Abstract
Objective:
Alcohol use among people living with HIV (PLWH) can reduce adherence and worsen health outcomes. We evaluated the economic cost of an effective smartphone application (HealthCall) to reduce drinking and improve antiretroviral adherence among heavy-drinking PLWH participating in a randomized trial.
Method:
Participants were randomized to receive a brief drinking-reduction intervention, either (a) the National Institute on Alcohol Abuse and Alcoholism (NIAAA) Clinician's Guide (CG-only, n = 37), (b) CG enhanced by HealthCall to monitor daily alcohol consumption (CG+HealthCall, n = 38), or (c) motivational interviewing delivered by a nonclinician enhanced by HealthCall (MI+HealthCall, n = 39). We used micro-costing techniques to evaluate start-up costs and incremental costs per participant incurred from the health care sector perspective in 2018 U.S. dollars. We also investigated potential cost offsets using participant-reported health care utilization.
Results:
Participants attended three intervention visits, and each visit cost on average $29 for CG-only, $32 for CG+HealthCall, and $15 for MI+HealthCall. The total intervention cost per participant was $94 for CG-only, $114 for CG+HealthCall, and $57 for MI+HealthCall; the incremental cost of CG+HealthCall compared with CG-only was $20 per participant, and the incremental savings of MI+HealthCall compared with CG-only was $37 per participant. No significant differences in health care utilization occurred among the three groups over 12 months.
Conclusions:
The cost of enhancing CG with the HealthCall application for heavy-drinking PLWH was modestly higher than using the CG alone, whereas MI enhanced with HealthCall delivered by a nonclinician had a lower cost than CG alone. HealthCall may be a low-cost enhancement to brief interventions addressing alcohol use and antiretroviral adherence among PLWH.
Alcohol use among people living with HIV (PLWH) influences HIV outcomes at every stage of the care continuum, including worse care retention and poor antiretroviral therapy (ART) adherence (Williams et al., 2016). PLWH are significantly more likely to meet diagnostic criteria for alcohol dependence compared to people without HIV (Shiau et al., 2017). Alcohol interventions among PLWH have typically relied on multi-session face-to-face counseling, which is efficacious in reducing alcohol consumption but may be unrealistic and costly in real-world HIV care settings given the number of sessions and the duration of the intervention (Bray et al., 2012, 2014; Samet et al., 2003; Scott-Sheldon et al., 2017). As with other chronic diseases, patient self-management for alcohol use and continuing care are essential for effective treatment. Motivational interviewing (MI) can change alcohol consumption by using a client-centered, directive therapeutic style to enhance client readiness and motivation for change (Frost et al., 2018; Guy et al., 2022; Hettema et al., 2005; Stein et al., 2021). MI is effective for health behavior change, but evidence suggests that more extensive intervention is necessary for patients with complex problems such as PLWH who use alcohol (Aharonovich et al., 2012; Magill et al., 2018). The National Institute on Alcohol Abuse and Alcoholism (NIAAA) Clinician's Guide (CG) also offers a validated, structured screening and intervention tool to reduce alcohol use, but more extensive intervention may be necessary for this complex patient population (Friedmann et al., 2001; Hasin et al., 2013; NIAAA, 2005). Smartphone interventions to reduce alcohol use among PLWH offer a potentially lower cost, scalable, and effective option to extend the dose of MI or the CG without placing undue burden on the provider (Hasin et al., 2014).
Multiple mobile and smartphone technologies have demonstrated efficacy for chronic disease management and alcohol use. Several types of alcohol mobile health interventions exist, including text-messaging monitoring and reminder systems or interactive voice response (Farren et al., 2022; Leightley et al., 2022; Quanbeck et al., 2014). A pilot trial of the HealthCall intervention to reduce drinking among PLWH recruited patients from an HIV clinic in New York City and asked participants to call into an interactive voice response system to report the number of drinks consumed per drinking day (Aharonovich et al., 2012). The HealthCall intervention demonstrated effectiveness in reducing drinking in this trial and in a larger three-arm randomized clinical trial (Hasin et al., 2013). HealthCall was subsequently translated into a smartphone application (Hasin et al., 2014) and used in a HealthCall-S pilot trial (Aharonovich et al., 2017) and a three-arm randomized clinical trial (Hasin et al., 2022). These studies showed that the HealthCall smartphone application was effective in reducing heavy drinking when paired with MI and with CG.
Text-messaging and mobile interventions present scalable, low barrier, and low-cost interventions for behavior change and are cost-effective for smoking cessation, physical activity, and other chronic disease management (Badawy & Kuhns, 2016; Iribarren et al., 2017; Stanczyk et al., 2014). The COVID-19 pandemic has emphasized the need for mobile and telehealth options, especially for vulnerable populations with problematic alcohol use (Attonito et al., 2021). However, although the costs of alcohol brief interventions are well documented (Bray et al., 2012, 2014), evidence is limited on the cost of delivering mobile interventions for alcohol use, particularly among PLWH. Our objectives were to estimate and compare the cost of delivering the smartphone-based alcohol reduction intervention with MI or CG in the HealthCall-S trial, and the likely costs of these interventions in nontrial settings.
