Abstract
Background
Convective dialysis modalities (haemofiltration (HF), haemodiafiltration (HDF), and acetate‐free biofiltration (AFB)) removed excess body fluid across the dialysis membrane with positive pressure and accumulated middle‐ and larger‐size accumulated solutes more efficiently than haemodialysis (HD). This increased larger solute removal combined with use of ultra‐pure dialysis fluid in convective dialysis is hypothesised to reduce the frequency and severity of symptoms during dialysis as well as improve clinical outcomes. Convective dialysis therapies (HDF and HF) are associated with lower mortality compared to diffusive therapy (HD) in observational studies. This is an update of a review first published in 2006.
Objectives
To compare convective (HF, HDF, or AFB) with diffusive (HD) dialysis modalities on clinical outcomes (mortality, major cardiovascular events, hospitalisation and treatment‐related adverse events) in men and women with end‐stage kidney disease (ESKD).
Search methods
We searched the Cochrane Renal Group's Specialised Register (to 18 February 2015) through contact with a Trials' Search Co‐ordinator using search terms relevant to this review.
Selection criteria
We included randomised controlled trials comparing convective therapy (HF, HDF, AFB) with another convective therapy or diffusive therapy (HD) for treatment of ESKD.
Data collection and analysis
Two independent authors identified studies, extracted data and assessed study risk of bias. We summarised treatment effects using the random effects model. We reported results as a risk ratio (RR) for dichotomous outcomes and mean difference (MD) for continuous data together with 95% confidence intervals (CI). We assessed for heterogeneity using the Chi2 test and explored the amount of variation in treatment estimates beyond that expected by chance using the I2 statistic.
Main results
Twenty studies comprising 667 participants were included in the 2006 review. In that review, there was insufficient evidence of treatment effects on major clinical outcomes to draw clinically meaningful conclusions. Searching to February 2015 identified 40 eligible studies comprising 3483 participants overall. In total, 35 studies (4039 participants) compared HF, HDF or AFB with HD, three studies (54 participants) compared AFB with HDF, and three studies (129 participants) compared HDF with HF.
Risks of bias in all studies were generally high resulting in low confidence in estimated treatment effects. Convective dialysis had no significant effect on all‐cause mortality (11 studies, 3396 participants: RR 0.87, 95% CI 0.72 to 1.05; I2 = 34%), but significantly reduced cardiovascular mortality (6 studies, 2889 participants: RR 0.75, 95% CI 0.61 to 0.92; I2 = 0%). One study reported no significant effect on rates of nonfatal cardiovascular events (714 participants: RR 1.14, 95% CI 0.86 to 1.50) and two studies showed no significant difference in hospitalisation (2 studies, 1688 participants: RR 1.23, 95% CI 0.93 to 1.63; I2 = 0%). One study reported rates of hypotension during dialysis were significantly reduced with convective therapy (906 participants: RR 0.72, 95% CI 0.66 to 0.80). Adverse events were not systematically evaluated in most studies and data for health‐related quality of life were sparse. Convective therapies significantly reduced predialysis levels of B2 microglobulin (12 studies, 1813 participants: MD ‐5.55 mg/dL, 95% CI ‐9.11 to ‐1.98; I2 = 94%) and increased dialysis dose (Kt/V urea) (14 studies, 2022 participants: MD 0.07, 95% CI ‐0.00 to 0.14; I2 = 90%) compared to diffusive therapy, but results across studies were very heterogeneous. Sensitivity analyses limited to studies comparing HDF with HD showed very similar results. Directly comparative data for differing types of convective dialysis were insufficient to draw conclusions.
Studies had important risks of bias leading to low confidence in the summary estimates and were generally limited to patients who had adequate dialysis vascular access.
Authors' conclusions
Convective dialysis may reduce cardiovascular but not all‐cause mortality and effects on nonfatal cardiovascular events and hospitalisation are inconclusive. However, any treatment benefits of convective dialysis on all patient outcomes including cardiovascular death are unreliable due to limitations in study methods and reporting. Future studies which assess treatment effects of convection dose on patient outcomes including mortality and cardiovascular events would be informative.
Plain language summary
Haemodiafiltration, haemofiltration and haemodialysis for end‐stage kidney disease
People who have severe loss of kidney function are treated with dialysis or a kidney transplant to remove toxins and fluid. Dialysis removes waste products and fluid by filtering these across a membrane in the dialysis machine (for haemodialysis) or within the body (for peritoneal dialysis). Toxins that build‐up in the body when the kidneys fail vary in size and larger molecules are removed less well by standard haemodialysis. Newer dialysis types 'push' water across the dialysis membrane which allows the removal of unwanted molecules more efficiently. Larger molecules are removed better and the dialysis fluid has fewer impurities, leading to the potential for convective dialysis to improve the ways patients feel and survive on dialysis. The three types of convective dialysis therapy are haemodiafiltration, haemofiltration, and acetate‐free biofiltration. Use of convective therapy for dialysis is higher in Europe and lower in the USA. Given the difference between regions for uptake of this treatment and the potential benefits on patient outcomes, we have updated this Cochrane review to new additional studies available in 2015.
We identified 40 studies enrolling 4137 adult participants. Of these, 35 studies in 4039 adults compared convective dialysis with standard haemodialysis. Overall the evidence in the studies was low or very low quality due to limitations in the methods used in the research leading to low confidence in the results. Overall, there was no evidence convective dialysis lowered risk of death from any cause but may reduce death due to heart or vascular disease. Overall treating 1000 men and women who have end‐stage kidney disease with convective dialysis rather than standard haemodialysis may prevent 25 dying from heart disease. Convective therapy may reduce blood pressure falls during dialysis but there was no evidence that convective dialysis influenced chances of hospital admission or other side‐effects, or improved quality of life.
Summary of findings
for the main comparison.
| Convective compared with diffusive dialysis modalities for men and women with end‐stage kidney disease | ||||||
|
Patient or population: men and women with end‐stage kidney disease Intervention: convective dialysis Comparison: diffusive dialysis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Diffusion | Convection | |||||
| All‐cause mortality | 200 per 1000 | Not significant | RR 0.87 (0.72 to 1.05) |
11 (3396) | ⊕⊕⊝⊝ low | Convective therapy has little or no effect on all‐cause mortality |
| Cardiovascular mortality | 100 per 1000 | 75 per 1000 | RR 0.75 (0.81 to 0.92) |
6 (2889) | ⊕⊕⊝⊝ low | Convective therapy may reduce cardiovascular mortality |
| Nonfatal cardiovascular events | 130 per 1000 | Not significant | RR 1.23 (0.93‐1.63) | 2 (1688) | ⊕⊝⊝⊝ very low | Convective therapy has uncertain effects on non‐fatal cardiovascular events |
| Health‐related quality of life | Not estimable | Not estimable | Not estimable | 8 (988) | ⊕⊕⊝⊝ very low | Convective therapy has uncertain effects on health‐related quality of life |
| *The assumed risk (e.g. the median control group risk across studies) is derived from data within dialysis registries for all‐cause mortality and cardiovascular mortality and the reported event rate in the available study for nonfatal cardiovascular events (CONTRAST (Dutch) Study 2005). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | ||||||
| GRADE (Grading of Recommendations Assessment, Development, and Evaluation) Working Group grades of evidence (Guyatt 2011). Low quality: Indicates that our confidence in the effect estimate is limited: The true effect may be substantially difference from the estimated effect. Very low quality: Indicated that we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimated effect. | ||||||
Background
Description of the condition
Dialysis, kidney transplantation or supportive care are available treatment options for end‐stage kidney disease (ESKD). Dialysis therapies include peritoneal dialysis as well as standard haemodialysis (HD) or convective dialysis (haemofiltration (HF), haemodiafiltration (HDF), and acetate‐free biofiltration (AFB)) to remove accumulated fluid and metabolites from the blood when kidney function is severely impaired. Despite effective removal of solutes and water by standard HD to avoid life‐threatening complications, long‐term dialysis patients reported markedly impaired quality of life, sleep disturbance, nausea, depressive symptoms, anxiety, thirst, and pain (Murtagh 2007). In addition, survival remains poor; 10 to 20% of men and women treated with long‐term dialysis die each year (USRDS 2011).
Description of the intervention
Standard HD removes accumulated metabolites and fluid from the patient's blood by diffusion across a semi‐permeable membrane into the dialysate fluid for removal into the dialysis waste. Convective dialysis (HDF, HF and AFB) clears water (convection) using positive pressure across the dialysis membrane for removal. Accumulated solutes follow the movement of water in a phenomenon known as 'solvent drag' (Henderson 2004). Water and electrolytes are replaced as required into the blood circulation. The replacement fluid can be added to the patient's blood before the membrane filter ("pre‐dilution") or after the dialysis filter along with the blood returning into the patient's blood circulation ("post‐dilution").
Accumulated metabolites have different molecular weights and are cleared differently depending on the type of molecular transport used in dialysis (diffusive versus convective). HF may remove higher molecular weight molecules whereas standard HD may be more effective at removing smaller solutes such as urea (Locatelli 2000). A hybrid system that includes both convection and diffusion, known as HDF combines convection with diffusion (Schmidt 1986). In HD and HDF, the dialysate contains acetate, which buffers circulating acids which cannot be buffered sufficiently by kidney function. Acetate depresses myocardial contractility and may cause haemodynamic instability during dialysis (Daugirdas 1991; Sztajzel 1993). AFB, a HDF technique, uses a hypertonic sodium bicarbonate solution in place of acetate to manage acidosis (Zucchelli 1990).
How the intervention might work
While standard HD clears smaller, water‐soluble metabolites efficiently by diffusion, poor clinical outcomes might be explained in part by inadequate removal of middle‐ and larger‐sized waste products of metabolism which are implicated in the pathogenesis of atherosclerosis and dialysis‐related amyloidosis (Guerin 2000). In uncontrolled studies, convective dialysis therapies are associated with lower mortality (Locatelli 1999; Vilar 2009) and lower circulating levels of middle‐size molecules such as vitamin B12 or B2 microglobulin in dialysis patients are associated with reduced cardiovascular and infection‐related mortality (Liabeuf 2012). In addition, HDF is associated with less frequent hypotension during dialysis (Vilar 2009) and the enhanced biocompatibility of ultrapure dialysate fluids used in convective technologies may reduce inflammation, oxidative stress and infection.(Arizono 2004; Calo 2007) Removal of larger metabolites by newer convective dialysis strategies is therefore a potential strategy to improve dialysis outcomes and greater convection volumes during HDF might be a principal determinant of clinical effectiveness with HDF because of greater clearances of middle and large uraemic solutes during treatment.
Why it is important to do this review
In our previous meta‐analysis, published in 2006, that included 17 randomised studies (600 participants) comparing convective with diffusive dialysis (Rabindranath 2006), convective modalities had uncertain effects on mortality in low‐quality evidence and data for adverse events were sparse. Since 2006, 18 additional studies (3439 participants) comparing convection with diffusion modalities have been published, but results have been inconsistent, and consequently there has been variable uptake of HDF into clinical practice; in 2010 the proportion of incident dialysis patients treated with HDF in Europe varied between 0.7% in Finland and 18.9% in the Catalonian region of Spain (ERA‐EDTA Registry 2010). In Australia and New Zealand, the proportion of dialysis patients treated with HDF in 2011 was 21.5% and 10.9%, respectively (ANZDATA 2012). The European Best Practice Guidelines that were published in 2007 suggest that HDF is a suitable treatment strategy to delay complications of ESKD and exchange volumes should be as high as possible (EBPG 2007).
Objectives
To compare convective (HF, HDF, or AFB) with diffusive (HD) dialysis modalities on clinical outcomes (mortality, major cardiovascular events, hospitalisation and treatment‐related adverse events) in men and women with ESKD
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) comparing convective therapies (HF, HDF, AFB) and HD.
Types of participants
Adults with ESKD treated with dialysis.
Types of interventions
Convective therapy (HDF/ HF/AFB) compared with diffusive therapy (HD)
Direct comparisons of different convective therapies (HDF/HF/AFB)
Types of outcome measures
Primary outcomes
All‐cause mortality.
Secondary outcomes
Clinical outcomes
Cardiovascular mortality
Hypotension (symptomatic hypotension, hypotension requiring treatment and post‐dialysis hypotension, recorded as number of events/person‐years follow‐up or number of patients experiencing one or more episodes)
Hospitalisation (days of hospitalisation, one or more episodes, or number of events/person‐years of follow‐up)
Change of dialysis modality (from convective to diffusive dialysis modality or vice versa)
Symptoms (headaches, nausea, vomiting) occurring during or after dialysis (recorded as number of treatment sessions at which event occurred or number of patients experiencing one or more episodes of headaches, nausea or vomiting)
"Any adverse symptoms" or number of patients experiencing "Any adverse symptoms" (number of events/person‐years of follow‐up or patients experiencing one or more events)
Health‐related quality of life (any instrument used)
Amyloid‐related complications (amyloidosis, carpal tunnel syndrome, amyloid‐related arthropathy).
Surrogate outcomes
Adequacy of dialysis (assessed by Kt/V values or by urea reduction ratio (URR))
End of treatment blood pressure (measured as systolic, diastolic or mean arterial pressure, in mm Hg)
End of treatment predialysis B2 microglobulin levels (mg/L).
Dialysis adequacy measures and B2 microglobulin measures were not regarded as key clinical outcomes in this review. However, they were included to facilitate the determination of their usefulness as secondary outcome measures for the interventions compared.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Renal Group's Specialised Register (to 18 February 2015) through contact with the Trials' Search Co‐ordinator using search terms relevant to this review. The Cochrane Renal Group’s specialised register contains studies identified from the following sources.
Quarterly searches of the Cochrane Central Register of Controlled Trials CENTRAL
Weekly searches of MEDLINE OVID SP
Handsearching of renal‐related journals & the proceedings of major renal conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected renal‐journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal & ClinicalTrials.gov
Studies contained in the specialised register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of the Cochrane Renal Group. Details of these strategies as well as a list of handsearched journals, conference proceedings and current awareness alerts are available in the 'specialised register' section of information about the Cochrane Renal Group.
See Appendix 1 for search terms used in strategies for this review.
Data collection and analysis
Selection of studies
The search strategies described were used to obtain titles and abstracts of studies that might be relevant to the review. The 2006 review was undertaken by six authors (Rabindranath 2006). Two authors independently assessed and retrieved titles and abstracts. The full text of all potentially relevant studies were retrieved by the same authors and independently assessed in detail. Two authors carried out data extraction independently using standardised data extraction forms. Disagreements were resolved in consultation among the authors.
The review update was undertaken by six authors. Two authors independently assessed and retrieved titles and abstracts. The full text (if published) of all potentially relevant studies were retrieved and independently assessed for inclusion by two authors. Two authors extracted data which was cross‐checked by a third author. Discrepancies were resolved by discussion among the authors.
Data extraction and management
Two authors carried out data extraction independently using standard data extraction forms and data were entered into RevMan. We extracted the number of events and participants as risk of events or when these data were not provided, the number of events/person‐years of follow‐up for dichotomous outcomes and mean (SD) and numbers of participants at risk for continuous outcomes. We translated studies not reported in English before assessment and data extraction. Where more than one publication of a study existed, only the publication with the most complete data was included.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
We considered the study to be at high risk of selective outcome reporting when investigators did not report data for all‐cause mortality and cardiovascular mortality and adverse events or patient symptoms.
We rated the quality of the evidence for convection versus diffusion interventions using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system for systematic reviews considering study limitations, precision and consistency of treatment estimates, directness of available evidence and publication bias for the clinical outcomes of quality of all‐cause and cardiovascular mortality, nonfatal cardiovascular events, and health‐related quality of life (Guyatt 2011) to generate a Table 1. To estimate the absolute number of men and women with ESKD who had mortality or nonfatal cardiovascular events avoided or incurred with convective dialysis therapy, we used the risk estimate and 95% CI obtained from the corresponding meta‐analysis together with the absolute population risk derived from previously published registry data (USRDS 2011) or from the control group of available RCTs.
Measures of treatment effect
We summarised treatment effects using random‐effects meta‐analysis and expressed results as relative risks (RR) or rate ratios with 95% confidence intervals (CI) for binary outcomes for all studies reporting one or more events (all‐cause mortality, cardiovascular mortality, rate of nonfatal cardiovascular events, rate of hospitalisation, rate of hypotension during dialysis, change in dialysis modality) and mean difference (MD) with 95% CI for continuous outcomes (serum B2 microglobulin, Kt/V, URR, blood pressure).
Data from cross‐over studies were included when authors reported results for the first phase of the study (Mandolfo 2008; Schiffl 2007; Vaslaki 2006). The studies of Schiffl 2007 and Vaslaki 2006 presented the results separately for the two phases and we included in our analysis data from the first phase. For Cristofano 2004, the results of first phase of the study were obtained after contacting the authors. For all the other cross‐over studies, results were extracted and presented in narrative form in a tabular format when available.
Dealing with missing data
Any additional unpublished information or clarification required from the authors was requested by written or electronic correspondence and relevant data obtained in this manner were included in the review.
Assessment of heterogeneity
Heterogeneity of treatment effects between studies was formally tested using the Cochran Q test. The I2 statistic was used to determine the proportion of variation in treatment estimates present that was attributable to heterogeneity beyond the level of that expected by chance (Higgins 2003).
Data synthesis
Data were pooled using a random effects model. For each analysis, the fixed effects model was also evaluated to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses to explore how possible sources of heterogeneity (type of treatment (different types of convective therapy (HF, HDF, AFB)); membrane flux (high versus low); age; allocation concealment) might modify treatment effects were not conducted as there was either no evidence for heterogeneity in analyses or insufficient numbers of studies available to conduct analysis.
To assess for potential bias from small study effects, we constructed funnel plots for the log risk ratio against its variance (standard error) for individual studies and formally assessed for plot asymmetry by using the Egger regression test.(Egger 1997).
Results
Description of studies
Results of the search
The results of the search processes are shown in Figure 1.
1.
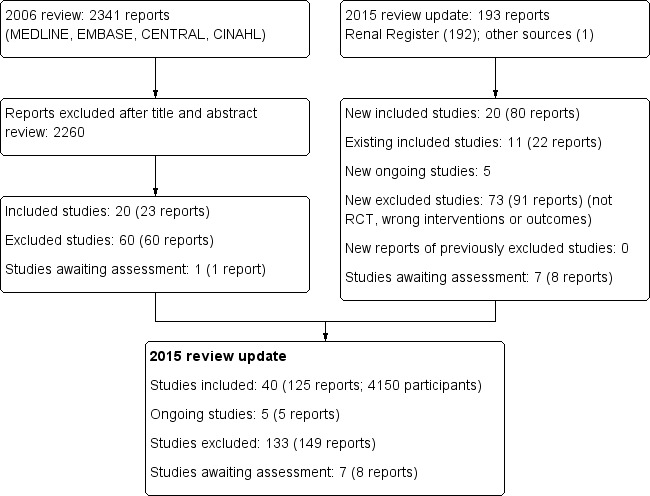
Study flow diagram.
2006 review
The combined search of MEDLINE, EMBASE, CINAHL, CENTRAL, American Journal of Physicians Database, and Database of Abstracts of Reviews of Effectiveness in May 2006 identified 2341 potentially relevant studies. After reviewing titles and abstracts, 2260 studies were excluded. The full text‐versions of 81 studies were retrieved, 59 which were excluded and one study (Ohyama 1981) in Japanese was waiting assessment. The major reason for exclusion was that the identified studies were not randomised. In total 20 studies (Altieri 2004; Bammens 2004; Basile 2001; Beerenhout 2005; Ding 2002; Eiselt 2000; Fox 1993; Lin 2001; Locatelli 1994; Lornoy 1998; Movilli 1996; Noris 1998; Schiffl 1992; Schrander vd Meer 1998; Teo 1987; Todeschini 2002; Tuccillo 2002; Verzetti 1998; Ward 2000; Wizemann 2000) reported in 23 publications enrolling 654 participants were included in the first version of this review.
2015 review update
The updated search of the Cochrane Renal Group's Specialised Register (18 February 2015) identified 193 new reports. After review 91 reports (73 studies) were excluded.
Five citations were ongoing studies with protocols available in the on‐line clinical studies registries (NCT01098149; NCT01327391; NCT01396863; NCT01445366; NCT02374372. We included 20 additional studies in this update (80 reports; 3483 participants) (Bolasco 2003; Coll 2009; CONTRAST (Dutch) Study 2005; Cristofano 2004; ESHOL Study 2011; Karamperis 2005; Kantartzi 2013; Mandolfo 2008; Meert 2009; Ohtake 2012; Pedrini 2011a; PROFIL Study 2011; Righetti 2010; Santoro 2005a; Schiffl 2007; Selby 2006a; Stefansson 2012; Santoro 1999; TURKISH HDF 2013; Vaslaki 2006).
Forty studies (4137 participants) could be included in the review. Thirty‐five studies (4039 participants) compared HF, HDF or AFB with HD, three studies (54 participants) compared AFB with HDF, and three studies (120 participants) compared HDF with HF (Table 2). For the studies that have been reported more than once, only data from the latest versions were used. The characteristics of the populations and interventions in the included studies are reported in the Characteristics of included studies.
1. Categories of interventions used in individual studies and duration of follow‐up.
| Study ID | Intervention | Duration | Number of patients |
| Altieri 2004 | HDF versus HF | 12 months | 39 |
| Bammens 2004 | HDF versus HD | 2 weeks | 14 |
| Basile 2001 | AFB versus HD | 12 months | 11 |
| Beerenhout 2005 | HF versus HD | 12 months | 40 |
| Bolasco 2003 | HF versus HDF versus HD | 18 months | 146 |
| Coll 2009 | AFB versus HDF | 15 months | 30 |
| CONTRAST (Dutch) Study 2005 | HDF versus HD | 36 months | 714 |
| Cristofano 2004 | HDF versus HD | 1 session | 12 |
| Ding 2002 | HDF versus AFB | 36 weeks | 12 |
| Eiselt 2000 | AFB versus HD | 12 months | 20 |
| ESHOL Study 2011 | HDF versus HD | 36 months | 906 |
| Fox 1993 | HF versus HD | 1 session | 9 |
| Kantartzi 2013 | HDF versus HD | 3 months | 24 |
| Karamperis 2005 | HDF versus HD | 2 sessions | 12 |
| Lin 2001 | HDF versus HD | 15 months | 67 |
| Locatelli 1994 | HDF versus HD | 24 months | 205 |
| Lornoy 1998 | HDF versus HD | 1 session | 8 |
| Mandolfo 2008 | HDF versus HD | 6 weeks | 8 |
| Meert 2009 | HDF versus HF | 9 weeks | 14 |
| Movilli 1996 | HDF versus AFB | 6 months | 12 |
| Noris 1998 | AFB versus HD | 1 week | 5 |
| Ohtake 2012 | HDF versus HD | 12 months | 22 |
| Pedrini 2011a | HDF versus HD | 12 months | 69 |
| PROFIL Study 2011 | HF versus HD | 24 months | 48 |
| Righetti 2010 | HDF versus HD | 18 months | 24 |
| Santoro 1999 | AFB versus HD | 48 months | 371 |
| Santoro 2005a | HF versus HD | 36 months | 64 |
| Schiffl 1992 | HF versus HD | 48 months | 32 |
| Schiffl 2007 | HDF versus HD | 48 months | 76 |
| Schrander vd Meer 1998 | AFB versus HD | 12 months | 24 |
| Selby 2006a | AFB versus HD | 4 weeks | 12 |
| Stefansson 2012 | HDF versus HD | 4 months | 20 |
| Teo 1987 | HDF versus HD | 8 months | 13 |
| Todeschini 2002 | AFB versus HD | 3 sessions | 9 |
| Tuccillo 2002 | HDF versus HD | 3 months | 12 |
| TURKISH HDF 2013 | HDF versus HD | 24 months | 782 |
| Vaslaki 2006 | HDF versus HD | 48 weeks | 129 |
| Verzetti 1998 | AFB versus HD | 12 months | 41 |
| Ward 2000 | HDF versus HD | 12 months | 50 |
| Wizemann 2000 | HDF versus HD | 24 months | 44 |
AFB ‐ acetate‐free biofiltration; HDF ‐ haemodiafiltration; HD ‐ haemodialysis; HF ‐ haemofiltration
Prior to publication of this review update a final search of the Specialised Register identified seven new potential studies (Beerenhout 2004; Bellien 2014; Cornelis 2014; de Sequera 2013; Francisco 2013; Gonzales‐Diez 2012; Krieter 2010a). These studies will be assessed for inclusion in a future update of this review.
Included studies
The 40 included studies were grouped into five subsets (Characteristics of included studies; Table 3).
2. Description of included studies according with the interventions used.
| Categories of intervention | Study | Total number of studies | Total number of patients |
| HDF versus HD | Bammens 2004; Lin 2001; Locatelli 1994; Lornoy 1998; Teo 1987; Tuccillo 2002; Ward 2000; Wizemann 2000; Bolasco 2003; CONTRAST (Dutch) Study 2005; Cristofano 2004; Karamperis 2005; Mandolfo 2008; Pedrini 2011a; Righetti 2010; Schiffl 2007; Stefansson 2012; TURKISH HDF 2013; Vaslaki 2006ESHOL Study 2011; Kantartzi 2013; Ohtake 2012 | 22 | 3299 |
| HF versus HD | Beerenhout 2005; Fox 1993; Schiffl 1992; Bolasco 2003; Santoro 2005a; PROFIL Study 2011 | 6 | 325 |
| AFB versus HD | Basile 2001; Santoro 1999; Eiselt 2000; Noris 1998; Schrander vd Meer 1998; Selby 2006a; Todeschini 2002; Verzetti 1998 | 8 | 487 |
| HDF versus AFB | Coll 2009; Ding 2002; Movilli 1996 | 3 | 59 |
| HDF versus HF | Altieri 2004; Bolasco 2003; Meert 2009 | 3 | 199 |
| More than two treatment arms | Bolasco 2003; Locatelli 1994; Schiffl 1992 | 3 | 383 |
AFB ‐ acetate‐free biofiltration; HDF ‐ haemodiafiltration; HD ‐ haemodialysis; HF ‐ haemofiltration
Convective versus diffusive therapy
HF versus HD (Beerenhout 2005; Bolasco 2003; Fox 1993; PROFIL Study 2011; Santoro 2005a; Schiffl 1992)
HDF versus HD (Bammens 2004; Bolasco 2003; CONTRAST (Dutch) Study 2005; Cristofano 2004; ESHOL Study 2011; Karamperis 2005; Kantartzi 2013; Lin 2001; Locatelli 1994; Lornoy 1998; Mandolfo 2008; Ohtake 2012; Pedrini 2011a; Righetti 2010; Schiffl 2007; Stefansson 2012; Teo 1987; Tuccillo 2002; TURKISH HDF 2013; Vaslaki 2006; Ward 2000; Wizemann 2000)
AFB versus HD (Basile 2001; Eiselt 2000; Noris 1998; Schrander vd Meer 1998; Selby 2006a; Santoro 1999; Todeschini 2002; Verzetti 1998)
Study characteristics
Of the 22 studies evaluating HDF, all but three (Locatelli 1994; Teo 1987; Tuccillo 2002) reported convection methods using fluid generated on‐line (on‐line HDF). In the HD control group, 12 studies (34%) used high‐flux membranes, 16 studies (46%) used low‐flux membranes, four studies (11%) used either low or high‐flux membrane and in three studies (9%) the membrane flux was unclear. Convection strategies were highly heterogeneous and no study randomised participants to specific targeted convection volumes. In 16 (46%) studies, adequate vascular access for high volume dialysis was required. Most studies included patients who were anuric or had minimal kidney function.
