Abstract
INTRODUCTION
The impact of adjuvant chemotherapy (ACT) using regimens including gemcitabine and platinum on the improvement of the prognosis of patients with locally advanced upper tract urothelial carcinoma (UTUC) has been recently demonstrated. This study aimed to determine the utility of ACT for patients with locally advanced UTUC in real-world clinical practice and the differences in efficacy among regimens.
METHODS
Of 206 UTUC patients who underwent radical nephroureterectomy, 78 were pathologically diagnosed as T3 or higher and/or had pathologically identified lymph node metastasis; 36 in the ACT group and 42 in the non-ACT group were evaluated for patient background, recurrence, and prognosis. In the ACT group, either cisplatin (GC group, 12 cases) or carboplatin (GCa group, 24 cases) was administered as the platinum agent to be combined with gemcitabine.
RESULT
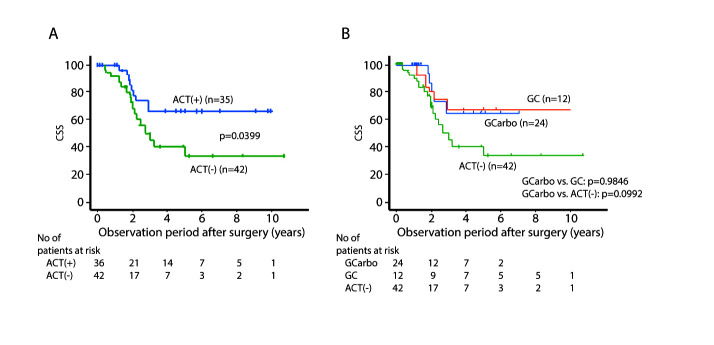
The median patient age in the ACT group and that in the non-ACT group was 71 and 79 years, respectively (p<0.0001). There was no significant difference between these two groups in terms of other patient parameters. The two- and five-year cancer-specific survival (CSS ) and the two- and five-year disease-free survival (DFS) for the ACT group were 81.7%, 66.0%, 60.6%, and 56.6%, respectively, and for the non-ACT group were 68.4%, 40.5%, 42.8%, and 29.3%, respectively (p=0.0399 for CSS and p=0.0814 for DFS). There was no significant difference in CSS and DFS between the GC group and GCa group (p=0.9846 and p=0.9389, respectively).
CONCLUSIONS
In real-world clinical practice in Japan, UTUC patients who receive ACT after radical nephroureterectomy may be expected to have better cancer control than those who do not receive ACT.
INTRODUCTION
Upper tract urothelial carcinoma (UTUC) is a relatively rare malignant disease compared with bladder cancer, and it accounts for 5–10% of urothelial carcinomas.1 Radical nephroureterectomy (RNU) has been a preferred surgical option for UTUC; however, recent findings indicate that high-risk or locally advanced UTUC rapidly progresses again after RNU. Compared with bladder cancer, some UTUC patients show much faster progression, with metastases occurring much earlier.2 The five-year cancer-specific survival (CSS ) is <50% for cases with pT2–3 and <10% for those with pT4.3 Thus, it is important for urologists to identify a therapeutic option that suppresses this rapid progression and to further improve the prognosis of high-risk or locally advanced UTUC.
While neoadjuvant systemic therapy using anti-cancer agents has been performed for muscle-invasive bladder cancer,4 using it for UTUC has been controversial because of the difficulty of accurate preoperative staging diagnosis for primary tumors. Recent randomized control trials (RCTs) have demonstrated that adjuvant chemotherapy (ACT) for improved survival in high-risk urothelial carcinoma (UC). One type of ACT is combination chemotherapy, used for for UTUC,5 and another is immune checkpoint therapy used for both bladder cancer and UTUC.6 It is important to obtain information on the efficacy and safety of ACT, which has been used in real-world clinical practice for some time, to consider appropriate strategies for sequential therapy. Herein, we investigate the utility of ACT for patients with UTUC in real-world clinical practice and the differences in efficacy among regimens.
METHODS
Patients
The Ethics Committee of Kobe City Medical Center West Hospital approved this study (authorization number: 22-019). The medical records of patients who underwent RNU for unilateral UTUC at Kobe City Medical Center West Hospital between January 2009 and December 2019 were retrospectively reviewed. A subgroup analysis of the RCT for ACT after RNU showed that ACT was particularly significant in patients with pT3 or higher,5 and another reported that ACT in patients with pT3 or higher or pN+ reduced the postoperative recurrence.7 Therefore, based on these studies, those who were pathologically diagnosed as T3 or higher and/or had pathologically identified regional lymph node metastasis were included in this study. Clinicopathologic data were obtained from medical records, including age, sex, tumor location, pathological TNM stage, histologic grade, lymphovascular invasion, and information on postoperative ACT.
