Abstract
In patients with interstitial lung disease (ILD), the risk of pulmonary embolism (PE) is increased; however, distinguishing between PE and ILD exacerbation can be difficult. Therefore, this study investigated the usefulness of the Wells criteria and revised Geneva score and predictive factors for diagnosing PE in ILD patients with worsening respiratory symptoms. We retrospectively collected the data of 65 patients with ILD who underwent computed tomography pulmonary angiography at Fukujuji Hospital and Kyorin University Faculty of Medicine from January 2018 to March 2023, including 18 patients in the PE group and 47 patients in the non-PE group, and the data were compared between the 2 groups. The Wells score (P = .165) and revised Geneva score (P = .140) were not useful for distinguishing between the PE and non-PE groups. Patients in the PE group showed higher D-dimer, total protein (TP), and globulin levels than those in the non-PE group (D-dimer median 24.5 µg/mL [range 3.0–79.3] vs 9.3 µg/mL [range 0.5–80.8], P = .016; TP median 7.2 g/dL [range 5.1–8.7] vs 6.4 g/dL [range 5.0–8.2], P = .002; globulin median 3.8 g/dL [range 2.6–5.5] vs 3.2 g/dL [range 3.0–5.3], P = .041). Using cutoff values of TP ≥ 7.0 g/dL and D-dimer ≥ 11.8 µg/mL, the odds ratios for predicting PE were 10.5 and 4.90, respectively. This study demonstrates that high TP and D-dimer levels are useful indicators for predicting PE in ILD patients with worsening respiratory symptoms, while the Wells score and revised Geneva score are not reliable in diagnosing PE.
Keywords: acute exacerbation, D-dimer, interstitial lung disease, pulmonary embolism, total protein
1. Introduction
Pulmonary embolism (PE) is a significant cause of cardiovascular death worldwide.[1,2] In patients with interstitial lung disease (ILD), the risk of thromboembolic events, including PE, is increased,[3–6] with reported prevalence rates ranging from 1.7% to 14.6%.[5–8] PE can lead to severe deterioration, including respiratory failure,[9] making it essential to investigate PE when ILD patients experience acute or gradual worsening of respiratory status.[6] In particular, PE should be suspected when acute deterioration occurs in the absence of imaging features or when lung function test findings are suggestive of ILD progression.[10] However, accurately diagnosing PE becomes challenging when patients have concurrent illnesses, and distinguishing between PE and ILD exacerbation can be difficult.[2,11] Furthermore, some patients even receive simultaneous diagnoses of ILD exacerbation and acute PE[6] Generally, the Wells score and revised Geneva score are typically employed to assess the likelihood of PE for patients with suspected PE[12,13]; however, we have observed many ILD patients who cannot be accurately classified as having or not having PE using these criteria. Despite the importance of distinguishing between the 2 conditions, no reports have demonstrated the characteristics of ILD patients with PE compared to those with ILD exacerbation. Therefore, this study investigated predictive factors for diagnosing PE in patients with ILD experiencing worsening respiratory symptoms, with a specific focus on the presence of PE.
2. Materials and methods
2.1. Study design and setting
We retrospectively collected the data of 71 patients with ILD who underwent computed tomography (CT) pulmonary angiography (CTPA) for the evaluation of PE at Fukujuji Hospital and Kyorin University Faculty of Medicine from January 2018 to March 2023. Four patients who underwent CTPA 1 week or more after initiation of anticoagulant therapy and 2 patients who underwent CTPA for the assessment of pulmonary hypertension rather than PE were excluded. Ultimately, 65 patients were reviewed, including 18 patients with PE (the PE group) and 47 patients with no PE (the non-PE group). All patients were admitted suffering from worsening respiratory symptoms. Data regarding symptoms, laboratory test results, radiological findings, and other relevant findings were collected and compared between the PE group and the non-PE group. The study was approved by the Institutional Review Board of Fukujuji Hospital (Study number: 23010, IRB approval dates: June 6th, 2023) and Kyorin University Faculty of Medicine (Study number: 2208, IRB approval dates: July 21st, 2023). The requirement for patient consent was waived because the study did not include any identifiable information for patients, and we applied the opt-out method. The decisions made by this board were based on and in accordance with the Declaration of Helsinki.
