Abstract
Although surgery is considered the first choice of treatment for patients diagnosed with tracheal cancer, the prediction of overall survival (OS) in patients undergoing surgical intervention is poor. To address this issue, we developed a nomogram that combined a risk classification system to estimate the OS of patients with tracheal cancer who underwent surgical intervention. A total of 525 qualified patients were selected from the surveillance, epidemiology, and end results database between 1975 and 2018 and were randomly divided into training and validation cohorts (7:3). The parameters of independent prognostic ability were determined using Cox regression analysis, and a nomogram was formed. The predictive ability of the nomogram was tested using the area under the curve of receiver operating characteristic curves and calibration curves. Survival curves were assessed between the different risk classification groups using the Kaplan–Meier method. The results indicated that Age, stage, histology, and tumor size were independent prognostic factors and were included in the predictive model. The calibration plots demonstrated good agreement between the nomogram prediction and actual observation for 24- and 36-month OS. The receiver operating characteristic curves suggested that the predictive model had good discrimination ability, with the area under the curves (training group 0.817, 0.785, and 0.801, respectively) and validation group (0.744, 0.794, and 0.822, respectively). Furthermore, the low-risk group had a better prognosis than the high-risk group in the total, training, and validation cohorts (all P < .001). This study established a novel nomogram system to predict OS and identify independent prognostic factors in patients with tracheal cancer who underwent surgical intervention. This model has the potential to assist doctors in making decisions regarding treatment options.
Keywords: nomogram, prediction, surgical intervention, survival, tracheal cancer
1. Introduction
Primary tracheal malignancy is a relatively rare tumor, with an estimated incidence about 0.1 per 100,000 individuals annually.[1] Tracheal tumors have the risk of blocking the airway and reducing oxygen supply. The main symptoms of tracheal tumors include dyspnea, increased airway secretions, hemoptysis, and cough, which can easily result in misdiagnosis of chronic obstructive pulmonary disease, bronchial asthma, and other airway obstructive diseases. Moreover, tracheal tumors are not easily detected in the early stages without a chest CT scan. Squamous cell carcinoma is the most common type of primary tracheal tumor, followed by adenoid cystic carcinoma.[2] Surgery remains the first-line treatment for tracheal tumors. Previous studies have shown that patients undergoing surgery have longer survival times.[3,4] It is worth mentioning that with the development of tracheoscopy techniques in recent years, patients have more surgical options, such as open resection surgery and endoscopic surgery, which reduces complications, operative time, and post-operative recovery period. Airway surgery with tracheoscopy can effectively remove tumors as a more available therapeutic method that can greatly improve the chances of patients receiving surgical treatment[5,6]
Although the preferred treatment for tracheal tumors is surgical management whenever possible, the degree of difficulty of the surgical technique can affect the decision to perform the surgery. Epidemiological studies conducted over the past 20 years have suggested that the number of patients undergoing resection is lower.[7,8] However, as surgical techniques continue to advance, it is becoming increasingly possible to achieve improved long-term survival rates in patients with tracheal tumors. In the hands of experienced surgeons, the mortality rate following tracheal resection is typically < 1%.[9] Fortunately, tracheoscopic surgery for tracheal tumors has developed rapidly in recent years, especially the use of rigid bronchoscopy, which can replace many thoracotomy operations and avoid various complications. Rigid bronchoscopy, in conjunction with an ultrasonically activated device, has emerged as a highly effective approach for alleviating tracheal stenosis and resecting intratracheal tumors. Treatment of primary tracheal cancer should be based on the patient’s condition. Open and endoscopic interventions are justified.[10–12] The increased availability of diverse surgical methods provides patients with greater options to effectively eliminate tumors. However, despite these advancements, there remains a dearth of studies analyzing the prognosis of tracheal cancer patients who undergo surgical management, and a model established to predict the prognosis of patients with tracheal cancer is still lacking. As such, it is of great value to develop a novel tool to predict the prognosis of patients diagnosed with tracheal cancer who underwent surgical intervention, including both tracheoscopic intervention and operative approaches.
The use of nomograms to forecast the survival rates of cancer patients has become prevalent. These visual tools can simplify complex regression equations into a comprehensible graph, rendering predictions accessible and clinically valuable. Nomograms are widely used in medical research and in clinical practice.[13,14] Survival analysis charts can be employed to predict the prognosis of patients with various treatments or cancer classifications, which can offer more targeted guidance to clinicians.[15] Hence, we aimed to analyze data obtained from the surveillance, epidemiology, and end results (SEER) database of patients with tracheal cancer treated with surgical intervention. Our objective was to develop a nomogram and risk classification system that can provide monitoring values and a stratified management approach for doctors.
