To the Editor:Primary ciliary dyskinesia (PCD) is a rare autosomal recessive inherited disease characterized by dysfunction of the motile cilia. DNAH5, the most frequently mutated gene in PCD, encodes a heavy chain of the ciliary outer dynein arms (ODAs). Herein, we report two patients with Kartagener syndrome (sinusitis, bronchiectasis, and situs inversus) in two consanguineous families carrying two novel homozygous DNAH5 variants [Supplementary Figures 1A and 1B, http://links.lww.com/CM9/B598].
Patient I was a 30-year-old male. He had recurrent respiratory infections since childhood and had visited our outpatient respiratory clinic. Lung function tests showed obstructive impairment (forced expiratory volume in one second to forced vital capacity ratio [FEV1/FVC] was 65.96% after bronchodilator administration). His nasal nitric oxide (nNO) concentration was significantly lower than normal: 10.8 nL/min (normal >77 nL/min). Computed tomography (CT) showed sinusitis, bronchiectasis, and situs inversus [Figure 1A and Supplementary Figures 1C and 1E, http://links.lww.com/CM9/B598]. The results suggested that the patient may have PCD; therefore, we performed whole-exome sequencing for him. A novel homozygous variant, c.7979A >G (p.Asn2660Ser), in DNAH5 (NM_001369.3) was identified [Figure 1B]. Although the variant is located in exon 48, the splicing prediction software (Varseak, https://varseak.bio/index.php) analysis suggested that this variant may cause abnormal splicing. We extracted RNA from the airway mucosa of the patient and validated its splicing effect (r.7977_8012del, p.Asn2660_Val2671del) [Figures 1C and 1D]. High-speed video analysis (HSVA) showed immotile cilia of the patient [Supplementary Video 1, http://links.lww.com/CM9/B640], and electron microscopy of the cilia showed a significant ODA defect [Figure 1E]. Based on these results, the patient was diagnosed with PCD. Although the patient had one son, we performed a routine semen analysis to verify whether the variant affected reproductive function, and found that the patient's sperm morphology and motility were normal [Figure 1F].
Figure 1.
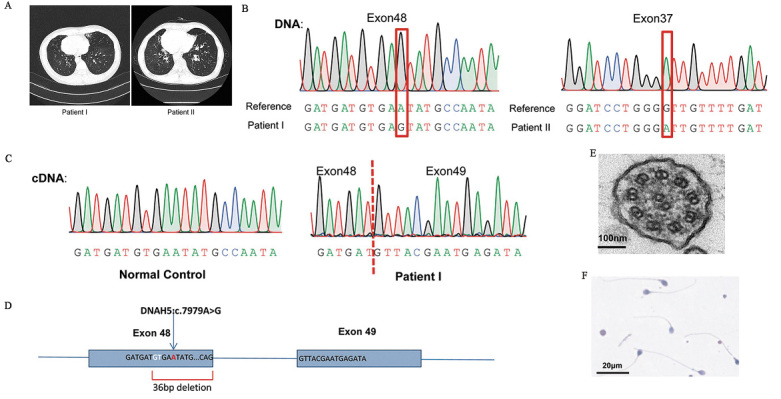
(A) The CT scan of patients showed bronchiectasis and situs inversus. (B) The result of the Sanger sequencing for two patients. (C) The cDNA sequence of patient I and normal control. (D) The variant c.7979A>G caused a 36-bp deletion of DNAH5 RNA. (E) Transmission electron microscopy showed an outer dynein arm defect of the patient's cilia. (F) Sperm morphology and motility are normal in Patient I. cDNA: Complementary DNA; CT: computed tomography; DNAH5: Dynein axonemal heavy chain 5.
Patient II was a 48-year-old man who had experienced recurrent coughing for more than 40 years. The lung function test showed obstructive impairment (FEV1/FVC was 69.96% after bronchodilator administration) and restrictive ventilation dysfunction (FEV1 percent predicted [FEV1 pred%] of 42.9%). The patient's nNO was 2.4 nL/min. CT also showed sinusitis, bronchiectasis, and situs inversus [Figure 1A, Supplementary Figures 1D and 1F, http://links.lww.com/CM9/B598]. Whole-exome sequencing revealed a homozygous variant in exon37 of DNAH5, c.6086G > A (p.Gly2029Asp) [Figure 1B]. The patient had two offspring and refused any other PCD-related examinations.
