Abstract
Background:
Kiwi root is a Chinese herb clinically used in the treatment of lung neoplasm; however, the multi-target mechanism of kiwi root in the treatment of non-small cell lung cancer (NSCLC) remains to be elucidated. Thus, this study aimed to investigate the molecular mechanisms of kiwi root in the treatment of NSCLC through network pharmacology and molecular docking techniques.
Methods:
The active components and targets of kiwi root were obtained from the TCMSP database, and NSCLC-related targets were obtained from the GeneCards, OMIM, and DrugBank databases. The intersection targets of NSCLC and kiwi root were obtained from VENNY 2.1.0. Then, the common targets were imported into the STRING database, and by using the Cytoscape 3.7.1 software, drug-disease network diagrams were created. Afterwards, the DAVID database was utilized to perform bioinformatic annotation. Finally, molecular docking of key components and key targets was performed by Autodock Tools.
Results:
A total of 4083 NSCLC-related disease genes were collected from the GeneCards, OMIM,and DrugBank databases, and 177 non-duplicated drug targets were acquired from the TCMSP database. A total of 138 intersection target genes were obtained, in which TP53, AKT1, and TNF were the key targets.
Conclusion:
Through network pharmacology techniques, the mechanism of kiwi root in the treatment of NSCLC has been uncovered and provides a theoretical basis for the clinical treatment of NSCLC with kiwi root, which requires further experimental validation.
Keywords: kiwi root, molecular docking, network pharmacology, non-small cell lung cancer
1. Introduction
Lung cancer is a malignant tumor with the highest morbidity and mortality in the world. According to the statistics, in 2020, 2.21 million new cases were diagnosed with lung cancer worldwide, with 1.8 million deaths. Unfortunately, the incidence and death of lung cancer is still rising.[1] According to the histopathological characteristics, lung cancer can be divided into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), among which NSCLC is the most common type of lung cancer, accounting for about 80%-85% of the total number of lung cancers.[2] Smoking, radon, asbestos, family history of lung cancer, and cumulative exposure to polycyclic aromatic hydrocarbons are risk factors for lung cancer, and among these factors, smoking is the foremost cause of lung cancer.[3] Studies have shown that metastatic disease is the cause of most cancer-related deaths.[4] NSCLC metastasizes early; nearly 70% of NSCLC patients were diagnosed at an advanced stage, with a 5-year survival rate of only 15%.[5] Therefore, there is an urgent need to develop highly effective, low-toxicity drugs to treat NSCLC.
In recent years, the study of herbal components has extensively attracted researchers’ interest. More and more traditional Chinese medicines and their components have been found to have significant anti-NSCLC effects. Zhang et al found that Cinobufagin has the effects of promoting apoptosis and inhibiting proliferation, migration, and invasion of A549 cells by inhibiting G9a, and has low toxicity and less effect on normal cells.[6] Liu et al found that berberine exerts its antitumor activity through its immunomodulatory function.[7]
Network pharmacology is a new discipline integrated with multiple disciplines, traditional Chinese medicine (TCM) is characterized by multi-component, multi-pathway, and multi-target, and the use of network pharmacology can systematically reveal the mechanism of action of TCM.[8] In recent years, researchers have conducted network pharmacology analysis of TCM to uncover the mechanism of TCM on NSCLC. Qi et al uncovered the anti-NSCLC effects and mechanisms of gypenosides from Gynostemma pentaphyllum by network pharmacology and metabolomics.[9] Network pharmacology can reveal the mechanism of TCM comprehensively.
Kiwi root has a long history of medicinal use. The TCM theory believes that the main effect of kiwi root is to clear heat. Studies have shown that kiwi root and kiwi fruit have an inhibitory effect on a variety of tumors, including lung cancer,[10] liver cancer,[11] gastric cancer,[12] and breast cancers.[13] Clinically, kiwi root is widely utilized instead of kiwi fruit. Professor Zhu frequently uses kiwi root in treating lung cancer, thus, we chose kiwi root for our study. However, the mechanism of its main components against NSCLC is still unclear. Therefore, this study was conducted to investigate the efficacy of kiwi root against NSCLC using network pharmacology techniques.
2. Materials and methods
2.1. Screening the main active ingredients and potential targets in kiwi root
Based on the framework of systems pharmacology, the traditional Chinese medicine systems pharmacology database and analysis platform (TCMSP) contains 499 herbal medicines and 12 crucial ADME-related properties. It aims to promote the development of herbal medicine, the integration of modern and traditional medicine, and drug discovery and development.[14] Thus, we utilized the TCMSP database to collect potential active ingredients of kiwi root. Oral bioavailability (OB) ≥ 30% indicates that the compound is well absorbed orally, drug-likeness (DL) represents the similarity of the compound to known drugs, and DL ≥ 0.18 is often used as a selection criterion for compounds in the class of traditional Chinese medicines. Therefore, we screened the active compounds that fulfill both OB ≥ 30% and DL ≥ 0.18, and then the targets were collected. Afterwards, these targets were imported into the UniProt (https://www.uniprot.org/) database to gain gene symbols.
2.2. Screening the potential targets of NSCLC
GeneCards (https://www.genecards.org/) is a comprehensive database that supplies researchers with annotative information about human genes, it is mined and integrated from more than 80 data sources, including UniProtKB, and HGNC.[15] OMIM is a database of human genes and genetic disorders, it contains a variety of genes and phenotypes.[16] Drugbank is a database containing comprehensive molecular information about drugs, their mechanisms, and targets.[17] Thus, the targets of NSCLC were obtained from the GeneCards, OMIM, and Drugbank databases.
2.3. The intersection targets between drug and disease
The intersection targets of NSCLC and kiwi root were acquired from VENNY 2.1.0 (http://bioinfogp.cnb.csic.es/tools/venny/index.html). Then, the intersection targets between drug and disease were acquired.
2.4. Construction of drug–compound–target gene network
The components and targets related to the kiwi root we obtained were imported into the Cytoscape 3.7.1 software to construct a drug-component-target visualization network diagram. Among them, different shapes were used to describe a drug, some components, and gene targets.