2. Methods
2.1. Database
We selected individuals who were diagnosed with tracheal cancer from the SEER database(https://seer.cancer.gov/seerstat/), which is a collection of population-based cancer statistics in the USA maintained by the National Cancer Institute. We use SEER Stat 8.4.1 to identify the data from 1975 to 2018 covering 18 registries.As the data obtained from the SEER database did not include any identifiable personal information, obtaining informed consent or ethical approval was unnecessary.
3. Patient selection
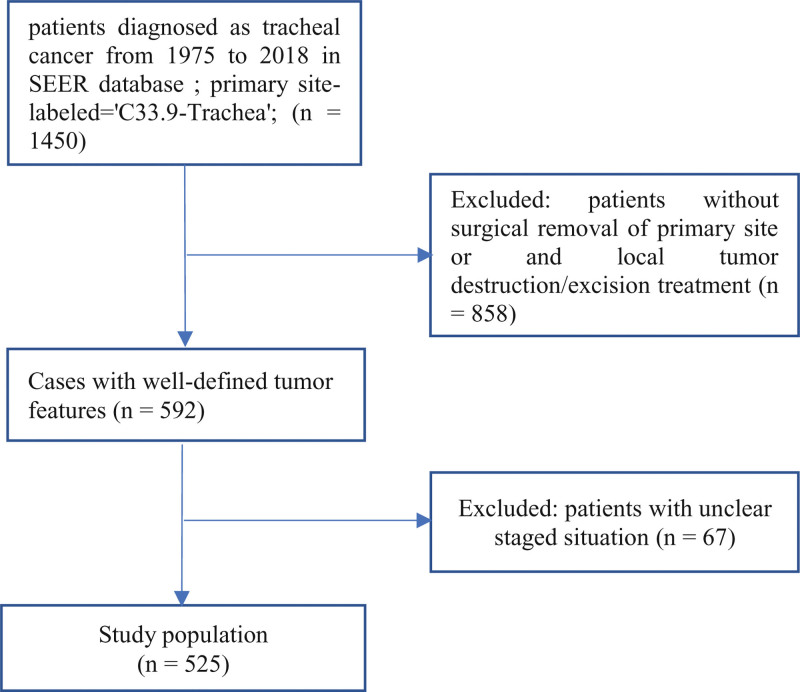
The following inclusion criteria were employed: Patients were pathologically diagnosed as tracheal cancer; Primary site-labeled=“C33.9-Trachea”; Treated with surgical intervention. The exclusion criteria included: Individuals with incomplete clinical data; Patients treated without tumor excision, tumor destruction, surgical removal, or debulking. Nineteen cases of missing marital status data were addressed by imputation. Ultimately, 525 eligible patients were selected for retrospective analysis and randomly assigned to the training and validation cohort (ratio = 7:3). The selection criteria are presented in a flow chart (Fig. 1). Variables incorporated into the patients characteristics were as follows: age, race, sex, marital status, histology, tumor size, stage, and treatment, including radiotherapy and chemotherapy(Table 1). The primary outcome of this study was overall survival (OS), defined as the time interval between the day of diagnosis and death.
Figure 1.
A graphical representation outlining the detailed inclusion and exclusion criteria, as well as the process of selecting individuals from the SEER database. SEER = the surveillance, epidemiology, and end results database.
Table 1.
Baseline and clinical pathological features of all patients.
