Abstract
Background
Interstitial lung diseases (ILD) comprise a heterogeneous group of mainly chronic lung diseases with more than 200 entities and relevant differences in disease course and prognosis. Little data is available on hospitalisation patterns in ILD.
Methods
The EXCITING-ILD (Exploring Clinical and Epidemiological Characteristics of Interstitial Lung Diseases) registry was analysed for hospitalisations. Reasons for hospitalisation were classified as all cause, ILD-related and respiratory hospitalisations, and patients were analysed for frequency of hospitalisations, time to first non-elective hospitalisation, mortality and progression-free survival. Additionally, the risk for hospitalisation according to GAP index and ILD subtype was calculated by Cox proportional-hazard models as well as influencing factors on prediction of hospitalisation by logistic regression with forward selection.
Results
In total, 601 patients were included. 1210 hospitalisations were recorded during the 6 months prior to registry inclusion until the last study visit. 800 (66.1%) were ILD-related, 59.3% of admissions were registered in the first year after inclusion. Mortality was associated with all cause, ILD-related and respiratory-related hospitalisation. Risk factors for hospitalisation were advanced disease (GAP Index stages II and III) and CTD (connective tissue disease)-ILDs. All cause hospitalisations were associated with pulmonary hypertension (OR 2.53, p = 0.005). ILD-related hospitalisations were associated with unclassifiable ILD and concomitant emphysema (OR = 2.133, p = 0.001) as well as with other granulomatous ILDs and a positive smoking status (OR = 3.082, p = 0.005).
Conclusion
Our results represent a crucial contribution in understanding predisposing factors for hospitalisation in ILD and its major impact on mortality. Further studies to characterize the most vulnerable patient group as well as approaches to prevent hospitalisations are warranted.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-023-02588-y.
Keywords: ILD, IPF, Hospitalisation, Prognosis, Risk factors
Background
Interstitial lung diseases (ILD) comprise a heterogeneous group of more than 200 mainly chronic diseases [1–3]. The diagnosis of an ILD is made in a multidisciplinary context considering clinical, radiological, and pathological aspects [2, 3]. With the advent of new therapeutic options including antifibrotic therapies for pulmonary fibrosis [4] or new approaches in inflammatory diseases such as systemic sclerosis [5], ILD-associated morbidity and mortality have reduced, but are still relevant. A recent global burden of disease study describes that incidence, mortality, and disability-adjusted life-years of different ILDs are increasing [6]. Furthermore, ILDs play a decisive role regarding the economic burden on healthcare systems, mainly due to costs associated with the pharmacological management and due to hospitalisations [7]. Hospitalisations have also a great impact on quality of life and prognosis, mainly hospitalisations for respiratory reasons [8]. These include e.g. pulmonary infections, acute exacerbations, or ILD related spontaneous pneumothoraces. A large study with almost 600 patients suffering from idiopathic pulmonary fibrosis (IPF) reported that all cause and respiratory-related hospitalisations were strongly associated with mortality [8]. Especially, acute exacerbations have a poor outcome in many ILDs as shown for IPF [9], hypersensitivity pneumonitis (HP) [10] and rheumatoid arthritis (RA) [11], as well as for progressive fibrosing ILDs [12]. Risk factors for hospitalisations are mainly unknown, but one study suggested that different severities of exacerbations exist [9]. In addition, an association between lung functional decline and an increased risk of hospitalisation in systemic sclerosis-associated interstitial lung disease (SSc-ILD) has recently been reported [13] and according to Cottin et al. also comorbidities, mainly cardiovascular events, are associated with frequent hospitalisations [14].
Important insights on these aspects could be obtained through registries. While several registries collecting data on IPF are available, e.g. the German INSIGHTS IPF registry, only few registries including all different ILD subtypes are existing worldwide [15]. The “Exploring Clinical and Epidemiological Characteristics of Interstitial Lung Diseases” (EXCITING-ILD) registry, a multicentre noninterventional, prospective, and observational disease and outcomes registry conducted by the German Center for Lung Research aimed to assess outcomes across all ILD subtypes [16]. Aim of the current work was to assess hospitalisations in different ILD subtypes, impact on mortality and influencing factors, e.g. comorbidities.
Methods
Study design
The EXCITING-ILD registry provides sociodemographic and medical data on ILDs in Germany from different healthcare facilities including ambulatory, in-patient, scientific pulmonology organisations, as well as patient support groups [16]. The study was approved by the Ethics Committee of the Medical Faculty of the University of Heidelberg, Germany (S-525/2013) as well as by all local ethics committees of the participating centres.
The registry protocol has been published elsewhere [16]. Shortly, incident and prevalent patients with any ILD were included and followed prospectively for at least 36 months and a maximum of five years. All patients with a minimum of one documented post-baseline visit were entered into the full analysis set (FAS) [16]. Data collected included baseline and follow up variables. Furthermore, demographic data, ILD subtypes, diagnostic procedures, distinct comorbidities, ILD management, and outcomes including data on hospitalisation and associated factors were assessed [16].
Statistical analysis
For our analyses, the following definitions applied: All Hospitalisations were further divided into ILD-related and ILD-unrelated. ILD-related hospitalisations were caused by or associated with ILD including hospitalisations for elective procedures. For further analyses (time-to-event endpoints) only non-elective ILD-hospitalisation from date of inclusion were considered. A respiratory hospitalisation was defined as exclusively non-elective caused by pneumonia, ILD exacerbation or pneumothorax. Multiple entries for the reason of admission were possible, which means that one hospitalisation can be associated with several of the listed reasons (e.g. pneumonia and ILD exacerbation). For time to first hospitalisation only non-elective hospitalisations were considered. In particular, time to first non-elective hospitalisation was defined as the first non-elective hospitalisation from inclusion to the registry. Hospitalisations were included into the following definitions of progression free survival (PFS): (A) ΔForced vital capacity (FVC) ≥ 10% or respiratory hospitalisation or death and (B) ΔFVC ≥ 10% or all cause hospitalisation or death. The GAP index, in the present study considered as a possible risk factor for hospitalisations, is a point scoring stage model based on clinical and physiologic variables to predict mortality in patients with pulmonary fibrosis. Higher GAP scores indicate worse health state [17].
