Abstract
Background
Patients with influenza-related acute respiratory distress syndrome (ARDS) are critically ill and require mechanical ventilation (MV) support. Prolonged mechanical ventilation (PMV) is often seen in these cases and the optimal management strategy is not established. This study aimed to investigate risk factors for PMV and factors related to weaning failure in these patients.
Methods
This retrospective cohort study was conducted by eight medical centers in Taiwan. All patients in the intensive care unit with virology-proven influenza-related ARDS requiring invasive MV from January 1 to March 31, 2016, were included. Demographic data, critical illness data and clinical outcomes were collected and analyzed. PMV is defined as mechanical ventilation use for more than 21 days.
Results
There were 263 patients with influenza-related ARDS requiring invasive MV enrolled during the study period. Seventy-eight patients had PMV. The final weaning rate was 68.8% during 60 days of observation. The mortality rate in PMV group was 39.7%. Risk factors for PMV were body mass index (BMI) > 25 (kg/m2) [odds ratio (OR) 2.087; 95% confidence interval (CI) 1.006–4.329], extracorporeal membrane oxygenation (ECMO) use (OR 6.181; 95% CI 2.338–16.336), combined bacterial pneumonia (OR 4.115; 95% CI 2.002–8.456) and neuromuscular blockade use over 48 h (OR 2.8; 95% CI 1.334–5.879). In addition, risk factors for weaning failure in PMV patients were ECMO (OR 5.05; 95% CI 1.75–14.58) use and bacteremia (OR 3.91; 95% CI 1.20–12.69).
Conclusions
Patients with influenza-related ARDS and PMV have a high mortality rate.
Risk factors for PMV include BMI > 25, ECMO use, combined bacterial pneumonia and neuromuscular blockade use over 48 h. In addition, ECMO use and bacteremia predict unsuccessful weaning in PMV patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-023-02648-3.
Introduction
The advancement of high-quality critical care has saved numerous lives worldwide, allowing patients to survive; however, certain patients experience incomplete recovery, necessitating prolonged mechanical ventilation (PMV) [1]. PMV is defined as mechanical ventilation lasting 21 days or more for a minimum of 6 h daily [2]. The global population of PMV patients continues to rise, placing a burden on health care systems.
In a meta-analysis concerning post-acute care hospitals in the United States and other countries, the success rate of ventilator liberation for patients with a duration of mechanical ventilation (MV) exceeding 14 days was 47 and 63%, respectively. However, the one-year mortality rates were 73 and 47%, respectively. This study suggests that nearly half of the patients who are discharged alive from these facilities do not survive beyond one year [3].
Influenza infection can lead to severe pulmonary complications, including acute respiratory distress syndrome (ARDS) and respiratory failure [4]. Most of the related fatal cases involve young individuals who were previously healthy and without any underlying complications. [5]. Although patients with ARDS might improve rapidly, some patients had substantial injury over respiratory and other vital organs, leading to prolonged mechanical ventilation times and increased mortality rates [6, 7].
To date, most published studies on influenza-related ARDS have primarily focused on investigating risk factors associated with hospital mortality. However, there is limited information available regarding risk factors for PMV in ARDS. The primary objective of this study was to investigate risk factors for PMV in critically ill patients with influenza. The secondary objective was to evaluate risk factors associated with unsuccessful weaning among PMV patients.
Methods
Study design
This is a retrospective study that analyzed cohorts in Taiwan Severe Influenza Research Consortium (TSIRC). The study received approval from the institutional review boards of all participating hospitals (Taipei Veterans General Hospital, 2016–05-020CC; Taichung Veterans General Hospital, CE16093A; National Taiwan University Hospital, 201605036RIND; Tri-Service General Hospital, 1–105-05–086; Chang Gung Memorial Hospital, 201600988B0; China Medical University Hospital, 105-REC2-053(FR); Kaohsiung Medical University Hospital, KUMHIRB-E(I)-20170097; Kaohsiung Chang Gung Memorial Hospital, 201600988B0). Given that all patient information during the data recording period was anonymized and deidentified, informed consent was not needed.
The data collection period for this study spans January 1, 2016, to March 31, 2016. Patients diagnosed with ARDS attributed to influenza and requiring admission to the intensive care unit during this timeframe were included in the study. The inclusion criterion was patients diagnosed with influenza-associated ARDS who received invasive mechanical ventilation.
The severity of ARDS was classified according to the Berlin definition, which involves acute onset of respiratory distress within one week, radiographic confirmation of bilateral diffuse opacities, absence of evidence of heart failure as the primary cause of pulmonary edema, and arterial partial pressure of oxygen/fraction of inspired oxygen ratio < 300, with positive end-expiratory pressure ≥ 5 cm H2O[8]. We collected data for patients with influenza-induced ARDS requiring mechanical ventilation support while excluding those under 18 years old.
The initial cohort of 263 individuals was used for calculating overall ventilator weaning rates. Patients who died before the 21st day of mechanical ventilation were excluded. Patients in the PMV cohort remained on a ventilator wean plan beyond the 21st day of ventilation and constituted the group with unsuccessful weaning from ventilatory support post PMV.
Data collection
We recorded demographic data, including sex, age, body mass index, and comorbidities, as well as clinical data during the intensive care unit stay, such as laboratory results and APACHE II severity scores [9]. Key invasive treatment measures and events and their timing following the onset of influenza-induced ARDS were analyzed, including ECMO, prone positioning, renal replacement therapy, vasopressor use, sedatives, neuromuscular blockers, and steroids. The occurrence and timing of bacterial pneumonia and bacteremia were also documented.
Treatment outcome assessment
The main treatment outcomes assessed in this study were ventilator weaning rate, length of stay in the intensive care unit, hospital length of stay, duration of mechanical ventilation, and successful weaning from the ventilator or weaning failure.
The primary outcome of interest was PMV, as defined as invasive mechanical ventilation exceeding 21 days. In the PMV group, reintubation or death within 48 h or the need for mechanical ventilation at discharge was considered unsuccessful weaning from ventilation.
The primary objective of this study was to investigate risk factors for PMV in critically ill patients with influenza, with a secondary aim to evaluate factors associated with failure to wean from ventilation post PMV.
