Abstract
While the illicit use and misuse of stimulants like cocaine and methylphenidate (MP) has increased, there remains no FDA approved treatments for psychostimulant use disorders (PSUD). Oxytocin (OT) has shown promise as a potential pharmacotherapy for PSUD. Dopamine (DA) neurotransmission plays a significant role in PSUD. We have recently shown that OT blunts the reinforcing effects of MP but, surprisingly, enhanced MP-induced stimulation of DA levels. Such effects have been suggested as a result of activation of OT receptors or, alternatively, could be mediated by direct actions of OT on MP blockade of the DA transporter. Here, we employed fast scan cyclic voltammetry (FSCV) to investigate the effects of systemic OT on MP-induced changes in the dynamics of DA, phasic release and uptake, in the nucleus accumbens shell (NAS) of Sprague-Dawley rats. We also tested the systemic effects of an antagonist of OT receptors, atosiban, to counteract the OT enhancement of dopaminergic effects of MP under microdialysis procedures in the NAS in rats. Administration of OT alone (2 mg/kg; i.p.) did not significantly modify evoked NAS DA dynamics measured by FSCV, and when administered 10 minutes before MP (0.1, 0.3, 1.0 mg/kg; i.v.), OT did not potentiate MP-induced increases in phasic DA release and did not alter DA clearance rate, suggesting no direct interactions of OT with the MP-induced blockade of DA uptake. Also, OT alone did not elicit significant changes in tonic, extracellular NAS DA levels measured by microdialysis. However, consistent with previous studies, we observed that OT pretreatments (2 mg/kg; i.p.) potentiated MP-induced (0.1, 0.3, 1.0 mg/kg; i.v.) efflux of extracellular NAS DA levels. This effect was abolished when rats were pretreated with atosiban (2 mg/kg; i.p.), suggesting that OT receptors mediate this OT action. Overall, our results suggest that OT receptors mediated OT potentiation of MP-induced stimulation of extracellular NAS DA levels, likely driven by modulation of DA receptor signaling pathways, without affecting MP blockade of DAT.
Keywords: Dopamine, psychostimulant use disorder, oxytocin, microdialysis, fast scan cyclic voltammetry, methylphenidate
Graphical Abstract
Introduction
Psychostimulant use disorder (PSUD), as defined in DSM-V (Hasin et al. 2013), is a condition affecting a rapidly growing number of individuals and is associated with significant medical and public health consequences worldwide. The United Nations estimated in their 2021 World Drug Report that about 20 million people used cocaine and 27 million people used amphetamine-like stimulants in 2019 (U.N. 2021). Of note, overdose deaths from cocaine are also increasing (Hedegaard et al. 2020).
Lack of approved, efficacious medications for PSUD has driven the search for the development of new therapeutic strategies, including preclinical and clinical assessment of newly synthesized lead molecules, as well as repurposing medications approved for other clinical indications and that show promise as PSUD treatments (Hersey et al. 2021; Lee et al. 2016). Among the latter drugs, oxytocin (OT) has emerged as a potential therapeutic option for psychostimulant use disorder (Lee et al. 2017). OT is a naturally occurring posterior pituitary peptide hormone, best known for its elevated levels post-partum and influence on maternal behaviors (Russell et al. 2003). However, its effects on social bonding (Olff et al. 2013; Romero et al. 2014), social behavior (Heinrichs et al. 2009), learning (Sarnyai & Kovacs 2014), and memory (Rimmele et al. 2009) have also been well documented. OT is believed to exert its effects on the mesocorticolimbic reward circuit of the brain, allowing its influence on motivation, reward, and associated behaviors (Love 2014). In this respect, the mesolimbic dopamine (DA) systems are also a primary target of addictive substances, including psychostimulants (Young et al. 2011; Di Chiara et al. 1999).
Among the behavioral changes induced by systemic administration of OT , decreased self-administration of both depressants (i.e., alcohol and opioids) (Ibragimov et al. 1987; King et al. 2017; Kovacs et al. 1985) and stimulants (Carson et al. 2010; Everett et al. 2020; Lee et al. 2019; Leong et al. 2017), reduced tolerance for addictive drugs (Sarnyai & Kovacs 1994), and attenuated withdrawal symptoms (King et al. 2020) have all been observed. These OT-induced behavioral effects have prompted research into the therapeutic potential of OT to combat substance use disorders in humans (McGregor & Bowen 2012; Lee et al. 2016). Preliminary OT studies in humans have been conducted and some of them show promise that OT may represent a novel pharmacotherapeutic approach for alcohol and substance use disorders (Flanagan et al. 2015; Kirkpatrick et al. 2014; McRae-Clark et al. 2013; Pedersen et al. 2013; Lee et al. 2014; Stauffer et al. 2016).
One stimulant that is of particular interest is methylphenidate (MP), which is approved for use in the treatment of attention-deficit/hyperactivity disorder (ADHD) and narcolepsy, and is frequently misused because of its psychostimulant properties (Shellenberg et al. 2020). In addition, its mechanism of action is analogous to that of cocaine. Indeed, like many other psychostimulants, but unlike amphetamines, MP elicits its effect by binding to and inhibiting the neuronal membrane dopamine transporter (DAT) and increasing extracellular DA in striatal brain regions like the nucleus accumbens (reviewed in Challman & Lipsky 2000; Faraone 2018).
The preclinical and clinical behavioral studies testing OT in drug-seeking behaviors have shown promise, but the neurochemical mechanisms that produce these effects are still under investigation. Many psychostimulant use studies to date have focused on the mesocorticolimbic DA system due to its participation in the neurochemical and behavioral effects elicited by addictive drugs (Young et al. 2011; Di Chiara et al. 1993). In this work, we focused on the nucleus accumbens shell (NAS) due to the abundance of OT receptors in this region (De Kloet et al. 1985), which are thought to play a role in OT-mediated behavioral changes, as well as the unique ability of psychostimulants to produce neurochemical effects after an acute dose (Di Chiara 2002; Di Chiara et al. 1998). Moreover, in our previous study systemic (i.p.) OT pretreatment in male rats decreased MP self-administration and unexpectedly potentiated MP-induced increases in extracellular DA levels in the NAS but not in the accumbens core as measured by microdialysis (Lee et al. 2019). In addition, these systemic OT actions were also observed after local (reverse dialysis) OT administration in the NAS, suggesting that OT receptors in the NAS were involved in OT-potentiation of MP-induced stimulation of DA extracellular levels. An alternative explanation for such effects could be a direct action of OT on DA dynamics elicited by MP after blockade of DAT. Thus, the present study aims to further elucidate the mechanism related to OT’s actions on MP-induced increases in rat NAS DA levels. Using fast scan cyclic voltammetry (FSCV), which allows the assessment of phasic, evoked release of DA and its clearance rate (an index of DA reuptake), we determined the ability of OT to directly interfere with MP blockade of DAT and related changes in phasic NAS DA dynamics. We also utilized microdialysis to study OT potentiation of MP-induced stimulation of NAS DA efflux under pharmacological blockade of OT receptors, using the OT-receptor antagonist atosiban.
Materials and Methods
Chemicals and Reagents
Oxytocin acetate salt hydrate (Sigma-Aldrich, St. Louis, MO; cat. 06379), atosiban (Adoop Bioscience, Irvine, CA; cat. A14334), and methylphenidate HCL (Mallinckrodt, Washington, DC; cat. 0406-1571-55) were prepared in solutions of sterile saline (Hospira, Lake Forest, IL; cat. 0409-4888-03) and injected at a volume of 1 mL/kg solution. Electrodes calibrated using dopamine hydrocholoride (Sigma-Aldrich, St. Louis, MO; cat. H8502) solutions.
