Abstract
Background:
There are limited studies and no surveillance protocols on pituitary dysfunction for adults who underwent anterior skull base radiation.
Methods:
Cross-sectional study of 50 consecutive patients with sinonasal or nasopharyngeal cancer who underwent definitive radiotherapy. The mean radiation doses, prevalence of pituitary dysfunction, and associated factors were calculated.
Results:
Pituitary hormone levels were abnormal in 23 (46%) patients, including 6 (12%) with symptomatic abnormalities requiring treatment. The most common hormonal abnormality was hyperprolactinemia (30%), central hypothyroidism (8%) and central hypogonadism (6%). Patients with abnormal pituitary hormone values received higher mean radiation doses to the pituitary gland (1143 cGy, P = 0.04), pituitary stalk (1129 cGy, P = 0.02), optic chiasm (1094 cGy, P = 0.01), and hypothalamus (900 cGy, P = 0.01).
Conclusions:
Nearly half of the patients had abnormal pituitary function, including over a tenth requiring treatment. There may be a dose-dependent association between hormonal dysfunction and radiation.
Keywords: dysfunction, nasopharyngeal, pituitary, radiation, sinonasal
1 |. INTRODUCTION
The treatment of patients with sinonasal and nasopharyngeal cancers is complex, requiring a multimodality treatment approach. High-dose radiation is a necessary adjunct to achieving locoregional control. The hypothalamic–pituitary (HP) axis is frequently included within the radiation field. Radiation-induced pituitary dysfunction is common and recognized to have a negative impact on quality of life as reported by cancer survivors.1,2 Most data on radiation-induced HP axis dysfunction have been derived from childhood brain cancer survivors and nasopharyngeal cancers from endemic areas in Southeast Asia. Current estimates of pituitary dysfunction in adults after anterior skull base radiation range from 25% to 100%.2–5
The risk of radiation-related endocrine dysfunction for head and neck cancers has been recognized in the National Comprehensive Cancer Network (NCCN) Guidelines.6 This includes a recommendation for thyroid-stimulating hormone testing every 6–12 months to monitor for primary thyroid dysfunction in patients who have undergone radiation of the neck. Likewise, there are recommendations for monitoring pituitary function in childhood brain cancer survivors.7,8 However, there are currently no similar recommendations or protocols for pituitary dysfunction or central endocrine disorders after sinonasal and nasopharyngeal radiation. Further, the relationship between pituitary dysfunction and radiation dose to the pituitary gland and adjacent structures, including the pituitary stalk, optic chiasm, and hypothalamus, are not well understood for adults treated with high-risk radiation therapy.
This study aims to evaluate pituitary dysfunction in a cross-section of patients who previously underwent radiotherapy for sinonasal or nasopharyngeal cancer. We aimed to determine the prevalence of pituitary dysfunction and explore potential associations between HP axis dysfunction and radiotherapy dose to the pituitary gland, pituitary stalk, optic chiasm, and hypothalamus. This study serves to support guideline development for prospective endocrine screening after anterior skull base radiation and further investigation of radiation dose relationships to reduce the risk of HP axis dysfunction.
2 |. MATERIALS AND METHODS
2.1 |. Population
Institutional review board approval was obtained with a waiver of informed consent to retrospectively review the charts of 50 consecutive patients seen between April 2019 and April 2020 in the senior author’s clinic for routine surveillance after radiotherapy for sinonasal or nasopharyngeal malignancies. Demographic, disease, treatment data, and endocrine laboratory values were collected.
Patients were included in the study if they had (i) a pathological diagnosis of a sinonasal or nasopharyngeal cancer; (ii) undergone treatment with definitive or adjuvant radiotherapy during adulthood (age ≥18 years); and (iii) no existing hormone replacement therapy before endocrinology evaluation.
2.2 |. Endocrine assessments
Blood samples for pituitary hormone laboratory tests were drawn at patients’ routine follow-up visits per NCCN guidelines.6 These included tests for insulin-like growth factor 1 (IGF-1), adrenocorticotropic hormone (ACTH), total cortisol, free thyroxine (FT4), total triiodothyronine (T3), thyroid-stimulating hormone (TSH), follicle-stimulating hormone (FSH), luteinizing hormone (LH), total testosterone for men and estradiol for women.
Patients with abnormal hormonal levels were evaluated by or had their cases discussed with an endocrinologist. Therapeutic interventions were determined by the treating endocrinologist based on patient symptom burden and severity of hormonal abnormality. Primary (nonpituitary) hormonal dysfunction was defined as failure in an endocrine gland itself (thyroid, gonads, adrenals), while secondary or central (pituitary) hormonal dysfunction was defined as failure of normal HP axis function resulted in the lack of stimulation of the target gland. For women older than 45 years with an elevated FSH, if we were unable to attribute the cause of gonadal failure to chemotherapy or menopause, we defaulted to menopause unrelated to treatment.
