Abstract
Cannabis use is rapidly increasing among older adults in the United States, in part to treat symptoms of common health conditions (e.g., chronic pain, sleep problems). Longitudinal studies of cannabis use and cognitive decline in aging populations living with chronic disease are lacking. We examined different levels of cannabis use and cognitive and everyday function over time among 297 older adults with HIV (ages 50–84 at baseline). Participants were classified based on average cannabis use: frequent (> weekly) (n = 23), occasional (≤ weekly) (n = 83), and non-cannabis users (n=191) and were followed longitudinally for up to 10 years (average years of follow-up = 3.9). Multi-level models examined the effects of average and recent cannabis use on global cognition, global cognitive decline, and functional independence. Occasional cannabis users showed better global cognitive performance overall compared to non-cannabis users. Rates of cognitive decline and functional problems did not vary by average cannabis use. Recent cannabis use was linked to worse cognition at study visits when participants had THC+ urine toxicology—this short-term decrement in cognition was driven by worse memory and did not extend to reports of functional declines. Occasional (≤ weekly) cannabis use was associated with better global cognition over time in older adults with HIV, a group vulnerable to chronic inflammation and cognitive impairment. Recent THC exposure may have a temporary adverse impact on memory. To inform safe and efficacious medical cannabis use, the effects of specific cannabinoid doses on cognition and biological mechanisms must be investigated in older adults.
Keywords: Aging, Elderly, Marijuana, Memory, Cognitive decline, HIV/AIDS
Resumen
El consumo de cannabis está aumentando rápidamente entre los adultos mayores en los Estados Unidos, en parte para tratar síntomas de afecciones de salud comunes (p. ej. dolor crónico, problemas de dormir). Actualmente, hay pocos estudios longitudinales sobre el consumo de cannabis y el deterioro cognitivo en poblaciones que envejecen y viven con enfermedades crónicas. Examinamos diferentes niveles del consumo de cannabis y funciones cognitivas a lo largo del tiempo entre 297 adultos mayores con VIH (de 50 a 84 años al principio de la investigación). Los participantes se clasificaron según el consumo promedio de cannabis: consumidores de cannabis frecuentes (> semanal) (n = 23) ocasionales (≤ semanal) (n = 83), y no consumidores de cannabis (n=191) fueron seguidos longitudinalmente hasta por 10 años (promedio = 3,9 años). Los modelos multinivel investigaron los efectos del consumo promedio y reciente de cannabis en la cognición global, el deterioro cognitivo global, y la independencia funcional. Los consumidores ocasionales de cannabis mostraron un mejor rendimiento cognitivo global en comparación con los no consumidores. El nivel de deterioro cognitivo y problemas funcionales no estuvieron asociado con el uso de cannabis. El consumo reciente de cannabis se vinculó con una peor cognición en las visitas del estudio cuando los participantes tenían toxicología de orina de THC positivo–esta disminución a corto plazo de la cognición se debió a una peor memoria, pero no se extendió a los informes de deterioros funcionales. El consumo ocasional (≤ semanal) de cannabis se asoció con una mejor cognición global a lo largo del tiempo en adultos mayores con VIH, un grupo susceptible a la inflamación crónica y la disfunción cognitiva. La exposición reciente al THC puede tener un impacto negativo temporal en la memoria. Los efectos de dosis específicas de cannabinoides en la cognición y sus mecanismos de acción biológicos deben ser investigados en personas mayores con el fin de informar el uso seguro y eficaz del cannabis medicinal.
Introduction
Cannabis use is increasing among older adults faster than any other age group in the United States [1–3]. This trend is expected to continue [4] given the expansion of state-based medical and recreational legalization, shifting cultural attitudes, and the widespread marketing of cannabis products to treat a host of conditions common among aging populations. There is an urgent public health need to identify risks and benefits of cannabis use in older adults, as well as individual-level and cannabis-level factors that may moderate its effects [5]. In particular, there is growing interest in clarifying the influence of cannabis use on cognitive function in older adults living with chronic disease, who are vulnerable to cognitive decline and late-life dementias such as Alzheimer’s disease [6, 7].
Older adults are more likely to use cannabis for medical purposes compared to younger populations [8], and mixed medical/recreational use is also reported [9]. Older cannabis users are more likely to use products with higher cannabidiol (CBD) content and lower Δ9-tetrahydrocannabinol (THC) content compared to younger age groups. Older adults report they use cannabis to ameliorate a range of health conditions common in aging, with those most frequently endorsed including chronic pain, sleep disturbances, weight loss, anxiety, and depression [10]. However, robust evidence to support therapeutic benefit of cannabis products on these conditions is limited [5, 11]. Age-related changes such as slowed metabolism and high rates of polypharmacy may complicate safe and efficacious medical cannabis use, increasing drug sensitivity and risk for drug-drug interactions. Acute sedation and dizziness have been observed in older cannabis users, which could increase fall risk [12]. National survey data show older adults who use cannabis medically are more likely to report functional impairments, while recreational cannabis use did not predict reports of such impairment. Authors hypothesized that functionally-impairing symptoms may have motivated initiation of medical cannabis use, but could not determine whether cannabis use also exacerbated functional problems such as impaired thinking and walking [13].
Recent reviews highlight the scarcity of research that directly examines the effect of late-life cannabis use on cognitive health and brain aging [6, 14, 15]. Only one cross-sectional study (n = 38, ages 60–80) examined current cannabis use and cognitive performance in a healthy older adult cohort, finding no cognitive differences by cannabis use, but analyses were underpowered [16]. Two other cross-sectional studies found no association between heavy cannabis use in adolescence and late-life cognitive performance (n = 50, ages 58–72) [17], or between active cannabis use at age 42 and cognitive performance at age 50 (n = 8,992) [18]. Two mid-life cohort studies have linked current ≥ weekly cannabis use (n = 1,897) and separately, greater cumulative cannabis exposure over adulthood (n = 3,385) to a small decrement in verbal memory performance, which was not considered clinically significant [19, 20]. Longitudinal analyses showed no differences by cannabis use in verbal memory rates of change over eight years [19]; however, there was no evidence of cognitive decline in this mid-life cohort. In contrast to these findings, in small intervention studies (n = 22, n = 11), adults showed improvements in executive functioning after initiation of medical cannabis use [21, 22]. Study findings suggest that medicinal users experience physical symptom relief, improvements in mood and function, and reductions in prescription opioid and benzodiazepine use, which authors hypothesize could contribute indirectly to better cognition [23]. In sum, the current evidence is inadequate to address whether cannabis use is a risk factor for cognitive decline in aging populations, and it appears cannabis may demonstrate divergent effects at specific levels and contexts of use.
Evidence from animal studies and investigation of the human body’s natural endocannabinoid system show that cannabinoids (e.g. chronic low dose THC) demonstrate anti-inflammatory and neuroprotective effects [24, 25]. While there is no research in humans to suggest cannabis compounds decrease risk for cognitive decline, there is interest in examining the therapeutic potential of cannabinoid products on brain function under conditions of high inflammation [26]. For older adults with pro-inflammatory health conditions such as HIV, late-life cannabis use may exert distinct effects on cognition. In HIV, chronic inflammation and immune system activation persist despite virally suppressive antiretroviral therapy (ART), and elevated immune biomarkers have been linked to greater cognitive impairment [27, 28]. Older adults with HIV show steeper rates of cognitive decline compared to their older HIV-negative counterparts [29], and the immunomodulatory properties of cannabis may be particularly beneficial in this context. In vitro studies show activation of cannabinoid receptors attenuates HIV replication in T-cells, microglia, and macrophages [30], while studies in humans link current cannabis use to lower plasma HIV RNA viral load [31] and accelerated HIV DNA decay [32]. In recent HIV studies, including work from our group, current cannabis use has been associated with lower levels of peripheral and central inflammation [33–36], lower rates of global cognitive impairment, and better performance in verbal fluency and learning domains in adults with HIV [37]. However, to our knowledge, no studies have examined the longitudinal impact of current cannabis use on global cognitive decline among older adults with HIV.