Statistical analysis
Results are presented as means ± standard deviations, medians with interquartile ranges, or percentages. Pearson’s χ2 test or Fisher's exact test was used to compare categorical variables. The normality of continuous variables was assessed using the Kolmogorov‒Smirnov and Shapiro‒Wilk tests. Independent sample t tests or Mann‒Whitney U tests were used to compare differences between groups for continuous variables, depending on the distribution’s normality. Comparison of categorical variables was performed using exact tests.
Model building was carried out through initial screening using univariate analysis with a threshold of p < 0.1, followed by enter selection based on variable choice, with an entry criterion of 0.05. Both univariate and multivariate binary logistic regression analyses were conducted to identify variables showing significant differences between the two groups and to determine independent predictors of PMV. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated.
All p values were two-tailed, and p < 0.05 was considered significant. Forest plots were employed to visually depict effect sizes, with odds ratios reported along with their 95% confidence intervals. Finally, Kaplan‒Meier survival curves were plotted for each factor in multivariate models, and comparisons were made using the log-rank test.
The analyses were conducted using MedCalc version 19.2.5 (MedCalc Software Ltd, Ostend, Belgium) and IBM SPSS Statistics for Windows/Macintosh, Version 24.0 (IBM Corp., Armonk, NY, USA).
Results
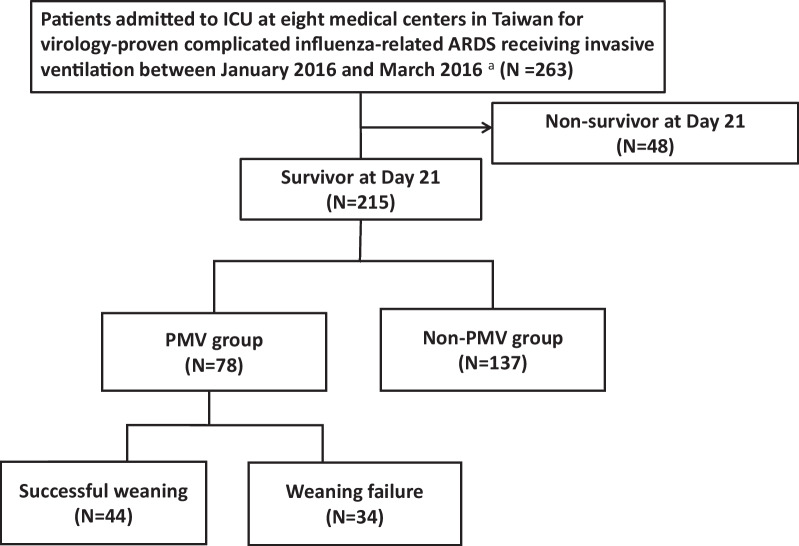
During the course of the study, 263 patients with influenza-induced acute respiratory distress syndrome (ARDS) were included, with exclusion criteria being patients who by 21 post initiation of mechanical ventilation 48 patients had died. Forty-eight patients (18.3%) died up to day 21. The hospital mortality for the whole group of patients was 30.4% and 39.7% of patients requiring PMV eventually died in hospital before discharge. Ultimately, 215 patients met the criteria and were included in the analysis of PMV. Among them, 78 patients who underwent PMV and subsequently underwent risk assessment for inability to be weaned from the ventilator were further evaluated (Fig. 1). In our cohorts, all ECMO patients received sedatives and neuromuscular blockage agents. The PMV group presented higher BMI and APACHE II score, and a higher frequency of ECMO, bacterial pneumonia, bacteremia, neuromuscular blockade for more than 48 h, and vasopressor usage when compared with non-PMV group. Also, PMV group has worse outcome, including longer MV days, longer ICU stay, longer hospital days and higher in-hospital mortality (Table 1).
Fig. 1.
Flow chart of the study. a Virology-proven methods include the rapid influenza diagnostic test, reverse transcription-polymerase chain reaction and virus culture. ARDS acute respiratory distress syndrome; ICU intensive care unit; MV mechanical ventilator
Table 1.
Characteristics of the 215 subjects with influenza-related ARDS
| All patients | Prolonged mechanical ventilation (PMV) | Weaning in PMV | |||||
|---|---|---|---|---|---|---|---|
| Characteristics | Yes | No | p Value | Success | Failure | p Value | |
| (n = 215) | (n = 78) | (n = 137) | (n = 44) | (n = 34) | |||
| Baseline data | |||||||
| Age (years) | 59.27(± 14.48) | 58.22(± 11.74) | 59.88(± 15.83) | 0.38 | 59.25(± 12.12) | 56.88(± 11.27) | 038 |
| Male sex | 134(62.3%) | 50(64.1%) | 84(61.3%) | 0.69 | 31(70.5%) | 19(55.9%) | 0.18 |
| Body mass index BMI (kg/m2) | 25.67(± 5.78) | 26.84(± 4.77) | 25.53(± 5.70) | 0.087 | 26.65(± 4.19) | 27.09(± 5.48) | 0.68 |
| BMI > 25 (kg/m2) | 124(57.7%) | 56(71.8%) | 68(49.6%)** | 0.002 | 23(67.6%) | 33(75%) | 0.47 |
| Malignancy | 26(12.1%) | 12(15.4%) | 14(10.2%) | 0.26 | 7(15.9%) | 5(14.7%) | 0.88 |
| Type II diabetes mellitus | 63(29.3%) | 22(28.2%) | 41(29.9%) | 0.79 | 11 (25%) | 11(32.4%) | 0.47 |
| Cerebrovascular disease | 14(6.5%) | 4(5.1%) | 10(7.3%) | 0.54 | 4 | 0 | 0.13 |
| Liver disease | 22(10.2%) | 9(11.5%) | 13(9.5%) | 0.63 | 4(11.8%) | 5(11.4%) | 1.0 |
| Cardiac disease | 25(11.6%) | 5(6.4%) | 20(14.6%) | 0.072 | 3(8.8%) | 2(4.5%) | 0.65 |