Method
Analytic overview
Institutional review boards at the New York State Psychiatric Institute–Columbia University Department of Psychiatry, Montefiore Medical Center/Albert Einstein College of Medicine, and Weill Cornell Medical College reviewed and approved this cost study. All clinical trial participants and staff who provided data for this analysis gave informed consent.
We used a micro-costing approach from the health care sector perspective to determine the cost of the three strategies to reduce heavy drinking and improve antiretroviral adherence among PLWH tested in the HealthCall-S trial: (a) the NIAAA CG with standard care (CG-only), (b) the NIAAA CG enhanced with the HealthCall smartphone application (CG+HealthCall), and (c) motivational interviewing enhanced with the HealthCall smartphone application (MI+HealthCall). We also explored potential cost savings using participant self-reported data to compare health care utilization costs among the three arms over 12 months. All costs are reported in 2018 U.S. dollars. Data were analyzed using Microsoft Excel 2016 (Microsoft Core; Redmond, WA) and Stata/IC 16.0 (Stata Corp LP, College Station, TX).
Overview of the intervention study
Participants were enrolled from an outpatient HIV clinic in Bronx, NY. Eligible patients were at least 18 years old, met criteria for current alcohol dependence according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; American Psychiatric Association, 1994), self-reported four or more alcoholic drinks on at least 1 day in the past 30 days, had HIV infection confirmed in their medical record, and spoke English or Spanish. The study sample was 58% male with a median age of 50 years (interquartile range: 41–54 years). Participants were 75% Black/African American, 17% White, and 9% American Indian/Alaska Native; 28% of participants were Hispanic and 19% spoke Spanish as their native language. Most participants were insured by Medicaid (89%) and received public assistance in the form of food stamps (93%), Supplemental Security Income (SSI; 50%), Social Security (36%), or cash assistance (Transitional Aid to Families with Dependent Children [TAFDC]/Employment Services Plan [ESP]/Emergency Advance Payment [EAP]; 26%) in the 12 months before enrollment. Most participants were unemployed (83%), and participants either had no high school degree (32%), had a high school diploma or General Educational Development (GED) credential (31%), or attended some college or technical school (27%).
Enrolled participants were randomized to receive either CG-only (n = 37), CG+HealthCall (n = 38), or MI+HealthCall (n = 39). No MI-only arm was tested, as this was inferior to MI+HealthCall in a previous trial (Hasin et al., 2013). All intervention groups were offered three in-person visits: baseline, 30 days, and 60 days. Primary outcomes for the clinical trial included mean drinks per drinking day in the past 30 days, frequency of drinking days in the past 30 days, and ART adherence. Outcomes were assessed at 30 and 60 days during the intervention, and at 3, 6, and 12 months after baseline as follow-up; 86% of participants were retained at 12 months across all arms.
The NIAAA CG was delivered to participants in the CG-only and CG+HealthCall arms by either a nurse practitioner or a physician at the baseline visit. This involved a discussion about alcohol use and ART adherence, which included providing feedback to participants about their alcohol use and setting drinking reduction goals. Participants were given a “Rethinking Drinking” booklet published by NIAAA (2009) that provided information and tips to reduce alcohol drinking. Follow-up intervention visits at 30 and 60 days involved a brief meeting with the same clinician to discuss alcohol drinking and ART adherence.
MI was delivered to participants in the MI+HealthCall arm by a substance use counselor with prior training and experience in MI. At baseline, the counselor discussed with participants the implications of alcohol use on health and the possibility of reducing drinking. Participants were asked to describe the pros and cons of alcohol drinking and develop a plan to reduce drinking if they wished. The counselor discussed drinking reduction goals with participants in a brief in-person meeting at 30 and 60 days.
The HealthCall smartphone application was added to the baseline, 30-day, and 60-day visits by the clinician or counselor for participants in the CG+HealthCall and MI+HealthCall arms to enhance these brief interventions. Participants received a study smartphone with a calling plan and HealthCall installed, or they received assistance to install the application on their personal smartphones. The baseline visit included instructions on using HealthCall daily and an opportunity to practice using HealthCall to record alcohol use. At the 30- and 60-day follow-up visits, the clinician or counselor reviewed a graph of the drinking patterns exported from HealthCall for the prior 30 days and provided feedback to participants on their drinking. CG+HealthCall and MI+HealthCall participants also received HealthCall technical assistance and troubleshooting from the study project manager between visits. Participants who did not open HealthCall on their phone at least every 2 days received a reminder from the project manager by phone call, text message, or email to use the application.
One-time start-up activities included in-person training in CG, MI, and HealthCall for each interventionist, as applicable. The substance use counselor participated in a 4-hour, one-on-one refresher course with a doctoral-level MI Network Trainer. Clinicians administering the CG participated in a 60-minute in-person training led by a study investigator who was a doctoral-trained psychologist experienced in substance use disorder. The MI counselor, clinicians, and project manager received a 60-minute training on using HealthCall with the study investigator.
The HealthCall smartphone app added to CG or MI demonstrated efficacy in reducing alcohol intake compared with CG alone (Hasin et al., 2022). At 30 days, participants in the MI+HealthCall arm had fewer drinks per drinking day (3.80) compared with CG-only (5.28) and CG+HealthCall (5.67) (incidence rate ratios [IRRs] = 0.62, 95% CI [0.46, 0.84], and 0.64, 95% CI [0.48, 0.87], respectively). At the 12-month follow-up visit, participants in the CG+HealthCall arm demonstrated long-term reduction in drinking with significantly fewer drinks per drinking day compared with CG-only (5.31 vs. 6.79, respectively; IRR = 0.71, 95% CI [0.51, 0.98]) (Hasin et al., 2022). The difference in mean drinks per drinking day at 12 months between MI+HealthCall (5.73) and CG+HealthCall (5.31) was not significant.