The current study sought to describe cannabis user profiles in later-life and examine benefits and risks of current cannabis use on cognitive and everyday function in a longitudinal cohort. First, we identified cannabis use patterns of older users and their demographic, clinical, and HIV disease correlates. In aim 1, we examined three levels of cannabis-cognition relationships: (1a) long-term effects of average cannabis use on overall global cognition and (1b) global cognitive declines; (1c) short-term effects of recent cannabis use on visit-specific cognitive performance. Average cannabis use was characterized by each participant’s pattern of use over study follow-up (between-person effect) and recent cannabis use was characterized by use at each study visit (within-person effect). Given previously observed anti-inflammatory effects of cannabis in HIV, we hypothesized that average occasional or frequent cannabis use over study follow-up would relate to better cognitive function overall and less steep cognitive declines over time compared to no cannabis use. For recent cannabis use, we hypothesized that some measures of heavy, recent use would relate to worse cognitive performance at specific visits. To aid interpretation of global findings, we also explored cognitive domain-specific outcomes. In aim 2, to determine the functional relevance of our findings, we examined the effects of recent and average cannabis use on everyday functioning difficulties and declines over study follow-up. Cognitive domain and functional analyses were exploratory.
Methods
Participants were 297 community-dwelling older adults with HIV who were followed longitudinally with comprehensive assessments every 6–12 months for up to 10 years (average total visits = 3.4, average total years of follow up = 3.9, average years between visits = 1.1). All participants were enrolled in NIH-funded research studies at UC San Diego’s HIV Neurobehavioral Research Program (HNRP). Study design and cohort selection have been described in detail previously [38]. All study procedures were approved by the UC San Diego Institutional Review Board, and all participants provided written, informed consent. Longitudinal study visits took place from 2000 to 2020.
Inclusion criteria for the current study were availability of detailed cognitive, medical, and substance use data at all visits. Exclusion criteria for HNRP studies include diagnosis of a psychotic disorder or a neurological or medical condition that may significantly affect cognitive test results (e.g., schizophrenia, epilepsy). Exclusion criteria for the current analyses were [1] positive drug screening test for non-prescription, addictive substances other than cannabis; [2] report of any substance use disorder in the past year other than cannabis; [3] reported use in the past year of cocaine, methamphetamine, amphetamine, other stimulants, heroin, other opioids, sedatives, hallucinogens, phencyclidine, ketamine, or inhalants.
Demographics.
Demographic information (age, education, sex, race, ethnicity, employment) was collected by self-report. Reading level was assessed with the Wide Range Achievement Test (WRAT) [39].
Cannabis Use.
Cannabis use was characterized at each study visit by self-report and THC-positive (THC+) urine toxicology which was determined by the presence of THC metabolites in urine. Frequency, quantity, and recency of cannabis use were captured via timeline follow-back (TLFB) interview [40]. Average cannabis use over study follow-up and recent cannabis use at each study visit were assessed to characterize long-term and short-term effects of cannabis on outcomes of interest. To define average cannabis use, frequency of cannabis use in the past six months was reported at each study visit and averaged across all visits to create a person-specific longitudinal average cannabis use score. Consistent with prior published longitudinal studies of cannabis use and cognition in mid-life adults [19], we next categorized cannabis users by greater or less than weekly use: frequent cannabis users (2–7 days per week) (n = 23) and occasional cannabis users (once weekly or less) (n = 83). The occasional cannabis user group included a range of older adult cannabis users who used once per week (n = 21, 25.3%), on a monthly basis (n = 27, 32.5%) and less than monthly (n = 35, 42.2%). To assess recent cannabis use, we examined days of cannabis use in the past month, estimated grams of cannabis used in the past month, and THC + urine toxicology. To assess lifetime cannabis use, we examined history of cannabis use disorder.
Cognition.
Participants completed a standardized and validated battery of neuropsychological tests at each study visit, assessing global cognition and seven domains: verbal fluency, executive function, learning, memory/delayed recall, attention/working memory, processing speed, and motor skills [41]. Raw neuropsychological test scores were converted to practice-effect corrected scaled scores (M = 10; SD = 3) and averaged across the entire battery and within cognitive domains to generate global and domain-specific scaled scores, which were used as cognitive trajectory outcome variables [42]. Scaled scores were not adjusted for demographics in order to allow for examination of the influence of age, sex, race/ethnicity, and education on cognitive change over time.
Everyday Function.
Three measures of everyday function were assessed at each study visit, capturing functional status, experience of cognitive difficulties, and declines in independent functioning. Functional status was assessed by a certified neuromedical interviewer via the Karnofsky Scale of Performance, which has been validated in people with HIV [43], and ranges from 0 (death) to 100 (unimpaired/normal functional status). Perceived cognitive difficulties in everyday life were assessed by the Patient’s Assessment of Own Functioning Inventory (PAOFI), a 33-item self-report measure [44]. PAOFI score represents the number of cognitive symptoms in daily life that participants endorsed experiencing “fairly often” or more, and are considered clinically significant. Declines in instrumental activities of daily living (IADLs) were assessed by a modified version of the 1969 Lawton & Brody Scale, a self-report measure in which participants rate their current functioning relative to their previous highest level of functioning on 13 IADL domains (e.g., medication management, transportation, financial management, housekeeping) [45]. The total score is the number of IADL domains for which functional declines and a need for increased assistance are reported.
Clinical and HIV Disease.
Participants completed a comprehensive neuromedical examination, including a structured, clinician-administered questionnaire and collection of blood and urine. Clinical factors assessed included psychiatric, medical, and substance use comorbidities. Current depressive symptoms were assessed by the Beck Depression Inventory (BDI-II) [46]. Diabetes, hypertension, and hyperlipidemia diagnoses were collected by self-report. Alcohol and tobacco use were assessed by TLFB interview and defined by use in the past month. HIV infection was confirmed by enzyme-linked immunosorbent assay with Western blot confirmation, and detailed ART use history was obtained by a standardized questionnaire. Duration of HIV disease was estimated by time since first positive HIV test. Routine clinical chemistry panels, complete blood counts, rapid plasma regain, hepatitis C virus antibody, and CD4 + T cells (flow cytometry) assays were performed. Levels of HIV RNA in plasma were measured using reverse transcriptase-polymerase chain reaction (Amplicor, Roche Diagnostics, Indianapolis, IN), with a lower limit of quantification (LLQ) of 50 copies/ml. HIV RNA concentration was dichotomized as detectable vs. undetectable at the LLQ.
Covariate Selection.
For multi-level models examining cognitive outcomes, demographic, clinical, and HIV disease variables were selected for inclusion as covariates if they differed by cannabis use group at baseline at p < 0.05. Variables selected as covariates using this method were years of education, race/ethnicity, and estimated duration of HIV. Additional covariates were selected if they were associated with average overall global cognition across the study at p < 0.05. Variables selected as covariates using this method were age, years of education, race/ethnicity, diabetes, hypertension, and tobacco use. Across the study, older age, fewer years of education, identifying as Black, Latino, or Other (compared to White), diabetes, hypertension, and use of tobacco products in the past month were associated with worse overall global cognition. To adjust for historical and current HIV disease severity, we included nadir CD4 + T-cell count < 200/μL and current CD4 + T-cell count < 200/μL as covariates.