Observational data were analysed descriptively using means with standard deviations (SD) and percentages. For time-to-event endpoints (hospitalisations, mortality, PFS), Kaplan–Meier curves were used to estimate event-free survival times. Confidence intervals were set with a two-sided level of 95% [16]. For the comparison of two or more Kaplan–Meier curves a log-rank test was performed. To quantify the difference between groups, hazard ratios and corresponding two-sided 95% confidence intervals were estimated based on a Cox proportional hazards model. The p-value of the corresponding Wald-test was calculated. Logistic regression was used to develop a model to predict hospitalisation and to identify variables with a significant influence on hospitalisation. The model was generated using forward selection. By this, the only essential predictors were selected to explain the target variables hospitalisation for any reason, due to ILD and due to respiratory causes. Sex, age, body mass index (BMI), pulmonary function parameters, ILD subtype, pulmonary hypertension, reflux, concomitant emphysema, and smoking behaviour were considered as possible predictors. First, univariable regression models were set up to predict hospitalisation (5% significance level) and second multiple regression models were generated. Data analysis was performed using the statistics software R (R version 4.1.2).
Results
Study population
In total, 601 patients were included in 31 centres. 60.7% were male and the mean age was 64.3 years (Table 1). All different ILD subtypes were included: 26.6% sarcoidosis, 25.3% IPF, 9.7% HP, 7.2% CTD- and RA-ILD, 7% NSIP, 5.7% unclassifiable ILD (uILD), 4.2% cryptogenic organizing pneumonia (COP), 2.7% drug-related ILDs (DI-ILD), 2.3% fibrosis in emphysema patients without signs of other ILDS (CPFE), 1.8% pneumoconiosis, 1.2% pulmonary lymphangioleiomyomatosis (LAM), 1.0% eosinophilic pneumonia, 1.0% radiotherapy associated-ILD (RTX-ILD), 0.8% other granulomatous lung disease (other GRAN-ILD), 0.8% desquamative interstitial pneumonia (DIP), 0.7% respiratory bronchiolitis-associated interstitial lung disease (RB-ILD), 0.5% pulmonary alveolar proteinosis (PAP), 0.3% pulmonary Langerhans´ cell histiocytosis (PLCH), and 1.2% others. Mean FVC was 76.4% predicted and most patients were in GAP stage I (Table 1).
Table 1.
Baseline characteristics of the full analysis set
| Characteristics | Total (n = 601) |
|---|---|
| Male Sex, n (%) | 365 (60.7) |
| Age [years], mean (SD) | 64.3 (14.2) |
| BMI, mean (SD) | 27.3 (4.84) n = 588 |
| Familial ILD, n (%) | 22 (3.7) |
| Current or Ex-smoker, n (%) | 322 (53.6) |
| FVC [% predicted], mean (SD) | 76.4 (20.8) n = 349 |
| FEV1 [% predicted], mean (SD) | 79.2 (20.0) n = 458 |
| FEV1/FVC [% predicted], mean (SD) | 105 (13.5) n = 347 |
| DLCo-SB [% predicted], mean (SD) | 54.1 (21.6) n = 329 |
| Walking distance in 6-min walk [m], mean (SD) | 371 (120) n = 149 |
|
GAP-Index, n (%) I II III Missing |
275 (45.8) |
| 164 (27.3) | |
| 128 (21.3) | |
| 34 (5.7) |
The total numbers for which the respective values were available are indicated within the rows. Where nothing is indicated, the numbers refer to the total of n=601. SD standard deviation, BMI body mass index, ILD interstitial lung disease, FVC forced vital capacity, FEV1 forced expiratory volume in 1 second, DLCO diffusing capacity for carbon monoxide (CO)
Characterisation of hospitalisations
During a median follow-up of 3 years, 1210 hospitalisations were recorded during the 6 months prior to registry inclusion until the last study visit. 66.1% of these hospitalisations were ILD-related. ILD-related admissions mainly included elective diagnostic procedures (n = 293, 33.5%) and acute exacerbations (n = 182, 20.8%). Other reasons were pulmonary infections (n = 165, 18.9%), initiation of non-invasive ventilation (n = 30, 3.4%), lung cancer or other malignancies (n = 18, 2.1%), pulmonary embolism (n = 14, 1.6%), and pneumothorax (n = 5, 0.6%) (Table 2).
Table 2.
