Abstract
The role of cyclophosphamide in the upfront light chain (AL) amyloidosis remains to be elucidated. We conducted a retrospective review of 136 patients at Moffitt Cancer Center. The addition of cyclophosphamide to bortezomib and dexamethasone did not significantly improve outcome. Our finding suggests that the addition of cyclophosphamide may not be beneficial in upfront setting, especially in transplant illegible patients.
Introduction:
Before 2021, the combination of bortezomib, cyclophosphamide, and dexamethasone (VCd) was one of the most used upfront therapy for systemic immunoglobulin light chain (AL) amyloidosis. Recently, daratumumab in combination with VCd resulted in improved outcomes compared to VCd. However, it’s still unclear the role of cyclophosphamide in this combination.
Materials and Methods:
We conducted this retrospective single-institutional study to compare the outcomes of upfront bortezomib and dexamethasone with or without cyclophosphamide (VD vs. VCd).
Results:
Of 136 total patients, 62 received VD and 74 received VCd. The median age was 64 and the median number of organs involved was 2. Hematologic response was achieved among 73.4% patients in the VD arm and 85.9% in the VCd arm at 3 months (P = .15). Best organ response was not different between 2 arms (34.1% vs. 52.9% for VD and VCd arms, respectively; P = .28). After a median follow-up of 24.4 months, 2-year OS for VD and VCd arm was 70.6% and 84.6% respectively. The median overall survival was 70 months for VD arm and not reached for VCd arm (P = .30). There was no statistically significant difference in median time to next therapy (9.3 vs. 13.5 months for VD and VCd arms, respectively. P = .99).
Conclusion:
the addition of cyclophosphamide to VD was not associated with improved outcomes of patients with AL amyloidosis in this retrospective study.
Keywords: Immunoglobulin light chain amyloidosis, Alkylators, AL amyloidosis, Long-term outcome, Outcomes
Introduction
Systemic immunoglobulin light chain (AL) amyloidosis is a rare plasma cell neoplasm characterized by the production of a monoclonal immunoglobulin light chain (LC) that leads to the aggregation of insoluble amyloid fibrils in the tissue, thereby causing progressive organ dysfunction. The most commonly affected organs include the heart, kidney, and liver. 1 AL amyloidosis is often fatal, especially among patients with cardiac involvement, when the diagnosis is delayed, or when treatment is ineffective. 2
Given the absence of therapy proven to disrupt fibril deposition, the mainstay of AL amyloidosis therapy involves anti–plasma cell therapy aimed at stopping or decreasing LC production. Compared to multiple myeloma, advances in therapy for AL amyloidosis have occurred at a slower pace, in large part because the incidence of AL amyloidosis is lower and few large, randomized trials of AL amyloidosis have been conducted.
Prior to 2009, the therapy involved the use of alkylating agents and dexamethasone (specifically the combination of melphalan with either prednisone or dexamethasone), or high dose melphalan and autologous stem cell transplant in patients who were deemed to be eligible. 3,4 The introduction of bortezomib, a proteasome inhibitor, in 2009 resulted in higher hematological response rates compared to historical controls among patients treated with alkylator-based therapy. 3,5–7 Kastritis et al compared the combination melphalan and dexamethasone with or without bortezomib as primary therapy in a phase III trial that enrolled 109 patients and demonstrated improved hematologic response rates and a 2-fold reduction in mortality with the addition of bortezomib to the alkylating agent backbone therapy. 4 Based on this study and others, bortezomib-alkylating agent-dexamethasone regimen became a standard treatment for patients prior to 2020. 5,6 However, the role of alkylating agent remains unclear in this combination, as prospective head-to-head comparison between upfront 2 drug bortezomib-dexamethasone combination, and bortezomib-alkylating agent-dexamethasone combination is lacking.
