Abstract
In Escherichia coli, transcription of the degP locus, which encodes a heat-shock-inducible periplasmic protease, is controlled by two parallel signal transduction systems that each monitor extracytoplasmic protein physiology. For example, the heat-shock-inducible sigma factor, ςE, controls degP transcription in response to the overproduction and folded state of various extracytoplasmic proteins. Similarly, the CpxA/R two-component signal transduction system increases degP transcription in response to the overproduction of a variety of extracytoplasmic proteins. Since degP transcription is attuned to the physiology of extracytoplasmic proteins, we were interested in identifying negative transcriptional regulators of degP. To this end, we screened for null mutations that increased transcription from a strain containing a degP-lacZ reporter fusion. Through this approach, we identified null mutations in the wecE, rmlAECA, and wecF loci that increase degP transcription. Interestingly, each of these loci is responsible for synthesis of the enterobacterial common antigen (ECA), a glycolipid situated on the outer leaflet of the outer membrane of members of the family Enterobacteriaceae. However, these null mutations do not stimulate degP transcription by eliminating ECA biosynthesis. Rather, the wecE, rmlAECA, and wecF null mutations each impede the same step in ECA biosynthesis, and it is the accumulation of the ECA biosynthetic intermediate, lipid II, that causes the observed perturbations. For example, the lipid II-accumulating mutant strains each (i) confer upon E. coli a sensitivity to bile salts, (ii) confer a sensitivity to the synthesis of the outer membrane protein LamB, and (iii) stimulate both the Cpx pathway and ςE activity. These phenotypes suggest that the accumulation of lipid II perturbs the structure of the bacterial outer membrane. Furthermore, these results underscore the notion that although the Cpx and ςE systems function in parallel to regulate degP transcription, they can be simultaneously activated by the same perturbation.
In Escherichia coli, transcription of the degP gene, which encodes a heat-shock-inducible periplasmic protease, is modulated by at least two signal transduction systems that function in parallel with respect to each other. The Cpx signal transduction pathway and the ςE regulatory system each control degP transcription in response to extracytoplasmic signals (4, 6, 15, 19, 21, 22). For example, overproduction of outer membrane proteins increases ςE activity, while mutations that decrease the production of outer membrane proteins concomitantly decrease ςE activity. The signal transduction system that senses these extracytoplasmic changes has recently been defined, and it consists of an inner membrane anti-sigma factor (RseA) and a periplasmic protein (RseB) that both monitor extracytoplasmic protein physiology (7, 18). A second signal transduction system, comprised of the CpxA-CpxR two-component proteins, also regulates degP transcription in response to extracytoplasmic protein physiology (4, 6, 9, 19, 32).
To further characterize the transcriptional regulation of degP by Cpx and ςE, we screened for negative regulators of degP transcription. Through this approach, we identified null mutations in three genes of the wec gene cluster that stimulate degP transcription.
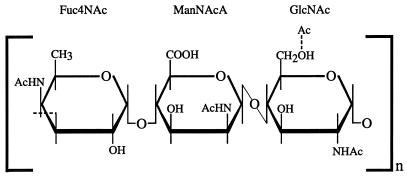
The wec gene cluster is required for the synthesis of the enterobacterial common antigen (ECA), a glycolipid found in the outer leaflet of the outer membrane in all species of the family Enterobacteriaceae (reviewed in references 11, 12, and 24). The polysaccharide portion of ECA consists of three sugar moieties: N-acetyl-d-glucosamine (GlcNAc), N-acetyl-d-mannosaminuronic acid (ManNAcA), and 4-acetamido-4,6-dideoxy-d-galactose (Fuc4NAc). These sugars form a linear trimeric repeat with the following structure: →3)α-d-Fuc4NAc-(1→4)-β-d-ManNAcA-(1→4)-α-d-GlcNAc-(1→) (Fig. 1) (13). Each polysaccharide chain is ultimately linked to phosphatidic acid via a phosphodiester linkage.
FIG. 1.
The trisaccharide repeat unit of the ECA polysaccharide moiety. This repeat unit, α-d-Fuc4NAc-(1→4)-β-d-ManNAcA-(1→4)-α-d-GlcNAc, constitutes the amino sugar polymer of ECA. N-Acetylglucosamine (GlcNAc) also serves as the attachment site for the lipid anchor. N-Acetyl-d-mannosaminuronic acid and 4-acetamido-4,6-dideoxy-d-galactose are abbreviated as ManNAcA and Fuc4NAc, respectively. The dashed line representing the bond between the number 6 oxygen and the acetyl group of GlcNAc indicates that the acetyl group is not present in stoichiometric amounts in ECA.
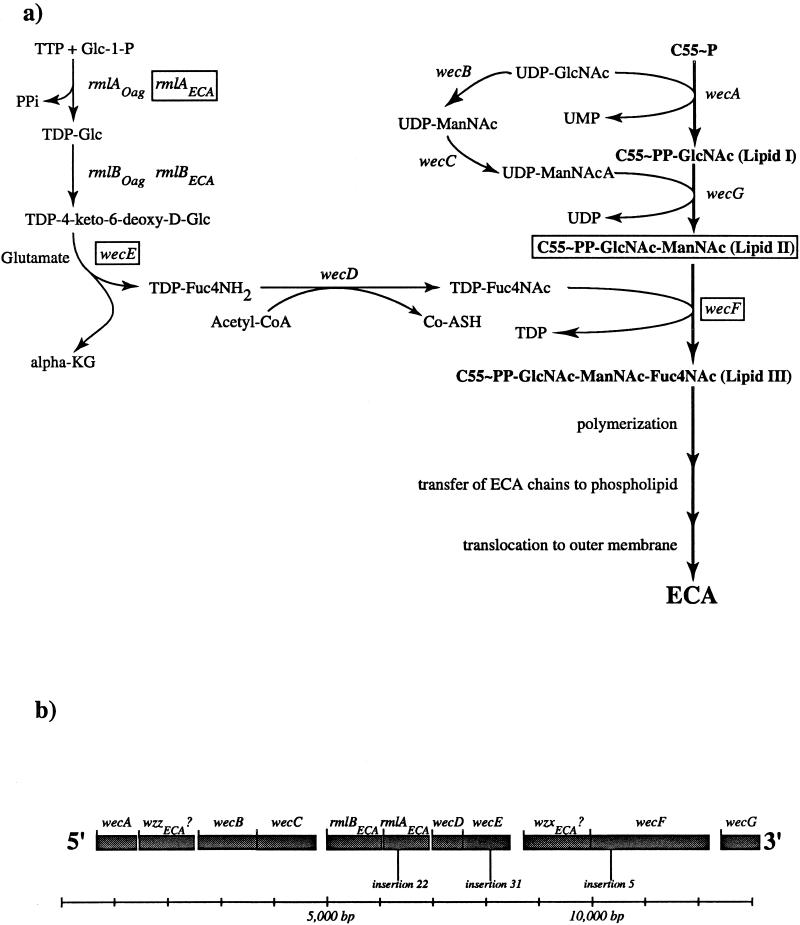
The ECA polysaccharide trimer is synthesized in stepwise fashion at the inner membrane by the successive transfer of the component amino sugars to the lipid carrier, undecaprenyl monophosphate (Fig. 2a). The first step in ECA synthesis is the transfer of GlcNAc-1-phosphate from UDP-GlcNAc to undecaprenyl monophosphate (C55-P) to yield C55-PP-GlcNAc, also known as lipid I. ManNAcA is then transferred from UDP-ManNAcA to lipid I to yield C55-PP-GlcNAc-ManNAcA (lipid II). Finally, Fuc4NAc is transferred from TDP-Fuc4NAc to lipid II to yield a complete lipid-linked trimer, C55-PP-GlcNAc-ManNAcA-Fuc4NAc (lipid III) (1, 16, 25). Subsequent steps involve polymerization, transfer of the polymer to a phospholipid aglycone, and translocation to the outer membrane. The nature and chronology of these last three events have not yet been established.