Seventeen studies had a parallel study design (Beerenhout 2005; CONTRAST (Dutch) Study 2005; Cristofano 2004; Eiselt 2000; ESHOL Study 2011; Lin 2001; Locatelli 1994; Bolasco 2003; Ohtake 2012; PROFIL Study 2011; Santoro 2005a; Schiffl 1992; Schrander vd Meer 1998; Santoro 1999; TURKISH HDF 2013; Ward 2000; Wizemann 2000). Ten studies evaluated short‐term outcomes conducted over follow‐up that varied between one dialysis sessions and two weeks of treatment (Bammens 2004; Cristofano 2004; Fox 1993; Karamperis 2005; Lornoy 1998; Mandolfo 2008; Noris 1998; Selby 2006a; Todeschini 2002; Tuccillo 2002). The remaining 24 studies evaluated outcomes during treatment of between two and 48 months (median 12 months) except one in which treatment duration was unclear (Lin 2001). Sample size varied between five and 906 participants (median 24). Of the overall participants, 2402 (59%) were derived from three large studies evaluating on‐line HDF (CONTRAST (Dutch) Study 2005; ESHOL Study 2011; TURKISH HDF 2013).
Direct comparisons of different convective therapies
HDF versus AFB (Coll 2009; Ding 2002; Movilli 1996)
HDF versus HF (Altieri 2004; Bolasco 2003; Meert 2009).
Three studies (Locatelli 1994; Schiffl 1992; Bolasco 2003) had three or more treatment arms. These studies had a parallel study design. In the study by Locatelli 1994, HDF was compared with cuprophane HD, low‐flux polysulfone HD and high ‐flux polysulfone HD. Schiffl 1992 compared HF with both low‐flux HD and high‐flux polysulfone HD. Bolasco 2003 assigned participants to HF, HDF or HD. When analysing data from these studies for dichotomous outcomes, convective modalities were compared with the combined results of all the HD treatment arms. For continuous data analysis, data for HDF compared with HD were used.
Study characteristics
Of the six studies comparing convection with another convective strategy, five reported convection methods using fluid generated on‐line. Two studies used high‐flux (33%) membranes for HD, one reported low‐flux membranes (17%), and in the remainder the HD flux was unclear. Convection strategies were highly heterogeneous and no study randomised participants to specific targeted convection volumes. In four (66%) studies, adequate vascular access for high volume dialysis was required. Most studies included patients who were anuric or had minimal renal function.
One study had a parallel study design (Bolasco 2003) and the remainder were cross‐over studies. Follow‐up duration ranged between two and 24 months (median six months) with only one study reporting follow‐up for 12 months or more. Study sample sizes were small (12 to 76 participants; median 21).
Excluded studies
In total 133 studies (149 reports) were excluded. The reasons for exclusion were; not randomised (69); wrong population (1); wrong intervention (43); or outcomes not relevant to this review (23).
Risk of bias in included studies
Risk of bias in individual studies is shown in Figure 2 and the summary risk of bias in included studies is shown in Figure 3. According to standard criteria (Appendix 2), studies generally had very serious limitations due to risks of bias in most evaluated domains leading to down‐grading of overall evidence quality.
2.
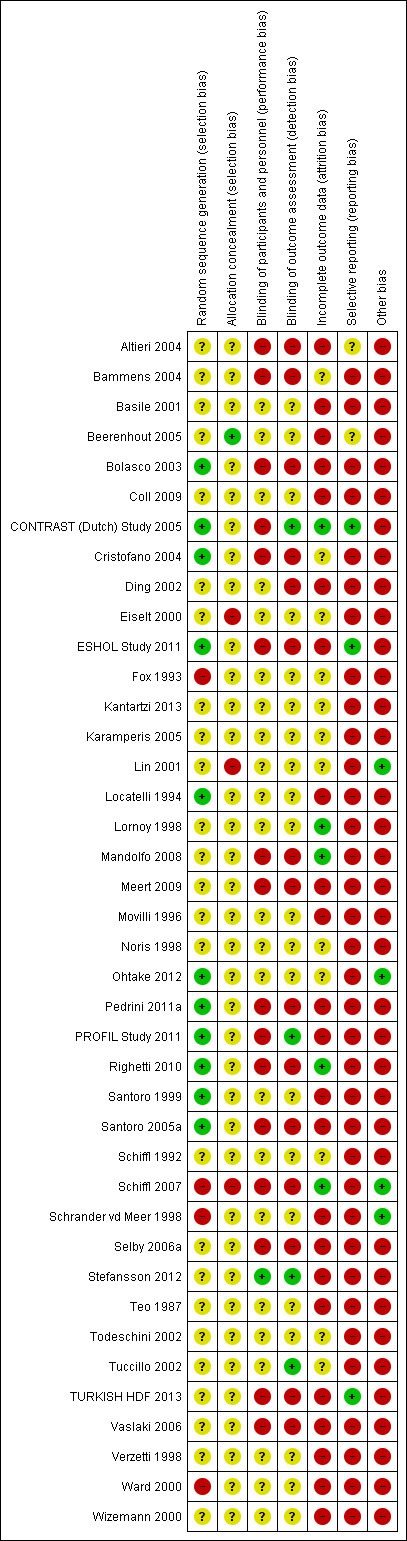
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
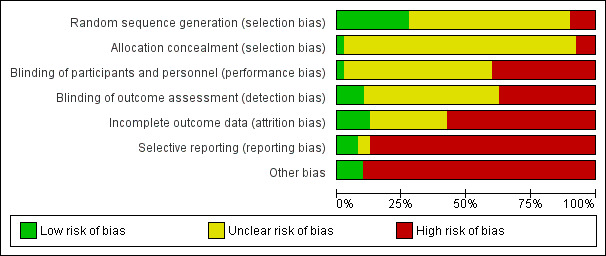
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All studies stated that patients were randomly allocated to treatment groups. Sequence generation methods were at low risk of bias in 11 studies (Bolasco 2003; CONTRAST (Dutch) Study 2005; Cristofano 2004; ESHOL Study 2011; Locatelli 1994; Ohtake 2012; Pedrini 2011a; PROFIL Study 2011; Righetti 2010; Santoro 1999; Santoro 2005a), high risk in four studies (Fox 1993; Schiffl 2007; Schrander vd Meer 1998; Ward 2000), and unclear in the remainder.
Allocation concealment was at low risk of bias in one study (Beerenhout 2005), high risk in three studies (Eiselt 2000; Lin 2001; Schiffl 2007) and unclear in the remainder.
Blinding
Two studies reported blinding of participants (Karamperis 2005; Stefansson 2012) but not investigators for all outcomes and three (CONTRAST (Dutch) Study 2005; PROFIL Study 2011; Stefansson 2012) reported blinding of outcome assessment. In Tuccillo 2002, we considered that the lack of blinding will not influence the results. In Karamperis 2005, the authors did not blind the investigators but used electronic devices for assessing the outcomes.
Incomplete outcome data
A key risk of bias present in all but five studies (CONTRAST (Dutch) Study 2005; Lornoy 1998; Mandolfo 2008; Righetti 2010; Schiffl 2007) was the lack of complete outcome data for at least 90% of randomised patients and/or loss of patients to follow‐up was not similar between treatment groups for reasons unrelated to the outcomes of interest. Of the three largest studies contributing to the meta‐analyses, ESHOL Study 2011 did not include 39% of randomised patients in their analyses, and in the TURKISH HDF 2013, 21% of participants left the study for reasons other than death including 10% of the participants allocated to HDF due to vascular access problems. Twenty‐three studies were at high risk of incomplete follow‐up (Altieri 2004; Basile 2001; Beerenhout 2005; Bolasco 2003; Coll 2009; Ding 2002; ESHOL Study 2011; Locatelli 1994; Meert 2009; Movilli 1996; Pedrini 2011a; PROFIL Study 2011; Santoro 1999; Santoro 2005a; Schrander vd Meer 1998; Selby 2006a; Stefansson 2012; Teo 1987; TURKISH HDF 2013; Vaslaki 2006; Verzetti 1998; Ward 2000; Wizemann 2000) and the risk was unclear in the remainder.
Selective reporting
Three studies were at low risk of bias for selective outcome reporting (CONTRAST (Dutch) Study 2005; ESHOL Study 2011; TURKISH HDF 2013), in two studies it was unclear (Altieri 2004; Beerenhout 2005) and the remainder were at high risk.
Other potential sources of bias
Twenty‐one studies (52%) had a cross‐over design which was considered as a potential source of bias because of the carry over effect. All but four studies (Lin 2001; Ohtake 2012; Schiffl 2007; Schrander vd Meer 1998) were at high risk of other sources of bias including: commercial sponsor on authorship, or data management, or both; interventions or baseline participant characteristics or both were not matched; data not extractable for meta‐analysis; abstract‐only publication; or early termination of the study.
Effects of interventions
See: Table 1
Convective transport (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis
All‐cause and cardiovascular mortality
In eleven studies (3396 participants), convective dialysis treatment had no significant effect on all‐cause mortality compared to HD (Analysis 1.1 (11 studies, 3396 participants): RR 0.87, 95% CI 0.72 to 1.05; I2 = 34%). There was a moderate level heterogeneity between the studies.
1.1. Analysis.
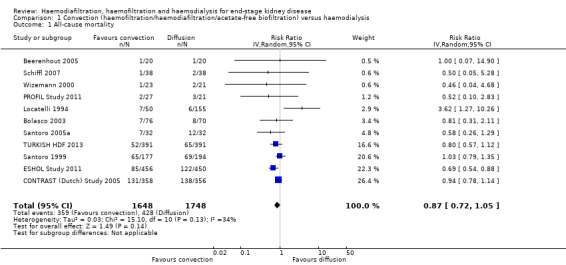
Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 1 All‐cause mortality.
Convective therapy significantly reduced death from cardiovascular causes compared with HD (Analysis 1.2 (6 studies, 2889 participants): RR 0.75, 95% CI 0.61 to 0.92; I2 = 0%). In absolute terms, convective therapy might prevent 25 cardiovascular deaths for every 1000 patients treated for one year, but has no significant effect on death overall.
1.2. Analysis.
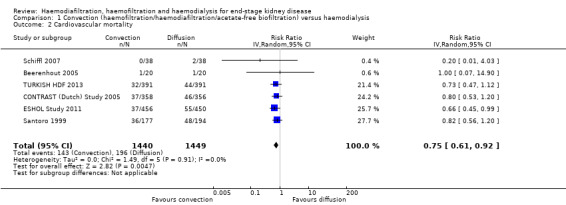
Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 2 Cardiovascular mortality.
Nonfatal cardiovascular events and hospitalisation
CONTRAST (Dutch) Study 2005 reported no significant effect on nonfatal cardiovascular events for convective dialysis versus HD (Analysis 1.3 (1 study, 714 participants): RR 1.14, 95% CI 0.86 to 1.50).
1.3. Analysis.
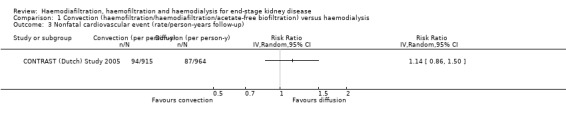
Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 3 Nonfatal cardiovascular event (rate/person‐years follow‐up).
Locatelli 1994 reported no significant difference between convective therapy and HD for the number of hospitalisations/year (Analysis 1.4.1 (1 study, 45 participants): MD 0.20 hospitalisations/y, 95% CI ‐0.07 to 0.47).
1.4. Analysis.
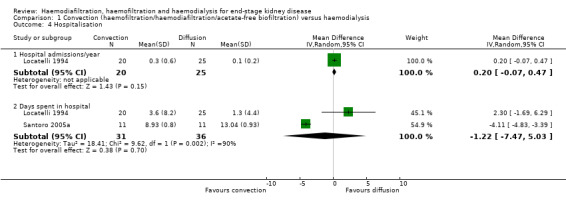
Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 4 Hospitalisation.
There was no significant difference between convective therapy and HD for the number of days spent in hospital (Analysis 1.4.2 (2 studies, 67 participants): MD ‐1.22 days, 95% CI ‐7.47 to 5.03; I2 = 90%), and rate of hospitalisation (Analysis 1.5 (2 studies, 1688 participants): RR 1.23, 95% CI 0.93 to 1.63; I2 = 0%). Verzetti 1998 (cross‐over study) reported less hospitalisation in the convective therapy group compared to the HD group (data presented in Analysis 1.17.1). The substantial level of heterogeneity observed in the analysis of days spent in hospital might be possibly due to the 10 year difference in the date of publication of contributing studies.
1.5. Analysis.
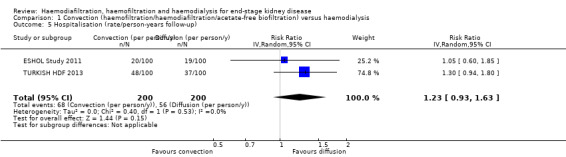
Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 5 Hospitalisation (rate/person‐years follow‐up).
1.17. Analysis.
Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 17 Data from cross‐over studies.
| Data from cross‐over studies | |||
|---|---|---|---|
| Study | Convective therapy | Diffusive therapy | P value from paper |
| Hospitalisation | |||
| Verzetti 1998 | 8 | 17 | Not reported |
| Patients experiencing hypotension | |||
| Fox 1993 | 1/9 | 0/9 | Not reported |
| Karamperis 2005 | 0/12 | 0/12 | Not significant |
| Pedrini 2011a | 2/62 | 5/62 | Not reported |
| Teo 1987 | 0/10 | 0/10 | Not reported |
| Intradialytic hypotensive events | |||
| Selby 2006a | 23 | 37 | Not significant |
| Stefansson 2012 | 32 dialysis sessions with hypotension from a total of 520 sessions | 28 dialysis sessions with hypotension from a total of 520 sessions | Not significant |
| Symptomatic intradialytic hypotensive events | |||
| Selby 2006a | 2 | 2 | Not significant |
| Predialysis systolic blood pressure (mm Hg) | |||
| Karamperis 2005 | 12 patients Mean (± SE): 145.0 (7) | 12 patients Mean (± SE): 144.0 (6) | Not significant |
| Noris 1998 | 5 patients Mean (± SE): 136.3 (2.7) | 5 patients Mean (± SE): 128.3 (3.6) | P > 0.05 |
| Pedrini 2011a | 62 patients Mean (± SE): 140 (22) | 62 patients Mean (± SE): 147 (22) | P = 0.014 |
| Stefansson 2012 | 20 patients Mean (± SE): 161.2 (29.9) | 20 patients Mean (± SE): 157.5 (26.1) | Not reported |
| Todeschini 2002 | 9 patients Mean (± SE): 153 (8) | 9 patients Mean (± SE): 153 (6) | P > 0.05 |
| Predialysis diastolic blood pressure (mm Hg) | |||
| Karamperis 2005 | 12 patients Mean (± SE): 81.0 (3) | 12 patients Mean (± SE): 83.0 (3) | Not significant |
| Noris 1998 | 5 patients Mean (± SE): 78.0 (2.7) | 5 patients Mean (± SE): 75.3 (3.4) | P > 0.05 |
| Pedrini 2011a | 62 patients Mean (± SE): 75.0 (13) | 62 patients Mean (± SE): 80.0 (13) | P = 0.05 |
| Stefansson 2012 | 20 patients Mean (± SE): 88.9 (12.6) | 20 patients Mean (± SE): 86.4 (10.8) | Not reported |
| Todeschini 2002 | 9 patients Mean (± SE): 83 (2) | 9 Mean (± SE): 88 (2) | P > 0.05 |
| Predialysis mean arterial pressure (mm Hg) | |||
| Karamperis 2005 | 12 patients Mean (± SE): 103.0 (4) | 12 patients Mean (± SE): 104.0 (4) | Not significant |
| Teo 1987 | 10 patients Mean (± SEM): 94.4 (6.7) | 10 patients Mean (± SEM): 94.7 (6.1) | "Statistically insignificant" |
| Postdialysis systolic blood pressure (mm Hg) | |||
| Karamperis 2005 | 12 patients Mean (± SE): 128.0 (8) | 12 patients Mean (± SE): 129.0 (5) | Not significant |
| Noris 1998 | 5 patients Mean (± SE): 136.3 (4.2) | 5 patients Mean (± SE): 127.1 (3.6) | P > 0.05 |
| Pedrini 2011a | 62 patients Mean (± SE): 138 (25) | 62 patients Mean (± SE): 138 (21) | "not differ significantly" |
| Stefansson 2012 | 20 patients Mean (± SE): 161.6 (25.1) | 20 patients Mean (± SE): 157.1 (22.8) | Not reported |
| Todeschini 2002 | 9 patients Mean (± SE): 114 (4) | 9 patients Mean (± SE): 121 (3) | P > 0.05 |
| Postdialysis diastolic blood pressure (mm Hg) | |||
| Karamperis 2005 | 12 patients Mean (± SE): 73.0 (4) | 12 patients Mean (± SE): 77.0 (4) | Not significant |
| Pedrini 2011a | 62 patients Mean (± SE): 77.0 (14) | 62 patients Mean (± SE): 76.0 (13) | "not differ significantly" |
| Stefansson 2012 | 20 patients Mean (± SE): 86.8 (12.8) | 20 patients Mean (± SE): 85.3 (10.3) | Not reported |
| Postdialysis fall in systolic blood pressure (mm Hg) | |||
| Todeschini 2002 | 9 patients Mean (± SE): ‐39 (8) | 9 patients Mean (± SE): ‐32 (6) | P > 0.05 |
| Postdialysis mean arterial pressure (mm Hg) | |||
| Karamperis 2005 | 12 patients Mean (± SE): 91.0 (5) | 12 patients Mean (± SE): 94.0 (3) | Not significant |
| Teo 1987 | 10 patients Mean (± SEM): 90.7 (3.8) | 10 patients Mean (± SEM): 96.3 (5.9) | "Statistically insignificant" |
| Difference between pre‐ and postdialysis systolic blood pressure (mm Hg) | |||
| Noris 1998 | 5 patients Mean (± SE): 0 (4.8) | 5 patients Mean (± SE): ‐0.3 (4.6) | P > 0.05 |
| Difference between pre‐ and postdialysis diastolic blood pressure (mm Hg) | |||
| Noris 1998 | 5 patients Mean (± SE): ‐1.4 (2.7) | 5 patients Mean (± SE): ‐3.1 (2.8) | P > 0.05 |
| Todeschini 2002 | 9 patients Mean (± SE): ‐8 (6) | 9 patients Mean (± SE): ‐13 (3) | P > 0.05 |
| Intradialysis mean systolic blood pressure (mm Hg) | |||
| Selby 2006a | 12 patients Mean (± SEM): 137.8 (5.3) | 12 patients Mean (± SEM): 145.5 (8.0) | P < 0.0001 |
| Intradialysis mean diastolic blood pressure (mm Hg) | |||
| Selby 2006a | 12 patients Mean (± SEM): 79.2 (1.9) | 12 patients Mean (± SEM): 80.8 (3.5) | P = 0.005 |
| Intradialysis mean arterial pressure (mm Hg) | |||
| Selby 2006a | 12 patients Mean (± SEM): 104.1(5.2) | 12 patients Mean (± SEM): 100.5 (2.9) | P < 0.0001 |
| Teo 1987 | 10 patients Mean (± SEM): 89.5 (5.6) | 10 patients Mean (± SEM): 95.3 (5.5) | "statistically insignificant decrease" |
| Kt/V | |||
| Basile 2001 | 10 patients Mean (± SD): .28 (0.05) | 10 patients Mean (± SD): 1.30 (0.05) | No significant difference |
| Kantartzi 2013 | 48 patients Mean (± SD): 1.45 (0.16) | 48 patients Mean (± SD): 1.42 (0.02) | P = 0.33 |
| Karamperis 2005 | 12 patients Mean (± SD): 1.8 (0.20) | 12 patients Mean (± SD): 1.70 (0.00) | No significant difference |
| Noris 1998 | 5 patients Mean (± SE) = 1.28 (0.08) | 5 patients Mean (± SE): 1.16 (0.11) | P > 0.05 |
| Pedrini 2011a | 62 patients Mean (± SE): 1.60 (0.31) |
62 patients Mean (± SE): 1.44 (0.26) |
P < 0.0001 |
| Righetti 2010 | 24 patients Mean (± SE): 1.6 (0.02) | 24 patients Mean (± SE): 1.51 (0.02) | P < 0.01 |
| Selby 2006a | 12 patients Mean (± SE): 1.37 (0.28) |
12 patients Mean (± SE): 1.38 (0.32) |
P = 0.91 |
| Stefansson 2012 | 20 patients Mean (± SE): 1.51 (0.2) | 20 patients Mean (± SE): 1.47 (0.24) | Not reported |
| Todeschini 2002 | 9 patients Mean (± SE): 1.54 (0.09) | 9 patients Mean (± SE): 1.46 (0.05) | P > 0.05 |
| Tuccillo 2002 | 12 patients Mean (± SD): 1.49 (0.20) | 12 patients Mean (± SD): 1.41 (0.24) | P > 0.05 |
| Urea reduction ratio | |||
| Righetti 2010 | 24 patients Mean (± SE): 73.1 (0.5) | 24 patients Mean (± SE): 70.9 (0.5) | P < 0.01 |
| Predialysis serum B2 microglobulin level (mg/L) | |||
| Kantartzi 2013 | 48 patients Mean (± SE): 31.9 (7.64) | 48 patients Mean (± SE): 47.36 (12.21) | P < 0.01 |
| Pedrini 2011a | 62 patients Mean (± SE): 22.2 (7.8) | 62 patients Mean (± SE): 33.5 (11.8) | P < 0.0001 |
| Righetti 2010 | 24 patients Mean (± SE): 26.0 (0.5) | 24 patients Mean (± SE): 30.9 (0.6) | P < 0.01 |
| Stefansson 2012 | 20 patients Mean (± SE): 23.7 (8.1) | 20 patients Mean (± SE): 34.6 (17) | Not reported |
Change in dialysis modality
The proportion of participants crossing over to another form of dialysis was not significantly different between treatment modalities (Analysis 1.6 (5 studies, 2919 participants): RR 0.87, 955 CI 0.41 to 1.84; I2 = 47%). There was a moderate level heterogeneity between the studies.
1.6. Analysis.
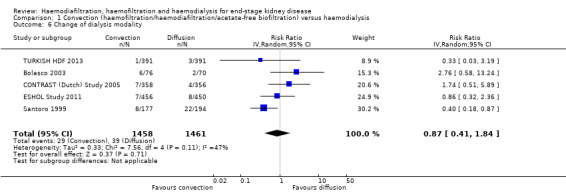
Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 6 Change of dialysis modality.
Blood pressure
ESHOL Study 2011 reported the number of hypotensive events/ person‐years of follow‐up; convective dialysis significantly reduced the rate of hypotension during dialysis (Analysis 1.7 (906 participants): RR 0.72, 95% CI 0.66 to 0.80). In two studies, the percentage of dialysis sessions complicated by hypotension was not significantly different (Analysis 1.8 (2 studies, 42 participants): MD ‐4.05%, 95% CI ‐15.39 to 7.30; I2 = 0%). Seven cross‐over studies reported data on number of patients experiencing hypotension, intradialytic hypotensive events or symptomatic intradialytic hypotensive events and reported uncertain effects of convective therapy (data presented in Analysis 1.17.2; Analysis 1.17.3; Analysis 1.17.4)
1.7. Analysis.
Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 7 Hypotension during dialysis (rate/person‐years follow‐up).
| Hypotension during dialysis (rate/person‐years follow‐up) | ||
|---|---|---|
| Study | Treatment effect | No. of participants |
| ESHOL Study 2011 | In this study which reporting the number of hypotensive events/person‐years follow‐up, convective dialysis reduced the rate of hypotension during dialysis (906 participants: RR 0.72, 95% CI 0.66 to 0.80) | 906 |
1.8. Analysis.
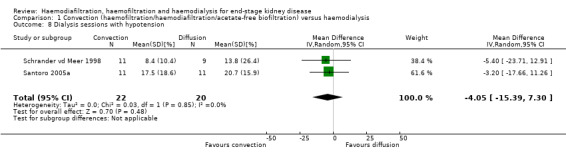
Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 8 Dialysis sessions with hypotension.
There was no significant difference between convective therapy and HD on predialysis systolic (Analysis 1.9.1 (7 studies, 1859 participants): MD 1.19 mm Hg, 95% CI ‐1.46 to 3.84; I2 = 44%) or diastolic blood pressure (Analysis 1.9.2 (6 studies, 1154 participants): MD ‐0.25 mm Hg, 95% CI ‐1.06 to 0.56; I2 = 0%). There was moderate heterogeneity between studies in treatment effects on predialysis systolic blood pressure.
1.9. Analysis.
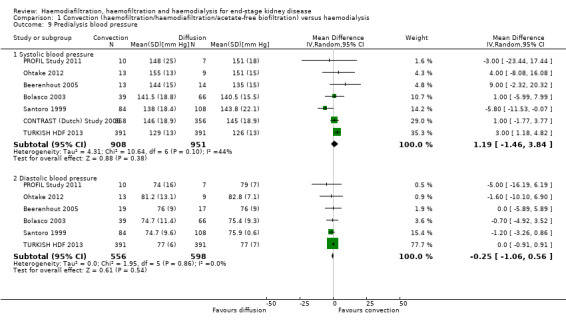
Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 9 Predialysis blood pressure.
Lin 2001 reported that the maximal drop in blood pressure during dialysis was significantly less with convection therapy compared to HD (Analysis 1.10 (1 study, 67 participants): MD ‐28.70 mm Hg, 95% CI ‐38.60 to ‐18.80).
1.10. Analysis.
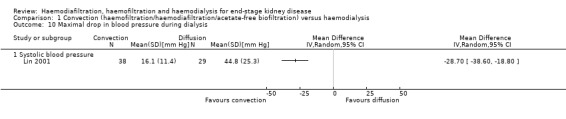
Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 10 Maximal drop in blood pressure during dialysis.
In cross‐over studies convective therapy was not significantly different from HD for predialysis systolic, diastolic, and mean arterial blood pressure (Analysis 1.17,5; Analysis 1.17.6; Analysis 1.17.7). Similarly, in cross‐over studies, convective therapy was not significantly different from HD for postdialysis systolic blood pressure, diastolic blood pressure, fall in systolic blood pressure, and mean arterial blood pressure (Analysis 1.17.8; Analysis 1.17.9; Analysis 1.17.10; Analysis 1.17.11). There was no difference between before‐ and after‐dialysis systolic or diastolic blood pressure in cross‐over studies Analysis 1.17.12; Analysis 1.17.13). Blood pressure during dialysis was lower with convective therapy in one cross‐over study (Selby 2006a; Analysis 1.17.14; Analysis 1.17.15; Analysis 1.17.16).
Quality of life
Data for health‐related outcomes were reported in eight studies (988 participants) (Beerenhout 2005; CONTRAST (Dutch) Study 2005; Kantartzi 2013; Lin 2001; Schiffl 2007; Stefansson 2012; Verzetti 1998; Ward 2000) and described in Table 4. Of these, 50% (four studies) were a parallel group design (Beerenhout 2005; CONTRAST (Dutch) Study 2005; Lin 2001; Ward 2000). In the CONTRAST (Dutch) Study 2005, changes in quality of life scores for all domains assessed were not statistically different between the groups. Lin 2001 reported that participants treated with convective therapy had significantly higher end‐of‐treatment "patient well‐being" scores although results for tolerance and mental alertness were not reported (Analysis 1.11.6 (1 study, 67 patients): MD 0.60, 95% CI 0.30 to 0.90). In the study of Ward 2000, patients in both treatment groups had similar perceptions of quality of life. Patients showed a significant improvement in physical symptoms during the study, irrespective of treatment allocation. There was little or no effect on the quality of life dimensions assessed by the Kidney Diseases Questionnaire including fatigue, depression, relationships, frustration, and between‐dialysis patient well‐being score (Ward 2000; Analysis 1.11). Similarly, in Beerenhout 2005, physical symptoms improved from baseline in the convection group but no direct comparison in scores comparing convection with diffusion treatment was reported for physical scores and other domains (frustration, depression) did not change significantly. The remaining four studies were cross‐over design and disaggregated data for the end of the first phase of treatment were not available.