On January 15, 2021, Food and Drug Administration granted accelerated approval to daratumumab in combination with bortezomib, cyclophosphamide and dexamethasone for newly diagnosed AL amyloidosis based on ANDROMEDA study. This approval further raises questions about the role of alkylating agent in this four-drug upfront combination. The ANDROMEDA study showed that the addition of daratumumab improved hematologic response rate and progression-free survival. The study randomized 388 treatment-naïve patients with AL amyloidosis to receive cyclophosphamide, bortezomib, and dexamethasone (VCd) with or without daratumumab. 7,10,11 The hematologic complete response rate was 53.3% in the daratumumab + VCd group versus 18.1% in the VCd alone group, and major organ deterioration progression-free survival favored the daratumumab group (hazard ratio, 0.58; P = .02). 7,10,11 However, the efficacy of this upfront 4-drug regimen comes with increased toxicity. Serious adverse events occurred in 43% of the patients in the daratumumab group including grade 3 or 4 infections in 16.6% pneumonia in 7.3%, cardiac failure in 6.2%, diarrhea in 5.7%, neutropenia in 5.2%, and syncope in 5.2%. 7 As daratumumab and bortezomib have shown potent activities toward AL amyloidosis, the benefit and toxicity of alkylating agent in this combination warrant further evaluation.
We conducted this retrospective single-institutional study to compare long-term outcomes between patients with AL amyloidosis treated with upfront bortezomib and dexamethasone alone (VD) and those who received bortezomib and dexamethasone with cyclophosphamide (VCd).
Materials and Methods
We reviewed medical records of 136 patients with confirmed systemic AL amyloidosis treated with up front therapy, VD with or without cyclophosphamide at our institution between January 2008 and January 2020. Of the 136 patients, 62 received VD and 74 received VCd. The diagnosis of systemic AL amyloidosis was confirmed with immunohistochemistry and/or proteomics analyses of a tissue biopsy sample.
Organ involvement, hematologic response, and best organ response were defined using the 10th International Symposium on Amyloid and Amyloidosis consensus criteria from 2005. 8 Cytogenetics abnormalities were assessed with fluorescence in situ hybridization. The hematologic response was assessed at 3-months follow-up after the therapy initiation. Hematologic response was evaluated using the difference in free LC (dFLC). Partial response was defined as a 50%–90% reduction of dFLC, and a very good partial response was defined as < 40 mg/L. Complete response (CR) was defined as normalization of serum free LC ratio and negative serum and urine immunofixation. 9–11 Patients who had dFLC < 5 mg/dL were assessed for response criteria of CR only. 12, 13 The best organ response was assessed at 6-month follow-up after the upfront therapy initiation. 14–17 Patients who died before the response assessment was considered no response. In patients who received ASCT consolidation, the hematological and organ response were assessed prior to ASCT.
The primary objective was OS, which was calculated from diagnosis until time of death or last follow-up. The secondary objective was time to next therapy (TTNT), which was calculated from the date of upfront treatment until the date of next treatment or date of death, whichever occurred first.
Statistical Analyses
Differences of continuous variables between treatment arms were compared using the Kruskal-Wallis test, and differences of categorical variables were compared using the χ2 test or Fisher exact test when the expected frequency was less than 5 in each category. OS and TTNT comparisons between arms were evaluated using Kaplan-Meier estimates and log-rank tests. Regression analyses were performed using multivariable Cox proportional hazards models. A backward stepwise elimination using Akaike information criteria was used to remove variables. All analyses were performed using R v3.6.3.
Results
Patients
Of 136 patients, 62 received VD and 74 received VCd. At least 20 mg of dexamethasone doses weekly were given in all patients. The median age at diagnosis was older in the VD arm than the VCd arm (median age: 65 vs. 62 years; interquartile range [59,72] and [57,69] respectively).
Before January 2014, patients were more frequently treated with VD than with VCd (50% vs. 20.3%; P <.001). Mayo stage (2012) I, II, III, and IV diseases were found in 25 (18.1%), 27 (19.6%), 33 (23.9%), and 24 (17.4%) patients, and disease staging was not statistically different between treatment arms.
Of the whole cohort, 70.6% of patients had renal involvement, 49% had cardiac involvement, 18.1% had gastrointestinal involvement, 12.5% had liver involvement, and 10.3% had peripheral nerve involvement. The median number of organs involved was 2 in both arms. Additionally, both arms had a similar percentage of cardiac and renal involvement. Other clinical characteristics evaluated at the time of diagnosis included sex, race, estimated glomerular filtration rate (eGFR), log of N-terminal pro-brain natriuretic peptide (NT ProBNP), troponin, dFLC, serum FLC ratio, beta 2 microglobulin, the percentage of plasma cell in bone marrow, and cytogenetics abnormalities, which include t(11;14), monosomy 1, hypodiploidy, del17p, and changes in 1q21. There were no statistically significant differences in those characteristics between patients in the 2 arms (Table 1).
