Abstract
Rationale:
Acamprosate (calcium-bis N-acetylhomotaurinate) is the leading medication approved for the maintenance of abstinence, shown to reduce craving and relapse in animal models and human alcoholics. Acamprosate can improve executive functions that are impaired by chronic intermittent ethanol (CIE) exposure. Recent work has suggested that acamprosate’s effects on relapse prevention are due to its calcium component, which raises the question whether its pro-cognitive effects are similarly mediated by calcium.
Objectives:
This study examined the effects of acamprosate on alcohol-induced behavioral deficits and compared them with the effects of the sodium salt version of N-acetylhomotaurinate or calcium chloride, respectively.
Methods:
We exposed mice to alcohol via three cycles of CIE and measured changes in alcohol consumption in a limited-access paradigm. We then compared the effects of acamprosate and calcium chloride (applied subchronically for 3 days during withdrawal) in a battery of cognitive tasks that have been shown to be affected by chronic alcohol exposure.
Results:
CIE-treated animals showed deficits in attentional set-shifting, and deficits in novel object recognition. Alcohol-treated animals showed no impairments in social novelty detection and interaction, or delayed spontaneous alternation. Both acamprosate and calcium chloride ameliorated alcohol-induced cognitive deficits to comparable extents. In contrast, the sodium salt version of N-acetylhomotaurinate did not reverse the cognitive deficits.
Conclusions:
These results add evidence to the notion that acamprosate produces its anti-relapse effects through its calcium moiety. Our results also suggest that improved regulation of drug intake by acamprosate after withdrawal might at least in part be related to improved cognitive function.
Keywords: Acamprosate, Calcium, Attentional set-shifting, Novel object recognition, Alcohol addiction
Introduction:
Addiction to alcohol is characterized by inappropriate decision making and loss of control over drinking in spite of negative outcomes (Koob, 2000). Drugs of abuse diminish cognitive control over impulsive behaviors that contribute to drug seeking and relapse to drug taking. Studies in human alcoholics have shown that several cognitive domains, particularly executive functions regulated by the prefrontal cortex (PFC), are impaired early during abstinence and then slowly recover with continued abstinence (Rourke and Grant, 1999; Sullivan et al., 2000; Moselhy et al., 2001; Wölwer et al., 2001; Tedstone and Coyle, 2004; Goldstein and Volkow, 2011; Wiers et al., 2014). Alcohol abusers show poorer performance on measures of response inhibition and cognitive flexibility (Parsons, 1983; Errico et al., 2002; Verdejo-García et al., 2006). Using an attentional set-shifting task that requires an intact PFC, we have previously shown that chronic intermittent ethanol (CIE) exposure similarly disrupts cognitive flexibility in mice. These changes correlate with alterations in N-methyl-D-aspartate receptor (NMDAR) function, causing aberrant synaptic plasticity in the PFC (Kroener et al., 2012; Hu et al., 2015). Altered glutamatergic neurotransmission in prefrontal-limbic circuits has been implicated in the development of addiction and the control of craving and extinction memory (Ishikawa et al., 2009; Moussawi et al., 2009; Holmes et al., 2013). The widely prescribed anti-craving medication acamprosate (Campral; calcium-bis (N-acetylhomotaurinate), abbreviated as Ca-AOTA) has been shown to normalize the increased levels of extracellular glutamate caused by alcohol exposure, suggesting that it may reduce craving by normalizing a hyper-glutamatergic state during withdrawal (Zornoza et al., 2003; De Witte, 2004; Kiefer and Mann, 2010; Umhau et al., 2010; Mason and Lehert, 2012). However, the mechanisms that underlie these effects are unknown and controversial (Spanagel et al., 2014; Mann et al., 2016; Spanagel et al, 2016). In mice, acamprosate reversed CIE-induced deficits in the attentional set-shifting task, but apparently did so without directly affecting previously proposed glutamate receptor mechanisms in the PFC (Hu et al., 2015). Moreover, a recent paper by Spanagel and colleagues (2014) suggested that acamprosate does not interact directly with NMDARs or metabotropic glutamate group I receptors, but instead that calcium by itself is the active moiety of acamprosate.
Here, we further investigated the ability of acamprosate to improve alcohol-induced cognitive deficits in a rodent model of chronic alcohol exposure, and we assessed the importance of calcium in these behavioral effects. We used CIE exposure to induce robust escalation of alcohol intake in mice and then compared performance in several behavioral tasks after animals had received subchronic (3 day) treatment with either Ca-AOTA (200 mg/kg), calcium chloride (CaCl2; 73.4 mg/kg), or a version of acamprosate in which calcium was replaced by sodium (sodium-N-acetylhomotaurinate, Na-AOTA; 200 mg/kg). Chronic alcohol exposure impaired performance in an attentional set-shifting task and it also affected memory in a novel object recognition (NOR) task, but it did not alter delayed spontaneous alternation in a T-maze, or social recognition memory. The deficits in attentional set-shifting and NOR were ameliorated by both Ca-AOTA and CaCl2, but not by Na-AOTA. These results indicate that acamprosate can restore alcohol-induced deficits in cognitive functions that rely on intact prefrontal-hippocampal networks, and importantly, they lend further support to the idea that modulation of calcium homeostasis is responsible for acamprosate’s behavioral effects.
Materials and methods
Subjects:
C57BL/6 adult male mice were singly housed in a temperature- and humidity-controlled animal facility under a 12-h light/dark cycle (lights on at 6:00 AM) with free access to food and water. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas at Dallas.
Limited-access drinking procedure and chronic intermittent ethanol exposure:
Animals were exposed to alcohol as previously described (Becker and Lopez, 2004; Kroener et al., 2012). Mice in the alcohol-exposed experimental groups were trained to drink alcohol on a daily limited access (2 h) schedule via a modified sucrose fading procedure (Becker and Lopez, 2004). Therefore, ethanol (10% v/v) was adulterated with sucrose (5% w/v) for 2 days. This was followed by 12% ethanol/5% sucrose for 2 days, 15% ethanol/5% sucrose for 2 days, 15% ethanol/2% sucrose for 2 days, and 15% ethanol/1% sucrose for 2 days. For the remainder of the experiment, animals drank 15% ethanol/0% sucrose. Alcohol intake (g/kg) was measured in a two-bottle choice paradigm for 5 consecutive days before the first CIE cycle (baseline drinking) and after each of three CIE cycles. In order to increase overall fluid consumption during the restricted-access period (and thus to minimize the contribution of small measuring errors), animals were briefly water deprived immediately before the two-bottle choice period. Therefore, 2 h before the beginning of the dark cycle, the water bottles were removed from the cages, and 1 h into the dark cycle (7:00 PM), they were replaced with two 15-ml pipettes that contained either water or alcohol. For animals in the control groups (H2O + saline and H2O + Ca-AOTA), both pipettes contained water. The placement of the pipettes alternated daily. After 2 h, the pipettes were removed and replaced by water bottles. The amount of solution consumed was recorded daily (± 0.1 ml), along with the animals’ body weights. After 5 days of baseline drinking, mice underwent CIE as previously described (Kroener et al., 2012). The mice were exposed to ethanol vapor (95% ethanol mixed with air) or air (control animals) in inhalation chambers for 16 h/day for 4 days. Alcohol intoxication was initiated by the administration of 1.53 g/kg ethanol (8% w/v; IP) together with the alcohol dehydrogenase inhibitor pyrazole (1 mmol/kg). Mice in the control groups received equivalent injections of pyrazole in saline. The ethanol level in the chambers was maintained to achieve a blood alcohol content in the mice between 0.15 – 0.2 g/dl, and ethanol levels in the chambers were checked daily using a breathalyzer (Lifeloc FC10 Breath Alcohol Tester, FC10B-05). Following 4 days of CIE exposure, mice underwent 2 days of withdrawal. After the withdrawal period, mice again drank 15% alcohol for 5 days. This pattern of CIE and limited-access drinking was repeated for three cycles (Fig. 1). Previous work has shown that this pattern of intermittent exposure and withdrawal leads to escalation of alcohol intake and the development of dependence (Becker and Lopez, 2004; Lopez and Becker, 2005). No animals had to be excluded from this study because they showed adverse reactions to the CIE treatment and/or alcohol consumption. Our study design did not consider the effects of alcohol drinking by itself (i.e., we did not include CIE-air groups which had access to alcohol during the limited-access period).
Fig. 1.
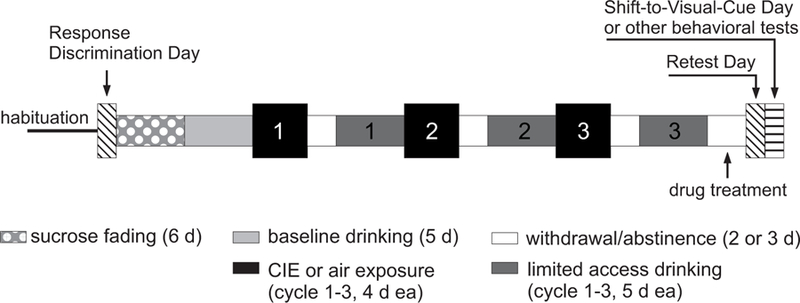
Schematic representation of the study design. Animals used in the attentional set-shifting task were first trained on an egocentric response discrimination task (Response Discrimination Day). Animals in these cohorts were then assigned to treatment groups based on their performance on the response discrimination task to ensure comparable average baseline performance scores. Mice in the chronic intermittent ethanol (CIE) exposed groups were then gradually (sucrose fading) exposed to 15% alcohol in a 2-bottle choice limited access paradigm (daily 2-hr drinking sessions). Mice in the control groups drank only water. After a baseline drinking period of 5 days, mice underwent 3 cycles of CIE exposure in vapor chambers. Control animals were exposed to air in the chambers and only drank water for the duration of the study. Fluid consumption during limited access drinking was measured for all animals over 5 days following each CIE cycle. The last CIE cycle was followed by 3 days of withdrawal or abstinence. During the last 2 days of this period animals in the different treatment groups received twice daily injections of saline, calcium-acamprosate (Ca-AOTA), sodium-acamprosate (Na-AOTA), or calcium chloride (CaCl2), respectively (indicated in the figure as “drug treatment”). Animals received their last injection on the morning of the third day. Animals used for the attentional set-shifting task were then re-tested on the original response discrimination (Retest Day). On the following day (the fourth day of withdrawal), animals in these groups had to acquire a new response strategy (Shift-to-Visual-Cue Day). Testing for all other behavioral tasks (using different cohorts of animals) also started on this day and was performed over three days.