| Variables | Level | All cohorts (n = 525) |
Training cohort (n = 366) |
Validation cohort (n = 159) |
|---|---|---|---|---|
| Age (%) | 0–49 | 132 (25.14) | 94 (25.68) | 38 (23.90) |
| 50–70 | 245 (46.67) | 165 (45.08) | 80 (50.31) | |
| >70 | 148 (28.19) | 107 (29.23) | 41 (25.79) | |
| Race (%) | White | 424 (80.76) | 297 (81.15) | 127 (79.87) |
| Black | 59 (11.24) | 39 (10.66) | 20 (12.58) | |
| Others | 42 (8.00) | 30 (8.20) | 12 (7.55) | |
| Sex (%) | Male | 277 (52.76) | 194 (53.01) | 83 (52.20) |
| Female | 248 (47.24) | 172 (46.99) | 76 (47.80) | |
| Marriage (%) | Unmarried | 194 (36.95) | 138 (37.70) | 56 (35.22) |
| Married | 331 (63.05) | 228 (62.30) | 103 (64.78) | |
| Histology (%) | Squamous carcinoma | 222 (42.29) | 155 (42.35) | 67 (42.14) |
| ACC | 150 (28.57) | 106 (28.96) | 44 (27.67) | |
| Others | 153 (29.14) | 105 (28.69) | 48 (30.19) | |
| Stage (%) | Localized | 265 (50.48) | 185 (50.55) | 80 (50.31) |
| Regional | 209 (39.81) | 146 (39.89) | 63 (39.62) | |
| Distant | 51 (9.71) | 35 (9.56) | 16 (10.06) | |
| Radiotherapy (%) | Performed | 309 (58.86) | 218 (59.56) | 91 (57.23) |
| NO | 216 (41.14) | 148 (40.44) | 68 (42.77) | |
| Chemotherapy (%) | Performed | 108 (20.57) | 74 (20.22) | 34 (21.38) |
| NO | 417 (79.43) | 292 (79.78) | 125 (78.62) | |
| Tumor size (%) | <2 cm | 111 (21.14) | 81 (22.13) | 30 (18.87) |
| 2–5 cm | 214 (40.76) | 151 (41.26) | 63 (39.62) | |
| >5 cm | 44 (8.38) | 33 (9.02) | 11 (6.92) | |
| Unknown | 156 (29.71) | 101 (27.60) | 55 (34.59) |
ACC = adenoid cystic carcinoma.
4. Nomogram construction and validation
The training cohort was used to develop the predictive model, construct the nomogram, and establish the risk classification system. The validation cohort was used to test and verify the developed model, and to identify independent prognostic variables affecting OS, both univariate and multivariate Cox regression analyses were conducted. First, 9 variables that may be relevant to the outcome based on clinical considerations were chosen. Second, univariate Cox regression analysis was performed, and variables with a P value of < .1 was obtained for further analysis in the multivariate Cox regression. Variables with P values < .5 in the multivariate Cox regression were used to establish a nomogram as independent prognostic factors. Calibration plots were used to determine the efficacy of the prediction model by comparing the predicted probability of the OS rates to the actual probability. Additionally, the discrimination ability of the nomogram was assessed using receiver operating characteristic (ROC) curves and the area under the curve (AUC).
5. Risk classification system and survival analyses
The risk classification system was established according to the total score of each patient calculated using the nomogram in the training cohort, with the function of R statistical analysis to determine the optimal cutoff point. Patients were categorized into either a low- or high-risk prognosis group based on the cutoff point. Survival curves were generated using the Kaplan–Meier method and compared using the log-rank test.
6. Statistical analysis
Statistical analyses were performed using R v4.1.2 and ibm spss statistics v25. All statistical tests were 2-tailed, and a P value of < .05 was deemed significant, except for univariate Cox regression analysis, where a P value of < .1 was used.
7. Results
7.1. Patients characteristics
The study population consisted of 525 patients who were randomly assigned to training (n = 366, 70%) and validation (n = 159, 30%) cohorts. Among the entire cohort as well as in the training and validation cohorts, the largest number of patients (n = 245, n = 165, and n = 80, respectively) fell within the age range of 50 to 70 years, representing a proportion of 46.67%. In all cohorts, the numbers of male and female individuals were similar. Patients were predominantly white (80.76%), whereas black and other patients represented 11.24% and 8% of the overall cohort, respectively. The majority of the patients were married (63.05%). In terms of histology, squamous carcinoma was the largest proportion in the overall, training, and validation cohorts (42.29%, 42.35%, and 42.14%, respectively), followed by adenoid cystic carcinoma (ACC) (28.57%, 28.96%, and 27.67%, respectively) and other histological types (29.14%, 28.69%, and 30.19%, respectively). Overall, in the training and validation cohorts, the highest proportion was in the localized stage (50.48%, 50.55%, and 50.31%, respectively), followed by the regional stage (39.81%, 39.89%, and 39.62%, respectively). Regarding tumor size, among the 3 cohorts, the highest proportion was the part 2 to 5 cm (40.78%, 41.26%, and 39.62%, respectively). As presented in Table 1, the baseline features of the patients in both the training and validation cohorts were well-matched.