Followup regimen
All patients were followed up every 3–6 months for at least five years on the basis of a protocol that consisted of urine analysis and chest-abdomen-pelvis computed tomography (CT) scans, cystoscopy, and urinary cytology. If negative, cystoscopy and cytology were repeated every three months for a period of two years, every six months thereafter until five years, and then annually. Disease progression was defined as local failure at the operative site, regional lymph node metastasis, or distant metastasis. Intravesical recurrence was not considered disease progression in this study.
Statistical analysis
Differences in the distribution of variables among groups were evaluated by a Mann Whitney test for continuous variables and Chi-squared test for categorical variables. Disease-free survival (DFS) and CSS probabilities were analyzed using the Kaplan-Meier method, and differences between groups were assessed using log-rank testing. The Cox proportional hazards regression model was used for multivariate analyses. All statistical analyses were conducted using the StatView 5.0 software (Abacus Concepts, Inc., Berkeley, CA, U.S.), and p<0.05 was determined to be statistically significant.
RESULTS
Of 206 UTUC patients who underwent RNU, 78 were pathologically diagnosed as T3 or higher and/or had pathologically identified lymph node metastasis. Of these, 28 had disease recurrence and 36 died because of disease progression. There were neither early postoperative deaths nor severe complications in either the ACT or non-ACT groups. The median of observation period for survivors was 34.3 months.
Thirty-six patients in the ACT group and 42 in the non-ACT group were retrospectively evaluated for patient background, recurrence, and prognosis. In the ACT group, either cisplatin (GC group, 12 cases) or carboplatin (GCa group, 24 cases) was administered as the platinum agent to be combined with gemcitabine. This study cohort consisted of 78 cases undergoing RNU for UTUC and pathologically diagnosed as T3 or higher and/or N positive. Characteristics of the patients are shown in Table 1.
Table 1.
Characteristics of patients
| ACT (−) (n=42) | ACT (+) (n=36) | p | Total (n=78) | |
|---|---|---|---|---|
|
| ||||
| Age (median) | 64–93 (81) | 54–77 (72) | <0.0001 | 54–93 (76) |
|
| ||||
| Sex (%) | ||||
| Male | 18 (42.9) | 23 (63.9) | 0.0637 | 41 (52.6) |
| Female | 24 (57.1) | 13 (36.1) | 37 (47.4) | |
|
| ||||
| Site of tumor (%) | ||||
| Renal pelvis | 20 (47.6) | 14 (38.9) | 0.4383 | 34 (43.6) |
| Ureter | 22 (52.4) | 22 (61.1) | 44 (56.4) | |
|
| ||||
| pT stage (%) | ||||
| 2 | 0 (0) | 1 (2.8) | 0.2770 | 1 (1.3) |
| 3 | 39 (92.9) | 34 (94.4) | 73 (93.6) | |
| 4 | 3 (7.2) | 1 (2.8) | 0.3836 | 4 (5.1) |
|
| ||||
| pN stage (%) | ||||
| Negative | 21 (50.0) | 20 (55.6) | 0.6242 | 41 (52.6) |
| Positive | 7 (16.7) | 10 (27.8) | 17 (21.8) | |
| Unknown | 14 (33.3) | 6 (16.7) | 0.0929 | 20 (25.6) |
|
| ||||
| Grade (%) | ||||
| High (3) | 21 (50.0) | 21 (58.3) | 0.4617 | 42 (53.8) |
| Low (1, 2) | 21 (50.0) | 15 (41.7) | 36 (46.2) | |
|
| ||||
| Hydronephrosis (%) | ||||
| (+) | 28 (66.7) | 26 (72.2) | 0.5961 | 54 (69.2) |
| (− ) | 14 (33.3) | 10 (27.8) | 24 (30.8) | |
|
| ||||
| CKD grade (%) | ||||
| ≤2 | 12 (28.6) | 17 (47.2) | 0.0893 | 29 (37.2) |
| ≥3 | 30 (71.4) | 19 (52.8) | 49 (62.8) | |
|
| ||||
| CRP (mg/dL) (%) | ||||
| <0.3 | 16 (38.1) | 12 (33.3) | 0.6621 | 28 (35.9) |
| ≥ 0.3 | 26 (61.9) | 24 (66.7) | 50 (64.1) | |
|
| ||||
| Type of surgery (%) | ||||
| Laparoscopic/robotic | 35 (83.3) | 31 (86.1) | 0.7346 | 66 (84.6) |
| Open | 7 (16.7) | 5 (13.9) | 12 (15.4) | |
ACT: adjuvant chemotherapy; CKD: chronic kidney disease; CRP C-reactive protein.
