Abstract
Background
Healthcare workers are at risk of acquiring viral diseases such as hepatitis B, hepatitis C and HIV through exposure to contaminated blood and body fluids at work. Most often infection occurs when a healthcare worker inadvertently punctures the skin of their hand with a sharp implement that has been used in the treatment of an infected patient, thus bringing the patient's blood into contact with their own. Such occurrences are commonly known as percutaneous exposure incidents.
Objectives
To determine the benefits and harms of extra gloves for preventing percutaneous exposure incidents among healthcare workers versus no intervention or alternative interventions.
Search methods
We searched CENTRAL, MEDLINE, EMBASE, NHSEED, Science Citation Index Expanded, CINAHL, NIOSHTIC, CISDOC, PsycINFO and LILACS until 26 June 2013.
Selection criteria
Randomised controlled trials (RCTs) with healthcare workers as the majority of participants, extra gloves or special types of gloves as the intervention, and exposure to blood or bodily fluids as the outcome.
Data collection and analysis
Two authors independently assessed study eligibility and risk of bias, and extracted data. We performed meta‐analyses for seven different comparisons.
Main results
We found 34 RCTs that included 6890 person‐operations as participating units and reported on 46 intervention‐control group comparisons. We grouped interventions as follows: increased layers of standard gloves, gloves manufactured with special protective materials or thicker gloves, and gloves with puncture indicator systems. Indicator gloves show a coloured spot when they are perforated. Participants were surgeons in all studies and they used at least one pair of standard gloves as the control intervention. Twenty‐seven studies also included other surgical staff (e.g. nurses). All but one study used perforations in gloves as an indication of exposure. The median control group rate was 18.5 perforations per 100 person‐operations. Seven studies reported blood stains on the skin and two studies reported self reported needlestick injuries. Six studies reported dexterity as visual analogue scale scores for the comparison double versus single gloves, 13 studies reported outer glove perforations. We judged the included studies to have a moderate to high risk of bias.
We found moderate‐quality evidence that double gloves compared to single gloves reduce the risk of glove perforation (rate ratio (RR) 0.29, 95% confidence interval (CI) 0.23 to 0.37) and the risk of blood stains on the skin (RR 0.35, 95% CI 0.17 to 0.70). Two studies with a high risk of bias also reported the effect of double compared to single gloves on needlestick injuries (RR 0.58, 95% CI 0.21 to 1.62).
We found low‐quality evidence in one small study that the use of three gloves compared to two gloves reduces the risk of perforation further (RR 0.03, 95% CI 0.00 to 0.52). There was similar low‐quality evidence that the use of one fabric glove over one normal glove reduces perforations compared to two normal gloves (RR 0.24, 95% CI 0.06 to 0.93). There was moderate‐quality evidence that this effect was similar for the use of one special material glove between two normal material gloves. Thicker gloves did not perform better than thinner gloves.
There was moderate to low‐quality evidence in two studies that an indicator system does not reduce the total number of perforations during an operation even though it reduces the number of perforations per glove used.
There was moderate‐quality evidence that double gloves have a similar number of outer glove perforations as single gloves, indicating that there is no loss of dexterity with double gloves (RR 1.10, 95% CI 0.93 to 1.31).
Authors' conclusions
There is moderate‐quality evidence that double gloving compared to single gloving during surgery reduces perforations and blood stains on the skin, indicating a decrease in percutaneous exposure incidents. There is low‐quality evidence that triple gloving and the use of special gloves can further reduce the risk of glove perforations compared to double gloving with normal material gloves. The preventive effect of double gloves on percutaneous exposure incidents in surgery does not need further research. Further studies are needed to evaluate the effectiveness and cost‐effectiveness of special material gloves and triple gloves, and of gloves in other occupational groups.
Plain language summary
Extra gloves or special types of gloves for preventing sharps injuries in healthcare workers
Background
Healthcare workers can hurt themselves accidentally with needles or sharp instruments that have been used in patient care. This carries a small risk that the healthcare worker becomes infected with a viral disease such as hepatitis or HIV. Therefore it is important to prevent blood contact to prevent infection. We evaluated whether the use of gloves, more than one layer of gloves or special gloves can prevent needles or sharp instruments from piercing the skin. Up until June 2013, we found 34 studies that evaluated 6890 operations. There were no studies in non‐surgical staff.
Two pairs of gloves compared to one pair only
In 12 studies, two pairs of gloves reduced the number of perforations in gloves by 71% compared to the use of one pair of gloves. In three studies, two pairs of gloves reduced blood stains on the skin by 65%. The reduction in self reported needlestick injuries was less clear.
Three pairs of gloves compared to two pairs of ordinary gloves
One low‐quality study showed that triple gloves compared to double gloves can further reduce perforations.
A pair of thicker or special gloves compared to a pair of ordinary gloves
Five low‐quality studies showed that the number of perforations was similar for thicker and thinner gloves. In two low‐quality studies, the use of one pair of fabric gloves over one pair of normal gloves reduced perforations compared to two pairs of normal gloves. This was similar for gloves made from special material such as fabric or steel, used in between normal gloves.
Indicator gloves
Indicator gloves show a coloured spot when they are pierced. Two studies showed that they reduced the number of perforations per glove but not the total amount of perforations.
Sensitivity of the fingers
There were no indications that using more layers of gloves decreased sensitivity of the fingers.
Conclusions
Surgeons and surgical staff can reduce their risk of contracting a serious viral infection by wearing two pairs of gloves instead of one pair of gloves. The use of three glove layers or gloves made from special material probably reduces the risk further but these need better evaluation. We need further studies to evaluate whether gloves have a similar preventive effect in other healthcare professionals outside the operating theatre.
Summary of findings
Summary of findings for the main comparison. Double gloves compared to single gloves for preventing percutaneous exposure injuries in healthcare personnel.
| Double gloves compared to single gloves for preventing percutaneous exposure injuries in healthcare personnel | ||||||
| Patient or population: healthcare personnel Settings: operating theatre Intervention: double gloves Comparison: single gloves | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Single gloves | Double gloves | |||||
| Inner glove perforations Water leak test or air test Follow‐up: median 1 operation | Study population | Rate ratio 0.29 (0.23 to 0.37) | 3437 (12 studies) | ⊕⊕⊕⊝ moderate1,2 | Includes 10 studies which measured the number of perforations and 2 studies which measured the number of gloves with 1 or more perforation Assumed risk calculated as the mean across the 10 studies that measured the number of perforations Risk expressed as the number of perforations per 1000 person‐operations | |
| 172 per 1000 | 50 per 1000 (40 to 64) | |||||
| Low | ||||||
| 7 per 1000 | 2 per 1000 (2 to 3) | |||||
| High | ||||||
| 280 per 1000 | 81 per 1000 (64 to 104) | |||||
| Dexterity: outer glove perforations ‐ number of perforations Water leak test or air test Follow‐up: median 1 operation | Study population | Rate ratio 1.10 (0.93 to 1.31) | 2817 (8 studies) | ⊕⊕⊕⊝ moderate2,3 | Includes 6 studies that measured the number of perforations and 2 studies that measured the number of gloves with 1 or more perforations
Assumed risk calculated as the mean across the 6 studies that measured the number of perforations Risk expressed as the number of perforations per 1000 person‐operations |
|
| 178 per 1000 | 195 per 1000 (165 to 233) | |||||
| Low | ||||||
| 8 per 1000 | 9 per 1000 (7 to 10) | |||||
| High | ||||||
| 290 per 1000 | 319 per 1000 (270 to 380) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1In most studies the outcome assessor was not blinded (n = 5) or it was unclear if the outcome assessors were blinded (n = 6). Only one study used a combined air and water test for the outcome assessment. In most studies the randomisation (sequence generation, concealment) was unclear (n = 9) and two studies had a high risk of bias. 2Heterogeneity: I² = 0%. 3In most studies the outcome assessor was not blinded (n = 5) or it was unclear if the outcome assessors were blinded (n = 2). Only one study used a combined air and water test for the outcome assessment. In most studies the randomisation (sequence generation, concealment) was unclear (n = 5) and two studies had a high risk of bias.
Background
Healthcare workers are at risk of acquiring infectious diseases through exposure at work. Exposure to blood or bodily fluids from infected patients can lead to infection with hepatitis B, hepatitis C and human immunodeficiency virus (HIV), among other pathogens. These are serious viral infections that may cause a chronic disease process or initiate cancer and eventually lead to death. According to Pruss‐Ustun et al, 16,000 hepatitis C, 66,000 hepatitis B and 1000 HIV infections may have occurred worldwide among healthcare workers in the year 2000 due to occupational exposure to blood and bodily fluids (Pruss‐Ustun 2005). The World Health Organization (WHO) reports that two million healthcare workers across the world experience percutaneous exposure to infectious diseases each year (WHO 2007). In Europe it is estimated that there are more than one million needlestick injuries annually (European Biosafety Network 2010). A European Union directive on prevention of sharp injuries in the hospital and healthcare sector was agreed upon in 2009, and member states were bound to implement the directive into their national legislation by May 2013.
Description of the condition
The risk of acquiring an infection is proportional to the prevalence of the infections in the patient population. Thus, in areas where hepatitis B, hepatitis C and HIV are highly prevalent, such as in certain countries in Africa, Asia and Eastern Europe, the risks are much higher than in Northern and Western Europe, Australia or in North America (Centers for Disease Control 2012; Shepard 2005). This situation has a significant impact on the health of workers and also on the healthcare system as a whole. The transmission of occupational blood‐borne infectious diseases leads to absenteeism, morbidity and, in some cases, mortality among healthcare workers. This leads to a reduction in the healthcare workforce and consequently affects patients’ quality of care and safety. The risk of acquiring an infectious disease at work means that healthcare workers may also suffer from psychological stress, which affects both their work and personal life (Fisman 2002; Sohn 2006). There is also an economic burden imposed on hospitals due to managing occupational exposure to blood‐borne diseases, such as costs related to blood tests, treatment, outpatient visits and lost working hours.
Description of the intervention
Exposure to blood or bodily fluids, also called percutaneous exposure, occurs when healthcare workers are injured with sharp needles or instruments, or when body fluids including blood are splashed during medical procedures. These incidents are called sharps or needlestick injuries, or percutaneous exposure incidents (PEIs). The actual causes of PEIs are multifactorial and include elements such as, but not limited to, types of devices and procedures, lack of access to or availability of personal protective equipment for the healthcare workers, sub‐optimal use of personal protective equipment, professional inexperience and lack of training and education on infection control and occupational health principles, improper management of sharps, poor organisational climate, high workload and fatigue, working alternate shifts, and high mental pressure and subjective perception of risk (Akduman 1999; Ansa 2002; Clarke 2002; Doebbeling 2003; Fisman 2007; Ilhan 2006; Oh 2005; Orji 2002; Roberts 1999; Smith 2006; Smith 2006b; Wallis 2007). Most of these causes can be addressed by specific interventions.
There are several types of interventions to prevent infection from PEI. For hepatitis B, vaccination has been successful (Chen 2005), but vaccination is not yet possible for hepatitis C or HIV (Mast 2004). Exposure reduction therefore remains the main preventive strategy. In general, there are several strategies for reducing or eliminating exposure, such as elimination of hazards at the source (for example, the elimination of unnecessary injections) or along the path (for example, safety medical devices or workplace practices, use of personal protective equipment, etc.) (Ellenbecker 1996; Roelofs 2003). The intervention examined in this Cochrane review, the use of gloves, is in the category of personal protective equipment.
How the intervention might work
The effectiveness of intact latex gloves as protection from HIV, for instance, has been shown in mechanical tests in the laboratory (Dalgleish 1988). Wearing multiple gloves (two or more pairs of gloves) is thought to provide increased resistance to needle penetration and thus protection against the transmission of body fluids (Edlich 2003). Special materials, such as gloves made from stainless steel wire weave, are expected to have a similar effect, as demonstrated by mechanical puncture tests with a needle penetration machine (Diaz‐Buxo 1991; Leslie 1996; Manson 1995; Mansouri 2010). Lefebvre 2008 demonstrated that double gloving significantly decreases the transmission of aqueous contaminant with cutting surgery needles as compared to a single glove layer. Finally, an indicator system attached to gloves might also decrease exposure to blood because it warns the user about glove punctures. Even though this would not prevent needlestick injuries as such, it could influence the person's behaviour in performing the task more safely and thus have a preventive effect.
Why it is important to do this review
There are several strategies available to reduce PEIs among healthcare workers and these are widely used. It is therefore important to know whether these preventive interventions are effective. Retrospective studies indicate that PEIs can be reduced by more than 50% by behavioural interventions, either education or the adoption of new techniques (Bryce 1999; Castella 2003). The use of safety devices may also have a significant effect (Bryce 1999; Castella 2003; Waclawski 2004). Even though the protective effect of double gloving has been shown for a long time in individual studies, it has been reported that single gloving still occurs (see also Cicconi 2010 and Haines 2011). However, the use of single gloves among healthcare workers is inconsistent and may be influenced by several factors including risk perception, healthcare culture and the availability and accessibility of supplies (Fadeyi 2011; Kinlin 2010; Timilshina 2011). Glove use should be emphasised as a key element of multimodal sharps injury reduction programmes. A systematic review might help in the better implementation of an effective intervention. Extra gloves would also help to reduce transmission of infections from healthcare workers to patients. This topic has been studied in another Cochrane Review (Tanner 2009) and the authors found two trials that reported fewer patient infections with double gloving. Needlestick injuries sustained by surgical staff were, however, not a primary outcome in that review. Our review is one of a group of Cochrane Reviews that address interventions to prevent PEIs: one on blunt needles by Parantainen 2011, one on safe devices by Lavoie 2012 and another on education and training, which is ongoing.
Objectives
To determine the benefits and harms of extra gloves for preventing percutaneous exposure incidents among healthcare workers versus no intervention or alternative interventions.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) including cluster‐randomised controlled trials (c‐RCTs), irrespective of language of publication, publication status or blinding.
Types of participants
Participants are healthcare workers, who are all persons professionally involved in providing health care to patients. We decided that at least 75% of the participants needed to fulfil this criterion.
Types of interventions
Inclusion criteria
We included all interventions that aim to reduce exposure to bodily fluids, including blood, by using extra gloves or special types of gloves.
We categorised interventions according to mode of action:
increasing the number of layers of gloves;
using thick gloves or gloves manufactured with special protective materials;
using glove puncture indicator systems which warn the worker about glove perforations; and
interventions with combinations of two or more of the above.
Exclusion criteria
We excluded studies carried out in the laboratory without direct (human) patient contacts.
Types of outcome measures
Primary outcomes
Exposure of healthcare workers to potentially contaminated bodily fluids, including blood, was our primary outcome measure. Exposure could be either needlestick injury, sharps injury, blood stains inside the gloves or on the skin, or glove perforations. We considered all reports of such exposure as valid measures of the outcome, including self reports, reports by the employer or empirical observations of blood stains by researchers.
Secondary outcomes
We used dexterity as a secondary outcome. We used the ratio of the number of perforations in the most outer gloves as an indication of loss of dexterity when wearing two glove layers compared to one glove layer. This is based on the assumption that a loss of dexterity as a result of double gloving would lead to a higher number of perforations in the outer gloves, whereas the inner gloves could still protect against skin perforation. In addition, we took visual analogue scales (VAS) as indicators of loss of dexterity.
Search methods for identification of studies
The search until September 2010 was part of a larger search for all interventions to prevent percutaneous exposure incidents (PEIs) in healthcare personnel. For interventions that are difficult to randomise we then also included non‐randomised studies. After the division of the original review into four separate reviews in 2011, we used an updated search strategy that was restricted to randomised glove studies only.
Electronic searches
For the original search we first applied search terms for percutaneous exposure incident (PEI). We then combined these terms for PEI with the recommended search strings for randomised trials (Robinson 2002) and for non‐randomised studies (Verbeek 2005).
We used the strategy to search CENTRAL, MEDLINE, EMBASE, NHSEED, Science Citation Index Expanded, CINAHL, OSH‐update (NIOSHTIC and CISDOC), LILACS and PsycINFO from the earliest record to September 2010. In addition, we searched the databases of WHO, the UK National Health Service (NHS) and the International Healthcare Worker Safety Center until 2009 (Appendix 1).
For the updated search we simplified the original search and used only a filter for randomised studies. We searched the same databases up until June 2013, except for LILACS, because the initial search did not reveal any studies (Appendix 2).
Searching other resources
We screened the reference lists of all relevant studies for additional studies. We contacted authors of intervention studies to obtain information missing from their published reports.
Data collection and analysis
Selection of studies
Using the inclusion and exclusion criteria, authors worked independently in pairs (AS and JV, M‐CL and MP) to screen the identified titles and abstracts of the references that were identified by the search strategy for potential studies. We obtained the full texts of those references that appeared to meet the inclusion criteria. We did not blind the full‐text articles because we felt that this would not increase validity. We resolved disagreements between pairs by consensus. The pairs consulted a third author when disagreements persisted.
Data extraction and management
Authors worked independently in pairs to extract data from the included studies into a form (AS and JV, M‐CL and MP, CM and SI). The form included the essential characteristics of the study, participants, interventions, outcomes and results. We also noted any adverse events and the sponsorship of the study. The two pairs of authors (AS and JV, M‐CL and MP, CM and SI) independently assessed the risk of bias of each study, using consensus when disagreements occurred. The pairs consulted a third author when disagreements persisted. We did not mask trial names because we did not believe that this would have increased validity.
Assessment of risk of bias in included studies
For the assessment of risk of bias in studies, we used the 'Risk of bias' tool as provided in RevMan 5 (RevMan 2012). We used the items on randomisation, allocation concealment, blinding of participants and outcome assessors, incomplete outcome data and selective outcome reporting, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
To rate the overall risk of bias in studies, we used random sequence generation, allocation concealment and blinding of the outcome assessor as the most important domains. We rated studies that had a high risk of bias in one of these three items as having a high risk of bias. We rated studies with low risk of bias in all three items as having a low risk of bias.
Measures of treatment effect
Authors reported the outcome of their studies in many different ways. We assumed that the most valid estimate of the risk of exposure for healthcare personnel was provided by the number of holes in gloves used by one person during one operation. It would have been more precise if 'operation' could have been defined as 'the number of hours engaged in an operation of average difficulty', comparable to a number of person‐years at risk, but the data were not sufficient to calculate this.
We treated the results of all trials as being dichotomous and used rate ratios (RR) rather than odds ratios, because of the high prevalence of most outcomes. Some studies reported rates that were larger than one per unit because needlestick injuries or glove perforations can be sustained more than once. To address this issue, we calculated the natural logarithm (ln) of the RRs and their standard errors from the number of glove perforations and the number of surgeon‐operations in an Excel spreadsheet, as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used the natural log of the rate ratios and their standard errors as input in RevMan where we combined them using the generic inverse variance method. We provide the raw data for all studies in additional tables (Table 2; Table 3; Table 4).
1. Raw data: increasing glove layers.
| Intervention | Control | Rate ratio | 95% CI | ||||||||||
| Study | # Events |
# Persons per operation |
# Operations (or glove pairs) |
Rate per person per operation |
# Events |
# Persons per operation |
# Operations (or glove pairs) |
Rate per person per operation |
RR | lnRR | SE1 | Lower | Upper |
| Intervention: increasing glove layers Comparison: double versus single | |||||||||||||
| Outcome: matched perforations per person‐operation | |||||||||||||
| Kovavisarach 1998 | 2 | 1.00 | 329 | 0.0061 | 53 | 1.00 | 371 | 0.1429 | 0.043 | ‐3.157 | 0.7203 | — | — |
| Kovavisarach 2002 | 1 | 1.00 | 82 | 0.0122 | 20 | 1.00 | 88 | 0.2273 | 0.054 | ‐2.925 | 1.0247 | — | — |
| Punyatanasakchai 2004 | 3 | 1.00 | 150 | 0.0200 | 27 | 1.00 | 150 | 0.1800 | 0.111 | ‐2.197 | 0.6086 | — | — |
| Thomas 2001 | 4 | 1.00 | 66 | 0.0606 | 19 | 1.00 | 66 | 0.2879 | 0.211 | ‐1.558 | 0.5501 | — | — |
| Outcome: inner glove perforations per person‐operation | |||||||||||||
| Gani 1990 | 22 | 3.86 | 103 | 0.0553 | 108 | 3.85 | 115 | 0.2439 | 0.227 | ‐1.4835 | 0.2339 | — | — |
| Rudiman 1999 | 3 | 1.00 | 60 | 0.0500 | 15 | 1.00 | 60 | 0.2500 | 0.200 | ‐1.609 | 0.6325 | — | — |
| Avery 1999a | 0.5 | 1.85 | 67 | 0.0040 | 1 | 1.83 | 71 | 0.0077 | 0.524 | ‐0.646 | 1.7321 | — | — |
| Kovavisarach 1998 | 19 | 1.00 | 329 | 0.0578 | 53 | 1.00 | 371 | 0.1429 | 0.404 | ‐0.906 | 0.2674 | — | — |
| Kovavisarach 1999 | 3 | 1.00 | 150 | 0.0200 | 16 | 1.00 | 150 | 0.1067 | 0.188 | ‐1.674 | 0.6292 | — | — |
| Kovavisarach 2002 | 5 | 1.00 | 82 | 0.0610 | 20 | 1.00 | 88 | 0.2273 | 0.268 | ‐1.316 | 0.5000 | — | — |
| Laine 2004b 2R | 6 | 1.41 | 90 | 0.0473 | 14 | 1.36 | 81.0 | 0.1271 | 0.372 | ‐0.989 | 0.4880 | — | — |
| Punyatanasakchai 2004 | 7 | 1.00 | 150 | 0.0467 | 27 | 1.00 | 150 | 0.1800 | 0.259 | ‐1.3499 | 0.4241 | — | — |
| Thomas 2001 | 10 | 1.00 | 66 | 0.1515 | 19 | 1.00 | 66 | 0.2879 | 0.526 | ‐0.642 | 0.3907 | — | — |
| Wilson 1996a | 2 | 1.00 | 96 | 0.0208 | 4.7 | 1.00 | 32 | 0.1458 | 0.143 | ‐1.946 | 0.8452 | — | — |
| Wilson 1996b | 2 | 1.00 | 96 | 0.0208 | 4.7 | 1.00 | 32 | 0.1458 | 0.143 | ‐1.946 | 0.8452 | — | — |
| Wilson 1996c | 3 | 1.00 | 96 | 0.0313 | 4.7 | 1.00 | 32 | 0.1458 | 0.214 | ‐1.540 | 0.7400 | — | — |
| Outcome: matched perforations per glove pair | |||||||||||||
| Jensen 1997 | 8 | — | 100 | 0.0800 | 40 | — | 100 | 0.4000 | 0.200 | ‐1.609 | 0.3873 | 0.09 | 0.43 |
| Outcome: inner glove perforations per glove pair/gloves | |||||||||||||
| Aarnio 2001 | 0.5 | — | 196 (gloves) | 0.0026 | 12 | — | 204 (gloves) | 0.0588 | 0.043 | ‐3.138 | 1.443 | 0.00 | 0.73 |
| Jensen 1997 | 12 | — | 100 | 0.1200 | 40 | — | 100 | 0.4000 | 0.3 | ‐1.204 | 0.329 | 0.16 | 0.57 |
| Marín Bertolin 1997 | 14 | — | 338 | 0.0414 | 31 | — | 328 | 0.0945 | 0.438 | ‐0.825 | 0.322 | 0.23 | 0.82 |
| Doyle 1992 | 3 | — | 79 | 0.0380 | 24 | — | 68 | 0.3529 | 0.108 | ‐2.229 | 0.612 | 0.03 | 0.36 |
| Outcome: number of inner glove perforations | |||||||||||||
| Berridge 1998 | 4 | — | — | — | 18 | — | — | — | — | — | — | — | — |
| Laine 2001 | 6 | — | — | — | 38 | — | — | — | — | — | — | — | — |
| Outcome: outer glove perforations per person‐operation | |||||||||||||
| Avery 1999a | 3 | 1.85 | 67 | 0.0242 | 1 | 1.83 | 71 | 0.0077 | 3.1447 | 1.1457 | 1.1547 | — | — |
| Gani 1990 | 117 | 3.86 | 103 | 0.2943 | 108 | 3.85 | 115 | 0.2439 | 1.2064 | 0.1877 | 0.1334 | — | — |
| Kovavisarach 1998 | 41 | 1.00 | 329 | 0.1246 | 53 | 1.00 | 371 | 0.1429 | 0.8723 | ‐0.1366 | 0.2080 | — | — |
| Kovavisarach 1999 | 20 | 1.00 | 150 | 0.1333 | 16 | 1.00 | 150 | 0.1067 | 1.2500 | 0.2231 | 0.3354 | — | — |
| Kovavisarach 2002 | 16 | 1.00 | 82 | 0.1951 | 20 | 1.00 | 88 | 0.2273 | 0.8585 | ‐0.1525 | 0.3354 | — | — |
| Punyatanasakchai 2004 | 34 | 1.00 | 150 | 0.2267 | 27 | 1.00 | 150 | 0.1800 | 1.2593 | 0.2305 | 0.2578 | — | — |
| Rudiman 1999 | 12 | 1.00 | 60 | 0.2000 | 15 | 1.00 | 60 | 0.2500 | 0.8000 | ‐0.2231 | 0.3873 | — | — |
| Thomas 2001 | 22 | 1.00 | 66 | 0.3333 | 19 | 1.00 | 66 | 0.2879 | 1.1579 | 0.1466 | 0.3132 | — | — |
| Outcome: outer glove perforations per glove pair | |||||||||||||
| Aarnio 2001 | 3 | — | 196 | 0.0153 | 12 | — | 204 | 0.0588 | 0.2602 | ‐1.3463 | 0.6455 | 0.07 | 0.92 |
| Doyle 1992 | 21 | — | 79 | 0.2658 | 24 | — | 68 | 0.3529 | 0.7532 | ‐0.2835 | 0.2988 | 0.42 | 1.35 |
| Jensen 1997 | 47 | — | 100 | 0.4700 | 40 | — | 100 | 0.4000 | 1.1750 | 0.1613 | 0.2151 | 0.77 | 1.79 |
| Marín Bertolin 1997 | 38 | — | 343 | 0.1108 | 31 | — | 335 | 0.0925 | 1.1972 | 0.1800 | 0.2420 | 0.75 | 1.92 |
| Outcome: number of outer glove perforations | |||||||||||||
| Berridge 1998 | 28 | — | — | — | 18 | — | — | — | — | — | — | — | — |
| Outcome: blood stains on the skin per person‐operation | |||||||||||||
| Avery 1999a | 0 | 1.85 | 67 | — | 0 | 1.83 | 71 | — | — | — | — | — | — |
| Naver 2000 | 2 | 2.66 | 98 | 0.0077 | 15 | 2.66 | 115 | 0.0490 | 0.156 | ‐1.8549 | 0.7528 | — | — |
| Rudiman 1999 | 4 | 1.00 | 60 | 0.0667 | 13 | 1.00 | 60 | 0.2167 | 0.308 | ‐1.1787 | 0.5718 | — | — |
| Thomas 2001 | 5 | 1.00 | 66 | 0.0758 | 8 | 1.00 | 66 | 0.1212 | 0.625 | ‐0.4700 | 0.5701 | — | — |
| Outcome: number of blood stains on the skin | |||||||||||||
| Berridge 1998 | 4 | — | — | — | 8 | — | — | — | — | — | — | — | — |
| Outcome: needlestick injuries per used glove pair | |||||||||||||
| Doyle 1992 | 1 | — | 79 | 0.0127 | 3 | — | 68 | 0.0441 | 0.287 | ‐1.2486 | 1.1547 | 0.03 | 2.76 |
| Marin‐Bertolin 1997 | 5 | — | 343 | 0.0146 | 7 | — | 335 | 0.0209 | 0.698 | ‐0.3601 | 0.5855 | 0.22 | 2.20 |
| Intervention: increasing glove layers Comparison: triple versus double | |||||||||||||
| Outcome: inner glove perforations per person‐operation | |||||||||||||
| Pieper 1995 l‐l‐l | 0.5 | 1.00 | 15 | 0.0333 | 16 | 1.00 | 15 | 1.0667 | 0.031 | ‐3.466 | 1.4361 | — | — |
1Calculation: SE = SQRT(1/intervention events + 1/control events)
CI: confidence interval; RR: rate ratio; lnRR natural logarithm of the rate‐ratio; SE: standard error