Animals
Male Sprague Dawley rats (CD sub-strain 001; Charles River, Wilmington, MA; Research Resource Identifier (RRID): RGD 70508) weighing 290-320 g and approximately 8-10 weeks old were housed in pairs with ad libitum access to food and water on a traditional 12/12 hr light/dark schedule with all experiments being performed during the light phase. Animals were given one week to acclimate to the animal facility before experiments were conducted. All animal procedures were approved and performed in accordance with guidelines from the Animal Care and Use Committee of the National Institute on Drug Abuse, Intramural Research Program, Baltimore, MD, USA which is fully accredited by the AAALAC International (19-MDRB-13, 21-MTMD-9). Anesthetics were selected with approval by the NIDA ACUC in accordance with NIH guidelines based on their efficacy. During surgical procedures, Bupivacaine (Hospira, Lake Forest, IL; cat. 0409-1163-18) was administered as a local anesthetic when making skin incisions or applying sutures. Pain medications (carprofen) were appropriately administered after surgery.
Fast Scan Cyclic Voltammetry
Surgery.
Sprague-Dawley rats were anesthetized with 0.5 g/mL urethane (Sigma-Aldrich, St. Louis, MO; cat. U2500; and dissolved in sterile saline) administered intraperitoneally. A silastic catheter was implanted into the external jugular vein as previously described (Garces-Ramirez et al. 2011; Tanda et al. 2008; Solinas et al. 2003; Tanda et al. 2015; Solinas et al. 2006). Briefly, a skin incision was made above the right external jugular vein, which was exposed by blunt dissection. The vein was isolated from the connective tissue, the silastic portion of the catheter (2.5 cm of silastic tubing, 0.51-mm i.d., 0.94-mm o.d., connected to a PE50 tubing, 0.58-mm i.d., 0.965-mm o.d. using 1 cm of a 22-gauge stainless steel blunt needle) was inserted into the vein and then sutured into place. During the same surgery session, the rats were placed in a stereotaxic frame and a carbon fiber microelectrode was placed into the NAS using the following coordinates (AP = +1.7 mm, ML = ±0.8 mm, DV = −6.5 to 7.5 mm)(Paxinos & Watson 1998). A bipolar tungsten stimulating electrode was inserted to target the medial forebrain bundle (MFB) (AP = −4.6 mm, ML = ±1.0 mm lateral, DV = −6.5 mm) (Paxinos & Watson 1998). Last, an Ag/AgCl reference electrode was implanted on the contralateral side of the brain.
Electrochemistry.
Carbon fiber microelectrodes were fabricated by threading a single carbon fiber (diameter of 0.007mm, Goodfellow Cambridge Limited, UK) through a glass capillary (1.2 mm in diameter, A-M Systems, Sequim, WA) and a micro-pipet puller (Narishige, Tokyo, JPN) was used to make a glass seal. Carbon fiber was trimmed to 50-100 μm from the glass seal. Electrodes were pre-calibrated using 0, 0.5, 1, 2, 3 μM DA hydrocholoride (Sigma-Aldrich, St. Louis, MO) solutions. DA was detected using a DA-specific waveform (scanning −0.4 V to 1.3 V and back to −0.4 V at 400 V/s). An electrical stimulation (24 pulses, 180 μA, 60 Hz, 4 ms duration) was applied to evoke DA release. FSCV data were collected using a UEI potentiostat and breakout box running Tarheel-CV (UNC electrical shop, Chapel Hill, NC) and Digitimer Neurolog NL800A (Ft. Lauderdale, FL). DA was identified by an oxidation event at 0.6 V and data was excluded if the signal was not consistent or the animals did not survive through the duration of the experiment.
FSCV data was analyzed using HDCV (UNC, Chapel Hill, NC) and maximum DA release (DAmax) and DA clearance (t1/2 and k) were determined using a custom macro written in Igor Pro (Wavemetrics, Portland, OR), further described in Keighron et al. (2019). Area under the curve of the evoked DA release was calculated using Graphpad Prism (San Diego, CA).
Microdialysis
Probe preparation and surgery.
Concentric dialysis probes, fabricated with an active dialyzing surface of 1.8–2.0 mm, were implanted into brain tissue as previously described (Garces-Ramirez et al. 2011; Tanda et al. 2008; Tanda et al. 2005; Tanda et al. 2007; Tanda et al. 2016; Tanda et al. 1997). This procedure was performed using ketamine (60.0 mg/kg; i.p.) and xylazine (12.0 mg/kg; i.p.) anesthesia. Probes were implanted into the rat NAS (AP = +2.0; ML = ±1.0; DV = −7.9 mm from bregma) (Paxinos & Watson 1998). At the time of surgery, a jugular vein catheter was also inserted as described above for intravenous (i.v.) administration of drug agents. Post-operatively, rats were housed in hemispherical CMA-120 cages (CMA/Microdialysis AB, Solna, Sweden) for recovery (>20 hrs) and for microdialysis test sessions. Local anesthetics and pain medications were appropriately administered as described in the FSCV section above.
Sample collection and analytical procedures.
Microdialysis parameters were set as follows, Ringer’s solution (147.0 mM NaCl, 2.2 mM CaCl2, and 4.0 mM KCl), delivered by a Bee Syringe Pump Controller (BASi, West Lafayette, IN), was infused at a flow rate of 1 μL/min through the microdialysis probes connected to fluid swivels (375/D/22QM, Instech, Plymouth Meeting, PA), in freely moving rats, as previously described (Garces-Ramirez et al. 2011; Tanda et al. 2008; Tanda et al. 2005; Tanda et al. 2007; Tanda et al. 2016; Tanda et al. 1997). Dialysate samples (10 μL every 10 min) were collected and analyzed immediately via a high-performance liquid chromatography system (chromatographic column MD-150 × 3.2, ThermoFisher, Waltham, MA), equipped with a coulometric detector (Coulochem II, or Coulochem III, ESA, ThermoFisher), and analytical cell (5014B; ESA, ThermoFisher), whose electrodes were set at +125 and −125 mV for DA quantification. The mobile phase (100 mM NaH2 PO4, 0.1 mM Na2EDTA, 0.5 mM n-octyl sulfate, 18% methanol; pH adjusted to 5.5 with Na2HPO4) was delivered at 0.50 mL/min by an isocratic pump (ESA model 582, ESA, ThermoFisher).
Histology
Proper probe or electrode placement was confirmed via histological analysis of brain tissue collected after the humane sacrifice of the rats following both microdialysis and FSCV experiments. For FSCV, brain tissue was lesioned by applying a high voltage to the electrode at the end of the experiment. Animals were humanely sacrificed by an overdose of urethane anesthetic (after FSCV; Sigma-Aldrich, St. Louis, MO; cat. U2500; approximately 0.5 g/kg i.v. or 1.5 g/kg i.p.) or Euthasol (after microdialysis; Virbac; cat. 051311-050-01, 100 mg/kg either i.p. or i.v.). Brain tissue was then collected and stored in a 4% formalde-fresh solution (Fisher Scientific, Waltham, MA; cat. SF93-4) for a few days. Brains were then transferred to a 30% sucrose solution (Sigma-Aldrich, St. Louis, MO; cat. S7903) for 3 days prior to tissue slicing. Tissue was sectioned at 30 μm using a cryostat (Leica Biosystems, Deer Park, IL). Tissue was analyzed under magnification for correct electrode placement and data were excluded if the electrode did not hit the target brain region. For microdialysis, brains were collected, left to fix in 4% formalde-fresh solution (Fisher Scientific, Waltham, MA; cat. SF93-4) for at least 5 days, and then sliced on a Vibratome 1000 Plus (the Vibratome Company, St. Louis, MO) in serial coronal slices (Paxinos & Watson 1998). Probe track locations were verified using atlas brain sections (Paxinos & Watson 1998) as templates. Only the data obtained in animals showing a correct probe track within the boundaries of the targeted brain areas were included in the statistical analysis.