7.2. Univariate Cox regression and multivariate Cox regression analyses for determining the prognostic factors
Univariate and multivariate Cox regression analyses were performed to identify the independent prognostic factors for OS in the training cohort. Univariate Cox regression analysis revealed the effects of age (50–70) (P < .001), age ( > 70 years) (P < .001), histology of ACC (P < .001), others(P < .001), chemotherapy (P = .003), stage of regional (P = .04), distant (P < .001), and tumor size of 2 to 5 cm (P = .065) and > 5 cm (P = .013) on the prognosis of patients. Multivariate Cox regression analysis further determined the factors with P < .1 in univariate Cox regression analysis. Although chemotherapy improved survival in univariate Cox regression analysis, it did not achieve statistical significance in the multivariate Cox regression model (P = .991). Multivariate Cox regression analysis demonstrated that age, stage, tumor size, and histology were independent prognostic factors incorporated into the predictive model. No statistically significant benefits were observed with radiotherapy. The prognostic factors for OS were evaluated using univariate and multivariate cox regression analyses, and the results are presented in Table 2.
Table 2.
Univariate Cox regression and multivariate Cox regression analyses to assess the prognostic factors for OS.
| Variable | Univariate analyses | Multivariate analyses | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age | ||||||
| 0–49 | Reference | Reference | ||||
| 50–70 | 2.22 | 1.545–3.2 | <.001 | 1.49 | 1.01–2.21 | .042 |
| >70 | 4.27 | 2.90–6.29 | <.001 | 3.20 | 2.12–4.84 | <.001 |
| Race | ||||||
| White | Reference | |||||
| Black | 0.89 | 0.59–1.35 | .612 | |||
| Others | 0.83 | 0.52–1.32 | .452 | |||
| Marriage | ||||||
| Unmarried | Reference | |||||
| Married | 0.85 | 0.65–1.10 | .226 | |||
| Sex | ||||||
| Male | Reference | |||||
| Female | 1.04 | 0.80–1.34 | .764 | |||
| Histology | ||||||
| Squamous carcinoma | Reference | Reference | ||||
| ACC | 0.35 | 0.25–0.49 | <.001 | 0.43 | 0.31–0.62 | <.001 |
| Others | 0.50 | 0.36–0.68 | <.001 | 0.56 | 0.41–0.78 | .001 |
| Stage | ||||||
| Localized | Reference | Reference | ||||
| Regional | 1.33 | 1.01–1.75 | .04 | 1.40 | 1.04–1.89 | .024 |
| Distant | 3.97 | 2.66–5.93 | <.001 | 2.61 | 1.73–3.93 | <.001 |
| Chemotherapy | ||||||
| Performed | Reference | Reference | ||||
| NP/NA | 0.62 | 0.45–0.84 | .003 | 1.00 | 0.71–1.39 | .991 |
| Radiotherapy | ||||||
| Performed | Reference | |||||
| NP/NA | 0.91 | 0.69–1.18 | .493 | |||
| Tumor size | ||||||
| <2 cm | Reference | Reference | ||||
| 2–5 cm | 1.42 | 0.97–2.08 | .065 | 1.24 | 0.84–1.83 | .264 |
| >5 cm | 1.91 | 1.14–3.18 | .013 | 1.96 | 1.15–3.34 | .013 |
| Unknown | 2.46 | 1.67–3.62 | <.001 | 2.40 | 1.61–3.58 | <.001 |
ACC = adenoid cystic carcinoma, OS = overall survival.