3. Summary of quality of life findings.
| Study ID | Comparison | Quality of Life scale used | Time of assessment | End of study result | Selective reporting of quality of life dimensions |
| Beerenhout 2005 | HF versus HD | Kidney Disease Questionnaire | Before randomisation, at 6 months and at 1 year | No significant difference in scores in all five components of the scoring system between interventions | Yes |
| CONTRAST (Dutch) Study 2005 | HDF versus HD | Kidney Disease Quality of Life‐Short Form | Median follow‐up of 2 years | There were no significant differences in changes in health‐related quality of life over time between groups (generic or kidney‐disease specific domains) | No |
| Kantartzi 2013 | HDF versus HD | SF‐36 | At 3 months | There were statistical significant differences in QoL for the total SF‐36 (36.1 (26.7 to 45.7) and 40.7 (30.2 to 62.8)), for classic low‐flux HD and high‐flux HDF, for bodily pain (45 (26.9 to 66.9) and 55 (35.6 to 87.5)), and
for role limitations due to emotional functioning (0 (0 to 33.3) and 33.3 (0 to 100)), respectively No data were available for the end of the first phase of treatment |
No |
| Lin 2001 | HDF versus HD | Patient well‐being score | Once weekly for 15 months | Patients on HDF had significantly better scores ((physical well‐being score) MD 0.60, 95% CI 0.30 to 0.90). | No |
| Schiffl 2007 | HDF versus HD | Kidney Disease Questionnaire | after 52 weeks | None of the other dimensions of the KDQ showed a change during the course of the study No data were available for the end of the first phase of treatment |
Yes |
| Stefansson 2012 | HDF versus HD | Physical functioning domain of IQOLA SF‐36 questionnaire | At day 60 | With the exception of a lower score for social functioning with HDF (P < 0.05), there was no significant difference in quality of life between HD and HDF No data were available for the end of the first phase of treatment |
No |
| Verzetti 1998 | AFB | Subjective well‐being | Monthly | Reported well‐being significantly higher in patients receiving AFB in multivariate analysis although unclear whether between‐groups comparison was reported No data were available for the end of the first phase of treatment |
No |
| Ward 2000 | HDF versus HD | Kidney Disease Questionnaire | At 6 months and 1 year | No significant difference in scores in all five components of the scoring system between interventions | No |
AFB ‐ acetate‐free biofiltration; HDF ‐ haemodiafiltration; HD ‐ haemodialysis; HF ‐ haemofiltration; SF‐36 ‐ Short‐Form Health Survey with 36 questions
1.11. Analysis.
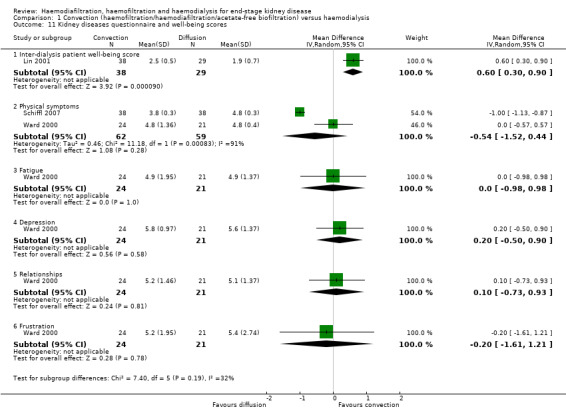
Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 11 Kidney diseases questionnaire and well‐being scores.
Urea clearance and B2 microglobulin
Convective therapy increased Kt/V (Analysis 1.12 (14 studies, 2022 participants): MD 0.07, 95% CI ‐0.00 to 0.14; I2 = 90%) and URR (Analysis 1.13 (3 studies, 879 participants): SMD 0.39, 95% CI 0.06 to 0.72; I2 = 48%). There were moderate to high levels of heterogeneity between studies. Ten cross‐over studies reported heterogeneous treatment effects for convective therapy on predialysis Kt/V (Analysis 1.17.17). A single cross‐over study reported a higher URR with convective therapy (Analysis 1.17.18).
1.12. Analysis.
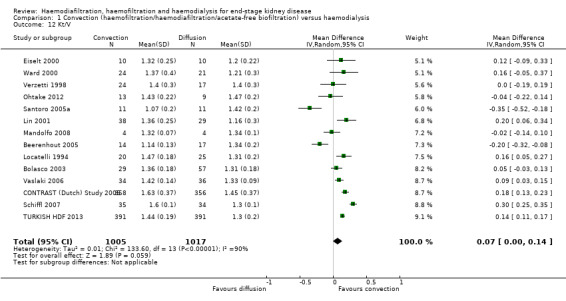
Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 12 Kt/V.
1.13. Analysis.
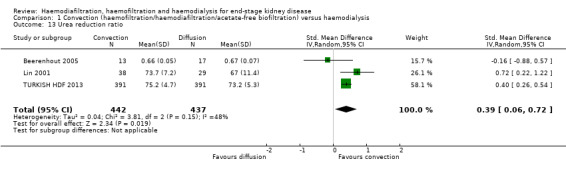
Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 13 Urea reduction ratio.
Convective therapy significantly lowered pre‐dialysis serum B2 microglobulin compared with diffusive therapies (Analysis 1.14 (12 studies, 1813 participants): MD ‐5.55 mg/L, 95% CI ‐9.11 to ‐1.98; I2 = 94%). Heterogeneity was significant across the studies. There was no significant difference between convective therapy and HD on end of treatment B2 microglobulin clearance (Analysis 1.15 (3 studies, 65 participants): MD 13.05 mg/L, 95% CI ‐5.94 to 32.04; I2 = 85%). Heterogeneity was high between the studies. Schiffl 1992 reported data for dialysate B2 microglobulin levels and found significantly higher dialysate B2 microglobulin concentrations in the dialysate with convection therapy (Analysis 1.16 (1 study, 20 patients): MD 133.00 mg/L, 95% CI 71.46 to 194.54). Four cross‐over studies (Kantartzi 2013; Pedrini 2011a; Righetti 2010; Stefansson 2012) reported lower levels of predialysis serum B2 microglobulin levels with convective therapy (Analysis 1.17.19).
1.14. Analysis.
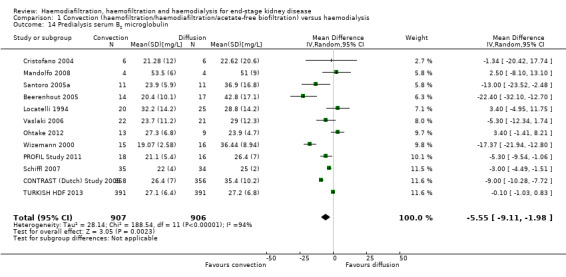
Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 14 Predialysis serum B2 microglobulin.
1.15. Analysis.
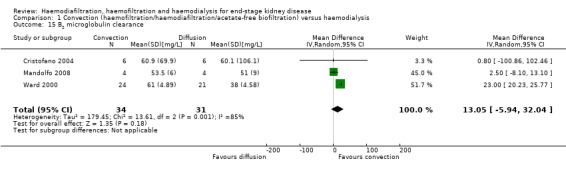
Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 15 B2 microglobulin clearance.
1.16. Analysis.
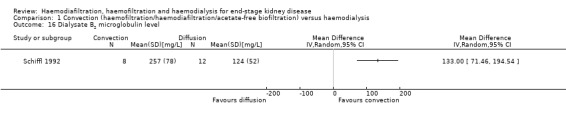
Comparison 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, Outcome 16 Dialysate B2 microglobulin level.
Symptoms (headaches, nausea, vomiting) related to dialysis
Symptoms of headaches, nausea/vomiting were not reported in any of the included studies.
Amyloid‐related complications
Only Lin 2001 reported this outcome with respect to carpal tunnel syndrome. The authors reported that 8/38 participants in the convective treatment group had carpal tunnel syndrome compared with 3/29 patients in the HD group within 13 dialysis sessions after commencing the study. Details of the number of patients in each treatment arm with carpal tunnel syndrome at baseline were not provided. Considering the long duration needed to develop carpal tunnel syndrome, it is probable that at baseline there may have been more participants in the convective modality group with carpal tunnel syndrome. Due to this potential imbalance in participant characteristics, we did not analyse this outcome based on the results of this study.
Convective therapy versus convective therapy: haemofiltration versus haemodiafiltration
All‐cause and cardiovascular mortality
Bolasco 2003 reported no significant difference in all‐cause mortality between HF and HDF (Analysis 2.1 (1 study, 76 participants): RR 2.78, 95% CI 0.57 to 13.44).
2.1. Analysis.
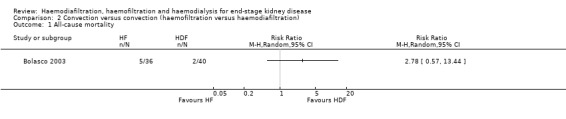
Comparison 2 Convection versus convection (haemofiltration versus haemodiafiltration), Outcome 1 All‐cause mortality.
Cardiovascular mortality was not reported in any of the included studies.
Hospitalisation
No data were available from the parallel RCTs.
Altieri 2004 reported no significant difference between HF and HDF for the number of days spent in hospital (Analysis 2.4.1).
2.4. Analysis.
Comparison 2 Convection versus convection (haemofiltration versus haemodiafiltration), Outcome 4 Data from cross‐over studies.
| Data from cross‐over studies | |||
|---|---|---|---|
| Study | Haemodiafiltraton | Haemofiltration | P value from paper |
| Days spent in hospital | |||
| Altieri 2004 | 30 patients Mean (± SD): 1.3 (4.7) | 30 patients Mean (± SD)L 1.9 (4.9) | Not significant |
| Average number of episodes of hypotension/patient/month | |||
| Altieri 2004 | 30 patients Mean (± SD): 1.1 (1.5) | 30 patients Mean (± SD): 0.5 (0.7) | P = 0.0169 |
| Number of patients experiencing hypotension | |||
| Altieri 2004 | 2/30 | 0/30 | P > 0.05 |
| Predialysis systolic blood pressure (mm Hg) | |||
| Altieri 2004 | 30 patients Mean (± SD): 130.9 (18.5) | 30 patients Mean (± SD): 140.2 (16.2) | P = 0.044 |
| Predialysis diastolic blood pressure (mm Hg) | |||
| Altieri 2004 | 30 patients Mean (± SD): 75.3 (9.7) | 30 patients Mean (± SD): 77.5 (10.4) | P > 0.05 |
| Predialysis mean arterial pressure (mm Hg) | |||
| Altieri 2004 | 30 patients Mean (± SD): 93.8 (11.5) | 30 patients Mean (± SD): 98.4 (10.8) | P > 0.05 |
| Postdialysis systolic blood pressure (mm Hg) | |||
| Altieri 2004 | 30 patients Mean (± SD): 129 (19.8) | 30 patients Mean (± SD): 1135.3 (15.7) | P > 0.05 |
| Postdialysis diastolic blood pressure (mm Hg) | |||
| Altieri 2004 | 30 patients Mean (± SD): 75.3 (9.3) | 30 patients Mean (± SD): 74.5 (7.9) |
P > 0.05 |
| Postdialysis mean arterial blood pressure (mm Hg) | |||
| Altieri 2004 | 30 patients Mean (± SD): 93.2 (11.6) | 30 patients Mean (± SD): 94.8 (9.3) | P > 0.05 |
| Number of patients experiencing hypertension | |||
| Altieri 2004 | 6/30 | 7/30 | P > 0.05 |
| Kt/V | |||
| Altieri 2004 | 30 patients Mean (± SD): 1.3 (0.1) | 30 patients Mean (± SD): 1.2 (0.1) | P < 0.001 |
| Predialysis serum B2 microglobulin (mg/L) | |||
| Altieri 2004 | 30 patients Mean (± SD): 17.8 (5.0) | 30 patients Mean (± SD): 19.3 (6.1) | Not significant |
| B2 microglobulin clearance (mL/min) | |||
| Meert 2009 | 14 patients Mean (± SD): 67.2 (18.5) | 14 patients Mean (± SD): 87.5 (9.6) | P < 0.017 |
Change in dialysis modality
Data for change in dialysis modality comparing HF with HDF were not available.
Blood pressure
No data were available from the parallel RCTs for hypotension.
Altieri 2004 reported no significant difference in the number of patients experiencing hypotension but the rate of hypotension episodes/patient/month was significantly lower with HF compared to HDF (Analysis 2.4.2).
Bolasco 2003 reported no significant difference between HF and HDF for systolic (Analysis 2.2.1 (1 study, 70 participants): MD ‐4.20 mm Hg, 95% CI ‐13.01 to 4.61) and diastolic blood pressure (Analysis 2.2.2 (1 study, 70 participants): MD ‐2.10 mm Hg, 95% CI ‐6.97 to 2.77). Altieri 2004 reported HDF lowered predialysis systolic blood pressure (Analysis 2.4.4) but had no significant effects on predialysis diastolic blood pressure (Analysis 2.4.5) and mean arterial pressure (Analysis 2.4.6). Similarly, Altieri 2004 also reported no significant differences between HF and HDF on postdialysis systolic (Analysis 2.4.7), diastolic (Analysis 2.4.8) and mean arterial pressure (Analysis 2.4.9).
2.2. Analysis.
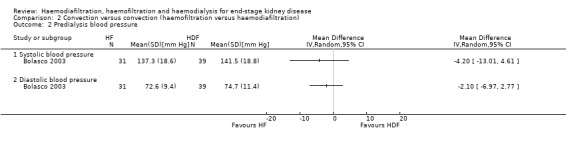
Comparison 2 Convection versus convection (haemofiltration versus haemodiafiltration), Outcome 2 Predialysis blood pressure.
Altieri 2004 reported no significant difference in the number of patients experiencing hypertension between HF and HDF (Analysis 2.4.10).
Quality of life
Data for quality of life comparing HF with HDF were not available from any of the included studies.
Urea clearance and B2 microglobulin
Bolasco 2003 reported a significantly higher Kt/V with HDF compared with HF (Analysis 2.3 (1 study, 58 participants): MD ‐0.24, 95% CI ‐0.33 to ‐0.15). Similarly, Altieri 2004 reported HDF resulted in a higher Kt/V compared to HF (Analysis 2.4.11).
2.3. Analysis.
Comparison 2 Convection versus convection (haemofiltration versus haemodiafiltration), Outcome 3 Kt/V.
No data were available from the parallel RCTs for B2 microglobulin. Altieri 2004 reported no significant difference between HDF and HF on predialysis B2 microglobulin levels (Analysis 2.4.12). Meert 2009 reported HF resulted in higher B2 microglobulin clearance compared to HDF (Analysis 2.4.13).
Symptoms (headaches, nausea, vomiting) related to dialysis
Altieri 2004 reported that patients tended to experience less headache on HF than HDF (P = 0.06) and that there was no significant differences for other intradialytic symptoms between convective therapies.
Convective therapy versus convective therapy: haemodiafiltration versus acetate‐free biofiltration
Data for outcomes comparing HDF with AFB were not reported in any parallel RCTs.
Mortality
Data for mortality comparing HDF with AFB were not reported.
Hospitalisation
Movilli 1996 reported no significant difference between HDF and AFB on number of hospitalisations and length of hospital stay (Analysis 3.1.1; Analysis 3.1.2).
3.1. Analysis.
Comparison 3 Convection versus convection (haemodiafiltration versus acetate‐free biofiltration), Outcome 1 Data from cross‐over studies.
| Data from cross‐over studies | |||
|---|---|---|---|
| Study | Haemodiafiltration | Acid‐free biofiltration | P value from paper |
| Number of hospitalisations/patient during observation period | |||
| Movilli 1996 | 12 patients Mean (± SD): 0.33 (0.71) | 12 patients Mean (± SD): 0.78 (0.93) | Not significant |
| Length of hospitalisation stay/patient (days/patient) | |||
| Movilli 1996 | 12 patients Mean (± SD): 2.70 (5.7) | 12 patients Mean (± SD): 3.60 (5.2) | Not significant |
| Number of dialysis sessions with hypotension | |||
| Coll 2009 | 21 patients 7/545 sessions |
21 patients 46/545 sessions |
"On‐line HDF was associated with fewer hypotensive episodes than treatment with on‐line HDF without acetate (P=0.019)" |
| Movilli 1996 | 12 patients 10/72 sessions | 12 patients 9/72 sessions | Not significant |
| Predialysis systolic blood pressure (mm Hg) | |||
| Ding 2002 | 9 patients Mean (± SD): 142.0 (10.0) | 9 patients Mean (± SD): 142.0 (11.0) | Not significant |
| Predialysis mean arterial pressure (mm Hg) | |||
| Ding 2002 | 9 patients Mean (± SD): 94.0 (16.5) | 9 patients Mean (± SD): 89.2 (17.7) | Not reported |
| Postdialysis systolic blood pressure (mm Hg) | |||
| Ding 2002 | 9 patients Mean (± SD): 141.0 (8.0) | 9 patients Mean (± SD): 141.00 (12.1) | Not significant |
| Interdialysis symptom score | |||
| Ding 2002 | 9 patients Mean (± SD): 1.99 (2.49) | 9 patients Mean (± SD): 2.57 (2.93) | Not significant |
| Kt/V | |||
| Movilli 1996 | 12 patients Mean (± SD): 1.32 (0.12) | 12 patients Mean (± SD): 1.32 (0.13) | Not significant |
| Urea reduction ratio | |||
| Ding 2002 | 9 patients Mean (± SD): 71.0 (7.9) | 9 patients Mean (± SD): 67.0 (6.5) | Not significant |
| Predialysis B2 microglobulin (mg/L) | |||
| Coll 2009 | 21 patients Mean (± SD): 27.7 (7.2) |
21 patients Mean (± SD): 27.4 (6.7) |
Not significant |
| Ding 2002 | 9 patients Mean (± SD): 26.3 (7.9) | 9 patients Mean (± SD): 25.9 (6.3) | Not significant |
| Number of dialysis sessions with side effects (nausea, vomiting, headaches) | |||
| Movilli 1996 | 12 patients 1/72 sessions | 12 patients 1/72 sessions | Not significant |
Change in dialysis modality
Data for change in dialysis modality comparing HDF with AFB were not reported.
Blood pressure
Coll 2009 and Movilli 1996 reported data for this outcome with inconsistent findings between studies. Movilli 1996 reported no significant difference in number of dialysis sessions complicated by hypotension (Analysis 3.1.3), while Coll 2009 reported a significant reduction in hypotensive events in the AFB arm (Analysis 3.1.3).
Ding 2002 reported no significant difference between HDF and AFB on predialysis systolic blood pressure (Analysis 3.1.4) and mean arterial pressure (Analysis 3.1.5) and postdialysis systolic blood pressure (Analysis 3.1.6).
Quality of life
Ding 2002 reported no significant difference between HDF and AFB on interdialysis symptom score (Analysis 3.1.7).
Urea clearance and B2 microglobulin
Movilli 1996 reported no significant difference between HDF and AFB on Kt/V (Analysis 3.1.8). Ding 2002 reported no significant difference between HDF and AFB on URR (Analysis 3.1.9). Ding 2002 and Movilli 1996 both reported no significant difference between HDF and AFB on pre‐dialysis B2 microglobulin values (Analysis 3.1.10).
Symptoms (headaches, nausea, vomiting) related to dialysis
Movilli 1996 reported no significant difference between HDF and AFB on headache, nausea or vomiting (Analysis 3.1.11).
Bias from small‐study effects and sensitivity analysis
For the patient‐level outcomes with sufficient extractable data (all‐cause mortality, cardiovascular mortality and change in dialysis modality), there was no evidence of funnel plot asymmetry (Figure 4; Figure 5; Figure 6) suggestive of bias from small‐study effects.
4.
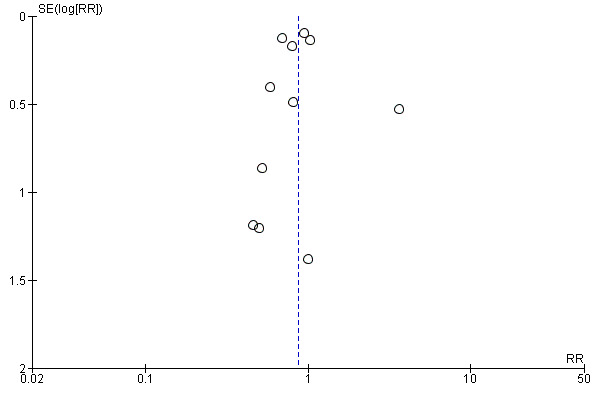
Funnel plot of comparison: 1 Convection (haemofiltration/HDF/acetate‐free biofiltration) versus haemodialysis, outcome: 1.1 All‐cause mortality.
5.
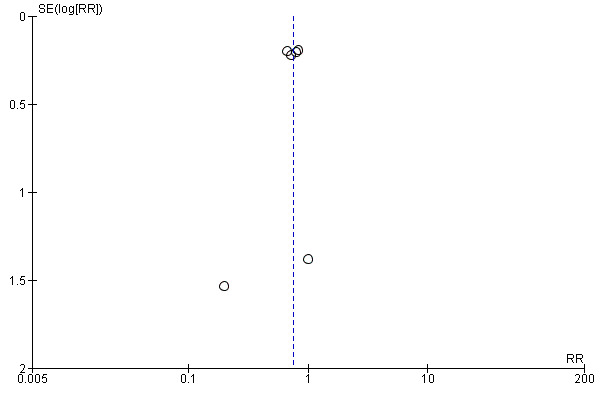
Funnel plot of comparison: 1 Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis, outcome: 1.2 Cardiovascular mortality.
6.
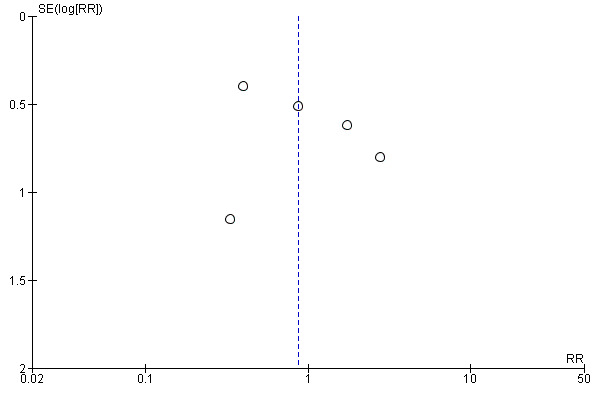
Funnel plot of comparison: 1 Convection (haemofiltration/HDF/acetate‐free biofiltration) versus haemodialysis, outcome: 1.6 Change of dialysis modality.
When analyses of convection versus diffusion modalities were limited to studies comparing HDF with HD, we found very similar treatment effects. Compared to HD, HDF had no significant effect on all‐cause mortality (7 studies, 2837 participants; RR 0.86, 95% CI 0.65 to 1.13) (Bolasco 2003; CONTRAST (Dutch) Study 2005; ESHOL Study 2011; Locatelli 1994; Schiffl 2007; TURKISH HDF 2013; Wizemann 2000) albeit with evidence of moderate heterogeneity (I2= 53%, P = 0.046) HDF reduced cardiovascular mortality (4 studies, 2512 participants; RR 0.72, 95% CI 0.57 to 0.91)(CONTRAST (Dutch) Study 2005; ESHOL Study 2011; Schiffl 2007; TURKISH HDF 2013), but not change in dialysis modality (4 studies, 2512 participants; RR 0.99, 95% CI 0.49 to 2.00) (CONTRAST (Dutch) Study 2005; ESHOL Study 2011; Bolasco 2003; TURKISH HDF 2013). Data for rates of nonfatal cardiovascular events, hospitalisation and hypotension during dialysis were already limited to studies comparing HDF with HD.
Discussion
Summary of main results
Thirty‐five studies in 4039 dialysis patients compared convective dialysis treatment (HDF, HF or AFB) with HD and six studies (174 participants) compared convective therapy directly with another convective therapy. Convective dialysis modalities had little or no effect on all‐cause mortality, may reduce cardiovascular death and hypotension during dialysis, but had uncertain effects on rates of nonfatal cardiovascular events and hospitalisation and serious limitations in study methodology markedly reduced our confidence in any treatment benefits of HDF. There was no difference in risks of changing dialysis modality between convective and diffusive modalities and treatment effects on clearances of urea and B2 microglobulin were markedly inconsistent between studies.
Overall completeness and applicability of evidence
First, while there were statistical reductions in cardiovascular mortality and rates of hypotension with HDF treatment, serious limitations in study methodologies reduced confidence in the conclusions that might be drawn from the available evidence for convective and diffusive dialysis modalities. Allocation concealment, blinding of outcome assessment, complete reporting of patient‐relevant outcomes and inclusion of patients in outcome analyses was inadequate in most studies, and therefore the evidence quality for clinical outcomes was downgraded to low or very low. Data for adverse events and quality of life were insufficient to draw clinically meaningful conclusions. Due to the limitations of existing data, the confidence in estimated effects of convective dialysis is low and the true effects of treatment might be substantially different from those summarized from existing studies.
Second, higher achieved convection volumes for HDF were associated with lower mortality in post‐hoc non‐randomised analyses within CONTRAST (Dutch) Study 2005, TURKISH HDF 2013, and ESHOL Study 2011. However, caution is needed before concluding this is evidence for the need to deliver high convection volumes to reduce all‐cause mortality with HDF. Convection volumes were not randomised treatment targets in any study (in other words, patients at randomisation were not equally likely to be assigned to specific convection volume targets) and as such the relationship observed between convection volume and mortality is also likely to be strongly confounded in important ways. Participants with insufficient vascular access and those with lower achieved blood flows (both known to be linked to poorer outcomes) would have been less likely to achieve higher convection volumes and those with longer treatment times would have been more likely to achieve convection targets. It is known that these specific patient and treatment characteristics independently predict mortality and may, as such, offer more viable and alternative explanations for the association between convection volume and risk of death.
Third, there was considerable heterogeneity in the dialysis interventions across studies including differences in flux, vascular access requirements, blood flows, and treatment times as well as convection volumes, resulting in uncertainty about which specific aspects of the convection process might be responsible for lower cardiovascular mortality. Future standardisation of therapy patterns might improve our understanding of the features of convective dialysis care that might lead to improved outcomes and patient acceptability.
Fourth, data for adverse events were sparse and no studies evaluated serious adverse events according to international definitions. Understanding hazards of treatment are essential to balancing desirable and undesirable outcomes when considering new therapies and questions about potential harms from convection therapy cannot be answered with confidence using the existing randomised study evidence.
Finally, the generalisability of the findings from RCTs to wider dialysis populations, including those in regions other than Europe might be limited. The absolute treatment benefits of convection on cardiovascular mortality might not be applicable to many dialysis patients, as data were frequently limited to participants who had vascular access sufficient to sustain high convection volumes.
Potential biases in the review process
The strengths of this review include the searching of conference proceedings and inclusions of additional unpublished data to provide a comprehensive analysis of all available evidence and the rigorous assessment of quality which has been incorporated into review conclusions using the methods of the GRADE guidelines. The limitations in this review are largely due to the reporting adequacy of the primary included studies.
Agreements and disagreements with other studies or reviews
This is an update of a review reported in 2006, which found insufficient evidence to provide robust recommendations for convective therapy to improve clinical outcomes for men and women who have ESKD (Rabindranath 2006). Despite the addition of 20 new studies including 3483 participants between 2006 and 2015, evidence for the benefits and harms of convective dialysis therapy remains low or very low quality.
The European Best Practice Guidelines on Haemodialysis (EBPG 2007) suggest on‐line HDF or HF should be considered to delay long‐term complications of dialysis therapy and that exchange volumes should be as high as possible. Current utilisation of HDF varies widely. Prevalence of HDF ranges between 0.3 and 232 per million of population within countries and regions of Europe (ERA‐EDTA Registry 2010) and the proportion of HDF as the treatment modality for patients in Australasia ranges between 0.8% and 23% (ANZDATA 2012). This review suggests the benefits of HDF or HF from contemporaneous RCTs are limited to reducing cardiovascular mortality and hypotension during dialysis and that evidence limitations lead to low confidence in these treatment benefits. None of the available studies randomised participants treated with HDF to different targeted convection volumes and accordingly robust evidence supportive of higher convection volumes to improve dialysis outcomes is not available.
This review draws similar conclusions to a recent systematic review and meta‐analysis that compared convective dialysis therapies (including high‐flux HD, HF, or HDF) with low‐flux HD alone (Susantitaphong 2013). In that review which included 36 parallel arm studies showed that convective therapies had little or no effect on all‐cause mortality (RR 0.88, 95% CI 0.76 to 1.02), and reduced risks of cardiovascular mortality (RR 0.84, 95% 0.71 to 0.98) and hypotension (RR 0.55, 95% CI 0.35 to 0.87). Unlike the present review, the quality of the available evidence was not incorporated into the review conclusions.