2.2. Definition
PE was diagnosed when an intraluminal filling defect was seen within a pulmonary artery by CTPA.[14] Deep vein thrombosis (DVT) was diagnosed based on lower-limb CT angiography and/or venous ultrasonography. In patients who were diagnosed with DVT, thrombi in veins and/or noncompressible veins were found by lower-limb venous ultrasonography, and/or lower-limb CT angiography showed an intraluminal filling defect in lower-limb veins.[1]
AE is defined by the following: previous or concurrent diagnosis of idiopathic pulmonary fibrosis (IPF), acute worsening or development of dyspnea typically of less than 1 month duration, computed tomography with new diffuse bilateral ground-glass opacity and/or consolidation superimposed on a background pattern consistent with the usual interstitial pneumonia pattern, and deterioration not fully explained by cardiac failure or fluid overload.[15]
The types of interstitial pneumonia were classified into IPF, nonspecific interstitial pneumonia, combined pulmonary fibrosis and emphysema (CPFE), cryptogenic organizing pneumonia, unclassified ILD, fibrotic hypersensitive pneumonitis, collagen vascular disease-interstitial pneumonia, and drug-induced interstitial pneumonia based on chest CT, laboratory, and histopathological findings. In particular, CPFE was diagnosed according to the criteria established by V. Cottin et al, which needed the presence of both emphysema in the upper zones and diffuse parenchymal lung disease with fibrosis in the lower zones of the lungs on chest CT.[16]
2.3. Assessment of PE using the Wells score, revised Geneva score, and pulmonary embolism rule-out criteria (PERC) rule
The Wells score and revised Geneva score are the prediction criteria for pulmonary embolism.[12,13] The Wells score consists of 7 variables: clinical signs and symptoms of deep vein thrombosis (3.0 points), alternative diagnosis less likely than pulmonary embolism (3.0 points), heart rate > 100/min (1.5 points), immobilization (3 days) or surgery in the previous 4 weeks (1.5 points), previous pulmonary embolism or deep vein thrombosis (1.5 points), hemoptysis (1.0 point), and malignancy (1.0 point).[12] Patients were considered low probability if the score was < 2.0, moderate if the score was 2.0 to 6.0 and high if the score was over 6.0.[12]
The revised Geneva score consists of 8 variables: age older than 65 years (1 point), previous deep venous thrombosis or PE (3 points), surgery or fracture within 1 month (2 points), active malignant condition (2 points), unilateral lower limb pain (3 points), hemoptysis (2 points), heart rate of 75 to 94 beats/min (3 points) or ≥ 95 beats/min (5 points), and pain on lower-limb deep venous palpation and unilateral oedemaedema (4 points).[13] Patients were considered low-probability if the score was 0 to 3 points, intermediate-probability if the score was 4 to 10 points, and high-probability if the score was ≥ 11 points.[13]
The PERC rule is an 8-factor decision rule to support the decision not to order a diagnostic test for PE in patients for whom the clinician already had a low clinical suspicion for PE.[17] Eight variables were included in a block rule: age < 50 years, pulse < 100 beats/min, SaO2 > 94%, no unilateral leg swelling, no hemoptysis, no recent trauma or surgery, no prior PE or DVT, and no contraceptive/hormone replacement/estrogenic hormone use.[17] The definition of fulfilled PERC rule is when all 8 variables were met.
2.4. Statistical methods
All data were analyzed and processed using EZR, version 1.53.[18] The Mann–Whitney U test and Fisher exact test were used for group comparisons. The Cochran–Armitage trend test was used to compare the probability of PE in the Wells score and revised Geneva score. The sensitivity, specificity, and odds ratios were calculated. The receiver operating characteristic (ROC) curve was constructed and used to determine the cutoff values detected by a point of maximum sensitivity and specificity. Multivariate analysis (binomial logistic regression analysis) was conducted based on the variables with significant differences in the univariate analysis. The level of statistical significance was set at P = .05 (2-tailed).
3. Results
The baseline characteristics of the study subjects are shown in Table 1. There were no significant differences in age or sex between patients in the PE group and the non-PE/DVT group (age median 81 years [range 49–85] vs 80 years [54–93], P = .349; male n = 13 [72.2%] vs n = 37 [78.7%], P = .743). There were no significant differences in the Wells score (median 2.5 [range 0.0–6.0] vs 1.0 [range 0.0–5.5], P = .165) or revised Geneva score (median 4.0 [range 0.0–6.0] vs 5.0 [range 1.0–9.0], P = .140) between patients in the PE group and the non-PE group. The probability categories of PE in the Wells score were not significantly different between patients in the PE group and the non-PE group (P = .180). The probability categories of PE in the revised Geneva score significantly differed (P = .048); however, the PE group showed lower probability categories compared to the non-PE group. There was no significant difference in the number of patients who fulfilled the PERC rule between the PE group and the non-PE group (n = 1 [5.6%] vs n = 0 [0.0%], P = .277). No significant differences were observed between the PE group and the non-PE group concerning factors such as having AE (P = 1.000), receiving home oxygen therapy (P = .753), having diabetes (P = 1.000), undergoing steroid/immunosuppressant therapy before admission (P = .353), having antifibrotic therapy before admission (P = .507), and intensifying treatments with steroids/immunosuppressants after hospitalization (P = .387).
Table 1.
Baseline characteristics of the study subjects.
