Abstract
Myeloid malignancies, a group of hematopoietic disorders that includes acute myeloid leukemia (AML), myelodysplastic syndromes (MDS), and myeloproliferative neoplasms (MPN), are caused by the accumulation of genetic and epigenetic changes in hematopoietic stem and progenitor cells over time. Despite the relatively low number of genomic drivers compared to other forms of cancer, the process by which these changes shape the genomic architecture of myeloid malignancies remains elusive. Recent advancements in clonal hematopoiesis research and the use of cutting-edge single-cell technologies have shed new light on the developmental process of myeloid malignancies. This review aims to delve into the intricacies of clonal evolution in myeloid malignancies and its implications for the development of new diagnostic and therapeutic approaches.
Clonal evolution in myeloid malignancies
Myeloid malignancies are a group of hematopoietic disorders characterized by the aberrant proliferation and differentiation of hematopoietic stem or progenitor cells (HSPCs) [1]. The common types of myeloid malignancies include acute myeloid leukemia (AML), myelodysplastic syndromes (MDS), and myeloproliferative neoplasms (MPNs) but also include other rare disorders such as chronic myelomonocytic leukemia (CMML), and MDS/MPN.
Myeloid malignancies, much like other forms of cancer, are the consequence of the accumulation of genetic and epigenetic alterations within HSPCs over an extended period of time. These alterations, which include gene mutations, copy number alterations, chromosome aberrations, and epigenetic changes, result in the transformation of HSPCs into malignant cells. However, compared to other cancer types such as melanoma or colorectal cancer, where a median of 10 driver mutations are detected per case [2], the numbers of genetic driver events found in myeloid malignancies is relatively small, with a median of just 3, 2, and 1–2 driver mutations per case in AML, MDS, and MPN, respectively [3–7]. Despite this relative simplicity of the genomic abnormalities, the evolutionary process that shapes the genomic architecture of myeloid malignancies has remained elusive. The complexity of this process is further compounded by the dynamic and heterogeneous nature of the genetic and epigenetic changes that occur, often in non-linear fashion, making it challenging to discern a unified evolutionary pathway that is common to all myeloid malignancies. This dynamic and heterogeneous nature of the genetic and epigenetic changes not only poses a challenge to identifying common therapeutic targets but also further complicates the identification of effective strategies for early detection and prevention of the disease.
The recent discovery of clonal hematopoiesis (see Glossary) as a preleukemic state [9], coupled with the technological advancements in massively parallel sequencing and single-cell genomics, have begun to shed new light on the developmental process of myeloid malignancies. These studies have provided a deeper understanding of the underlying mechanism of myeloid malignancy development and have led to the identification of novel therapeutic targets and refinement of the diagnostic classifications of myeloid diseases. The application of these technologies has also allowed for the characterization of the genetic and epigenetic changes that occur in these diseases at a single-cell resolution, providing new insights into the clonal evolution of myeloid malignancies.
The purpose of this review is to summarize these recent findings and examine how clonal evolution specifically pertains to the development of myeloid malignancies. While the overarching principle of clonal evolution in cancer has been the subject of numerous previous reviews [10–13], the current analysis will delve into the specific intricacies of clonal evolution as it pertains to myeloid malignancies. By focusing on the specific nuances of clonal evolution in myeloid malignancies, this review aims to provide a contemporary understanding of the evolutionary process in myeloid malignancies and its implications for the development of new diagnostic and therapeutic approaches.
Genomic architecture of myeloid malignancies
The democratization of next-generation sequencing techniques, such as massively parallel sequencing has greatly propelled our understanding of the genetic landscape of positively selected mutations, also known as driver mutations, in cancers [14]. One of the pioneering applications of whole genome sequencing (WGS) in cancer research was in a single case of AML [15], which paved the way for a plethora of subsequent studies using unbiased genetic sequencing to profile driver mutations across cancers [14, 16]. These studies have since revealed that AML, MDS, MPN each has driver mutations in 76, 43, and 33 genes, respectively, creating an ever-expanding catalog of driver mutations involved in myeloid diseases [3, 5, 6]. The mutation landscape among myeloid malignancies overlaps significantly, although certain mutations have specificity against a specific disease, for example NPM1 and CEBPA mutations for AML, CALR mutation for MPN, that could be also used as a diagnostic biomarker [17].