This review diverges from the conclusions of the largest HDF study to date (ESHOL Study 2011). This review finds no treatment effects of HDF in mortality, compared to a 30% relative reduction in risk of death from any causes during 36 months of follow‐up in the ESHOL Study 2011. We suggest that the consistent findings across all studies in the meta‐analysis for mortality in this review (variation in the estimates was not beyond that expected by chance) indicate the overall summary estimate is robust even accounting for the findings of the ESHOL Study 2011. Notably in that study, patients were withdrawn and not included in analyses if they did not receive the allocated treatment modality for two months or more, including those who did not achieve the minimum requested replacement volume (18L/session). As it is probable these participants might have had poorer outcomes (such as poorer vascular access due to other comorbidities including vascular disease or diabetes) and may have been preferentially excluded from follow‐up, the ESHOL Study 2011 might unreliably estimate mortality risk. It would be informative to re‐evaluate mortality data from ESHOL Study 2011 using intention‐to‐treat analyses that include mortality outcome data for all randomised patients according to their original treatment assignment.
Authors' conclusions
Implications for practice.
While there is increasing but variable uptake of HDF in Europe, the current body of evidence provides low or very‐low quality evidence suggesting confidence in estimated treatment effects of convective dialysis therapies is limited or very limited. Convective dialysis may reduce cardiovascular events or rates of hypotension during dialysis but effects on mortality, nonfatal cardiovascular events and hospitalisation in addition to quality of life and adverse events are inconclusive and the overall confidence in these results is low or very low. Until there are additional robust studies, widespread uptake of convective therapies including HDF is not warranted and targeting higher convection volumes to improve outcomes is not supported by high‐quality evidence.
Implications for research.
A significant number of studies have now been conducted to assess the effectiveness of convective dialysis therapies to improve clinical outcomes in people treated with HD. However, given the methodological limitations in the available studies, additional studies that are powered to evaluate treatment effects of HDF on mortality, major cardiovascular events, and adverse treatment effects are needed before widespread uptake of HDF is warranted. In addition, given the hypothesis that higher convection volumes might improve clinical outcomes from convective therapy, future studies targeting specific convection volumes and using intention‐to‐treat analysis that show benefit for clinical outcomes are required before higher convection volumes can be recommended.
What's new
| Date | Event | Description |
|---|---|---|
| 18 February 2015 | New search has been performed | Updated incorporating data |
| 18 February 2015 | New citation required and conclusions have changed | Review update ‐ 20 new studies added |
| 22 November 2011 | Amended | Search strategies & search methods revised |
Acknowledgements
2006 review
The 2006 version of this review was funded by the National Kidney Research Fund (UK). We thank Drs C Basile, J Eiselt, LW Henderson, W Lornoy, E Moville, M Noris, H Schiffl, and V Wizemann for supplying data relating to their studies on request. Dr Strippoli was part‐funded through a University of Sydney School of Public Health, non‐established PhD scholarship.
2015 review
Drs Rabindranath, Daly, Roderick, Wallace and MacLeod were authors on the first version of this review. We thank Drs Alvestrand (PROFIL Study 2011), Asci (TURKISH HDF 2013), Coll (Coll 2009), Grooteman (CONTRAST (Dutch) Study 2005), Locatelli (Bolasco 2003), Mancini (Santoro 2005a), Mandolfo (Mandolfo 2008), Mostovaya (CONTRAST (Dutch) Study 2005), Ok (TURKISH HDF 2013), Pedrini (Pedrini 2011a), Righetti (Righetti 2010), Santoro (Santoro 2005a; Santoro 1999), Selby (Selby 2006a), Stefansson (Stefansson 2012), Tessitore (Santoro 1999), Vaslaki (Vaslaki 2006),and Vernaglione (Cristofano 2004) for supplying data for their studies on request.
Dr Nistor is a fellow of the Methods Support Team of European Renal Best Practice (ERBP), supported by a grant of the European Renal Association‐European Dialysis Transplantation Association (ERA‐EDTA).
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms used |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Convection (haemofiltration/haemodiafiltration/acetate‐free biofiltration) versus haemodialysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 11 | 3396 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.72, 1.05] |
| 2 Cardiovascular mortality | 6 | 2889 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.61, 0.92] |
| 3 Nonfatal cardiovascular event (rate/person‐years follow‐up) | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 4 Hospitalisation | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Hospital admissions/year | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.07, 0.47] |
| 4.2 Days spent in hospital | 2 | 67 | Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐7.47, 5.03] |
| 5 Hospitalisation (rate/person‐years follow‐up) | 2 | 400 | Risk Ratio (IV, Random, 95% CI) | 1.23 [0.93, 1.63] |
| 6 Change of dialysis modality | 5 | 2919 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.41, 1.84] |
| 7 Hypotension during dialysis (rate/person‐years follow‐up) | Other data | No numeric data | ||
| 8 Dialysis sessions with hypotension | 2 | 42 | Mean Difference (IV, Random, 95% CI) | ‐4.05 [‐15.39, 7.30] |
| 9 Predialysis blood pressure | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Systolic blood pressure | 7 | 1859 | Mean Difference (IV, Random, 95% CI) | 1.19 [‐1.46, 3.84] |
| 9.2 Diastolic blood pressure | 6 | 1154 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐1.06, 0.56] |
| 10 Maximal drop in blood pressure during dialysis | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1 Systolic blood pressure | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Kidney diseases questionnaire and well‐being scores | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 Inter‐dialysis patient well‐being score | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.60 [0.30, 0.90] |
| 11.2 Physical symptoms | 2 | 121 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.52, 0.44] |
| 11.3 Fatigue | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.98, 0.98] |
| 11.4 Depression | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.50, 0.90] |
| 11.5 Relationships | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.73, 0.93] |
| 11.6 Frustration | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.61, 1.21] |
| 12 Kt/V | 14 | 2022 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.00, 0.14] |
| 13 Urea reduction ratio | 3 | 879 | Std. Mean Difference (IV, Random, 95% CI) | 0.39 [0.06, 0.72] |
| 14 Predialysis serum B2 microglobulin | 12 | 1813 | Mean Difference (IV, Random, 95% CI) | ‐5.55 [‐9.11, ‐1.98] |
| 15 B2 microglobulin clearance | 3 | 65 | Mean Difference (IV, Random, 95% CI) | 13.05 [‐5.94, 32.04] |
| 16 Dialysate B2 microglobulin level | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 17 Data from cross‐over studies | Other data | No numeric data | ||
| 17.1 Hospitalisation | Other data | No numeric data | ||
| 17.2 Patients experiencing hypotension | Other data | No numeric data | ||
| 17.3 Intradialytic hypotensive events | Other data | No numeric data | ||
| 17.4 Symptomatic intradialytic hypotensive events | Other data | No numeric data | ||
| 17.5 Predialysis systolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 17.6 Predialysis diastolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 17.7 Predialysis mean arterial pressure (mm Hg) | Other data | No numeric data | ||
| 17.8 Postdialysis systolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 17.9 Postdialysis diastolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 17.10 Postdialysis fall in systolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 17.11 Postdialysis mean arterial pressure (mm Hg) | Other data | No numeric data | ||
| 17.12 Difference between pre‐ and postdialysis systolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 17.13 Difference between pre‐ and postdialysis diastolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 17.14 Intradialysis mean systolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 17.15 Intradialysis mean diastolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 17.16 Intradialysis mean arterial pressure (mm Hg) | Other data | No numeric data | ||
| 17.17 Kt/V | Other data | No numeric data | ||
| 17.18 Urea reduction ratio | Other data | No numeric data | ||
| 17.19 Predialysis serum B2 microglobulin level (mg/L) | Other data | No numeric data |
Comparison 2. Convection versus convection (haemofiltration versus haemodiafiltration).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Predialysis blood pressure | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Systolic blood pressure | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Diastolic blood pressure | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Kt/V | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Data from cross‐over studies | Other data | No numeric data | ||
| 4.1 Days spent in hospital | Other data | No numeric data | ||
| 4.2 Average number of episodes of hypotension/patient/month | Other data | No numeric data | ||
| 4.3 Number of patients experiencing hypotension | Other data | No numeric data | ||
| 4.4 Predialysis systolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 4.5 Predialysis diastolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 4.6 Predialysis mean arterial pressure (mm Hg) | Other data | No numeric data | ||
| 4.7 Postdialysis systolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 4.8 Postdialysis diastolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 4.9 Postdialysis mean arterial blood pressure (mm Hg) | Other data | No numeric data | ||
| 4.10 Number of patients experiencing hypertension | Other data | No numeric data | ||
| 4.11 Kt/V | Other data | No numeric data | ||
| 4.12 Predialysis serum B2 microglobulin (mg/L) | Other data | No numeric data | ||
| 4.13 B2 microglobulin clearance (mL/min) | Other data | No numeric data |
Comparison 3. Convection versus convection (haemodiafiltration versus acetate‐free biofiltration).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Data from cross‐over studies | Other data | No numeric data | ||
| 1.1 Number of hospitalisations/patient during observation period | Other data | No numeric data | ||
| 1.2 Length of hospitalisation stay/patient (days/patient) | Other data | No numeric data | ||
| 1.3 Number of dialysis sessions with hypotension | Other data | No numeric data | ||
| 1.4 Predialysis systolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 1.5 Predialysis mean arterial pressure (mm Hg) | Other data | No numeric data | ||
| 1.6 Postdialysis systolic blood pressure (mm Hg) | Other data | No numeric data | ||
| 1.7 Interdialysis symptom score | Other data | No numeric data | ||
| 1.8 Kt/V | Other data | No numeric data | ||
| 1.9 Urea reduction ratio | Other data | No numeric data | ||
| 1.10 Predialysis B2 microglobulin (mg/L) | Other data | No numeric data | ||
| 1.11 Number of dialysis sessions with side effects (nausea, vomiting, headaches) | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Altieri 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Total substitution volume: 60 L/patient/session Treatment duration: 12 months |
|
| Outcomes | Intradialytic problems
Interdialytic problems
Other
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated; probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not stated; probably not done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not using intention‐to‐treat analysis; loss to follow‐up 6/39 (23%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol of the study available |
| Other bias | High risk | Carry over effect might be present because of the cross‐over design; data at the end of first phase of treatment not available; commercial sponsor listed as author |
Bammens 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Both treatment groups
Control group
Co‐interventions: NS |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated; probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not stated; probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data about drop‐outs provided after different cross‐over phases; lost to follow‐up: 0/14 |
| Selective reporting (reporting bias) | High risk | Data at the end of first phase of treatment not available |
| Other bias | High risk | Carryover effect present because of the cross‐over design; data not extractable for meta‐analysis |
Basile 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: NS |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated; probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated; probably not done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 33% loss to follow‐up (5/15 patients whose Hb dropped at monthly checks were withdrawn from the study) |
| Selective reporting (reporting bias) | High risk | Data at the end of first phase of treatment not available |
| Other bias | High risk | Carry over effect present because of the cross‐over design; data not available for meta‐analysis |
Beerenhout 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Centrally and envelopes were used |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing outcome data balanced across groups with similar reasons for missing data across groups but with 13/40 patients (32%) not included in the final analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | Imbalanced ratio men/women between groups; interventions not matched; funded by industry |
Bolasco 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer generated: " randomisation list that was stratified by centre and prepared in advance by one author" |
| Allocation concealment (selection bias) | Unclear risk | Patients were centrally randomised using an email assignment from one of the authors |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10/146 (14%) withdrew from study due to transfer to another technique, thrombosis or vascular access infection, withdrawal of consent, transfer to another centre, transfer to another study, infection) |
| Selective reporting (reporting bias) | High risk | Key patient relevant outcomes not reported |
| Other bias | High risk | Interventions and patient characteristics not matched; industry support provided |
Coll 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information, the method of concealment is not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "no difference for dialysis tolerance between the groups"; no intention‐to‐treat analysis; lost to follow‐up (9; no clear description of drop‐outs, reasons or belonging) |
| Selective reporting (reporting bias) | High risk | Not all of the study's pre‐specified primary outcomes have been reported; data at the end of first phase of treatment not available |
| Other bias | High risk | Important difference between selected and analysed patients (e.g. dialysis vintage 249 months in the selected initial group versus 164 months in the analysed group); carry over effect present because of the cross‐over design |
CONTRAST (Dutch) Study 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally by blocks |
| Allocation concealment (selection bias) | Unclear risk | Centrally |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not done: "Because of the nature of the intervention, it was not possible to blind the patients, the local study nurses, or the investigators to the treatment assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adjudication committee unaware of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All the results are available |
| Selective reporting (reporting bias) | Low risk | All important outcomes are reported |
| Other bias | High risk | Interventions not matched between treatment groups; early termination due to futility; funded by industry |
Cristofano 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | The allocation was made by means of a computer generated sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated. probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not stated, probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not sufficiently detailed |
| Selective reporting (reporting bias) | High risk | Insufficient information |
| Other bias | High risk | Abstract‐only publication |
Ding 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: not stated |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "A second patient refused to finish post‐HDF and insisted that AFB be tried because of his unbearable shoulder" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No intention‐to‐treat analysis; 3 patients violated the study protocol (one patient's fistula failed during the second month of pre‐HDF; one refused to finish post‐HDF; one patient had severe headache accompanied by poorly controlled hypertension at the end of pre‐HDF shift and dropped out of the study before starting post‐HDF modality) |
| Selective reporting (reporting bias) | High risk | Data for end of first phase of treatment not available |
| Other bias | High risk | Carry over effect present because of the cross‐over design |
Eiselt 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomised according to the cause of their underlying CKD |
| Allocation concealment (selection bias) | High risk | Inadequate: names were drawn from individual subgroups at the following ratios 1:1 |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient data; no loss to follow‐up |
| Selective reporting (reporting bias) | High risk | Not all patient important outcomes reported |
| Other bias | High risk | Interventions and baseline patient characteristics not matched |
ESHOL Study 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A central computerised random‐generator |
| Allocation concealment (selection bias) | Unclear risk | Centrally |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 355/906 discontinued the study, 39% from the total number of included patients, 41% in the HDF arm |
| Selective reporting (reporting bias) | Low risk | All the prespecified outcomes were reported |
| Other bias | High risk | Commercial sponsor on authorship or involved in data management; interventions and baseline patient characteristics not matched |
Fox 1993.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: not stated |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "by coin toss", an insecure method |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient data; no loss to follow‐up |
| Selective reporting (reporting bias) | High risk | Data for end of first phase of treatment not available, no protocol of the study available |
| Other bias | High risk | Carry over effect present because of the cross‐over design; data not extractable for meta‐analysis |
Kantartzi 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up 2/24; no clear description of drop‐outs or reasons |
| Selective reporting (reporting bias) | High risk | Data at the end of first phase of treatment not available |
| Other bias | High risk | Carry over effect due to cross‐over design, interventions not matched |
Karamperis 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The treatment modality was blinded to the patient by use of filter types unknown to the patients and not ordinarily used in the department. The tubing was mounted as to haemodiafiltration in all sessions, and the indicators showing the treatment modality on the console were covered." Investigators not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated, probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clearly stated that all patients that performed one dialysis have done the second dialysis session as well |
| Selective reporting (reporting bias) | High risk | Data for end of first phase of treatment not available |
| Other bias | High risk | Study conducted in two consecutive dialysis sessions, "wash out effect" insufficient, carry over effect might be present because of the cross‐over design; data not extractable for meta‐analysis; interventions not matched |
Lin 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: not stated |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | High risk | Inadequate: "We used partially randomised patient preference (PRPP) design by incorporating patient preferences into this randomised trials" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated, insufficient information |
| Selective reporting (reporting bias) | High risk | Outcome/s of interest reported incompletely and cannot be used in meta‐analysis; protocol of the study unavailable; failure to report a key/expected outcome (mortality, major CV events) |
| Other bias | Low risk | Not additional risks apparent |
Locatelli 1994.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Treatment group 4
Co‐interventions: not stated |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate: "Randomization was centralized at the Department of Nephrology at Lecco Hospital, using separate lists for each Center that were randomly divided into blocks of four for the assignment of two or four treatments (depending on the treatments available in the different Centers)." |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 108/205 analysed (46%) (34% due to technical reasons, acute clinical reason, fistula‐related reason, treatment inadequacy) |
| Selective reporting (reporting bias) | High risk | Key outcomes not reported |
| Other bias | High risk | Interventions not matched and patient characteristics not matched at baseline; interventions and patient characteristics not matched; commercial sponsor involved in the conduct of this study |
Lornoy 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Co‐interventions: not stated |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 0/8 lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Insufficient information; protocol of the study not available; only data about B2 microglobulin were available |
| Other bias | High risk | Carry over effect present because of the cross‐over design; data not extractable for meta‐analysis |
Mandolfo 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Centrally randomised |
| Allocation concealment (selection bias) | Unclear risk | Centrally randomised |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | High risk | Key patient outcomes not provided |
| Other bias | High risk | Carry over effect present because of the cross‐over design; commercial sponsor involved in authorship or data management |
Meert 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group 1
Control group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Insufficient information. probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Insufficient information. probably not done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing outcome data; loss to follow‐up 3/17 (18%) (transplantation (2); lack of compliance (1)) |
| Selective reporting (reporting bias) | High risk | Data for end of first phase of treatment not available |
| Other bias | High risk | Carry over effect present because of the cross‐over design; in the reported results is included a comparison between random and non‐randomly allocated groups; commercial sponsor involved in authorship or data management |
Movilli 1996.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: not stated |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3/12 patients died |
| Selective reporting (reporting bias) | High risk | Data for end of first phase of treatment not available |
| Other bias | High risk | Carry over effect present because of the cross‐over design |
Noris 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: not stated |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing outcome data balanced across groups; 0/5 lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Data for end of first phase of treatment not available; no protocol of the study available |
| Other bias | High risk | Carry over effect present because of the cross‐over design; data not extractable for meta‐analysis and interventions not matched |
Ohtake 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear; insufficient information provided about losses to follow‐up |
| Selective reporting (reporting bias) | High risk | Data about mortality events were missing |
| Other bias | Low risk | No additional risks identified |
Pedrini 2011a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised by central telephone into a 1:1 ratio" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not performed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not performed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10% lost for follow‐up, 4% due to access failures (unclear whether imbalanced between groups) |
| Selective reporting (reporting bias) | High risk | Data for end of first phase of treatment not available |
| Other bias | High risk | Carry over effect present because of the cross‐over design; commercial sponsorship on authorship or data management; interventions not matched |
PROFIL Study 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "patients were randomised to treatment with either HF or HD using an online computer‐based program stratified by age and diabetes " |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Echocardiograms read by an observer blinded to treatment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing outcome data balanced across groups but attrition is 29%; randomised (48), analysed (34), finished the 24 months follow‐up (17, 35%) |
| Selective reporting (reporting bias) | High risk | Study protocol available (ISRCTN83264534) and all pre‐specified outcomes have been reported. All key patient outcomes not provided |
| Other bias | High risk | Commercial sponsor on authorship and/or involved in data management, interventions not matched |
Righetti 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally: "An independent person performed randomisation for the sequence of treatment. " |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced across groups but no intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | Data for end of first phase of treatment not available; insufficient information, no study protocol available |
| Other bias | High risk | Carry over effect present because of the cross‐over design; interventions not matched |
Santoro 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was done centrally (with a computerized random‐number generator) using the balanced block randomisation technique with a 1:1 ratio, stratification according to the clinical centre concerned and a block size of eight" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated, probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated, probably not done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 21% loss to follow‐up and dropouts censored at time of termination |
| Selective reporting (reporting bias) | High risk | Outcomes of interest are not reported |
| Other bias | High risk | Interventions not matched and patient baseline characteristics not matched |
Santoro 2005a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were centrally randomised, 1:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "There was no blinding of participants, investigators, or outcome assessors." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "There was no blinding of participants, investigators, or outcome assessors." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20% withdrew from study due to personal reasons, transferred to another centre to centre not able to offer treatment (HF); imbalance between arms |
| Selective reporting (reporting bias) | High risk | Study protocol unavailable; outcomes of interest reported incompletely |
| Other bias | High risk | Baseline patient characteristics not matched |
Schiffl 1992.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: not stated |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | High risk | Limited amount of data reported for a 2 year study |
| Other bias | High risk | Data not extractable for data analysis, intervention not matched |
Schiffl 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "by coin flip", an insecure method |
| Allocation concealment (selection bias) | High risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Unblinded' |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Unblinded' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3% loss to follow‐up due to move away from dialysis centre; similar in both groups |
| Selective reporting (reporting bias) | High risk | Outcomes of interest not reported |
| Other bias | Low risk | Carry over effect present because of the cross‐over design but we included in our analysis only the data available about the first phase of the study |
Schrander vd Meer 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: not stated |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Each pair of patients was randomised to either AFB or HD |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13% lost to follow‐up due to allergy, refusal or side‐effects. Unclear whether imbalance between groups |
| Selective reporting (reporting bias) | High risk | Not all the expected outcomes were reported |
| Other bias | Low risk | No additional risks identified |
Selby 2006a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Probably not done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Insufficient reporting |
| Selective reporting (reporting bias) | High risk | Study protocol unavailable and data for end of first phase of treatment not available |
| Other bias | High risk | Patients included were selected using two different inclusion criteria (prone to hypotension or stable patients) with no clear description of the initial number of analysed number. Carry over effect present because of the cross‐over design; data not extractable for meta‐analysis and interventions not matched |
Stefansson 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The study was patient‐blinded and partially investigator‐blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The interviewers did not know which treatment was performed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20% loss to follow‐up (pain, intracerebral bleeding, patient request, and dialysis access problems) |
| Selective reporting (reporting bias) | High risk | Study protocol unavailable; outcomes of interest not all reported |
| Other bias | High risk | Carry over effect present because of the cross‐over design; interventions not matched |
Teo 1987.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: not stated |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 15% loss to follow‐up (declined by patient and personal reasons) |
| Selective reporting (reporting bias) | High risk | Study protocol unavailable but no data available to be included |
| Other bias | High risk | Carry over effect present because of the cross‐over design; data not extractable for meta‐analysis, interventions not matched |
Todeschini 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: not stated |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear; no loss to follow‐up |
| Selective reporting (reporting bias) | High risk | Data for end of first phase of treatment not available |
| Other bias | High risk | Carry over effect present because of the cross‐over design; data not extractable for meta‐analysis |
Tuccillo 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: not stated |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding but review authors judge that outcome measurement not likely influenced |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Short duration of study, < 10% attrition |
| Selective reporting (reporting bias) | High risk | Data for end of first phase of treatment not available |
| Other bias | High risk | Carry over effect present because of the cross‐over design; data not extractable for meta‐analysis; abstract only publication |
TURKISH HDF 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20% loss to follow‐up plus imbalance in loss to follow‐up due to vascular access problems |
| Selective reporting (reporting bias) | Low risk | Study protocol available and all patient important outcomes were reported |
| Other bias | High risk | Commercial sponsorship of study |
Vaslaki 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A random code was used, with a separate list for each study centre |
| Allocation concealment (selection bias) | Unclear risk | Centrally performed by an independent institute |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Open" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Open" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 46% lost and dropped out patients "not replaced"; lost to follow‐up: 20 drop‐outs (4 died, 11 were transplanted and other reasons for 5) and 49 withdrawn patients |
| Selective reporting (reporting bias) | High risk | Insufficient information about patient important outcomes |
| Other bias | High risk | Commercial sponsor involved in authorship and data management; interventions not matched |
Verzetti 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: not stated |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20% loss to follow‐up |
| Selective reporting (reporting bias) | High risk | Outcome/s of interest reported incompletely, cannot be used in meta‐analysis; "Intradialysis status P = 0.003"; study protocol unavailable |
| Other bias | High risk | Carry over effect present because of the cross‐over design; data not extractable for meta‐analysis and interventions not matched |
Ward 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: not stated |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Each pair of patients was randomised to either AFB or HD |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 22% patients lost‐to‐follow‐up |
| Selective reporting (reporting bias) | High risk | All outcomes of interest not reported |
| Other bias | High risk | Patient baseline characteristics not matched |
Wizemann 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: not stated |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 18% loss to follow‐up |
| Selective reporting (reporting bias) | High risk | Study protocol unavailable and outcomes of interest reported incompletely, cannot be used in meta‐analysis (e.g. BP) |
| Other bias | High risk | Sponsor involved in authorship and/or data management; interventions not matched |
ACEi ‐ angiotensin‐converting enzyme inhibitor; AFB ‐ acetate‐free biofiltration; BD ‐ conventional bicarbonate dialysis; BP ‐ blood pressure; CKD ‐ chronic kidney disease; CrCl ‐ creatinine clearance; CV ‐ cardiovascular; EPO ‐ erythropoietin; ESKD ‐ end‐stage kidney disease; Hb ‐ haemoglobin; HCT ‐ haematocrit; HD ‐ haemodialysis; HF ‐ haemofiltration; HDF ‐ haemodiafiltration; HTN ‐ hypertension; LVMi ‐ left ventricular mass index; MAP ‐ mean arterial pressure; MI ‐ myocardial infarction; QB ‐ blood flow rate; QD ‐ dialysate flow rate; QoL ‐ quality of life; rHuEPO: recombinant human EPO; RRT ‐ renal replacement therapy; TIA ‐ transient Ischaemic attack; URR ‐ urea reduction ratio
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adam 1996 | Population included not relevant to this review (patients with acute kidney injury |
| Ahrenholz 1997 | Not RCT |
| Ahrenholz 1998 | Not RCT |
| Ahrenholz 2004 | Not RCT; interventions not relevant to this review |
| Altieri 1997 | Not RCT |
| Altieri 1999 | Not RCT |
| Altieri 2000 | Not RCT |
| Altieri 2001 | Not RCT |
| Baldamus 1980 | Not RCT |
| Baldamus 1982 | Not RCT |
| Baldamus 1985 | Not RCT |
| Baragett 2003 | Not RCT |
| Basile 1985 | Not RCT |
| Basile 1985a | Not RCT |
| Basile 1987 | Interventions not relevant to this review |
| Basile 1988 | Outcomes not relevant to this review |
| Bazzatto 1988 | Outcomes not relevant to this review |
| Beerenhout 2002 | Not RCT |
| Bolasco 2000 | Not RCT |
| Bonaudo 1998 | Interventions not relevant to this review |
| Bonomini 2004 | Outcomes not relevant to this review |
| Bordin 2002 | Not RCT |
| Bosc 1997 | Interventions not relevant to this review |
| Bosc 1998 | Interventions not relevant to this review |
| Boscticardo 1981 | Not RCT |
| Brink 1995 | Not RCT |
| Calo 2007 | Outcomes not relevant to this review |
| Canaud 1994 | Not RCT |
| Canaud 2000 | Not RCT |
| Canaud 2001 | Not RCT |
| Cappelli 1985 | Not RCT |
| Carozzi 1992 | Not RCT |
| Cavalcanti 2004 | Not an RCT |
| Cerulli 2000 | Outcomes not relevant to this review |
| Champagne 2008 | Outcomes not relevant to this review |
| Chanard 1988 | Not RCT |
| Chang 1979 | Not RCT |
| Chauveau 1993 | Outcomes not relevant to this review |
| Chen 2005c | Not RCT |
| Chiappini 2004 | Interventions not relevant to this review |
| Cirillo 2011 | Interventions not relevant to this review |
| Collins 1985 | Not RCT |
| David 2000 | Not RCT |
| Duranti 2004 | Not RCT |
| Feliciani 2007 | Interventions not relevant to this review |
| Fu 2006 | Not RCT |
| Gerdemann 2002 | Interventions not relevant to this review |
| Giannattasio 2006 | Interventions not relevant to this review |
| Harzallah 2008 | Interventions not relevant to this review |
| Hdez‐Jaras 1994 | Outcomes not relevant to review |
| Henderson 1980 | Not RCT |
| Higuchi 2004 | Outcomes not relevant to review |
| Hillion 1997 | Interventions not relevant to this review |
| Hmida 2002 | Not RCT |
| Ikebe 2006 | Interventions not relevant to this review |
| Jahn 1981 | Not RCT |
| Jose 2008 | Outcomes not relevant to this review |
| Joyeux 2009 | Outcomes not relevant to this review |
| Kanter 2008 | Interventions not relevant to this review |
| Katschnig 1980 | Not RCT |
| Kim 2009 | Interventions not relevant to this review |
| Kishimoto 1980 | Not RCT |
| Klemm 1997 | Not RCT |
| Klingel 2004 | Outcomes not relevant to review |
| Krieter 2005 | Interventions not relevant to this review |
| Krieter 2008a | Interventions not relevant to this review |
| Krieter 2010 | Interventions not relevant to this review |
| Kuno 1994 | Not RCT |
| Leber 1980 | Not RCT |
| Li 1997 | Interventions not relevant to this review |
| Lin 2003a | Outcomes not relevant to this review (serum AGE level reduction rates) |
| Liomin 1984 | Not RCT |
| Locatelli 1998 | Not RCT |
| Locatelli 1999 | Not RCT |
| Locatelli 2001 | Not RCT |
| Locatelli 2002 | Not RCT (review article) |
| Lornoy 1998a | Not RCT |
| Lornoy 2001 | Interventions not relevant to this review |
| Maeda 1990 | Not RCT |
| Maggiore 2000 | Not RCT (review article) |
| Maheshwari 2012 | Interventions not relevant to this review |
| Malberti 1991 | Not RCT |
| Mastrangelo 1986 | Not RCT |
| Mesic 2011 | Interventions not relevant to this review |
| Minutolo 2002 | Interventions not relevant to this review |
| Mioli 1986 | Not RCT |
| Mishkin 2002 | Not RCT |
| Mohini 1989 | Interventions not relevant to this review |
| Morena 2006 | Interventions not relevant to this review |
| Movilli 2011 | Not RCT |
| Mrowka 1993 | Interventions not relevant to this review |
| Nakazawa 1997 | Not RCT |
| Ohyama 1981 | Not RCT |
| Pacitti 1993 | Not RCT |
| Panichi 1994 | Not RCT |
| Panichi 1998 | Not RCT |
| Panichi 2006 | Interventions not relevant to this review |
| Pedrini 1999 | Interventions not relevant to this review |
| Pedrini 2006 | Interventions not relevant to this review |
| Pedrini 2009 | Interventions not relevant to this review |
| Pedrini 2011 | Interventions not relevant to this review |
| Petras 2005 | Interventions not relevant to this review |
| Pizzarelli 2004 | Outcomes not relevant to this review |
| Quellhorst 1983 | Not RCT |
| Quellhorst 1983a | Not RCT |
| Ragazzoni 2004 | Interventions not relevant to this review |
| Ramunni 2006 | Interventions not relevant to this review |
| Rius 2007 | Interventions not relevant to this review |
| Ronco 2000 | Interventions not relevant to this review |
| Sakurai 1990 | Interventions not relevant to this review |
| Santoro 2005 | Interventions not relevant to this review |
| Santoro 2008 | Interventions and outcomes not relevant to this review |
| Savoldi 2004 | Outcomes not relevant to this review |
| Shaldon 1998 | Not RCT |
| Sidoti 2004 | Interventions not relevant to this review |
| Sirolli 2004 | Outcomes not relevant to review |
| Spongano 1992 | Not RCT |
| Strujic 2006 | Not RCT |
| Susantitaphong 2008 | Interventions not relevant to this review |
| Timio 1986 | Outcomes not relevant to this review |
| Tomo 2004 | Outcomes not relevant to review |
| Umimoto 2000 | Not RCT |
| Vantelon 1977 | Not RCT |
| Vaslaki 1998 | Outcomes not relevant to review |
| Vaslaki 2000 | Not RCT |
| Vaslaki 2002 | Outcomes not relevant to review |
| Vaslaki 2003 | Outcomes not relevant to review |
| Vaslaki 2005 | Outcomes not relevant to review |
| Wang 2004d | Outcomes not relevant to review |
| Wizemann 2001 | Not RCT (review article) |
| Zehnder 1999 | Not RCT |
| Zimmerman 2003 | Not RCT |
| Zucchelli 1988 | Not RCT |
Characteristics of studies awaiting assessment [ordered by study ID]
Beerenhout 2004.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Bellien 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Cornelis 2014.