For the thyroid and male gonadal axes, participants with low or normal pituitary hormone levels (TSH, FSH, and LH) and primary hormone levels (FT4 and testosterone) below the reference range were categorized as having secondary or central hypothyroidism and secondary or central hypogonadism, respectively. Patients with elevated pituitary hormone levels (TSH, FSH, or LH) combined with low peripheral hormone levels (FT4 or testosterone) were categorized as having primary hormonal dysfunction. For the thyroid axis, primary thyroid dysfunction included overt (low FT4 and elevated TSH) and subclinical (normal FT4 and elevated TSH) hypothyroidism. Secondary (pituitary/hypothalamic) thyroid dysfunction was defined as low FT4 and normal or low TSH.
2.3 |. Dosimetric parameter assessments
Of the 50 patients, 9 received irradiation at other institutions, and their treatment plans were not available for evaluation. The radiotherapy records of the remaining 41 patients were imported into one treatment planning system (RayStation version 9A; RaySearch Laboratories) to allow for standardized calculation of dosimetric parameters. Comprehensive dosimetric evaluation, including volume, mean dose, maximum dose (Dmax) to 0.01 mL, Dmax to 0.1 mL, and Dmax to 0.5 mL to the pituitary gland, pituitary stalk, optic chiasm, and hypothalamus, was performed. A template was created to automatically tabulate the dosimetric parameters (volume, mean dose, Dmax to 0.01 mL, Dmax to 0.1 mL, and Dmax to 0.5 mL) for the various organs at risk (hypothalamus, optic chiasm, pituitary, and stalk). For patients with multiple treatment courses, including re-irradiation, a composite plan was generated by the following steps: rigid image registration, deformable image registration, dose deformation of multiple CT image sets to the primary CT image set, dose summation on the primary CT image set, and integrity check by verifying the summation of a structure’s mean dose.
2.4 |. Statistical analysis
Descriptive statistics for values and frequencies of study patients within the categories for each of the dosimetric parameters were determined. Comparisons of dosimetric parameters obtained from patients with or without abnormal pituitary hormone levels were done via the Student t test. Univariable logistic regression was completed to evaluate for factors associated with pituitary dysfunction. Patients with missing data were excluded from individual analyses. P values of <0.05 were considered statistically significant. Statistical analyses were performed using the Statistica 13 (TIBCO Software Inc) statistical software application.
3 |. RESULTS
A total of 50 consecutive patients who received radiation for sinonasal or nasopharyngeal cancer were included in this study. Patient and tumor characteristics are described in Table 1. All patients had comprehensive pituitary hormone testing, and 41 patients had a dosimetric evaluation. Pituitary hormone assessment was performed at a median of 20 months (range 3–145 months) after the completion of radiotherapy. All but 6 (12%) patients underwent pituitary hormone testing within 5 years of completing radiotherapy. Definitive radiotherapy included 38 (76%) patients who received intensity-modulated radiotherapy (IMRT) and 12 (24%) patients who received intensity-modulated proton therapy (IMPT). The median target prescription dose was 64 Gy (range 45–70 Gy), and the median dose per fraction was 2.06 Gy. Concurrent chemoradiotherapy (CCRT) with cisplatin or carboplatin was given to 35 (70%) patients; the remainder received radiotherapy alone. Recurrent tumors were treated with re-irradiation to the skull base in 5 (10%) patients. There was no statistically significant difference in the proportion of patients with pituitary hormonal abnormalities between those treated with IMRT versus IMPT, CCRT versus radiotherapy only, or primary radiotherapy versus re-irradiation (data not shown).
TABLE 1.
Patient demographic and clinical characteristics.
| Characteristic | No. (%) |
|---|---|
| Gender | |
| Male | 31 (62) |
| Female | 19 (38) |
| Age at radiotherapy, mean (range), years | 54 (31–81) |
| Site of tumor | |
| Nasopharyngeal carcinoma | 20 (40) |
| Sinonasal carcinoma | 30 (60) |
| Histology | |
| Nasopharyngeal carcinoma | 18 (36) |
| Squamous cell carcinoma | 7 (14) |
| Adenoid cystic carcinoma | 6 (12) |
| Olfactory neuroblastoma | 6 (12) |
| Sinonasal undifferentiated carcinoma | 5 (10) |
| Sarcoma | 3 (6) |
| Neuroendocrine carcinoma | 2 (4) |
| Melanoma | 2 (4) |
| Adenocarcinoma | 1 (2) |
A total of 30 (60%) patients had any abnormal hormone level on laboratory testing. Of these, 23 (46%) patients had abnormal pituitary hormone levels possibly attributed to radiotherapy, including 6 (12%) patients who required treatment (Table 2). The most common hormonal abnormality was hyperprolactenemia (30%), followed by central hypothyroidism (8%), central hypogonadism (6%), and central adrenal insufficiency (2%). Abnormalities affecting more than 1 axis was seen in 13 (26%) patients. In addition, 7 (14%) patients had isolated primary endocrine abnormalities (2 with primary hypogonadism, 3 with primary hypothyroidism, and 2 with both). In patients with abnormal pituitary function, the median time to diagnosis of pituitary dysfunction was 14 months after the completion of radiotherapy.