Reasons for ILD-related admissions
| Reason for ILD-related admissions | N = 874 (%) |
|---|---|
| Pneumonia / pulmonary infection | 165 (18.9) |
| ILD Exacerbation | 182 (20.8) |
| Pneumothorax | 5 (0.6) |
| Suspicion on/diagnosis of lung cancer or other ILD-related malignancies | 18 (2.1) |
| Pulmonary embolism | 14 (1.6) |
| Elective for diagnostic procedure | 293 (33.5) |
| Initiation of NIV | 30 (3.4) |
| Others | 167 (19.1) |
This table lists all reasons for ILD-related admission. For one hospitalisation several reasons for admission could be considered. ILD= interstitial lung disease, NIV non-invasive ventilation
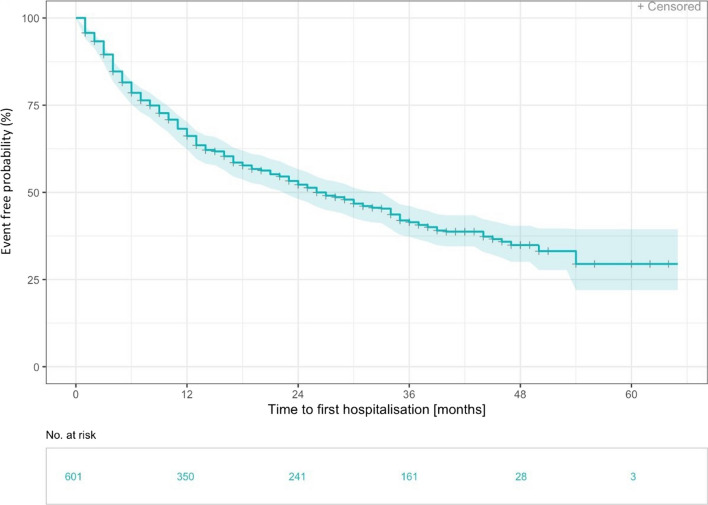
Non-elective hospitalisations (n = 322) occurred most frequently with 59.3% (n = 191) in the 1st year after inclusion into the registry, while in the second year 21.4% of hospitalisations (n = 69) were recorded. 208 events of first hospitalisation due to ILD (34.6%) were reported. Median time to first hospitalisation was 50.8 months (range 0.1 to 50.8 months, Additional file 1: Fig. S1) compared to 26 months (range 0,1 to 50.8 months) in all cause hospitalisations (Fig. 1).
Fig. 1.
Time to first non-elective hospitalisation in months—Kaplan Meier curve. Time to first non-elective hospitalisation was defined as the first non-elective hospitalisation from inclusion to the registry (n= 322). Median time to first hospitalisation was 26 months
Progression free survival and mortality
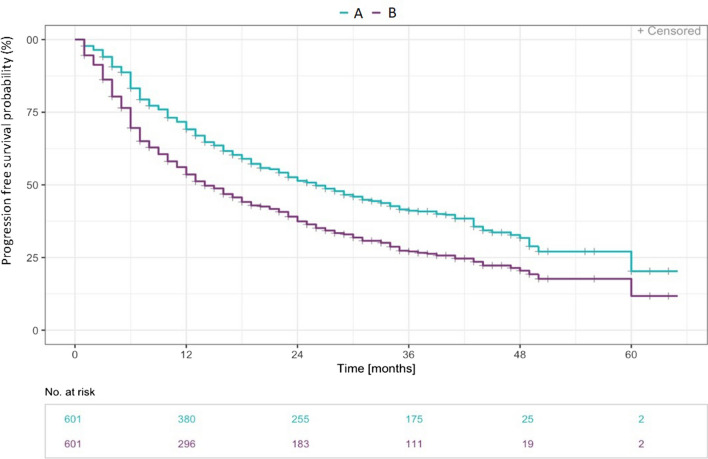
Median progression free survival time was 25.7 months (range 0.1 to 59.63 months) for PFS definition A (i.d. ΔFVC ≥ 10% or respiratory hospitalisation or death) and 13.9 months (range 0.1 to 59.63 months) for PFS definition B (ΔFVC ≥ 10% or all cause hospitalisation or death).
The probability of progression free survival in association with a respiratory hospitalisation was 69.1% in the first 12 months and 51.4% in the second year compared to 53.6% within the first year and 37.4% in second for all cause hospitalisations (Fig. 2).
Fig. 2.
Time to ILD progression in months after definition A and B—Kaplan Meier curve. A ΔForced vital capacity (FVC) ≥ 10% or respiratory hospitalisation or death (n= 340); B ΔFVC ≥ 10% or all cause hospitalisation or death (n= 415)
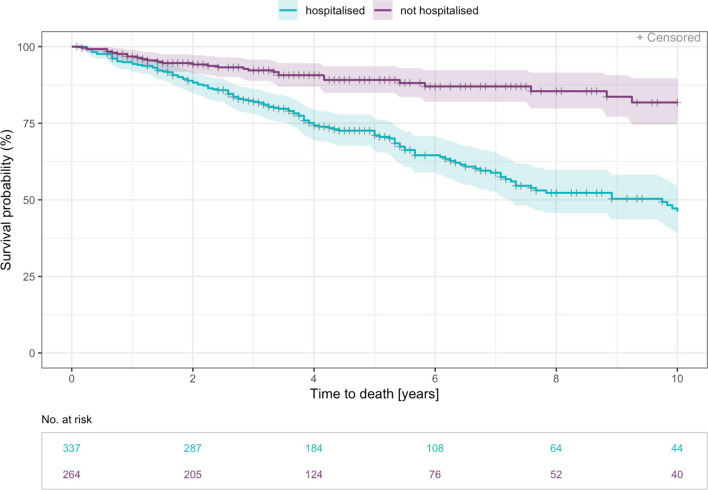
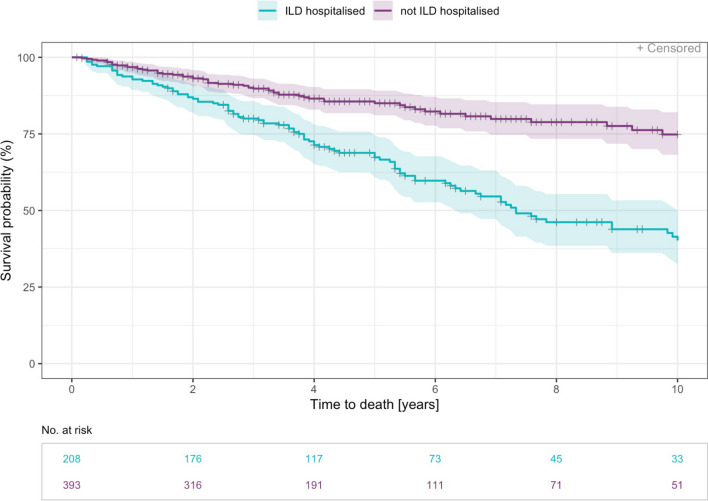
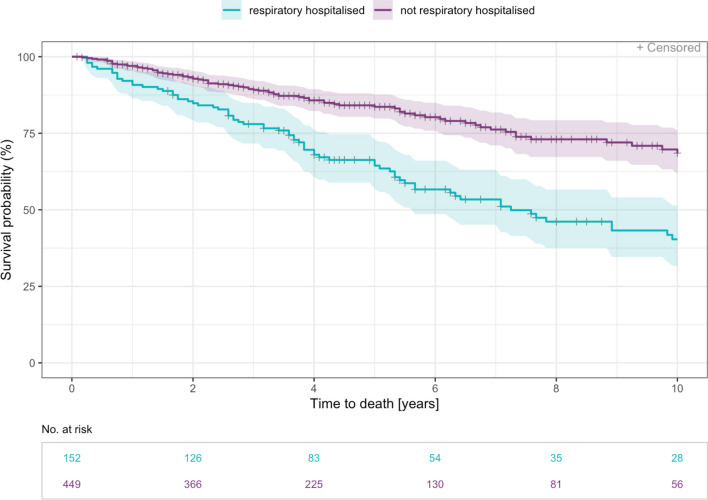
Time to death was significantly increased in patients without all cause hospitalisations (HR 0.30 [0.20, 0.44], p < 0.001). The risk of death was also significantly decreased for patients without hospitalisations due to ILD (HR 0.36 [0.27, 0.49], p < 0.001) and without respiratory hospitalisations (HR 0.40 [0.30, 0.54], p < 0.001) (Figs. 3, 4 and 5).