| Hypertension | 93(43.3%) | 35(44.9%) | 58(42.3%) | 0.72 | 19(43.2%) | 16(47.1%) | 0.73 |
| Immunosuppressantb use before influenza infection | 10(4.7%) | 5(6.4%) | 5(3.6%) | 0.5 | 1(2.3%) | 3(8.8%) | 0.31 |
| Autoimmune disease | 14(6.5%) | 6(7.7%) | 8(5.8%) | 0.60 | 2(4.5%) | 4(11.8%) | 0.40 |
| End-stage renal disease | 14(6.5%) | 7(9.0%) | 7(5.1%) | 0.27 | 4(9.1%) | 3(8.8%) | 1.0 |
| Severity scores | |||||||
| APACHE II score | 22.59(± 8.13) | 24.14(± 8.52) | 21.7(± 7.8)* | 0.034 | 23.30 ± 8.74 | 25.24(± 8.23) | 0.32 |
| ARDS a Severity | 0.18 | 0.76 | |||||
| Severe | 122(56.7%) | 49(62.8%) | 73(53.3%) | 27(61.4%) | 22(64.7%) | ||
| Mild to moderate | 93(43.3%) | 29(37.2%) | 64(46.7%) | 17(38.6%) | 12(35.3%) | ||
| Treatments and clinical outcome | |||||||
| Prone | 49(22.8%) | 23(29.5%) | 26(19%) | 0.08 | 14(31.8%) | 9(26.5%) | 0.61 |
| ECMO | 34(15.8%) | 26(33.3%) | 8(5.8%)** | < 0.01 | 8(18.2%) | 18(52.9%)** | < 0.01 |
| Combined with bacterial pneumonia onset before D21 | 67(31.2%) | 39(50%) | 28(20.4%)** | < 0.01 | 26(59.1%) | 22(64.7%) | 0.61 |
| Bacteremia onset before D21 | 34(15.8%) | 19(24.4%) | 15(10.9%)** | 0.01 | 6(13.6%) | 13(38.2%)* | 0.012 |
| Steroid user | 127(59.1%) | 46(59%) | 81(59.1%) | 0.98 | 23(52.3%) | 23(67.6%) | 0.17 |
| Sedation | 159(74%) | 60(76.9%) | 99(72.3%) | 0.45 | 32(72.7%) | 28(82.4%) | 0.32 |
| Neuromuscular blockade > 48 h | 119(55.3%) | 59(75.6%) | 60(43.8%)*** | < 0.001 | 32(72.7%) | 27(79.4%) | 0.5 |
| Need for vasopressor agents | 103(47.9%) | 49(62.8%) | 54(39.4%)*** | < 0.01 | 25(56.8%) | 24(70.6%) | 0.21 |
| Renal replacement therapy c | 19(8.8%) | 11(14.1%) | 8(5.8%)* | 0.04 | 6(13.6%) | 5(14.7%) | 1.0 |
| Ventilator-duration (days) | 21.18(± 17.74) | 39.24(± 17.77) | 10.90(± 4.77)*** | < 0.001 | 36.12(± 14.60) | 43.29(± 20.72) | 0.09 |
| ICU stay (days) | 22.32(± 18) | 38.18(± 20.65) | 13.22(± 6.24)*** | < 0.001 | 36(± 19.22) | 41(± 22.34) | 0.29 |
| Hospital-stay (days) | 37.59(± 27.38) | 54.12(± 32.73) | 28.11(± 18)*** | < 0.001 | 58.90(± 29.70) | 47.92(± 35.77) | 0.14 |
| In hospital Mortality | 42(19.5%) | 31(39.7%) | 11(8%)*** | < 0.001 | 5(11.4%) | 26(76.5%)*** | < 0.001 |
Data are presented as the mean ± standard deviation and number (%)
APACHE II Acute Physiology and Chronic Health Evaluation, ARDS acute respiratory distress syndrome, ECMO extracorporeal membrane oxygenation
aIn accordance with Berlin definition
bOral prednisolone equivalent dosage > 5 mg/day or > 150 mg cumulative dose within 1 month before influenza infection; or regular treatment using other immunosuppressants within 1month before influenza infection
cExcluding those with end-stage renal disease receiving regular hemodialysis
*< 0.05
**0 < 0.01
***0 < 0.001
To further elucidate risk factors for PMV in influenza-associated ARDS patients, univariate and multivariate logistic regression analyses were conducted (Table 2). Ultimately, it was determined that in ARDS patients with influenza complications, BMI > 25, ECMO use, combined with bacterial pneumonia, and neuromuscular blockade exceeding 48 h were significant independent variables associated with PMV. To reduce the selection bias, we also compared the PMV or death before MV day 21 group to non-PMV group (Additional file 2: Figure S1). In PMV or death before MV day 21 group, they had higher APACHE II score, more patients with severe ARDS, more use of ECMO before MV day 7, more patients with bacterial pneumonia before MV day 7, more use of neuromuscular blockade over 48 h, more need of vasopressors, and more need of renal replacement therapy compared with survived non-PMV group (Additional file 1: Table S1). Moreover, in PMV or death before MV day 21 group, they had longer days of MV support, longer ICU and hospital days and higher in-hospital mortality compared with survived non-PMV group. After a multivariate regression analysis, higher APACHE II score, ECMO before MV day 7, combined with bacterial pneumonia before MV day 7, neuromuscular blockade over 48 h, and the need for vasopressor agents were associated with patients with PMV or death before MV day 21 (Additional file 1: Table S2).
Table 2.