TABLE 2.
Hormonal abnormalities after anterior skull base radiation.
| Type | No. (%) |
|---|---|
| Overall abnormal pituitary hormone levels | 23 (46) |
| Hyperprolactinemia | 15 (30) |
| Central hypothyroidism | 4 (8) |
| Central hypogonadism | 3 (6) |
| Central adrenal insufficiency | 1 (2) |
| Abnormal pituitary hormone levels requiring treatment | 6 (12) |
| Central hypothyroidism, hyperprolactinemia | 1 (2) |
| Central hypothyroidism, central hypogonadism | 1 (2) |
| Central hypothyroidism, central hypogonadism, hyperprolactinemia | 2 (4) |
| Central hypothyroidism | 1 (2) |
| Hyperprolactinemia | 1 (2) |
| Primary hormonal abnormality (isolated vs. concurrent) | 16 (32) |
| Isolated primary hormone abnormality | 7 (14) |
| Concurrent with pituitary hormone abnormality | 8 (16) |
| Type of hormonal abnormality | |
| Primary hypothyroidism | 9 (18) |
| Primary hypogonadism | 6 (12) |
| Number of axes involved | |
| One axis | 17 (34) |
| Two axis | 11 (22) |
| Three axis | 2 (4) |
Regression analysis was performed to evaluate for factors associated with pituitary dysfunction attributed to skull base radiation (Table 3). No variables were found to be significantly associated with hormone dysfunction. Patients with radiation doses <60 Gy (odds ratio [OR] 0.32, 95% confidence interval [CI] 0.10–1.04, P = 0.081) and no regional metastases (OR 0.36, 95% CI 0.08–1.55, P = 0.19) tended to be less likely to have pituitary dysfunction.
TABLE 3.
Regression for pituitary dysfunction.
| Variable | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Age <50 vs. 50–69 vs. 70+ | 0.69 | ||
| Gender (men vs. women) | 0.78 | 0.24–2.48 | 0.77 |
| Race (white vs. others) | 1.95 | 0.58–6.51 | 0.37 |
| Histology | 0.66 | ||
| Subsite (nasopharynx vs. others) | 1.54 | 0.49–4.84 | 0.56 |
| T category 3–4 vs. 1–2 | 1.23 | 0.39–4.17 | 0.77 |
| N0 vs. N+ | 0.35 | 0.10–1.27 | 0.13 |
| Primary vs. re-irradiation | 0.38 | 0.06–2.30 | 0.40 |
| Chemoradiation | 1.95 | 0.58–6.51 | 0.37 |
| Radiation dose <60 vs. 60+ | 0.36 | 0.08–1.55 | 0.19 |
Figures 1 and 2 depict radiotherapy doses and hormonal levels for the 41 patients with dosimetry data. The mean dose to the pituitary, pituitary stalk, optic chiasm, and hypothalamus with normal pituitary function was 4094, 2878, 2004, and 1133 cGy, respectively. In patients with abnormal pituitary hormone levels, the mean dose to the pituitary, pituitary stalk, optic chiasm, and hypothalamus in patients was 5237, 4007, 3098, and 2033 cGy, respectively. Compared with patients with normal pituitary hormone levels, patients with abnormal pituitary hormone levels received higher mean radiation doses to the pituitary gland (1143 cGy, P = 0.04), pituitary stalk (1129 cGy, P = 0.02), optic chiasm (1094 cGy, P = 0.01), and hypothalamus (900 cGy, P = 0.02). There was a non-significant trend toward higher radiotherapy dose between patients with pituitary dysfunction requiring treatment, pituitary dysfunction not requiring treatment, and normal hormone levels.
FIGURE 1.