Statistical analyses.
Group differences on baseline characteristics by cannabis use group were examined using analysis of variance or Wilcoxon tests for continuous variables and χ2 tests for categorical variables. Pairwise comparisons were examined using Tukey’s HSD or chi-square χ2 tests with Bonferroni adjustments. Cannabis use over study follow-up did not predict length of study follow-up (p > 0.05), so data were assumed to be missing at random.
Multi-level models examined: (1a) the long-term effects of average cannabis use on overall global cognition (scaled scores) and (1b) global cognitive declines (i.e., between-person effects); and (1c) the short-term effect of recent cannabis use on visit-specific global cognition (i.e., within-person effect). To accomplish this, we first examined a model that was unadjusted for covariates, and next we examined a model that was adjusted for age, sex, education, race/ethnicity, diabetes, hypertension, tobacco use, estimated duration of HIV, nadir CD4 + T-cell count < 200/μL, and current CD4 + T-cell count < 200/μL. The effect of person-mean centered age was used as a time scale and modeled as a random slope, allowing us to examine average cannabis use as a between-person predictor of global cognitive trajectory for each participant. Between-person effects capture the association of average cannabis use (i.e., overall pattern of cannabis use across study-follow up) on overall cognition (i.e., average cognition over the total study follow up period) and cognitive decline, while within-person effects capture the effect of visit-to-visit variation in cannabis use within an individual and estimate the influence of recent, heavy cannabis use on cognition at a specific time-point. To first identify which metric of within-person cannabis use best predicted variability in global cognition from visit-to-visit, we ran three separate multi-level models each examining a measure of recent cannabis use: days of cannabis use in the past month, grams of cannabis used in the past month, and THC + urine drug screening. Any recent cannabis use measure that was associated with global cognition was included in the study’s primary multi-level model that simultaneously examined the effects of average and recent cannabis use on cognitive outcomes. Random intercepts were specified, and unstandardized model estimates and 95% confidence intervals are reported. Multi-level models were repeated for each cognitive domain to explore domain-specific effects of any relationships observed on global cognition.
To investigate whether cannabis effects extend to everyday functioning, we first examined whether the three recent measures of cannabis use predicted visit-to-visit variability in measures of everyday function (functional status, cognitive difficulties, IADL declines), and separately, whether average patterns of cannabis use predicted overall levels of functional decline over study follow-up.
Results
The study cohort of 297 older adults with HIV had an average baseline age of 56.2 years (range = 50–84). 89% of participants were men, 72% were gay or bisexual, and 62% were White or European American, 19% were Black or African American, 15% were Latino or Hispanic, 1% were Asian American, and 3% were Other. Baseline demographic, clinical, and HIV disease characteristics by cannabis user group are shown in Table 1. Years of follow-up since baseline ranged from 0.5 to 10 years (M = 3.9; SD = 2.8).
Table 1.
Baseline characteristics of older adults with HIV by cannabis use (n = 297)
| Characteristic | No cannabis use n = 191 |
Occasional cannabis use n = 83 |
Frequent cannabis use n = 23 |
F or χ1 (df) | p-value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 56.4 (6.0) | 56.7 (6.1) | 53.8 (4.0) | 2.2 (2, 294) | 0.11 |
| Education (years) | 13.7 (3.0) | 14.9 (2.3) | 13.1 (2.6) | 7.0 (2, 294) | 0.001 |
| Sex (% men) | 81.1% | 91.6% | 87.0% | 4.9 (2) | 0.10 |
| Race/ethnicity | 18.4 (2) | 0.02 | |||
| White | 56.5% | 71.1% | 78.3% | ||
| Black | 20.4% | 15.7% | 13.0% | ||
| Latino | 20.4% | 6.0% | 8.7% | ||
| Asian | 0.5% | 1.2% | 0% | ||
| Other | 2.1% | 6.0% | 0% | ||
| Employed | 30.5% | 33.7% | 34.8% | 0.4 (2) | 0.83 |
| Reading level (WRAT) | 100.1 (15.1) | 103.6 (14.6) | 100.8 (11.9) | 2.1 (2, 277) | 0.13 |
| Clinical | |||||
| Depression symptoms | 9 (3–16) | 7 (4–13) | 12 (4–24) | 1.0 (2, 272) | 0.35 |
| Diabetes | 23.0% | 14.6% | 19.1% | 2.6 (2) | 0.27 |
| Hypertension | 52.4% | 43.9% | 38.1% | 2.7 (2) | 0.25 |
| Hyperlipidemia | 54.0% | 50.0% | 52.4% | 0.4 (2) | 0.83 |
| Hepatitis C Virus | 23.0% | 12.2% | 14.3% | 4.6 (2) | 0.10 |
| Past month tobacco use1 | 13.1% | 16.9% | 20.0% | 2.9 (2) | 0.23 |
| Past month alcohol use1 | 18.3% | 31.3% | 34.8% | 5.4 (2) | 0.07 |
| HIV Disease | |||||
| AIDS Diagnosis | 68.6% | 66.3% | 65.2% | 0.2 (2) | 0.90 |
| Duration of HIV (years) | 15.9 (7.4) | 18.6 (7.7) | 17.5 (8.2) | 3.6 (2, 293) | 0.03 |
| Nadir CD4 + T-cell count | 133 (33–270) | 170 (46–294) | 138 (24–362) | 0.77 (2, 294) | 0.47 |
| Current CD4 + T-cell count | 556 (386–748) | 636 (461–823) | 603 (297–956) | 2.2 (2, 290) | 0.11 |
| ART status (% on) | 91.5% | 92.8% | 87.0% | 0.7 (2) | 0.70 |
| HIV RNA in plasma (% undetectable) | 90.5% | 90.7% | 90.0% | 0.2 (2) | 0.91 |
Data are presented as Mean (SD), Median (IQR), or %
Use in the past month
Cannabis User Profiles.
35.7% of the cohort (n = 106) endorsed cannabis use at some point during study follow-up per self-report over the prior six months at each study visit. 7.7% (n = 23) reported frequent cannabis use (> weekly use) and 28.0% (n = 83) reported occasional cannabis use (≤ weekly use) on average over study follow-up. Baseline cannabis use characteristics of occasional and frequent users are compared in Table 2. At baseline, occasional cannabis users averaged 3.5 days of cannabis use in the past month, with median 0.1 g per day of use. Frequent cannabis users averaged of 26.1 days of use in the past month, with median 0.5 g per day of use. In terms of lifetime cannabis use, 37.5% of occasional users and 65.2% of frequent users had a history of cannabis use disorder. Occasional compared to frequent cannabis users differed significantly by all cannabis use characteristics examined in Table 2 at baseline.
Table 2.