Data collection
We estimated start-up training costs using time estimates from semi-structured interviews with the study interventionists and the project manager. In the semi-structured interview with the project manager, we also estimated time spent creating and adapting HealthCall intervention materials. We calculated the number of in-person visits and number of weeks between the baseline and final intervention visit for each participant from study records. The interventionists provided an overall estimate of the average duration of initial and follow-up/study visits for participants in each trial arm via semi-structured interviews. Time spent screening potential participants for alcohol dependence, scheduling intervention visits, and rescheduling appointments was calculated using estimates from interviews with study staff, and the frequency of these activities was estimated using study screening, recruitment, and enrollment logs. We calculated weekly time providing HealthCall reminders and troubleshooting from study logs that tracked each HealthCall reminder or troubleshooting encounter by participant.
Participants in all three groups completed an audio computer-assisted self-interview (ACASI) questionnaire on substance use and health care utilization (emergency department visits, inpatient hospital stays, outpatient visits, mental health visits, substance use treatment, and support groups) at baseline and at 30 days, 60 days, 3 months, 6 months, and 12 months after baseline. The cost of the ACASI sessions was excluded from the analysis because these were considered research costs that would not occur in a real-world scenario (Charles et al., 2013).
Unit costs
Table 1 summarizes the unit cost inputs for our analysis. We assigned the relevant published national mean wage and fringe benefit rates from the U.S. Bureau of Labor Statistics (BLS) to clinician, staff, and trainer time calculated based on time estimates and study records. We applied an overhead rate calculated from a previous study that was conducted at the same hospital (Teixeira et al., 2018). We estimated the cost for emergency department visits, inpatient hospital stays, outpatient visits, mental health visits, and substance use treatment using published sources (Agency for Health-care Research and Quality, 2018; Centers for Medicare & Medicaid Services, 2018; Dunlap et al., 2018; Substance Abuse and Mental Health Services Administration [SAMHSA], 2003; U.S. Department of Veterans Affairs, 2016).
Table 1.
Cost inputs
| Cost input | Unit cost, 2018 USD | Source |
|---|---|---|
| Personnel time, $ per hour | ||
| Project manager | 34 46 | (U.S. Bureau of Labor Statistics, 2018) |
| Trainer, psychologist | 41.63 | (U.S. Bureau of Labor Statistics, 2018) |
| Substance use counselor | 23.04 | (U.S. Bureau of Labor Statistics, 2018) |
| Nurse practitioner | 52.90 | (U.S. Bureau of Labor Statistics, 2018) |
| Fringe and overhead | ||
| –Fringe rate | 0.463 | (U.S. Bureau of Labor Statistics, 2019) |
| –Overhead rate | 0.488 | (Teixeira et al., 2018) |
| Health care costs | ||
| Emergency department visit | 1,008.39 | (Agency for Healthcare Research and Quality, 2018) |
| Inpatient hospital stay (per night) | 4,336.08 | (Agency for Healthcare Research and Quality, 2018) |
| Outpatient hospital or community clinic visit | 1,149.35 | (Agency for Healthcare Research and Quality, 2018) |
| Outpatient mental health provider visit | 318.78 | (Centers for Medicare & Medicaid Services, 2018) |
| Outpatient substance use treatment visit | 45.01 | (Substance Abuse and Mental Health Services Administration, 2003) |
| Residential substance use treatment program (per day) | 128.23 | (Substance Abuse and Mental Health Services Administration, 2003) |
| Support group or group counseling session | 8.67 | (Dunlap et al., 2018) |
Note: USD = U.S. dollars.
Analysis
We calculated the weekly cost of screening for alcohol dependence, scheduling intervention visits, appointment reminder calls, and rescheduling appointments by multiplying the total estimated weekly time spent on these activities by the wage and fringe rate corresponding to the study team member responsible for each activity. The total weekly screening, scheduling, and retention costs were attributed to each intervention arm proportionally to the number of participants enrolled in each arm. We then estimated the weekly cost per participant by arm and multiplied this cost by the number of weeks each participant spent in the intervention period to estimate the average total cost of these activities per participant by arm over the intervention period. Although the intervention protocol called for the last intervention visit to be 60 days from baseline, we used the actual time participants spent in the intervention period to reflect real-world scheduling challenges and delays.
The cost of in-person intervention visits was calculated by applying the corresponding wage and fringe rates to the overall time estimates reported by study staff in interviews. We estimated the total and weekly cost of the project manager's time providing HealthCall reminders and troubleshooting to participants in each study arm by applying the project manager's wage rate to the time for these activities recorded in the study logs for each participant. The cost of providing HealthCall reminders and troubleshooting was included in the average cost per participant for the relevant study arms. Study-provided smartphones and calling plans for participants who did not install HealthCall on their own smartphones were assumed to be research costs that would not be incurred by the health care sector in the real world and were therefore excluded from our analyses. In a real-world scenario, having a smartphone likely would be required before a patient could be enrolled in the program, and it is unlikely that an HIV clinic would choose to provide smartphones to patients. We assumed that no incremental costs would be incurred for clinic space because this intervention is intended to be delivered during a clinical visit.