2.5. Construction of protein–protein interaction (PPI) network
Much of the intracellular complexity comes from functional and regulatory interactions between proteins, thus the discovery of interrelationships between the proteins is crucial in our study, the STRING database systematically collects and integrates physical interactions and functional associations between proteins and proteins from different database resources, contains protein–protein interaction networks for more than 10,000 distinct genomes.[18] According to the corresponding calculation method, the species was set to “Homo sapiens,” the confidence score was set to “highest confidence (0.900),” the disconnected nodes in the network were hidden, then the file of the network was downloaded, and imported into the Cytoscape 3.7.1 software. The parts that unattached to the main part of the PPI network were eliminated, and afterwards, the proteins were sorted according to the degree value.
2.6. Bioinformatic annotation
The common targets of drug and disease were imported into the Database for Annotation, Visualization, and Integrated Discovery database (DAVID, https://david.ncifcrf.gov/), then gene ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of common targets were performed.
2.7. Molecular docking
Based on the results of the PPI network and drug–compound–target gene network, we selected the top 3 ranked targets and components for molecular docking. The PubChem database (https://pubchem.ncbi.nlm.nih.gov/) provides components’ names, 2D structures, molecular formulas, and molecular weight. The top 3 ligands (2D format) were downloaded from the database, then the structures of ligands were optimized using the Chem 3D software and Auto Dock Tools 1.5.7 software, and then prepared for molecular docking. Protein Sequence Database (https://www.rcsb.org) provides proteins’ 3D structure, the structures of the core targets were downloaded, and then, excess chains, ions, and water molecules were removed by the PyMOL software. The Auto Dock Tools 1.5.7 software was utilized to add hydrogen atoms and convert their format for docking. Afterwards, the ligands and receptors were imported in sequence in the AutoDock Tools 1.5.7 software, grid box parameters were configured and saved, and molecular docking was accomplished through the AutoDock Vina software. Finally, the PyMOL software was utilized to display the molecular docking results. It is generally accepted that a docking score less than or equal to -5.0 kcal/mol indicates a strong affinity between the docked compound and the target.[19]
3. Results
3.1. Active compounds of kiwi root
The active compounds of kiwi root were retrieved from the TCMSP database, with OB ≥ 30% and DL ≥ 0.18 as inclusion criteria, then a total of 6 components and 177 non-duplicate targets were acquired.
3.2. Main targets of NSCLC
We searched 3 publicly available databases: GeneCards, OMIM, and Drugbank. A total of 3683 targets with a relevance score greater than the median value from the GeneCards database, 469 targets from the OMIM database, and 52 targets from the Drugbank database. After removing duplicate values, we finally obtained 4083 disease targets.
3.3. Targets of kiwi root in the treatment of NSCLC
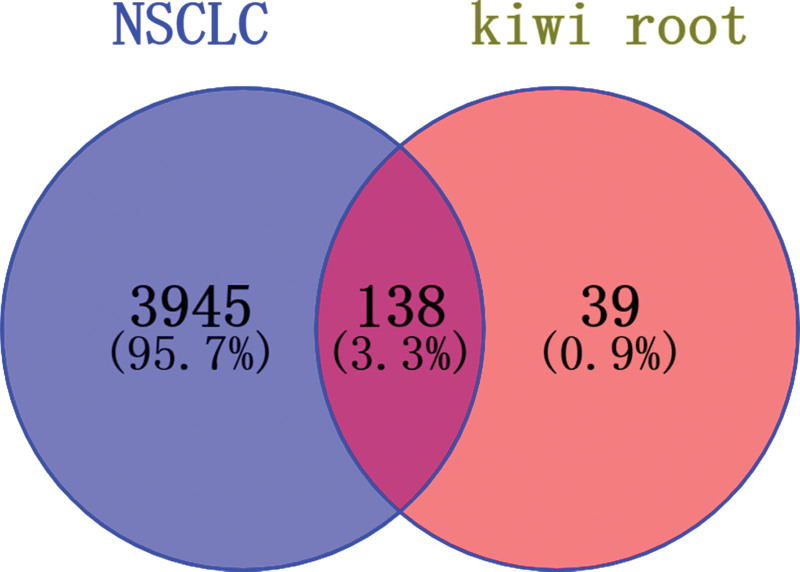
The targets of kiwi root and NSCLC were imported into Venny 2.1.0, and a total of 138 intersection targets for kiwi root in the treatment of NSCLC were obtained, as shown in Figure 1. The targets are listed in Table 1.
Figure 1.
Screening of potential targets of NSCLC with kiwi root. NSCLC = non-small cell lung cancer.
Table 1.
Potential targets of kiwi root in treating NSCLC.