2. Raw data: special gloves.
| Intervention | Control | Rate ratio | 95% CI | ||||||||||
| Study | # Events |
# Persons per operation |
# Operations (or glove pairs) |
Rate per person per operation |
# Events |
# Persons per operation |
# Operations (or glove pairs) |
Rate | RR | lnRR | SE | Lower | Upper |
| Intervention: special gloves Comparison: double special versus double normal | |||||||||||||
| Outcome: inner glove perforations per person‐operation | |||||||||||||
| Hester 1992 o‐c | 7 | 3.50 | 25 | 0.0800 | 8 | 3.50 | 25 | 0.0914 | 0.875 | ‐0.134 | 0.5176 | — | — |
| Louis 1998 | 16 | 2.00 | 25 | 0.3200 | 22 | 2.00 | 25 | 0.4400 | 0.727 | ‐0.319 | 0.3286 | — | — |
| Sanders 1990 | 2 | 1.00 | 25 | 0.0800 | 26 | 1.00 | 25 | 1.0400 | 0.077 | ‐2.565 | 0.7338 | — | — |
| Underwood 1993 | 5 | 2.00 | 40 | 0.0625 | 30 | 2.00 | 40 | 0.3750 | 0.167 | ‐1.791 | 0.4830 | — | — |
| Outcome: inner glove perforations per glove pair | |||||||||||||
| Tanner 2006 | 12 | — | 73 | 0.1644 | 42 | — | 110 | 0.3818 | 0.431 | ‐0.843 | 0.3273 | 0.23 | 0.82 |
| Intervention: special gloves Comparison: thicker versus thinner gloves | |||||||||||||
| Outcome: matched perforations per person‐operation | |||||||||||||
| Liew 1995 Double | 2 | 3.00 | 32 | 0.020833 | 7 | 3.00 | 32 | 0.0729 | 0.286 | ‐1.253 | 0.8018 | — | — |
| Outcome: inner glove perforations per person‐operation | |||||||||||||
| Carter 1996 | 61 | 1.00 | 140 | 0.4357 | 77 | 1.00 | 140 | 0.5500 | 0.792 | ‐0.233 | 0.1714 | — | — |
| Chua 1996 | 9 | 1.00 | 119 | 0.0756 | 69 | 1.00 | 238 | 0.2899 | 0.261 | ‐1.344 | 0.3544 | — | — |
| Liew 1995 Double | 12 | 3.00 | 32 | 0.1250 | 19 | 3.00 | 32 | 0.1979 | 0.632 | ‐0.459 | 0.3687 | — | — |
| Liew 1995 Single | 9 | 3.00 | 22 | 0.1364 | 4 | 3.00 | 21 | 0.0635 | 2.148 | 0.764 | 0.6009 | — | — |
| Sebold 1993 | 8 | 1.00 | 25 | 0.3200 | 14 | 1.00 | 22 | 0.6364 | 0.503 | ‐0.687 | 0.4432 | — | — |
| Intervention: special gloves Comparison: triple special versus double normal | |||||||||||||
| Outcome: inner glove perforations per person‐operation | |||||||||||||
| Hester 1992 o‐c‐o | 4 | 3.50 | 25 | 0.0457 | 8 | 3.50 | 25 | 0.0914 | 0.500 | ‐0.693 | 0.6124 | — | — |
| Pieper 1995 l‐k‐k | 1 | 1.00 | 15 | 0.0667 | 8 | 1.00 | 7.5 | 1.0667 | 0.063 | ‐2.773 | 1.0607 | — | — |
| Pieper 1995 l‐s‐s | 2 | 1.00 | 15 | 0.1333 | 8 | 1.00 | 7.5 | 1.0667 | 0.125 | ‐2.079 | 0.7906 | — | — |
| Sebold 1993 | 0.5 | 1.00 | 24 | 0.0208 | 14 | 1.00 | 22 | 0.6364 | 0.033 | ‐3.419 | 1.4392 | — | — |
| Sutton 1998 | 5 | 1.00 | 56 | 0.0893 | 17 | 1.00 | 62 | 0.2742 | 0.326 | ‐1.122 | 0.5088 | — | — |
| Intervention: special gloves Comparison: thick glove versus glove combination | |||||||||||||
| Outcome: inner glove perforations per person‐operation | |||||||||||||
| Sebold 1993 | 8 | 1.00 | 25 | 0.3200 | 0.5 | 1.00 | 24 | 0.0208 | 15.360 | 2.732 | 1.458 | — | — |
| Outcome: matched perforations per glove pair | |||||||||||||
| Turnquest 1996 | 12 | — | 172 | 0.0698 | 12 | — | 169 | 0.0710 | 0.983 | ‐0.018 | 0.4082 | 0.44 | 2.19 |
| Outcome: blood stains on the skin per glove pair | |||||||||||||
| Turnquest 1996 | 2 | — | 172 | 0.0116 | 2 | — | 169 | 0.0118 | 0.983 | ‐0.0176 | 1.0000 | 0.14 | 6.98 |
See footnotes Table 2.
3. Raw data: indicator gloves.
| Intervention | Control | Rate Ratio | 95% CI | ||||||||||
| Study | # Events |
# Persons per operation |
# Operations (or glove pairs) |
Rate per person per operation |
# Events |
# Persons per operation |
# Operations (or glove pairs) |
Rate per person per operation |
RR | lnRR | SE | Lower | Upper |
| Intervention: indicator gloves Comparison: first glove: indicator glove versus standard glove | |||||||||||||
| Outcome: matched perforations per person‐operation | |||||||||||||
| Laine 2004a | 1 | 1.00 | 115 | 0.0087 | 38 | 1.00 | 156 | 0.2436 | 0.036 | ‐3.333 | 1.0131 | — | — |
| Naver 2000 | 6 | 2.66 | 98 | 0.0230 | 53 | 2.66 | 115 | 0.1733 | 0.133 | ‐2.019 | 0.4307 | — | — |
| Outcome: inner glove perforations per person‐operation | |||||||||||||
| Laine 2004b DI (versus double) | 1 | 1.38 | 88.5 | 0.0082 | 6 | 1.41 | 90 | 0.0473 | 0.173 | ‐1.753 | 1.0801 | — | — |
| Laine 2004b DI (versus single) | 1 | 1.38 | 88.5 | 0.0082 | 14 | 1.36 | 81 | 0.1271 | 0.064 | ‐2.742 | 1.0351 | — | — |
| Outcome: number of glove perforations | |||||||||||||
| Laine 2001 (versus single) | 6 | — | — | 38 | — | — | — | — | — | ||||
| Laine 2001 (versus double) | 6 | — | — | 6 | — | — | — | — | — | ||||
| Intervention: indicator gloves Comparison: all gloves: double indicator versus double standard | |||||||||||||
| Outcome: inner glove perforations per person‐operation | |||||||||||||
| Duron 1996 | 1 | 1.00 | 54 | 0.0185 | 0.5 | 1.00 | 46 | 0.0109 | 1.7037 | 0.5328 | 1.7321 | — | — |
| Nicolai 1997 | 16 | 3.00 | 13 | 0.4103 | 16 | 3.00 | 9 | 0.5926 | 0.6923 | ‐0.3677 | 0.3536 | — | — |
See footnotes Table 2.
Unit of analysis issues
We intended to calculate the design effect for studies that employed a cluster‐randomised design but did not make an allowance for the design effect. In studies where the operators were randomised or where the unit of randomisation was the operation or the patient and where there was only one surgeon, we assumed that there was no unit of analysis issue. In studies where the unit of analysis was the patient or operation and where there was more than one person who could sustain needlestick injuries in one operation, the outcomes could be clustered at the operator level. To avoid these issues, we calculated all outcomes per surgeon and per operation. We called this unit of analysis a surgeon‐operation to indicate that this was the risk for one surgeon performing one operation.
Dealing with missing data
We contacted the authors of seven included studies but did not receive an answer or authors could not provide us with the additional information needed for the meta‐analysis. We could calculate the number of operations for three studies (Carter 1996; Chua 1996; Liew 1995 Single) and the number of persons participating in the operation for three other studies (Laine 2004b 2R; Laine 2004b DI; Naver 2000) from the data presented in the article. We based our calculation on the assumption that only one pair of gloves was collected per person per procedure.
Assessment of heterogeneity
We defined studies as clinically homogeneous when they had similar populations, interventions and outcomes measured at the same follow‐up point. We judged interventions to be sufficiently homogeneous when they fit into one of the categories defined in Types of interventions. We regarded all healthcare professionals as sufficiently similar to assume a similar preventive effect from glove interventions. We also considered studies similar for participants with a high and a low exposure level.
We divided outcomes into inner, outer and matched glove perforations, reported needlestick injuries, observable blood stains on the hands and dexterity reported on a VAS scale. We judged studies falling within these categories to be conceptually similar and sufficiently homogeneous to be combined in a meta‐analysis.
We assessed statistical heterogeneity by means of the I2 statistic. We used the values of < 40%, between 30% and 60%, between 50% and 90%, and > 75% as indicating not important, moderate, substantial and considerable heterogeneity, respectively, as proposed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of reporting biases
We assessed publication bias with a funnel plot and with Egger's test for comparisons that had more than five studies available for inclusion.
Data synthesis
We pooled studies with sufficient data, which we judged to be clinically and statistically homogeneous, with RevMan 5 software (RevMan 2012). Where studies were statistically heterogeneous, we used a random‐effects model; otherwise we used a fixed‐effect model.
For studies with multiple study arms that belong to the same comparison, we divided the number of events and participants equally over the study arms to prevent double counting of study participants in the meta‐analysis (e.g. Analysis 7.1; Laine 2004b DI).
7.1. Analysis.
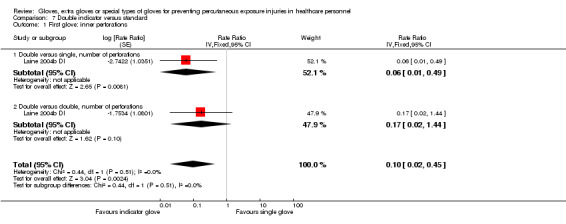
Comparison 7 Double indicator versus standard, Outcome 1 First glove: inner perforations.
Finally, we used the GRADE approach to assess the quality of the evidence per comparison and per outcome, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Starting from an assumed level of high quality, we reduced the quality of the evidence by one or more levels if there were one or more limitations in the following domains: risk of bias, consistency, directness of the evidence, precision of the pooled estimate and the possibility of publication bias. Thus, we rated the level of evidence as high, moderate, low or very low depending on the number of limitations. For the most important comparisons and outcomes, we used the program GRADEpro to generate 'Summary of findings' tables (GRADEpro 2008).
Subgroup analysis and investigation of heterogeneity
We re‐analysed the data to determine whether there was a difference in effect in studies with high exposure in the control group. We also re‐analysed subgroups from low and middle‐income countries for the year of study publication since limited resources create special challenges for preventive care, as reported by World Bank 2013. We also regrouped studies that were carried out in countries with a high HIV or hepatitis C prevalence among adults, as reported by the Centers for Disease Control and Prevention (Centers for Disease Control 2012).
Sensitivity analysis
We conducted two sensitivity analyses to find out if risk of bias led to changes in the findings. We first re‐analysed the results including only studies with a low risk of bias. In a second re‐analysis we included all studies with a low and unknown risk of bias.
Results
Description of studies
Results of the search
With the search strategy described in Appendix 1 and Appendix 2, after removal of duplicates, we had a total of 11,337 references (11,239 from our search in 2010 plus 98 from the search update in 2013). We selected 336 references for full‐text reading (322 plus 14 from 2013). Out of these, we excluded those that did not fulfil our inclusion criteria or that were duplicate publications. In case the article did not provide enough data, we contacted the authors and asked them to send the missing information. If we did not receive sufficient information to judge if the study should be included, we classified the study as awaiting classification. This resulted in 34 articles eligible for inclusion in our review. Five of them had three study arms and two had two study arms. Hence the total number of intervention‐control comparisons was 46 (Figure 1).
1.
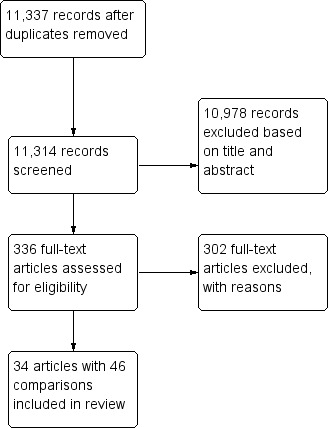
Study flow diagram.
In the last update before publication, we located one extra study for possible inclusion. We classified the study as awaiting classification (Guo 2012).
Included studies
Interventions
1. Increasing the number of layers of gloves
Eighteen studies evaluated whether two glove layers offer more protection than one glove layer. Since all studies were carried out among surgeons, the minimum control intervention was at least one pair of standard gloves. There were no studies that compared gloves versus no gloves in healthcare staff other than surgeons. There were no studies that compared three versus one glove layer. One study evaluated whether three glove layers are better than two. We defined wearing one glove layer as single gloving, two layers as double gloving and three layers as triple gloving. An extra layer of gloves could be both standard gloves or indicator gloves. Indicator gloves are coloured gloves, which are usually green and worn as inner gloves under a standard glove. The green colour will show an outer glove perforation when liquid leaks between both layers and the colour of the inner glove becomes highly visible for the glove wearer. Besides the different colour, indicator and standard gloves are similar in thickness and material and the protection with increased glove layers works in the same way. We only included studies using indicator gloves in the comparisons of extra gloves if they had collected all gloves used during one procedure.
1.1 Double versus single gloving
Seventeen studies used double standard gloves (Berridge 1998; Doyle 1992; Gani 1990; Jensen 1997; Kovavisarach 1998; Kovavisarach 1999; Kovavisarach 2002; Laine 2001; Laine 2004b 2R; Marín Bertolin 1997; Punyatanasakchai 2004; Quebbeman 1992; Rudiman 1999; Thomas 2001; Wilson 1996a; Wilson 1996b; Wilson 1996c). One study used double standard and indicator gloves (Avery 1999a).
Three studies specified the size of the gloves used. In Wilson 1996a, one half size larger glove was worn over one normal sized glove, Wilson 1996b used the larger glove inside and in Wilson 1996c, two normal sized gloves were used.
The control intervention was single gloving with standard gloves (single standard) in all 18 studies (Avery 1999a; Berridge 1998; Doyle 1992; Gani 1990; Jensen 1997; Kovavisarach 1998; Kovavisarach 1999; Kovavisarach 2002; Laine 2001; Laine 2004b 2R; Marín Bertolin 1997; Punyatanasakchai 2004; Quebbeman 1992; Rudiman 1999; Thomas 2001; Wilson 1996a; Wilson 1996b; Wilson 1996c).
1.2 Triple versus double gloving
One study compared three layers of standard gloves to two layers of standard gloves (Pieper 1995 l‐l‐l).
2. Gloves manufactured from special protective materials
Studies used all types of gloves made from special material (e.g. wire or cotton). Studies also evaluated thicker gloves, which are meant to increase protection as a result of thicker glove membranes. Special material gloves are usually permeable to liquids. Unlike thicker gloves that can also be worn as single gloves, in healthcare settings gloves made out of special material are usually worn together with normal material gloves (latex, nitrile rubber or vinyl) in double or triple gloving.
Studies could be combined into three different comparisons. For the first comparison (special or thicker gloves versus normal gloves) we included studies that evaluated whether special material gloves are better than standard gloves or thicker gloves are better than comparable thinner gloves. Those studies compared single to single, double to double and triple to triple gloves. For the second comparison (special or thicker gloves versus a combination of gloves) we used studies that evaluated whether special material or thicker gloves are equally as effective as two other gloves. Those studies compared single to double and double to triple gloves. For the third comparison (an extra or special glove layer versus no extra layer) we used studies that evaluated whether an extra glove layer of special material or thicker gloves adds additional protection compared to not wearing this extra layer. Those studies compared double to single and triple to double gloves.
2.1 Special or thicker gloves versus normal gloves
2.1.1 Double special gloves versus double normal gloves
Five studies compared double gloving with one special material glove and one normal material glove (double special) to double gloving with two normal material gloves (double normal). Four studies used special material gloves made out of knitted fabric (cloth: Sanders 1990; Tanner 2006; cotton: Hester 1992 o‐c; Underwood 1993 and one study used wire weave gloves: Louis 1998). Normal material gloves were standard gloves (standard thickness) in four studies and thicker gloves (orthopaedic gloves) in one study (Hester 1992 o‐c).
The comparison was double gloving with two standard gloves (double standard) in four studies and double gloving with one standard and one thicker glove (double thicker) in one study (Hester 1992 o‐c).
2.1.2 Thicker gloves versus thinner gloves
Five studies compared thicker gloves to thinner gloves. Three studies compared single thicker gloves to single thinner gloves (Carter 1996; Chua 1996; Liew 1995 Single) and two studies compared double thicker (one thicker and one standard glove) to double thinner gloves (two standard gloves) (Liew 1995 Double; Sebold 1993).
The studies compared orthopaedic gloves or other gloves designed for heavy duty to standard gloves (standard thickness) (Chua 1996; Sebold 1993), standard gloves to thinner gloves (designed to increase sensitivity) (Carter 1996) or thicker versus thinner gloves as stated by the study authors (Liew 1995 Double; Liew 1995 Single).
2.2 Special or thicker gloves versus glove combinations
2.2.1 Thicker gloves versus glove combinations
Two studies evaluated whether thicker gloves are equivalent to glove combinations. The studies compared thicker gloves to two standard gloves and thicker gloves to the combination of standard and special material gloves. Turnquest 1996 compared one layer of orthopaedic gloves (thicker than standard gloves) to two layers of standard gloves. Another study compared double thicker (inner standard, outer orthopaedic glove) to triple special gloves (inner standard glove, middle knitted fabric glove, outer standard glove) (Sebold 1993).
2.3 Extra glove layer of special or thicker gloves versus no extra layer
2.3.1 Triple special gloves versus double normal gloves
Five studies compared three layers of two normal material and one special material glove (triple special) to two layers of normal material gloves (double normal). Studies used special material gloves between two standard gloves or between one standard and one thicker glove. We included the former as triple special standard and the latter as triple special thicker. Four studies compared triple special standard to double standard gloves (Pieper 1995 l‐k‐k; Pieper 1995 l‐s‐s; Sebold 1993; Sutton 1998) and one study compared triple special thicker to double thicker (outer standard gloves, inner orthopaedic gloves) (Hester 1992 o‐c‐o).
Special material gloves were made out of knitted fabric (cotton: Hester 1992 o‐c‐o; cloth: Sebold 1993), spectra polyethylene fibres (Sutton 1998), long molecule chains of poly paraphenylene terephtalamide (Kevlar; Pieper 1995 l‐k‐k) or stainless steel and polyester weave (Pieper 1995 l‐s‐s).
3. Glove puncture indicator systems
Indicator gloves are coloured standard gloves and worn under an outer standard glove (double indicator). When an outer glove perforation occurs, moisture from the operating site leaks between both layers and the colour of the inner glove (usually green) becomes highly visible for the glove wearer. The glove wearer can react faster when a perforation occurs and replace the perforated glove with a new glove.
We included two comparisons to standard gloves. The first comparison includes studies that evaluate the theory that the use of indicator gloves reduces the number of perforations in one glove and thus lowers the risk of exposure to bodily fluids (lower number of perforations per glove). The second comparison includes studies that evaluate the theory that the immediate feedback of indicator gloves enables the glove wearer to change their behaviour and protects them from getting additional perforations during the remaining surgical procedure (lower total number of perforations during one procedure).
3.1 First glove: double indicator gloves versus standard gloves
Six studies compared double indicator gloves to standard gloves (single or double) and only collected the first pair of gloves used during the procedure. Four studies compared double indicator gloves to single standard gloves (Laine 2001; Laine 2004a; Laine 2004b DI; Naver 2000). Two studies compared double indicator gloves to double standard gloves (Laine 2001; Laine 2004b DI).
3.2 All gloves: double indicator versus double standard
Two studies compared double indicator gloves to double standard gloves and analysed all gloves used during the surgical procedures (Duron 1996; Nicolai 1997).
Types of study design
All included studies were randomised controlled trials. For every surgical procedure, studies randomised operations, patients, operating teams or individual team members to the type of gloving. In all studies the intervention lasted for the duration of one operation as none of the included studies randomised participants to one type of gloving for the whole duration of the study (e.g. one surgeon has to use double gloves in all procedures during the next four months). Most studies presented the effect per number of gloves used and not per operation per person, which is the unit of randomisation. None of those studies adjusted for the cluster effect. However, we calculated the effect per person‐operation and included the number of persons and operations as the denominator in the outcome measure. Hence, we included all studies as individual randomised trials.
Participants
The majority of studies included only surgeons or surgeons and their assistants. Scrub or theatre nurses were included in nine studies. One study also included dental hygienists, one study surgical technicians and three dental studies included surgeons and surgical staff. Twenty‐six studies included procedures that are related to obstetrics, orthopedics or abdominal surgery. Seven studies did not specify the type of operation but two of these studies stated that the operations lasted longer than one hour. Six studies took place in dentistry workplaces.
We included thirty‐one studies in the meta‐analysis with an average of 115 person‐operations in the intervention group (range 15 to 398) and 119 person‐operations in the control group (range 8 to 443). These studies included 6890 person‐operations in total.
We included two studies with the number of glove pairs used in the meta‐analysis. The total number of glove pairs was 825, with 343/335 intervention/control pairs in one and 68/79 in the other study.
Outcomes
Exposure was reported as the number of glove perforations in all but one study (Quebbeman 1992). Seven studies reported incidences of blood contamination (blood stains on the skin) (Avery 1999a; Berridge 1998; Naver 2000; Quebbeman 1992; Rudiman 1999; Thomas 2001; Turnquest 1996). Two studies reported the number of self reported needlestick injuries (Doyle 1992; Marín Bertolin 1997).
Perforations were recorded with two different measures. Most studies reported any perforation to the innermost glove and some studies reported matched perforations. The first outcome considers every perforation to the innermost glove as a break of the protective barrier and therefore includes all possible exposures. The second outcome, matched perforations, considers perforations as a break in the protective barrier only if the inner glove and the outer glove are perforated in the same area (same spot, finger or side). We reported both outcomes as it is unclear which outcome measurement represents a more valid measure of the risk.
To calculate the risk, most authors used either the number of gloves or pairs of gloves as denominators. We calculated the outcome as risk per person during one operation for the risk of perforations and blood stains. If the study did not report the number of operations but the number of persons involved per operation (or the other way around) and reported the number of gloves or pairs used, we assumed that per person one pair of glove equals one operation and calculated the missing number. We calculated the number of operations for three studies (Carter 1996; Chua 1996; Liew 1995 Single) and the number of persons involved in the operation for three other studies (Laine 2004b 2R; Laine 2004b DI; Naver 2000). This might result in an underestimation of the risk, but this would happen equally in the control and intervention group and not influence the rate ratio. Further, some studies reported inner glove perforations not as the total number of perforations but as the number of gloves or glove pairs with one or more perforations (Aarnio 2001; Carter 1996; Chua 1996; Gani 1990; Jensen 1997; Laine 2004a; Liew 1995 Double; Liew 1995 Single; Louis 1998; Marín Bertolin 1997; Naver 2000; Rudiman 1999; Tanner 2006; Turnquest 1996; Underwood 1993). This might result in an underestimation of the risk and trials are accordingly grouped in subgroups.
Glove perforations were detected by filling the gloves with water and observing the jets of water in 36 studies, including one study that added ink to the water. In four studies the gloves were filled with air and then immersed in water, after which the perforations were noted as air bubbles. In one study they were examined visually.