The median patient age in the ACT group and that in non-AC group was 71 and 79 years old, respectively (p<0.0001). Regarding other parameters of the patients’ background, there was no significant difference between these two groups. The two- and five-year CSS for the ACT group were 81.7% and 66.0%, respectively, and for the non-ACT group were 68.4% and 40.5%, respectively (p=0.0399) (Figure 1A). The two- and five-year DFS for the ACT group were 60.6% and 56.6%, respectively, and for the non-ACT group were 42.8% and 29.3%, respectively (p=0.0814) (Figure 2A). There was no significant difference in CSS and DFS between the GC group and GCa group (Figures 1B, 2B). Multivariate analysis showed that the absence of ACT was one of the independent predictive factors for a worse CSS , along with lymphovascular invasion (LVI), positive surgical margin, and ureteral primary tumor (Figure 3).
Figure 1.
Cancer-specific survival (CSS) stratified by (A) presence of adjuvant chemotherapy (ACT); and (B) regimen. GC: gemcitabine/cisplatin; GCarbo: gemcitabine/carboplatin.
Figure 2.
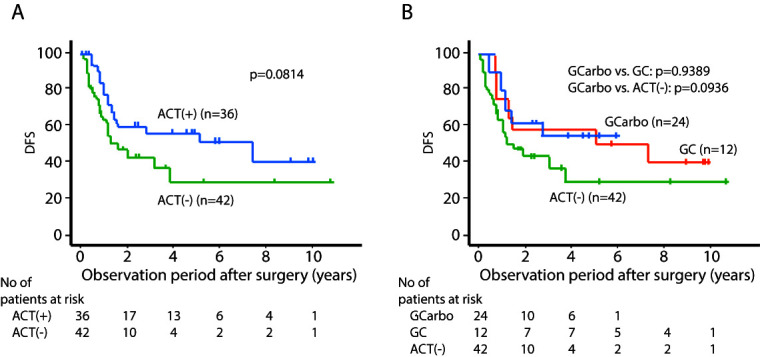
Disease-free survival (DFS) stratified by (A) presence of adjuvant chemotherapy (ACT); and (B) regimen. GC: gemcitabine/cisplatin; GCarbo: gemcitabine/carboplatin.
Adverse events (AEs) in the ACT group are listed in Table 2. Although grade 3 or higher AEs related to bone marrow suppression, such as thrombocytopenia and neutropenia, were relatively common in both regimens, no fatal AEs occurred in any of the patients.
Table 2.
Adverse events of adjuvant chemotherapy
| GC (n=12) | GCa (n=24) | Total (n=36) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any grade | Grade ≤3 | Any grade | Grade ≤3 | Any grade | Grade ≤3 | |||||||
|
| ||||||||||||
| Appetite loss | 2 | (16.7) | 0 | (0) | 1 | (4.2) | 0 | (0) | 3 | (8.3) | 0 | (0) |
|
| ||||||||||||
| Nausea | 0 | (0) | 0 | (0) | 4 | (16.7) | 0 | (0) | 4 | (11.2) | 0 | (0) |
|
| ||||||||||||
| General fatigue | 0 | (0) | 0 | (0) | 3 | (12.5) | 0 | (0) | 3 | (8.3) | 0 | (0) |
|
| ||||||||||||
| Neutropenia | 3 | (25.0) | 1 | (8.3) | 15 | (62.5) | 6 | (25.0) | 18 | (50.0) | 7 | (19.5) |
|
| ||||||||||||
| Thrombocytopenia | 5 | (41.7) | 5 | (41.7) | 14 | (58.3) | 12 | (50.0) | 19 | (52.8) | 17 | (47.2) |
|
| ||||||||||||
| Eruption | 1 | (8.3) | 0 | (0) | 1 | (4.2) | 0 | (0) | 2 | (5.6) | 0 | (0) |
|
| ||||||||||||
| Orthostatic hypotension | 0 | (0) | 0 | (0) | 1 | (4.2) | 0 | (0) | 1 | (2.8) | 0 | (0) |
|
| ||||||||||||
| Diarrhea | 0 | (0) | 0 | (0) | 1 | (4.2) | 0 | (0) | 1 | (2.8) | 0 | (0) |
GC: gemcitabine/cisplatin; GCa: gemcitabine/carboplatin.