Drug treatment:
Following the last CIE cycle, all animals were treated for two and a half days with one of several drugs (Ca-AOTA, n = 35; CaCl2, n = 32; or Na-AOTA, n = 15) or vehicle (EtOH + saline, n = 45; H2O + saline, n = 15; H2O + Ca-AOTA, n = 21) before behavioral testing took place. All drugs were dissolved in saline (Ca-AOTA 200 mg/kg; CaCl2 73.4 mg/kg; Na-AOTA 200 mg/kg) and injected subcutaneously twice a day, 12 h apart, at a volume of 10 ml/kg for 2 consecutive days and on the morning of the following day 1 h before behavioral testing. Animals that did not receive drug treatment after CIE (or air) treatment were injected with equivalent amounts of saline. The calcium salts used here contained equivalent amounts of Ca2+ ions (0.499 mmol/kg), and the bioavailability and pharmacokinetic profile of the different salt formulations of N-acetylhomotaurinate has been previously verified (Spanagel et al., 2014). Ca-AOTA was a generous gift from Dr. Foster Olive, and Na-AOTA was a generous gift from Dr. Rainer Spanagel.
After the final period of withdrawal and drug treatment, animals performed a series of behavioral tests as described below. Animals used for the attentional set-shifting experiments had to be food restricted and the vast majority of these animals therefore only performed this one task. All other animals were tested on two to three of the other behavioral tasks over 3 days. For these animals, experiments proceeded in the following order: novel object recognition, spontaneous alternation, and social interaction. The focus of our studies was to examine the effect of acamprosate and CaCl2 on alcohol-induced cognitive deficits and therefore, animals did not resume drinking after completion of the task.
Attentional set-shifting:
Procedures followed our previously described protocols (Kroener et al., 2012; Hu et al., 2015). Mice were first food restricted to 85% of their free-feeding weight over 3 weeks, and during this time they were handled for 5 min each day. Mice were then habituated to a plus-maze (each arm was 35 cm long and 10 cm wide, with 14 cm high walls) and trained to seek food rewards (~ 1/16 of a Honey Nut Cheerio) at the end of the arms. On the first day of habituation, 4 reward pellets were placed in each of the arms and mice were allowed to freely explore the maze and to consume the food pellets for 20 min. If a mouse consumed all 16 pellets prior to 20 min, it was briefly removed from the maze while the maze was re-baited. On the second day of habituation, arms were only baited with 2 pellets each (in the middle and at the end of the arms) and mice were again allowed to explore the maze for a total of 20 min. All arms were re-baited whenever a mouse consumed all 8 food pellets before the end of the 20-min period. On the third day of habituation, only 1 food pellet was placed at the end of each arm. To reach habituation criterion, animals were required to consume all 4 food pellets on the maze at least four times within the 20-min period. All animals in this study reached this criterion on the third habituation day. On day four, the maze was converted to a T-maze by placing a barrier into the arm opposite of the stem arm, and the propensity of mice to turn left or right (turn bias) was assessed. In addition, a visual cue (a 13×10 cm plastic sheet with alternating black and white stripes) was placed on the wall of one of the choice arms, close to the entry of the arm. The placement of this cue varied in a pseudorandom manner (see below). After the mouse chose an arm and consumed the reward, it was returned to the stem arm and allowed to make another choice. If the mouse chose the same arm as on the initial choice, it was returned to the stem arm until it chose the other arm and consumed the food pellet. After choosing both arms, the mouse was placed in the holding cage and the barrier was moved to a different arm (labeled North, South, East, and West) to start a new trial. The initial turn that a mouse made during each trial was counted toward its turn bias, and the direction (right or left) that a mouse turned four or more times over 7 trials was considered its turn bias. On day five (Response Discrimination Day), the mice were trained on an egocentric response strategy that required them to always turn to one side (chosen opposite of their previously established turn bias) to obtain the food reward. For each trial, the starting point varied among one of three arms (the North arm was not used as the stem arm) to discourage the use of an allocentric response strategy to locate the reward. The order of the start arms was changed so that the frequency of the arms was balanced across blocks of 12 trials. Training on the egocentric response strategy took place in the presence of the visual cue, which had to be ignored at this stage of the task. Placement of the visual cue into the right or left arm varied pseudorandomly to balance the frequency of occurrences in each arm across blocks of 12 consecutive trials. Mice continued the task until they reached a criterion of nine correct choices over 10 consecutive trials. Once mice reached criterion, they were tested on a probe trial, in which they started from the up until then unused fourth arm (the North arm). If the mice performed the probe trial correctly, the task was complete. If the mice failed, response training was continued until the mice reached a criterion of 4 correct turns over 5 consecutive trials, and then another probe trial was administered. This procedure was continued until the mouse reached the criterion and performed the probe trial correctly. Animals were then divided into treatment groups based on their performance on the Response Discrimination to eliminate any potential baseline performance bias between the groups. Animals then underwent limited-access drinking and CIE procedures as described above. Mice were 160–170 days old at the end of the last alcohol drinking period. After the third drinking cycle, mice underwent 2 days of withdrawal and food restriction (2 g of food per day). During these 2 days they also received their respective drug treatments in the morning and evening. Three days after the last drinking day, animals were re-tested on the response discrimination (Retest Day) to control for potential effects of the CIE and drug treatment on long-term memory. In the next day, animals were then trained to shift their strategy and now always enter the arm that contained the visual cue (Shift to Visual-Cue Day). The criteria for successful completion of the task were identical to those on Response Discrimination and Retest Day. For all the test days, the total number of trials to criterion was analyzed. Errors were scored as entries into arms that did not contain the visual cue, and they were further broken down into three error subtypes to determine whether treatments altered the ability to either shift from the previously learned strategy (perseverative errors), or the ability to maintain the new strategy (regressive or never-reinforced errors). A perseverative error occurred when a mouse made the same egocentric response as required during the Response Discrimination Day, but which was opposite to the direction of the arm containing the visual cue. Six of 12 trials per block required the mouse to respond in this manner. A perseverative error was scored when the mouse entered the incorrect arm on 3 or more trials per block of 4 trials. Errors that followed the pattern of perseverative errors, but did not occur in groups of three or more, were scored as “other errors”. Once the animal made less than three perseverative errors in a block, all subsequent errors were scored as regressive errors, as animal was now following the new strategy at least half of the trials. Finally, all other errors were counted as never-reinforced errors, because the strategy that animals followed on these trials had never been rewarded before.
Novel object recognition:
Procedures followed those previously described (Jeevakumar et al., 2015). Mice were first habituated to an empty open chamber (39 × 19 × 30.5 cm) for 2 days in 10-min sessions. On the third day, the 10-min habituation session was immediately followed by a training trial during which mice were allowed to investigate two wooden objects for 3 min. Animals were then placed into their home cage for 2 min before they were placed back into the testing chamber, which now contained one familiar object from the training trial and one novel wooden object. Animals again were allowed to investigate both objects for 2 min. The objects and the chamber were cleaned with 70% ethanol between all trials, and configurations of the objects during each trial were changed for each animal in all the treatment groups. Trials were video-recorded and the amount of time the mice spent investigating the objects during both the training trial and the novel object trial were recorded and scored offline by three investigators who were blind to the experimental condition of the animals. We report both the total time that animals spend with the familiar and novel object, as well as a “recognition index”, which is obtained by calculating the ratio of time spent investigating the novel object over the total investigation time for both objects.
Delayed spontaneous alternation:
Procedures were adapted from Deacon and Rawlins (2006). Mice were placed into the stem arm of a T-maze and a central partition wedge was placed in between the horizontal goal arms. Mice were left to choose between the left and the right open goal arm. Once a mouse had entered an arm, it was confined to this arm for 30 s by blocking off the entrance of the arm. After 30 s, the barrier was removed and the animal was placed back into the stem arm and allowed to once more choose between the left and right arms. Each animal completed a total of 6 trials with a 20-min interval between trials. The maze was cleaned with 70% ethanol after each trial. Each trial was timed and the direction of the turns was recorded to calculate a percentage of spontaneous alternations between left and right arm entries.
Social interaction:
Experimental mice and two stimulus mice were singly housed for 24 h prior to the task. On test day, the experimental mice were placed in a new cage for 1 h prior to experimentation. A stimulus mouse was placed in a custom cylindrical cage (height 20 cm, steel bars separated by 1 cm, acrylic base and lid) and introduced into the cage of the experimental mouse for 4 consecutive trials, with each exposure lasting 1 min. In between exposures, the stimulus mouse was removed for 10 min. The cylindrical cages were cleaned with 20% ethanol after each exposure. For the fifth exposure, a novel stimulus mouse was introduced into the home cage of the experimental mouse for 1 min. All sessions were video-recorded and interaction times (defined as sniffing and investigating at close proximity) were scored offline by three experimenters who were blinded to the treatment condition of the experimental animal.