| NO | Gene name | NO | Gene name | NO | Gene name | NO | Gene name |
|---|---|---|---|---|---|---|---|
| 1 | ABCG2 | 36 | CXCL8 | 71 | IL10 | 106 | PPARG |
| 2 | ACACA | 37 | CYP1A1 | 72 | IL1A | 107 | PRKACA |
| 3 | ACHE | 38 | CYP1A2 | 73 | IL1B | 108 | PRKCA |
| 4 | ADRB2 | 39 | CYP1B1 | 74 | IL2 | 109 | PRKCB |
| 5 | AHR | 40 | CYP3A4 | 75 | IL6 | 110 | PRKCD |
| 6 | AKT1 | 41 | DPP4 | 76 | INSR | 111 | PRKCE |
| 7 | ALOX5 | 42 | E2F1 | 77 | IRF1 | 112 | PRSS1 |
| 8 | AR | 43 | E2F2 | 78 | JUND | 113 | PTEN |
| 9 | BAX | 44 | EGF | 79 | MAP2 | 114 | PTGER3 |
| 10 | BCL2 | 45 | EGFR | 80 | MAPK1 | 115 | PTGS1 |
| 11 | BCL2L1 | 46 | ELK1 | 81 | MMP1 | 116 | PTGS2 |
| 12 | BIRC5 | 47 | ERBB2 | 82 | MMP2 | 117 | RAF1 |
| 13 | CALM1 | 48 | ERBB3 | 83 | MMP3 | 118 | RASA1 |
| 14 | CASP3 | 49 | ESR1 | 84 | MMP9 | 119 | RASSF1 |
| 15 | CASP8 | 50 | F10 | 85 | MPO | 120 | RB1 |
| 16 | CASP9 | 51 | F2R | 86 | MYC | 121 | RELA |
| 17 | CAT | 52 | F3 | 87 | NCOA2 | 122 | RUNX1T1 |
| 18 | CAV1 | 53 | FASN | 88 | NFE2L2 | 123 | RUNX2 |
| 19 | CCL2 | 54 | FOS | 89 | NFKBIA | 124 | RXRA |
| 20 | CCNB1 | 55 | GABRA3 | 90 | NOS3 | 125 | SELE |
| 21 | CCND1 | 56 | GJA1 | 91 | NQO1 | 126 | SERPINE1 |
| 22 | CD40LG | 57 | GSTM1 | 92 | NR1I2 | 127 | SOD1 |
| 23 | CDK1 | 58 | GSTP1 | 93 | NR3C2 | 128 | SPP1 |
| 24 | CDKN1A | 59 | HIF1A | 94 | ODC1 | 129 | STAT1 |
| 25 | CDKN2A | 60 | HK2 | 95 | OPRM1 | 130 | TGFB1 |
| 26 | CHEK2 | 61 | HMOX1 | 96 | PARP1 | 131 | THBD |
| 27 | CHRM3 | 62 | HSF1 | 97 | PCNA | 132 | TNF |
| 28 | CHRNA2 | 63 | HSP90AB1 | 98 | PGR | 133 | TOP1 |
| 29 | CHRNA7 | 64 | HSPA5 | 99 | PIK3CG | 134 | TOP2A |
| 30 | CHUK | 65 | HSPB1 | 100 | PLAT | 135 | TP53 |
| 31 | CLDN4 | 66 | ICAM1 | 101 | PLAU | 136 | VCAM1 |
| 32 | COL1A1 | 67 | IFNG | 102 | PON1 | 137 | VEGFA |
| 33 | CRP | 68 | IGF2 | 103 | POR | 138 | XDH |
| 34 | CTSD | 69 | IGFBP3 | 104 | PPARA | ||
| 35 | CXCL10 | 70 | IGHG1 | 105 | PPARD |
3.4. Construction of drug-compound-target network
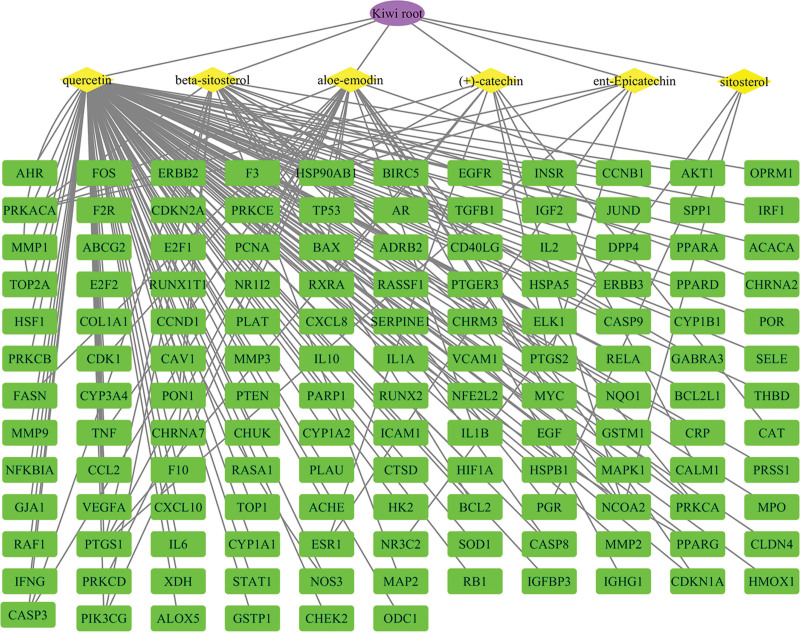
The drug, ingredients, and targets were imported into the Cytoscape 3.7.1 software. As shown in Figure 2, the purple oval was used to represent the drug, the yellow diamonds were used to represent the ingredients, and the green rectangles represent the gene targets, in the drug-compound-target network, according to the degree value, the top 3 compounds were quercetin, beta-sitosterol and aloe-emodin.
Figure 2.
The “drug-compound-target” network of kiwi root in the treatment of NSCLC. The purple node indicates the kiwi root, the yellow nodes indicate ingredients, the green nodes indicate potential gene targets, and the edges indicate their interactions. NSCLC = non-small cell lung cancer.