Risk factors for PMV in patients with influenza-related ARDS
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% confidence interval | p value | Odds ratio | 95% confidence interval | p value | |
| Body mass index (kg/m2) | 1.046 | 0.992 ~ 1.103 | 0.095 | |||
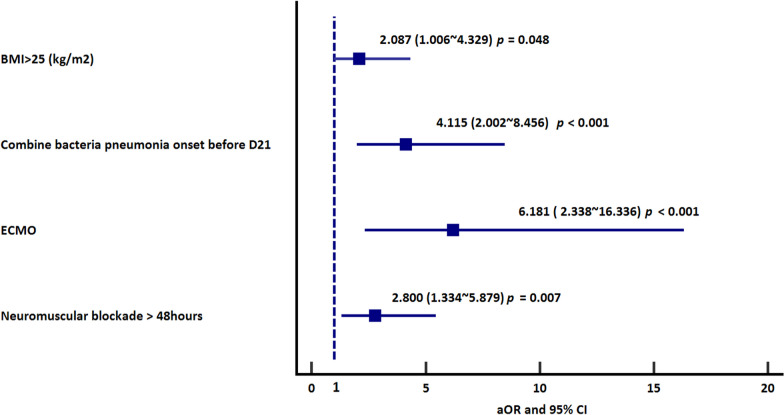
| BMI > 25 (kg/m2) | 2.583 | 1.423 ~ 4.688 | 0.002 | 2.087 | 1.006 ~ 4.329 | 0.048 |
| Cardiac disease | 0.401 | 0.144 ~ 1.114 | 0.08 | |||
| Combined with bacterial pneumonia onset before D21 | 3.893 | 2.120 ~ 7.149 | < 0.001 | 4.115 | 2.002 ~ 8.456 | < 0.001 |
| Bacteremia onset before D21 | 2.619 | 1.244 ~ 5.517 | 0.011 | 1.821 | 0.755 ~ 4.391 | 0.18 |
| APACHE II score | 1.038 | 1.003 ~ 1.075 | 0.036 | 1.014 | 0.97 ~ 1.060 | 0.55 |
| ECMO | 8.062 | 3.428 ~ 18.964 | < 0.001 | 6.181 | 2.338 ~ 16.336 | < 0.001 |
| Prone | 1.785 | 0.934 ~ 3.411 | 0.079 | |||
| Neuromuscular blockade > 48 h | 3.985 | 2.149 ~ 7.389 | < 0.001 | 2.800 | 1.334 ~ 5.879 | 0.007 |
| Need for vasopressor agents | 2.597 | 1.464 ~ 4.606 | < 0.001 | 1.528 | 0.753 ~ 3.099 | 0.24 |
| Acute kidney injury requiring renal replacement therapy a | 2.647 | 1.016 ~ 6.896 | 0.046 | 2.578 | 0.778 ~ 8.543 | 0.121 |
ARDS acute respiratory distress syndrome, ECMO extracorporeal membrane oxygenation, APACHE II Acute Physiology and Chronic Health Evaluation
aExcluding those with end-stage renal disease receiving regular hemodialysis
To reduce the survival bias, we compared the PMV or death between MV D7 and D21 group with non-PMV group in patients with MV use more than 7 days (Additional file 2: Figure S2). In PMV or death between MV day 8 and MV day 21 group, they had higher APACHE II score, more use of ECMO before MV day 7, more patients with bacterial pneumonia before MV day 7, more bacteremia before MV day 7, more use of neuromuscular blockade over 48 h, more need of vasopressors, and more need of renal replacement therapy compared with survived non-PMV group (Additional file 1: Table S3). We also observed longer MV days, longer ICU and hospital days and higher in-hospital mortality in PMV or death between MV day 8 and MV day 21 group compared with survived non-PMV group. In addition, ECMO before MV day 7, patients with bacterial pneumonia and use of neuromuscular blockade over 48 h were risk factors for PMV or death between MV day 8 and MV day 21 (Additional file 1: Table S4).
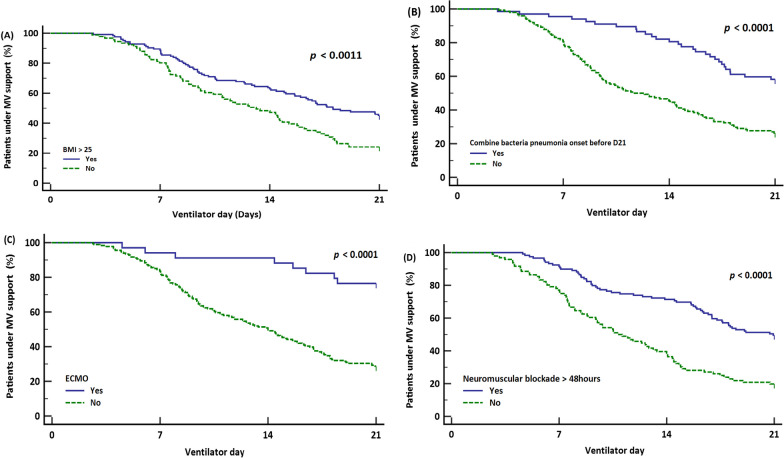
The results of binary logistic regression analysis—forest plot of variables independently associated with PMV are shown in Fig. 2. confirming that patients with BMI > 25 kg/m2, ECMO use, concomitant bacterial pneumonia, and neuromuscular blockade use over 48 h had substantial risks for PMV. In survival analysis, we found that BMI > 25 kg/m2, patients with bacterial pneumonia, ECMO and use of neuromuscular blockade more than 48 h were also associated with longer duration of MV support (Fig. 3A–D).
Fig. 2.
Factors associated with PMV. Forest plot of significant variables included in multivariable regression analysis. Odds ratios are reported with 95% confidence intervals
Fig. 3.
Kaplan‒Meier survival analysis for PMV risk factors. A In estimation for prolonged mechanical ventilation (PMV) occurrence in influenza-induced ARDS, stratification was conducted based on BMI > 25 (kg/m2). Individuals with BMI > 25 (kg/m2) exhibited a significantly higher prevalence of PMV. B In estimation of prolonged mechanical ventilation (PMV) occurrence in influenza-induced ARDS, stratification was performed based on the presence or absence of combined bacterial pneumonia within 21 days prior to ventilator support. Individuals with combined bacterial pneumonia showed a significantly higher incidence of PMV. C In estimation for prolonged mechanical ventilation (PMV) in influenza-induced ARDS, stratification was performed based on the presence or absence of ECMO utilization. Individuals receiving ECMO support demonstrated a significantly higher incidence of PMV. D In estimation for prolonged mechanical ventilation (PMV) occurrence in influenza-induced ARDS, stratification was performed based on the duration of neuromuscular blockade agent (NMBA) use exceeding 48 h. Individuals with NMBA use exceeding 48 h showed a significantly higher incidence of PMV
For secondary outcomes, we assessed 78 patients with Prolonged Mechanical Ventilation (PMV), analyzing risk factors associated with unsuccessful extubation in PMV patients (Table 3). The mean duration of mechanical ventilation was 39.24 (± 17.77) days. Out of these PMV patients, 44 were eventually successfully weaned from MV.