Data analysis:
Data were analyzed using one-way analyses of variance (ANOVA), two-way ANOVAs, or a repeated-measure ANOVAs, followed by Bonferroni post hoc tests, using SPSS Statistics 20 (IBM Corp., USA). For all measures the data is presented as mean ± standard error of the mean (SEM). An alpha level of p < 0.05 was considered significant.
Results
Voluntary alcohol consumption in the two-bottle choice paradigm:
Voluntary alcohol consumption during limited-access drinking increased over the course of our CIE-treatment. Individual one-way ANOVAs showed a main effect of time for all CIE groups (CIE + saline: F(3, 176) = 12.111, p < 0.001; CIE + Ca-AOTA: F(3, 136) = 6.275, p < 0.001; CIE + CaCl2: F(3, 124) = 3.223, p = 0.025; CIE + Na-AOTA: F(3, 56) = 20.254, p < 0.001). Post hoc tests showed that alcohol intake was significantly increased following CIE exposure, compared to baseline drinking levels (Fig. 2). In contrast, CIE treatment did not significantly affect water consumption (ml/kg) in the same animals (CIE + saline: F(3, 176) = 0.866, p = 0.460; CIE + Ca-AOTA: F(3, 136) = 0.047, p = 0.986; CIE + CaCl2: F(3, 124) = 0.817, p = 0.487; CIE + Na-AOTA: F(3, 56) = 1.083, p = 0.364). Alcohol intake after the final CIE cycle (CIE 3, Fig. 2) did not differ between the CIE-treated groups (F(3,123) = 0.934, p = 0.427). Thus, our null hypothesis was that all CIE-treated animals would show similar behavioral impairments following the final withdrawal period during which drug treatment with Ca-AOTA, Na-AOTA, and CaCl2, respectively, took place.
Fig. 2.
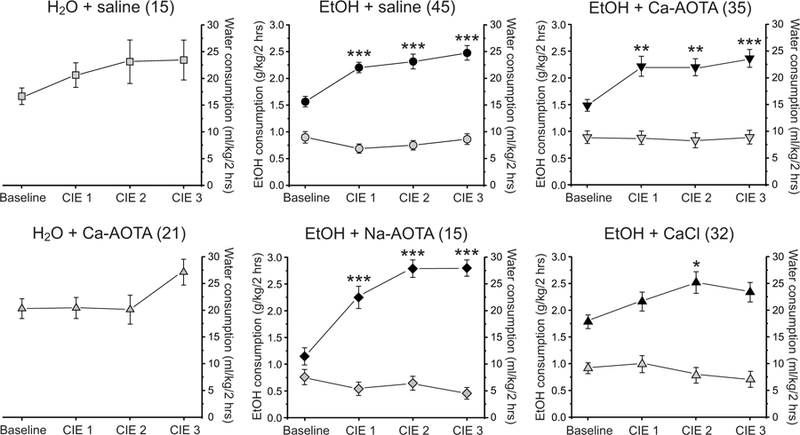
Chronic intermittent ethanol (CIE) treatment increases alcohol intake in the 2-bottle choice paradigm. The average alcohol intake (black symbols; g/kg, left axis) of CIE-treated animals (middle and right columns) was significantly increased following CIE, indicating that CIE treatment induced alcohol dependence in these animals. Water consumption (grey symbols; ml/kg, right axis) was not influenced by CIE treatment in the same animals. Similarly, water consumption of alcohol-naïve control animals (H2O + saline and H2O + Ca-AOTA, left column) remained relatively stable over time and was not systematically affected by air exposure in the vapor chambers. * p < 0.05, **p < 0.01, ***p < 0.001 compared to baseline drinking, following Bonferroni correction.
Attentional set-shifting:
Flexibility in directing cognitive resources is a common index of executive functions, which in rodents are associated with the medial PFC (Birrell and Brown, 2000; Bissonette et al., 2013). We have previously shown that CIE impairs cognitive flexibility in mice (Kroener et al., 2012) and that acamprosate can reverse some of these changes (Hu et al., 2015). Here we used the same attentional set-shifting task, which requires animals to switch from an egocentric strategy to a visual-cue based strategy (Fig. 3A), to test whether subchronic administration of CaCl2 can mimic the effects of acamprosate on cognitive flexibility. Animals were assigned to the treatment groups based on performance on Response Discrimination Day in an effort to eliminate baseline differences between treatment groups before they were exposed to alcohol. After CIE and drug treatment, animals were retested on the same strategy (Retest Day). A two-way ANOVA with the factors drug-treatment and test-day found no main effect of treatment across Response Discrimination Day and Retest Day (F(5,72) = 1.904, p = 0.104). In addition, animals reached criterion on Retest Day faster than during the initial Response Day (F(1,72) = 66.332, p < 0.001). This indicates that CIE exposure did not affect long-term memory of the original training over 54 days of CIE treatment, and that similarly drug treatment did not affect performance of the original response strategy. In contrast, a one-way ANOVA revealed a main effect of drug-treatment on performance on Shift-to-Visual-Cue Day (F(5,72) = 33.925, p < 0.001). Post-hoc tests showed that the CIE + saline group and the CIE + Na-AOTA group required more trials to reach criterion than all other treatment groups. Further analysis revealed that the treatment groups also significantly differed in the total number of errors committed on Shift-to-Visual-Cue Day (F(5,72) = 53.772, p < 0.001, Fig. 3C). When we analyzed the different types of errors committed, there was a significant difference between the treatment groups for all 4 types of errors (perseverative errors: F(5,72) = 8.550, p < 0.001; regressive errors: F(5,72) = 12.957, p < 0.001; never reinforced errors: F(5,72) = 30.665, p < 0.001; other errors: F(5,72) = 6.697, p < 0.001). Both CIE + saline- and CIE + Na-AOTA-treated animals committed a larger number of total errors and made more Perseverative, Regressive, Never Reinforced, and Other errors than any of the other treatment groups. In contrast, CIE + Ca-AOTA- and CIE + CaCl2-treated animals required a comparable number of trials to criterion and committed similar numbers of errors as the alcohol-naive control groups (Fig. 3D). The treatment groups did not differ in the average number of reinforcers (i.e. correct responses resulting in food rewards) obtained during either Response Discrimination or Retest Day (not shown), suggesting that differences in set-shifting performance were not due to the difference in the strength of the association established during training. Importantly, these data suggest that CaCl2, but not Na-AOTA, can mimic the positive effects of Ca-AOTA on attentional deficits that are caused by chronic EtOH exposure.
Fig. 3.
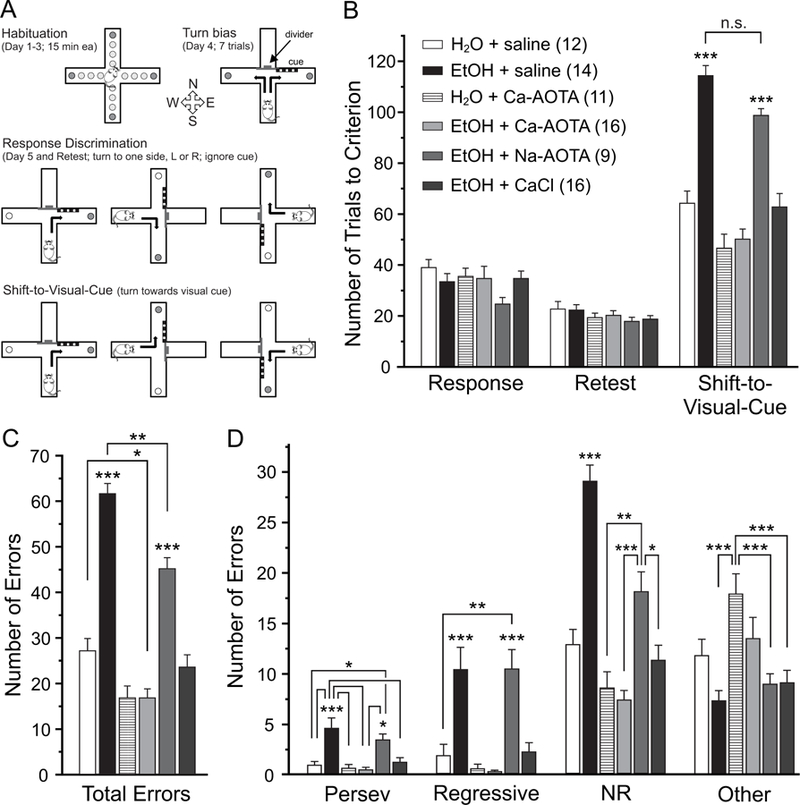
Chronic ethanol exposure impairs attentional set-shifting, which can be reversed by subchronic treatment with calcium-acamprosate (Ca-AOTA) or calcium-chloride (CaCl2), but not sodium acamprosate (Na-AOTA). A) Illustration of the experimental setup and design. Animals were habituated to the maze over 3 days. On the fourth day, animals were tested for an innate preference to turn left or right in the T-maze (Turn bias). On the next day, animals were then trained against their turn bias to perform an egocentric response discrimination in order to retrieve a food reward in one of the two arms of the T-maze. Training took place in the presence of a visual cue that was placed pseudorandomly in a balanced manner into one of the two arms. Animals then underwent 3 cycles of CIE or air exposure followed by limited access drinking over 32 days as outlined in Figure 1. Three days after the last drinking day, animals were re-tested on the original Response Discrimination (Retest day). On the final day, the animals were required to shift their strategy, so that they now always had to turn into the arm that contained the visual cue to obtain the reward (Shift-to-Visual-Cue). B) Chronic ethanol exposure impairs the intra-dimensional shift in an attentional set-shifting task without affecting long-term memory of the original strategy (Retest). Compared to alcohol-naïve control animals, mice in the ethanol group (CIE + saline), and ethanol animals that received Na-AOTA treatment, required more trials to reach criterion during the Shift-to-Visual-Cue Day, suggesting impaired attentional control in ethanol-exposed mice. Ethanol-exposed animals that were treated with either Ca-AOTA or CaCl2 during the last withdrawal period performed on the same level as alcohol-naïve control animals. C and D) Error type analyses showed that alcohol-exposed (CIE + saline and CIE + Na-AOTA) animals had a higher total number of errors than mice in the alcohol-naïve control groups or animals treated with Ca-AOTA or CaCl2. While saline and Na-AOTA treated animals committed more errors of all types, perseverative errors and regressive errors are most indicative of deficits in cognitive flexibility and dysfunctions of the medial PFC. Abbreviations: Persev, perseverative; NR, never reinforced; *p < 0.05, **p < 0.01; ***p < 0.001, following Bonferroni correction. Unless individual comparisons are indicated by lines, the levels of significance apply to comparisons with all other groups.