Six studies (Avery 1999a; Avery 1999b; Tanner 2006; Wilson 1996a; Wilson 1996b; Wilson 1996c) reported the loss of dexterity measured on a visual analogue scale but none of those studies reported the data in sufficient detail to be used in a meta‐analysis. Five studies reported median scores and one study reported mean scores (Tanner 2006). However, in most studies it was unknown how many participants were included; it was very likely that the same participants were included more than once in the evaluation and interquartile range values were missing. However, 13 trials reported perforations to the outermost glove for the comparison double versus single gloves (Avery 1999a; Gani 1990; Kovavisarach 1998; Kovavisarach 1999; Kovavisarach 2002; Punyatanasakchai 2004; Rudiman 1999; Thomas 2001). If there were more perforations in the outer glove of the double gloving arm than in the single glove, this could be taken as an indication that double gloving leads to an impairment of dexterity. Therefore, we included this outcome as an adverse outcome, as a proxy measure for loss of dexterity.
Control group outcome rates
A sample of non‐used gloves was investigated for perforations in several studies as a preliminary control. The number of perforations was always found to be zero or very small. Needlestick injuries or blood contamination incidences were presumed to be zero before the start of the procedure in question.
We present the control rates for studies which are included in the meta‐analysis.
1. Glove perforations
The number of perforations in control groups varied greatly.
1.1 Single standard gloves
The mean control group rate in single standard gloves across 14 studies was 0.185 perforations per person‐operation (range 0.008 to 0.290).
1.2 Single thinner gloves
The mean control group rate for single thinner gloves (thinner than standard gloves) was 0.307 perforations per person‐operation (0.063 to 0.550) across two studies.
1.3 Double standard gloves
The mean control group rate across nine studies for double standard gloves was 0.515 inner glove perforations per person‐operation (range 0.011 to 1.067) and 0.210 matched perforations per person‐operation (range 0.143 to 0.288) across four studies.
1.4 Double thicker
The mean control group rate for double thicker gloves (one standard, one thick glove) was 0.091 perforations per person‐operation (one study).
1.5 Double fabric
The mean control group rate for double fabric gloves (one standard over one fabric glove) was 0.021 perforations per person‐operation (one study).
2. Blood stains on the skin
The average control group rate across three studies in single gloves was 0.129 blood stains per person‐operation (range 0.049 to 0.217, three studies).
3. Needlestick injuries
The mean control group rate across two studies in single gloves was 0.033 needlestick injuries per glove‐pairs used (range 0.021 to 0.044).
Geographical location
More than half of the studies were conducted in Europe (19) or the USA (10). Most studies from Europe were conducted in the UK (10). Other European countries were Finland (five), Denmark (two), France (one) and Spain (one). Nine studies were from Asia (Thailand (four), India (one), Indonesia (one) and Oman (three)). Other studies were from Australia (three).
Year of study
Most studies (n = 31) were published in the 1990s. The earliest two studies were published in 1990 and the latest study in 2006.
Excluded studies
Based on the full‐text articles, we mainly excluded studies because they either did not have a primary outcome measure or the methods or comparison were inadequate. For instance, two studies used behavioural changes such as glove use as outcome measure (Duerink 2006; Jeffe 1999) and one study reported alarms by an electronic device meant to detect barrier breakdowns (Caillot 1999). In one study the randomisation was unclear and the patients in the intervention and control group were significantly different (Kelly 1993). Brunton 2000 compared two single, non‐sterile, powder‐free gloves and Gaujac 2007 compared sterile to non‐sterile double gloving. Some studies reported results from technical laboratory tests without patients involved. Many studies were of a descriptive nature and as such did not include an intervention.
Studies awaiting classification
Five studies are not yet included or excluded because the interventions are unclear. All these studies compared different types of single gloves but did not provide information on the characteristics of the gloves used. Four studies refer to the gloves only as A, B, C or D (Bliss 1992; Hwang 1999b; Hwang 1999c; Hwang 1999d) and one study reports the name of the gloves used (Newsom 1998). We contacted the authors or manufacturer but did not receive an answer. We located one study in the last search update which is still awaiting full‐text assessment and data extraction (Guo 2012).
Risk of bias in included studies
Risk of bias varied considerably over studies (Figure 2). Overall, we considered only one study to have a low risk of bias based on adequate allocation procedures (randomisation and concealment) and blinding of the outcome assessor (Tanner 2006). We judged 17 studies to have a high risk of bias, according to their high risk in at least one of these three items (Figure 3). Other studies had both low and unknown risks of bias in these three items (n = 23).
2.
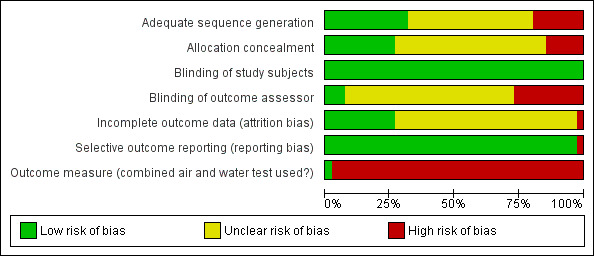
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
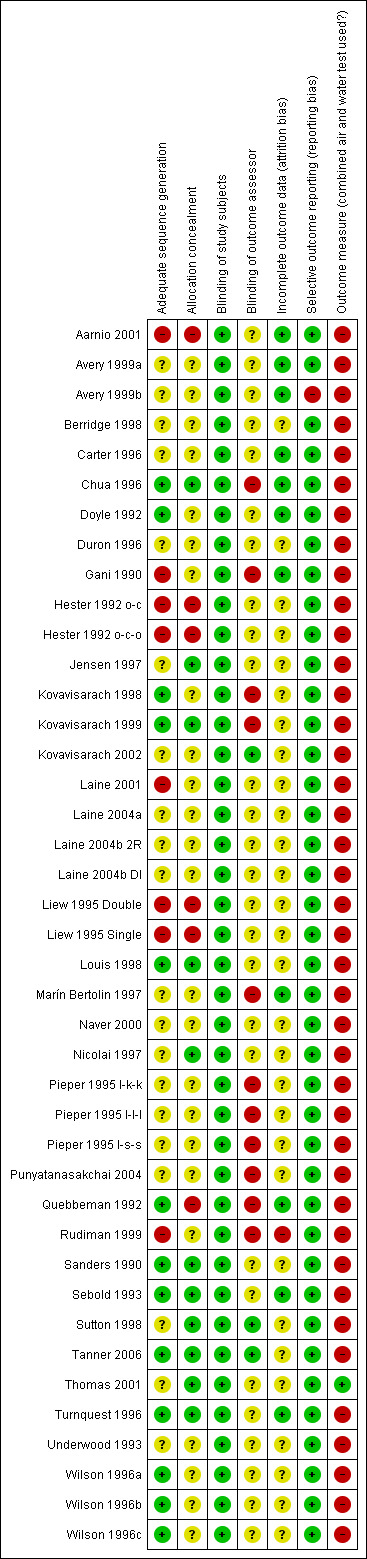
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Twenty studies did not describe the randomisation procedure and we judged the risk of bias as unknown (Figure 3). Studies that provided information reported mostly valid procedures. Thirteen studies used a draw or random number tables and here we assessed the risk of bias as low. Eight studies used inappropriate procedures and randomised according to the date of birth, hospital record numbers, hospital admission day or unit record numbers and we assessed the risk of bias as high (Aarnio 2001; Gani 1990; Hester 1992 o‐c; Hester 1992 o‐c‐o; Laine 2001; Liew 1995 Double; Liew 1995 Single; Rudiman 1999).
The majority of studies did not report information about allocation concealment (n = 24) and we judged the risk of bias as unknown (Figure 3). Eleven studies used envelopes which were opened just before the procedure or concealment was done by the involvement of a research nurse and we judged the risk of bias as low. Six studies did not conceal the allocation and we judged the risk of bias as high (Aarnio 2001; Hester 1992 o‐c; Hester 1992 o‐c‐o; Liew 1995 Double; Liew 1995 Single; Quebbeman 1992).
Blinding
It is impossible to be blind the glove user to extra or different types of gloves. In spite of this lack of blinding, we assessed the risk of bias as low because it would be difficult for a surgeon to use this knowledge to change the number of needlestick injuries, blood contaminations on the skin or perforations of gloves.
However, not blinding the outcome assessor is considered a potential risk of bias, when evaluating the effect of the type of glove or gloving. For 27 studies it was unclear if the outcome assessor was blinded and we assessed the risk of bias as unclear (Figure 3). The outcome assessment should be done blind in order to be of low risk of bias and this was reported for three studies only (Kovavisarach 2002; Sutton 1998; Tanner 2006). In 11 studies, the outcome was assessed by the participants themselves or the gloves were labelled as being used for double gloving and we judged the risk of bias as high.
Incomplete outcome data
In 29 studies the risk of bias is unknown because information was lacking as to whether all gloves were reported or it was unknown how many gloves were excluded (Figure 3). Eleven studies reported perforations or incidences for at least 90% of all gloves used during the procedures and cases randomised and we judged the risk of bias as low. One study reported that 17% of the gloves were excluded from the analysis and we judged the risk of bias as high (Rudiman 1999).
Selective reporting
All but one study reported all outcomes that were described in the methods section of the report and we judged the risk of bias as low. Avery 1999b only reported the number of outer glove perforations per type of glove and the number of inner glove perforations was missing, therefore we judged the risk of bias as high.
Other potential sources of bias
Outcome measure
The measurement of needlestick injuries was a source of bias in all studies that used this outcome (Doyle 1992; Marín Bertolin 1997; Quebbeman 1992). Needlestick injuries can only be based on self report because there are no other methods of ascertaining that an injury has occurred. Like any occupational injury, the reporting of needlestick injuries increases when workers are more aware of the problem, for example due to an awareness campaign. Any intervention has the same effects as an awareness campaign and is likely to raise the number of reported injuries. This will probably lead to an underestimation of the true intervention effect.
We considered the measurement of perforations to be at low risk of bias for studies using both a water leak test and an air inflation test. Only one study used both (Thomas 2001). Other studies used either only one of the two or visually inspected the gloves and we therefore judged them to be at high risk of bias.
Effects of interventions
See: Table 1
There was no study that compared the use of gloves versus no intervention in healthcare staff other than surgeons.
1. Extra layers of gloves
1.1 Double versus single gloves
1.1.1 Perforations
Outcome: all perforations in the innermost glove
Eighteen trials reported this outcome. We could combine 12 of these trials in a meta‐analysis (Avery 1999a; Gani 1990; Kovavisarach 1998; Kovavisarach 1999; Kovavisarach 2002; Laine 2004b 2R; Punyatanasakchai 2004; Rudiman 1999; Thomas 2001; Wilson 1996a; Wilson 1996b; Wilson 1996c) (Analysis 1.1). The use of double gloves significantly reduced the number of perforations per person‐operation in the inner glove by 71% compared to single gloves (rate ratio (RR) 0.29, 95% confidence interval (CI) 0.23 to 0.37). Both subgroups showed similar results (P value = 0.15 for subgroup differences).
1.1. Analysis.
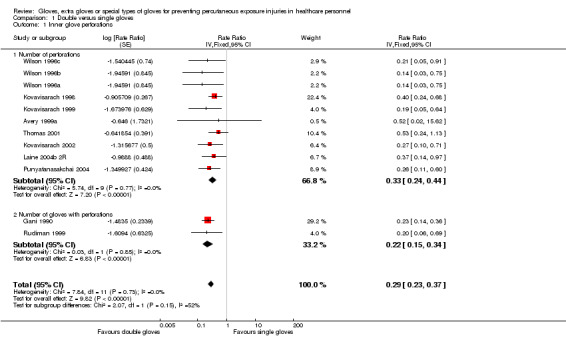
Comparison 1 Double versus single gloves, Outcome 1 Inner glove perforations.
Six trials did not report sufficient information on the number of persons per operation and could not be included in the meta‐analysis. All of those trials reported a positive effect of the intervention. Significant results ranged from 96% to 56% fewer perforations in double standard gloves compared to single standard gloves. Four trials reported enough information to show the effect as the number of inner glove perforations per total number of gloves or glove pairs used. The rate ratio in Aarnio 2001 was 0.04 (95% CI 0.00 to 0.73), in Doyle 1992 0.15 (95% CI 0.04 to 0.48), in Jensen 1997 0.30 (95% CI 0.16 to 0.57) and in Marín Bertolin 1997 0.44 (95% CI 0.23 to 0.82). Two trials did not provide information on the number of gloves used and only reported the number of perforations (Berridge 1998; Laine 2001). Both studies reported that the number of perforations to the inner glove was reduced with the double gloving method.
Outcome: matched perforations
Five trials reported matched perforations. We could combine four trials in a meta‐analysis (Kovavisarach 1998; Kovavisarach 2002; Punyatanasakchai 2004; Thomas 2001) (Analysis 1.2). The combined effect shows a 89% reduction of inner glove perforations when using double standard or indicator gloves compared to single standard gloves (RR 0.11, 95% CI 0.05 to 0.20).
1.2. Analysis.
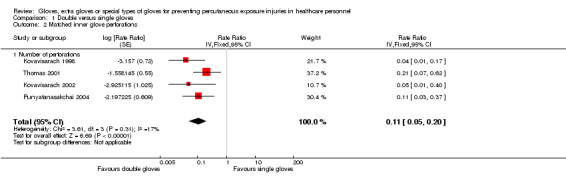
Comparison 1 Double versus single gloves, Outcome 2 Matched inner glove perforations.
One trial did not report sufficient data to be included in the meta‐analysis and the outcome is calculated per pairs of gloves used. Jensen 1997 reported a 80% reduction of perforations using double standard gloves compared to single standard gloves (RR 0.2, 95% CI 0.09 to 0.43).
1.1.2 Needlestick injuries
Only two trials reported needlestick injuries (Doyle 1992; Marín Bertolin 1997) and showed a statistically non‐significant reduction when using double standard gloves (RR 0.58, 95% CI 0.21 to 1.62) (Analysis 1.3).
1.3. Analysis.
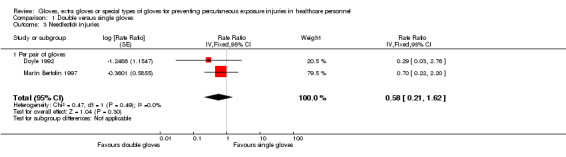
Comparison 1 Double versus single gloves, Outcome 3 Needlestick injuries.
1.1.3 Blood stains
Six trials reported the incidence of blood stains on the skin. Three trials (Naver 2000; Rudiman 1999; Thomas 2001) showed a 65% statistically significant reduction of blood contamination incidents on the skin for double gloves compared to single gloves (RR 0.35, 95% CI 0.17 to 0.70) (Analysis 1.4).
1.4. Analysis.
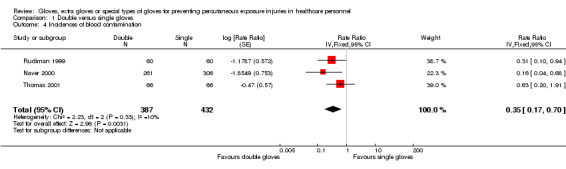
Comparison 1 Double versus single gloves, Outcome 4 Incidences of blood contamination.
Three other trials could not be included in the meta‐analysis. Avery 1999a could not be included because the study did not have any events, either in the double gloving or in the single gloving group. We had to exclude Quebbeman 1992 from the analysis because 28% of the participants switched from the intervention to the control group or vice versa. Berridge 1998 did not provide information on the number of gloves used but reported that blood contamination to the skin was reduced by half when wearing two pairs of gloves.
1.1.4 Dexterity
Outcome: from visual analogue scales (VAS)
There were four studies that reported the loss of dexterity for double versus single gloves, measured with a visual analogue scale, but none of these studies reported the data sufficiently to be combined in a meta‐analysis. All four studies reported less dexterity with the use of double compared to single gloves. Because of the lack of a standardised way of measuring dexterity and the lack of sufficient statistical testing, it is difficult to judge if this decreased dexterity is clinically important or not. We report their published data in Analysis 1.5.
1.5. Analysis.
Comparison 1 Double versus single gloves, Outcome 5 Dexterity: VAS score.
| Dexterity: VAS score | |||
|---|---|---|---|
| Study | Notes | Double gloves | Single gloves |
| Avery 1999a | VAS 1 to 10 (1‐2 = very poor, 3‐4 = poor, 5‐6 = average, 7‐8 = good, 9‐10 = very good) |
Median (interquartile range): | Median (interquartile range): |
| Avery 1999a | Comfort: | Double standard: 4 (3 to 6) (poor) Double indicator: 5 (4 to 6) (average) |
Single surgical: 7 (6 to 8) (good) Single Biogel: 8 (7 to 9) (good) |
| Avery 1999a | Sensitivity: | Double standard: 4 (3 to 5) (poor) Double indicator 5 (4 to 6) (average) |
Single surgical 7 (6 to 9) (good) Single Biogel 8 (7 to 8) (good) |
| Avery 1999a | |||
| Avery 1999a | |||
| Avery 1999a | |||
| Avery 1999a | |||
| Wilson 1996a | VAS 1 to 5: (1 = poor, 2 = fair, 3 = good, 4 = very good, 5 = excellent) |
Median | Median |
| Wilson 1996a | Comfort: | Double larger outside: 3 (good) | Single standard: 4 (very good) |
| Wilson 1996a | Instrument handling: | Double larger outside: 3 (good) | Single standard: 4 (very good) |
| Wilson 1996a | Needle loading: | Double larger outside: 3 (good) | Single standard: 4 (very good) |
| Wilson 1996a | Knot tying: | Double larger outside: 3 (good) | Single standard: 4 (very good) |
| Wilson 1996a | Tissue handling: | Double larger outside: 3 (good) | Single standard: 4 (very good) |
| Wilson 1996a | Hand sensitivity: | Double larger outside: 3 (good) | Single standard: 4 (very good) |
| Wilson 1996b | VAS 1 to 5: (1 = poor, 2 = fair, 3 = good, 4 = very good, 5 = excellent) |
Median | Same group as Wilson 1996a |
| Wilson 1996b | Comfort: | Double larger inside: 3 (good) | |
| Wilson 1996b | Instrument handling: | Double larger inside: 3 (good) | |
| Wilson 1996b | Needle loading: | Double larger inside: 3 (good) | |
| Wilson 1996b | Knot tying: | Double larger inside: 3 (good) | |
| Wilson 1996b | Tissue handling: | Double larger inside: 3 (good) | |
| Wilson 1996b | Hand sensitivity: | Double larger inside: 3 (good) | |
| Wilson 1996c | VAS 1 to 5: (1 = poor, 2 = fair, 3 = good, 4 = very good, 5 = excellent) |
Median | Same group as Wilson 1996a |
| Wilson 1996c | Comfort: | Double normal size: 2 (fair) | |
| Wilson 1996c | Instrument handling: | Double normal size: 3 (good) | |
| Wilson 1996c | Needle loading: | Double normal size: 3 (good) | |
| Wilson 1996c | Knot tying: | Double normal size: 3 (good) | |
| Wilson 1996c | Tissue handling: | Double normal size: 3 (good) | |
| Wilson 1996c | Hand sensitivity: | Double normal size: 2 (fair) | |
Outcome: outer glove perforations
Thirteen trials reported outer glove perforations. Eight trials are included in the meta‐analysis (Avery 1999a; Gani 1990; Kovavisarach 1998; Kovavisarach 1999; Kovavisarach 2002; Punyatanasakchai 2004; Rudiman 1999; Thomas 2001). The difference between double and single gloves was non‐significant (RR 1.10, 95% CI 0.93 to 1.31) (Analysis 1.6).
1.6. Analysis.
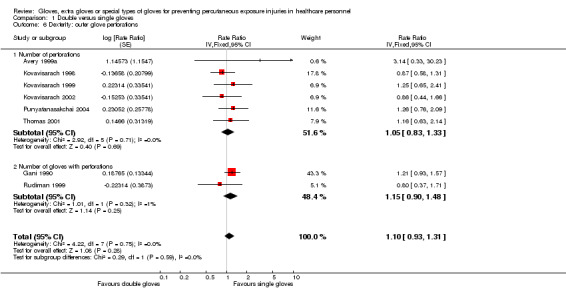
Comparison 1 Double versus single gloves, Outcome 6 Dexterity: outer glove perforations.
We could not include five trials in the meta‐analysis due to missing data and the results were inconsistent. Four studies reported the number of perforations per glove pairs used. Three studies showed non‐significant results, with two of them showing an increase and one showing a decrease in outer glove perforations with double gloves (Doyle 1992, RR 0.75, 95% CI 0.42 to 1.35; Jensen 1997, RR 1.75, 95% CI 0.77 to 1.79; Marín Bertolin 1997, RR 1.20, 95% CI 0.75 to 1.92). One study showed a significant reduction of outer glove perforations with double gloves (Aarnio 2001, RR 0.26, 95% CI 0.07 to 0.92). The study Berridge 1998 did not provide information about the number of gloves used and reported more perforations for double gloves (28 compared to 18).
1.2. Triple versus double gloves
1.2.1 Perforations
Outcome: all perforations in the inner glove
One small study showed a 97% reduction of inner glove perforations with the use of three glove layers compared to two glove layers (RR 0.03, 95% CI 0.00 to 0.52) (Pieper 1995 l‐l‐l) (Analysis 2.1).
2.1. Analysis.

Comparison 2 Triple versus double gloves, Outcome 1 Inner glove perforations.
2. Special and thicker gloves
2.1 Double special versus double normal gloves
2.1.1 Perforations
Outcome: all perforations in the inner glove
We had four studies that compared double special gloves versus normal gloves (Hester 1992 o‐c; Louis 1998; Sanders 1990; Underwood 1993) (Analysis 3.1). The difference between subgroups was high (P value = 0.003) and therefore, we did not combine studies using different special material gloves.
3.1. Analysis.
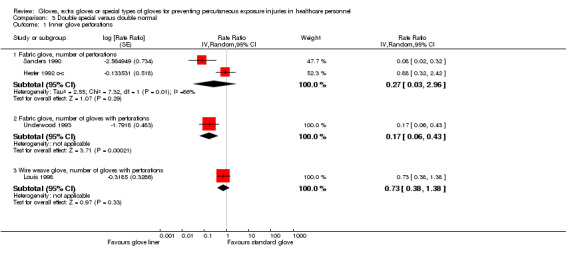
Comparison 3 Double special versus double normal, Outcome 1 Inner glove perforations.
We combined three studies that used fabric gloves and they showed an 87% reduction of inner glove perforations when wearing one fabric and one standard glove (double special) compared to double standard gloves (RR 0.24, 95% CI 0.06 to 0.93). One study showed a non‐significant reduction of inner glove perforations when using wire weave gloves in double gloving compared to double standard gloves (RR 0.73, 95% CI 0.38 to 1.38) .
One study could not be included in the meta‐analysis. The study reported a 57% reduction per glove pairs used when wearing one standard and one cloth glove compared to two standard gloves (Tanner 2006, RR 0.43, 95% CI 0.23 to 0.82).
2.1.2 Dexterity
There was one study that reported the VAS scores for the loss of dexterity for double with fabric glove versus double latex gloves. Participants (n = 18) reported double latex gloves to be less good for tactile sensation, general dexterity, precision with instruments, grip and power, cement handling and comfort compared to double latex gloves (Analysis 3.2).
3.2. Analysis.
Comparison 3 Double special versus double normal, Outcome 2 Dexterity: VAS score.
| Dexterity: VAS score | |||
|---|---|---|---|
| Study | Notes | Double gloves | Single gloves |
| Tanner 2006 | VAS 0 to 1: double with fabric glove versus double latex (0 = better than latex; 0.5 = same as latex, 1 = worse than latex) |
Mean score (range) (N = 18) | |
| Tanner 2006 | Tactile sensation | 0.83 (0.5 to 1) | |
| Tanner 2006 | General dexterity | 0.76 (0.5 to 1) | |
| Tanner 2006 | Precision instrumentation | 0.78 (0.25 to 1) | |
| Tanner 2006 | Grip and power | 0.63 (0.25 to 1) | |
| Tanner 2006 | Handling cement | 0.62 (0.43 to 1) | |
| Tanner 2006 | Comfort | 0.62 (0.5 to 1) | |
2.2 Triple special versus double normal gloves
2.2.1 Perforations
Outcome: all perforations in the inner glove
Five studies reported perforations in the inner glove and all studies are included in the meta‐analysis (Hester 1992 o‐c‐o; Pieper 1995 l‐k‐k; Pieper 1995 l‐s‐s; Sebold 1993; Sutton 1998). The result shows a 76% reduction in inner glove perforations when using an additional layer of special material gloves (RR 0.24, 95% CI 0.13 to 0.45) (Analysis 4.1).
4.1. Analysis.
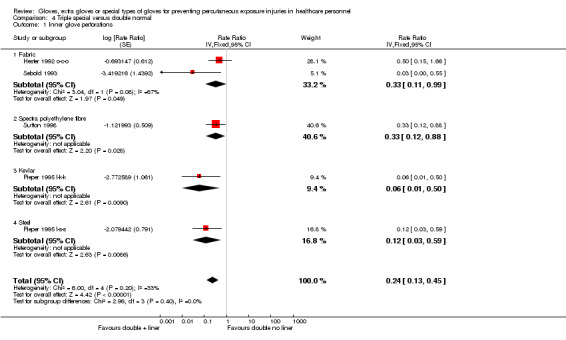
Comparison 4 Triple special versus double normal, Outcome 1 Inner glove perforations.
2.3 Thicker versus thinner gloves
2.3.1 Perforations
Outcome: all perforations in the innermost glove
Five studies reported the number of perforations to the inner glove (Chua 1996; Sebold 1993) or the number of inner gloves with perforations (Carter 1996; Liew 1995 Double; Liew 1995 Single). Study results are inconsistent, with one study favouring thicker gloves, three studies showing non‐significant results favouring thicker gloves and one study favouring thinner gloves (Analysis 5.1). We did not combine studies in the meta‐analysis due to high subgroup differences (P value = 0.02; I² = 80.3%).