FIG. 2.
The ECA polysaccharide biosynthetic pathway and the wec gene cluster. (a) The ECA polysaccharide biosynthetic pathway. Gene names are listed in italics adjacent to the reactions that their products catalyze. The genes affected by the mutational analysis employed in this study are enclosed within rectangles. Abbreviations: C55-P, undecaprenyl monophosphate; GlcNAc, N-acetylglucosamine; ManNAcA, N-acetyl-d-mannosaminuronic acid; Fuc4NAc, 4-acetamido-4,6-dideoxy-d-galactose; Glc-1-P, glucose-1-phosphate; PPi, pyrophosphate; TDP-Glc, TDP-glucose; TDP-4-keto-6-deoxy-d-Glc, TDP-4-keto-6-deoxy-d-glucose; α-KG, α-ketoglutarate; TDP-FucN, TDP-fucosamine; acetyl-CoA, acetyl coenzyme A; Co-ASH, coenzyme A; ManNAc, N-acetyl mannose. (b) The wec gene cluster. The open reading frames of this cluster are shown in shaded boxes. All open reading frames are transcribed from 5′ to 3′ as shown. The positions of the insertions described in this study are also depicted. A 13,000-bp scale is depicted below the gene cluster for reference.
The mutations identified in this study all interfere with the conversion of lipid II to lipid III (Fig. 2). Indeed, the results described here indicate that the accumulation of lipid II stimulates degP transcription. Specifically, the lipid II-accumulating mutants can (i) confer upon E. coli a sensitivity to bile salts, (ii) confer a sensitivity to the synthesis of the outer membrane protein LamB, and (iii) stimulate both the Cpx pathway and ςE activity. These phenotypes suggest that the wec mutations perturb the structure of the bacterial outer membrane. Moreover, analysis of these mutations underscores the notion that although the Cpx and ςE systems function in parallel to regulate degP transcription, they can be simultaneously activated by the same perturbation (4, 9, 19).
MATERIALS AND METHODS
Media and reagents.
Media were prepared as described by Silhavy et al. (28). Liquid cultures were grown in Luria broth. Unless specifically noted, the final concentrations of antibiotics used in growth media were as follows: ampicillin, 50 μg/ml; spectinomycin, 50 μg/ml; and chloramphenicol, 20 μg/ml. Standard microbiological techniques were used for strain construction and bacterial growth (28). 5-Bromo-4-chloro-3-indolyl-d-galactoside (X-Gal) was purchased from Fischer.
Strains and phage.
All strain genotypes are provided in the text and figure legends. λRS88, λRS45, and λNK1324 have been described elsewhere (10, 29). The degP-lacZ and rpoHP3-lacZ operon fusions have been described elsewhere (6, 15).
Screening for negative regulators of degP transcription.
Nine independent cultures of MC4100 were infected with λNK1324 (which delivers the Tn10cam transposon) as described by Kleckner et al. (10). Infected cells were then plated on Luria agar containing chloramphenicol to select for cells carrying Tn10cam insertions. The resulting transductants were grouped into nine independent pools, with each pool containing at least 1,200 colonies.
P1vir lysates were prepared on each pool as described by Kleckner et al. (10). The P1 lysates were then used to transduce the Tn10cam insertions into PND257 (MC4100; ompR::Tn10 λRS88[degP-lacZ]). Transductants were plated on Luria agar containing chloramphenicol and 1.4 μg of X-Gal per ml. The parent strain (PND257) is phenotypically Lac− (white) on this medium, providing a simple screen for mutants with increased degP transcription. Eight Lac+ (blue) colonies were isolated from each of the nine pools and further analyzed.
The Tn10cam insertions within each Lac+ mutant were reintroduced into the parent strain (PND257) to determine whether the increase in Lac activity was due to the specific Tn10cam insertion. Those mutant strains whose increased Lac activity resulted from the Tn10cam insertion were further analyzed. These rebuilt strains were used for subsequent analyses. Each insertion mutation (except insertion 5) was numbered XY. The X value indicates the pool number (1 to 9) from which the mutation was isolated, while the Y value indicates the isolate number (1 to 8) from the given pool. Insertion 5 was isolated in a preliminary pilot experiment, and as a consequence, it was not given a designated pool number.
Determination of β-galactosidase activity.
Cells were grown overnight in Luria broth, then subcultured (1:40) into 2 ml of the same medium, and grown to mid-exponential phase. β-Galactosidase activities were determined by a microtiter plate assay (29). β-Galactosidase activities are expressed as (units/A600) × 103, where units = micromoles of product formed per minute. Assays were performed on a minimum of four independent isolates of each strain, and the results were averaged to obtain the indicated activities. Error bars indicate the standard deviations. The absence of error bars indicates that the standard deviation fell below the resolution limit of the graphing program.
Passive hemagglutination assay for the presence of ECA.
Determination of the presence of ECA was performed as described elsewhere (24).
Determination of chromosomal insertion sites of the Tn10cam insertions.
The precise sites of Tn10cam insertions were determined in the following manner. Strains carrying the wecF::cam, rmlAECA::cam, and wecE::cam mutations were subjected to arbitrarily primed PCR (2) using the CAM-5′ primer (5′ CTG ACG GGG TGG TGC GTA ACG GC 3′) and the ARB1 primer (5′ GG CCA CGC GTC GAC TAG TAC NNN NNN NNN NGA TAT 3′). The PCR products generated by these two primers were subjected to a secondary amplification step with the CAM-5′ primer and the ARB2 primer (5′ GGC CAC GCG TCG ACT AGT AC 3′). PCR products generated from the second amplification step were sequenced, and the junction between the Tn10cam sequence and the host chromosomal DNA was determined from this sequence information.
Specifically, the Tn10cam of mutant 5 (wecF::cam) is inserted between nucleotides 7863 and 7864 of the published wec gene cluster sequence (accession no. AE000455). The Tn10cam of mutant 31 (wecE::cam) is inserted between nucleotides 5707 and 5708 of the published wec gene cluster sequence, while the Tn10cam of insertion 22 (rmlAECA::cam) is situated between nucleotides 3894 and 3895 of the same sequence.
Assay for lipid II accumulation.
The incorporation of [3H]GlcNAc into lipid II was determined as previously described (26). Briefly, bacteria were grown with vigorous aeration at 37°C in 60 ml of Proteose Peptone-beef extract medium supplemented with glucose (0.2% final concentration) to an A600 of 0.4. The cells were then harvested by centrifugation, resuspended in fresh Proteose Peptone–beef extract–0.2% glucose (6 ml), and incubated at 37°C with [3H]GlcNAc (75 μCi, 5.2 Ci/mmol) for 30 min. The labeled cells were subsequently poured over crushed ice, harvested by centrifugation, and washed with cold 0.9% saline. The washed cells were then successively extracted with 95% ethanol (6 ml) and acetone (6 ml) and then dried in vacuo. The dried cells were extracted with chloroform-methanol (3:2 [vol/vol]), and the extracts were analyzed by ascending paper chromatography on EDTA-treated silica-gel-impregnated paper with chloroform-methanol-water-concentrated ammonium hydroxide (88:48:10:1 [vol/vol/vol/vol]) as the developing solvent. The material in the region of the chromatogram corresponding to lipid II was eluted, and the amount of radioactivity in this material was determined. In addition, the identity of this material as lipid II disaccharide was verified by treatment of the material with mild acid followed by gel filtration chromatography of the water-soluble fraction on a Bio-Gel P2 column.