These driver mutations can be detected in combination or independently, resulting in a wide variety of mutational patterns. Certain specific patterns of mutations are recurrently observed (e.g., DNMT3A, NPM1, FLT3 combination for AML, SRSF2, TET2, ASXL1 combination in MDS/MPN), leading to the identification of novel molecular subtypes, some of which are associated with clinical prognosis and response to therapy [3, 4, 18–20]. Furthermore, the sequence in which the mutations occur adds an additional layer of complexity to the mutational subtypes of myeloid malignancies.
The mutation order inferred from population-based sequencing studies
As cancer cells replicate, they pass on any somatic mutations present in the parental cells to their daughter cells, while also acquiring additional mutations. These somatic mutations can serve as a cellular “barcode” and “molecular clock”, allowing for the reconstruction of the clonal evolution history [21–23]. The temporal sequence in which mutations are acquired, whether early or late in the progression of the disease, establishes a hierarchical pattern of genetic mutations. Early mutations are present in the majority of cancer cells and are referred to as clonal mutations, whereas late mutations, found in only a small fraction of cancer cells, are known as subclonal mutations.
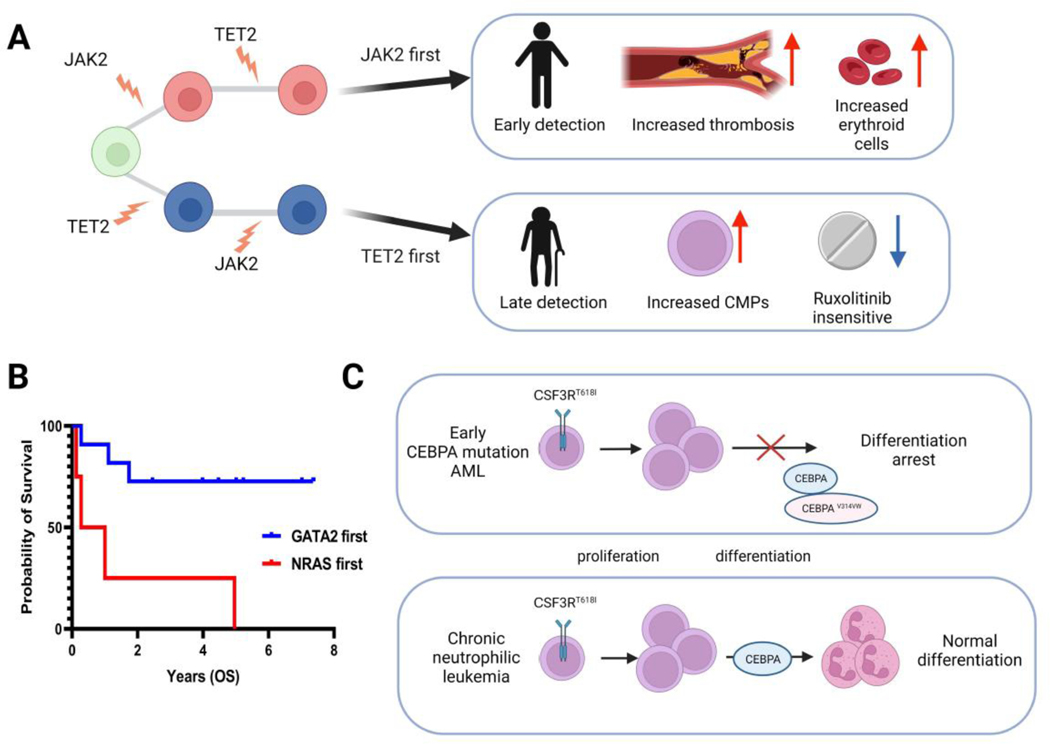
The relative order of mutation acquisition in myeloid malignancies can be inferred from DNA sequencing data by analyzing the variation in variant allele frequency (VAF) among somatic mutations. This approach is based on the principle that early mutations have a higher VAF than later mutations (Figure 1A). Using a large cohort of sequencing data, Papaemmannuil and colleagues have estimated the relative timing of mutation acquisition in MDS and AML [3, 5]. They found that mutations in genes involved in epigenetic regulation, such as those associated with DNA methylation (DNMT3A, TET2, IDH1/2), RNA splicing (SRSF2, U2AF1, and SF3B1), and chromatin modification (ASXL1, EZH2, BCOR), were frequently acquired early, while mutations in signaling genes like NRAS, KRAS, KIT, PTPN11, and FLT3 were acquired later. Similarly, Grinfeld and colleagues used this approach to study a large cohort of MPN and described the relative order of mutation acquisition [6]. However, it should be noted that the relative order of mutation acquisition inferred from population-level studies is a mere tendency and may not be universally applicable. A subset of patients with AML, MDS, and MPN exhibit a distinct order of mutations, deviating from a typical pattern observed in population-level studies, which potentially affects clinical phenotype. For example, Ortmann and colleagues in their study, identified two distinct mutation orders in MPN patients, in which one group of patients presented with JAK2 mutation as the founding event, followed by the acquisition of TET2 mutations, whereas the other group of patients acquired TET2 mutations prior to JAK2 mutation [24]. They found that JAK2-first patients were more likely to present with polycythemia vera than essential thrombocythemia and had an increased risk of developing thrombosis; on the other hand, TET2-first patients were 12.3 years older than JAK2-first patients and there was a predominance of common myeloid progenitors over other progenitors within the CD34+CD38+ compartment. These findings were also validated in a larger cohort [6] (Figure 2A). This finding highlights the importance of understanding the specific nuances of clonal evolution in myeloid malignancies, as it can have a significant impact on the diagnosis, prognosis, and treatment decisions.