Novel object recognition:
Next, we tested how CIE affects the ability of mice to distinguish between a familiar and a novel object (Fig. 4A). Following a training phase where animals had the opportunity to investigate two objects, they were then exposed to a novel object and one of the familiar objects from the training phase, and we scored the investigation times for each of the two objects. A two-way mixed ANOVA with drug-treatment as the between-subjects factor and object-investigation time as the within-subjects factor revealed a main effect for the objects (F(1,58) = 106.312, p < 0.001), but no main effect of treatment group (F(5,58) = 1.011, p = .420). The absence of a main effect for treatment group indicates that there was no significant difference in the total object investigation time between groups (Fig. 4B). However, this was qualified by an interaction between object investigation time (object preference) and treatment group (F(5,58) = 4.652, p = 0.001). Further analysis of simple main effects revealed that this interaction was due to the CIE + saline and CIE + Na-AOTA groups, which showed no significant preference for the novel object (Fig. 4c). For each animal, we also calculated a recognition index, which normalized the investigation time for the novel object to a percent of the total investigation time. A one-way ANOVA revealed a main effect of treatment on this recognition index (F(5,58) = 4.187, p = 0.003). Post-hoc comparisons revealed that CIE + saline and CIE + Na-AOTA-treated animals showed significantly lower recognition indices than animals in the other treatment groups. On the other hand, the recognition index of Ca-AOTA- and CaCl2-treated animals did not differ significantly from that of mice in the alcohol-naive control groups, indicating that these treatment were able to prevent the deficits in NOR that result from chronic EtOH exposure.
Fig. 4.
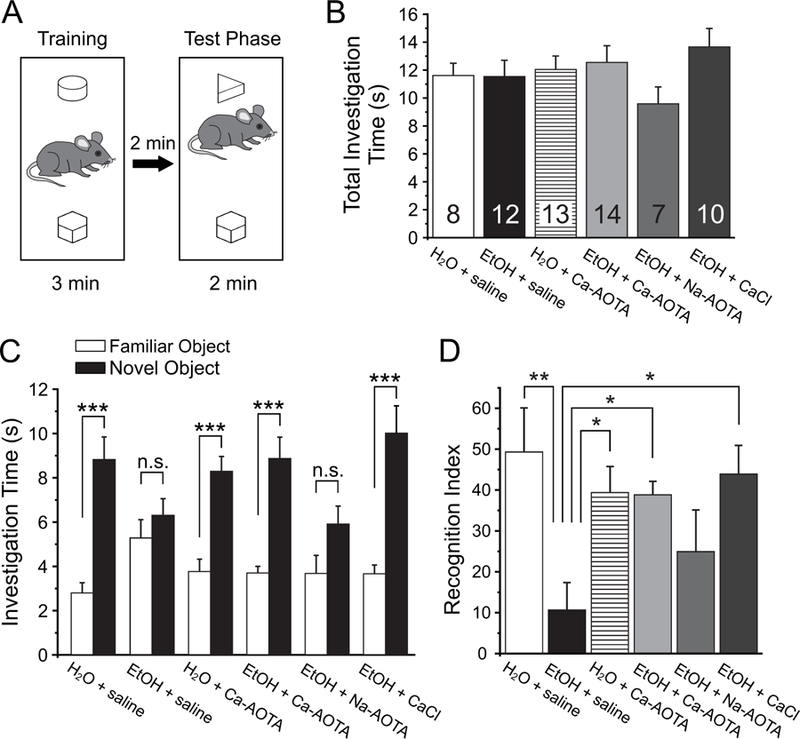
Treatment with calcium-acamprosate (Ca-AOTA) or calcium-chloride (CaCl2) reverses deficits in novel object recognition induced by chronic ethanol exposure. A) Mice were placed in an open box and allowed to investigate two wooden objects for 3 minutes before they were placed back into their home cage for 2 minutes. One of the two objects was replaced with a novel object and animals were allowed to explore both objects for an additional 2 minutes. B) Total investigation time during the training phase did not differ significantly between treatment groups, and animals showed no inherent preferences for any one object. C) During the test phase, animals in all groups spent significantly more time with the novel object, with the exception of mice exposed to EtOH and treated with saline or sodium acamprosate (Na-AOTA), which showed no significant preference for the novel object. D) A recognition index was calculated by dividing the amount of time spent with the novel object over the total time spent investigating both objects. Ethanol exposed animals that were treated with saline scored lower recognition indices, suggesting a deficit in their ability to differentiate between familiar and novel objects. Treatment with Ca-AOTA or CaCl2 prevented these deficits, resulting in recognition indices comparable to those of alcohol-naïve control mice. Significance is indicated as * for p < 0.05, ** for p < 0.01, and *** for p < 0.001, following Bonferroni correction.
Delayed spontaneous alternation:
Next, we assessed alcohol-induced changes in short-term memory and spatial learning by measuring the frequency of spontaneous alternations between entries into a left or right goal-arm during free-choice trials in a modified T-maze (Fig. 5). A one-way ANOVA revealed no significant effect of CIE or drug treatment on delayed spontaneous alternations (F(5,59)= 0.624, p = 0.682), indicating that CIE did not affect short-term memory.
Fig 5.
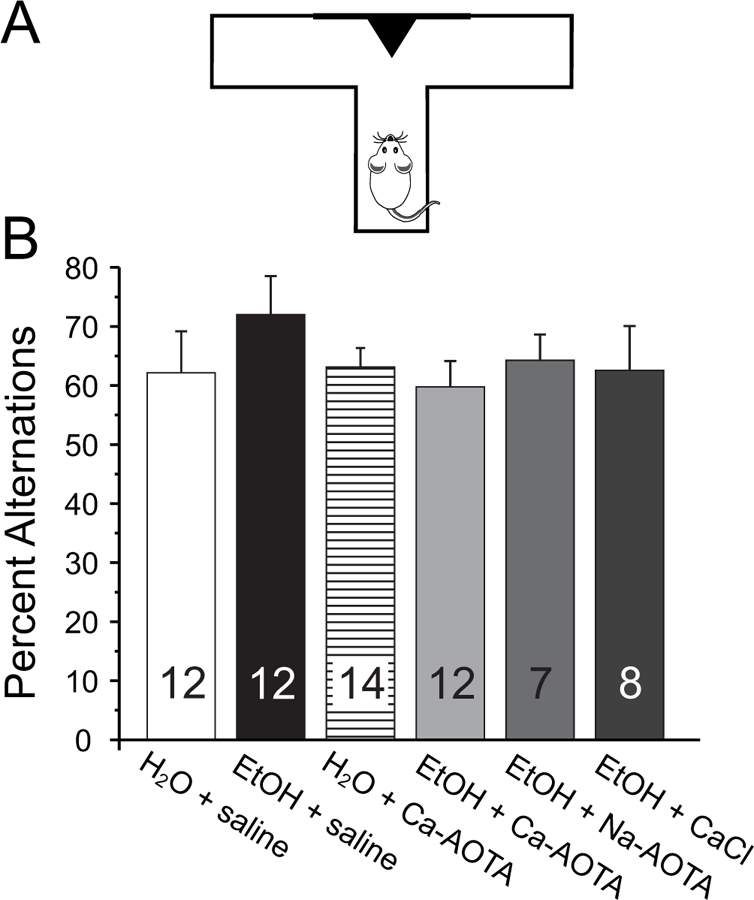
Chronic ethanol exposure (CIE) does not impair normal foraging behavior and short-term memory. A) Animals were placed in the stem arm of a modified T-maze and allowed to enter the left or right arm, respectively. After the choice, animals were confined to that arm for 30 seconds and then placed back immediately into the stem arm for a second free-choice trial. If the animal selected the arm opposite to its initial choice this was considered a spontaneous alternation. Pairs of free-choice runs were repeated for a total of 6 trials, with 20 minutes separating each trial. B) Chronic ethanol exposure did not affect the number of spontaneous alternations compared to alcohol-naïve control animals. Similarly, treatment with calcium-acamprosate (Ca-AOTA) by itself did not affect alternation behavior.
