Abstract
Background:
The gut microbiota-dependent metabolite trimethylamine N-oxide (TMAO) has recently been recognized to be one of the risk factors for cardiovascular disease (CVD). However, there is a scarcity of data on the relationship between circulating TMAO levels and hypertension in patients with CVD. Meta analysis and a dose-response relationship were used in this study to assess the relationship between circulating trimethylamine N-oxide levels and the risk of hypertension in patients with CVD.
Methods:
CNKI, Wanfang Database, Pubmed, Embase, Cochrane Library, and Web of Science were searched up to June 01, 2023. Meta-analysis and dose-response analysis of relative risk data from prospective cohort studies reporting on the relationship between circulating TMAO levels and hypertension risk in patients with CVD were conducted.
Results:
Fifteen studies with a total of 15,498 patients were included in the present meta-analysis. Compared with a lower circulating TMAO level, a higher TMAO level was associated with a higher risk of hypertension in patients with CVD (RR = 1.14,95%CI (1.08, 1.20)). And the higher the TMAO level, the greater the risk of hypertension. The dose-response analysis revealed a linear dose-response relationship between circulating TMAO levels and the risk of hypertension in patients with CVD. The risk of hypertension increased by 1.014% when the circulating TMAO level increased by 1 μ mol/L.
Conclusion:
In patients with CVD, the level of circulating TMAO is significantly related to the risk of hypertension. The risk of hypertension increased by 1.014% for every 1 μ mol/L increase in circulating TMAO levels.
Keywords: cardiovascular disease, dose-response relationship, hypertension, meta-analysis, trimethylamine N-oxide
1. Introduction
Cardiovascular disease (CVD), as a growing public health issue, has become one of the world diseases with the highest morbidity and mortality rates.[1] Despite the fact that methods for diagnosing and treating CVD are constantly being updated and developed, the mortality rate of patients with CVD remains high. As a result, more research into the pathogenesis and risk factors of CVD is required. A large number of studies in recent years have found a link between the occurrence and progression of CVD and the gut microbiota.[2] The human intestinal tract contains a large number of bacteria, which together form the gut microbiota, which can symbiotically interact with the host and maintain intestinal homeostasis.[3] When the balance of the gut microbiota is disrupted due to dietary habits, environmental factors, drug use, or other factors, the risk of CVD will increase significantly.[4–6] Trimethylamine N-oxide (TMAO), a gut microbiota-dependent metabolite, has been shown to be involved in the occurrence and development of CVD, either directly or indirectly, and is an important risk factor for CVD.[7]
One of the risk factors for CVD is hypertension. The 2017 AHA/ACC hypertension guidelines defined grade 1 hypertension as 130/80mmHg,[8] resulting in a significant increase in hypertension prevalence. People with systolic blood pressure >130mmhg had a significantly higher risk of developing CVD and all-cause mortality when compared to the general population, and this risk increased as systolic blood pressure increased.[9] Long-term hypertension can result in life-threatening complications such as CVD, stroke, renal failure, and fundus lesions. The pathogenesis of hypertension is complicated, with environmental, dietary, mental, and genetic factors all playing a role.[10] Recent research has discovered that the gut microbiota and its metabolites can cause the onset and progression of hypertension in a variety of ways.[11,12] Elevated circulating TMAO levels have been linked to an increased risk of MACE and all-cause death in patients with CVD.[13,14] However, there is a scarcity of data on the relationship between circulating TMAO levels and hypertension in patients with CVD. Therefore, we performed the present dose–response meta-analysis of published articles to examine the relationship between circulating trimethylamine N-oxide levels and the risk of hypertension in patients with CVD.
2. Methods
2.1. Search strategy
We (L.G. and X.C.) searched several electronic databases (CNKI, Wanfang Database, Pubmed, Embase, Cochrane Library, and Web of Science) up to June 01,2023 for prospective cohort studies, reporting on the relationship between circulating TMAO levels and hypertension risk in patients with CVD. Indexing terms included (“Trimethylamine N-oxide” or “TMAO”) AND (“cardiovascular disease” OR “coronary heart diseases” OR “heart failure” OR “arrhythmia” OR “hypertension”) without language restrictions. At the same time, the references in the retrieved literature are searched for additional information. The study protocol was registered in the PROSPERO database (available from: https://www.crd.york.ac.uk/PROSPERO. ID: CRD42023450026).
2.2. Study selection
All titles and abstracts were initially screened to ensure they met the eligibility requirements. We (L.G. and X.C.) only included prospective cohort studies with a study population that included patients with CVD (including coronary heart disease, heart failure, and arrhythmia); patients who have higher levels of circulating TMAO as exposure; and incidence of hypertension as outcome. Meanwhile, studies without original data, animal experiment studies, review articles, case reports and commentaries were excluded.
2.3. Data extraction
Two reviewers (L.G. and X.C.) worked independently to extract the data. A third reviewer (Y.M.) was invited to participate in the discussion to reach a consensus. The following information was extracted from each study: the first author name, publication data, country, disease status, sample size, average age, sex ratio, circulating TMAO levels, BMI, proportion of smokers, proportion of hypertension, proportion of diabetes, proportion of dyslipidemia, proportion of aspirin users, proportion of statins users, proportion of β-blockers users, proportion of ACEI or ARB users.
2.4. Assessment of methodological quality
The Newcastle-Ottawa Quality Assessment Scale (NOS) was used to evaluate the included studies’ quality.[15] The scale assesses the study quality based on the sum of 3 scores: selection, comparability, and outcome. The quality of the included studies was classified as low (scores 1–4), moderate (scores 5–6), or high (scores 7–9) based on the NOS scores. Low-quality studies are excluded from meta-analysis.
