Abstract
Proper cell–cell communication is necessary to orchestrate the cell fate determination, proliferation, movement, and differentiation that occurs during the development of a complex, multicellular organism. Members of the Wnt family of secreted signaling molecules regulate these processes in virtually every embryonic tissue and during the homeostatic maintenance of adult tissues. Mammalian genetic studies have been particularly useful in illustrating the specific roles that Wnt signaling pathways play in embryonic development, and in the etiology of diseases such as cancer. This chapter will largely focus on the functional roles that Wnts, signaling through the Wnt/ -catenin pathway, play during early mammalian development.
Keywords: Wnt3a, Beta-catenin, Gastrulation, Mouse, Axis formation, Somitogenesis
1. Introduction
Wnt genes encode a large family of highly conserved secreted glycoproteins that play critical roles in development and disease (1, 2). Starting with the discovery of the first Wnt gene, Wnt1 (Int-1), as a common site of integration for the Mouse Mammary Tumor Virus in breast cancers (3), mice have played a central role in the study of Wnt function. Considerable effort has been devoted to the elucidation of the biochemical pathways that transduce Wnt signals, at least in part because of the role that Wnt pathways play in initiating cancer (see the Wnt homepage http://www.stanford.edu/~rnusse/wntwindow.html for details on Wnt genes, pathways, and links to the many reviews on Wnt signaling). These pathways include the Wnt/β-catenin pathway, and lesser-understood pathways such as the Wnt/PCP and the Wnt/Ca2+ pathways (see Chapters 1 and 10 in this volume for detailed reviews of these pathways). Although studies in many different model organisms and cell lines have established the framework for investigations into the mechanisms of Wnt signaling, the ability to precisely manipulate the germline, coupled with the suitability of the mouse as a model mammalian organism for the study of human disease, makes the mouse a particularly attractive system to study Wnt function in vivo.
2. Brief Overview of the Wnt/β-Catenin Signaling Pathway
β-Catenin is the primary transducer of Wnt signals in the canonical Wnt/β-catenin signaling pathway. In the absence of Wnt ligand, cytoplasmic β-catenin is maintained at low levels due to the activity of the destruction complex. This multiprotein complex, which includes the tumor suppressors adenomatous polyposis coli (APC) and Axin, and members of the glycogen synthase kinase (GSK)-3 and casein kinase (CK)-1 families, binds β-catenin and phosphorylates it on N-terminal residues to target it for ubiquitylation and destruction by the proteasome (4). The binding of Wnt ligands to the seven-pass transmembrane receptor Frizzled (Fz) and the single-pass transmembrane coreceptor lipoprotein receptor-related proteins 5 and 6 (Lrp5/6) promotes the recruitment of Axin to Lrp and Fz-bound Dishevelled, presumably affecting the stability or conformation of the destruction complex. Although the precise mechanisms are not clear, the reduced activity of the destruction complex is thought to be due to the inhibition of GSK3-mediated phosphorylation of β-catenin. Nonphosphorylated β-catenin is stable, accumulates, and translocates into the nucleus where it binds to T-cell factor (Tcf)/lymphoid enhancer factor (Lef) transcription factors. Tcf/Lef proteins bind to specific sequences in the regulatory elements of target genes. When the Wnt/β-catenin pathway is inactive, Tcf/Lef factors repress the transcription of target genes through interactions with the Groucho/TLE repressors. The binding of β-catenin to Tcf/Lef displaces Groucho and recruits transcriptional coactivators, converting Tcf/Lef proteins from repressors to activators of target genes (5, 6). Thus stimulation of the Wnt/β-catenin signaling pathway results in the activation of transcriptional programs of gene expression.
The 19 known Wnt ligands have been traditionally classified as canonical Wnts if they have been shown to stimulate the Wnt/β-catenin pathway, or as non-canonical Wnts if they have not. Assays for Wnt/β-catenin pathway activation are numerous and varied and include β-catenin stabilization, cell transformation, and β-catenin/Tcf-luciferase reporter assays in vitro, axis duplication assays in Xenopus embryos, and assessment of cells or embryos for the expression of characterized Wnt/β-catenin target genes. Fewer assays are available for the assessment of Wnt activity in alternative signaling pathways. Probably the most widely accepted assay for ligand activity in the vertebrate PCP pathway remains the axis extension assay in Xenopus and Zebrafish embryos. Overexpression of noncanonical Wnt ligands interferes with convergent-extension (CE) cell movements and results in shorter, broader embryos (reviewed in ref. (7)), a phenotype distinguishable from the axis duplications observed upon injection of canonical Wnts. Recent studies have blurred the line that distinguishes Wnt ligands from one another. For instance, Wnt5a and Wnt11, long considered non-canonical Wnts, can indeed signal via β-catenin if the appropriate receptor combination is present on the responding cell (8, 9). These results led to the suggestion that pathway selection is not an intrinsic property of the ligand, but rather is determined by receptor context. Nevertheless, lipid modification of Wnts is necessary for canonical Wnt signaling, leaving open the possibility that differential palmitoylation of Wnts could contribute to pathway selectivity (discussed in ref. (10)).
Mammalian genetic studies have contributed substantially to our understanding of the function of Wnt ligands during development. Wnt genes are generally expressed in a highly localized and temporally regulated fashion in virtually all tissues throughout embryogenesis. The majority of Wnt genes have been mutated by conventional knockout studies (11). Although many of these mutations lead to dramatic developmental defects, a significant number result in viable, fertile animals when homozygous null. This is presumably due to genetic redundancy, since many Wnt genes are expressed in overlapping spatial and temporal domains in numerous tissues. Surprisingly few of these viable Wnt mutants (discussed below) have been published, or examined directly in double mutant tests for redundancy, likely due to the insur-mountable depression that the developmental biologist experiences following the discovery of a viable phenotype. Targeted null mutations of genes encoding central components of Wnt signaling pathways have led to interesting phenotypes that either manifest as early embryonic lethal, necessitating the use of conditional alleles to address function in later tissues, or as highly specific phenotypes that are only apparent at relatively late developmental stages due to genetic redundancy. This broad range of phenotypes has frequently made the assignment of a given Wnt to a specific signaling pathway somewhat ambiguous. This chapter reviews recent progress in our understanding of the genetic networks underlying Wnt signaling during early mammalian preimplantation and postimplantation development.