Figure 1.
Schematic diagram summarizing the methods used to reconstruct mutation order and hierarchy. (A) The mutational hierarchy can be inferred from variant allele frequency (VAF) data obtained from bulk DNA sequencing. (B) The relative mutation order and clonal evolution pattern can be inferred from single-cell targeted DNA sequencing data by analyzing the cellular-level of mutation co-occurrence, enabling the description of parallel or branching evolution patterns even with single-timepoint samples. (C) When genome-wide mutation data is available from single cells, by using somatic mutations as a molecular clock, the absolute timing of mutation acquisition can be estimated. Abbreviation: HSC, hematopoietic stem cell.
Figure 2.
This figure summarizes how mutation order affects the clinical phenotype of myeloid malignancies. (A) Myeloproliferative neoplasms (MPN) that start with JAK2 and TET2 mutations have different clinical phenotypes. CMP: common myeloid progenitor (B) The prognosis of acute myeloid leukemia (AML) patients is different between those who start with GATA2 mutation vs. NRAS mutation. OS: overall survival (C) The oncogenic cooperation between CEBPA and CSF3R mutations depends on the order of the mutations.
Clonal hematopoiesis of indeterminate potential (CHIP) and early myeloid mutations
In parallel with these investigations, whole exome sequencing studies of large cohorts of healthy individuals have revealed that a subset of normal individuals, particularly the elderly, carry somatic driver mutations in their blood cells at low VAF [9, 25]. These mutations, collectively referred to as clonal hematopoiesis of indeterminate potential (CHIP) or simply clonal hematopoiesis, confer an elevated risk of developing hematologic malignancies, with a relative bias toward myeloid malignancies [26, 27]. The list of mutations frequently found in CHIP overlaps with the mutations deemed as early mutations in the studies previously discussed, such as DNMT3A, TET2, ASXL1, SRSF2, and U2AF1, thereby supporting the hypothesis that CHIP represents the early developmental process of myeloid malignancies. The fact that the majority of individuals with CHIP possess a single driver mutation, and that they have essentially normal blood indices, suggests that the acquisition of early driver mutations alone is not sufficient to transform HSCs into malignancy, which is consistent with the observation in various genetically engineered mouse models [28–32]. Therefore, it is likely that the successive acquisition of secondary and tertiary mutations (often described as ‘backseat drivers’ and ‘aggressive drivers’) plays a crucial role in determining the disease phenotype and malignant transformation of HSCs [33].
Inference of mutation order in myeloid malignancies using single-cell genomics
The hierarchical analysis of VAF data and the identification of CHIP have provided a general framework for understanding the temporal order of mutations and clonal architecture in myeloid malignancies. However, VAF data obtained through bulk sequencing alone is not sufficient to fully determine the subclonal structure [34]. Specifically, without the incorporation of multiple samples collected from different regions of the tumor or at different time points, it is challenging to determine whether subclonal mutations were acquired sequentially or in parallel. While obtaining multi-timepoint or multi-region samples can partially alleviate this limitation [35–39], it is often not a practical solution [40].
Recent advancements in single-cell DNA sequencing have enabled a more precise characterization of the subclonal structure by providing insights into mutation co-occurrence at the single-cell level [41–46]. These methods allow for a description of parallel evolution or branching evolution patterns even with single timepoint samples (Figure 1B). There are various methodologies for single-cell DNA sequencing, and a detailed review of the technological aspects of these methods can be found elsewhere[47–50].