Maltose sensitivity disc assays.
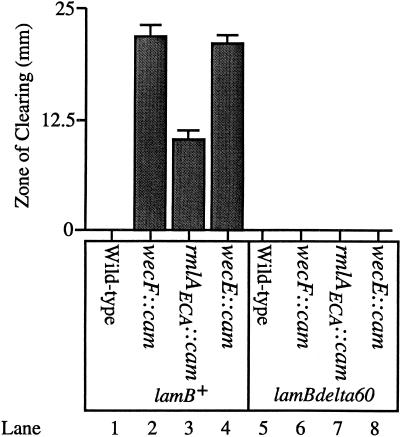
The maltose sensitivity disc assays, whose results are shown in Fig. 6, were performed as follows. Each strain was grown to saturation overnight at 37°C in 5 ml of Luria broth. Three milliliters of molten Luria top agar (55°C) was mixed with 100 μl of an overnight culture and immediately spread onto Luria agar (warmed to 23°C). The top agar was allowed to solidify for 2 min. A Schleicher and Schuell analytical paper filter disc (7-mm diameter) was then placed in the middle of the Luria agar plate. Ten microliters of 20% (wt/vol) maltose was placed on the filter disc, and the plates were incubated overnight at 37°C. The zone of clearing, which is defined as the diameter of inhibited growth minus the diameter of the filter disc, was measured 18 h after the inception of incubation. Each value shown in Fig. 6 is the average of four replicate experiments. The error bars represent the standard deviations from each average.
FIG. 6.
High-level export of the LamB maltoporin in lipid II-accumulating strains is toxic. Ten microliters of 20% maltose was added to filter discs that had been placed on lawns of the following strains: SP299 (MC4100; ompR101 zja::Tn10 linked to lamB+ λRS88[degP-lacZ]) (lane 1), SP301 (SP299; wecF::cam) (lane 2), SP303 (SP299; rmlAECA::cam) (lane 3), SP305 (SP299; wecE::cam) (lane 4), SP298 (MC4100; ompR101 zja::Tn10 linked to lamBΔ60 λRS88[degP-lacZ]) (lane 5), SP300 (SP298; wecF::cam) (lane 6), SP302 (SP298; rmlAECA::cam) (lane 7), and SP304 (SP298; wecE::cam) (lane 8). The ompR101 allele is a deletion within the ompR open reading frame, rendering the strains described in this figure null for ompR. The lamBΔ60 mutation deletes part of the coding sequence for the LamB signal sequence (8). Thus, strains containing the lamBΔ60 mutation cannot export LamB across the inner membrane. The values displayed along the y axis (zone of clearing) represent the amount of growth inhibition caused by the addition of maltose. The zone of clearing value is defined as the diameter of growth inhibition around the maltose-saturated filter disc, minus the diameter of the filter disc itself (7 mm). All strains were grown at 37°C as described in Materials and Methods.
Genetic nomenclature.
Genes and gene products involved in ECA and O-antigen synthesis and assembly are designated in accordance with the recently formulated bacterial polysaccharide gene nomenclature scheme (23). The following are the relevant new designations, each of which is accompanied by the former designation in parentheses: wbb (rfb), wec (rfe/rff), wecA (rfe), wzzECA? (o349), wecB (rffE), wecC (rffD), rmlB (o355), rmlA (o292), wecD (rffC), wecE (rffA), wzxECA? (o416), wecF (rffT), and wecG (rffM). In addition, the subscripts ECA and Oag have been included where appropriate to distinguish between homologs involved in ECA and O-antigen synthesis, respectively.
RESULTS
Rationale and mutant isolation.
We sought to identify negative regulators of degP transcription by generating null mutations that increased transcription from a degP-lacZ reporter fusion. To facilitate this analysis, we created strain PND257 (MC4100; ompR::Tn10 λRS88[degP-lacZ]), which contains the degP-lacZ fusion as well as the ompR::Tn10 null mutation. ompR null strains do not synthesize the major outer membrane proteins OmpC and OmpF (30), and consequently, ςE activity is reduced in such strains (15). Because of the reduction in ςE activity, PND257 is phenotypically Lac− (white) on Luria agar containing 1.4 μg of X-Gal per ml. This Lac− phenotype provides a simple screen for mutants with increased degP transcription. By generating null mutations throughout the chromosome of PND257, we hoped to identify mutants with increased Lac activity, and by extension, mutations that impaired the function of negative regulators of degP transcription.
We used λNK1324, which delivers the Tn10cam transposon (10), to perform transposon mutagenesis on strain PND257. The chloramphenicol-resistant colonies generated by this procedure were screened for those with increased degP transcription. Approximately 15,000 chloramphenicol-resistant colonies were screened, and 11 colonies with increased degP transcription were analyzed. Of these 11 isolates, 9 grew poorly on lactose-MacConkey agar, while the remaining two grew as well as the parent (PND257) on this medium. Since sensitivity to MacConkey agar is often an indicator of a structural perturbation in the outer membrane (20), and since degP transcription is attuned to such perturbations (15, 22, 27), we chose to analyze the nine MacConkey agar-sensitive mutants.
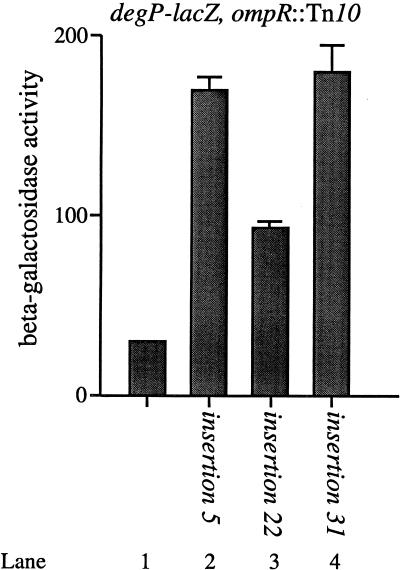
Figure 3 shows that each transposon-generated insertion increases degP transcription approximately three- to sixfold compared to the parent strain. For example, insertions 5 and 31 increase degP transcription approximately sixfold (Fig. 3, compare lanes 2 and 4 with lane 1), while insertion 22 increases degP transcription approximately threefold (Fig. 3, compare lanes 1 and 3). The six remaining transposon-generated mutations increase degP transcription to the same extent as that observed with insertions 5 and 31 (data not shown).
FIG. 3.
The Tn10cam insertions stimulate degP-lacZ transcription. The β-galactosidase activities of PND257 (MC4100; ompR::Tn10 λRS88[degP-lacZ]) (lane 1), PND788 (PND257; Tn10cam insertion 5) (lane 2), PND789 (PND257; Tn10cam insertion 22) (lane 3), and PND790 (PND257; Tn10cam insertion 31) (lane 4) were determined. Tn10cam insertions 5 and 31 each stimulate degP-lacZ transcription approximately sixfold, while insertion 22 stimulates degP-lacZ transcription approximately threefold compared to the parent strain. All strains were grown as described in Materials and Methods.