Fig. 3.
Time to death—Kaplan Meier curve for years 0–10 since diagnosis by all cause hospitalisation. Patients who have reported at least one hospitalisation that started after inclusion date are counted as hospitalised (n= 337). Median time to event was 9.7 years (range 0.2–34.4 years)
Fig. 4.
Time to death—Kaplan Meier curve for years 0–10 since diagnosis by ILD-hospitalisation. Non-elective ILD-hospitalisation from date of inclusion were considered (n= 208). Median time to event was 7.3 years (range 0.2–34.4 years)
Fig. 5.
Time to death—Kaplan Meier curve for years 0–10 since diagnosis by respiratory hospitalisation. A respiratory hospitalisation was defined as exclusively non-elective caused by pneumonia, ILD exacerbation or pneumothorax (n= 152). Median time to event was 7.2 years (range 0.2–34.4 years)
Factors influencing hospitalisations
Two analyses were performed to determine factors influencing hospitalisations. First, time to first hospitalisation was compared between different values of GAP index as well as for different ILD subtypes using cox regression models. Secondly, to analyse a wider range of possible influencing factors, multiple logistic regression was used to determine predictors of hospitalisation in general. The analyses using cox regression models showed that GAP index had a significant effect on time to first hospitalisation. In higher GAP stages II and III hospitalisations occurred earlier compared to GAP stage I, e.g. for GAP index III vs. I with a Hazard ratio of 2.99 (p < 0.001; Table3). Time to first hospitalisation was analysed in different ILD subtypes compared to IPF. Time to first hospitalisation as well as progression-free survival were lowest in connective tissue diseases with pulmonary involvement (CTD-ILD) with a median time to first hospitalisation of 9,8 months (range 0.5 to 53.45) as compared to 12.2 months (range 0.2 to 49.15) in IPF (p = 0.014). Significant effects on time to first hospitalisation and time to first ILD-hospitalisation were also found in granulomatous lung diseases compared to IPF (HR 0.35 and 0.23, p < 0.001; Table3). Kaplan Meier curves for time to first non-elective hospitalisation by ILD subtype are provided within the supplement (Additional file 1: Fig. S2).
Table 3.
Output of Cox regression model of time to first hospitalisation and time to first ILD-hospitalisation analysis by GAP Index (n=601) and ILD subtype (n=468)
| Time to first hospitalisation | Time to first ILD-hospitalisation | |||
|---|---|---|---|---|
| HR [95% CI] | p value (Wald test) |
HR [95% CI] | p value (Wald test) |
|
| GAP Index | ||||
| II vs. I |
2.5 [1.91, 3.27] |
< 0.001 |
2.36 [1.67, 3.33] |
< 0.001 |
| III vs. I |
2.99 [2.24, 3.99] |
< 0.001 |
3.13 [2.2, 4.47] |
< 0.001 |
| No PFT vs. I |
2.14 [1.36, 3.36] |
< 0.001 |
2.34 [1.35, 4.05] |
0.002 |
| ILD Subtype | ||||
| Granulomatous lung diseases vs. IPF |
0.35 [0.25, 0.48] |
< 0.001 |
0.23 [0.15, 0.36] |
< 0.001 |
| HP vs. IPF |
0.82 [0.58, 1.18] |
0.287 |
0.96 [0.64, 1.44] |
0.844 |
| CTD-ILD vs. IPF |
1.59 [1.1, 2.3] |
0.014 |
1.53 [0.99, 2.36] |
0.055 |
| Pneumoconiosis vs. IPF |
0.68 [0.3, 1.54] |
0.353 |
0.14 [0.02, 1.02] |
0.052 |
| Drug related ILD vs. IPF |
0.97 [0.51, 1.85] |
0.936 |
0.89 [0.41, 1.9] |
0.758 |
| Radiotherapy associated ILD vs. IPF |
0.92 [0.34, 2.49] |
0.873 |
0.34 [0.05, 2.44] |
0.283 |
| CPFE vs. IPF |
1.29 [0.7, 2.38] |
0.412 |
0.97 [0.43, 2.2] |
0.936 |
For these analyses, Hazard Ratios were drawn from a comparison of the most relevant ILD subtypes and IPF. HR= Hazard Ratio, CI= confidence interval, No PFT= No pulmonary function testing, IPF= Idiopathic pulmonary fibrosis, HP= Hypersensitivity pneumonitis, CTD-ILD= Rheumatic and connective tissue diseases with pulmonary involvement, CPFE= Fibrosis in emphysema patients without signs of other ILDs
In the second step, multiple logistic regressions with forward selection were set to develop a model for the prediction of all cause, ILD-related and respiratory hospitalisations. In univariate analysis, baseline pulmonary function parameters showed an association both to all cause (FVC: OR = 0.98, p < 0.001; DLCO-SB: OR = 0.97, p < 0.001) and ILD-related hospitalisations (FVC: OR = 0.98, p < 0.001; DLCO-SB: OR = 0.96, p < 0.001). For all cause hospitalisations pulmonary hypertension (OR = 2.53, p = 0.005) and age (OR = 1.05, p < 0.001) were significant risk factors, while there was a non-significant association to reflux (OR = 1.23, p = 0.297), male sex (OR = 1.23, p = 0.217), concomitant emphysema (OR = 1.30, p = 0.323) and smoking (OR = 1.22, p = 0.220). ILD-related hospitalisations were also associated with pulmonary hypertension (OR = 2.84, p < 0.001).