We compared the incremental cost or savings of the CG+HealthCall and MI+HealthCall arms compared with the CG-only arm. The added cost of HealthCall reflects additional time for intervention visits, reminders to use HealthCall, and technical troubleshooting. Savings primarily reflect differences in labor costs between clinicians and nonclinicians.
In sensitivity analyses we adjusted start-up and intervention costs based on the type of provider that delivered the intervention to account for real-world variations in clinic staffing. We report three different scenarios for the type of provider administering the interventions. In the base case scenario, we estimated costs if a nurse practitioner administered the CG and a counselor administered MI, which largely reflects the study staffing. Although a physician provided occasional coverage in the study, this accounted for less than 10% of visits, so we assumed a nurse practitioner wage for all clinician visits. In the low-cost scenario, we estimated the cost per participant if the CG and MI were both administered by a substance use counselor. In the high-cost scenario, we estimated costs if the CG and MI were both administered by a nurse practitioner.
We used the resource-costing method to estimate the costs associated with participant health care utilization outside of the study. Participant self-reported health care utilization was multiplied by relevant resource unit costs for each participant over the 12-month study period (Table 1). We calculated the predicted mean, per-participant costs, by arm, for each resource category using three multivariable generalized linear model regressions (CG-only vs. CG+HealthCall; CG-only vs. MI+HealthCall; MI+HealthCall vs. CG+HealthCall) for the 60-day intervention period and the entire 12-month observation period. The health care utilization analysis is described in detail in Appendix 1. (Supplemental material appears as an online-only addendum to this article on the journal's website.)
Results
Start-up costs
The total estimated start-up cost using the base case assumptions of a nurse practitioner delivering CG and a counselor delivering MI was $190 for CG-only, $490 for CG+HealthCall, and $520 for MI+HealthCall, including fringe benefits and overhead. Creating and adapting the training and intervention materials cost $2,520 in total across all study arms (Supplemental Table 1).
Intervention costs
Table 2 presents the details of the intervention costs for each study arm. Participants were in the study for an average of 9.5 weeks (SD = 1.65) from baseline to the 60-day visit in the CG-only arm, 9.8 weeks (SD = 1.44) in the CG+HealthCall arm, and 8.9 weeks (SD = 1.04) in the MI+HealthCall arm. Screening participants for alcohol use disorder cost approximately $12 per week for all participants over the course of the study. Scheduling cost approximately $38 per week, whereas retention (rescheduling and reminder calls) cost approximately $75 per week for all participants. The total weekly screening, scheduling, and retention cost attributed to each study arm was approximately $40 for CG-only, $41 for CG+HealthCall, and $43 for MI+HealthCall. The average weekly cost per participant was approximately $1.10; accordingly, the average screening, scheduling, and retention cost for participants over the intervention period was approximately $10.40, $10.70, and $9.60 for CG-only, CG+HealthCall, and MI+HealthCall, respectively.
Table 2.
Average total cost per participant per study arm
| Cost category per study arm | Cost per scenario, 2018 USD | |||
|---|---|---|---|---|
| Time estimate, in minutes | Nurse practitioner for CG; counselor for MI | Counselor for CG and MI | Nurse practitioner for CG and MI | |
| CG-only (n = 37) | ||||
| Screening, scheduling, and retention | 10 | 10 | 10 | 10 |
| Face-to-face visits | 50 | 65 | 28 | 65 |
| Visit documentation | 15 | 19 | 8 | 19 |
| Total | 75 | 94 | 47 | 94 |
| CG+HealthCall (n = 38) | ||||
| Screening, scheduling, and retention | 10 | 11 | 11 | 11 |
| Face-to-face visits | 60 | 77 | 34 | 77 |
| Visit documentation | 15 | 19 | 8 | 19 |
| Reminders to use HealthCall | 5 | 4 | 4 | 4 |
| HealthCall troubleshooting | 3 | 2 | 2 | 2 |
| Total | 93 | 114 | 59 | 114 |
| MI+HealthCall (n = 39) | ||||
| Screening, scheduling, and retention | 9 | 10 | 10 | 10 |
| Face-to-face visits | 60 | 34 | 34 | 77 |
| Visit documentation | 15 | 8 | 8 | 19 |
| Reminders to use HealthCall | 3 | 3 | 3 | 3 |
| HealthCall troubleshooting | 2 | 2 | 2 | 2 |
| Total | 89 | 57 | 57 | 111 |
Notes: USD = U.S. dollars; CG = Clinician's Guide; MI = motivational interviewing; time estimates are per interview data; per-protocol time for face-to-face visits was 55 minutes for CG-only, 65 minutes for CG+HealthCall, and 65 minutes for MI+HealthCall.
The baseline visit for the CG-only arm was estimated to take 20 minutes per participant, whereas the baseline visits for CG+HealthCall and MI+HealthCall participants were estimated to take 30 minutes, indicating an additional 10 minutes to introduce HealthCall. Follow-up visits were estimated to take 15 minutes per participant for all three study arms. Interventionists reported spending an additional 5 minutes per study visit on documentation.
