes Relat Dis. 2021; 17:1799–1823. [DOI] [PubMed] [Google Scholar]
- 10.Coleman KJ, Brookey J. Gender and racial/ethnic background predict weight loss after Roux-en-Y gastric bypass independent of health and lifestyle behaviors. Obes Surg. 2014; 24:1729–1736. [DOI] [PubMed] [Google Scholar]
- 11.Arterburn D, Livingston EH, Schifftner T, Kahwati LC, Henderson WG, Maciejewski ML. Predictors of long-term mortality after bariatric surgery performed in Veterans Affairs medical centers. Arch Surg. 2009; 144:914–920. [DOI] [PubMed] [Google Scholar]
- 12.Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E, Mehran A, Ko CY, Gibbons MM. Does weight loss immediately before bariatric surgery improve outcomes: a systematic review. Surg Obes Relat Dis. 2009; 5:713–721. [DOI] [PubMed] [Google Scholar]
- 13.Toussi R, Fujioka K, Coleman KJ. Pre- and postsurgery behavioral compliance, patient health, and postbariatric surgical weight loss. Obes. 2009; 17:996–1002. [DOI] [PubMed] [Google Scholar]
- 14.Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E, Mehran A, Ko CY, Gibbons MM. Preoperative predictors of weight loss following bariatric surgery: systematic review. Obes Surg. 2012; 22:70–89. [DOI] [PubMed] [Google Scholar]
- 15.van Hout GC, Verschure SK, van Heck GL. Psychosocial predictors of success following bariatric surgery. Obes Surg. 2005; 15:552–560. [DOI] [PubMed] [Google Scholar]
- 16.Clark SM, Saules KK, Schuh LM, Stote J, Creel DB. Associations between relationship stability, relationship quality, and weight loss outcomes among bariatric surgery patients. Eat Behav. 2014; 15:670–672. [DOI] [PubMed] [Google Scholar]
- 17.Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E, Mehran A, Ko CY, Gibbons MM. Patient behaviors associated with weight regain after laparoscopic gastric bypass. Obes Res Clin Pract. 2011; 5:e169–266. [DOI] [PubMed] [Google Scholar]
- 18.Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E, Mehran A, Ko CY, Shekelle PG, Gibbons MM. Is social support associated with greater weight loss after bariatric surgery?: a systematic review. Obes Rev. 2011; 12:142–148. [DOI] [PubMed] [Google Scholar]
- 19.Sockalingam S, Hawa R, Wnuk S, et al. Psychosocial predictors of quality of life and weight loss two years after bariatric surgery: Results from the Toronto Bari-PSYCH study. Gen Hosp Psychiatry. 2017; 47:7–13. [DOI] [PubMed] [Google Scholar]
- 20.Wykowski K, Krouse HJ. Self-care predictors for success post-bariatric surgery: a literature review. Gastroenterology Nursing. 2013; 36:129–135. [DOI] [PubMed] [Google Scholar]
- 21.Devlin MJ, King WC, Kalarchian MA, et al. Eating pathology and associations with long-term changes in weight and quality of life in the longitudinal assessment of bariatric surgery study. Int J Eat Disord. 2018;51(12):1322–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noar SM, Zimmerman RS. Health Behavior Theory and cumulative knowledge regarding health behaviors: are we moving in the right direction? Health Educ Res. 2005; 20:275–290. [DOI] [PubMed] [Google Scholar]
- 23.Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychol. 2009; 28:690–701. [DOI] [PubMed] [Google Scholar]
- 24.Wing RR, Papandonatos G, Fava JL, Gorin AA, Phelan S, McCaffery J, Tate DF. Maintaining large weight losses: the role of behavioral and psychological factors. J Consult Clinl Psychol. 2008; 76:1015–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metzgar CJ, Preston AG, Miller DL, Nickols-Richardson SM. Facilitators and barriers to weight loss and weight loss maintenance: a qualitative exploration. J Hum Nutr Diet. 2015; 28:593–603. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell JE, Selzer F, Kalarchian MA, Devlin MJ, Strain GW, Elder KA, Marcus MD, Wonderlich S, Christian NJ, Yanovski SZ. Psychopathology before surgery in the longitudinal assessment of bariatric surgery-3 (LABS-3) psychosocial study. Surg Obes Relat Dis. 2012; 8:533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavender JM, King WC, Kalarchian MA, Devlin MJ, Hinerman A, Gunstad J, Marcus MD, Mitchell JE. Examining emotion-, personality-, and reward-related dispositional tendencies in relation to eating pathology and weight change over seven years in the Longitudinal Assessment of Bariatric Surgery (LABS) study. J Psychiatr Res. 2020; 120:124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belle SH, Berk PD, Chapman WH, Christian NJ, Courcoulas AP, Dakin GF, Flum DR, Horlick M, King WC, McCloskey CA, Mitchell JE, Patterson EJ, Pender JR, Steffen KJ, Thirlby RC, Wolfe BM, Yanovski SZ; LABS Consortium. Baseline characteristics of participants in the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study. Surg Obes Relat Dis. 2013; 9:926–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coleman KJ, Paz SR, Bhakta BB, Taylor B, Liu J, Yoon TK, Macias M, Arterburn DE, Crawford CL, Drewnowksi A, Figueroa Gray MS, Hansell LD, Ji M, Lewis KH, Moore DD, Murali SB, Young DR. Cohort profile: The Bariatric Experience Long Term (BELONG): a long-term prospective study to understand the psychosocial, environmental, health and behavioural predictors of weight loss and regain in patients who have bariatric surgery. BMJ Open. 2022; 12:e059611. doi: 10.1136/bmjopen-2021-059611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore DD, Arterburn DE, Bai Y, Cornejo M, Crawford CL, Drewnowski A, Gray MF, Ji M, Lewis KH, Paz S, Taylor B, Yoon TK, Young DR, Coleman KJ. The Bariatric Experience Long Term (BELONG): Factors Related to Having Bariatric Surgery in a Large Integrated Healthcare System. Obes Surg. 2021; 31:847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NIDDK. Potential Candidates for Weight Loss Surgery. https://www.niddk.nih.gov/health-information/weight-management/bariatric-surgery/potential-candidates. Accessed on May 2, 2023.