8. Calibration and validation of the nomogram
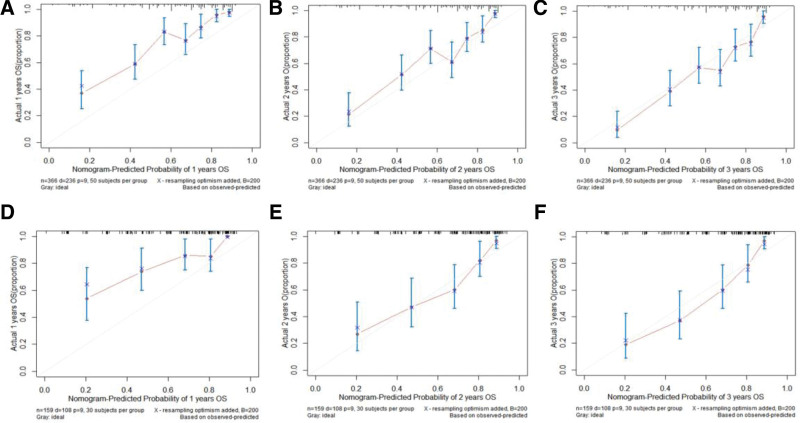
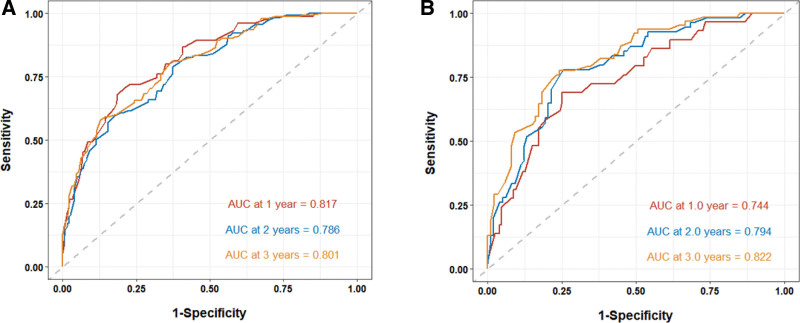
The prediction model was then transformed into a visualizable nomogram (Fig. 2). Each prognostic factor was assigned a score on a point scale. The calibration plots demonstrated a high level of consistency between the predictive nomogram and the observed outcomes for 24- and 36-month OS among the 3 cohorts. (Fig. 3). The calibration plots for 12-month displayed a minor discrepancy from the actual observations, indicating a slightly elevated level of prediction (Fig. 3). ROC curves were used to determine the predictive accuracy and discriminatory ability of the model. As shown in Figure 4, the AUCs for the 12-, 24-, and 36-month survival rates in the training cohort were 0.817 (95% CI: 0.765–0.869), 0.785 (95% CI: 0.736–0.834), and 0.801 (95% CI: 0.755–0.847), respectively. In the validation cohort, the AUCs for 12-, 24-, and 36-month survival rates were 0.744 (95% CI, 0.655–0.833), 0.794 (95% CI, 0.726–0.862), and 0.822 (95% CI, 0.758–0.886), respectively.
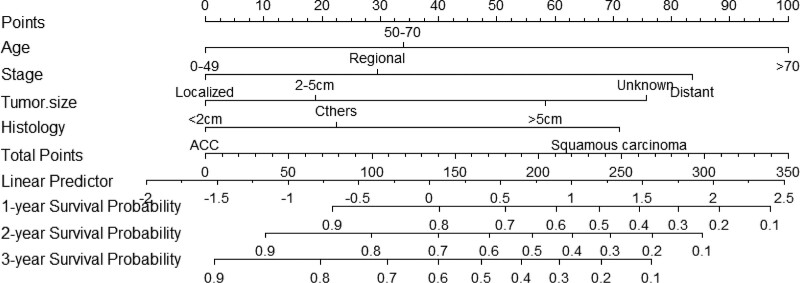
Figure 2.
Predictive nomogram for estimating the 12-, 24-, and 36-month OS rates of tracheal cancer patients treated with surgical intervention. OS = overall survival.
Figure 3.
Calibration curves of the nomogram for both training cohort at 12 (A), 24 (B) and 36 (C) month and validation cohort at 12 (D), 24 (E) and 36 (F) month.
Figure 4.
The prediction and actual probabilities of the nomogram for OS were plotted on the X- and Y-axis, respectively. (A) The ROC curves of training cohort for estimating 12-, 24-, and 36-month OS, with AUCs of 0.817, 0.786, and 0.801, respectively. (B) The ROC curves of validation cohort for estimating 12-, 24-, and 36-month OS, with AUCs of 0.744, 0.794, and 0.822, respectively. AUC = the area under the curve, OS = overall survival, ROC = receiver operating characteristic.