3. Wnt Signaling During Preimplantation Development
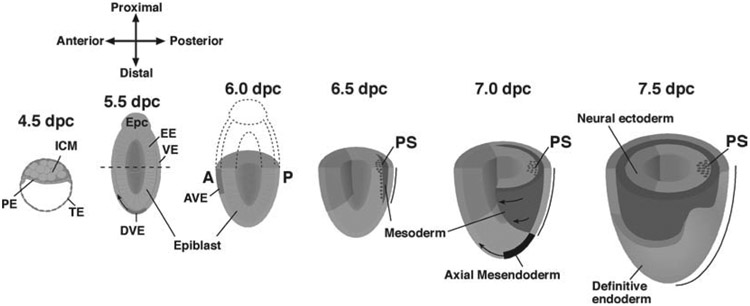
The fertilized egg is totipotent, that is, it possesses the capacity to generate all differentiated cell types. Shortly after fertilization, the conceptus undergoes a series of reductive cleavage divisions that lead to the first establishment of polarized cells, and to the first cellular differentiation events. These early events result in the formation of the blastocyst by embryonic day (E) 3.5 (reviewed in ref. (12)), which consists of an outer epithelial extraembryonic trophectoderm (TE), and an internal clump of inner cell mass (ICM) cells (Fig. 23.1). The ICM is asymmetrically located within the TE due to the formation of the blastocoel cavity, leading to a distinct proximal–distal axis defined by the ICM at the proximal pole. The TE interacts directly with the maternal uterine wall during implantation (E4.5), and subsequently contributes to the trophoblast lineages of the placenta. The TE that surrounds the blastocoelic cavity is termed the mural TE, while the TE directly in contact with the epiblast is known as the polar TE. The ICM gives rise to the epiblast (which produces all of the cells of the embryo proper) and the overlying primitive endoderm (PE) around the time of implantation, and the PE subsequently generates the visceral endoderm (VE) and parietal endoderm.
Fig. 23.1.
Perimplantation and postimplantation stages of mammalian development. Schematic diagrams illustrating perimplantation (4.5 days post-coitum (dpc)) and postimplantation (5.5 dpc onward) mouse embryos. The dashed line overlying the 5.5-dpc embryo demarcates the extraembryonic ectoderm above, from the contiguous epiblast below. Please see the text for details. PE, primitive endoderm; TE, trophectoderm; ICM, inner cell mass; Epc, ectoplacental cone; EE, extraembryonic ectoderm; VE, visceral endoderm; DVE, distal visceral endoderm; AVE, anterior visceral endoderm; PS, primitive streak.
At least 6 Wnt genes are expressed in overlapping but tissue-specific patterns during preimplantation development (13, 14). For instance, Wnt1 expression appears restricted to the ICM, while Wnt3a messenger RNA (mRNA) is detected throughout the blastocyst. Although presumed null mutations have been generated in all 19 Wnt genes (11), preimplantation phenotypes have not been observed in any single Wnt mutant. Genetic redundancy may explain the lack of an early phenotype given that many components of the Wnt/β-catenin pathway are expressed in the preimplantation embryo (15, 16). However, nuclear localization of β-catenin has not been observed in preimplantation embryos (17), nor has the expression of β-catenin/Tcf-lacZ reporter transgenes been detected (17-21), suggesting that the Wnt/β-catenin pathway is not active in the embryo at these stages. Consistent with these studies, preimplantation phenotypes were not observed in either loss-of-function (LOF) or gain-of-function (GOF) studies of β-catenin, nor in single or double mutants of any other component of the Wnt/β-catenin or Wnt/PCP pathway examined to date, including Lrp5, Lrp6; Dvl1, Dvl2, or Dvl3; Tcf1, Tcf3, Tcf4; and Lef1, Vangl2, and Celsr1, among others (11). These results strongly suggest that the mammalian embryo does not require the Wnt/β-catenin or Wnt/PCP signaling pathways for proper preimplantation development.
Interestingly, Wnt ligand expression by the blastocyst may serve to coordinate the embryo–uterus interactions necessary for implantation. A β-catenin/Tcf lacZ reporter is expressed in the maternal uterine epithelium at sites of embryo implantation, and expression is dependent upon the presence of the blastocyst (22). This activation can be mimicked by injection of beads coated with Wnt7a- but not Wnt5a-expressing cells into the uterine lumen. Lumenal injections of the Wnt antagonist sFRP2 significantly inhibited blastocyst implantation, suggesting that a Wnt ligand(s) is necessary for successful implantation. While these results are intriguing, formal proof that β-catenin is required in the uterine epithelium for embryo implantation is lacking. Future studies aimed at the identification of relevant target genes in the epithelium will be necessary to understand the underlying mechanisms.
4. Wnt Signaling and the Establishment of the Body Plan
Wnt signaling pathways play crucial roles in the development of the postimplantation embryo. Since the role of the Wnt/β-catenin pathway in body plan formation has been reviewed (23, 24), this section provides a brief overview of the important developmental processes that occur after blastocyst implantation, and then focuses on recent advances in our understanding of the role of Wnt signaling in these processes.
The embryo undergoes major morphogenetic changes after implantation as the spherical blastocyst transforms into an elongated, cup-shaped structure known as the egg cylinder. The egg cylinder can be considered as two regions, an embryonic and extraembryonic region that is composed of three distinct tissues, the embryonic epiblast, the extraembryonic ectoderm located proximally to the epiblast, and an overlying sheet of VE that cocoons the epiblast and extraembryonic ectoderm (Fig. 23.1). Recent studies suggest that morphogenetic rearrangements of the polar TE occurring shortly after implantation lead to the folding and formation of the extraembryonic ectoderm (25). This morphogenetic folding, together with increased proliferation of polar TE (26), drives the growth of the epiblast and extraembryonic ectoderm in a distal direction, resulting in their envelopment by the VE.
Although the extraembryonic ectoderm and VE contribute only to extraembryonic tissues, both tissues are in direct contact with the epiblast and are crucial for the establishment of the anterior–posterior (AP) body axis. The epiblast is an epithelium of pluripotent stem cells that gives rise to all the cells of the embryo proper including the germ line. The imparting of positional information to the epiblast is crucial for the establishment of the body plan. The formation of the AP axis is a fundamental first step in the establishment of this plan as the anterior pole defines where the head will form, while the posterior pole defines the site of primitive streak (PS) and ultimately tail formation. The VE plays a particularly important role in the specification of the AP axis. Around E5.5, VE cells located at the distal end of the egg cylinder (DVE; Fig. 23.1) migrate proximally and asymmetrically to become the anterior VE (AVE) (27, 28). The AVE defines the prospective anterior pole, as surgical removal of the AVE results in the loss of anterior epiblast and neurectoderm fates (29). Concomitantly, the expression of powerful developmental signaling molecules including the Tgf-β family member, Nodal, Wnt3, and Wnt8a in the proximal epiblast shifts posteriorly to define the prospective site of PS formation (and consequently, mesoderm formation) at the posterior pole (Fig. 23.1). The restriction of the AVE to the distal tip of the embryo, AVE migration, and the expression of proximal posterior epiblast markers such as Nodal appears to be under the control of signals secreted by the extraembryonic ectoderm (30). Thus interactions between extraembryonic ectoderm, VE, and the epiblast, are responsible for the formation of the AVE and the posterior localization of gene expression in the epiblast, thereby converting proximal–distal polarity into AP polarity. This phenomenon is frequently referred to as axis conversion or rotation.