| Methods | Country: Netherlands
Setting: unclear, Netherlands centres
Study time frame: October 2011 to October 2012
Study design: cross‐over RCT Enrolment: not reported Duration of follow‐up: at 15, 30, 60,1 20, 240 minutes (4‐hour and 8‐hour sessions) and at 360 and 480 minutes (8‐hour sessions) |
| Participants | Ages eligible for study: 18 to 80 years Inclusion criteria prevalent conventional HD patients; AV‐fistula enabling double‐needle vascular access with blood flow rate of at least 350 mL/min; informed consent; age more than 18 years Exclusion criteria withdrawal of consent; acute intercurrent illness (infection, malignancy, cardiovascular event, uncontrolled diabetes) |
| Interventions | Assigned interventions 4‐hour HD, 4‐hour HDF, 8‐hour HD and 8‐hour HDF Prevalent conventional HD (CHD) patients (dialysing 3 days a week during 4 hours per dialysis session) will undergo, in random order, a mid‐week 4‐hour HD session, a mid‐week 4‐hour HDF session, a mid‐week 8‐hour HD session, and a mid‐week 8‐hour HDF session with a 2‐week interval between every session to assess the influence of treatment duration and of convection on the removal of uraemic toxins and on the haemodynamic responses and autonomic nervous regulation In between the study dialysis sessions these patients will receive routine CHD treatments |
| Outcomes | Primary outcome measures Removal of uraemic toxins Secondary outcome measures Haemodynamic response: BP, heart rate, heart rate variability, cardiac output and systemic vascular resistance will be measured. Skin microcirculation will be measured with laser Doppler flowmetry. |
| Notes | source: http://clinicaltrials.gov/ct2/show/study/NCT01328119?term=NCT01328119&rank=1 |
de Sequera 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Francisco 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Gonzales‐Diez 2012.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Krieter 2010a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
NCT01098149.
| Trial name or title | Tolerance to hemodialysis in insulin‐requiring diabetic patients: BD vs AFB with blood volume biofeedback (THIRD) |
| Methods | Country: Italy
Setting: multi‐centre, 5 Italian centres
Study time frame: 2006 to March 2010
Study design: cross‐over RCT Enrolment: 55 patients Duration of follow‐up: 3 months |
| Participants | Ages eligible for study: 18 to 85 years Inclusion criteria End stage renal disease patients; patients affected by diabetic nephropathy with insulin therapy, for, at least, 6 months; patients with renal replacement therapy with haemodialysis three time a week, for, at least, 6 months; age between 18 and 85 years Exclusion criteria Patients affected by neoplasm and/or mental illness; patients with residual diuresis > 500 mL/d; patients in single needle bicarbonate dialysis |
| Interventions | The study, 9 months long, is aimed to verify the treatment tolerance of insulin requiring diabetic patients, by using standard bicarbonate dialysis (BD), or acetate free biofiltration (AFB) and/or a blood volume control (BVC). The study is divided in three phases: the first one, three months long, is the baseline in standard bicarbonate dialysis, then all the patients are shifted to AFB with BVC, for other three months, while the last three months long phase, after a randomisation, has the aim to identify the relative contribution of each factor (absence of acetate in the bath or BVC) in the treatment tolerance improvement (if any). The treatment tolerance will be evaluated considering the frequency of intradialytic hypotensive events. |
| Outcomes | Primary outcome measures tolerance to dialysis. (time frame: 3 months) The treatment tolerance is measured by the number of intra dialytic hypotensive events Secondary outcome measures The secondary outcome measure is to evaluate the relative efficiency of each factor (AFB in the bath and blood volume control) to reach this result. (time frame: 3 months) The evaluation will be done on: frequency of hypotensive events; number of nurse interventions (defined as ultrafiltration rate stop, or saline infusion); antihypertensive drugs. |
| Starting date | March 2006 |
| Contact information | Dott. Ezio Movilli, Dept of Nephrology ‐Brescia, Italy |
| Notes | Study details as provided by Università deg li Studi di Brescia. source: http://clinicaltrials.gov/ct2/show/NCT01098149 |
NCT01327391.
| Trial name or title | Tolerance of "on Line" hemodiafiltration in chronic renal failure patients (on‐line‐HDF) |
| Methods | Country: France
Setting: multi‐centre, 36 French centres
Study time frame: 2005 to December 2012
Study design: parallel RCT Enrolment: 600 patients Duration of follow‐up: 2 years |
| Participants | Ages eligible for study: 65 to 90 years Inclusion criteria: patient who has signed the written consent form; aged > 65 and < 90 years; creatinine clearance < 10 mL/min; on dialysis for a minimum of 3 months; with 3 times/week haemodialysis sessions; erythropoietin dosage needed to maintain haemoglobin at a constant level (range of haemoglobin: 9 to 13 g/dL without any variation of more than 2g/dL for less than 3 months); without any problem of vascular access Exclusion criteria: patient aged < 65 and > 90 years; presence of severe malnutrition (albumin < 20 g/L);, unstable clinical condition; unipuncture or failed vascular access flow; known problems of coagulation |
| Interventions | Active arm: on‐line haemodiafiltration Haemodialysis patients treated with on line HDF technic Procedure: on line haemodiafiltration 3 sessions/week; 3‐4 hours per session Comparator: haemodialysis Haemodialysis patients treated with conventional haemodialysis technic using high‐flux dialyzers Procedure: haemodialysis 3 sessions/week; 3‐4 hours per session; high‐flux dialyzers |
| Outcomes | Primary outcome measures Tolerance of "on line" HDF treatment versus conventional high‐flux haemodialysis in terms of adverse events occurring during dialysis sessions (time frame: between day 30 and day 120 of treatment) Secondary outcome measures Quality of life evaluated with the KDQOL questionnaire (time frame: day 0, 180, 365, 730) Incidence of cardiovascular events (time frame: day 180, 365, 730) Influence of the technic on mineral metabolism disturbances (time frame: day 180, 365, 730); measure of mineral metabolism parameters (Ca, PO4, PTH) All‐cause and cardiovascular mortality (time frame: day 180, 365, 730) Influence of the technic on inflammatory parameters (time frame: day 180, 365, 730) measure of pro‐inflammatory cytokines and acute phase reactant proteins Influence of the technic on microbiological safety (time frame: day 180, 365, 730); measure of microbiological purity of dialysate Influence of the technic on oxidative stress parameters (time frame: day 180, 365, 730) measure of oxidative stress markers (AOPP, AGE) and antioxidant systems (vitamin E) |
| Starting date | May 2005 |
| Contact information | Prof Bernard CANAUD, Centre Hospitalier Universitaire Montpellier France Sponsors and Collaborators: University Hospital, Montpellier Ministry of Health, France |
| Notes | Estimated study completion date: December 2012 source: http://clinicaltrials.gov/ct2/results?term=NCT01327391&Search=Search |
NCT01396863.
| Trial name or title | Acute brain volume changes in haemodialysis: comparison of low flux haemodialysis with pre‐dilution haemodiafiltration |
| Methods | Country: Denmark
Setting: single centre
Study time frame: July 2011 to February 2012
Study design: cross‐over RCT Enrolment: 12 patients Duration of follow‐up: 4.5 hours after one haemodialysis session and 4.5 hours after one session of HDF |
| Participants | Ages eligible for study: 18 years and older Inclusion criteria Age ≥ 18 years Informed consent; patient with end‐stage renal disease (ESRD); stabile haemodialysis treatment (Kt/V ≥ 1.3); no contraindications against MRI (pacemaker or other metal implants, claustrophobia, severe adiposity); weight < 140 kg Exclusion criteria Clinical signs of new structural, thromboembolic or vascular brain disease the last 3 month before entering the study; changes in corticosteroid treatment during the last two weeks; change in diuretics during the last two weeks; non‐compliant with regard to salt and fluid intake; acute disease |
| Interventions | Assigned intervention Procedure: HDF during the first examination The patient will receive treatment with pre‐dilution HDF during the first examination. During the second examination the patient will receive treatment with low‐flux hemodialysis. MRI of the brain will be performed before and after the treatment. The MRI‐data will later be processed to determine the degree of brain volume change due to the treatment. Assigned comparison Procedure: HD during the first examination. The patient will receive treatment with low‐flux haemodialysis during the first examination. During the second examination the patient will receive treatment with pre‐dilution hemodiafiltration. MRI of the brain will be performed before and after the treatment. The MRI‐data will later be processed to determine the degree of brain volume change due to the treatment. |
| Outcomes | Primary outcome measures Percent brain volume change (PBVC), brain volume before and after one haemodialysis session (4,5 hours) and one session of HDF (4,5 hours) |
| Starting date | July 2011 |
| Contact information | Study director: Jens D. Jensen, MD, PhD, Department of Renal Medicine C, Aarhus University Hospital, Skejby, Denmark Principal Investigator: Niels Johansen, Department of Renal Medicine C, Aarhus University Hospital, Skejby, Denmark |
| Notes | Study completion date: February 2012 source: http://clinicaltrials.gov/ct2/show/NCT01396863?term=NCT01396863&rank=1 |
NCT01445366.
| Trial name or title | Solute removal with high volume hemodiafiltration versus long high flux hemodialysis |
| Methods | Country: Belgium
Setting: single centre
Study time frame: April 2012 to July 2012
Study design: cross‐over RCT Enrolment: 10 patients Duration of follow‐up: 2 weeks |
| Participants | Ages eligible for study: 18 years and older Inclusion criteria Chronic kidney disease (CKD) stage 5 with haemodialysis or HDF treatment for more than three months; no vascular access related problems (Arteriovenous (A/V) fistula, graft or bi‐flow catheter); double needle/lumen vascular access; no ongoing infection; signed informed consent form Exclusion criteria Inclusion criteria not met; known HIV or active hepatitis B or C infection (Positive Polymerisation Chain Reaction (PCR)); pregnancy; unstable clinical condition (e.g. cardiac or vascular instability); known coagulation problems; patients participating in another study interfering with the planned study |
| Interventions | Intervention: high volume post dilution HDF
Comparator: high‐flux haemodialysis This is a prospective cross‐over study including 10 stable haemodialysis patients with chronic kidney disease stage 5. The cross‐over study lasts 2 weeks with the study dialysis sessions at midweek. During one session, the patient will be dialyzed during 4 hours with high volume post dilution haemodiafiltration (HDF) with an FX800 haemodialyser (Fresenius Medical Care) and a blood flow of 300mL/min, dialysate flow of 500mL/min, and substitution flow of 75 mL/min. During the other midweek session, the patient will be dialyzed during 8 hours with high‐flux haemodialysis (HD) with an FX80 haemodialyser (Fresenius Medical Care) and a blood flow of 200mL/min and a dialysate flow of 500mL/min. |
| Outcomes | Primary outcome measures Uraemic retention solute concentrations from pre and post dialysis blood samples, dialyzer inlet and outlet blood samples, and spent dialysate samples. (time frame: during 4 hours) Uraemic retention solute concentrations from pre and post dialysis blood samples, dialyzer inlet and outlet blood samples, and spent dialysate samples. (time frame: during 8 hours) |
| Starting date | April 2012 |
| Contact information | Raymond Vanholder, PhD, MD University Hospital Ghent, Ghent, Belgium, 9000 |
| Notes | Estimated study completion date: December 2012 |
NCT02374372.
| Trial name or title | Prospective randomized study comparing the hemodiafiltration on‐line and conventional hemodialysis in terms of cost‐benefit |
| Methods | Country: Canada Allocation: randomised Endpoint classification: efficacy study Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants | Chronic renal failure; haemodialysis |
| Interventions | Active comparator: conventional haemodialysis Active comparator: haemodiafiltration on‐line haemodiafiltration |
| Outcomes | Compare the medication cost between the 2 groups (HD and HDF) (time frame: 3 years) Demonstrate lower cost of erythropoietin in HDF, with same control of anaemia to HD group (time frame: 3 years) Demonstrate lower cost of phosphate binder in HDF, with same control of phospho‐calcium balance to HD group (time frame: 3 years) Demonstrate lower need of erythropoietin and best control of anaemia in HDF (time frame: 3 years) Demonstrate lower need of phosphate binder and best control of phospho‐calcium balance in HDF (time frame: 3 years) Demonstrate less hospitalisation stay and cost related in HDF group (time frame: 3 years) Stabilisation or regression of left ventricular hypertrophy (time frame: 3 years) |
| Starting date | January 2011 |
| Contact information | Renée Lévesque, MD, renee.levesque@sympatico.ca Marie‐Line Caron, B.Sc, marie‐line.caron.chum@ssss.gouv.qc.ca |
| Notes | Estimated completion date: June 2016 Source: clinicaltrials.gov/ct2/show/study/NCT02374372 |
Differences between protocol and review
Risk of bias assessment tool has replaced the quality assessment checklist (Rabindranath 2005).
Contributions of authors
Original review (2006)
Giovanni FM Strippoli: Design, conduct, data‐analysis, writing review
Alison M MacLeod: Design and writing the review
Conal Daly: Designing, screening search results, selecting relevant studies and writing the review
Paul Roderick: Design and writing the review.
Sheila Wallace: Develop search strategy
Kannaiyan S Rabindranath: Develop search strategy, screen search titles, select studies, data extraction and analysis, writing review
Updated review (2015)
Ionut Nistor: Screening and identification of additional studies for inclusion, development of database, data extraction, completion of tables and figures, drafting of first version of updated manuscript
Suetonia Palmer: Data checking and analysis, revision of first and subsequent drafts, generation of additional tables
Valeria Saglimbene: Screening and identification of additional studies for inclusion, data extraction and checking
Jonathan C. Craig: Data analysis and revision of first and subsequent drafts
Giovanni FM Strippoli: Screening and identification of additional studies for inclusion, data analysis and revision of first and subsequent drafts
Sources of support
Internal sources
University of Sydney School of Public Health, non‐established PhD scholarship, Australia.
External sources
National Kidney Research Fund, UK.
-
European Renal Best Practice and ERA‐EDTA, Other.
Ionut Nistor was the recipient of a grant from European Renal Best Practice (ERBP) and the European Renal Association‐ European Dialysis Transplantation Association (ERA‐EDTA).
Declarations of interest
The 2006 review was funded by the National Kidney Research Fund (UK).
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Altieri 2004 {published data only}
- Altieri P, Sorba G, Bolasco P, Ledebo I, Ganadu M, Ferrara R, et al. Comparison between hemofiltration and hemodiafiltration in a long‐term prospective cross‐over study. Journal of Nephrology 2004;17(3):414‐22. [MEDLINE: ] [PubMed] [Google Scholar]
Bammens 2004 {published data only}
- Bammens B, Evenepoel P, Claes K, Kuypers D, Maes B, Verbeke K, et al. Middle molecule and protein bound toxin removal by an FX‐class dialyser in haemodialysis and haemodiafiltration: a randomised cross‐over study [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):757. [CENTRAL: CN‐00444322] [Google Scholar]
- Bammens B, Evenepoel P, Verbeke K, Vanrenterghem Y. Protein bound solutes: explaining the dissociation between urea‐reduction ratio and patient outcome? [abstract no: SU‐PO911]. Journal of the American Society of Nephrology 2003;14(Nov):736A. [CENTRAL: CN‐00644357] [Google Scholar]
- Bammens B, Evenepoel P, Verbeke K, Vanrenterghem Y. Removal of protein bound solutes by convective transport: a randomized cross‐over study [abstract no: SU‐PO905]. Journal of the American Society of Nephrology 2003;14(Nov):734A. [CENTRAL: CN‐00644358] [Google Scholar]
- Bammens B, Evenepoel P, Verbeke K, Vanrenterghem Y. Removal of the protein‐bound solute p‐cresol by convective transport: a randomized crossover study. American Journal of Kidney Diseases 2004;44(2):278‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Basile 2001 {published data only}
- Basile C, Giordano R, Montanaro A. Effect of acetate‐free biofiltration (AFB) on renal anemia: a prospective cross‐over study [abstract]. Journal of the American Society of Nephrology 2001;12:352A. [DOI] [PubMed] [Google Scholar]
- Basile C, Giordano R, Montanaro A, Maio PD, Padova FD, Marangi AL, et al. Effect of acetate‐free biofiltration on the anaemia of haemodialysis patients: a prospective cross‐over study. Nephrology Dialysis Transplantation 2001;16(9):1914‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Basile C, Giordano R, Montanaro A, Padova F, Marangi AL. Effect of acetate‐free biofiltration (AFB) on renal anemia: a prospective cross‐over study [abstract]. 38th Congress. European Renal Association. European Dialysis and Transplantation Association; 2001 Jun 24‐27; Vienna, Austria. 2001:206. [CENTRAL: CN‐00444349] [DOI] [PubMed]
Beerenhout 2005 {published data only}
- Beerenhout CH, Luik AJ, Jeuken‐Mertens SG, Bekers O, Menheere P, Hover L, et al. Pre‐dilution on‐line haemofiltration vs low‐flux haemodialysis: a randomized prospective study. Nephrology Dialysis Transplantation 2005;20(6):1155‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kooman J, Beerenhout C, Luik A, Sande F, Leunissen K. Predilution on‐line hemofiltration versus hemodialysis: a randomised study [abstract no: SA‐PO458]. Journal of the American Society of Nephrology 2004;15(Oct):403A. [CENTRAL: CN‐00626039] [Google Scholar]
- Meert N, Beerenhout C, Smet R, Kooman J, Vanholder R. Removal of protein‐bound uremic solutes by predilution on‐line hemofiltration [abstract no: MP401]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v334. [Google Scholar]
- Meert N, Beerenhout C, Smet R, Kooman J, Vanholder R. Removal of protein‐bound uremic solutes by predilution on‐line hemofiltration [abstract no: SA‐PO285]. Journal of the American Society of Nephrology 2004;15(Oct):363A. [Google Scholar]
Bolasco 2003 {published and unpublished data}
- Bolasco P, Altieri P, Andrulli S, Basile C, Filippo S, Feriani M, et al. Convection versus diffusion in dialysis: an Italian prospective multicentre study. Nephrology Dialysis Transplantation 2003;18 Suppl 7:vii50‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Locatelli F, Altieri P, Andrulli S, Bolasco P, Sau G, Pedrini LA, et al. Hemofiltration and hemodiafiltration reduce intradialytic hypotension in ESRD. Journal of the American Society of Nephrology 2010;21(10):1798‐807. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locatelli F, Altieri P, Andrulli S, Sau G, Bolasco P, Pedrini LA, et al. Phosphate levels in patients treated with low‐flux haemodialysis, pre‐dilution haemofiltration and haemodiafiltration: post hoc analysis of a multicentre, randomized and controlled trial. Nephrology Dialysis Transplantation 2014;29(6):1239‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Locatelli F, Altieri P, Andrulli S, Sau G, Bolasco P, Pedrini LA, et al. Predictors of haemoglobin levels and resistance to erythropoiesis‐stimulating agents in patients treated with low‐flux haemodialysis, haemofiltration and haemodiafiltration: results of a multicentre randomized and controlled trial. Nephrology Dialysis Transplantation 2012;27(9):3594‐600. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coll 2009 {published data only (unpublished sought but not used)}
- Coll E, Perez‐Garcia R, Martin de Francisco AL, Galceran J, Garcia‐Osuna R, Martin‐Malo A, et al. Acetate‐free on‐line PHF: how to improve hyperacetatemia and haemodynamic tolerance [PHF on‐line sin acetato: como mejorar la hiperacetatemia y la tolerancia hemodinamica]. Nefrologia 2009;29(2):156‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CONTRAST (Dutch) Study 2005 {published and unpublished data}
- Bots ML, Hoedt C, Pc Grooteman M, Weerd NC, Mazairac AH, Levesque R, et al. The effect of online hemodiafiltration on systemic inflammation in a randomized controlled trial: Results from the convective transport study (CONTRAST) [abstract]. Nephrology Dialysis Transplantation 2012;27(Suppl 2):ii206. [EMBASE: 70765903] [Google Scholar]
- Gritters M, Schoorl M, Schoorl M, Bartels PC, Grooteman MP, Nube MJ, et al. Platelet activation is increased in hemodiafiltration (HDF): a subgroup analysis of the CONvective TRAnsport STudy (CONTRAST) [abstract no: F‐FC313]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):70A. [Google Scholar]
- Gritters M, Vroling L, Grooteman MP, Haas RR, Broxterman HJ, Nube MJ, et al. Hemodiafiltration (HDF) increases the number of circulating hematopoietic progenitor cells (HPC) and lowers numbers of circulating cells with endothelial markers: a subgroup analysis of CONTRAST (CONvective TRAnsport Study) [abstract no: F‐PO680]. Journal of the American Society of Nephrology 2007;18(Abstracts):249A. [Google Scholar]
- Gritters‐van den Oever M, Grooteman MP, Bartels PC, Blankestijn PJ, Bots ML, Dorpel MA, et al. Post‐dilution haemodiafiltration and low‐flux haemodialysis have dissimilar effects on platelets: a side study of CONTRAST. Nephrology Dialysis Transplantation 2009;24(11):3461‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Grooteman MP, Dorpel MA, Bots ML, Penne EL, Weerd NC, Mazairac AH, et al. Effect of online hemodiafiltration on all‐cause mortality and cardiovascular outcomes. Journal of the American Society of Nephrology 2012;23(6):1087‐96. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grooteman MP, Dorpel MA, Bots ML, Penne EL, Weerd NC, Mazairac AH, et al. Online hemodiafiltration versus low‐flux hemodialysis: effects on all‐cause mortality and cardiovascular events in a randomized controlled trial. The Convective Transport Study (CONTRAST) [abstract]. NDT Plus 2011;4(Suppl 2):4.2S.1. [Google Scholar]
- Mazairac AH, Blankestijn PJ, Grooteman MP, Penne EL, Weerd NC, Hoedt CH, et al. The cost‐utility of haemodiafiltration versus haemodialysis in the Convective Transport Study. Nephrology Dialysis Transplantation 2013;28(7):1865‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mazairac AH, Bots ML, Wit GA, Jong B, Boeschoten W, Weerd NC, et al. Improvement of health‐related quality of life (QoL) in hemodialysis (HD) patients over the past decade in the Netherlands [abstract no: TH‐PO811]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):293A. [Google Scholar]
- Mazairac AH, Grooteman MP, Blankestijn PJ, Penne EL, Weerd NC, Hoedt CH, et al. Differences in quality of life of hemodialysis patients between dialysis centers. Quality of Life Research 2012;21(2):299‐307. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazairac AH, Wit GA, Grooteman MP, Penne EL, Weerd NC, Hoedt CH, et al. Clinical performance targets and quality of life in hemodialysis patients. Blood Purification 2012;33(1‐3):73‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mazairac AH, Wit GA, Grooteman MP, Penne EL, Weerd NC, Hoedt CH, et al. Effect of hemodiafiltration on quality of life over time. Clinical Journal of the American Society of Nephrology: CJASN 2013;8(1):82‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazairac AH, Wit GA, Grooteman MP, Penne EL, Weerd NC, Dorpel MA, et al. A composite score of protein‐energy nutritional status predicts mortality in haemodialysis patients no better than its individual components. Nephrology Dialysis Transplantation 2011;26(6):1962‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mostovaya IM, Bots ML, Dorpel MA, Goldschmeding R, Hoedt CH, Kamp O, et al. Left ventricular mass in dialysis patients, determinants and relation with outcome. Results from the COnvective TRansport STudy (CONTRAST). PLoS ONE [Electronic Resource] 2014;9(2):e84587. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostovaya IM, Bots ML, Dorpel MA, Grooteman MP, Kamp O, Levesque R, et al. A randomized trial of hemodiafiltration and change in cardiovascular parameters. Clinical Journal of the American Society of Nephrology: CJASN 2014;9(3):520‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penne EJ, Blankestijn PJ, Bots ML, Dorpel MA, Grooteman MP, Nube MJ, et al. New study evaluating online haemodiafiltration for the reduction of cardiovascular morbidity and mortality in patients undergoing chronic haemodialysis [Nieuw onderzoek naar online‐hemodiafiltratie voor de vermindering van cardiovasculaire morbiditeit en mortaliteit bij patienten die chronisch worden behandeld met hemodialyse]. Nederlands Tijdschrift voor Geneeskunde 2006;150(28):1583‐5. [MEDLINE: ] [PubMed] [Google Scholar]
- Penne EL, Blankestijn PJ, Bots ML, Dorpel MA, Grooteman MP, Nube MJ, et al. Effect of increased convective clearance by on‐line hemodiafiltration on all cause and cardiovascular mortality in chronic hemodialysis patients ‐ the Dutch CONvective TRAnsport STudy (CONTRAST): rationale and design of a randomised controlled trial [ISRCTN38365125]. Current Controlled Trials in Cardiovascular Medicine 2005;6(1):8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penne EL, Blankestijn PJ, Bots ML, Dorpel MA, Grooteman MP, Nube MJ, et al. Effect of increased convective clearance by on‐line hemodiafiltration on all cause and cardiovascular mortality in chronic hemodialysis patients: design and rationale of the Dutch CONvective TRAnsport STudy (CONTRAST) [abstract no: SA‐PO471]. Journal of the American Society of Nephrology 2004;15(Oct):406A. [CENTRAL: CN‐00550574] [Google Scholar]
- Penne EL, Blankestijn PJ, Bots ML, Dorpel MA, Grooteman MP, Nube MJ, et al. Resolving controversies regarding hemodiafiltration versus hemodialysis: the Dutch Convective Transport Study. Seminars in Dialysis 2005;18(1):47‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Penne EL, Weerd NC, Blankestijn PJ, Dorpel MA, Grooteman MP, Nube MJ, et al. Role of residual kidney function and convective volume on change in beta2‐microglobulin levels in hemodiafiltration patients. Clinical Journal of the American Society of Nephrology: CJASN 2010;5(1):80‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penne EL, Weerd NC, Grooteman MP, Nube MJ, Bots ML, Dorpel MA, et al. Pronounced decrease of beta‐2 microglobulin by online hemodiafiltration in patients without residual renal function [abstract no: TH‐PO634]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):249A. [Google Scholar]
- Penne EL, Weerd NC, Dorpel MA, Grooteman MP, Levesque R, Nube MJ, et al. Short‐term effects of online hemodiafiltration on phosphate control: a result from the randomized controlled Convective Transport Study (CONTRAST). American Journal of Kidney Diseases 2010;55(1):77‐87. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Penne L, Blankestijn P, Bots M, Dorpel R, Grooteman M, Nube M, et al. The CONvective TRAnsport STudy (CONTRAST) is designed to detect differences in mortality and cardiovascular disease between online hemodiafiltration and hemodialysis: preliminary findings on proof of principle [abstract no: SA‐PO811]. Journal of the American Society of Nephrology 2005;16:734A. [Google Scholar]
- Penne L, Blankestijn P, Bots M, Dorpel MA, Grooteman M, Nube M, et al. Effect of increased convective clearance by on‐line hemodiafiltration on all cause and cardiovascular mortality in chronic dialysis patients: design and preliminary baseline characteristics of the CONvective TRAnsport STudy (CONTRAST) [abstract no: T‐PO40054]. Nephrology 2005;10(Suppl 1):A189. [CENTRAL: CN‐00601971] [Google Scholar]
- Weerd NC, Blankestijn PJ, Bots ML, Dorpel RA, Grooteman MP, Nube MJ, et al. Dose dependent decrease in beta‐2‐microglobulin levels in patients treated with online hemodiafiltration in the Dutch CONvective TRAnsport STudy (CONTRAST) [abstract no: S‐PO‐0130]. 4th World Congress of Nephrology.19th International Congress of the International Society of Nephrology (ISN); 2007 Apr 21‐25; Rio de Janeiro, Brazil. 2007:83.