2.5. Statistical analysis
All the data were analyzed by STATA17.0 software, and Q and I2 statistics were used to evaluate the heterogeneity of the included study (Low, moderate, and high heterogeneity were defined as I2 values of 0 to 25, 25 to 49, and > 50%, respectively.). If I2 < 50%, a fixed-effects model would be used to compute the pooled RR estimates. Otherwise, the pooled RR estimates would be calculated using a random-effects model. To investigate the source of heterogeneity, the following variables were used: country, disease status, sample size, average age, sex ratio, circulating TMAO concentration, BMI, proportion of smokers, proportion of hypertension, proportion of diabetes, proportion of dyslipidemia, proportion of aspirin users, proportion of statins users, proportion of β-blockers users, proportion of ACEI or ARB users. Begg and Egger tests were used to determine whether there was publication bias, and a sensitivity analysis was performed to test the results’ reliability by eliminating each of the included studies sequentially.
The dose-response relationship between circulating TMAO levels and the risk of hypertension was evaluated by collecting the following data from the study: the average circulating TMAO level in each group; the total number of cases in each group; the total number of hypertension cases in each group. When the mean value of TMAO is not reported in the study, the average of the upper and lower intervals is used; when the TMAO level is an open interval, the interval lowest limit is multiplied by 1.5. The dose-response relationship between circulating TMAO level and the risk of hypertension was analyzed using the data presented above, and a dose-response diagram was created.
3. Result
3.1. Characteristics of the included studies
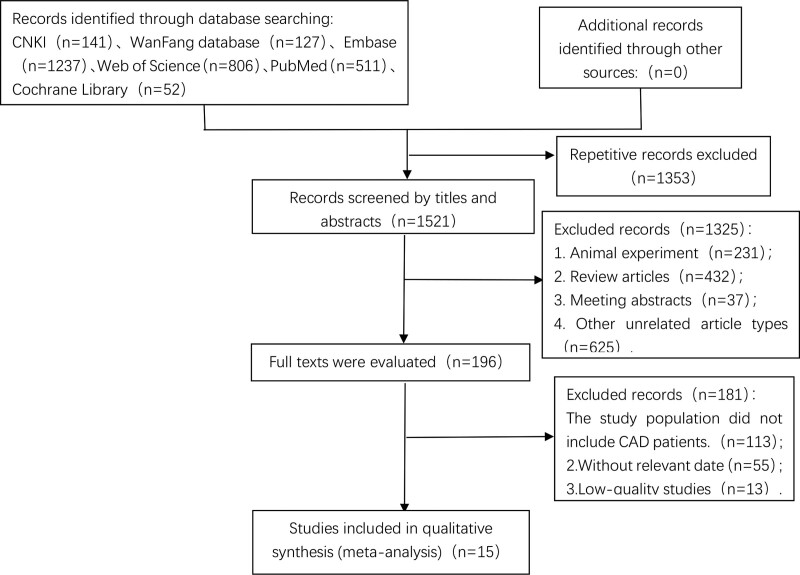
A total of 2874 potentially relevant publications were identified, including CNKI (n = 141), WanFang database (n = 127), Embase (n = 1237), PubMed (n = 511), Web of science (n = 806), Cochrane library (n = 52). After screening the titles and abstracts, our meta-analysis included 15 studies with a total of 15,498 participants.[16–30] (the flow chart for the study selection is shown in Figure 1).
Figure 1.
Flow chart of the study selection for the meta-analysis.
The average age of the included studies’ participants ranged from 59 to 80 years, the average TMAO level ranged from 2.57 to 20.37 μ mol/L, and the proportion of men ranged from 44 to 88 percent. A total of twelve studies on the dose-response relationship between circulating TMAO levels and the risk of hypertension in patients with CVD were included.[16–18,20–23,25,26,28–30] The results of study quality revealed that the NOS scores of 3 studies were 7,[16,25,27] 6 studies had NOS scores of 8,[19,21–24,29] and 6 studies had NOS scores of 9.[17,18,20,26,28,30] (the general characteristics of the included studies are illustrated in Table 1).
Table 1.
Characteristics of included studies
| Study | Yr | Country | Disease status | Sample | Age (y) | Male, % | TMAO, μ mol/L | BMI, kg/m2 | Smoking, % | Hypertension, % | Diabetes, % | Dyslipidemia, % | Aspirin use, % | Beta-blocker use, % | Statin use, % | ACEI or ARB use, % | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LI[16] | 2022 | China | Patients with ACS | 985 | 63.0 (54.0–70.0) | 77.8 | 6.7 (4.0–11.7) | 25.7 (23.3–27.8) | 70.8 | 66.4 | 36.3 | 92.8 | 94.7 | 85.3 | 94.7 | 69.5 | 7 |
| Qiu[17] | 2022 | China | Patients with IHF | 189 | 64 ± 10.5 | 83.1 | 4.92 (2.55–6.84) | N/A | 28.6 | 52.4 | 33.3 | 60.8 | N/A | N/A | N/A | N/A | 9 |
| Eyileten[18] | 2021 | Austria | Patients with ACS | 292 | 59 ± 12 | 80 | 2.57 (1.77–3.85) | 27.8 | 7 | 63 | 21 | 57 | 100 | 93 | 93 | 88 | 9 |