The Wnt/β-catenin pathway plays an important role in the initial establishment of the AP axis. Embryos homozygous for a null allele of β-catenin do not initiate AP axis formation due to the abnormal specification of the DVE, and the failure of the DVE to rotate anteriorly (31). Chimera studies demonstrate that β-catenin is required in epiblast, and not in VE cells, suggesting that β-catenin regulates the specification and movement of VE cells in a non-cell autonomous fashion. One way that the β-catenin pathway may indirectly regulate DVE specification and movement may be via the Nodal signaling pathway. Nodal signaling from the epiblast is essential for the formation of the DVE (32). The Nodal co-receptor Cripto, which is also required for the anterior movement of the DVE (33), is a direct target gene of the Wnt/β-catenin pathway and is not expressed in β-catenin null mutants (34). These and other results suggest that β-catenin regulates DVE migration by facilitating Nodal signaling in the epiblast through the activation of Cripto.
Wnt/β-catenin signaling also has important roles in DVE migration and AP axis formation that are independent of the Nodal pathway. The paired-type homeobox gene Otx2 is another important regulator of AP axis formation. The DVE forms in Otx2−/− embryos but it does not rotate anteriorly (35, 36). Expression of dickkopf1 (Dkk1), which encodes a secreted negative regulator of Wnt signaling, is normally found in a proximal subset of wild-type DVE cells and then becomes asymmetrically localized as DVE cells migrate anteriorly. Although several DVE markers were expressed in Otx2−/− embryos, Dkk1 expression was not initiated, suggesting that it might play a role in directional migration of DVE cells as a downstream target of Otx2 (37). Gel shift assays and promoter–reporter analyses in transgenic mice demonstrated that Otx2 indeed directly activates the Dkk1 promoter. Remarkably, the forced expression of Dkk1 in the Otx2 spatial domain, by “knocking-in” a Dkk1 complementary DNA (cDNA) into the Otx2 locus, led to the partial rescue of axis rotation defects. These results suggest that Otx2 regulates anterior specification, at least in part, by activating Dkk1 and thereby inhibiting the Wnt/β-catenin pathway. This is supported by the observation that genetically reducing Wnt/β-catenin signaling in Otx2−/− mutants by deleting one copy of the β-catenin gene (Ctnnb1), also partially rescues the anterior migration defect of DVE cells. Moreover, cultures of whole E5.5 embryos with beads coated with recombinant protein demonstrated that Dkk1 attracted migrating DVE cells, while Wnt3a repelled them (37). Clearly, Dkk1 plays an essential role in anterior specification since a targeted mutation of Dkk1 leads to the loss of anterior head structures (38). Together, these results suggest that asymmetrical Wnt/β-catenin signaling promotes the anteriorly directed migration of DVE cells necessary for the specification of the AP body axis.
5. Gastrulation and the Regulation of Mesoderm Formation by Wnt Signaling
Gastrulation is a complex morphogenetic process that is responsible for the formation of the mesoderm and definitive endoderm germ layers and for the elaboration of the body plan. Gastrulation begins at E6.5 with the formation of the PS, a transient developmental structure that forms at the posterior terminus (Fig. 23.1) and converts pluripotent epiblast stem cells into mesoderm and endoderm progenitors. Many powerful inducing factors, including members of the Wnt, Fgf, and Tgfb/Bmp families, are expressed in the PS where they play important roles in the regulation of cell fates. Epiblast cells fated to give rise to mesoderm cells undergo an epithelial-to-mesenchymal transition and generally ingress and migrate away from the PS to generate the mesodermal germ layer.
The Wnt/β-catenin pathway plays a major role in the formation of the PS during gastrulation. Both Wnt3 and Wnt8a are radially expressed in the proximal epiblast prior to gastrulation and become posteriorly localized, forecasting the site of PS formation, after the DVE migrates anteriorly to orient the AP axis (37, 39). Wnt3 but not Wnt8a plays an essential role in PS formation since Wnt3−/− embryos lack a PS and mesoderm (39), while Wnt8a mutants appear normal (T. Yamaguchi and A. McMahon, unpublished observations). The lack of a phenotype in the Wnt8a mutants is presumably due to redundancy with Wnt3, although this has not been tested directly. Analyses of AVE markers indicate that Wnt3−/− mutants are able to establish a correctly oriented AP axis, however the overlying ectoderm does not acquire anterior neural identity. Thus the absence of both anterior neural identity and posterior structures demonstrates that Wnt3 is necessary for patterning of the epiblast along the entire AP axis. Wnts appear to function as posteriorizing signals since ectopic expression of Wnt8a leads to the enhanced formation of posterior structures while reducing the formation of anterior neuroectoderm (40).
Recent experiments have shed light on the molecular signals responsible for the activation of Wnt3 in the proximal posterior epiblast and for the positioning of the PS in the posterior embryo. It has been known for some time that Nodal is required in the epiblast for gastrulation and PS formation (41, 42). Nodal activity likely determines the site of PS formation since combined mutations in the Nodal antagonists Cerberus-like and Lefty1, which are expressed in the AVE, lead to ectopic Nodal signaling and the formation of multiple PS (43). Nodal appears to regulate PS formation by regulating Wnt3, but does so indirectly through the activation of Bmp4 in the extraembryonic ectoderm, which in turn signals back to the epiblast to induce Wnt3 (32,44). Wnt3 completes a positive feedback loop by stimulating Nodal expression in the epiblast. It has been suggested that Nodal is a direct target gene of Wnt3 since Tcf binding sites were identified in the proximal epiblast enhancer (44), however the functional relevance of these sites has not yet been tested by mutational analyses. Nevertheless, these studies clearly demonstrate that reciprocal inductive interactions between the epiblast and contiguous extraembryonic tissues are important for the transcriptional activation of Wnt3 and the subsequent formation of the PS.
Although not formally demonstrated through genetic means, in vitro studies and comparisons of phenotypes and gene expression profiles of Wnt3-null and β-catenin-null mutants are consistent with Wnt3 signaling via β-catenin (31, 34, 45, 46). In general, antibody reagents detect active β-catenin protein at, or near, sites of Wnt3 and Wnt8a expression in the pregastrulation embryo, however the precise locations are often inconsistent with reported sites of β-catenin/Tcf-lacZ reporter activity. For instance, cytoplasmic dephosphorylated β-catenin is most strongly expressed in the extraembryonic VE at pregastrulation and early gastrulation stages, and is poorly detected, if at all, in the nucleus and cytoplasm of epiblast cells at these stages, despite the strong expression of Wnt ligands there (17, 37). In contrast, β-catenin/Tcf lacZ reporters (see ref. (18) for review) are only weakly expressed in extraembryonic tissues, but are strongly activated in the posterior epiblast as the PS forms (17, 19, 20). These reporter studies, together with the previously mentioned chimera analyses demonstrating that β-catenin function is required in the epiblast and not in the VE (31), call into question the relevance of the β-catenin expression detected in the VE.