Study Design and Statistical Analyses
The studies included in this work were not pre-registered. Drug treatments were arbitrarily assigned, the experimenters were not blinded to treatment, and only one experiment was performed on each animal. A standard sample size of 5-6 animals was used based on previously published work (Lee et al. 2019; Keighron et al. 2019) and confirmed with a post-hoc power analysis (minimum sample size of n = 3, α = 0.05, power = 0.80) (ClinCalc:Kane 2019). Fig. 1 contains a graphical summary of the experimental procedure. For the FSCV study, 21 rats were used and 4 were excluded. For the microdialysis tests, 40 rats were used for this study and 9 were excluded. Animals were excluded if they were statistically significant outliers from the mean, if an incomplete data set was collected, or if probe/electrode placement was outside of the NAS boundaries. In the neurochemical studies, results are presented as group means (± SEM), normalized, and expressed as a percentage of basal DA values, which were calculated as means of 2-4 consecutive samples (microdialysis) or files (FSCV) immediately preceding drug administration (less than 15% variability). Assessment of the normality of our neurochemical data was performed by applying the Shapiro–Wilk test, which template/calculator is available at Statistics Kingdom website (https://www.statskingdom.com/320ShapiroWilk.html). Data from our neurochemistry tests, microdialysis and FSCV, were found to be normally distributed. No randomization was performed to allocate subjects in this study but animals were arbitrarily assigned to each drug treatment. Either acute OT (OT acetate salt hydrate, Sigma-Aldrich, St. Louis, MO) (2 mg/kg; OT concentration: 2 mg/ml, administered i.p. at 1ml/kg) or saline (Hospira, Lake Forest, IL) treatments were arbitrarily assigned and administered i.p. as a pretreatment 10 min before the start (time = 0 in the figures) of the first dose of MP (Mallinckrodt, St. Louis, MO). Increasing doses of MP were administered (i.v.) at the time points of 0 (0.1 mg/kg), 30 (0.32 mg/kg), and 60 min (1.0 mg/kg). Doses of OT and MP, as well as the administration timeline, were determined based on previously published data (Lee et al. 2019). Using the same experimental conditions and the same schedule of MP injections, the above experiment was repeated with atosiban (2 mg/kg; i.p.) pretreatments given 10 min before OT pretreatments. Atosiban dosing was based on effective doses in the literature (Broadbear et al. 2011). Importantly, OT was only administered as an acute dose prior to neurochemical studies (2mg/kg; i.p.; at 1mL/kg).
Figure 1: Experimental procedure.
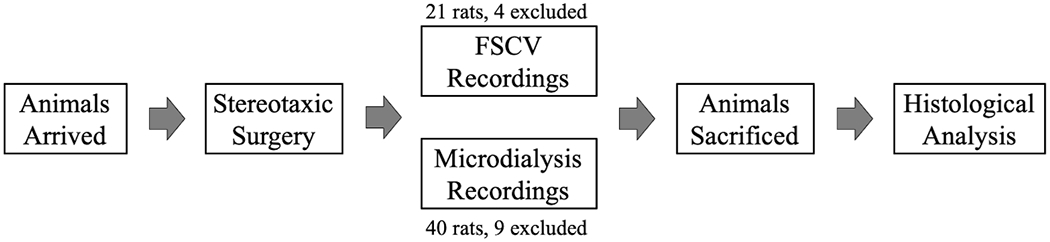
Animals arrived and underwent stereotaxic surgery for fast scan cyclic voltammetry (FSCV) analysis or microdialysis probe implantation. After neurochemical experiments were completed animals were humanly sacrificed and histological analyses were completed to confirm proper electrode or probe placement.
Raw FSCV data with representative color plots are shown in this manuscript but due to the high individual variability in control evoked NAS DA release we have chosen to normalize the data when comparing over the full 2-hour time course post drug administration. Microdialysis data were normalized as percentage of basal values for each treatment group to facilitate comparison with our previously published results (Lee et al. 2019). As stated above, our data were found to be normally distributed.
Statistical analysis (Statistica, New York City, NY, or Graphpad Prism, San Diego, CA) was carried out using a t-test, a mixed media analysis or a two-way ANOVA for repeated measures, over time, applied to the data obtained from analysis of successive evoked DA release or serial assays of dialysate DA normalized as percentage of basal values of each group. The Grubbs test for outliers was used to confirm that no data was statistically different than the mean.
Results
FSCV reveals that OT administration does not significantly modify phasic NAS DA evoked release or clearance rate
An example of color plot for DA FSCV data generated by a carbon fiber microelectrode implanted in the NAS of rats before and after OT treatment (2 mg/kg; i.p.) is shown in Fig. 2A and B. In Fig. 2C we show the average baseline or control evoked release of NAS DA in gray. In comparison, we show the averaged evoked release of NAS DA 5-20 min following administration of OT (2 mg/kg; i.p.) (Fig. 2C). OT did not elicit significant changes in evoked maximum DA release (DAmax) [paired t-test: t=1.318; df=4; p=0.2580] (Fig. 2D) or DA clearance rate (t1/2) [paired t-test: t=1.039; df=4; p=0.3574] (Fig. 2E) in comparison to baseline NAS evoked DA. The rate of DA clearance in FSCV studies is dependent on diffusion of DA away from the electrode surface, enzymatic breakdown of DA, as well as DA clearance by DAT (Sabeti et al. 2002).
Figure 2: Oxytocin (OT) administration does not change phasic nucleus accumbens shell (NAS) dopamine (DA) dynamics.
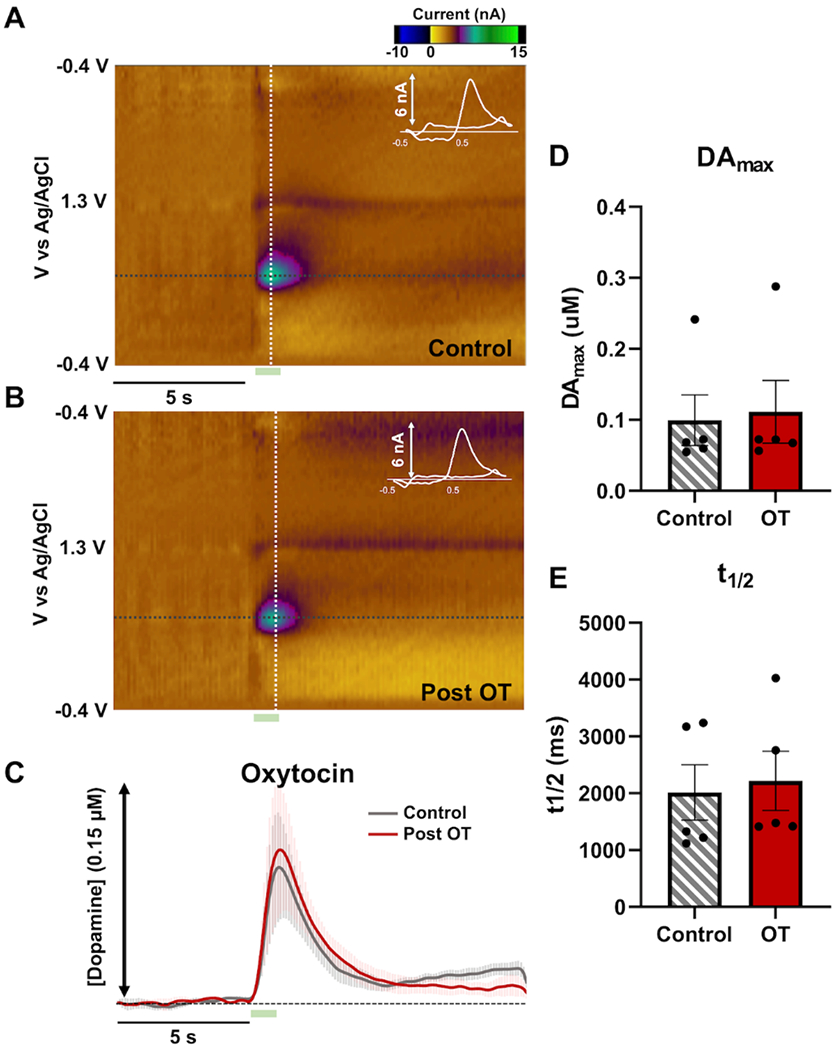
(A) An example color plot and cyclic voltammogram (shown in white and inlayed in the color plot) of a control evoked release of DA in the rat NAS are shown with a dopamine event visible at ~0.6 V. Stimulation is marked by a green box below the color plot (5-7s). (B) An example color plot and cyclic voltammogram post OT administration (2 mg/kg; i.p). (C) Average evoked NAS DA before (control; gray; n=5 rats) and after OT administration (5-20 min post OT; red; n=5 rats). Error is shown in lighter colors above and below the traces. (D) Maximum DA release and (E) clearance rat (t1/2) for control (gray; n=5 rats) and post OT (red; n=5 rats).