| Kinugasa[19] | 2021 | Japan | Patients with AHF | 146 | 80 (73–85) | 46.4 | 20.37 (10.45–38.31) | 21.13 (18.44–23.44) | N/A | 80.1 | 40.4 | 44.5 | N/A | 76.7 | N/A | 83.6 | 8 |
| Gencer[20] | 2020 | Multiple countries | Patients with SCAD | 1803 | 68 (60–74) | 74.4 | 4.75 | 28.7 (25.3–33.7) | 68.7 | 80.2 | 33.9 | 82.1 | 99.8 | 85.8 | 93.5 | 79 | 9 |
| Zhou[21] | 2020 | China | Patients with ACS | 1208 | 73 (64–80) | 68.5 | 4.5 | 28.1 (25.4–30.2) | N/A | 44.7 | 28.6 | 39.7 | 86.3 | 76.5 | 81.5 | 72.8 | 8 |
| Matsuzawa[22] | 2019 | Japan | Patients with ACS | 112 | 63 (56–71) | 88 | 5.63 (3.20–10.38) | 24.1 (22.0–26.6) | 74 | 48 | 29 | 62 | 100 | 86 | 98 | 96 | 8 |
| Svingen[23] | 2018 | Norway | Patients with SCAD | 4141 | 62 (51–73) | 71.9 | 7.57 (2.2–23.5) | 26.3 (24.1–29.1) | 31.7 | 46.7 | 14 | N/A | N/A | N/A | N/A | N/A | 8 |
| Zhang[24] | 2018 | China | Patients with CAD and AF | 200 | 62.7 | 54.5 | 5.02 | 23.1 | 46 | 34.5 | 14 | 9 | N/A | N/A | N/A | N/A | 8 |
| Suzuki[25] | 2017 | UK | Patients with ACS | 1079 | 67 (57–77) | 72 | 3.70 (4.60–6.40) | N/A | 42 | 52 | 23 | N/A | 85 | 81 | 89 | 84 | 7 |
| Tang[26] | 2017 | USA | Patients with SCAD and T2DM | 1216 | 64.4 ± 10.2 | 58 | 4.4 (2.8–7.7) | N/A | 63 | 79 | N/A | N/A | 75 | 66 | 64 | 59 | 9 |
| Xu[27] | 2017 | China | Patients with ACS | 200 | 67.4 ± 12.8 | 44 | 5.94 ± 1.93 | 24.08 | 41.5 | 70.5 | 27.5 | N/A | N/A | N/A | N/A | N/A | 7 |
| Senthong[28] | 2016 | USA | Patients with SCAD | 2235 | 63 ± 11 | 71 | 3.8 (2.5–6.5) | N/A | 70 | 76 | 35 | N/A | 81 | 70 | 71 | N/A | 9 |
| Suzuki[29] | 2016 | UK | Patients with AHF | 972 | 78 (69–84) | 61 | 5.6 (3.4–10.5) | N/A | 9.6 | 58.3 | 33.8 | 24.4 | N/A | N/A | N/A | N/A | 8 |
| Tang[30] | 2014 | USA | Patients with CHF | 720 | 66 ± 10 | 59 | 5.0 (3.0–8.5) | 28.4 (25.1–33.1) | N/A | 78 | 41 | N/A | 64 | 69 | 61 | 69 | 9 |
ACS = acute coronary syndrome, AF = atrial fibrillation, AHF = acute heart failure, BMI = body mass index, CAD = coronary artery disease, CHF = Chronic heart failure, IHF = ischemic heart failure, N/A = no applicable, NOS = Newcastle-Ottawa Scale, SCAD = stable coronary artery disease, T2DM = type 2 diabetes mellitus, TMAO = trimethylamine N-oxide.
3.2. The association between circulating TMAO levels and hypertension risk in patients with cardiovascular disease
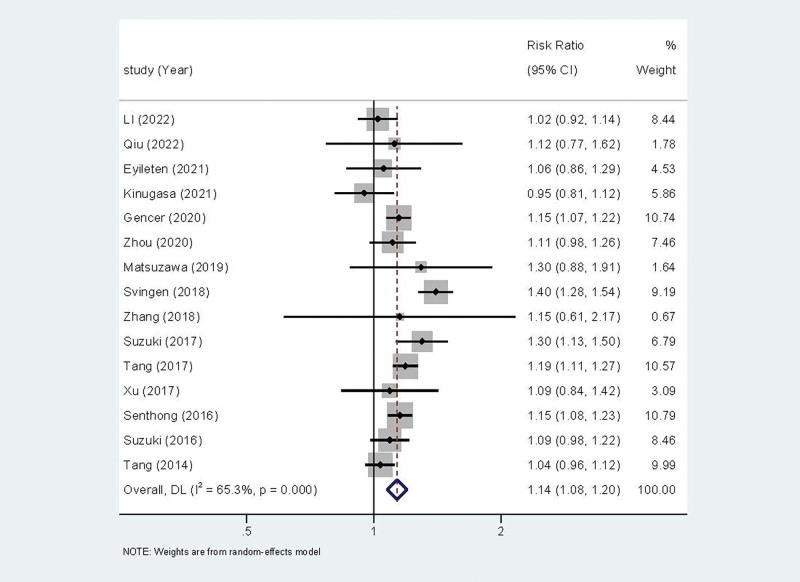
The relationship between circulating TMAO levels and the risk of hypertension in patients with CVD was reported in fifteen of the included studies. A random effect model was used for Meta analysis due to the statistical heterogeneity among the studies (I2 = 65.3% P < .001). The results revealed that circulating TMAO levels were associated with the risk of hypertension in patients with CVD (RR = 1.14, 95%CI (1.08, 1.20))(Fig. 2). And the higher the level of TMAO in the patient, the greater the risk of developing hypertension.
Figure 2.
Forest plot of the relationship between circulating TMAO levels and the risk of hypertension in patients with cardiovascular disease. TMAO = trimethylamine N-oxide.
Subgroup analysis is required to identify the source of heterogeneity in studies on the relationship between circulating TMAO levels and the risk of hypertension in patients with CVD due to statistical heterogeneity (I2 = 65.3% P < .001). To investigate the source of heterogeneity, the following variables were used: country, disease status, sample size, average age, sex ratio, circulating TMAO concentration, BMI, proportion of smokers, proportion of hypertension, proportion of diabetes, proportion of dyslipidemia, proportion of aspirin users, proportion of statins users, proportion of β-blockers users, proportion of ACEI or ARB users. Following the subgroup analysis, the results revealed that no significant source of heterogeneity was discovered, indicating that additional sensitivity analysis is required (Table 2).