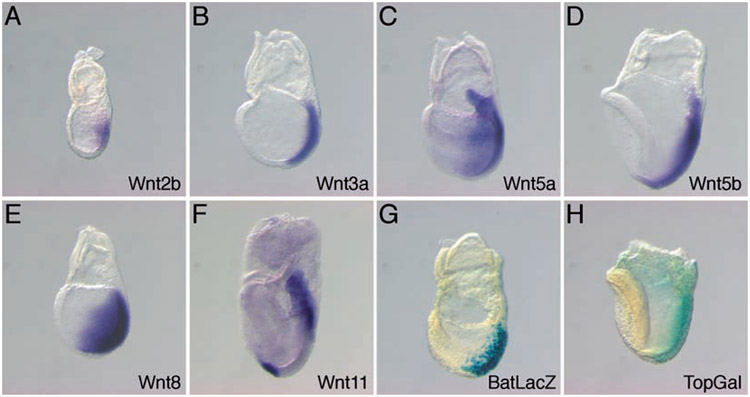
While it is clear that Wnt3 is essential for the formation of the earliest mesoderm to arise in the PS, Wnt3 expression is down regulated in the PS by E7.5 (47), 6 days before mesoderm formation ceases in the PS-derived tailbud at E13.5. Several other Wnt genes are expressed in the PS at E7.5, including Wnt2b, Wnt3a, Wnt5a, Wnt5b, and Wnt11 (Fig. 23.2) (48), and presumably compensate for the absence of Wnt3 expression. Wnt3a is first expressed in the PS at E7.5, and appears to function as the primary mesoderm inducer at this stage since Wnt3a null embryos die at mid-gestation with no apparent posterior mesoderm, PS, or tailbud, and instead display ectopic neural tubes (48, 49). The construction of a genetic series of Wnt3a alleles, through the breeding of animals carrying null and hypomorphic mutations, clearly demonstrate that Wnt3a plays a central role in the formation of all trunk and tail mesoderm (50).
Fig. 23.2.
Expression of Wnt genes and β-catenin/Tcf–lacZ reporter transgenes during gastrulation. Whole-mount in situ hybridization demonstrates that the expression of Wnt2b, Wnt3a, Wnt5a, Wnt5b, Wnt8, and Wnt11 is posteriorly localized and largely centered on the primitive streak. The expression of the BATlacZ and TOPgal reporters indicate that the Wnt/β-catenin signaling pathway is active in the primitive streak.
Multiple lines of genetic evidence convincingly demonstrate that Wnt3a regulates mesoderm fates by signaling through the Wnt/β-catenin pathway. Conditional inactivation of Ctnnb1 (β-catenin) in the PS at E7.5 (the stage at which Wnt3a is first expressed there) results in a loss of posterior mesoderm that is similar to the mesoderm deficit observed in Wnt3a mutants (51). Importantly, expression of a stabilized form of β-catenin in Wnt3a−/− embryos was sufficient to rescue the posterior mesoderm deficit, providing formal genetic proof that β-catenin mediates the mesoderm-inducing activity of Wnt3a. Numerous studies provide additional support: a constitutively active form of Lef1 rescues the phenotypes observed in the hypomorphic Wnt3a mutant vestigial tail (vt) (52), Tcf1;Lef1 double mutants phenocopy the Wnt3a mutants (53), Lrp6 mutants genetically interact with vt (54, 55), and, finally, β-catenin/Tcf-responsive β-gal reporters and endogenous β-catenin/Tcf target genes (see below) are downregulated by LOF alleles of Wnt3a and Ctnnb1, and upregulated by GOF alleles of Ctnnb1 (21, 51, 52, 56-60). Together, the data unequivocally demonstrates that Wnt3a utilizes the canonical β-catenin/Tcf-Lef pathway to induce the formation of all streak-derived mesoderm.
With the exception of Wnt5a, null mutations in the remaining Wnt genes expressed during gastrulation have not yielded gastrulation phenotypes. For instance, embryos homozygous for a targeted disruption of Wnt2b are viable and fertile (T. Tsukiyama and T. Yamaguchi, unpublished data). Again, the lack of an apparent phenotype is likely due to genetic redundancy with canonical Wnts such as Wnt3a. Although Wnt5a expression in the PS overlaps considerably with other Wnts (Fig. 23.2), embryos homozygous for a targeted null mutation of Wnt5a display shortened trunks and do not form tails, resulting in a posterior truncation that is milder than that observed in Wnt3a−/− mutants (61). Molecular and morphological analyses of Wnt5a mutants demonstrate that this posterior truncation phenotype is not due to an aberrant specification of posterior mesodermal fates but instead likely arises from defects in CE cell movements in or near the PS (ref. (61) and unpublished data).
Despite recent observations in vitro that suggest that Wnt5a can signal through β-catenin (9), little evidence exists to support a role for β-catenin in transducing Wnt5a signals in vivo. For instance, the expression of Wnt/β-catenin target genes and β-catenin/Tcf–lacz reporters in the PS is unaffected by the absence of Wnt5a (ref. (61) and unpublished data). Moreover, genetic experiments demonstrate that Wnt5a interacts with core components of the Wnt/PCP pathway such as Vangl2/Looptail during axis extension (62). Thus Wnt5a appears to control PS morphogenesis by regulating the Wnt/PCP pathway, however the precise cellular and molecular mechanisms underlying Wnt5a activity in the PS remain largely unknown. It is intriguing to note that embryos lacking the Wnt antagonists sFRP1 and (FRP2 display a phenotype remarkably similar to the Wnt5a mutant phenotype (63). Wnt5a has been reported to inhibit the activation of the Wnt/β-catenin pathway (7), leading to the expectation that this pathway will be hyperactivated in Wnt5a mutants, however no supporting evidence for this was found in the Wnt5a mutants nor in the (zFRP1;sFRP2 double mutants. The relationship between Wnt5a and sFRP1/2 may be complex since Wnt5a does not appear to simply bind directly to sFRP1 (64, 65).
6. The Wnt3a/β-Catenin Pathway and Mesodermal Segmentation
Fate mapping, transplantation, and gene expression studies of the E7.5 gastrulating embryo indicate that different mesodermal lineages arise from different AP regions of the PS (66, 67). Paraxial presomitic mesoderm (PSM) progenitors arise in the anterior PS, while intermediate, lateral, and extraembryonic mesoderm cells arise from successively posterior regions of the streak. Transplantation studies performed in the chick suggest that while these mesodermal fates are determined in the PS, mesodermal progenitors are not irreversibly committed to these fates until after they have left the PS (68, 69). Different mesodermal fates likely arise from the combinatorial activities of multiple secreted signaling molecules, however the mechanism controlling the commitment and differentiation of mesodermal progenitors to mature cell types is not well understood.
The continuous formation of new mesodermal progenitors in the PS, together with the morphogenetic movements of gastrulation and CE, drive the anteriorly directed movement of mesodermal cells out of the PS. Once immature PSM cells, for example, get a prescribed distance from the PS, they undergo a dramatic transition in gene expression, initiating a poorly understood maturation process that results in the termination of early mesoderm specification genes, and the activation of a segment boundary formation program that cleaves the anterior mesoderm every 2 hours, resulting in the periodic formation of somites.