Table 3.
Risk factors for weaning failure in PMV patients
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% confidence interval | p value | Odds ratio | 95% confidence interval | p value | |
| ECMO | 5.06 | 1.83 ~ 14.04 | 0.002 | 5.05 | 1.75 ~ 14.58 | 0.003 |
| Bacteremia | 3.92 | 1.30 ~ 11.83 | 0.015 | 3.91 | 1.20 ~ 12.69 | 0.023 |
ECMO extracorporeal membrane oxygenation
Thirty-four patients either died or required ongoing ventilator support after 21 days of ventilation. Following univariate and multivariate regression analyses in these two patient groups (Table 3), we confirmed that the use of ECMO support (OR: 5.05; 95% CI 1.75–14.58) and bacteremia (OR: 3.91; 95% CI 1.20–12.69) were risk factors associated with failed ventilator liberation after 21 days of ventilation.
Throughout the follow-up period, 181 patients achieved successful ventilator weaning, resulting in a ventilator weaning rate of 68.8%.
Discussion
Patients with influenza related ARDS have independent risk factors to develop PMV, including BMI > 25, combined bacterial pneumonia, neuromuscular blockade use over 48 h during treatment, and ECMO support. Additionally, we found that risk factors for unsuccessful weaning after 21 days of ventilation are ECMO support and presence of bacteremia.
In our cohort, an average BMI > 25 (overweight and obese) was observed, with a higher prevalence of patients with BMI > 25 in the PMV group. The most recent studies have described that individuals with obesity who contract influenza are more likely to require mechanical ventilation and experience longer stays in the intensive care unit (ICU), along with an increased risk of mortality [10, 11]. One of clinical studies on ARDS have noted higher BMI in ARDS patients than in non-ARDS patients. Development of ARDS increases significantly with higher body weight [12]. A meta-analysis demonstrated a significant association between obesity in critically ill patients and prolonged mechanical ventilation duration [13]. Over the past two years, the literature has begun to highlight that the proportion of patients with BMI > 25 of COVID-19 is nearly twice that of those with influenza [14]. There was a trend towards a positive association between the BMI (normal weight, overweight and obesity) and the risk of serious events linked to COVID-19, with a marked increase from 8.1% to 20% and 30.6% respectively [15]. A COVID-19 study also indicated that patients with higher BMI spent more days on ventilators than those with normal weight, aligning with our cases and the results of this study [16].
The combination of influenza virus and bacterial pneumonia can exacerbate the severity of ARDS, infectious shock, and multiorgan failure. [17] In patients requiring hospitalization, bacterial pneumonia is more commonly seen in influenza than in COVID-19. [18, 19]. During influenza virus infection, changes in respiratory epithelial cells and host immune responses lead to exposure of the epithelial surface. As the infection progresses, bacteria can adhere, and respiratory bacteria can accumulate in the airway epithelial mucus, thereby promoting secondary bacterial pneumonia [20]. In the 2009 H1N1 pandemic, bacterial pneumonia as a pulmonary complication was associated with prolonged duration of mechanical ventilation. [21] In our cohort, the presence of bacterial pneumonia prolonged the duration of mechanical ventilation, and in another study conducted by our team, we found that severe influenza-related ARDS hospital-acquired lower respiratory tract infections are associated with prolonged mechanical ventilation and worse prognosis [22]. Another multicenter Italian study on COVID-19 patient weaning from ventilators also identified late-onset ventilator-associated pneumonia as one of the factors influencing ventilator liberation. [23]。In another study on COVID-19 with superinfections, bacteremia accounted for 47.4% of cases, and patients with secondary infections had a prolonged mechanical ventilation time of up to 37 days. [24] Bacteremia can progress to systemic inflammatory response syndrome (SIRS), sepsis, septic shock, and multiple organ dysfunction syndrome (MODS) [25, 26]. The occurrence of sepsis can affect diaphragmatic stability and lead to failed ventilator liberation. The presence of sepsis is associated with evident diaphragmatic weakness. [27–31] Currently, diaphragm dysfunction in critically ill patients is believed to occur primarily through two mechanisms: ventilator-induced diaphragmatic dysfunction (VIDD) [32] and sepsis-induced dysfunction. [33] Sepsis typically impairs oxygen consumption, increases anaerobic metabolism, and leads to metabolic acidosis. The need to compensate for acidemia increases ventilation requirements and may result in failed ventilator liberation [34].
In recent years, research has indicated that spontaneous ventilation can help to improve hypoxemia and lung compliance and reduce diaphragm atrophy in patients with acute respiratory distress syndrome (ARDS).
[35–37] However, spontaneous breathing can lead to elevated respiratory drive and vigorous inspiratory efforts, causing uneven pressure distribution and potentially resulting in patient self-inflicted lung injury (P-SILI) [38]. Multiple animal studies have demonstrated detrimental cycles, such as asynchrony with the ventilator, elevated transpulmonary pressure, and double triggering, which further worsen lung injury. [28, 39–41] A trial in 2010 reported improved 90-day survival rates in severe ARDS patients receiving neuromuscular blockade (NMBAs) [42], but a larger trial in 2019 contradicted these findings. [43] Therefore, use of NMBAs in ARDS patients remains controversial. Although current evidence does not support routine early use of neuromuscular blockade in all adult patients with moderate to severe ARDS, utilizing NMBAs in the early stages of ARDS to ensure good synchrony with the ventilator and promote lung-protective strategies remains a reasonable treatment option. There are formal guidelines that recommend continuous infusion of NMBAs for less 48 h [44] and evaluation daily by specialized physicians for ongoing use. [45, 46] Clinical data indicate that using neuromuscular blocking agents can reduce barotrauma and improve physiological and clinical outcomes. [47] However, there are potential adverse effects on diaphragmatic contractile function and delayed extubation. [48] Several studies and a meta-analysis on acute respiratory distress syndrome (ARDS) have confirmed that neuromuscular blocking agents (NMBAs) do not improve mortality rates, ventilator-free days (VFDs), or the duration of mechanical ventilation. [49–51]. However, it is important to note that the incongruences in research methodologies lead to an inability to reach a definitive consensus on this matter.