Null mutations within the wec gene cluster stimulate degP transcription.
The transposon insertions in each of the MacConkey agar-sensitive isolates were all tightly linked to the wec gene cluster, which is located at approximately 85 min on the E. coli chromosome. Since the wec gene cluster is involved in the biosynthesis of ECA, we were interested in determining whether the Tn10cam insertions affected the biosynthesis of ECA. Using a passive hemagglutination assay for ECA biosynthesis (25), we determined that eight of the nine insertions did not produce ECA (ECA−). Only one insertion (no. 22) remained ECA+. Interestingly, insertion 22 causes the weakest induction of degP transcription (Fig. 3).
Fine-structure mapping of each insertion using previously described Tn10 insertions within the wec gene cluster (16) indicated that five of the nine Tn10cam insertions (no. 5, 41, 45, 47, and 51) were tightly linked to the wecE locus. In addition, three of the nine Tn10cam insertions (no. 31, 32, and 33) were tightly linked to the wecE locus, while the remaining insertion (no. 22) was tightly linked to the wecD locus (Fig. 2b).
Using this linkage information, we then determined the precise sites of insertion of three representative Tn10cam insertions (no. 5, 22, and 31). The Tn10cam of mutant 5 is inserted within the 140th codon of the wecF open reading frame. The Tn10cam of mutant 31 is situated within the 215th codon of the wecE coding sequence, while the Tn10cam of mutant 22 is inserted within the 123rd codon of rmlAECA (see Materials and Methods for the precise nucleotide insertion sites). Thus, our screen identified three distinct loci in the wec gene cluster, wecF, wecE, and rmlAECA. Accordingly, Tn10cam insertions 5, 22, and 31 are referred to as wecF::cam, rmlAECA::cam, and wecE::cam, respectively.
The rmlAECA::cam mutation.
The rmlAECA::cam mutation was anomalous in two major regards. First, unlike the other eight mutations, the rmlAECA::cam mutation did not confer an ECA− phenotype, although it mapped within the wec gene cluster. Second, the rmlAECA::cam mutation conferred a relatively weak increase in degP transcription (approximately 50% of that observed with the other Tn10cam insertions).
A potential explanation of the phenotypes conferred by the rmlAECA::cam mutation was provided by previously described biochemical analysis of the rmlAECA gene product (14). Specifically, the predicted rmlAECA gene product has 65% identity with RmlAOag, a glucose-1-phosphate thymidylyltransferase that plays a role in the initial stages of TDP-Fuc4NAc biosynthesis (33). Marolda and Valvano (14) have demonstrated that the rmlAECA gene product does indeed display glucose-1-phosphate thymidylyltransferase activity, thus confirming what the sequence homology between RmlAECA and RmlAOag had suggested. Given the activity of the rmlAECA gene product, we predicted that a lesion in this locus would reduce production of TDP-glucose and, hence, slow Fuc4NAc synthesis (Fig. 2a). As a consequence, the rmlAECA::cam mutation would slow the production of ECA by impeding the conversion of lipid II to lipid III. However, since a functional rmlAOag gene product is still present in the E. coli chromosome (33), Fuc4NAc synthesis and ECA synthesis would be reduced but not abolished. According to this model, a lesion in rmlAECA would (i) not confer an ECA− phenotype and (ii) display an attenuated increase in degP transcription compared with the wecF and wecE mutations, which completely abolish the synthesis of lipid III. These predictions are borne out in the data described above (Fig. 3).
One additional prediction is that all of the Tn10cam insertions should impede the conversion of lipid II to lipid III (Fig. 2). Indeed, analysis of the PND788 (PND257; wecF::cam), PND789 (rmlAECA::cam), and PND790 (wecE::cam) strains shows that they accumulate significant amounts of lipid II, while their parent strain, PND257, does not accumulate lipid II in detectable quantities (Table 1). This information indicates that (i) the mutations in wecF and wecE are loss-of-function mutations and (ii) this study provides the first evidence indicating that the rmlAECA gene product is actually involved in ECA biosynthesis.
TABLE 1.
Accumulation of lipid II in Tn10cam insertion mutants defective in the synthesis of lipid III
| Strain | Relevant genotype | Accumulation of lipid IIa |
|---|---|---|
| PND257 | Parent | − |
| PND788 | wecF::Tn10cam | + |
| PND789 | rmlAECA::Tn10cam | + |
| PND790 | wecE::Tn10cam | + |
+, >1,000 cpm of [3H]GlcNAc-labeled lipid II was recovered from extracts prepared from approximately 108 cells. −, [3H]GlcNAc-labeled lipid II was not detectable in extracts prepared from approximately 108 cells.
The accumulation of the lipid II ECA biosynthetic intermediate increases degP transcription and confers sensitivity to bile salts.
From the data presented, it is clear that all of the mutations identified in our screen affect the same general step in the biosynthesis of ECA. Specifically, the insertions within wecF and wecE cause the accumulation of lipid II while the rmlAECA::cam mutation impedes the conversion of lipid II to lipid III (Fig. 2a).
What is not clear, however, is whether it is the absence of ECA or the accumulation of the lipid II intermediate that stimulates degP transcription and confers sensitivity to MacConkey agar. Various tests of epistasis were performed to distinguish between these two possibilities. For example, the wecA::Tn10 mutation was introduced into the wecF::cam, wecE::cam, and rmlAECA::cam strains, and the resulting amounts of degP transcription were determined (Fig. 4). The wecA::Tn10 mutation blocks ECA synthesis at its earliest step, preventing the transfer of GlcNAc phosphate from UDP-GlcNAc to undecaprenyl monophosphate (Fig. 2a). If the lack of ECA is the cause of the observed phenotypes, the wecA::Tn10 wecE::cam double mutant strain should display at least the same degree of degP transcriptional induction and MacConkey agar sensitivity as observed with the wecE::cam mutation alone. If the accumulation of lipid II is responsible for the transcriptional induction of degP, then the double mutant strain should not display the transcriptional induction observed with the single chloramphenicol-resistant insertion mutations. These predictions also hold true for the wecF::cam and rmlAECA::cam mutant strains.
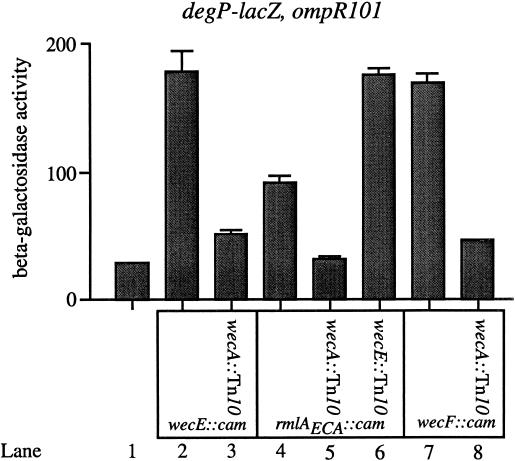
FIG. 4.