Remarkably, the Wnt3a/β-catenin pathway directly, and indirectly, controls the majority of these developmental events. In addition to the roles already described for Wnt3a in the specification of mesoderm fates, Wnt3a also plays a major role in the subsequent segmentation of mesoderm. Although it was originally observed that only the first 7–10 (of the approximately 60–65 expected) pairs of somites form in Wnt3a null mutants, the lack of posterior somites was primarily attributed to the impaired formation of mesoderm in the PS and tailbud (48). It was not until it was shown that the direct Wnt3a target gene, Axin2, is expressed in a graded and oscillating fashion in the PSM that Wnt3a was directly implicated in segmentation (57). This led to the development of the clock and gradient model, modified from preexisting clock and wavefront models, that proposed that the Wnt3a/β-catenin (and Notch) pathways are core components of a molecular oscillator or segmentation clock that controls periodic somite formation, and, together with Fgf8, establishes a morphogen gradient that sets the segment boundary position (reviewed in refs. (70, 71)). Recent studies using Wnt3a nulls, and conditional Ctnnb1 LOF and GOF alleles refutes the proposed role for Wnt3a/β-catenin signaling in the segmentation clock, suggesting instead that the Wnt3a gradient maintains posterior PSM cells in an immature progenitor state that is permissive but not instructive for the cycling segmentation clock (51). Conditional stabilization of β-catenin in the PSM did not disrupt molecular oscillations but led to a dramatic expansion of immature PSM and a delay in the formation of segment boundaries, clearly supporting the proposed role for Wnt3a in setting the position of segment boundaries. Thus Wnt3a plays a central role in eliciting proper posterior development, functioning to coordinate PSM maturation with the segmentation clock and boundary formation.
7. Complex Transcriptional Networks Activated by Wnt3a/β-Catenin Signaling
Attempts to elucidate the transcriptional networks activated by the Wnt3a/β-catenin pathway during early mammalian development have led to the identification of several important direct target genes. The T-box transcription factor and early panmesodermal marker, T (also known as Brachyury), was one of the first developmentally relevant direct target genes to be identified (52, 56, 60). Embryos homozygous for this classic mouse mutation display a posterior truncation phenotype that is remarkably similar to the Wnt3a−/− phenotype, suggesting that Wnt3a and T may function in the same pathway. Indeed, T is downstream of Wnt3a/β-catenin signaling since its expression is downregulated in the PS of Wnt3a null mutants, completely absent in embryos conditionally lacking β-catenin in the PS, and upregulated in embryos expressing a stabilized form of β-catenin in the PS (51, 60). Molecular analyses demonstrated that Tcf/Lef binding sites found in the T promoter bind Lef1 and are essential for the promoter to drive expression in reporter assays in vitro, and in the PS in transgenic animals (52, 56, 60). These results indicate that T is a major transcriptional effector of Wnt3a/β-catenin signaling during posterior development. Surprisingly little is known about the target genes of T itself.
Targeted mutations in several other developmental genes lead to phenotypes that are similar to the Wnt3a and T mutant phenotypes, suggesting their potential involvement in this pathway. For instance, the lack of posterior somites and the ectopic neural tubes observed in embryos lacking Tbx6, a PSM-specific Tbox transcription factor closely related to T, closely resembles the Wnt3a−/− phenotype (72). However, in contrast to Wnt3a and T mutants that lack tailbuds, Tbx6 mutants display a grossly enlarged tailbud, indicating that Tand Tbx6 have divergent functions. Analysis of Tbx6 expression in Wnt3a and T mutants, coupled with genetic analyses of T and Tbx6 mutants, suggests that Tbx6 is likely a direct target of T, and therefore an indirect target of the Wnt3a/β-catenin pathway (59, 60, 73, 74). Tbx6 may play an important role in integrating inputs from multiple signaling pathways since Tbx6 transcription is also directly regulated by the Notch pathway (75).
Interestingly, many of the target genes of Tbx6 that have been described to date are also directly co-regulated by the Wnt/β-catenin or Notch pathways, i.e., the target gene promoters possess functional binding sites for both Tbx6 and Tcf or RBPJκ (the transcriptional effector of the Notch pathway). For example, Tbx6 and the Wnt/β-catenin pathway synergistically activate the expression of the bHLH transcription factor Mesogenin1 in the PSM (ref. (76); W.C. Dunty and T. Yamaguchi, unpublished). Mesogenin1 and Tbx6 are expressed in similar domains in the PSM and targeted mutations of Mesogenin1 reveal a mutant phenotype that is remarkably similar to the Tbx6 mutant phenotype (77). Analysis of gene expression in the enlarged tailbuds of Tbx6 and Mesogenin mutants indicates that the tailbuds are largely composed of immature mesodermal progenitors. This suggests that Tbx6 and Mesogenin, under the control of Wnt3a, promote the progression or maturation of posterior mesodermal progenitors to anterior, segmented somites.
Several other Wnt3a/β-catenin target genes help to illustrate the diverse activities of this pathway during posterior development. The Delta-like1 (Dll1) gene encodes a Notch ligand that is necessary for the activation of the segmentation clock and proper segmentation (78, 79). Dll1 expression is dependent upon Tbx6 and the Wnt3a/β-catenin pathway, indicating that Wnt3a controls segmentation in part through the activation of Dll1, and therefore, Notch activity (21, 58, 59). Wnt3a also controls segmentation through the repression of the segment boundary determination gene Ripply2 (51). In addition, Wnt3a plays important roles in imparting positional information to the PSM. Wnt3a−/− mice display homeotic transformations of the vertebrae that are similar to transformations observed in Hox mutants (80). Indeed, Wnt3a indirectly regulates Hox gene expression through the activation of members of the Cdx family of homeodomain encoding genes, which are known regulators of Hox gene activity (ref. (81), and references therein). Remarkably, Wnt3a also participates in the determination of the left–right (LR) body axis, indirectly regulating the LR determinant and Dll1/Notch target gene, Nodal, through the activation of Dll1 (21, 82, 83). This suggests that Dll1 links Wnt3a to segmentation and the orientation of the LR axis.
8. Summary
The Wnt/β-catenin pathway regulates multiple events crucial for early mammalian embryogenesis, including the specification of the AP and LR body axes, PS and mesoderm formation, mesodermal patterning, and segmentation. Once gastrulation has been initiated and the body axes have been established, Wnt3a plays a particularly important role in the coordination of the many developmental events that are necessary for proper posterior development. Wnt3a functions at the top of an interacting transcriptional network of genes that starts with the induction of the transcription factors T and Tbx6, both of which play central roles in integrating the inputs from multiple signaling pathways. Wnt3a subsequently activates numerous other developmental regulator genes in cooperation with T and Tbx6. Although significant progress has been made in the identification of direct and indirect target genes of the Wnt3a/β-catenin pathway, relatively little is known about how these molecular targets control cellular behavior. Recently, Wnts have emerged as important homeostatic regulators of adult tissues, stimulating the self-renewal of hematopoietic, skin, and gastrointestinal stem cells (1, 2). Genetic mutations that cause aberrant activation of Wnt/β-catenin signaling lead to hematopoietic and gastrointestinal malignancies, suggesting that these cancers arise from the co-opting of normal, physiological regulators of stem cell self-renewal. It stands to reason then that achieving a mechanistic understanding of Wnt/β-catenin signaling in normal embryonic and adult tissues will provide valuable insights into the formation of cancers caused by dysregulated Wnt signaling. Future studies addressing how Wnt/β-catenin signals and their target genes elicit mesoderm progenitors from pluripotent epiblast stem cells during gastrulation will be particularly enlightening in this regard.