In contrast, our study findings indicate a significant impact of utilizing NMBAs for more than 48 h on the extension of mechanical ventilation duration, known as prolonged mechanical ventilation (PMV).
ECMO provides circulatory or respiratory support in cases of refractory cardiogenic shock or ARDS. Multiple studies have indicated that ECMO is feasible and effective for ARDS patients caused by H1N1 infection in 2009[52–54].One study assessing ECMO-related mortality risk reported that the mortality rate for influenza-induced ARDS patients receiving ECMO support was the lowest observed thus far, despite an average duration of mechanical ventilation support of up to 40 days. [55]。
In a multicenter retrospective study conducted in Italy, COVID-19 and influenza-related ARDS receiving ECMO patients had longer durations of invasive mechanical ventilation than influenza patients, with durations of 33 days and 25 days[56], respectively. However, the mortality rate during COVID-19 was higher than that during the 2009 H1N1 period, potentially due to more frequent use of noninvasive ventilation (HFNC) forms before endotracheal intubation, leading to more severe self-inflicted lung injury. [38, 57]。In studies involving ECMO usage for H1N1 influenza patients, the duration of mechanical ventilation before ECMO initiation was identified as an important prognostic factor. [54] A multicenter study conducted in COVID-19 patients yielded similar results. [58] Numerous international multicenter studies have substantiated the elevated mortality rates associated with ARDS, which are estimated to be approximately 40% [59–62]. In another study focusing on the association of higher tidal volumes with increased mortality conducted by our group, a 30-day mortality rate of 23.2% was observed [63], but the estimated mortality rate for patients after PMV increased to 39.7%. This similarity with large international studies confirms the association between PMV and a higher mortality rate.
Our study has several limitations. First, it is a retrospective study, which may have resulted in missing statistical and medical data, leading to variations. Second, there was a lack of consistency in the treatment strategies for influenza-related ARDS among the different study sites. The treatment policies of participating centers were not standardized, which increased potential confounding factors. Last, our study focused on patients with ARDS caused by influenza. Therefore, whether the findings apply to ARDS caused by other factors needs to be confirmed through future, more rigorous prospective clinical studies. Despite these limitations, to the best of our knowledge, this is the first multicenter study that elucidates risk factors associated with prolonged mechanical ventilation in patients with ARDS caused by influenza. Our study may aid clinicians with regard to treatment directions and decision-making for critically ill patients.
Conclusion
Patients with influenza-related ARDS and subsequent PMV have a high mortality rate. The present study identified several independent predictors of prolonged mechanical ventilation (PMV) in influenza-associated ARDS. These predictors included BMI > 25, combine bacterial pneumonia, the use of neuromuscular blockers more than 48 h during ICU stay, and ECMO support. Additionally, unsuccessfully weaning from mechanical ventilation was independently associated with ECMO support during hospitalization or development of bacteremia.
Supplementary Information
Additional file 1: Table S1. Characteristics of the 263 subjects with influenza-related ARDS. Table S2. Risk factors for PMV or death before MV D21 in subjects with influenza-related ARDS. Table S3.Characteristics of the subjects with influenza-related ARDS and MV use > 7 days. Table S4. Risk factors for PMV in patients with influenza-related ARDS.
Additional file 2: Figure S1. Flow chart of the study. a.Virology-proven methods include the rapid influenza diagnostic test, reverse transcription-polymerase chain reaction and virus culture. ARDS, acute respiratory distress syndrome; ICU, intensive care unit; MV, mechanical ventilator. Figure S2.
Author contributions
Authors’ contributions: P.C Hsu, W.C Chen and K.Y Yang had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Data collection and conceptualization: P.C Hsu, K-C Kao, C-K Peng, C-C Sheu, S-J Liang M-C Chan , H-C Wang , Y-M Chen, W.C Chen, K.Y Yang. Formal Analysis: P.C Hsu, K-C Kao, C-K Peng, C-C Sheu, S-J Liang M-C Chan , H-C Wang , Y-M Chen,W.C Chen, K.Y Yang. Methodology: P.C Hsu,W.C Chen, Y.T Lin , K.Y Yang. Investigation: P.C Hsu, K-C Kao, C-K Peng, C-C Sheu, S-J Liang M-C Chan , H-C Wang , Y-M Chen,W.C Chen, K.Y Yang. Writing – Review & Editing:P.C Hsu, Y.T Lin, W.C Chen, K.Y Yang. Writing – Original Draft Preparation: P.C Hsu, W.C Chen. Supervision: W.C Chen, Y.T Lin and K.Y Yang. Funding acquisition:W.C Chen, K.Y Yang. All authors read and approved the final manuscript.
Funding
This research was funded by grants from Taipei Veterans General Hospital (V111B-024, V112B-031, V112C-068, V112D65-003-MY2-1), and National Science and Technology Council (Taiwan) (NSTC 112-2314-B-A49-040, NSTC 112-2314-B-075-050). This work was supported by grants from the Ministry of Education, Higher Education SPROUT Project for Cancer Progression Research Center (111W31101) and Cancer and Immunology Research Center (112W31101).
Availability of data and materials
The data presented in this study are available on request from the corresponding author.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Boards of all eight hospitals (Taipei Veterans General Hospital, 2016-05-020CC; Taichung Veterans General Hospital, CE16093A; Tri-Service General Hospital, 1-105- 05-086; National Taiwan University Hospital, 201605036RIND; Chang Gung Memorial Hospital, 201600988B0; China Medical University Hospital, 105-REC2-053(FR); Kaohsiung Medical University Hospital, KUMHIRB-E(I)- 20170097; and Kaohsiung Chang Gung Memorial Hospital, 201600988B0.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wei-Chih Chen and Kuang-Yao Yang contributed equally to this work.
Contributor Information
Kuang-Yao Yang, Email: kyyang@vghtpe.gov.tw.