In multiple regression models regarding ILD-related hospitalisations the combination of an emphysema in unclassifiable ILD (OR = 2.13, p = 0.001) and a current or ex-smoking status in other granulomatous ILD (OR = 3.08, p = 0.005) were identified as the most important risk factors. Also, DLCO-SB showed an association (OR = 1.00, p < 0.001; Additional file 1: Table S1). The strongest predictor for a respiratory hospitalisation was the combination of CTD-ILD and a concomitant emphysema (OR = 2.16, p = 0.006; Table4). An increased risk was also shown for male sex and HP of unknown origin (OR = 1.69, p = 0.008; Table4).
Table 4.
Results of multiple logistic regression with forward selection to predict respiratory hospitalisation (n=601)
| Predictor variable | Odds Ratio | Standarderror | 95%-CI | p-value |
|---|---|---|---|---|
| VCmax:DLCO-SB | 1.00 | 0.000 | [0.000; 0.000] | < 0.001 |
| Pulmonary hypertension [yes]:Age | 1.00 | 0.001 | [0.002; 0.007] | 0.001 |
| BMI:ILD/Vasc | 1.02 | 0.007 | [0.009; 0.036] | 0.001 |
| Concomitant emphysema:ILD/CTD | 2.16 | 0.276 | [0.229; 1.313] | 0.006 |
| Sex [male]:ILD/u-HP | 1.69 | 0.196 | [0.140; 0.908] | 0.008 |
Logistic regression with forward selection was used to develop a model to predict respiratory hospitalisation (yes/no), relevant interactions are presented in this table. Vasc Vasculits, u-HP HP of unknown origin
Discussion
In the present study we report a high rate of hospitalisations in patients with various ILDs with a large share of ILD-related hospitalisations. Notably, hospitalisations were associated with impaired prognosis. Besides elective diagnostic procedures relevant reasons for admission were acute ILD exacerbations and pulmonary infections. This is in line with a large German claims data study, which reported among 36.816 patients a hospitalisation rate of 71.2% for non-ILD-related and 56.6% for ILD-related reasons [18]. For IPF, Brown et al. and Cottin et al. reported an even higher rate of ILD-related hospitalisations compared to our data, potentially due to more frequent acute exacerbations in IPF than in other ILDs [8, 14].
To identify factors influencing hospitalisation, time to first hospitalisation was compared between different values of the GAP index and between different ILD subtypes. In addition, sex, age, BMI, pulmonary function parameters, ILD subtype, pulmonary hypertension, reflux, concomitant emphysema, and smoking behaviour were analysed in multiple logistic regression models to determine which of these variables are predictors of hospitalisation in general. Risk factors for hospitalisations included advanced disease, reflected by higher GAP stages associating with more frequent hospitalisations. Thus, the GAP Index, originally developed to predict prognosis in patients with IPF [17], was identified here to also predict hospitalisations in all ILDs. Baseline pulmonary function parameters were also associated with hospitalisations as described in SSc-ILD [13] or IPF [19], as well as in AE-IPF where disease severity as assessed by lung function associates with the probability of an acute exacerbation [20, 21].
Other predictors for non-elective hospitalisations were pulmonary hypertension and emphysema, the former in line with GAP stage most probably reflecting disease severity. The impact of emphysema is only poorly understood. Potential reasons might be a higher probability of pulmonary infections in a severely impaired lung due to both, ILD and emphysema; it is however unknown whether acute exacerbations of the co-existing COPD might also act as a co-factor.
Disease entity plays a decisive role in predicting hospitalisation with relevant differences e.g. between IPF and granulomatous lung diseases. This is in line with German claims data by Wälscher et al. reporting a lower frequency in non-ILD-related hospitalisations for sarcoidosis [18]. Possible reasons are younger age, less comorbidities and usually a relatively good prognosis in sarcoidosis and other granulomatous lung diseases [18]. A higher risk for hospitalisation and impaired survival was seen in rheumatic and connective tissue diseases with pulmonary involvement (CTD-ILD). These findings may partly be due to hospitalisations for the rheumatic manifestations, complications like pulmonary hypertension or therapy associated pulmonary infections related hospitalisations [22, 23].