In myeloid malignancies, the application of the microfluidics-based, high-throughput targeted DNA single-cell sequencing platform developed by Mission Bio, Inc. has provided a comprehensive single-cell landscape of myeloid driver mutations by enabling single-cell genotyping of several thousand cells for targeted DNA regions [51]. Through the collective analysis of over 200 AML samples by three independent groups, insights into the temporal order of mutation acquisition and the branching evolution patterns in AML have been uncovered[52–54]. These studies have not only confirmed earlier findings from bulk sequencing that mutations in epigenetic genes (that are also CHIP genes) are often initiating events, but also that different types of signaling mutations are often acquired in parallel, creating multiple subclones with distinct mutational combinations. Interestingly, while genetically different, these subclonal mutations converge toward a common functional outcome, such as the activation of RAS/MAPK signaling pathways. This observation of convergent evolution in AML suggests that the genetic diversity among subclones that arises through clonal evolution is driven by a selective pressure for the activation of specific oncogenic pathways. Moreover, this highlights the idea that clonal evolution is not just a random process but is shaped by the selective pressures that favors the development of certain genetic aberrations that confer growth advantage to cancer cells.
Understanding the absolute timing of clonal evolution in myeloid malignancies
Despite the advancements in high-throughput single-cell genotyping techniques that have provided insights into the relative timing of the mutation acquisition and branching evolution patterns in AML, the absolute timing of these mutational events, such as the precise moment of mutation acquisition within the lifetime of the patient and the duration between each successive mutational event, remains elusive. This limitation is in part due to the targeted nature of the genotyping platform used in these studies, which does not provide genome-wide mutation information for individual cells.
The rate of mutation acquisition in hematopoietic stem cells (HSCs) has been shown to be remarkably linear over time, with estimates suggesting that a single HSC acquires on average 18–20 mutations per year [22, 55–57]. This linearity in mutation acquisition enables the use of somatic mutations as a molecular clock to estimate the precise timing of mutation acquisition (Figure 1C). However, this approach requires genome-wide mutation profiling in each single cell, which was not employed in the studies discussed earlier.
Recent studies by Williams et al. and others have estimated the absolute timing of mutation acquisition in HSPCs from patients with myeloproliferative neoplasms and normal individuals by utilizing the linear and fixed mutation rate of HSCs [22, 57–60]. By performing whole-genome sequencing on genomic DNA obtained from single-HSPC colonies, they estimated that the JAK2 V617F mutation, a common driver mutation in MPNs, was acquired at the very beginning of their life, potentially in utero, in some MPN patients [58, 60]. While the experimental evidence to support this inference is lacking at this point, the estimate aligns with the mathematical model of clonal hematopoiesis development, which also suggests an early origin for some of the mutations [61]. However, other driver mutations, such as mutations in splicing genes SRSF2, SF3B1, and U2AF1, may arise later in life and yet rapidly expand due to the increased fitness they confer [59]. This is in accordance with the observed enrichment of these mutations in myeloid malignancies among the elderly population [62]. Nonetheless, these studies establish a proof of concept that estimating the absolute timing of mutation acquisition is feasible through single-cell whole genome profiling.
Phenotypic hierarchy and clonal evolution
The recognition of phenotypic diversity has long been a crucial aspect in the classification and prognostication of myeloid malignancies, predating the knowledge of their genetic heterogeneity. For example, the French-American-British (FAB) system subdivides AML based on the differentiation state of the AML cells [63]. Additionally, the coexistence of cellular populations with varying phenotypes within individual cases of AML has been previously documented through the use of flowcytometry. However, the advancement of single-cell RNA sequencing techniques and the application of computational deconvolution method to bulk RNA sequencing data have enabled a more detailed understanding of the composition of phenotypic heterogeneity in AML. van Galen et al. conducted a study utilizing single-cell RNA sequencing on a cohort of 16 AML samples and 5 normal bone marrow samples to gain insight into the cellular composition of AML based on their phenotypic states [64]. They found that the cellular composition of AML samples varied, with some samples primarily composed of HSC-like cells, while others exhibited a spectrum of progenitor-like cells in addition to more differentiated leukemia cells, such as monocytic- and dendritic-like cells. This variation in cellular composition was found to be associated with distinct response to therapy and prognosis. This observation was corroborated by a similar study by Zeng et al., who employed computational deconvolution of bulk RNA sequencing data from over 1,000 AML samples to identify four major subtypes of AML defined by the composition of cellular differentiation state, namely primitive, mature, granulocyte-macrophage progenitor (GMP)-like, and intermediate subtypes, which also predicted the response to the therapy [65]. However, the underlying mechanisms that shape the observed phenotypic composition remain unclear. In particular, the relationship between phenotypic heterogeneity and the genetic evolution in AML is yet to be fully deciphered and requires further investigation. The use of cutting-edge single-cell multi-omics technology holds the potential to uncover the intricate relationship between phenotypic heterogeneity and genetic evolution in AML [64, 66, 67].