5.1. Analysis.
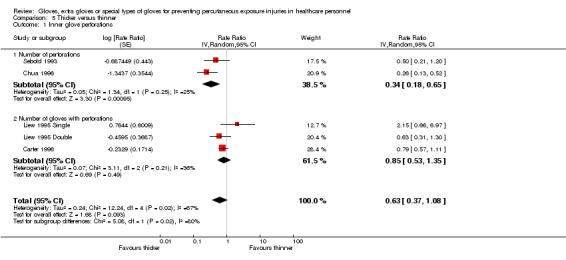
Comparison 5 Thicker versus thinner, Outcome 1 Inner glove perforations.
Outcome: matched perforations
One small study also reported the number of matched perforations (Liew 1995 Double) and the beneficial effect of thicker gloves compared to thinner gloves was non‐significant (RR 0.29, 95% CI 0.06 to 1.38) (Analysis 5.2).
5.2. Analysis.

Comparison 5 Thicker versus thinner, Outcome 2 Matched perforations.
2.4 Thicker gloves versus combinations of gloves
Two studies compared thicker gloves to combinations of gloves.
2.4.1 Perforations
Outcome: all perforations in the innermost glove
One study (Sebold 1993) showed a non‐significant increase in perforations when using thick gloves instead of wearing one standard glove and one special material glove (RR 15.36, 95% CI 0.88 to 267.57) (Analysis 6.1). Another study (Turnquest 1996) reported the outcome per pairs of gloves and did not find a difference in perforations when wearing one layer of thick gloves compared to two layers of standard gloves (RR 0.98, 95% CI 0.44 to 2.19).
6.1. Analysis.

Comparison 6 Thick versus glove combinations, Outcome 1 Inner glove perforations.
2.4.2 Blood stains
Turnquest 1996 reported the number of blood stains on the skin and could not show a difference in the number of blood stains per gloves used between the two gloving methods (RR 0.98, 95% CI 0.14 to 6.98).
3. Indicator gloves
3.1 First pair: double indicator versus standard (single or double)
3.1.1 Perforations
Outcome: all perforations in first pair of inner glove
Two studies with four study arms reported any perforation to the first inner glove (Laine 2001; Laine 2004b DI). One study with two study arms is included in the meta‐analysis. The number of perforations to the first inner glove was reduced by 90% when using double indicator gloves compared to standard gloves (RR 0.10, 95% CI 0.02 to 0.45) (Analysis 7.1). The effect was larger when comparing double indicator gloves with the single gloving method (94%) than when comparing with the double standard gloving method (83%).
Laine 2001 did not provide sufficient information to be included in the meta‐analysis. For the first gloves the authors reported no difference in the number of perforations to the inner glove in intervention and double standard gloves groups (six perforations in both groups) but a big difference in inner glove perforations in the intervention and single standard gloves groups (six compared to 76). However, it is unclear how many indicator and standard gloves were included in the study.
Outcome: matched perforations in first pair of gloves
Two studies reported matched perforations (Laine 2004a; Naver 2000). Using double indicator gloves reduced the number of matched perforations in the first glove pair by 91% compared to single gloves (RR 0.09, 95% CI 0.03 to 0.29) (Analysis 7.2).
7.2. Analysis.
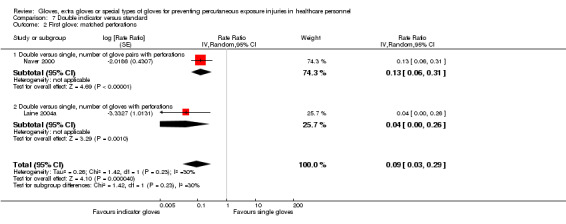
Comparison 7 Double indicator versus standard, Outcome 2 First glove: matched perforations.
3.2 All gloves: double indicator versus double standard
3.2.1 Perforations
Outcome: all perforations in all inner gloves
Two studies compared double indicator gloves with double standard gloves (Duron 1996; Nicolai 1997). The number of perforations to the inner glove during one operation was non‐significantly lower when wearing indicator gloves compared to standard gloves (RR 0.72, 95% CI 0.36 to 1.42) (Analysis 7.3).
7.3. Analysis.
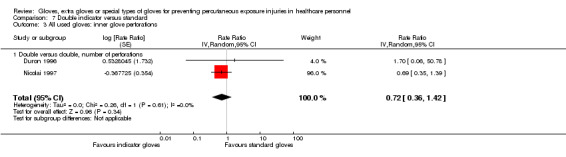
Comparison 7 Double indicator versus standard, Outcome 3 All used gloves: inner glove perforations.
3.2.2 Dexterity
There was one study that reported the loss of dexterity, rated with VAS scores for double gloves with indicator systems versus double gloves without the system, but sensitivity and dexterity were still rated as adequate for both gloving types (Analysis 7.4).
7.4. Analysis.
Comparison 7 Double indicator versus standard, Outcome 4 Dexterity: VAS score.
| Dexterity: VAS score | |||
|---|---|---|---|
| Study | Notes | Double indicator | Double standard |
| Avery 1999b | VAS 1 to 10 (values not provided for all scores; 7 = good, 6 = adequate) |
Median (interquartile range): | Median (interquartile range): |
| Avery 1999b | Comfort | Double indicator: 6 (5 to 7) (= adequate) | Double standard: 6 (5 to 7) (= adequate) |
| Avery 1999b | Sensitivity | Double indicator: 5 (4 to 6) (= adequate) | Double standard: 5 (4 to 6) (= adequate) |
5. Sensitivity analyses and explanation of heterogeneity
We carried out a sensitivity analysis for the comparisons that yielded positive results by leaving out all studies that we judged as being at high risk of bias for at least one of the items: random sequence generation, allocation concealment or blinding of outcome assessor. A sensitivity analysis including only low‐risk studies was not possible, as only one study had a low risk of bias for all three items (Tanner 2006).
For seven studies without a high risk of bias double gloving versus single gloving yielded a rate ratio of 0.33 (95% CI 0.21 to 0.51) which is almost exactly the same as the overall result of 0.29 (95% CI 0.23 to 0.37). Heterogeneity remained the same (I² = 0%).
We left out one study with a high risk of bias from the meta‐analysis that compared double special with double normal gloves. The pooled rate ratio for the two subgroups using fabric gloves increased significantly from 0.24 (95% CI 0.06 to 0.93) to 0.13 (95% CI 0.06 to 0.29) and the heterogeneity dropped from 78% to 0% (I² statistic).
For two studies without a high risk or bias triple special versus double normal gloves yielded a rate ratio of 0.25 (95% 0.10 to 0.65), which is similar to 0.24 (95% CI 0.13 to 0.45), but the heterogeneity measured with the I² statistic increased from 33% to 56%.
We also carried out a sensitivity analysis in comparisons with high heterogeneity, leaving out studies with a high risk of bias. For two studies comparing thicker with thinner gloves the rate ratio of 0.75 (95% CI 0.55 to 1.02) remained non‐significant and almost the same as 0.63 (95% CI 0.37 to 1.08), however the heterogeneity dropped from 67% to 0% (I² statistic).
6. Subgroup analyses
For the main comparison, which had clear significant results and included most of the studies (Analysis 1.1), we grouped studies from countries with a high HIV or hepatitis C prevalence in one subgroup. Ten of 12 studies belonged to the category with a HIV prevalence of more than 1% or a hepatitis C prevalence of more than 2%. For double gloving, the effect in the studies from the high prevalence countries was slightly better (RR 0.28, 95% CI 0.22 to 0.37) than in countries with a lower prevalence (RR 0.38, 95% CI 0.15 to 0.96) (Analysis 8.1). The subgroup difference was non‐significant (P value = 0.54).
8.1. Analysis.
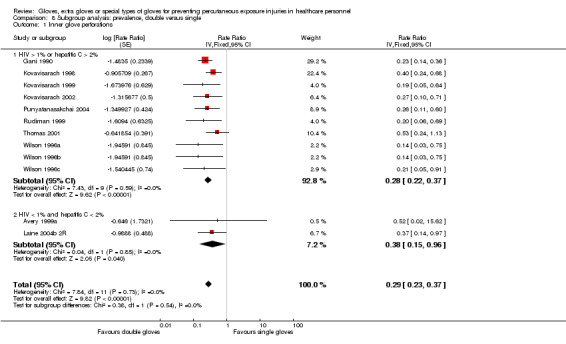
Comparison 8 Subgroup analysis: prevalence, double versus single, Outcome 1 Inner glove perforations.
In a second analysis for the same comparison, we grouped studies from low and middle‐income countries and studies from high‐income countries in different subgroups. For double gloving the effect for high‐income countries was slightly better, with a rate ratio of 0.23 (95% CI 0.16 to 0.34) compared to 0.34 (95% CI 0.24 to 0.47) for low and middle‐income countries (Analysis 9.1). The difference between subgroups was non‐significant (P value = 0.15).
9.1. Analysis.
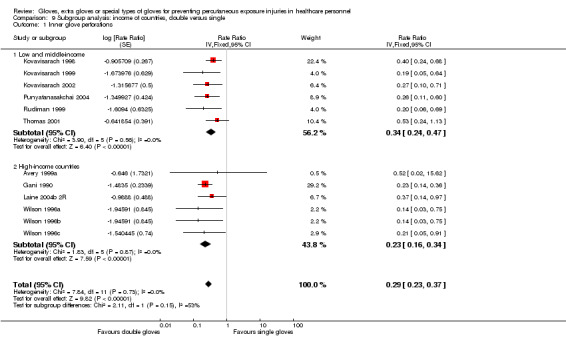
Comparison 9 Subgroup analysis: income of countries, double versus single, Outcome 1 Inner glove perforations.
In a third subgroup analysis for the same comparison, we grouped studies according to the exposure in the control groups. We labelled studies with more than 0.2 perforations per person‐operation in the control group as high‐exposure studies (n = 4). For double gloving the effect in the high‐exposure studies was slightly better (RR 0.27, 95% CI 0.19 to 0.39) than in studies with lower exposure rates (RR 0.31, 95% CI 0.22 to 0.43) (Analysis 10.1). The subgroup difference was non‐significant (P value = 0.66).
10.1. Analysis.
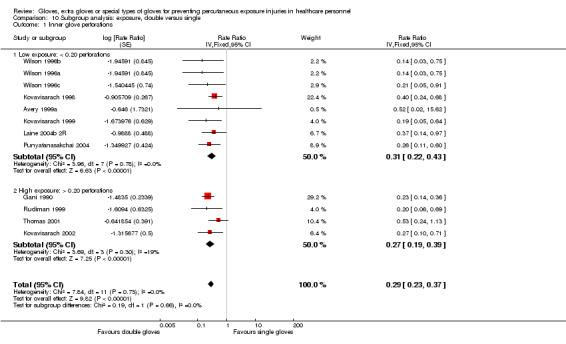
Comparison 10 Subgroup analysis: exposure, double versus single, Outcome 1 Inner glove perforations.
7. Publication bias
We did not detect publication bias in our results. We generated a funnel plot for the comparison of double versus single gloves (Figure 4). The result of the Egger's test was non‐significant.
4.
Funnel plot of comparison: 1 Double versus single, outcome: 1.3 Inner glove perforations
Discussion
Summary of main results
No study compared the effect of gloves versus no intervention in healthcare staff other than surgical staff.
There was moderate‐quality evidence that double gloving in the form of using two pairs of standard thickness gloves instead of one pair reduced the risk of inner glove perforation for one person for one operation by 71% (rate ratio (RR) 0.29, 95% confidence interval (CI) 0.23 to 0.37). The effect was larger when measuring only matched perforations (89%). The use of two glove layers also reduced the risk of blood contamination by 65% (RR 0.35, 95% CI 0.17 to 0.70) compared to one glove layer. Double gloves reduced the number of reported needlestick injuries by 42% in two studies, but the result was not statistically significant (RR 0.58, 95% CI 0.21, 1.62) because this was based on 16 events only. The effect of double gloves compared to single gloves was similar when restricted to studies from low‐income countries, to studies from countries with a high prevalence of HIV or hepatitis C and to studies with high exposure rates.
Three layers of standard gloves or gloves made out of special material in combination with standard gloves can provide additional protection. There was low‐quality evidence for a 97% reduction in the number of perforations with three layers of standard gloves compared to two layers but this is based on one small study only.
The use of double fabric gloves (one fabric and one standard material glove) compared to two standard material gloves reduced the risk of inner glove perforations by 76% (95% CI 6% to 94%) and is based on low‐quality evidence from three studies only. Our sensitivity analysis (excluding one study at high risk of bias) increased the effect of double fabric gloves compared to double normal gloves from 76% to 87% (RR 0.13 95% CI 0.06 to 0.29) and the I² statistic for heterogeneity dropped from 78% to 0%.
The effect of double fabric gloves compared to double normal gloves is similar to the effect of triple special gloves (two standard and one special material glove). There was moderate‐quality evidence that triple special gloves compared to double normal gloves reduced the risk of perforations by 76% (RR 0.24, 95% CI 0.13 to 0.45). This was shown for fabric, Kevlar, steel and spectra polyethylene fibre gloves.
There was moderate‐quality evidence that double indicator gloves compared to standard gloves (single and double gloves) reduced the number of perforations in one glove on average by 90% (RR 0.10, 95% CI 0.02 to 0.45). When perforations occur, the glove is replaced faster with an intact glove layer. However, the use of indicator gloves did not significantly reduce the total number of inner glove perforations for one person during one operation compared to double gloves without the indicator system (RR 0.72, 95% CI 0.36 to 1.42).
In five low‐quality studies, thicker gloves had a similar risk of inner glove perforations compared to thinner gloves (RR 0.63, 95% CI 0.37 to 1.08).
As a proxy measure of loss of dexterity due to double gloving, eight studies compared the number of perforations in the outer glove of the double gloves to the number of perforations in single gloves and found this to be similar (RR 1.10, 95% CI 0.93 to 1.31).
Overall completeness and applicability of evidence
Perforations are a proxy measure for actual needlestick injuries. We assume that needlestick injuries are proportional to the number of perforations even though we do not have good evidence to underpin this assumption. There were two studies that measured both perforations and needlestick injuries in this review. The rate ratio for these two outcomes differed substantially (RR 0.32 and RR 0.57, respectively). In the review by Parantainen 2011 there were four studies that measured both these outcomes and there was no difference between the RRs, but this could also be due to the use of blunt needles as the intervention. However, laboratory studies (Edlich 2003) showed that the penetration through two glove layers requires considerably more force than the penetration of one layer only and, thus, supports the idea that extra glove layers mechanically prevent needlestick injuries.
The measurement of needlestick injuries is notoriously difficult because it relies on self report, which is easily biased by awareness of the problem (Boal 2008). Thus it could be that reporting is more frequent with double gloving because this would draw more attention to the problem of needlestick injuries. It could also be that some needlestick injuries with single gloves do go unnoticed because less force is needed to perforate the glove barrier. Given these measurement problems it is not likely that the effect on needlestick injuries can be assessed with more certainty.
The effect of double gloving on dexterity was not fully clear in this review because only three studies measured and reported this adverse effect, with two studies showing a slight decrease in dexterity, but one study rating dexterity for double gloves as poor. As a proxy but objective measure for loss of dexterity, we assumed that needlestick injuries would be more frequent in the outer glove of double gloves than in single gloves. However, this was not the case. Additionally, we found one study that evaluated dexterity in the laboratory under standard conditions and found that double gloves did not influence dexterity (Fry 2010). We believe, therefore, that there is no serious impairment of dexterity from double gloving.
Studies included in this review covered a time period from 1990 to 2007 and apparently were initiated by the HIV epidemic and the risk of contamination for surgical staff. The number of glove studies reached a peak in the 1990s and then decreased again. We found nine studies from low and middle‐income countries (at the time of the study) (World Bank 2013). Four studies were from Thailand (1998, 1999, 2002, 2004), one from Indonesia (1999), one from India (2001) and three from Oman (1996). Only three studies were from an area with more than 1% prevalence of HIV (Thailand) and 12 studies were from an area with more than 2% prevalence of hepatitis C among the adult population (Indonesia, Australia, Thailand, Oman) (Centers for Disease Control 2012).
Most studies could be described as pragmatic trials because they were carried out by the healthcare staff who were themselves at risk. This increases the applicability of the evidence but at the same time has probably decreased the quality of studies. This has also led to a lack of trials with nurses as study participants. Studies included only operations or dental procedures, excluding other healthcare procedures with exposure risks, such as blood sampling by phlebotomists. Some authors have argued that double gloves protect against sharps injuries with surgery needles but protect much less against injuries with hollow‐bore needles (Bouvet 2009). It could therefore be that the effects are less in other participants with tasks different from surgery. Although one study with nurses as study participants is awaiting classification, all participants were part of the operating staff and were thus not very different from the participants of the studies included in this review.
Quality of the evidence
We included only randomised controlled trials, even though there were also many non‐randomised studies available. Therefore, the studies included are the better‐quality studies. Nevertheless, we rated the quality of the included studies as at best moderate. This could have resulted from most studies being performed by surgeons themselves and not being set up by a research institute. The reporting of randomisation methods and allocation concealment was especially unclear and because many studies were over 20 years old, it was impossible to get clarification from the authors.
Even though glove perforations form a fairly objective method of assessing potential exposure to blood, the differences in reporting this outcome decreased its validity. It is unclear in which direction this bias would go. Ideally one would like to know the number of perforations per physician/nurse per unit of exposure time, for example the number of perforations per 100 physician/nurse hours at risk. For operations, this would not be very difficult to calculate as was shown by Meyers 2008. Consensus on this measure is needed. We also rated the risk of bias from outcome assessment as high when the authors had not used a combined water and air test to assess perforations. Current European standards also specify that, to test gloves for perforations, both a specific water test and air test should be used to assess whether a glove achieves the acceptance quality level (level 2 for surgical gloves which means that perforations are allowed in 1.5% of new gloves) (CEN 2003). However, we believe that this bias has not influenced the results of the review to a great extent because the same measurement error would apply to both the intervention and the control group.
In spite of these limitations, and given the relatively large effect size and the consistency of the evidence, we believe there is no need for more and better studies on double gloving.
For triple gloving, special material gloves and thicker gloves, the evidence was less clear and we do see a need for more and better‐quality studies.
Potential biases in the review process
We did not exclude articles in languages other than English. Therefore, even though few were found, we are confident that there is no language bias in our review. We carried out all selection and data extraction processes in duplicate and involved a third assessor if we could not easily reach consensus.
We did not see publication bias in the funnel plot of the double gloving studies and also the Egger's test was non‐significant. Even though glove manufacturers must have a financial interest in the results of double gloving studies, because double gloving will increase the amount of gloves sold, we did not see involvement of the manufacturers in the included studies.
Agreements and disagreements with other studies or reviews
Several reviews have been published on the prevention of percutaneous exposure incidents in the past but only two included gloves as the intervention. Compared to the earlier review of Rogers 2000, the number of included studies increased enormously. Probably due to an non‐comprehensive search strategy the authors found only four studies, whereas we located 45. Tanner 2009 is a Cochrane Review on the prevention of surgical cross‐infection and glove perforations are only included as a secondary outcome. The review limited the inclusion of studies to surgical team members and included 31 randomised controlled trials. We included three more studies but did not find studies outside surgery. Two trials included in the review by Tanner 2009 assessed infections but did not find any. Based on the same studies as were included in this review, the authors concluded that double gloving protects against glove perforations even though they calculated odds ratios to assess the treatment effect. Given the high incidence of perforations odds ratios will provide an overestimate of the effect. Therefore, we believe that the rate ratios that we calculated for this review are a more appropriate estimate of the treatment effect. A more recent review by Yang identified only 10 studies, eight RCTs and two cohort studies that evaluated double gloving (Yang 2011). The authors did not perform a meta‐analysis but still concluded that double gloving was effective.
Authors' conclusions
Implications for practice.
We found moderate‐quality evidence that double gloves reduce the risk of percutaneous exposure incidents compared to single gloves for surgeons and surgical staff. The risk of inner glove perforations was reduced by 71% (rate ratio (RR) 0.29, 95% confidence interval (CI) 0.23 to 0.37) and the risk of blood contamination by 65% (RR 0.35, 95% CI 0.17 to 0.70). The effect was less clear for needlestick injuries but this was measured in two studies only and could be biased by measurement error. Even though loss of dexterity was reported in two studies, based on measurement with visual analogue scales, double gloves were still rated as good in one study and average in another. An increase in outer glove perforations could indicate a loss of dexterity but none was found (RR 1.10, 95% CI 0.93 to 1.31).
Surgeons can achieve a further reduction in the risk of percutaneous exposure by using three pairs of gloves or extra gloves made from special material. Fabric gloves over single gloves compared to two layers of normal material gloves reduced the risk of perforations by 76% (RR 0.24, 95% CI 0.06 to 0.93). Special material gloves (fabric, Kevlar, steel or spectra polyethylene fibre gloves) between two normal material gloves or the use of three standard material gloves did have the same preventive effect. However, we rated the evidence for the use of three glove layers or special gloves as low to moderate and it needs to be balanced against the additional costs and the influence on dexterity, which is unknown.
Evidence for thicker gloves, comparing one thick glove to two layers of normal gloves, was missing. However, the comparison of thicker gloves to comparably thinner gloves did not show a significant difference in the number of perforations (RR 0.63, 95% CI 0.37 to 1.08) and one study showed higher but non‐significant rates in thicker gloves compared to the glove combination of one fabric glove over one standard glove (RR 15.36, 95% CI 0.88 to 267.57).
We conclude that the prevention of percutaneous exposure incidents can be successfully achieved with an increase in the number of glove layers, rather than by increasing the thickness of gloves. The preventive effect can be increased when using more than two layers or when using special material gloves. However, evidence is missing for the use of gloves or for the effect of extra gloves for other tasks than surgery. It is also difficult to say which type of special material glove is the best.
Implications for research.
No further studies are needed to show the preventive effect of double gloving during surgery. However, it is unclear whether the results apply to healthcare professionals who are doing tasks outside the operating theatre, such as blood collection. The use of gloves or double gloves does not seem to be common practice here, therefore randomised trials can still be carried out. These should take into account proper randomisation procedures and measurement of the outcome. Injuries occur less frequently in this setting than in the operating theatre, therefore larger sample sizes are needed. In addition, the cost‐effectiveness of double gloving for other occupational groups should be evaluated. Similar trials to evaluate effectiveness and cost‐effectiveness are needed for the use of gloves made from special materials.
What's new
| Date | Event | Description |
|---|---|---|
| 10 October 2013 | Amended | The original version of this protocol was published with the title: "Prevention of percutaneous injuries with risk of hepatitis B, hepatitis C, or other viral infections for health‐care workers". However, it turned out that the scope was far too wide and would result in an unmanageable amount of studies for one review. Therefore the decision was taken to split the protocol into four new ones. The other three new titles are: "Blunt versus sharp suture needles for preventing percutaneous exposure incidents in surgical staff", "Devices for preventing percutaneous exposure injuries caused by needles in health care personnel" and "Education and training for preventing percutaneous exposure injuries in health care personnel". |
Notes
The protocol for this review was first published as "Prevention of percutaneous injuries with risk of hepatitis B, hepatitis C, or other viral infections for healthcare workers" (Parantainen 2008). Our initial idea was to include all interventions used to prevent needlestick injuries. However, after the publication of the protocol it became apparent that very many studies would be eligible for inclusion. The decision was therefore made to split the protocol up into four new protocols. The other protocols are titled: "Blunt versus sharp suture needles for preventing percutaneous exposure incidents in surgical staff" (Parantainen 2011), "Devices to prevent needle recapping for preventing percutaneous exposure injuries in health care personnel" (Lavoie 2012) and "Education and training for preventing percutaneous exposure injuries in healthcare personnel".
The original protocol was hosted by the Hepato‐Biliary Group but due to the heavy involvement of Jos Verbeek and the Occupational Safety and Health Group, the new titles were registered under their aegis. The Hepato‐Biliary Group continues to be involved in an advisory capacity.
Acknowledgements
We would like to thank Minna Anthoni and Ulla‐Maija Hellgren, who participated in the writing of an early version of the first protocol. We extend our gratitude to Ms Leena Isotalo, the Trials Search Coordinator of the Cochrane Occupational Safety and Health Review Group, for designing the systematic search strategies and for running them in all the chosen electronic reference databases. We would also like to thank Dimitrinka Nikolova and Christian Gluud from the Hepato‐Biliary Group, for their comments on an early version of our protocol, and Jani Ruotsalainen from the Occupational Safety and Health Group, Janet Wale from the Bone, Joint and Muscle Trauma Group and Jenny Bellorini from the Ear, Nose and Throat Group for copy editing the text.