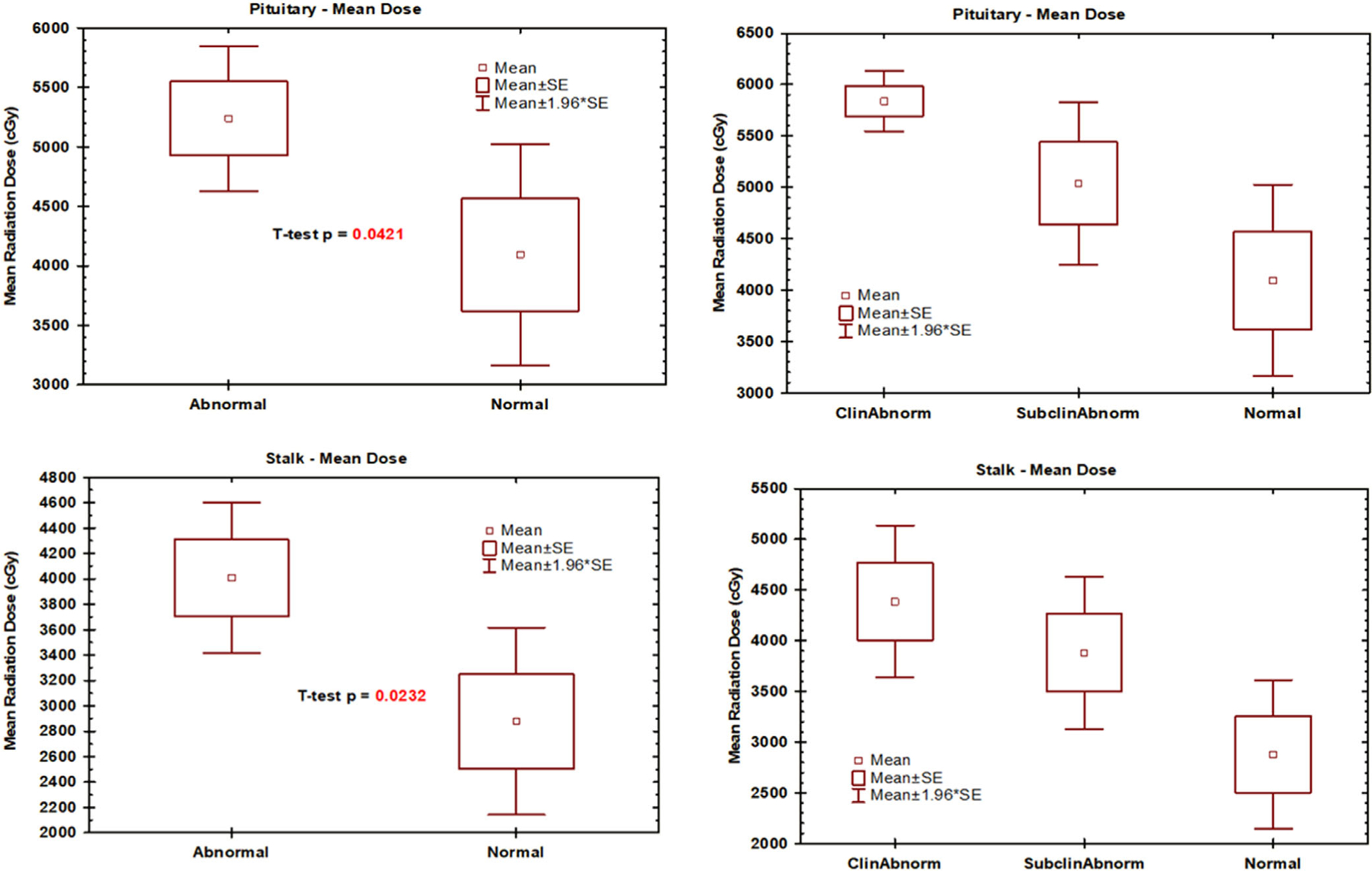
Mean pituitary gland and pituitary stalk radiotherapy dose and hormonal levels (N = 41): Abnormal is all pituitary dysfunction; Normal is no pituitary dysfunction; ClinAbnorm pituitary dysfunction requiring treatment; SubclinAbnorm is pituitary dysfunction not requiring treatment.
FIGURE 2.