Table 1.
Baseline Characteristics of Patients Treated With Bortezomib and Dexamethasone With and Without Cyclophosphamide (VD vs. VCd).
| Clinical Factor | Bortezomib and Dexamethasone (N = 62) | Bortezomib, Dexamethasone, and Cyclophosphamide (N = 74) | P value |
|---|---|---|---|
| Male Gender, n (%) | 37 (59.7) | 48 (64.9) | .60 |
| Age at diagnosis, median (IQR) | 65.0 (59.0; 72) | 62.0 (57.0; 69.0) | .027 |
| No. patients diagnosed before January 1, 2014 (%) | 31 (50.0) | 15 (20.3) | < .001 |
| Race, no. (%)* | .24 | ||
| White | 44 (71.0) | 61 (82.4) | |
| Black | 8 (12.9) | 4 (5.4) | |
| Other | 1 (1.61) | 2 (2.8) | |
| No. organs involved, median (min, max) | 2 (1, 5) | 2 (1, 6) | .05 |
| Liver, (%) | 7 (11.3) | 10 (13.5) | .80 |
| Kidney, (%) | 44 (71.0) | 52 (70.3) | > .999 |
| Heart, (%) | 29 (46.8) | 38 (51.4) | .61 |
| Peripheral Neural System, (%) | 3 (4.84) | 11 (14.9) | .09 |
| Gastrointestinal, (%) | 10 (16.1) | 15 (20.3) | .66 |
| Biomarkers at time of diagnosis | |||
| eGFR, mL/min/1.73m2, median (min; max), n = 123 | 49.0 (5.00; 119) | 53.0 (6.00; 175) | .99 |
| NT-pro-BNP, pg/mL, median (min; max), n = 115 | 1112 (37.0; 56600) | 2051 (35.0; 87163) | .23 |
| cTnT, ng/mL, median (min; max), n = 102 | 0.02 (0.00; 0.34) | 0.04 (0.00; 1.24) | .19 |
| 24-h urine protein, g/24 h, median (min; max), n = 90 | 4.80 (0.13; 1414) | 4.80 (0.02; 7533) | .74 |
| dFLC, mg/dL, median (min; max), n = 127 | 103 (2; 22579) | 153 (3.2; 5818) | .31 |
| Lambda light chain involvement, no (%) | 40 (66) | 49 (66) | .94 |
| Beta 2 microglobulin, mg/L, median (min; max), n = 112 | 4.30 (0.80; 52.0) | 4.00 (1.70; 61.4) | .97 |
| Bone marrow plasma cell, %, median (min; max), n = 128 | 10.0 (0.40; 80.0) | 13.0 (0.00; 80.0) | .44 |
| Abnormal cytogenetics, no. (%) | |||
| t(11,14) | 7/14 (50) | 15/28 (29.4) | .83 |
| Mayo 2012 Stage, no. (%)* | N = 47 | N = 52 | .56 |
| Stage I | 14 (22.6) | 11 (14.5) | |
| Stage II | 10 (16.1) | 17 (22.4) | |
| Stage III | 13 (21.0) | 20 (26.3) | |
| Stage IV | 10 (16.1) | 14 (18.4) | |
| ASCT after upfront therapy | |||
| Received, n (%) | 2 (3.2) | 12 (16.2) | .02 |
Percentages may not sum to 1 because of missing data.
Abbreviations: cTnT = cardiac troponin T; dFLC = difference between involved and uninvolved free light chains; eGFR = estimated glomerular filtration rate; IQR = interquartile range; NT-pro-BNP = N-terminal pro hormone B-type natriuretic peptide; sFLC = serum free light chains. ASCT = autologous hematopoietic stem cell transplant.
The alkylating agent-based therapies were the most common second-line therapy among patients in the VD group, whereas immunomodulatory drug– (iMID-) based therapy and daratumumab were the most common among patients in the VCd group.
Hematologic and Organ Responses
Of 136 total patients, hematologic response was evaluable among 113 patients, including 49 in the VD arm and 64 in the VCd arm (Table 2). The remaining 23 patients were not evaluable due to lack of data. Hematologic response (PR or better) was achieved among 70.6% of patients in the VD arm and 84.6% in the VCd arm at the 3-month follow-up visit; however, this difference was not statistically significant (P = .07). Complete response rates at 3 months were not statistically significant between arms (25.5% vs. 21.5% in VD and VCd arms, respectively; P = .62). The combined VGPR and CR rates at 3 months were 29.4% and 38.4% in VD arm and VCd arm respectively (P = .31).