3.5. Construction of PPI network
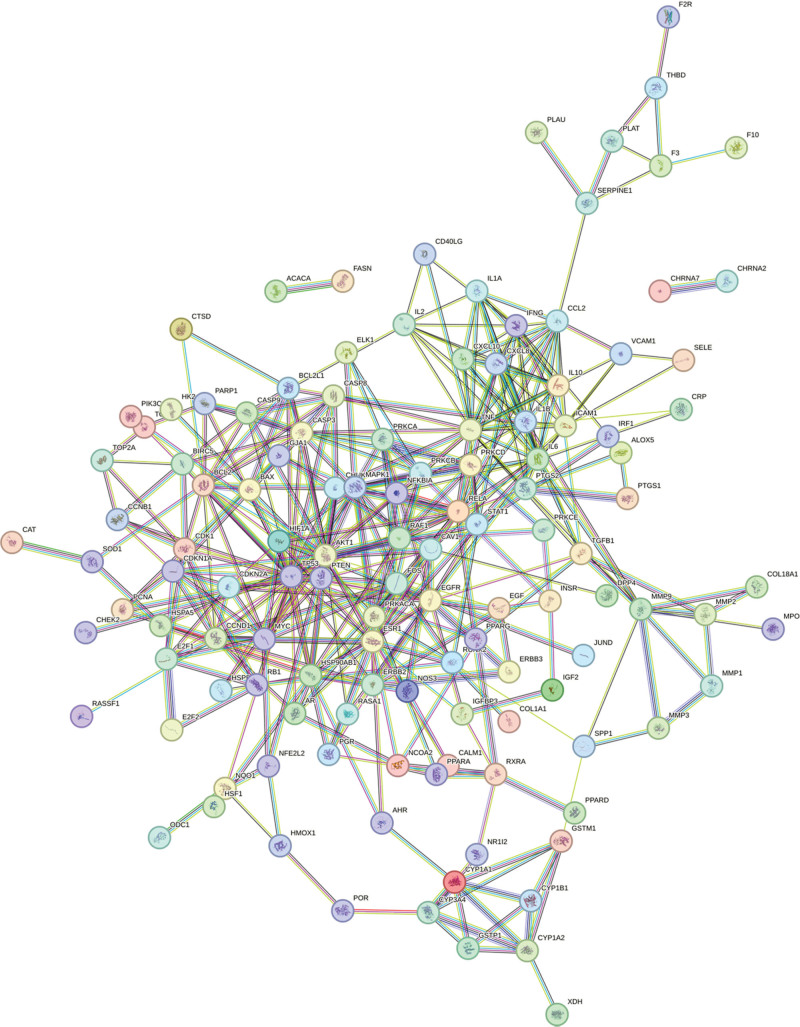
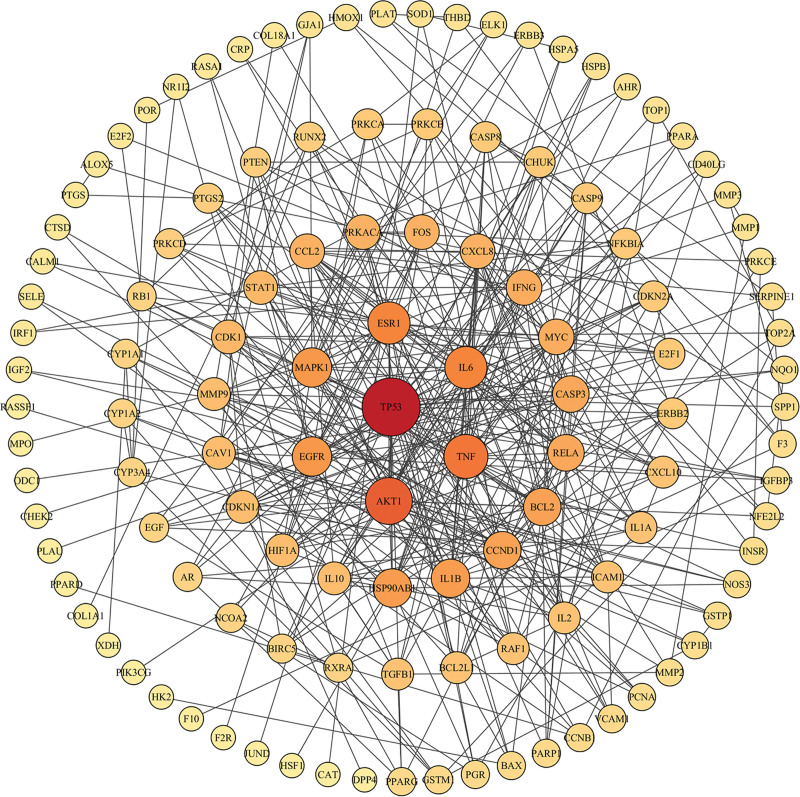
The intersection targets of kiwi root and NSCLC were obtained by Venny 2.1.0. A total of 138 intersection targets were imported into the STRING database, then the PPI network was constructed, the species was set as “Homo Sapiens,” and the disconnected targets were hidden, as shown in Figure 3. Then the file was imported into the Cytoscape 3.7.1 software for visualization and analysis. By using the network analyzer function of the software, topology analysis was performed. After eliminating the parts unconnected to the main part of this network, as shown in Figure 4, according to the degree value, the top 3 key gene targets of kiwi root were TP53, AKT1, and TNF.
Figure 3.
The PPI network of kiwi root in treating NSCLC. NSCLC = non-small cell lung cancer.
Figure 4.
The PPI network of kiwi root in treating NSCLC. The PPI network was selected by the Cytoscape 3.7.1 software. The nodes in red have the highest degree of interaction. NSCLC = non-small cell lung cancer, PPI = protein–protein interactions.
3.6. GO enrichment
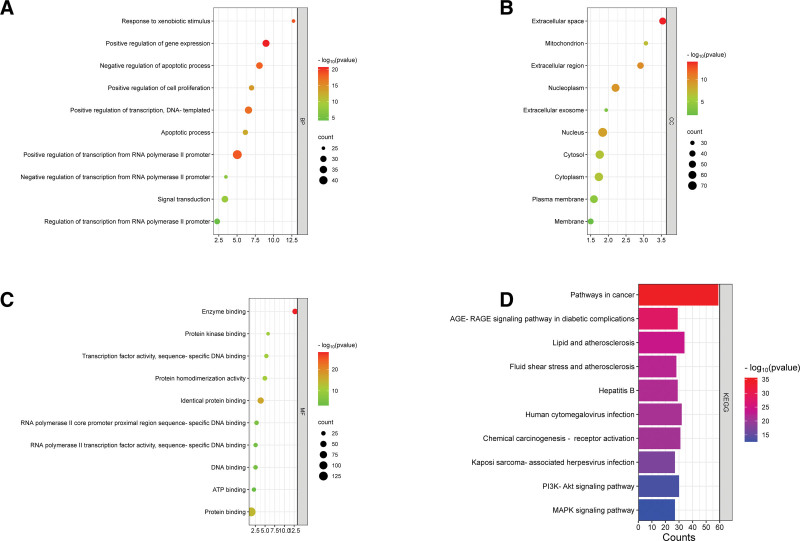
GO enrichment analysis and KEGG pathway enrichment analysis were performed on 138 intersection targets of kiwi root and NSCLC. In the GO enrichment analysis, A total of 723 biological processes (BP), 82 cellular components (CC), and 138 molecular functions (MF) were found. The biological process of potential targets mainly included positive regulation of gene expression, positive regulation of transcription, DNA-templated, positive regulation of transcription from RNA polymerase II promoter (Fig. 5A). Cellular components included extracellular space, extracellular region, nucleoplasm, nucleus, cytoplasm, plasma membrane and other cell structures (Fig. 5B). Molecular function included enzyme binding, identical protein binding, protein binding and so on (Fig. 5C).