TSIRC (Taiwan Severe Influenza Research Consortium):
Han-Chung Hu, Wann-Cherng Perng, Ming-Ju Tsai, Chieh-Liang Wu, Ying-Chun Chien, and Wen-Feng Fang
References
- 1.Boles JM, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29(5):1033–1056. doi: 10.1183/09031936.00010206. [DOI] [PubMed] [Google Scholar]
- 2.MacIntyre NR, et al. Management of patients requiring prolonged mechanical ventilation: report of a NAMDRC consensus conference. Chest. 2005;128(6):3937–3954. doi: 10.1378/chest.128.6.3937. [DOI] [PubMed] [Google Scholar]
- 3.Damuth E, et al. Long-term survival of critically ill patients treated with prolonged mechanical ventilation: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(7):544–553. doi: 10.1016/S2213-2600(15)00150-2. [DOI] [PubMed] [Google Scholar]
- 4.Matthay MA, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5(1):18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar A, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302(17):1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 6.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377(6):562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 7.Short KR, et al. Pathogenesis of influenza-induced acute respiratory distress syndrome. Lancet Infect Dis. 2014;14(1):57–69. doi: 10.1016/S1473-3099(13)70286-X. [DOI] [PubMed] [Google Scholar]
- 8.Ranieri VM, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 9.Knaus WA, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Morgan OW, et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PLoS One. 2010;5(3):e9694. doi: 10.1371/journal.pone.0009694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Kerkhove MD, et al. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med. 2011;8(7):e1001053. doi: 10.1371/journal.pmed.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong MN, et al. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax. 2010;65(1):44–50. doi: 10.1136/thx.2009.117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akinnusi ME, Pineda LA, El Solh AA. Effect of obesity on intensive care morbidity and mortality: a meta-analysis*. Crit Care Med. 2008;36(1):151–158. doi: 10.1097/01.CCM.0000297885.60037.6E. [DOI] [PubMed] [Google Scholar]
- 14.Piroth L, et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med. 2021;9(3):251–259. doi: 10.1016/S2213-2600(20)30527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pietri L, et al. Excess body weight is an independent risk factor for severe forms of COVID-19. Metabolism. 2021;117:154703. doi: 10.1016/j.metabol.2021.154703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Son J, et al. Overweight and obesity are associated with acute kidney injury and acute respiratory distress syndrome, but not with increased mortality in hospitalized COVID-19 patients: a retrospective cohort study. Front Endocrinol. 2021;12:747732. doi: 10.3389/fendo.2021.747732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma S, et al. Clinical characteristics of critically ill patients co-infected with SARS-CoV-2 and the influenza virus in Wuhan. China Int J Infect Dis. 2020;96:683–687. doi: 10.1016/j.ijid.2020.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hedberg P, et al. Bacterial co-infections in community-acquired pneumonia caused by SARS-CoV-2, influenza virus and respiratory syncytial virus. BMC Infect Dis. 2022;22(1):108. doi: 10.1186/s12879-022-07089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rouzé A, et al. Early bacterial identification among intubated patients with COVID-19 or influenza pneumonia: a European multicenter comparative clinical trial. Am J Respir Crit Care Med. 2021;204(5):546–556. doi: 10.1164/rccm.202101-0030OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denney L, Ho LP. The role of respiratory epithelium in host defence against influenza virus infection. Biomed J. 2018;41(4):218–233. doi: 10.1016/j.bj.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moretti M, et al. Ventilator-associated bacterial pneumonia in coronavirus 2019 disease, a retrospective monocentric cohort study. J Infect Chemother. 2021;27(6):826–833. doi: 10.1016/j.jiac.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen WC, et al. Risk factor analysis of nosocomial lower respiratory tract infection in influenza-related acute respiratory distress syndrome. Ther Adv Respir Dis. 2020;14:1753466620942417. doi: 10.1177/1753466620942417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gamberini L, et al. Factors influencing liberation from mechanical ventilation in coronavirus disease 2019: multicenter observational study in fifteen Italian ICUs. J Intensive Care. 2020;8:80. doi: 10.1186/s40560-020-00499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buehler PK, et al. Bacterial pulmonary superinfections are associated with longer duration of ventilation in critically ill COVID-19 patients. Cell Rep Med. 2021;2(4):100229. doi: 10.1016/j.xcrm.2021.100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler-Laporte G, et al. MRSA colonization status as a predictor of clinical infection: a systematic review and meta-analysis. J Infect. 2018;77(6):489–495. doi: 10.1016/j.jinf.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Bullock, B. and M.D. Benham, Bacterial Sepsis, in StatPearls. 2023, StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC.: Treasure Island (FL). [PubMed]
- 27.Demoule A, et al. Diaphragm dysfunction on admission to the intensive care unit. Prevalence, risk factors, and prognostic impact-a prospective study. Am J Respir Crit Care Med. 2013;188(2):213–9. doi: 10.1164/rccm.201209-1668OC. [DOI] [PubMed] [Google Scholar]
- 28.Ebihara S, et al. Mechanical ventilation protects against diaphragm injury in sepsis: interaction of oxidative and mechanical stresses. Am J Respir Crit Care Med. 2002;165(2):221–228. doi: 10.1164/ajrccm.165.2.2108041. [DOI] [PubMed] [Google Scholar]
- 29.Jung B, et al. Sepsis is associated with a preferential diaphragmatic atrophy: a critically ill patient study using tridimensional computed tomography. Anesthesiology. 2014;120(5):1182–1191. doi: 10.1097/ALN.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 30.Lanone S, et al. Diaphragmatic fatigue during sepsis and septic shock. Intensive Care Med. 2005;31(12):1611–1617. doi: 10.1007/s00134-005-2748-4. [DOI] [PubMed] [Google Scholar]
- 31.Supinski GS, Callahan LA. Diaphragm weakness in mechanically ventilated critically ill patients. Crit Care. 2013;17(3):R120. doi: 10.1186/cc12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powers SK, et al. Ventilator-induced diaphragm dysfunction: cause and effect. Am J Physiol Regul Integr Comp Physiol. 2013;305(5):R464–R477. doi: 10.1152/ajpregu.00231.2013. [DOI] [PubMed] [Google Scholar]
- 33.Scheiermann P, Pischke SE. Lipid peroxidation in multidrug-resistant gram-negative sepsis: translating science to the septic patient? Crit Care. 2013;17(2):120. doi: 10.1186/cc12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Artigas A, et al. The American-European consensus conference on ARDS, part 2 ventilatory, pharmacologic, supportive therapy, study design strategies and issues related to recovery and remodeling. Intensive Care Med. 1998;24(4):378–98. doi: 10.1007/s001340050585. [DOI] [PubMed] [Google Scholar]