Table 2.
Clinical Outcome of Systemic Immunoglobulin Light Chain Amyloidosis Patients Treated With Bortezomib and Dexamethasone With and Without Cyclophosphamide.
| Variable | Bortezomib and Dexamethasone | Bortezomib, Dexamethasone, and Cyclophosphamide | P value |
|---|---|---|---|
| Hematologic response, no. | N = 51 | N = 65 | .18 |
| Complete response, no. (%) | 13 (25.5%) | 14 (21.5%) | |
| Very good partial response, no. (%) | 2 (3.9%) | 11 (16.9%) | |
| Partial response, no. (%) | 21 (41.2%) | 30 (46.2%) | |
| No response, no. (%) | 15 (29.4%) | 10 (15.4%) | |
| Any organ response, no. | N = 46 | N = 52 | .17 |
| Response, no. (%) | 15 (32.6%) | 24 (46.2%) | |
| No response, no. (%) | 31 (67.4%) | 28 (53.8.1%) | |
| Early mortality | |||
| 90-d mortality, no. (%) | 1 (2%) | 3 (4.6%) | .99 |
Of 136 total patients, best organ response was evaluable among 95 patients, including 44 in the VD arm and 51 in the VCd arm (Table 2). Among the remaining 31 patients with non–evaluable organ response, 23 patients lacked data and 8 patients did not have baseline organ assessment. The organ response rate was a higher among those in the VCd arms (32.6% vs. 46.21% for VD and VCd arms, respectively, P = .17).
Of 136 total patients, 14 patients underwent ASCT after achieving response on induction therapy. VCd arm had significantly higher proportion of patients who underwent ASCT as compared to Vd arm (16.2% vs. 3.2%, P =. 02).
OS and TTNT
After a median follow-up of 24.4 months, 35 patients had died. There was no statistical difference in OS between both arms (70 months vs. not reached for VD and VCd arms, respectively; P = .30). The 2-year overall survival rate was 76% for VD arm (95% CI, 62%–86%) as compared with 82% in the VCd arms (95% CI, 67%–91%) (Figure 1). The 90-day mortality rate was similar between arms (2% vs. 4.6% for Vd and VCd arms respectively, P =. 99).