Appendices
Appendix 1. Search strategies 2010
| Database | Period of search | Search strategy |
| EMBASE | 1974 to September 2010 |
#6 #5 AND [humans]/lim AND [embase]/lim #5 #3 AND #4 #4 [randomized controlled trial]/lim OR [controlled clinical trial]/lim OR random* OR 'double blind' OR 'single blind' OR (singl* OR doubl* OR trebl* OR tripl* AND (blind* OR mask*)) OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'triple blind procedure'/exp OR placebo* OR 'controlled study'/exp OR 'cross sectional study'/exp OR 'crossover procedure'/exp OR 'latin square design'/exp OR 'follow up'/exp OR 'comparative study'/exp OR 'evaluation studies'/exp OR 'evaluation study' OR prospectiv* OR volunteer*] #3 #1 AND #2 #2 'health care personnel'/exp OR 'health care personnel' OR 'health care worker'/exp OR 'health care worker' OR 'health care workers' OR 'health care facilities and services'/exp OR 'medical profession'/exp OR 'nursing as a profession'/exp OR ('virus transmission'/exp AND 'patient'/exp AND professional) #1 'needlestick injury'/exp OR needlestick* OR 'needle stick'/exp OR 'sharp injury' OR 'sharp injuries' OR 'sharp medical' OR 'sharp instrument' OR 'sharp needle' OR 'sharp needles' OR sharps OR 'percutaneous exposure' OR 'percutaneous injury' OR 'percutaneous injuries' OR 'percutaneous trauma' OR 'stick injury' OR 'stick injuries' OR 'stab wound'/exp OR 'face injury'/de OR 'eye injury'/de OR 'arm injury'/de OR 'hand injury'/de OR 'needle'/exp OR (splash* AND ('blood'/exp OR blood OR secretion* OR fluid* OR 'body fluid'/exp OR 'body fluids'/exp)) |
| Wiley InterScience: The Cochrane Library databases: CENTRAL and NHSEED | 1993 to September 2010 |
#3 #1 AND #2 #2 EXP Needlestick Injuries (MeSH) OR needlestick* OR "needle stick OR "needle sticks" OR "percutaneous exposure" OR "percutaneous exposures" OR "percutaneous injury" OR "percutaneous injuries2 OR "stick injury" OR "stick injuries" OR Wounds, Stab (MeSH) OR Wounds, Penetrating (MeSH) OR Facial injuries (MeSH) OR EXP Eye Injuries, Penetrating (MeSH) OR Forearm Injuries (MeSH) OR EXP Hand Injuries (MeSH) OR [splash* AND blood OR secretion* OR fluid* OR EXP Body Fluids (MeSH) OR EXP Bodily Secretions (MeSH)] #1 EXP Health Occupations (MeSH) OR EXP Health Personnel (MeSH) OR EXP Health Facilities (MeSH) OR "health care worker" OR "health care workers" OR Disease Transmission, patient‐to‐Professional (MeSH) |
| Science Citation Index Expanded | 1986 to 5 October 2010 |
#4 #1 AND #2 AND #3 #3 TS=(random* OR control* OR trial OR trials OR "single blind" OR "double blind" OR "triple blind" OR "latin square" OR placebo* OR comparative OR "follow up" OR prospectiv* OR "cross over" OR volunteer*) #2 TS=(needlestick* OR "needle stick" OR "needle sticks" OR "stick injury" OR "stick injuries" OR "wound stab" OR "stab wound" OR "penetrating wound" OR "penetrating wounds") OR TS=(sharp* AND ( injury OR injuries OR medical OR instrument*)) OR TS=(percutaneous AND (exposure OR exposures OR injury OR injuries)) OR TS=(injur* AND (facial OR eye OR eyes OR arm OR hand OR finger OR fingers)) OR TS=(splash* AND (blood OR secretion* OR fluid OR fluids)) OR TS="blood borne infection" #1 TS=("health care worker" OR "health care workers" OR "health occupations" OR "health personnel" OR physician* OR nurse* OR hospital* OR clinic OR clinics) |
| CINAHL | 1982 to September 2010 |
#5 #3 AND #4 #4 "randomized controlled trial" or "clinical trials" or "clinical trial" or "random allocation" or "double blind". or "single blind" or ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)) or "latin square" or placebo# or random* or "research design" or "comparative study" or "comparative studies" or "evaluation study" or "evaluation studies" or "follow up study" or "follow up studies" or "prospective study" or "prospective studies" or "cross over study" or "cross over studies" or control* or prospective* or volunteer or (MH "Clinical Trials+") or (MH "Nonrandomized Trials") or (MH "Crossover Design") #3 #1 AND #2 #2 TX "needlestick injury" or needlestick# or "needle stick" or "needle sticks" or "sharp injury" or "sharp injuries" or "sharp medical device" or "sharp medical devices" or "sharp instrument" or "sharp instruments" or "sharp needle" or "sharp needles" or "percutaneous exposure" or "percutaneous exposures" or "percutaneous injury" or "percutaneous injuries" or "stick injury" or "stick injuries" or "wounds, stab" or "wounds, penetrating" or "facial injuries" or "eye injuries, penetrating" or "arm injuries" or "forearm injuries" or "hand injuries" or "finger injuries" or (splash# and (blood or secretion# or fluid#)) or ("occupational exposure" and ("body fluid" or "body fluids" or blood)) #1 (MH "Health Occupations") OR health occupations or (MH "Health Personnel+") or (MH "Health Facilities+") OR health facilities or TX "health care worker" or TX "health care workers" or (MH "Personnel, Health Facility+") or (MH "Occupational Health Services+") or (MH "Occupational Hazards+") or (MH "Occupational Exposure") or TX "health care personnel" or (MH "Health Personnel+") or (MH "HIV Infections+") |
| OSH UPDATE (NIOSHTIC‐2 and CISDOC) | NIOSHTIC‐2: 1900 to September 2010 CISDOC: 1987 to September 2010 |
#15 #13 AND #14 #14 PY{2007} OR PY{2008} OR PY{2009} #13 #7 AND #12 #12 #8 OR #11 #11 #9 AND #10 #10 GW{blind* OR mask*} #9 GW{singl* OR doubl* OR tripl* OR trebl*} #8 GW{random* OR control* OR trial OR trials OR comparativ* OR evaluation* OR "latin square" OR placebo OR "follow up" OR prospectiv* OR "cross over" OR volunteer*} #7 #1 AND #6 #6 #2 OR #5 #5 #3 AND #4 #4 GW{splash*} #3 GW{blood OR fluid* OR secretion*} #2 GW{"sharp medical" OR "sharp instrument" OR "sharp instruments" OR needlestick* OR "needle stick" OR "needle sticks" OR "sharp injury" OR "sharp injuries" OR "stab wound" OR "stab wounds" OR "wound penetrating" OR "stick injury" OR "stick injuries" OR "percutaneous injury" OR "percutaneous injuries" OR "percutaneous exposure" OR "percutaneous exposures" OR "sharp needle" OR "sharp needles"} #1 GW{nurse OR nurses OR physician OR physicians OR hospital* OR "health occupation" OR "health occupations" OR "health personnel" OR "health care personnel" OR "health care worker" OR "health care workers" OR "health worker" OR "health workers"} |
| MEDLINE in PubMed | From 1950 to September 2010 |
#5 Search #1 AND #2 AND (#3 OR #4) #4 Search effect*[tw] OR control[tw] OR controls*[tw] OR controla*[tw] OR controle*[tw] OR controli*[tw] OR controll*[tw] OR control'*[tw] OR evaluation*[tw] OR program*[tw] #3 ("Randomized Controlled Trial"[pt] OR "Controlled Clinical Trial"[pt] OR "Randomized Controlled Trials as Topic"[mh] OR "Random Allocation"[mh] OR "Double‐Blind Method"[mh] OR "Single‐Blind Method"[mh] OR "Clinical Trial"[pt] OR "Clinical Trials as Topic"[mh] OR "clinical trial"[tw] OR ((singl*[tw] OR doubl*[tw] OR trebl*[tw] OR tripl*[tw]) AND (mask*[tw] OR blind*[tw])) OR "latin square"[tw] OR Placebos[mh] OR placebo*[tw] OR random*[tw] OR "Research Design"[mh:noexp] OR "Comparative Study"[pt] OR "Evaluation Studies as Topic"[mh] OR "Follow‐up Studies"[mh] OR "Prospective Studies"[mh] OR "Cross‐over Studies"[mh] OR control[tw] OR controls*[tw] OR controla*[tw] OR controle*[tw] OR controli*[tw] OR controll*[tw] OR control'*[tw] OR prospectiv*[tw] OR volunteer*[tw]) NOT (Animals[mh] NOT Humans[mh) #2 "Needlestick injuries"[mh] OR needlestick*[tw] OR "needle stick"[tw] or "needle sticks"[tw] OR "sharp injury"[tw] OR "sharp injuries"[tw] OR sharps[tw] OR "sharp medical device"[tw] OR "sharp medical devices"[tw] OR "sharp instrument"[tw] OR "sharp instruments"[tw] OR "sharp medical instrument"[tw] OR "sharp medical instruments"[tw] OR "sharp needle"[tw] OR "sharp needles"[tw] OR "percutaneous exposure"[tw] OR "percutaneous exposures"[tw] OR "percutaneous injury"[tw] OR "percutaneous injuries"[tw] OR "stick injury"[tw] OR "stick injuries"[tw] OR "Wounds, Stab"[mh:noexp] OR "Wounds, Penetrating"[mh:noexp] OR "Facial injuries"[mh:noexp] OR "Eye Injuries, Penetrating"[mh] OR "Arm Injuries"[mh:noexp] OR "Forearm Injuries"[mh:noexp] OR "Hand Injuries"[mh] OR (splash* AND (blood[tw] or secretion*[tw] OR fluid*[tw] OR "Body Fluids"[mh])) #1 "Health Occupations"[mh] OR "Health Personnel"[mh] OR "Health Facilities"[mh] OR "health care worker"[tw] OR "health care workers"[tw] OR "Infectious Disease Transmission, Patient‐to‐Professional"[mh] |
| PsycINFO (OvidSP) | 1967 to September 2010 |
#5 limit 4 to all journals #4 #1 AND #2 AND #3 #3 random* OR control* OR trial OR trials OR comparativ* OR evaluation* OR ((singl* OR doubl* OR tripl* OR trebl*) AND (blind* OR mask*)) OR "latin square" OR placebo* OR "follow up" OR prospectiv* OR "cross over" OR volunteer* #2 (splash* AND (blood OR secretion* OR fluid OR fluids)) OR ("eye injuries" AND penetrating) OR (wound* AND (stab OR penetrating)) OR "percutaneous exposure" OR "percutaneous exposures" OR "percutaneous injury" OR "percutaneous injuries" OR "stick injury" OR "stick injuries" OR "sharp injury" OR "sharp injuries" OR "sharp medical" OR "sharp instrument" OR "sharp instruments" OR "sharp needle" OR "sharp needles" OR needlestick* OR "needle stick" OR "needle sticks" #1 (nursing or nurse or nurses or physician or physicians or "health care personnel" or "health personnel" or "health care worker" or "health care workers" or "Clinicians*" or "Dentist*" or "Health‐Personnel" or "Medical Personnel" or "Military‐Medical‐Personnel" or "Nurses*" or "Physician*" or "Psychiatric‐Hospital‐Staff*" or "medical students" or "hospitals" or "occupational exposure" or "occupational exposures").mp. [mp=title, abstract, heading word, table of contents, key concepts] |
| LILACS | September 2010 | "Health Occupations" or "Health Personnel" OR "Health Facilities" OR "health care worker" OR "health care workers" OR "Disease Transmission, Patient‐to‐Professional" OR "INJURIES" or "WOUNDS AND INJURIES/PC" or "accidents, OCCUPATIONAL" or "injuries, poisonings, and OCCUPATIONAL diseases" or "OCCUPATIONAL exposure" or "OCCUPATIONAL health policy" or "OCCUPATIONAL risks" OR "INJURIES" or "WOUNDS AND INJURIES/PC" or "accidents, OCCUPATIONAL" or "injuries, poisonings, and OCCUPATIONAL diseases" or "OCCUPATIONAL exposure" or "OCCUPATIONAL health policy" or "OCCUPATIONAL risks" [Descritor de assunto] and "CLINICAL TRIAL" OR "CLINICAL TRIAL, PHASE I" OR "CLINICAL TRIAL, PHASE II" OR "CLINICAL TRIAL, PHASE III" OR "CLINICAL TRIAL, PHASE IV" OR "COMPARATIVE STUDY" OR "CONTROLLED CLINICAL TRIAL" OR "EVALUATION STUDIES" OR "META‐ANALYSIS" OR "MULTICENTER STUDY" OR "RANDOMIZED CONTROLLED TRIAL" OR "REVIEW" [Tipo de publicação] and not "ANIMALS" or "HUMANS" [Palavras] |
Appendix 2. Search strategy update 2010 to 2013
| Database | Period of search | Search strategy |
| EMBASE | September 2010 to June 2013 |
#1 [randomized controlled trial]/lim OR [controlled clinical trial]/lim OR random* OR 'double blind' OR 'single blind' OR (singl* OR doubl* OR trebl* OR tripl* AND (blind* OR mask*)) OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'triple blind procedure'/exp OR placebo* #2 'needlestick injury'/exp OR needle* OR 'sharp injury' OR 'sharp injuries' OR 'sharp medical' OR 'sharp instrument' OR 'sharp needle' OR 'sharp needles' OR sharps OR 'percutaneous exposure' OR 'percutaneous injury' OR 'percutaneous injuries' OR 'percutaneous trauma' OR 'stick injury' OR 'stick injuries' OR 'stab wound'/de OR 'needle'/exp OR (splash* AND ('blood'/exp OR blood OR secretion* OR fluid* OR 'body fluid'/exp)) #3 glove* OR 'double gloving' #4 #1 AND #2 AND #3 (69) #5 #4 AND [1‐9‐2010]/sd (13) |
| Wiley InterScience: The Cochrane Library databases: CENTRAL and NHSEED | 2007 to June 2013 (CENTRAL Issue 5/NHSEED Issue 2) |
#1 MeSH descriptor: [Needlestick Injuries] explode all trees (83) #2 MeSH descriptor: [Wounds, Stab] explode all trees (103) #3 MeSH descriptor: [Wounds, Penetrating] this term only (167) #4 needle* or "percutaneous exposure" or "percutaneous exposures" or "percutaneous injury" or "percutaneous injuries" or "stick injury" or "stick injuries":ti,ab,kw (Word variations have been searched) (4766) #5 MeSH descriptor: [Bodily Secretions] explode all trees (7011) #6 MeSH descriptor: [Body Fluids] explode all trees (4000) #7 blood or secretion* or fluid*:ti,ab,kw (Word variations have been searched) (128034) #8 #5 or #6 or #7 (134181) #9 splash*:ti,ab,kw (Word variations have been searched) (15) #10 #8 and #9 (5) #11 #1 or #2 or #3 or #4 or #10 (4943) #12 MeSH descriptor: [Gloves, Protective] explode all trees (162) #13 glove* or "double gloving":ti,ab,kw (Word variations have been searched) (414) #14 #12 or #13 (414) #15 #11 and #14 (41 Cochrane Library records) |
| Science Citation Index Expanded | 2010 to June 2013 |
#1 TS=(random* OR control* OR trial OR trials OR "single blind" OR "double blind" OR "triple blind" OR "latin square" OR placebo* OR comparative OR "follow up" OR prospectiv* OR "cross over" OR volunteer*) #2 TS=(needle* OR "stick injury" OR "stick injuries" OR "wound stab" OR "stab wound" OR "penetrating wound" OR "penetrating wounds") OR TS=(sharp* AND ( injury OR injuries OR medical OR instrument*)) OR TS=(percutaneous AND (exposure OR exposures OR injury OR injuries)) OR TS=(splash* AND (blood OR secretion* OR fluid OR fluids)) OR TS="blood borne infection" #3 TS=(glove* OR "double gloving") #4 #1 AND #2 AND #3 (23) |
| CINAHL plus with full text through EBSCO | Up until October 2013 | (MH “gloves”) OR “glov*” plus relevant filter for randomised controlled trials as implemented in CINAHL |
| OSH UPDATE (NIOSHTIC‐2 and CISDOC) | 2010 to June 2013 |
#1 GW{random* OR control* OR trial OR trials OR comparativ* OR evaluation* OR "latin square" OR placebo OR "follow up" OR prospectiv* OR "cross over" OR volunteer*} (188990) #2 GW{blind* OR mask*} AND GW{singl* OR doubl* OR tripl* OR trebl*} (1240) #3 #1 OR #2 (189162) #4 GW{needle* OR "sharp medical" OR "sharp instrument" OR "sharp instruments" OR "sharp injury" OR "sharp injuries" OR "stab wound" OR "stab wounds" OR " penetrating wound " OR "stick injury" OR "stick injuries" OR "percutaneous injury" OR "percutaneous injuries" OR "percutaneous exposure" OR "percutaneous exposures" } (2858) #5 GW{blood OR fluid* OR secretion*} AND GW{splash*} (365) #6 #4 OR #5 (3109) #7 GW{glove* OR "double gloving"} (7440) #8 #3 AND #6 AND #7 (388) #9 PY{2010} OR PY{2011} OR PY{2012} OR PY{2013} (24042) #10 #8 AND #9 (70) #11 DC{OUNIOS OR OUCISD} #12 #10 AND #11 (3) |
| MEDLINE in PubMed | September 2010 to June 2013 |
#1 ("Randomized Controlled Trial"[pt] OR "Controlled Clinical Trial"[pt] OR "Randomized Controlled Trials as Topic"[mh] OR "Random Allocation"[mh] OR "Double‐Blind Method"[mh] OR "Single‐Blind Method"[mh] OR "Clinical Trial"[pt] OR "Clinical Trials as Topic"[mh] OR "clinical trial"[tw] OR ((singl*[tw] OR doubl*[tw] OR trebl*[tw] OR tripl*[tw]) AND (mask*[tw] OR blind*[tw])) OR "latin square"[tw] OR Placebos[mh] OR placebo*[tw] OR random*[tw] OR "Research Design"[mh:noexp] OR "Comparative Study"[pt] OR "Evaluation Studies as Topic"[mh] OR "Follow‐up Studies"[mh] OR "Prospective Studies"[mh] OR "Cross‐over Studies"[mh] OR control[tw] OR controls*[tw] OR controla*[tw] OR controle*[tw] OR controli*[tw] OR controll*[tw] OR control'*[tw] OR prospectiv*[tw] OR volunteer*[tw]) NOT (Animals[mh] NOT Humans[mh]) (5114514) #2 "Needlestick injuries"[mh] OR needle*[tw] OR "sharp injury"[tw] OR "sharp injuries"[tw] OR sharps[tw] OR "sharp medical device"[tw] OR "sharp medical devices"[tw] OR "sharp instrument"[tw] OR "sharp instruments"[tw] OR "sharp medical instrument"[tw] OR "sharp medical instruments"[tw] OR "sharp needle"[tw] OR "sharp needles"[tw] OR "percutaneous exposure"[tw] OR "percutaneous exposures"[tw] OR "percutaneous injury"[tw] OR "percutaneous injuries"[tw] OR "stick injury"[tw] OR "stick injuries"[tw] OR "Wounds, Stab"[mh:noexp] OR "Wounds, Penetrating"[mh:noexp] OR (splash* AND (blood[tw] or secretion*[tw] OR fluid*[tw] OR "Body Fluids"[mh])) (127087) #3 "Gloves, Protective"[Mesh] OR glove*[tw] OR "double gloving" (8631) #4 #1 AND #2 AND #3 #5 #4 AND "2010/09/01"[Date ‐ Entrez] : "3000" [Date ‐ Entrez] (41) |
| PsycINFO (ProQuest) | 2009 to 2013 |
#1 random* OR control* OR trial OR trials OR comparativ* OR evaluation* OR ((singl* OR doubl* OR tripl* OR trebl*) AND (blind* OR mask*)) OR "latin square" OR placebo* OR "follow up" OR prospectiv* OR "cross over" OR volunteer* (956861) #2 (splash* AND (blood OR secretion* OR fluid OR fluids)) OR (wound* AND (stab OR penetrating)) OR "percutaneous exposure" OR "percutaneous exposures" OR "percutaneous injury" OR "percutaneous injuries" OR "stick injury" OR "stick injuries" OR "sharp injury" OR "sharp injuries" OR "sharp medical" OR "sharp instrument" OR "sharp instruments" OR needle* (4311) #3 glove* OR "double gloving" (1881) #4 #1 AND #2 AND #3 (7) Limit to Publication year 2009 – (4) |
Data and analyses
Comparison 1. Double versus single gloves.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Inner glove perforations | 12 | Rate Ratio (Fixed, 95% CI) | 0.29 [0.23, 0.37] | |
| 1.1 Number of perforations | 10 | Rate Ratio (Fixed, 95% CI) | 0.33 [0.24, 0.44] | |
| 1.2 Number of gloves with perforations | 2 | Rate Ratio (Fixed, 95% CI) | 0.22 [0.15, 0.34] | |
| 2 Matched inner glove perforations | 4 | Rate Ratio (Fixed, 95% CI) | 0.11 [0.05, 0.20] | |
| 2.1 Number of perforations | 4 | Rate Ratio (Fixed, 95% CI) | 0.11 [0.05, 0.20] | |
| 3 Needlestick injuries | 2 | Rate Ratio (Fixed, 95% CI) | 0.58 [0.21, 1.62] | |
| 3.1 Per pair of gloves | 2 | Rate Ratio (Fixed, 95% CI) | 0.58 [0.21, 1.62] | |
| 4 Incidences of blood contamination | 3 | 819 | Rate Ratio (Fixed, 95% CI) | 0.35 [0.17, 0.70] |
| 5 Dexterity: VAS score | Other data | No numeric data | ||
| 6 Dexterity: outer glove perforations | 8 | Rate Ratio (Fixed, 95% CI) | 1.10 [0.93, 1.31] | |
| 6.1 Number of perforations | 6 | Rate Ratio (Fixed, 95% CI) | 1.05 [0.83, 1.33] | |
| 6.2 Number of gloves with perforations | 2 | Rate Ratio (Fixed, 95% CI) | 1.15 [0.90, 1.48] |
Comparison 2. Triple versus double gloves.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Inner glove perforations | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 1.1 Number of perforations | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Double special versus double normal.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Inner glove perforations | 4 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Fabric glove, number of perforations | 2 | Rate Ratio (Random, 95% CI) | 0.27 [0.03, 2.96] | |
| 1.2 Fabric glove, number of gloves with perforations | 1 | Rate Ratio (Random, 95% CI) | 0.17 [0.06, 0.43] | |
| 1.3 Wire weave glove, number of gloves with perforations | 1 | Rate Ratio (Random, 95% CI) | 0.73 [0.38, 1.38] | |
| 2 Dexterity: VAS score | Other data | No numeric data |
Comparison 4. Triple special versus double normal.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Inner glove perforations | 5 | Rate Ratio (Fixed, 95% CI) | 0.24 [0.13, 0.45] | |
| 1.1 Fabric | 2 | Rate Ratio (Fixed, 95% CI) | 0.33 [0.11, 0.99] | |
| 1.2 Spectra polyethylene fibre | 1 | Rate Ratio (Fixed, 95% CI) | 0.33 [0.12, 0.88] | |
| 1.3 Kevlar | 1 | Rate Ratio (Fixed, 95% CI) | 0.06 [0.01, 0.50] | |
| 1.4 Steel | 1 | Rate Ratio (Fixed, 95% CI) | 0.12 [0.03, 0.59] |
Comparison 5. Thicker versus thinner.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Inner glove perforations | 5 | Rate Ratio (Random, 95% CI) | 0.63 [0.37, 1.08] | |
| 1.1 Number of perforations | 2 | Rate Ratio (Random, 95% CI) | 0.34 [0.18, 0.65] | |
| 1.2 Number of gloves with perforations | 3 | Rate Ratio (Random, 95% CI) | 0.85 [0.53, 1.35] | |
| 2 Matched perforations | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 2.1 Number of gloves with matched perforations | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Thick versus glove combinations.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Inner glove perforations | 1 | Rate Ratio (Random, 95% CI) | Totals not selected | |
| 1.1 Standard glove + fabric glove, number of perforations | 1 | Rate Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. Double indicator versus standard.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 First glove: inner perforations | 1 | Rate Ratio (Fixed, 95% CI) | 0.10 [0.02, 0.45] | |
| 1.1 Double versus single, number of perforations | 1 | Rate Ratio (Fixed, 95% CI) | 0.06 [0.01, 0.49] | |
| 1.2 Double versus double, number of perforations | 1 | Rate Ratio (Fixed, 95% CI) | 0.17 [0.02, 1.44] | |
| 2 First glove: matched perforations | 2 | Rate Ratio (Random, 95% CI) | 0.09 [0.03, 0.29] | |
| 2.1 Double versus single, number of glove pairs with perforations | 1 | Rate Ratio (Random, 95% CI) | 0.13 [0.06, 0.31] | |
| 2.2 Double versus single, number of gloves with perforations | 1 | Rate Ratio (Random, 95% CI) | 0.04 [0.00, 0.26] | |
| 3 All used gloves: inner glove perforations | 2 | Rate Ratio (Random, 95% CI) | 0.72 [0.36, 1.42] | |
| 3.1 Double versus double, number of perforations | 2 | Rate Ratio (Random, 95% CI) | 0.72 [0.36, 1.42] | |
| 4 Dexterity: VAS score | Other data | No numeric data |
Comparison 8. Subgroup analysis: prevalence, double versus single.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Inner glove perforations | 12 | Rate Ratio (Fixed, 95% CI) | 0.29 [0.23, 0.37] | |
| 1.1 HIV > 1% or hepatitis C > 2% | 10 | Rate Ratio (Fixed, 95% CI) | 0.28 [0.22, 0.37] | |
| 1.2 HIV < 1% and hepatitis C < 2% | 2 | Rate Ratio (Fixed, 95% CI) | 0.38 [0.15, 0.96] |
Comparison 9. Subgroup analysis: income of countries, double versus single.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Inner glove perforations | 12 | Rate Ratio (Fixed, 95% CI) | 0.29 [0.23, 0.37] | |
| 1.1 Low and middle‐income | 6 | Rate Ratio (Fixed, 95% CI) | 0.34 [0.24, 0.47] | |
| 1.2 High‐income countries | 6 | Rate Ratio (Fixed, 95% CI) | 0.23 [0.16, 0.34] |
Comparison 10. Subgroup analysis: exposure, double versus single.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Inner glove perforations | 12 | Rate Ratio (Fixed, 95% CI) | 0.29 [0.23, 0.37] | |
| 1.1 Low exposure: < 0.20 perforations | 8 | Rate Ratio (Fixed, 95% CI) | 0.31 [0.22, 0.43] | |
| 1.2 High exposure: > 0.20 perforations | 4 | Rate Ratio (Fixed, 95% CI) | 0.27 [0.19, 0.39] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aarnio 2001.