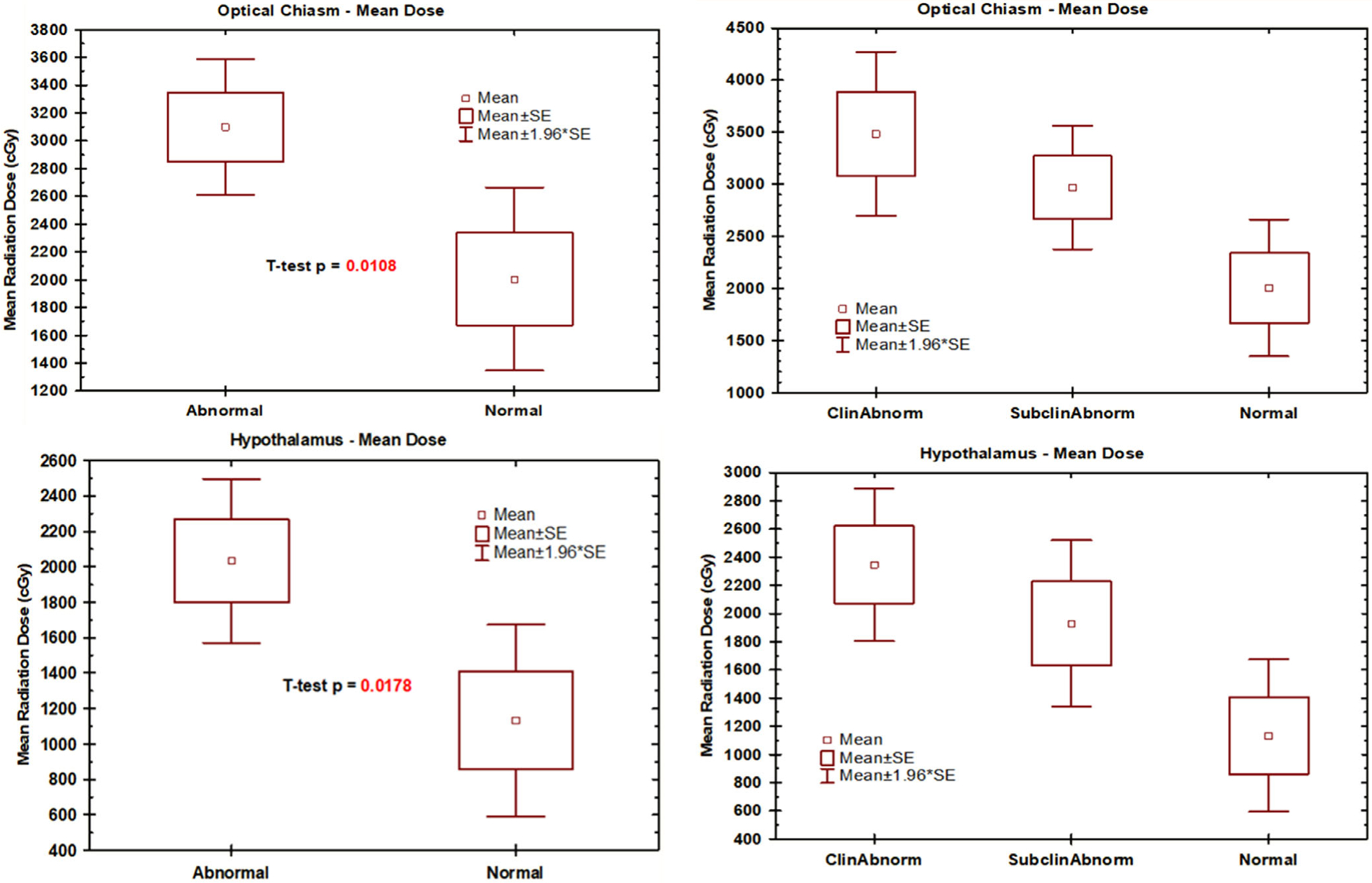
Mean optic chiasm and hypothalamus radiotherapy dose and hormonal levels (N = 41): Abnormal is all pituitary dysfunction; Normal is no pituitary defunction; ClinAbnorm pituitary dysfunction requiring treatment; SubclinAbnorm is pituitary dysfunction not requiring treatment.
4 |. DISCUSSION
This study provides the first estimation of the prevalence of pituitary hormone dysfunction as a correlative of radiotherapy dose in adults who underwent radiotherapy for anterior skull base tumors. Nearly half the patients had pituitary dysfunction, with just over a tenth requiring treatment. Despite previous research highlighting this problem, there is no accepted screening protocol or national guidelines as there are for primary hypothyroidism after neck radiation.6 These data can inform evidence-based screening protocols and provide guidance on dose modification to limit the risk of untreated pituitary dysfunction after sinonasal radiation.
4.1 |. Prevalence
We report a prevalence of 46% pituitary hormone dysfunction, with 12% of patients requiring treatment. One study to date has assessed the incidence of pituitary hormone dysfunction after modern anterior skull base radiation.5 VanKoevering et al. evaluated pituitary serologies in patients aged 15–79 years who underwent external beam radiation for anterior skull base malignancies. The cohort of 81 patients was derived from a sample of 508 patients with the exclusion of patients without hormone testing, among other factors, making their calculation of incidence prone to selection bias. Although they did not specify the percentage of patients with pituitary dysfunction requiring treatment, they reported an incidence of pituitary dysfunction of 37%, slightly less than what we found.