Figure 1.
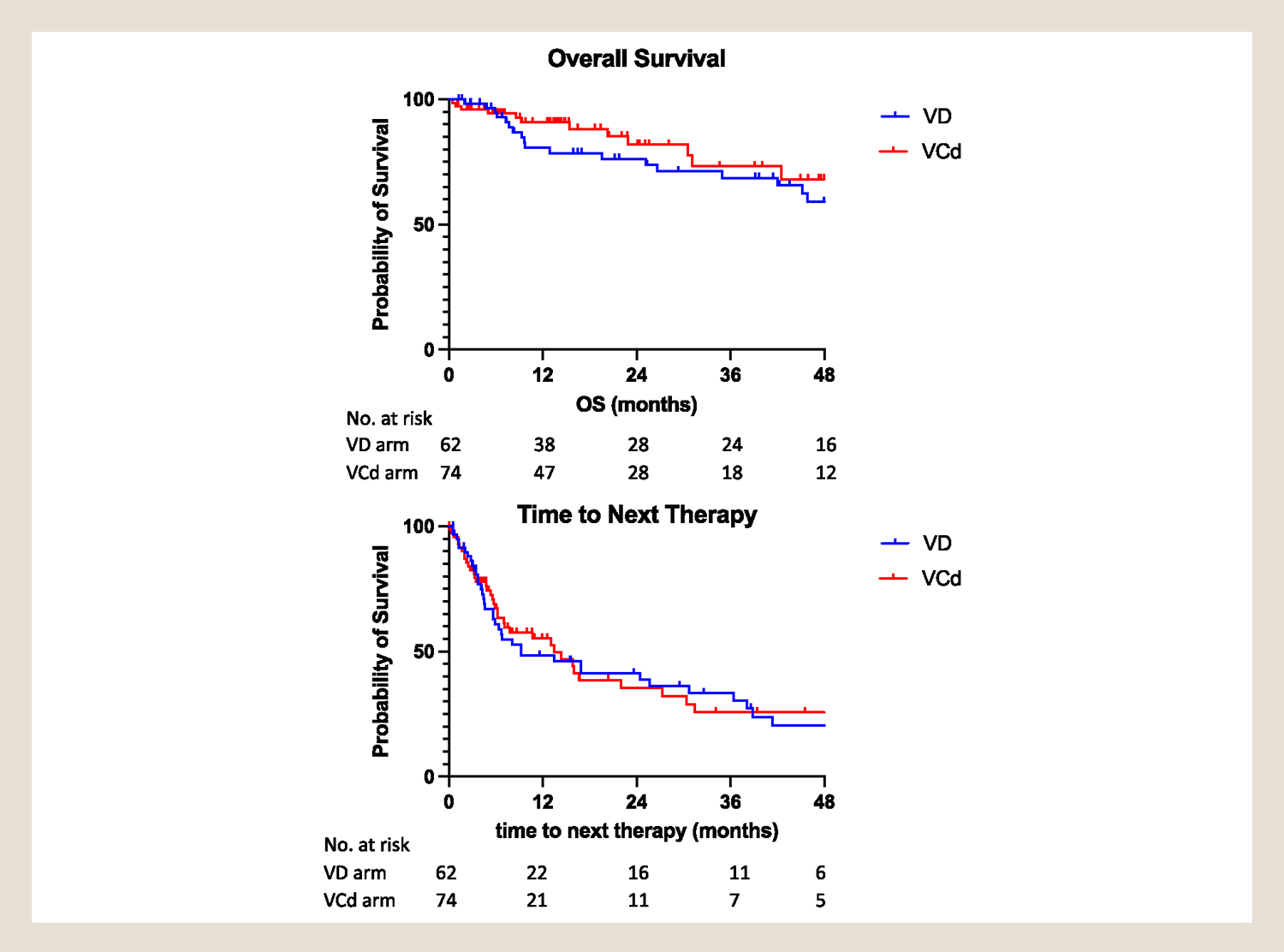
Top: Overall survival of AL amyloidosis patients treated with upfront Bortezomib and dexamethasone with/without cyclophosphamide. Bottom: Time to next therapy of AL amyloidosis patients treated with upfront Bortezomib and dexamethasone with/without alkylators.
The median TTNT was not statistically different between both arms (9.1 vs. 13.3 months for the VD and VCd arms, respectively; P = .98) (Figure 1). The difference became even smaller when patients who received autologous stem cell transplant (ASCT) as consolidation were excluded (9.2 vs. 7.7 months for VD and VCd arms, respectively; P = .12) (Figure S1). Among patients who achieved hematologic response, more patients in the VCd arm received ASCT as a consolidation therapy (16% vs. 5% in VCd and VD arms, respectively; P = .054).
In the univariate analysis, the addition of cyclophosphamide to VD was associated 31% risk reduction in death, though statistical significance was not reached (HR 0.69, 95% CI 0.35–1.4, P =. 3). However, when adjusted for sex, race sex, age at diagnosis, race, log of NT Pro-BNP, eGFR and consolidation with ASCT, VCd was no longer associated with improved survival as compared to VD (HR 1.22, 95% CI 0.5–3.0). TTNT was similar between the 2 treatments group (HR 0.99, 95% CI 0.80–1.3).
Black race, eGFR < 60 mL/min/1.73m2, and proBNP > 1800 pg/mL were associated with poor survival in the multivariate COX proportional hazard regression analysis. Receiving ASCT as were associated with improved survival and longer TTNT [HR 0.38 (95% CI 0.05, 3.1), 0.23 (95% CI 0.066, 0.819), respectively]. (Table 3 and Table S1).
Table 3.
Multivariable Analysis of Factors Associated With Overall Survival and time to Next Therapy.