| Methods | Study design: randomised controlled trial Object of randomisation: patient | |
| Participants | Finland; September to October 1999 Surgeon and sometimes assistant surgeon All vascular surgical operations during 2‐month trial period (n = 73) |
|
| Interventions | Intervention: double gloving with indicator glove (Biogel Indicator, Regent Medical, Malaysia) Control group: single gloving (Gammex or Nutex, Ansell Medical, Malaysia) | |
| Outcomes | Included in the review: number of perforations in inner gloves per gloves used Additional: number of perforations detected during surgery |
|
| Notes | Missing information per gloving type: number of operations, number of persons per operation; no response to emails | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | High risk | According to year of birth of patient |
| Allocation concealment | High risk | Year of birth unlikely concealed |
| Blinding of study subjects | Low risk | Subjects not blinded, low risk of influencing outcome |
| Blinding of outcome assessor | Unclear risk | Unknown |
| Incomplete outcome data (attrition bias) | Low risk | No information about excluded data (gloves nor patients); author stated 73 operations were performed and reported data for all of them; more glove pairs than operations which means likely all gloves included in analysis |
| Selective outcome reporting (reporting bias) | Low risk | Number of inner and outer glove perforations per number of gloves used reported |
| Outcome measure (combined air and water test used?) | High risk | Standardised water filling test method (EN 455‐1), air test missing |
Avery 1999a.
| Methods | Study design: randomised controlled trial Object of randomisation: workers | |
| Participants | UK Two senior dental surgeons and three qualified dental hygienists performing routine dental treatments on HIV‐positive patients Number of operations: 67 with double gloves, 71 with single gloves; approximated two persons per operation on average |
|
| Interventions | Intervention: two gloves (two surgical gloves or Regent 'reveal' glove system) Control: one glove (Biogel D, Regent or Surgical glove) |
|
| Outcomes | Outcome: number of glove perforations The total number of gloves in each group not reported Perforation detection: the gloves were filled with water and perforations were noted as a jet of water Secondary outcomes: subjective opinions of the ease of glove donning, comfort and sensitivity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information provided |
| Allocation concealment | Unclear risk | No information provided |
| Blinding of study subjects | Low risk | — |
| Blinding of outcome assessor | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | 4 out of 138 patients were excluded |
| Selective outcome reporting (reporting bias) | Low risk | Perforations |
| Outcome measure (combined air and water test used?) | High risk | Water test only (water inflation technique, visual detection for contamination with blood) |
Avery 1999b.
| Methods | Study design: randomised controlled trial Object of randomisation: workers | |
| Participants | UK Two principal surgeons and their assistants performing maxillofacial surgery Number studied: 1061 gloves (113 patients) Intervention group n = 453 (113 patients); control group n = 608 (113 patients) | |
| Interventions | The workers used one of two methods of double gloving: standard Regent surgical gloves or the Reveal perforation identification system. Inner glove perforations were used as control | |
| Outcomes | Outcome: number of outer glove perforations per total number of gloves Perforation detection: the gloves were filled with water and perforations were noted as a jet of water Secondary outcome: glove comfort and sensibility | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Randomly allocated, no information provided on how |
| Allocation concealment | Unclear risk | No information |
| Blinding of study subjects | Low risk | — |
| Blinding of outcome assessor | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Low risk | All randomised cases analysed; probably all gloves included |
| Selective outcome reporting (reporting bias) | High risk | Only number of unnoticed outer glove perforations per type of glove reported; number of inner glove perforations missing |
| Outcome measure (combined air and water test used?) | High risk | Inflating with water only |
Berridge 1998.
| Methods | Study design: randomised controlled trial Object of randomisation: not reported | |
| Participants | UK Surgeons, assistants and scrub nurses performing elective and emergency peripheral vascular surgical operations Number studied: 88 operations Intervention group n = 43; control group n = 45 Number of gloves not reported | |
| Interventions | Double gloving The control group wore single gloves | |
| Outcomes | Outcome: proportion of participants with blood‐contaminated hand or digit detected by macroscopic evidence; proportion of participants with glove perforation(s) Perforation detection: the gloves were filled with water and perforations were noted as a jet of water | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Not described |
| Allocation concealment | Unclear risk | Not reported |
| Blinding of study subjects | Low risk | — |
| Blinding of outcome assessor | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear if all gloves used were collected |
| Selective outcome reporting (reporting bias) | Low risk | Perforations in single and double gloves and contamination reported |
| Outcome measure (combined air and water test used?) | High risk | Water test only; not reported whether both inner and outer gloves were tested |
Carter 1996.
| Methods | Study design: randomised controlled trial Object of randomisation: the order of gloves used | |
| Participants | UK One surgeon performing most common anorectal procedures Number studied: 280 operations (690 gloves) Intervention group n = 140 (351 gloves); control group n = 140 (339 gloves) | |
| Interventions | Use of Biogel Super‐Sensitive gloves which are thinner but theoretically as strong as standard Biogel gloves. Standard Biogel gloves were used as control | |
| Outcomes | Outcome: number of perforated gloves per total number of gloves Perforation detection: the gloves were filled with water and perforations were noted as a jet of water | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information |
| Allocation concealment | Unclear risk | Not reported; likely decided by surgeon |
| Blinding of study subjects | Low risk | Not possible; knowledge about the gloving method judged as low risk of changing the outcome |
| Blinding of outcome assessor | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Low risk | All gloves used reported as analysed |
| Selective outcome reporting (reporting bias) | Low risk | Number of gloves with punctures reported |
| Outcome measure (combined air and water test used?) | High risk | Water test only |
Chua 1996.
| Methods | Study design: randomised controlled trial Object of randomisation: the order of gloves used | |
| Participants | UK Two consultants, two senior registrars and two postgraduate students performing orthodontic procedures Number studied: 60 patients (716 gloves) Group 1: n = 238 gloves; Group 2: n = 238 gloves; Group 3: n = 240 gloves | |
| Interventions | Intervention: single gloving with latex glove designed for heavy duty (Biogel D, Regent hospital product) Control group either single gloving with latex standard (lightweight micro‐touch glove, Johnson & Johnson) or single latex‐free (N‐Dex, Best Manufacturing Europe N.V.) | |
| Outcomes | Outcome: number of perforations per total number of gloves Perforation detection: the gloves were filled with water and perforations were noted as a jet of water. Permanent black ink was added to the water to improve the detection Secondary outcome: user satisfaction | |
| Notes | Intervention: Biogel D Control: micro‐touch | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Order of use based on randomisation code |
| Allocation concealment | Low risk | Not concealed until OP; OP were similar; each surgeon used both types of gloves |
| Blinding of study subjects | Low risk | Not possible; knowledge of gloving type judged as low risk of changing the outcome |
| Blinding of outcome assessor | High risk | Not blinded; 1 investigator assessed all gloves; investigator was one of the surgeons |
| Incomplete outcome data (attrition bias) | Low risk | Only 4 of 720 gloves excluded |
| Selective outcome reporting (reporting bias) | Low risk | Perforations reported |
| Outcome measure (combined air and water test used?) | High risk | Only water test |
Doyle 1992.
| Methods | Study design: randomised controlled trial Object of randomisation: per operation; two operators were individually randomised to use either one or two standard gloves | |
| Participants | UK
Surgeons and their assistants performing operative obstetric and gynaecological procedures involving use of sharp instruments
Number randomised: 150 glove sets (number followed 147)
Intervention group n = 79 (glove sets); control group n = 68 (pairs of gloves) Total number of operations: 75 (not separately reported for control and intervention) |
|
| Interventions | Double gloves worn by surgeons and/or their assistants in the intervention group The control group wore single gloves | |
| Outcomes | Outcome: the number of perforations per total number of (inner) glove pairs; needlestick injuries; presence of blood on the skin Measurement: not reported for presence of blood on the skin. Perforation detection: the gloves were filled with water and perforations were noted as a jet of water Secondary outcome: subjective views on impairment of dexterity when double gloved | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random number tables |
| Allocation concealment | Unclear risk | At the time of operation |
| Blinding of study subjects | Low risk | — |
| Blinding of outcome assessor | Unclear risk | Gloves were placed in a bag together with a questionnaire (type and other information); unclear if assessor saw the questionnaire |
| Incomplete outcome data (attrition bias) | Low risk | — |
| Selective outcome reporting (reporting bias) | Low risk | three of four outcomes reported: perforations, needlestick injuries, subjective impairment of dexterity Not reported: presence of blood |
| Outcome measure (combined air and water test used?) | High risk | Water test only; each glove was filled with approximally 500 ml of water and tested for leaks |
Duron 1996.
| Methods | Study design: randomised controlled trial Object of randomisation: patient | |
| Participants | France One surgeon and two theatre nurses performing operations which comprised central venous cannulation and insertion of implantable catheters with ports Number studied: 100 operations (216 double glove sets) Intervention group n = 216 inner gloves; control group n = 216 outer gloves | |
| Interventions | Double gloving All participants double gloved and outer gloves were used as controls | |
| Outcomes | Outcome: number of (matching) perforations per total number of gloves Perforation detection: the gloves were filled with water and perforations were noted as a jet of water | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Not described |
| Allocation concealment | Unclear risk | Not described |
| Blinding of study subjects | Low risk | Not possible; knowledge about the gloving method judged as low risk of changing the outcome |
| Blinding of outcome assessor | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | No information about excluded gloves |
| Selective outcome reporting (reporting bias) | Low risk | All outcomes reported (perforations recognised during OP and with test) |
| Outcome measure (combined air and water test used?) | High risk | Water test only |
Gani 1990.
| Methods | Study design: cluster‐randomised controlled trial Object of randomisation: patients (corresponding to operating teams) | |
| Participants | Australia Surgeons, assistants and scrub nurses performing other than microsurgical operations Number studied: 218 operations (1761 gloves) Intervention group n = 846 gloves; control group n = 915 gloves | |
| Interventions | Double gloving The control group was single gloved | |
| Outcomes | Outcome: number of perforated gloves. Only matching outer‐inner glove perforations were included in the intervention group. Perforation detection: the gloves were filled with water and perforations were noted as a jet of water | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | High risk | Hospital record numbers (odd versus even numbers) |
| Allocation concealment | Unclear risk | No information provided |
| Blinding of study subjects | Low risk | Not possible |
| Blinding of outcome assessor | High risk | Each member of the operating team was trained and responsible for testing all the gloves worn by him/her during the case |
| Incomplete outcome data (attrition bias) | Low risk | 15 out of 233 cases excluded |
| Selective outcome reporting (reporting bias) | Low risk | All outcomes reported |
| Outcome measure (combined air and water test used?) | High risk | Water‐filling and individual digital distension (no air test) |
Hester 1992 o‐c.
| Methods | Study design: randomised controlled trial Object of randomisation: patients | |
| Participants | USA Surgeons and their assistants in consecutive orthopaedic surgeries Number randomised: 75 Intervention group 1: n = 25; intervention group 2: n = 25; control group: 25 | |
| Interventions | Intervention group 1: double gloving with one thicker glove and one cotton glove (outer cotton Protek, inner orthopaedic glove) Control group was double gloving with one thicker glove (latex inner, orthopaedic glove outer layer) | |
| Outcomes | Outcome: the number of inner glove perforations per total number of glove sets Perforation detection: water leak test | |
| Notes | Hester 1992 o‐c: double latex versus latex‐cotton. Hester 1992 o‐c‐l: double latex versus latex‐cotton‐latex | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | High risk | Based on hospital admission day |
| Allocation concealment | High risk | Not possible to conceal |
| Blinding of study subjects | Low risk | Not possible; knowledge about the gloving method judged as low risk of changing the outcome |
| Blinding of outcome assessor | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | No information |
| Selective outcome reporting (reporting bias) | Low risk | Perforation and needlestick, skin contamination |
| Outcome measure (combined air and water test used?) | High risk | Water test only |
Hester 1992 o‐c‐o.
| Methods | Same as Hester 1992 o‐c | |
| Participants | Same as Hester 1992 o‐c | |
| Interventions | Intervention group 2: double gloving plus one cotton glove (outer standard latex, middle Protek cotton, inner orthopaedic glove) Control group was double gloving including one thicker glove (latex inner, orthopaedic glove outer layer) | |
| Outcomes | Same as Hester 1992 o‐c | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | High risk | Same as Hester 1992 o‐c |
| Allocation concealment | High risk | — |
| Blinding of study subjects | Low risk | — |
| Blinding of outcome assessor | Unclear risk | — |
| Incomplete outcome data (attrition bias) | Unclear risk | — |
| Selective outcome reporting (reporting bias) | Low risk | — |
| Outcome measure (combined air and water test used?) | High risk | — |
Jensen 1997.
| Methods | Study design: randomised controlled trial Object of randomisation: two participants per OP, each individually randomised to type of glove | |
| Participants | Denmark Principal surgeons and first assistants in consecutive intra‐abdominal operations in a county hospital Number randomised: 400 glove sets Intervention group n = 200 (glove sets); control group n = 200 (pairs of gloves) | |
| Interventions | Double gloves worn by surgeons and/or first assistants in the intervention group The control group wore single gloves | |
| Outcomes | Outcome: the number of perforations per total number of (inner) glove pairs Measurement: double glove barrier was recorded as perforated only if both the inner and the outer glove had one or more leaks Perforation detection: the gloves were filled with water and perforations were noted as a jet of water Secondary outcome: participants' opinions about the use of double gloves | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information |
| Allocation concealment | Low risk | Envelope was opened just before the beginning of the operation |
| Blinding of study subjects | Low risk | Knowledge about the gloving method judged as low risk of changing the outcome |
| Blinding of outcome assessor | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Unclear risk | Only gloves from the beginning were included; unknown how many gloves were changed and not included |
| Selective outcome reporting (reporting bias) | Low risk | Perforated glove barriers reported |
| Outcome measure (combined air and water test used?) | High risk | Water test only (filling glove with water and manipulating each digit) |
Kovavisarach 1998.
| Methods | Study design: randomised controlled trial Object of randomisation: worker | |
| Participants | Thailand Surgeons performing perineorrhaphies Number studied: 1400 gloves (700 patients) Intervention group n = 658 gloves; control group n = 742 gloves | |
| Interventions | Double gloving Control group was single gloved | |
| Outcomes | Outcome: number of (matching) perforations per total number of gloves Perforation detection: the gloves were filled with air, immersed in water and perforations were noted as air bubbles Secondary outcome: user satisfaction | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Randomly selected 1 out of 2 envelopes |
| Allocation concealment | Unclear risk | No information provided |
| Blinding of study subjects | Low risk | Knowledge about the gloving method judged as low risk of changing the outcome |
| Blinding of outcome assessor | High risk | Bags with gloves were labelled with method and other information |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported how many operations or gloves were excluded or missing |
| Selective outcome reporting (reporting bias) | Low risk | Perforation rates |
| Outcome measure (combined air and water test used?) | High risk | Air test only; filling with air and immersing in water |
Kovavisarach 1999.
| Methods | Study design: randomised controlled trial Object of randomisation: patients | |
| Participants | Thailand Primary surgeons in 300 caesarean sections in an antenatal clinic Number randomised: 300 Intervention group n = 150; control group n = 150 | |
| Interventions | Double gloves worn by surgeons in the intervention group (150 glove sets) The control group wore single gloves (150 glove pairs) | |
| Outcomes | Outcome: the number of perforations per total number of glove pairs Measurement: both matching inner‐outer perforations and double‐inner perforations were recorded in the intervention group Perforation detection: the gloves were filled with air and then immersed in water and perforations were noted as air bubbles | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Sealed envelopes, 1 out of 2 |
| Allocation concealment | Low risk | Randomisation at the time of operation |
| Blinding of study subjects | Low risk | Knowledge about the gloving method judged as low risk of changing the outcome |
| Blinding of outcome assessor | High risk | Bags with gloves were labelled with method and other information |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective outcome reporting (reporting bias) | Low risk | Glove perforation rates |
| Outcome measure (combined air and water test used?) | High risk | Air test only, filling with air and immersing in water |
Kovavisarach 2002.
| Methods | Study design: randomised controlled trial Object of randomisation: workers | |
| Participants | Thailand Primary surgeons and specialist assistants performing total abdominal hysterectomy Number studied: 544 gloves (170 operations) Intervention group n = 368 gloves; control group n = 176 | |
| Interventions | Double gloving Control group was single gloved | |
| Outcomes | Outcome: number of (matching) perforations per total number of gloves Perforation detection: the gloves were filled with air and then immersed in water and perforations were noted as air bubbles | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Not described; comment: probably done as earlier study from the same investigator clearly describes use of random sequences (Kovavisarach 1999) |
| Allocation concealment | Unclear risk | No information; comment: earlier studies showed low risk (Kovavisarach 1999) |
| Blinding of study subjects | Low risk | Knowledge about the gloving method judged as having a low risk of changing the outcome |
| Blinding of outcome assessor | Low risk | After OP each glove in separate bag; information coded via number on bag and recorded in separate book; authors were unaware of the kind of gloving method during testing |
| Incomplete outcome data (attrition bias) | Unclear risk | Visible perforated gloves were changed during OP and only original gloves were used for the study (missing data); insufficient reporting of the number of exclusions |
| Selective outcome reporting (reporting bias) | Low risk | Glove perforation in single and double gloving methods |
| Outcome measure (combined air and water test used?) | High risk | Air test only (filling with air and immersing in water) |
Laine 2001.
| Methods | Study design: randomised controlled trial Object of randomisation: patients | |
| Participants | Finland Surgeons and assistants performing different types of surgeries Number studied: 2462 gloves (885 operations) Intervention group n = 1442; control group n = 1020 | |
| Interventions | 1) Double gloving with an indication system: control group 1 was single gloved, control group 2 was double gloved with two standard gloves 2) Double gloving with two standard gloves; control group was single gloved |
|
| Outcomes | Outcome: number of (matching) perforations per total number of (inner) gloves Perforation detection: the gloves were filled with water and perforations were noted as a jet of water | |
| Notes | Length of surgery: glove perforations significantly higher in surgeries longer than two hours compared to those less than two hours. The number of double inner gloves was the sum of indicator system and combination glove inner gloves. The number of double outer gloves was not used, only single gloves |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | High risk | According to year of birth of patient (even or uneven) |
| Allocation concealment | Unclear risk | No information |
| Blinding of study subjects | Low risk | Knowledge of gloving type judged as low risk |
| Blinding of outcome assessor | Unclear risk | Gloves were labelled with identification labels; unknown what they were |
| Incomplete outcome data (attrition bias) | Unclear risk | Only first gloves collected; unknown how many excluded |
| Selective outcome reporting (reporting bias) | Low risk | Glove perforations |
| Outcome measure (combined air and water test used?) | High risk | Water test only (EN455‐1) |
Laine 2004a.
| Methods | Study design: randomised controlled trial Object of randomisation: workers | |
| Participants | Finland Surgeons and assistant surgeons performing conventional and laparoscopic abdominal operations Number studied: 806 gloves (271 procedures) Intervention group n = 358; control group n = 448 | |
| Interventions | Double gloving Control group was single gloved | |
| Outcomes | Outcome: number of perforated gloves per total number of gloves. Only matching outer‐inner glove perforations were included in the intervention group Perforation detection: the gloves were filled with water and perforations were noted as a jet of water | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Procedure not reported |
| Allocation concealment | Unclear risk | Not reported |
| Blinding of study subjects | Low risk | Knowledge of gloving type judged as low risk |
| Blinding of outcome assessor | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported how many operations or gloves were excluded or missing |
| Selective outcome reporting (reporting bias) | Low risk | Perforations of gloves |
| Outcome measure (combined air and water test used?) | High risk | Water test only: water‐leak test (EN 455‐1) |
Laine 2004b 2R.
| Methods | Study design: randomised controlled trial Object of randomisation: workers | |
| Participants | Finland Principal and assistant surgeons performing orthopaedic and trauma operations Number studied: 1769 gloves (349 operations) Intervention group n = 1516; control group n = 224 | |
| Interventions | Double gloving Control group was single gloved | |
| Outcomes | Outcome: number of perforated gloves per total number of gloves. Only matching outer‐inner glove perforations were included in the intervention group Perforation detection: the gloves were filled with water and perforations were noted as a jet of water | |
| Notes | In RevMan analyses we have divided the control group evenly over the 2 study arms with 7/112 each | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information |
| Allocation concealment | Unclear risk | No information |
| Blinding of study subjects | Low risk | Knowledge of gloving type judged as low risk |
| Blinding of outcome assessor | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Unclear risk | Total does not include gloves changed during surgery; unknown how many excluded |
| Selective outcome reporting (reporting bias) | Low risk | Perforations |
| Outcome measure (combined air and water test used?) | High risk | Water test only (EN455‐1) |
Laine 2004b DI.
| Methods | Study design: randomised controlled trial Object of randomisation: workers | |
| Participants | Finland Principal and assistant surgeons performing orthopaedic and trauma operations Number studied: 1769 gloves (349 operations) Intervention group n = 1516; control group n = 224 | |
| Interventions | Double gloving with an indicator glove Control group 1 was single gloved Control group 2 was double gloved with two standard gloves | |
| Outcomes | Outcome: number of perforated gloves per total number of gloves. Only matching outer‐inner glove perforations were included in the intervention group Perforation detection: the gloves were filled with water and perforations were noted as a jet of water | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Same as Laine 2004b 2R |
| Allocation concealment | Unclear risk | — |
| Blinding of study subjects | Low risk | — |
| Blinding of outcome assessor | Unclear risk | — |
| Incomplete outcome data (attrition bias) | Unclear risk | — |
| Selective outcome reporting (reporting bias) | Low risk | — |
| Outcome measure (combined air and water test used?) | High risk | — |
Liew 1995 Double.
| Methods | Study design: cluster‐randomised controlled trial Object of randomisation: patients | |
| Participants | Australia Surgeons, assistants and scrub nurses performing elective orthopaedic operations Number studied: 579 gloves (107 patients) Intervention group n = 392; control group n = 187 | |
| Interventions | Double gloving including thicker latex glove (Triflex, Baxter) Control group: double gloving including thinner latex glove (Gammex, Ansell) | |
| Outcomes | Outcome: number of perforated gloves per total number of gloves. Only matching outer‐inner glove perforations were included in the intervention group Perforation detection: the gloves were filled with water and perforations were noted as a jet of water | |
| Notes | We have used the thicker Baxter glove as the comparison glove. Comparison is perforations in inner gloves only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | High risk | According to unit record number |
| Allocation concealment | High risk | Not concealed |
| Blinding of study subjects | Low risk | Not possible; knowledge about the gloving method judged as having a low risk of changing the outcome |
| Blinding of outcome assessor | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective outcome reporting (reporting bias) | Low risk | Perforations |
| Outcome measure (combined air and water test used?) | High risk | Water test only |
Liew 1995 Single.
| Methods | Study design: cluster‐randomised controlled trial Object of randomisation: patients | |
| Participants | Australia Surgeons, assistants and scrub nurses performing elective orthopaedic operations Number studied: 579 gloves (107 patients) Intervention group n = 392; control group n = 187 | |
| Interventions | Single gloving with thicker latex glove (Triflex, Baxter) Control group was single gloved with thinner latex glove (Gammex, Ansell) | |
| Outcomes | Outcome: number of perforated gloves per total number of gloves. Only matching outer‐inner glove perforations were included in the intervention group Perforation detection: the gloves were filled with water and perforations were noted as a jet of water | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | High risk | Same as Liew 1995 Double |
| Allocation concealment | High risk | — |
| Blinding of study subjects | Low risk | — |
| Blinding of outcome assessor | Unclear risk | — |
| Incomplete outcome data (attrition bias) | Unclear risk | — |
| Selective outcome reporting (reporting bias) | Low risk | — |
| Outcome measure (combined air and water test used?) | High risk | — |
Louis 1998.
| Methods | Study design: randomised controlled trial Object of randomisation: patients | |
| Participants | US Surgeons and assistants performing orthopaedic procedures Number studied: 223 inner gloves (50 operations) Intervention group n = 106; control group n = 117 | |
| Interventions | Double gloving with polyester/stainless steel wire weave gloves as outer gloves and latex gloves as inner gloves The control group was double latex gloved | |
| Outcomes | Outcome: number of perforated gloves per total number of gloves Perforation detection: the gloves were filled with water and perforations were noted as a jet of water | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Cards indicating glove type in shuffled envelopes, numbered from 1 to 50 |
| Allocation concealment | Low risk | Sealed envelopes, opened at time of operation |
| Blinding of study subjects | Low risk | Not possible; knowledge about the gloving method judged as having a low risk of changing the outcome |
| Blinding of outcome assessor | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | Not enough data provided |
| Selective outcome reporting (reporting bias) | Low risk | Glove perforations |
| Outcome measure (combined air and water test used?) | High risk | Water test only |
Marín Bertolin 1997.
| Methods | Study design: randomised controlled trial Object of randomisation: not reported | |
| Participants | Spain Surgeons and scrub nurses performing plastic and reconstructive surgery Number studied: 666 gloves (107 operations) Intervention group n = 338; control group n = 328 | |
| Interventions | Double gloving Control group was single gloved | |
| Outcomes | Outcome: number of (matching) perforations per total number of (inner) gloves Perforation detection: the gloves were filled with water and perforations were noted as a jet of water Secondary outcome: peroperatively detected needlestick injuries | |
| Notes | Length of surgery: in surgeries lasting more than two hours, there were many more perforations than in those lasting less than two hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Procedure not reported |
| Allocation concealment | Unclear risk | No information |
| Blinding of study subjects | Low risk | — |
| Blinding of outcome assessor | High risk | Bags were labelled with name and method; unlikely that bags got relabeled before testing, e.g. into numbers from another person then the tester |
| Incomplete outcome data (attrition bias) | Low risk | 12 out 1092 gloves excluded |
| Selective outcome reporting (reporting bias) | Low risk | Perforation rates for single, double‐outer and double‐inner gloves |
| Outcome measure (combined air and water test used?) | High risk | Water test only; 500 ml water and gently squeezed |
Naver 2000.