The burden of hospitalisations was reflected by an association to survival. A study with almost 600 patients suffering from IPF showed that both all-cause and respiratory-related hospitalisations were strongly associated with mortality [8]. However, our data suggest an association beyond IPF for all ILDs. Presumably, non-elective hospitalisations appear mainly in progressive disease and thus represent a disease progression on its own [8, 18]. In line with this, the progression criteria reported here might represent future endpoints in clinical trials. However, possible regional differences with regard to the hospitalisation of patients with ILDs must be taken into account. Precisely because hospitalisations have such a major impact on mortality, approaches for prevention are warranted. Vaccinations could contribute in this respect. This was shown recently by an epidemiological claims data analysis for the vaccination against influenza in the sense of a reduced all-cause mortality in half of the seasons observed. However, Marijic et al. reported also an overall low vaccination rate in ILD patients [24]. Additionally, early detection and optimised treatment of comorbidities are essential.
Strengths of our analyses include the large number of patients recruited from different healthcare facilities as well as the prospective design. This enables to reflect the “real world”-situation of patients with ILDs and is therefore of high value. In particular, our results highlight the great impact of hospitalisations on mortality. Noteworthy, possible risk factors were identified, which could help to improve patient care.
However, also some limitations applied. Due to the study character as observational and nonrandomised, no causal associations may be derived. In addition, both prevalent and incident patients were included. The diagnoses were mostly, but not exclusively, made on an interdisciplinary basis. CT imaging was not considered in the registry. Additionally, clinical decisions of the physicians may differ, especially because patients were treated in centres with different levels of expertise in ILD [16]. So, potentially allocation or channelling bias could be caused and confound the association between treatment and outcomes [16]. The higher number of admissions in the first year of the registry compared to the following years might be caused by a decreasing number of patients in the registry over the years. Therefore, this point should be interpreted with caution. Nevertheless, it illustrates the high burden of disease in interstitial lung diseases. As only pulmonary hypertension, emphysema and GERD were recorded as comorbidities, no statements about cardiac or other comorbidities can be made.
Conclusion
In the EXCITING-ILD registry hospitalisations were frequent and occurred mainly early. Non-elective hospitalisations are strongly correlated with prognosis and thus may serve as a surrogate endpoint in clinical trials. Our results represent a crucial contribution in understanding predisposing factors for hospitalisation in ILD and its major impact on mortality. Further studies to characterize the most vulnerable patient group as well as approaches to prevent hospitalisations such as vaccinations are warranted.
Supplementary Information
Additional file 1: Figure S1. Time to first ILD-hospitalisation in months—Kaplan Meier curve Non-elective ILD-hospitalisation from date of inclusion were considered (n=208). Figure S2. Time to first non-elective hospitalisation in months by ILD subtypes—Kaplan Meier curve. Time to first non-elective hospitalisation was defined as the first non-elective hospitalisation from inclusion to the registry (n=322). The most relevant ILD subtypes were considered. IPF Idiopathic pulmonary fibrosis, NSIP non-specific interstitial pneumonia, COP cryptogenic organizing pneumonia, uILD not classifiable IIP, Sarc sarcoidosis, CTD rheumatic and connective tissue diseases with pulmonary involvement, RA rheumatoid arthritis-associated ILD, LAM pulmonary lymphangioleiomyomatosis, PLCH pulmonary langerhans´ cell histiocytosis, PAP pulmonary alveolar proteinosis, EP eosinophilic pneumonia. Table S1. Results of multiple logistic regression with forward selection to predict ILD hospitalisation (n=601). Logistic regression with forward selection was used to develop a model to predict ILD hospitalisation (yes/no), relevant interactions are presented in this table. DLCO-SB diffusing capacity for carbon monoxide (CO) – single breath, ILD interstitial lung disease, u-HP HP of unknown origin, IPF idiopathic pulmonary fibrosis, CTD connective tissue disease, Vasc Vasculits, uILD not classifiable idiopathic interstitial pneumonia, NSIP non-specific interstitial pneumonia, other GRAN-ILD: other e.g. involvement in chronic inflammatory liver and gut diseases, except hypersensitivity pneumonitis [EAA].
Acknowledgements
The registry is located at the Translational Lung Research Centre Heidelberg (TLRC), a member of the German Centre for Lung Research (DZL), and supported by an unrestricted grant from InterMune (now Roche). Financial support is independent of the scientific concept of the registry, data analysis, interpretation, or publication other than an acknowledgement of financial support. We would like to thank Phillen Maqhuzu and Larissa Schwarzkopf from Helmholtz Centre Munich for their support regarding database and statistics. Furthermore, we would like to thank Eva Brammen from Chrestos Concept GmbH & Co. KG for support regarding the database, tables and figures.