Social interaction:
Finally, we used a social interaction task (Jeevakumar et al., 2015; Phensy et al., 2017) to assess whether CIE or drug treatment alter sociability or affect social recognition memory (Fig. 6A). A two-way mixed ANOVA, with drug-treatment as the between-groups factor revealed that there was a main effect of trial number (F(4,216) = 20.952, p < 0.001), but no main effect of treatment (F(5,54)= 0.515, p = 0.764). Moreover, no significant interaction was found (F(4,216)= 0.794, p = 0.719), suggesting that CIE did not cause a deficit in sociability or social novelty preference.
Fig 6.
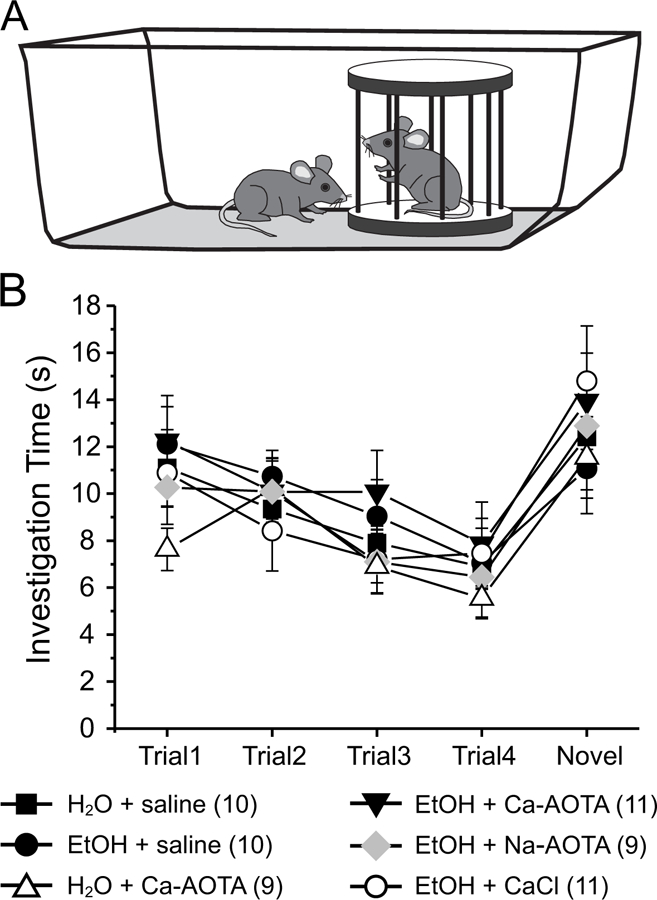
Chronic ethanol exposure (CIE) does not impair normal social investigation patterns. A) Stimulus mice were introduced repeatedly into the home cage of experimental mice in order to test social memory and interaction patterns. Four exposures to the same stimulus mouse (trials 1–4) were followed by presentation of a novel stimulus mouse (trial 5). B) All treatment groups showed normal investigation patterns, which are characterized by a progressive decrease in investigation time with repeated presentation of the initial stimulus mouse. In all groups investigation times increased again in trial 5 upon presentation of the novel stimulus mouse, indicating social recognition memory of the novel mouse.
Discussion
We used an animal model of alcohol dependence to examine the effects of chronic ethanol exposure and acamprosate on cognitive functions. CIE impaired the ability to shift strategies in an attentional set-shifting task and impaired novel object recognition memory, but did not affect delayed spontaneous alternation or measures of social interaction and novelty detection. As previously shown, subchronic application of Ca-AOTA improved performance in the attentional set-shifting task (Hu et al., 2015), and it also reversed the deficits in NOR. Most importantly, we show that these effects of Ca-AOTA on CIE-induced behavioral deficits are mimicked by CaCl2, but not by Na-AOTA, raising important questions about the mechanism of action of acamprosate.
Alcohol addiction is characterized by impulsive and compulsive behaviors, such as inappropriate decision making and loss of control over drinking despite negative outcomes. Attentional control and response inhibition are regulated by the PFC (Birrell and Brown, 2000; Floresco et al., 2006; Radke et al., 2017). Accordingly, aberrations in PFC function are related to reduced executive control and poor decision making in alcoholics, forming a vicious cycle that contributes to alcohol abuse and dependence (Moselhy et al., 2001; George and Koob, 2010; Chen et al., 2013). To evaluate the effectiveness of different drugs for the treatment of CIE-induced deficits in executive functions we tested animals on an attentional set-shifting task, which in rodents requires an intact mPFC (Birrell and Brown, 2000; Floresco et al., 2006, 2009; Dalton et al., 2011). Consistent with our previous results (Kroener et al., 2012; Hu et al., 2015), CIE animals treated with saline during the last withdrawal cycle required significantly more trials to reach criterion on Shift-to-Visual-Cue Day. Sodium acamprosate-treated animals showed similar deficits. An analysis of error types furthermore indicated that CIE + saline- and CIE + Na-AOTA-treated animals committed more perseverative errors, indicating a deficit in the ability to shift attention to the new task demands. In addition, CIE + saline- and CIE + Na-AOTA-treated animals also committed more regressive and never-reinforced errors, suggesting that these animals had difficulties to maintain the new strategy once they made the switch. Subchronic treatment with Ca-AOTA or CaCl2 for 3 days during withdrawal improved both overall performance and the number of errors committed. Thus, acamprosate can restore at least some executive functions of the mPFC that are impaired by heavy alcohol use, and this effect is mimicked by CaCl2, but not a formulation of acamprosate that lacks calcium. For the interpretation of this and all our other results, it is important to note that the pharmacokinetic profiles for IP injections of equimolar solutions of Ca-AOTA and Na-AOTA do not differ, and that the amounts of N-acetylhomotaurinate detected in brain tissue are also comparable for both compounds (Spanagel et al., 2014). However, we did not measure calcium concentrations in the brain, and thus, our evidence that calcium affected alcohol-induced changes in behavior by acting directly in the brain remains indirect.
Chronic alcohol consumption and binge drinking also impair declarative memory functions in human patients (Junghans et al., 2009; Parada et al., 2011). In rodents, these memory processes are often assessed via variants of recognition memory tasks (Kinnavane et al., 2015). The process of recognition memory, which describes the ability to distinguish novel from familiar stimuli, is fundamental to an organism’s ability to record events and to guide prospective behavior. In tests which measure spontaneous preference, recognition memory is inferred by the greater lengths of time spent exploring novel rather than familiar stimuli (Ennaceur and Delacur, 1988; Ennaceur, 2010). Chronic and binge-like alcohol exposures have previously been shown to disrupt novel object recognition in both male and female mice (Meunier et al., 2005; Stragier et al., 2015; Golub et al., 2015) and rats (Cippitelli et al., 2010; Zhao et al., 2013). Here, we show that repeated cycles of CIE also impair object-based short-term memory, and that both Ca-AOTA and CaCl2, but not Na-AOTA, improve these deficits. Prolonged, excessive alcohol consumption leads to neurodegeneration in corticolimbic brain structures, including the hippocampus and entorhinal cortex, which together with the mPFC enable object recognition (Cohen and Stackman, 2015; Tanimizu et al., 2017). Together with our current and previous (Hu et al., 2015) data regarding attentional set-shifting, these results suggest that Ca-AOTA and CaCl2 improve cognitive function at least partially by acting on the mPFC and hippocampus.
When exploring their environment, drug-naïve rodents show a strong tendency to alternate entries into the arms of a maze. In order to measure treatment-induced changes in spatial learning and short-term memory, we measured the frequency of spontaneous alternations between entries into a left or right goal-arm during free-choice trials in a T-maze. Impaired performance in the spontaneous alternation task can result from dysfunctions of the mPFC or corticolimbic pathways that lead to behavioral disinhibition (Lalonde, 2002; Phensy et al., 2017), and lower alternation scores have been taken to indicate impairments in spatial working memory (Stefani and Moghaddam, 2005; Castañé et al., 2015; Grayson et al., 2016). In both rats and mice, chronic and subchronic exposures to high doses of ethanol have previously been shown to impair spontaneous alternation behavior (Götesson et al., 2012; Pickering et al., 2015; Vedder et al., 2015; Dominguez et al., 2016). In our current study we found no significant ethanol-induced changes in spontaneous alternation; however, the reasons for this discrepancy are not clear. In rats, even a relatively brief period of ethanol injections (2.5g/kg for 5 days) induces deficits in continuous (10 min) alternation behavior in a plus-maze (Götesson et al., 2012; Pickering et al., 2015). In C57/BL6 mice, similar alternation deficits were also observed in a T-maze when brief (30 s) delays separated individual choice trials (Dominguez et al., 2016). However, while the study by Dominguez and coworkers (2016) is similar to ours in the choice of animal model and the experimental procedure used for testing spontaneous alternation, mice in the latter study also exclusively drank alcohol (12% v/v) for 6 months (compared to 2 h choice sessions or CIE for a total of 32 days in our study). Thus, it is likely that the differences between our current data and the aforementioned previous studies reflect either species differences or the duration of alcohol exposure.