Acknowledgments
I apologize to the many authors whose work was omitted due to time and space constraints. I thank Kristin Biris for assistance with manuscript and figure preparation, and members of the laboratory for comments on the manuscript. Work originating in my laboratory was supported by the Intramural Research Program of the National Institutes of Health (NIH), the National Cancer Institute, and the Center for Cancer Research.
References
- 1.Clevers H. (2006) Wnt/beta-catenin signaling in development and disease. Cell 127, 469–480. [DOI] [PubMed] [Google Scholar]
- 2.Reya T and Clevers H (2005) Wnt signalling in stem cells and cancer. Nature 434, 843–850. [DOI] [PubMed] [Google Scholar]
- 3.Nusse R and Varmus HE (1982) Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 31, 99–109. [DOI] [PubMed] [Google Scholar]
- 4.Kimelman D and Xu W (2006) beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene 25, 7482–7491. [DOI] [PubMed] [Google Scholar]
- 5.Stadeli R, Hoffmans R, and Basler K (2006) Transcription under the control of nuclear Arm/beta-catenin. Curr Biol 16, R378–385. [DOI] [PubMed] [Google Scholar]
- 6.Willert K and Jones KA (2006) Wnt signaling: is the party in the nucleus? Genes Dev 20, 1394–1404. [DOI] [PubMed] [Google Scholar]
- 7.Veeman MT, Axelrod JD, and Moon RT (2003) A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell 5, 367–377. [DOI] [PubMed] [Google Scholar]
- 8.Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie CC, Lin X, and Heasman J (2005) Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell 120, 857–871. [DOI] [PubMed] [Google Scholar]
- 9.Mikels AJ and Nusse R (2006) Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol 4, e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikels AJ and Nusse R (2006) Wnts as ligands: processing, secretion and reception. Oncogene 25, 7461–7468. [DOI] [PubMed] [Google Scholar]
- 11.van Amerongen R and Berns A (2006) Knockout mouse models to study Wnt signal transduction. Trends Genet 22, 678–689. [DOI] [PubMed] [Google Scholar]
- 12.Rossant J and Tam PP (2004) Emerging asymmetry and embryonic patterning in early mouse development. Dev Cell 7, 155–164. [DOI] [PubMed] [Google Scholar]
- 13.Mohamed OA, Dufort D, and Clarke HJ (2004) Expression and estradiol regulation of Wnt genes in the mouse blastocyst identify a candidate pathway for embryo-maternal signaling at implantation. Biol Reprod 71, 417–424. [DOI] [PubMed] [Google Scholar]
- 14.Kemp C, Willems E, Abdo S, Lambiv L, and Leyns L (2005) Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Dev Dyn 233, 1064–1075. [DOI] [PubMed] [Google Scholar]
- 15.Hamatani T, Carter MG, Sharov AA, and Ko MS (2004) Dynamics of global gene expression changes during mouse preimplantation development. Dev Cell 6, 117–131. [DOI] [PubMed] [Google Scholar]
- 16.Wang QT, Piotrowska K, Ciemerych MA, Milenkovic L, Scott MP, Davis RW, and Zernicka-Goetz M (2004) A genome-wide study of gene activity reveals developmental signaling pathways in the preimplantation mouse embryo. Dev Cell 6, 133–144. [DOI] [PubMed] [Google Scholar]
- 17.Mohamed OA, Clarke HJ, and Dufort D (2004) Beta-catenin signaling marks the prospective site of primitive streak formation in the mouse embryo. Dev Dyn 231, 416–424. [DOI] [PubMed] [Google Scholar]
- 18.Barolo S (2006) Transgenic Wnt/TCF pathway reporters: all you need is Lef? Oncogene 25, 7505–7511. [DOI] [PubMed] [Google Scholar]
- 19.Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, and Piccolo S (2003) Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A 100, 3299–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merrill BJ, Pasolli HA, Polak L, Rendl M, Garcia-Garcia MJ, Anderson KV, and Fuchs E (2004) Tcf3: a transcriptional regulator of axis induction in the early embryo. Development 131, 263–274. [DOI] [PubMed] [Google Scholar]
- 21.Nakaya MA, Biris K, Tsukiyama T, Jaime S, Rawls JA, and Yamaguchi TP (2005) Wnt3a links left-right determination with segmentation and anteroposterior axis elongation. Development 132, 5425–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohamed OA, Jonnaert M, Labelle-Dumais C, Kuroda K, Clarke HJ, and Dufort D (2005) Uterine Wnt/beta-catenin signaling is required for implantation. Proc Natl Acad Sci U S A 102, 8579–8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marikawa Y. (2006) Wnt/beta-catenin signaling and body plan formation in mouse embryos. Semin Cell Dev Biol 17, 175–184. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi TP (2001) Heads or tails: Wnts and anterior-posterior patterning. Curr Biol 11, R713–724. [DOI] [PubMed] [Google Scholar]
- 25.Perea-Gomez A, Meilhac SM, Piotrowska-Nitsche K, Gray D, Collignon J, and Zernicka-Goetz M (2007) Regionalization of the mouse visceral endoderm as the blastocyst transforms into the egg cylinder. BMC Dev Biol 7, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Copp AJ (1979) Interaction between inner cell mass and trophectoderm of the mouse blastocyst. II. The fate of the polar trophectoderm. J Embryol Exp Morphol 51, 109–120. [PubMed] [Google Scholar]
- 27.Srinivas S, Rodriguez T, Clements M, Smith JC, and Beddington RS (2004) Active cell migration drives the unilateral movements of the anterior visceral endoderm. Development 131, 1157–1164. [DOI] [PubMed] [Google Scholar]
- 28.Thomas T, Yamagishi H, Overbeek PA, Olson EN, and Srivastava D (1998) The bHLH factors, dHAND and eHAND, specify pulmonary and systemic cardiac ventricles independent of left-right sidedness. Dev Biol 196, 228–236. [DOI] [PubMed] [Google Scholar]
- 29.Thomas P and Beddington R (1996) Anterior primitive endoderm may be responsible for patterning the anterior neural plate in the mouse embryo. Curr Biol 6, 1487–1496. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez TA, Srinivas S, Clements MP, Smith JC, and Beddington RS (2005) Induction and migration of the anterior visceral endoderm is regulated by the extra-embryonic ectoderm. Development 132, 2513–2520. [DOI] [PubMed] [Google Scholar]