Figure 5.
GO enrichment analysis of key targets and KEGG enrichment analysis. (A) The top 10 significant P values of BP; (B) The first 10 significant P values of CC; (C) The top 10 significant P values of MF; (D) The top 10 significant P values of KEGG pathways. BP = biological process, CC = cellular component, MF = molecular function, KEGG = Kyoto encyclopedia of genes and genomes.
3.7. KEGG enrichment
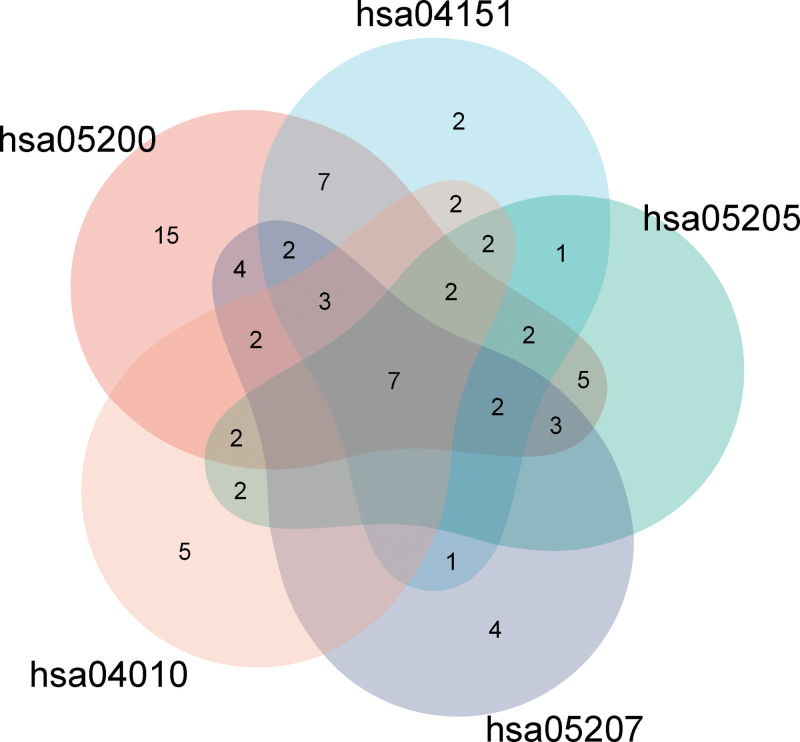
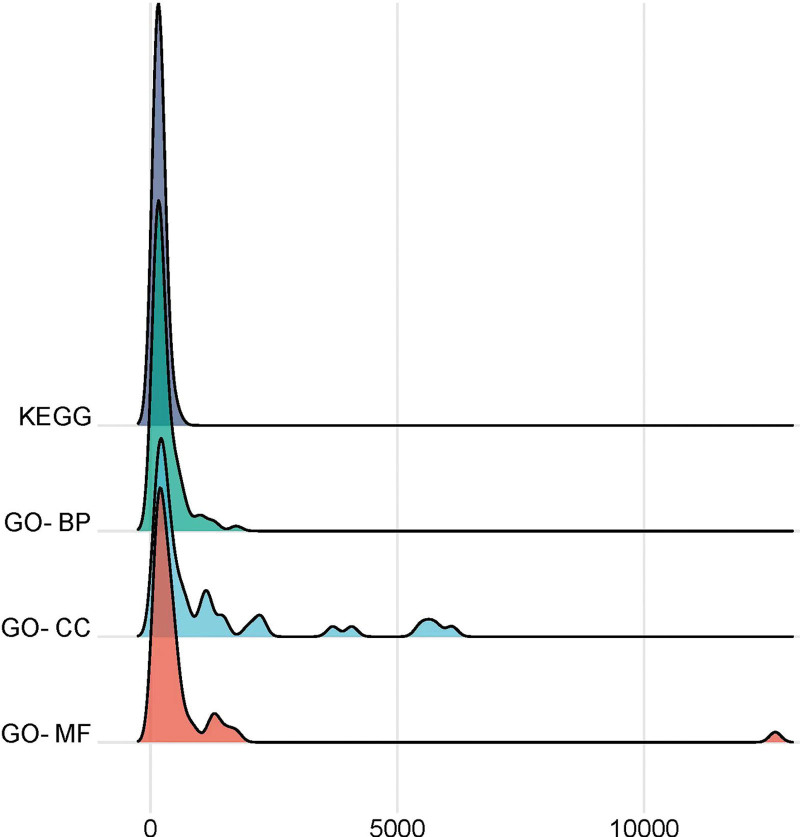
Through KEGG analysis, 175 main signaling pathways were found, according to the size of the P value, the top 20 pathways were visualized, as shown in Figure 5D, the main signaling pathways were related to pathways in cancer, AGE-RAGE signaling pathway in diabetic complications, lipid and atherosclerosis, fluid shear stress and atherosclerosis, PI3K- Akt signaling pathway, MAPK signaling pathway, etc. It suggested that kiwi root in the treatment of NSCLC is mainly involved in the above signaling pathways. We analyzed 5 top-ranked cancer-related signaling pathways, including pathways in cancer, PI3K-Akt signaling pathway, proteoglycans in cancer, chemical carcinogenesis - receptor activation, and MAPK signaling pathway. Afterwards, 7 common targets in 5 signaling pathways were screened out, including MAPK3, PRKCA, EGFR, AKT1, MAPK1, VEGFA, and MYC, as shown in Figure 6. We selected the peak map made of Pop hit values greater than or equal to 100 in the 3 items of GO and KEGG analysis, and it can be seen from Figure 7 that the 4 groups of Pop hit values were centrally distributed around 1000, and some of them were as high as 5000 or more, which indicated that these pathways or biological processes are representative.
Figure 6.
The common targets in the top 5 cancer-related signaling pathways.