Cannabis use characteristics of occasional and frequent use groups at baseline
| Characteristic | Occasional cannabis use n = 83 |
Frequent cannabis use n = 23 |
F or χ2 (df) | p-value |
|---|---|---|---|---|
| Age of first use | 19.4 (7.0) | 13.6 (4.2) | 14.0 (1, 96) | < 0.001 |
| Total days used1 | 3.5 (7.3) | 26.1 (7.1) | 173.3 (1, 104) | < 0.001 |
| Total grams used1 | 0.3 (0–0.5) | 7.5 (3–15) | 31.9 (1, 104) | < 0.001 |
| Median grams/day1,2 | 0.1 (0–0.2) | 0.5 (0.2–0.5) | 13.6 (1, 104) | < 0.001 |
| Days since last use | 21 (4–98) | 1 (0.9–2) | 12.1 (1, 104) | < 0.001 |
| THC + urine toxicology | 21.3% | 86.4% | 32.2 (1) | < 0.001 |
| History of cannabis use disorder | 37.5% | 65.2% | 5.6 (1) | 0.02 |
Data are presented as Mean (SD) or Median (IQR)
Over the past month;
Per day of cannabis use
Occasional cannabis users had more years of education than non-cannabis users and frequent cannabis users (F (2, 294) = 6.96, p = 0.001). Occasional and frequent cannabis users were more likely to be White than non-users (χ2 [2] = 18.44, p = 0.02). Occasional cannabis users had longer duration of HIV than non-users (F (2, 294) = 3.64, p = 0.03). All other demographic, clinical, and HIV disease characteristics were comparable across cannabis use groups (Table 1).
Short-term Effects of Recent Cannabis Use on Global Cognition.
Days of cannabis use in the past month and grams of cannabis used in the past month were not associated with worse global cognitive performance at each study visit (ps > 0.05). THC + urine toxicology was associated with worse global cognitive performance (b = − 0.33, 95% CI = [−0.59, − 0.07], p = 0.01), indicating that at visits when a participant was THC-positive they performed worse relative to visits when they were THC-negative.
Unadjusted Cannabis Effects on Global Cognition and Global Cognitive Decline
In a multi-level model unadjusted for covariates, at the between-person level, occasional cannabis use and frequent cannabis use were associated with better global cognition compared to the non-user group (occasional: b = 0.77, 95% CI = [0.27, 1.27], p = 0.003; frequent: b = 1.02, 95% CI = [0.14, 1.91], p = 0.02). Rates of global cognitive decline did not differ by cannabis use group (ps > 0.05). At the within-person level, the negative association between a THC-positive urine drug screen and visit-specific cognitive performance remained significant (b = − 0.37, 95% CI = [−0.61, − 0.13], p = 0.002).
Adjusted Cannabis Effects on Global Cognition and Global Cognitive Decline
Results of the multi-level model examining effects of average and recent cannabis use on global cognitive function and global cognitive decline adjusted for covariates are presented in Table 3. Covariates included age, sex, race/ethnicity, education, diabetes, hypertension, tobacco use, HIV disease duration, nadir CD4 + T-cell count < 200/μL, and current CD4 + T-cell count < 200/μL. At the between-person level, occasional cannabis use remained significantly associated with better global cognition compared to the non-user group (b = 0.50, 95% CI = [0.03, 0.96], p = 0.03), while after covariate adjustment, frequent cannabis use was no longer associated with better global cognition (p = 0.50) compared to non-users. The effect of cannabis use group on rates of global cognitive decline remained non-significant (ps > 0.05) (Fig. 1). At the within-person level, the negative effect of a THC-positive urine drug screen remained significant; when participants tested positive for THC, they performed worse cognitively (b = − 0.27, 95% CI = [−0.52, − 0.02], p = 0.03), compared to visits when they did not test positive for THC.
Table 3.
Effects of average and recent cannabis use on global cognition and global cognitive decline among older adults with HIV
| Estimate | SE | p-value | |
|---|---|---|---|
| Between-person level (long-term effects of average use) | |||
| Outcome: Average Global Cognition Scaled Score | |||
| Occasional cannabis use (vs. no use) | 0.50 | 0.24 | 0.03 |
| Frequent cannabis use (vs. no use) | 0.29 | 0.43 | 0.50 |
| Average age | −0.08 | 0.02 | < 0.001 |
| Sex | −0.28 | 0.15 | 0.06 |
| Education | 0.14 | 0.04 | < 0.001 |
| Race/ethnicity | −0.44 | 0.11 | < 0.001 |
| Diabetes | 0.02 | 0.26 | 0.93 |
| Hypertension | −0.48 | 0.23 | 0.04 |
| Tobacco Use | −0.54 | 0.35 | 0.12 |
| Average HIV duration | 0.003 | 0.01 | 0.80 |
| Nadir CD4 + T-cell count < 200/μL | 0.09 | 0.19 | 0.62 |
| Outcome: Global Cognitive Decline | |||
| Slope of global cognition (for non-users) | −0.04 | 0.02 | 0.04 |
| Occasional cannabis use (vs. no use) | 0.02 | 0.03 | 0.52 |
| Frequent cannabis use (vs. no use) | 0.03 | 0.08 | 0.71 |
| Within-person level (short-term effects of recent use) | |||
| Outcome: Visit-to-visit Change in Global Cognition | |||
| THC + urine toxicology | −0.27 | 0.13 | 0.03 |
| Current CD4 + T-cell count < 200/μL | −0.09 | 0.20 | 0.66 |
Fig. 1.
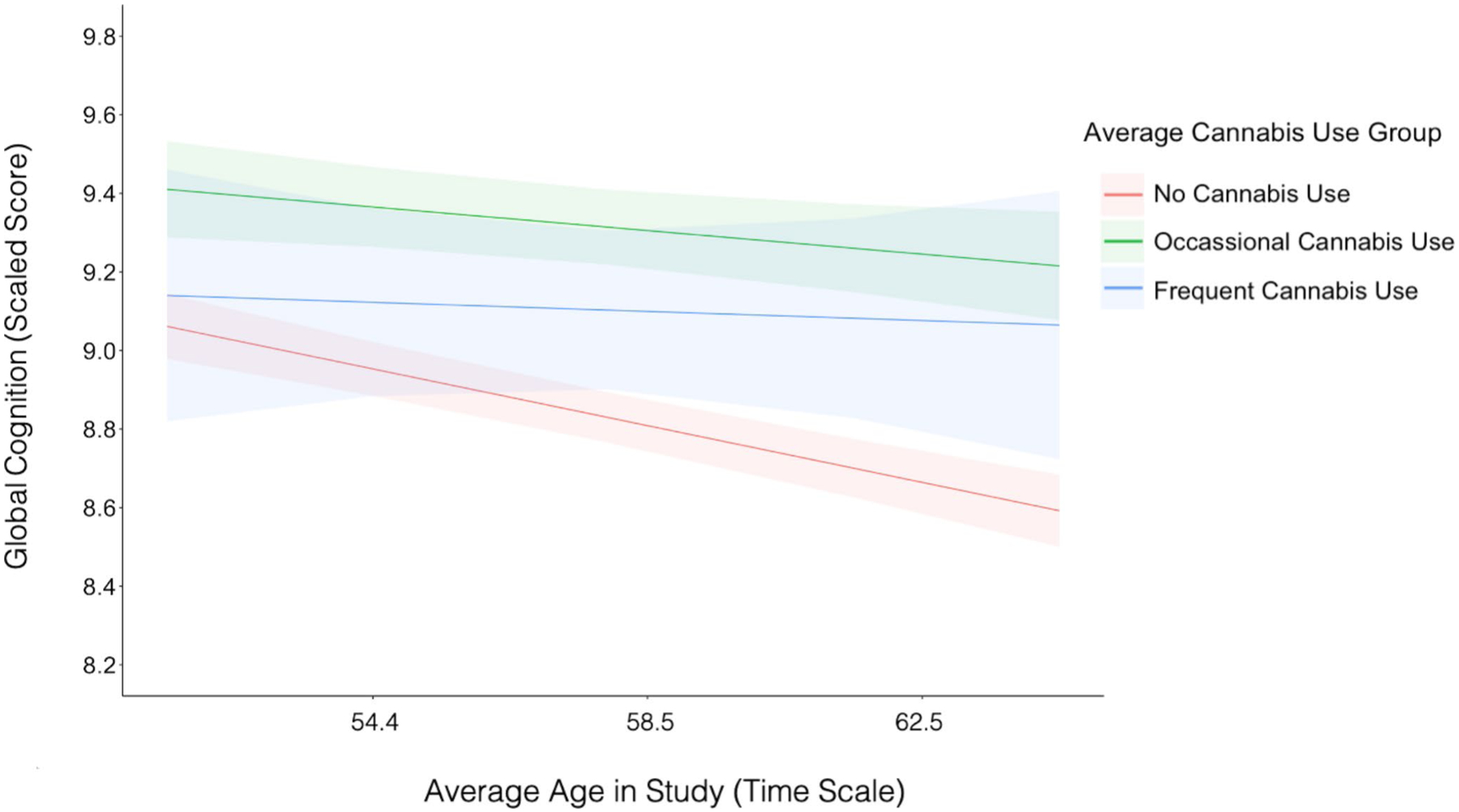
Occasional cannabis users demonstrated better global cognitive performance over time compared to non-users (b = 0.50, p = 0.03). Frequent cannabis users and non-users did not differ in global cognitive performance (b = 0.29, p = 0.50), nor did occasional cannabis users and frequent cannabis users (b = 0.21, p = 0.65). Rates of global cognitive decline did not differ between cannabis use groups (ps > 0.05) with similar trajectories of global cognitive decline between occasional cannabis users and non-users (b = 0.02, p = 0.52), frequent cannabis users and non-users (b = 0.03, p = 0.71), and occasional users and frequent users (b = 0.009, p = 0.91). Notes: Intercepts, slopes, and 95% CI bands were derived from multi-level model estimates
Adjusted Cannabis Effects on Cognitive Domains
Examination of cognitive domain-specific outcomes revealed at the between-person level, that the better average global cognitive performance observed among occasional cannabis users compared to non-users was primarily driven by the domain of attention/working memory (b = 0.82, 95% CI = [0.19, 1.44], p = 0.01), with trend-level associations observed for verbal fluency (b = 0.56, 95% CI = [−0.06, 1.17], p = 0.07) and learning (b = 0.56, 95% CI = [−0.08, 1.20] p = 0.09). At the within-person level, the short-term adverse effect of recent THC use on global cognition at specific study visits was driven by the domain of memory/delayed recall (b = −0.70, 95% CI = [−1.23, − 0.17], p = 0.009). Cannabis use group was not related to rates of decline in any of the seven cognitive domains (ps > 0.05).