- 35.Putensen C, et al. Spontaneous breathing during ventilatory support improves ventilation-perfusion distributions in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1241–1248. doi: 10.1164/ajrccm.159.4.9806077. [DOI] [PubMed] [Google Scholar]
- 36.Putensen C, et al. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med. 2001;164(1):43–49. doi: 10.1164/ajrccm.164.1.2001078. [DOI] [PubMed] [Google Scholar]
- 37.Carvalho NC, et al. Higher levels of spontaneous breathing reduce lung injury in experimental moderate acute respiratory distress syndrome. Crit Care Med. 2014;42(11):e702–e715. doi: 10.1097/CCM.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 38.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195(4):438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 39.Goligher EC, et al. Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am J Respir Crit Care Med. 2018;197(2):204–213. doi: 10.1164/rccm.201703-0536OC. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida T, et al. Spontaneous breathing during lung-protective ventilation in an experimental acute lung injury model: high transpulmonary pressure associated with strong spontaneous breathing effort may worsen lung injury. Crit Care Med. 2012;40(5):1578–1585. doi: 10.1097/CCM.0b013e3182451c40. [DOI] [PubMed] [Google Scholar]
- 41.Beitler JR, et al. Quantifying unintended exposure to high tidal volumes from breath stacking dyssynchrony in ARDS: the breathe criteria. Intensive Care Med. 2016;42(9):1427–1436. doi: 10.1007/s00134-016-4423-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papazian L, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 43.Moss M, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380(21):1997–2008. doi: 10.1056/NEJMoa1901686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papazian L, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9(1):69. doi: 10.1186/s13613-019-0540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. Lancet. 2021;398(10300):622–637. doi: 10.1016/S0140-6736(21)00439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rhoney DH, Murry KR. National survey of the use of sedating drugs, neuromuscular blocking agents, and reversal agents in the intensive care unit. J Intensive Care Med. 2003;18(3):139–145. doi: 10.1177/0885066603251200. [DOI] [PubMed] [Google Scholar]
- 47.Chang W, et al. Validation of neuromuscular blocking agent use in acute respiratory distress syndrome: a meta-analysis of randomized trials. Crit Care. 2020;24(1):54. doi: 10.1186/s13054-020-2765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shehabi Y, et al. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186(8):724–731. doi: 10.1164/rccm.201203-0522OC. [DOI] [PubMed] [Google Scholar]
- 49.Ho ATN, Patolia S, Guervilly C. Neuromuscular blockade in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. J Intensive Care. 2020;8:12. doi: 10.1186/s40560-020-0431-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Bassi G, et al. Early short course of neuromuscular blocking agents in patients with COVID-19 ARDS: a propensity score analysis. Crit Care. 2022;26(1):141. doi: 10.1186/s13054-022-03983-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lyu T, et al. The effect of neuromuscular blocking agents uses in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Minerva Anestesiol. 2021;87(3):341–350. doi: 10.23736/S0375-9393.20.14783-7. [DOI] [PubMed] [Google Scholar]
- 52.Davies A, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302(17):1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 53.Roch A, et al. Extracorporeal membrane oxygenation for severe influenza A (H1N1) acute respiratory distress syndrome: a prospective observational comparative study. Intensive Care Med. 2010;36(11):1899–1905. doi: 10.1007/s00134-010-2021-3. [DOI] [PubMed] [Google Scholar]
- 54.Patroniti N, et al. The Italian ECMO network experience during the 2009 influenza A(H1N1) pandemic: preparation for severe respiratory emergency outbreaks. Intensive Care Med. 2011;37(9):1447–1457. doi: 10.1007/s00134-011-2301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmidt M, et al. The preserve mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med. 2013;39(10):1704–1713. doi: 10.1007/s00134-013-3037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fanelli V, et al. Extracorporeal membrane oxygenation for COVID-19 and influenza H1N1 associated acute respiratory distress syndrome: a multicenter retrospective cohort study. Crit Care. 2022;26(1):34. doi: 10.1186/s13054-022-03906-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barbaro RP, et al. Extracorporeal membrane oxygenation for COVID-19: evolving outcomes from the international extracorporeal life support organization registry. Lancet. 2021;398(10307):1230–1238. doi: 10.1016/S0140-6736(21)01960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lebreton G, et al. Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID-19 pandemic in Greater Paris, France: a multicentre cohort study. Lancet Respir Med. 2021;9(8):851–862. doi: 10.1016/S2213-2600(21)00096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bellani G, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 60.Villar J, et al. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 2011;37(12):1932–1941. doi: 10.1007/s00134-011-2380-4. [DOI] [PubMed] [Google Scholar]
- 61.Esteban A, et al. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med. 2013;188(2):220–230. doi: 10.1164/rccm.201212-2169OC. [DOI] [PubMed] [Google Scholar]
- 62.Wang CY, et al. One-year mortality and predictors of death among hospital survivors of acute respiratory distress syndrome. Intensive Care Med. 2014;40(3):388–396. doi: 10.1007/s00134-013-3186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chan MC, et al. First tidal volume greater than 8 mL/kg is associated with increased mortality in complicated influenza infection with acute respiratory distress syndrome. J Formos Med Assoc. 2019;118(1 Pt 2):378–385. doi: 10.1016/j.jfma.2018.06.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Characteristics of the 263 subjects with influenza-related ARDS. Table S2. Risk factors for PMV or death before MV D21 in subjects with influenza-related ARDS. Table S3.Characteristics of the subjects with influenza-related ARDS and MV use > 7 days. Table S4. Risk factors for PMV in patients with influenza-related ARDS.
Additional file 2: Figure S1. Flow chart of the study. a.Virology-proven methods include the rapid influenza diagnostic test, reverse transcription-polymerase chain reaction and virus culture. ARDS, acute respiratory distress syndrome; ICU, intensive care unit; MV, mechanical ventilator. Figure S2.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.