OT does not alter MP-induced increases in phasic NAS DA
Every 5 min, NAS DA was evoked and observed in a 15 s sampling file (experimental paradigm outlined in Fig. 3). After 4 consistent baseline sampling files with consistent evoked DAmax (collected every 5 min within a 20 min period), rats were administered either saline (i.p.) or OT (2 mg/kg; i.p.). Ten min later, three increasing doses of MP (0.1, 0.32, 1.0 mg/kg; i.v.) spaced 30 min apart were administered. After the final injection, DA dynamics were observed for an hour to ensure that DA returned to baseline/control levels. In Fig. 3 we also show an example of an individual animal’s evoked NAS DA response to the saline/MP and OT/MP treatment for visualization. We qualitatively note that no large change in evoked DAmax or DA clearance are observed following treatment with saline or OT, but we see a dose-dependent increase in evoked NAS DA with MP administration. Analysis of FSCV results from Fig. 4 showed that saline or OT pretreatment produced no distinct, significant differences in NAS DA dynamics, including the maximum evoked DA release (DAmax) (Fig. 4A), area under the curve of the DA release event (AUC) (Fig. 4B), and rate of DA clearance, t1/2 (Fig. 4C). [Two-way repeated measures ANOVAs: main effect treatment: F1,10=0.290, p=0.602; main effect time: F29,290=55.070, p<0.0001; interaction: F29,290=0.445, p=0.995], area under the DA evoked release and reuptake curve (AUC) [Two-way repeated measures ANOVAs: main effect treatment: F1,10=0.001, p=0.972; main effect time: F29,290=19.410, p<0.0001; interaction: F29,290=0.551, p=0.972], and DA clearance (t1/2: time it takes for half of the evoked release to be re-uptaken/cleared from the extracellular space) [Mixed-effects analysis: main effect treatment: F1,10=0.936, p=0.356; main effect time: F29,288=15.090, p<0.0001; interaction: F29,288=0.968, p=0.516] (Fig. 4 A–C).
Figure 3: Monitoring phasic nucleus accumbens shell (NAS) dopamine (DA) dynamics with fast scan cyclic voltammetry (FSCV).
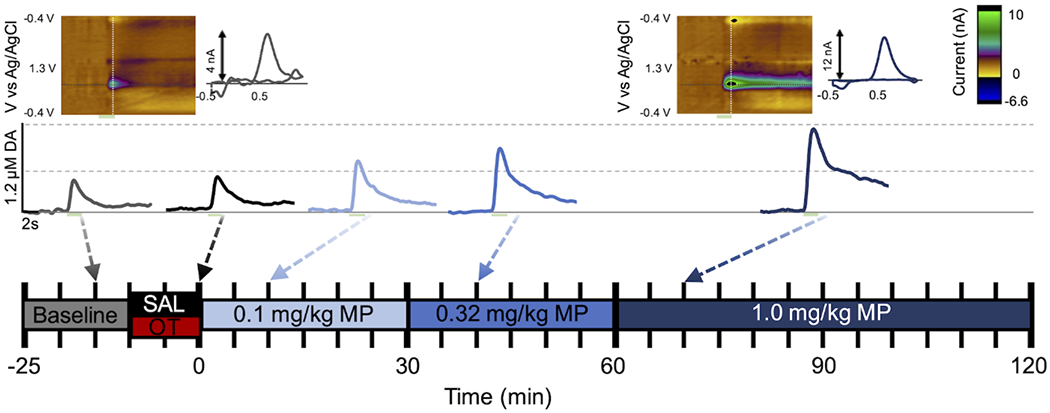
A schematic showing the experimental timeline in the middle and an individual animal’s evoked NAS DA release treated with saline (SAL) and methylphenidate (MP) above. Example baseline and post-treatment color plots and cyclic voltammograms are shown at the top. Stimulation is marked by a green box below the plot. FSCV files are collected for 15 s every 5 min and after 4 stable baseline files. Next, either SAL or oxytocin (OT) pretreatment is administered (0 or 2 mg/kg; i.p.) 2 more files are collected and then 3 increasingly large doses of MP (0.1, 0.3, 1.0 mg/kg; i.v.) are administered 30 min apart (6 files) and data is collected for 60 min (12 files) after the last and largest dose of MP.
Figure 4: Oxytocin (OT) administration does not alter methylphenidate (MP)-induced increases in phasic nucleus accumbens shell (NAS) dopamine (DA).
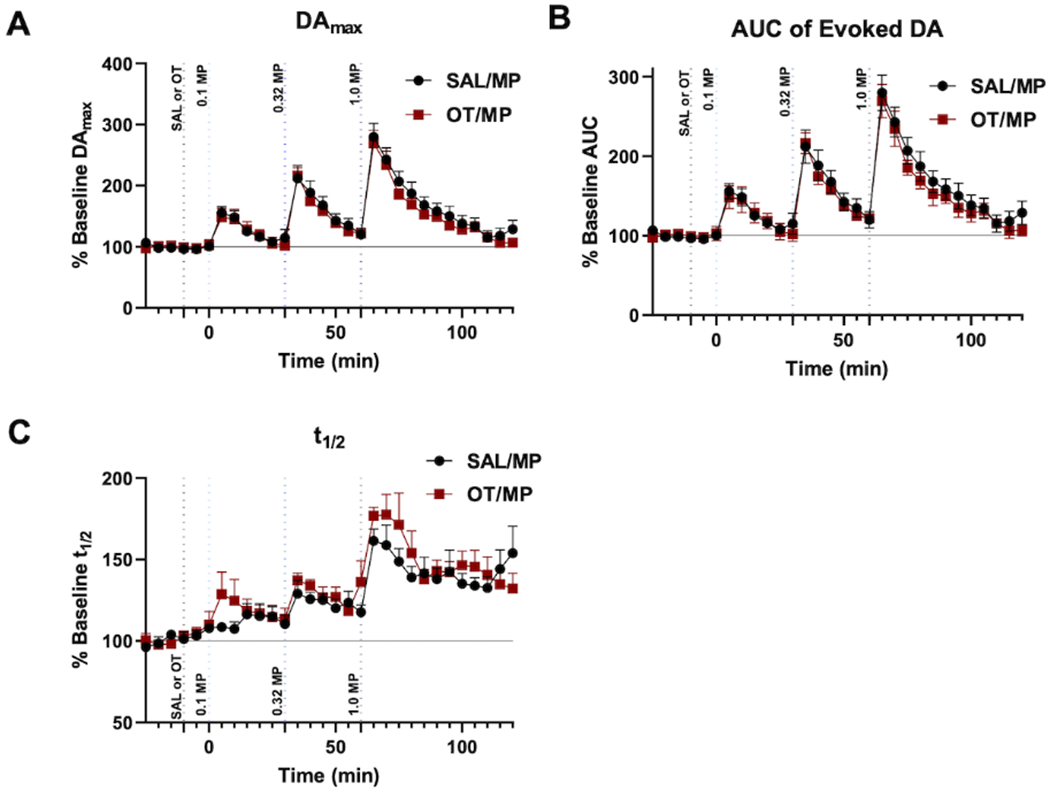
Analysis of fast scan cyclic voltammetry (FSCV) results of evoked NAS DA at baseline/control, 10 min after saline or OT (2mg/kg; i.p.) and 10 min after increasing doses of methylphenidate (MP) (0.1, 0.32, 1.0 mg/kg; i.v.). Data are shown as the average of each animal’s results normalized to their baseline for (A) Maximum evoked DA (DAmax), (B) area under the curve (AUC), (C) t1/2 throughout the experimental paradigm and error bars indicate SEM (n=6 rats for each).