| Methods | Study design: cluster‐randomised controlled trial Object of randomisation: patient (operation) | |
| Participants | Denmark Surgeons, assistants and scrub nurses performing elective gastrointestinal surgery Number studied: 566 glove pairs Intervention group n = 260; control group n = 306 | |
| Interventions | Double gloving with indicator system Control group was single gloved | |
| Outcomes | Outcome: number of perforations per total number of gloves Perforation detection: the gloves were filled with water and perforations were noted as a jet of water Secondary outcomes: incidence of blood contamination of the hands, self detection of glove perforations during surgery | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Procedure not described |
| Allocation concealment | Unclear risk | No information |
| Blinding of study subjects | Low risk | Not possible; knowledge about the gloving method judged as having a low risk of changing the outcome |
| Blinding of outcome assessor | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Unclear risk | Some people used more than one pair of gloves but only the first pair was included in analysis; number of excluded cases not reported |
| Selective outcome reporting (reporting bias) | Low risk | Punctures in inner and outer gloves |
| Outcome measure (combined air and water test used?) | High risk | Water test only, filled with water (EN 455‐1) |
Nicolai 1997.
| Methods | Study design: cluster‐randomised controlled trial Object of randomisation: patients | |
| Participants | England Surgeons, first assistants and scrub nurses performing total hip or knee arthroplasty Number studied: 362 gloves (22 operations) Intervention group n = 209; control group n = 153 | |
| Interventions | Double gloving with indicator system Control group used standard double gloves | |
| Outcomes | Outcome: number of glove perforations per total number of gloves Perforation detection: the gloves were filled with water and perforations were noted as a jet of water Secondary outcome: self detection of glove perforations | |
| Notes | Exact numbers of inner gloves in both groups are not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | No information |
| Allocation concealment | Low risk | Sealed envelopes |
| Blinding of study subjects | Low risk | Not possible; knowledge about the gloving method judged as having a low risk of changing the outcome |
| Blinding of outcome assessor | Unclear risk | Information on labels not described |
| Incomplete outcome data (attrition bias) | Unclear risk | No information |
| Selective outcome reporting (reporting bias) | Low risk | Glove perforations reported |
| Outcome measure (combined air and water test used?) | High risk | Water test |
Pieper 1995 l‐k‐k.
| Methods | Study design: randomised controlled trial Object of randomisation: workers | |
| Participants | US Oral and maxillofacial surgery residents and staff performing application of Erich arch bars Number studied: 270 gloves (30 procedures) Group 1: n = 60, Group 2: n = 90, Group 3: n = 60, Group 4: n = 60 | |
| Interventions | Group 1: double latex gloving Group 2: triple gloving including Kevlar glove Group 3: triple gloving including stainless steel glove Group 4: triple layer latex gloving | |
| Outcomes | Outcome: number of inner glove perforations per total number of gloves Perforation detection: the gloves were filled with water and perforations were noted as a jet of water | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Procedure not described |
| Allocation concealment | Unclear risk | Not reported |
| Blinding of study subjects | Low risk | Not possible; knowledge about the gloving method judged as having a low risk of changing the outcome |
| Blinding of outcome assessor | High risk | All gloves used for testing purposes were placed in separate bags marked outer glove, inner glove, middle triple, inner triple, inner Kevlar, inner stainless steel |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective outcome reporting (reporting bias) | Low risk | Glove perforations |
| Outcome measure (combined air and water test used?) | High risk | Water test only |
Pieper 1995 l‐l‐l.
| Methods | Same as Pieper 1995 l‐k‐k | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Same as Pieper 1995 l‐k‐k |
| Allocation concealment | Unclear risk | — |
| Blinding of study subjects | Low risk | — |
| Blinding of outcome assessor | High risk | — |
| Incomplete outcome data (attrition bias) | Unclear risk | — |
| Selective outcome reporting (reporting bias) | Low risk | — |
| Outcome measure (combined air and water test used?) | High risk | — |
Pieper 1995 l‐s‐s.
| Methods | Same as Pieper 1995 l‐k‐k | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Same as Pieper 1995 l‐k‐k |
| Allocation concealment | Unclear risk | — |
| Blinding of study subjects | Low risk | — |
| Blinding of outcome assessor | High risk | — |
| Incomplete outcome data (attrition bias) | Unclear risk | — |
| Selective outcome reporting (reporting bias) | Low risk | — |
| Outcome measure (combined air and water test used?) | High risk | — |
Punyatanasakchai 2004.
| Methods | Study design: randomised controlled trial Object of randomisation: workers | |
| Participants | Thailand Surgeons performing episiotomy repairs after vaginal delivery during a 7‐month period Number studied: 900 gloves Intervention group n = 600; control group n = 300 | |
| Interventions | Double gloving Control group was single gloved | |
| Outcomes | Outcome: number of perforated glove pairs per total number of glove pairs (also in RevMan analyses) Perforation detection: the gloves were filled with water and perforations were noted as a jet of water | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Procedure not described |
| Allocation concealment | Unclear risk | No information provided |
| Blinding of study subjects | Low risk | Not possible; knowledge about the gloving method judged as having a low risk of changing the outcome |
| Blinding of outcome assessor | High risk | Gloves in bags were labelled; re‐labelling before testing unlikely |
| Incomplete outcome data (attrition bias) | Unclear risk | Gloves got changed if visibly perforated and only original gloves were used for study; number of excluded gloves unknown |
| Selective outcome reporting (reporting bias) | Low risk | Glove perforation rates |
| Outcome measure (combined air and water test used?) | High risk | Water test only |
Quebbeman 1992.
| Methods | Study design: randomised controlled trial Object of randomisation: workers | |
| Participants | US Surgeons and first assistants performing operations which were predicted to last more than 2 hours and to include blood loss of more than 100 ml Number studied: 284 exposures (involvement of individual surgical team member) (143 procedures) Intervention group n = 130; control group n = 154 | |
| Interventions | Double gloving Control group was single gloved | |
| Outcomes | Outcome: 1. Number of glove failures per total number of exposures; 2. number of glove cuts per total number of exposures; 3. number of needlestick injuries per total number of exposures Secondary outcome: ease of use Measurement: visual detection | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Randomisation chart |
| Allocation concealment | High risk | Participants could refuse randomisation and wear desired glove type |
| Blinding of study subjects | Low risk | Not possible; knowledge about the gloving method judged as having a low risk of changing the outcome |
| Blinding of outcome assessor | High risk | Nurse inspected finger of surgeons, knowing type of gloves used |
| Incomplete outcome data (attrition bias) | Low risk | All cases analysed |
| Selective outcome reporting (reporting bias) | Low risk | Blood contamination of the finger, compliance |
| Outcome measure (combined air and water test used?) | High risk | Visual inspection |
Rudiman 1999.
| Methods | Study design: randomised controlled trial Object of randomisation: patients | |
| Participants | Indonesia Surgeons and first assistants performing laparotomies Number studied: 180 gloves (60 operations) Intervention group n = 60 (27 operations); control group n = 120 (33 operations) | |
| Interventions | Double gloving Control group was single gloved | |
| Outcomes | Outcome measure: 1. Number of glove pairs with 1 or more inner glove perforations; 2. incidence of blood contamination Perforation detection: the gloves were filled with water and perforations were noted as a jet of water Secondary outcome: self detection of glove perforations | |
| Notes | Operations that were 2 hours or longer were significantly associated with a higher incidence of glove perforation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | High risk | Based on hospital record numbers (odd versus even) |
| Allocation concealment | Unclear risk | No information |
| Blinding of study subjects | Low risk | Not possible; knowledge about the gloving method judged as having a low risk of changing the outcome |
| Blinding of outcome assessor | High risk | Self evaluation by the user of the glove |
| Incomplete outcome data (attrition bias) | High risk | 12 out of 72 excluded |
| Selective outcome reporting (reporting bias) | Low risk | Glove perforation rates for double and single glove method |
| Outcome measure (combined air and water test used?) | High risk | Water test only (glove was filled with water to the wrist and each finger was individually pressured) |
Sanders 1990.
| Methods | Study design: randomised controlled trial Object of randomisation: workers | |
| Participants | US Surgeons performing bone manipulation and/or application of implants Number studied: 110 inner gloves (50 operations) Intervention group n = 52; control group n = 58 | |
| Interventions | Double gloving with cotton cloth outer gloves and latex inner gloves Control group used double latex gloves | |
| Outcomes | Outcome: number of inner glove perforations per total number of inner gloves Perforation detection: the gloves were filled with water and perforations were noted as a jet of water Secondary outcome: self reported glove perforations | |
| Notes | In the control group, the number of punctures increased with the duration of the operation. A puncture was found in all operations longer than 3 hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Cards indicating glove type in shuffled envelopes, numbered from 1 to 50 |
| Allocation concealment | Low risk | Sealed envelopes, opened at time of operation |
| Blinding of study subjects | Low risk | Not possible; knowledge about the gloving method judged as having a low risk of changing the outcome |
| Blinding of outcome assessor | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective outcome reporting (reporting bias) | Low risk | Number of inner glove perforations |
| Outcome measure (combined air and water test used?) | High risk | Water test only |
Sebold 1993.
| Methods | Study design: randomised controlled trial Object of randomisation: operations | |
| Participants | US Surgeons performing major joint arthroplasty Number studied: 284 gloves inner or outer gloves (71 operations) Intervention 1: n = 100; Intervention 2: n = 96; control group: n = 88 | |
| Interventions | 1) Double gloving with thicker glove (orthopaedic outer gloves and latex inner gloves)
Control group was double latex gloves 2) Triple gloving with latex outer gloves, cloth middle gloves and latex inner gloves Control group 1 was double latex gloved; control group 2 was double gloving with thicker glove |
|
| Outcomes | Outcome: number of inner glove perforations per total number of inner gloves Perforation detection: the gloves were filled with water and perforations were noted as a jet of water Secondary outcome: self detection of glove perforations | |
| Notes | Double versus double: Sebold 1993; triple versus double Sebold 1993 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Cards indicating glove type in envelopes, sealed and shuffled, numbered from 1 to 75 |
| Allocation concealment | Low risk | Envelopes sealed until beginning of OP |
| Blinding of study subjects | Low risk | Not possible; knowledge about the gloving method judged as having a low risk of changing the outcome |
| Blinding of outcome assessor | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | 3 out of 25 cases in control excluded; 1 out of 25 in cloth glove group excluded |
| Selective outcome reporting (reporting bias) | Low risk | Perforations reported for inner, outer, changed gloves |
| Outcome measure (combined air and water test used?) | High risk | Water test only |
Sutton 1998.
| Methods | Study design: randomised controlled trial Object of randomisation: patients | |
| Participants | UK One surgeon performing hip and knee arthroplasty Number studied: 118 procedures Intervention group n = 56; control group n = 62 | |
| Interventions | Use of spectra polyethylene fibre gloves (Paraderm) between two layers of latex gloves (Ansell Nutex as inner, Regent Biogel as outer) Control group used two pairs of latex gloves (Ansell Nutex as inner, Regent Biogel as outer gloves) | |
| Outcomes | Outcome: number of inner and outer glove perforations per total number of procedures Perforation detection: the gloves were filled with water and perforations were noted as a jet of water | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Procedure not described: "randomised (using sealed envelopes) into two groups" |
| Allocation concealment | Low risk | Sealed envelopes |
| Blinding of study subjects | Low risk | Not possible; knowledge about the gloving method judged as having a low risk of changing the outcome |
| Blinding of outcome assessor | Low risk | Investigator testing for punctures was blind to which group the gloves belonged |
| Incomplete outcome data (attrition bias) | Unclear risk | All used gloves were tested; unknown if cases (OPs) were excluded |
| Selective outcome reporting (reporting bias) | Low risk | Perforations |
| Outcome measure (combined air and water test used?) | High risk | Water test only |
Tanner 2006.
| Methods | Study design: randomised controlled trial Object of randomisation: operating teams | |
| Participants | UK Consultants, specialist registrars, senior house officers and scrub nurses performing hip or knee arthroplasty during a 4‐month period Number studied: 406 gloves Intervention group n = 220; control group n = 186 | |
| Interventions | Double gloving with knitted outer gloves and latex inner gloves Control group was double latex gloved | |
| Outcomes | Outcome: number of inner glove perforations per total number of inner gloves Perforation detection: the gloves were filled with water and perforations were noted as a jet of water Secondary outcome: self detection of glove perforations | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random number tables |
| Allocation concealment | Low risk | Sealed envelopes until time of OP |
| Blinding of study subjects | Low risk | Not possible; knowledge about the gloving method judged as having a low risk of changing the outcome |
| Blinding of outcome assessor | Low risk | Testers checking were blind to which group the gloves belonged |
| Incomplete outcome data (attrition bias) | Unclear risk | Not enough information provided |
| Selective outcome reporting (reporting bias) | Low risk | Inner glove perforations |
| Outcome measure (combined air and water test used?) | High risk | Water test only |
Thomas 2001.
| Methods | Study design: randomised controlled trial Object of randomisation: workers | |
| Participants | India Surgeons and first assistants performing surgical procedures which lasted more than 1 hour Number studied: 66 procedures (396 gloves) Intervention group n = 33; control group n = 33 | |
| Interventions | Double gloving Control group was single gloved | |
| Outcomes | Outcome: 1. Number of (matching inner) glove perforations per total number of procedures; 2. number of visible blood contamination cases of the participant's hands per total number of procedures Perforation detection: firstly, the gloves were filled with air, immersed in water and perforations were noted as air bubbles. Secondly, the gloves were filled with water and perforations were noted as a jet of water | |
| Notes | Matching outer‐inner glove perforations used in RevMan analyses. Total numbers of inner and outer gloves are not reported. We have therefore presumed that the number of double inner gloves is 1/3 of the total number of gloves and that the number of single gloves is 1/3 of the total number of gloves | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Procedure not described |
| Allocation concealment | Low risk | Sealed envelopes until start of OP |
| Blinding of study subjects | Low risk | Not possible; knowledge about the gloving method judged as having a low risk of changing the outcome |
| Blinding of outcome assessor | Unclear risk | Not reported (author contacted, awaiting answer) |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported (author contacted, awaiting answer) |
| Selective outcome reporting (reporting bias) | Low risk | Outer and inner glove perforations per gloving type; matched holes for double gloving |
| Outcome measure (combined air and water test used?) | Low risk | Air and water test used |
Turnquest 1996.
| Methods | Study design: randomised controlled trial Object of randomisation: workers | |
| Participants | US
Primary surgeons, first and second assistants, and surgical technicians performing obstetrical procedures during a 6‐month period Number of glove pairs: intervention group: 169 double glove pairs; control group: 172 single glove pairs |
|
| Interventions | Double gloving Control group was single gloved | |
| Outcomes | Outcome: number of (matching) perforations per total number of gloves Perforation detection: the gloves were filled with water and perforations were noted as a jet of water Secondary outcome: recognition of punctures during the procedures | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Computer‐generated set of random numbers |
| Allocation concealment | Low risk | Sealed envelopes, opened at the beginning of OP |
| Blinding of study subjects | Low risk | Not possible; knowledge about the gloving method judged as having a low risk of changing the outcome |
| Blinding of outcome assessor | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Less than 10% of gloves excluded: intervention: 44 out of 720; control: 20 out of 364 |
| Selective outcome reporting (reporting bias) | Low risk | Number of gloves with perforations |
| Outcome measure (combined air and water test used?) | High risk | Water test only |
Underwood 1993.
| Methods | Study design: randomised controlled trial Object of randomisation: patient (n = 80) | |
| Participants | UK
Surgeons (first and assistant) performing sternal wiring (following cardiac surgery) Number of inner gloves: intervention group: 80 pairs; control group: 80 pairs |
|
| Interventions | Intervention group: double gloving including special material glove (inner latex and outer cotton) Control group: double standard (inner and outer standard latex glove) |
|
| Outcomes | Outcome: inner gloves with perforations Perforation detection: the gloves were filled with water and perforations were noted as a jet of water |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Not reported |
| Allocation concealment | Unclear risk | Not reported |
| Blinding of study subjects | Low risk | Not possible; knowledge about the gloving method judged as having a low risk of changing the outcome |
| Blinding of outcome assessor | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of used, collected and excluded gloves not reported |
| Selective outcome reporting (reporting bias) | Low risk | Number of inner gloves with perforations |
| Outcome measure (combined air and water test used?) | High risk | Water leaking test only |
Wilson 1996a.
| Methods | Study design: randomised controlled trial Object of randomisation: workers | |
| Participants | Oman 32 surgeons preforming different types of operations Number studied: 384 operations Intervention 1: n = 96; intervention 2: n = 96; intervention 3: n = 96; control group: n = 96 | |
| Interventions | Comparison of 3 different double gloving combinations: Intervention 1: normal size inner gloves and larger outer gloves Intervention 2: larger inner gloves and normal outer gloves Intervention 3: normal sized inner and outer gloves Control group was single gloved | |
| Outcomes | Outcome measure: number of (inner) glove perforations per total number of operations (also in RevMan analyses) Perforation detection: the gloves were filled with water and perforations were noted as a jet of water Secondary outcome: user comfort, dexterity | |
| Notes | Wilson 1996a normal size inner gloves and larger outer gloves versus single gloving. Wilson 1996b larger inner gloves and normal outer gloves versus single gloving. Wilson 1996c normal sized inner and outer gloves versus single gloving | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | The order of the gloving method was randomised by drawing the letters A, B, C, D out of a sealed envelope |
| Allocation concealment | Unclear risk | No information |
| Blinding of study subjects | Low risk | Not possible; knowledge about the gloving method judged as having a low risk of changing the outcome |
| Blinding of outcome assessor | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported how many operations or gloves were excluded or missing |
| Selective outcome reporting (reporting bias) | Low risk | All outcomes reported |
| Outcome measure (combined air and water test used?) | High risk | Inflating with water, trying to cuff and squeezing the palm and each finger in turn, looking for the fine spray of water (water test only, no air test) |
Wilson 1996b.
| Methods | Same as Wilson 1996a | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Same as Wilson 1996a |
| Allocation concealment | Unclear risk | — |
| Blinding of study subjects | Low risk | — |
| Blinding of outcome assessor | Unclear risk | — |
| Incomplete outcome data (attrition bias) | Unclear risk | — |
| Selective outcome reporting (reporting bias) | Low risk | — |
| Outcome measure (combined air and water test used?) | High risk | — |
Wilson 1996c.
| Methods | Same as Wilson 1996a | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Same as Wilson 1996a |
| Allocation concealment | Unclear risk | — |
| Blinding of study subjects | Low risk | — |
| Blinding of outcome assessor | Unclear risk | — |
| Incomplete outcome data (attrition bias) | Unclear risk | — |
| Selective outcome reporting (reporting bias) | Low risk | — |
| Outcome measure (combined air and water test used?) | High risk | — |
OP = operation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brunton 2000 | The aim of the intervention was not to reduce perforations ‐ two single, non‐sterile, powder‐free gloves compared |
| Caillot 1999 | Double versus single gloving; outcome: number of electronic alarms rather than exposure injuries |
| Duerink 2006 | No injury outcome |
| Gaujac 2007 | The aim of the intervention was not to reduce perforations ‐ double gloving with sterile gloves compared to double gloving with one sterile and one non‐sterile glove |
| Jeffe 1999 | No injury outcome |
| Kelly 1993 | Unclear randomisation and significant differences between intervention and control group |
Characteristics of studies awaiting assessment [ordered by study ID]
Bliss 1992.
| Methods | Study design: cluster‐randomised controlled trial Object of randomisation: calendar day |
| Participants | UK Surgeons, assistants and scrub nurses Number studied: 2604 gloves Group 1: n = 1378; Group 2: n = 1226 Not reported which group is intervention and which is control |
| Interventions | Comparison of 2 different glove types in single gloving situations |
| Outcomes | Outcome: number of glove perforations per total number of gloves Perforation detection: the gloves were filled with air, immersed in water and perforations were noted as air bubbles |
| Notes | The comparison was between cheaper and more expensive gloves; the authors did not report which is which |
Guo 2012.
| Methods | Study design: randomised controlled trial |
| Participants | Nurses |
| Interventions | Double versus single gloves |
| Outcomes | Perforations |
| Notes |
Hwang 1999b.
| Methods | Study design: randomised controlled trial Object of randomisation: workers |
| Participants | Taiwan 35 physicians, 30 nurses and 1 technician routinely wearing rubber gloves Number studied: 336 gloves Group 1: n = 80; Group 2: n = 148; Group 3: n = 46; Group 4: n = 62 |
| Interventions | Comparison of four different types of (single) gloves |
| Outcomes | Outcome: number of perforated or torn gloves per total number of gloves Perforation detection: visual examination Secondary outcome: user satisfaction, allergic reactions |
| Notes | Not reported which were intervention gloves and which were control gloves |
Hwang 1999c.
| Methods | Study design: randomised controlled trial Object of randomisation: workers |
| Participants | Taiwan 35 physicians, 30 nurses and 1 technician routinely wearing rubber gloves Number studied: 336 gloves Group 1: n = 80; Group 2: n = 148; Group 3: n = 46; Group 4: n = 62 |
| Interventions | Comparison of four different types of (single) gloves |
| Outcomes | Outcome: number of perforated or torn gloves per total number of gloves Perforation detection: visual examination Secondary outcome: user satisfaction, allergic reactions |
| Notes | Not reported which were intervention gloves and which were control gloves |
Hwang 1999d.
| Methods | Study design: randomised controlled trial Object of randomisation: workers |
| Participants | Taiwan 35 physicians, 30 nurses and 1 technician wearing routinely rubber gloves Number studied: 336 gloves Group 1: n = 80; Group 2: n = 148; Group 3: n = 46; Group 4: n = 62 |
| Interventions | Comparison of four different types of (single) gloves |
| Outcomes | Outcome: number of perforated or torn gloves per total number of gloves Perforation detection: visual examination Secondary outcome: user satisfaction, allergic reactions |
| Notes | Not reported which were intervention gloves and which were control gloves |
Newsom 1998.
| Methods | Study design: randomised controlled trial Object of randomisation: operations |
| Participants | UK General surgeons and urologists performing open and endoscopic surgery Number studied: 670 gloves (317 operations) Intervention group n = 348; control group n = 322 |
| Interventions | Use of powder‐free, non‐latex surgical gloves Control group used standard surgical gloves |
| Outcomes | Outcome: 1. Number of perforated gloves per total number of gloves; 2. number of perforated glove pairs per total number of glove pairs; 3. number of perforated gloves per total number of operations Perforation detection: the gloves were filled with water and perforations were noted as a jet of water |
| Notes | Unclear if intervention intends to prevent needlestick injuries; comparison is possibly single thicker versus single thin; awaiting answer from manufacturer |
Differences between protocol and review
According to the original review protocol, at least 75% of the healthcare workers in a study had to work in direct patient care, whereas the rest could be assisting personnel. In practice, it proved difficult to judge whether this criterion was fulfilled. We therefore applied a more global judgement as to whether the majority of participants were healthcare workers with direct patient contacts.
We originally planned to reanalyze data to determine whether there was a difference in effect in workers with high exposure, such as surgeons. However, all included studies included surgeons and we decided to group studies, according to the rate in the control groups, into high and low‐risk studies.
Contributions of authors
Jos Verbeek and Christina Mischke are the co‐ordinators of the review work. Annika Saarto (previously Parantainen), Marie‐Claude Lavoie and Manisha Pahwa participated in the writing of the review and protocol. Sharea Ijaz participated in the data extraction and the writing of the Discussion section.
Sources of support
Internal sources
-
Finnish Institute of Occupational Health, Finland.
Provided salaries and office facilities and resources for Christina Mischke, Sharea Ijaz, Annika Parantainen and Jos Verbeek
-
Pan American Health Organization, USA.
Provided salaries and office facilities and resources as well as support to attend Cochrane Collaboration training sessions for Manisha Pahwa and Marie‐Claude Lavoie
External sources
No sources of support supplied
Declarations of interest
Christina Mischke none known.
Jos H Verbeek none known.
Annika Saarto none known.
Marie‐Claude Lavoie none known.
Manisha Pahwa none known.
Sharea Ijaz none known.