Abbreviations
- BMI
Body mass index
- CI
Confidence interval
- COP
Cryptogenic organizing pneumonia
- CPFE
Fibrosis in emphysema patients without signs of other ILDS
- CTD
Connective tissue disease
- DLCO-SB
Diffusing capacity for carbon monoxide (CO) single breath
- EP
Eosinophilic pneumonia
- EXCITING-ILD
Exploring Clinical and Epidemiological Characteristics of Interstitial Lung Diseases
- FAS
Full analysis set
- FEV1
Forced expiratory volume in 1s
- FVC
Forced vital capacity
- HP
Hypersensitivity pneumonitis
- HR
Hazard ratios
- ILD
Interstitial lung disease
- IPF
Idiopathic pulmonary fibrosis
- LAM
Pulmonary lymphangioleiomyomatosis
- NIV
Non-invasive ventilation
- NSIP
Non-specific interstitial pneumonia
- OR
Odd’s ratio
- PAP
Pulmonary alveolar proteinosis
- PFT
Pulmonary function testing
- PLCH
Pulmonary langerhans´ cell histiocytosis
- RA
Rheumatoid arthritis
- SD
Standard deviation
- SSc
Systemic sclerosis
- u-HP
HP of unknown origin
- uILD
Not classifiable IIP
- Vasc
Vasculits
Author contributions
MK and PM were responsible for the study design. HJK, LH, PH, AE, CW, DS, CS, MJ, SV, MH, AH, MP, JB, RK, AG, MP, VS, CL, and PH had contributions to the conception of the work and were involved in recruiting the patients and documentation in the full analysis set. PM and LS were responsible for the statistical analyses. KB was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by an unrestricted grant from InterMune (now Roche). Financial support is independent of the scientific concept of the registry, data analysis, interpretation, or publication other than an acknowledgement of financial support.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki and was approved by the Ethics Committee of the Medical Faculty of the University of Heidelberg, Germany (S-525/2013) as well as by all local ethics committees of the participating centres. Informed consent to participate in the study was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
KB received payment for lectures from Boehringer Ingelheim and a grant from Sarkoidose-Netzwerk e.V. DS reports fees for lectures or consultations from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Janssen, MSD, Sanofi, all outside the submitted work. CS reports fees for lectures GSK, AstraZeneca, Boehringer Ingelheim, Berlin Chemie. MJ reports fees for lectures or consultations from AstraZeneca, Bencard, Boehringer Ingelheim, GSK, HAL Allergy, Sanofi, all outside the submitted work. SV reports fees from Berlin Chemie, GSK, Lilly and Boehringer-Ingelheim. JB reports personal fees for lectures and consulting from Astra-Zeneca, Biogen, Boehringer-Ingelheim, BMS, Ferrer, Novartis, Roche, and Sanofi-Genzyme. MP has received payment or honoraria for lectures, presentations or educational events from Boehringer Ingelheim and AstraZeneca. VS received support for attending meetings from CSL Behring. PM received fees for consulting and lectures from Boehringer-Ingelheim and Roche. MK reports grants, consulting fees, and payment for lectures from Boehringer Ingelheim and Roche. All other authors have nothing to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rainer Karpavicius—Deceased.
Contributor Information
Katharina Buschulte, Email: katharina.buschulte@med.uni-heidelberg.de.
Michael Kreuter, Email: Michael.Kreuter@marienhaus.de.
References
- 1.Valeyre D, Duchemann B, Annesi-Maesano I, et al. Interstitial lung diseases, in Respiratory Epidemiology, T. Welte, I. Annesi-Maesano, G. Viegi, and B. Lundbäck,Eds., vol. 65 of ERSMonograph, chapter 6, ERS, 2014.
- 2.American Thoracic Society and European Respiratory Society American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2002;165(2):277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 3.Travis WD, Costabel U, Hansell D. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raghu G, Remy-Jardin M, Richeldi L, Thomson CC, Inoue Y, Johkoh T, Kreuter M, Lynch DA, Maher TM, Martinez FJ, Molina-Molina M, Myers JL, Nicholson AG, Ryerson CJ, Strek ME, Troy LK, Wijsenbeek M, Mammen MJ, Hossain T, Bissell BD, Herman DD, Hon SM, Kheir F, Khor YH, Macrea M, Antoniou KM, Bouros D, Buendia-Roldan I, Caro F, Crestani B, Ho L, Morisset J, Olson AL, Podolanczuk A, Poletti V, Selman M, Ewing T, Jones S, Knight SL, Ghazipura M, Wilson KC. Idiopathic pulmonary fibrosis (an Update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2022;205(9):e18–e47. doi: 10.1164/rccm.202202-0399ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khanna D, Lin CJF, Furst DE, Goldin J, Kim G, Kuwana M, Allanore Y, Matucci-Cerinic M, Distler O, Shima Y, van Laar JM, Spotswood H, Wagner B, Siegel J, Jahreis A, Denton CP, focuSSced investigators Tocilizumab in systemic sclerosis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2020;8(10):963–974. doi: 10.1016/S2213-2600(20)30318-0. [DOI] [PubMed] [Google Scholar]
- 6.Ma X, Zhu L, Kurche JS, Xiao H, Dai H, Wang C. Global and regional burden of interstitial lung disease and pulmonary sarcoidosis from 1990 to 2019: results from the Global Burden of Disease study 2019. Thorax. 2022;77(6):596–605. doi: 10.1136/thoraxjnl-2020-216732. [DOI] [PubMed] [Google Scholar]
- 7.Maqhuzu PN, Kreuter M, Bahmer T, Kahn N, Claussen M, Holle R, Schwarzkopf L. Cost drivers in the pharmacological treatment of interstitial lung disease. Respir Res. 2021;22(1):218. doi: 10.1186/s12931-021-01807-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown AW, Fischer CP, Shlobin OA, Buhr RG, Ahmad S, Weir NA, Nathan SD. Outcomes after hospitalization in idiopathic pulmonary fibrosis: a cohort study. Chest. 2015;147(1):173–179. doi: 10.1378/chest.13-2424. [DOI] [PubMed] [Google Scholar]
- 9.Kreuter M, Koegler H, Trampisch M, Geier S, Richeldi L. Differing severities of acute exacerbations of idiopathic pulmonary fibrosis (IPF): insights from the INPULSIS® trials. Respir Res. 2019;20(1):71. doi: 10.1186/s12931-019-1037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hariri LP, Mino-Kenudson M, Mark EJ, et al. Distinct histopathology of acute onset or abrupt exacerbation of hypersensitivity pneumonitis. Hum Pathol. 2012;43(5):660–668. doi: 10.1016/j.humpath.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Hozumi H, Nakamura Y, Chida K, et al. Acute exacerbation in rheumatoid arthritis-associated interstitial lung disease: a retrospective case control study. BMJ Open. 2013;3(9):e003132. doi: 10.1136/bmjopen-2013-003132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreuter M, Bendstrup E, Kondoh Y et al., on behalf of the INBUILD trial investigators “Acute exacerbations in patients with progressive fibrosing interstitial lung diseases: data from the INBUILD trial” B94. Learning from registries and clinical trials in ild. Am Thorac Soc, 2022. A3428-A3428.