DISCUSSION
In this study, we demonstrated that ACT using gemcitabine plus cisplatin or carboplatin improved survival for patients with UTUC who underwent RNU in real-world clinical practice in the Japanese population.
A CheckMate 274 trial demonstrated a longer DFS with adjuvant therapy using nivolumab, and thus, nivolumab is recommended for adjuvant therapy for high-risk UC.6 In addition to conventional platinum-based systemic chemotherapy regimens for advanced UC, a variety of systemic treatment options are now available, including maintenance therapy with avelumab,8 pembrolizumab for chemotherapy-resistant patients,9 and EV for patients who are refractory to these regimens.10 In this context, it is important to evaluate the efficacy of conventional postoperative ACT with platinum-based agents.
According to several retrospective studies, the impact of ACT for UTUC after RNU has remained controversial. Some retrospective studies demonstrated that there was no remarkable difference in postoperative outcome, including overall survival (OS ) and CSS , for patients with high-risk UTUC who underwent RNU regardless of the use of ACT.11–14 Others reported that ACT made no improvement to CSS and OS , while a decrease in intravesical recurrence was shown in patients who received ACT.15,16 On the other hand, there have been several reports that ACT could improve recurrence-free survival (RFS), OS , and/or CSS .17–20 Considering the positive effects of ACT in these studies, it is possible that there may be some relationship between the effect of ACT and worse pathologic factors, such as high tumor staging and positive LVI.
Recently, the POUT study, a RCT designed to compare oncologic outcomes between gemcitabine-platinum combination chemotherapy for ACT and surveillance in UTUC patients, demonstrated that ACT could remarkably improve DFS in locally advanced UTUC patients who underwent with RNU;6 however, only 149 of 709 patients with UTUC were included in this study, so the effect of nivolumab may differ depending on the primary tumor, and the effect of adjuvant nivolumab in patients who have not received neoadjuvant chemotherapy (NAC) is unknown. Furthermore, while NAC for UTUC has the advantage of providing a sufficient dose before surgical loss of renal functioning; the difficulty in accurately evaluating the invasion of UTUC by preoperative imaging may lead to overtreatment, and no large RCTs have demonstrated its usefulness.
While the evidence of GCa therapy as ACT for UTUC has not been established, its non-inferiority has been demonstrated in a subanalysis of the POUT study.6 In our study, consistent with the POUT results, there was no difference in CSS or DFS based on the use of GC or GCa regimen. There was no difference in renal function between the two groups, which may be due to bias in dose reductions and dose interval adjustments made at the discretion of the treating physicians, especially in patients who received GC therapy.
Limitations
There are several limitations to the current study.
Because it is a small, retrospective one, bias due to differences in the decisions or management of each physician is inevitable. We have recommended ACT for patients with pT3 or higher or pN+; however, in many cases, patients did not agree to ACT in the short postoperative period, and in those cases, we only followed up with observation. The backgrounds of the patients in the ACT and non-ACT groups were similar for most items, but the age was significantly lower in the ACT group.
Further, significant results were obtained for CSS , but there was no significant difference in DFS, although the ACT group tended to have slightly better DFS. This is in contrast to past studies, which seem to have found more significant results for DFS than for CSS or OS . The reason for this may be that the ease of introducing ACT is related to the choice and management of sequential therapy after recurrence. Although the effects of each regimen appear to be equivalent, further study with a larger number of cases is essential to confirm our results.
CONCLUSIONS
In real-world clinical practice in Japan, UTUC patients who receive ACT after RNU may be expected to have better cancer control than those who do not receive ACT. Although it needs to be validated in future large RCTs, ACT post-RNU is still considered a useful treatment option, along with nivolumab for those patients who are eligible for it.
Footnotes
COMPETING INTERESTS: The authors do not report any competing personal or financial interests related to this work.
This paper has been peer-reviewed.