OT administration does not significantly modify tonic NAS DA levels
Extracellular levels of NAS DA were analyzed using microdialysis following an acute administration of OT (2 mg/kg; i.p.). Results show that no statistically significant change in normalized NAS DA levels were seen in comparison with vehicle administration [Two-way ANOVA, main effect treatment: F1,9=1.014, p=0.340; main effect time: F12,108=1.156, p=0.323; treatment by time interaction: F12,108=0.973, p=0.479] (Fig. 5).
Figure 5: Time course showing that oxytocin (OT) administration does not significantly modify extracellular nucleus accumbens shell (NAS) dopamine (DA) levels.
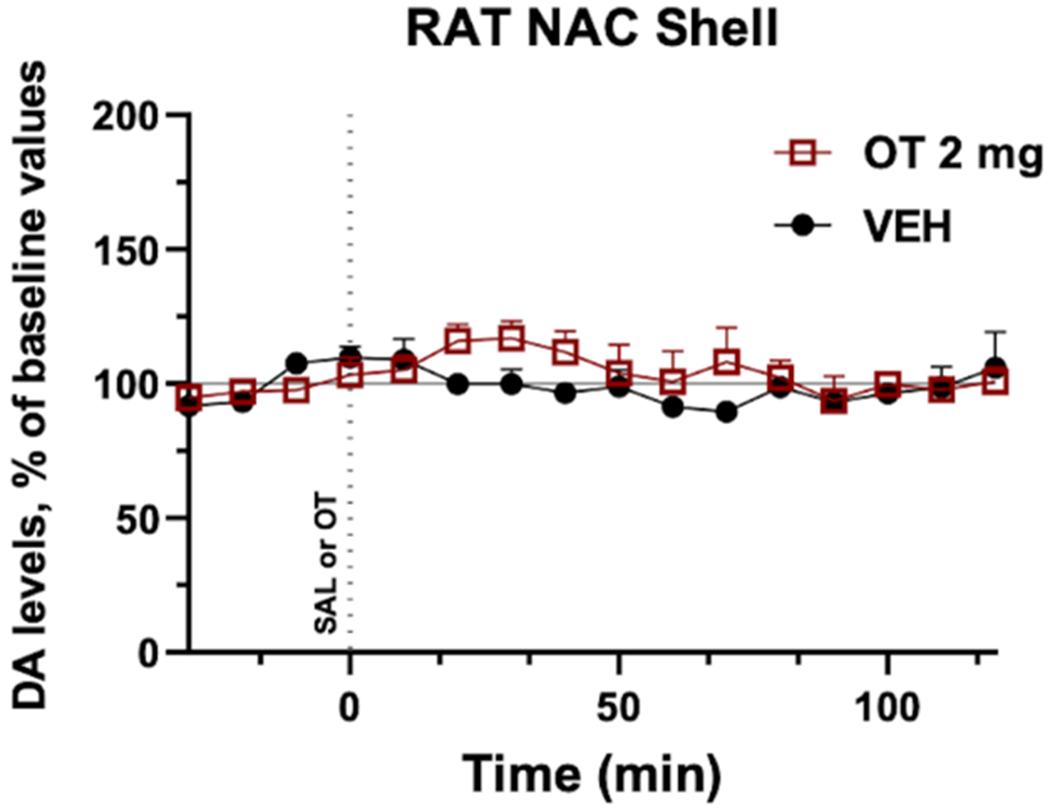
DA levels were measured every 10 min and, after a consistent baseline was established, either vehicle (VEH, saline; i.p., shown in black, n=5 rats) or OT (2 mg/kg; i.p., shown in red, n=6 rats) was administered. Data are shown as the average of each animal’s results normalized to their baseline and error bars indicate SEM.
OT potentiates MP-induced increases in tonic NAS DA
Next, we employed microdialysis to probe NAS DA in response to OT pretreatment (10 minutes) before administration of three increasing doses of MP administered 30 min apart (see experimental paradigm in Fig. 6A). Microdialysis results (Fig. 6) confirm our previous work (Lee et al. 2019), showing that oxytocin administration, 2 mg/kg i.p., significantly potentiates MP stimulation of DA levels. Further, we find that this effect can be significantly blunted by a pretreatment with the oxytocin receptor antagonist atosiban (2 mg/kg; i.p.), 10 min before administration of oxytocin [Two-way ANOVA, main effect treatment: F2,17=11.738, p<0.001; main effect time: F12,204=56.692, p<0.001; treatment by time interaction: F12,204=2.115, p<0.005].
Figure 6: Atosiban, an oxytocin-receptor antagonist, blunted oxytocin potentiation of methylphenidate (MP)-induced increases in extracellular dopamine (DA) levels in dialysates from the nucleus accumbens shell (NAS).
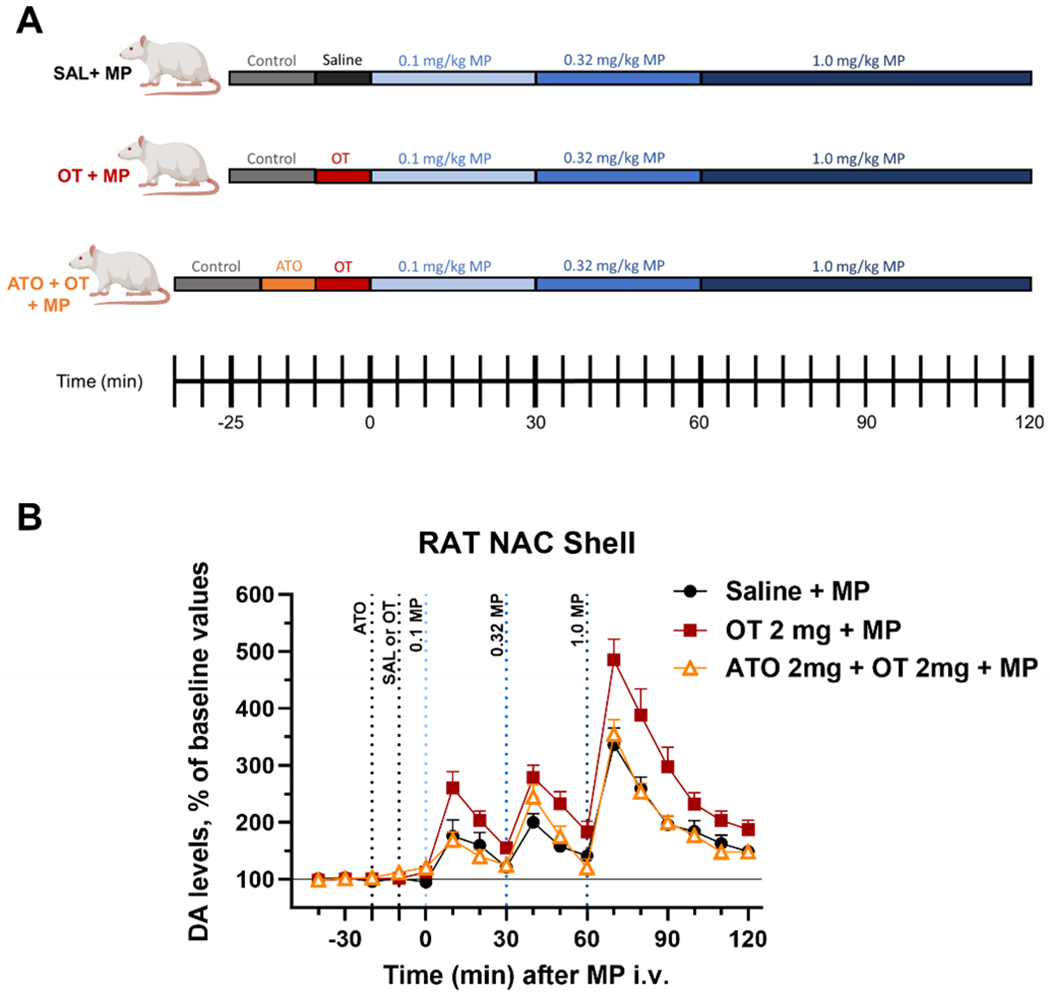
(A) A schematic showing the experimental paradigm for both treatment groups. (B) Time course of the effects of atosiban (ATO; 2 mg/kg; i.p.) administered 10 min before OT (2mg/kg; i.p.), and 10 min after OT administration, 3 increasing doses of MP (0.1, 0.3, and 1 mg/kg; i.v.), spaced 30 min apart, were administered. (Saline + MP, n=6 rats; OT + MP, n=8 rats; ATO + OT + MP, n=6 rats). Data are shown as the average of each animal’s results normalized to their baseline and error bars indicate SEM.