- 13.Kreuter M, Del Galdo F, Miede C, Khanna D, Wuyts WA, Hummers LK, Alves M, Schoof N, Stock C, Allanore Y. Impact of lung function decline on time to hospitalisation events in systemic sclerosis-associated interstitial lung disease (SSc-ILD): a joint model analysis. Arthritis Res Ther. 2022;24(1):19. doi: 10.1186/s13075-021-02710-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cottin V, Schmidt A, Catella L, Porte F, Fernandez-Montoya C, Le Lay K, Bénard S. Burden of Idiopathic Pulmonary Fibrosis Progression: A 5-Year Longitudinal Follow-Up Study. PLoS ONE. 2017;12(1):e0166462. doi: 10.1371/journal.pone.0166462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behr J, Prasse A, Wirtz H, Koschel D, Pittrow D, Held M, Klotsche J, Andreas S, Claussen M, Grohé C, Wilkens H, Hagmeyer L, Skowasch D, Meyer JF, Kirschner J, Gläser S, Kahn N, Welte T, Neurohr C, Schwaiblmair M, Bahmer T, Oqueka T, Frankenberger M, Kreuter M. Survival and course of lung function in the presence or absence of antifibrotic treatment in patients with idiopathic pulmonary fibrosis: long-term results of the INSIGHTS-IPF registry. Eur Respir J. 2020;56(2):1902279. doi: 10.1183/13993003.02279-2019. [DOI] [PubMed] [Google Scholar]
- 16.Kreuter M, Herth FJ, Markart P, et al. Exploring clinical and epidemiological characteristics of interstitial lung diseases: rationale, aims, and design of a nationwide prospective registry-the EXCITING-ILD registry. Biomed Res Int. 2015;2015:123876. doi: 10.1155/2015/123876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryerson CJ, Vittinghoff E, Collard HR, et al. Predicting survival across chronic interstitial lung disease: the ILD-GAP model. Chest. 2014;145(4):723–728. doi: 10.1378/chest.13-1474. [DOI] [PubMed] [Google Scholar]
- 18.Wälscher J, Witt S, Schwarzkopf L, Kreuter M. Hospitalisation patterns of patients with interstitial lung disease in the light of comorbidities and medical treatment—a German claims data analysis. Respir Res. 2020;21(1):73. doi: 10.1186/s12931-020-01335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lassenius MI, Toppila I, Pöntynen N, Kasslin L, Kaunisto J, Kilpeläinen M, Laitinen T. Forced vital capacity (FVC) decline, mortality and healthcare resource utilization in idiopathic pulmonary fibrosis. Eur Clin Respir J. 2019;7(1):1702618. doi: 10.1080/20018525.2019.1702618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collard HR, Yow E, Richeldi L, Anstrom KJ, Glazer C, IPFnet Investigators Suspected acute exacerbation of idiopathic pulmonary fibrosis as an outcome measure in clinical trials. Respir Res. 2013;14:73. doi: 10.1186/1465-9921-14-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song JW, Hong SB, Lim CM, Koh Y, Kim DS. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J. 2011;37(2):356–363. doi: 10.1183/09031936.00159709. [DOI] [PubMed] [Google Scholar]
- 22.Borchers AT, Leibushor N, Cheema GS, Naguwa SM, Gershwin ME. Immune-mediated adverse effects of biologicals used in the treatment of rheumatic diseases. J Autoimmun. 2011;37(4):273–288. doi: 10.1016/j.jaut.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Xing NS, Fan GZ, Yan F, Liu YP, Zhang R. Safety and efficacy of rituximab in connective tissue disease-associated interstitial lung disease: a systematic review and meta-analysis. Int Immunopharmacol. 2021;95:107524. doi: 10.1016/j.intimp.2021.107524. [DOI] [PubMed] [Google Scholar]
- 24.Marijic P, Schwarzkopf L, Maier W, Trudzinski F, Schwettmann L, Kreuter M. Effects of influenza vaccination in patients with interstitial lung diseases: an epidemiological claims data analysis. Ann Am Thorac Soc. 2022;19(9):1479–1488. doi: 10.1513/AnnalsATS.202112-1359OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Time to first ILD-hospitalisation in months—Kaplan Meier curve Non-elective ILD-hospitalisation from date of inclusion were considered (n=208). Figure S2. Time to first non-elective hospitalisation in months by ILD subtypes—Kaplan Meier curve. Time to first non-elective hospitalisation was defined as the first non-elective hospitalisation from inclusion to the registry (n=322). The most relevant ILD subtypes were considered. IPF Idiopathic pulmonary fibrosis, NSIP non-specific interstitial pneumonia, COP cryptogenic organizing pneumonia, uILD not classifiable IIP, Sarc sarcoidosis, CTD rheumatic and connective tissue diseases with pulmonary involvement, RA rheumatoid arthritis-associated ILD, LAM pulmonary lymphangioleiomyomatosis, PLCH pulmonary langerhans´ cell histiocytosis, PAP pulmonary alveolar proteinosis, EP eosinophilic pneumonia. Table S1. Results of multiple logistic regression with forward selection to predict ILD hospitalisation (n=601). Logistic regression with forward selection was used to develop a model to predict ILD hospitalisation (yes/no), relevant interactions are presented in this table. DLCO-SB diffusing capacity for carbon monoxide (CO) – single breath, ILD interstitial lung disease, u-HP HP of unknown origin, IPF idiopathic pulmonary fibrosis, CTD connective tissue disease, Vasc Vasculits, uILD not classifiable idiopathic interstitial pneumonia, NSIP non-specific interstitial pneumonia, other GRAN-ILD: other e.g. involvement in chronic inflammatory liver and gut diseases, except hypersensitivity pneumonitis [EAA].
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.