| Overall Survival | |||
|---|---|---|---|
|
| |||
| Variable | Hazard Ratio (95% CI) | P value | N (events) |
| Treatment group | |||
| VD | 1.0 (reference) | - | N = 95 (27) |
| VCd | 1.22 (0.50, 3.0) | .66 | |
| Sex (Male) | 0.91 (0.38, 2.2) | .82 | |
| Race | |||
| White | 1.0 (reference) | - | |
| Black | 4.0 (1.5, 10) | .006 | |
| Other | 6.0 (0.64,56) | .12 | |
| Age >= 65 y | 1.3 (0.57, 3.0) | .52 | |
| eGFR < 60 mL/min/1.73m2 | 2.27 (0.85, 6.1) | .10 | |
| proBNP > 1800 pg/mL | 1.6 (0.72, 3.7) | .23 | |
| Received ASCT as consolidation | 0.38 (0.05, 3.1) | .37 | |
| Time to next treatment | |||
| Variable | Hazard Ratio (95% CI) | P value | N (events) |
| Treatment group | |||
| VD | 1.0 (reference) | - | N = 91 (53) |
| VCd | 1.5 (.79, 1.7) | .23 | |
| Sex (Male) | 1.4 (0.78, 2.7) | .24 | |
| Race | |||
| White | 1.0 (reference) | - | |
| Black | 1.3 (0.56, 3.1) | .54 | |
| Other | 7.5 (1.5, 37) | .013 | |
| Age >= 65 y | 0.84 (0.47,1.5) | .55 | |
| eGFR < 60 mL/min/1.73m2 | 1.1 (0.64,2.1) | .65 | |
| proBNP > 1800 pg/mL | 0.55 (0.31,1.0) | .05 | |
| Received ASCT as consolidation | 0.23(0.066, 0.819) | .023 | |
Abbreviations: VD = bortezomib and dexamethasone; VCd = bortezomib = dexamethasone and cyclophosphamide; CI = confidence interval; eGFR = estimated glomerular filtration rate; NT-pro-BNP = N-terminal pro hormone B-type natriuretic peptide; ASCT: autologous stem cell transplant.
Black patients had a shorter survival as compared to white patients (HR 3.3, 95% CI 1.4–8.2, P =. 009), but not significantly shorter TTNT for first-line therapy. Patients with NT proBNP level > 1800 pg/mL at diagnosis had shorter median OS (45.8 months vs. 64.4 months, P =. 042).
Discussion
In this retrospective study, we compared long-term outcomes of patients with AL amyloidosis who were treated either with upfront VD or VCd therapy at our institution between 2008 and 2020. We found that the addition of cyclophosphamide to VD doublet therapy did not significantly improve long-term OS or TTNT among patients with newly diagnosed AL amyloidosis. This is especially true for patients who were illegible for transplant, as the margin of benefits was smaller and likely be outweighed by the risk of added cyclophosphamide. Though additional therapy can provide better hematologic response, it may not translate to survival benefits.
Though this was a retrospective study, patients’ characteristics were well matched between the 2 study arms, except for the diagnosis time period and age at diagnosis. VD therapy was more commonly used before Mikhael et al and Venner et al’s study in January 2012, which reported high hematologic response rates using VCd therapy among patients with either newly diagnosed or relapsed AL amyloidosis. 13,14 In our study, the earlier date of diagnosis and older cohort in the VD arm could have resulted in a worse outcome compared to a more contemporary treated VCd arm. 3 As such, the comparable outcomes noted in our study between the 2 arms are noteworthy.
Our results are in line with those reported by Kastritis et al, who retrospectively reviewed outcomes of 101 patients with AL amyloidosis treated with VD or VCd. The authors reported a similar hematologic response rate (68% and 78%; P = .26) and overall survival (33 months and 36 months) between patients treated with VD and VCd, respectively. 18 Compared to Kastritis et al, we reported higher median OS (70 months and not reached for VD and VCd arms, respectively). The improvement in our study compared Kastritis’s group is possibly due to the inclusion of patients who subsequently received ASCT. The OS and hematologic response rates in our study were comparable to those seen in other recently reported prospective studies of patients receiving upfront bortezomib combination therapy. 19
In our cohort, significantly more patients in the VCd arm received ASCT as consolidation therapy than those in the VD arm, despite similar clinical characteristics in both arms. This difference could potentially reflect that more ASCTs were performed after 2012 because of reduced transplant-related mortality and better selection of patients for ASCT.
Limitations
The retrospective and nonrandomized nature of this study represented the principal limitation, and the analysis might be underpowered to identify the real differences between groups if they exist. Ideally, a randomized controlled study with first-line daratumumab + VD with and without cyclophosphamide would be needed to unequivocally address the role of alkylating agents in the upfront management of patients with AL amyloidosis. However, our present retrospective study predated the use of daratumumab and was unable to answer this question. In our study, thirty-four patients (33.9%) in the VD arm received subsequent alkylating agent therapy. This may possibly cloud the OS benefit from the initial lack of alkylating agents given to patients in the VD arm. The ffollow-upin VD arm was shorter than VCd, which could potentially underestimate the benefit of the addition of cyclophosphamide.