Cannabis Effects on Everyday Function
Average patterns of occasional and frequent cannabis use did not predict overall levels of functional status, reported experience of cognitive difficulties, nor IADL declines across study follow-up (ps > 0.05). More days of cannabis use in the past month, however, was associated with greater self-reported cognitive difficulties on the PAOFI (b = 0.07, 95% CI = [0.01, 0.13], p = 0.01) compared to fewer days of cannabis use. Days of cannabis use in the past month was unrelated to functional status and IADL declines at each study visit (ps > 0.05). THC-positiveurine drug screening and grams of cannabis used in the past month were unrelated to visit-to-visit variability in the functional status, cognitive difficulties, and IADL declines (ps > 0.05).
Discussion
In a longitudinal, well-characterized cohort of older adults with HIV, we found that occasional cannabis use in later-life was associated with better overall global cognition compared to no cannabis use, a potentially important finding given this population’s increased vulnerability to cognitive impairment. Further, frequent cannabis use did not relate to worse global cognition over study follow-up. There was no evidence that cannabis use moderates rates of global cognitive decline over the course of six months to 10 years, suggesting that cannabis use within the ranges observed in this study is not a risk factor for early decline in any cognitive domain. Short-term decrements in global cognitive performance were detected at visits when older adults had THC + urine toxicology, but this effect did not extend to reports of functional problems. The putative beneficial effect of average occasional cannabis use across follow-up was driven by better attention, learning, and verbal fluency performance, while the deleterious short-term effect of recent THC exposure was driven by poor memory performance. Together, these results suggest a dynamic relationship between cannabis use and cognition, and domain-specific findings suggest differing mechanisms may underlie the short-term versus longer term cannabis effects observed. Functionally, greater frequency of recent cannabis use was linked to greater self-reported cognitive difficulties, while all other average and short-term cannabis use metrics showed no effect on overall level nor visit-to-visit variability in functional status, self-reported cognitive difficulties, or IADL declines.
The present study provides several novel contributions to the current sparse literature that examines cannabis use and cognitive function among older adults [6, 14, 47]. Occasional and frequent cannabis users did not show higher burden of medical comorbidities such as diabetes or hypertension compared to non-users, nor did occasional or frequent cannabis users show worse historical or current HIV disease outcomes with similar levels of current CD4 + T-cell counts, current ART use, and undetectable plasma HIV RNA. Overall, examination of demographic, clinical, and HIV disease variables showed that later-life cannabis use was not associated with poor health outcomes. However, it is important to note that cannabis users in both the occasional and frequent cannabis user groups endorsed relatively moderate doses of daily cannabis use (as measured by grams/day because we lacked cannabinoid content and potency data). Thus, it is possible that our findings are not generalizable to older adults who have heavier cannabis consumption patterns.
The more acute finding that memory performance was worse on visits when participants had recently used THC is consistent with the notion that commonly observed cannabis-associated deficits in verbal memory [48] are due to the short-term pharmacological effects of THC, and these deficits resolve after short periods of cannabis abstinence (i.e., 25 days) [49, 50]. While worse performance appears to be restricted to periods of greater frequency THC use in our analyses, even a short-term decrement in memory functioning could lead to challenges in everyday functioning such as medication and financial management. However, THC-positive urine toxicology was unrelated to variability in functional outcomes, suggesting these memory decrements may not substantially impair everyday function. An alternative explanation is that our self-report and clinically-rated functioning measures may not be sensitive to subtle changes in functioning as they are proxies of impairment in real-world functioning and subject to reporter bias [51]. Performance-based and direct observation everyday functioning measures may be advantageous to employ in future studies of cannabis use to improve the ecological validity of everyday functioning assessment. Future research is also needed to determine whether the biphasic, age- and dose-dependent effects of THC on memory observed in animal studies (i.e., low dose THC administration improves memory function selectively in older mice, while high dose THC induces memory impairment) translate to older human populations [52, 53]. Such work will be critical for establishing cannabinoid-specific recommendations for older adult medical and recreational use to limit any possible negative cognitive outcomes due to use of THC. Our results also underscore the importance of delineating short-term vs. long-term effects of cannabis on various indicators of cognitive performance, including cognitive variability from visit-to-visit, overall performance over time, and cognitive declines.
The short-term negative association between THC and memory performance did not extend to overall cognitive performance across the study. In fact, a pattern of occasional cannabis use across the study (≤ weekly use) was associated with better global cognitive performance compared to no cannabis use, above and beyond demographic, clinical, and HIV disease predictors of cognition and correlates of cannabis use. This relationship was driven by performance in attention, learning, and verbal fluency, highlighting cognitive domains that are commonly affected in HIV, and are clinically relevant in predicting everyday functioning outcomes [45]. However, in this cohort, occasional cannabis did not predict improved functional outcomes as assessed by interviewer-determined functional status, self-reported cognitive difficulties, or self-reported IADL declines. As noted above, our metrics of everyday function could lack sensitivity to detect cannabis effects.