- Hoedt CH, Bots ML, Grooteman MP, Mazairac AH, Penne EL, Weerd NC, et al. Should we still focus that much on cardiovascular mortality in end stage renal disease patients? The CONvective TRAnsport STudy. PLoS ONE [Electronic Resource] 2013;8(4):e61155. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoedt CH, Bots ML, Grooteman MP, Weerd NC, Penne EL, Mazairac AH, et al. Clinical predictors of decline in nutritional parameters over time in ESRD. Clinical Journal of the American Society of Nephrology: CJASN 2014;9(2):318‐25. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerd N, Blankestijn P, Bots M, Dorpel R, Grooteman M, Nube M, et al. Dose dependent decrease in beta‐2‐microglobulin (b2M) levels in patients treated with online hemodiafiltration (HDF) in the Dutch CONvective TRAnsport STudy (CONTRAST) [abstract no: F‐FC077]. Journal of the American Society of Nephrology 2006;17(Abstracts):53A. [CENTRAL: CN‐00601973] [Google Scholar]
- Weerd N, Penne E, Blankestijn P, Bots M, Dorpel R, Grooteman M, et al. No increase in erythropoietin (EPO) sensitivity despite increased middle molecular weight (MMV) clearance in patients treated with online hemodiafiltration (HDF) in the Dutch CONvective TRAnsport STudy (CONTRAST) [abstract no: F‐PO826]. Journal of the American Society of Nephrology 2007;18(Abstracts):283A. [Google Scholar]
- Weerd N, Penne L, Blankestijn P, Bots M, Dorpel R, Grooteman M. Determinants of epoetin (EPO) sensitivity in patients treated with online hemodiafiltration (HDF) or conventional hemodialysis (HD) in the Dutch CONvective TRAnsport STudy (CONTRAST) [abstract no: TH‐PO722]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):270A. [Google Scholar]
- Weerd N, Penne L, Grooteman M, Levesque R, Dorpel R, Blankestijn P, et al. Resistance to erythropoiesis stimulating agents (ESA) in a randomized controlled trial (CONTRAST study) [abstract no: OSU052]. NDT Plus 2010;3(Suppl 3):iii296. [Google Scholar]
- Weerd NC, Grooteman MP, Bots ML, Dorpel MA, Hoedt CH, Mazairac AH, et al. Hepcidin‐25 in chronic hemodialysis patients is related to residual kidney function and not to treatment with erythropoiesis stimulating agents. PLoS ONE [Electronic Resource] 2012;7(7):e39783. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerd NC, Grooteman MP, Bots ML, Dorpel MA, Hoedt CH, Mazairac AH, et al. Hepcidin‐25 is related to cardiovascular events in chronic haemodialysis patients. Nephrology Dialysis Transplantation 2013;28(12):3062‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Weerd NC, Hoedt CH, Blankestijn PJ, Bots ML, Dorpel MA, Levesque R, et al. Resistance to erythropoiesis stimulating agents in patients treated with online hemodiafiltration and ultrapure low‐flux hemodialysis: results from a randomized controlled trial (CONTRAST). PLoS ONE [Electronic Resource] 2014;9(4):e94434. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cristofano 2004 {published and unpublished data}
- Cristofano C, Vernaglione L, Perniola MA, Lo Barco C, Muscogiuri P, Chimienti S. Cystatin C, beta2microglobulin (B2MCG) and C reactive protein (CRP) in two separate chambers hemodiafiltration and online endogenous liquid reinfusion (HFR) and in low flux polysulphone bicarbonate conventional hemodialysis (LFHD) [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:371. [CENTRAL: CN‐00550765]
- Vernaglione L, Cristofano C, Chimienti S. Cystatin C (CYS), b2‐microglobulin (MIC) and C‐reactive protein (CRP) behaviours during hemodiafiltration with online endogenous liquid reinfusion (HFR) and low‐flux polysulphone bicarbonate dialysis (BD) [abstract no: SA‐PO702]. Journal of the American Society of Nephrology 2006;17(Abstracts):722A. [CENTRAL: CN‐00644356] [Google Scholar]
Ding 2002 {published data only}
- Ding F, Ahrenholz P, Winkler RE, Ramlow W, Tiess M, Michelsen A. On‐line hemodiafiltration versus acetate‐free biofiltration: a prospective cross‐over study [abstract]. 38th Congress. European Renal Association. European Dialysis and Transplantation Association; 2001 Jun 24‐27; Vienna, Austria. 2001:284. [CENTRAL: CN‐00460648]
- Ding F, Ahrenholz P, Winkler RE, Ramlow W, Tiess M, Michelsen A, et al. Online hemodiafiltration versus acetate‐free biofiltration: A prospective crossover study. Artificial Organs 2002;26(2):169‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eiselt 2000 {published data only}
- Eiselt J, Racek J, Opatrny K Jr. The effect of hemodialysis and acetate‐free biofiltration on anemia. International Journal of Artificial Organs 2000;23(3):173‐80. [MEDLINE: ] [PubMed] [Google Scholar]
ESHOL Study 2011 {published data only (unpublished sought but not used)}
- Maduell F, Moreso F, Pons M, Ramos R, Mora‐Macia J, Carreras J, et al. High‐efficiency postdilution online hemodiafiltration reduces all‐cause mortality in hemodialysis patients. Journal of the American Society of Nephrology 2013;24(3):487‐97. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maduell F, Moreso F, Pons M, Ramos R, Mora‐Macia J, Foraster A, et al. Design and patient characteristics of ESHOL study, a Catalonian prospective randomized study. Journal of Nephrology 2011;24(2):196‐202. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fox 1993 {published data only}
- Fox SD, Henderson LW. Cardiovascular response during hemodialysis and hemofiltration: thermal, membrane and catecholamine influences. Blood Purification 1993;11(4):224‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kantartzi 2013 {published data only}
- Kantartzi K, Panagoutsos S, Mourvati E, Roumeliotis A, Leivaditis K, Devetzis V, et al. Can dialysis modality influence quality of life in chronic hemodialysis patients? low‐flux hemodialysis versus high‐flux hemodiafiltration: a cross‐over study. Renal Failure 2013;35(2):216‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Karamperis 2005 {published data only}
- Jensen D, Karamperis N, Jensen JD. Hemodynamic stability during pre‐HDF and lowflux HD at temperature controlled conditions using high calcium‐ion dialysis and replacement fluid. A blinded randomized controlled study [abstract no: SU‐PO275]. Journal of the American Society of Nephrology 2004;15(Oct):593A‐4A. [CENTRAL: CN‐00583242] [Google Scholar]
- Jensen JD, Karamperis N, Sloth E. Hemodynamic stability in predilution hemodiafiltration (HDF) compared with lowflux hemodialysis (HD) at temperature controlled conditions ‐ a blind randomized controlled trial [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):192‐3. [CN‐00445915] [Google Scholar]
- Karamperis N, Jensen D, Sloth E, Jensen JD. Comparison of predilution hemodiafiltration and low‐flux hemodialysis at temperature‐controlled conditions using high calcium‐ion concentration in the replacement and dialysis fluid. Clinical Nephrology 2007;67(4):230‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Karamperis N, Sloth E, Jensen JD. Predilution hemodiafiltration displays no hemodynamic advantage over low‐flux hemodialysis under matched conditions. Kidney International 2005;67(4):1601‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lin 2001 {published data only}
- Lin CL, Huang CC, Chang CT, Wu MS, Hong JJ, Chien CC, et al. Clinical improvement by increased frequency of on‐line hemodialfiltration [abstract]. 8th Asian Pacific Congress of Nephrology; 2000 Mar 26‐30; Taipei, Taiwan. 2000:194. [CENTRAL: CN‐00461180]
- Lin CL, Huang CC, Chang CT, Wu MS, Hung CC, Chien CC, et al. Clinical improvement by increased frequency of on‐line hemodialfiltration. Renal Failure 2001;23(2):193‐206. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lin CL, Yang CW, Chiang CC, Chang CT, Huang CC. Long‐term on‐line hemodiafiltration reduces predialysis beta‐2‐microglobulin levels in chronic hemodialysis patients. Blood Purification 2001;193(3):301‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lin CL, Yang CW, Chiang CC, Chang CT, Huang CC. On‐line hemodiafiltration reduces pre‐dialysis b2‐microglobulin levels in chronic hemodialysis patients [abstract]. 8th Asian Pacific Congress of Nephrology; 2000 Mar 26‐30; Taipei, Taiwan. 2000:313. [CENTRAL: CN‐00461181]
Locatelli 1994 {published data only}
- Locatelli F, Italian Cooperative Dialysis Study Group. Effect of hemodialysis membranes on serum albumin. American Journal of Kidney Diseases 2001;37(2):455‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Locatelli F, Mastrangelo F, Redaelli B, Ronco C, Marcelli D, Greca G, et al. Effects of different membranes and dialysis technologies on patient treatment tolerance and nutritional parameters. The Italian Cooperative Dialysis Study Group. Kidney International 1996;50(4):1293‐302. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Locatelli F, Mastrangelo F, Redaelli B, Ronco C, Marcelli D, Greca G, et al. The effects of different membranes and dialysis technologies on the treatment tolerance and nutritional parameters of hemodialysis patients [abstract no: A1031]. Journal of the American Society of Nephrology 1996;7(9):1454. [CENTRAL: CN‐00583453] [Google Scholar]
- Locatelli F, Mastrangelo F, Redaelli B, Ronco C, Marcelli D, Orlandini G. The effects of different membranes and dialysis technologies on the treatment tolerance and nutritional parameters of hemodialysed patients. Design of a prospective randomised multicentre trial. Journal of Nephrology 1994;7(2):123‐9. [EMBASE: 1994156257] [Google Scholar]
Lornoy 1998 {published data only (unpublished sought but not used)}
- Lornoy W, Becaus I, Billiouw JM, Sierens L, Malderen P, D'Haenens P. On‐line haemodiafiltration. Remarkable removal of beta2‐microglobulin. Long‐term clinical observations. Nephrology Dialysis Transplantation 2000;15 Suppl 1:49‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lornoy W, Becaus I, Billiouw JM, Sierens L, Malderen P, Vrouwziekenhuis OL. Remarkable removal of b2‐microglobulin, but not of small molecules by on‐line hemodiafiltration (HDF). Nephrology Dialysis Transplantation 1997;12(3):631. [CENTRAL: CN‐00626022] [Google Scholar]
- Lornoy W, Becaus I, Billiouw JM, Sierens L, Vrouwziekenhuis OL. Remarkable removal of b2‐microglobulin, but not of small molecules by on‐line hemodiafiltration (HDF) [abstract]. Journal of the American Society of Nephrology 1995;6(3):546. [CENTRAL: CN‐00484892] [Google Scholar]
- Lornoy W, Becaus I, Billiouw JM, Sierens L, Malderen P. Remarkable removal of beta‐2‐microglobulin by on‐line hemodiafiltration. American Journal of Nephrology 1998;18(2):105‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mandolfo 2008 {published and unpublished data}
- Mandolfo S, Borlandelli S, Imbasciati E, Badalamenti S, Graziani G, Sereni L, et al. Pilot study to assess increased dialysis efficiency in patients with limited blood flow rates due to vascular access problems. Hemodialysis International 2008;12(1):55‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Meert 2009 {published data only}
- Meert N, Dhondt A, Eloot S, Vanholder R. Effective removal of uremic solutes by different convective strategies: a prospective cross‐over trial [abstract no: F‐PO672]. Journal of the American Society of Nephrology 2007;18(Abstracts):247A‐8A. [Google Scholar]
- Meert N, Eloot S, Waterloos MA, Landschoot M, Dhondt A, Glorieux G, et al. Effective removal of protein‐bound uraemic solutes by different convective strategies: a prospective trial. Nephrology Dialysis Transplantation 2009;24(2):562‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Movilli 1996 {published data only}
- Movilli E, Camerini C, Zein H, D'Avolio G, Sandrini M, Strada A, et al. A prospective comparison of bicarbonate dialysis, hemodiafiltration, and acetate‐free biofiltration in the elderly. American Journal of Kidney Diseases 1996;27(4):541‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Noris 1998 {published data only}
- Noris M, Todeschini M, Casiraghi F, Minetti L, Imberti B, Cereda C, et al. Effect of acetate (AHD), bicarbonate (BHD) dialysis and acetate free biofiltration (AFB) on nitric oxide synthesis: implications for dialysis hypotension [abstract]. Journal of the American Society of Nephrology 1996;7(9):1493. [CENTRAL: CN‐00446974] [DOI] [PubMed] [Google Scholar]
- Noris M, Todeschini M, Casiraghi F, Roccatello D, Martina G, Minetti L, et al. Effect of acetate, bicarbonate dialysis and acetate free biofiltration on nitric oxide synthesis: implications for dialysis hypotension. American Journal of Kidney Diseases 1998;32(1):115‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ohtake 2012 {published data only}
- Ohtake T, Oka M, Ishioka K, Honda K, Mochida Y, Maesato K, et al. Cardiovascular protective effects of on‐line hemodiafiltration: comparison with conventional hemodialysis. Therapeutic Apheresis & Dialysis 2012;16(2):181‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pedrini 2011a {published data only (unpublished sought but not used)}
- Casino F, Pedrini LA, Lopez T, Cristofaro V, Conte F. Comparing dialysis dose in haemodialysis and online haemodiafiltration. A multicentre randomized cross‐over study [abstract no: SP701]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv251. [Google Scholar]
- Cristofaro V, Pedrini LA, Casino F, Campolo A, Borzumati M, Prencipe MA, et al. High efficiency on‐line haemodiafiltration improves control of secondary hyperparathyroidism. A multicentre randomized cross‐over study [abstract no: SP699]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv251. [Google Scholar]
- Pedrini LA, Cristofaro V, Casino F, Conte F, Borzumati M, Campolo A, et al. Effects of online haemodiafiltration on cardiovascular risk factors. A multicentre randomized cross‐over study [abstract no: MP335]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv411. [Google Scholar]
- Pedrini LA, Cristofaro V, Comelli M, Casino FG, Prencipe M, Baroni A, et al. Long‐term effects of high‐efficiency on‐line haemodiafiltration on uraemic toxicity. A multicentre prospective randomized study. Nephrology Dialysis Transplantation 2011;26(8):2617‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PROFIL Study 2011 {published data only (unpublished sought but not used)}
- Alvestrand A, Gutierrez A, Hagerman I, Ledebo I, Mattsson E, Dam Jensen J, et al. Hemofiltration delays deterioration of left ventricular hypertrophy in incident dialysis patients. Initial report from PROFIL, a prospective randomized study comparing hemofiltration with hemodialysis [abstract no: SAP302]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi333. [CENTRAL: CN‐00653728] [Google Scholar]
- Alvestrand A, Ledebo I, Hagerman I, Wingren K, Mattsson E, Qureshi AR, et al. Left ventricular hypertrophy in incident dialysis patients randomized to treatment with hemofiltration or hemodialysis: results from the ProFil study. Blood Purification 2011;32(1):21‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gutierrez A, Alvestrand A, Ledebo I, Dam Jensen J, Wingren K, the PROFIL Study Group. Hemofiltration is adequate at low Kt/V. Initial report from PROFIL, a prospective, randomized study comparing hemofiltration with hemodialysis [abstract no: FP340]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi132. [Google Scholar]
Righetti 2010 {published data only (unpublished sought but not used)}
- Righetti M, Filiberti O, Ranghino A, Ferrario G, Milani S, Serbelloni P, et al. Internal hemodiafiltration versus low‐flux bicarbonate dialysis: Results from a long‐term prospective study. International Journal of Artificial Organs 2010;33(11):796‐802. [MEDLINE: ] [PubMed] [Google Scholar]
- Righetti M, Filiberti O, Ranghino A, Ferrario G, Servelloni P, Milani S, et al. Internal haemodiafiltration improves many cardiovascular risk factors: results from a long‐term multicenter prospective trial [abstract no: SU375]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009.
Santoro 1999 {published and unpublished data}
- Poli A, Tessitore N, Panzetta O, Mantovani W, Esteban J, London G, et al. Acetate‐free biofiltration (AFB) and cardiovascular case‐fatality rate (CFR) in incident hemodialysis patients (pts) [abstract no: SU‐PO562]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):706A. [Google Scholar]
- Santoro A, Panzetta G, Tessitore N, Atti M, Mancini E, Esteban J, et al. A prospective randomised European multicentre study of medium‐long run mortality and morbidity comparing acetate‐free biofiltration and bicarbonate dialysis. Journal of Nephrology 1999;12(6):375‐82. [MEDLINE: ] [PubMed] [Google Scholar]
- Santoro A, Panzetta O, Tessitore N, Wizemann V, Perez R, London G, et al. Cardiovascular effects of acetate‐free biofiltration (AFB) and conventional bicarbonate dialysis (BD) in chronic dialysis (CD) patients: a controlled randomized European multicenter study [abstract no: FP360]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi139. [Google Scholar]
- Santoro A, Panzetta O, Tessitore N, Wizemann V, Perez R, Neumann K, et al. A European multicentre RCT on cardiovascular effects of acetate‐free biofiltration (AFB) and conventional bicarbonate dialysis (BD) in chronic dialysis (CD) patients [abstract no: SU‐FC051]. Journal of the American Society of Nephrology 2007;18(Abstracts):78A. [Google Scholar]
- Santoro A, Tessitore N, Panzetta O, Wizemann V, Neumann K, London G, et al. A Controlled Randomized European Multicenter Study (CREMS) on mortality in chronic dialysis (CD) patients: a comparison between acetate‐free biofiltration (AFB) and conventional bicarbonate dialysis (BD) [abstract no: FO033]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi15. [Google Scholar]
- Santoro A, Tessitore N, Wizemann V, Panzetta O, Perez R, Ara JM, et al. Comparison between acetate‐free biofiltration (AFB) and bicarbonate dialysis (BD) on mortality in chronic dialysis (CD) patients: a Controlled Randomized European Multicenter Study (CREMS) [abstract no: 767]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):511A‐2A. [Google Scholar]
- Tessitore N, Santoro A, Panzetta GO, Wizemann V, Perez‐Garcia R, Martinez AJ, et al. Acetate‐free biofiltration reduces intradialytic hypotension: a European multicenter randomized controlled trial. Blood Purification 2012;34(3‐4):354‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Santoro 2005a {published and unpublished data}
- Santoro A, Mancini E, Bibiano L, Specchio A, Francioso A, Robaudo C, et al. Online convective therapies: results from a hemofiltration trial. Contributions to Nephrology 2005;149:51‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Santoro A, Mancini E, Bolzani R, Boggi R, Cagnoli L, Francioso A, et al. The effect of on‐line high‐flux hemofiltration versus low‐flux hemodialysis on mortality in chronic kidney failure: a small randomized controlled trial. American Journal of Kidney Diseases 2008;52(3):507‐18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Santoro A, Mancini E, MAMHEBI Study Group. Effects of online hemofiltration (OL‐HF) versus bicarbonate dialysis (BD) on mortality and morbidity in hemodialysis (HD) patients: a prospective, randomized, multicenter trial (MAMHEBI Study) [abstract no: TH‐FC106]. Journal of the American Society of Nephrology 2006;17(Abstracts):24A. [CENTRAL: CN‐00653816] [Google Scholar]
Schiffl 1992 {published data only}
- Schiffl H, D'Agostini B, Held E. Removal of beta‐2 microglobulin by hemodialysis and hemofiltration: a four year follow‐up. Biomaterials, Artificial Cells, & Immobilization Biotechnology 1992;20(5):1223‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schiffl 2007 {published data only}
- Schiffl H. Prospective randomized cross‐over long‐term comparison of online haemodiafiltration and ultrapure high‐flux haemodialysis. European Journal of Medical Research 2007;12(1):26‐33. [MEDLINE: ] [PubMed] [Google Scholar]
Schrander vd Meer 1998 {published data only}
- Schrander‐Van der Meer AM, Wee PM, Donker AJ, Dorp WT, Gasthuis K. Improved dialysis efficiency during acetate free biofiltration (AFB) [abstract no: A0858]. Journal of the American Society of Nephrology 1996;7(9):1419. [CENTRAL: CN‐00583169] [Google Scholar]
- Schrander‐vd Meer AM, ter Wee PM, Donker AJ, Dorp WT. Dialysis efficacy during acetate‐free biofiltration. Nephrology Dialysis Transplantation 1998;13(2):370‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schrander‐vd Meer AM, ter Wee PM, Donker AJ, Dorp WT. Improved dialysis efficiency during acetate free biofiltration [abstract]. Nephrology 1997;3(Suppl 1):S538. [CENTRAL: CN‐00461692] [Google Scholar]
- Schrander‐vd Meer AM, ter Wee PM, Kan G, Donker AJ, Dorp WT. Cardiovascular effects of acetate free biofiltration [abstract]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):172A. [CENTRAL: CN‐00447637] [Google Scholar]
- Schrander‐vd Meer AM, ter Wee PM, Kan G, Donker AJ, Dorp WT. Improved cardiovascular variables during acetate free biofiltration. Clinical Nephrology 1999;51(5):304‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Selby 2006a {published data only (unpublished sought but not used)}
- Selby NM, Fluck RJ, Taal MW, McIntyre CW. Acetate‐free double chamber haemodiafiltration (PHF) is associated with improved systemic haemodynamics and lower troponin‐T levels as compared with standard dialysis [abstract no: SP298]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v119. [Google Scholar]
- Selby NM, Fluck RJ, Taal MW, McIntyre CW. Effects of acetate‐free double‐chamber hemodiafiltration and standard dialysis on systemic hemodynamics and troponin T levels. ASAIO Journal 2006;52(1):62‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stefansson 2012 {published and unpublished data}
- Stefansson BV, Abramson M, Nilsson U, Haraldsson B. Hemodiafiltration improves plasma 25‐hepcidin levels: a prospective, randomized, blinded, cross‐over study comparing hemodialysis and hemodiafiltration. Nephron Extra 2012;2(1):55‐65. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson BV, Haraldsson B. A prospective, double blinded, randomized, cross‐over study comparing hemodialysis and hemodiafiltration regarding dialysis complications, quality of life and non‐traditional cardiovascular risk factors [abstract no: TH‐PO626]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):248A. [Google Scholar]
Teo 1987 {published data only}
- Teo KK, Basile C, Ulan RA, Hetherington MD, Kappagoda T. Effects of hemodialysis and hypertonic hemodiafiltration on cardiac function compared. Kidney International 1987;32(3):399‐407. [EMBASE: 1987215388] [DOI] [PubMed] [Google Scholar]
Todeschini 2002 {published data only}
- Todeschini M, Macconi D, Fernandez NG, Ghilardi M, Anabaya A, Binda E, et al. Effect of acetate‐free biofiltration and bicarbonate hemodialysis on neutrophil activation. American Journal of Kidney Diseases 2002;40(4):783‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tuccillo 2002 {published data only}
- Tuccillo S, Bellizzi V, Catapano F, Iorio B, Esposito L, Giannattasio P, et al. Acute and chronic effects of standard hemodialysis and soft hemodiafiltration on interdialytic serum phosphate levels [Effetti acuti e cronici della emodialisi standard e dell'emodiafiltrazione‐soft sui livelli interdialitici della fosforemia]. Giornale Italiano di Nefrologia 2002;19(4):439‐45. [MEDLINE: ] [PubMed] [Google Scholar]
TURKISH HDF 2013 {published and unpublished data}
- Keller DM. Hemodiafiltration, hemodialysis show similar survival rates. 2011. http://www.medscape.com/viewarticle/746131 (accessed 26 March 2015).