Other studies of radiation-related pituitary dysfunction have been limited to alternative populations. One series with adult patients with radiated brain tumors found hypopituitarism in 41% of individuals.3 The frequency of growth hormone, gonadotropin, ACTH, and TSH deficiencies were 32%, 27%, 21%, and 9%, respectively. In a meta-analysis of studies evaluating nonpituitary cranial radiation, the prevalence of hypopituitarism ranged from 25% to 100% for head and neck cancer patients and 37%–77% for intracerebral tumor patients.2 The point prevalence of any degree of hypopituitarism for all patients was 66%. Other studies included radiation techniques dating well over a decade prior and do not represent modern radiation treatment fields.4,9–12 More recent studies are limited to patients treated for nasopharyngeal cancers.13–16 Similar reports have also been completed in pediatric populations.17,18
4.2 |. Pituitary hormone dysfunction
Elevated prolactin levels were seen in 30% of the patients in our study. In contrast, the posterior pituitary is known to be resistant to radiotherapy, and none of our patients had symptoms suggestive of posterior pituitary dysfunction, such as diabetes insipidus. Radiation has a unique effect on the HP-prolactin axis compared with other hormones. Radiation-induced hyperprolactinemia occurs because the hypothalamic neuroinhibitory neurotransmitter dopamine becomes attenuated or depleted.2,14,19 Although prolactin levels initially increase with time, they may gradually normalize or decrease after loss of lactotrophs. Because of our study’s cross-sectional nature, the prevalence of prolactin dysfunction could vary. The prevalence could be greater if patients with normoprolactinemia previously hypoprolactinemia, implying that we may be overlooking the. Alternatively, the prevalence could be less if patients had non-radiation induced hyperprolactinemia (e.g., prolactinoma, medications, and health-related).
Due to the high radiation exposure and field that surrounds the base of the brain, the HP axis is at a far greater risk of injury with radiotherapy to the anterior skull base than with other head and neck tumors. Although prior studies have associated growth hormone deficiency with cranial radiation, no patients in this study had growth hormone deficiency.3,20–22 Growth hormone–deficient adults can experience subtle changes in their quality of life such as loss of muscle mass, increased visceral fat, insulin resistance, decreased bone mineral density, and elevated risk of cardiovascular disease.23
Previous studies evaluating cranial radiation have suggested that the somatotroph and gonadotroph axes are much more radiosensitive than the thyrotroph and corticotroph axes.20,24–26 In contrast, we found thyrotroph deficiency (8%) to be more common than gonadotroph deficiency (6%). While our study found that patients who undergo anterior skull base radiation can have central hypothyroidism, prior studies have also found that nasopharyngeal radiation can have a component of mixed or primary hypothyroidism due to direct radiation damage to the thyroid gland.27,28 This complexity highlights the limitations of current NCCN guidelines, which recommend TSH testing alone every 6–12 months for patients who have received cervical radiation.6 The incidence of primary hypothyroidism after radiotherapy for head and neck cancer is estimated to be 15%–33% in the first 1–2 years after treatment.29,30 Our study suggests that the addition of thyroxine measurement is vital for recognizing central hypothyroidism, which occurs in nearly a tenth of patients who undergo skull base radiation. In these patients, TSH alone will not be reliable as primary hypothyroidism can be disguised by simultaneous central hypothyroidism. We and others would therefore advocate for an update to the NCCN guidelines with the addition of thyroxine testing in at-risk patients.5
4.3 |. Radiation dose and timing
This study is unique in evaluating pituitary hormone abnormalities together with radiation doses in patients treated for anterior skull base malignancies. Our analysis found a strong association between HP dysfunction and the amount of radiation to the pituitary gland, stalk, optic chiasm, and hypothalamus. Patients in this study tended to be less likely to have pituitary dysfunction with less than 60 Gy of radiation. Other studies have demonstrated a similar relationship for patients who received direct cranial radiation.2,3 The earliest signs of dysfunction can appear within the first year of treatment but have been identified as late as 11 years after radiotherapy.2,31 Ratnasingam et al. reported that hypopituitarism doubled every 2 years after radiotherapy and suggested screening twice a year for patients at high risk.14
4.4 |. Limitations
This study does not offer a temporal assessment of the effects of radiotherapy to the skull base due to heterogeneity in patient treatment and timing. Pituitary dysfunction tended to be associated with radiation dose, but the type of radiation varied between patients, including definitive versus adjuvant, IMRT versus IMPT, CCRT versus radiotherapy only, or primary radiation versus re-irradiation. The accuracy of the dosimetric values obtained has intrinsic shortcomings because they depend on the accuracy of the treatment planning system’s dose and volume calculations, and the planned dose may not be the exact delivered dose. Dynamic and provocative tests were not used to detect somatotroph and corticotroph dysfunction in all our patients. We anticipate that the prevalence of overt pituitary dysfunction will be greater with longer follow-up. In addition, we did not conduct pre-radiotherapy pituitary laboratory testing and other causes of hormonal dysfunction cannot be ruled out. Further cohort and prospective studies are required to determine whether there is a radiation dose threshold for hormonal dysfunction and the chronology of pituitary dysfunction after radiotherapy for skull base tumors. It is currently unknown whether dose de-escalation reduces the prevalence of pituitary hormone deficiency.