Our study’s finding that occasional cannabis use is consistently linked to better overall global cognition in older age advances prior cross-sectional studies in adults with HIV which have observed positive associations between past or current cannabis use and cognition [37, 54, 55]. Cross-sectionally, null effects [56–58] and adverse effects of cannabis are also observed, in particular on learning and memory performance, selectively among moderate-to-heavy cannabis users [59] and those with more advanced symptomatic HIV disease [60], indicating that harmful effects may be contingent on specific high levels of use or more severe HIV disease. It is possible that occasional cannabis users may have started with better cognition function as they had on average 1.2 more years of formal education than non-cannabis users. While we adjusted for education in our analyses and reading level, a proxy for educational quality, did not differ by cannabis use group, residual confounding related to educational experiences or other unmeasured sociocultural factors could have influenced our analyses. Any protective relationship observed between cannabis use and cognitive function may be due to the direct effects of cannabis and cannabinoids on cognition (e.g., via mitigation of chronic neuroinflammation and neural injury that contribute to cognitive dysfunction), or indirect effects (e.g., via amelioration of clinical symptoms such as chronic pain or sleep disturbances which can interfere with daily cognitive functioning). Evidence for direct cannabis effects are supported by animal and human studies which show cannabinoids can disrupt and attenuate age-related neuroinflammation [61, 62], and HIV-associated pro-inflammatory processes that are associated with cognitive impairment [36, 63, 64]. It is important to note that heavier cannabis consumption may disrupt endocannabinoid system homeostasis [65], and eclipse any possible neuroprotective effects that cannabis may show in inflammatory conditions. A structural neuroimaging study showed that heavier quantities of cannabis use (grams used in the past month) of cannabis use were associated with smaller entorhinal cortex and fusi-form gyrus volumes in HIV + adults [66]. While many older adults and people with HIV report cannabis use to treat conditions such as insomnia, anxiety, and depression that can negatively impact cognitive function [10, 67], future mechanistic studies are necessary to explore to what degree clinical symptom reductions and/or neuroinflammatory and brain functional or connectivity changes mediate cannabis-cognition relationships.
Cannabis use was unrelated to rates of decline in global cognition in our cohort, suggesting that while cannabis use may positively influence overall cognitive level and/or contribute to maintained cognitive function, its effects do not extend to slowing the process of age-related cognitive decline. While similar analyses that investigate the potential of cannabis products to attenuate or accelerate cognitive declines in older adults with and without HIV are scarce [6], one large longitudinal study of men with and without HIV found current daily and monthly cannabis use were associated with declines in processing speed selectively among men with HIV [68]. The authors note the magnitude of the effect was small and not clinically meaningful. Our study’s cognitive domain specific analyses were inconsistent with this prior study, revealing null results across all seven domains, including processing speed. Our lack of findings on cognitive slope may also be influenced by the younger age distribution of our older adult cohort, with a mean age of 58.8 across all study visits. Older cohorts with greater variability in rates of cognitive decline may increase statistical power to detect cannabis effects on cognitive slope.
Our study has notable strengths and limitations. Our analytic approach was comprehensive in examining both average between-person and short-term within-person cannabis effects on overall cognition, cognitive declines, and visit-to-visit changes in cognitive performance. Further, we took a robust approach to covariate adjustment for demographic, clinical, and HIV disease predictors of cognition and correlates of cannabis use. Lastly, our cannabis use measures were defined both by self-report and urine drug screening, while previous population-based investigations of cannabis use and cognitive function in older adults and adults with HIV relied solely on retrospective self-report which is vulnerable to recall bias [69], and these previous studies could not examine THC-specific effects.
Our results should be interpreted in light of several limitations. While we employed robust statistical methods in a longitudinal cohort, we cannot infer causality based on the observational design of the study. We also cannot discount the possibility that unmeasured third variables underlie the relationships observed. Our study lacked an HIV-negative older adult comparison group, which would enable us to examine whether the cannabis-cognition relationships we observed are restricted to people with HIV or extend to older adults without HIV. Demographically, our older adult sample is predominantly male (89%), majority White (62%), and does not reflect the gender, ethnic, and racial diversity of the national population of people with HIV, which is 22% women and 70% people of color [70]. Future analyses with greater representation of women, and ethnic and racial minorities who are disproportionately affected by the HIV epidemic are needed. While we initially aimed to examine cannabis use in vulnerable older adults, and the age of participants ranged from 50 to 84 years at baseline, the majority of our available cohort may be more appropriately described as middle-aged to older adults. Thus, our findings may not generalize to older adults ages 65 and older, who may be more susceptible to adverse cannabis effects and comprise the fastest growing cannabis user group in the U.S. While we considered the effects of sociocultural (e.g., education) and medical (e.g., diabetes, hypertension) factors on older adults’ cognitive performance, our study was limited by the lack of data on cognitive risk factors related to major medical events such as medical surgeries involving general anesthesia. When characterizing the influence of substance use on cognition (e.g., past month alcohol and tobacco use), we lacked data that could capture frequency and severity of alcohol and tobacco use, which may have underestimated their influences on cognition. Finally, we did not collect detailed information about older adults’ medical or recreational motivations for cannabis use (e.g., specific anxiety, sleep, or pain symptoms medical users may be attempting to treat with cannabis products), the potency and cannabinoid profiles of products used from 2000 to 2020, nor route of administration (e.g., smoking, vaping, edibles, tinctures). We also lacked continuous data characterizing THC concentration, which would have allowed us more variability to assess whether specific levels of THC urine toxicology were related to cognitive outcomes. Detailed characterization of cannabinoid content, direct measurement of cannabis metabolites, and thorough characterization of sociocultural, medical, and substance use covariates are needed in future cannabis research. Specifically, the potential divergent effects on cognition by dose and cannabinoid should be investigated (e.g., high THC vs. high CBD vs. 1:1 THC + CBD profiles, as well as less-studied cannabinoids).
To our knowledge, this study is the first to characterize longitudinal patterns of current cannabis use and global cognitive performance over time in a cohort of older adults with HIV. We found no evidence that cannabis use influences risk for cognitive nor functional decline. Our findings suggest a short-term cognitive risk of THC-based cannabis products on memory performance, but a possible long-term global cognitive benefit from occasional (once weekly or less) later-life cannabis use. Further mechanistic work is needed to probe this positive finding to inform whether cannabinoids show therapeutic potential in treating chronically elevated neuroinflammation and reducing downstream cognitive problems in people with HIV.
Acknowledgements
This work was supported by the National Institute of Health (NIH): (1) the HIV Neurobehavioral Research Center (HNRC) is supported by award P30MH062512 from the National Institute of Mental Health (NIMH); (2) The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) study is supported by awards N01MH22005, HHSN271201000036C, and HHSN271201000030C from NIMH; (3) the California NeuroAIDS Tissue Network (CNTN) is supported by awards U01MH083506, R24MH59745, and U24MH100928 from NIMH; and (4) The Multi-Dimensional Successful Aging Among HIV-Infected Adults Study is supported by award R01MH099987 from the NIMH, and (5) The Translational Methamphetamine AIDS Research Center (TMARC) is supported by awards P50DA026306 and P01DA12065 from the National Institute on Drug Abuse (NIDA). CWW was supported by training grant T32DA031098 from NIDA and training grant T32AG078115 from the National Institute on Aging (NIA). ES was supported by training grant R25MH108389.
Funding
This work was supported by NIH funding. See Acknowledgements for full details.
Footnotes
Code Availability weiming.watson@ucsf.edu.
Conflict of Interest The authors declare no conflicts of interest.