The accumulation of lipid II stimulates transcription of degP-lacZ. β-Galactosidase activities were determined for the following strains: PND423 (MC4100; ompR101 λRS88[degP-lacZ]) (lane 1), SP173 (PND423; wecE::cam) (lane 2), SP190 (PND423; wecE::cam wecA::Tn10) (lane 3), SP172 (PND423; rmlAECA::cam) (lane 4), SP185 (PND423; rmlAECA::cam wecA::Tn10) (lane 5), SP188 (PND423; rmlAECA::cam wecE::Tn10) (lane 6), SP171 (PND423; wecF::cam) (lane 7), and SP179 (PND423; wecF::cam wecA::Tn10) (lane 8). The wecA::Tn10 mutation blocks the transcriptional induction of degP-lacZ that is conferred by the wecE::cam, rmlAECA::cam, and wecF::cam insertions (compare lanes 2 and 3, 4 and 5, and 7 and 8). The weak transcriptional induction of degP-lacZ conferred by the rmlAECA::cam mutation can be increased by the wecE::Tn10 mutation (compare lanes 4 and 6). The ompR101 mutation is a deletion within the ompR open reading frame, rendering the strains described in this figure null for ompR. All strains were grown as described in Materials and Methods.
Figure 4 indicates that the accumulation of lipid II is responsible for the observed phenotypes. For example, when the wecA::Tn10 mutation is introduced into strains containing either the wecE::cam, rmlAECA::cam, or wecF::cam mutation, the double mutant strain shows only a minimal induction of degP transcription (Fig. 4, compare lanes 2 and 3, 4 and 5, and 7 and 8). In addition, these double mutant strains are no longer MacConkey agar sensitive.
Finally, based on the biochemical analysis of RmlAECA (14) and on the homology between the rmlAECA gene product and the RmlAOag protein, we have suggested that the rmlAECA::cam mutation attenuates, but does not abolish, the conversion of lipid II to lipid III. This notion explains the weak induction of degP transcription conferred by this mutation, and it also explains why the rmlAECA::cam lesion does not confer an ECA− phenotype. If this model is correct, introduction of the wecE::Tn10 mutation into a strain that contains the rmlAECA::cam insertion should raise degP transcription to the level observed with the wecE mutation alone. Again, this prediction is verified. Specifically, the rmlAECA wecE double mutant strain displays the same degree of transcriptional induction of degP as the wecE mutation does in isolation (compare lanes 2, 4, and 6 in Fig. 4).
There are two general conclusions that can be drawn from Fig. 4. First, the accumulation of lipid II stimulates degP transcription and confers MacConkey agar sensitivity in these strains. Second, the rmlAECA::cam mutation impedes, but does not abolish, the conversion of lipid II to lipid III in the biosynthesis of ECA.
The induction of degP transcription by accumulation of the lipid II intermediate is decreased in an ompR+ background.
Since the experiments described above have all utilized strains that were ompR null, we were also interested in determining the effects of lipid II accumulation in an ompR+ background. Figure 5 shows that while the wec mutations still stimulate degP transcription in the ompR+ background, the magnitude of the induction of degP transcription conferred by these mutations is significantly reduced compared to the analogous ompR strains. These ompR+, lipid II-accumulating strains are also no longer as sensitive to growth on MacConkey agar as their ompR counterparts.
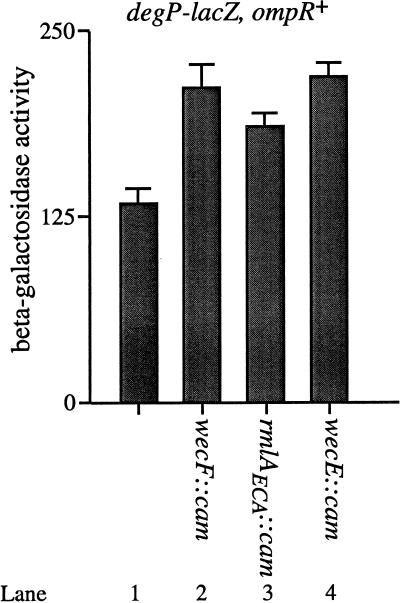
FIG. 5.
Accumulation of lipid II stimulates transcription of degP-lacZ in the ompR+ background. β-Galactosidase activities were determined for the following strains: PND2000 (MC4100; λRS88[degP-lacZ]) (lane 1), SP332 (PND2000; wecF::cam) (lane 2), SP333 (PND2000; rmlAECA::cam) (lane 3), and SP334 (PND2000; wecE::cam) (lane 4). The wecF::cam and wecE::cam mutations stimulate degP-lacZ transcription approximately 1.6-fold. The rmlAECA::cam mutation stimulates degP-lacZ transcription approximately 1.4-fold compared to the parent strains. All strains were grown as described in Materials and Methods.
Since the major proteins whose synthesis is controlled by OmpR are the outer membrane proteins OmpF and OmpC, it seemed likely that the enhanced degP transcriptional induction observed in the ompR background was due to the lack of these proteins. Consistent with this observation, production of OmpC in an ompR, lipid II-accumulating background reduced the magnitude of transcriptional induction of the degP locus (data not shown). Thus, at a minimum, it is the lack of OmpC that heightens the MacConkey agar sensitivity and the increase in degP transcription that is observed in the lipid II-accumulating strains.
Accumulation of the lipid II intermediate can confer a sensitivity to high-level synthesis of the outer membrane protein LamB.
During the course of this study, we noted that the lipid II-accumulating mutant strains were exquisitely sensitive to growth on maltose and maltodextrins. As a consequence, we wanted to determine if these strains had difficulty growing in the presence of all types of sugars or whether their sensitivity was restricted to growth in the presence of maltose and its oligomers. To this end, we assayed the growth of the lipid II-accumulating strains on Luria agar in the presence of high concentrations of maltose, lactose, and glucose. The lipid II-accumulating strains were sensitive only to maltose, indicating that this sensitivity is not simply a sugar-mediated effect (data not shown).
Since the various phenotypes described for these strains appear to be associated with perturbations in the outer membrane, the observed maltose sensitivity might be due to high-level synthesis and export of the outer membrane porin LamB (LamB synthesis is induced in the presence of maltose). To address this issue, the lamBΔ60 mutation, which deletes a portion of the LamB signal sequence (and prevents its export across the inner membrane [8]), was introduced into the lipid II-accumulating strains. We then determined if these lamBΔ60 strains were also sensitive to high levels of maltose. Figure 6 indicates that the lamBΔ60 mutation abolishes the sensitivity of the lipid II-accumulating strains to high concentrations of maltose (compare lanes 2 and 6, 3 and 7, and 4 and 8). Thus, the maltose sensitivity observed with these strains is due to high-level export of wild-type LamB, further suggesting that the accumulation of lipid II perturbs the physiology of the outer membrane.
Accumulation of lipid II does not interfere with incorporation or assembly of LamB in the outer membrane.
Because of the observed toxicity associated with high-level synthesis of LamB in the lipid II-accumulating mutants, we wanted to determine if these strains displayed defects in the incorporation and assembly of LamB into the outer membrane. To address this issue, we performed two experiments. (i) We examined the amount of LamB protein associated with membrane fractions of a parental strain, PND257 (MC4100; ompR::Tn10 λRS88[degP-lacZ]), as well as derivative strains that accumulate lipid II, PND788 (PND257; wecF::cam), PND789 (PND257; rmlAECA::cam), and PND790 (PND257; wecE::cam). (ii) We also examined the kinetics of LamB trimerization in PND257, PND788, PND789, and PND790. None of the lipid II-accumulating strains displayed defects in the incorporation of LamB into membrane fractions, nor did these mutants display defects in the trimerization of LamB (data not shown). Thus, the toxicity associated with high-level synthesis of LamB in lipid II-accumulating mutants is not the result of a gross structural defect in LamB assembly.
The wecF::cam, wecE::cam, and rmlAECA::cam mutations increase ςE activity.