9. Risk classification system
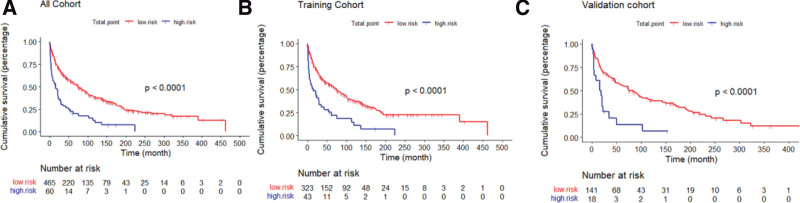
A risk classification system was established based on the total score determined using the nomogram. The system segregated all patients into 2 groups based on their scores: low-risk (risk score: 0–205) and high-risk (risk score:205–350). Subsequently, Kaplan–Meier survival curves were plotted for the low- and high-risk groups in each cohort (Fig. 5). Our analysis indicated that in the training cohort, the low-risk group had better prognosis than the high-risk group (P < .001). Similarly, in the validation cohort, the low-risk group exhibited a superior OS (P < .001). Furthermore, the results demonstrated that the low-risk group had better prognosis in the overall cohort (P < .001) (Fig. 5).
Figure 5.
Survival curves of low-risk and high-risk groups in all cohort (A), training cohort (B) and validation cohort (C) (all P < .001).
10. Discussion
In this study, we performed a Cox regression analysis of patients with tracheal cancer receiving surgical treatment, and 4 independent prognostic factors were included. We also constructed a nomogram by expanding the patient cohort from 1975 to 2018 and sourced it from 18 SEER registries. Our analysis confirmed the robustness of the model, demonstrating its ability to predict outcomes with high accuracy. Specifically, we observed a strong correlation between the predicted 24- and 36-month OS curves generated by the nomogram and actual observed outcomes.
Our results demonstrated that age, stage, tumor size, and histology were independent prognostic factors, and the prognosis of patients with primary tracheal malignancies was influenced by several independent risk factors, including age, lymph node, metastasis, multiple primary tumors, histology, tumor size, and extension. the surgical method used, radiotherapy.[16,17] Survival outcomes in surgically treated patients with primary tracheal malignancies may be affected by factors such as tumor location, extent, lymph node metastasis, and post-operative complications, as studied by Li et al[18], yet with fewer cases and among both ACC and SCC who underwent surgical resection. For malignant tumors, radiation therapy and chemotherapy can serve as supplementary treatments, preparatory treatments prior to surgery, or standalone treatments.[19] Surgery involving the trachea is frequently accompanied by radiation therapy. However, radiation therapy is typically recommended for patients who are not candidates for surgery, or to minimize the risk of recurrence following surgery. Zheng et al[20] have indicated that radiation therapy can have beneficial therapeutic effects. High-dose radiotherapy may increase local tumor control rates and survival time. However, the efficacy of radiation therapy in conjunction with surgical intervention increases the risk of complications, such as tracheobronchial fistulas and airway stenosis. One study also showed that there was no statistically significant difference in survival between patients who received radiotherapy and those who did not receive radiotherapy in patients who were able to undergo surgery, while radiotherapy had a significant impact on survival when the tissue type was squamous carcinoma and the tumor was staged as non-local lesions.[21] In a study by Mallick et al[22], the role of post-operative radiotherapy in the treatment of tracheal tumors remained undefined. The use of post-operative radiotherapy was not a significant factors affecting overall survival. Agrawal et al[23] also reported that patients who underwent combined radiotherapy and surgery therapy had lower overall and cancer-specific survival than those who only underwent surgery. Yet Junmiao W et al showed a favorable survival benefit impact of post-operative radiotherapy patients compared to those who received surgery, which was only found for SCC patients.[24]These results seem to indicate that radiotherapy lacks evidence of definitely beneficial outcomes post-operation. Typically, neoadjuvant treatment comprising chemotherapy alone is the preferred approach; however, it is not still a first-line treatment for tracheal tumors. However, there have been successful cases in which this approach was employed.[25,26] Interestingly, in our study, for patients who underwent surgical treatment, chemotherapy and radiotherapy were not included as independent prognostic factors in the multivariate Cox regression analysis. Although chemotherapy was found to have a statistically significant difference in the univariate Cox regression analysis, it did not demonstrate an obvious difference in the multivariate Cox regression analysis. Therefore, it was not included in the predictive model index.