PCD is an inherited genetic motile ciliopathy that may present as Kartagener syndrome (sinusitis, bronchiectasis, and situs inversus). To date, more than 50 genes have been found to be related to PCD.[1] PCD is hetergenous and most pathogenic genes have autosomal recessive inheritance, many patients with PCD are found in consanguineous families. DNAH5 is one of the most common PCD-associated genes. Dynein axonemal heavy chain 5 (DNAH5) is an important component of the ODAs of cilia. Mutations in DNAH5 cause defects in the ODAs of the cilia, leading to cilia movement dysfunction and resulting in PCD symptoms such as sinusitis, bronchiectasis, and situs inversus.[2] According to guidelines, genetic testing, immunofluorescence, nNO measurement, HSVA, and transmission electron microscopy (TEM) are important diagnostic methods for PCD.[3] In our Patient I, the results of nNO, HSVA, TEM, and genetic testing confirmed the diagnosis of PCD. Patient II had significantly reduced nNO levels and a homozygous variant of DNAH5, which also suggested a diagnosis of PCD.
Infertility is a common symptom of PCD due to the dysfunction of sperm flagella. However, in the previous literature, it is rare to see patients with DNAH5 variants with infertility problems. Consistent with most of the DNAH5 cases reported previously, both of our patients had offspring without assisted reproductive technology. Routine semen analysis is the most common way to assess male reproductive function; however, many factors affect this examination.[4] Accidental semen routine abnormalities are common in healthy individuals, and repeated experiments are important in determining infertility.
Whole-exome sequencing is a popular objective method to identify inherited diseases, especially PCD. With the development of sequencing technology and cost reduction, genetic testing is becoming an important method for PCD diagnosis in bronchiectasis patients.[5] Measurement of nNO is an acceptable non-invasive examination for most patients. Most patients with PCD have low levels of nNO. Immunofluorescence, HSVA, and TEM are important examinations for PCD diagnosis that can directly detect defects in the cilia. As these examinations require invasive access to the respiratory mucosa, many patients do not accept them. Furthermore, not all hospitals are equipped with relevant examination equipment, increasing the difficulty of diagnosis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patients consent forms. In the form, the patients have given consent for their images and other clinical information to be reported in the journal.
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Yang BY, Lei C, Xu YJ, Yang DH, Lu CY, Liu Y, Guo T, Luo H. Whole-exome sequencing identified novel DNAH5 homozygous variants in two consanguineous families with primary ciliary dyskinesia. Chin Med J 2024;137:115–116. doi: 10.1097/CM9.0000000000002736
References
- 1.Wallmeier J Nielsen KG Kuehni CE Lucas JS Leigh MW Zariwala MA, et al. . Motile ciliopathies. Nat Rev Dis Primers 2020;6: 77. doi: 10.1038/s41572-020-0209-6. [DOI] [PubMed] [Google Scholar]
- 2.Hornef N Olbrich H Horvath J Zariwala MA Fliegauf M Loges NT, et al. . DNAH5 mutations are a common cause of primary ciliary dyskinesia with outer dynein arm defects. Am J Respir Crit Care Med 2006;174: 120–126. doi: 10.1164/rccm.200601-084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucas JS Barbato A Collins SA Goutaki M Behan L Caudri D, et al. . European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia. Eur Respir J 2017;49. doi: 10.1183/13993003.01090-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verón GL Tissera AD Bello R Beltramone F Estofan G Molina RI, et al. . Impact of age, clinical conditions, and lifestyle on routine semen parameters and sperm kinematics. Fertil Steril 2018;110: 68–75.e4. doi: 10.1016/j.fertnstert.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Shoemark A Griffin H Wheway G Hogg C Lucas JS, Genomics England Research Consortium, et al. . Genome sequencing reveals underdiagnosis of primary ciliary dyskinesia in bronchiectasis. Eur Respir J 2022;60: 2200176. doi: 10.1183/13993003.00176-2022. [DOI] [PubMed] [Google Scholar]