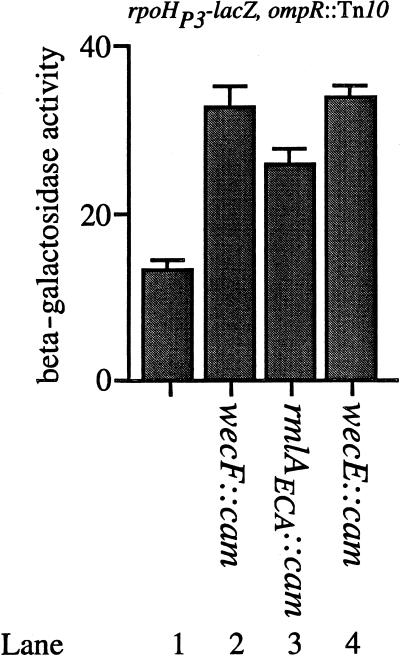
Finally, we were interested in the mechanism(s) by which degP transcription was being stimulated as a result of lipid II accumulation. Two regulatory pathways are known to modulate degP transcription. As mentioned elsewhere, degP transcription can be increased by increasing ςE activity, through the RseA/B signal transduction system (15). In addition, degP transcription is also regulated by the Cpx two-component signal transduction pathway (6, 22), which functions in parallel with the ςE signal transduction pathway (4, 6). To determine the specific route by which the wecF::cam, rmlAECA::cam, and wecE::cam mutations functioned to increase degP transcription, we introduced each insertion into SP245 (MC4100; ompR::Tn10 rpoHP3-lacZ). The rpoHP3 promoter is recognized solely by RNA polymerases containing the ςE subunit. Hence, this fusion provides an assay for only ςE activity (6, 15). Figure 7 shows that rpoHP3-lacZ transcription is induced by the Tn10cam insertions in a qualitatively similar fashion as that observed with the induction of degP transcription. For example, the wecF::cam and wecE::cam insertions stimulate rpoHP3-lacZ transcription approximately 2.6-fold (Fig. 7, compare lane 1 with lanes 2 and 4). The rmlAECA::cam insertion stimulates rpoHP3-mediated transcription approximately 2.3-fold (compare lanes 1 and 3 of Fig. 7).
FIG. 7.
The wecF::cam, rmlAECA::cam, and wecE::cam mutations stimulate ςE activity. The β-galactosidase activities of SP245 (MC4100; ompR::Tn10 λRS45[rpoHP3-lacZ]) (lane 1), SP282 (SP245; wecF::cam) (lane 2), SP283 (SP245; rmlAECA::cam) (lane 3), and SP284 (SP245; wecE::cam) (lane 4) were determined. The wecF::cam and wecE::cam mutations stimulate rpoHP3-lacZ transcription approximately 2.6-fold. The rmlAECA::cam mutation stimulates rpoHP3-lacZ transcription approximately 2.3-fold. All strains were grown as described in Materials and Methods.
The wecF, wecE, and rmlAECA mutations activate the Cpx signal transduction pathway.
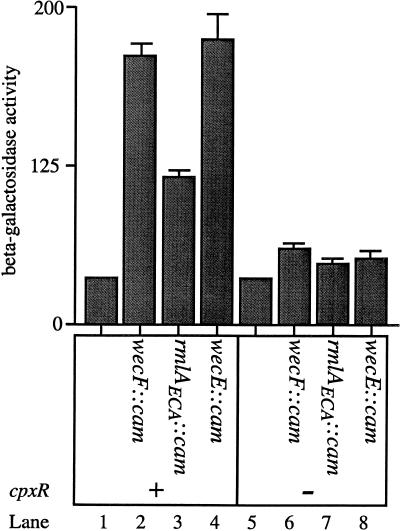
The results presented in Fig. 7 demonstrate that the wecF, rmlAECA, and wecE mutations activate degP transcription, at least in part by stimulating ςE activity. However, we were also interested in determining if these mutations stimulated degP transcription via the Cpx pathway as well. Accordingly, the cpxR null mutation was introduced into the parent strain (PND257) as well as the wecF, rmlAECA, and wecE mutant strains (PND788, PND789, and PND790, respectively), and the amount of degP-lacZ transcription generated from these strains was quantified. Interestingly, elimination of the Cpx pathway by a cpxR null mutation decreases, but does not abolish, the transcriptional induction of degP conferred by the wecF, rmlAECA, and wecE mutations (Fig. 8). For example, in a cpxR background, the wecF::cam and wecE::cam mutations stimulate degP transcription only 1.6- to 1.7-fold (Fig. 8, compare lane 5 with lanes 6 and 8). Similarly, the rmlAECA::cam mutation stimulates degP transcription approximately 1.4-fold in the cpxR background (Fig. 8, compare lanes 5 and 7). Although the transcriptional induction of degP is qualitatively similar in the cpxR+ and cpxR background, CpxR is clearly responsible for the majority of the degP transcriptional induction in the parental background (PND257). For instance, the wecF::cam mutation stimulates degP transcription approximately 6-fold in the cpxR+ background, but only 1.7-fold in the cpxR background (Fig. 8, compare lanes 1 and 2 with lanes 5 and 6).
FIG. 8.
The wecF::cam, rmlAECA::cam, and wecE::cam mutations activate the Cpx signal transduction pathway. The β-galactosidase activities of PND257 (MC4100 ompR::Tn10 λRS88[degP-lacZ]) (lane 1), PND788 (PND257 wecF::cam) (lane 2), PND789 (PND257 rmlAECA::cam) (lane 3), PND790 (PND257 wecE::cam) (lane 4), SP150 (PND257 cpxR::spec) (lane 5), SP231 (SP150 wecF::cam) (lane 6), SP232 (SP150 rmlAECA::cam) (lane 7), and SP233 (SP150 wecE::cam) (lane 8) were determined. The wecF::cam and wecE::cam mutations stimulate degP-lacZ transcription 1.6- to 1.7-fold in the absence of CpxR. Similarly, the rmlAECA::cam mutation stimulates degP-lacZ transcription approximately 1.4-fold in the absence of CpxR. This is in contrast to the three- to sixfold stimulation conferred by these mutations in the presence of CpxR (lanes 1 to 4). All strains were grown as described in Materials and Methods.
Since the transcriptional induction of degP is partially blocked by the cpxR null mutation, and since the wecF, rmlAECA, and wecE mutations also increase transcription of rpoHP3-lacZ, these mutations must activate both signal transduction pathways that are known to control degP transcription.
DISCUSSION
In our search for negative regulators of degP transcription, we have shown that mutations that cause the accumulation of the lipid II intermediate in the pathway for ECA biosynthesis cause several envelope-associated perturbations. First, these mutations confer a sensitivity to growth in the presence of bile salt detergents, which is a classic indicator of an outer membrane permeability defect (20). Second, these mutations confer a sensitivity to high-level export of the wild-type LamB porin, also suggesting a perturbation in outer membrane physiology. Third, these mutations increase degP transcription by stimulating both the ςE modulatory signal transduction system (15) and the Cpx signal transduction system (6, 22). Since degP encodes a periplasmic protease that destroys misfolded extracytoplasmic proteins (see reference 17), this is also an indicator of a perturbation in the physiology of periplasmic and/or outer membrane proteins. Taken together, these results imply that lipid II accumulation perturbs the physiology of envelope proteins, thus causing an increase in degP transcription.
Accumulation of lipid II.