Clinical relevance of clonal evolution
The central premise of studying clonal evolution is to better understand the underlying mechanisms that shape the clinical phenotype of the disease and translate those findings into clinical practice. As previously discussed, the difference in the order of mutations such as JAK2 and TET2, lead to different clinical phenotypes and prognosis in MPN [6, 24] (Figure 2A). Replicating this observation in AML and MDS has proven to be more challenging because these diseases have more complex mutations patterns compared to MPN. Nevertheless, two studies utilized VAF data from extensive bulk DNA sequencing data to investigate the connection between mutation order and clinical phenotype in AML and MDS, respectively [19, 68]. Benard et al. studied VAF data from the aggregated cohort of 2,929 patients with AML and found that the specific orders in which mutations occur can have an impact on both prognosis and the response to therapy. For instance, they identified that patients who acquired GATA2 mutation earlier than NRAS mutation had a better survival compared to the patients with the opposite mutation order (Figure 2B). Additionally, Braun et al. employed an inducible recombinase system to demonstrate that the oncogenic cooperation between CEBPA and CSF3R mutations is contingent on the order of mutation acquisition. Specifically, they observed that the development of AML requires the acquisition of CEBPA mutation before CSF3R mutation. Conversely, when CSF3R mutation precedes CEBPA mutation, it typically results in the phenotype similar to chronic neutrophilic leukemia [69] (Figure 2C). These findings indicated that specific mutation order has a significant impact on clinical presentation, disease phenotype, prognosis, and response to therapy, offering additional evidence that clonal evolution defines clinical phenotype in myeloid malignancies (Figure 2B).
Precise reconstruction of the clonal evolution history has the potential to inform strategies for early detection and prevention of the disease. The study by Williams et al. estimated the clonal growth of JAK2 mutated HSCs, enabling predictions of the age at which the clone becomes detectable in peripheral blood [58, 59]. This information can be used to design evidence-based approaches for early detection and potentially time the interventions to halt disease progression.
Moreover, a deeper understanding of the genetic and phenotypic heterogeneity of myeloid malignancies have the potential to inform treatment decision and predict therapy response. Tracking the dynamics of individual clones or cell types during specific therapies can help identify cells that are responsive or resistant to therapy and uncover novel mechanisms of resistance [46, 64, 65, 70–74].
Concluding remarks and future perspectives
The advent of next-generation sequencing technologies, single-cell analysis, and advanced computational analytics has led to a more in-depth understanding of myeloid malignancies as complex and diverse ecosystems composed of genetically and phenotypically heterogeneous cells. Despite these promising developments, the clinical impact of understanding clonal evolution and heterogeneity is still in its infancy, with the required tools and methods not yet widely accessible in routine clinical settings. Moreover, to fully harness the potential of tumor evolution in improving patient care, additional research and investigation are necessary to fully understand its scope and significance. Several outstanding questions are yet to be answered (see Outstanding questions). These include identifying the mutation that driver the transition from a preleukemic state to malignant leukemia, understanding the intervals between successive mutations in order to design an effective disease monitoring strategy, and exploring the potential of perturbing the typical order of mutations. Addressing these knowledge gaps through the integration of a multidisciplinary approach- utilizing gene-editing tools, single-cell multi-omics, and cellular and animal model systems- could potentially inform the design of an evolutionary-guided strategy for early detection, disease monitoring, and treatment decision-making.
Outstanding questions.
Which mutations drive the transition from preleukemic state to malignant leukemia?
What is the interval between first and second mutations, and third?
What happens to the phenotype if we reverse the typical order of mutations?
Does mutation acquisition occur in deterministic way or by chance?
Does the same mutation occur only once in a tumor lifetime (infinite site assumption) or does it occur multiple times independently?
How do germline mutations affect the acquisition of somatic mutations?
Is phenotypic heterogeneity driven by genetic heterogeneity?