Ethics Approval/Consent to Participate All study procedures were approved by the UC San Diego Institutional Review Board, and all participants provided written, informed consent.
Data Availability
weiming.watson@ucsf.edu.
References
- 1.Kuerbis A, Sacco P, Blazer DG, Moore AA. Substance abuse among older adults. Clin Geriatr Med. 2014;30(3):629–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd SL, Striley CW. Marijuana use among adults 50 years or older in the 21st Century. Gerontol Geriatr Med. 2018;4:2333721418781668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han BH, Palamar JJ. Marijuana use by middle-aged and older adults in the United States, 2015–2016. Drug Alcohol Depend. 2018;191:374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han BH, Palamar JJ. Trends in cannabis use among older adults in the United States, 2015–2018. JAMA Intern Med. 2020;180(4):609–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minerbi A, Häuser W, Fitzcharles M-A. Medical cannabis for older patients. Drugs Aging. 2019;36(1):39–51. [DOI] [PubMed] [Google Scholar]
- 6.Scott EP, Brennan E, Benitez A. A systematic review of the Neurocognitive Effects of Cannabis Use in older adults. Curr Addict Rep. 2019;6(4):443–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sagar KA, Gruber SA. Marijuana matters: reviewing the impact of marijuana on cognition, brain structure and function, & exploring policy implications and barriers to research. Int Rev Psychiatry. 2018;30(3):251–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi NG, DiNitto DM, Marti CN. Nonmedical versus medical marijuana use among three age groups of adults: associations with mental and physical health status. Am J Addictions. 2017;26(7):697–706. [DOI] [PubMed] [Google Scholar]
- 9.Sexton M, Cuttler C, Mischley LK. A survey of cannabis acute effects and withdrawal symptoms: differential responses across user types and age. J Altern Complement Med. 2019;25(3):326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hazekamp A, Ware MA, Muller-Vahl KR, Abrams D, Groten-hermen F. The medicinal use of cannabis and cannabinoids—an international cross-sectional survey on administration forms. J Psychoactive Drugs. 2013;45(3):199–210. [DOI] [PubMed] [Google Scholar]
- 11.National Academies of Sciences E. Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. National Academies Press; 2017. [PubMed] [Google Scholar]
- 12.van den Elsen GA, Ahmed AI, Lammers M, Kramers C, Verkes RJ, van der Marck MA, et al. Efficacy and safety of medical cannabinoids in older subjects: a systematic review. Ageing Res Rev. 2014;14:56–64. [DOI] [PubMed] [Google Scholar]
- 13.Han BH, Le A, Funk-White M, Palamar JJ. Cannabis and prescription drug Use among older adults with functional impairment. American Journal of Preventive Medicine; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinstein G, Sznitman SR. The implications of late-life cannabis use on brain health: a mapping review and implications for future research. Ageing Res Rev. 2020;59:101041. [DOI] [PubMed] [Google Scholar]
- 15.Pocuca N, Walter TJ, Minassian A, Young JW, Geyer MA, Perry W. The Effects of Cannabis Use on Cognitive Function in Healthy Aging: A Systematic Scoping Review. Archives of Clinical Neuropsychology. 2020;Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thayer RE, YorkWilliams SL, Hutchison KE, Bryan AD. Preliminary results from a pilot study examining brain structure in older adult cannabis users and nonusers. Psychiatry Research: Neuroimaging. 2019;285:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burggren AC, Siddarth P, Mahmood Z, London ED, Harrison TM, Merrill DA, et al. Subregional hippocampal thickness abnormalities in older adults with a history of heavy cannabis use. Cannabis and cannabinoid research. 2018;3(1):242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dregan A, Gulliford MC. Is illicit drug use harmful to cognitive functioning in the midadult years? A cohort-based investigation. Am J Epidemiol. 2012;175(3):218–27. [DOI] [PubMed] [Google Scholar]
- 19.McKetin R, Parasu P, Cherbuin N, Eramudugolla R, Anstey KJ. A longitudinal examination of the relationship between cannabis use and cognitive function in mid-life adults. Drug Alcohol Depend. 2016;169:134–40. [DOI] [PubMed] [Google Scholar]
- 20.Auer R, Vittinghoff E, Yaffe K, Künzi A, Kertesz SG, Levine DA, et al. Association between lifetime marijuana use and cognitive function in middle age: the coronary artery Risk Development in Young adults (CARDIA) study. JAMA Intern Med. 2016;176(3):352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gruber SA, Sagar KA, Dahlgren MK, Gonenc A, Smith RT, Lambros AM, et al. The grass might be greener: medical marijuana patients exhibit altered brain activity and improved executive function after 3 months of treatment. Front Pharmacol. 2018;8:983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gruber SA, Sagar KA, Dahlgren MK, Racine MT, Smith RT, Lukas SE. Splendor in the grass? A pilot study assessing the impact of medical marijuana on executive function. Front Pharmacol. 2016;7:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colizzi M, Bhattacharyya S. Does cannabis composition matter? Differential effects of delta-9-tetrahydrocannabinol and cannabidiol on human cognition. Curr Addict Rep. 2017;4(2):62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bilkei-Gorzo A, Albayram O, Draffehn A, Michel K, Piyanova A, Oppenheimer H, et al. A chronic low dose of Δ 9-tetrahydrocannabinol (THC) restores cognitive function in old mice. Nat Med. 2017;23(6):782. [DOI] [PubMed] [Google Scholar]
- 25.Cristino L, Bisogno T, Di Marzo V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat Reviews Neurol. 2020;16(1):9–29. [DOI] [PubMed] [Google Scholar]
- 26.Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456–73. [DOI] [PubMed] [Google Scholar]
- 27.Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun. 2015;45:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, et al. Persistence of hiv – associated cognitive impairment, inflammation and neuronal injury in era of highly active antiretroviral treatment. AIDS. 2011;25(5):625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canizares S, Cherner M, Ellis RJ. HIV and aging: effects on the central nervous system. Semin Neurol. 2014;34(1):27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramirez SH, Reichenbach NL, Fan S, Rom S, Merkel SF, Wang X, et al. Attenuation of HIV-1 replication in macrophages by cannabinoid receptor 2 agonists. J Leukoc Biol. 2013;93(5):801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milloy MJ, Marshall B, Kerr T, Richardson L, Hogg R, Guil-lemi S, et al. High-intensity cannabis use associated with lower plasma human immunodeficiency virus‐1 RNA viral load among recently infected people who use injection drugs. Drug Alcohol Rev. 2015;34(2):135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaillon A, Nakazawa M, Anderson C, Christensen-Quick A, Ellis RJ, Franklin D, et al. Effect of cannabis use on human immunodeficiency virus DNA during suppressive antiretroviral therapy. Clin Infect Dis. 2020;70(1):140–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manuzak JA, Gott TM, Kirkwood JS, Coronado E, Hensley-McBain T, Miller C, et al. Heavy cannabis use associated with reduction in activated and inflammatory immune cell frequencies in antiretroviral therapy–treated human immunodeficiency virus–infected individuals. Clin Infect Dis. 2018;66(12):1872–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rizzo MD, Crawford RB, Henriquez JE, Aldhamen YA, Gulick P, Amalfitano A, et al. HIV-infected cannabis users have lower circulating CD16 + monocytes and IFN-γ-inducible protein 10 levels compared with nonusing HIV patients. Aids. 2018;32(4):419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castro FdO, Silva JM, Dorneles GP, Barros J, Ribeiro CB, Noronha I, et al. Distinct inflammatory profiles in HIV-infected individuals under ART using cannabis, cocaine or cannabis plus cocaine. AIDS (London, England; 2019. [DOI] [PubMed] [Google Scholar]