New
References
References to studies included in this review
Aarnio 2001 {published data only}
- Aarnio P, Laine T. Glove perforation rate in vascular surgery ‐ a comparison between single and double gloving. Vasa 2001;30:122‐4. [DOI] [PubMed] [Google Scholar]
Avery 1999a {published data only}
- Avery CME, Gallagher P, Birnbaum W. Double gloving and a glove perforation indication system during the dental treatment of HIV‐positive patients: are they necessary?. British Dental Journal 1999;186(1):27‐9. [DOI] [PubMed] [Google Scholar]
Avery 1999b {published data only}
- Avery CME, Taylor J, Johnson PA. Double gloving and a system for identifying glove perforation in maxillofacial surgery. British Journal of Oral and Maxillofacial Surgery 1999;37:316‐9. [DOI] [PubMed] [Google Scholar]
Berridge 1998 {published data only}
- Berridge DC, Starky G, Jones NAG, Chamberlain J. A randomised controlled trial of double‐versus single‐gloving in vascular surgery. Journal of the Royal College of Surgeons of Edinburgh 1998;43(1):9‐10. [PubMed] [Google Scholar]
Carter 1996 {published data only}
- Carter S, Choong S, Marino A, Sellu D. Can surgical gloves be made thinner without increasing their liability to puncture?. Annals of The Royal College of Surgeons of England 1996;78:186‐7. [PMC free article] [PubMed] [Google Scholar]
Chua 1996 {published data only}
- Chua KL, Taylor GS, Bagg J. A clinical and laboratory evaluation of three types of operating gloves for use in orthodontic practice. British Journal of Orthodontics 1996;23:115‐20. [DOI] [PubMed] [Google Scholar]
Doyle 1992 {published data only}
- Doyle PM, Alvi S, Johanson R. The effectiveness of double‐gloving in obstetrics and gynaecology. British Journal of Obstetrics and Gynaecology 1992;99:83‐4. [DOI] [PubMed] [Google Scholar]
Duron 1996 {published data only}
- Duron J‐J, Keilani K, Elian NG. Efficacy of double gloving with a coloured inner pair for immediate detection of operative glove perforations. European Journal of Surgery 1996;162:941‐4. [PubMed] [Google Scholar]
Gani 1990 {published data only}
- Gani JS, Anseline PF, Bissett RL. Efficacy of double versus single gloving in protecting the operating team. Australian and New Zealand Journal of Surgery 1990;60:171‐5. [PubMed] [Google Scholar]
Hester 1992 o‐c {published data only}
- Hester RA, Nelson CL, Harrison S. Control of contamination of the operative team in total joint arthroplasty. Journal of Arthroplasty 1992;7(3):267‐9. [DOI] [PubMed] [Google Scholar]
Hester 1992 o‐c‐o {published data only}
- Hester RA, Nelson CL, Harrison S. Control of contamination of the operative team in total joint arthroplasty. Journal of Arthroplasty 1992;7(3):267‐9. [DOI] [PubMed] [Google Scholar]
Jensen 1997 {published data only}
- Jensen SL, Kristensen B, Fabrin K. Double gloving as self protection in abdominal surgery. European Journal of Surgery 1997;163:163‐7. [PubMed] [Google Scholar]
Kovavisarach 1998 {published data only}
- Kovavisarach E, Jaravechson S. Comparison of perforation between single and double gloving in perineorrhaphy after vaginal delivery. Australian and New Zealand Journal of Obstetrics and Gynaecology 1998;38(1):58‐60. [DOI] [PubMed] [Google Scholar]
Kovavisarach 1999 {published data only}
- Kovavisarach E, Vanitchanon P. Perforation in single‐ and double‐gloving methods for cesarean section. International Journal of Gynecology and Obstetrics 1999;67:157‐61. [DOI] [PubMed] [Google Scholar]
Kovavisarach 2002 {published data only}
- Kovavisarach E, Seedadee C. Randomised controlled trial of glove perforation in single and double‐gloving methods in gynaecologic surgery. Australian and New Zealand Journal of Obstetrics and Gynaecology 2002;42(5):519‐21. [DOI] [PubMed] [Google Scholar]
Laine 2001 {published data only}
- Laine T, Aarnio P. How often does glove perforation occur in surgery? Comparison between single gloves and a double‐gloving system. American Journal of Surgery 2001;181(6):564‐6. [DOI] [PubMed] [Google Scholar]
Laine 2004a {published data only}
- Laine T, Kaipia A, Santavirta J, Aarnio P. Glove perforations in open and laparoscopic abdominal surgery: the feasibility of double gloving. Scandinavian Journal of Surgery 2004;93:73‐6. [DOI] [PubMed] [Google Scholar]
Laine 2004b 2R {published data only}
- Laine T, Aarnio P. Glove perforation in orthopedic and trauma surgery: a comparison between single, double indicator and double gloving with two regular gloves. Journal of Bone and Joint Surgery 2004;86‐B(6):898‐90. [DOI] [PubMed] [Google Scholar]
Laine 2004b DI {published data only}
- Laine T, Aarnio P. Glove perforation in orthopedic and trauma surgery: a comparison between single, double indicator and double gloving with two regular gloves. Journal of Bone and Joint Surgery 2004;86‐B(6):898‐90. [DOI] [PubMed] [Google Scholar]
Liew 1995 Double {published data only}
- Liew SM, Stoney JD, Falkenberg MP, Leitl SM. A comparative study of two types of latex surgical gloves in elective orthopaedic surgery. Australian and New Zealand Journal of Surgery 1995;65:406‐8. [DOI] [PubMed] [Google Scholar]
Liew 1995 Single {published data only}
- Liew SM, Stoney JD, Falkenberg MP, Leitl SM. A comparative study of two types of latex surgical gloves in elective orthopaedic surgery. Australian and New Zealand Journal of Surgery 1995;65:406‐8. [DOI] [PubMed] [Google Scholar]
Louis 1998 {published data only}
- Louis SS, Steinberg EL, Gruen OA, Bartkett CS, Helfet DL. Outer gloves in orthopaedic procedures: a polyester stainless steel wire weave glove liner compared with latex. Journal of Orthopaedic Trauma 1998;12(2):101‐5. [DOI] [PubMed] [Google Scholar]
Marín Bertolin 1997 {published data only}
- Marín‐Bertolin S, Gonzalez‐Martinez R, Gimenez CN, Vila PM, Amorrortu‐Velayos J. Does double gloving protect surgical staff from skin contamination during plastic surgery?. Plastic and Reconstructive Surgery 1997;99(4):956‐60. [DOI] [PubMed] [Google Scholar]
Naver 2000 {published data only}
- Naver LP, Gottrup F. Incidence of glove perforations in gastrointestinal surgery and the protective effect of double gloves: a prospective randomised controlled study. European Journal of Surgery 2000;166(4):293‐5. [DOI] [PubMed] [Google Scholar]
Nicolai 1997 {published data only}
- Nicolai P, Aldam CH, Allen PW. Increased awareness of glove perforation in major joint replacement. A prospective, randomised study of Regent Biogel Reveal gloves. Journal of Bone and Joint Surgery 1997;79(3):371‐3. [DOI] [PubMed] [Google Scholar]
Pieper 1995 l‐k‐k {published data only}
- Pieper SP, Schimmele SR, Johnson JA, Harper JL. A prospective study of the efficacy of various gloving techniques in the application of Erich Arch Bars. Journal of Oral and Maxillofacial Surgery 1995;53:1174‐6. [DOI] [PubMed] [Google Scholar]
Pieper 1995 l‐l‐l {published data only}
- Pieper SP, Schimmele SR, Johnson JA, Harper JL. A prospective study of the efficacy of various gloving techniques in the application of Erich Arch Bars. Journal of Oral and Maxillofacial Surgery 1995;53:1174‐6. [DOI] [PubMed] [Google Scholar]
Pieper 1995 l‐s‐s {published data only}
- Pieper SP, Schimmele SR, Johnson JA, Harper JL. A prospective study of the efficacy of various gloving techniques in the application of Erich Arch Bars. Journal of Oral and Maxillofacial Surgery 1995;53:1174‐6. [DOI] [PubMed] [Google Scholar]
Punyatanasakchai 2004 {published data only}
- Punyatanasakchai P, Chittacharoen A, Ayudhya NIN. Randomised controlled trial of glove perforation in single and double gloving in episiotomy repair after vaginal delivery. Journal of Obstetrics and Gynaecology Research 2004;30(5):354‐7. [DOI] [PubMed] [Google Scholar]
Quebbeman 1992 {published data only}
- Quebbeman EJ, Telford GL, Wadsworth K, Hubbard S, Goodman H, Gottlieb MS. Double gloving: protecting surgeons from blood contamination in the operating room. Archives of Surgery 1992;127:213‐7. [DOI] [PubMed] [Google Scholar]
Rudiman 1999 {published data only}
- Rudiman R, Karnadihardja W, Ruchiyat Y, Hanafi B. Efficacy of double versus single gloving in laparotomy procedures. Asian Journal of Surgery 1999;22:85‐8. [Google Scholar]
Sanders 1990 {published data only}
- Sanders R, Fortin P, Ross E, Helfet D. Outer gloves in orthopaedic procedures. Cloth compared with latex. Journal of Bone and Joint Surgery 1990;72(6):914‐7. [PubMed] [Google Scholar]
Sebold 1993 {published data only}
- Sebold EJ, Jordan LR. Intraoperative glove perforation: a comparative analysis. Clinical Orthopaedics and Related Research 1993;297:242‐4. [PubMed] [Google Scholar]
Sutton 1998 {published data only}
- Sutton PM, Greene T, Howell FR. The protective effect of a cut‐resistant glove liner. Journal of Bone and Joint Surgery 1998;80‐B:411‐3. [DOI] [PubMed] [Google Scholar]
Tanner 2006 {published data only}
- Tanner J, Wraighte P, Howard P. Knitted outer gloves in primary hip and knee arthroplasty. Hip International 2006;16(1):62‐5. [DOI] [PubMed] [Google Scholar]
Thomas 2001 {published data only}
- Thomas S, Agarwal M, Mehta G. Intraoperative glove perforation‐‐single versus double gloving in protection against skin contamination. Postgraduate Medical Journal 2001;77:458‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turnquest 1996 {published data only}
- Turnquest MA, How HY, Allen SA, Voss DH, Spinnato JA. Perforation rates using a single pair of orthopedic gloves vs. a double pair of gloves in obstetric cases. Journal of Maternal‐Fetal Medicine 1996;5:362‐5. [DOI] [PubMed] [Google Scholar]
Underwood 1993 {published data only}
- Underwood MJ, Weerasena N, Graham TR, Hosie K, Dunning J, Bailey JS, et al. Prevalence and prevention of glove perforation during cardiac operations. Journal of Thoracic and Cardiovascular Surgery 1993;106(2):375‐7. [PubMed] [Google Scholar]
Wilson 1996a {published data only}
- Wilson SJ, Sellu D, Uy A, Jaffer MA. Subjective effects of double gloves on surgical performance. Annals of The Royal College of Surgeons of England 1996;78(1):20‐2. [PMC free article] [PubMed] [Google Scholar]
Wilson 1996b {published data only}
- Wilson SJ, Sellu D, Uy A, Jaffer MA. Subjective effects of double gloves on surgical performance. Annals of The Royal College of Surgeons of England 1996;78(1):20‐2. [PMC free article] [PubMed] [Google Scholar]
Wilson 1996c {published data only}
- Wilson SJ, Sellu D, Uy A, Jaffer MA. Subjective effects of double gloves on surgical performance. Annals of The Royal College of Surgeons of England 1996;78(1):20‐2. [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Brunton 2000 {published data only}
- Brunton PA, Abidia R, Macfarlane TV, Wilson NHF. An evaluation of powder‐free gloves in general dental practice. Primary Dental Care 2000;7(3):125‐8. [DOI] [PubMed] [Google Scholar]
Caillot 1999 {published data only}
- Caillot J‐L, Cote C, Abidi H, Fabry J. Electronic evaluation of the value of double gloving. British Journal of Surgery 1999;86:1387‐90. [DOI] [PubMed] [Google Scholar]
Duerink 2006 {published data only}
- Duerink DO, Farida H, Nagelkerke NJD, Waihyono H, Keuter M, Lestari ES, et al. Preventing nosocomial infections: improving compliance with standard precautions in an Indonesian teaching hospital. Journal of Hospital Infection 2006;64:36‐43. [DOI] [PubMed] [Google Scholar]
Gaujac 2007 {published data only}
- Gaujac C, Ceccheti MM, Yonezaki F, Garcia IR, Peres MPSM. Comparative analysis of 2 techniques of double‐gloving protection during arch bar placement for intermaxillary fixation. Journal of Oral and Maxillofacial Surgery 2007;65:1922‐5. [DOI] [PubMed] [Google Scholar]
Jeffe 1999 {published data only}
- Jeffe DB, Mutha S, Kim LE, Evanoff BA, Fraser VJ. Evaluation of a preclinical, educational and skills‐training program to improve students' use of blood and body fluid precautions: one‐year follow up. Preventive Medicine 1999;29:365‐73. [DOI] [PubMed] [Google Scholar]
Kelly 1993 {published data only}
- Kelly KE, Lee KC, Tami TA. Surgical glove perforations in otolaryngology: prevention with cut‐resistant gloves. Otolaryngology ‐ Head and Neck Surgery 1993;108(1):91‐5. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Bliss 1992 {published data only}
- Bliss S, Alexander‐Williams J. Surgical gloves ‐ a comparative study of the incidence and site of punctures. British Journal of Theatre Nursing 1992;1(11):16‐7. [PubMed] [Google Scholar]
Guo 2012 {published data only}
- Guo YP, Wong PM, Li Y, Or PPL. Is double‐gloving really protective? A comparison between the glove perforation rate among perioperative nurses with single and double gloves during surgery. American Journal of Surgery 2012;204:210‐5. [DOI] [PubMed] [Google Scholar]
Hwang 1999b {published data only}
- Hwang K‐L, Kou S‐J, Lu Y‐M, Yang N‐C. Evaluation of the quality of surgical gloves among four different manufacturers. Annals of Occupational Hygiene 1999;43(4):275‐81. [PubMed] [Google Scholar]
Hwang 1999c {published data only}
- Hwang K‐L, Kou S‐J, Lu Y‐M, Yang N‐C. Evaluation of the quality of surgical gloves among four different manufacturers. Annals of Occupational Hygiene 1999;43(4):275‐81. [PubMed] [Google Scholar]
Hwang 1999d {published data only}
- Hwang K‐L, Kou S‐J, Lu Y‐M, Yang N‐C. Evaluation of the quality of surgical gloves among four different manufacturers. Annals of Occupational Hygiene 1999;43(4):275‐81. [PubMed] [Google Scholar]
Newsom 1998 {published data only}
- Newsom SW, Smith MO, Shaw P. A randomised trial of the durability of non‐allergenic latex free surgical gloves versus latex gloves. Annals of The Royal College of Surgeons of England 1998;80(4):288‐92. [PMC free article] [PubMed] [Google Scholar]
Additional references
Akduman 1999
- Akduman D, Kim LE, Parks RL, L'Ecuyer PB, Mutha S, Jeffe DB, et al. Use of personal protective equipment and operating room behaviors in four surgical subspecialties: personal protective equipment and behaviors in surgery. Infection Control and Hospital Epidemiology 1999;20(2):110‐4. [DOI] [PubMed] [Google Scholar]
Ansa 2002
- Ansa VO, Udoma EJ, Umoh MS, Anah MU. Occupational risk of infection by human immunodeficiency and hepatitis B viruses among health workers in south‐eastern Nigeria. East African Medical Journal 2002;79(5):254‐6. [DOI] [PubMed] [Google Scholar]
Boal 2008
- Boal WL, Leiss JK, Sousa S, Lyden JT, Li J, Jagger J. The national study to prevent blood exposure in paramedics: exposure reporting. American Journal of Industrial Medicine 2008;51(3):213‐22. [doi: 10.1002/ajim.20558; PUBMED: PMID: 18213637] [DOI] [PubMed] [Google Scholar]
Bouvet 2009
- Bouvet E, Pellissier G, Abiteboul D, L'Hériteau F. Is double gloving an effective barrier to protect surgeons against blood exposure due to needlestick injury?. Infection Control and Hospital Epidemiology 2009;30(9):928‐9. [DOI] [PubMed] [Google Scholar]
Bryce 1999
- Bryce EA, Ford J, Chase L, Taylor C, Scharf S. Sharps injuries: defining prevention priorities. American Journal of Infection Control 1999;27(5):447‐52. [DOI] [PubMed] [Google Scholar]
Castella 2003
- Castella A, Vallino A, Argentero PA, Zotti CM. Preventability of percutaneous injuries in healthcare workers: a year‐long survey in Italy. Journal of Hospital Infection 2003;55(4):290‐4. [DOI] [PubMed] [Google Scholar]
CEN 2003
- European Committee for Standardization (CEN). European standard EN 374‐3:2003 Protective gloves against chemicals and micro‐organisms. European Committee for Standardization, Brussels 2003.
Centers for Disease Control 2012
- Centers for Disease Control and Prevention. Chapter 3: Infectious diseases related to travel. CDC Health Information for International Travel ‐ the Yellow Book. Centers for Disease Control and Prevention, 2012. [Google Scholar]
Chen 2005
- Chen W, Gluud C. Vaccines for preventing hepatitis B in health‐care workers. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD000100.pub3] [DOI] [PubMed] [Google Scholar]
Cicconi 2010
- Cicconi L, Claypool M, Stevens W. Prevention of transmissible infections in the perioperative setting. AORN Journal 2010;92(5):519‐27. [DOI] [PubMed] [Google Scholar]
Clarke 2002
- Clarke SP, Rockett JL, Sloane DM, Aiken LH. Organizational climate, staffing, and safety equipment as predictors of needlestick injuries and near‐misses in hospital nurses. American Journal of Infection Control 2002;30(4):207‐16. [DOI] [PubMed] [Google Scholar]
Dalgleish 1988
- Dalgleish AG, Malkovsky M. Surgical gloves as a mechanical barrier against human immunodeficiency viruses. British Journal of Surgery 1988;75:171‐2. [DOI] [PubMed] [Google Scholar]
Diaz‐Buxo 1991
- Diaz‐Buxo JA. Cut resistant glove liners for medical use. Surgery Gynecology and Obstetrics 1991;172(4):312‐4. [PubMed] [Google Scholar]
Doebbeling 2003
- Doebbeling BN, Vaughn TE, McCoy KD, Beekmann SE, Woolson RF, Ferguson KJ, et al. Percutaneous injury, blood exposure, and adherence to standard precautions: are hospital‐based health care providers still at risk?. Clinical Infectious Diseases 2003;37(8):1006‐13. [DOI] [PubMed] [Google Scholar]
Edlich 2003
- Edlich RF, Wind TC, Hill LG, Thacker JG, Walter M. Reducing accidental injuries during surgery. Journal of Long‐Term Effects of Medical Implants 2003;13(1):1‐10. [DOI] [PubMed] [Google Scholar]
Ellenbecker 1996
- Ellenbecker MJ. Engineering controls as an intervention to reduce worker exposure. American Journal of Industrial Medicine 1996;29(4):303‐7. [DOI] [PubMed] [Google Scholar]
European Biosafety Network 2010
- The European Biosafety Network. Prevention of Sharps Injuries in the Hospital and Healthcare Sector Implementation. Guidance for the EU Framework Agreement, Council Directive and Associated National Legislation. http://www.europeanbiosafetynetwork.eu/EU%20Sharps%20Injuries%20Implementation%20Guidance.pdf 2010.
Fadeyi 2011
- Fadeyi A, Fowotade A, Abiodun MO, Jimoh AK, Nwabuisi C, Desalu OO. Awareness and practice of safety precautions among healthcare workers in the laboratories of two public health facilities in Nigeria. Nigerian Postgraduate Medical Journal 2011;18(2):141‐6. [PubMed] [Google Scholar]
Fisman 2002
- Fisman DN, Mittleman AM, Sorock GS, Harris AD. Willingness to pay to avoid sharps‐related injuries: a study in injured health care workers. American Journal of Infection Control 2002;30(5):283‐7. [DOI] [PubMed] [Google Scholar]
Fisman 2007
- Fisman DN, Harris AD, Rubin M, Sorock GS, Mittleman AM. Fatigue increases the risk of injury from sharp devices in medical trainees: results from a case‐crossover study. Infection Control and Hospital Epidemiology 2007;28(1):10‐7. [DOI] [PubMed] [Google Scholar]
Fry 2010
- Fry DE, Harris WE, Kohnke EN, Twomey CL. Influence of double‐gloving on manual dexterity and tactile sensation of surgeons. Journal of the American College of Surgeons 2010;210(3):325‐30. [doi: 10.1016/j.jamcollsurg.2009.11.001; PUBMED: PMID: 20193896] [DOI] [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows. GRADE working group, 2008.
Haines 2011
- Haines T, Stringer B, Herring J, Thoma A, Harris KA. Surgeons' and residents' double‐gloving practices at 2 teaching hospitals in Ontario. Canadian Journal of Surgery 2011;54(2):95‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ilhan 2006
- Ilhan MN, Durukan E, Aras E, Turkcuoglu S, Aygun R. Long working hours increase the risk of sharp and needlestick injury in nurses: the need for new policy implication. Journal of Advanced Nursing 2006;56(5):563‐8. [DOI] [PubMed] [Google Scholar]
Kinlin 2010
- Kinlin LM, Mittleman MA, Harris AD, Rubin MA, Fisman DN. Use of gloves and reduction of risk of injury caused by needles or sharp medical devices in healthcare workers: results from a case‐crossover study. Infection Control and Hospital Epidemiology 2010;31(9):908‐17. [DOI] [PubMed] [Google Scholar]
Lavoie 2012
- Lavoie MC, Verbeek JH, Parantainen A, Pahwa M. Devices for preventing percutaneous exposure injuries caused by needles in health care personnel. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD009740] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2008
- Lefebvre DR, Strande LF, Hewitt CW. An enzyme‐mediated assay to quantify inoculation volume delivered by suture needlestick injury: two gloves are better than one. Journal of the American College of Surgeons 2008;206(1):113‐22. [DOI] [PubMed] [Google Scholar]
Leslie 1996
- Leslie FL, Woods JA, Thacker JG, Morgan RF, McGregor W, Edlich RF. Needle puncture resistance of surgical gloves, finger guards and glove liners. Journal of Biomedical Materials Research (Applied Biomaterials) 1996;33:41‐6. [DOI] [PubMed] [Google Scholar]
Manson 1995
- Manson TT, Bromberg WG, Thacker JG, McGregor W, Morgan RF, Edlich RF. A new glove puncture detection system. Journal of Emergency Medicine 1995;13:357‐64. [DOI] [PubMed] [Google Scholar]
Mansouri 2010
- Mansouri M, Tidley M, Sanati KA, Roberts C. Comparison of blood transmission through latex and nitrile glove materials. Occupational Medicine 2010;60(3):205‐10. [DOI] [PubMed] [Google Scholar]
Mast 2004
- Mast E, Mahoney F, Kane M, Margolis H. Hepatitis B vaccines. In: Plotkin OW editor(s). Vaccines. Philadelphia: Saunders, 2004:299‐337. [Google Scholar]
Meyers 2008
- Myers DJ, Epling C, Dement J, Hunt D. Risk of sharp device‐related blood and body fluid exposure in operating rooms. Infection Control and Hospital Epidemiology 2008;29(12):1139‐48. [doi: 10.1086/592091; PUBMED: PMID: 18991506] [DOI] [PubMed] [Google Scholar]
Oh 2005
- Oh HS, Yi SE, Choe KW. Epidemiological characteristics of occupational blood exposures of healthcare workers in a university hospital in South Korea for 10 years. Journal of Hospital Infection 2005;60(3):269‐75. [DOI] [PubMed] [Google Scholar]
Orji 2002
- Orji EO, Fasubaa OB, Onwudiegwu U, Dare FO, Ogunniyi SO. Occupational health hazards among health care workers in an obstetrics and gynaecology unit of a Nigerian teaching hospital. Journal of Obstetrics and Gynaecology 2002;22(1):75‐8. [DOI] [PubMed] [Google Scholar]
Parantainen 2011
- Parantainen A, Verbeek JH, Lavoie MC, Pahwa M. Blunt versus sharp suture needles for preventing percutaneous exposure incidents in surgical staff. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD009170.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pruss‐Ustun 2005
- Pruss‐Ustun A, Rapiti E, Hutin Y. Estimation of the global burden of disease attributable to contaminated sharps injuries among health‐care workers. American Journal of Industrial Medicine 2005;48(6):482‐90. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Roberts 1999
- Roberts RB, Lincoff AS, Frankel RE. Occupational exposure to HIV in an urban university hospital setting. Brazilian Journal of Infectious Diseases 1999;3(2):50‐62. [PubMed] [Google Scholar]
Robinson 2002
- Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. International Journal of Epidemiology 2002;31(1):150‐3. [DOI] [PubMed] [Google Scholar]
Roelofs 2003
- Roelofs CR, Barbeau EM, Ellenbecker MJ, Moure‐Eraso R. Prevention strategies in industrial hygiene: a critical literature review. American Industrial Hygiene Association Journal 2003;64(1):62‐7. [DOI] [PubMed] [Google Scholar]
Rogers 2000
- Rogers B, Goodno L. Evaluation of interventions to prevent needlestick injuries in health care occupations. American Journal of Preventive Medicine 2000;18(4 Suppl):90‐8. [DOI] [PubMed] [Google Scholar]
Shepard 2005
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infectious Diseases 2005;5:558‐67. [DOI] [PubMed] [Google Scholar]
Smith 2006
- Smith DR, Choe MA, Jeong JS, Jeon MY, Chae YR, An GJ. Epidemiology of needlestick and sharps injuries among professional Korean nurses. Journal of Professional Nursing 2006;22(6):359‐66. [DOI] [PubMed] [Google Scholar]
Smith 2006b
- Smith DR, Mihashi M, Adachi Y, Nakashima Y, Ishitake T. Epidemiology of needlestick and sharps injuries among nurses in a Japanese teaching hospital. Journal of Hospital Infection 2006;64(1):44‐9. [DOI] [PubMed] [Google Scholar]
Sohn 2006
- Sohn JW, Kim BG, Kim SH, Han C. Mental health of healthcare workers who experience needlestick and sharps injuries. Journal of Occupational Health 2006;48(6):474‐9. [DOI] [PubMed] [Google Scholar]
Tanner 2009
- Tanner J, Parkinson H. Double gloving to reduce surgical cross‐infection. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD003087.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Timilshina 2011
- Timilshina N, Ansari MA, Dayal V. Risk of infection among primary health workers in the Western Development Region, Nepal: knowledge and compliance. Journal of Infection in Developing Countries 2011;1(5):18‐22. [DOI] [PubMed] [Google Scholar]
Verbeek 2005
- Verbeek J, Salmi J, Pasternack I, Jauhiainen M, Laamanen I, Schaafsma F, et al. A search strategy for occupational health intervention studies. Journal of Occupational and Environmental Medicine 2005;62(10):682‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waclawski 2004
- Waclawski ER. Evaluation of potential reduction in blood and body fluid exposures by use of alternative instruments. Occupational Medicine 2004;54(8):567‐9. [DOI] [PubMed] [Google Scholar]
Wallis 2007
- Wallis GC, Kim WY, Chaudhary BR, Henderson JJ. Perceptions of orthopaedic surgeons regarding hepatitis C viral transmission: a questionnaire survey. Annals of the Royal College of Surgeons of England 2007;89(3):276‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2007
- World Health Organization. Needlestick injuries: implementation of pilot projects to reduce sharps injuries in health care workers. http://www.who.int/occupational_health/topics/needinjuries/en/index.html (accessed 5 September 2007).
World Bank 2013
- World Bank. Data: GNI per capita, Atlas method (current US$). http://data.worldbank.org/indicator/NY.GNP.PCAP.CD?page=1 (accessed June 1, 2013).
Yang 2011
- Yang L, Mullan B. Reducing needle stick injuries in healthcare occupations: an integrative review of the literature. ISRN Nursing 2011;2011:315432. [doi: 10.5402/2011/315432] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Parantainen 2008
- Parantainen A, Lavoie MC, Pahwa M, Verbeek JH. Prevention of percutaneous injuries with risk of hepatitis B, hepatitis C, or other viral infections for health care workers. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD007197] [DOI] [Google Scholar]