- 32.van Buuren S, Groothuis-Oudshoorn K. MICE: Multivariate Imputation by Chained Equations in R.” Journal of Statistical Software. 2011; 45:1–67. [Google Scholar]
- 33.The Comprehensive R Network. Previous Releases of R for Windows. https://cran.r-project.org/bin/windows/base/old/. Accessed on May 2, 2023.
- 34.El Ansari W, Elhag W. Weight regain and insufficient weight loss after bariatric surgery: Definitions, prevalence, mechanisms, predictors, prevention and management strategies, and knowledge gaps-a scoping review. Obes Surg. 2021; 31: 1755–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hecht LM, Pester B, Braciszewski JM, Graham AE, Mayer K, Martens K, Miller-Matero LR. Socioeconomic and racial disparities in bariatric surgery. Obes Surg. 2020; 30:2445–2449. [DOI] [PubMed] [Google Scholar]
- 36.Kitamura R, Chen R, Trickey A, Eisenberg D. Positive and negative independent predictive factors of weight loss after bariatric surgery in a veteran population. Obesity Surgery. 2020; 30:2124–2130. [DOI] [PubMed] [Google Scholar]
- 37.Chang WW, Hawkins DN, Brockmeyer JR, Faler BJ, Hoppe SW, Prasad BM. Factors influencing long-term weight loss after bariatric surgery. SOARD. 2021; 15:456–461. [DOI] [PubMed] [Google Scholar]
- 38.Jambhekar A, Maselli A, Robinson S, Kabata K, Gorecki P. Demographics and socioeconomic status as predictors of weight loss after laparoscopic sleeve gastrectomy: A prospective cohort study. Int J Surg. 2018; 54:163–169. [DOI] [PubMed] [Google Scholar]
- 39.Sockalingam S, Hawa R, Wnuk S, Santiago V, Kowgier M, Jackson T, Cassin S. Psychosocial predictors of quality of life and weight loss two years after bariatric surgery: results from the Toronto Bari-PSYCH study. Gen Hosp Psych. 2017; 47, 7–13. [DOI] [PubMed] [Google Scholar]
- 40.Dawes AJ, Maggard-Gibbons M, Maher AR, Booth MJ, Miake-Lye I, Beroes JM, Shekelle PG. Mental health conditions among patients seeking and undergoing bariatric surgery: a meta-analysis. JAMA. 2016; 315:150–163. [DOI] [PubMed] [Google Scholar]
- 41.King WC, Belle SH, Hinerman AS, Mitchell JE, Steffen KJ, Courcoulas AP. Patient behaviors and characteristics related to weight regain after Roux-en-Y Gastric Bypass: A multicenter prospective cohort study. Ann Surg. 2020; 272:1044–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Field AE, Inge TH, Belle SH, Johnson GS, Wahed AS, Pories WJ, Courcoulas AP. Association of obesity subtypes in the Longitudinal Assessment of Bariatric Surgery Study and 3-year postoperative weight change. Obesity. 2018; 26:1931–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang WW, Hawkins DN, Brockmeyer JR, Faler BJ, Hoppe SW, Prasad BM. Factors influencing long-term weight loss after bariatric surgery. SOARD. 2019; 15:456–461. [DOI] [PubMed] [Google Scholar]
- 44.Sarwer DB, Allison KC, Wadden TA, Ashare R, Spitzer JC, McCuen-Wurst C, Wu J. Psychopathology, disordered eating, and impulsivity as predictors of outcomes of bariatric surgery. SOARD. 2019; 15:650–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Courcoulas AP, Johnson E, Arterburn DE, Haneuse S, Herrinton LJ, Fisher DP, et al. Reduction in Long-term Mortality After Sleeve Gastrectomy and Gastric Bypass Compared to Nonsurgical Patients with Severe Obesity. Ann Surg. 2023; 277:442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coleman KJ, Shu YH, Fischer H, Johnson E, Yoon TK, Taylor B, et al. Bariatric Surgery and Risk of Death in Persons with Chronic Kidney Disease. Ann Surg. 2022; 276:e784–e791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022; 145:e18–e114. Erratum in: Circulation. 2022; 145:e772. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