The average total cost, per participant, including screening, scheduling, retention, and in-person visits, was approximately $94 for CG-only, $114 for CG+HealthCall, and $57 for MI+HealthCall. The incremental cost of HealthCall added to CG compared with CG-only was $20 and the incremental savings for HealthCall added to MI compared with CG-only was $36.
Sensitivity analysis for different interventionists
If a substance use counselor delivered all interventions instead of using a nurse practitioner for CG, the total estimated start-up cost would decrease to $150 for CG-only and $260 for CG+HealthCall compared with $520 for MI+HealthCall. If a nurse practitioner administered both the CG and MI, instead of using a counselor for MI, the start-up cost would remain $190 for CG-only and $490 for CG+HealthCall but would increase to $850 for MI+HealthCall.
The total intervention cost for each scenario is presented in Table 2. In the low-cost scenario in which a substance use counselor delivers CG, MI, and HealthCall, the incremental cost of HealthCall would decrease to $12 per participant compared with CG-only. In the high-cost scenario the incremental cost of HealthCall would remain $20 per participant.
Cost offsets
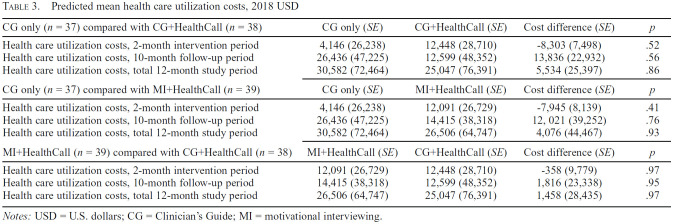
The estimated costs of nonstudy health care utilization during the 60-day intervention period are reported in Table 3 (see Supplemental Table 2 for unadjusted health care utilization counts). We observed no significant differences in health care utilization costs between arms during the intervention period, during follow-up, or over the entire 12-month data collection period.
Table 3.
Predicted mean health care utilization costs, 2018 USD
| CG only (n = 37) compared with CG+HealthCall (n = 38) | CG only (SE) | CG+HealthCall (SE) | Cost difference (SE) | p |
|---|---|---|---|---|
| Health care utilization costs, 2-month intervention period | 4,146 (26,238) | 12,448 (28,710) | -8,303 (7,498) | .52 |
| Health care utilization costs, 10-month follow-up period | 26,436 (47,225) | 12,599 (48,352) | 13,836 (22,932) | .56 |
| Health care utilization costs, total 12-month study period | 30,582 (72,464) | 25,047 (76,391) | 5,534 (25,397) | .86 |
| CG only (n = 37) compared with MI+HealthCall (n = 39) | CG only (SE) | MI+HealthCall (SE) | Cost difference (SE) | p |
| Health care utilization costs, 2-month intervention period | 4,146 (26,238) | 12,091 (26,729) | -7,945 (8,139) | .41 |
| Health care utilization costs, 10-month follow-up period | 26,436 (47,225) | 14,415 (38,318) | 12, 021 (39,252) | .76 |
| Health care utilization costs, total 12-month study period | 30,582 (72,464) | 26,506 (64,747) | 4,076 (44,467) | .93 |
| MI+HealthCall (n = 39) compared with CG+HealthCall (n = 38) | MI+HealthCall (SE) | CG+HealthCall (SE) | Cost difference (SE) | p |
| Health care utilization costs, 2-month intervention period | 12,091 (26,729) | 12,448 (28,710) | -358 (9,779) | .97 |
| Health care utilization costs, 10-month follow-up period | 14,415 (38,318) | 12,599 (48,352) | 1,816 (23,338) | .95 |
| Health care utilization costs, total 12-month study period | 26,506 (64,747) | 25,047 (76,391) | 1,458 (28,435) | .97 |
Notes: USD = U.S. dollars; CG = Clinician's Guide; MI = motivational interviewing.
Discussion
Implementing the HealthCall smartphone application to enhance either the NIAAA CG or MI for drinking reduction among PLWH added just $12–$20 per participant to health care sector costs over a 60-day intervention period, excluding start-up costs. The difference in intervention cost between these approaches was largely a factor of the wage rate of the clinic staff member delivering the intervention; if a substance use counselor delivers the intervention enhanced with HealthCall, the cost is substantially less than if it is delivered by a nurse practitioner.
Although MI was less expensive than CG in the base case, the cost disparity was negligible in the sensitivity analyses when staffing was equalized. Considering MI requires more training than CG and may necessitate ongoing monitoring to maintain fidelity, CG may be less costly overall after accounting for start-up costs for an organization with numerous staff or frequent turnover. NIAAA originally developed the CG for use by primary care clinicians, but it has since been updated for use by both primary care and mental health providers, is available from the NIAAA webpage, and requires very little instruction or training to use (NIAAA, 2005). Trial results also support the potential attractiveness of delivering CG+HealthCall with substance use counselors if results are comparable to when delivered by clinicians.