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Leow JJ, Chong KT, Chang SL, et al. Upper tract urothelial carcinoma: A different disease entity in terms of management. ESMO Open. 2017;1:e000126. doi: 10.1136/esmoopen-2016-000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.EAU Guidelines. Edn presented at the EAU Annual Congress; Amsterdam. 2022; [Accessed Sept 29, 2023]. Available at: https://uroweb.org/news/new-eau-guidelines. [Google Scholar]
- 4.Kubota Y, Hatakeyama S, Tanaka T, et al. Oncological outcomes of neoadjuvant chemotherapy in patients with locally advanced upper tract urothelial carcinoma: A multicenter study. Oncotarget. 2017;8:101500–8. doi: 10.18632/oncotarget.21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birtle A, Johnson M, Chester J, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomized controlled trial. Lancet. 2020;395:1268–77. doi: 10.1016/S0140-6736(20)30415-3. Epub 2020 Mar 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab vs. placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021;384:2102–14. doi: 10.1056/NEJMoa2034442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bamias A, Deliveliotis C, Fountzilas G, et al. Adjuvant chemotherapy with paclitaxel and carboplatin in patients with advanced carcinoma of the upper urinary tract: A study by the Hellenic Cooperative Oncology Group. J Clin Oncol. 2004;22:2150–4. doi: 10.1200/JCO.2004.09.043. [DOI] [PubMed] [Google Scholar]
- 8.Powles T, Park SH, Voog E, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383:1218–30. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 9.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376:1015–26. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med. 2021;384:1125–35. doi: 10.1056/NEJMoa2035807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellenthal NJ, Shariat SF, Margulis V, et al. Adjuvant chemotherapy for high-risk upper tract urothelial carcinoma: results from the Upper Tract Urothelial Carcinoma Collaboration. J Urol. 2009;182:900–6. doi: 10.1016/j.juro.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Cohen A, Kuchta K, Park S. Neoadjuvant and adjuvant chemotherapy use in upper tract urothelial carcinoma. Urol Oncol. 2017;35:322–7. doi: 10.1016/j.urolonc.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Lee SE, Byun SS, Park YH, et al. Adjuvant chemotherapy in the management of pT3N0M0 transitional cell carcinoma of the upper urinary tract. Urol Int. 2006;77:22–6. doi: 10.1159/000092930. [DOI] [PubMed] [Google Scholar]
- 14.Necchi A, Lo Vullo S, Mariani L, et al. Adjuvant chemotherapy after radical nephroureterectomy does not improve survival in patients with upper tract urothelial carcinoma: A joint study by the European Association of Urology-Young Academic Urologists and the Upper Tract Urothelial Carcinoma Collaboration. BJU Int. 2018;121:252–9. doi: 10.1111/bju.14020. [DOI] [PubMed] [Google Scholar]
- 15.Soga N, Arima K, Sugimura Y. Adjuvant methotrexate, vinblastine, adriamycin, and cisplatin chemotherapy has potential to prevent recurrence of bladder tumors after surgical removal of upper urinary tract transitional cell carcinoma. Int J Urol. 2010;15:800–3. doi: 10.1111/j.1442-2042.2008.02114.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim TS, Oh JH, Rhew HY. The efficacy of adjuvant chemotherapy for locally advanced upper tract urothelial cell carcinoma. J Cancer. 2013;4:686–90. doi: 10.7150/jca.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seisen T, Krasnow RE, Bellmunt J, et al. Effectiveness of adjuvant chemotherapy after radical nephroureterectomy for locally advanced and/or positive regional lymph node upper tract urothelial carcinoma. J Clin Oncol. 2017;35:852–60. doi: 10.1200/JCO.2016.69.4141. [DOI] [PubMed] [Google Scholar]
- 18.Lee KS, Kim KH, Yoon YE, et al. Impact of adjuvant chemotherapy in patients with upper tract urothelial carcinoma and lymphovascular invasion after radical nephroureterectomy. Korean J Urol. 2015;56:41–7. doi: 10.4111/kju.2015.56.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang YC, Chen MF, Shi CS, et al. The efficacy of postoperative adjuvant chemotherapy for patients with pT3N0M0 upper tract urothelial carcinoma. J Urol. 2015;194:323–9. doi: 10.1016/j.juro.2015.03.077. [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa T, Komemushi Y, Kawai T, et al. Efficacy of post-nephroureterectomy cisplatin-based adjuvant chemotherapy for locally advanced upper tract urothelial carcinoma: A multi-institutional retrospective study. World J Urol. 2017;35:1569–75. doi: 10.1007/s00345-017-2032-6. [DOI] [PubMed] [Google Scholar]