What is the role of epigenetic evolution in myeloid malignancy development?
What is the role of microenvironment in shaping the clonal evolution?
Highlights.
Myeloid malignancies develop through a sequential acquisition of driver mutations, typically starting with epigenetic gene mutations followed by mutations in signaling genes.
Advancements in single-cell technologies have facilitated a comprehensive characterization of the mutational landscape during the development of myeloid malignancies, providing insights into the branching evolution patterns and precise timing of mutation acquisition.
The order in which mutations are acquired significantly impacts the clinical phenotype of myeloid malignancies, highlighting the importance of understanding clonal evolution and heterogeneity for guiding clinical decision-making.
Acknowledgments
K.T. is supported by NIH National Cancer Institute grants R01CA237291, R01CA262636, P01CA265748, the Leukemia and Lymphoma Society Scholar Award, Dresner Foundation Early Investigator Award, Andrew Sabin Family Foundation Award, American Society of Hematology Scholar Award, Break Through Cancer Grant, Physician Scientist Program at MD Anderson Cancer Center, and generous philanthropic contributions to MD Anderson Moon Shot Program Grant.
Glossary
- Clonal hematopoiesis
clonally expanded subpopulations of hematopoietic stem cells (HSCs) or progenitors that are commonly driven by somatically acquired gene mutations or chromosome abnormalities
- Computational deconvolution method
a mathematical technique used to separate a signal from a mixture of signals, enabling the extraction of information from a signal distorted or obscured by noise or other signals
- Massively parallel sequencing
also known as next generation sequencing, a high-throughput method that allows sequencing of millions of DNA or RNA (often in a form of cDNA) molecules simultaneously, enabling rapid, comprehensive analysis of genomic data
- Normal blood indices
a blood test result indicating normal range in red blood cell count, hemoglobin, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW), platelet count, white blood cell count, and differential white blood cell count
- Variant allele frequency (VAF)
the proportion of sequencing reads that represent a specific genetic variant in a DNA sample over the total read counts of the same locus
Footnotes
Declaration of interests
K.T. receives consulting fee from Symbio Pharmaceuticals, honoraria from Mission bio, Illumina, and Otsuka Pharmaceuticals. T.T. has no interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shih AH et al. (2012) The role of mutations in epigenetic regulators in myeloid malignancies. Nature Reviews Cancer 12 (9), 599–612. [DOI] [PubMed] [Google Scholar]
- 2.Martincorena I. et al. (2017) Universal Patterns of Selection in Cancer and Somatic Tissues. Cell 171 (5), 1029–1041.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papaemmanuil E. et al. (2016) Genomic Classification and Prognosis in Acute Myeloid Leukemia. New England Journal of Medicine 374 (23), 2209–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Research, N. et al. (2013) Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 368 (22), 2059–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papaemmanuil E. et al. (2013) Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood 122 (22), 3616–3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grinfeld J. et al. (2018) Classification and Personalized Prognosis in Myeloproliferative Neoplasms. New England Journal of Medicine 379 (15), 1416–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martincorena I. and Campbell PJ (2015) Somatic mutation in cancer and normal cells. Science 349 (6255), 1483–1489. [DOI] [PubMed] [Google Scholar]
- 8.Martínez-Jiménez F. et al. (2020) A compendium of mutational cancer driver genes. Nat Rev Cancer 20 (10), 555–572. [DOI] [PubMed] [Google Scholar]
- 9.Jaiswal S. et al. (2014) Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 371 (26), 2488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greaves M. and Maley CC (2012) Clonal evolution in cancer. Nature 481 (7381), 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGranahan N. and Swanton C. (2017) Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell 168 (4), 613–628. [DOI] [PubMed] [Google Scholar]
- 12.Yates LR and Campbell PJ (2012) Evolution of the cancer genome. Nature Reviews Genetics 13 (11), 795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGranahan N. and Swanton C. (2015) Biological and Therapeutic Impact of Intratumor Heterogeneity in Cancer Evolution. Cancer Cell 27 (1), 15–26. [DOI] [PubMed] [Google Scholar]
- 14.Watson IR et al. (2013) Emerging patterns of somatic mutations in cancer. Nat Rev Genet 14 (10), 703–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ley TJ et al. (2008) DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature 456 (7218), 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garraway Levi A. and Lander Eric S. (2013) Lessons from the Cancer Genome. Cell 153 (1), 17–37. [DOI] [PubMed] [Google Scholar]