5 |. CONCLUSIONS
Nearly half of patients who underwent radiotherapy for sinonasal and nasopharyngeal cancer experienced hormonal abnormalities, including pituitary–hypothalamus and primary organ dysfunction. Over a tenth of patients required treatment. There may be a direct dose-dependent relationship between the amount of radiation and pituitary gland, pituitary stalk, optic chiasm, and hypothalamus dysfunction. These results suggest the need for standardized screening protocols for pituitary dysfunction in patients receiving high-dose radiotherapy to the anterior skull base.
ACKNOWLEDGMENTS
Editorial support was provided by Bryan Tutt, Scientific Editor, Research Medical Library.
FUNDING INFORMATION
David Fuller received/receives funding and salary support germane to this project during the period of study execution from: the National Institutes of Health (NIH) National Cancer Institute (NCI) Head and Neck Program and Radiation Oncology and Cancer Imaging Program of the MD Anderson Cancer Center Support Grant (P30CA016672).
Funding information
National Institutes of Health (NIH) National Cancer Institute (NCI) Institutional Training Program, Grant/Award Number: T32CA261856; The Head and Neck Program and Radiation Oncology and Cancer Imaging Program of the MD Anderson Cancer Center Support Grant, Grant/Award Number: P30CA016672
CONFLICT OF INTEREST STATEMENT
David Fuller has received direct industry grant support, honoraria, and travel funding from Elekta AB unrelated to this project. The other authors made no disclosures.
Footnotes
ETHICS STATEMENT
Institutional review board approval was obtained with a waiver of informed consent.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.Ajmal A, McKean E, Sullivan S, Barkan A. Decreased quality of life (QoL) in hypopituitary patients: involvement of glucocorticoid replacement and radiation therapy. Pituitary. 2018; 21(6):624–630. [DOI] [PubMed] [Google Scholar]
- 2.Appelman-Dijkstra NM, Kokshoorn NE, Dekkers OM, et al. Pituitary dysfunction in adult patients after cranial radiotherapy: systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(8):2330–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agha A, Sherlock M, Brennan S, et al. Hypothalamic-pituitary dysfunction after irradiation of nonpituitary brain tumors in adults. J Clin Endocrinol Metab. 2005;90(12):6355–6360. [DOI] [PubMed] [Google Scholar]
- 4.Lam KS, Tse VK, Wang C, Yeung RT, Ho JH. Effects of cranial irradiation on hypothalamic-pituitary function – a 5-year longitudinal study in patients with nasopharyngeal carcinoma. Q J Med. 1991;78(286):165–176. [PubMed] [Google Scholar]
- 5.VanKoevering KK, Sabetsarvestani K, Sullivan SE, Barkan A, Mierzwa M, McKean EL. Pituitary dysfunction after radiation for anterior Skull Base malignancies: incidence and screening. J Neurol Surg B Skull Base. 2020;81(1):75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfister DG, Spencer S, Adelstein D, et al. Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(7):873–898. [DOI] [PubMed] [Google Scholar]
- 7.van Iersel L, Mulder RL, Denzer C, et al. Hypothalamic-pituitary and other endocrine surveillance among childhood cancer survivors. Endocr Rev. 2022;43(5):794–823. [DOI] [PubMed] [Google Scholar]
- 8.Hudson MM, Bhatia S, Casillas J, Landier W, Section On Hematology/Oncology CSOGASOFPHO. Long-term follow-up care for childhood, adolescent, and young adult cancer survivors. Pediatrics. 2021;148(3):e2021053127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhandare N, Kennedy L, Malyapa RS, Morris CG, Mendenhall WM. Hypopituitarism after radiotherapy for extracranial head and neck cancers. Head Neck. 2008;30(9): 1182–1192. [DOI] [PubMed] [Google Scholar]
- 10.Snyers A, Janssens GO, Twickler MB, et al. Malignant tumors of the nasal cavity and paranasal sinuses: long-term outcome and morbidity with emphasis on hypothalamic-pituitary deficiency. Int J Radiat Oncol Biol Phys. 2009;73(5):1343–1351. [DOI] [PubMed] [Google Scholar]