- 36.Watson CW-M, Campbell LM, Sun-Suslow N, Hong S, Umlauf A, Ellis R et al. Daily Cannabis Use is Associated With Lower CNS Inflammation in People With HIV. Journal of the International Neuropsychological Society. 2021(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson CW-M, Paolillo EW, Morgan EE, Umlauf A, Sundermann EE, Ellis RJ, et al. Cannabis exposure is Associated with a lower likelihood of neurocognitive impairment in people living with HIV. JAIDS J Acquir Immune Defic Syndr. 2020;83(1):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilkinson GS, Robertson GJ. Wide range achievement test 4 (WRAT4). Lutz, FL: Psychological Assessment Resources; 2006. [Google Scholar]
- 40.Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav. 2014;28(1):154. [DOI] [PubMed] [Google Scholar]
- 41.Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26(3):307–19. [DOI] [PubMed] [Google Scholar]
- 42.Cysique LA, Franklin D Jr, Abramson I, Ellis RJ, Letendre S, Collier A, et al. Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change. J Clin Exp Neuropsychol. 2011;33(5):505–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gandhi NS, Skolasky RL, Peters KB, Moxley RT, Creighton J, Roosa HV, et al. A comparison of performance-based measures of function in HIV-associated neurocognitive disorders. J Neurovirol. 2011;17(2):159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chelune GJ, Heaton RK, Lehman RA. Neuropsychological and personality correlates of patients’ complaints of disability. Advances in clinical neuropsychology. Springer; 1986. pp. 95–126. [Google Scholar]
- 45.Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10(3):317–31. [DOI] [PubMed] [Google Scholar]
- 46.Beck AT, Steer RA, Brown GK. Beck depression inventory-II. San Antonio. 1996;78(2):490–8. [Google Scholar]
- 47.Pocuca N, Walter T, Minassian A, Young J, Geyer M, Perry W. The Effects of Cannabis Use on cognitive function in healthy aging: a systematic scoping review. Archives of clinical neuropsychology. the Official Journal of the National Academy of Neuropsychologists; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Broyd SJ, van Hell HH, Beale C, Yücel M, Solowij N. Acute and chronic effects of cannabinoids on human cognition—a systematic review. Biol Psychiatry. 2016;79(7):557–67. [DOI] [PubMed] [Google Scholar]
- 49.Tait RJ, Mackinnon A, Christensen H. Cannabis use and cognitive function: 8-year trajectory in a young adult cohort. Addiction. 2011;106(12):2195–203. [DOI] [PubMed] [Google Scholar]
- 50.Schreiner AM, Dunn ME. Residual effects of cannabis use on neurocognitive performance after prolonged abstinence: a meta-analysis. Exp Clin Psychopharmacol. 2012;20(5):420–9. [DOI] [PubMed] [Google Scholar]
- 51.Schmitter-Edgecombe M, Parsey C, Cook DJ. Cognitive correlates of functional performance in older adults: comparison of self-report, direct observation, and performance-based measures. J Int Neuropsychological Society: JINS. 2011;17(5):853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calabrese EJ, Rubio-Casillas A. Biphasic effects of THC in memory and cognition. Eur J Clin Invest. 2018;48(5):e12920. [DOI] [PubMed] [Google Scholar]
- 53.Sarne Y, Toledano R, Rachmany L, Sasson E, Doron R. Reversal of age-related cognitive impairments in mice by an extremely low dose of tetrahydrocannabinol. Neurobiol Aging. 2018;61:177–86. [DOI] [PubMed] [Google Scholar]
- 54.Kallianpur KJ, Birn R, Ndhlovu LC, Souza SA, Mitchell B, Paul R, et al. Impact of Cannabis Use on Brain structure and function in suppressed HIV infection. J Behav brain Sci. 2020;10(8):344. [PMC free article] [PubMed] [Google Scholar]
- 55.Crook CL, Savin MJ, Byrd D, Summers AC, Guzman VA, Morris EP et al. The neurocognitive effects of a past cannabis use disorder in a diverse sample of people living with HIV. AIDS care. 2020:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang L, Cloak C, Yakupov R, Ernst T. Combined and independent effects of chronic marijuana use and HIV on brain metabolites. J Neuroimmune Pharmacol. 2006;1(1):65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang L, Wang H, Liang H, Ernst T, Oishi K. Microstructural Brain Abnormalities in HIV + Individuals with or without Chronic Marijuana Use. Journal of Neuroinflammation. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Francesco D, Underwood J, Bagkeris E, Boffito M, Post F, Mallon P, et al. Depression, lifestyle factors and cognitive function in people living with HIV and comparable HIV-negative controls. HIV Med. 2019;20(4):274–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thames AD, Mahmood Z, Burggren AC, Karimian A, Kuhn TP. Combined effects of HIV and marijuana use on neurocognitive functioning and immune status. AIDS Care. 2016;28(5):628–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cristiani SA, Pukay-Martin ND, Bornstein RA. Marijuana use and cognitive function in HIV-infected people. J Neuropsychiatry Clin Neurosci. 2004;16(3):330–5. [DOI] [PubMed] [Google Scholar]
- 61.Di Marzo V, Stella N, Zimmer A. Endocannabinoid signalling and the deteriorating brain. Nat Rev Neurosci. 2015;16(1):30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aso E, Ferrer I. Cannabinoids for treatment of Alzheimer’s disease: moving toward the clinic. Front Pharmacol. 2014;5:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Henriquez JE, Bach AP, Matos-Fernandez KM, Crawford RB, Kaminski NE. Δ 9-Tetrahydrocannabinol (THC) Impairs CD8 + T Cell-Mediated Activation of Astrocytes. Journal of Neuroimmune Pharmacology. 2020:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rizzo MD, Crawford RB, Bach A, Sermet S, Amalfitano A, Kaminski NE. Δ9-Tetrahydrocannabinol suppresses monocyte-mediated astrocyte production of Monocyte chemoattractant protein 1 and Interleukin-6 in a toll-like receptor 7–Stimulated Human coculture. J Pharmacol Exp Ther. 2019;371(1):191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rotter A, Bayerlein K, Hansbauer M, Weiland J, Sperling W, Kornhuber J, et al. CB1 and CB2 receptor expression and promoter methylation in patients with cannabis dependence. Eur Addict Res. 2013;19(1):13–20. [DOI] [PubMed] [Google Scholar]
- 66.Thames AD, Kuhn TP, Williamson TJ, Jones JD, Mahmood Z, Hammond A. Marijuana effects on changes in brain structure and cognitive function among HIV + and HIV− adults. Drug Alcohol Depend. 2017;170:120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woolridge E, Barton S, Samuel J, Osorio J, Dougherty A, Hold-croft A. Cannabis use in HIV for pain and other medical symptoms. J Pain Symptom Manag. 2005;29(4):358–67. [DOI] [PubMed] [Google Scholar]
- 68.Okafor CN, Plankey MW, Li M, Chen X, Surkan PJ, Shoptaw S, et al. Association of marijuana use with changes in cognitive processing speed and flexibility for 17 years in HIV-seropositive and HIV-seronegative men. Subst Use Misuse. 2019;54(4):525–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van der Pol P, Liebregts N, de Graaf R, Korf DJ, van den Brink W, van Laar M. Validation of self-reported cannabis dose and potency: an ecological study. Addiction. 2013;108(10):1801–8. [DOI] [PubMed] [Google Scholar]
- 70.Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2010–2015. 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
weiming.watson@ucsf.edu.