- Ok E, Asci G, Ok ES, Kircelli F, Yilmaz M, Demirci MS, et al. Comparison of post dilution on‐line hemodiafiltration and hemodialysis (Turkish HDF study) [abstract]. NDT Plus 2011;4(Suppl 2):4.2S.1. [Google Scholar]
- Ok E, Asci G, Toz H, Ok ES, Kircelli F, Yilmaz M, et al. Mortality and cardiovascular events in online haemodiafiltration (OL‐HDF) compared with high‐flux dialysis: results from the Turkish OL‐HDF Study. Nephrology Dialysis Transplantation 2013;28(1):192‐202. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vaslaki 2006 {published data only (unpublished sought but not used)}
- Vaslaki L, Berta K, Ladanyi E, Pethoe F, Karatson A, Misz M, et al. Less need for erythropoietin in on‐line haemodiafiltration compared to haemodialysis [abstract no: MP406]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v336. [Google Scholar]
- Vaslaki L, Major L, Berta K, Karatson A, Misz M, Pethoe F, et al. On‐line haemodiafiltration versus haemodialysis: stable haematocrit with less erythropoietin and improvement of other relevant blood parameters. Blood Purification 2006;24(2):163‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Verzetti 1998 {published data only}
- Verzetti G, Navino C, Bolzani R, Galli G, Panzetta G. Acetate‐free biofiltration versus bicarbonate haemodialysis in the treatment of patients with diabetic nephropathy: a cross‐over multicentric study. Nephrology Dialysis Transplantation 1998;13(4):955‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Verzetti G, Navino C, Panzetta G, Galli G, Ortensia A, Odone P, et al. Multicentric trial on acetate‐free biofiltration (AFB) and diabetes [abstract]. Nephrology Dialysis Transplantation 1995;10(6):1031. [CENTRAL: CN‐00261128] [Google Scholar]
Ward 2000 {published data only}
- Ward RA, Schmidt B, Hillebrand GF, Samtleben W. On‐line hemodiafiltration (HDF) provides better solute removal than high‐flux hemodialysis (hfHD) [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):186A. [CENTRAL: CN‐00448283] [DOI] [PubMed] [Google Scholar]
- Ward RA, Schmidt B, Hullin J, Hillebrand GF, Samtleben W. A comparison of on‐line hemodiafiltration and high‐flux hemodialysis: a prospective clinical study. Journal of the American Society of Nephrology 2000;11(12):2344‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wizemann 2000 {published data only}
- Wizemann V, Lotz C, Techert F, Uthoff S. On‐line haemodiafiltration versus low‐flux haemodialysis. A prospective randomized study. Nephrology Dialysis Transplantation 2000;15 Suppl 1:43‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adam 1996 {published data only}
- Adam S, Gretz N, Hafner G, Ragaller M, Abreu M, Bender HJ. Influence of haemofiltration, ‐diafiltration and ‐dialysis on the elimination of urophanic substances under continuous renal‐replacement‐therapy with high‐reflux‐membranes [abstract]. Wiener Klinische Wochenschrift 1996;108(Suppl 1):28. [CENTRAL: CN‐00251993] [Google Scholar]
Ahrenholz 1997 {published data only}
- Ahrenholz P, Winkler RE, et al. On‐line hemodiafiltration with pre‐ and post‐dilution: a comparison of efficacy and compatibility [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A125. [CENTRAL: CN‐00261396] [Google Scholar]
Ahrenholz 1998 {published data only}
- Ahrenholz P, Winkler RE, Ramlow W, Tiess M, Thews O. On‐line hemodiafiltration with pre‐ and postdilution: impact on the acid‐base status. International Journal of Artificial Organs 1998;21(6):321‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Ahrenholz 2004 {published data only}
- Ahrenholz PG, Winkler RE, Michelsen A, Lang DA, Bowry SK. Dialysis membrane‐dependent removal of middle molecules during hemodiafiltration: the beta2‐microglobulin/albumin relationship. Clinical Nephrology 2004;62(1):21‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Altieri 1997 {published data only}
- Altieri P, Sorba GB, Bolasco PG, Bostrom M, Asproni E, Ferrara R, et al. On‐line predilution hemofiltration versus ultrapure high‐flux hemodialysis: a multicenter prospective study in 23 patients. Sardinian Collaborative Study Group of On‐Line Hemofiltration. Blood Purification 1997;15(3):169‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Altieri 1999 {published data only}
- Altieri P, Sorba GB, Bolasco PG, Ledebo I, Asproni E, Ferrara R, et al. Predilution hemofiltration vs hemodialysis. Results from prospective crossover multicenter study [abstract]. Nephrology Dialysis Transplantation 1999;14(9):A224. [CENTRAL: CN‐00520313] [Google Scholar]
Altieri 2000 {published data only}
- Altieri P, Sorba G, Bolasco P, Asproni E, Ledebo I, Bostrom M, et al. Pre‐dilution haemofiltration‐‐the Sardinian multicentre studies: Present and future. The Sardinian Collaborative Study Group on Haemofiltration On‐Line. Nephrology Dialysis Transplantation 2000;15 Suppl 2:55‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Altieri 2001 {published data only}
- Altieri P, Sorba G, Bolasco P, Asproni E, Ledebo I, Cossu M, et al. Predilution haemofiltration‐‐the Second Sardinian Multicentre Study: comparisons between haemofiltration and haemodialysis during identical Kt/V and session times in a long‐term cross‐over study. Nephrology Dialysis Transplantation 2001;16(6):1207‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Baldamus 1980 {published data only}
- Baldamus CA, Knobloch M, Schoeppe, Koch KM. Hemodialysis/hemofiltration: a report of a controlled cross‐over study. International Journal of Artificial Organs 1980;3(4):211‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Baldamus 1982 {published data only}
- Baldamus CA, Ernst W, Frei U, Koch KM. Sympathetic and hemodynamic response to volume removal during different forms of renal replacement therapy. Nephron 1982;31(4):324‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Baldamus 1985 {published data only}
- Baldamus CA, Quellhorst E. Outcome of long‐term hemofiltration. Kidney International ‐ Supplement 1987;17:S41‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Baragett 2003 {published data only}
- Baragett I, Bamonti F, Patrosso C, Corghi E, Furiani S, D'Aloja G, et al. Effect of acetate free biofiltration on hyperhomocysteinemia in uremic patients: A cross‐sectional multicenter study. International Journal of Artificial Organs 2003;26(3):256‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Basile 1985 {published data only}
- Basile C, Maggio A, Scatizzi A. Acid‐base imbalance correction and dialysis hypoxemia in hypertonic hemodiafiltration (HHDF) [abstract]. Kidney International 1985;28(2):364. [CENTRAL: CN‐00550391] [Google Scholar]
Basile 1985a {published data only}
- Basile C, Maggio A, Scatizzi A. Small and middle molecules removal in hypertonic hemodiafiltration [abstract]. Kidney International 1985;28(2):364. [CENTRAL: CN‐00550396] [Google Scholar]
Basile 1987 {published data only}
- Basile C, Miller JD, Koles ZJ, Grace M, Ulan RA. The effects of dialysis on brain water and EEG in stable chronic uremia. American Journal of Kidney Diseases 1987;9(6):462‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Basile 1988 {published data only}
- Basile C, Maggio A, Ulan RA, Scatizzi A. Hypertonic hemodiafiltration: a preliminary report on a cross‐over study. Kidney International ‐ Supplement 1988;24:S132‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Bazzatto 1988 {published data only}
- Bazzato G, Coli U, Landini S, Fracasso A, Righetto F, Scanferla F, et al. Removal of phosphate either by bicarbonate dialysis or biofiltration in uremics. Kidney International ‐ Supplement 1988;24:S180‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Beerenhout 2002 {published data only}
- Beerenhout CH, Kooman JP, Luik AJ, Jeuken‐Mertens SG, Sande FM, Leunissen KM. Optimizing renal replacement therapy‐‐a case for online filtration therapies?. Nephrology Dialysis Transplantation 2002;17(12):2065‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bolasco 2000 {published data only}
- Bolasco P, Altieri P, Sorba G, Cabiddu G, Ferrara R, Serra G, et al. Adequacy in pre‐dilution haemofiltration: Kt/V or infusion volume? The Sardinian Collaborative Study Group on Haemofiltration On‐line. Nephrology Dialysis Transplantation 2000;15 Suppl 2:60‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bonaudo 1998 {published data only}
- Bonaudo R, Pacitti A, Amore A, Gianoglio B, Perugin L, Goldoni M, et al. Hemofiltration without heparin [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):167A. [CENTRAL: CN‐00444479] [Google Scholar]
Bonomini 2004 {published data only}
- Bonomini M, Ballone E, Stante S, Bucciarelli T, Dottori S, Arduini A, et al. Removal of uraemic plasma factor(s) using different dialysis modalities reduces phosphatidylserine exposure in red blood cells. Nephrology Dialysis Transplantation 2004;19(1):68‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bordin 2002 {published data only}
- Bordin V, Catalano C, Berto A, Silvestrin G, Landro D, Fabbian F. Determining the prescription for online predilution hemofiltration: Our experience and a review of the literature. Dialysis & Transplantation 2002;31(6):375‐9. [EMBASE: 2002191148] [Google Scholar]
Bosc 1997 {published data only}
- Bosc JY, LeBlanc M, et al. Comparison between high flux cellulose triacetate and high flux polysulfone: a clinical study in hemodialysis and hemodiafiltration [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A150. [CENTRAL: CN‐00261407] [Google Scholar]
Bosc 1998 {published data only}
- Bosc JY, LeBlanc M, Garred LJ, Marc JM, Foret M, Babinet F, et al. Direct determination of blood recirculation rate in hemodialysis by a conductivity method. ASAIO Journal 1998;44(1):68‐73. [MEDLINE: ] [PubMed] [Google Scholar]
Boscticardo 1981 {published data only}
- Bosticardo GM, Triolo G, Basolo B, Belardi P, Bossi P, Aprato A, et al. Hemofiltration: present and future [Emofiltrazione: presente e futuro]. Archivio Per Le Scienze Mediche 1981;138(1):1‐10. [MEDLINE: ] [PubMed] [Google Scholar]
Brink 1995 {published data only}
- Brink HS, Friemann AT, Huisman RM, Jong PE. Acetate free biofiltration versus haemodiafiltration: a prospective cross‐over trial [abstract]. Nephrology Dialysis Transplantation 1995;10(4):572. [CENTRAL: CN‐00444541] [Google Scholar]
Calo 2007 {published data only}
- Calo L, Naso A, Carraro G, Wratten ML, Pagnin E, Bertipaglia L, et al. Effect of hemodiafiltration with on‐line regeneration of ultrafiltrate (HFR) on oxidative stress in dialysis patients [abstract no: SP679]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv244. [DOI] [PubMed] [Google Scholar]
- Calo LA, Naso A, Carraro G, Wratten ML, Pagnin E, Bertipaglia L, et al. Effect of haemodiafiltration with online regeneration of ultrafiltrate on oxidative stress in dialysis patients. Nephrology Dialysis Transplantation 2007;22(5):1413‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Canaud 1994 {published data only}
- Canaud B, Flavier JL, Argiles A, Stec F, NGuyen QV, Bouloux C, et al. Hemodiafiltration with on‐line production of substitution fluid: long‐term safety and quantitative assessment of efficacy. Contributions to Nephrology 1994;108:12‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Canaud 2000 {published data only}
- Canaud B, Bosc JY, Leray‐Moragues H, Stec F, Argiles A, Leblanc M, et al. On‐line haemodiafiltration. Safety and efficacy in long‐term clinical practice. Nephrology Dialysis Transplantation 2000;15 Suppl 1:60‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Canaud 2001 {published data only}
- Canaud B, Wizemann V, Pizzarelli F, Greenwood R, Schultze G, Weber C, et al. Cellular interleukin‐1 receptor antagonist production in patients receiving on‐line haemodiafiltration therapy. Nephrology Dialysis Transplantation 2001;16(11):2181‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cappelli 1985 {published data only}
- Cappelli G, Icardi A, Lamperi S. Biofiltration (BF) versus hemofiltration (HF) in the treatment of chronic uremia [abstract]. Kidney International 1985;28(2):365. [CENTRAL: CN‐00550380] [PubMed] [Google Scholar]
Carozzi 1992 {published data only}
- Carozzi S, Nasini MG, Caviglia PM, Schelotto C, Santoni O, Atti M. Acetate free biofiltration. Effects on peripheral blood monocyte activation and cytokine release. ASAIO Journal 1992;38(1):52‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Cavalcanti 2004 {published data only}
- Cavalcanti S, Ciandrini A, Severi S, Badiali F, Bini S, Gattiani A, et al. Model‐based study of the effects of the hemodialysis technique on the compensatory response to hypovolemia. Kidney International 2004;65(4):1499‐510. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cerulli 2000 {published data only}
- Cerulli N, Greca G, Laville M, Palla R, Ramello A, Ghezzi PM, et al. The effect of hemodiafiltration with on‐line endogenous reinfusion (on‐line HFR) on anemia: Design of a European, open, randomised, multicentre trial. European Collaborative Study. Journal of Nephrology 2000;13(1):34‐42. [MEDLINE: ] [PubMed] [Google Scholar]
Champagne 2008 {published data only}
- Champagne K, Barre P, Tangri N, Iqbal S, Kimoff RJ. Effect of hemodiafiltration vs conventional hemodialysis on sleep apnea severity and 24‐hour blood pressure level [abstract no: M545]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009.
- Champagne K, Tangri N, Barre P, Kimoff R. Effect of hemodiafiltration (HDF) vs. conventional hemodialysis (CHD) on sleep apnea (SA) severity: findings of a pilot randomized cross‐over study [abstract no: J14]. American Thoracic Society International Conference; 2008 May 16‐21; Toronto, Canada. 2008:A937.
Chanard 1988 {published data only}
- Chanard J, Toupance O, Gillery P, Lavaud S. Evaluation of protein loss during hemofiltration. Kidney International ‐ Supplement 1988;24:S114‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Chang 1979 {published data only}
- Chang TM, Chirito E, Barre P, Cole C, Lister C, Resurreccion E. Long‐term clinical assessment of combined ACAC hemoperfusion‐ultrafiltration in uremia. Artificial Organs 1979;3(2):127‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chauveau 1993 {published data only}
- Chauveau P, Houillier P, Ramdane M, Zins B, Poignet JL, Naret C, et al. Serial determinations of bone mineral density in hemodialyzed patients on acetate free biofiltration [abstract]. 12th International Congress of Nephrology; 1993 Jun 13‐18; Jerusalem, Israel. 1993:468.
Chen 2005c {published data only}
- Chen X, Li Z, Wu P, Zhang D, Shen Q, Yu X. B2‐microglobulin and iPTH removal in on‐line hemodiafiltration versus low‐ and high‐flux hemodialysis [abstract no: T‐PO40041]. Nephrology 2005;10(Suppl 1):A185. [CENTRAL: CN‐00782409] [Google Scholar]
Chiappini 2004 {published data only}
- Chiappini MG, Ammann T, Selvaggi G, Bravi M, Traietti P. Effects of different dialysis membranes and techniques on the nutritional status, morbidity and mortality of hemodialysis patients [Effetti di diverse membrane e metodiche dialitiche sullo stato nutrizionale, morbilita e mortalita di pazienti emodializzati]. Giornale Italiano di Nefrologia 2004;21 Suppl 30:S190‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Cirillo 2011 {published data only}
- Cirillo I, Vaccaro N, Balis D, Redman R, Matzke GR. Influence of continuous venovenous hemofiltration and continuous venovenous hemodiafiltration on the disposition of doripenem. Antimicrobial Agents & Chemotherapy 2011;55(3):1187‐93. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 1985 {published data only}
- Collins AJ, Keshaviah P, Ilstrup KM, Shapiro F. Clinical comparison of hemodialysis and hemofiltration. Kidney International ‐ Supplement 1985;17:S18‐22. [MEDLINE: ] [PubMed] [Google Scholar]
David 2000 {published data only}
- David S, Triolo G, Tessitore N, Gruppo di Studio Collaborativo Europeo HFR on line. EPO response in patients treated by hemodiafiltration with infusion of regenerated ultrafiltrate (on line HFR): a protocol for a multicenter study [abstract]. Nephrology Dialysis Transplantation 2000;15(9):A163. [CENTRAL: CN‐00460611] [Google Scholar]
Duranti 2004 {published data only}
- Duranti E. Acetate‐free hemodialysis: a feasibility study on a technical alternative to bicarbonate dialysis. Blood Purification 2004;22(5):446‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Feliciani 2007 {published data only}
- Feliciani A, Riva MA, Zerbi S, Ruggiero P, Plati AR, Cozzi G, et al. New strategies in haemodiafiltration (HDF): prospective comparative analysis between on‐line mixed HDF and mid‐dilution HDF. Nephrology Dialysis Transplantation 2007;22(6):1672‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pedrini LA, Feliciani A, Riva A, Zerbi S, Cozzi G. Middle molecular uremic toxins removal. Prospective comparative study between two new on‐line haemodiafiltration (HDF) techniques: mixed HDF and mid‐dilution HDF [abstract no: SP695]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv249. [CENTRAL: CN‐00615864] [Google Scholar]
Fu 2006 {published data only}
- Fu R, Gui B, Wang L, Li R, Guo R, Zhang Y. The clinical study on dialysis effect and nutritional status of different dialysis modes. Journal of Xi'an Jiaotong University Medical Sciences 2006;27(4):414‐6. [EMBASE: 2006447629] [Google Scholar]
Gerdemann 2002 {published data only}
- Gerdemann A, Wagner Z, Solf A, Bahner U, Heidland A, Vienken J, et al. Plasma levels of advanced glycation end products during haemodialysis, haemodiafiltration and haemofiltration: Potential importance of dialysate quality. Nephrology Dialysis Transplantation 2002;17(6):1045‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Giannattasio 2006 {published data only}
- Giannattasio P, Minutolo R, Bellizzi V, Iorio BR, Scigliano R, Zamboli P, et al. Effects of efficiency and length of acetate‐free biofiltration session on postdialysis solute rebound. American Journal of Kidney Diseases 2006;47(6):1045‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harzallah 2008 {published data only}
- Harzallah K, Hichri N, Mazigh C, Tagorti M, Hmida A, Hmida J. Variability of acid‐base status in acetate‐free biofiltration 84% versus bicarbonate dialysis. Saudi Journal of Kidney Diseases & Transplantation 2008;19(2):215‐21. [MEDLINE: ] [PubMed] [Google Scholar]
Hdez‐Jaras 1994 {published data only}
- Hdez‐Jaras J, Galan A, Martin J. Kinetics of bicarbonate and acetate: a comparative study between haemodiafiltration in double chamber and high‐flow haemodialysis [abstract]. Nephrology Dialysis Transplantation 1994;9(10):1512. [CENTRAL: CN‐00261075] [Google Scholar]
Henderson 1980 {published data only}
- Henderson LW. Hemofiltration for the treatment of hypertensions associated with end‐stage renal failure. Artificial Organs 1980;4(2):103‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higuchi 2004 {published data only}
- Higuchi T, Yamamoto C, Kuno T, Okada K, Soma M, Fukuda N, et al. A comparison of bicarbonate hemodialysis, hemodiafiltration, and acetate‐free biofiltration on cytokine production. Therapeutic Apheresis & Dialysis 2004;8(6):460‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hillion 1997 {published data only}
- Hillion D, Robine M, et al. Evaluation of a new triacetate membrane in hemodiafiltration (HDF) compared with 5 high performance hemodiafilters [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A167. [CENTRAL: CN‐00261415] [Google Scholar]
Hmida 2002 {published data only}
- Hmida J, Balma A, Lebben I, Hichri N, Dhahri M. Clinical evaluation of acetate‐free biofiltration at 84 0/00 in patients with chronic renal insufficiency [Evaluation clinique a moyen terme de la technique de biofiltration sans acetate a 84 0/00 chez les malades insuffisants renaux chroniques]. Tunisie Medicale 2002;80(8):473‐84. [MEDLINE: ] [PubMed] [Google Scholar]
Ikebe 2006 {published data only}
- Ikebe N, Eguchi K, Suzuki K, Miwa N, Mineshima M, Akiba T. Trial of a new haemodiafiltration (HDF): intermittent infusion HDF [abstract no: SP685]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv246. [Google Scholar]
Jahn 1981 {published data only}
- Jahn H, Schohn D, Schmitt R. Hemodynamic studies in chronic terminal renal insufficiency‐‐Effects of hemodialysis (Hd), hemofiltration (Hf) and ultrafiltration (Uf) procedures [Etudes hemodynamiques au cours de l'insuffisance renale chronique terminale‐‐effets des techniques d'epuration extrarenale]. Nephrologie 1981;2(2):53‐62. [MEDLINE: ] [PubMed] [Google Scholar]
Jose 2008 {published data only}
- Jose M, Yu R, Kirkland G. The influence of haemodiafiltration on inflammation, insulin resistance and adipokines [abstract no: 044]. Nephrology 2008;13(Suppl 3):A110. [Google Scholar]
Joyeux 2009 {published data only}
- Joyeux V, Sijpkens Y, Nilsson L, Haddj‐Elmrabet A, Bijvoet AJ. Automated pressure control mode improves middle molecule removal in hemodiafiltration [abstract no: M450]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009.
Kanter 2008 {published data only}
- Kanter J, Puerta MC, Garcia RP, Gomez JM, Jofre R, Rodriguez PB. On‐line sequential hemodiafiltration (HDF‐OL‐S): a new therapeutic option [Hemodiafiltracion en linea secuencial (HDF‐OL‐S): una nueva opcion terapeutica]. Nefrologia 2008;28(4):433‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Katschnig 1980 {published data only}
- Katschnig H, Pogglitsch H, Holzer H. Hemofiltrate dialysis: first experiences with hemofiltrate regeneration by means of dialysis [Hamofiltratdialyse: klinisch‐experimentelle daten zur hamofiltratregeneration mittels dialyse]. Nieren‐und Hochdruckkrankheiten 1980;9(3):132‐5. [EMBASE: 1980220746] [Google Scholar]
Kim 2009 {published data only}
- Kim S, Oh KH, Chin HJ, Na KY, Kim YS, Chae DW, et al. Effective removal of leptin via hemodiafiltration with on‐line endogenous reinfusion therapy. Clinical Nephrology 2009;72(6):442‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kishimoto 1980 {published data only}
- Kishimoto T, Yamagami S, Tanaka H, Ohyama T, Yamamoto T, Yamakawa M, et al. Superiority of hemofiltration to hemodialysis for treatment of chronic renal failure: comparative studies between hemofiltration and hemodialysis on dialysis disequilibrium syndrome. Artificial Organs 1980;4(2):86‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Klemm 1997 {published data only}
- Klemm A, Sperschneider H, Winnefeld K, Gunther K, Stein G. Comparison of aluminum removal between hemodialysis with polycarbonate low flux membrane and hemofiltration with polysulfone high flux membrane in end‐stage renal failure patients. Clinical Nephrology 1997;47(2):133‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Klingel 2004 {published data only}
- Klingel R, Schaefer M, Schwarting A, Himmelsbach F, Altes U, Uhlenbusch‐Korwer I, et al. Comparative analysis of procoagulatory activity of haemodialysis, haemofiltration and haemodiafiltration with a polysulfone membrane (APS) and with different modes of enoxaparin anticoagulation. Nephrology Dialysis Transplantation 2004;19(1):164‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Krieter 2005 {published data only}
- Krieter DH, Falkenhain S, Chalabi L, Collins G, Lemke HD, Canaud B. Clinical cross‐over comparison of mid‐dilution hemodiafiltration using a novel dialyzer concept and post‐dilution hemodiafiltration. Kidney International 2005;67(1):349‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Krieter DH, Falkenhain S, Chalabi L, Collins G, Summerton J, Spence E, et al. Improved middle molecule removal in mid‐dilution HDF (HDF) with the nephros olpur™ md 190 filter compared to post‐dilution HDF (post‐HDF) [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:366. [CN‐00509293]
Krieter 2008a {published data only}
- Krieter DH, Hunn E, Morgenroth A, Lemke HD, Wanner C. Matching efficacy of high‐efficiency HDF in simple HD mode with PUREMA [abstract no: PUB297]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):894A. [Google Scholar]
- Krieter DH, Hunn E, Morgenroth A, Lemke HD, Wanner C. Matching efficacy of online hemodiafiltration in simple hemodialysis mode. Artificial Organs 2008;32(12):903‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Krieter 2010 {published data only}
- Krieter DH, Hackl A, Rodriguez A, Chenine L, Moragues HL, Lemke HD, et al. Protein‐bound uraemic toxin removal in haemodialysis and post‐dilution haemodiafiltration. Nephrology Dialysis Transplantation 2010;25(1):212‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kuno 1994 {published data only}
- Kuno T, Kikuchi F, Yanai M, Nagura Y, Takahashi S. Clinical advantages of acetate‐free biofiltration. Contributions to Nephrology 1994;108:121‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Leber 1980 {published data only}
- Leber HW, Wizemann V, Techert F. Simultaneous hemofiltration/hemodialysis (HF/HD): short‐ and long‐term tolerance. Introduction of a system for automatic fluid replacement. Artificial Organs 1980;4(2):108‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Li 1997 {published data only}
- Li X, Li M, Liu T, Li L, Duan L, Li Y, et al. Hemofiltration or hemodiafiltration with on‐line production of substitution fluid: clinical observation of safety and effectiveness. Chinese Medical Journal 1997;110(7):520‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Lin 2003a {published data only}
- Lin CL, Huang CC, Yu CC, Yang HY, Chuang FR, Yang CW. Reduction of advanced glycation end product levels by on‐line hemodiafiltration in long‐term hemodialysis patients. American Journal of Kidney Diseases 2003;42(3):524‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Liomin 1984 {published data only}
- Liomin E, Schneider H, Streicher E. Hemodynamics during hemodialysis, hemofiltration and hemodiafiltration using bicarbonate vs acetate buffers [Hamodynamik bei hamodialyse, hamofiltration und hamodiafiltration bikarbonat‐vs azetatpufferung]. Klinische Wochenschrift 1984;62(19):911‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Locatelli 1998 {published data only}
- Locatelli F, Vecchio L, Manzoni C. Morbidity and mortality on maintenance haemodialysis. Nephron 1998;80(4):380‐400. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Locatelli 1999 {published data only}
- Locatelli F, Marcelli D, Conte F, Limido A, Malberti F, Spotti D. Comparison of mortality in ESRD patients on convective and diffusive extracorporeal treatments. The Registro Lombardo Dialisi E Trapianto. Kidney International 1999;55(1):289‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Locatelli 2001 {published data only}
- Locatelli F, Vecchio L, Andrulli S. Dialysis: its role in optimizing recombinant erythropoietin treatment. Nephrology Dialysis Transplantation 2001;16 Suppl 7:29‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Locatelli 2002 {published data only}
- Locatelli F, Manzoni C, Filippo S. The importance of convective transport. Kidney International ‐ Supplement 2002;80:115‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lornoy 1998a {published data only}
- Lornoy W, Becaus I, Billiouw JM, Sierens L, Malderen P, Rampelbergh S, et al. B2 microglobulin kinetics in on‐line hemodiafiltration with high volume of replacement solution versus high flux hemodialysis. Is there a limit in the amount of replacement volume? [abstract]. 35th Congress. European Renal Association. European Dialysis and Transplantation Association; 1998 Jun 6‐9; Rimini, Italy. 1998:302. [CENTRAL: CN‐00484891]
Lornoy 2001 {published data only}
- Lornoy W, Becaus I, Billiouw J, Beckers F, Malderen P, Langenhove P. Comparison of different high flux membranes with on‐line hemodialfiltration [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):453A. [CENTRAL: CN‐00626021] [Google Scholar]
- Lornoy W, Becaus I, Billiouw JM, Beckers F, Malderen P, Langenhove P. Comparison of different high flux membranes with on‐line haemodiafiltration (HDF) [abstract]. 38th Congress. European Renal Association. European Dialysis and Transplantation Association; 2001 Jun 24‐27; Vienna, Austria. 2001:253. [CENTRAL: CN‐00461209]
Maeda 1990 {published data only}
- Maeda K, Kobayakawa H, Fujita Y, Takai I, Morita H, Emoto Y, et al. Effectiveness of push/pull hemodiafiltration using large‐pore membrane for shoulder joint pain in long‐term dialysis patients. Artificial Organs 1990;14(5):321‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Maggiore 2000 {published data only}
- Maggiore Q, Pizzarelli F, Dattolo P, Maggiore U, Cerrai T. Cardiovascular stability during haemodialysis, haemofiltration and haemodiafiltration. Nephrology Dialysis Transplantation 2000;15 Suppl 1:68‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Maheshwari 2012 {published data only}
- Maheshwari V, Samavedham L, Rangaiah GP, Loy Y, Ling LH, Sethi S, et al. Comparison of toxin removal outcomes in online hemodiafiltration and intra‐dialytic exercise in high‐flux hemodialysis: a prospective randomized open‐label clinical study protocol. BMC Nephrology 2012;13:156. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Malberti 1991 {published data only}
- Malberti F, Surian M, Farina M, Vitelli E, Mandolfo S, Guri L, et al. Effect of hemodialysis and hemodiafiltration on uremic neuropathy. Blood Purification 1991;9(5‐6):285‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mastrangelo 1986 {published data only}
- Mastrangelo F, Rizzelli S, Passione A, Procaccini DA, Gigante B, Gallucci M, et al. Puglia cooperative study on biofiltration. International Journal of Artificial Organs 1986;9 Suppl 3:25‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Mesic 2011 {published data only}
- Mesic E, Bock A, Major L, Vaslaki L, Berta K, Wikstrom B, et al. Dialysate saving by automated control of flow rates: comparison between individualized online hemodiafiltration and standard hemodialysis. Hemodialysis International 2011;15(4):522‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Minutolo 2002 {published data only}
- Minutolo R, Bellizzi V, Cioffi M, Iodice C, Giannattasio P, Andreucci M, et al. Postdialytic rebound of serum phosphorus: pathogenetic and clinical insights. Journal of the American Society of Nephrology 2002;13(4):1046‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mioli 1986 {published data only}
- Mioli V, Albertazzi A, Baroni C, Bordoni E, Buoncristiani U, Capponi E, et al. Polycentric 384‐month study of biofiltration (BF) with AN69s. International Journal of Artificial Organs 1986;9 Suppl 3:15‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Mishkin 2002 {published data only}
- Mishkin MA, Mishkin GJ, Lew SQ. Solute removal in hemodiafiltration compared to standard high flux dialysis: combined analysis of 2 cross over studies [abstract no: F‐P0870]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):238A. [CENTRAL: CN‐00446789] [Google Scholar]
Mohini 1989 {published data only}
- Mohini R, Michaels R, Trost C, Gron D, Zasuwa G, Dumler F, et al. Comparison of effect of recombinant human erythropoietin (rHEPO) in patients on high flux (HF) vs conventional (C) hemodialysis [abstract]. Kidney International 1989;35(1):256. [CENTRAL: CN‐00782715] [Google Scholar]
Morena 2006 {published data only}
- Morena M, Dupuy A, Taamma R, Tetta C, Cristol J, Canaud B, et al. Convective techniques improve middle substances removal: a randomized cross‐over study comparing hemodialysis and on line hemodiafiltration using HF80 versus FX80 [abstract no: SP697]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv251. [Google Scholar]
Movilli 2011 {published data only}
- Movilli E, Camerini C, Gaggia P, Poiatti P, Pola A, Viola BF, et al. Effect of post‐dilutional on‐line haemodiafiltration on serum calcium, phosphate and parathyroid hormone concentrations in uraemic patients. Nephrology Dialysis Transplantation 2011;26(12):4032‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mrowka 1993 {published data only}
- Kuchle C, Fricke H, Held E, Schiffl H. High‐flux hemodialysis postpones clinical manifestation of dialysis‐related amyloidosis. American Journal of Nephrology 1996;16(6):484‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kuchle C, Fricke H, et al. High‐flux hemodialysis postpones clinical manifestation of dialysis associated amyloidosis [abstract]. Nephrology Dialysis Transplantation 1996;11(6):A232. [CN‐00261301] [DOI] [PubMed] [Google Scholar]
- Kuchle C, Lang SM, Held E, Schiffl H. Biocompatibility, b2‐microglobulin and dialysis‐associated amyloidosis [abstract]. Nephrology 1997;3(Suppl 1):S404. [CENTRAL: CN‐00461113] [Google Scholar]
- Mrowka C, Schiffl H. Comparative evaluation of beta 2‐microglobulin removal by different hemodialysis membranes: a six‐year follow‐up. Nephron 1993;63(3):368‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schiffl H, Kuchle C, Held E. Beta‐2‐microglobulin removal by different hemodialysis membranes. Contributions to Nephrology 1995;112:156‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nakazawa 1997 {published data only}
- Nakazawa R, Nakamura M, et al. A comparison of b2‐microglobulin removal during haemodialysis, haemodiafiltration and direct haemoperfusion [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A175. [CENTRAL: CN‐00261422] [Google Scholar]
Ohyama 1981 {published data only}
- Ohyama T. Study on guanidine compounds in chronic renal failure: comparison of hemofiltration (HF) and hemodialysis (HD). Journal of the Osaka City Medical Center 1981;30(3):533‐43. [EMBASE: 1982218680] [Google Scholar]
Pacitti 1993 {published data only}
- Pacitti A, Tetta C, Mangiarotti G, Canavese C, Segoloni GP. Beta‐2‐microglobulin serum profiles in different settings of mass transport and fluid pyrogen content. Kidney International ‐ Supplement 1993;41:S96‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Panichi 1994 {published data only}
- Panichi V, Parrini M, Bianchi AM, Andreini B, Cirami C, Finato V, et al. Mechanisms of acid‐base homeostasis in acetate and bicarbonate dialysis, lactate hemofiltration and hemodiafiltration. International Journal of Artificial Organs 1994;17(6):315‐21. [MEDLINE: ] [PubMed] [Google Scholar]
Panichi 1998 {published data only}
- Panichi V, Pietro S, Andreini B, Migliori M, Tessore V, Taccola D, et al. Cytokine production in haemodiafiltration: a multicentre study. Nephrology Dialysis Transplantation 1998;13(7):1737‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Panichi 2006 {published data only}
- Panichi V, Manca‐Rizza G, Paoletti S, Filippi C, Consani C, Sidoti A, et al. Effects of inflammatory and nutritional markers of hemodiafiltration with online regeneration of ultrafiltrate (HFR) versus on‐line hemodiafiltration: a cross‐over randomized multicenter trial [abstract no: MO13]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v191. [DOI] [PubMed] [Google Scholar]
- Panichi V, Manca‐Rizza G, Paoletti S, Taccola D, Consani C, Filippi C, et al. Effects on inflammatory and nutritional markers of haemodiafiltration with online regeneration of ultrafiltrate (HFR) vs online haemodiafiltration: a cross‐over randomized multicentre trial. Nephrology Dialysis Transplantation 2006;21(3):756‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pedrini 1999 {published data only}
- Pedrini L, Cristafaro V. Hemodiafiltration with simultaneous pre and post dilution (pre‐post‐HDF) [abstract]. Nephrology Dialysis Transplantation 1999;14(9):A177. [CENTRAL: CN‐00583434] [Google Scholar]
Pedrini 2006 {published data only}
- Pedrini LA, Cozzi G, Faranna P, Mercieri A, Ruggiero P, Zerbi S, et al. Transmembrane pressure modulation in high‐volume mixed hemodiafiltration to optimize efficiency and minimize protein loss. Kidney International 2006;69(3):573‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pedrini 2009 {published data only}
- Feliciani A. Reverse mid‐dilution HDF: technical optimization for clearance optimization [abstract no: SP255]. XLV ERA‐EDTA Congress; 2008 May 10‐13; Stockholm, Sweden. 2008.