Secondary to missing data in this retrospective study, dFLC, troponin, status of t (11;14), plasma cell percentage in the bone marrow and 24-hour urine protein excretion were not accounted for in the multivariable cox proportional hazards regression analysis. Only the upfront treatment arms, sex, age at diagnosis, race, log of N-terminal pro-brain natriuretic peptide (NT Pro-BNP), estimated glomerular filtration rate (eGFR) and consolidation with ASCT were included in the full model. Larger sample size with multi-institutional study and longer follow-up will be needed to confirm our observation.
Lastly, our center is a tertiary referral center. Therefore, the study selected patients who were considered candidates for systemic therapies and referral. Our cohort had less cardiac involvement and a lower percentage of lower death than typically seen in AL amyloidosis.
Conclusion
In our experience, the addition of cyclophosphamide to bortezomib and dexamethasone therapy was not associated with improved outcomes in patients with newly diagnosed AL amyloidosis, especially in patients who were not candidate for transplant consolidation. However, the addition of cyclophosphamide likely would benefit transplant-eligible patients given higher combined hematologic very good partial response and complete response rate. Future studies are needed to evaluate the role of cyclophosphamide in the recently FDA-approved upfront combination of daratumumab, bortezomib, cyclophosphamide, and dexamethasone to balance the toxicities associated with cyclophosphamide and the potential clinical benefit on overall survival in the regimen.
Supplementary Material
Clinical Practice Points.
Before 2021, cyclophosphamide, dexamethasone, and bortezomib (VCd) was standard of care for newly diagnosed with AL amyloidosis. However, a head-to-head comparison was not evaluated between bortezomib/dexamethasone (VD) with and without cyclophosphamide. Recently, quadruplet therapy, daratumumab and VCd, became the new standard of care, which puts the role of cyclophosphamide further in question when balancing toxicity and benefit.
This single-center, retrospective study included 136 patients; 62 received VD and 74 received VCd for newly diagnosed AL amyloidosis. The median age of diagnosis was 65 and 62 years for VD and VCd, respectively. Fifty percent and 15% of patients in VD and VCd arm were diagnosed before 2014. Despite being older and treated at an earlier time, the patients treated with VD had similar TTNT and OS compared to those treated with VCd. After a median follow-up of 24.4 months, the 2-year overall survival rate was 76% for the VD arm (95% CI, 62%−86%) compared with 82% in the VCd arm (95% CI, 67%−91%). The combined very good partial and complete responses were 32.7% and 40.6% for VD and VCd, respectively (P = .38).
Our study did not find an improvement in outcomes when adding cyclophosphamide to bortezomib and dexamethasone in patients with newly diagnosed AL amyloidosis. However, the addition of cyclophosphamide would likely benefit transplant-eligible patients given improved combined hematologic very good partial response and complete response rate. Future studies are needed to evaluate the role of cyclophosphamide in the recently FDA-approved upfront combination of daratumumab, bortezomib, cyclophosphamide, and dexamethasone.
Acknowledgments
Editorial assistance was provided by the Moffitt Cancer Center’s Office of Scientific Publishing by Daley Drucker and y Mikael Michalik (fieldwork student). No compensation was given beyond their regular salaries. This work has been supported in part by the Biostatistics & Bioinformatics Shared Resource at the H. Lee Moffitt Cancer Center & Research Institute, a comprehensive cancer center designated by the National Cancer Institute and funded in part by Moffitt’s Cancer Center Support Grant (P30-CA076292).
Disclosure
J.B. receives Speakers’ Bureau from Bristol-Myers Squibb and Janssen and has a consulting role in Celgene and Seattle Genetics. M.A. receives honoraria from Janssen and Speakers’ Bureau from GlaxoSmithKline and Janssen Oncology, research funding from BMS (inst) and has a consulting role in BMS, Janssen Oncology and oncopeptides. T.N. receives research funding from Celgene (Inst), Karyopharm Therapeutics (Inst), and Novartis (Inst). R.B. receives research funding from Abbvie (inst), Celgene (Inst), Karyopharm Therapeutics (Inst), Merck Sharp & Dohme (Inst), Sanofi (Inst), Signal Genetics (Inst), Takeda (Inst) and has a consulting role for Celgene and Karyopharm Therapeutics. The remaining authors declare no conflict of interest.