Table 2.
Subgroup analysis of the relationship between circulating TMAO levels and the risk of hypertension in patients with cardiovascular disease
| Subgroups | Studies, (n) | Overall effect | Heterogeneity | ||
|---|---|---|---|---|---|
| RR (95% CI) | P value | I2, % | P value | ||
| All | 15 | 1.14 (1.08, 1.20) | <.001 | 65.3 | <.001 |
| Study location | |||||
| Asia | 7 | 1.05 (0.98, 1.12) | .156 | 0 | .698 |
| Europe | 5 | 1.20 (1.08, 1.33) | .001 | 78.4 | .001 |
| North America | 3 | 1.13 (1.04, 1.22) | .002 | 71.9 | .028 |
| Disease status | |||||
| Patients with CAD | 11 | 1.16 (1.09, 1.23) | <.001 | 70.7 | <.001 |
| Patients with HF | 3 | 1.05 (0.96, 1.15) | .305 | 5.4 | .348 |
| Patients with arrhythmia | 1 | 1.15 (0.61, 2.17) | .657 | — | — |
| Year of publication | |||||
| ≥2019 | 7 | 1.09 (1.02, 1.15) | .005 | 18.8 | .287 |
| <2019 | 8 | 1.18 (1.09, 1.27) | <.001 | 75.1 | <.001 |
| Participants, n | |||||
| ≥1000 | 6 | 1.21 (1.13, 1.29) | <.001 | 70.3 | .005 |
| <1000 | 9 | 1.05 (1.00, 1.10) | .072 | 0 | .883 |
| Male, % | |||||
| ≥70% | 8 | 1.18 (1.09, 1.28) | <.001 | 71.6 | <.001 |
| <70% | 7 | 1.09 (1.02, 1.16) | .007 | 42 | .111 |
| Mean/median, yr | |||||
| ≥65 | 7 | 1.10 (1.04, 1.17) | .002 | 51.8 | .052 |
| <65 | 8 | 1.17 (1.08, 1.28) | <.001 | 68.3 | .002 |
| Mean circulating TMAO, μ mol/L | |||||
| ≥5 | 8 | 1.11 (0.99, 1.24) | .074 | 79.2 | <.001 |
| <5 | 7 | 1.16 (1.12, 1.20) | <.001 | 0 | .6 |
| Smoking, % | |||||
| ≥50 | 5 | 1.14 (1.09, 1.20) | <.001 | 31.8 | .209 |
| <50 | 7 | 1.19 (1.07, 1.34) | .002 | 63.3 | .012 |
| N/A | 3 | 1.04 (0.97, 1.11) | .251 | 10 | .329 |
| BMI (kg/m2) | |||||
| ≥26 | 4 | 1.19 (1.05, 1.34) | .007 | 80.9 | .001 |
| <26 | 6 | 1.03 (0.97, 1.09) | .31 | 0 | .754 |
| N/A | 5 | 1.17 (1.12, 1.22) | <.001 | 5.7 | .374 |
| Hypertension, % | |||||
| ≥60 | 8 | 1.10 (1.04, 1.16) | <.001 | 53.6 | .035 |
| <60 | 7 | 1.22 (1.09, 1.35) | <.001 | 62.5 | .014 |
| Diabetes, % | |||||
| ≥30 | 7 | 1.09 (1.03, 1.14) | .001 | 45 | .091 |
| <30 | 7 | 1.22 (1.09, 1.35) | <.001 | 55.5 | .036 |
| N/A | 1 | 1.19 (1.11, 1.27) | <.001 | — | — |
| Dyslipidemia, % | |||||
| ≥60 | 4 | 1.11 (1.03, 1.19) | .008 | 21.1 | .283 |
| <60 | 5 | 1.07 (1.00, 1.14) | .061 | 0 | .626 |
| N/A | 6 | 1.19 (1.09, 1.30) | <.001 | 81 | <.001 |
| Aspirin use, % | |||||
| ≥90 | 4 | 1.10 (1.02, 1.19) | .012 | 26.4 | .253 |
| <90 | 5 | 1.15 (1.07, 1.22) | <.001 | 62.9 | .029 |
| N/A | 6 | 1.13 (0.97, 1.33) | .125 | 77.7 | <.001 |
| Beta-blocker use, % | |||||
| ≥80 | 5 | 1.14 (1.04, 1.24) | .005 | 52.1 | .08 |
| <80 | 5 | 1.10 (1.03, 1.18) | .005 | 65.1 | .022 |
| N/A | 5 | 1.19 (1.02, 1.40) | .032 | 70.2 | .009 |
| Statin use, % | |||||
| ≥90 | 4 | 1.10 (1.02, 1.19) | .012 | 26.4 | .253 |
| <90 | 5 | 1.15 (1.07, 1.22) | <.001 | 62.9 | .029 |
| N/A | 6 | 1.13 (0.97, 1.33) | .125 | 77.7 | <.001 |
| ACEI or ARB use, % | |||||
| ≥80 | 4 | 1.12 (0.94, 1.34) | .191 | 67.5 | .026 |
| <80 | 5 | 1.11 (1.04, 1.17) | .001 | 59.6 | .042 |
| N/A | 6 | 1.19 (1.07, 1.32) | .002 | 68.5 | .007 |
| NOS | |||||
| ≥8 | 12 | 1.14 (1.07, 1.21) | <.001 | 66.4 | .001 |
| <8 | 3 | 1.13 (0.95, 1.35) | .154 | 72.2 | .027 |
NOS = Newcastle-Ottawa Scale, TMAO = trimethylamine N-oxide.