The total costs of all three arms of this study ($57–$114) are comparable to other brief interventions for alcohol use. In a literature review of the costs of alcohol screening and brief intervention in medical settings, Bray and colleagues found the cost of brief interventions to range from $3 to $196, with a median cost of $48 in 2009 USD, equivalent to $62 in 2018 USD (BLS, 2022; Bray et al., 2012). Furthermore, the U.S. Medicaid reimbursement rate for alcohol and/or drug screening and brief intervention is $48 per 15 minutes (SAMHSA, 2022). Considering the HealthCall intervention paired with CG and MI includes three 15- to 30-minute sessions totaling $57–59, the cost of HealthCall added to either CG and MI in the low-cost scenario would presumably fall within the Medicaid reimbursement range in the United States (SAMHSA, 2022).
Additional costs incurred for the study arms receiving HealthCall included extra time at the baseline visit (approximately 10 minutes) to install and introduce the smartphone application and periodic technical assistance and reminders by study staff to use HealthCall to log daily alcohol intake. In a real-world scenario, smartphone troubleshooting may be provided through the phone carrier. Staff-generated reminders to use the HealthCall application (as opposed to automated reminders) also may not occur in real-world practice; however, in this trial staff-generated reminders may have affected the primary outcomes, and therefore we included them in the cost analysis.
Clinicians and counselors adding HealthCall to their brief interventions require one-time training in the technology, at approximately $145 for a counselor and $300 for a nurse practitioner. These costs are comparable to those reported for smartphone interventions for smoking cessation and chronic conditions (Badawy & Kuhns, 2016; Stanczyk et al., 2014). Retention activities, including appointment reminders and rescheduling in-person visits to accommodate participant needs, added approximately $1 per participant per week. Considering the high retention rate across all three study arms, this cost appears low to engage and retain each patient in this vulnerable population.
Limitations
Some previously identified limitations to this trial include use of the DSM-IV criteria for inclusion rather than DSM-5 (American Psychiatric Association, 2013) to maintain continuity between studies and not including heavy drinkers who do not have a dependence or moderate-to-severe alcohol use disorder diagnosis (Hasin et al., 2022).
This cost analysis has several limitations. First, time spent on in-person intervention visits was estimated as an average for all visits of the same type, as we did not have data about the duration of each visit; this may result in an over- or under-estimation of the cost of individual visits. Prior literature has indicated that semi-structured interviews may lead to an overestimate of patient contact time and an underestimate of noncontact time (e.g., documentation), resulting in a slightly higher total intervention time; thus, the costs presented here are likely conservative estimates (Bratt et al., 1999; Bray et al., 2012, 2014; Cowell et al., 2017). We did not include the cost of smartphones and phone plans in our analysis because in a real-world setting implementation of HealthCall would likely be limited to individuals who already have a smartphone. Additional subsidies may be required in some settings to improve access to this technology. We were unable to conduct a cost analysis from the patient or societal perspectives because of lack of data on patient time spent on the intervention, and on societal outcomes such as productivity-related cost savings.
Although we did not find any significant cost offsets from changes in health care utilization, this finding may be attributable to the small sample size, limited data collection period, or large variation among the few participants who reported health care utilization costs. It is possible that cost offsets could be observed in a larger sample or longer observation period, although prior research similarly has not demonstrated a strong relationship between brief alcohol interventions and reduction in health care utilization costs (Bray et al., 2011).
Conclusion
This is the first study to estimate the cost of delivering a smartphone application to reduce alcohol use among heavy-drinking PLWH, and to compare the costs of two different brief drinking-reduction interventions delivered with technological enhancement. These brief interventions have substantially lower costs when delivered by a counselor compared with being delivered by a prescriber-level clinician. The cost of adding HealthCall to the brief interventions was modest and the total cost was consistent with Medicaid reimbursement, indicating that HealthCall may be a low-cost option for addressing alcohol use and antiretroviral adherence among PLWH.
Conflict-of-Interest Statement
The authors have no conflicts of interest to declare.
Footnotes
Research reported in this article was supported by National Institute on Alcohol Abuse and Alcoholism Award Number R01AA023163; National Institute on Drug Abuse Award Numbers P30DA040500, T32DA031099, and K01DA051348; and National Institute of Nursing Research Award Number T32NR014205. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Agency for Healthcare Research and Quality. Medical Expenditure Panel Survey. 2018. Retrieved February 21, 2020, from https://meps.ahrq.gov/mepsweb/ [PubMed]
- Aharonovich E., Greenstein E., O’Leary A., Johnston B., Seol S. G., Hasin D. S. HealthCall: Technology-based extension of motivational interviewing to reduce non-injection drug use in HIV primary care patients—A pilot study. AIDS Care. 2012;24(12):1461–1469. doi: 10.1080/09540121.2012.663882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharonovich E., Stohl M., Cannizzaro D., Hasin D. HealthCall delivered via smartphone to reduce co-occurring drug and alcohol use in HIV-infected adults: A randomized pilot trial. Journal of Substance Abuse Treatment. 2017;83:15–26. doi: 10.1016/j.jsat.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (4th ed.). Washington, DC: Author; 1994. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- American Psychiatric Association. (5th ed.). Arlington, VA: Author; 2013. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- Attonito J., Villalba K., Fontal S. Priorities for alcohol use disorder treatment and prevention during COVID-19's second wave. American Journal of Public Health. 2021;111(3):359–362. doi: 10.2105/AJPH.2020.306070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy S. M., Kuhns L. M. Economic evaluation of text-messaging and smartphone-based interventions to improve medication adherence in adolescents with chronic health conditions: A systematic review. JMIR mHealth and uHealth. 2016;4(4):e121. doi: 10.2196/mhealth.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratt J. H., Foreit J., Chen P. L., West C., Janowitz B., de Vargas T. A comparison of four approaches for measuring clinician time use. Health Policy and Planning. 1999;14(4):374–381. doi: 10.1093/heapol/14.4.374. [DOI] [PubMed] [Google Scholar]
- Bray J. W., Cowell A. J., Hinde J. M. A systematic review and meta-analysis of health care utilization outcomes in alcohol screening and brief intervention trials. Medical Care. 2011;49(3):287–294. doi: 10.1097/MLR.0b013e318203624f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray J. W., Mallonee E., Dowd W., Aldridge A., Cowell A. J., Vendetti J. Program- and service-level costs of seven screening, brief intervention, and referral to treatment programs. Substance Abuse and Rehabilitation. 2014;5:63–73. doi: 10.2147/SAR.S62127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray J. W., Zarkin G. A., Hinde J. M., Mills M. J. Costs of alcohol screening and brief intervention in medical settings: a review of the literature. Journal of Studies on Alcohol and Drugs. 2012;73(6):911–919. doi: 10.15288/jsad.2012.73.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Medicare & Medicaid Services. Physician Fee Schedule search. 2018. Retrieved February 21, 2020, from https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx.