Figure 7.
The peak map of Hop hit values.
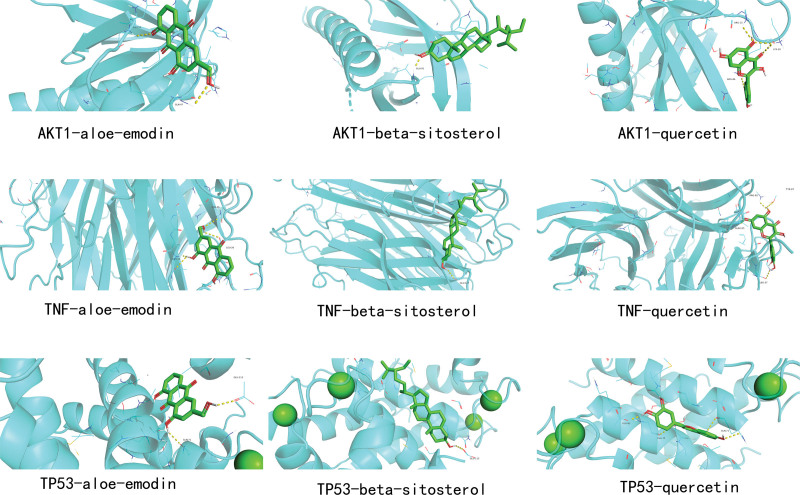
3.8. Molecular docking analysis
We selected the top 3 ranked components and targets for molecular docking, quercetin, beta-sitosterol, and aloe-emodin were picked from the drug-compound-target network as the 3 core components, TP53, AKT1, and TNF were selected from the PPI network as the 3 key targets. A docking score less than or equal to -5.0 kcal/mol indicates a strong affinity between the docked compound and the target, among them, the docking binding energy between TP53 and aloe-emodin was the lowest (−7.9 kcal·mol−1), indicating that the interaction between TP53 bound the most tightly to aloe-emodin (Table 2). Thus, it was suggested that TP53 and aloe-emodin might be the core target of kiwi root for the treatment of NSCLC. The results are shown in Figure 8.
Table 2.
Docking results between core components and key targets.
| Component | Target | Binding energy (kcal·mol−1) |
|---|---|---|
| Quercetin | AKT1 | -6.2 |
| TNF | -7.1 | |
| TP53 | -7.5 | |
| Beta-sitosterol | AKT1 | -7.0 |
| TNF | -6.3 | |
| TP53 | -7.6 | |
| Aloe-emodin | AKT1 | -6.9 |
| TNF | -6.5 | |
| TP53 | -7.9 |
AKT1 = protein kinase B, TNF = Tumor necrosis factor, TP53 = tumor antigen p53.
Figure 8.
Diagram of molecular docking patterns. Blue bands represent protein receptors, green conformations represent ligands, and yellow lines represent hydrogen bonds.
4. Discussion
Lung cancer is a common and serious disease that can lead to death and bring serious economic burdens. NSCLC metastasizes early, most of the patients are often diagnosed in advanced stages, and the chance of surgery therapy is often lost, leaving them with radiation and chemotherapy, which have various side effects, so many patients are reluctant to undergo radiotherapy and chemotherapy. Traditional Chinese medicine has been used in China for thousands of years in treating lung cancer. Therefore, the utilization of TCM helps us to develop new anti-cancer drugs. In this study, we investigated the mechanism of the main components of kiwi root in the treatment of NSCLC by network pharmacology techniques and found that the main compounds of kiwi root were quercetin, beta-sitosterol, and aloe-emodin, and the main targets of their action were TP53, AKT1, and TNF. Through GO and KEGG enrichment analysis, it was suggested that kiwi root might exert its anti-NSCLC effects through the PI3K-Akt signaling pathway, MAPK signaling pathway, and AGE-RAGE signaling pathway, and the results of molecular docking suggested that the main components of kiwi root were well-bound to the core targets of the proteins, which revealed that the kiwifruit root against NSCLC involved in multi-component, multi-pathway, and multi-target.
Quercetin has anti-inflammatory, antioxidant, and anti-tumor properties,[20–22] quercetin was found to against various cancers through several pathways. Quercetin may induce apoptosis in MGMTGBM cells through Wnt3a/β-Catenin and Akt/NF-κB signaling pathways,[23] and facilitates cell death and chemosensitivity in human pancreatic cancer cells through RAGE/PI3K/AKT/mTOR axis.[24] Experimental studies have shown that quercetin can inhibit DNA damage responses to induce apoptosis via SIRT5/PI3K/AKT pathway in NSCLC,[25] and cause G2-M phase arrest in lung cancer cells, induce apoptosis through BAX, BCL-2, and caspase3 and suppress oxidative stress.[26] Beta-sitosterol is a component found in the medicinal plant Grewia tiliaefolia, it can be considered a potential drug candidate for lung cancer treatment due to its inhibition of human lung cancer cell lines A549,[27] the inhibition effect of beta-sitosterol in NSCLC involves in ROS mediated mitochondrial dysregulation and p53 activation.[28] Aloe-emodin induces autophagy and apoptotic cell death through Akt/mTOR and MAPK signaling.[29] Therefore, the core active compounds of kiwi root in NSCLC may be involved in multiple pathways and induced cell death.