| PE group (n = 18) | Non-PE group (n = 47) | P value | |
|---|---|---|---|
| Age, median (range), yr | 81 (49–85) | 80 (54–93) | .349 |
| Sex (male/female) | 13/5 | 37/10 | .743 |
| Smoking history, n (%) | 13 (72.2) | 30 (63.8) | .873 |
| Obesity (BMI ≥ 25 kg/m2), n (%) | 1 (5.6) | 4 (8.5) | 1.000 |
| Presence of symptoms, n (%) | 18 (100.0) | 47 (100.0) | N.A. |
| Dyspnoea, n (%) | 17 (94.4) | 45 (95.7) | 1.000 |
| Chest pain, n (%) | 2 (11.1) | 2 (4.3) | .305 |
| Bloody sputum, n (%) | 0 (0.0) | 2 (4.3) | 1.000 |
| Unilateral lower extremity edema, n (%) | 1 (5.6) | 2 (4.3) | 1.000 |
| Mortality, n (%) | 6 (33.3) | 12 (25.5) | .548 |
| Type of ILD | |||
| IPF, n (%) | 7 (38.9) | 11 (23.4) | .230 |
| NSIP, n (%) | 0 (0.0) | 1 (2.1) | 1.000 |
| CPFE, n (%) | 1 (5.6) | 8 (17.0) | .425 |
| COP, n (%) | 4 (22.2) | 4 (8.5) | .202 |
| Unclassified ILD, n (%) | 4 (22.2) | 12 (25.5) | 1.000 |
| Fibrotic HP, n (%) | 1 (5.6) | 3 (6.4) | 1.000 |
| CVD-IP, n (%) | 1 (5.6) | 5 (10.6) | 1.000 |
| Drug-induced IP | 0 (0.0) | 3 (6.4) | .555 |
| Acute exacerbation, n (%) | 10 (55.6) | 26 (55.3) | 1.000 |
| Having home oxygen therapy, n (%) | 5 (27.8) | 11 (23.4) | .753 |
| Having steroid/immunosuppressant therapy before admission, n (%) | 3 (16.7) | 15 (31.9) | .353 |
| Having anti-fibrotic therapy before admission, n (%) | 5 (27.8) | 9 (19.1) | .507 |
| Intensification of treatment with steroids and immunosuppressants after hospitalization, n (%) | 11 (61.1) | 34 (72.3) | .387 |
| Having anticoagulant/antiplatelet therapy before admission, n (%) | 3 (16.7) | 7 (14.9) | 1.000 |
| Underlying disease | |||
| Lung cancer, n (%) | 2 (11.1) | 5 (10.6) | 1.000 |
| Pulmonary hypertension, n (%)a | 9 (75.0) | 24 (72.7) | 1.000 |
| COPD, n (%) | 2 (11.1) | 4 (8.5) | 1.000 |
| Heart disease | 4 (22.2) | 10 (21.3) | 1.000 |
| SAS, n (%) | 0 (0.0) | 0 (0.0) | 1.000 |
| ADL (bedridden/wheelchair-bound/walkable), n | 1/4/13 | 6/5/36 | .382 |
| Wearing compression stockings or having intermittent leg compression devices, n (%) | 1 (5.6) | 2 (4.3) | 1.000 |
| Having diabetes, n (%) | 2 (11.1) | 5 (10.6) | 1.000 |
| Vital sign | |||
| Disturbance of consciousness, n (%) | 1 (5.6) | 2 (4.3) | 1.000 |
| Body temperature, median (range) | 36.6 (35.6–39.0) | 36.8 (35.4–39.8) | .449 |
| Heart rate, median (range) | 84 (50–133) | 94 (58–185) | .592 |
| Diastolic blood pressure, median (range) | 127 (98–162) | 131 (90–175) | .450 |
| Respiratory rate, median (range) | 24 (15–56) | 20 (12–40) | .287 |
| Respiratory failure, n (%) | 13 (72.2) | 38 (80.9) | .507 |
| Wells score, median (range) | 2.5 (0.0–6.0) | 1.0 (0.0–5.5) | .165 |
| The probability categories of PE (low/moderate/high), n | 7/11/0 | 27/20/0 | .180* |
| Revised Geneva score, median (range) | 4.0 (0.0–6.0) | 5.0 (1.0–9.0) | .140 |
| The probability categories of PE (low/moderate/high), n | 7/10/1 | 5/42/0 | .048* |
| Fulfilled the PERC Rule, n (%) | 1 (5.6) | 0 (0.0) | .277 |
| Presence of DVT, n (%) | 10 (55.6) | 11 (23.4) | .019 |
| Laboratory findings | |||
| Haematocrit, median (range), % | 36.1 (24.5–47.9) | 34.0 (25.1–45.4) | .272 |
| WBCs, median (range), cells/µL | 8450 (3100–14,480) | 9770 (4890–19,600) | .250 |
| Platelet count, median (range), ×104/μL | 20.5 (9.1–38.5) | 21.4 (7.5–73.5) | .587 |
| PT-INR, median (range)b | 1.09 (0.95–1.39) | 1.08 (0.85–20.3) | .539 |
| APTT, median (range), secb | 29.1 (24.0–43.1) | 29.2 (22.9–43.0) | .764 |
| D-dimer, median (range), µg/mL | 24.5 (3.0–79.3) | 9.3 (0.5–80.8) | .016 |
| CRP, median (range), mg/dL | 4.62 (0.24–17.24) | 3.94 (0.05–38.45) | .775 |
| LDH, median (range), IU/L | 300 (170–724) | 308 (135.2–768) | .775 |
| Total protein, median (range), g/dL | 7.2 (5.1–8.7) | 6.4 (5.0–8.2) | .002 |
| Albumin, median (range), g/dL | 3.2 (2.2–4.0) | 3.0 (1.7–4.1) | .076 |
| Globulin, median (range), g/dL | 3.8 (2.6–5.5) | 3.2 (3.0–5.3) | .041 |
| Albumin/globulin ratio, median (range), % | 0.89 (0.44–1.29) | 0.94 (0.43–1.57) | .769 |
| KL-6, median (range), IU/Lc | 1285 (278–2184) | 760 (195–17503) | .083 |
ADL = activities of daily living, APTT = activated partial thromboplastin time, BMI = body mass index, COP = cryptogenic organizing pneumonia, COPD = chronic obstructive pulmonary disease, CPFE = combined pulmonary fibrosis and emphysema, CRP = C-reactive protein, CVD = collagen vascular disease, DVT = deep vein thrombosis, HP = hypersensitive pneumonitis, ILD = interstitial lung disease, IP = interstitial pneumonia, IPF = idiopathic pulmonary fibrosis, KL-6 = Krebs von den Lungen-6, LDH = lactate dehydrogenase, NSIP = nonspecific interstitial pneumonia, PE = pulmonary embolism, PERC = pulmonary embolism rule out criteria, PT-INR = prothrombin time-international normalized ratio, SAS = sleep apnea syndrome.