It is well known that alcohol consumption facilitates interactions with peers and alleviates anxiety in humans (Varlinskaya and Spear, 2002; Kirchner et al., 2006); however, symptoms of alcohol withdrawal also include dysfunctional social and aggressive behavior (Overstreet et al., 2002; Varlinskaya and Spear 2004; Broadwater et al., 2011). Similarly, in rodent models of social interaction, acute ethanol administration at low doses facilitates social interaction (Nadal et al., 1993; Varlinskaya and Spear, 2009; Lopez-Cruz et al., 2016), but dose-related decrements in social interaction and recognition have also been observed after high doses of alcohol (Lister and Hilakivi, 1988; Hilakivi et al., 1989; Lopez-Cruz et al., 2016). Heavy EtOH consumption and deprivation can cause a hyperexcitable state that encompasses hypermotility (Poldrugo and Snead, 1984) and impaired social interactions between animals during EtOH withdrawal (File et al., 1989), and these effects can be augmented by repeated withdrawal cycles (Overstreet et al., 2002; Hwa et al., 2015). Persistent deficits in social behavior and social recognition memory are also major negative consequences of ethanol exposure during prenatal and early postnatal development in rodent models (Kelly et al., 1997; Hamilton et al., 2014; Diaz et al., 2016; Rodriguez et al., 2016; Bird et al., 2017), and these deficits are related to structural changes in the frontal cortex (Hamilton et al., 2010). Here, we used a social approach test which allows the controlled measure of social interactions initiated by the test mouse, providing a replicable measure of sociability in mice. In addition, the repeated presentation of the first stimulus mouse, and a novel mouse, respectively, allows for a multimodal measure of social recognition memory (Moy et al., 2004; Jeevakumar et al., 2015; Phensy et al., 2017). Our results indicate that three cycles of CIE and 2-h daily sessions of voluntary alcohol consumption in the two-bottle choice paradigm do not induce long-lasting changes in social approach behavior or social recognition memory, as the behavioral patterns and overall interaction times did not significantly differ between our treatment groups.
Alcohol produces a variety of (non-specific) actions in the brain, and current FDA-approved therapies for alcohol dependence target several different systems. Meta-analyses have concluded that both acamprosate and the opioid receptor blocker naltrexone (Revia, Vivitrol) can significantly reduce heavy drinking and promote abstinence relative to placebo in both men and women, with better outcomes typically seen for acamprosate (Kranzler and Gage, 2008; Mason and Lehert, 2012; Maisel et al., 2013; but see Anton et al., 2006; Mason et al., 2006). Acamprosate is a synthetic GABA analog and presumed functional NMDA antagonist (see below). Synaptic plasticity of the glutamatergic system has been implicated in almost all stages of alcohol abuse and dependence, from the initiation and maintenance of alcohol consumption to reinstatement of alcohol-seeking behavior and relapse to alcohol use. Chronic alcohol abuse produces a hyper-glutamatergic state, characterized by elevated extracellular glutamate and altered glutamate receptors and transporters (Dahchour et al., 1998; Rao et al., 2015). Measures of central glutamate in humans are reduced across time when acamprosate therapy is initiated at the onset of alcohol abstinence (Umhau et al., 2010). Similarly, acamprosate can decrease EtOH preference in rats and suppress the elevated glutamatergic tone during repeated withdrawal (Spanagel et al., 1996; Dahchour et al., 1998). Although its precise mechanisms of action are still unknown (Reilly et al., 2008; Kiefer and Mann, 2010; Spanagel et al., 2014), evidence suggests that acamprosate restores the balance between excitatory and inhibitory amino acid neurotransmission that gets disrupted by chronic alcohol consumption (Al-Qatari et al., 1998; Rammes et al., 2001; Yahn et al., 2013). Specifically, it has been suggested that acamprosate can attenuate the hyper-glutamatergic states that occur during early withdrawal by modulating transmission at both NMDA and mGluR5 glutamate receptors, and by augmenting intracellular calcium release (Zeise et al., 1990, 1993; Al-Qatari et al., 1998; Naassila et al., 1998; Rammes et al., 2001; Harris et al., 2002; Dahchour and De Witte, 2003; DeWitte et al., 2005; Spanagel et al., 2005; Reilly et al., 2008; Mann et al., 2008; Kiefer and Mann, 2010). However, a recent report found strong evidence that the modulatory effects of acamprosate on drinking behavior are in fact not due to a direct interaction with NMDARs, but instead can be mimicked by elevations in calcium levels alone (Spanagel et al., 2014). Here we tested this idea further by exploring the effects of calcium acamprosate (Ca-AOTA) on a variety of cognitive behaviors that might be altered by chronic ethanol exposure, and we compared these effects with those of equivalent doses of CaCl2. As outlined above, CaCl2, but not Na-AOTA, was equally effective in reversing CIE-induced cognitive deficits as Ca-AOTA, lending further support to the claim that calcium is the active moiety in acamprosate. A recent report that tested patients with high alcohol intake also found lowered plasma calcium concentrations in that population and specifically in those patients with increased craving as a risk factor for relapse. These changes were associated with lowered calcitonin and vitamin D concentrations in the high-risk population (Schuster et al., 2017). Alterations in vitamin D levels and bone density (which reflect deficits in calcium homeostasis) have long been noted in alcoholic patients (Luisier et al., 1977; Lopez-Larramona et al., 2013). How altered calcium homeostasis affects behavior, and specifically the cognitive functions that we tested here, is still unclear, but ethanol can alter intracellular calcium levels and affect voltage and receptor-operated calcium channels, as well as G protein-mediated calcium responses that regulate neuronal excitability and glutamatergic transmission (Catlin et al., 1999; Mulholland et al., 2011; Varodayan et al., 2017). Despite the clear need for more mechanistic studies, our finding that calcium improves cognitive function and attentional control in alcohol-dependent animals supports the implication of earlier preclinical studies (O’Brien, 1952, 1964; Spanagel et al., 2014; Schuster et al., 2017), which indicated that calcium supplementation might provide a useful intervention for decreasing craving and relapse in alcohol-dependent subjects.
Acknowledgments
Funding: NIAAA 1RO3AA023268 (SK)
References:
- Al-Qatari M, Bouchenafa O, Littleton J (1998) Mechanism of action of acamprosate. Part II. Ethanol dependence modifies effects of acamprosate on NMDA receptor binding in membranes from rat cerebral cortex. Alcohol Clin Exp Res 22:810–14. [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A; COMBINE Study Research Group (2006) Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 295(17):2003–17. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF (2004) Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res 28:1829–38. [DOI] [PubMed] [Google Scholar]
- Bird CW, Barto D, Magcalas CM, Rodriguez CI, Donaldson T, Davies S, Savage DD, Hamilton DA (2017) Ifenprodil infusion in agranular insular cortex alters social behavior and vocalizations in rats exposed to moderate levels of ethanol during prenatal development. Behav Brain Res 320:1–11. doi: 10.1016/j.bbr.2016.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ (2000) Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci 20:4320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Powell EM, Roesch MR (2013) Neural structures underlying set-shifting: roles of medial prefrontal cortex and anterior cingulate cortex. Behav Brain Res 250:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI, Spear LP (2011) Chronic intermittent ethanol exposure in early adolescent and adult male rats: effects on tolerance, social behavior, and ethanol intake. Alcohol Clin Exp Res 35(8):1392–403. doi: 10.1111/j.1530-0277.2011.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañé A, Santana N, Artigas F (2015) PCP-based mice models of schizophrenia: differential behavioral, neurochemical and cellular effects of acute and subchronic treatments. Psychopharmacology 232, 4085–4097. 10.1007/s00213-015-3946-6. [DOI] [PubMed] [Google Scholar]
- Catlin MC, Guizzetti M, Costa LG (1999) Effects of ethanol on calcium homeostasis in the nervous system: implications for astrocytes. Mol Neurobiol 19(1):1–24. [DOI] [PubMed] [Google Scholar]
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A (2013) Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature 496:359–62. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Zook M, Bell L, Damadzic R, Eskay RL, Schwandt M, Heilig M (2010) Reversibility of object recognition but not spatial memory impairment following binge-like alcohol exposure in rats. Neurobiol Learn Mem 94(4):538–46. doi: 10.1016/j.nlm.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SJ, Stackman RW Jr (2015) Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav Brain Res 285:105–17. doi: 10.1016/j.bbr.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahchour A, De Witte P (2003) Effects of acamprosate on excitatory amino acids during multiple ethanol withdrawal periods. Alcohol Clin Exp Res 27:465–70. [DOI] [PubMed] [Google Scholar]
- Dahchour A, De Witte P, Bolo N, Nédélec JF, Muzet M, Durbin P, Macher JP (1998) Central effects of acamprosate: part 1. Acamprosate blocks the glutamate increase in the nucleus accumbens microdialysate in ethanol withdrawn rats. Psychiatry Res 82:107–14. [DOI] [PubMed] [Google Scholar]
- Dalton GL, Ma LM, Phillips AG, Floresco SB (2011) Blockade of NMDA GluN2B receptors selectively impairs behavioral flexibility but not initial discrimination learning. Psychopharmacology 216:525–35. [DOI] [PubMed] [Google Scholar]
- De Witte P (2004) Imbalance between neuroexcitatory and neuroinhibitory amino acids causes craving for ethanol. Addict Behav 29:1325–39. [DOI] [PubMed] [Google Scholar]