The results presented in Fig. 4 demonstrate that it is the accumulation of lipid II that stimulates degP transcription. Moreover, previous studies have also noted a toxicity associated with the accumulation of lipid II. For example, Rick et al. (26) have observed that Salmonella typhimurium strains containing a lesion in rmlAOag (which encodes a homolog of RmlAECA) accumulate the lipid II intermediate and display sensitivity to sodium dodecyl sulfate (SDS). This SDS-sensitive phenotype can be suppressed by mutations that block the accumulation of lipid II.
Despite the body of evidence indicating that lipid II accumulation confers a host of envelope-associated defects, it is unclear why lipid II accumulation exerts these effects. While ECA is not essential for the viability of E. coli, the undecaprenyl carrier lipid that is used to synthesize the ECA trisaccharide is essential. We considered the possibility that lipid II accumulation indirectly impeded the synthesis of the peptidoglycan layer and/or lipopolysaccharide by sequestering undecaprenyl monophosphate (C55-P). However, overproduction of the BacA protein, which is believed to increase the pool of free C55-P (3), had no ameliorative effect on the lipid II-associated phenotypes (6a). Thus, it seems unlikely that the accumulation of lipid II exerts its effects by sequestering C55-P.
An alternative model posits that the partially completed ECA trisaccharide may interfere with the biogenesis of envelope proteins, thus altering the permeability of the outer membrane and signaling for increased levels of the envelope protease, DegP. This interference would most likely occur at or near the inner membrane since the lipid II disaccharide remains attached to its undecaprenyl carrier lipid in the inner membrane. According to this view, the accumulation of lipid II in the inner membrane affects some process(es) that is important for outer membrane biogenesis.
The involvement of rmlAECA in ECA biosynthesis.
The results presented here also represent the first mutational analysis of rmlAECA. Previous studies that have sought mutations in genes involved in ECA biosynthesis have uncovered several loci in the wec and wbb gene clusters. For example, mutations in wecB, wecC, wecD, wecE, wecF, and wecG have all been identified because of their abolition of ECA biosynthesis (Fig. 2) (16).
Indeed, rmlAOag mutations (Fig. 2a) have also been shown to radically reduce (but not abolish) ECA biosynthesis in S. typhimurium (26). However, despite the homology between RmlAECA and RmlAOag, no mutations were ever identified in rmlAECA. Based on the previous analyses and on the results presented here, we suggest that RmlAECA and RmlAOag perform redundant functions for the biosynthesis of ECA. The reasons for suggesting this are threefold. First, RmlAECA and RmlAOag have 65% amino acid sequence identity, and they each display glucose-1-phosphate thymidylyltransferase activity (14). Second, rmlAOag null strains do not completely abolish ECA biosynthesis in S. typhimurium. Finally, Fig. 4 clearly demonstrates that the accumulation of lipid II stimulates degP transcription. If RmlAECA partially contributes to the conversion of lipid II to lipid III, then inactivation of this locus should display an attenuated increase in degP transcription and should remain ECA+. Moreover, when the rmlAECA null mutation is combined with a second mutation that completely abolishes the conversion of lipid II to lipid III (i.e., wecE), the second mutation should raise the transcriptional induction of degP from the attenuated response observed with only the rmlAECA mutation to the strong induction observed with the wecE mutation. All of these predictions are verified by the results presented here.
Thus, although the rmlAECA locus appears to be involved in the biosynthesis of ECA, its identification in this role has been lacking because mutational inactivation of this locus is phenotypically masked by its functional homolog, rmlAOag.
The lack of porin enhances the susceptibility of E. coli to lipid II accumulation.
From the results of Fig. 3 and 5, it is clear that strains lacking the outer membrane porins OmpF and OmpC are more susceptible to the toxic effects of lipid II accumulation. The reasons for this enhanced susceptibility are at present unclear. It is possible that the lack of OmpF and OmpC directly alters the structure of the outer membrane, perhaps making this membrane more susceptible to the perturbations caused by the accumulation of lipid II. For example, lipid II accumulation may increase the ability of the outer membrane to be solubilized by bile salts when OmpF and OmpC are absent.
Alternatively, we note that the lack of porins in an ompR background decreases the expression of the ςE regulon (including degP) by approximately fourfold (15). Since ςE regulation is involved in responding to extracytoplasmic protein stresses, ompR strains (i.e., ςE attenuated) may not be equipped to properly cope with large-scale perturbations, such as those caused by the accumulation of lipid II. However, since the precise biochemical effect(s) of lipid II accumulation is not known, we cannot at present distinguish among these possibilities.
The export-associated toxicity of LamB.
To our knowledge, this study describes the first instance in which export of the wild-type LamB protein is toxic. The requirement for export of LamB is specific, as expression of the nonexportable LamBΔ60 mutant is not toxic. Interestingly, this toxicity is not due to a gross structural defect in the assembly of LamB. However, we cannot exclude the possibility that a minor structural alteration in LamB assembly (not detectable by membrane fractionation or trimerization studies) confers this toxicity. Alternatively, the transit of large amounts of LamB protein en route to the outer membrane may confer the toxicity observed in the lipid II-accumulating strains. Suppressor analysis may ultimately be informative as to the precise molecular nature of this toxicity.
Transcriptional induction of degP.
Finally, we note that the lipid II-mediated induction of degP transcription is mediated by increases in both Cpx and ςE activity (Fig. 7 and 8).
While lipid II accumulation manifests several phenotypes, all of these phenotypes intimate a perturbation in the structure of the outer membrane (e.g., SDS sensitivity, bile salt sensitivity, toxicity of export of LamB, and increased transcription of degP). The fact that lipid II accumulation stimulates both signal transduction pathways that control degP transcription further supports the hypothesis that both of these signal transduction pathways monitor extracytoplasmic stress (5, 6, 9, 15, 32). Moreover, the activation of both pathways indicates that under some circumstances both Cpx and ςE are affected by the same types of extracytoplasmic stresses.
ACKNOWLEDGMENTS
We thank Susan DiRenzo for manuscript preparation and members of the Silhavy laboratory (especially Scott Hande) for comments and suggestions throughout the course of this work.
P.N.D. gratefully acknowledges support from a National Institutes of Health (NIH) training grant (GM07388). This work was supported by an NIGMS grant to T.J.S. (GM34821) and to P.D.R. (GM52882).
REFERENCES
- 1.Barr K, Rick P D. Biosynthesis of enterobacterial common antigen in Escherichia coli. In vitro synthesis of lipid-linked intermediates. J Biol Chem. 1987;262:7142–7150. [PubMed] [Google Scholar]
- 2.Caetano-Annoles G. Amplifying DNA with arbitrary oligonucleotide primers. PCR Methods Appl. 1993;3:85–92. doi: 10.1101/gr.3.2.85. [DOI] [PubMed] [Google Scholar]
- 3.Cain B D, Norton P J, Eubanks W, Nick H S, Allen C M. Amplification of the bacA gene confers bacitracin resistance to Escherichia coli. J Bacteriol. 1993;175:3784–3789. doi: 10.1128/jb.175.12.3784-3789.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connolly L, de las Peñas A, Alba B M, Gross C A. The response to extracytoplasmic stress in Escherichia coli is controlled by partially overlapping pathways. Genes Dev. 1997;11:2012–2021. doi: 10.1101/gad.11.15.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cosma C L, Danese P N, Carlson J H, Silhavy T J, Snyder W B. Activation of the Cpx two-component signal transduction pathway in Escherichia coli suppresses envelope-associated stresses. Mol Microbiol. 1995;18:491–505. doi: 10.1111/j.1365-2958.1995.mmi_18030491.x. [DOI] [PubMed] [Google Scholar]
- 6.Danese P N, Snyder W B, Cosma C L, Davis L J B, Silhavy T J. The Cpx two-component signal transduction pathway of Escherichia coli regulates transcription of the gene specifying the stress-inducible periplasmic protease, DegP. Genes Dev. 1995;9:387–398. doi: 10.1101/gad.9.4.387. [DOI] [PubMed] [Google Scholar]
- 6a.Danese, P. N., G. R. Oliver, and T. J. Silhavy. Unpublished observation.