Cochran-Armitage test.
PE group n = 12, non-PE group n = 33.
PE group n = 18, non-PE group n = 43.
PE group n = 17, non-PE group n = 47.
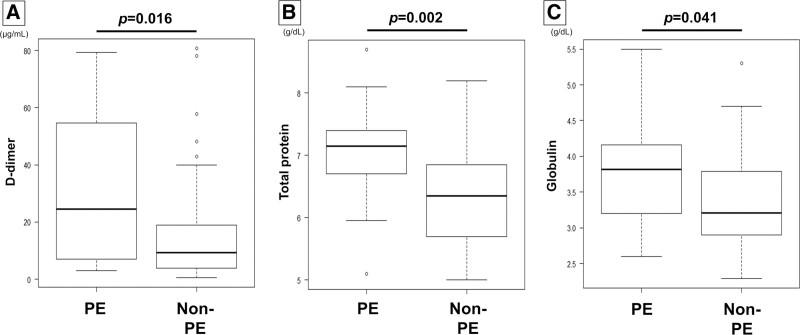
Patients in the PE group showed higher D-dimer, total protein (TP), and globulin levels than those in the non-PE group (D-dimer median 24.5 µg/mL [range 3.0–79.3] vs median 9.3 µg/mL [range 0.5–80.8], P = .016; TP median 7.2 g/dL [range 5.1–8.7] vs median 6.4 g/dL [range 5.0–8.2], P = .002; globulin median 3.8 g/dL [range 2.6–5.5] vs median 3.2 g/dL [range 3.0–5.3], P = .041) (Fig. 1). A multivariable analysis of the variables with significant differences in the univariate analysis was conducted. The variables included D-dimer, serum TP, and serum globulin levels. D-dimer and serum TP were significantly associated with the diagnosis of PE (Table 2), and the odds ratios were 1.05 (95% confidence interval [Cl] 1.02–1.08, P = .003) for D-dimer and 6.09 (95% Cl 1.50–24.8, P = .012) for serum TP.
Figure 1.
Comparisons of D-dimer, total protein (TP), and globulin levels between patients in the PE group and non-PE group. Patients in the PE group showed higher D-dimer (A), TP (B), and globulin levels (C) than those in the non-PE group (D-dimer median 24.5 µg/mL [range 3.0–79.3] vs median 9.3 µg/mL [range 0.5–80.8], P = .016; TP median 7.2 g/dL [range 5.1–8.7] vs median 6.4 g/dL [range 5.0–8.2], P = .002; globulin median 3.8 g/dL [range 2.6–5.5] vs median 3.2 g/dL [range 3.0–5.3], P = .041).
Table 2.
Multivariate analysis of the presence of PE in patients with ILD.
| Odds ratio | 95% Cl | P value | ||
|---|---|---|---|---|
| Upper limit | Lower limit | |||
| D-dimer | 1.05 | 1.02 | 1.08 | .003 |
| Total protein | 6.09 | 1.50 | 24.8 | .012 |
| Globulin | 0.56 | 0.13 | 2.35 | .428 |
Cl = confidence interval, ILD = interstitial lung disease, PE = pulmonary embolism.
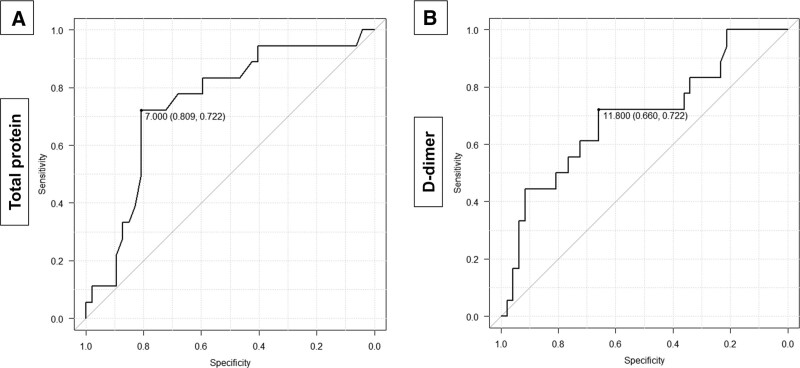
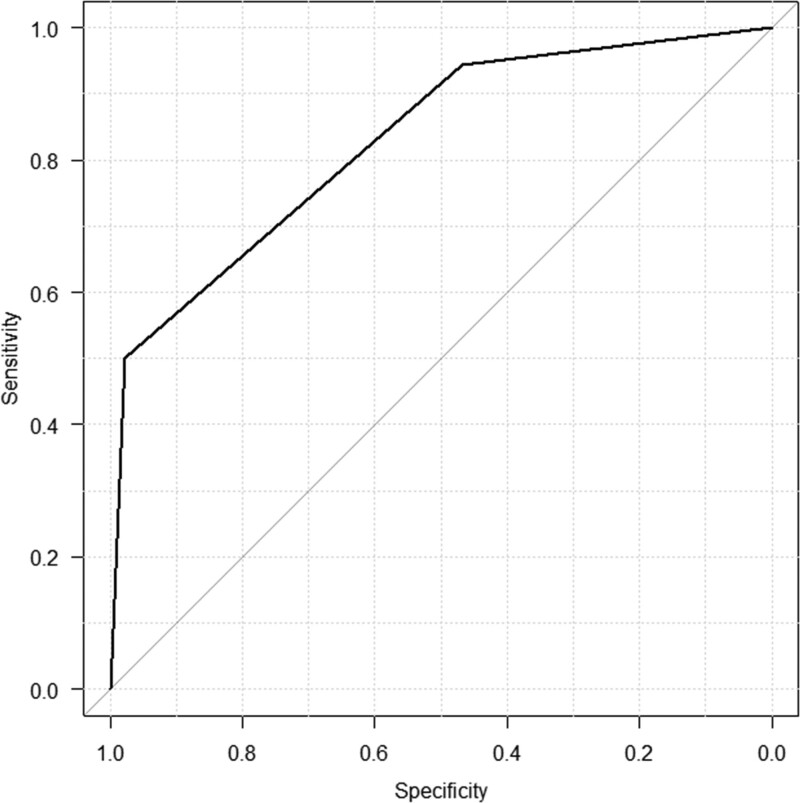