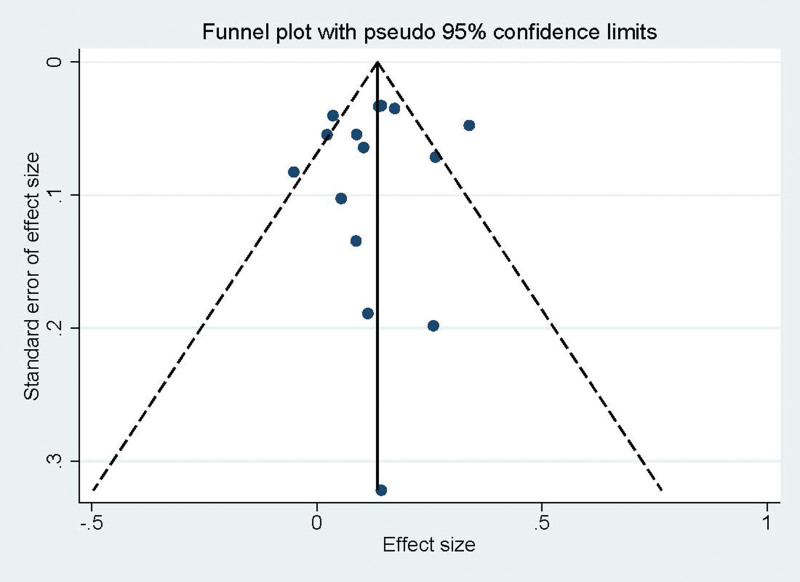
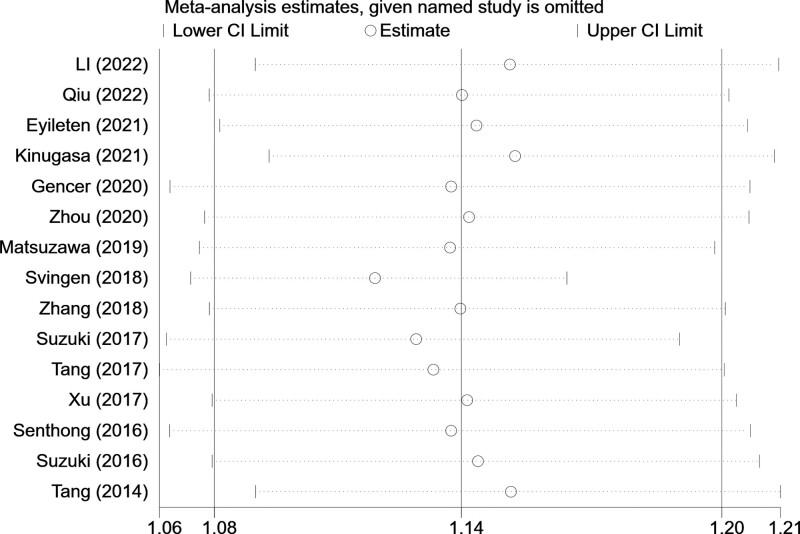
3.3. Publication bias and sensitivity analysis
The Egger test (t = -0.36, P = .724) and Begg test (Z = 0.20, P = .843) indicated no statistically significant publication bias in this meta-analysis (Fig. 3). The funnel diagram depicts a roughly symmetrical distribution. The sensitivity analysis revealed that the consolidated results were all stable (Fig. 4).
Figure 3.
Funnel diagram of the relationship between circulating TMAO levels and risk of hypertension in patients with cardiovascular disease. TMAO = trimethylamine N-oxide.
Figure 4.
Sensitivity analysis of the relationship between circulating TMAO levels and the risk of hypertension in patients with cardiovascular disease. TMAO = trimethylamine N-oxide.
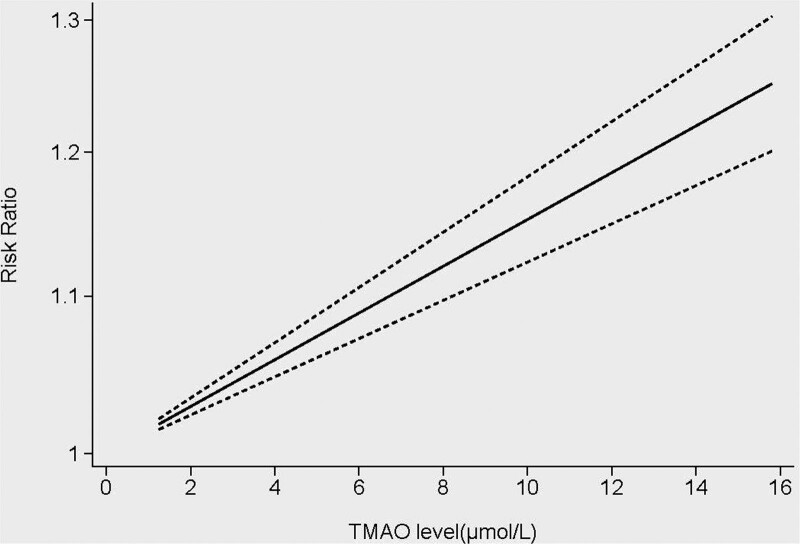
3.4. Dose-response analysis
The dose-response relationship between circulating TMAO levels and the incidence of hypertension was studied using 12 studies and 14,952 patients.[16–18,20–23,25,26,28–30] The results revealed a linear dose-response relationship between circulating TMAO levels and the risk of hypertension in patients with CVD. The risk of hypertension increased by 1.014% when the circulating TMAO level increased by 1 μ mol/L. The risk of hypertension increased by 7.34% when the circulating TMAO level was 5 μ mol/L, and by 15.22% when the circulating TMAO level was 10 μ mol/L (Fig. 5).
Figure 5.
Dose-response relationship between circulating TMAO levels and the risk of hypertension in patients with cardiovascular disease. TMAO = trimethylamine N-oxide.