- 31.Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, and Birchmeier W (2000) Requirement for beta-catenin in anterior-posterior axis formation in mice. J Cell Biol 148, 567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brennan J, Lu CC, Norris DP, Rodriguez TA, Beddington RS, and Robertson EJ (2001) Nodal signalling in the epiblast patterns the early mouse embryo. Nature 411, 965–969. [DOI] [PubMed] [Google Scholar]
- 33.Ding J, Yang L, Yan YT, Chen A, Desai N, Wynshaw-Boris A, and Shen MM (1998) Cripto is required for correct orientation of the anterior-posterior axis in the mouse embryo. Nature 395, 702–707. [DOI] [PubMed] [Google Scholar]
- 34.Morkel M, Huelsken J, Wakamiya M, Ding J, van de Wetering M, Clevers H, Taketo MM, Behringer RR, Shen MM, and Birchmeier W (2003) Beta-catenin regulates Cripto- and Wnt3-dependent gene expression programs in mouse axis and mesoderm formation. Development 130, 6283–6294. [DOI] [PubMed] [Google Scholar]
- 35.Kimura C, Yoshinaga K, Tian E, Suzuki M, Aizawa S, and Matsuo I (2000) Visceral endoderm mediates forebrain development by suppressing posteriorizing signals. Dev Biol 225, 304–321. [DOI] [PubMed] [Google Scholar]
- 36.Perea-Gomez A, Lawson KA, Rhinn M, Zakin L, Brulet P, Mazan S, and Ang SL (2001) Otx2 is required for visceral endoderm movement and for the restriction of posterior signals in the epiblast of the mouse embryo. Development 128, 753–765. [DOI] [PubMed] [Google Scholar]
- 37.Kimura-Yoshida C, Nakano H, Okamura D, Nakao K, Yonemura S, Belo JA, Aizawa S, Matsui Y, and Matsuo I (2005) Canonical Wnt signaling and its antagonist regulate anterior-posterior axis polarization by guiding cell migration in mouse visceral endoderm. Dev Cell 9, 639–650. [DOI] [PubMed] [Google Scholar]
- 38.Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward DW, Glinka A, Grinberg A, Huang SP, Niehrs C, Belmonte JC, and Westphal H (2001) Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell 1, 423–434. [DOI] [PubMed] [Google Scholar]
- 39.Liu P, Wakamiya M, Shea MJ, Albre-cht U, Behringer RR, and Bradley A (1999) Requirement for Wnt3 in vertebrate axis formation. Nat Genet 22, 361–365. [DOI] [PubMed] [Google Scholar]
- 40.Popperl H, Schmidt C, Wilson V, Hume CR, Dodd J, Krumlauf R, and Beddington RS (1997) Misexpression of Cwnt8C in the mouse induces an ectopic embryonic axis and causes a truncation of the anterior neuroectoderm. Development 124, 2997–3005. [DOI] [PubMed] [Google Scholar]
- 41.Zhou X, Sasaki H, Lowe L, Hogan BL, and Kuehn MR (1993) Nodal is a novel TGF-beta-like gene expressed in the mouse node during gastrulation. Nature 361, 543–547. [DOI] [PubMed] [Google Scholar]
- 42.Conlon FL, Lyons KM, Takaesu N, Barth KS, Kispert A, Herrmann B, and Robertson EJ (1994) A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development 120, 1919–1928. [DOI] [PubMed] [Google Scholar]
- 43.Perea-Gomez A, Vella FD, Shawlot W, Oulad-Abdelghani M, Chazaud C, Meno C, Pfister V, Chen L, Robertson E, Hamada H, Behringer RR, and Ang SL (2002) Nodal antagonists in the anterior visceral endoderm prevent the formation of multiple primitive streaks. Dev Cell 3, 745–756. [DOI] [PubMed] [Google Scholar]
- 44.Ben-Haim N, Lu C, Guzman-Ayala M, Pescatore L, Mesnard D, Bischofberger M, Naef F, Robertson EJ, and Constam DB (2006) The nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4. Dev Cell 11, 313–323. [DOI] [PubMed] [Google Scholar]
- 45.Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, and Kemler R (1995) Lack of beta-catenin affects mouse development at gastrulation. Development 121, 3529–3537. [DOI] [PubMed] [Google Scholar]
- 46.Shimizu H, Julius MA, Giarre M, Zheng Z, Brown AM, and Kitajewski J (1997) Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth Differ 8, 1349–1358. [PubMed] [Google Scholar]
- 47.Liu T, Liu X, Wang H, Moon RT, and Malbon CC (1999) Activation of rat frizzled-1 promotes Wnt signaling and differentiation of mouse F9 teratocarcinoma cells via pathways that require Galpha(q) and Galpha(o) function. J Biol Chem 274, 33539–33544. [DOI] [PubMed] [Google Scholar]
- 48.Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, and McMahon AP (1994) Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev 8, 174–189. [DOI] [PubMed] [Google Scholar]
- 49.Yoshikawa Y, Fujimori T, McMahon AP, and Takada S (1997) Evidence that absence of Wnt-3a signaling promotes neuralization instead of paraxial mesoderm development in the mouse. Dev Biol 183, 234–242. [DOI] [PubMed] [Google Scholar]
- 50.Greco TL, Takada S, Newhouse MM, McMahon JA, McMahon AP, and Camper SA (1996) Analysis of the vestigial tail mutation demonstrates that Wnt-3a gene dosage regulates mouse axial development. Genes Dev 10, 313–324. [DOI] [PubMed] [Google Scholar]
- 51.Dunty WC Jr., Biris KK, Chalamalasetty RB, Taketo MM, Lewandoski M, and Yamaguchi TP (2008) Wnt3a/ -catenin signaling controls posterior body development by coordinating mesoderm formation and segmentation. Development 135, 85–94. [DOI] [PubMed] [Google Scholar]
- 52.Galceran J, Hsu SC, and Grosschedl R (2001) Rescue of a Wnt mutation by an activated form of LEF-1: regulation of maintenance but not initiation of Brachyury expression. Proc Natl Acad Sci U S A 98, 8668–8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galceran J, Farinas I, Depew MJ, Clevers H, and Grosschedl R (1999) Wnt3a−/−-like phenotype and limb deficiency in Lef1(−/−)Tcf1(−/−) mice. Genes Dev 13, 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pinson KI, Brennan J, Monkley S, Avery BJ, and Skarnes WC (2000) An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 407, 535–538. [DOI] [PubMed] [Google Scholar]
- 55.Kokubu C, Heinzmann U, Kokubu T, Sakai N, Kubota T, Kawai M, Wahl MB, Galceran J, Grosschedl R, Ozono K, and Imai K (2004) Skeletal defects in ringelschwanz mutant mice reveal that Lrp6 is required for proper somitogenesis and osteogenesis. Development 131, 5469–5480. [DOI] [PubMed] [Google Scholar]