The ROC curve for the diagnosis of PE is shown in Figure 2. The area under the ROC curve (AUC) of TP was 0.746 (95% Cl 0.611–0.882). The cutoff value of TP was determined using the ROC curve, and it was found to be 7.0 g/dL or higher. The sensitivity, specificity, and odds ratio for this cutoff value were 72.2%, 80.9%, and 10.5 (95% CI 2.69–48.2), respectively. The AUC of D-dimer was 0.696 (95% CI 0.545–0.847). The cutoff value of D-dimer was determined to be 11.8 µg/mL or higher. The sensitivity, specificity, and odds ratio for this cutoff value were 72.2%, 66.0%, and 4.90 (95% CI 1.35–20.9), respectively. Figure 3 shows the ROC curve of the combination of TP ≥ 7.0 g/dL and D-dimer ≥ 11.8 µg/mL for the presence of PE. Each variable was assigned a value of 1 point, thus totaling 2 points. The AUC was 0.829 (95% Cl 0.727–0.931). When the cutoff value was 2 points, the sensitivity, specificity, and odds ratio for this cutoff value were 50.0%, 97.9%, and 42.2 (95% CI 4.91–2030.4), respectively. When the cutoff value was 1 point, the sensitivity, specificity, and odds ratio for this cutoff value were 94.4%, 46.7%, and 14.5 (95% CI 1.95–650.9), respectively.
Figure 2.
The ROC curve of total protein (A) and D-dimer (B) for the diagnosis of PE. A: The AUC was 0.746 (95% Cl 0.611–0.882). The cutoff value of total protein was determined using the ROC curve, and it was found to be 7.0 g/dL or higher. The sensitivity, specificity, and odds ratio for this cutoff value were 72.2%, 80.9%, and 10.5 (95% CI 2.69–48.2), respectively. B: The AUC was 0.696 (95% CI 0.545–0.847). The cutoff value of D-dimer was determined to be 11.8 µg/mL or higher. The sensitivity, specificity, and odds ratio for this cutoff value were 72.2%, 66.0%, and 4.90 (95% CI 1.35–20.9), respectively. AUC = area under the ROC curve, Cl = confidence interval, ROC = receiver operating characteristic.
Figure 3.
The ROC curve of the combination of TP ≥ 7.0 g/dL and D-dimer ≥ 11.8 µg/mL for the presence of PE. Each variable was assigned a value of 1 point, thus totaling 2 points. The AUC was 0.829 (95% Cl 0.727–0.931). When the cutoff value was 2 points, the sensitivity, specificity, and odds ratio for this cutoff value were 50.0%, 97.9%, and 42.2 (95% CI 4.91–2030.4), respectively. When the cutoff value was 1 point, the sensitivity, specificity, and odds ratio for this cutoff value were 94.4%, 46.7%, and 14.5 (95% CI 1.95–650.9), respectively. AUC = area under the ROC curve, Cl = confidence interval, ROC = receiver operating characteristic.