- Charles J. M., Edwards R. T., Bywater T., Hutchings J. Micro-costing in public health economics: Steps towards a standardized framework, using the Incredible Years Toddler Parenting Program as a worked example. Prevention Science. 2013;14(4):377–389. doi: 10.1007/s11121-012-0302-5. [DOI] [PubMed] [Google Scholar]
- Cowell A. J., Dowd W. N., Landwehr J., Barbosa C., Bray J. W. A time and motion study of screening, brief intervention, and referral to treatment implementation in health-care settings. Addiction. 2017;112(Supplement 2):65–72. doi: 10.1111/add.13659. [DOI] [PubMed] [Google Scholar]
- Dunlap L. J., Zarkin G. A., Orme S., Meinhofer A., Kelly S. M., O’Grady K. E., Gryczynski J., Mitchell S. G., Schwartz R. P. Re-engineering methadone—Cost-effectiveness analysis of a patient-centered approach to methadone treatment. Journal of Substance Abuse Treatment. 2018;94:81–90. doi: 10.1016/j.jsat.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farren C., Farrell A., Hagerty A., McHugh C. A 6-month randomized trial of a smartphone application, U Control Drink, in aiding recovery in alcohol use disorder. European Addiction Research. 2022;28(2):122–133. doi: 10.1159/000519945. [DOI] [PubMed] [Google Scholar]
- Friedmann P. D., Saitz R., Gogineni A., Zhang J. X., Stein M. D. Validation of the screening strategy in the NIAAA “Physicians’ Guide to Helping Patients with Alcohol Problems. Journal of Studies on Alcohol. 2001;62(2):234–238. doi: 10.15288/jsa.2001.62.234. [DOI] [PubMed] [Google Scholar]
- Frost H., Campbell P., Maxwell M., O’Carroll R. E., Dombrowski S. U., Williams B., Cheyne H., Coles E., Pollock A. Effectiveness of motivational interviewing on adult behaviour change in health and social care settings: A systematic review of reviews. PLoS One. 2018;13(10):e0204890. doi: 10.1371/journal.pone.0204890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick H. A., Doshi J. A., Sonnad S. S., Polsky D. (2nd ed.) Oxford University Press; 2014. Economic evaluation in clinical trials. [DOI] [Google Scholar]
- Guy A. A., Zelaya D. G., Surace A., Mastroleo N. R., Pantalone D. W., Monti P. M., Mayer K. H., Kahler C. W. Discrimination and alcohol problems among heavy drinking HIV-positive men who have sex with men: The buffering effect of a brief motivational intervention to reduce alcohol use. Drug and Alcohol Dependence. 2022;233:109384. doi: 10.1016/j.drugalcdep.2022.109384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin D. S., Aharonovich E., Greenstein E. HealthCall for the smartphone: Technology enhancement of brief intervention in HIV alcohol dependent patients. Addiction Science & Clinical Practice. 2014;9(1):5. doi: 10.1186/1940-0640-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin D. S., Aharonovich E., O’Leary A., Greenstein E., Pavlicova M., Arunajadai S., Waxman R., Wainberg M., Helzer J., Johnston B. Reducing heavy drinking in HIV primary care: A randomized trial of brief intervention, with and without technological enhancement. Addiction. 2013;108(7):1230–1240. doi: 10.1111/add.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin D. S., Aharonovich E., Zingman B. S., Stohl M., Walsh C., Elliott J. C., Fink D. S., Knox J., Durant S., Menchaca R., Sharma A. HealthCall: A randomized trial assessing a smartphone enhancement of brief interventions to reduce heavy drinking in HIV care. Journal of Substance Abuse Treatment. 2022;138:108733. doi: 10.1016/j.jsat.2022.108733. https://doi.rg/10.1016/j.jsat.2022.108733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema J., Steele J., Miller W. R. Motivational interviewing. Annual Review of Clinical Psychology. 2005;1(1):91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- Iribarren S. J., Cato K., Falzon L., Stone P. W. What is the economic evidence for mHealth? A systematic review of economic evaluations of mHealth solutions. PLoS One. 2017;12(2):e0170581. doi: 10.1371/journal.pone.0170581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leightley D., Williamson C., Rona R. J., Carr E., Shearer J., Davis J. P., Simms A., Fear N. T., Goodwin L., Murphy D. Evaluating the efficacy of the Drinks: Ration mobile app to reduce alcohol consumption in a help-seeking military veteran population: Randomized controlled trial. JMIR mHealth and uHealth. 2022;10(6):e38991. doi: 10.2196/38991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill M., Apodaca T. R., Borsari B., Gaume J., Hoadley A., Gordon R. E. F., Tonigan J. S., Moyers T. A meta-analysis of motivational interviewing process: Technical, relational, and conditional process models of change. Journal of Consulting and Clinical Psychology. 2018;86(2):140–157. doi: 10.1037/ccp0000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. Washington, DC: National Institutes of Health; 2005. Helping patients who drink too much: A clinician's guide. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. Rethinking drinking. 2009. Retrieved February 18, 2020, from https://www.rethinking-drinking.niaaa.nih.gov/