However, few studies have constructed a nomogram for predicting the OS of patients with tracheal cancer.[20,24] It is worth mentioned that up to now the nomogram in our study is the first tool for patients with tracheal cancer receiving surgical intervention. The nomogram is a practical tool for predicting disease survival and can be used to calculate survival probability to patients in a visual manner. The calibration plots and discrimination are uesd to evaluate the performance of the model.[27] In our study,t he calibration plots showed a high level of agreement between the nomogram predictions and actual observations for the 24- and 36-month OS in the training cohort. Moreover, the calibration plots indicated fairly good agreement in the validation cohort and for 24- and 36-month OS. However, the agreement was slightly higher than the actual observation for 1-year OS, which may be due to the limited sample size of the study. Furthermore, we used ROC to assess the discrimination capacity. As shown in Figure 4, the AUCs of the 12-, 24-, and 36-month OS rates were 0.817, 0.786, and 0.801 in the training cohort, In order to verify this result, the model was applied to the the validation cohort, the AUCs of the 12-, 24-, and 36-month OS rates were 0.744, 0.794, and 0.822, respectively. The high AUCs showed good discrimination capacity. The findings indicated that the nomogram, consisting of 4 prognostic factors, exhibited the highest level of consistency between predicted prognosis and actual observations. Hence, the nomogram developed in this study provides a reliable prediction model for estimating the probability of survival among patients with tracheal cancer undergoing surgical intervention. Additionally, an effective risk classification system was developed using a nomogram prediction model. The results demonstrated that patients in the high-risk group had a significantly worse prognosis than those in the low-risk group, and the nomogram based on the 4 factors successfully identified high-risk and low-risk populations in the training and validation cohorts.Using our risk classification system, we can identify high-risk patients more accurately and provide them with more rigorous follow-up and care.
This study had certain limitations. First, the data were obtained from the SEER database, and the individual patient information was not comprehensive enough and lacked more general health information. Second, the study relied on a retrospective analysis and lacked an analysis of different types of surgery prognosis, which may have introduced some degree of deviation. Third, the sample size of the classification was not sufficiently large and there was no further analysis of the data regarding the type of surgery. Fourth, an external validation was not performed. Nonetheless, this study is the first to analyze independent risk factors, develop a prognostic model, and compare survival outcomes across different risk classifications in patients with tracheal tumors who have undergone surgical intervention. Utilizing this prediction model, the survival probability information of patients with tracheal cancer undergoing surgical intervention is likely to be obtained.
11. Conclusion
A novel nomogram and associated risk classification system were developed to predict the OS of patients with tracheal tumors who underwent surgical intervention. The performance of the model was verified, and it demonstrated a good accuracy. This model can assist clinicians in making informed clinical decisions and tailoring treatments for patients.
Acknowledgements
We would like to thank the SEER team for providing the data.
Author contributions
Data curation: Chunjuan Zhao.
Software: Xin Liu, Hang Liu, Liyun Wu.
Writing – original draft: Wei Shi, Yanhong Ning, Xin Liu, Hang Liu.
Writing – review & editing: Wei Shi, Yanhong Ning, Xin Liu, Hang Liu.
Abbreviations:
- ACC
- adenoid cystic carcinoma
- AUC
- the area under the curve
- OS
- overall survival
- ROC
- receiver operating characteristic
- SEER
- the surveillance, epidemiology, and end results database
WS, YN, and LX contributed equally to this work.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
The authors have no funding and conflicts of interest to disclose.
Written informed consent was obtained from all the participants.
How to cite this article: Shi W, Ning Y, Liu X, Liu H, Zhao C, Wu L. Prognostic factors and constructing a nomogram in tracheal cancer patients treated with surgical intervention: A study based on SEER database. Medicine 2024;103:1(e36787).
Contributor Information
Yanhong Ning, Email: 89569243@qq.com.
Xin Liu, Email: nnliuhang@163.com.
Hang Liu, Email: nnliuhang@163.com.
Chunjuan Zhao, Email: 214846702@qq.com.
Liyun Wu, Email: 401874289@qq.com.