- 7.de las Peñas A, Connolly L, Gross C A. The ςE-mediated response to extracytoplasmic stress in Escherichia coli is transduced by RseA and RseB, two negative regulators of ςE. Mol Microbiol. 1997;24:373–385. doi: 10.1046/j.1365-2958.1997.3611718.x. [DOI] [PubMed] [Google Scholar]
- 8.Emr S D, Silhavy T J. Mutations affecting localization of an Escherichia coli outer membrane protein, the bacteriophage lambda receptor. J Mol Biol. 1980;141:63–90. doi: 10.1016/s0022-2836(80)80029-5. [DOI] [PubMed] [Google Scholar]
- 9.Jones C H, Danese P N, Pinkner J S, Silhavy T J, Hultgren S J. The chaperone-assisted membrane release and folding pathway is sensed by two signal transduction systems. EMBO J. 1997;16:6394–6406. doi: 10.1093/emboj/16.21.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleckner N, Bender J, Gottesman S. Uses of transposons with emphasis on Tn10. Methods Enzymol. 1991;204:139–180. doi: 10.1016/0076-6879(91)04009-d. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn H M, Meier-Dieter U, Mayer H. ECA, the enterobacterial common antigen. FEMS Microbiol Rev. 1988;4:195–222. doi: 10.1111/j.1574-6968.1988.tb02743.x. [DOI] [PubMed] [Google Scholar]
- 12.Mäkelä P H, Mayer H. Enterobacterial common antigen. Bacteriol Rev. 1976;40:591–632. doi: 10.1128/br.40.3.591-632.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Männel D, Mayer H. Isolation and chemical characterization of the enterobacterial common antigen. Eur J Biochem. 1978;86:361–370. doi: 10.1111/j.1432-1033.1978.tb12318.x. [DOI] [PubMed] [Google Scholar]
- 14.Marolda C L, Valvano M A. Genetic analysis of the dTDP-rhamnose biosynthesis region of the Escherichia coli VW187 (O7:K1) rfb gene cluster: identification of functional homologs of rfbB and rfbA in the rff cluster and correct location of the rffE gene. J Bacteriol. 1995;177:5539–5546. doi: 10.1128/jb.177.19.5539-5546.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mecsas J, Rouvière P E, Erickson J W, Donohue T J, Gross C A. The activity of ςE, an Escherichia coli heat-inducible ς-factor, is modulated by expression of outer membrane proteins. Genes Dev. 1993;7:2618–2628. doi: 10.1101/gad.7.12b.2618. [DOI] [PubMed] [Google Scholar]
- 16.Meier-Dieter U, Starman R, Barr K, Mayer H, Rick P D. Biosynthesis of enterobacterial common antigen in Escherichia coli. Biochemical characterization of Tn10 insertion mutants defective in enterobacterial common antigen synthesis. J Biol Chem. 1990;265:13490–13497. [PubMed] [Google Scholar]
- 17.Misra R, Peterson A, Ferenci T, Silhavy T J. A genetic approach for analyzing the pathway of LamB assembly into the outer membrane of Escherichia coli. J Biol Chem. 1991;266:13592–13597. [PubMed] [Google Scholar]
- 18.Missiakas D, Mayer M P, Lemaire M, Georgopoulos C, Raina S. Modulation of the Escherichia coli ςE (RpoE) heat-shock transcription-factor activity by the RseA, RseB and RseC proteins. Mol Microbiol. 1997;24:355–371. doi: 10.1046/j.1365-2958.1997.3601713.x. [DOI] [PubMed] [Google Scholar]
- 19.Missiakas D, Raina S. Signal transduction pathways in response to protein misfolding in the extracytoplasmic compartments of E. coli: role of two new phosphoprotein phosphatases PrpA and PrpB. EMBO J. 1997;16:1670–1685. doi: 10.1093/emboj/16.7.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pogliano J, Lynch A S, Belin D, Lin E C, Beckwith J. Regulation of Escherichia coli cell envelope proteins involved in protein folding and degradation by the Cpx two-component system. Genes Dev. 1997;11:1169–1182. doi: 10.1101/gad.11.9.1169. [DOI] [PubMed] [Google Scholar]
- 22.Raina S, Missiakas D, Georgopoulos C. The rpoE gene encoding the sigma E (sigma 24) heat shock sigma factor of Escherichia coli. EMBO J. 1995;14:1043–1055. doi: 10.1002/j.1460-2075.1995.tb07085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeves P R, Hobbs M, Valvano M A, Skurnik M, Whitfield C, Coplin D, Kido N, Klena J, Maskell D, Raetz C, Rick P D. Bacterial polysaccharide synthesis and gene nomenclature. Trends Microbiol. 1996;4:495–503. doi: 10.1016/s0966-842x(97)82912-5. [DOI] [PubMed] [Google Scholar]
- 24.Rick P D, Silver R P. Enterobacterial common antigen and capsular polysaccharides. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 104–122. [Google Scholar]
- 25.Rick P D, Mayer H, Neumeyer B A, Wolski S, Bitter-Suermann D. Biosynthesis of enterobacterial common antigen. J Bacteriol. 1985;162:494–503. doi: 10.1128/jb.162.2.494-503.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rick P D, Wolski S, Barr K, Ward S, Ramsay-Sharer L. Accumulation of a lipid-linked intermediate in enterobacterial common antigen synthesis in mutants lacking dTDP-glucose pyrophosphorylase. J Bacteriol. 1988;170:4008–4014. doi: 10.1128/jb.170.9.4008-4014.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rouvière P E, de las Peñas A, Mecsas J, Lu C Z, Rudd K E, Gross C A. rpoE, the gene encoding the second heat-shock sigma factor, ςE, in Escherichia coli. EMBO J. 1995;14:1032–1042. doi: 10.1002/j.1460-2075.1995.tb07084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1984. [Google Scholar]
- 29.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusion. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 30.Slauch J M, Silhavy T J. cis-acting ompF mutations that result in OmpR-dependent constitutive expression. J Bacteriol. 1991;173:4039–4048. doi: 10.1128/jb.173.13.4039-4048.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slauch J M, Garrett S, Jackson D E, Silhavy T J. EnvZ functions through OmpR to control porin gene expression in Escherichia coli K-12. J Bacteriol. 1988;170:439–441. doi: 10.1128/jb.170.1.439-441.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snyder W B, Davis L J B, Danese P N, Cosma C L, Silhavy T J. Overproduction of NlpE, a new outer-membrane lipoprotein, suppresses the toxicity of periplasmic LacZ by activation of the Cpx signal transduction pathway. J Bacteriol. 1995;177:4216–4223. doi: 10.1128/jb.177.15.4216-4223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevenson G, Neal B, Liu D, Hobbs M, Packer N H, Batley M, Redmond J W, Lindquist L, Reeves P. Structure of the O antigen of Escherichia coli K-12 and the sequence of its rfb gene cluster. J Bacteriol. 1994;176:4144–4156. doi: 10.1128/jb.176.13.4144-4156.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]