- Quanbeck A., Chih M.-Y., Isham A., Gustafson D. Mobile delivery of treatment for alcohol use disorders: A review of the literature. Alcohol Research: Current Reviews. 2014;36(1):111–122. [PMC free article] [PubMed] [Google Scholar]
- Samet J. H., Larson M. J., Horton N. J., Doyle K., Winter M., Saitz R. Linking alcohol- and drug-dependent adults to primary medical care: A randomized controlled trial of a multi-disciplinary health intervention in a detoxification unit. Addiction. 2003;98:509–516. doi: 10.1046/j.1360-0443.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- Scott-Sheldon L. A. J., Carey K. B., Johnson B. T., Carey M. P. MASH Research Team. Behavioral interventions targeting alcohol use among people living with HIV/AIDS: A systematic review and meta-analysis. AIDS and Behavior. 2017;21:126–143. doi: 10.1007/s10461-017-1886-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiau S., Arpadi S. M., Yin M. T., Martins S. S. Patterns of drug use and HIV infection among adults in a nationally representative sample. Addictive Behaviors. 2017;68:39–44. doi: 10.1016/j.addbeh.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanczyk N. E., Smit E. S., Schulz D. N., de Vries H., Bolman C., Muris J. W. M., Evers S. M. A. A. An economic evaluation of a video- and text-based computer-tailored intervention for smoking cessation: A cost-effectiveness and cost-utility analysis of a randomized controlled trial. PLoS One. 2014;9(10):e110117. doi: 10.1371/journal.pone.0110117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M. D., Herman D. S., Kim H. N., Howell A., Lambert A., Madden S., Moitra E., Blevins C. E., Anderson B. J., Taylor L. E., Pinkston M. M. A randomized trial comparing brief advice and motivational interviewing for persons with HIV–HCV co-infection who drink alcohol. AIDS and Behavior. 2021;25(4):1013–1025. doi: 10.1007/s10461-020-03062-2. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. The Alcohol and Drug Services (ADSS) cost study: Costs of substance abuse treatment in the specialty sector. 2003. Retrieved February 21, 2020, from https://www.datafiles.samhsa.gov/study-publication/adss-cost-study-costs-substance-abuse-treatment-specialty-sector-nid15215.
- Substance Abuse and Mental Health Services Administration. Coding for screening and brief intervention reimbursement. 2022. Apr 14, Retrieved August 24, 2022, from https://www.samhsa.gov/sbirt/coding-reimbursement.
- Teixeira P. A., Bresnahan M. P., Laraque F., Litwin A. H., Shukla S. J., Schwartz J. M., Reynoso S., Perumalswami P. V., Weiss J. M., Wyatt B., Schackman B. R. Telementoring of primary care providers delivering hepatitis C treatment in New York City: Results from Project INSPIRE. Learning Health Systems. 2018;2(3):e10056. doi: 10.1002/lrh2.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau U.S. of Labor Statistics. Occupational employment and wage statistics: National occupational employment and wage estimates. 2018, May. https://www.bls.gov/oes/current/oes_nat.htm Retrieved February 21, 2020, from.
- Bureau U.S. of Labor Statistics. Employer costs for employee compensation. 2019. Jan, Retrieved February 21, 2020, from https://www.bls.gov/bls/news-release/ecec.htm.
- Bureau U.S. of Labor Statistics. Consumer Price Index. 2022. Sep 13, Retrieved September 16, 2022, from https://www.bls.gov/cpi/
- Department U.S. of Veterans Affairs. Office of Procurement, Acquisition and Logistics (OPAL): Pharmaceutical prices. 2016. Retrieved March 12, 2020, from https://www.va.gov/opal/nac/fss/pharmPrices.asp.
- Williams E. C., Hahn J. A., Saitz R., Bryant K., Lira M. C., Samet J. H. Alcohol use and human immunodeficiency virus (HIV) infection: Current knowledge, implications, and future directions. Alcoholism: Clinical and Experimental Research. 2016;40(10):2056–2072. doi: 10.1111/acer.13204. [DOI] [PMC free article] [PubMed] [Google Scholar]