Discussion
Our results demonstrate that systemic OT administered alone produced no significant changes in NAS tonic and phasic DA as assessed by microdialysis and FSCV, respectively. OT pretreatment did not alter MP-stimulated increases in phasic, evoked NAS DA compared to a saline pretreatment using FSCV (Fig. 4). This finding suggests that, under our experimental conditions, OT pretreatment does not alter neuronal dopamine release in response to the stimulant MP. This observation is interesting because dopamine receptors have also been found in close proximity to and/or coupled to OT receptors, suggesting this interaction may be a natural process for modulating neurochemistry (Romero-Fernandez et al. 2013; Fuxe et al. 2012). However, it does not appear to have an effect under the current conditions. It is worth noting that during the present FSCV experiments, animals were anesthetized with urethane which largely halts spontaneous firing of DA neurons and may obstruct any DA firing effects potentially produced by interaction of DA and OT receptors. Thus, it is possible that under non-anesthetized conditions the results could be different. We also observed using FSCV that OT pretreatment did not significantly alter the rate of dopamine clearance, as compared to that of saline pretreated animals, indicating lack of direct interactions of OT on DAT function in a way that interferes with MP-induced inhibition of DA uptake.
Next, using microdialysis, we confirmed that OT pretreatment potentiates MP-induced increases in tonic, extracellular NAS DA levels (Lee et al. 2019). Further, the systemic effect of OT was blunted by a preemptive administration of the OT receptor antagonist, atosiban. Previous work suggested that OT’s enhancement of MP-stimulated NAS DA levels could be the result of OT activation of NAS OT receptors. For example, systemic administration of OT selectively potentiated the dopaminergic effects of MP in the NAS, but not in the accumbens core, and local infusion of OT into the NAS produced similar results (Lee et al. 2019). Alternatively, OT could directly interact with DAT and/or MP to produce systemic and local effects. Our present FSCV results, showing lack of significant changes in baseline or MP-stimulated evoked DA release or clearance rate, however, do not support such an alternative explanation. Moreover, our microdialysis tests with atosiban strongly suggest that OT potentiation of MP-induced stimulation of NAS DA levels is mediated by activation of OT receptors, because OT effects are antagonized by pretreating the animals with an OT receptor antagonist.
In particular, these studies tease out the differences in effects of OT on MP-induced increases in NAS DA in tonic (microdialysis) vs. phasic (FSCV) DA. From these studies, we postulate that (1) OT is not directly modulating dopaminergic neuron neurotransmitter release or electrically evoked release events, (2) nor is OT directly acting at DAT and/or interfering with MP-induced blockade of DAT and slowing DA clearance rate.
Our neurochemical results of OT potentiating MP-induced increases in NAS DA appear to be in sharp contrast to the ability of OT to blunt the behavioral reinforcing effects of MP under self-administration procedures (Tanda et al. 2017; Lee et al. 2019). Herein, we delve into the molecular underpinnings of oxytocin’s actions on NAS DA via OT receptors.
If DA transmission plays a role in psychostimulant self-administration behavior (Wise et al. 1995; Volkow et al. 2019), the effects of OT pretreatment aimed to block MP-maintained self-administration behavior shown in our previous study would suggest a possible impairment of post-synaptic DA transmission by OT. Such impairment would result in attenuated or blunted behavior even under conditions of increased DA signaling, as shown in our previous study (Lee et al. 2019). We would suggest that this OT-induced impairment in translating the post-synaptic DA transmission signal might also result in a potentially attenuated DA signal to pathways that play a role in maintaining the steady-state of DA in the terminal regions of DA neurons (Imperato & Di Chiara 1985; Imperato et al. 1988; Valenti & Grace 2010). This effect would produce activation of known DA neuronal feedback loops to restore DA levels, thus increasing DA release and DA extracellular levels (Imperato & Di Chiara 1985; Valenti & Grace 2010).
Our neurochemical results suggest that OT directly or indirectly interferes with post-synaptic DA transmission (stimulation/inhibition of DA firing, negative DA receptor feedback, etc.) producing the behavioral effects observed with OT administration without the expected blunting in NAS DA we probe ‘pre-synaptically’ (Lee et al. 2019). Additionally, OT appears to produce changes in NAS DA only in states of DA neurotransmission activation (such as that produced by MP administration). Under these conditions, increased receptor activation is present. This response may be further exacerbated by increases in negative DA receptor feedback, as well as increases in negative GABAergic input, both of which are activated by high dopaminergic tone. Our results suggest that OT might interfere with the recruitment of these feedback pathways via OT-induced post-synaptic changes in NAS DA signaling. However, we cannot exclude that systemic administration of OT may also modulate the endogenous oxytocinergic system, its pathways and receptors at the central and periphery levels. Systemic administration of OT may potentially affect the extracellular levels of OT in different brain areas (Neumann et al. 2013), thus potentially modulating oxytocin functions/activities (Moos et al. 1984; Carson et al. 2010). It is important to note that OT has been shown to differentially modulate distinct dopaminergic brain areas which may result in facilitation or inhibition of specific behavioral effects (Xiao et al. 2017).
It is well documented that OT receptors are also present on glutamatergic and GABAergic projections to mesolimbic regions like the NAS (Peris et al. 2017; Xiao et al. 2017). For example, infusion of OT into the nucleus accumbens was shown to increase glutamate levels in that region (Weber et al. 2018), without producing enhancements in NAS DA (Lee et al. 2019). OT has also been shown to increase GABA in multiple brain regions, including the hypothalamus, via a mechanism that is sensitive to atosiban (Thakur et al. 2019). Microdialysis studies on prefrontal cortex and dorsal hippocampus showed OT did not alter baseline glutamate, but did blunt methamphetamine induced increases in glutamate, while OT increased baseline GABA and blunted methamphetamine-induced decreases in the hippocampus (Qi et al. 2012). Therefore, it is possible that OT may be indirectly mediating NAS DA via actions on glutamatergic and GABAergic neurons, in order to produce the neurochemical changes on MP-induced increases that we observed.
In conclusion, we show that OT increases MP-induced stimulation of NAS DA in a manner that is dependent upon the activation of OT receptors. Further studies are needed to determine the mechanism by which OT modulates NAS DA after administration of MP. Our study is currently limited to male rats and to pharmacologic modulation of the OT receptor, thus additional studies are needed to provide a more encompassing mechanistic understanding of the actions of OT in PSUD. However, based on both clinical and preclinical studies showing OT’s ability to interfere with the behavioral, reinforcing actions of psychostimulants (Lee et al. 2017; McGregor & Bowen 2012; Sarnyai & Kovacs 2014), we postulate that OT could serve as a promising potential pharmacotherapeutic for PSUD.
Acknowledgments
The authors would like to thank Mark A. Coggiano for experimental support, Jian Jing Cao for figure assistance, Claudio Zanettini for statistical guidance, Gail Seabold for helpful revisions, and Amy H. Newman for thoughtful discussion. Figures created with BioRender.com. This work was supported in part by the Medication Development Program (Z1A DA000611, GT), National Institute on Drug Abuse (NIDA) Intramural Research Program, NIH/DHHS.