- 56.Arnold SJ, Stappert J, Bauer A, Kispert A, Herrmann BG, and Kemler R (2000) Brachyury is a target gene of the Wnt/beta-catenin signaling pathway. Mech Dev 91, 249–258. [DOI] [PubMed] [Google Scholar]
- 57.Aulehla A, Wehrle C, Brand-Saberi B, Kemler R, Gossler A, Kanzler B, and Herrmann BG (2003) Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev Cell 4, 395–406. [DOI] [PubMed] [Google Scholar]
- 58.Galceran J, Sustmann C, Hsu SC, Folberth S, and Grosschedl R (2004) LEF1-mediated regulation of Delta-like1 links Wnt and Notch signaling in somitogenesis. Genes Dev 18, 2718–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hofmann M, Schuster-Gossler K, Watabe-Rudolph M, Aulehla A, Herrmann BG, and Gossler A (2004) WNT signaling, in synergy with T/TBX6, controls Notch signaling by regulating Dll1 expression in the presomitic mesoderm of mouse embryos. Genes Dev 18, 2712–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamaguchi TP, Takada S, Yoshikawa Y, Wu N, and McMahon AP (1999) T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes Dev 13, 3185–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamaguchi TP, Bradley A, McMahon AP, and Jones S (1999) A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 126, 1211–1223. [DOI] [PubMed] [Google Scholar]
- 62.Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, Steel KP, Dai X, and Chen P (2007) Wnt5a functions in planar cell polarity regulation in mice. Dev Biol 306, 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Satoh W, Gotoh T, Tsunematsu Y, Aizawa S, and Shimono A (2006) Sfrp1 and Sfrp2 regulate anteroposterior axis elongation and somite segmentation during mouse embryogenesis. Development 133, 989–999. [DOI] [PubMed] [Google Scholar]
- 64.Xu Q, D’Amore PA, and Sokol SY (1998) Functional and biochemical interactions of Wnts with FrzA, a secreted Wnt antagonist. Development 125, 4767–4776. [DOI] [PubMed] [Google Scholar]
- 65.Dennis S, Aikawa M, Szeto W, d’Amore PA, and Papkoff J (1999) A secreted frizzled related protein, FrzA, selectively associates with Wnt-1 protein and regulates wnt-1 signaling. J Cell Sci 112 (Pt 21), 3815–3820. [DOI] [PubMed] [Google Scholar]
- 66.Tam PP and Beddington RS (1987) The formation of mesodermal tissues in the mouse embryo during gastrulation and early organogenesis. Development 99, 109–126. [DOI] [PubMed] [Google Scholar]
- 67.Kinder SJ, Tsang TE, Quinlan GA, Hadjantonakis AK, Nagy A, and Tam PP (1999) The orderly allocation of mesodermal cells to the extraembryonic structures and the anteroposterior axis during gastrulation of the mouse embryo. Development 126, 4691–4701. [DOI] [PubMed] [Google Scholar]
- 68.Garcia-Martinez V and Schoenwolf GC (1992) Positional control of mesoderm movement and fate during avian gastrulation and neurulation. Dev Dyn 193, 249–256. [DOI] [PubMed] [Google Scholar]
- 69.Schoenwolf GC, Garcia-Martinez V, and Dias MS (1992) Mesoderm movement and fate during avian gastrulation and neurulation. Dev Dyn 193, 235–248. [DOI] [PubMed] [Google Scholar]
- 70.Aulehla A and Herrmann BG (2004) Segmentation in vertebrates: clock and gradient finally joined. Genes Dev 18, 2060–2067. [DOI] [PubMed] [Google Scholar]
- 71.Pourquie O. (2003) The segmentation clock: converting embryonic time into spatial pattern. Science 301, 328–330. [DOI] [PubMed] [Google Scholar]
- 72.Chapman DL and Papaioannou VE (1998) Three neural tubes in mouse embryos with mutations in the T-box gene Tbx6. Nature 391, 695–697. [DOI] [PubMed] [Google Scholar]
- 73.Chapman DL, Garvey N, Hancock S, Alexiou M, Agulnik SI, Gibson-Brown JJ, Cebra-Thomas J, Bollag RJ, Silver LM, and Papaioannou VE (1996) Expression of the T-box family genes, Tbx1-Tbx5, during early mouse development. Dev Dyn 206, 379–390. [DOI] [PubMed] [Google Scholar]
- 74.Chapman DL, Cooper-Morgan A, Harrelson Z, and Papaioannou VE (2003) Critical role for Tbx6 in mesoderm specification in the mouse embryo. Mech Dev 120, 837–847. [DOI] [PubMed] [Google Scholar]
- 75.White PH, Farkas DR, and Chapman DL (2005) Regulation of Tbx6 expression by Notch signaling. Genesis 42, 61–70. [DOI] [PubMed] [Google Scholar]
- 76.Wittler L, Shin EH, Grote P, Kispert A, Beckers A, Gossler A, Werber M, and Herrmann BG (2007) Expression of Msgn1 in the presomitic mesoderm is controlled by synergism of WNT signalling and Tbx6. EMBO Rep 8, 784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoon JK and Wold B (2000) The bHLH regulator pMesogenin1 is required for maturation and segmentation of paraxial mesoderm. Genes Dev 14, 3204–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hrabe de Angelis M, McIntyre J 2nd, and Gossler A (1997) Maintenance of somite borders in mice requires the Delta homologue DII1. Nature 386, 717–721. [DOI] [PubMed] [Google Scholar]
- 79.Barrantes IB, Elia AJ, Wunsch K, Hrabe de Angelis MH, Mak TW, Rossant J, Conlon RA, Gossler A, and de la Pompa JL (1999) Interaction between Notch signalling and Lunatic fringe during somite boundary formation in the mouse. Curr Biol 9, 470–480. [DOI] [PubMed] [Google Scholar]
- 80.Ikeya M and Takada S (2001) Wnt-3a is required for somite specification along the anteroposterior axis of the mouse embryo and for regulation of cdx-1 expression. Mech Dev 103, 27–33. [DOI] [PubMed] [Google Scholar]
- 81.Pilon N, Oh K, Sylvestre JR, Savory JG, and Lohnes D (2007) Wnt signaling is a key mediator of Cdx1 expression in vivo. Development 134, 2315–2323. [DOI] [PubMed] [Google Scholar]
- 82.Krebs LT, Iwai N, Nonaka S, Welsh IC, Lan Y, Jiang R, Saijoh Y, O’Brien TP, Hamada H, and Gridley T (2003) Notch signaling regulates left-right asymmetry determination by inducing Nodal expression. Genes Dev 17, 1207–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Raya A, Kawakami Y, Rodriguez-Esteban C, Buscher D, Koth CM, Itoh T, Morita M, Raya RM, Dubova I, Bessa JG, de la Pompa JL, and Belmonte JC (2003) Notch activity induces Nodal expression and mediates the establishment of left-right asymmetry in vertebrate embryos. Genes Dev 17, 1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]