The target interaction network of the kiwi root in NSCLC may involve in TP53, AKT1, and TNF. TP53 is an important tumor suppressor gene, it is frequently mutated in human cancers,[30] the TP53 gene is involved in preventing and suppressing the growth of tumors by regulating cell cycle and apoptosis in NSCLC patients.[31] The mutations of TP53 are associated with a worsened prognosis and cause resistance to cancer therapy.[32] Akt is a serine/threonine kinase and is involved in the PI3K signaling pathway, capable of phosphorylating a variety of downstream effectors including proteins central to regulate apoptosis, transcription factors, and oncogenic factors, it participates in cellular survival, proliferation, migration, metabolism, and angiogenesis.[33] TCM also acts on Akt1 in the treatment of NSCLC, like Lycium barbarum.[34] Tumor necrosis factor, also known as TNF, is widely expressed in NSCLC, it could be a biomarker in NSCLC.[35]
In this study, we also analyzed the BP, MF, CC, and pathways involved in the potential targets of kiwi root against NSCLC, KEGG analysis showed that PI3K-AKT and MAPK signaling pathway may be the central pathways in NSCLC therapy. The phosphoinositide 3-kinase (PI3K)-AKT signaling pathway is always activated in various types of cancer, aberrant stimulation of the PI3K-AKT signaling pathway is linked to tumor growth, angiogenesis, and survival.[36] Under physiological conditions, the PI3K-AKT signaling pathway regulates key metabolic processes, such as glucose metabolism, maintenance of redox balance, and biosynthesis of macromolecules, in cancer cells, the PI3K-AKT signaling pathway regulates cellular metabolism by modulating the activities of nutrient transporters and metabolic enzymes to support the aberrant needs of cancer cells.[37] The hyperactivation of the Ras/RAF/MEK/ERK (MAPK) signaling pathway is responsible for more than 40% of cases of human cancers.[38] MAPK signaling is crucial in cell communication and regulates cell survival, growth, and differentiation,[39] some potential ingredients against NSCLC like Salvianolic acid B may inhibit MAPK signaling pathways. The binding energies of 3 key ingredients and targets are less than -5kcal/mol, which indicates that the key ingredients have high binding activity with receptor proteins. The results of network pharmacology are further verified.
The present study still has some limitations, for instance, complex chemical reactions will happen when decocting medicine, and different dosages of different components may exert different influences in our study. Conceivably, network pharmacology combined with biological experiments can reveal the mechanism of TCM more comprehensively, only data analysis does not truly reflect its complete mechanism of action.
5. Conclusion
In this study, the key components, gene targets, protein–protein interactions, biological functions, signaling pathways, and possible mechanisms were systematically analyzed by network pharmacology and molecular docking techniques, the main compounds of kiwi root were screened out, including quercetin, β-sitosterol, and aloe-emodin, and the main targets of kiwi root against NSCLC were TP53, AKT1, and TNF. Kiwi root might inhibit the growth of NSCLC through PI3K-Akt and MAPK signaling pathways, which indicates that kiwifruit root may exert its anti-NSCLC effect through multi-component, multi-pathway, and multi-target. In the coming period, we will conduct further experiments to verify the mechanism of the core components of kiwi root against NSCLC.
Author contributions
Conceptualization: Ruochen Li.
Data curation: Ruochen Li.
Funding acquisition: Xun Zhou.
Investigation: Mingxiao Wang.
Software: Jin Tian.
Visualization: Minghui Liu, Gaigai Li.
Writing – original draft: Ruochen Li.
Writing – review & editing: Xun Zhou.
Abbreviations:
- DL
- drug-likeness
- GO
- gene ontology
- KEGG
- Kyoto encyclopedia of genes and genomes
- NSCLC
- non-small cell lung cancer
- OB
- oral bioavailability
- PPI
- protein–protein interactions
- TCM
- Traditional Chinese Medicine
- TCMSP
- The traditional Chinese medicine systems pharmacology database and analysis platform
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors have no conflicts of interest to disclose.
The authors gratefully acknowledge the financial support by The Project of National Famous Traditional Chinese Medicine Practitioner Zhusheng Zhu Studio (No.2022-75).
Because we use public databases, according to the ethics guidelines, neither informed consent nor approval of the ethics committee is required.
How to cite this article: Li R, Wang M, Tian J, Liu M, Li G, Zhou X. Exploration of kiwi root on non-small cell lung cancer based on network pharmacology and molecular docking. Medicine 2024;103:1(e36852).
Contributor Information
Ruochen Li, Email: 280169672@qq.com.
Mingxiao Wang, Email: 479382231@qq.com.
Jin Tian, Email: 995022819@qq.com.
Minghui Liu, Email: 708931918@qq.com.
Gaigai Li, Email: 280169672@qq.com.
References
- [1].Sharma R. Mapping of global, regional and national incidence, mortality and mortality-to-incidence ratio of lung cancer in 2020 and 2050. Int J Clin Oncol. 2022;27:665–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Xie S, Wu Z, Qi Y, et al. The metastasizing mechanisms of lung cancer: recent advances and therapeutic challenges. Biomed Pharmacother. 2021;138:111450. [DOI] [PubMed] [Google Scholar]
- [3].Lahiri A, Maji A, Potdar PD, et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer. 2023;22:1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Al Bakir M, Huebner A, Martínez-Ruiz C, et al. The evolution of non-small cell lung cancer metastases in TRACERx. Nature. 2023;616:534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang H, Jiang H, Zhu L, et al. Cancer-associated fibroblasts in non-small cell lung cancer: recent advances and future perspectives. Cancer Lett. 2021;514:38–47. [DOI] [PubMed] [Google Scholar]
- [6].Zhang L, Liang B, Xu H, et al. Cinobufagin induces FOXO1-regulated apoptosis, proliferation, migration, and invasion by inhibiting G9a in non-small-cell lung cancer A549 cells. J Ethnopharmacol. 2022;291:115095. [DOI] [PubMed] [Google Scholar]
- [7].Liu Y, Liu X, Zhang N, et al. Berberine diminishes cancer cell PD-L1 expression and facilitates antitumor immunity via inhibiting the deubiquitination activity of CSN5. Acta Pharm Sin B. 2020;10:2299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhao Li, Zhang H, Li N, et al. Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula. J Ethnopharmacol. 2023;309:116306. [DOI] [PubMed] [Google Scholar]