List of Abbreviations
- ADHD
Attention-deficit/hyperactivity disorder
- ATO
Atosiban
- AUC
Area under the curve
- DA
Dopamine
- DAmax
Maximum evoked dopamine release
- DAT
Dopamine transporter
- FSCV
Fast scan cyclic voltammetry
- i.p.
intraperitoneal
- i.v.
intravenous
- NAS
Nucleus accumbens shell
- MFB
Medial forebrain bundle
- MP
Methylphenidate
- OT
Oxytocin
- PSUD
Psychostimulant use disorder
- RRID
Research Resource Identifier
- t1/2
Clearance rate
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Data Availability
All data is available upon reasonable request.
References
- Broadbear JH, Tunstall B and Beringer K (2011) Examining the role of oxytocin in the interoceptive effects of 3, 4-methylenedioxymethamphetamine (MDMA,’ecstasy’) using a drug discrimination paradigm in the rat. Addiction biology 16, 202–214. [DOI] [PubMed] [Google Scholar]
- Carson DS, Hunt GE, Guastella AJ, Barber L, Cornish JL, Arnold JC, Boucher AA and McGregor IS (2010) Systemically administered oxytocin decreases methamphetamine activation of the subthalamic nucleus and accumbens core and stimulates oxytocinergic neurons in the hypothalamus. Addict Biol 15, 448–463. [DOI] [PubMed] [Google Scholar]
- Challman TD and Lipsky JJ (2000) Methylphenidate: Its Pharmacology and Uses. Mayo Clinic Proceedings 75, 711–721. [DOI] [PubMed] [Google Scholar]
- De Kloet E, Rotteveel F, Voorhuis TA and Terlou M (1985) Topography of binding sites for neurohypophyseal hormones in rat brain. European journal of pharmacology 110, 113–119. [DOI] [PubMed] [Google Scholar]
- Di Chiara G (2002) Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behavioural brain research 137, 75–114. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Acquas E, Tanda G and Cadoni C (1993) Drugs of abuse: biochemical surrogates of specific aspects of natural reward? Biochem Soc Symp 59, 65–81. [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Bassareo V, Pontieri F, Acquas E, Fenu S, Cadoni C and Carboni E (1999) Drug addiction as a disorder of associative learning. Role of nucleus accumbens shell/extended amygdala dopamine. Ann N Y Acad Sci 877, 461–485. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Cadoni C, Acquas E, Bassareo V and Carboni E (1998) Homologies and differences in the action of drugs of abuse and a conventional reinforcer (food) on dopamine transmission: an interpretative framework of the mechanism of drug dependence. Adv Pharmacol 42, 983–987. [DOI] [PubMed] [Google Scholar]
- Everett NA, Baracz SJ and Cornish JL (2020) The effect of chronic oxytocin treatment during abstinence from methamphetamine self-administration on incubation of craving, reinstatement, and anxiety. Neuropsychopharmacology 45, 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV (2018) The pharmacology of amphetamine and methylphenidate: Relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neuroscience & Biobehavioral Reviews 87, 255–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JC, Baker NL, McRae-Clark AL, Brady KT and Moran-Santa Maria MM (2015) Effects of adverse childhood experiences on the association between intranasal oxytocin and social stress reactivity among individuals with cocaine dependence. Psychiatry Res 229, 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Ciruela F, Manger P, Leo G, Díaz-Cabiale Z and Agnati LF (2012) On the role of volume transmission and receptor–receptor interactions in social behaviour: focus on central catecholamine and oxytocin neurons. Brain research 1476, 119–131. [DOI] [PubMed] [Google Scholar]
- Garces-Ramirez L, Green JL, Hiranita T et al. (2011) Sigma Receptor Agonists: Receptor Binding And Effects On Mesolimbic Dopamine Neurotransmission Assessed By Microdialysis. Biological Psychiatry 69, 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, O’brien CP, Auriacombe M et al. (2013) DSM-5 criteria for substance use disorders: recommendations and rationale. American Journal of Psychiatry 170, 834–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard H, Spencer MR and Garnett MF (2020) Increase in drug overdose deaths involving cocaine: United States, 2009–2018. NCHS data brief. [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B and Domes G (2009) Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol 30, 548–557. [DOI] [PubMed] [Google Scholar]
- Hersey M, Bacon AK, Bailey LG, Coggiano MA, Newman AH, Leggio L and Tanda G (2021) Psychostimulant Use Disorder, an Unmet Therapeutic Goal: Can Modafinil Narrow the Gap? Frontiers in Neuroscience 15, 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibragimov R, Kovacs GL, Szabo G and Telegdy G (1987) Microinjection of oxytocin into limbic-mesolimbic brain structures disrupts heroin self-administration behavior: a receptor-mediated event? Life Sci 41, 1265–1271. [DOI] [PubMed] [Google Scholar]
- Imperato A and Di Chiara G (1985) Dopamine release and metabolism in awake rats after systemic neuroleptics as studied by trans-striatal dialysis. J Neurosci 5, 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato A, Tanda G, Frau R and Di Chiara G (1988) Pharmacological profile of dopamine receptor agonists as studied by brain dialysis in behaving rats. J Pharmacol Exp Ther 245, 257–264. [PubMed] [Google Scholar]
- Kane S (2019) Sample Size Calculator. Vol. 2022. ClinCalc: https://clincalc.com/Stats/SampleSize.aspx. [Google Scholar]
- Keighron JD, Giancola JB, Shaffer RJ, DeMarco EM, Coggiano MA, Slack RD, Newman AH and Tanda G (2019) Distinct effects of (R)-modafinil and its (R)-and (S)-fluoro-analogs on mesolimbic extracellular dopamine assessed by voltammetry and microdialysis in rats. European Journal of Neuroscience 50, 2045–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CE, Gano A and Becker HC (2020) The role of oxytocin in alcohol and drug abuse. Brain Res 1736, 146761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CE, Griffin WC, Luderman LN, Kates MM, McGinty JF and Becker HC (2017) Oxytocin Reduces Ethanol Self-Administration in Mice. Alcohol Clin Exp Res 41, 955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Lee R, Wardle MC, Jacob S and de Wit H (2014) Effects of MDMA and Intranasal oxytocin on social and emotional processing. Neuropsychopharmacology 39, 1654–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GL, Borthaiser Z and Telegdy G (1985) Oxytocin reduces intravenous heroin self-administration in heroin-tolerant rats. Life Sci 37, 17–26. [DOI] [PubMed] [Google Scholar]
- Lee MR, Glassman M, King-Casas B, Kelly DL, Stein EA, Schroeder J and Salmeron BJ (2014) Complexity of oxytocins effects in a chronic cocaine dependent population. Eur Neuropsychopharmacol 24, 1483–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Rohn MC, Tanda G and Leggio L (2016) Targeting the oxytocin system to treat addictive disorders: rationale and progress to date. CNS drugs 30, 109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Rohn MCH, Tanda G and Leggio L (2017) Oxytocin’s Effects in Cocaine and Other Psychostimulant Addictions. In: The Neuroscience of Cocaine: Mechanisms and Treatment, (Preedy VR ed.), pp. 227–234. Elsevier Inc. [Google Scholar]
- Lee MR, Rohn MCH, Zanettini C, Coggiano MA, Leggio L and Tanda G (2019) Effect of systemically administered oxytocin on dose response for methylphenidate self-administration and mesolimbic dopamine levels. Ann N Y Acad Sci 1455, 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong KC, Freeman LR, Berini CR, Ghee SM, See RE and Reichel CM (2017) Oxytocin Reduces Cocaine Cued Fos Activation in a Regionally Specific Manner. Int J Neuropsychopharmacol 20, 844–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love TM (2014) Oxytocin, motivation and the role of dopamine. Pharmacol Biochem Behav 119, 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor IS and Bowen MT (2012) Breaking the loop: oxytocin as a potential treatment for drug addiction. Horm Behav 61, 331–339. [DOI] [PubMed] [Google Scholar]