4. Discussion
The results of this study show a direct link between circulating TMAO concentration and an increased risk of hypertension in CVD patients. CVD patients with high TMAO concentrations had a 14% increased risk of developing hypertension compared to CVD patients with low TMAO concentrations. Simultaneously, there was a linear dose-response relationship between circulating TMAO concentration and hypertension risk in CVD patients. The risk of hypertension increased by 1.014% for every 1 μ mol/L increase in circulating TMAO concentration
TMAO is a small molecular organic compound (75.11g/mol) produced primarily by the oxidation of trimethylamine (TMA) in the gut microbiota. When the human body consumes foods containing L-carnitine, choline, and betaine, these substances are converted into TMA by various enzymes in the gut microbiota. The majority of TMA is passively absorbed into the portal circulation and oxidized to TMAO in the liver by flavin-containing monooxygenases (FMOS).[31] The high concentration of TMAO in the bloodstream increases the risk of MACE and all-cause death in CVD patients. First, TMAO inhibits cholesterol reverse transport and increases cholesterol accumulation in macrophages, resulting in increased foam cell formation and dyslipidemia, as well as atherosclerotic plaques[32,33]; Furthermore, TMAO can impair endothelial function and cause vascular inflammation, leading to vascular dysfunction.[34–36] According to the latest research, TMAO also promotes platelet aggregation, which increases the risk of blood clots and makes atherosclerotic plaques more likely to rupture.[37,38] In addition, circulating TMAO concentration have been independently associated with subclinical myocardial damage.[39]
The latest evidence suggests that the gut-brain-bone marrow (BM) axis, which includes gut microbiota, gut epithelial wall permeability, increased production of pro-inflammatory BM cells, and neuroinflammation, plays an important role in hypertension. Hypertension can cause an imbalance in the gut microbiota, a weakening of the intestinal barrier, and inflammation. Inflammatory mediators produced in the intestine can easily cross the weakened intestinal barrier and circulate to the brain, causing neuroinflammation and mild systemic inflammation. The interaction of these 3 organs strengthens their individual responses, resulting in hypertension maintenance and increase, creating a vicious circle.[40] A proven triangle exists between salt, high blood pressure, and gut microbiota. The gut microbiota produces 2 major products: short-chain fatty acids (SCFAs) and TMAO. SCFAs play an important role in blood pressure regulation, while TMAO is also involved in the development of atherosclerosis and hypertension. High salt consumption can cause an increase in TMAO and a decrease in SCFAs in the intestine, causing intestinal disorders and promoting the onset and progression of hypertension.[41] TMAO may also play a role in the pathogenesis of hypertension in a variety of ways and can help predict the severity of hypertension.[42] The renin-angiotensin-aldosterone system (RAAS) impacts cardiovascular homeostasis via direct actions on peripheral blood vessels and via modulation of the autonomic nervous system. Angiotensin (Ang) II is the main effect substance of RAAS, which increases blood pressure through various pathways.[43] TMAO can prolong the hypertensive effect of Ang II, lengthen the duration of hypertension, and increase susceptibility to hypertension.[44] Furthermore, TMAO promotes Ang II-induced vasoconstriction, which promotes Ang II-induced hypertension and damages glomerular filtration rate via the PERK/ROS/CaMKII/PLC3 axis.[45] According to the findings of a recent animal experimental study, an increase in TMAO levels can lead to higher plasma osmotic pressure and trigger the regulation of the “TMAO-AVP-AQP-2” axis in rats, which causes increased water reabsorption and eventually leads to hypertension.[46] It is well known that the risk of CVD increases exponentially with age, and the level of circulating TMAO has also been shown to increase with age. High levels of TMAO can cause oxidative stress, which can lead to endothelial dysfunction and eventually lead to hypertension.[47] Furthermore, an increase in TMAO could be a new mechanism causing aortic stiffness. When the aorta hardens, its elasticity weakens and the velocity of blood flow in the blood vessels increases, resulting in an increase in blood pressure and eventually lead to hypertension.[48] TMAO-induced advanced glycation end-products (AGEs) accumulation, as well as superoxide-related oxidative stress, which causes aortic hardening and increases systolic blood pressure.[49]
The studies discussed above reveal a link between TMAO and hypertension and provide preliminary evidence that circulating TMAO levels can predict the risk of hypertension in CVD patients. However, there are some unanswered questions about the relationship between circulating TMAO levels and hypertension. First, there is a lack of long-term monitoring of TMAO concentration in CVD patients, which makes it impossible to determine the long-term average concentration and source of TMAO in CVD patients, and it is impossible to determine whether high levels of TMAO cause hypertension. Second, while the strategy for reducing TMAO levels through dietary intervention to reduce the risk of CVD has been confirmed,[50] the intervention for TMAO is still in the animal experimental stage, and there is no effective treatment in clinic. As a result, more research is needed to investigate the link between circulating TMAO concentrations and hypertension in CVD patients.
5. Study limitations
When interpreting the results, many potential limitations of our study must be considered. First, there are some differences between research groups, such as research objects, countries, regions, races, and eating habits, which may cause results to differ. Second, there is no systematic correction of various factors of the research objects for the study of hypertension, and the results may be influenced by multiple factors. Third, the setting of the boundary value of the TMAO level interval varies greatly between research cohorts, which may have an effect on the results. Given these limitations, additional research is needed to validate our finding of a link between circulating TMAO levels and hypertension in patients with CVD.
6. Conclusions
The results of this study show that circulating TMAO levels are associated with the incidence of hypertension in patients with CVD. In patients with CVD, there is a linear dose-response relationship between circulating TMAO levels and the risk of hypertension. The risk of hypertension increased by 1.014% when the circulating TMAO level increased by 1 μ mol/L. Elevated circulating TMAO levels may indicate an increased risk of hypertension in patients with CVD.
Acknowledgments
The authors’ contributions were as follows. Y.M.: Devised the study; L.G., X.C.: contributed to data collection, analysis, and interpretation of data; X.S., Y.M.: carried out the quality assessment; J.H.: wrote the initial draft of the manuscript; J.H., L.G., X.C., Q.X., X.S., Y.M.: participated in the discussion and approved the final manuscript.