- 17.Khoury JD et al. (2022) The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 36 (7), 1703–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tazi Y. et al. (2022) Unified classification and risk-stratification in Acute Myeloid Leukemia. Nature Communications 13 (1), 4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagata Y. et al. (2019) Invariant patterns of clonal succession determine specific clinical features of myelodysplastic syndromes. Nature Communications 10 (1), 5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernard E. et al. (2022) Molecular International Prognostic Scoring System for Myelodysplastic Syndromes. NEJM Evidence 1 (7), EVIDoa2200008. [DOI] [PubMed] [Google Scholar]
- 21.Alexandrov LB et al. (2015) Clock-like mutational processes in human somatic cells. Nature Genetics 47 (12), 1402–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee-Six H. et al. (2018) Population dynamics of normal human blood inferred from somatic mutations. Nature 561 (7724), 473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee-Six H. and Kent DG (2020) Tracking hematopoietic stem cells and their progeny using whole-genome sequencing. Experimental Hematology 83, 12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ortmann CA et al. (2015) Effect of Mutation Order on Myeloproliferative Neoplasms. New England Journal of Medicine 372 (7), 601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Genovese G. et al. (2014) Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med 371 (26), 2477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steensma DP et al. (2015) Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 126 (1), 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niroula A. et al. (2021) Distinction of lymphoid and myeloid clonal hematopoiesis. Nature Medicine 27 (11), 1921–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Challen GA et al. (2012) Dnmt3a is essential for hematopoietic stem cell differentiation. Nature Genetics 44 (1), 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moran-Crusio K. et al. (2011) Tet2 Loss Leads to Increased Hematopoietic Stem Cell Self-Renewal and Myeloid Transformation. Cancer Cell 20 (1), 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagase R. et al. (2018) Expression of mutant Asxl1 perturbs hematopoiesis and promotes susceptibility to leukemic transformation. Journal of Experimental Medicine 215 (6), 1729–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim E. et al. (2015) SRSF2 Mutations Contribute to Myelodysplasia by Mutant-Specific Effects on Exon Recognition. Cancer Cell 27 (5), 617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirai Cara L. et al. (2015) Mutant U2AF1 Expression Alters Hematopoiesis and Pre-mRNA Splicing In Vivo. Cancer Cell 27 (5), 631–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadigh S. and Kim AS (2021) Molecular Pathology of Myeloid Neoplasms: Molecular Pattern Recognition. Surgical Pathology Clinics 14 (3), 517–528. [DOI] [PubMed] [Google Scholar]
- 34.Malikic S. et al. (2019) Integrative inference of subclonal tumour evolution from single-cell and bulk sequencing data. Nature Communications 10 (1), 2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding L. et al. (2012) Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature 481 (7382), 506–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walter MJ et al. (2012) Clonal Architecture of Secondary Acute Myeloid Leukemia. New England Journal of Medicine 366 (12), 1090–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J. et al. (2014) Intratumor heterogeneity in localized lung adenocarcinomas delineated by multiregion sequencing. Science 346 (6206), 256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Bruin EC et al. (2014) Spatial and temporal diversity in genomic instability processes defines lung cancer evolution. Science 346 (6206), 251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerlinger M. et al. (2012) Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. New England Journal of Medicine 366 (10), 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caravagna G. et al. (2018) Detecting repeated cancer evolution from multi-region tumor sequencing data. Nature Methods 15 (9), 707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paguirigan AL et al. (2015) Single-cell genotyping demonstrates complex clonal diversity in acute myeloid leukemia. Science Translational Medicine 7 (281), 281re2–281re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Potter N. et al. (2019) Single cell analysis of clonal architecture in acute myeloid leukaemia. Leukemia 33 (5), 1113–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klco Jeffery M. et al. (2014) Functional Heterogeneity of Genetically Defined Subclones in Acute Myeloid Leukemia. Cancer Cell 25 (3), 379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saito Y. et al. (2017) Overcoming mutational complexity in acute myeloid leukemia by inhibition of critical pathways. Science Translational Medicine 9 (413), eaao1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hughes AE et al. (2014) Clonal architecture of secondary acute myeloid leukemia defined by single-cell sequencing. PLoS Genet 10 (7), e1004462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quek L. et al. (2018) Clonal heterogeneity of acute myeloid leukemia