- McRae-Clark AL, Baker NL, Maria MM and Brady KT (2013) Effect of oxytocin on craving and stress response in marijuana-dependent individuals: a pilot study. Psychopharmacology (Berl) 228, 623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos F, Freund-Mercier MJ, Guerne Y, Guerne JM, Stoeckel ME and Richard P (1984) Release of oxytocin and vasopressin by magnocellular nuclei in vitro: specific facilitatory effect of oxytocin on its own release. J Endocrinol 102, 63–72. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI, Lukas M and Landgraf R (2013) Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology 38, 1985–1993. [DOI] [PubMed] [Google Scholar]
- Olff M, Frijling JL, Kubzansky LD, Bradley B, Ellenbogen MA, Cardoso C, Bartz JA, Yee JR and van Zuiden M (2013) The role of oxytocin in social bonding, stress regulation and mental health: an update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology 38, 1883–1894. [DOI] [PubMed] [Google Scholar]
- Paxinos G and Watson C (1998) The Rat Brain in Stereotaxic Coordinates. . Academic Press, Sydney. [Google Scholar]
- Pedersen CA, Smedley KL, Leserman J, Jarskog LF, Rau SW, Kampov-Polevoi A, Casey RL, Fender T and Garbutt JC (2013) Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcohol Clin Exp Res 37, 484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris J, MacFadyen K, Smith JA, de Kloet AD, Wang L and Krause EG (2017) Oxytocin receptors are expressed on dopamine and glutamate neurons in the mouse ventral tegmental area that project to nucleus accumbens and other mesolimbic targets. Journal of Comparative Neurology 525, 1094–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Han WY, Yang JY, Wang LH, Dong YX, Wang F, Song M and Wu CF (2012) Oxytocin regulates changes of extracellular glutamate and GABA levels induced by methamphetamine in the mouse brain. Addiction biology 17, 758–769. [DOI] [PubMed] [Google Scholar]
- Rimmele U, Hediger K, Heinrichs M and Klaver P (2009) Oxytocin makes a face in memory familiar. J Neurosci 29, 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Fernandez W, Borroto-Escuela D, Agnati L and Fuxe K (2013) Evidence for the existence of dopamine d2-oxytocin receptor heteromers in the ventral and dorsal striatum with facilitatory receptor–receptor interactions. Molecular psychiatry 18, 849–850. [DOI] [PubMed] [Google Scholar]
- Romero T, Nagasawa M, Mogi K, Hasegawa T and Kikusui T (2014) Oxytocin promotes social bonding in dogs. Proceedings of the National Academy of Sciences of the United States of America 111, 9085–9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA, Leng G and Douglas AJ (2003) The magnocellular oxytocin system, the fount of maternity: adaptations in pregnancy. Front Neuroendocrinol 24, 27–61. [DOI] [PubMed] [Google Scholar]
- Sabeti J, Adams CE, Burmeister J, Gerhardt GA and Zahniser NR (2002) Kinetic analysis of striatal clearance of exogenous dopamine recorded by chronoamperometry in freely-moving rats. Journal of neuroscience methods 121, 41–52. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z and Kovacs GL (1994) Role of oxytocin in the neuroadaptation to drugs of abuse. Psychoneuroendocrinology 19, 85–117. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z and Kovacs GL (2014) Oxytocin in learning and addiction: From early discoveries to the present. Pharmacol Biochem Behav 119, 3–9. [DOI] [PubMed] [Google Scholar]
- Shellenberg TP, Stoops WW, Lile JA and Rush CR (2020) An update on the clinical pharmacology of methylphenidate: therapeutic efficacy, abuse potential and future considerations. Expert Review of Clinical Pharmacology 13, 825–833. [DOI] [PubMed] [Google Scholar]
- Solinas M, Justinova Z, Goldberg SR and Tanda G (2006) Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. Journal of neurochemistry 98, 408–419. [DOI] [PubMed] [Google Scholar]
- Solinas M, Panlilio L, Antoniou K, Pappas L and Goldberg S (2003) The cannabinoid CB1 antagonist N-piperidinyl-5-(4-chlorophenyl)-1-(2, 4-dichlorophenyl)-4-methylpyrazole-3-carboxamide (SR-141716A) differentially alters the reinforcing effects of heroin under continuous reinforcement, fixed ratio, and progressive ratio schedules of drug self-administration in rats. Journal of Pharmacology and Experimental Therapeutics 306, 93–102. [DOI] [PubMed] [Google Scholar]
- Stauffer CS, Musinipally V, Suen A, Lynch KL, Shapiro B and Woolley JD (2016) A two-week pilot study of intranasal oxytocin for cocaine-dependent individuals receiving methadone maintenance treatment for opioid use disorder. Addict Res Theory 24, 490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Ebbs A, Newman AH and Katz JL (2005) Effects of 4′-chloro-3α-(diphenylmethoxy)-tropane on mesostriatal, mesocortical, and mesolimbic dopamine transmission: comparison with effects of cocaine. Journal of Pharmacology and Experimental Therapeutics 313, 613–620. [DOI] [PubMed] [Google Scholar]
- Tanda G, Ebbs AL, Kopajtic TA, Elias LM, Campbell BL, Newman AH and Katz JL (2007) Effects of muscarinic M1 receptor blockade on cocaine-induced elevations of brain dopamine levels and locomotor behavior in rats. Journal of Pharmacology and Experimental Therapeutics 321, 334–344. [DOI] [PubMed] [Google Scholar]
- Tanda G, Kopajtic TA and Katz JL (2008) Cocaine-like neurochemical effects of antihistaminic medications. Journal of neurochemistry 106, 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Mereu M, Hiranita T, Quarterman JC, Coggiano M and Katz JL (2016) Lack of specific involvement of (+)-naloxone and (+)-naltrexone on the reinforcing and neurochemical effects of cocaine and opioids. Neuropsychopharmacology 41, 2772–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE and Di Chiara G (1997) Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science 276, 2048–2050. [DOI] [PubMed] [Google Scholar]
- Tanda G, Rohn MC, Newman AH, Coggiano M, Zanettini C, Katz JL, Leggio L and Lee MR (2017) Systemic Oxytocin Attenuates Methylphenidate Self-Administration and Potentiates Its Effects on Dopamine in the Nucleus Accumbens Shell in Rats. The FASEB Journal 31, 986.989–986.989. [Google Scholar]
- Tanda G, Valentini V, De Luca MA, Perra V, Serra GP and Di Chiara G (2015) A systematic microdialysis study of dopamine transmission in the accumbens shell/core and prefrontal cortex after acute antipsychotics. Psychopharmacology 232, 1427–1440. [DOI] [PubMed] [Google Scholar]
- Thakur P, Shrivastava R and Shrivastava VK (2019) Effects of exogenous oxytocin and atosiban antagonist on GABA in different region of brain. IBRO reports 6, 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.N. (2021) World Drug Report
- Valenti O and Grace AA (2010) Antipsychotic drug-induced increases in ventral tegmental area dopamine neuron population activity via activation of the nucleus accumbens-ventral pallidum pathway. Int J Neuropsychopharmacol 13, 845–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Michaelides M and Baler R (2019) The neuroscience of drug reward and addiction. Physiological reviews 99, 2115–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber RA, Logan CN, Leong K-C, Peris J, Knackstedt L and Reichel CM (2018) Regionally specific effects of oxytocin on reinstatement of cocaine seeking in male and female rats. International Journal of Neuropsychopharmacology 21, 677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Leeb K, Pocock D, Newton P, Burnette B and Justice J (1995) Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology 120, 10–20. [DOI] [PubMed] [Google Scholar]
- Xiao L, Priest MF, Nasenbeny J, Lu T and Kozorovitskiy Y (2017) Biased oxytocinergic modulation of midbrain dopamine systems. Neuron 95, 368–384. e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL and Wang Z (2011) The role of mesocorticolimbic dopamine in regulating interactions between drugs of abuse and social behavior. Neurosci Biobehav Rev 35, 498–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data is available upon reasonable request.