Author contributions
Conceptualization: Yu-Lan Ma.
Data curation: Lu Guo, Xian-Hui Chen, Qian Xie, Xiu-Ying Song.
Formal analysis: Lu Guo, Xian-Hui Chen, Qian Xie, Xiu-Ying Song.
Supervision: Yu-Lan Ma.
Validation: Yu-Lan Ma.
Writing – original draft: Jia-Ming Han.
Writing – review & editing: Jia-Ming Han.
Abbreviations:
- CVD
- cardiovascular disease
- NOS
- Newcastle-Ottawa Quality Assessment Scale
- TMAO
- trimethylamine N-oxide
The datasets generated during and/or analyzed during the current study are publicly available.
This study was supported by the National Natural Science Foundation of China (81760084).
The authors have no conflicts of interest to disclose.
All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
How to cite this article: Han J-M, Guo L, Chen X-H, Xie Q, Song X-Y, Ma Y-L. Relationship between trimethylamine N-oxide and the risk of hypertension in patients with cardiovascular disease: A meta-analysis and dose-response relationship analysis. Medicine 2024;103:1(e36784).
References
- [1].Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics–2022 update: a report from the American Heart Association[. Circulation. 2022;145:e153–639. [DOI] [PubMed] [Google Scholar]
- [2].Kumar T, Dutta RR, Velagala VR, et al. Analyzing the complicated connection between intestinal microbiota and cardiovascular diseases. Cureus. 2022;14:e28165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhou B, Yuan Y, Zhang S, et al. Intestinal flora and disease mutually shape the regional immune system in the intestinal tract. Front Immunol. 2020;11:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Alhajri N, Khursheed R, Ali MT, et al. Cardiovascular health and the intestinal microbial ecosystem: the impact of cardiovascular therapies on the gut microbiota. Microorganisms. 2021;9:2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Murphy K, O’Donovan AN, Caplice NM, et al. Exploring the gut microbiota and cardiovascular disease. Metabolites. 2021;11:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Al Samarraie A, Pichette M, Rousseau G. Role of the gut microbiome in the development of atherosclerotic cardiovascular disease. Int J Mol Sci. 2023;24:5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhen J, Zhou Z, He M, et al. The gut microbial metabolite trimethylamine N-oxide and cardiovascular diseases. Front Endocrinol. 2023;14:1085041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. [DOI] [PubMed] [Google Scholar]
- [9].Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2:775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xiong Y, Xiong Y, Zhu P, et al. The role of gut microbiota in hypertension pathogenesis and the efficacy of antihypertensive drugs. Curr Hypertens Rep. 2021;23:4040. [DOI] [PubMed] [Google Scholar]
- [11].Avery EG, Bartolomaeus H, Maifeld A, et al. The gut microbiome in hypertension: recent advances and future perspectives. Circ Res. 2021;128:934–50. [DOI] [PubMed] [Google Scholar]
- [12].Kumar T, Dutta RR, Velagala VR, et al. Analyzing the complicated connection between intestinal microbiota and cardiovascular diseases. Cureus. 2022;14:e28165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li X, Fan Z, Cui J, et al. Trimethylamine N-oxide in heart failure: a meta-analysis of prognostic value trimethylamine N-oxide in heart failure: a meta-analysis of prognostic value. Front Cardiovasc Med. 2022;9:817396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yao ME, Liao PD, Zhao XJ, et al. Trimethylamine-N-oxide has prognostic value in coronary heart disease: a meta-analysis and dose-response analysis. BMC Cardiovasc Disord. 2020;20:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [16].Li N, Zhou J, Wang Y, et al. Association between trimethylamine N-oxide and prognosis of patients with acute myocardial infarction and heart failure. ESC Heart Fail. 2022;09:3846–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Qiu WD, Xiao XJ, Xia S, et al. Predictive value of plasma TMAO combined with NT-proBNP on the prognosis and length of hospitalization of patients with ischemic heart failure. Zhonghua Xin Xue Guan Bing Za Zhi. 2022;50:684–9. [DOI] [PubMed] [Google Scholar]
- [18].Eyileten C, Jarosz-Popek J, Jakubik D, et al. Plasma trimethylamine-N-oxide is an independent predictor of long-term cardiovascular mortality in patients undergoing percutaneous coronary intervention for acute coronary syndrome. Front Cardiovasc Med. 2021;8:728724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kinugasa Y, Nakamura K, Kamitani H, et al. Trimethylamine N-oxide and outcomes in patients hospitalized with acute heart failure and preserved ejection fraction. ESC Heart Fail. 2021;8:2103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gencer B, Li XS, Gurmu Y, et al. Gut microbiota-dependent trimethylamine N-oxide and cardiovascular outcomes in patients with prior myocardial infarction: a nested case control study from the PEGASUS-TIMI 54 trial. J Am Heart Assoc. 2020;9:e015331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhou X, Jin M, Liu L, et al. Trimethylamine N-oxide and cardiovascular outcomes in patients with chronic heart failure after myocardial infarction. ESC Heart Fail. 2020;7:189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Matsuzawa Y, Nakahashi H, Konishi M, et al. Microbiota-derived trimethylamine N-oxide predicts cardiovascular risk after STEMI. Sci Rep. 2019;9:11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Svingen GFT, Zuo H, Ueland PM, et al. Increased plasma trimethylamine-N-oxide is associated with incident atrial fibrillation. Int J Cardiol. 2018;267:100–6. [DOI] [PubMed] [Google Scholar]
- [24].Yu-Lan Z, Pei-Sheng Z. The connection between trimethylamine oxide, an intestinal flora metabolite, and atrial fibrillation. World J Complex Med. 2018;4:30–3. [Google Scholar]