References
- [1].Mukkamalla SKR, Winters R, Chandran AV. Tracheal Cancer. Treasure Island (FL); StatPearls Publishing; 2023. [PubMed] [Google Scholar]
- [2].Junker K. Pathology of tracheal tumors. Thorac Surg Clin. 2014;24:7–11. [DOI] [PubMed] [Google Scholar]
- [3].Klepetko W. Surgerical treatment of tracheal tumours. J Thoracic Oncol. 2019;14:S99–S100. [Google Scholar]
- [4].Marchant F, Mäkitie A, Salo J, et al. Tracheal and laryngotracheal resections and reconstructions-a single-centre experience. J Thorac Dis. 2022;14:2053–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Alkhars HF, Al Muhaimid T, Al Abdulwahid F, et al. Endoscopic excision of primary tracheal schwannoma: a case report. Am J Case Rep. 2023;24:e939823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lee JK, Kho BG, Kim TO, et al. Three cases of rigid bronchoscopic removal of carinal masses: case report. Respir Med Case Rep. 2022;40:101759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Benissan-Messan DZ, Merritt RE, Bazan JG, et al. National utilization of surgery and outcomes for primary tracheal cancer in the United States. Ann Thorac Surg. 2020;110:1012–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Macchiarini P. Primary tracheal tumours. Lancet Oncol. 2006;7:83–91. [DOI] [PubMed] [Google Scholar]
- [9].Auchincloss HG, Wright CD. Complications after tracheal resection and reconstruction: prevention and treatment. J Thorac Dis. 2016;8(Suppl 2):S160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Iwasaki M, Ishihara S, Shimomura M, et al. Endoscopic surgery using ultrasonic energy device for tracheal metastatic tumor. Ann Thorac Surg. 2022;114:e189–91. [DOI] [PubMed] [Google Scholar]
- [11].Sakaguchi Y, Matsumoto K, Nishioka K, et al. Bronchoscopic surgery for a solitary tracheal tumor of tracheobronchopathia osteochondroplastica. Ann Thorac Surg. 2020;109:e419–21. [DOI] [PubMed] [Google Scholar]
- [12].Parshin VD, Rusakov MA, Parshin AV, et al. [Surgery of primary tracheal tumors]. Khirurgiia (Mosk). 2022;8:12–24. [DOI] [PubMed] [Google Scholar]
- [13].Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26:1364–70. [DOI] [PubMed] [Google Scholar]
- [14].Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol. 2015;33:861–9. [DOI] [PubMed] [Google Scholar]
- [16].Gao H, He X, Du J, et al. Competing risk analysis of primary tracheal carcinoma based on SEER database. Cancer Manag Res. 2019;24:1059–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jiaxi H, Jianfei S, Jun H. Prognosis of primary tracheal tumor: a population-based analysis. J Surg Oncol. 2017;115:1004–10. [DOI] [PubMed] [Google Scholar]
- [18].Li J, Tan F, Wang Y, et al. Clinical characteristics, surgical treatments, prognosis, and prognostic factors of primary tracheal cancer patients: 20-year data of the National Cancer Center, China. Transl Lung Cancer Res 2022;11:735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Diaz-Mendoza J, Debiane L, Peralta AR, et al. Tracheal tumors. Curr Opin Pulm Med. 2019;25:336–43. [DOI] [PubMed] [Google Scholar]
- [20].Zheng Z, Du Z, Fang Z, et al. Survival benefit of radiotherapy and nomogram for patients with primary tracheal malignant tumors: a propensity score-matched SEER database analysis. J Cancer Res Clin Oncol. 2023;149:9919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xie L, Fan M, Sheets NC, et al. The use of radiation therapy appears to improve outcome in patients with malignant primary tracheal tumors: a SEER-based analysis. Int J Radiat Oncol Biol Phys. 2012;84:464–70. [DOI] [PubMed] [Google Scholar]
- [22].Mallick S, Benson R, Giridhar P, et al. Demography, patterns of care and survival outcomes in patients with malignant tumors of trachea: a systematic review and individual patient data analysis of 733 patients. Lung Cancer. 2019;132:87–93. [DOI] [PubMed] [Google Scholar]
- [23].Agrawal S, Jackson C, Celie KB, et al. Survival trends in patients with tracheal carcinoma from 1973 to 2011. Am J Otolaryngol. 2017;38:673–7. [DOI] [PubMed] [Google Scholar]
- [24].Wen J, Liu D, Xu X, et al. Nomograms for predicting survival outcomes in patients with primary tracheal tumors: a large population-based analysis. Cancer Manag Res. 2018;10:6843–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jiang BY, Zhang JT, Yan LX, et al. Neoadjuvant immune checkpoint inhibitor plus chemotherapy in rare tracheal tumors. Cancer Commun (Lond). 2021;41:1243–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Heuermann M, Bekker S, Czeczok T, et al. Tracheal chondrosarcoma: a case report, systematic review, and pooled analysis. Cancer Rep (Hoboken). 2022;5:e1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Alba AC, Agoritsas T, Walsh M, et al. Discrimination and calibration of clinical prediction models: users guides to the medical literature. JAMA. 2017;318:1377–84. [DOI] [PubMed] [Google Scholar]