4. Discussion
This study demonstrates that the Wells score, the revised Geneva score, and the PERC rule are not useful in patients with ILD for predicting the presence of PE and provides insights into the characteristics of PE in patients with ILD. A previous report showed that the Wells score and revised Geneva score can be used for categorizing PE risk in patients with ILD [11]; however, our study found that these criteria were not reliable in diagnosing PE. The probability categories of PE in the Wells score were not effective in distinguishing between patients in the PE group and the non-PE group, and those in the revised Geneva score were lower in the PE group than in the non-PE group. The PERC rule could also not rule out PE in ILD patients. Furthermore, there was no notable difference in the proportion of patients with AEs between the PE and non-PE groups, and approximately 61% of patients in the PE group received intensified treatment due to the provoked AE triggered by PE or the AE that coincidentally occurred with PE. Interestingly, patients in the PE group showed higher D-dimer, serum TP, and serum globulin levels than those in the non-PE groups. Furthermore, a multivariable analysis showed that serum TP and D-dimer were associated with the diagnosis of PE, and the cutoff values were TP ≥ 7.0 g/dL and D-dimer ≥ 11.8 µg/mL for predicting the presence of PE. PE could be ruled out when both variables were not fulfilled, and PE could be diagnosed when both variables were fulfilled. Even though serum globulin levels did not exhibit statistical significance in the multivariable analysis, this may be attributed to the potential confounding effect of serum TP, which comprises both serum albumin and globulin levels. Accordingly, previously reported criteria, such as the Wells score, revised Geneva score, and PERC rule, were not useful for diagnosing or ruling out PE in ILD patients, and high serum TP levels and D-dimer levels were indicated to be valuable indicators for predicting the presence of PE.
The Wells score and revised Geneva score have been developed to predict the presence of PE for patients with suspected PE,[12,13] and it is recommended to combine those scores with D-dimer.[1,12,19,20] Among patients with ILD, a previous study reported that the Wells score and revised Geneva score can be used for categorizing PE risk, especially dyspnea, lower extremity edema, and palpitations, which are associated with venous thromboembolism risk.[11] However, this study included many patients who did not suffer from worsening respiratory symptoms; indeed, only 36.8% of patients had dyspnea.[11] Our study investigated patients with worsening respiratory symptoms, and many patients showed abnormalities in vital signs and physical examinations in both the PE group and the non-PE group. Therefore, it is challenging to predict the presence of venous thromboembolism in ILD patients with worsening respiratory symptoms by using the Wells score and revised Geneva score. In addition, D-dimer is associated with the risk of developing AE.[21] In many ILD patients with AE, negative D-dimer results may not be observed. Therefore, among patients experiencing worsening respiratory symptoms, it might not be possible to rule out PE solely based on negative D-dimer results. Generally, the pathogenesis of venous thromboembolism (VTE) and ILD may share common pathways,[4] and ILD patients have underlying lung damage and pulmonary vascular abnormalities.[5,22–24] Therefore, it can be very difficult to distinguish between AE and PE in patients with ILD experiencing worsening respiratory symptoms, and other predictive factors for diagnosing PE in those patients are needed.
Our study demonstrates that TP ≥ 7.0 g/dL and D-dimer ≥ 11.8 µg/mL were useful for predicting the presence of PE. It is well known that PE in ILD could be the presence of an enhanced prothrombotic state,[25,26] and increasing biomarkers such as thrombin, factor VIII, and D-dimer might be associated with hypercoagulability in ILD, especially in patients with more severe disease.[25–27] The usefulness of D-dimer levels for diagnosing VTE, including PE, has been reported,[28] and our study showed that D-dimer levels were higher in the PE group than in the non-PE group. However, 34% of patients in the non-PE group showed D-dimer ≥ 11.8 µg/mL, making it difficult to predict PE using D-dimer alone. On the other hand, our study demonstrates that the AUC of serum TP for predicting PE was higher than that of D-dimer. Serum TP, including albumin and globulins, can influence blood viscosity, which is related to hypercoagulability[29,30]; in particular, immunoglobulins may directly affect red cell aggregation.[30] Therefore, the risk of thromboembolic events, including PE, might be associated with serum TP levels in ILD patients. To our knowledge, there is no previous report investigating the relationship between PE and serum TP. A previous report indicated a lower incidence of thromboembolism in patients with hypogammaglobulinemia (2.6%) than in those without hypogammaglobulinemia (5.7%) among individuals with coronavirus disease 2019 infection, although statistical significance was not reached due to the small sample size.[31] While we hypothesize that low serum TP levels, including globulin, might suppress the development of VTE in patients with ILD, there is currently insufficient evidence to substantiate this hypothesis. Further research is needed to elucidate the specific mechanisms underlying the association between PE and serum TP levels.
The study findings suggest that ILD patients with high serum TP and D-dimer levels should undergo CTPA to assess for the presence of PE when those patients suffer from worsening respiratory symptoms. However, some patients cannot undergo CTPA due to contrast allergy or renal insufficiency.[2] When clinical suspicion of PE is high and the necessary diagnostic tests are not immediately feasible, empirical treatment with anticoagulation may be considered.[1,2] Early empiric anticoagulation has been associated with reduced mortality in patients with PE.[1] On the other hand, it is crucial to be cautious about the potential adverse effects of anticoagulation, particularly the risk of hemorrhage.[2] Additionally, in the context of IPF treatment, warfarin has been associated with increased mortality and lack of efficacy, likely due to manipulation of the clotting cascade,[32] not due to complications of anticoagulation.[25] In particular, the combination of TP ≥ 7.0 g/dL and D-dimer ≥ 11.8 µg/mL can be valuable for predicting the presence of PE. When both of these variables were fulfilled, the specificity was notably high at 97.9%, which means that it can be used for ruling in PE in patients with ILD. When either variable was fulfilled, the sensitivity was notably high at 94.4%, which means that it can be used for ruling out PE in patients with ILD. Therefore, the combination of serum TP and D-dimer levels might help in deciding whether to initiate empiric anticoagulation therapy.