- 11.Woo E, Lam K, Yu YL, Ma J, Wang C, Yeung RT. Temporal lobe and hypothalamic-pituitary dysfunctions after radiotherapy for nasopharyngeal carcinoma: a distinct clinical syndrome. J Neurol Neurosurg Psychiatry. 1988;51(10):1302–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samaan NA, Schultz PN, Yang KP, et al. Endocrine complications after radiotherapy for tumors of the head and neck. J Lab Clin Med. 1987;109(3):364–372. [PubMed] [Google Scholar]
- 13.Zhai R, Lyu Y, Ni M, et al. Predictors of radiation-induced hypothyroidism in nasopharyngeal carcinoma survivors after intensity-modulated radiotherapy. Radiat Oncol. 2022;17(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratnasingam J, Karim N, Paramasivam SS, et al. Hypothalamic pituitary dysfunction amongst nasopharyngeal cancer survivors. Pituitary. 2015;18(4):448–455. [DOI] [PubMed] [Google Scholar]
- 15.Lin Z, Wang X, Xie W, Yang Z, Che K, Wu VW. Evaluation of clinical hypothyroidism risk due to irradiation of thyroid and pituitary glands in radiotherapy of nasopharyngeal cancer patients. J Med Imaging Radiat Oncol. 2013;57(6):713–718. [DOI] [PubMed] [Google Scholar]
- 16.Ipekci SH, Cakir M, Kiyici A, Koc O, Artac M. Radiotherapy-induced hypopituitarism in nasopharyngeal carcinoma: the tip of an iceberg. Exp Clin Endocrinol Diabetes. 2015;123(7): 411–418. [DOI] [PubMed] [Google Scholar]
- 17.Bhandare N, Kennedy L, Malyapa RS, Morris CG, Mendenhall WM. Hypopituitarism after radiotherapy for extracranial head and neck cancers in pediatric patients. Am J Clin Oncol. 2008;31(6):567–572. [DOI] [PubMed] [Google Scholar]
- 18.Xie C, Li J, Weng Z, et al. Decreased pituitary height and stunted linear growth after radiotherapy in survivors of childhood nasopharyngeal carcinoma cases. Front Endocrinol. 2018; 9:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samaan NA, Bakdash MM, Caderao JB, Cangir A, Jesse RH Jr, Ballantyne AJ. Hypopituitarism after external irradiation. Evidence for both hypothalamic and pituitary origin. Ann Intern Med. 1975;83(6):771–777. [DOI] [PubMed] [Google Scholar]
- 20.Darzy KH, Shalet SM. Hypopituitarism after cranial irradiation. J Endocrinol Invest. 2005;28(5 Suppl):78–87. [PubMed] [Google Scholar]
- 21.Brennan BM, Rahim A, Mackie EM, Eden OB, Shalet SM. Growth hormone status in adults treated for acute lymphoblastic leukaemia in childhood. Clin Endocrinol (Oxf). 1998;48(6): 777–783. [DOI] [PubMed] [Google Scholar]
- 22.Toogood AA. Endocrine consequences of brain irradiation. Growth Horm IGF Res. 2004;14:S118–S124. [DOI] [PubMed] [Google Scholar]
- 23.Bates AS, Van’t Hoff W, Jones PJ, Clayton RN. The effect of hypopituitarism on life expectancy. J Clin Endocrinol Metab. 1996;81(3):1169–1172. [DOI] [PubMed] [Google Scholar]
- 24.Darzy KH, Shalet SM. Hypopituitarism as a consequence of brain tumours and radiotherapy. Pituitary. 2005;8(3–4):203–211. [DOI] [PubMed] [Google Scholar]
- 25.Littley MD, Shalet SM, Beardwell CG, Ahmed SR, Applegate G, Sutton ML. Hypopituitarism following external radiotherapy for pituitary tumours in adults. Q J Med. 1989; 70(262):145–160. [PubMed] [Google Scholar]
- 26.Taphoorn MJ, Heimans JJ, van der Veen EA, Karim AB. Endocrine functions in long-term survivors of low-grade supratentorial glioma treated with radiation therapy. J Neurooncol. 1995;25(2):97–102. [DOI] [PubMed] [Google Scholar]
- 27.Rosenthal MB, Goldfine ID. Primary and secondary hypothyroidism in nasopharyngeal carcinoma. Jama. 1976;236(14): 1591–1593. [PubMed] [Google Scholar]
- 28.Lin Z, Wu VW, Lin J, Feng H, Chen L. A longitudinal study on the radiation-induced thyroid gland changes after external beam radiotherapy of nasopharyngeal carcinoma. Thyroid. 2011;21(1):19–23. [DOI] [PubMed] [Google Scholar]
- 29.Boomsma MJ, Bijl HP, Christianen ME, et al. A prospective cohort study on radiation-induced hypothyroidism: development of an NTCP model. Int J Radiat Oncol Biol Phys. 2012; 84(3):e351–e356. [DOI] [PubMed] [Google Scholar]
- 30.Sinard RJ, Tobin EJ, Mazzaferri EL, et al. Hypothyroidism after treatment for nonthyroid head and neck cancer. Arch Otolaryngol Head Neck Surg. 2000;126(5):652–657. [DOI] [PubMed] [Google Scholar]
- 31.Taku N, Gurnell M, Burnet N, Jena R. Time dependence of radiation-induced hypothalamic-pituitary axis dysfunction in adults treated for non-pituitary, intracranial neoplasms. Clin Oncol (R Coll Radiol). 2017;29(1):34–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