- De Witte P, Littleton J, Parot P, Koob G (2005) Neuroprotective and abstinence-promoting effects of acamprosate: elucidating the mechanism of action. CNS Drugs 19:517–37. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Rawlins JN (2006) T-maze alternation in the rodent. Nat Protoc 1(1):7–12. [DOI] [PubMed] [Google Scholar]
- Diaz MR, Mooney SM, Varlinskaya EI (2016) Acute prenatal exposure to ethanol on gestational day 12 elicits opposing deficits in social behaviors and anxiety-like behaviors in Sprague Dawley rats. Behav Brain Res 310:11–9. doi: 10.1016/j.bbr.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez G, Dagnas M, Decorte L, Vandesquille M, Belzung C, Béracochéa D, Mons N (2016) Rescuing prefrontal cAMP-CREB pathway reverses working memory deficits during withdrawal from prolonged alcohol exposure. Brain Struct Funct 221(2):865–77. doi: 10.1007/s00429-014-0941-3. [DOI] [PubMed] [Google Scholar]
- Ennaceur A (2010) One-trial object recognition in rats and mice: methodological and theoretical issues. Behav Brain Res 215(2):244–54. doi: 10.1016/j.bbr.2009.12.036 [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J (1988) A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav Brain Res 31:47–59. [DOI] [PubMed] [Google Scholar]
- Errico AL, King AC, Lovallo WR, Parsons OA (2002) Cortisol dysregulation and cognitive impairment in abstinent male alcoholics. Alcohol Clin Exp Res 26:1198–204. [DOI] [PubMed] [Google Scholar]
- File SE, Baldwin HA, Hitchcott PK (1989) Flumazenil but not nitrendipine reverses the increased anxiety during ethanol withdrawal in the rat. Psychopharmacology (Berl) 98(2):262–4. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT (2006) Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate Set-Shifting. Neuropsychopharmacology 31:297–309. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Zhang Y, and Enomoto T (2009) Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behav Brain Res 204:396–409. [DOI] [PubMed] [Google Scholar]
- George O, Koob GF (2010) Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev 35(2): 232–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND (2011) Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12:652–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub HM, Zhou QG, Zucker H, McMullen MR, Kokiko-Cochran ON, Ro EJ, Nagy LE, Suh H (2015) Chronic alcohol exposure is associated with decreased neurogenesis, aberrant integration of newborn neurons, and cognitive dysfunction in female mice. Alcohol Clin Exp Res 39(10):1967–77. doi: 10.1111/acer.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götesson J, Ericson M, Söderpalm B, Pickering C (2012) Repeated ethanol but not phencyclidine impairs spontaneous alternation behaviour in the Y-maze. Basic Clin Pharmacol Toxicol 110(4):347–52. doi: 10.1111/j.1742-7843.2011.00819.x [DOI] [PubMed] [Google Scholar]
- Grayson B, Barnes SA, Markou A, Piercy C, Podda G, Neill JC (2016) Postnatal phencyclidine (PCP) as a neurodevelopmental animal model of schizophrenia pathophysiology and symptomatology: A review. Curr Top Behav Neurosci 29, 403–428. 10.1007/7854_2015_403. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Akers KG, Rice JP, Johnson TE, Candelaria-Cook FT, Maes LI, Rosenberg M, Valenzuela CF, Savage DD (2010) Prenatal exposure to moderate levels of ethanol alters social behavior in adult rats: Relationship to structural plasticity and immediate early gene expression in frontal cortex. Behav Brain Res 207:290–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DA, Barto D, Rodriguez CI, Magcalas CM, Fink BC, Rice JP, Bird CW, Davies S, Savage DD (2014) Effects of moderate prenatal ethanol exposure and age on social behavior, spatial response perseveration errors and motor behavior. Behav Brain Res 269:44–54. doi: 10.1016/j.bbr.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris BR, Prendergast MA, Gibson DA, Rogers DT, Blanchard JA, Holley RC, Fu MC, Hart SR, Pedigo NW, Littleton JM (2002) Acamprosate inhibits the binding and neurotoxic effects of trans-ACPD, suggesting a novel site of action at metabotropic glutamate receptors. Alcohol Clin Exp Res 26:1779–93. [DOI] [PubMed] [Google Scholar]
- Hilakivi LA, Durcan MJ, Lister RG (1989) Effects of caffeine on social behavior, exploration and locomotor activity: interactions with ethanol. Life Sci 44(8):543–53. [DOI] [PubMed] [Google Scholar]
- Holmes A, Spanagel R, Krystal JH (2013) Glutamatergic targets for new alcohol medications. Psychopharmacol (Berl) 229(3):539–54. doi: 10.1007/s00213-013-3226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Morris B, Carrasco A, Kroener S (2015) Effects of acamprosate on attentional set–shifting and cellular function in the prefrontal cortex of chronic alcohol–exposed mice. Alcohol Clin Exp Res 39(6):953–61. doi: 10.1111/acer.12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS1, Nathanson AJ, Shimamoto A, Tayeh JK, Wilens AR, Holly EN, Newman EL, DeBold JF, Miczek KA (2015) Aggression and increased glutamate in the mPFC during withdrawal from intermittent alcohol in outbred mice. Psychopharmacology (Berl) 232(16):2889–902. doi: 10.1007/s00213-015-3925-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Mu P, Moyer JT, Wolf JA, Quock RM, Davies NM, Hu XT, Schlüter OM, Dong Y (2009) Homeostatic synapse-driven membrane plasticity in nucleus accumbens neurons. J Neurosci 29:5820–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeevakumar V, Driskill C, Paine A, Sobhanian M, Vakil H, Morris B, Ramos J, Kroener S (2015) Ketamine administration during the second postnatal week induces enduring schizophrenia–like behavioral symptoms and reduces parvalbumin expression in the medial prefrontal cortex of adult mice. Behav Brain Res 282C:165–175. doi: 10.1016/j.bbr.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Junghanns K, Horbach R, Ehrenthal D, Blank S, Backhaus J (2009) Chronic and high alcohol consumption has a negative impact on sleep and sleep-associated consolidation of declarative memory. Alcohol Clin Exp Res 33(5):893–7. doi: 10.1111/j.1530-0277.2009.00909.x. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Tran TD (1997) Alcohol exposure during development alters social recognition and social communication in rats. Neurotoxicol Teratol 19:383–9. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Mann K (2010) Acamprosate: How, where, and for whom does it work? Mechanism of action, treatment targets, and individualized therapy. Curr Pharm Des 16:2098–102. [DOI] [PubMed] [Google Scholar]
- Kinnavane L, Albasser MM, Aggleton JP (2015) Advances in the behavioural testing and network imaging of rodent recognition memory. Behav Brain Res 285:67–78. doi: 10.1016/j.bbr.2014.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner TR, Sayette MA, Cohn JF, Moreland RL, Levine JM (2006) Effects of alcohol on group formation among male social drinkers. J Stud Alcohol 67(5):785–93. [DOI] [PubMed] [Google Scholar]
- Koob GF (2000) Animal models of craving for ethanol. Addiction (Abingdon, England), 95 Suppl 2:S73–81. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Gage A (2008) Acamprosate efficacy in alcohol-dependent patients: summary of results from three pivotal trials. Am J Addict 17:70–6. [DOI] [PubMed] [Google Scholar]
- Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, Chandler LJ (2012) Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PloS One 7:e37541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde R (2002) The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev 26, 91–104. 10.1016/S0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- Lister RG, Hilakivi LA (1988) The effects of novelty, isolation, light and ethanol on the social behavior of mice. Psychopharmacology (Berl) 96(2):181–7. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Becker HC (2005) Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (2005) 181: 688–696. doi 10.1007/s00213-005-0026-3 [DOI] [PubMed] [Google Scholar]
- López-Cruz L, San-Miguel N, Bayarri P, Baqi Y, Müller CE, Salamone JD, Correa M (2016) Ethanol and caffeine effects on social interaction and recognition in mice: Involvement of adenosine A2A and A1 receptors. Front Behav Neurosci 10:206. eCollection 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Larramona G, Lucendo AJ, Gonzalez-Delgado L (2013) Alcoholic liver disease and changes in bone mineral density. Rev Esp Enferm Dig 105:609–621. [DOI] [PubMed] [Google Scholar]
- Luisier M, Vodoz JF, Donath A, Courvoisier B, Garcia B (1977) 25-hydroxyvitamin D deficiency with reduction of intestinal calcium absorption and bone density in chronic alcoholism. Schweiz Med Wochenschr 107:1529–1533. [PubMed] [Google Scholar]
- Mann K, Hoffmann S, Pawlak CR (2016) Does acamprosate really produce its anti-relapse effects via calcium? No support from the PREDICT study in human alcoholics. Neuropsychopharmacology 1(3):659–60. doi: 10.1038/npp.2015.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Kiefer F, Spanagel R, Littleton J (2008) Acamprosate: recent findings and future research directions. Alcohol Clin Exp Res 32:1105–10. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Goodman AM, Chabac S, Lehert P (2006) Effect of oral acamprosate on abstinence in patients with alcohol dependence in a double-blind, placebo-controlled trial: the role of patient motivation. J Psychiatr Res 40(5):383–93. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Lehert P (2012) Acamprosate for alcohol dependence: a sex-specific meta-analysis based on individual patient data. Alcohol Clin Exp Res 36:497–508. doi: 10.1111/j.1530-0277.2011.01616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier J, Demeilliers B, Célérier A, Maurice T (2006) Compensatory effect by sigma1 (sigma1) receptor stimulation during alcohol withdrawal in mice performing an object recognition task. Behav Brain Res 166(1):166–76. [DOI] [PubMed] [Google Scholar]