- [9].Qi Y-S, Xie J-B, Xie P, et al. Uncovering the anti-NSCLC effects and mechanisms of gypenosides by metabolomics and network pharmacology analysis. J Ethnopharmacol. 2021;281:114506. [DOI] [PubMed] [Google Scholar]
- [10].Gorinstein S, Haruenkit R, Poovarodom S, et al. The comparative characteristics of snake and kiwi fruits. Food Chem Toxicol. 2009;47:1884–91. [DOI] [PubMed] [Google Scholar]
- [11].Fang T, Zhao Z, Yuan F, et al. Actinidia Chinensis Planch Root extract attenuates proliferation and metastasis of hepatocellular carcinoma by inhibiting the DLX2/TARBP2/JNK/AKT pathway. J Ethnopharmacol. 2020;251:112529. [DOI] [PubMed] [Google Scholar]
- [12].Gao Z, Deng G, Li Y, et al. Actinidia chinensis Planch prevents proliferation and migration of gastric cancer associated with apoptosis, ferroptosis activation and mesenchymal phenotype suppression. Biomed Pharmacother. 1100;126:92. [DOI] [PubMed] [Google Scholar]
- [13].Gan C, Jin Z, Wei X, et al. Actinidia chinensis Planch root extract inhibits the proliferation, migration and invasion of breast cancer cells via the AKT/GSK-3β signaling pathway. Folia Histochem Cytobiol. 2021;59:226–35. [DOI] [PubMed] [Google Scholar]
- [14].Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminf. 2014;6:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Safran M, Dalah I, Alexander J, et al. GeneCards Version 3: the human gene integrator. Database. 2010;2010:baq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Amberger JS, Bocchini CA, Scott AF, et al. OMIM org: leveraging knowledge across phenotype–gene relationships. Nucleic Acids Res. 2019;47:D1038–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wishart DS, Feunang YD, Guo AC, et al. DrugBank 50: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46:D1074–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Szklarczyk D, Kirsch R, Koutrouli M, et al. The STRING database in 2023: protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023;51:D638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hsin K-Y, Ghosh S, Kitano H. Combining machine learning systems and multiple docking simulation packages to improve docking prediction reliability for network pharmacology. PLoS One. 2013;8:e83922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jin Q, Chen M, Jin Z, et al. Quercetin alleviates gliotoxin-induced duckling tissue injury by inhibiting oxidative stress, inflammation and increasing heterophil extracellular traps release. Food Chem Toxicol. 1137;176:48. [DOI] [PubMed] [Google Scholar]
- [21].Al-Ansari MM, Al-Humaid L, Aldawsari M, et al. Quercetin extraction from small onion skin (Allium cepa L var aggregatum Don) and its antioxidant activity. Environ Res. 2023;224:115497. [DOI] [PubMed] [Google Scholar]
- [22].Huang K-Y, Wang T-H, Chen C-C, et al. Growth suppression in lung cancer cells harboring EGFR-C797S mutation by quercetin. Biomolecules. 2021;11:1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang W, Yuan X, Mu J, et al. Quercetin induces MGMT+ glioblastoma cells apoptosis via dual inhibition of Wnt3a/β-Catenin and Akt/NF-κB signaling pathways. Phytomedicine. 2023;118:154933. [DOI] [PubMed] [Google Scholar]
- [24].Lan C-Y, Chen S-Y, Kuo C-W, et al. Quercetin facilitates cell death and chemosensitivity through RAGE/PI3K/AKT/mTOR axis in human pancreatic cancer cells. J Food Drug Anal. 2019;27:887–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhou B, Yang Y, Pang X, et al. Quercetin inhibits DNA damage responses to induce apoptosis via SIRT5/PI3K/AKT pathway in non-small cell lung cancer. Biomed Pharmacother. 2023;165:115071. [DOI] [PubMed] [Google Scholar]
- [26].Farrag IM, et al. Antiproliferative, apoptotic effects and suppression of oxidative stress of quercetin against induced toxicity in lung cancer cells of rats: in vitro and in vivo study J Cancer 12 (17): 5249. Biochem Eng J. 2021;163:107758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rajavel T, Mohankumar R, Archunan G, et al. Beta sitosterol and Daucosterol (phytosterols identified in Grewia tiliaefolia) perturbs cell cycle and induces apoptotic cell death in A549 cells. Sci Rep. 2017;7:3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rajavel T, Packiyaraj P, Suryanarayanan V, et al. β-Sitosterol targets Trx/Trx1 reductase to induce apoptosis in A549 cells via ROS mediated mitochondrial dysregulation and p53 activation. Sci Rep. 2018;8:2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shen F, Ge C, Yuan P. Aloe-emodin induces autophagy and apoptotic cell death in non-small cell lung cancer cells via Akt/mTOR and MAPK signaling. Eur J Pharmacol. 2020;886:173550. [DOI] [PubMed] [Google Scholar]
- [30].Chen X, Zhang T, Su W, et al. Mutant p53 in cancer: from molecular mechanism to therapeutic modulation. Cell Death Dis. 2022;13:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fan Z, Zhang Q, Feng Li, et al. Genomic landscape and prognosis of patients with TP53-mutated non-small cell lung cancer. Ann Transl Med. 2022;10:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Xu F, Lin H, He P, et al. A TP53-associated gene signature for prediction of prognosis and therapeutic responses in lung squamous cell carcinoma. Oncoimmunology. 2020;9:1731943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Revathidevi S, Munirajan AK. Akt in cancer: mediator and more. Seminars in Cancer Biology. Vol. 59. Academic Press. 2019. [DOI] [PubMed] [Google Scholar]
- [34].Zhang L, Gong Y, Zhang L, et al. Gou Qi Zi inhibits proliferation and induces apoptosis through the PI3K/AKT1 signaling pathway in non-small cell lung cancer. Front Oncol. 2022;12:1034750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gong K, Guo G, Beckley N, et al. Tumor necrosis factor in lung cancer: complex roles in biology and resistance to treatment. Neoplasia. 2021;23:189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Noorolyai S, Shajari N, Baghbani E, et al. The relation between PI3K/AKT signalling pathway and cancer. Gene. 2019;698:120–8. [DOI] [PubMed] [Google Scholar]
- [37].Hoxhaj G, Manning BD. The PI3K–AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer. 2020;20:74–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yuan J, Dong X, Yap J, et al. The MAPK and AMPK signalings: interplay and implication in targeted cancer therapy. J Hematol Oncol. 2020;13:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Degirmenci U, Mei W, Jiancheng H. Targeting aberrant RAS/RAF/MEK/ERK signaling for cancer therapy. Cells. 2020;9:198. [DOI] [PMC free article] [PubMed] [Google Scholar]