This investigation has several limitations. First, this study was conducted retrospectively and specifically focused on identifying the presence of PE in patients with ILD. The investigation did not explore factors contributing to exacerbation or solely latent conditions. Second, some medical data were not recorded. Additionally, this study collected patients who underwent CTPA to search for PE, and patients who were not suspected to have PE clinically were not investigated. The decision to perform CTPA was made by the attending physician. The D-dimer level might be associated with the decision to perform CTPA. Therefore, a further prospective study is needed. Although 5 patients underwent CTPA after starting anticoagulant therapy (3 patients in the PE group and 2 patients in the non-PE group), the duration from initiation of anticoagulant therapy to the CT scan was within 1 week, minimizing potential biases related to this aspect.
5. Conclusion
This study demonstrates the characteristics of PE in patients with ILD and revealed predictive factors such as TP ≥ 7.0 g/dL and D-dimer ≥ 11.8 µg/mL for the presence of PE, while the Wells score, revised Geneva score, and PERC rule were not reliable in diagnosing or ruling out PE. In addition, PE could be ruled out when both of those variables were not fulfilled, and PE could be diagnosed when both of those variables were fulfilled.
Author contributions
Conceptualization: Masafumi Shimoda.
Data curation: Masafumi Shimoda, Hiroki Nunokawa, Yoshiaki Tanaka, Kozo Morimoto, Iori Moue, Kozo Yoshimori, Takeshi Saraya.
Formal analysis: Masafumi Shimoda.
Investigation: Masafumi Shimoda.
Methodology: Masafumi Shimoda, Haruyuki Ishii.
Project administration: Ken Ohta, Haruyuki Ishii.
Software: Masafumi Shimoda.
Supervision: Yoshiaki Tanaka, Haruyuki Ishii.
Visualization: Masafumi Shimoda.
Writing – original draft: Masafumi Shimoda.
Writing – review & editing: Masafumi Shimoda, Yoshiaki Tanaka, Haruyuki Ishii.
Abbreviations:
- AE
- acute exacerbation
- AUC
- the area under the ROC curve
- CI
- confidence interval
- CT
- computed tomography
- CTPA
- computed tomography pulmonary angiography
- DVT
- deep vein thrombosis
- ILD
- interstitial lung disease
- IPF
- idiopathic pulmonary fibrosis
- PE
- pulmonary embolism
- PERC
- pulmonary embolism rule-out criteria
- ROC
- receiver operating characteristic
- TP
- total protein
- VTE
- venous thromboembolism
The study was approved by the Institutional Review Board of Fukujuji Hospital (Study number: 23010, IRB approval dates: June 6th, 2023) and Kyorin University Faculty of Medicine (Study number: 2208, IRB approval dates: July 21st, 2023). The requirement for patient consent was waived because the study did not include any identifiable information for patients, and we applied the opt-out method. The decisions made by this board were based on and in accordance with the Declaration of Helsinki.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
The authors have no funding and conflicts of interest to disclose.
How to cite this article: Shimoda M, Nunokawa H, Tanaka Y, Morimoto K, Moue I, Yoshimori K, Saraya T, Ohta K, Ishii H. Predictive factors of the presence of pulmonary embolism in patients with interstitial lung disease: Observational study. Medicine 2024;103:1(e36828).
Contributor Information
Hiroki Nunokawa, Email: hrk910@ks.kyorin-u.ac.jp.
Yoshiaki Tanaka, Email: tanakay@fukujuji.org.
Kozo Morimoto, Email: yoshimorik@fukujuji.org.
Iori Moue, Email: mouei@fukujuji.org.
Kozo Yoshimori, Email: yoshimorik@fukujuji.org.
Takeshi Saraya, Email: saraya@ks.kyorin-u.ac.jp.
Ken Ohta, Email: ohtak@fukujuji.org.
Haruyuki Ishii, Email: h141@ks.kyorin-u.ac.jp.