treated with the IDH2 inhibitor enasidenib. Nature Medicine 24 (8), 1167–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim B. et al. (2020) Advancing Cancer Research and Medicine with Single-Cell Genomics. Cancer Cell 37 (4), 456–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Navin NE (2015) The first five years of single-cell cancer genomics and beyond. Genome Research 25 (10), 1499–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Navin NE (2014) Cancer genomics: one cell at a time. Genome Biology 15 (8), 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y. and Navin Nicholas E. (2015) Advances and Applications of Single-Cell Sequencing Technologies. Molecular Cell 58 (4), 598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pellegrino M. et al. (2018) High-throughput single-cell DNA sequencing of acute myeloid leukemia tumors with droplet microfluidics. Genome Res 28 (9), 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morita K. et al. (2020) Clonal evolution of acute myeloid leukemia revealed by high-throughput single-cell genomics. Nat Commun 11 (1), 5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miles LA et al. (2020) Single-cell mutation analysis of clonal evolution in myeloid malignancies. Nature 587 (7834), 477–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ediriwickrema A. et al. (2020) Single-cell mutational profiling enhances the clinical evaluation of AML MRD. Blood Advances 4 (5), 943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Welch John S. et al. (2012) The Origin and Evolution of Mutations in Acute Myeloid Leukemia. Cell 150 (2), 264–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abascal F. et al. (2021) Somatic mutation landscapes at single-molecule resolution. Nature 593 (7859), 405–410. [DOI] [PubMed] [Google Scholar]
- 57.Mitchell E. et al. (2022) Clonal dynamics of haematopoiesis across the human lifespan. Nature 606 (7913), 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams N. et al. (2022) Life histories of myeloproliferative neoplasms inferred from phylogenies. Nature 602 (7895), 162–168. [DOI] [PubMed] [Google Scholar]
- 59.Fabre MA et al. (2022) The longitudinal dynamics and natural history of clonal haematopoiesis. Nature 606 (7913), 335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Egeren D. et al. (2021) Reconstructing the Lineage Histories and Differentiation Trajectories of Individual Cancer Cells in Myeloproliferative Neoplasms. Cell Stem Cell 28 (3), 514–523.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watson CJ et al. (2020) The evolutionary dynamics and fitness landscape of clonal hematopoiesis. Science 367 (6485), 1449–1454. [DOI] [PubMed] [Google Scholar]
- 62.Lindsley RC et al. (2015) Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood 125 (9), 1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bennett JM et al. (1985) Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann Intern Med 103 (4), 620–5. [DOI] [PubMed] [Google Scholar]
- 64.van Galen P. et al. (2019) Single-Cell RNA-Seq Reveals AML Hierarchies Relevant to Disease Progression and Immunity. Cell 176 (6), 1265–1281.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zeng AGX et al. (2022) A cellular hierarchy framework for understanding heterogeneity and predicting drug response in acute myeloid leukemia. Nature Medicine 28 (6), 1212–1223. [DOI] [PubMed] [Google Scholar]
- 66.Rodriguez-Meira A. et al. (2019) Unravelling Intratumoral Heterogeneity through High-Sensitivity Single-Cell Mutational Analysis and Parallel RNA Sequencing. Molecular Cell 73 (6), 1292–1305.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nam AS et al. (2019) Somatic mutations and cell identity linked by Genotyping of Transcriptomes. Nature 571 (7765), 355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benard BA et al. (2021) Clonal architecture predicts clinical outcomes and drug sensitivity in acute myeloid leukemia. Nature Communications 12 (1), 7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Braun TP et al. (2019) Myeloid lineage enhancers drive oncogene synergy in CEBPA/CSF3R mutant acute myeloid leukemia. Nature Communications 10 (1), 5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pei S. et al. (2020) Monocytic Subclones Confer Resistance to Venetoclax-Based Therapy in Patients with Acute Myeloid Leukemia. Cancer Discovery 10 (4), 536–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McMahon CM et al. (2019) Clonal Selection with RAS Pathway Activation Mediates Secondary Clinical Resistance to Selective FLT3 Inhibition in Acute Myeloid Leukemia . Cancer Discovery 9 (8), 1050–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Choe S. et al. (2020) Molecular mechanisms mediating relapse following ivosidenib monotherapy in IDH1-mutant relapsed or refractory AML. Blood Advances 4 (9), 1894–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DiNardo CD et al. (2020) Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood 135 (11), 791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Daver NG et al. (2023) Venetoclax and idasanutlin in relapsed/refractory AML: a nonrandomized, open-label phase 1b trial. Blood 141 (11), 1265–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]