- Pedrini LA, Feliciani A, Zerbi S, Cozzi G, Ruggiero P. Optimization of mid‐dilution haemodiafiltration: technique and performance. Nephrology Dialysis Transplantation 2009;24(9):2816‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pedrini 2011 {published data only}
- Pedrini LA, Gmerek A, Wagner J. Efficiency of post‐dilution hemodiafiltration with a high‐flux alpha‐polysulfone dialyzer. International Journal of Artificial Organs 2011;34(5):397‐404. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Petras 2005 {published data only}
- Petras D, Fortunato A, Soffiati G, Brendolan A, Bonello M, Crepaldi C, et al. Sequential convective therapies (SCT): a prospective study on feasibility, safety, adequacy and tolerance of on‐line hemofiltration and hemodiafiltration in sequence. International Journal of Artificial Organs 2005;28(5):482‐8. [EMBASE: 2005265716] [DOI] [PubMed] [Google Scholar]
Pizzarelli 2004 {published data only}
- Pizzarelli F, Cerrai T, Dattolo P, Ferro G. On‐line haemodiafiltration with and without acetate. Nephrology Dialysis Transplantation 2006;21(6):1648‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pizzarelli F, Cerrai T, Ferro G, Dattolo P. On‐line hemodiafiltration without acetate. Giornale Italiano di Nefrologia 2004;21 Suppl 30:S97‐101. [MEDLINE: ] [PubMed] [Google Scholar]
Quellhorst 1983 {published data only}
- Quellhorst E. Long‐term follow up in chronic hemofiltration. International Journal of Artificial Organs 1983;6(3):115‐20. [MEDLINE: ] [PubMed] [Google Scholar]
Quellhorst 1983a {published data only}
- Quellhorst EA, Schuenemann B, Hildebrand U. Morbidity and mortality in long‐term hemofiltration. ASAIO Journal 1983;6(4):185‐91. [EMBASE: 1984102779] [Google Scholar]
Ragazzoni 2004 {published data only}
- Ragazzoni E, Carpani P, Agliata S, Ciranna G, Cusinato S, Albini M, et al. HFR vs HDF‐ON line: plasmatic amino acids loss evaluation [HFR vs HDF on‐line: valutazione della perdita aminoacidica plasmatica]. Giornale Italiano di Nefrologia 2004;21 Suppl 30:S85‐90. [MEDLINE: ] [PubMed] [Google Scholar]
Ramunni 2006 {published data only}
- Ramunni A, Piccolo T, Saracino A, Bitonto G, Coratelli P. Efficacy of hemodiafiltration with endogenous reinfusion (HFR) in reducing amino acids depletion: comparison with bicarbonate dialysis [abstract no: SP693]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv249. [Google Scholar]
Rius 2007 {published data only}
- Rius A, Hernandez‐Jaras J, Pons R, Garcia Perez H, Torregrosa E, Sanchez Canel JJ, et al. Kinetic of calcium, phosphate, magnesium and PTH variations during hemodiafiltration [Cineica del calcio, fosforo, magnesio y variaciones de la parathormona (PTH) en pacientes en hemodiafiltracion]. Nefrologia 2007;27(5):593‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Ronco 2000 {published data only}
- Ronco C, Brendolan A, Milan M, Rodeghiero MP, Zanella M, Greca G. Impact of biofeedback‐induced cardiovascular stability on hemodialysis tolerance and efficiency. Kidney International 2000;58(2):800‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sakurai 1990 {published data only}
- Sakurai S, Yoshiyama N, Takeuchi H. Acetate‐free biofiltration (AFBF) vs. bicarbonate hemodialysis (BHD): evaluation of short‐time AFBF with various doses of hypertonic bicarbonate solution [abstract]. 11th International Congress of Nephrology; 1990 Jul 15‐20; Tokyo, Japan. 1990:20A.
Santoro 2005 {published data only}
- Ferramosca E, Conz P, Cristofaro V, Gaggi R, Grammatico F, Wratten ML, et al. Mid‐dilution hemodiafiltration: a new technique able to maximize the middle molecules removal [abstract no: TH‐PO640]. Journal of the American Society of Nephrology 2005;16:258A. [CENTRAL: CN‐00644157] [Google Scholar]
- Santoro A, Conz PA, De C, Acquistapace I, Gaggi R, Ferramosca E, et al. Mid‐dilution: the perfect balance between convection and diffusion. Contributions to Nephrology 2005;149:107‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Santoro 2008 {published data only}
- Santoro A, London G, Cagnoli L, Mercadal L, Fessy H, Perrone B, et al. Intra‐dialytic potassium (K+) profiling may reduce the risk of cardiac arrhytmias in hemodialysi (HD) patients [abstract no: FP353]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi136. [CENTRAL: CN‐00644249] [Google Scholar]
- Santoro A, Mancini E, London G, Mercadal L, Fessy H, Perrone B, et al. Patients with complex arrhythmias during and after haemodialysis suffer from different regimens of potassium removal. Nephrology Dialysis Transplantation 2008;23(4):1415‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Savoldi 2004 {published data only}
- Savoldi S, Sereni L, Bertok S, Ianche M, Bianco F, Marega A, et al. The hemodiafiltration with infusion of acetate‐free dialysis fluid can modify the inflammatory response in patients "high responders" to inflammatory stimuli? [Il trattamento in emodiafiltrazione con infusione di liquido di dialisi (PHF acetate free) puo modificare la risposta infiammatoria in pazienti gia identificati come "high responders" a stimoli infiammatori?]. Giornale Italiano di Nefrologia 2004;21 Suppl 30:S122‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Shaldon 1998 {published data only}
- Shaldon S, Beau MC, Claret G, Deschodt G, Oules R, Ramperez P, et al. Hemofiltration with regeneration of the ultrafiltrate by adsorption: preliminary clinical experience in the treatment of the terminal stage of chronic renal insufficiency [Homofiltration avec regeneration de l'ultrafiltrat par adsorption: premiere experience clinique chez l'insuffisant renal chronique au stade ultim]. Journal d Urologie et de Nephrologie 1978;84(12):878‐83. [MEDLINE: ] [PubMed] [Google Scholar]
Sidoti 2004 {published data only}
- Sidoti A, Borracelli D, Biagioli M, Ghezzi PM. Bicarbonate balance in hemodiafiltration (HDF): a comparison between two infusion methods of on‐line prepared solution [Bilancio dei bicarbonati in emodiafiltrazione (HDF): confronto fra due metodiche di reinfusione di liquido prodotto on‐line]. Giornale Italiano di Nefrologia 2004;21 Suppl 30:S177‐80. [MEDLINE: ] [PubMed] [Google Scholar]
Sirolli 2004 {published data only}
- Sirolli V, Cappelli P, Amoroso L, Liberato L, Muscianese P, Brummer U, et al. Haemodiafiltration with on‐line reinfusion of endogenous ultrafiltrate (HFR on‐line) removes uraemic retained solutes causing increased exposure of phosphatidylserine on erythrocyte membrane [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:383‐4. [CENTRAL: CN‐00509484]
- Sirolli V, Cappelli P, Amoroso L, Liberato L, Muscianese P, Santarelli P, et al. On‐line HFR and removal of uremic toxins inducing the loss of phospholipidic asymmetry of the erythrocyte membrane [HFR on‐line e rimozione di tossine uremiche inducenti la perdita dell'asimmetria fosfolipidica della membrana eritrocitaria]. Giornale Italiano di Nefrologia 2004;21 Suppl 30:S208‐11. [MEDLINE: ] [PubMed] [Google Scholar]
Spongano 1992 {published data only}
- Spongano M, Santoro A, Bolzani R, Ferrari G, Aucella F, Borghi M, et al. Analysis of plasma bicarbonate determinants in acetate‐free biofiltration [Analisi dei determinanti della bicarbonatemia post‐dialitica in biofiltrazione senza acetato (AFB)]. Giornale Italiano di Nefrologia 1992;9(3):131‐7. [EMBASE: 1992221794] [Google Scholar]
Strujic 2006 {published data only}
- Strujic BJ, Vitunjski B, Majer L, Juric K, Crnjakovic J, Gudel J, et al. The effect of hemodiafiltration (HDF) and paired hemodiafiltration on‐line (PHF) on the level of serum b2 microglobulin compared to conventional hemodialysis (HD) [abstract no: SP690]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv248. [Google Scholar]
Susantitaphong 2008 {published data only}
- Susantitaphong P, Tiranathanagul K, Kanjanabuch T, Avihingsanon Y, Praditpornsilpa K, Tungsanga K, et al. Efficacy of mid‐dilution on‐line hemodiafiltration compared with two standard pre‐dilution and post‐dilution on‐line hemodiafiltration [abstract no: THPO627]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):248A. [Google Scholar]
Timio 1986 {published data only}
- Timio M, Ronconi M, Venanzi S, Lazzaroni P, Lori G. Effect of biofiltration on cardiac arrhythmias. International Journal of Artificial Organs 1986;9 Suppl 3:129‐32. [MEDLINE: ] [PubMed] [Google Scholar]
Tomo 2004 {published data only}
- Tomo T, Matsuyama K, Nasu M. Effect of hemodiafiltration against radical stress in the course of blood purification. Blood Purification 2004;22 Suppl 2:72‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Umimoto 2000 {published data only}
- Umimoto K, Hirai Y, Hayashi T, Tanaka H. The effect of biofiltration on red blood cells 2.3‐diphosphoglycerate and pH. Artificial Organs 2000;24(12):981‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vantelon 1977 {published data only}
- Vantelon J, Lauriat F, Perrone B, Jeannot F. Is it possible to treat uremia by hemofiltration? Initial clinical results obtained with a poly‐acrylo‐nytril membrane [Peut‐on traiter l'uremie par hemofiltration? Premiers resultats cliniques obtenus sur membrane poly‐acrylo‐nitrile]. Nouvelle Presse Medicale 1977;6(13):1117‐20. [MEDLINE: ] [PubMed] [Google Scholar]
Vaslaki 1998 {published data only}
- Vaslaki L, Weber C, Mitteregger R, Falkenhagen D. No difference in cytokine induction between patients on on‐line hemodiafiltration (HDF) and low‐flux HD [abstract]. International Journal of Artificial Organs 1998;21(10):609. [CENTRAL: CN‐00321994] [Google Scholar]
Vaslaki 2000 {published data only}
- Vaslaki L, Weber C, Mitteregger R, Falkenhagen D. Cytokine induction in patients undergoing regular online hemodiafiltration treatment. Artificial Organs 2000;24(7):514‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vaslaki 2002 {published data only}
- Vaslaki L, Major L, Berta K, Karatson A, Misz M, Ladanyi E, et al. Impact of convection on online hemodiafiltration on blood concentration of lipids [abstract no: PUB237]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):718A. [CENTRAL: CN‐00448171] [Google Scholar]
- Vaslaki L, Major L, Berta K, Karatson A, Misz M, Ladanyi E, et al. The impact of convection in online hemodiafiltration on blood concentration of advanced glycation end products [abstract no: M383]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):154. [CENTRAL: CN‐00509536] [Google Scholar]
Vaslaki 2003 {published data only}
- Vaslaki L, Major L, Berta K, Karatson A, Misz M, Pethoe F, et al. Decrease of serum phosphate with on‐line haemodiafiltration [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):194. [CENTRAL: CN‐00448172] [Google Scholar]
Vaslaki 2005 {published data only}
- Vaslaki LR, Berta K, Major L, Weber V, Weber C, Wojke R, et al. On‐line hemodiafiltration does not induce inflammatory response in end‐stage renal disease patients: results from a multicenter cross‐over study. Artificial Organs 2005;29(5):406‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wang 2004d {published data only}
- Wang C, Lou TQ, Tang H, Chen ZJ, Yin PD, Yu XQ. Clearance effect of different blood purification techniques on parathyroid hormone in renal function failure patients on maintenance hemodialysis. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue [Chinese Critical Care Medicine] 2004;16(12):753‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Wizemann 2001 {published data only}
- Wizemann V, Kulz M, Techert F, Nederlof B. Efficacy of haemodiafiltration. Nephrology Dialysis Transplantation 2001;16 Suppl 4:27‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zehnder 1999 {published data only}
- Zehnder C, Gutzwiller JP, Renggli K. Hemodiafiltration‐‐a new treatment option for hyperphosphatemia in hemodialysis patients. Clinical Nephrology 1999;52(3):152‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Zimmerman 2003 {published data only}
- Zimmerman DL, Swedko PJ, Posen GA, Burns KD. Daily hemofiltration with a simplified method of delivery. ASAIO Journal 2003;49(4):426‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Zucchelli 1988 {published data only}
- Zucchelli P, Santoro A, Fusaroli M, Borghi M. Biofiltration in uremia. Kidney International ‐ Supplement 1988;24:S141‐4. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies awaiting assessment
Beerenhout 2004 {published data only}
- Beerenhout C, Dejagere T, Sande FM, Bekers O, Leunissen KM, Kooman JP. Haemodynamics and electrolyte balance: a comparison between on‐line pre‐dilution haemofiltration and haemodialysis. Nephrology Dialysis Transplantation 2004;19(9):2354‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bellien 2014 {published data only}
- Bellien J, Freguin‐Bouilland C, Joannides R, Hanoy M, Remy‐Jouet I, Monteil C, et al. High‐efficiency on‐line haemodiafiltration improves conduit artery endothelial function compared with high‐flux haemodialysis in end‐stage renal disease patients. Nephrology Dialysis Transplantation 2014;29(2):414‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cornelis 2014 {published data only}
- Cornelis T, Sande FM, Eloot S, Cardinaels E, Bekers O, Damoiseaux J, et al. Acute hemodynamic response and uremic toxin removal in conventional and extended hemodialysis and hemodiafiltration: a randomized crossover study. American Journal of Kidney Diseases 2014;64(2):247‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Sequera 2013 {published data only}
- Sequera P, Albalate M, Perez‐Garcia R, Corchete E, Puerta M, Ortega M, et al. A comparison of the effectiveness of two online haemodiafiltration modalities: mixed versus post‐dilution. Nefrologia 2013;33(6):779‐87. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Francisco 2013 {published data only}
- Francisco RC, Aloha M, Ramon PS. Effects of high‐efficiency postdilution online hemodiafiltration and high‐flux hemodialysis on serum phosphorus and cardiac structure and function in patients with end‐stage renal disease. International Urology & Nephrology 2013;45(5):1373‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gonzales‐Diez 2012 {published data only}
- Gonzalez‐Diez B, Cavia M, Torres G, Abaigar P, Camarero V, Muniz P. The effects of 1‐year treatment with a haemodiafiltration with on‐line regeneration of ultrafiltrate (HFR) dialysis on biomarkers of oxidative stress in patients with chronic renal failure. Molecular Biology Reports 2012;39(1):629‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Krieter 2010a {published data only}
- Krieter DH, Fischer R, Lemke HD, Merget K, Canaud B, Wanner C. Endothelial progenitor cells (EPCs) in different dialysis procedures [abstract no: TH‐PO399]. Journal of the American Society of Nephrology 2009;20(Abstracts):206A. [Google Scholar]
- Krieter DH, Fischer R, Merget K, Lemke HD, Morgenroth A, Canaud B, et al. Endothelial progenitor cells in patients on extracorporeal maintenance dialysis therapy. Nephrology Dialysis Transplantation 2010;25(12):4023‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01098149 {published data only}
- Cancarini G. Tolerance to hemodialysis in insulin‐requiring diabetic patients: a prospective randomized, cross‐over multicenter study between bicarbonate dialysis (BD) and blood volume controlled acetate‐free biofiltration (BVC‐AFB). clinicaltrials.gov/ct2/show/NCT01098149 (accessed 18 February 2015).
NCT01327391 {published data only}
- Canaud B. Tolerance of "on Line" hemodiafiltration and impact on morbidity and cardiovascular risk factors in chronic renal failure patients. clinicaltrials.gov/ct2/show/NCT01327391 (accessed 18 February 2015).
NCT01396863 {published data only}
- Johansen N, Jensen D. Brain swelling during hemodialysis a comparison of how low flux hemodialysis and pre‐dilution hemodiafiltration affects acute brain volume changes. clinicaltrials.gov/ct2/show/NCT01396863 (accessed 18 February 2015).
NCT01445366 {published data only}
- Vanholder R. Solute removal with high volume hemodiafiltration versus long high flux hemodialysis. clinicaltrials.gov/ct2/show/NCT01445366 (accessed 18 February 2015).
NCT02374372 {published data only}
- Levesque R, Caron L. Prospective randomized study comparing the hemodiafiltration on‐line and conventional hemodialysis in terms of cost‐benefit. clinicaltrials.gov/ct2/show/NCT02374372 (accessed 31 March 2015).
Additional references
ANZDATA 2012
- Australia, New Zealand Dialysis, Transplant Registry. 35th Annual Report 2012. Chapter 5. Haemodialysis (including home haemodialysis). www.anzdata.org.au/anzdata/AnzdataReport/35thReport/2012c05_haemodialysis_v2.11.pdf (accessed 28 March 2015).
Arizono 2004
- Arizono K, Nomura K, Motoyama T, Matsushita Y, Matsuoka K, Miyazu R, et al. Use of ultrapure dialysate in reduction of chronic inflammation during hemodialysis. Blood Purification 2004;22 Suppl 2:26‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Daugirdas 1991
- Daugirdas JT. Dialysis hypotension: a hemodynamic analysis. Kidney International 1991;39(2):233‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
EBPG 2007
- Tattersall J, Martin‐Malo A, Pedrini L, Basci A, Canaud B, Fouque D, et al. EBPG guidelines on dialysis strategies. Nephrology Dialysis Transplantation 2007;22(Suppl 2):ii5‐ii21. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315(7109):629‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ERA‐EDTA Registry 2010
- ERA‐EDTA Registry. ERA‐EDTA Registry Annual Report 2010. Academic Medical Centre, Department of Medical Informatics, Amsterdam, The Netherlands. 2010. www.era‐edta‐reg.org/files/annualreports/pdf/AnnRep2010.pdf (accessed 28 March 2105).
Guerin 2000
- Guerin AP, London GM, Marchais SJ, Metivier F. Arterial stiffening and vascular calcifications in end‐stage renal disease. Nephrology Dialysis Transplantation 2000;15(7):1014‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Henderson 2004
- Henderson LW, Besarab A, Michaels A, Bluemle Jr LW. Blood purification by ultrafiltration and fluid replacement (diafiltration). Hemodialysis International 2004;8(1):10‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Liabeuf 2012
- Liabeuf S, Lenglet A, Desjardins L, Neirynck N, Glorieux G, Lemke HD, et al. Plasma beta‐2 microglobulin is associated with cardiovascular disease in uremic patients. Kidney International 2012;82(12):1297‐303. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Locatelli 2000
- Locatelli F, Filippo S, Manzoni C. Removal of small and middle molecules by convective techniques. Nephrology Dialysis Transplantation 2000;15 Suppl 2:37‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Murtagh 2007
- Murtagh FE, Addington‐Hall J, Higginson IJ. The prevalence of symptoms in end‐stage renal disease: a systematic review. Advances in Chronic Kidney Disease 2007;14(1):82‐99. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schmidt 1986
- Schmidt M. Haemodiafiltration. Haemofiltration. Berlin: Springer, 1986:265‐71. [Google Scholar]
Susantitaphong 2013
- Susantitaphong P, Siribamrungwong M, Jaber BL. Convective therapies versus low‐flux hemodialysis for chronic kidney failure: a meta‐analysis of randomized controlled trials. Nephrology Dialysis Transplantation 2013;28(11):2859‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sztajzel 1993
- Sztajzel J, Ruedin P, Stoermann C, Monin C, Schifferli J, Leski M, et al. Effects of dialysate composition during hemodialysis on left ventricular function. Kidney International ‐ Supplement 1993;41:S60‐6. [MEDLINE: ] [PubMed] [Google Scholar]
USRDS 2011
- US Renal Data System. USRDS 2011 Annual Data Report. Atlas of End‐Stage Renal Disease in the United States, 2011. Bethesda MD National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. www.usrds.org/atlas11.aspx (accessed 28 March 2015).
Vilar 2009
- Vilar E, Fry AC, Wellsted D, Tattersall JE, Greenwood RN, Farrington K. Long‐term outcomes in online hemodiafiltration and high‐flux hemodialysis: a comparative analysis. Clinical Journal of The American Society of Nephrology: CJASN 2009;4(12):1944‐53. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zucchelli 1990
- Zucchelli P, Santoro A, Ferrari G, Spongano M. Acetate‐free biofiltration: hemodiafiltration with base‐free dialysate. Blood Purification 1990;8(1):14‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Rabindranath 2005
- Rabindranath KS, Strippoli GF, Roderick P, Wallace SA, MacLeod AM, Daly C. Comparison of hemodialysis, hemofiltration, and acetate‐free biofiltration for ESRD: systematic review. American Journal of Kidney Diseases 2005;45(3):437‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rabindranath 2006
- Rabindranath K S, Strippoli G F, Daly C, Roderick P J, Wallace S, MacLeod A M. Haemodiafiltration, haemofiltration and haemodialysis for end‐stage kidney disease. Cochrane Database of Systematic Reviews 2006, Issue CD006258. [DOI: 10.1002/14651858.CD006258] [DOI] [PubMed] [Google Scholar]