- [25].Suzuki T, Heaney LM, Jones DJ, et al. Trimethylamine N-oxide and risk stratification after acute myocardial infarction. Clin Chem. 2017;63:420–8. [DOI] [PubMed] [Google Scholar]
- [26].Tang WH, Wang Z, Li XS, et al. Increased trimethylamine N-oxide portends high mortality risk independent of glycemic control in patients with type 2 diabetes mellitus. Clin Chem. 2017;63:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xu K-Z, Lin L-M, Wu Y, et al. Relationship between the plasma level of trimethylamine N-oxide and complication risk in patients with acute myocardial infarction. Chin JArterioscler. 2017;25:1126–31. [Google Scholar]
- [28].Senthong V, Wang Z, Li XS, et al. Intestinal microbiota-generated metabolite trimethylamine-N-oxide and 5-year mortality risk in stable coronary artery disease: the contributory role of intestinal microbiota in a COURAGE-like patient cohort. J Am Heart Assoc. 2016;5:e002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Suzuki T, Heaney LM, Bhandari SS, et al. Trimethylamine N-oxide and prognosis in acute heart failure. Heart. 2016;102:841–8. [DOI] [PubMed] [Google Scholar]
- [30].Tang WH, Wang Z, Fan Y, et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64:1908–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Steele CN, Baugh ME, Griffin LE, et al. Fasting and postprandial trimethylamine N-oxide in sedentary and endurance-trained males following a short-term high-fat diet. Physiol Rep. 2021;9:e14970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Alhajri N, Khursheed R, Ali MT, et al. Cardiovascular health and the intestinal microbial ecosystem: the impact of cardiovascular therapies on the gut microbiota. Microorganisms. 2021;9:2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pathak P, Helsley RN, Brown AL, et al. Small molecule inhibition of gut microbial choline trimethylamine lyase activity alters host cholesterol and bile acid metabolism. Am J Physiol Heart Circ Physiol. 2020;318:H1474–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liu X, Tu J, Zhou Z, et al. TMAO-activated hepatocyte-derived exosomes are widely distributed in mice with different patterns and promote vascular inflammation. Cardiol Res Pract. 2022;2022:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liu X, Shao Y, Tu J, et al. TMAO-activated hepatocyte-derived exosomes impair angiogenesis via repressing CXCR4. Front Cell Dev Biol. 2021;9:804049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Casso AG, VanDongen NS, Gioscia-Ryan RA, et al. Initiation of 3,3-dimethyl-1-butanol at midlife prevents endothelial dysfunction and attenuates in vivo aortic stiffening with ageing in mice. J Physiol. 2022;600:4633–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Krüger-Genge A, Jung F, Hufert F, et al. Effects of gut microbial metabolite trimethylamine N-oxide (TMAO) on platelets and endothelial cells. Clin Hemorheol Microcirc. 2020;76:309–16. [DOI] [PubMed] [Google Scholar]
- [38].Koay YC, Chen YC, Wali JA, et al. Plasma levels of trimethylamine-N-oxide can be increased with “healthy” and “unhealthy” diets and do not correlate with the extent of atherosclerosis but with plaque instability. Cardiovasc Res. 2020;117:435–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Senthong V, Kiatchoosakun S, Wongvipaporn C, et al. Gut microbiota-generated metabolite, trimethylamine-N-oxide, and subclinical myocardial damage: a multicenter study from Thailand. Sci Rep. 2021;11:14963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Li J, Raizada MK, Richards EM. Gut-brain-bone marrow axis in hypertension. Curr Opin Nephrol Hypertens. 2020;30:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Naqvi S, Asar TO, Kumar V, et al. A cross-talk between gut microbiome, salt and hypertension. Biomed Pharmacother. 2021;134:111156. [DOI] [PubMed] [Google Scholar]
- [42].Wu Y, Wu Y, Duan L, et al. Clinical meaning of serum trimethylamine oxide, N-terminal-pro-brain natriuretic peptide, hypoxia-inducible factor-1a and left ventricular function and pregnancy outcome in patients with pregnancy-induced hypertension. J Med Biochem. 2023;42:265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shanks J, Ramchandra R. Angiotensin II and the cardiac parasympathetic nervous system in hypertension. Int J Mol Sci. 2021;22:12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Marcin U, Radoslaw J, Michal D, et al. Trimethylamine-N-oxide: a carnitine-derived metabolite that prolongs the hypertensive effect of angiotensin II in rats. Can J Cardiol. 2014;30:1700–5. [DOI] [PubMed] [Google Scholar]
- [45].Jiang S, Shui Y, Cui Y, et al. Gut microbiota dependent trimethylamine N-oxide aggravates angiotensin II-induced hypertension. Redox Biol. 2021;46:102115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Liu M, Han Q, Yang J. Trimethylamine-N-oxide (TMAO) increased aquaporin-2 expression in spontaneously hypertensive rats. Clin Exp Hypertens. 2018;41:312–22. [DOI] [PubMed] [Google Scholar]
- [47].Brunt VE, Gioscia-Ryan RA, Casso AG, et al. Trimethylamine-N-oxide promotes age-related vascular oxidative stress and endothelial dysfunction in mice and healthy humans. Hypertension. 2020;76:101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Pierce GL, Roy SJ, Gimblet CJ. The gut-arterial stiffness axis: is TMAO a novel target to prevent age-related aortic stiffening? Hypertension. 2021;78:512–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Brunt VE, Casso AG, Gioscia-Ryan RA, et al. Gut microbiome-derived metabolite trimethylamine N-oxide induces aortic stiffening and increases systolic blood pressure with aging in mice and humans. Hypertension. 2021;78:499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Coutinho-Wolino KS, de F Cardozo LFM, de Oliveira Leal V, et al. Can diet modulate trimethylamine N-oxide (TMAO) production? What do we know so far? Eur J Nutr. 2021;60:3567–84. [DOI] [PubMed] [Google Scholar]