- Moselhy HF, Georgiou G, Kahn A (2001) Frontal lobe changes in alcoholism: a review of the literature. Alcohol and Alcoholism 36:357–68. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW (2009) N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci 12:182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN (2004) Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav 3, 287–302. 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Mulholland PJ, Becker HC, Woodward JJ, Chandler LJ (2011) Small conductance calcium-activated potassium type 2 channels regulate alcohol-associated plasticity of glutamatergic synapses. Biol Psychiatry 69:625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naassila M, Hammoumi S, Legrand E, Durbin P, Daoust M (1998) Mechanism of action of acamprosate. Part I. Characterization of spermidine-sensitive acamprosate binding site in rat brain. Alcohol Clin Exp Res 22:802–9. [PubMed] [Google Scholar]
- Nadal RA, Pallares MA, Ferré NS (1993) The effects of caffeine in the social interaction test and on exploration in rats: comparison with ethanol and clorazepate. Behav Pharmacol 4(5):501–508. [PubMed] [Google Scholar]
- O’Brien CC (1952) Experimental evidence in the treatment of alcoholism by intensive calcium therapy. J Am Osteopath Assoc 51:393–394. [PubMed] [Google Scholar]
- O’Brien CC (1964) Intensive calcium therapy as an initial approach to the psychotherapeutic relationship in the rehabilitation of the compulsive drinker. J Psychol 57:125–129. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR (2002) Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res 26(8):1259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada M, Corral M, Caamaño-Isorna F, Mota N, Crego A, Holguín SR, Cadaveira F (2011) Binge drinking and declarative memory in university students. Alcohol Clin Exp Res 35(8):1475–84. doi: 10.1111/j.1530-0277.2011.01484.x. [DOI] [PubMed] [Google Scholar]
- Parsons OA (1983) Cognitive dysfunction and recovery in alcoholics. Subst Alcohol Actions Misuse 4:175–90. [PubMed] [Google Scholar]
- Phensy A, Duzdabanian HE, Brewer S, Panjabi A, Driskill C, Berz A, Peng G, Kroener S (2017) Antioxidant treatment with N-acetyl cysteine prevents the development of cognitive and social behavioral deficits that result from perinatal ketamine treatment. Front Behav Neurosci 11:106. doi: 10.3389/fnbeh.2017.00106. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering C, Alsiö J, Morud J, Ericson M, Robbins TW, Söderpalm B (2015) Ethanol impairment of spontaneous alternation behaviour and associated changes in medial prefrontal glutamatergic gene expression precede putative markers of dependence. Pharmacol Biochem Behav 132:63–70. doi: 10.1016/j.pbb.2015.02.021. [DOI] [PubMed] [Google Scholar]
- Poldrugo F, Snead OC 3rd (1984) Electroencephalographic and behavioral correlates in rats during repeated ethanol withdrawal syndromes. Psychopharmacology (Berl) 83(2):140–6. [DOI] [PubMed] [Google Scholar]
- Radke AK, Jury NJ, Kocharian A, Marcinkiewcz CA, Lowery-Gionta EG, Pleil KE, McElligott ZA, McKlveen JM Kash TL, Holmes A (2017) Chronic EtOH effects on putative measures of compulsive behavior in mice. Addict Biol 22(2):423–434. doi: 10.1111/adb.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammes G, Mahal B, Putzke J, Parsons C, Spielmanns P, Pestel E, Spanagel R, Zieglgänsberger W, Schadrack J (2001) The anti-craving compound acamprosate acts as a weak NMDA-receptor antagonist, but modulates NMDA-receptor subunit expression similar to memantine and MK-801. Neuropharmacology 40:749–60. [DOI] [PubMed] [Google Scholar]
- Rao PS, Bell RL, Engleman EA, Sari Y (2015). Targeting glutamate uptake to treat alcohol use disorders. Front Neurosci 9:144. doi: 10.3389/fnins.2015.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly MT, Lobo I, McCracken LM, Borghese CM, Gong D, Horishita T, Harris RA (2008) Effects of acamprosate on neuronal receptors and ion channels expressed in Xenopus oocytes. Alcohol Clin Exp Res 32:188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez CI, Magcalas CM, Barto D, Fink BC, Rice JP, Bird CW, Davies S, Pentkowski NS, Savage DD, Hamilton DA (2016) Effects of sex and housing on social, spatial, and motor behavior in adult rats exposed to moderate levels of alcohol during prenatal development. Behav Brain Res 313:233–43. doi: 10.1016/j.bbr.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rourke SB, Grant I (1999) The interactive effects of age and length of abstinence on the recovery of neuropsychological functioning in chronic male alcoholics: a 2-year follow-up study. J Int Neuropsychol Soc 5:234–46. [DOI] [PubMed] [Google Scholar]
- Schuster R, Koopmann A, Grosshans M, Reinhard I, Spanagel R, Kiefer F (2017) Association of plasma calcium concentrations with alcohol craving: New data on potential pathways. Eur Neuropsychopharmacology 27(1):42–47. doi: 10.1016/j.euroneuro.2016.11.007. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, Schreiber S, Matsuda F, Lathrop M, Schumann G, Albrecht U (2005). The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med 11:35–42. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Vengeliene V, Jandeleit B, Fischer WN, Grindstaff K, Zhang X, Gallop MA, Krstew EV, Lawrence AJ, Kiefer F (2014) Acamprosate produces its anti-relapse effects via calcium. Neuropsychopharmacology 39:783–91. doi: 10.1038/npp.2013.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Vengeliene V, Kiefer F (2016) Reply to: Does acamprosate really produce its anti-relapse effects via calcium? No support from the PREDICT study in human alcoholics. Neuropsychopharmacology 41:661–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R1, Hölter SM, Allingham K, Landgraf R, Zieglgänsberger W (1996) Acamprosate and alcohol: I. Effects on alcohol intake following alcohol deprivation in the rat. Eur J Pharmacol 305(1–3):39–44. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B (2005) Transient N-methyl-D-aspartate receptor blockade in early development causes lasting cognitive deficits relevant to schizophrenia. Biol Psych 57:433–436. 10.1016/j.biopsych.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Stragier E, Martin V, Davenas E, Poilbout C, Mongeau R, Corradetti R, Lanfumey L (2015) Brain plasticity and cognitive functions after ethanol consumption in C57BL/6J mice. Transl Psychiatry 5:e696. doi: 10.1038/tp.2015.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A (2000) Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res 24:611–621. [PubMed] [Google Scholar]
- Tanimizu T, Kono K, Kida S (2017) Brain networks activated to form object recognition memory. Brain Res Bull pii: S0361–9230(17)30219–8. doi: 10.1016/j.brainresbull.2017.05.017. [DOI] [PubMed]
- Tedstone D, Coyle K (2004) Cognitive impairments in sober alcoholics: performance on selective and divided attention tasks. Drug Alcohol Depend 75:277–286. [DOI] [PubMed] [Google Scholar]
- Umhau JC, Momenan R, Schwandt ML, Singley E, Lifshitz M, Doty L, Adams LJ, Vengeliene V, Spanagel R, Zhang Y, Shen J, George DT, Hommer D, Heilig M (2010) Effect of acamprosate on magnetic resonance spectroscopy measures of central glutamate in detoxified alcohol-dependent individuals: a randomized controlled experimental medicine study. Arch Gen Psychiatry 67:1069–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP (2002) Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res 26(10):1502–11. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP (2004) Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcohol Clin Exp Res 28(1):40–50. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP (2009) Ethanol-induced social facilitation in adolescent rats: role of endogenous activity at mu opioid receptors. Alcohol Clin Exp Res 33(6):991–1000. doi: 10.1111/j.1530-0277.2009.00920.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, de Guglielmo G, Logrip ML, George O, Roberto M (2017) Alcohol dependence disrupts amygdalar L-type voltage-gated calcium channel mechanisms. J Neurosci 37(17):4593–4603. doi: 10.1523/JNEUROSCI.3721-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedder LC, Hall JM, Jabrouin KR, Savage LM (2015) Interactions between chronic ethanol consumption and thiamine deficiency on neural plasticity, spatial memory, and cognitive flexibility. Alcohol Clin Exp Res 39(11):2143–53. doi: 10.1111/acer.12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-García A, Bechara A, Recknor EC, Pérez-García M (2006) Executive dysfunction in substance dependent individuals during drug use and abstinence: an examination of the behavioral, cognitive and emotional correlates of addiction. J Int Neuropsychol Soc 12:405–15. [DOI] [PubMed] [Google Scholar]
- Wiers CE, Stelzel C, Park SQ, Gawron CK, Ludwig VU, Gutwinski S, Heinz A, Lindenmeyer J, Wiers RW, Walter H, Bermpohl F (2014) Neural correlates of alcohol approach bias in alcohol addiction: the spirit is willing but the flesh is weak for spirits. Neuropsychopharmacology 39:688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölwer W, Burtscheidt W, Redner C, Schwarz R, Gaebel W (2001) Outpatient behaviour therapy in alcoholism: impact of personality disorders and cognitive impairments. Acta Psychiatr Scand 103:30–37. [DOI] [PubMed] [Google Scholar]
- Yahn SL, Watterson LR, Olive MF (2013) Safety and efficacy of acamprosate for the treatment of alcohol dependence. Subst Abuse 6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeise ML, Kasparov S, Capogna M, Zieglgänsberger W (1993) Acamprosate (calciumacetylhomotaurinate) decreases postsynaptic potentials in the rat neocortex: possible involvement of excitatory amino acid receptors. Eur J Pharmacol 231:47–52. [DOI] [PubMed] [Google Scholar]
- Zeise ML, Kasparow S, Capogna M, Zieglgänsberger W (1990) Calciumdiacetylhomotaurinate (CA-AOTA) decreases the action of excitatory amino acids in the rat neocortex in vitro. Prog Clin Biol Res 351:237–42. [PubMed] [Google Scholar]
- Zhao YN, Wang F, Fan YX, Ping GF, Yang JY, Wu CF (2013) Activated microglia are implicated in cognitive deficits, neuronal death, and successful recovery following intermittent ethanol exposure. Behav Brain Res 236(1):270–82. doi: 10.1016/j.bbr.2012.08.052. [DOI] [PubMed] [Google Scholar]
- Zornoza T, Cano MJ, Polache A, Granero L (2003) Pharmacology of acamprosate: an overview. CNS Drug Rev 9:359–74. [DOI] [PMC free article] [PubMed] [Google Scholar]