References
- [1].Essien EO, Rali P, Mathai SC. Pulmonary embolism. Med Clin North Am. 2019;103:549–64. [DOI] [PubMed] [Google Scholar]
- [2].Goldhaber SZ. Pulmonary embolism. Lancet. 2014;364:244–305. [Google Scholar]
- [3].Hubbard RB, Smith C, Le Jeune I, et al. The association between idiopathic pulmonary fibrosis and vascular disease: a population-based study. Am J Respir Crit Care Med. 2008;178:1257–61. [DOI] [PubMed] [Google Scholar]
- [4].Sode BF, Dahl M, Nielsen SF, et al. Venous thromboembolism and risk of idiopathic interstitial pneumonia: a nationwide study. Am J Respir Crit Care Med. 2010;181:1085–92. [DOI] [PubMed] [Google Scholar]
- [5].Park SH. Pulmonary embolism is more prevalent than deep vein thrombosis in cases of chronic obstructive pulmonary disease and interstitial lung diseases. Springerplus. 2016;5:1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Alsilmi R. The prevalence of pulmonary embolism in patients with interstitial lung disease: a cross-sectional retrospective study. Cureus. 2022;14:e23063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Saydain G, Islam A, Afessa B, et al. Outcome of patients with idiopathic pulmonary fibrosis admitted to the intensive care unit. Am J Respir Crit Care Med. 2002;166:839–42. [DOI] [PubMed] [Google Scholar]
- [8].Collard HR, Ward AJ, Lanes S, et al. Burden of illness in idiopathic pulmonary fibrosis. J Med Econ. 2012;15:829–35. [DOI] [PubMed] [Google Scholar]
- [9].Sprunger DB, Olson AL, Huie TJ, et al. Pulmonary fibrosis is associated with an elevated risk of thromboembolic disease. Eur Respir J. 2012;39:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Margaritopoulos GA, Kokosi MA, Wells AU. Diagnosing complications and comorbidities of fibrotic interstitial lung disease. Expert Rev Respir Med. 2019;13:645–58. [DOI] [PubMed] [Google Scholar]
- [11].Luo Q, Xie J, Han Q, et al. Prevalence of venous thromboembolic events and diagnostic performance of the wells score and revised geneva scores for pulmonary embolism in patients with interstitial lung disease: a prospective study. Heart Lung Circ. 2014;23:778–85. [DOI] [PubMed] [Google Scholar]
- [12].Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83:416–20. [PubMed] [Google Scholar]
- [13].Wicki J, Perneger TV, Junod AF, et al. Assessing clinical probability of pulmonary embolism in the emergency ward: a simple score. Arch Intern Med. 2001;161:92–7. [DOI] [PubMed] [Google Scholar]
- [14].Leuschner G, Wenter V, Milger K, et al. Suspected pulmonary embolism in patients with pulmonary fibrosis: discordance between ventilation/perfusion SPECT and CT pulmonary angiography. Respirology. 2016;21:1081–7. [DOI] [PubMed] [Google Scholar]
- [15].Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis an international working group report. Am J Respir Crit Care Med. 2016;194:265–75. [DOI] [PubMed] [Google Scholar]
- [16].Cottin V, Nunes H, Brillet PY, et al. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J. 2005;26:586–93. [DOI] [PubMed] [Google Scholar]
- [17].Kline JA, Mitchell AM, Kabrhel C, et al. Clinical criteria to prevent unnecessary diagnostic testing in emergency department patients with suspected pulmonary embolism. J Thromb Haemost. 2004;2:1247–55. [DOI] [PubMed] [Google Scholar]
- [18].Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Freund Y, Cohen-Aubart F, Bloom B. Acute pulmonary embolism: a review. JAMA. 2022;328:1336–45. [DOI] [PubMed] [Google Scholar]
- [20].Klok FA, Mos IC, Nijkeuter M, et al. Simplification of the revised Geneva score for assessing clinical probability of pulmonary embolism. Arch Intern Med. 2008;168:2131–6. [DOI] [PubMed] [Google Scholar]
- [21].Ishikawa G, Acquah SO, Salvatore M, et al. Elevated serum D-dimer level is associated with an increased risk of acute exacerbation in interstitial lung disease. Respir Med. 2017;128:78–84. [DOI] [PubMed] [Google Scholar]
- [22].Renzoni EA, Walsh DA, Salmon M, et al. Interstitial vascularity in fibrosing alveolitis. Am J Respir Crit Care Med. 2003;167:438–43. [DOI] [PubMed] [Google Scholar]
- [23].Cosgrove GP, Brown KK, Schiemann WP, et al. Pigment epithelium-derived factor in idiopathic pulmonary fibrosis: a role in aberrant angiogenesis. Am J Respir Crit Care Med. 2004;170:242–51. [DOI] [PubMed] [Google Scholar]
- [24].Sakao S, Taraseviciene-Stewart L, Wood K, et al. Apoptosis of pulmonary microvascular endothelial cells stimulates vascular smooth muscle cell growth. Am J Physiol Lung Cell Mol Physiol. 2006;291:L362–8. [DOI] [PubMed] [Google Scholar]
- [25].Navaratnam V, Fogarty AW, McKeever T, et al. Presence of a prothrombotic state in people with idiopathic pulmonary fibrosis: a population-based case–control study. Thorax. 2014;69:207–15. [DOI] [PubMed] [Google Scholar]
- [26].Howell DC, Laurent GJ, Chambers RC. Role of thrombin and its major cellular receptor, protease-activated receptor-1, in pulmonary fibrosis. Biochem Soc Trans. 2002;30:211–6. [DOI] [PubMed] [Google Scholar]
- [27].Howell DC, Goldsack NR, Marshall RP, et al. Direct thrombin inhibition reduces lung collagen, accumulation, and connective tissue growth factor mRNA levels in bleomycin-induced pulmonary fibrosis. Am J Pathol. 2001;159:1383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Linkins LA, Takach Lapner S. Review of D-dimer testing: good, bad, and ugly. Int J Lab Hematol. 2017;39(Suppl 1):98–103. [DOI] [PubMed] [Google Scholar]
- [29].Somer T, Meiselman HJ. Disorders of blood viscosity. Ann Med. 1993;25:31–9. [DOI] [PubMed] [Google Scholar]
- [30].Kwaan HC. Role of plasma proteins in whole blood viscosity: a brief clinical review. Clin Hemorheol Microcirc. 2010;44:167–76. [DOI] [PubMed] [Google Scholar]
- [31].Scarpa R, Dell’Edera A, Felice C, et al. Impact of Hypogammaglobulinemia on the course of COVID-19 in a non-intensive care setting: a single-center retrospective cohort study. Front Immunol. 2022;13:842643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Noth I, Anstrom KJ, Calvert SB, et al. Idiopathic pulmonary fibrosis clinical research a placebo-controlled randomized trial of warfarin in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;186:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]