Footnotes
IRB Approval Status
Reviewed and approved by University of South Florida Institutional Review Board (Pro00033867).
Clinicaltrials.gov (or equivalent) listing (if applicable): none.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.clml.2022.04.003.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, (Brandon Blue, MD). The data are not publicly available due to restrictions eg, their containing information that could compromise the privacy of research participants.
References
- 1.Merlini G, Dispenzieri A, Sanchorawala V, et al. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers. 2018;4:38. [DOI] [PubMed] [Google Scholar]
- 2.Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387:2641–2654. [DOI] [PubMed] [Google Scholar]
- 3.Muchtar E, Gertz MA, Kumar SK, et al. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood.. 2017;129:2111–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kastritis E, Leleu X, Arnulf B, et al. Bortezomib, melphalan, and dexamethasone for light-chain amyloidosis. J Clin Oncol. 2020;38:3252–3260. [DOI] [PubMed] [Google Scholar]
- 5.Mikhael JR, Schuster SR, Jimenez-Zepeda VH, et al. Cyclophosphamide-bortezomib-dexamethasone (CyBorD) produces rapid and complete hematologic response in patients with AL amyloidosis. Blood. 2012;119:4391–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venner CP, Lane T, Foard D, et al. Cyclophosphamide, bortezomib, and dexamethasone therapy in AL amyloidosis is associated with high clonal response rates and prolonged progression-free survival. Blood. 2012;119:4387–4390. [DOI] [PubMed] [Google Scholar]
- 7.Kastritis E, Palladini G, Minnema MC, et al. Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med. 2021;385:46–58. [DOI] [PubMed] [Google Scholar]
- 8.Gertz MA, Comenzo R, Falk RH, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th international symposium on amyloid and amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol. 2005;79:319–328. [DOI] [PubMed] [Google Scholar]
- 9.Manwani R, Cohen O, Sharpley F, et al. A prospective observational study of 915 patients with systemic AL amyloidosis treated with upfront bortezomib. Blood. 2019;134:2271–2280. [DOI] [PubMed] [Google Scholar]
- 10.Sidana S, Dispenzieri A, Murray DL, et al. Revisiting complete response in light chain amyloidosis. Leukemia. 2020;34:1472–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muchtar E, Dispenzieri A, Leung N, et al. Optimizing deep response assessment for AL amyloidosis using involved free light chain level at end of therapy: failure of the serum free light chain ratio. Leukemia. 2019;33:527–531. [DOI] [PubMed] [Google Scholar]
- 12.Sidana S, Tandon N, Dispenzieri A, et al. Clinical presentation and outcomes in light chain amyloidosis patients with non-evaluable serum free light chains. Leukemia. 2018;32:729–735. [DOI] [PubMed] [Google Scholar]
- 13.Dittrich T, Bochtler T, Kimmich C, et al. AL amyloidosis patients with low amyloidogenic free light chain levels at first diagnosis have an excellent prognosis. Blood.. 2017;130:632–642. [DOI] [PubMed] [Google Scholar]
- 14.Palladini G, Hegenbart U, Milani P, et al. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood. 2014;124:2325–2332. [DOI] [PubMed] [Google Scholar]
- 15.Palladini G, Dispenzieri A, Gertz MA, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 2012;30:4541–4549. [DOI] [PubMed] [Google Scholar]
- 16.Palladini G, Dispenzieri A, Gertz MA, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 2012;30:4541–4549. [DOI] [PubMed] [Google Scholar]
- 17.Palladini G, Hegenbart U, Milani P, et al. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood, The Journal of the American Society of Hematology.. 2014;124:2325–2332. [DOI] [PubMed] [Google Scholar]
- 18.Kastritis E, Gavriatopoulou M, Roussou M, et al. Addition of cyclophosphamide and higher doses of dexamethasone do not improve outcomes of patients with AL amyloidosis treated with bortezomib. Blood Cancer J. 2017;7:e570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manwani R, Cohen O, Sharpley F, et al. A prospective observational study of 915 patients with systemic AL amyloidosis treated with upfront bortezomib. Blood. 2019;134:2271–2280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, (Brandon Blue, MD). The data are not publicly available due to restrictions eg, their containing information that could compromise the privacy of research participants.


